
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Malar., 28 March 2025
Sec. Case Management
Volume 3 - 2025 | https://doi.org/10.3389/fmala.2025.1544378
This article is part of the Research TopicGlobal Perspectives on Severe Vivax Malaria: Incidence, Outcomes, and Biological MechanismsView all articles
 Md Fahad Zamil1
Md Fahad Zamil1 Ching Swe Phru1
Ching Swe Phru1 Anamul Hasan1
Anamul Hasan1 Afrida Tabassum Trina1
Afrida Tabassum Trina1 Mohammad Shahbaz2
Mohammad Shahbaz2 Shahrear Tanvir Ahmed3
Shahrear Tanvir Ahmed3 Mohammad Sharif Hossain1
Mohammad Sharif Hossain1 Mohammad Shafiul Alam1*
Mohammad Shafiul Alam1*We report a case of Primaquine (PQ) induced hemoglobinuria in a patient with the glucose-6-phosphate dehydrogenase (G6PD) Mahidol variant from Bandarban, Bangladesh. The patient presented with mixed Plasmodium falciparum and Plasmodium vivax malaria and was recommended to be treated according to national guidelines with Artemether-Lumefantrine for three days and PQ for 14 days. Ten days later, the patient developed a fever and jaundice, followed by hemoglobinuria twelve days after the initial diagnosis. This highlighted the need for G6PD testing, which was subsequently confirmed by both Point-of-Care (POC) testing and spectrophotometry. The POC test showed a G6PD activity of 2.6 IU/g Hb, while spectrophotometry measured 1.47 IU/g Hb, both indicating G6PD deficiency (<30% activity). As a result, PQ was discontinued, and the patient received four units of blood transfusion. Additionally, genotyping was carried out, confirming the Mahidol variant. This case highlights the importance of routine G6PD screening before PQ administration, especially in malaria-endemic regions with different G6PD variants.
Malaria is a potentially fatal disease caused by the Plasmodium parasite. The World Malaria Report 2024 documented approximately 263 million reported cases along with an estimated 597,000 fatalities in 2023, marking an increase of approximately 11 million cases from the previous year (World Health Organization, 2024). Recently, the most geographically prevalent malaria parasite is Plasmodium vivax (P. vivax), accounting for approximately 45% of malaria cases in the WHO South-East Asia Region leading to a significant global increase in associated morbidity and mortality (Anwar et al., 2024). However, Plasmodium falciparum (P. falciparum) continues to be the main contributor to the majority of fatalities (World Health Organization, 2024). The coexistence of these two species in varying proportions and geographical areas is also occasionally observed (Kumar et al., 2024).
Although Bangladesh has reduced malaria by 93% between 2008 and 2020, approximately 19 million people remain at risk of infection. Three districts in Chittagong Hill Tracts (CHT) remain high-transmission areas: Bandarban, Rangamati, and Khagrachari, a region where 90% of malaria cases occur. Bandarban accounts for more than 50% of these cases. Three sub-districts of Bandarban - Thanchi, Alikadam, and Rowangachari - typically have the highest annual parasitological index (API), although the overall malaria burden is decreasing. P. falciparum is predominant in Bangladesh, accounting for 73% of cases, with P. vivax accounting for the remainder. A few documented cases of P. malariae and P. ovale have also been reported (National Malaria Control Programme, 2021; Haldar et al., 2023).
Currently, the only medication that has gametocytocidal and hypnozoitocidal activity against P. falciparum and P. vivax is an 8-aminoquinoline compound (Primaquine- PQ). Although PQ is generally well tolerated by recipients, in individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency, it can lead to severe hemolysis (Phru et al., 2020). The degree of hemolysis depends on the level of G6PD deficiency and the dose and duration of exposure to PQ (Beutler, 1994). The level of induced hemolysis associated with G6PD deficiency is influenced by the specific genetic variant, with over 230 clinically relevant variants reported to date, although not all of them are polymorphic and of clinical relevance. The clinical presentation of G6PD variants varies from no clinical manifestations to very mild with essentially no symptoms to severe acute (Howes et al., 2013; Geck et al., 2023)hemolytic anemia (Yi et al., 2019). The majority of variants are asymptomatic but can cause neonatal jaundice and acute hemolytic anemia when exposed to primaquine or tafenoquine, which are class B of the WHO G6PD variants. WHO recommends G6PD testing before administering these antimalarial drugs for certain G6PD variants (Luzzatto et al., 2024). G6PD variants are widespread worldwide. In Southeast Asia, numerous G6PD variants have been identified in different populations, with G6PD Viangchan and G6PD Mahidol being the most common (Phompradit et al., 2011a). Thus far, the Orissa, Mediterranean, Kerala-Kalyan, and Mahidol G6PD variants have been identified in Bangladesh (Phru et al., 2020).
G6PD deficiency, a genetic disorder that affects approximately 500 million people globally (Li et al., 2024), is particularly common in regions where malaria is endemic (Dechyotin et al., 2021). G6PD is a crucial enzyme in the pentose phosphate pathway that produces NADPH, which is in turn required for reactive oxygen species homeostasis and cellular biosynthesis are crucial for red blood cells (Shah et al., 2024). G6PD is an essential enzyme in the pentose phosphate pathway, producing NADPH. NADPH is necessary for maintaining reactive oxygen species homeostasis and regulating cellular biosynthesis, both of which are crucial for red blood cell function. In the majority of cases, G6PD deficiency is asymptomatic, but under certain circumstances such as exposure to fava beans, infections, or medications, it can also present as mild-to-severe hemolytic anemia (Gupta, 2024).
A 16-year-old man weighing 50 kg from Rowangchhari Upazila, Bandarban presented with a fever on 27 October 2023 and was diagnosed with a mixed malaria infection by P. falciparum and P. vivax by an NGO health worker using a rapid diagnostic test (RDT). The patient was started on antimalarial treatment with Artemether-Lumefantrine (AL) 20/120 mg, four tablets twice daily for three days (total of 24 tablets), and PQ 15 mg, one tablet daily for 14 days, according to National Guidelines (Directorate General of Health Services (DGHS), 2019). Despite 10 days of treatment, the patient’s fever persisted. He presented to the Rowangchhari Upazila Health Complex on 5 November 2023 with a temperature of 102°F and a blood pressure of 90/60 mmHg. A repeat RDT was negative for malaria. Given the unresolved fever and at the patient’s request, he was referred to Bandarban Sadar Hospital, the district referral hospital. Before referral, the patient received oral Paracetamol 500 mg and a single intramuscular dose of Ceftriaxone 1 gram.
On admission to Bandarban Sadar Hospital the same day, the patient’s vital signs were stable, with a body temperature of 99°F and blood pressure of 120/80 mmHg. However, the attending physician noted clinical jaundice. Initial management included intravenous dextrose 5% DNS (1 liter) and oral paracetamol for fever, intravenous ondansetron 8 mg for nausea, and intravenous Ceftriaxone 1 gram every 12 hours. PQ 15 mg was continued for 4 days according to National Guidelines.
Routine diagnostic tests were ordered on admission and the following day. Despite two additional negative malaria RDTs, the patient’s fever persisted at a moderate level (100-102°F) with fluctuating blood pressure (100/60 to 120/70 mmHg). The decision was made to complete the full course of PQ in accordance with the guidelines while continuing supportive care and the Ceftriaxone antibiotic.
On 7 November 2023, a urine examination revealed hemoglobinuria. The recorded temperature was 100°F and the BP level was 100/50 mmHg. The patient’s temperature was 100°F, and blood pressure dropped to 100/50 mmHg. A two-unit blood transfusion was administered. Given the suspicion of PQ-induced hemolysis, all quinine derivatives were withheld pending further evaluation. Baseline investigations and fever tests were unremarkable but unfortunately, the data were not documented in the patient’s medical file. A request for G6PD level testing was urgently sent to the local icddr,b (International Centre for Diarrhoeal Disease Research, Bangladesh) field office in Bandarban.
The patient’s legal guardian provided written, informed consent for retrieving his medical records and G6PD activity and genotyping testing. A total of 3 mL of venous blood was collected in a BD Vacutainer® EDTA Tubes (Becton, Dickinson and Company, Franklin Lakes, NJ) and transported to the Emerging Infections and Parasitology Laboratory of the icddr,b in Dhaka. Initially, the hematological history was evaluated before G6PD testing. The patient’s hematological parameters showed significant anomalies, which included severe anemia, as indicated by low hemoglobin (5.6 g/dL), hematocrit (19.2%), and RBC count (2.45 *10¹²/L), along with reduced MCV, MCH, and MCHC (Khan et al., 2013). To confirm the root cause of the hemolytic event, G6PD activity was measured using both a gold standard spectrophotometry assay (kits from Pointe Scientific, Canton, MI on a Shimadzu UV-1800 Spectrophotometer, Shimadzu Scientific Instruments, Kyoto, Japan) (Alam et al., 2018) and a point-of-care (POC) test (STANDARD G6PD Test, SD BIOSENSOR, ROK). The POC test showed G6PD activity levels of 2.6 IU/g Hb, which is considered deficient according to the IFU (Instructions for Use). Using the Adjusted Male Median (AMM) the G6PD level for spectrophotometry was defined as 100% G6PD activity, whereas G6PD deficiency was classified as <30% activity. A study conducted on 1,002 participants from the ethnic groups of CHT, Bangladesh previously set the AMM at 7.03 U/g Hb (Ley et al., 2020). The patient’s spectrophotometry result showed a G6PD value of 1.47 IU/g Hb, indicating <30% activity and defined as G6PD deficiency. A single unit of fresh blood was transfused on the same day following a rapid point-of-care G6PD testing and CBC testing. Vital signs were monitored closely. On 9 November, after confirmation of G6PD deficiency using reference laboratory data, a second blood transfusion was administered. The patient’s condition improved significantly during this period, with resolution of jaundice and stable vital signs (pulse 76 bpm, blood pressure 110/80 mmHg). There were no signs of recurrent hemoglobinuria post-transfusion. The patient remained hospitalized for an additional three days for observation before being discharged from Bandarban Sadar Hospital on 13 November 2023. A summarized timeline of the case history and treatment process described in Figure 1.
Genomic DNA was extracted from a whole blood sample using the QIAamp® DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. To exclude malaria as a potential cause of hemolysis, a real-time qPCR assay targeting five human malaria species was performed as previously described (Sazed et al., 2021). The results were negative confirming the absence of malaria parasites at the time of hemolysis.
To confirm the host G6PD variants we targeted known variants circulating in this region (Mahidol, Mediterranean, Orissa and Kalyan-Kerala) by PCR RFLP (Restriction fragment length polymorphism) using Primer and restriction enzyme sets for the corresponding variants reported elsewhere. The thermal cycling profile was followed as described in the reference literature (Ley et al., 2020). PCR was performed in a 20 μL reaction volume containing 10µL DreamTaq™ PCR Master Mix (2X) (Thermo Fisher Scientific, Massachusetts, United States), 1µL forward and reverse primer (10µM), 3 µL DNA and the rest of the volume filled with nuclease-free water. After thermal cycling, we performed enzyme digestion for the Mahidol, Mediterranean, and Orissa variants. Sequencing was required for the Kalyan-Kerala variant. A total of 5 µL of the PCR product was digested by specific restriction enzymes (Except for Kalyan-Kerala) in a 50 µL reaction by the manufacturer’s instruction (New England Biolabs, Massachusetts, United States). The DNA bands were then separated on a 3% agarose gel and visualized by the FastGene® FAS-V Imaging System. The sample was identified as a Mahidol variant. Mahidol (487 G > A) digestion should have resulted in the visualization of two distinct bands at 82 bp and 22 bp (Phompradit et al., 2011b). Our gel run showed two separate bands (82 + 22 bp) for the sample and confirmed it as a Mahidol variant (Figure 2). Enzyme digestion did not appear in the gel run for the two other variants, confirming them as negative. Since the sample tested positive for the Mahidol variant, we did not proceed with sequencing, which is necessary only for the Kalyan-Kerala variant.
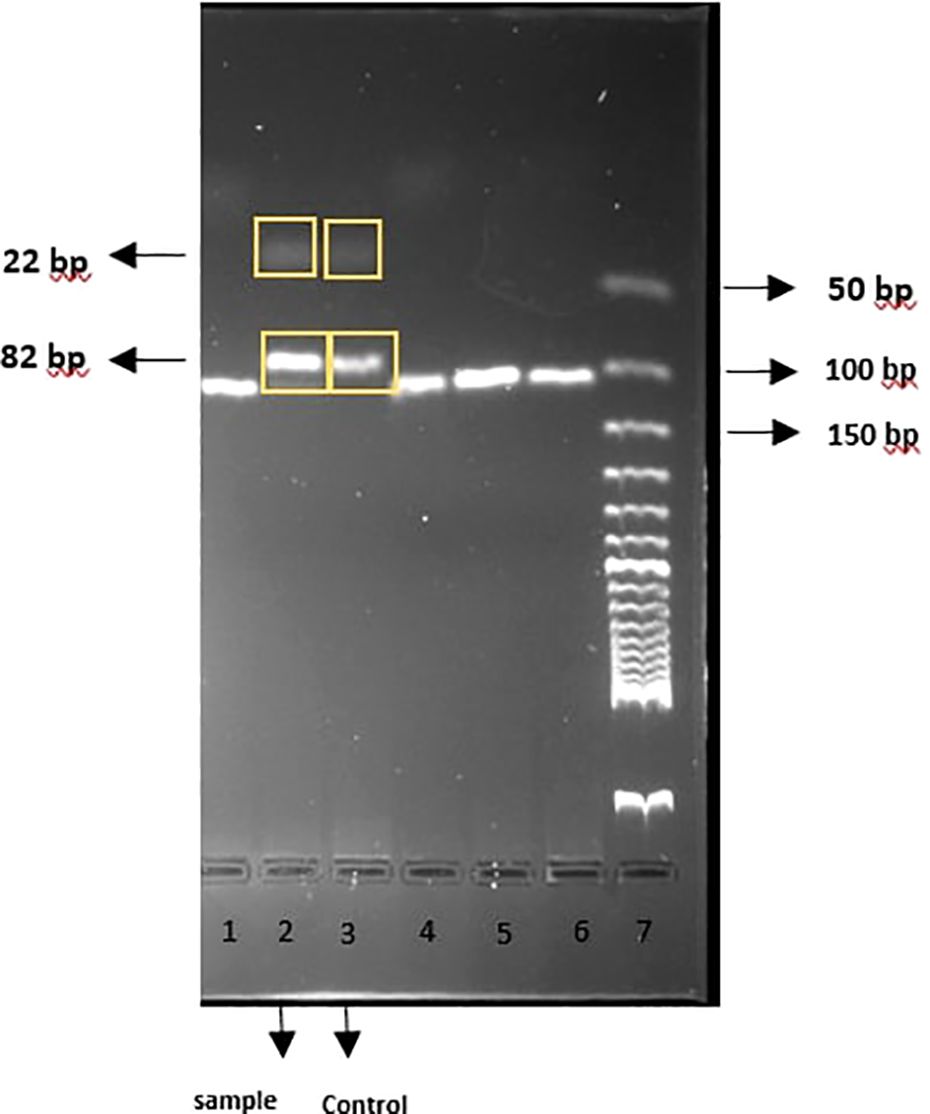
Figure 2. PCR-RFLP products visualized by 3% gel electrophoresis; Lane 2: Sample, Lane3: G6PD Mahidol Control.
This patient experienced severe hemolysis as a consequence of PQ treatment for presumed P. vivax infection in the setting of significant G6PD deficiency. The World Health Organization (WHO) recommends routine G6PD screening before PQ administration. While individuals with regular G6PD activity (≥30%) can receive daily PQ, those with G6PD deficiency require a modified regimen of 0.75 mg/kg PQ weekly for eight weeks instead of the standard 14-day course (WHO, 2022).
Bangladesh lacks a surveillance system for G6PD deficiency. The CHT region is known to have a high prevalence of G6PD deficiency, with significant variation among ethnic groups. This region is permanently inhabited by at least 12 major indigenous populations and a Bengali subpopulation (Hussain et al, 2015). A study conducted on 999 participants from both Bengali and ethnic groups of CHT showed that 9% of the individuals had G6PD deficiency, with 93.9% of those affected belonging to different ethnic groups. The Chak tribe had the highest prevalence (26%), while the Tripura tribe showed the lowest prevalence (2%) (Ley et al., 2020). Widespread and reliable testing remains a challenge due to budgetary and logistical constraints. However, this is likely an underestimate due to the lack of routine G6PD testing before PQ administration for malaria. A previous case with the Mediterranean G6PD variant resulted in severe PQ-induced hemolysis. This case was also from CHT with P. vivax mono-infection and was associated with severe hemolysis. The patient’s hemoglobin level dropped to 6.0 g/dL, and serum bilirubin was measured at 5.6 mg/dL, despite no evidence of malaria in laboratory tests. G6PD levels were subsequently determined using a spectrophotometer, which confirmed a deficiency, indicating that the hemolysis was induced by PQ in the context of G6PD deficiency. The patient recovered after receiving two units of blood transfusion. Subsequent whole genome sequencing identified the G6PD Mediterranean variant (Phru et al., 2020).
We documented a case of PQ-induced hemoglobinuria associated with the G6PD Mahidol variant in the CHT tribal population (Tripura). The Mahidol variant, commonly found in Myanmar and Thailand, exhibits enzyme activity ranging from 5 to 32% of normal levels. Its susceptibility to induced hemolysis falls between that of the G6PD A− and Mediterranean variants (Howes et al., 2013). Both cases highlight the adverse impact of PQ in individuals with G6PD deficiency. In contrast, the previous case involved an individual of Bengali origin with the G6PD Mediterranean variant and a P. vivax mono-infection. Our case underscores the potential for underdiagnosed severe hemolytic events due to misdiagnosis or insufficient G6PD testing. PQ should be administered with extra precautions to prevent serious adverse events by identifying early signs of hemolysis in settings where testing is unavailable. Most importantly, implementing routine G6PD screening before PQ administration in all healthcare facilities nationwide is essential to prevent such adverse outcomes.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving humans were approved by Ethical Review Committee (ERC) of icddr,b. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
MZ: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Writing – original draft, Writing – review & editing. CP: Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. AH: Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing. AT: Investigation, Methodology, Project administration, Resources, Validation, Writing – review & editing. MS: Investigation, Project administration, Resources, Supervision, Validation, Writing – review & editing. SA: Investigation, Project administration, Resources, Supervision, Validation, Writing – review & editing. MH: Data curation, Project administration, Resources, Supervision, Visualization, Writing – review & editing. MA: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article. The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
We are grateful to the National Malaria Elimination Programme of Bangladesh, for their excellent services to malaria patients across the country. The icddr,b is grateful to the governments of Bangladesh and Canada for providing core/unrestricted support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alam M. S., Kibria M. G., Jahan N., Price R. N., Ley B. (2018). Spectrophotometry assays to determine G6PD activity from Trinity Biotech and Pointe Scientific G6PD show good correlation. BMC Res. Notes. 11, 1–4. doi: 10.1186/s13104-018-3964-7
Anwar M. N., Smith L., Devine A., Mehra S., Walker C. R., Ivory E., et al. (2024). Mathematical models of Plasmodium vivax transmission: A scoping review. PloS Comput. Biol. 20, e1011931. doi: 10.1371/journal.pcbi.1011931
Dechyotin S., Sakunthai K., Khemtonglang N., Yamsri S., Sanchaisuriya K., Kitcharoen K., et al. (2021). Prevalence and molecular characterization of glucose-6-phosphate dehydrogenase (G6PD) deficiency in females from previously malaria endemic regions in Northeastern Thailand and identification of a novel G6PD variant. Mediterr. J. Hematol. Infect. Dis. 13. doi: 10.4084/MJHID.2021.029
Directorate General of Health Services (DGHS) (2019). Revised malaria treatment regimen-2017. Available online at: https://old.dghs.gov.bd/images/docs/Guideline/Malaria%20Treatment%20Regimen%202017.pdf (Accessed October 31, 2024).
Geck R. C., Powell N. R., Dunham M. J. (2023). Functional interpretation, cataloging, and analysis of 1,341 glucose-6-phosphate dehydrogenase variants. Am. J. Hum. Genet. 110, 228–239. doi: 10.1016/j.ajhg.2023.01.003
Gupta A. (2024). “G6PD deficiency,” in Decision making through problem based learning in hematology: A step-by-step approach in patients with anemia (Cham, Switzerland: Springer;), 121–133.
Haldar K., Alam M. S., Koepfli C., Lobo N. F., Phru C. S., Islam M. N., et al. (2023). Bangladesh in the era of malaria elimination. Trends Parasitology. 39, 760–773. doi: 10.1016/j.pt.2023.06.009
Howes R. E., Dewi M., Piel F. B., Monteiro W. M., Battle K. E., Messina J. P., et al. (2013). Spatial distribution of G6PD deficiency variants across malaria-endemic regions. Malaria J. 12, 1–15. doi: 10.1186/1475-2875-12-418
Hussain S., Ruano A. L., Rahman A., Rashid S. F., Hill P. S. (2015). From knowing our needs to enacting change: findings from community consultations with indigenous communities in Bangladesh. Int. J. Equity Health 14, 126. doi: 10.1186/s12939-015-0264-x
Khan Z., Nawaz M., Khan A., Bacha U. (2013). Hemoglobin, red blood cell count, hematocrit and derived parameters for diagnosing anemia in elderly males. Proc. Pakistan Acad. Sci. 50, 217–226.
Kumar A., Singh P. P., Tyagi S., Hari Kishan Raju K., Sahu S. S., Rahi M. (2024). Vivax malaria: a possible stumbling block for malaria elimination in India. Front. Public Health 11, 1228217. doi: 10.3389/fpubh.2023.1228217
Ley B., Kibria M. G., Khan W. A., Auburn S., Phru C. S., Jahan N., et al. (2020). Wide range of G6PD activities found among ethnic groups of the Chittagong Hill Tracts, Bangladesh. PloS Negl. Trop. Diseases. 14, e0008697. doi: 10.1371/journal.pntd.0008697
Li H., Ch’ih Y., Li M., Luo Y., Liu H., Xu J., et al. (2024). Newborn screening for G6PD deficiency in HeFei, FuYang and AnQing, China: Prevalence, cut-off value, variant spectrum. J. Med. Biochem. 43, 86–96. doi: 10.5937/jomb0-43078
Luzzatto L., Bancone G., Dugué P.-A., Jiang W., Minucci A., Nannelli C., et al. (2024). New WHO classification of genetic variants causing G6PD deficiency. Bull. World Health Organization. 102, 615. doi: 10.2471/BLT.23.291224
National Malaria Control Programme (2021). National strategic plan for malaria elimination 2021-2025 (Bangladesh: Ministry of Health and Family Welfare).
Phompradit P., Kuesap J., Chaijaroenkul W., Rueangweerayut R., Hongkaew Y., Yamnuan R., et al. (2011a). Prevalence and distribution of glucose-6-phosphate dehydrogenase (G6PD) variants in Thai and Burmese populations in malaria endemic areas of Thailand. Malaria J. 10, 1–8. doi: 10.1186/1475-2875-10-368
Phompradit P., Kuesap J., Chaijaroenkul W., Rueangweerayut R., Hongkaew Y., Yamnuan R., et al. (2011b). Prevalence and distribution of glucose-6-phosphate dehydrogenase (G6PD) variants in Thai and Burmese populations in malaria endemic areas of Thailand. Malaria J. 10, 368. doi: 10.1186/1475-2875-10-368
Phru C. S., Kibria M. G., Thriemer K., Chowdhury M. U., Jahan N., Aktaruzzaman M., et al. (2020). Case report: a case of primaquine-induced hemoglobinuria in glucose-6-phosphate dehydrogenase deficient malaria patient in southeastern Bangladesh. Am. J. Trop. Med. hygiene. 102, 156. doi: 10.4269/ajtmh.19-0643
Sazed S. A., Kibria M. G., Alam M. S. (2021). An optimized real-time qPCR method for the effective detection of human malaria infections. Diagnostics. 11, 736. doi: 10.3390/diagnostics11050736
Shah S. S., Stone E. F., Francis R. O., Karafin M. S. (2024). The global role of G6PD in infection and immunity. Front. Immunol. 15, 1393213. doi: 10.3389/fimmu.2024.1393213
WHO (2022). WHO guidelines for malaria, 25 november 2022 (Geneva, Switzerland: World Health Organization Geneva).
World Health Organization (2024). World Malaria report. Available online at: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (Accessed February 28, 2025).
Yi H., Li H., Liang L., Wu Y., Zhang L., Qiu W., et al. (2019). The glucose-6-phosphate dehydrogenase Mahidol variant protects against uncomplicated Plasmodium vivax infection and reduces disease severity in a Kachin population from northeast Myanmar. Infection Genet. Evolution. 75, 103980. doi: 10.1016/j.meegid.2019.103980
Keywords: malaria, G6PD deficiency, primaquine, hemoglobinuria, Mahidol variant
Citation: Zamil MF, Phru CS, Hasan A, Trina AT, Shahbaz M, Ahmed ST, Hossain MS and Alam MS (2025) Primaquine-induced hemoglobinuria: a case report of a G6PD deficient malaria patient with Mahidol trait from Bandarban, Bangladesh. Front. Malar. 3:1544378. doi: 10.3389/fmala.2025.1544378
Received: 12 December 2024; Accepted: 12 March 2025;
Published: 28 March 2025.
Edited by:
Dhanpat Kumar Kochar, Sardar Patel Medical College, IndiaReviewed by:
Adilson José DePina, CCS-SIDA/MoH, Cabo VerdeCopyright © 2025 Zamil, Phru, Hasan, Trina, Shahbaz, Ahmed, Hossain and Alam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Shafiul Alam, c2hhZml1bEBpY2RkcmIub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.