
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Malar., 14 March 2025
Sec. Vectors
Volume 3 - 2025 | https://doi.org/10.3389/fmala.2025.1540884
This article is part of the Research TopicAddressing Contemporary Threats to Global Malaria Control: New Tools and StrategiesView all 9 articles
 Alphonce A. Assenga1*
Alphonce A. Assenga1* Ludovic P. Ahoua Alou2
Ludovic P. Ahoua Alou2 Soromane Camara2
Soromane Camara2 Alphonsine A. Koffi2
Alphonsine A. Koffi2 Raphael N’Guessan2,3
Raphael N’Guessan2,3 Dismas S. Kamande1
Dismas S. Kamande1 Safina Ngonyani1
Safina Ngonyani1 Ummi A. Kibondo1
Ummi A. Kibondo1 Olukayode G. Odufuwa1
Olukayode G. Odufuwa1 Watson S. Ntabaliba1
Watson S. Ntabaliba1 Ruth G. Lekundayo1
Ruth G. Lekundayo1 Faraji Abilah1
Faraji Abilah1 Edith P. Madumla1
Edith P. Madumla1 Joseph B. Muganga1
Joseph B. Muganga1 Jason Moore1,4,5
Jason Moore1,4,5 Sarah J. Moore1,4,5,6
Sarah J. Moore1,4,5,6Background: The widespread development of pyrethroid-resistant Anopheles populations, has reduced the efficacy of pyrethroid insecticide-treated nets (ITNs), hindering malaria control efforts. This study tested PRONet Duo, a new ITN with two active ingredients-bifenthrin and chlorfenapyr. Bifenthrin is a fluorinated pyrethroid that is highly stable and more slowly detoxified by pyrethroid-resistant mosquitoes. Chlorfenapyr disrupts cellular energy production. The efficacy of PRONet Duo was compared to Interceptor® G2, an alpha-cypermethrin and chlorfenapyr ITN with proven efficacy in malaria reduction.
Methods: The study was conducted in two identical 9x9 Latin square experimental hut trials against wild free-flying Anopheles gambiae sensu lato in M’Bé, Côte d’Ivoire, and Lupiro, Tanzania using 18 experimental huts over 108 nights. The primary endpoint was the proportion of 72-hour mosquito mortality (M72) and the secondary endpoint was the proportion of mosquito blood-feeding. The study was done following World Health Organization guidelines. Data were analyzed using mixed-effect linear regression with a 7% margin of non-inferiority. Data were classified as non-inferior using delta and superior using the line of no difference.
Results: PRONet Duo demonstrated a non-inferior and superior mosquito mortality compared to Interceptor® G2 in both study sites. In Côte d’Ivoire, the M72 of PRONet Duo was 84% [81,88], higher than that of Interceptor® G2 (72% [68,76], OR: 1.54 [1.27,1.88]) and it was superior to MAGNet® (30% [27,34], OR: 13.74 [11.35,16.63], p<0.0001). In Tanzania, M72 of PRONet Duo was 68% [62,73], higher than that induced by Interceptor® G2 (44% [40,49], Odds Ratio (OR): 2.77 [2.31, 3.33]), and MAGNet® (36% [32,41], OR:4.82 [4.06,5.72] p<0.0001). PRONet Duo also induced non-inferior and superior prevention of blood-feeding compared to Interceptor® G2, with less than 11% feeding success observed in either trial site.
Conclusion: PRONet Duo ITNs are non-inferior and superior to the first-in-class Interceptor® G2 in terms of mosquito mortality and prevention of blood-feeding demonstrating the added benefit of bifenthrin for insecticide resistance management. Both chlorfenapyr nets offered superior mortality compared to the pyrethroid-only ITN. PRONet Duo offers an additional highly effective ITN for control of pyrethroid-resistant mosquitoes in malaria endemic regions.
The widespread distribution and use of insecticide-treated nets (ITNs) averted around 1.4 billion malaria cases between 2000 and 2022 (WHO, 2023a). The majority of these ITNs are treated with pyrethroids that are highly efficacious against pyrethroid susceptible mosquitoes. However, the prolonged use of pyrethroid ITNs as a monotherapy, and in concert with widespread agricultural pesticide use (Tepa et al., 2022) has exerted intense selection pressure on mosquito populations. Only mosquitoes that have adaptations allowing them to survive exposure to insecticides (and go on to reproduce) remain in the population, leading to widespread pyrethroid resistance among malaria vector populations (Liu, 2015; Riveron et al., 2018). Alongside insufficient (50%) coverage of ITNs, primarily driven by a median 2-year functional survival of ITNs (Bertozzi-Villa et al., 2021), insecticide resistance has also likely contributed to halting the decline in malaria cases that has been occurring since 2015 (WHO, 2023a). These challenges necessitate the need to develop new ITNs with additional chemistries to control pyrethroid-resistant malaria vectors.
The World Health Organization (WHO) Global Plan for Insecticide Resistance Management (GPIRM) which provides guidance for slowing the development of, and managing existing insecticide resistance, and The WHO Global Technical Strategy (GTS) which provides a roadmap for global malaria control through to elimination, have called for the manufacture of ITNs that utilize additional active ingredients (AI) with new modes of action aimed at controlling resistant malaria vectors (WHO, 2012; WHO, 2015). The first such nets were Olyset® Plus, which contains pyrethroid and a synergist piperonyl butoxide (PBO). PBO works by inhibiting the mixed function oxidases (such as cytochrome P450) responsible for metabolic pyrethroid resistance in Anopheles mosquitoes restoring their pyrethroid susceptibility (Pennetier et al., 2013)
However, PBO does not work against all resistance mechanisms in Anopheles mosquitoes (Zahouli et al., 2023) and can be rapidly lost from ITNs (Martin et al., 2023). Another new dual-AI net, Interceptor® G2, made of chlorfenapyr and alpha-cypermethrin, was developed and after demonstrating entomological (WHO, 2017) and epidemiological evidence (Accrombessi et al., 2023; Mosha et al., 2024) through laboratory, field evaluations and cluster randomized trials, received strong recommendations for use in areas of pyrethroid resistance by the WHO (2023b).
While pyrethroids target the mosquito nervous system (sodium-gated channels), the metabolites of the pyrole insecticide chlorfenapyr works effectively against pyrethroid-resistant mosquitoes by targeting the oxidative pathways in the insect’s mitochondria, thus disrupting the production of Adenosine Triphosphate (ATP) making the insect energy deficient, resulting in death (Black Bc et al., 1994; David, 2021; Kibondo et al., 2022). Metabolic resistance is one of the main mechanisms of resistance observed in malaria vectors (Vontas et al., 2020) where one or several detoxification gene families: cytochrome P450s (P450s), esterases, and glutathione S-transferases (GSTs) are overproduced to detoxify insecticides (Liu, 2015). While this metabolism is a detoxification process that successfully detoxifies pyrethroids, it increases the potency of chlorfenapyr by metabolizing it to tralopyril, which is highly insecticidal and may therefore be exploited as a means to control metabolically resistant insect populations (David, 2021). Chlorfenapyr has no known cross-resistance to the existing insecticide classes used in public health making it an ideal insecticide against resistant malaria mosquitoes (Ngufor et al., 2016; Accrombessi, 2024). Therefore, it is exploiting the resistance mechanisms that the mosquitoes have developed against pyrethroids, i.e., upregulation of mixed function oxidases and as it is not a neurotoxicant, it is unlikely to have cross-resistance (Ngufor et al., 2016).
Although it is a pyrethroid, bifenthrin may also be useful against pyrethroid resistant mosquitoes. Bifenthrin has a different structural motif than other pyrethroids, and is heavily fluorinated making it less vulnerable to metabolic attacks by key cytochrome P450s (Lissenden et al., 2021; Moyes et al., 2021). Bifenthrin is a contact insecticide that affects mosquitoes by keeping the para-homologous sodium channels open, thus causing neuro-excitation (Ashbrook, 2015). It is also a less irritant insecticide allowing the vector to spend more time on a treated net, increasing the probability that it will obtain a lethal dose (Hougard, 2002).
PRONet Duo is an ITN incorporated with chlorfenapyr and bifenthrin, designed to control pyrethroid resistant mosquitoes. It was tested in laboratory and experimental hut trials to demonstrate its comparative entomological efficacy to standard pyrethroid-only nets, and to the first-in-class chlorfenapyr ITN Interceptor® G2 by measuring how well the nets killed mosquitoes or prevented them from feeding on human blood. This experiment was conducted in Côte d’Ivoire and Tanzania to generate efficacy data required by the WHO prequalification (WHO, 2023f) using a non-inferiority experimental design required by Global Malaria Program (GMP) WHO (2024a) to give reassurance of public health benefits using entomological surrogates of clinical efficacy which includes mosquito mortality and blood feeding prevention (Sherrard-Smith et al., 2022). Both of these endpoints impact vectorial capacity (Brady et al., 2016) and data from these parameters from experimental hut trials can be used to predict the clinical impact of insecticide treated interventions (Churcher et al., 2024).
The study in both sites was a partially randomized, double blind experimental hut study of the investigational product, PRONet Duo, with positive controls 1) chlorfenapyr (CFP) ITN, Interceptor® G2 (IG2), which is a PQ listed ITN of the same product class as PRONet Duo and 2) standard pyrethroid only net, MAGNet®, which has the same pyrethroid as the other two nets but no CFP, and was used to demonstrate the additional benefit of CFP in this setting. A negative control (untreated net) was used to check the quality of the study. The study was conducted following the WHO 2023 Guidelines for conducting experimental hut tests, cone and tunnel tests and guidelines for the prequalification assessment of insecticide-treated nets and guidelines for laboratory and field testing of long-lasting insecticidal nets (WHO, 2023c; WHO, 2023d; WHO, 2023e; WHO, 2023f).
The study was carried out in two sites; one in Côte d’Ivoire and the other in Tanzania using the same design. Each trial was independently powered and used two simultaneous 9x9 Latin square design (LSD) using a total of 18 huts with nine unwashed nets in nine huts and nine 20x-washed nets in the remaining nine huts (Supplementary Figure S1). A total of 12 rounds i.e., N= 108 replicates per arm were conducted in both sites to ensure that the trial was well powered. Only the results of five arms are reported in this publication as several innovator nets were tested, but only PRONet Duo is going for further development. In each LSD one net was used as a negative control while five arms were the investigational (innovator) items and three arms were the active comparators (Supplementary Figure S1; Table 1). Both trials were conducted with the same 18 arms.
The primary endpoint was mortality measured after 72 hours (M72) as this is the convention for chlorfenapyr WHO (2024a). Secondary endpoints included mortality at 24-, 48-, 96- and 120-hours post exposure as well as blood feeding success (BF).
Côte d’Ivoire: The study was conducted at the M’Bé field site (5.209963 W and 7.970241 N), which is a large rice-irrigated valley producing year-round An. gambiae s.l., representing >99.8% of Anopheles. This species is highly resistant to pyrethroids but susceptible to chlorfenapyr (Camara et al., 2018a; Camara et al., 2018b) (Supplementary Table S1).
Tanzania: The study was conducted in Lupiro Village (8.385°S and 36.670°E) in Ulanga district, south-eastern Tanzania. The village lies 270m above sea level on the Kilombero River valley, 26 kilometers (km) south of Ifakara town, where Ifakara Health Institute (IHI) is located. The experimental huts are located between perennial river-fed rice agriculture and the village. The annual rainfall is 1200-1800 millimeters (mm) with a long rainy season between February and June and short rains between October and December, while temperatures range between 20 and 34 degrees centigrade (°C). The primary malaria vector is Anopheles arabiensis, that was PCR-confirmed at the time of the study to comprise 99.9% of An. gambiae complex species (Scott et al., 1993) and is abundant all year round. The WHO insecticide susceptibility tests (WHO, 2022a) conducted at the time of the study showed that this wild Anopheles arabiensis is resistant to all classes of pyrethroids, with a metabolic resistance mechanism and efficacy of pyrethroids was restored with pre-exposure to PBO. The mosquitoes were susceptible to chlorfenapyr (Supplementary Table S2).
Each ITN product was tested as two arms: unwashed and twenty times washed. The wash interval, wash resistance and net preparation was conducted before the experimental hut trial following WHO Guidelines (WHO, 2013). Washing is used to simulate ITN performance after loss of insecticide from three years of use.
The wash interval of the PRONet Duo nets was 1 day determined experimentally from the regeneration curve using metabolically resistant mosquitoes at the Vector Control Product Testing Unit (VCPTU) of the IHI following WHO Guidelines (WHO, 2013) in use at the time of testing (Supplementary Figure S2). The published 1 day wash interval for both MAGNet® (WHOPES, 2011) and Interceptor® G2 (WHOPES, 2017) was used. The wash resistance of PRONet Duo was confirmed as 20 standard washes in the laboratory following current WHO guidance (WHO, 2023f) (Supplementary Figure S3). Nets were prepared for field testing following current WHO guidance (WHO, 2023f) (Supplementary Data Sheet 1). Confirmatory bioassays (cone bioassays for PYR, tunnel tests for CFP) were conducted before and after the experimental hut trial. Cone bioassays were conducted on MAGNet® ITN as it only contains pyrethroid. 50 mosquitoes (10 cones) per strain were exposed to each net piece for the baseline quality check, regeneration time bioassays and wash resistance test. 25 per piece were used for pre and post hut quality checks (Supplementary Data Sheet 2). Tunnel tests were conducted on PRONet Duo and Interceptor® G2 as the cone test is not a suitable assay for chlorfenapyr nets. 50 mosquitoes per strain were exposed to each net piece for the baseline quality check, regeneration time bioassays and wash resistance test as well as pre and post hut quality checks. Therefore 100 mosquitoes were exposed per tunnel per replicate. (Supplementary Data Sheet 3). MAGNet® was assessed at 24 hours using cone test (Supplementary Data Sheet 2). Both PRONet Duo and Interceptor® G2 were assessed in tunnel tests at 72 hours post exposure (Supplementary Figure S3; Supplementary Data Sheet 3).
The Côte d’Ivoire trial used 18 West African huts. The Tanzania study used four types of hut designs: four East African huts, four West African huts, four Rapley huts and six Ifakara huts making a total of 18 huts. The description of these hut types is as provided elsewhere (WHO, 2023c).
In both sites, before the start of the study, a baseline study was conducted for two nights with untreated nets and with sleepers sleeping underneath untreated nets to asses mosquito retention. These were done by releasing 20 marked mosquitoes in each hut at 19:00 h and recapturing them in the morning at 6:00 h to determine the mosquito retention capacity of each hut. Furthermore, approximately 10 dead mosquitoes were placed into each experimental hut overnight to assess if ants scavenged them.
A total of 18 human volunteers and a total of 18 net arms were used in the 18 huts. The net arms and human volunteers were rotated between them an equal number of times to average possible biases introduced by differences in hut design or human attractiveness on outcomes measured across the trial (Nash, 2021). Volunteers were rotated sequentially among huts each night of the study using a pre-prepared roster. Each net type remained in a particular hut for nine nights before moving to a different hut in the following experiment round (nine nights makes a round). At the end of each nine-night round of the experiment and before the nets moved to a different hut, the experimental huts were cleaned and aired for one day to prevent carry-over of insecticide residuals.
Volunteers entered the huts in Côte d’Ivoire at 20:00 hours and remained inside until 05:00 hours while in Tanzania they were in huts between 19:00 hours and 06:00 hours. Each morning of the study, mosquitoes were collected from inside of the net, followed by dead mosquitoes on the floor, resting mosquitoes on walls and ceilings and those from the exit traps or veranda were collected using aspiration. Mosquitoes were sorted and scored by location as dead fed, dead unfed, alive fed, and alive unfed. The alive mosquitoes were held for up to 120 hours in a temperature-controlled room and provided with access to a 10% sugar solution to assess delayed mortality.
Testing of susceptibility to alpha-cypermethrin before and after exposure to PBO using WHO tube tests and chlorfenapyr in bottle bioassay with acetone solvent was conducted shortly after the experimental trial. For WHO tube tests, 25 Mosquitoes per tube were used for each treatment group (PBO-Only, PBO+Pyrethroid, pyrethroid-only and control) following WHO standard operating procedures (WHO, 2022a). Mortality was recorded after 24 hours holding period. For the bottle bioassay, 25 mosquitoes were placed in each of four treatment bottles and two control bottles, following WHO standard operating procedures (WHO, 2022b), and mortality was measured at 120 hours holding period to ensure complete conversion of the pro-insecticide (Black Bc et al., 1994; WHO, 2024b).
A short questionnaire was administered to the volunteering participants who slept in the experimental huts to record any side effects from sleeping under nets. In Tanzania, this questionnaire was offered three times during the first round of the experiment while in Cote d’Ivoire it was administered once at the end of the trial.
The study participants(sleepers) recruited provided written informed consent to volunteer in the experiment. Studies in both sites was approved by the respective institutional and national review boards; Tanzania National Institute for Medical Research certificate NIMR/HQ/R.8a/Vol.IX/4558 and Côte d’Ivoire certificate N/Réf: 081-22/MSHPCMU/CNESVS-km. The experiment was medically supervised (Gimnig et al., 2013). All sleepers were males aged 18 and over, and in Tanzania, all were offered malaria prophylaxis according to local guidance and screened weekly for malaria parasites. Due to the complications of malaria in pregnancy and cultural norms around women working alone at night, women were not recruited for the study. No one was found positive for Plasmodium for the duration of the trial. Sleepers in Côte d’Ivoire were rather advised to seek for prompt testing and treatment, free of charge if they observed signs/symptoms of malaria or other vector-borne diseases.
Data were recorded using paper forms and digital data entry. In Tanzania after the primary entry in paper forms, experimental hut data was also recorded digitally using the open data kit (ODK) employing tablets equipped with the ODK Collect app which contains a comprehensive electronic data form. Once tablets are connected to the internet, the collected data was transmitted to the Ifakara Health Institute (IHI) ODK Central Server.
Data was cleaned and analyzed using STATA 17 statistical software (Stata-Corp, College Station TX, USA). Outliers, and balancing of observations as per study design, were checked by graphing and tabulating variables. Two inferential analyses were carried out: 1) The non-inferiority of PRONet Duo nets to Interceptor® G2, and 2) The superiority of chlorfenapyr nets to MAGNet®. Binary outcomes (proportion dead or proportion blood fed) were analyzed using generalized linear regression with a binomial distribution and log link with fixed effects for net type, washing status (washed or unwashed), hut, volunteer and night. The non-inferiority margin was set at a fixed effect difference of 7% as an odds ratio (OR) based on the mortality estimate of the Interceptor® G2 WHO (2024a). Data were presented with the point estimates as a percentage with 95% confidence intervals (CI) in square brackets. Non-inferiority and superiority was interpreted and presented following the CONSORT standards for reporting non-inferiority trials (Piaggio et al., 2012). As four hut types were used in Tanzania, in order to check the effects of huts on the assessment of non-inferiority in the Tanzanian site, an additional sub-analysis was conducted using only Ifakara Huts. The results agreed with the main analysis and conclusions were unchanged.
The sample size was estimated in both sites using simulation-based power analysis in R statistical software before the trial began. Post-hoc calculations of actual study power were also performed using simulation-based power analysis in R version 4.3.2 http://www.r-project.org using lme4 package (Johnson et al., 2015). In both sites 1000 simulations for generalized linear mixed models were run using a Latin square design for 81 nights of data collection with one hut per treatment arm. For Côte d’Ivoire, an estimated mean number of 5 An. gambiae mosquitoes per night, mosquito variability of 0.42 was used for hut night and an estimated 72-hour mortality of 84% for PRONet Duo and 72% for Interceptor® G2 based on data from the trial. For Tanzania, an estimated mean number of 7 An. arabiensis mosquitoes per night, variability of 0.75 was used for hut night and an estimated 72-hour mortality of 44% for Interceptor® G2 based on previous data (Kibondo et al., 2022). Study power is presented in a supplementary table (Supplementary Table S5).
All test items were stored and tested under optimal conditions following WHO 2023 Guidance. Control mortality was acceptable in all tests at both sites. Temperature in the experimental huts and field laboratory were in acceptable range for the duration of the experiment (Supplementary Table S8). ITNs were highly effective in laboratory bioassays at all critical test phases: on receipt, after washing and after experimental hut trials (Supplementary Tables S3, S4, S9).
The study in both trial sites was powered with alpha of 0.05 and beta of >80% to detect the superiority of PRONet Duo. After nine rounds (81 nights) post-hoc power calculations showed the study to have over 95% % power in both sites (Supplementary Table S5).
The experiment in both sites was conducted for a total of 108 nights. A total of 6,338 (median 6 [interquartile range IQR: 3, 10]) female mosquitoes were collected in Côte d’Ivoire and all were Anopheles gambiae s.l. by PCR test. In Tanzania, a total of 9,383 (median 5 [IQR:1, 16]) female Anopheles gambiae s.l, were collected. PCR confirmed that 100% of all collected Anopheles gambiae s.l were Anopheles arabiensis.
In Côte d’Ivoire and Tanzania, both chlorfenapyr ITNs demonstrated superior efficacy compared to standard comparator pyrethroid only MAGNet® ITN. The magnitude of difference was greater in Côte d’Ivoire than Tanzania, both numerically and by Odds Ratio (OR). Superiority of chlorfenapyr ITNs was observed relative to the pyrethroid only arm at all holding times in both sites is provided in the supplementary file (Supplementary Table S6). Additionally, in both study sites, all treated nets were superior to the untreated negative control net at all holding times.
Côte d’Ivoire: PRONet Duo killed more than twice the proportion of mosquitoes on the combined arms compared to the pyrethroid only net. MAGNet® killed 30% [27, 34] An. gambiae s.l. PRONet Duo mortality was 84% [81, 88], OR =13.74 [11.35, 16.63] p<0.0001. Interceptor® G2 mortality was 72% [68, 76], OR= 8.91 [7.52, 10.54] p<0.0001.
Tanzania: PRONet Duo killed nearly twice the proportion of mosquitoes on the combined arms compared to the pyrethroid only net. MAGNet® killed 36% [32, 41] An. arabiensis. PRONet Duo mortality was 68% [62, 73], OR: 4.82 [4.06, 5.72], p<0.0001. Interceptor® G2 mortality was 44% [40, 49], OR:1.74 [1.54, 1.96], p<0.0001.
In both sites, both PRONet Duo and Interceptor® G2 ITNs demonstrated superior efficacy compared to standard comparator pyrethroid-only net MAGNet® net for the mortality endpoint when the unwashed and washed arms were considered separately (Supplementary Tables S6).
In comparison with MAGNet®, feeding success in the PRONet Duo and Interceptor® G2 arms was lower in Côte d’Ivoire and similar in Tanzania (Figures 1C, D). Blood feeding was lower in all ITN arms compared to the negative control in the two trial sites (Figures 1C, D).

Figure 1. Percent mosquito mortality (A, B) and percent blood feeding success (C, D) by PRONet Duo in comparison with Interceptor® G2 and pyrethroid-only MAGNet® ITN in Côte d’Ivoire (A, C) and Tanzania (B, D). Data represent arithmetic mean % control corrected mortality and blood feeding success with 95% confidence intervals.
Côte d’Ivoire: Feeding success in the MAGNet® arm was 19% [16, 22] PRONet Duo arm was10% [7,13] OR 0.40 [0.38, 0.60] p<0.0001 and Interceptor® G2 was 20% OR 1.14 [0.95, 1.36] p=0.173. (Figure 1C; Supplementary Table S6)
Tanzania: Feeding success in the MAGNet® arm was 5% [3,7] PRONet Duo arm was 3% [2,5] OR 0.81 [0.54, 1.22] p=0.322, Interceptor® G2 was 6% [4,8] OR 1.97 [1.48, 2.61] p<0.0001. (Figure 1D; Supplementary Tables S6).
PRONet Duo was non-inferior and superior to Interceptor® G2, in the combined unwashed and washed analysis as well as when the unwashed ITNs were compared or the washed ITNs were compared (Figure 2A). PRONet Duo was non-inferior to Interceptor® G2 because the lower confidence interval was above delta, and in addition, the lower confidence interval was above the line of no difference indicating superior mortality (Piaggio et al., 2012). In both study sites, PRONet Duo induced a higher proportion of mosquito mortality at 72 hours compared to the active comparator Interceptor® G2 that was greater than the 7% delta and also greater than the line of no difference, indicating superiority. Non-inferiority and superiority were seen at each holding time in both locations (Supplementary Table S7).
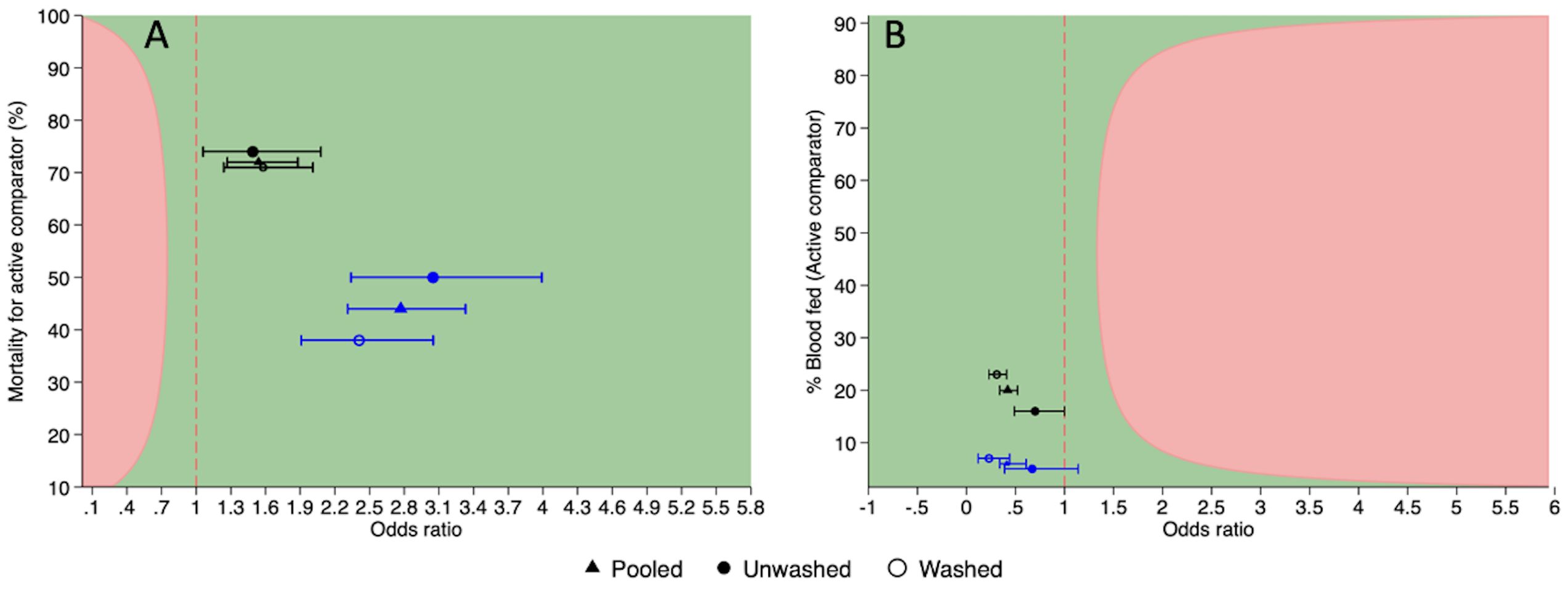
Figure 2. The efficacy of PRONet Duo ITNs in comparison to Interceptor® G2 against wild pyrethroid-resistant Anopheles gambiae sensu lato in Côte d’Ivoire (represented in black) and in Tanzania (represented in blue) on the primary outcome of mortality at 72hrs with 95% C.I (A) and blood feeding with 95% C.I (B). Pink line indicates the non-inferiority margin. Dashed line indicates the line of no difference.
In Côte d’Ivoire, PRONet Duo induced higher mortality compared to Interceptor® G2 for the combined arms. M72 in the PRONet Duo nets was 84% (95% CI:81, 88) while that of Interceptor® G2 was 72% (95% CI:68, 76). In Tanzania, M72 was 68% [62, 73] for PRONet Duo for the combined arms while that of Interceptor®G2 was 44% [40, 49]. Mosquitoes were more likely to die in the PRONet Duo nets compared to Interceptor® G2 (OR 2.77 (2.31, 3.33)
In both study sites, PRONet Duo induced greater reduction in blood feeding compared to Interceptor® G2 for the combined arms, unwashed arms as well as the washed arms (Figure 2B). In Côte d’Ivoire, PRONet Duo reduced more blood feeding compared to Interceptor® G2 for the combined arms. Feeding success in the PRONet Duo nets was 10% [95% CI: 7,14) while that of Interceptor® G2 was 20% [16, 23] (Supplementary Table S7). In Tanzania, the proportion of mosquitoes that blood fed was 3% [CI: 2, 5] for PRONet Duo for the combined arms while that of Interceptor® G2 was 6% [4, 8]. Mosquitoes were less likely to blood-feed in the PRONet Duo nets compared to Interceptor® G2 OR 0.41 [0.28,0.61].
In Côte d’Ivoire, the wild Anopheles gambiae s.l were resistant to pyrethroids but susceptible to chlorfenapyr (Supplementary Table S1).
In Tanzania, wild caught Anopheles arabiensis was susceptible to alpha-cypermethrin after pre-exposure to PBO. Bottle bioassay (with acetone as solvent) showed that the wild Anopheles arabiensis was 100% susceptible to chlorfenapyr at M120 (Supplementary Table S2).
The regeneration time study done in Tanzania showed that PRONet Duo had a regeneration time of 1 day with the metabolic resistant Anopheles arabiensis in a tunnel test using 72-hour mortality and 1 day with pyrethroid susceptible Anopheles gambiae sensu stricto using 24-hour mortality (Supplementary Figure S2). Tunnel tests using metabolically resistant Anopheles gambiae s.l in both trial sites showed that PRONet Duo was resistant to 20 washes and feeding inhibition was 100% (Supplementary Figure S3).
This study was a comparative efficacy trial of the candidate chlorfenapyr-bifenthrin incorporated net (PRONet Duo) compared to the active comparator Interceptor® G2 and the pyrethroid-only net MAGNet® (standard comparator). The study assessed the non-inferiority of PRONet Duo to Interceptor® G2 for the primary endpoints of 72-hour mortality and blood-feeding inhibition. The evaluations were conducted using pyrethroid-resistant Anopheles gambiae s.l in Côte d’Ivoire and Tanzania, and included both unwashed and 20-times washed nets. According to WHO guidelines, candidate ITNs must retain their efficacy after 20 washes and demonstrate non-inferiority to the first-in-class product as well as superiority to the standard comparator WHO (2024a). The study used a single protocol so the same study was conducted in both East and West Africa, and the data was presented in a pooled analysis (Figures 1, 2). This improves the generalizability of the results as WHO requires data from at least two ecologically distinct areas for decision-making (WHO, 2023f; WHO, 2024a)
The results of our study show that PRONet Duo is non-inferior and superior to the active comparator Interceptor® G2 (treated with alpha-cypermethrin and chlorfenapyr) as well as superior to the standard comparator MAGNet® net (pyrethroid-only net) in terms of inducing mortality on Anopheles mosquitoes at the standard 72 hour holding period and extended holding times beyond 72 hours. This was observed in the unwashed, and 20x washed arms and when the washed and unwashed nets data was combined during analysis.
The superiority of pyrethroid-chlorfenapyr ITNs to pyrethroid-only ITNs has been demonstrated in previous experimental hut studies (Bayili et al., 2017; Zahouli et al., 2023) and in this study, both Interceptor® G2 and PRONet Duo demonstrated superior efficacy compared to pyrethroid-only MAGNet® net on the mortality endpoint. The dual active ingredients in PRONet Duo and Interceptor® G2 explains the observed superiority to MAGNet® ITN. In both study settings mosquitoes were resistant to pyrethroids but susceptible to chlorfenapyr. Unlike pyrethroids, Chlorfenapyr is a non-neurological insecticide that prevents the production of Adenosine Triphosphate (ATP) in cell’s mitochondria leading to cellular death (Black Bc et al., 1994). The superiority of Interceptor® G2 to pyrethroid only ITNs for malaria control in areas of pyrethroid resistance has been demonstrated in randomized control trials in Tanzania (Mosha et al., 2022) and Benin (Accrombessi et al., 2023), leading to a recommendation of this product class for malaria control in areas of mosquito resistance to pyrethroids (WHO, 2023b).
PRONet Duo did not give a superior reduction in blood feeding compared to the pyrethroid only MAGNet®, which contains a high concentration of alphacypermethrin (5.8 g/kg). It is likely that the use of the less irritant pyrethroid bifenthrin explains the superior mosquito kill but not reduced blood feeding (Hougard, 2002). However, PRONet Duo was superior to Interceptor® G2 in reducing blood-feeding. This can be attributed to the lower content of alphacypermethrin (2.4g/kg) in Interceptor® G2 (Roberts et al., 2000).
The non-inferiority and superiority of PRONet Duo to Interceptor® G2 is likely due to the added benefit of bifenthrin. Bifenthrin has a higher proportion of fluorine atoms and a bulky structure compared to other pyrethroids (Lissenden et al., 2021). Fluorine atoms can enhance the uptake of insecticides by increasing cell membrane permeability and lipophilicity (Jeschke, 2004). They can also make insecticides less susceptible to enzymatic breakdown by mosquito detoxification enzymes (Zhu et al., 2023) by enhancing the compound’s stability (carbon-fluorine bonds are the strongest single bond in organic chemistry) (Mori et al., 2007). The unique structural motif of bifenthrin (rigid bi-phenyl alcohol moiety) means it is depleted more slowly by cytochrome P450 monooxygenase enzymes which are common resistance mechanisms in Anopheles mosquitoes (Yunta et al., 2019), and kills more resistant mosquitoes compared to other pyrethroids (Hougard, 2002; Moyes et al., 2021). The efficacy of bifenthrin was demonstrated 20 years ago (Hougard et al., 2003), and there is one bifenthrin IRS product PQ listed (WHO, 2018). Still, bifenthrin has not been used on an ITN to date, presumably due to the high cost of bifenthrin due to its fluorinated moiety (Champagne et al., 2015). There are several advantages to bifenthrin beyond its increased efficacy against resistant mosquitoes including low vapor pressure (1.81 10-7 mmHg) which will reduce evaporative loss of the AI, low water solubility (<1μg/liter) which will resist loss of AI through washing, and low photolysis which will resist loss of AI when ITNs are dried outdoors. Bifenthrin is a contact insecticide and is known to be less irritant pyrethroid compared to other pyrethroids (Hougard, 2002) increasing the likelihood of prolonged contact with the treated net and consequent increased toxicity (Hodjati et al., 2003).
The laboratory assays support the results of the two experimental hut trials well. PRONet Duo induced excellent 72-hour mortality 92% [89.5, 94.5] even after 20 washes. PRONet Duo is, to our knowledge, the first ITN incorporated with chlorfenapyr. It therefore offers an important development in ITN technology by providing a rugged and wash resistant product. Polyethylene nets can be made to be extremely strong and resistant to damage by using a high denier, high density polyethylene and strong knitting patterns (Skovmand and Bosselmann, 2011). This may offer an alternative for use in areas where rugged nets are required as a high resistance to damage is correlated with longer ITN survival (Kilian et al., 2021).
Even though a single protocol was used for the study there were differences in study conduct. In Côte d’Ivoire, only West African huts were used to conduct the study, but in Tanzania, four different hut types were used to evaluate ITN efficacy. All of the hut types are approved for use in ITN evaluations by WHO (WHO, 2023f), but using four hut types did increase heterogeneity in the data. This was overcome by running the trial for an additional three rounds so that 108 nights of data were collected. Sub-analysis was conducted to ensure the results were valid, however, we will in the future conduct these studies using only one standard hut type across sites.
The experimental hut studies in this trial demonstrated that the candidate ITN PRONet Duo is non-inferior and superior to the first-in-class product Interceptor® G2 on the primary endpoint of mortality at 72-hours against pyrethroid resistant Anopheles gambiae s.l in both Côte d’Ivoire and Tanzania, indicating that PRONet Duo is likely to have public health benefit based on entomological surrogates of clinical efficacy. Both chlorfenapyr ITNs (PRONet Duo and Interceptor® G2) were superior and to the pyrethroid-only MAGNet® net, adding additional data to support the use of chlorfenapyr nets in areas of pyrethroid resistance mediated by mixed-function oxidases. Non-inferiority and superiority were shown for the unwashed nets, washed nets as well as when unwashed and washed data were combined in the analysis.
The results underscore the added benefit of bifenthrin in controlling pyrethroid-resistant malaria vectors in endemic countries. Furthermore, the excellent performance of PRONet Duo after 20 washes (as a proxy for 3 years of use) suggests its potential for long-term field use, particularly in areas where the durability of ITNs is paramount. Given all these findings, a community trial of PRONet Duo nets with durability outcome indicators is required to establish its long-term efficacy and relative cost-effectiveness for operational use against malaria.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Tanzania National Institute for Medical Research, and Côte d’Ivoire Ministry of Health, Public Hygiene and Universal Health Coverage. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
AA: Conceptualization, Formal Analysis, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. LA: Formal Analysis, Investigation, Supervision, Writing – review & editing. SC: Formal Analysis, Investigation, Supervision, Writing – review & editing. AK: Investigation, Methodology, Supervision, Writing – review & editing. RN: Methodology, Software, Supervision, Writing – review & editing. DK: Supervision, Writing – review & editing. SN: Supervision, Writing – review & editing. UK: Formal Analysis, Writing – review & editing. OO: Formal Analysis, Writing – review & editing. WN: Supervision, Writing – review & editing. RL: Supervision, Writing – review & editing. FA: Writing – review & editing. EM: Methodology, Writing – review & editing. JBM: Data curation, Software, Writing – review & editing. JM: Resources, Writing – review & editing. SM: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study was funded by VKA Polymers Pvt Ltd of India. The funders had no role in study design, data collection, analysis, or manuscript preparation.
We are thankful to our funder, Anand Samiappan, and the team at VKA Polymers Pvt Ltd., India, for making this research possible. We are thankful to all technicians whose dedication was instrumental throughout this research. We thank the entire team in Lupiro village, especially participants who slept in the experimental huts and tirelessly collected mosquitoes. We also acknowledge all members of the vector control product testing unit (VCPTU) for their invaluable support during the execution of this work. Finally, special thanks to Dr. Jenny Stevenson for her insightful contributions during the preparation of this manuscript and Professor John Bradley for useful discussions on the analysis.
All authors with the exception of FA conduct evaluations of a range of vector control products for a range of manufacturers.
The authors declare that this study received funding from VKA Polymers Pvt Ltd of India. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmala.2025.1540884/full#supplementary-material
ITNs, Insecticide-Treated Nets; WHO, World Health Organization; IQR, Interquartile Range;
PBO, Piperonyl Butoxide; PYR, Pyrethroid; CFP, Chlorfenapyr.
Accrombessi M., Cook J, Dangbenon E, Sovi A, Yovogan B, Assongba L, et al (2024). Effectiveness of pyriproxyfen-pyrethroid and chlorfenapyr-pyrethroid long-lasting insecticidal nets (LLINs) compared with pyrethroid-only LLINs for malaria control in the third year post-distribution: a secondary analysis of a cluster-randomised controlled trial in Benin. Lancet Infect Dis. 24, 619–628.
Accrombessi M., Cook J., Dangbenon E., Yovogan B., Akpovi H., Sovi A., et al. (2023). Efficacy of pyriproxyfen-pyrethroid long-lasting insecticidal nets (LLINs) and chlorfenapyr-pyrethroid LLINs compared with pyrethroid-only LLINs for malaria control in Benin: a cluster-randomised, superiority trial. Lancet. 401, 435–446. doi: 10.1016/S0140-6736(22)02319-4
Ashbrook A. R. (2015). Chlorfenapyr and Bifenthrin Susceptibility Monitoring of Field Collected Bed Bug Populations from the United States. Open Access Theses. 1036. Available at: https://docs.lib.purdue.edu/open_access_theses/1036.
Bayili K., N’do S., Namountougou M., Sanou R., Ouattara A., Dabire R. K., et al. (2017). Evaluation of efficacy of Interceptor((R)) G2, a long-lasting insecticide net coated with a mixture of chlorfenapyr and alpha-cypermethrin, against pyrethroid resistant Anopheles. Malar J. 16, 190. doi: 10.1186/s12936-017-1846-4
Bertozzi-Villa A., Bever C. A., Koenker H., Weiss D. J., Vargas-Ruiz C., Nandi A. K., et al. (2021). Maps and metrics of insecticide-treated net access, use, and nets-per-capita in Africa from 2000-2020. Nat. Commun. 12, 3589. doi: 10.1038/s41467-021-23707-7
Black Bc H. R., Ahammadsahib K. I., Kukel C. D., Donovan S. (1994). Insecticidal Action and Mitochondrial Uncoupling Activity of AC-303,630 and Related Halogenated Pyrroles. Pesticide Biochem. Physiol. 50, 115–128. doi: 10.1006/pest.1994.1064
Brady O. J., Godfray H. C., Tatem A. J., Gething P. W., Cohen J. M., McKenzie F. E., et al. (2016). Vectorial capacity and vector control: reconsidering sensitivity to parameters for malaria elimination. Trans. R Soc. Trop. Med. Hyg. 110, 107–117. doi: 10.1093/trstmh/trv113
Camara S., Ahoua Alou L. P., Koffi A. A., Clegban Y. C. M., Kabran J. P., Koffi F. M., et al. (2018a). Efficacy of Interceptor((R)) G2, a new long-lasting insecticidal net against wild pyrethroid-resistant Anopheles gambiae s.s. from Cote d’Ivoire: a semi-field trial. Parasite 25, 42. doi: 10.1051/parasite/2018042
Camara S., Koffi A. A., Ahoua Alou L. P., Koffi K., Kabran J. K., Kone A., et al. (2018b). Mapping insecticide resistance in Anopheles gambiae (s.l.) from Cote d’Ivoire. Parasit Vectors 11, 19. doi: 10.1186/s13071-017-2546-1
Champagne P. A., Desroches J., Hamel J. D., Vandamme M., Paquin J. F. (2015). Monofluorination of Organic Compounds: 10 Years of Innovation. Chem. Rev. 115, 9073–9174. doi: 10.1021/cr500706a
Churcher T. S., Stopard I. J., Hamlet A., Dee D. P., Sanou A., Rowland M., et al. (2024). The epidemiological benefit of pyrethroid-pyrrole insecticide treated nets against malaria: an individual-based malaria transmission dynamics modelling study. Lancet Glob Health 12, e1973–e1e83. doi: 10.1016/S2214-109X(24)00329-2
David M. D. (2021). The potential of pro-insecticides for resistance management. Pest Manag Sci. 77, 3631–3636. doi: 10.1002/ps.v77.8
Gimnig J. E., Walker E. D., Otieno P., Kosgei J., Olang G., Ombok M., et al. (2013). Incidence of malaria among mosquito collectors conducting human landing catches in western Kenya. Am. J. Trop. Med. Hyg. 88, 301–308. doi: 10.4269/ajtmh.2012.12-0209
Hodjati M. H., Mousavi N., Curtis C. F. (2003). Irritant effect, prevention of blood feeding and toxicity of nets impregnated with different pyrethroids on An. stephensi. J. Vect Borne Dis. 40, 54–59.
Hougard J. M. (2002). Bifenthrin: A useful pyrethroid insecticide for treatment of mosquito nets. J Med Entomol. doi: 10.1603/0022-2585-39.3.526
Hougard J. M., Duchon S., Darriet F., Zaim M., Rogier C., Comparative performances P. G. (2003). under laboratory conditions, of seven pyrethroid insecticides. Bull. World Health Organization. 81, 324–333.
Jeschke P. (2004). The unique role of fluorine in the design of active ingredients for modern crop protection. Chembiochem. 5, 571–589. doi: 10.1002/cbic.200300833
Johnson P. C., Barry S. J., Ferguson H. M., Muller P. (2015). Power analysis for generalized linear mixed models in ecology and evolution. Methods Ecol. Evol. 6, 133–142. doi: 10.1111/mee3.2015.6.issue-2
Kibondo U. A., Odufuwa O. G., Ngonyani S. H., Mpelepele A. B., Matanilla I., Ngonyani H., et al. (2022). Influence of testing modality on bioefficacy for the evaluation of Interceptor((R)) G2 mosquito nets to combat malaria mosquitoes in Tanzania. Parasit Vectors. 15, 124. doi: 10.1186/s13071-022-05207-9
Kilian A., Obi E., Mansiangi P., Abílio A. P., Haji K. A., Guillemois E., et al. (2021). Correlation of textile ‘resistance to damage’ scores with actual physical survival of long-lasting insecticidal nets in the field. Malar J. 20, 29. doi: 10.1186/s12936-020-03570-5
Lissenden N., Kont M. D., Essandoh J., Ismail H. M., Churcher T. S., Lambert B., et al. (2021). Review and Meta-Analysis of the Evidence for Choosing between Specific Pyrethroids for Programmatic Purposes. Insects 12, 826. doi: 10.3390/insects12090826
Liu N. (2015). Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu. Rev. Entomol. 60, 537–559. doi: 10.1146/annurev-ento-010814-020828
Martin J. L., Messenger L. A., Rowland M., Mosha F. W., Bernard E., Kisamo M., et al. (2023). Bio-efficacy of field aged novel class of long-lasting insecticidal nets, against pyrethroid-resistant malaria vectors in Tanzania: A series of experimental hut trials. medRxiv. 2023, 10.21.23297289. doi: 10.1101/2023.10.21.23297289
Mori T., Ujihara K., Matsumoto O., Yanagi K., Matsuo N. (2007). Synthetic studies of fluorine-containing compounds for household insecticides. J. Fluorine Chem. 128, 1174–1181. doi: 10.1016/j.jfluchem.2007.07.016
Mosha J. F., Kulkarni M. A., Lukole E., Matowo N. S., Pitt C., Messenger L. A., et al. (2022). Effectiveness and cost-effectiveness against malaria of three types of dual-active-ingredient long-lasting insecticidal nets (LLINs) compared with pyrethroid-only LLINs in Tanzania_ a four-arm, cluster-randomised trial.pdf. Lancet. 399, 1227–1241. doi: 10.1016/S0140-6736(21)02499-5
Mosha J. F., Matowo N. S., Kulkarni M. A., Messenger L. A., Lukole E., Mallya E., et al. (2024). Effectiveness of long-lasting insecticidal nets with pyriproxyfen-pyrethroid, chlorfenapyr-pyrethroid, or piperonyl butoxide-pyrethroid versus pyrethroid only against malaria in Tanzania: final-year results of a four-arm, single-blind, cluster-randomised trial. Lancet Infect. Dis. 24, 87–97. doi: 10.1016/S1473-3099(23)00420-6
Moyes C. L., Lees R. S., Yunta C., Walker K. J., Hemmings K., Oladepo F., et al. (2021). Assessing cross-resistance within the pyrethroids in terms of their interactions with key cytochrome P450 enzymes and resistance in vector populations. Parasit Vectors. 14, 115. doi: 10.1186/s13071-021-04609-5
Nash R. K. (2021). Systematic review of the entomological impact of insecticide-treated nets evaluated using experimental hut trials in Africa. Curr. Res. Parasitol. Vector-Borne Dis. 1. doi: 10.1016/j.crpvbd.2021.100047
Ngufor C., Critchley J., Fagbohoun J., N’Guessan R., Todjinou D., Rowland M. (2016). Chlorfenapyr (A Pyrrole Insecticide) Applied Alone or as a Mixture with Alpha-Cypermethrin for Indoor Residual Spraying against Pyrethroid Resistant Anopheles gambiae sl: An Experimental Hut Study in Cove, Benin. PloS One 11, e0162210. doi: 10.1371/journal.pone.0162210
Pennetier C., Bouraima A., Chandre F., Piameu M., Etang J., Rossignol M., et al. (2013). Efficacy of Olyset(R) Plus, a new long-lasting insecticidal net incorporating permethrin and piperonyl-butoxide against multi-resistant malaria vectors [corrected. PloS One 8, e75134. doi: 10.1371/annotation/bed4305a-d665-4150-a682-a20d9cf9b79f
Piaggio G., Elbourne D. R., Pocock S. J., Evans S. J., Altman D. G. (2012). Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. Jama. 308, 2594–2604. doi: 10.1001/jama.2012.87802
Riveron J. M., Tchouakui M., Mugenzi L., Menze B. D., Chiang M.-C., Wondji C. S. (2018). “Insecticide Resistance in Malaria Vectors: An Update at a Global Scale,” in Towards Malaria Elimination - A Leap Forward. chapter 7. London, UK: IntechOpen.
Roberts D. R., Alecrim W. D., Hshieh P., Grieco J. P., Bangs M., Andre R. G., et al. (2000). A probability model of vector behavior: effects of DDT repellency, irritancy, and toxicity in malaria control. J. Vector Ecol. 25, 48–61.
Scott J. A., Brogdon W. G., Collins F. H. (1993). Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 49, 520–529. doi: 10.4269/ajtmh.1993.49.520
Sherrard-Smith E., Ngufor C., Sanou A., Guelbeogo M. W., N’Guessan R., Elobolobo E., et al. (2022). Inferring the epidemiological benefit of indoor vector control interventions against malaria from mosquito data. Nat. Commun. 13, 3862. doi: 10.1038/s41467-022-30700-1
Skovmand O., Bosselmann R. (2011). Strength of bed nets as function of denier, knitting pattern, texturizing and polymer. Malar J. 10, 87. doi: 10.1186/1475-2875-10-87
Tepa A., Kengne-Ouafo J. A., Djova V. S., Tchouakui M., Mugenzi L. M. J., Djouaka R., et al. (2022). Molecular Drivers of Multiple and Elevated Resistance to Insecticides in a Population of the Malaria Vector Anopheles gambiae in Agriculture Hotspot of West Cameroon. Genes (Basel) 13, 1206. doi: 10.3390/genes13071206
Vontas J., Katsavou E., Mavridis K. (2020). Cytochrome P450-based metabolic insecticide resistance in Anopheles and Aedes mosquito vectors: Muddying the waters. Pesticide Biochem. Physiol. 170, 104666. doi: 10.1016/j.pestbp.2020.104666
WHO (2012). Gobal plan for insecticide resistance management in malaria vectors. (Geneva, Switzerland: World Health Organization).
WHO (2013). Guidelines for laboratory and field testing of long-lasting insecticidal nets (Geneva, Switzerland: World Health Organization).
WHO (2015). Global technical strategy for malaria 2016-2030 (Geneva, Switzerland: World Health Organization).
WHO (2017). Report of the Twentieth WHOPES Working Group Meeting, WHO/HQ, Geneva, 20–24 March 2017: Review of Interceptor G2 LN, DawaPlus 3.0 LN, DawaPlus 4.0 LN, SumiLarv 2 MR, Chlorfenapyr 240 SC. (Geneva, Switzerland: World Health Organization).
WHO (2022a). Manual for monitoring insecticide resistance in mosquito vectors and selecting appropriate interventions (Geneva, Switzerland: World Health Organization).
WHO (2022b). Standard operating procedure for testing insecticide susceptibility of adult mosquitoes in WHO bottle bioassays. (Geneva, Switzerland: World Health Organization).
WHO (2023b). WHO guidelines for malaria WHO/UCN/GMP/2023 (Geneva, Switzerland: World Health Organization).
WHO (2023c). “Implementation guidance (Module 5),” in Semi-field methods for ITNs: Experimental hut tests (Geneva, Switzerland: World Health Organization).
WHO (2023d). “Implementation guidance (Module 3 and 5),” in Bioassay methods for insecticide-treated nets: Tunnel test (Geneva, Switzerland: World Health Organization).
WHO (2023e). “Implementation guidance (Module 3 and 5),” in Bioassay methods for insecticide-treated nets: Cone test (Geneva, Switzerland: World Health Organization).
WHO (2023f). WHO guideline for the prequalification assessment of insecticide- treated nets. (Geneva, Switzerland: World Health Organization)
WHO (2024a). WHO Data requirements and protocol for determining comparative efficacy of vector control products (Geneva: World Health Organization).
WHO (2024b). Generic risk assessment – Human Health: Chlorfenapyr (CAS No. 122453-73-0) An active ingredient in insecticide-treated nets. (Geneva, Switzerland: World Health Organization).
WHOPES (2011). Report of the fourteenth WHOPES working group meeting: WHO/HQ, Geneva (Geneva: WHO Pesticide Evaluation Scheme), 11–15. A pril 2011: review of spinosad EC, lifenet LN, magnet LN, royal sentry LN, yahe LN.WHO/HTM/NTD/WHOPES/2011.7.
WHOPES (2017). Report of the twentieth WHOPES working group meeting, WHO/HQ, Geneva (Geneva: WHO Pesticide Evaluation Scheme), 20–24. March 2017: Review Of: Interceptor G2 Ln Dawaplus 3.0 Ln Dawaplus 4.0 Ln Sumilarv 2 Mr Chlorfenapyr 240 Sc WHO/HTM/NTD/WHOPES/2017.04.
Yunta C., Hemmings K., Stevenson B., Koekemoer L. L., Matambo T., Pignatelli P., et al. (2019). Cross-resistance profiles of malaria mosquito P450s associated with pyrethroid resistance against WHO insecticides. Pestic Biochem. Physiol. 161, 61–67. doi: 10.1016/j.pestbp.2019.06.007
Zahouli J. Z. B., Edi C. A. V., Yao L. A., Lisro E. G., Adou M., Kone I., et al. (2023). Small-scale field evaluation of PermaNet((R)) Dual (a long-lasting net coated with a mixture of chlorfenapyr and deltamethrin) against pyrethroid-resistant Anopheles gambiae mosquitoes from Tiassale, Cote d’Ivoire. Malar J. 22, 36. doi: 10.1186/s12936-023-04455-z
Keywords: Anopheles gambiae, Anopheles coluzzii, Anopheles arabiensis, non-inferiority, chlorfenapyr, bifenthrin, PRONet Duo, Interceptor® G2
Citation: Assenga AA, Ahoua Alou LP, Camara S, Koffi AA, N’Guessan R, Kamande DS, Ngonyani S, Kibondo UA, Odufuwa OG, Ntabaliba WS, Lekundayo RG, Abilah F, Madumla EP, Muganga JB, Moore J and Moore SJ (2025) PRONet Duo insecticide-treated net incorporated with chlorfenapyr and bifenthrin is superior to Interceptor® G2 nets against pyrethroid-resistant Anopheles gambiae sensu lato: a randomized experimental hut trial in Côte d’Ivoire and Tanzania using non-inferiority design. Front. Malar. 3:1540884. doi: 10.3389/fmala.2025.1540884
Received: 06 December 2024; Accepted: 13 February 2025;
Published: 14 March 2025.
Edited by:
Louisa Alexandra Messenger, University of Nevada, Las Vegas, United StatesReviewed by:
Adilson José DePina, CCS-SIDA/MoH, Cabo VerdeCopyright © 2025 Assenga, Ahoua Alou, Camara, Koffi, N’Guessan, Kamande, Ngonyani, Kibondo, Odufuwa, Ntabaliba, Lekundayo, Abilah, Madumla, Muganga, Moore and Moore. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alphonce A. Assenga, YWFzc2VuZ2FAaWhpLm9yLnR6
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.