- 1Department of Computer Engineering, Faculty of Engineering and Technology, University of Buea, Buea, Cameroon
- 2Department of Health Economics Policy and Management, Catholic University of Cameroon, Bamenda, Cameroon
- 3School of Science, Navajo Technical University, Crownpoint, NM, United States
- 4Molecular Diagnostics Research Group, Biotechnology Centre, University of Yaoundé 1, Yaoundé, Cameroon
- 5Science Lab. Tech. Department, School of Science, Akanu Ibiam Federal Polytechnic, Unwana, Nigeria
- 6Noguchi Memorial Institute for Medical Research, University of Ghana, Accra, Ghana
- 7HIV/AIDS Prevention and Control Programme, Baptist Convention Health Service, Bamenda, Cameroon
- 8Medical Research Council, Banjul, The Gambia @ London School of Hygiene and Tropical Medicine, London, United Kingdom
- 9Biochemical Research and Clinical Centre, University of Cape Coast, Cape Coast, Ghana
Background: Malaria remains a serious health challenge; thus, the need for continual improvements in treatment regimens and the adoption of new treatment guidelines to enhance case management is vital. We report trends in malaria case management over 15 years at the Jakiri Health Centre in Cameroon, an area that has been plagued with civil unrest since 2016.
Methods: Data were collected retrospectively from consultation, laboratory, antenatal care (ANC) and prescription registers, and double entered into Excel. Analysis was conducted using SPSS statistics and Microsoft Excel.
Results: A total of 3,800 febrile patients visited the outpatient department (OPD), and patients received 8,324 prescriptions. Of these, 11.6% (964/8324) were anti-malarials, 3.5% (291/8324) were ferrous sulfate (FS), 40.8% (3,396/8324) were antibiotics (AB), and 44.1% (3,673/8324) were analgesics. The antimalarials prescribed were artemisinin-based combination therapies (ACTs) 83.2% (802/964) and quinine (Q) 16.8% (162/964). No ACTs were prescribed between 2006 and 2011. The 5 to <15 years age group received the most proportion of ACTs 42.3% (189/447), followed by the 1 to <5 years age group 31.8% (127/399). Males were more likely to be prescribed ACTs than females were (OR= 1.336, 95% CI 1.141–1.564; p<0.0001). Between 2006 and 2013, the number of quinine prescriptions increased from 17.2% (21/122) in 2006 to a peak of 26.1% (30/115) in 2009. Antibiotic prescription rates were high across all age groups. The prescription of antimalarials to malaria-negative patients was relatively low over time. ACTs were prescribed to all participants attending the ANC who were confirmed malaria-positive. 97.7% of SP was prescribed as intermittent preventive treatment in pregnancy (IPTp) with highest prescription rates observed among women in their third trimester 96.2% (76/79).
Conclusion: This study demonstrated that recommended malaria treatment guidelines have been adopted by the Jakiri Health Centre, which has transitioned from quinine as a first-line treatment to ACTs. ACTs were not used in the facility until 2012. Though anti-malaria prescription was largely appropriate, antibiotics and analgesics were highly over prescribed. There is great need for continuous monitoring and refresher training for health workers to enforce adherence to the guidelines.
Introduction
Malaria is still a significant public health challenge, especially in sub-Saharan Africa, where the disease burden is highest (Ndo et al., 2011; Ndong et al., 2015; Ssempiira et al., 2017). Cameroon is among the endemic countries plagued by this deadly disease, as the entire country is still at risk of transmission (Bowen, 2013; Kwenti et al., 2017; Massoda Tonye et al., 2018). Effective case management remains critical among possible approaches to reduce the malaria burden (Kimbi et al., 2014a). Over the years, several interventions have been implemented to reduce the burden of malaria (Ndo et al., 2011). Vector control is a vital component of malaria prevention and control and relies mainly on the use of long-lasting insecticidal nets (LLIN) (Ndo et al., 2011; Antonio-Nkondjio et al., 2019), insecticide-treated nets (Sumbele et al., 2014; Kwenti et al., 2017), and indoor residual spraying (Apinjoh et al., 2015; Ndong et al., 2019). From 2009 to 2019, over 20 million LLINs were distributed in Cameroon with the help of international partners such as global funds (Antonio-Nkondjio et al., 2019; Nlinwe et al., 2021), and between 2000 and 2015, the prevalence of malaria decreased from 41% to 24.3% due to the increase in the number of bed nets used across the country (MINSANTE, 2018). However, there is a great disparity between the possession and actual use of LLINs, which affects the efficiency of LLINs in vector control in different epidemiological settings in Cameroon (Bowen, 2013; Kimbi et al., 2014b; Fokam et al., 2016; Nlinwe et al., 2021). One approach to reducing the malaria burden is effective case management. Attendance and malaria case management in the Jakiri Health Centre have significantly been affected since 2016 due to ongoing civil unrest in the two English-speaking regions of the country.
In 2001, the WHO recommended using artemisinin-based combination therapies (ACTs) for treating uncomplicated malaria (WHO, 2001; Whegang Youdom et al., 2019). Cameroon adopted the new treatment guidelines in 2004and changed its policy from monotherapy to artemisinin-based combination therapies (ACTs) with artesunate-amodiaquine (ASAQ) as the first-line treatment for uncomplicated malaria (Mangham-Jefferies et al., 2015; Ndong et al., 2015). In 2006, artemether-lumefantrine (AL) was officially adopted in Cameroon as an alternative ACT (NMCP, 2008; Ndong et al., 2015; Whegang Youdom et al., 2019) due to increasing resistance to monotherapies such as chloroquine in several parts of Africa (Talisuna et al., 2004) and Cameroon in particular (Basco et al., 2006; Sayang et al., 2009a). Moreover, quinine (Q) and sulfadoxine-pyrimethamine (SP) were reserved for the treatment of severe malaria and intermittent preventive treatment during pregnancy (IPTp), respectively (Sayang et al., 2009a; Mangham et al., 2012; Ndong et al., 2015).
It has been reported that malaria is overtreated in Sub-Saharan Africa (Reyburn et al., 2007; Rowe et al., 2009; Juma and Zurovac, 2011), including Cameroon (Mangham et al., 2012). This is due to the presumptuous prescription of antimalarials and antibiotics to patients not confirmed to be positive for the malaria parasite at health facilities (Shillcutt et al., 2008; Mbonye et al., 2013). This is believed to be partly due to poor adherence to microscopy results. This led to adopting highly sensitive and specific rapid diagnostic test (RDT) devices in the African region and resource-constrained settings (Mangham et al., 2012). Furthermore, using RDTs reduces the cost of unnecessary treatment with ACTs, especially among those with nonmalarial febrile illness (Lubell et al., 2007; Shillcutt et al., 2008). Thus, the WHO recommended parasitological confirmation of all malaria cases before treatment (Shillcutt et al., 2008; Mangham et al., 2012).
We retrospectively assessed malaria case management at the Jakiri Health Centre over 15 years, starting with the official adoption of the ACT. We hypothesize that malaria was overtreated in Jakiri between 2006 and 2021 through the prescription and overuse of antimalarials, analgesics and antibiotics. This is the first attempt to assess malaria case management in Jakiri health Centre using the 2004 national malaria treatment guidelines in Cameroon. It is hoped that the findings of this study will inform healthcare policy in Cameroon, especially in areas affected by the civil unrest that began in 2016 which let to displacement of people and interruption to remote communities (Omam, 2021; Omam et al., 2021). This significantly affected the access to healthcare services as well as interrupted the supply of health interventions to urban and rural areas. The hard to reach areas were hardest hit with some health facilities closing down (Tanue et al., 2024).
Materials and methods
Study site
This study was conducted at the Jakiri Health Centre, a public health facility. Jakiri is situated in the Bui Division Northwest Region, Cameroon, with geocoordinates of 6° 6’ 0” North and 10° 39’ 0” East (Figure 1). Malaria transmission in Jakiri is perennial but occurs at a low intensity. The primary vectors responsible for spreading malaria are Anopheles gambiae (s.s) and Anopheles funestus. With a high landscape, Jakiri has a population of approximately 6,610 and experiences two main seasons: rainy and dry. The dry and wet seasons run from November to February and March to October respectively. The average annual rainfall ranges from 1500 mm–2000 mm. The average temperature in Jakiri during the warm season is above 24°C and lasts from February to March. The hottest month of the year is March, with an average increase of 25°C and a decrease of 15°C. The cool season lasts for 4.5 months, from June 22 to November 8, with an average daily high temperature below 22°C. The coldest month of the year in Jakiri is August, with an average temperature of 14°C and a peak temperature of 21°C (weatherspark.com, 2020). The main human activity in this area is agriculture—cattle rearing and farming.

Figure 1. Map of Cameroon showing the study site (Eric et al., 2018).
Data collection
The data were collected retrospectively through perusal of consultation, laboratory, and prescription registers of the Jakiri Health Centre. Information was gathered on demographic characteristics, time (months and years), diagnosis and treatment of malaria. The data were entered into Microsoft Excel 2016. Participants were classified into six different age groups: <1 year (0–11 months), 1 to <5 years, 5 to <15 years, 15 to <45 years, 45 to <65 years and ≥65 years. Apart from the data from febrile patients, data was also collected from the antenatal care registers in the Jakiri Health Centre.
Ethical clearance
Ethical clearance for this study was obtained from the Catholic University of Cameroon (CATUC) Bamenda Institutional Review Board (003/BCH/CATUC-IRB/WFM). Administrative authorization was obtained from the Regional Delegation of Public Health, Bamenda Cameroon.
Data analysis
The data were analyzed using the IBM-Statistical Package for Social Sciences (IBM-SPSS) version 26.0. Pearson’s chi-square test was utilized to compare prescription patterns between variables, specifically investigating the prescription of antimalarials and antibiotics across sex and age groups. Additionally, odds ratios were evaluated to determine the statistical and practical significance at p<0.05. Graphical representation of the data was performed using Microsoft Excel 2016.
Results
Malaria diagnoses
Before the year 2012, microscopy was the only tool used for malaria diagnosis in Jakiri Health Centre. Between 2012 and 2016, 40% and 60% of all suspected malaria cases were diagnosed using microscopy and rapid diagnostic tests (RDTs) respectively. Beyond 2016, RDTs were used to diagnose more than 95% of malaria cases (Ndang et al., 2024).
From 2006 to 2021, 3,800 febrile patients were registered at the Jakiri Health Center, out of which 26.6% (1,012/3,800) and 73.4% (2,788/3,800) of whom were suspected to suffer from severe and uncomplicated malaria, respectively. Laboratory analyses confirmed that 24.9% (945/3,800) of patients were infected with malaria of which 902 cases had uncomplicated malaria and 43 had severe malaria.
The proportion of confirmed malaria cases fluctuated over the years, increasing from 19.7% (24/122) in 2006 to 23.3% (21/90) in 2008 and 26.1% (30/115) in 2009. Between 2009 and 2014, the proportion decreased from 26.1% (30/115) to 16.0% (43/268), followed by a steady rise to a peak of 39.8% (129/324) in 2019, before declining to 24.8% (190/766) in 2021. Malaria prevalence was highest in Children below 15 years old. Trends in malaria prevalence in this area during the study period has been reported by Ndang et al. (2024).
A total of 566 pregnant women attended the antenatal care (ANC) at the Jakiri Health Centre between 2014 and 2021. Of these, 48.4% (274/566) aged 15–24 years, 45.1% (255/566) aged 25–35 years and 6.5% (37/566) were 36 to 45 years. The majority of the participants were in the second trimester 70.1% (397/566) while 18.4% (104/566) were in the first trimester and 11.5% (65/566) were in their third trimester. Of all pregnant women, 8.3%(47/566) were confirmed positive for malaria parasite. Malaria prevalence among pregnant women attending ANC seems to decrease with increase in age from 9.9% (27/274) for those 15–24 years to 7.1% (18/255) for the 25–35 years and 5.4% (2/37) for participants 36 to 46 years. Prevalence was 10.6%(11/104), 8.3% (33/397) and 4.6% (3/65) in the first, second and third trimesters respectively.
Malaria case management
Analysis revealed that 8,324 prescriptions were received by patients who sought treatment at the Jakiri Health Centre between 2006 and 2021. The prescribed drugs included antimalarials 11.6% (964/8324), ferrous sulfate (FS) 3.5% (291/8324), antibiotics 40.8% (3396/8324), and analgesics 44.1% (3673/8324). The anti-malaria prescribed were ACTs 83.2% (802/964) and quinine 16.8% (162/964). Patients received one of the following combinations of drugs: ACT only (1.7%), FS alone (0.11%), ACT + antibiotics (AB) (16.4%), quinine + AB (3.9%), FS + AB + ACT (3.0%), FS + AB + quinine (0.3%), or FS + AB (4.2%). A proportion of 61.5% (2,336) of the patients were prescribed only antibiotics, 8.6% were prescribed only analgesics, and 0.3% did not receive any treatment. Analgesics were prescribed to all patients who received either antibiotics or antimalarials. There were no combinations of quinine + ACTs, and quinine + ACTs + antibiotics.
Prescription of ACT
Among the patients who were prescribed ACT, 92.8% (744/802) were confirmed to be malaria positive, while 7.2% (58/802) had a negative result for malaria. No ACTs were prescribed from 2006 to 2011. From 2012 to 2021, the number of ACT prescriptions received by febrile patients fluctuated, with a general increase in prescriptions over time The percentage of prescriptions for ACTs was as follows: 5.1% in 2012, 2.9% in 2013, 15.7% in 2016, 44.7% in 2017, 30.0% in 2018, 39.2% in 2019, 36.8% in 2020, and 24.7% in 2021. The odds of being prescribed an ACT were greater in malaria-positive patients than in those with a negative result for malaria (OR= 178.501, 95% CI 131.83–241.70, p<0.0001), which was statistically significant and practically relevant. Males were more likely to be prescribed an ACT than females were (OR= 1.336, 95% CI 1.141–1.564, p<0.0001). 9.5% (96/1012) of patients diagnosed to have severe malaria were prescribed ACT while 25.3% (706/2788) of those diagnosed with uncomplicated malaria received ACT (Figure 2).
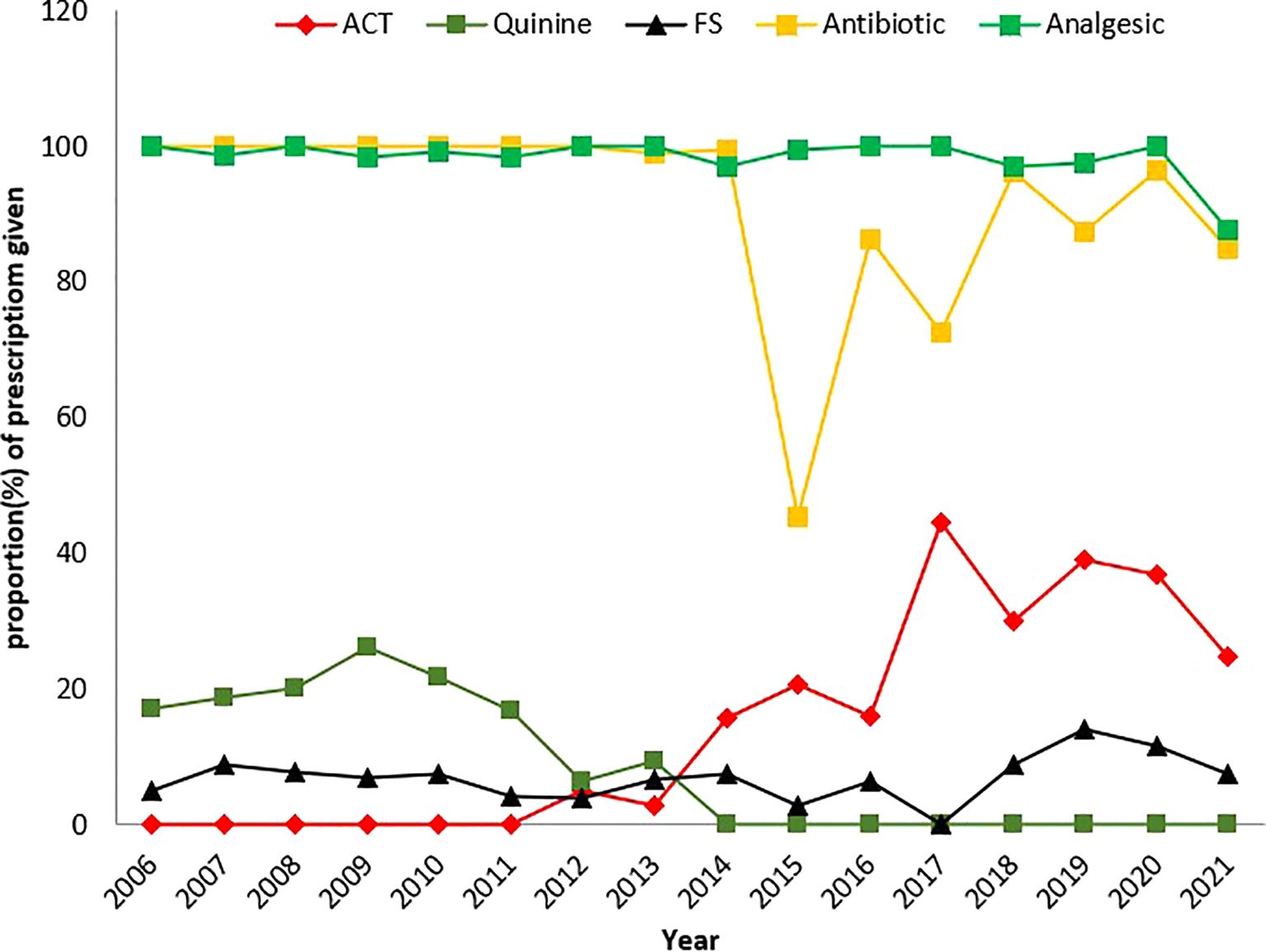
Figure 2. Graphical representation of trends in the prescription of treatment for the management of febrile illnesses (p-value<0.001). Each data point represents the annual proportion of patients treated with a particular drug regimen for a given year.
Prescription of quinine
Quinine was prescribed to 162 patients, 97.5% (158/162) of whom were confirmed to be malaria positive and 2.5% (4/162) of whom were malaria negative. The odds of being prescribed quinine were significantly greater in patients with a positive malaria result than in those with a negative result (OR = 143.09, 95% CI 58.88–387.20, p<0.0001). Quinine prescription increased from 2006 to 2009, but reduced from 2006 to 2013: it rose from 21 (17.2%) in 2006 to 15 (18.8%) in 2007 to 18 (20.0%) in 2008 and to a peak of 30 (26.1%) in 2009. The prescription of quinine decreased to 26 (21.7%) in 2010, 20 (16.8%) in 2011, 10 (6.4%) in 2012 and 19 (9.3%) in 2013 (Figure 2). Between 2014 and 2019, quinine was not prescribed to patients. In 2020 and 2021, two quinine prescriptions were recorded: 1 (0.2%) and 1 (0.1%), respectively. Prescription of quinine in males was 3.6% (54/1482) and that in females was 4.7%(108/2318), but the difference was not statistically significant (X2 = 2.284, p= 0.131) over the study period.
Prescription of FS
A total of 291 patients were prescribed FS, 41.6% (121/291) of whom were confirmed to be malaria positive, while 58.4% (170/291) were confirmed to be malaria negative. The odds of being prescribed FS were greater in malaria-negative patients than in those with a positive test for malaria (OR = 2.319, 95% CI 1.81–2.97, p<0.0001). In terms of gender, there were significantly more female patients (235 [80.8%]) than male patients (56 [19.2%]) who received FS (X2 = 51.701, p<0.0001). More FSs were prescribed to patients in 2019 14.2% (46/324), 2020 11.5% (59/495) and 2021 7.6% (58/766) than in the other years. No FS was prescribed to patients in 2017. Between 2006 and 2012, females were prescribed more FS than males were. There was no recorded FS prescription in 2006, 2008, or from 2010 to 2012 (Figure 2).
Prescription of antibiotics
Of the 3,396 patients who received antibiotics, 27.1% (921/3396) were malaria positive, while 72.9% (2475/3396) were malaria negative. The odds of being prescribed an antibiotic were significantly greater for patients with a negative result than those confirmed to be malaria positive (OR= 5.892, 95% CI 3.874–8.962, p<0.0001). Among those treated, females 60.8% (2066/3396) were prescribed more antibiotics than males 39.2% (1396/3396) [X2 = 0.360, p = 0.549]. Between 2006 and 2012, antibiotics were prescribed to all febrile patients. The annual prescription of antibiotics from 2013 to 2021 generally exceeded 70.0% each year except in 2015, when the proportions decreased to 45.0% (Figure 2).
Prescription patterns across different age groups and gender
Across the various age groups, the prescription of ACTs increased with age for patients <15 years: <1 yr 13.6% (12/88), 1 to <5 years 31.8% (127/399) and 5 to <15 years 42.3% (189/447). For patients 15 years and older, ACT prescription was observed to decrease: 15 to <45 years, 18.0% (363/2015); 45 to <65 years, 14.7% (92/627); and >65 years, 8.5% (19/224). Quinine prescriptions decreased as age increased, with prescriptions less than 6.0% within each age group. Patients aged less than 45 years had similar prescription patterns: 5.7% (5/88) for patients aged <1 yr, 5.0% (20/399) for patients aged 1 to <5 yrs, and 5.1% (23/447) for patients aged 5 to <15 yrs. FS mainly was prescribed to patients in the age groups 1 to <5 years 10.5% (42/399), 15 to <45 years 9.9% (199/2015) and 5 to <15 years 8.5% (38/447). It was least prescribed to patients aged 45 to <65 years 0.8% (5/627). The analysis revealed a decrease in the prescription of antibiotics as age increased. The prescription of antibiotics across all age groups exceeded 80.0%. Like antibiotics, analgesic prescription remained high in all age groups, exceeding 90.0% within each age group. For all confirmed malaria patients, a significant difference was observed between males and females across all the prescriptions received, except for antibiotics, for which the difference was not significant (Table 1A). The difference between prescriptions made for both male and female malaria-negative patients was not significant except for FS (p= 0.002) (Table 1B).
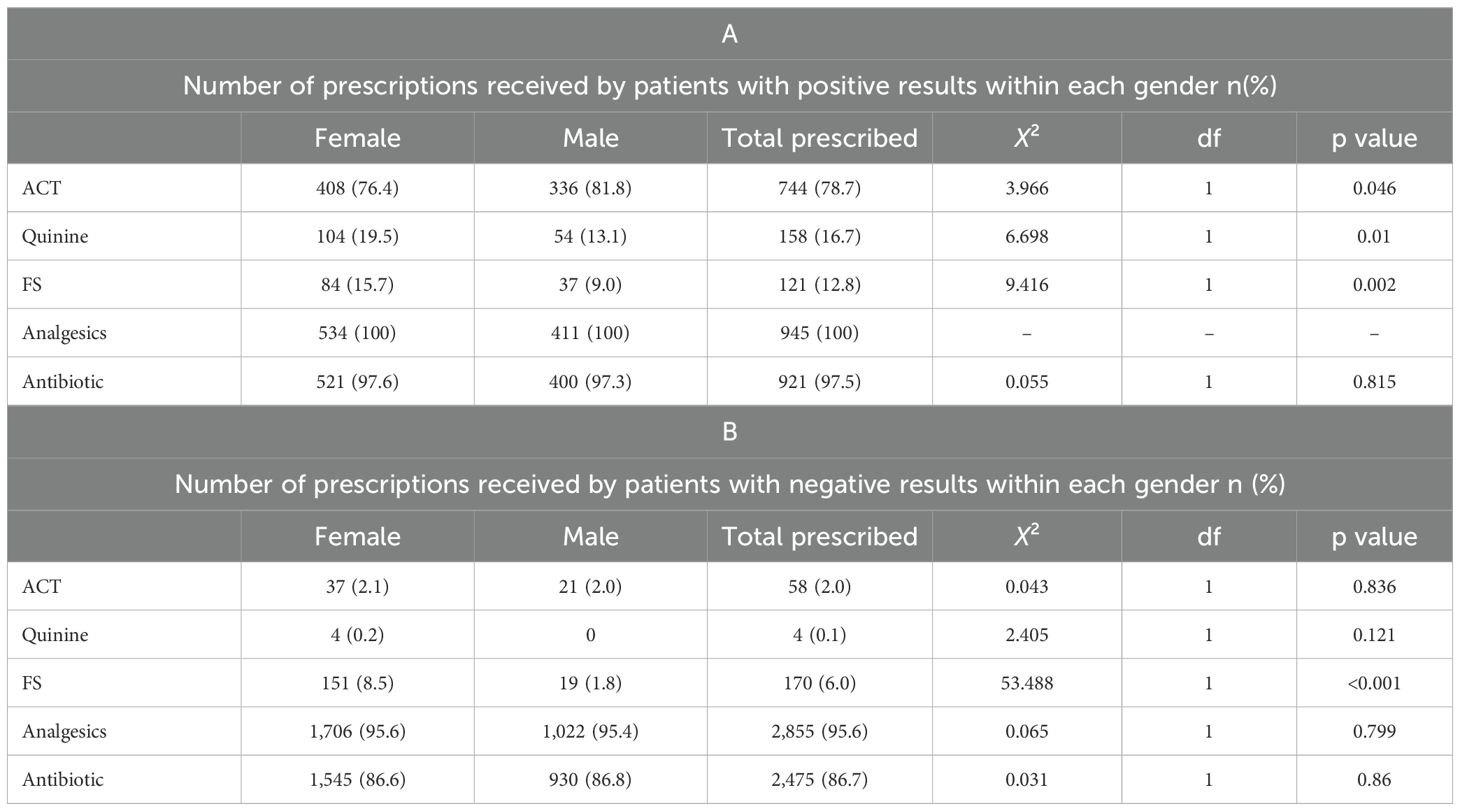
Table 1. Distribution of treatment prescribed within gender for febrile patients tested for malaria: A) confirmed positive malaria cases, B) confirmed negative malaria cases.
The proportions of ACTs prescribed to confirmed malaria patients were similar among the age groups: 1 to <5 years 84.4% (124/147), 5 to <15 years 84.3% (183/217) and 45 to < 65 years 84.0% (84/100). Patients <1 yr and 45 to <65 years were not prescribed FS. Antibiotic prescription was high across the age groups, and analgesics were prescribed to all patients in all age groups for malaria-positive patients. For confirmed malaria patients, quinine was mostly prescribed to children younger than 1 year 29.4% (5/17), followed by those aged 15 to < 45 years 20.5% (91/443) (Figure 3A). The prescription of antimalarials to malaria-negative patients was relatively low, with ACTs being the most commonly prescribed to patients aged 15 to <45 years 2.4% (37/1572). The prescription of antibiotics and analgesics remained high across all age groups (Figures 3A, B).

Figure 3. Pattern of treatment prescribed to patients tested for malaria. (A) Confirmed malaria-positive patients. (B) Confirmed malaria-negative patients. Each data point represents the proportion of prescriptions of a particular treatment given to a specific age group from 2006–2021.
Intermittent preventive treatment and malaria case management for women attending antenatal care
Intermittent preventive treatment using single-dose sulfadoxine–pyrimethamine (SP) to prevent malaria was prescribed to 77.7% (440/566) pregnant women. Of these 2.3% (10/440) were confirmed positive for malaria and 97.7% (430/440) were negative for malaria. Prescription of SP was 86.7% (221/255) and 89.2% (33/37) among women 25–35 years old and 36–46 years old respectively. Most of the pregnant women received SP in the second 93.2% (370/397) and third trimesters95.4% (62/65). Over time, SP was most prescribed in 2016 89.5% (34/38), 2015 85.2% (75/88), and 2021 82.7%(67/81) (Table 2). All participants received antibiotics and FS and no quinine was prescribed. All malaria positive patients were prescribed ACTs and Analgesics. Pregnant women in first trimester received the most ACT 10.6% (11/104).
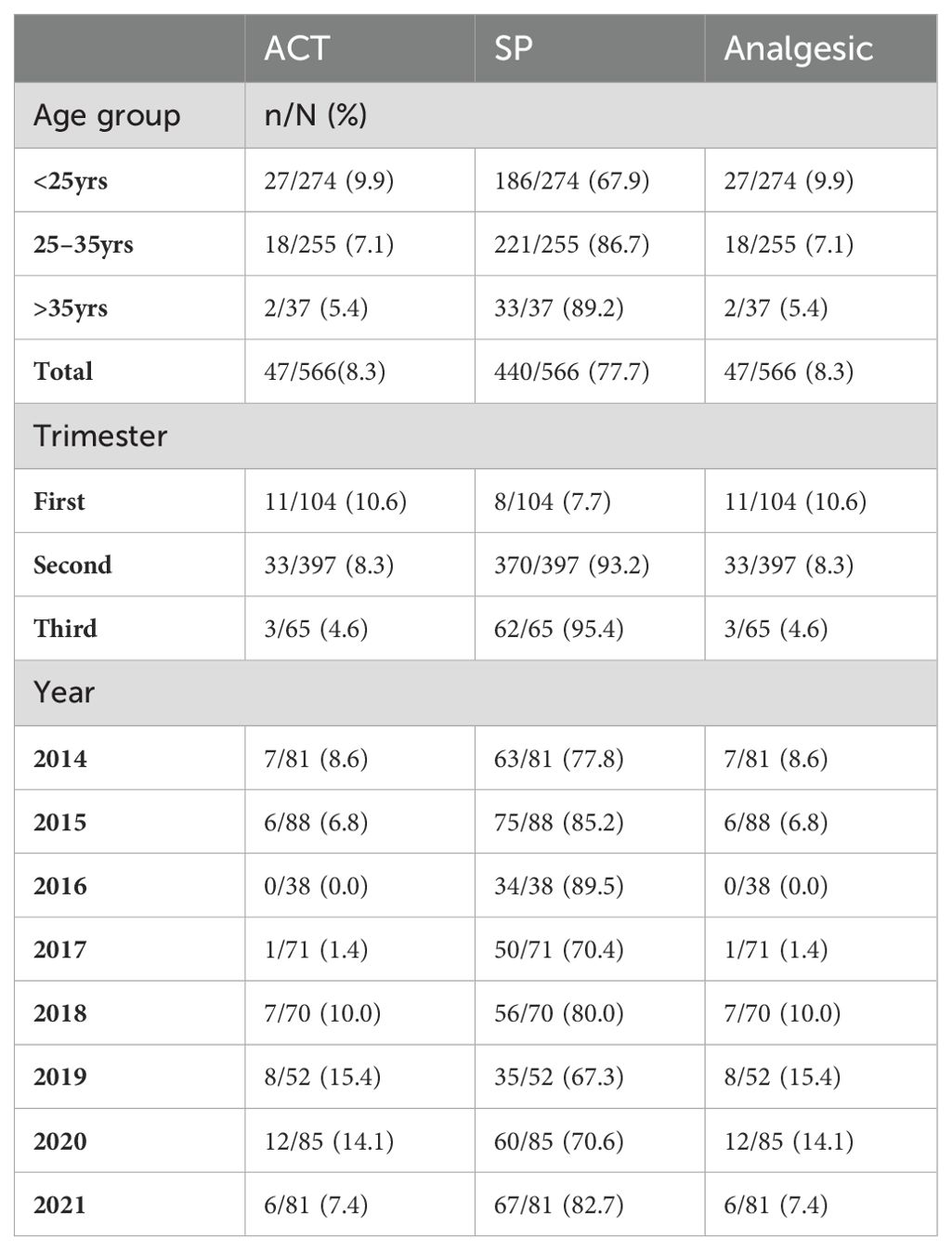
Table 2. Prescription patterns for intermittent preventive treatment for pregnant women (SP, ACTs and Analgesics) in the Jakiri Health District.
Discussion
The findings of this study suggest that, at 66% of the prevalence level, uncomplicated malaria was overdiagnosed in the Jakiri Health Centre from 2006 to 2021. This reflects what happens in Cameroon as reported by Mangham et al. (2012), Sayang et al. (2009) and Ndong et al. (2015). These acts of misdiagnosis led to unnecessary treatments being prescribed to patients who did not carry the parasite or who were not receiving beneficial treatment for other illnesses and infections (Mwangi et al., 2005; Rakotonirina et al., 2008; Parsel et al., 2017). The advent of RDTs and the need to confirm all suspected cases before treatment where possible, as recommended by the WHO, has led to a reduction in the level of overtreatment in many settings (Masanja et al., 2012).
This study revealed that anti-malaria agents and antibiotics were prescribed in different combinations, such as ACT+AB, Q+AB, FS+AB+ACT, FS+AB+Q, and FS+AB. However, no combinations of ACTs and quinine were recorded. The prescription of ACTs and quinine for malaria case management fluctuated over time. These results align with the reports of Ndong and colleagues (Ndong et al., 2015), except that in the latter, in some cases, both ACT and quinine were prescribed to some patients.
There has been no prescription of ACT for the treatment of uncomplicated malaria during the period from 2006 to 2011, as quinine was the only antimalarial agent prescribed along with antibiotics to febrile patients confirmed to have malaria. This is in contrast to the findings of Sayang et al. (2009), where 15% of febrile patients were prescribed ACTs, and another 2012 report, where ACTs were prescribed to 51% of febrile patients (Sayang et al., 2009a; Sayang et al., 2009b; Mangham et al., 2012). This finding might suggest that although the change in malaria treatment policy from monotherapy to ACTs was adopted in Cameroon in 2004, the implementation of the policy was much slower in some health facilities than in others despite the efforts of the National Malaria Control Programme. It is not clear whether this is because the health workers in this facility were not up to date with the new treatment guidelines and preferred to use quinine, which they were comfortable with, or because the cost of treatment with ACTs posed a challenge or because of patient preference (Sayang et al., 2009b). It could also be that confirmed cases were being treated for severe malaria. There is, however, no evidence that no ACTs were used in malaria case management between 2006 and 2011. Instead, cases of underuse and overuse of ACTs were recorded in various epidemiological settings in Cameroon, which was attributed to either nonadherence to the treatment guidelines or the presumptuous prescription of drugs to febrile patients, respectively (Sayang et al., 2009a; Sayang et al., 2009b; Mangham et al., 2012). There is a need for rigorous monitoring when implementing a new treatment policy.
Until 2011, only quinine was prescribed to febrile patients. In 2012, when the first doses of ACTs were prescribed, quinine prescriptions decreased drastically until 2013, after which no prescription was recorded until 2020 and 2021, when 0.2% and 0.1% of quinine prescriptions were recorded, respectively. This finding aligns with Ndong and colleagues, who reported that quinine and ACT prescription fluctuated alternatively (Ndong et al., 2015). These results suggest that after 2013, ten years after its adoption in 2004, the first-line malaria treatment guidelines for the use of ACTs for the treatment of uncomplicated malaria were increasingly being adhered to at the Jakiri Health Centre. Under this malaria treatment policy, quinine was reserved for severe cases. Another possible explanation for the sharp decline in quinine prescriptions over time could be the decrease in severe malaria cases in the area. This is reflected in the fact that in 2019 and 2020, a few cases of quinine use were recorded. Another possible explanation could be linked to the cost. The period after 2013 coincided with the introduction in 2014 of artesunate or artemether free of charge for under 5s and subsidized for older children and adults.
Over 90% of ACTs were prescribed to patients who were confirmed to be positive for malaria. Children between 5 and 15 years of age received the most ACTs (42.3%), followed by children aged 1 to <5 years (31.6%). This percentage is lower than that reported by Sayang and colleagues, who reported that 61% of patients <5 years old were prescribed ACTs. This is in line with findings from Zambia (50%) and Uganda (66%), where <5 children have been reported to receive higher proportions of ACT prescriptions (Zurovac et al., 2007). This, however, contrasts with the findings of Ndong and colleagues, who reported that patients aged <1 year received the most ACTs. These results show improvements in malaria treatment in this health facility, as more than three-quarters of anti-malaria prescriptions were received by patients who were confirmed to have malaria. This shows a gradual but steady shift from the earlier practice where prescribers do not adhere to test results, as reported in Cameroon (Mangham et al., 2012; Ndong et al., 2015), Ghana (Ansah et al., 2010) and Tanzania (Reyburn et al., 2007).
Approximately two-thirds of antibiotics were prescribed to patients with a negative result for malaria. This finding is consistent with reports by Ndong et al. (2014) and Reyburn et al. (2007). Between 2006 and 2012, all febrile patients who visited the Jakiri Health Centre were prescribed an antibiotic except in 2015 and 2017, when the proportions of antibiotics prescribed were 45% and 73%, respectively. The proportions of patients who were prescribed antibiotics ranged from 86 to 99%. The proportion of patients with confirmed malaria diagnosis who received antibiotics was lower than those who were negative for malaria. This suggests that antibiotics were highly overprescribed, especially for malaria patients, at the Jakiri Health Centre. However, it is not clear whether these confirmed malaria cases were coinfections of the malaria parasite and the bacteria.
The findings further suggest that there was excessive overprescription of analgesics (100%) to confirmed malaria-positive patients and more than 95% to patients confirmed to be negative for malaria. This results in inappropriate treatment and places a tremendous financial burden on patients and the health system.
ACTs were prescribed to all malaria patients attending antenatal care and quinine was not used in this health facility between 2014 and 2021 for the treatment of malaria in pregnancy. This contradicts the guidelines for malaria treatment in Cameroon for pregnant women. Malaria in pregnancy is considered and treated as severe malaria. According to the guidelines, pregnant women in their first trimesters confirmed to have malaria are prescribed quinine while ACTs are prescribed to patients as from the second trimester (Medicines for Malaria Venture, 2014). Over 93% of patients in the second and third trimesters received SP while 7.7% of patients in the first trimester were received SP. This is in line with the prescription guidelines for intermittent preventive treatment in pregnancy, as SP is to be administered only to pregnant women as from the second trimester (Medicines for Malaria Venture, 2014). The fact that highest proportion of pregnant women with a positive malaria test were observed in the first trimester points to the urgency to conduct malaria test for pregnant women early in their pregnancy to promptly clear the parasites.
The results presented in this study show malaria case management at the Jakiri Health Centre. The data used were collected retrospectively over a 15 years period and were not randomly selected; hence, the data may not represent the entire population. Additionally, these data were collected from one public health center, including data from confessional and private health facilities, which could further improve the findings. We did not assess bacterial or viral infections over the study period, which would have enhanced the findings. Another factor that could have affected the malaria prevalence and case management in this area is the instability observed since 2016 which led to displacement of the population and disruption of health system functioning, making access to health case very difficult (Folefac, 2022). However, it is not clear what the extend of the instability on the health of the population is as it was not assessed in the current report.
Conclusion
The study demonstrated that malaria treatment guidelines have been adopted in the Jakiri Health Centre with the transition from quinine as a first-line treatment to ACTs. ACTs were not used in the facility until 2012. Though prescription anti-malaria was largely appropriate, antibiotics and analgesics were highly over prescribed. There is a great need for continuous monitoring as well as refresher training for health workers to enforce adherence to the guidelines and health workers’ education and training on antimicrobial resistance to promote antimicrobial stewardship. This could lower the drug pressure and cost burden on the population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Catholic University of Cameroon (CATUC) Bamenda Institutional Review Board (003/BCH/CATUC-IRB/WFM). Administrative authorization was obtained from the Regional Delegation of Public Health, Bamenda Cameroon. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
NN: Writing – original draft, Formal analysis. NM: Conceptualization, Data curation, Methodology, Writing – review & editing. PM: Formal analysis, Writing – review & editing. OS: Writing – review & editing. CC: Writing – review & editing. EC: Writing – review & editing. AA: Formal analysis, Writing – review & editing. NC: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACT, Artemisinin-based combination therapy; IPTp, Intermettent preventive treatment in pregnancy; SP, Sulfadoxine-pyrimethamine; INTs, Insecticide-treated nets; LLINs, Long-lasting insecticidal nets; RDTs, Rapid diagnostic tests; ASAQ, Artesunate-amodiaquine; AL, Artemether-lumefantrine; FS, Ferrous sulfate.
References
Ansah E. K., Narh-Bana S., Epokor M., Akanpigbiam S., Quartey A. A., Gyapong J., et al. (2010). Rapid testing for malaria in settings where microscopy is available and peripheral clinics where only presumptive treatment is available: a randomised controlled trial in Ghana. BMJ 340, c930. doi: 10.1136/bmj.c930
Antonio-Nkondjio C., Ndo C., Njiokou F., Bigoga J. D., Awono-Ambene P., Etang J., et al. (2019). Review of malaria situation in Cameroon: technical viewpoint on challenges and prospects for disease elimination. Parasites Vectors 12, 501. doi: 10.1186/s13071-019-3753-8
Apinjoh T. O., Anchang-Kimbi J. K., Mugri R. N., Tangoh D. A., Nyingchu R. V., Chi H. F., et al. (2015). The effect of insecticide treated nets (ITNs) on plasmodium falciparum infection in rural and semi-urban communities in the south west region of Cameroon. PloS One 10, e0116300. doi: 10.1371/journal.pone.0116300
Basco L. K., Ngane V. F., Ndounga M., Same-Ekobo A., Youmba J.-C., Abodo R. T. O., et al. (2006). Molecular epidemiology of malaria in Cameroon. XXI. Baseline therapeutic efficacy of chloroquine, amodiaquine, and sulfadoxine-pyrimethamine monotherapies in children before national drug policy change. Am. J. Trop. Med. Hyg. 75, 388–395. doi: 10.4269/ajtmh.2006.75.388
Bowen H. L. (2013). Impact of a mass media campaign on bed net use in Cameroon. Malaria J. 12, 36. doi: 10.1186/1475-2875-12-36
Eric B., Agwafo T., Silas Lendzele S., Eric M., Jaidzemo S. (2018). Market gardening as A strategy for poverty alleviation in BUI division, north west region of Cameroon. Int. J. Bus. Manag.
Fokam E. B., Dzi K. T. J., Ngimuh L., Enyong P. (2016). The effect of long lasting insecticide bed net use on malaria prevalence in the tombel health district, south west region-Cameroon. Malar. Res. Treat 2016, 3216017. doi: 10.1155/2016/3216017
Folefac C. H. (2022). Assessing the impact of political crisis in Cameroon, 1972-2018. North-West University, South Africa.
Juma E., Zurovac D. (2011). Changes in health workers’ malaria diagnosis and treatment practices in Kenya. Malaria J. 10, 1. doi: 10.1186/1475-2875-10-1
Kimbi H. K., Nkesa S. B., Ndamukong-Nyanga J. L., Sumbele I. U., Atashili J., Atanga M. B. (2014a). Knowledge and perceptions towards malaria prevention among vulnerable groups in the Buea Health District, Cameroon. BMC Public Health 14, 883. doi: 10.1186/1471-2458-14-883
Kimbi H. K., Nkesa S. B., Ndamukong-Nyanga J. L., Sumbele I. U. N., Atashili J., Atanga M. B. S. (2014b). Socio-demographic factors influencing the ownership and utilization of insecticide-treated bed nets among malaria vulnerable groups in the Buea Health District, Cameroon. BMC Res. Notes 7, 624. doi: 10.1186/1756-0500-7-624
Kwenti T. E., Kwenti T. D. B., Latz A., Njunda L. A., Nkuo-Akenji T. (2017). Epidemiological and clinical profile of paediatric malaria: a cross sectional study performed on febrile children in five epidemiological strata of malaria in Cameroon. BMC Infect. Dis. 17, 499. doi: 10.1186/s12879-017-2587-2
Lubell Y., Reyburn H., Mbakilwa H., Mwangi R., Chonya K., Whitty C. J. M., et al. (2007). “The cost-effectiveness of parasitologic diagnosis for malaria-suspected patients in an era of combination therapy,” in Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives: Supplement to Volume 77(6) of American Journal of Tropical Medicine and Hygiene. American Society of tropical Medicine and Hygiene.
Mangham LJ, Cundill B, Achonduh OA, Ambebila JN, Lele AK, Metoh TN, et al. (2012). Malaria prevalence and treatment of febrile patients at health facilities and medicine retailers in Cameroon. Trop. Med. Int. Health 17, 330–342. doi: 10.1111/j.1365-3156.2011.02918.x
Mangham-Jefferies L., Hanson K., Mbacham W., Onwujekwe O., Wiseman V. (2015). Mind the gap: knowledge and practice of providers treating uncomplicated malaria at public and mission health facilities, pharmacies and drug stores in Cameroon and Nigeria. Health Policy Plann. 30, 1129–1141. doi: 10.1093/heapol/czu118
Masanja I. M., Selemani M., Amuri B., Kajungu D., Khatib R., Kachur S. P., et al. (2012). Increased use of malaria rapid diagnostic tests improves targeting of anti-malarial treatment in rural Tanzania: implications for nationwide rollout of malaria rapid diagnostic tests. Malar. J. 11, 221. doi: 10.1186/1475-2875-11-221
Massoda Tonye S. G., Kouambeng C., Wounang R., Vounatsou P. (2018). Challenges of DHS and MIS to capture the entire pattern of malaria parasite risk and intervention effects in countries with different ecological zones: the case of Cameroon. Malaria J. 17, 156. doi: 10.1186/s12936-018-2284-7
Mbonye A. K., Lal S., Cundill B., Hansen K. S., Clarke S., Magnussen P. (2013). Treatment of fevers prior to introducing rapid diagnostic tests for malaria in registered drug shops in Uganda. Malar. J. 12, 131. doi: 10.1186/1475-2875-12-131
Medicines for Malaria Venture (2014). Guidelines for the management of malaria in Cameroon: intended for health personnel. Available online at: https://www.scribd.com/document/807460011/Cameroon-Guide-de-Pec-Final-Anglais-2 (Accessed 21 Feb 2025).
MINSANTE (2018). (2018) minsante. XIième Journée mondiale de lutte contre le paludisme “prêt à vaincre le paludisme” Nous sommes la génération qui peut éliminer le paludisme (Dossier de Presse. Minsante), 1–20.
Mwangi T. W., Mohammed M., Dayo H., Snow R. W., Marsh K. (2005). Clinical algorithms for malaria diagnosis lack utility among people of different age groups. Trop. Med. Int. Health 10, 530–536. doi: 10.1111/j.1365-3156.2005.01439.x
Ndang N. H., Mengnjo N. C., Netongo P. M., Chuye N. H., Chu C. E., Hsiang M., et al. (2024). Trends in malaria prevalence over 15 years (2006–2021) in Jakiri North-West Region, Cameroon. Discovery Public Health 21, 227. doi: 10.1186/s12982-024-00361-0
Ndo C., Menze-Djantio B., Antonio-Nkondjio C. (2011). Awareness, attitudes and prevention of malaria in the cities of Douala and Yaoundé (Cameroon). Parasites Vectors 4, 181. doi: 10.1186/1756-3305-4-181
Ndong I. C., Okyere D., Enos J. Y., Mensah B. A., Nyarko A., Abuaku B., et al. (2019). Prevalence of asymptomatic malaria parasitaemia following mass testing and treatment in Pakro sub-district of Ghana. BMC Public Health 19, 1622. doi: 10.1186/s12889-019-7986-4
Ndong I. C., van Reenen M., Boakye D. A., Mbacham W. F., Grobler A. F. (2015). Trends in malaria case management following changes in the treatment policy to artemisinin combination therapy at the Mbakong Health Centre, Cameroon 2006–2012: A retrospective study. Acta Trop. 150, 100–106. doi: 10.1016/j.actatropica.2015.06.014
Nlinwe N. O., Singong Y. C., Florentine T. M. R. (2021). Evaluation of malaria preventive measures among adult patients attending the Bamendjou and Foumbot district hospitals of the West Region of Cameroon. Malaria J. 20, 60. doi: 10.1186/s12936-021-03592-7
NMCP (2008). (2008) National Malaria Control Programme. Guidelines for the management of malaria in Cameroon (Yaounde: Ministry of Public Health) (Accessed 30 Sep 2023).
Omam L.-A. (2021) Metuge A Rapid response mechanism in conflict-affected settings of Cameroon: lessons learned from a multisector intervention for internally displaced persons. J. Global Health Rep. 15, 15. doi: 10.1186/s13031-021-00427-9
Omam L.-A., Jarman E., Ekokobe W., Evon A., Omam E. N. (2021). Mobile clinics in conflict-affected communities of North West and South West regions of Cameroon: an alternative option for differentiated delivery service for internally displaced persons during COVID-19. Confl. Health 15, 90. doi: 10.1186/s13031-021-00427-9
Parsel SM, Gustafson SA, Friedlander E, Shnyra AA, Adegbulu AJ, Liu Y, et al. (2017). Malaria over-diagnosis in Cameroon: diagnostic accuracy of Fluorescence and Staining Technologies (FAST) Malaria Stain and LED microscopy versus Giemsa and bright field microscopy validated by polymerase chain reaction. Infect. Dis. Poverty. 6, 32. doi: 10.1186/s40249-017-0251-0
Rakotonirina H., Barnadas C., Raherijafy R., Andrianantenaina H., Ratsimbasoa A., Randrianasolo L., et al. (2008). Accuracy and reliability of malaria diagnostic techniques for guiding febrile outpatient treatment in malaria-endemic countries. Am. J. Trop. Med. Hyg. 78, 217–221. doi: 10.4269/ajtmh.2008.78.217
Reyburn H., Mbakilwa H., Mwangi R., Mwerinde O., Olomi R., Drakeley C., et al. (2007). Rapid diagnostic tests compared with malaria microscopy for guiding outpatient treatment of febrile illness in Tanzania: randomised trial. BMJ 334, 403. doi: 10.1136/bmj.39073.496829.AE
Rowe A. K., de León G. F. P., Mihigo J., Santelli A. C. F., Miller N. P., Van-Dúnem P. (2009). Quality of malaria case management at outpatient health facilities in Angola. Malar. J. 8, 275. doi: 10.1186/1475-2875-8-275
Sayang C., Gausseres M., Vernazza-Licht N., Malvy D., Bley D., Millet P. (2009a). Treatment of malaria from monotherapy to artemisinin-based combination therapy by health professionals in urban health facilities in Yaoundé, central province, Cameroon. Malar. J. 8, 176. doi: 10.1186/1475-2875-8-176
Sayang C., Gausseres M., Vernazza-Licht N., Malvy D., Bley D., Millet P. (2009b). Treatment of malaria from monotherapy to artemisinin-based combination therapy by health professionals in rural health facilities in southern Cameroon. Malar. J. 8, 174. doi: 10.1186/1475-2875-8-174
Shillcutt S., Morel C., Goodman C., Coleman P., Bell D., Whitty C. J., et al. (2008). Cost-effectiveness of malaria diagnostic methods in sub-Saharan Africa in an era of combination therapy. Bull. World Health Organ 86, 101–110. doi: 10.2471/BLT.07.042259
Ssempiira J., Nambuusi B., Kissa J., Agaba B., Makumbi F., Kasasa S., et al. (2017). Geostatistical modelling of malaria indicator survey data to assess the effects of interventions on the geographical distribution of malaria prevalence in children less than 5 years in Uganda. PloS One 12, e0174948. doi: 10.1371/journal.pone.0174948
Sumbele I. U., Ning T. R., Bopda O. S., Nkuo-Akenji T. (2014). Variation in malariometric and red cell indices in children in the Mount Cameroon area following enhanced malaria control measures: evidence from a repeated cross-sectional study. Malaria J. 13, 334. doi: 10.1186/1475-2875-13-334
Talisuna A. O., Bloland P., D’Alessandro U. (2004). History, dynamics, and public health importance of malaria parasite resistance. Clin. Microbiol. Rev. 17, 235–254. doi: 10.1128/CMR.17.1.235-254.2004
Tanue EA, Omam LA, Ayuk GT, Noukeme BM, Metuge A, Nganmou I, et al. (2024). A formative cross-sectional study to assess caregiver’s health-seeking behaviour and knowledge surrounding malaria, and understand the burden of malaria among children under-five in conflict-affected communities of Cameroon. Malar. J. 23, 99. doi: 10.1186/s12936-024-04902-5
weatherspark.com (2020). Jakiri Climate, Weather By Month, Average Temperature (Cameroon) - Weather Spark. Available online at: https://weatherspark.com/y/65568/Average-Weather-in-Jakiri-Cameroon-Year-Round (Accessed 18 Oct 2023).
Whegang Youdom S., Chiabi A., Basco L. K. (2019). Monitoring the efficacy and safety of artemisinin-based combination therapies: A review and network meta-analysis of antimalarial therapeutic efficacy trials in Cameroon. Drugs R. D. 19, 1–14. doi: 10.1007/s40268-018-0259-3
WHO (2001). (2001) World Health Organization. Antimalarial drug combination therapy. Report of a WHO technical consultation (Geneva: World Health Organization) (Accessed 30 Sep 2023).
Keywords: malaria, treatment, case management, Jakiri, Cameroon
Citation: Ndang NH, Mengnjo NC, Netongo PM, Soniran OT, Chu CE, Chiabi E, Amambua-Ngwa A and Cheng NI (2025) Malaria case management over 15 years (2006–2021) in Jakiri North-West Region Cameroon. Front. Malar. 3:1518778. doi: 10.3389/fmala.2025.1518778
Received: 14 November 2024; Accepted: 23 January 2025;
Published: 25 February 2025.
Edited by:
Andre Lin Ouedraogo, Bill and Melinda Gates Foundation, United StatesReviewed by:
Sant Muangnoicharoen, Mahidol University, ThailandAdilson José DePINA, CCS-SIDA/MoH, Cabo Verde
Sanie Sesay, Sanofi, France
Copyright © 2025 Ndang, Mengnjo, Netongo, Soniran, Chu, Chiabi, Amambua-Ngwa and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ndong Henry Ndang, bmRvbmdoZW5yeW5kYW5nQGdtYWlsLmNvbQ==; Ndong Ignatius Cheng, bmRvbmdpY2hlbmdAeWFob28uY29t
 Ndong Henry Ndang
Ndong Henry Ndang Njodzela Christian Mengnjo2
Njodzela Christian Mengnjo2 Palmer Masumbe Netongo
Palmer Masumbe Netongo