- 1Department of Medical Laboratory Science, College of Medicine and Health Science, Wachemo University, Hossana, Ethiopia
- 2Department of Medical Parasitology, School of Biomedical and Laboratory Science, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
- 3Department of Medical Laboratory Science, College of Health Science, Woldia University, Woldia, Ethiopia
- 4Department of Medical Laboratory Science, Hossana College of Health Science, Hossana, Ethiopia
- 5College of Health Science, Oda Bultum University, Chiro, Ethiopia
- 6Department of Medical Laboratory Science, College of Medicine and Health Science, Worabe University, Worabe, Ethiopia
Background: Ethiopia has faced a significant burden of malaria, enduring endemic transmission in various regions. Despite concerted efforts spanning years, malaria remains a persistent public health issue, particularly affecting underserved rural communities. Previous developments in vector control, diagnostic capabilities, and treatment protocols have led to notable reductions in malaria morbidity and mortality. However, challenges persist, including the emergence of drug and insecticide resistance, compounded by environmental and demographic factors. Understanding the historical context and contemporary challenges is crucial for navigating Ethiopia’s path toward malaria elimination.
Methods: From October 2023 to January 2024, a systematic search was conducted across open access journals such as PubMed, EMBASE, CINALH, Web of Science, Global Health, and Google Scholar using MeSH and Emtree terms for malaria. The focus was on challenges of malaria elimination in Ethiopia, such as drug resistance, vectors’ insecticide resistance and the loss of the diagnostic potential of rapid diagnostic kits.
Main text: Recent trend analyses and World Health Organization reports indicate resurgence in malaria cases in Ethiopia. Factors contributing to this resurgence include emerging drug resistance, insecticide resistance, and genetic mutations such as single nucleotide polymorphisms. Other challenges include the spreading resistance to effective antimalarial drugs, socio-cultural barriers to malaria elimination, the challenge posed by Plasmodium vivax in elimination efforts, and the occurrence of imported cases in previously low burden areas. To reverse the rising trend of malaria cases, it is necessary to customize intervention strategies through active community engagement, rigorous healthcare infrastructure, and learning from countries that have successfully eliminated malaria.
Conclusion: While past successes are notable, they do not guarantee current progress, highlighting the need for rigorous implementation of strategies and adaptation of intervention methods to ensure the success of malaria elimination efforts. However, recent data suggests a concerning resurgence in malaria cases, potentially fueled by emerging drug and insecticide resistance. To achieve sustained success in malaria elimination, there is an urgent need for continued vigilance, customization of intervention strategies, and robust collaboration between stakeholders to address these challenges effectively.
1 Introduction
Malaria, a mosquito-borne infectious disease caused by parasites of the Plasmodium genus, remains a significant global health challenge, particularly in tropical and subtropical regions (Kolawole et al., 2023). The disease exacts a heavy toll on human health and socioeconomic development, with millions of cases reported annually worldwide (Chima et al., 2003; Worrall et al., 2005). Malaria manifests clinically with a spectrum of symptoms, including fever, chills, headache, and muscle aches, often mimicking flu-like illness. Moreover, severe cases can progress rapidly, leading to life-threatening complications such as cerebral malaria, severe anemia, respiratory distress, and multi-organ failure (Warrell, 2017; Balaji et al., 2020).
The disease has deep roots in Ethiopia, with evidence of its presence dating back centuries. The country’s diverse ecological landscape provides suitable settings for malaria transmission, particularly in lowland areas with abundant mosquito breeding sites (Alelign and Dejene, 2016). Over the years, Ethiopia has implemented various malaria control initiatives, including vector control measures, case management strategies, and community-based interventions. Despite these efforts, malaria remains endemic in many parts of the country, posing significant health risks to vulnerable populations (Kassa, 2015; Mutero et al., 2020).
Despite substantial progress in malaria control over the years, the disease continues to exert a heavy burden on health systems and populations, contributing to morbidity, mortality, and economic losses. As Ethiopia strives to eliminate malaria by 2030, understanding the historical context, current challenges, and future perspectives is essential for adjustment and customizing effective elimination strategies (Adugna, 2011; Ayele et al., 2012).
2 Objective of the review
This review aimed to critically examine the burden of malaria in Ethiopia during the pre-elimination era, focusing on historical trends, current challenges, and future prospects. By analyzing epidemiological data, policy documents, and research studies, we identified key factors influencing malaria transmission dynamics and assess the feasibility of achieving malaria elimination by 2030.
3 Burden of malaria in Ethiopia
3.1 Overview of malaria prevalence in Ethiopia
Malaria continues to impose a significant health burden in Ethiopia, with an estimated 68% of the population living in areas with moderate to high malaria transmission rates (Kassa, 2015). The disease in humans is caused by five Plasmodium species globally, but in Ethiopia, Plasmodium falciparum and P. vivax are the primary parasites contributing to the burden (Girum et al., 2019; Aschale et al., 2023). Parasite transmission in the country is mainly driven by Anopheles arabiensis, with other species like An. pharoensis, An. coustani s.l., An. nili, An. funestus, An. stephensi and An. demeilloni playing smaller roles (Aschale et al., 2023).
The disease disproportionately affects rural communities, where access to healthcare services and preventive measures is limited (Abdishu et al., 2022; Graves et al., 2009; Hailu et al., 2017). In 2022, Ethiopia reported approximately 2.4 million confirmed malaria cases, making it one of the highest-burden countries in sub-Saharan Africa (WHO, 2023). In Ethiopia, the burden of malaria varied significantly across different locations. Reports indicated high prevalence rates in various regions, including Kola Diba in Northwest Ethiopia (75%) (Alemu et al., 2012), Benishangul-Gumuz regional state in Western Ethiopia (51.8%) (Alkadir et al., 2020), Woreta town (48%) (Alelign et al., 2018), and the Omo zone of Southern Ethiopia (41.5%).
The economic burden of malaria in Ethiopia is substantial, with direct and indirect costs estimated to exceed $200 million annually, constituting approximately 10% of its total health expenditure (Jobin, 2014; Eregata et al., 2019). These costs encompass expenses related to diagnosis, treatment, and productivity losses due to illness and absenteeism from work or school. Despite the rising collaboration funding by the Global Fund, PM/USAID, and the government from 2020 to 2022 (WHO, 2023), malaria-related expenditures continue to place a significant strain on household finances and national healthcare budgets, thereby undermining efforts to achieve sustainable development goals.
3.2 History of malaria epidemics in Ethiopia
Malaria has historically been endemic throughout the country, posing significant health risks to its population (Negash et al., 2004; Adugna, 2011; Yukich et al., 2013). Ethiopia commenced its battle against malaria over half a century ago. Initially, malaria control began as a pilot project in the 1950s, subsequently transitioning into a national eradication campaign in the 1960s, followed by a control strategy in the 1970s. This effort has witnessed alternating periods of success and failure. In 1976, the vertical organization known as the National Organization for the Control of Malaria and Other Vector-borne Diseases emerged from the Malaria Eradication Service. As is the case in other regions where malaria is endemic, the disease remains far from being conquered. The causative agent, Plasmodium, has developed resistance to several drugs, while the vector mosquito has evolved mechanisms to resist the chemical interventions (Merida, 1965; Vecchiato, 1991; Gish, 1992; Negash et al., 2004).
3.3 Elimination efforts in Ethiopia
In March 1966, systematic malaria eradication operations began in Ethiopia, with the ambitious goal of eliminating malaria from the country by 1980. These efforts were part of a global initiative led by the World Health Organization (WHO) to eradicate malaria worldwide. Ethiopia’s eradication campaign included a combination of vector control measures, such as Indoor Residual Spraying (IRS), and mass drug administration (Tesfaye, 1973; Britanak et al., 1974; Janssens and Wery, 1987).
Despite initial hopefulness, Ethiopia’s campaign to eliminate malaria encountered numerous challenges and setbacks. During the 1958 epidemic, the estimated number of malaria cases was about three million. The favorable climatic conditions in 1958 facilitated the propagation, longevity, and dispersal of the vector. These circumstances were compounded by the presence of insecticide-resistant vectors and Chloroquine-resistant P. falciparum, contributing to the severity of the situation. These challenges led to a significant toll, with approximately 150,000 deaths reported in Ethiopia in 1962 alone. The widespread use of dichlorodiphenyltrichloroethane (DDT) for IRS was met with resistance from mosquito populations, limiting its effectiveness. Furthermore, the failure to sustain elimination efforts in target areas, particularly in rural and remote regions, impeded progress toward achieving goals (Pankhurst, 1966; Farvar et al., 1971; Teklehaimanot, 1986).
3.4 Current trends of malaria cases (2019-2022)
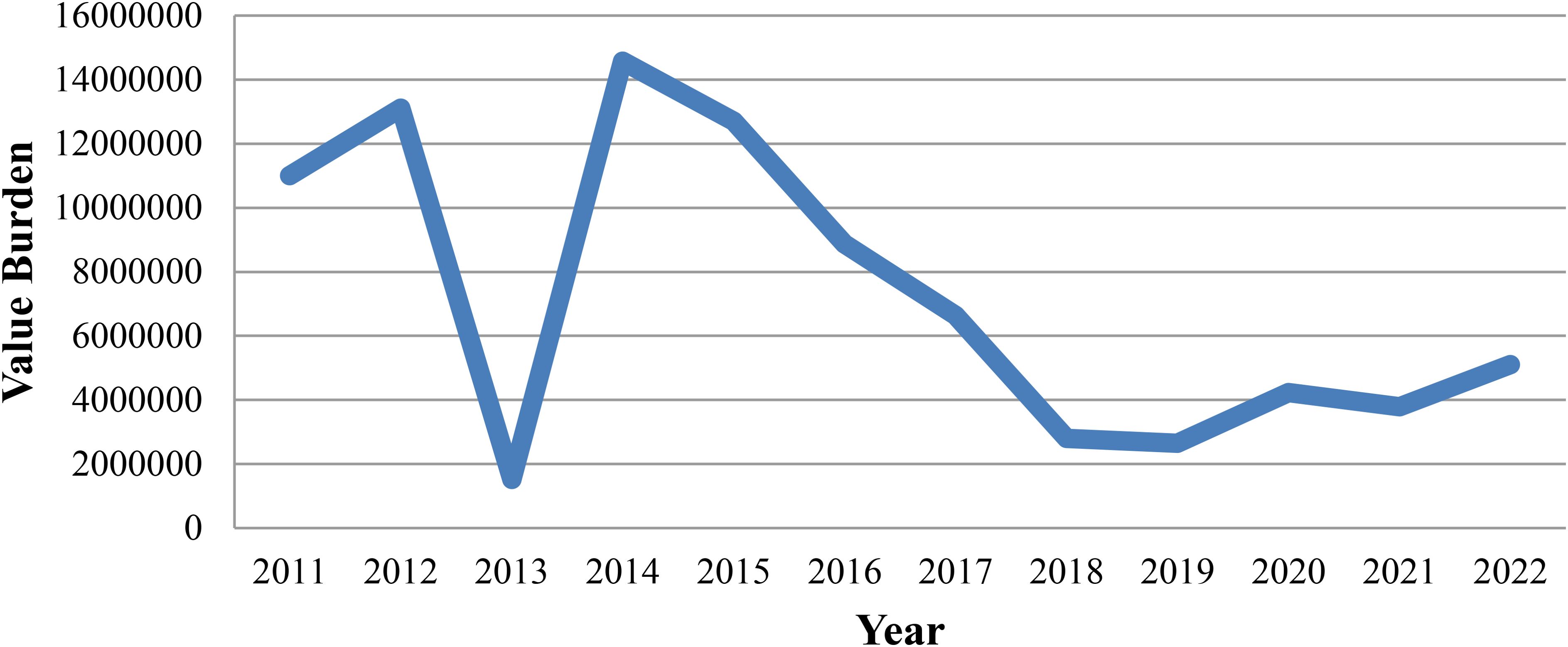
Despite on-going control efforts, Ethiopia has witnessed a troubling surge in malaria cases in recent years, marking a reversal of the declining trend observed until 2015 (Liu and Qin, 2023; WHO, 2023). Between 2016 and 2019, the number of confirmed malaria cases decreased by 47%. According to the Health Management Information System reports from 2016 to 2019, malaria-related admissions have significantly decreased. Particularly noteworthy is the decline observed in 2019, with only 15,307 admissions recorded compared to over 30,000 admissions in 2018. Similarly, malaria-attributed deaths have seen an annual decline. Deaths due to malaria were decreased by 58%, from 510 to 213 between 2016 and 2019. However, driven by the continued fallout of pandemic-related program disruptions, armed conflict, and displacement, Ethiopia experienced a 32.5% increase in confirmed malaria cases between 2021 and 2022, rising from 1.1 million to 1.5 million. As of 2023, reported malaria cases have increased by 150% and 120%, respectively, compared to the same periods in 2021 and 2022 (Nureye and Tekalign, 2023; WHO, 2023).
From 2019 to 2022, the number of reported malaria cases has shown a steady increase, indicative of the persistent challenges encountered in disease prevention and control efforts Figure 1 (WHO, 2023).
3.5 Malaria elimination roadmap in Ethiopia
In 2014, Ethiopia’s Ministry of Health introduced a Malaria Elimination Roadmap, targeting the elimination of malaria by 2030. By expanding key interventions since 2005, including Long Lasting Insecticidal Net (LLIN) distribution and IRS, malaria cases have notably decreased. This encouraged the Ethiopia Ministry of Health aims for nationwide elimination by 2030 (Aregawi et al., 2014; MoHE, 2017; MOHE, 2021).
The roadmap delineates with global malaria elimination objectives. These objectives comprise reducing malaria incidence, eliminating malaria hotspots, preventing malaria resurgence, and sustaining elimination gains (World Health Organization, 2015; MoHE, 2017). However, Ethiopia currently faces challenges in malaria control, leading to the possibility of failing to achieve the 2030 elimination goal (Yalew, 2022). Identifying the impeding hurdles and overcoming them requires extensive efforts and strategic improvements. Learning from past efforts and embracing innovative strategies can propel Ethiopia toward its goal of malaria elimination by 2030.
4 Challenges to malaria elimination in Ethiopia
4.1 Artemisinin resistance and single nucleotide polymorphisms
Artemisinin resistance, characterized by delayed parasite clearance following artemisinin treatment, has emerged in some regions of Ethiopia (Jaiteh et al., 2021; Fola et al., 2023). This resistance is associated with Single Nucleotide Polymorphisms (SNPs) in the parasite’s genome, particularly the kelch13 (K13) gene (Volkman et al., 2017).
The presence of the P. falciparum carrying the K13 622I mutation, associated with partial resistance to artemisinin, was detected in a significant proportion of samples (Fola et al., 2023). The 622I mutation had only been previously described in northwest Ethiopia, near the Sudan border in 2014 at 2.4% prevalence (Bayih et al., 2016); however, an increase of such mutation was reported in the same location to 9.5% (Alemayehu AA. et al., 2021). Noteworthy is that artemisinin-based combination therapy (ACT), specifically artemether-lumefantrine, has been the primary treatment for uncomplicated falciparum malaria in Ethiopia since 2004 (Ababa, 2004).
Several significant mutations were discovered in the P. falciparum multidrug resistance gene 1 (pfmdr1), including N86Y (wild), Y184F (mutant), and D1246Y (wild), as well as in other genes linked to lumefantrine resistance, the partner drug of artemisinin (Malmberg et al., 2013; Fola et al., 2023). A single case study reported a patient with P. falciparum malaria resistant to Ethiopia’s intended use of the longer half-life drug Dihydroartemisinin-Piperaquine (Russo et al., 2018).
4.2 Vector insecticide resistance
Mosquitoes, primarily Anopheles species, have developed resistance to commonly used insecticides, reducing the effectiveness of IRS and LLIN. In Ethiopia, resistance among vectors to insecticides has continued to rise despite the cessation of DDT spraying. An. arabiensis populations exhibit resistance to pyrethroids and organochlorine insecticides across much of the country. Additionally, wild populations of An. arabiensis have shown resistance to pyrethroid insecticides commonly used for net treatment (Messenger et al., 2017; Mekuriaw et al., 2019; Chanyalew et al., 2022; Demissew et al., 2022). Reduced susceptibility of An. arabiensis to malathion, pirimiphos-methyl, propoxur, and bendiocarb was also reported elsewhere. Knockdown resistance (kdr L1014F) was detected in all mosquito populations, with allele frequencies ranging from 42% to 91% (Alemayehu et al., 2017).
4.3 The spread of Anopheles stephensi
Since its detection in Djibouti in 2012, An. stephensi has spread to the Horn of Africa, including Ethiopia (Faulde et al., 2014). In eastern Ethiopia since 2016, additional locations of An. stephensi have been discovered in subsequent surveys (Balkew et al., 2020; Balkew et al., 2021). From 2021 to 2023, entomological monitoring in 26 urban areas revealed that An. stephensi, comprising 7.7%, was the second leading Anopheles species following An. arabiensis (79.5%). It has also continued to be identified in new areas for the first time in western Ethiopia, suggesting a possible on-going expansion of its distribution (Ashine et al., 2023).
Anopheles stephensi is a potent malaria vector in urban areas, thriving in artificial and contaminated water sources for breeding. Its spread poses a significant challenge to malaria control efforts. A high SNP was detected that confer resistance against insecticides (Acford-Palmer et al., 2023; Ashine et al., 2023; Whittaker et al., 2023). Every An. stephensi specimen gathered near Metehara exhibited resistance to all insecticides tested, including those utilized in IRS and LLINs (Teshome et al., 2023).
A study reported that all An. stephensi samples displayed resistance to carbamates, resulting in mortality rates of 23% for bendiocarb and 21% for propoxur. Moreover, adult An. stephensi exhibited resistance to pyrethroid insecticides, with mortality rates of 67% for deltamethrin and 53% for permethrin. Additionally, resistance to DDT and malathion was evident, with mortality rates of 32%, alongside resistance to pirimiphos-methyl, with a mortality rate of 14%. Notably, these resistances were observed in the absence of the kdr L1014F and L1014S mutations, as well as the ace1R G119S mutation (Yared et al., 2020).
4.4 Histidine-rich protein deletion
The deletion of the histidine-rich protein 2 (HRP2) genes, poses challenges to malaria diagnosis (Jejaw Zeleke et al., 2022). The deletions of the Pfhrp2/3 gene undoubtedly affect the accuracy of malaria diagnosis. The deletion is found in multiple areas and rising (Girma et al., 2019; Golassa et al., 2020; Alemayehu GS. et al., 2021; Tafa et al., 2023) and it undoubtedly hampers the effectiveness of current malaria control and elimination efforts in Ethiopia (Golassa et al., 2020; Alemayehu GS. et al., 2021; Feleke et al., 2021). The study conducted in Adama, Ethiopia, reported 100% deletions in both the pfhrp2 and pfhrp3 genes. In the pfhrp2 gene, deletions extended to the flanking region gene 228 in 4% of samples, while the flanking region gene 230 displayed deletions in 22% of the samples. According to the study, 95% of samples showed deletions around pfhrp3 gene 475, while 40% exhibited deletions around pfhrp3 gene 485 (Golassa et al., 2020).
4.5 Rise of Plasmodium vivax in high altitude areas
Eliminating P. vivax poses significant challenges due to its ability to form dormant liver stages, causing relapses even after treatment. Targeting these dormant forms requires specialized approaches, making its elimination more challenging compared to other malaria species (Habtamu et al., 2022). In addition, the evolution of P. vivax to infect Duffy-negative red blood cells was reported in Jimma, Ethiopia. The report indicated that two out of 94 Duffy-negative patients tested positive for vivax malaria (Lo et al., 2015).
Traditionally considered less prevalent in high-altitude areas, P. vivax malaria is increasingly being reported in such regions of Ethiopia (Tadesse et al., 2018; Ketema et al., 2021). The unique biology of P. vivax, including its preference for cooler temperatures, presents challenges for malaria control and elimination efforts (Hulden and Hulden, 2011; Schäfer et al., 2021).
4.6 Malaria resurgence in previously low burden areas
Despite progress in malaria control, certain regions of Ethiopia that previously experienced low malaria burden are witnessing a resurgence of the disease. Factors contributing to this resurgence include population movement, ecological changes, and weakened healthcare infrastructure (DePina et al., 2018; Endo and Eltahir, 2020; Ewnetu and Lemma, 2022).
5 Perspectives against the challenges
5.1 Case studies: successful malaria elimination strategies
In recent years, remarkable strides have been made in the global fight against malaria, with several countries showcasing successful elimination efforts. Among these exemplars, Azerbaijan, Tajikistan, Belize, El Salvador, and Cabo Verde stand out for their effective strategies in eliminating malaria and certified malaria free by WHO in 2023 (WHO, 2023).
Azerbaijan achieved malaria elimination after years of dedicated control initiatives. Central to its success was a robust commitment from political leaders to prioritize malaria elimination. The country implemented comprehensive vector control programs, ensuring access to healthcare services even in remote areas, and engaged in collaborative efforts with neighboring nations to combat cross-border transmission (Mammadov et al., 2016; Li et al., 2023; WHO, 2023; World Health Organization, 2023).
Tajikistan, has accomplished malaria elimination through strengthened surveillance systems, targeted interventions, cross-sectoral collaboration, and active community participation (Matthys et al., 2008; Kondrashin et al., 2017; WHO, 2023). Belize eliminate malaria via strategic planning, active case detection, vector control, and cross-border collaboration were instrumental in its success (Roberts and Rodriguez, 1994; World Health Organization, 2021; WHO, 2023).
El Salvador, eliminate malaria, propelled by strong political will, enhanced surveillance systems, integrated vector management, and health system strengthening efforts (Bennett and Smith, 2018; Burton et al., 2018; Gardellini et al., 2023; WHO, 2023). Meanwhile, Cabo Verde, an island nation off West Africa’s coast, attained malaria elimination certified in 2023, leveraging its geographic isolation, integrated surveillance and response systems, health promotion, and cross-sectoral collaboration (DePina et al., 2018; DePina et al., 2019; WHO, 2023; Kokori et al., 2024).
These case studies underscore several key lessons applicable to Ethiopia’s malaria elimination efforts, including the importance of strong political commitment, integrated approaches, cross-sectoral collaboration, and community participation. By adopting evidence-based strategies and leveraging successful experiences, Ethiopia can accelerate progress toward its goal of malaria elimination by 2030.
5.2 Strengthening healthcare infrastructure
In Ethiopia, the cornerstone of malaria elimination lies in augmenting healthcare infrastructure to ensure universal access to diagnosis and treatment services. This comprehensive endeavor encompasses upgrading health facilities with improved infrastructure, equipment, and personnel training. Additionally, it involves the critical aspect of human resource development, with a focus on training and deploying more healthcare workers, especially in rural and underserved regions. Furthermore, effective supply chain management is imperative to guarantee a consistent flow of essential malaria commodities, including diagnostic tests and antimalarial drugs, thus preventing stock outs and ensuring uninterrupted service delivery.
5.3 Enhanced surveillance and monitoring systems
The implementation of robust surveillance and monitoring systems is vital for detecting malaria cases, tracking transmission trends, and guiding targeted interventions. This involves the establishment of real-time reporting mechanisms to facilitate prompt data collection, analysis, and response to malaria outbreaks and hotspots. Active case detection activities, such as community-based surveillance and mobile health units, should be deployed to identify and treat malaria cases promptly. Furthermore, sentinel site surveillance networks should be established to monitor drug resistance, vector behavior, and malaria transmission dynamics in high-risk areas, enabling proactive intervention strategies.
5.4 Community engagement and education
Engaging communities and raising awareness about malaria prevention and control measures are pivotal for fostering behavior change and ownership of elimination efforts. To achieve this, targeted health education campaigns should be conducted to promote the use of preventive measures. Mobilizing community leaders, volunteers, and local organizations is essential to actively participate in malaria control activities, including environmental clean-up campaigns and distribution of preventive tools. Innovative communication strategies, such as radio broadcasts, community-theater, and mobile messaging, should be employed to disseminate malaria-related information and encourage positive health behaviors.
5.5 Vector control strategies
Conducting targeted campaigns to eliminate mosquito breeding sites and reduce vector density in malaria-endemic areas is vital. Additionally, distributing LLINs to vulnerable populations, particularly pregnant women and children, provides personal protection against mosquito bites. Implementing larval source management interventions, such as environmental modifications and biological control measures, further reduces mosquito breeding habitats and larval populations, contributing to overall vector control efforts.
5.6 Drug resistance prevention
Managing drug resistance and exploring alternative treatment options are critical components of malaria elimination strategy. Monitoring drug efficacy through therapeutic efficacy studies and molecular surveillance helps detect emerging resistance patterns and guides treatment policies. Promoting treatment adherence and the utilization of ACTs ensures effective treatment and helps prevent the spread of drug resistance.
5.7 Introducing malaria vaccine
Introducing the RTS,S/AS01 malaria vaccine, currently utilized in countries like Ghana, Kenya, Malawi, and Tanzania, could be a significant breakthrough for Ethiopia’s malaria elimination efforts. This vaccine has shown promise in reducing malaria cases among young children, providing an additional tool in the fight against the disease. By incorporating RTS,S/AS01 into its malaria control strategies, Ethiopia can bolster its efforts to achieve elimination targets and improve public health outcomes.
6 Conclusion
Ethiopia continues to tackle with a significant burden of malaria, especially in lowland regions where transmission rates remain high. Recent years have witnessed a concerning uptick in malaria cases, underscoring persistent challenges in control efforts, exacerbated by factors like climate variability and limited access to preventive measures and healthcare services. Despite these successes, Ethiopia faces overwhelming challenges, including drug and vector resistance, diagnostic limitations, and the emergence of Plasmodium vivax in high-altitude areas, alongside malaria resurgence in previously low-burden regions. Mobilizing communities and raising awareness through targeted health education campaigns. In addition, intensifying vector control strategies to reduce mosquito populations and interrupt transmission. Likewise, managing drug resistance effectively through monitoring, promotion of combination therapies, and research should be reinforced. Furthermore, fostering innovations in diagnostics and treatment to enhance malaria case management and surveillance should be strengthened. By implementing these recommendations in a coordinated and sustained manner, Ethiopia can conquer the challenges of malaria elimination and work toward a malaria-free future.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
DW: Conceptualization, Formal analysis, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing, Supervision. YT: Methodology, Supervision, Writing – review & editing. AM: Project administration, Supervision, Writing – review & editing. ET: Conceptualization, Writing – review & editing. KM: Project administration, Writing – review & editing. WA: Methodology, Writing – review & editing. MM: Project administration, Writing – review & editing. KB: Methodology, Writing – review & editing. HG: Methodology, Writing – review & editing. MS: Writing – review & editing, Project administration. MT: Conceptualization, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACT, Artemisinin Based Combination Therapy; DDT, Dichlorodiphenyltrichloroethane; HRP, Histidine Rich Protein; IRS, Indoor Residual Spraying; LLIN, Long Lasting Insecticidal Net; SNP, Single Nucleotide Polymorphism; WHO, World Health Organization.
References
Abdishu M., Gobena T., Damena M., Abdi H., Birhanu A. (2022). Determinants of malaria morbidity among school-aged children living in East Hararghe Zone, Oromia, Ethiopia: a community-based case–control study. Pediatr. Health Med. Ther. 183–193.
Acford-Palmer H., Phelan J. E., Tadesse F. G., Kristan M., Collins E., Spadar A., et al. (2023). Identification of two insecticide resistance markers in Ethiopian Anopheles stephensi mosquitoes using a multiplex amplicon sequencing assay. Sci. Rep. 13, 5612. doi: 10.1038/s41598-023-32336-7
Alelign A., Dejene T. (2016). Current status of malaria in Ethiopia: evaluation of the burden, factors for transmission and prevention methods. Acta Parasitologica Globalis 7, 1–6.
Alelign A., Tekeste Z., Petros B. (2018). Prevalence of malaria in Woreta town, Amhara region, Northwest Ethiopia over eight years. BMC Public Health 18, 1–6. doi: 10.1186/s12889-018-5913-8
Alemayehu E., Asale A., Eba K., Getahun K., Tushune K., Bryon A., et al. (2017). Mapping insecticide resistance and characterization of resistance mechanisms in Anopheles arabiensis (Diptera: Culicidae) in Ethiopia. Parasites Vectors 10, 1–11. doi: 10.1186/s13071-017-2342-y
Alemayehu G. S., Blackburn K., Lopez K., Cambel Dieng C., Lo E., Janies D., et al. (2021). Detection of high prevalence of Plasmodium falciparum histidine-rich protein 2/3 gene deletions in Assosa zone, Ethiopia: implication for malaria diagnosis. Malaria J. 20, 1–11. doi: 10.1186/s12936-021-03629-x
Alemayehu A. A., Castaneda-Mogollon D., Tesfa H., Getie S., Mohon A. N., Balasingam N., et al. (2021). Expansion of the Plasmodium falciparum Kelch 13 R622I mutation in Northwest Ethiopia. doi: 10.21203/rs.3.rs-171038/v1
Alemu A., Muluye D., Mihret M., Adugna M., Gebeyaw M. (2012). Ten year trend analysis of malaria prevalence in Kola Diba, North Gondar, Northwest Ethiopia. Parasites Vectors 5, 1–6. doi: 10.1186/1756-3305-5-173
Alkadir S., Gelana T., Gebresilassie A. (2020). A five year trend analysis of malaria prevalence in Guba district, Benishangul-Gumuz regional state, western Ethiopia: a retrospective study. Trop. Diseases Travel Med. Vaccines 6, 1–7. doi: 10.1186/s40794-020-00112-4
Aregawi M., Lynch M., Bekele W., Kebede H., Jima D., Taffese H. S., et al. (2014). Time series analysis of trends in malaria cases and deaths at hospitals and the effect of antimalarial interventions, 2001–2011, Ethiopia. PloS One 9, e106359. doi: 10.1371/journal.pone.0106359
Aschale Y., Getachew A., Yewhalaw D., De Cristofaro A., Sciarretta A., Atenafu G. (2023). Systematic review of sporozoite infection rate of Anopheles mosquitoes in Ethiopia, 2001–2021. Parasites Vectors 16, 437. doi: 10.1186/s13071-023-06054-y
Ashine T., Eyasu A., Asmamaw Y., Simma E., Zemene E., Epstein A., et al. (2023). Spatiotemporal distribution of Anopheles stephensi in different eco-epidemiological settings in Ethiopia. doi: 10.21203/rs.3.rs-3793340/v1
Ayele D. G., Zewotir T. T., Mwambi H. G. (2012). Prevalence and risk factors of malaria in Ethiopia. Malaria J. 11, 1–9. doi: 10.1186/1475-2875-11-195
Balaji S., Deshmukh R., Trivedi V. (2020). Severe malaria: Biology, clinical manifestation, pathogenesis and consequences. J. Vector Borne Dis. 57, 1–13. doi: 10.4103/0972-9062.308793
Balkew M., Mumba P., Dengela D., Yohannes G., Getachew D., Yared S., et al. (2020). Geographical distribution of Anopheles stephensi in eastern Ethiopia. Parasites Vectors 13, 1–8. doi: 10.1186/s13071-020-3904-y
Balkew M., Mumba P., Yohannes G., Abiy E., Getachew D., Yared S., et al. (2021). An update on the distribution, bionomics, and insecticide susceptibility of Anopheles stephensi in Ethiopia, 2018–2020. Malaria J. 20, 263. doi: 10.1186/s12936-021-03801-3
Bayih A. G., Getnet G., Alemu A., Getie S., Mohon A. N., Pillai D. R. (2016). A unique Plasmodium falciparum Kelch 13 gene mutation in northwest Ethiopia. Am. J. Trop. Med. Hyg 94, 132.
Bennett A., Smith J. L. (2018). Malaria elimination: lessons from El Salvador. Am. J. Trop. Med. Hygiene 99, 1. doi: 10.4269/ajtmh.18-0390
Britanak R., Davis J., Daly J. (1974). Syncrisis: the dynamics of health: Volume VIII, Ethiopia (Washington, DC: Office of International Health, Division of Planning and Evaluation, US Department of Health, Education, and Welfare).
Burton R. A., Chévez J. E. R., Sauerbrey M., Guinovart C., Hartley A., Kirkwood G., et al. (2018). Factors associated with the rapid and durable decline in malaria incidence in El Salvador, 1980–2017. Am. J. Trop. Med. Hygiene 99, 33. doi: 10.4269/ajtmh.17-0629
Chanyalew T., Natea G., Amenu D., Yewhalaw D., Simma E. A. (2022). Composition of mosquito fauna and insecticide resistance status of Anopheles Gambiae sensu lato in Itang special district, Gambella, Southwestern Ethiopia. Malaria J. 21, 125. doi: 10.1186/s12936-022-04150-5
Chima R. I., Goodman C. A., Mills A. (2003). The economic impact of malaria in Africa: a critical review of the evidence. Health Policy 63, 17–36. doi: 10.1016/S0168-8510(02)00036-2
Demissew A., Animut A., Kibret S., Tsegaye A., Hawaria D., Degefa T., et al. (2022). Evidence of pyrethroid resistance in Anopheles amharicus and Anopheles arabiensis from Arjo-Didessa irrigation scheme, Ethiopia. PloS One 17, e0261713. doi: 10.1371/journal.pone.0261713
DePina A. J., Dia A. K., de Ascenção Soares Martins A., Ferreira M. C., Moreira A. L., Leal S. V., et al. (2019). Knowledge, attitudes and practices about malaria in Cabo Verde: a country in the pre-elimination context. BMC Public Health 19, 1–14. doi: 10.1186/s12889-019-7130-5
DePina A. J., Niang E. H. A., Barbosa Andrade A. J., Dia A. K., Moreira A., Faye O., et al. (2018). Achievement of malaria pre-elimination in Cape Verde according to the data collected from 2010 to 2016. Malaria J. 17, 1–12. doi: 10.1186/s12936-018-2376-4
Endo N., Eltahir E. A. (2020). Increased risk of malaria transmission with warming temperature in the Ethiopian Highlands. Environ. Res. Lett. 15, 054006. doi: 10.1088/1748-9326/ab7520
Eregata G. T., Hailu A., Memirie S. T., Norheim O. F. (2019). Measuring progress towards universal health coverage: national and subnational analysis in Ethiopia. BMJ Global Health 4, e001843. doi: 10.1136/bmjgh-2019-001843
Ewnetu Y., Lemma W. (2022). Highland malaria transmission dynamics in space and time before pre-elimination era, northwest Ethiopia. J. Epidemiol. Glob Health 12, 362–371. doi: 10.1007/s44197-022-00034-8
Farvar M. T., Thomas M. L., Boksenbaum H., Soule T. N. (1971). The pollution of asia. Environment: Sci. Policy Sustain. Dev. 13, 10–17. doi: 10.1080/00139157.1971.9931126
Faulde M. K., Rueda L. M., Khaireh B. A. (2014). First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti, Horn of Africa. Acta tropica 139, 39–43. doi: 10.1016/j.actatropica.2014.06.016
Feleke S. M., Reichert E. N., Mohammed H., Brhane B. G., Mekete K., Mamo H., et al. (2021). Plasmodium falciparum is evolving to escape malaria rapid diagnostic tests in Ethiopia. Nat. Microbiol. 6, 1289–1299. doi: 10.1038/s41564-021-00962-4
Fola A. A., Feleke S. M., Mohammed H., Brhane B. G., Hennelly C. M., Assefa A., et al. (2023). Plasmodium falciparum resistant to artemisinin and diagnostics have emerged in Ethiopia. Nat. Microbiol. 8, 1911–1919. doi: 10.1038/s41564-023-01461-4
Gardellini T., Le N. K., Hoare I., Jacob B., Escobar E. A., Sanyaolu A., et al. (2023). Elimination strategy for malaria in El Salvador: a retrospective study. Curr. Trop. Med. Rep. 10, 300–308. doi: 10.1007/s40475-023-00294-9
Girma S., Cheaveau J., Mohon A. N., Marasinghe D., Legese R., Balasingam N., et al. (2019). Prevalence and epidemiological characteristics of asymptomatic malaria based on ultrasensitive diagnostics: a cross-sectional study. Clin. Infect. Dis. 69, 1003–1010. doi: 10.1093/cid/ciy1005
Girum T., Shumbej T., Shewangizaw M. (2019). Burden of malaria in Ethiopia, 2000-2016: findings from the Global Health Estimates 2016. Trop. Diseases Travel Med. Vaccines 5, 1–7. doi: 10.1186/s40794-019-0090-z
Gish O. (1992). Malaria eradication and the selective approach to health care: some lessons from Ethiopia. Int. J. Health Serv. 22, 179–192. doi: 10.2190/DUKB-DPGP-5W81-YKCW
Golassa L., Messele A., Amambua-Ngwa A., Swedberg G. (2020). High prevalence and extended deletions in Plasmodium falciparum hrp2/3 genomic loci in Ethiopia. PloS One 15, e0241807. doi: 10.1371/journal.pone.0241807
Graves P. M., Richards F. O., Ngondi J., Emerson P. M., Shargie E. B., Endeshaw T., et al. (2009). Individual, household and environmental risk factors for malaria infection in Amhara, Oromia and SNNP regions of Ethiopia. Trans. R. Soc. Trop. Med. Hygiene 103, 1211–1220. doi: 10.1016/j.trstmh.2008.11.016
Habtamu K., Petros B., Yan G. (2022). Plasmodium vivax: the potential obstacles it presents to malaria elimination and eradication. Trop. Diseases Travel Med. Vaccines 8, 27. doi: 10.1186/s40794-022-00185-3
Hailu A., Lindtjørn B., Deressa W., Gari T., Loha E., Robberstad B. (2017). Economic burden of malaria and predictors of cost variability to rural households in south-central Ethiopia. PloS One 12, e0185315. doi: 10.1371/journal.pone.0185315
Hulden L., Hulden L. (2011). Activation of the hypnozoite: a part of Plasmodium vivax life cycle and survival. Malaria J. 10, 1–6. doi: 10.1186/1475-2875-10-90
Jaiteh F. K., dhugassa Lemma S., Ngwa A. A., Wayasa L. G. (2021). Delayed parasite clearance rates and low clinical and parasitological responses following treatment of uncomplicated falciparum malaria with artemether-lumefantrine in Ethiopian-Sudan border (Western Ethiopia: Springer Nature).
Janssens P., Wery M. (1987). Malaria in africa south of the sahara. Ann. Trop. Med. Parasitol. 81, 487–498. doi: 10.1080/00034983.1987.11812151
Jejaw Zeleke A., Hailu A., Bayih A. G., Kefale M., Amare A. T., Tegegne Y., et al. (2022). P lasmodium falciparum histidine-rich protein 2 and 3 genes deletion in global settings (2010–2021): a systematic review and meta-analysis. Malaria J. 21, 26. doi: 10.1186/s12936-022-04051-7
Kassa D. H. (2015). Malaria prevention and control in Ethiopia (Addis Ababa, Ethiopia: University of South Africa).
Ketema T., Bacha K., Getahun K., Portillo H., Bassat Q. (2021). : Plasmodium vivax epidemiology in Ethiopia 2000-2020: a systematic review and meta-analysis. PloS Negl. Trop. Dis. 15, e0009781. doi: 10.1371/journal.pntd.0009781
Kokori E., Olatunji G., Umenzeakor K., Abraham I. C., Ogunbowale I., Uduigwome E. O., et al. (2024). Global malaria eradication: Insights from Cabo Verde and implications for sub-Saharan Africa. New Microbes New Infections 59. doi: 10.1016/j.nmni.2024.101244
Kolawole E. O., Ayeni E. T., Abolade S. A., Ugwu S. E., Awoyinka T. B., Ofeh A. S., et al. (2023). Malaria endemicity in Sub-Saharan Africa: Past and present issues in public health. Microbes Infect. Dis. 4, 242–251.
Kondrashin A. V., Sharipov A. S., Kadamov D. S., Karimov S. S., Gasimov E., Baranova A. M., et al. (2017). Elimination of Plasmodium falciparum malaria in Tajikistan. Malaria J. 16, 1–12. doi: 10.1186/s12936-017-1861-5
Li X., Snow R. W., Lindblade K., Noor A. M., Steketee R., Rabinovich R., et al. (2023). Border malaria: defining the problem to address the challenge of malaria elimination. Malaria J. 22, 239. doi: 10.1186/s12936-023-04675-3
Liu G. G., Qin X. (2023). Global health and development: Low-carbon economy and health innovation (Cham, Switzerland: Springer Nature).
Lo E., Yewhalaw D., Zhong D., Zemene E., Degefa T., Tushune K., et al. (2015). Molecular epidemiology of Plasmodium vivax and Plasmodium falciparum malaria among Duffy-positive and Duffy-negative populations in Ethiopia. Malaria J. 14, 1–10. doi: 10.1186/s12936-015-0596-4
Malmberg M., Ferreira P. E., Tarning J., Ursing J., Ngasala B., Björkman A., et al. (2013). Plasmodium falciparum drug resistance phenotype as assessed by patient antimalarial drug levels and its association with pfmdr1 polymorphisms. J. Infect. Dis. 207, 842–847. doi: 10.1093/infdis/jis747
Mammadov S., Gasimov E., Kurdova-Mintcheva R., Wongsrichanalai C. (2016). Elimination of Plasmodium vivax malaria in Azerbaijan. Am. J. Trop. Med. Hygiene 95, 78. doi: 10.4269/ajtmh.16-0173
Matthys B., Sherkanov T., Karimov S. S., Khabirov Z., Mostowlansky T., Utzinger J., et al. (2008). History of malaria control in Tajikistan and rapid malaria appraisal in an agro-ecological setting. Malaria J. 7, 1–11. doi: 10.1186/1475-2875-7-217
Mekuriaw W., Yewhalaw D., Woyessa A., Bashaye S., Massebo F. (2019). Distribution and trends of insecticide resistance in malaria vectors in Ethiopia (1986-2017): a review. Ethiopian J. Public Health Nutr. (EJPHN) 3, 51–61.
Messenger L. A., Shililu J., Irish S. R., Anshebo G. Y., Tesfaye A. G., Ye-Ebiyo Y., et al. (2017). Insecticide resistance in Anopheles arabiensis from Ethiopia (2012–2016): a nationwide study for insecticide resistance monitoring. Malaria J. 16, 1–14. doi: 10.1186/s12936-017-2115-2
MOHE (2021). National malaria elimination program (NMEP). Available online at: https://wwwmohgovet/en/initiatives-4-col/National_Malaria_Elimination_Program?language_content_entity=en (Accessed June 21, 2024).
Mutero C. M., Okoyo C., Girma M., Mwangangi J., Kibe L., Ng’ang’a P., et al. (2020). Evaluating the impact of larviciding with Bti and community education and mobilization as supplementary integrated vector management interventions for malaria control in Kenya and Ethiopia. Malaria J. 19, 1–17. doi: 10.1186/s12936-020-03464-6
Negash K., Jima D., Nafo-Traore F., Mukelabai K., Banda J., Medhin A., et al. (2004). Ethiopia roll back malaria consultative mission: essential actions to support the attainment of the Abuja targets. Ethiopia RBM Country Consultative Mission Final Rep. 39.
Nureye D., Tekalign E. (2023). Malaria can become a Big Threat in Urban Areas of Ethiopia and Somewhere Else in Africa. EC Pharmacol. Toxicol. 11, 30–35.
Pankhurst R. (1966). Some factors influencing the health of traditional Ethiopia. J. Ethiopian Stud. 4, 31–70.
Roberts D. R., Rodriguez M. H. (1994). The environment, remote sensing, and malaria control. Ann. New York Acad. Sci. 740, 396–402. doi: 10.1111/j.1749-6632.1994.tb19898.x
Russo G., L’Episcopia M., Menegon M., Souza S. S., Dongho B. G. D., Vullo V., et al. (2018). Dihydroartemisinin–piperaquine treatment failure in uncomplicated Plasmodium falciparum malaria case imported from Ethiopia. Infection 46, 867–870. doi: 10.1007/s15010-018-1174-9
Schäfer C., Zanghi G., Vaughan A. M., Kappe S. H. (2021). Plasmodium vivax latent liver stage infection and relapse: biological insights and new experimental tools. Annu. Rev. Microbiol. 75, 87–106. doi: 10.1146/annurev-micro-032421-061155
Tadesse F. G., Slater H. C., Chali W., Teelen K., Lanke K., Belachew M., et al. (2018). The relative contribution of symptomatic and asymptomatic Plasmodium vivax and Plasmodium falciparum infections to the infectious reservoir in a low-endemic setting in Ethiopia. Clin. Infect. Dis. 66, 1883–1891. doi: 10.1093/cid/cix1123
Tafa B., Dugassa S., Mekasha S., Dufera B., Ademu A., Gidisa B., et al. (2023). Widespread pfhrp2/3 deletions and HRP2-based false-negative results in southern Ethiopia. doi: 10.21203/rs.3.rs-3404831/v1
Teklehaimanot A. (1986). Chloroquine-resistant Plasmodium falciparum malaria in Ethiopia. Lancet 328, 127–129. doi: 10.1016/S0140-6736(86)91945-8
Tesfaye A. (1973). The training and development of manpower for the social services in Ethiopia (Addis Ababa, Ethiopia: University of Michigan).
Teshome A., Erko B., Golassa L., Yohannes G., Irish S. R., Zohdy S., et al. (2023). Resistance of Anopheles stephensi to selected insecticides used for indoor residual spraying and long-lasting insecticidal nets in Ethiopia. Malaria J. 22, 218. doi: 10.1186/s12936-023-04649-5
Vecchiato N. L. (1991). Ethnomedical beliefs, health education, and malaria eradication in Ethiopia. Int. Q. Community Health Educ. 11, 385–397. doi: 10.2190/LTMQ-Y081-UBGF-62TJ
Volkman S. K., Herman J., Lukens A. K., Hartl D. L. (2017). Genome-wide association studies of drug-resistance determinants. Trends Parasitol. 33, 214–230. doi: 10.1016/j.pt.2016.10.001
Warrell D. A. (2017). “Clinical features of malaria,” in Essential malariology, 4Ed (Oxford, UK: CRC Press), 191–205.
Whittaker C., Hamlet A., Sherrard-Smith E., Winskill P., Cuomo-Dannenburg G., Walker P. G., et al. (2023). Seasonal dynamics of Anopheles stephensi and its implications for mosquito detection and emergent malaria control in the Horn of Africa. Proc. Natl. Acad. Sci. 120, e2216142120. doi: 10.1073/pnas.2216142120
World Health Organization. (2015). Global technical strategy for malaria 2016-2030 (Geneva, Switzerland: World Health Organization).
World Health Organization. (2021). Zeroing in on malaria elimination: final report of the E-2020 initiative (Geneva, Switzerland: World Health Organization).
World Health Organization. (2023). Report of the first and second meetings of the technical advisory group on malaria elimination and certification, 13–14 September 2022 and 27 January 2023. Addis Ababa
Worrall E., Basu S., Hanson K. (2005). Is malaria a disease of poverty? A review of the literature. Trop. Med. Int. Health 10, 1047–1059. doi: 10.1111/j.1365-3156.2005.01476.x
Yalew A. W. (2022). Achievements, gaps, and emerging challenges in controlling malaria in Ethiopia. Front. Trop. Dis. 2, 771030. doi: 10.3389/fitd.2021.771030
Yared S., Gebressielasie A., Damodaran L., Bonnell V., Lopez K., Janies D., et al. (2020). Insecticide resistance in Anopheles stephensi in Somali Region, eastern Ethiopia. Malaria J. 19, 1–7. doi: 10.1186/s12936-020-03252-2
Keywords: malaria, Plasmodium, Ethiopia, trend analysis, elimination
Citation: Woldesenbet D, Tegegne Y, Mussema A, Tamene E, Mohamed K, Abebe W, Mekuria M, Bogale K, Geremew H, Shifa MM and Tegegne MA (2025) Can Ethiopia eliminate malaria? Malaria burden: insights from the pre-elimination era, current challenges and perspectives. Front. Malar. 3:1492444. doi: 10.3389/fmala.2025.1492444
Received: 06 September 2024; Accepted: 13 January 2025;
Published: 30 January 2025.
Edited by:
Anita Ghansah, Noguchi Memorial Institute for Medical Research, GhanaReviewed by:
Ramesh C. Dhiman, National Institute of Malaria Research (ICMR), IndiaCopyright © 2025 Woldesenbet, Tegegne, Mussema, Tamene, Mohamed, Abebe, Mekuria, Bogale, Geremew, Shifa and Tegegne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dagmawi Woldesenbet, ZGFnbWF3aTI5MjlAZ21haWwuY29t
 Dagmawi Woldesenbet
Dagmawi Woldesenbet Yalewayker Tegegne2
Yalewayker Tegegne2 Abdulhakim Mussema
Abdulhakim Mussema Kemal Mohamed
Kemal Mohamed Wagaw Abebe
Wagaw Abebe Kasahun Bogale
Kasahun Bogale Habtamu Geremew
Habtamu Geremew