
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Malar. , 08 April 2025
Sec. Vectors
Volume 3 - 2025 | https://doi.org/10.3389/fmala.2025.1478249
This article is part of the Research Topic Addressing Contemporary Threats to Global Malaria Control: New Tools and Strategies View all 8 articles
Background: Africa is still home to the highest number of malaria cases and deaths. To reduce the burden of malaria in Africa, different classes of insecticides have been used since the eradication era. However, the effectiveness of insecticides is reduced periodically. This study aimed to assess the susceptibility status of major African malaria vectors to different insecticides commonly used for public health.
Methods: To conduct this review, we used open-access global databases, i.e., PubMed, Google Scholar, Scopus, Web of Sciences, and Pro-Quest, to extract relevant articles published between January 2002 and 28 December 2023. Primary articles were searched using keywords such as “insecticide susceptibility status”, ‘insecticide resistance”,” malaria vectors”, “Africa”, and “Anopheles”. Articles published in English that met the inclusion criteria were included in this review. Data were extracted from the included article texts, tables, figures, and supplementary information. The validity of all included articles was checked before inclusion by critical evaluation using standardized methods. Finally, the results of the original articles are presented in tables, graphs, and maps.
Results: In total, 61 relevant articles were retrieved and extracted from 1,794 accessed articles. Of these, most articles documented resistance in Anopheles gambiae s.l. and An. funestus to organochlorines, i.e., DDT (4%); cyclodins, i.e., dieldrin (4%); pyrethroids, including lambda-cyhalothrin (0.05%), cyfluthrin (0.15%), permethrin (0.75%), and deltamethrin (0.05%); and carbamate, i.e., propoxur (0.1%), across Africa. These mosquito species have also developed knockdown resistance to different insecticide classes (pyrethroids and organochlorines) in Africa. However, the resistance of these malaria vectors varied in different areas of the continent and in different localities within the same country. The highest levels of insecticide resistance in Anopheles mosquitoes across Africa were recorded between 2011 and 2015. However, currently, mosquito populations are susceptible to candidate insecticides such as chlothianidin (neoncotinoid), chlorfenapyr (pyrole), and brofanilide (meta-diamide), which are newly introduced insecticides for vector control interventions.
Conclusion: This review revealed that the major African malaria vectors have developed resistance to most insecticides used for public health. However, they were susceptible to a few existing insecticides (pirimiphos-methyl) and new candidate insecticides such as clothianidin, chlorfenapyr, and brofanilide. This warrants the development and implementation of insecticide resistance monitoring and management strategies for malaria control and elimination programs in malaria endemic countries of Africa to extend the effective lifespan of insecticides to which populations of the major African malaria vectors are susceptible and to reduce the resistance frequency. We also recommend the use of integrated vector management to complement the chemical insecticide vector control interventions in the containment of major African malaria vectors.
Malaria is a major public health concern across the world. The disease is most common in tropical and subtropical regions of the world. It is caused by the genus Plasmodium, which is a single-celled protozoan parasite. Currently, five Plasmodium species infect humans worldwide: Plasmodium falciparum, P. vivax, P. ovalae, P. malariae, and P. knowlesi. The latter, a parasite of Macacus monkeys, can infect humans (Kolawole et al., 2022; Slater et al., 2022).
The World Malaria Report 2023 states that there were an estimated 249 million cases and 608,000 deaths in 2022 globally. Of these, 94% of the malaria cases and 96% of the deaths occurred in African regions, with children under the age of five accounting for 80% of the deaths. Plasmodium falciparum was the deadliest and most frequent malaria parasite in Africa (USAID, 2022; WHO, 2021).
Malaria is mainly transmitted by the bite of an infected female Anopheles mosquito. There are over 500 species of Anopheles mosquitoes, and approximately 100 of them can transmit malaria. Likewise, 30–40 species of Anopheles mosquitoes have been found to usually carry Plasmodium parasites. Among these, Anopheles gambiae s.l, Anopheles funestus, Anopheles darlingi, Anopheles dirus, Anopheles minimus, and Anopheles punctulatus are the primary vectors of malaria globally (Sinka, 2013; van de Straat et al., 2021). An. gambiae s.l. and the An. funestus group are the two main complexes that mostly transmit malaria in Africa. Although An. gambiae s.l. consists of at least seven morphologically indistinguishable species, An. gambiae sensu stricto (s.s), An. coluzzii, and An. arabiensis, all members of this complex, are the primary known malaria vectors in Africa alongside An. funestus (Sinka et al., 2012, 2010, 2020). However, an invasive malaria vector, An. stephensi, has been recently reported in different African countries (Sinka et al., 2020).
Efforts to reduce malaria transmission have evolved in response to changes in vector behavior and species composition. Historically, various metals such as arsenic, copper, lead, phosphorus, mercury, fluorine, boron, sulfur, and others, and plant-based pesticides such as pyrethrum sprays, have been employed for vector control (Carter, 1952; Roark, 1938). In the 19th century, several traditional insecticides, including Paris green, phenol and cresol, naphthalene, Bordeaux combination, rosin fish oil soap, calcium arsenate, nicotine, and sulfate, were introduced and effectively targeted both adult mosquitoes and larvae (Quilty and Cattle, 2011; Zacharia, 2011). In malaria-endemic areas, these insecticides were often supplemented with environmental management strategies (Raghavendra et al., 2011; Utzinger et al., 2001).
During World War II, the emergence of typhoid fever and malaria pandemics led to the large-scale deployment of dichloro-diphenyl-trichloroethane (DDT)-based vector control (Berry-Cabán, 2011; EPA, US, 1975). However, over time, resistance to DDT and the emergence of new pest populations necessitated the introduction of alternative insecticides, such as pyrethroids, carbamates, and organophosphates (Ibrahim et al., 1998; Ongono et al., 2020).
Malaria control strategies in Africa have primarily targeted mosquito vectors, complemented by chemoprevention, early diagnosis, and effective treatment (Mbabazi et al., 2021; Moyes et al., 2020). Other measures, such as environmental management, insect repellents, protective barriers like clothing, screens and curtains, and biological control strategies, have also been utilized. However, the most widely implemented interventions remain insecticide-treated nets (ITNs) and indoor residual spraying (IRS) (Moyes et al., 2020; Ranson and Lissenden, 2016). For many decades, the World Health Organization (WHO) recommended four classes of insecticides, namely, organochlorines, organophosphates, pyrethroids, and carbamates, for malaria vector control. More recently, in response to escalating insecticide resistance, the WHO has endorsed the use of neonicotinoids, a class of neuro-active (nicotine-like) insecticides, to enhance vector control effectiveness (WHO, 2023b).
IRS is predominantly employed in malaria-prone regions to prevent outbreaks, while ITNs are widely used in areas with sustained malaria transmission. The application of DDT in IRS campaigns significantly contributed to malaria reduction in many African countries (Baleta, 2009; Mabaso et al., 2004). However, resistance to DDT was first documented in 1957 (Brown, 1958; Zahar, 1984), prompting the adoption of alternative insecticides, such as malathion, in resistant regions from 1986 onward (Kinfe et al., 2021). Additionally, deltamethrin and permethrin-treated bed nets were introduced and distributed to malaria-prone countries on the continent (Carnevale et al., 1991), followed by the deployment of other insecticides, including fenitrothion, bendiocarb, lambda-cyhalothrin, and propoxur, between 1992 and 2015 (Yewhalaw et al., 2017).
Despite these efforts, the growing prevalence of insecticide resistance remains one of the most significant challenges to malaria vector control in Africa. In response, next-generation ITNs (such as PBO nets and dual nets) and novel IRS insecticide formulations (including SumiShield 50WG, Actellic 300CS, and Vectron T500) have been introduced to combat pyrethroid-resistant mosquito populations (Accrombessi et al., 2023; Mosha et al., 2022; Snetselaar et al., 2021; WHO, 2023a; Accrombessi et al., 2023). However, the long-term efficacy and sustainability of these interventions require continuous assessment.
Although ITNs and IRS remain the cornerstones of malaria prevention, the increasing resistance of malaria vectors to insecticides poses a serious challenge to malaria control efforts in Africa. To effectively address this issue, ongoing surveillance and monitoring of insecticide resistance patterns across different malaria-endemic regions are essential. Since susceptibility levels vary by country depending on insecticide use, this study aimed to provide a comprehensive analysis of pooled data from multiple African nations to assess the current susceptibility of major malaria vectors to public health insecticides.
To perform this review, relevant published articles and reports on the susceptibility of malaria vectors to various insecticides were used. This systemic review and meta-analysis was carried out according to the requirements of the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines (Supplementary Table 1) (Juache-Villagrana et al., 2022). The inclusion and exclusion criteria were established based on the relevance and alignment of the primary articles with the current study’s objectives.
To search relevant articles for this study, we used global electronic databases: PubMed, Google Scholar, Scopus, Web of Science, and Pro-Quest. During the search, we applied filters to restrict the publication years from 1 January 2002 to 28 December 2023 and confined the results to articles published in English. The search keywords for the literature review included both MeSH terms and test words combined using Boolean operators such as “OR” and “AND.” The keywords used were “Susceptibility status,” “Malaria vector,” “Insecticide resistance,” “Africa,” and “Anopheles mosquito.” The searched articles and syntax using these keywords in each electronic database are summarized in Supplementary Table 2.
Inclusion criteria: This study included preliminary experiments involving the susceptibility testing of malaria vectors to several insecticides done according to the WHO protocol (Corbel et al., 2023; WHO, 2022b). All studies and publications written exclusively in English were included. These primary articles provided the results of susceptibility tests, including knockdown rates every 15 minutes after exposure and after 60 minutes of exposure. Additionally, the inclusion criteria included data on the 50% and/or 95% knockdown times. Mortality rates recorded after 24 hours of exposure and those after 48 and 72 hours were part of the inclusion criteria for studies focusing on the insecticides broflanilide, clothianidin, and chlorfenapyr.
Exclusion criteria: Original publications with inadequate data, not written in English, and with no full text available; abstracts from scientific conferences; research conducted outside of Africa; and research with a mosquito mortality rate greater than 5% compared to the control group were excluded from this evaluation.
The quality of primary studies was assessed using the Joanna Briggs Institute (JBI) critical appraisal checklist (Zeng et al., 2015) with a slight modification to the mosquito susceptibility test based on WHO protocol. The quality assessment tools consisted of six criteria designed to evaluate the quality of the primary studies considered for inclusion in our review. These criteria focused on aspects such as the number of Anopheles mosquitoes tested and replicates, methods for identifying outcomes, study setting, response outcomes, and statistical analysis. Studies with a quality score above 50% were included, while those scoring 50% or below were excluded (Supplementary File 3). The authors (AM and GA) did the quality evaluation independently, and when various assumptions were presented, other co-authors actively contributed. To ensure the high quality of the primary data, we included articles that used a susceptibility test based on WHO-impregnated papers, and susceptible Kisumu (control) strains showed a 100% mortality rate (Williams et al., 2019). This suggests that the articles included were high quality. Additionally, we also used some studies that did not use susceptible Kisumu strains but used a mortality rate in the control test of less than 5%.
After analyzing the complete text of relevant published papers and obtaining reports on the insecticidal susceptibility of malaria vectors, data were extracted from original study texts, tables, figures, and additional files. An Excel spreadsheet was used to extract and record the results of tests conducted based on the WHO susceptibility test protocol, the type and discrimination dose of insecticide tested, the 50% and 95% knockdown time and/or knockdown results from 15 to 60 minutes exposure, and the 24-hour post-exposure mortality in Anopheles mosquitoes (Supplementary Table 4). The frequencies and percentages were then computed and presented.
Malaria vector susceptibility to several insecticides has been classified using WHO criteria (WHO, 2022a). The overall mortality rates range from 98%–100%, showing susceptibility, while the drug resistance suspects range from 90%–98%, and less than 90% indicates resistance. The knockdown times (KDT50 and KDT95) were calculated using Excel. After organizing data on the time spent on Anopheles mosquito knockdown and mortality from published sources in Excel and the Statistical Package for the Social Sciences (SPSS) version 25.0, the data were presented in tables, graphs, and maps.
To generate the means for knockdown time and mortality of Anopheles mosquitoes throughout the continent, we utilized the mean values of each article’s finding of the knockdown rate and mortality of An. gambiae s.l. or An. funestus for each insecticide, using the following formula:
where M = mean of dead An. gambiae s.l. or An. funestus pooled data, m = mean of dead An. gambiae s.l. or An. funestus for a single insecticide reported in each article, n = number of insecticides tested, and k = the number of Anopheles mosquitoes tested in each study.
To determine the total number of mosquitoes killed by each insecticide based on the outcomes of each primary data, we used the following formula:
where = mean number of An. gambiae s.l. or An. funestus knockdown to each insecticide at a given time interval, t = specific time of An. gambiae s.l. or An. funestus knockdown recorded at 15-minute intervals up to 1 hour; and y = quantity of a specific insecticide tested. The numbers 1, 2,3…n represent the number of original articles extracted and k = the number of Anopheles mosquitoes tested in each study.
Similarly, to calculate the 50% and 90% knockdown time of an insecticide to An. gambiae s.l. or An. funestus, we used the following formula:
where = mean of time in which an insecticide knockdown of 50% or 90% of An. gambiae s.l. or An. funestus, x=50% or 90% knockdown of An. gambiae s.l. or An. funestus for each insecticide in the primary studies, and 1, 2, 3,…n = the time an insecticide efficacy test takes for 50% or 90% knockdown in the original studies, and N = the total number of original efficacy tests of an insecticide.
Moreover, to calculate the 95% confidence interval of 50% and 90% knockdown time of an insecticide efficacy test, we used the following formula:
where T = 95% confidence interval of 50% and 90% knockdown time, = mean knockdown time of insecticide in original studies, Z = level of the confidence interval, S = variance of 50% and 90% knockdown time, and n = total number of original studies with an efficacy test of certain insecticides.
A total of 1,794 articles were automatically retrieved from the online databases. Due to duplication, 396 of these studies were excluded. The remaining 1,398 publications were retrieved and 461 publications were excluded because their titles and abstracts did not fulfill the inclusion criteria. The remaining 937 papers qualified for full-text review. Of these, 876 papers were excluded due to specified exclusion criteria, such as studies conducted outside of Africa, articles without comprehensive data, research with unclear methodology, and research studies focusing solely on species composition. Finally, only 61 full-text publications were eligible for inclusion in the final quantitative meta-analysis (Figure 1).
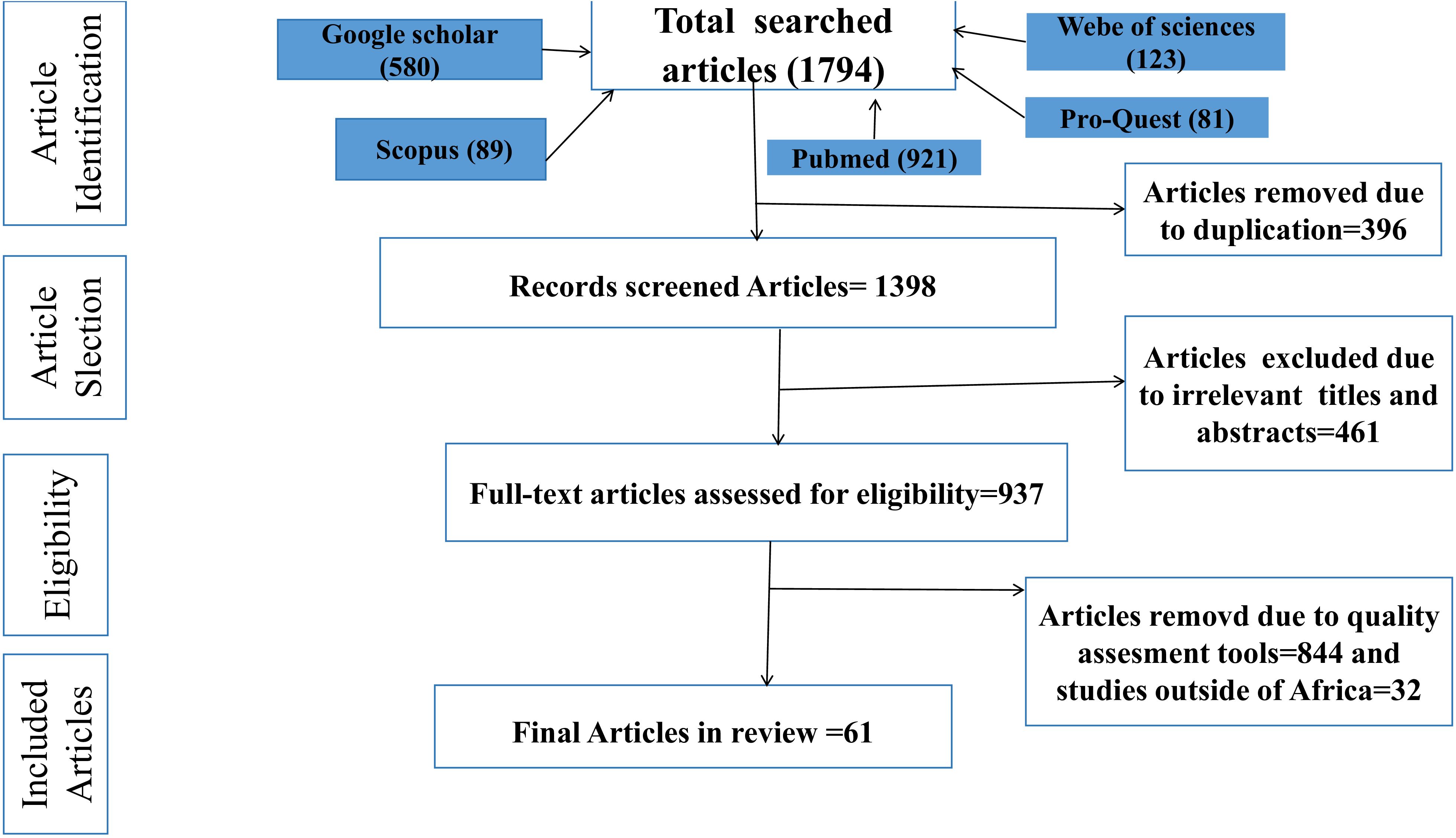
Figure 1. PRISMA flow diagram showing the selection process of eligible studies for this review, 2002-2023.
In different African countries, 162,760 adult female Anopheles mosquitoes from two major Anopheles mosquito species were tested for susceptibility to several insecticides. From the publications suitable for this evaluation, we found 88.02% (143,268/162,760) of An. gambiae s.l. and the remaining 19,492 An. funestus mosquitoes were tested for different insecticides (Supplementary Table 4). To conduct the test, larvae were collected from the field and reared to adults and 3–5-day-old non-blood-sucking adult females were used for the insecticide susceptibility tests.
A total of 15 different insecticide susceptibility tests were conducted on An. gambiae (s.l.) and the An. funestus group collected in different African countries: DDT 4%, dieldrin 4%, deltamethrin 0.05%, cyfluthrin 0.15%, lambda-cyhalothrin 0.05%, permethrin 0.75%, bendiocarb 0.1%, propoxur 0.1%, fenitrothion 1.0%, malathion 5%, pirimiphos methyl 0.25%, clothianidin (sumishield), alphacypermethrin 0.05%, chlorfenapyr and brofanilide. The total number of Anopheles mosquitoes knocked down was recorded after 15, 30, 45, and 60 minutes of exposure to organophosphate and pyrethroid. In addition, after 24 hours of exposure, 50% knockdown time (KDT), 95% KDT, and mortality of Anopheles mosquitoes were reported. In addition, the mortality rate of Anopheles mosquitoes after 48 and 72 hours of clothianidin, brofanilide 6 μg/mL, and chlorfenapyr 100 μg/mL was also recorded. The knockdown and mortality of Anopheles mosquitoes due to different insecticides in different African countries after 60 minutes and 24 hours of exposure is summarized in Figure 2; Supplementary Tables 5, 6, based on Equations 1 and 2.
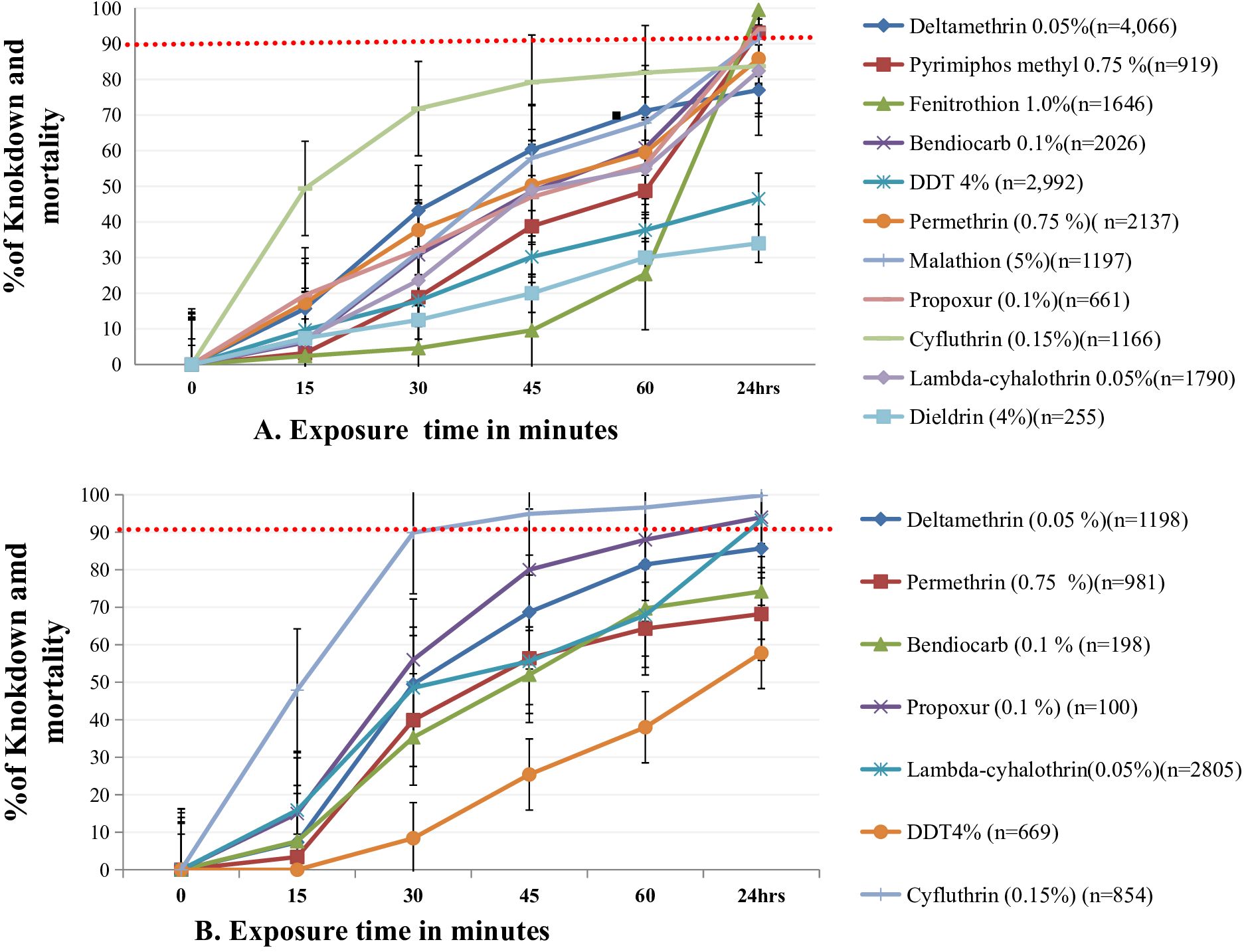
Figure 2. Knockdown and mortality of An. gambae s.l. (A) and An. funestus (B) after exposure to different insecticides, recorded every 15 minutes up to 60 minutes and after 24 hours, respectively. n indicates the number of exposed Anopheles mosquitoes.
Knockdown times of 50% and 95% (KDT50 and KDT95) of Anopheles mosquito populations differed by insecticides. Overall, all the pooled data demonstrated a shorter knockdown time for cyfluthrin, permethrin, and deltamethrin as compared to DDT (4%) and dieldrin for both An.gambiae s.l. and An.funestus. The mortality rate of An. gambiae s.l. after 24 hours of exposure to fenitrothion and cyfluthrin was 97.3% and 83.9%, respectively. The mortality rate of An. funestus 24 hours post exposure to cyfluthrin was 99.8% (Figure 2) based on Equation 2.
The KDT50 of cyfluthrin for An. gambiae s.l. and An. funestus was fast (approximately 16.2–21.3 min), and KDT95 for An. gambiae s.l. was faster than for An. funestus. The mean KDT50 for deltamethrin and permethrin ranged from 39.6 to 43.6 minutes for An. gambiae s.l. and An. funestus, whereas KDT95 was much longer, and the mortality rate 24 h post-exposure was lower than 90% for An. gambiae s.l. and An. funestus. Similarly, the mean KDT50 for lambda-cyhalothrin was 55.4 minutes for both An. gambiae s.l. and An. funestus, but the mean KDT95 was longer. The mortality rate of An. gambiae s.l., 24 hours post-exposure was between 90% and 98% for all except for lambda-cyhalothrin, which was less than 90%. Similarly, the mortality rates of An. gambiae s.l. and An. funestus 24 hours post-exposure to organophosphates was higher than 90%, as shown in Figure 2, based on Equations 3 and 4 and Supplementary Table 6.
The susceptibility status of An. gambiae s.l. and An. funestus to several insecticides in different localities of Africa is summarized in Figures 3A, B, 4; Supplementary Tables 7, 8. The mean KDT50 for populations of An. gambiae s.l. against deltamethrin was 44.3 minutes in southern Africa, followed by West Africa (45.2 minutes), Central Africa (46.3 minutes), and East Africa (48.3 minutes). In southern Africa, the mortality rate of An. gambiae s.l. 24 hours post-exposure to deltamethrin was above 90%. In contrast, the mean KDT50 of deltamethrin for An. funestus was 49.4 minutes, 53.2 minutes, and 76.2 minutes for populations from West Africa, East Africa, and Central Africa, respectively. The mean mortality rates of populations of An. gambiae s.l. and An. funestus 24 hours post-exposure to deltamethrin was considerably lower at all localities. The mean KDT50 for populations of An. gambiae s.l. from southern Africa exposed to permethrin was relatively faster (30.4 minutes), followed by populations from East Africa (45.4 minutes), Central Africa (49.4 minutes), and West Africa (58.5 minutes), whereas the mortality rate of populations of An. gambiae s.l. 24 hours post-exposure to deltamethrin was less than 90% at all sites. The mean KDT50 for populations of An. funestus in southern Africa, East Africa, West Africa, and Central Africa exposed to permethrin was 30.4 minutes, 38.7 minutes, 48.4 minutes, and 71.9 minutes, respectively. The mean mortality rate of populations of An. funestus 24 hours post-exposure to permethrin was above 98% in southern Africa, but lower elsewhere.
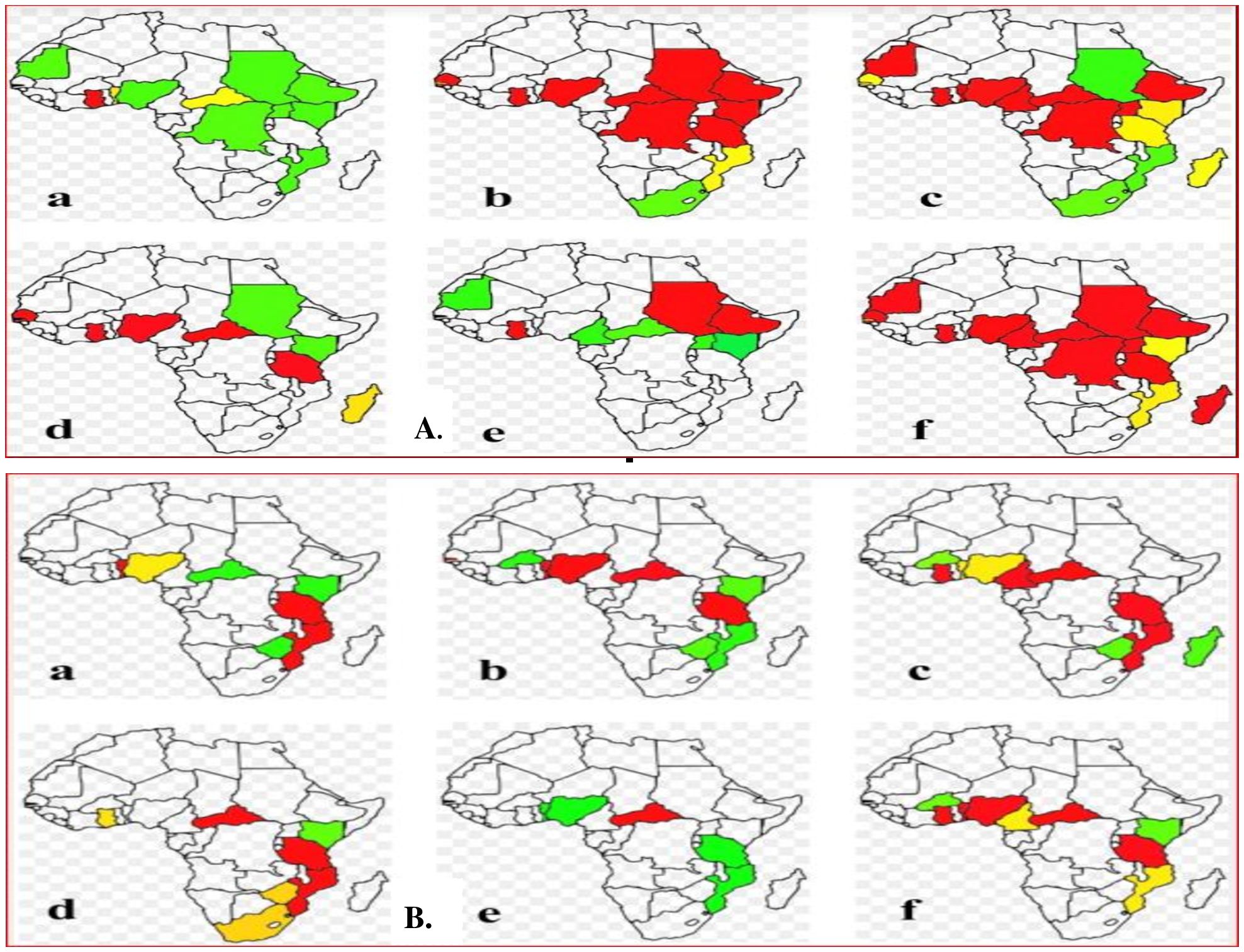
Figure 3. (A) The susceptibility status of An. gambiae s.l across Africa. (B) The susceptibility status of An.funestus across Africa. (a) Bendiocarb, (b) DDT, (c) deltamethrin, (d) lambda-cyhalothrin, (e) malathion, (f) permethrin. Lable red denotes that the Anopheles mosquitoes tested in these countries were developing resistance to the corresponding insecticides. Lable Yellow indicates that the Anopheles mosquitoes tested in these countries were suspected of resistance to the corresponding insecticides. Lable green indicates that the Anopheles mosquitoes tested in these countries were susceptible to the corresponding insecticides.
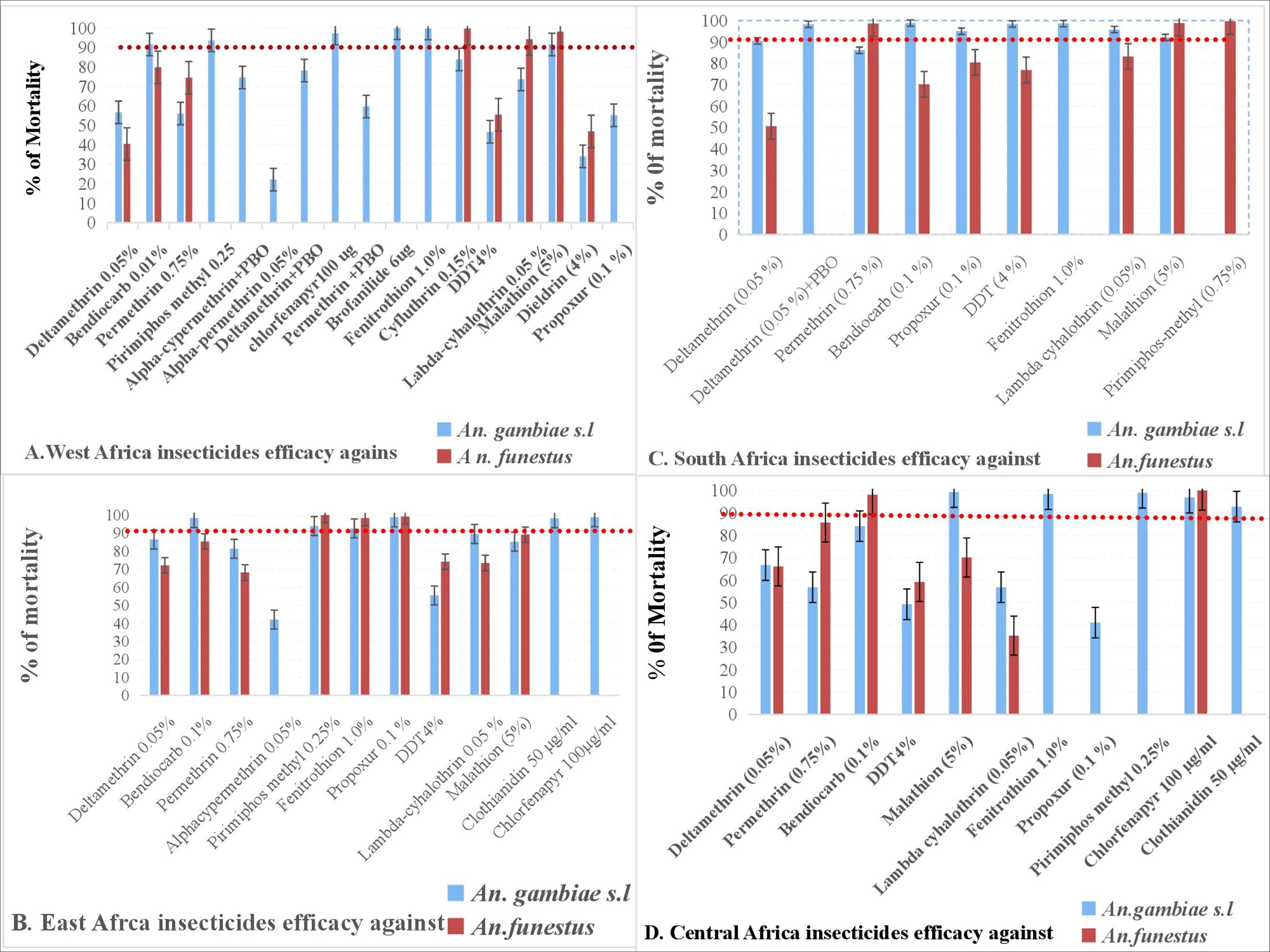
Figure 4. Mortality of An. gambiae s.l. and An. funestus after 24 hours of exposure to different insecticides at different locations.
The mortality rates of populations of An. gambiae s.l. from East Africa and southern Africa 24 hours post-exposure to propoxur was 98% and 90%, respectively, but lower than 90% for populations from West Africa. Similarly, the mortality rates of populations of An. gambiae s.l. from East Africa and West Africa 24 hours post-exposure to propoxur was 98% and 80.4%, respectively. The mean KDT50 for populations of An. gambiae s.l from Central Africa, East Africa, and West Africa exposed to lambda-cyhalothrin was 36.5 minutes, 42.1 minutes, and 89.8 minutes, respectively. Similarly, the mean KDT50 for populations of An. funestus from West Africa, East Africa, southern Africa, and Central Africa exposed to lambda-cyhalothrin was 28.7 minutes, 32.9 minutes, 37.6 minutes, and 69.4 minutes, respectively. The mean mortality rate of populations of An. funestus from West Africa, East Africa, southern Africa, and Central Africa against lambda-cyhalothrin was 90%, 73.4%, 83.2%, and 35%, respectively.
The mean KDT50 of both An. gambiae s.l. and An. funestus exposed to organochlorines (DDT and dieldrin) was quite high, and the mortality rate was less than 90% at many sites (West Africa, East Africa, and Central Africa), with the exception of the mean KDT50 for DDT against An. gambiae s.l., which was shorter (36 minutes), while the mortality rate of populations of both species from southern Africa 24 hours post-exposure to DDT was greater than 98%. Moreover, the mortality rates of populations of An. gambiae s.l. and An. funestus 24 hours post-exposure to pirimiphos methyl, fenitrothion, clothianidin, and chlorfenapyr were greater than 90% in all locations.
Although DDT resistance in mosquitoes was first detected globally in 1957, many insecticides have since been introduced and used for IRS. Insecticide efficacy is constantly studied and analyzed in malaria endemic areas of the world. However, the emergence of vector resistance to several insecticides has increased the monitoring and assessment of the susceptibility status of malaria vectors since 2002. Based on this, the susceptibility status of African malaria vectors to different insecticides in Africa has been evaluated in many African countries using the WHO tube test and CDC bottle assay. This chapter thus summarizes the susceptibility status of the major African malaria vectors to different insecticides in Africa from 2002 to 2023 (Figure 5; Supplementary Table 9).
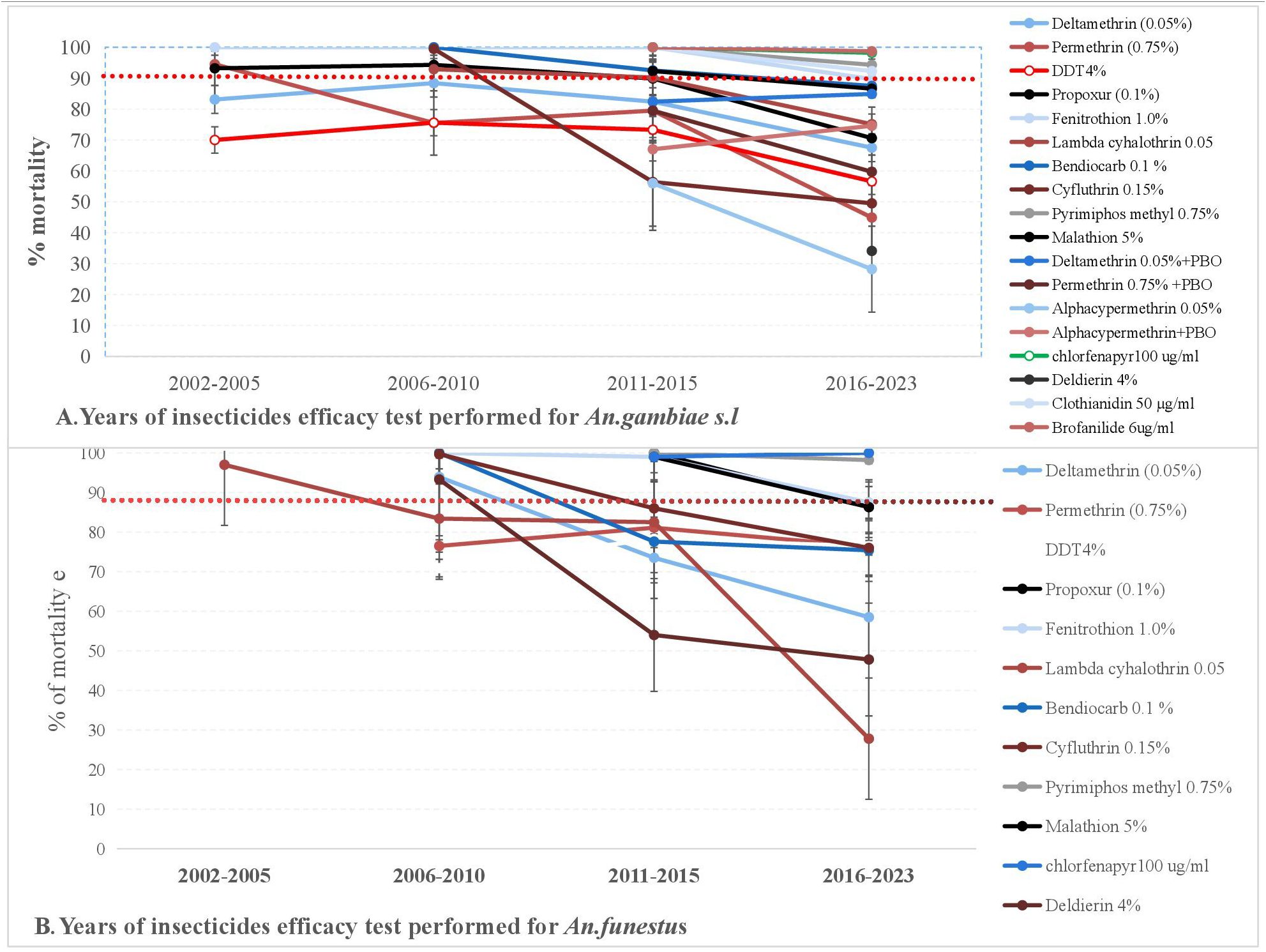
Figure 5. Trends of susceptibility status of An. gambiae s.l. (A) and An. Funestus (B) for various insecticides in Africa from 2002 to 2021.
The mortality rate of populations of An. gambiae s.l exposed to permethrin was greater (>90%) between 2002 and 2005, while the mortality rate was significantly reduced between 2016 and 2023. Similarly, the mortality rate of An. funestus exposed to permethrin was lower than the susceptibility thresholds. Furthermore, the mortality of An. gambiae s.l and An. funestus exposed to lambda-cyhalothrin and deltamethrin was greater than 90% between 2006 and 2010, but reduced between 2011 and 2015. After 2015, An. gambiae s.l. showed higher susceptibility to fenitrothion and pyrimiphos-methyl. Similarly, An. funestus was susceptible to pirimiphos-methyl. Between 2011 and 2015, cross-resistance to organochlorines and pyrethroids was observed in populations of An. gambiae s.l and An. funestus. After 2016, both populations of An. gambiae s.l. and An. funestus exhibited cross-resistance to organophosphate and carbamates. Generally, between 2002 to 2023, populations of An. gambiae s.l. and An. funestus showed reduced mortality to several insecticides (DDT, permethrin, delthamethrin, lambda-cyhalothrin, propoxur, and others) in Africa. However, the major African malaria vectors were susceptible to a few insecticides (pirimiphos methyl, clothianidin, chlorfenapyr, and brofanilide) with mortality rates greater than 90%.
The major malaria vectors in Africa primarily belong to the An. gambiae complex and An. funestus group (Coetzee and Koekemoer, 2013; Sougoufara et al., 2016). These vectors have shown significant changes in abundance and composition in response to the widespread use of ITNs and IRS (Sougoufara et al., 2016, 2020).
Recent research has revealed that a significant proportion of malaria vectors rest on surfaces not typically targeted by IRS, such as floors, furniture, and other household items. In metal-roofed houses, up to 60%–66% of An. arabiensis were found resting on these non-targeted surfaces (Msugupakulya et al., 2020). The effectiveness of ITNs and IRS is largely dependent on vector ecology and behavior, with these interventions primarily targeting indoor-biting mosquitoes (Huho et al., 2013; Sougoufara et al., 2016). This finding highlights a potential gap in the effectiveness of current vector control strategies.
Although the use of ITNs and IRS has significantly reduced the prevalence of malaria in Africa (Moyes et al., 2020), changes in vector behavior and composition pose new challenges. The emergence of outdoor-biting mosquito populations and insecticide resistance threatens the progress made in malaria control (Kafy et al., 2017; Msugupakulya et al., 2020). Furthermore, the rise of insecticide resistance among the major African malaria vectors has posed a growing obstacle to malaria control and elimination efforts (Moyes et al., 2020; Sougoufara et al., 2017). To address these issues, there is an urgent need for additional vector control tools that can target the full range of malaria vectors.
In this study, 50% KDT in both An. gambiae s.l. and An. funestus to pyrethroids such as cyfluthrin (0.15%) was faster (14.5–16.5 min) than organochlorines (both DDT 4% and dieldrin 4% were >60 min). Furthermore, the 90% KDT of An. funestus to pyrethroids was 32.5 minutes, but this was longer for An. gambiae s.l. Similar studies have reported that pyrethroids were relatively more toxic against Anopheles species (Vythilingam et al., 1992). This may be due to the higher toxicity, selective action, rapid knockdown effect, and lower environmental persistence of pyrethroids compared to organochlorines, particularly against Anopheles mosquitoes. However, it is crucial to use these insecticides judiciously and as a part of integrated vector management (IVM) practices to minimize environmental risks and delay the development of insecticide resistance.
Furthermore, the organochlorine DDT and dieldrin had 50% KDT and 90% KDT values above 60 minutes for An. gambiae s.l. and An. funestus. These insecticides were the most used in malaria endemic countries, and their use from the 1940s to the present has resulted in tremendous selective pressure and widespread insecticide resistance (Davidson, 1963; Mekuriaw et al., 2020). This may be due to the extensive and prolonged use of certain insecticide classes, such as organochlorine and pyrethroids, in both agriculture and public health, resulting in a decline in insecticide efficacy (Yadouleton et al., 2009, 2010). This decline in insecticide efficacy may be attributed to cross-resistance resulting from the widespread use of organochlorines and pyrethroids in both the agricultural and public health sectors.
The 50% KDTs of deltamethrin and permethrin against An. gambiae s.l. were between 38.6 and 43.6 minutes. Furthermore, the 50% KDT of permethrin against An. funestus was comparatively quicker (42.2 minutes) than DDT (54.2–56.7 minutes). However, the KDT50 of deltamethrin and lambda-cyhalothrin against An. funestus were above 60 minutes. This might be attributed to slowed vector absorption and behavioral adaptation and the subsequent suppression of vector cholinesterase (Agossa et al., 2014; Hayes and Laws, 1991). In contrast, the 90% KDTs of these insecticides was longer for both An. gambiae s.l. and An. funestus. Similar findings have been reported in other investigations (Bass et al., 2007; Martinez-Torres et al., 1998).
The mortality of An. gambiae s.l. was higher than 90% after 24 hours of exposure to pirimiphos-methyl (0.25%) and/or fenitrothion (1.0%). This is supported by the study conducted by Aïkpon et al. (2013). The early striking action of organophosphate is slow; the later paralytic and lethal effects are potent.
Likewise, the mortality rate of An. gambiae s.l. for bendiocarb, propoxur, and clothianidin 50 μg/mL 24-hour exposure was higher than 90%. Other studies in the Democratic Republic of the Congo and India suggest that clothianidin 50 μg/mL was a better option for IRS choice for vector control of insecticide-resistant mosquitoes (Ngwej et al., 2019; Sreehari et al., 2018). However, even though there were indications of resistance of An. gambiae s.l. to bendiocarb and propoxur in some African countries, while other countries still use it for IRS (Aïkpon et al., 2013). The big studies of residual efficacy assessment reports in favor of this finding (Dengela et al., 2018; Osman et al., 2019).
Similarly, even though the 50% and 90% KDTs of lambda-cyhalothrin for An. funestus were above 60 minutes, this insecticide and pirimiphos-methyl demonstrated higher mortality (over 90%) 24 hours post-exposure. Other insecticides, including DDT, dieldrin, deltamethrin, permethrin, lambda-cyhalothrin, and malathion, demonstrated less than 80% mortality 24 hours post-exposure for An. gambiae s.l. Another study conducted in Rwanda indicated that insecticide resistance to pyrethroids (lambda-cyhalothrin, deltamethrin, and permethrin) and organochlorine (DDT and dieldrin) was gradually increasing (Hakizimana et al., 2016). Similarly, previous studies in Africa revealed that, except for alpha-cypermethrin, none of the insecticides satisfy the minimal range of residual effectiveness specified by the WHO (Dengela et al., 2018; Moyes et al., 2020). This may be due to target site insensitivity.
In this review, insecticide susceptibility testing of African malaria vectors has revealed concerning trends in resistance development across the continent. Insecticide resistance in An. gambiae s.l. and An. funestus, the major malaria vectors in Africa, is widespread and poses a significant threat to malaria control efforts. Studies across sub-Saharan Africa have reported resistance to multiple insecticide classes, including pyrethroids, carbamates, organochlorines, and organophosphates (Ondeto et al., 2017). In Tanzania, An. funestus showed high levels of resistance to pyrethroids and DDT, with a sporozoite rate of 3.4% (Matowo et al., 2021). Similarly, in Uganda, An. gambiae s.l. exhibited high resistance to DDT (85.4% survival) and significant resistance to permethrin (38.5% survival) (Ramphul et al., 2009).
Interestingly, the distribution of insecticide resistance is not uniform across Africa. In Kenya, resistance to pyrethroids has been detected in An. gambiae s.s., An. arabiensis, and An. funestus s.s., while resistance to carbamates was limited to An. gambiae s.s. and An. Arabiensi (Ondeto et al., 2017). The intensity of resistance also varies geographically, with some areas showing alarming increases in pyrethroid and DDT resistance from 2005 to 2017, where mean mortality following insecticide exposure declined from almost 100% to less than 30% in some regions (Hancock et al., 2020).
In summary, the distribution of insecticide resistance in An. gambiae s.l. and An. funestus across Africa is complex and dynamic. The prevalence and intensity of resistance vary by species, insecticide class, and geographical location. This heterogeneity underscores the need for continuous monitoring and region-specific resistance management strategies to maintain the effectiveness of vector control interventions.
An. gambiae s.l. have developed resistance to organochlorines, carbamates, and pyrethroids in West Africa. Only the organophosphate (pyrimiphos-methyl) and the carbamate (bendiocarb) showed mortality rates above 90% after 24 hours of exposure for An. gambiae s.l. Studies have reported high levels of resistance to multiple insecticide classes in both species across the region (Matowo et al., 2021; Mugenzi et al., 2022). In Ghana, An. funestus s.s. and An. gambiae s.l. populations showed resistance to pyrethroids, organochlorines, and carbamates, with high-intensity resistance to pyrethroids observed in both species (Mugenzi et al., 2022). Similarly, in the Democratic Republic of Congo, both An. gambiae and An. funestus exhibited high and multiple resistance to major public health insecticide classes (Riveron et al., 2018).
The mechanisms of resistance differ between the two species. In An. gambiae s.l., both metabolic and target site insensitivity mechanisms drive resistance, while only metabolic mechanisms have been observed in An. funestus (Riveron et al., 2018). The kdr mutations (L1014S and L1014F) associated with pyrethroid and DDT resistance are frequently detected in An. gambiae s.s. and An. arabiensis populations (Ondeto et al., 2017). In contrast, An. funestus relies more heavily on metabolic resistance mechanisms, with overexpression of cytochrome P450 genes such as CYP6P9a and CYP6P9b playing a significant role (Odero et al., 2024).
An. gambiae s.l. was completely susceptible to fenitrothion 24 hours post-exposure and brofanilide 6 μg/mL and chlorfenapyr 100 µg/mL 48 and 72 hours post-exposure. Similarly, a study conducted by Tungu et al. (2022) and Accrombessi et al. (2023) in Benin about large-scale (Phase III) evaluation of brofanilide 50μg/mL for IRS use and efficacy of pyriproxyfen-pyrethroid and chlorfenapyr-pyrethroid Long lasting insecticidal bed nets (LLINs) supported this finding (Accrombessi et al., 2023; Tungu et al., 2022). In contrast, findings from West Africa revealed resistance of An. gambiae s.l. to different classes of insecticides (Cisse et al., 2015; Namountougou et al., 2019; Zouré et al., 2021). The resistance of An. gambiae s.l. in this region to pyrethroids and organochlorines was caused by target site insensitivity such as single sodium channel mutations and metabolic resistance. Decreased sensitivity to organophosphates and carbamates caused by mutations in acetylcholinesterase was also reported by Zouré et al. (2021).
In this study, An. funestus was also found to be resistant to organochlorine, carbamate, and pyrethroid. Suspected resistance to lambda-cyhalothrin has also been recorded. However, An. funestus was only susceptible to malathion (98.2% mortality) and cyfluthrin (99.7% mortality). Moreover, a study reported that An. funestus was susceptible to pyrethroids in West Africa, but other similar studies revealed resistance of An. funestus to pyrethroids in other regions of Ghana (Mugenzi et al., 2022; Pwalia et al., 2019). Studies from central Côte d’Ivoire documented that Anopheles species were susceptible to pirimiphos-methyl but resistant to pyrethroids (Baffour-Awuah et al., 2016). The use of synergists such as piperonyl butoxide (PBO) and the introduction of new insecticide classes, such as chlorfenapyr, may help mitigate the impact of resistance on vector control.
Populations of An. gambiae s.l. have developed resistance to organochlorines, pyrethroids, carbamates, and organophosphates in East Africa. However, mortality rates for bendiocarb, pirimiphos-methyl, and propoxur were higher than 90% 24 hours post-exposure. However, An. gambiae s.l. was shown to have complete susceptibility to bendiocarb and propoxur 24 hours post-exposure and clothianidin 50µg/mL and chlorfenapyr 100µg/mL 72 hours post-exposure. Mosha et al. (2022) reported on the effectiveness of chlorfenapyr 100µg/mL against malaria in Tanzania. Another finding in this region showed the resistance of An. gambiae s.l. to several insecticides such as carbamates, organochlorines, pyrethroids, and, to a lesser extent, organophosphates (Yewhalaw and Kweka, 2016). Similarly, another study demonstrated phenotypic resistance and genetic variation patterns to these insecticides (Hancock et al., 2018). Furthermore, a study conducted in Uganda indicated that the populations of An. gambiae s.l. from West Africa (L1014F) and East Africa (L1014S) had kdr mutations, which confer resistance to DDT and pyrethroids. Additionally, populations of An. gambiae resistant to pyrethroids and DDT exhibited enhanced esterase activity (Verhaeghen et al., 2006). These findings help us to better understand resistance patterns across Africa, and they can also help us to develop insecticide resistance monitoring and management strategies.
Populations of An. funestus have also demonstrated resistance to organochlorines, carbamates, and pyrethroids. However, they were susceptible to certain organophosphates (fenitrothion and pirimiphos-methyl) and a carbamate (propoxur). A similar finding was reported in East Africa (Yewhalaw and Kweka, 2016). The mechanisms of resistance detected in populations of An. gambiae s.l. and An. funestus were knockdown resistance (L1014S, and L1014F), mixed function oxidases, and cuticular resistance to DDT and pyrethroids in East Africa (Yewhalaw and Kweka, 2016), requiring the development of novel control strategies.
Populations of An. gambiae s.l. were susceptible to deltamethrin, DDT, bendiocarb, propoxur, fenitrothion, lambda-cyhalothrin, malathion, and fenitrothionin this region. In contrast, An. funestus was completely resistant to carbamates, organochlorines, and pyrethroids in Southern African regions, but susceptible to organophosphates (pirimiphos-methyl and malathion) and permethrin. However, a study in Malawi documented pyrethroid resistance in populations of An. funestus and An. arabiensis (Mzilahowa et al., 2016). Another comparable finding in the region demonstrated that mosquito populations respond with evolutionary resistance to tremendous selection pressure, with metabolic resistance having the highest operational impact in the region (Weedall et al., 2020).
In South Africa and Mozambique, An. funestus populations have shown evidence of resistance to pyrethroid insecticides, with elevated levels of mixed function oxidases responsible for detoxification (Brooke et al., 2001). This mechanism also confers cross-resistance to carbamate insecticides such as propoxur. In Malawi, An. funestus populations were found to be fully susceptible to dieldrin, an organochlorine insecticide (Wondji et al., 2011). This suggests extensive barriers to gene flow between populations from different regions.
In summary, insecticide resistance in An. gambiae s.l. and An. funestus is a growing concern in southern Africa, with pyrethroid resistance being particularly widespread. The geographical variations in resistance patterns highlight the need for region-specific resistance management strategies. Continued monitoring and research are crucial to inform effective vector control interventions in these countries.
An. gambiae s.l. was shown to be resistant to carbamates, organochlorines, and pyrethroids (DDT, deltamethrin, bendiocarb, propoxur, permethrin, and lambda-cyhalothrin) in Central Africa. However, they were fully susceptible to organophosphates (malathion, fenitrothion, and pirimiphos methyl) 24 hours post-exposure. Likewise, other studies revealed that populations of An. gambiae s.l. were resistant to carbamates, organochlorines, and pyrethroids. Anopheles funestus was only susceptible to chlorfenapyr 100 µg/mL and bendiocarb. Similarly, there was 100% mortality observed to organophosphates (Dadzie et al., 2016; Olé Sangba et al., 2017). This suggests that resistance in African malaria vectors may be attributed to cross-resistance due to target-site insensitivity and metabolic resistance.
Similarly, An. funestus was resistant to carbamates, organochlorines, and pyrethroids, with mortality rates ranging from 35% to 85.7%, except for bendiocarb, which showed 98.1% mortality in the area. Research conducted in Central Africa indicated resistance to permethrin, deltamethrin, DDT, lambda-cyhalothrin, and a malathion (Olé Sangba et al., 2016). Another study showed that An. funestus was highly resistant to the pyrethroids deltamethrin and permethrin in Malawi, with mortality rates of 0%–41% and 0%–44%, respectively, in 2015. Another study showed that An. funestus populations were susceptible to the organophosphates malathion and pirimiphos-methyl (Mzilahowa et al., 2016). Furthermore, in the Central African Republic, An. funestus showed mortality rates as low as 23%–35% for pyrethroids and DDT. The study also reported that 100% mortality to bendiocarb was observed in An. funestus (Olé Sangba et al., 2016). Furthermore, 100% susceptibility of An. funestus to malathion was recorded in Chad (Kerah-Hinzoumbé et al., 2008). This highlights the importance of continued monitoring and the potential for insecticide rotation strategies.
In general, major African malaria vectors’ mortality rates declined across the continent. Between 2002 and 2015, malaria vector control strategies such as IRS and ITNs were significantly strengthened, but the resistance of the Anopheles vector became widespread in African countries (Sougoufara et al., 2017). Other studies found that vector control strategies, including IRS and ITN, were significantly strengthened between 2000 and 2015, and consequently, resistance of malaria vectors to major classes of insecticides increased during this period (Hancock et al., 2020; Ranson and Lissenden, 2016).
Based on these findings, few insecticides, including pirimiphos-methyl from the organophosphates group and new candidate insecticides (clothianidin, chlorfenapyr, and broflanilide/tenebenal) demonstrated significant mortality rates in malaria vectors. This aligns with the 2023 WHO recommendations, which advocate the use of chlorfenapyr and its combination with pyrethroids for LLINs and broflanilide for IRS (WHO, 2023a).
From 2011 to 2015, significant cross-resistance within and between organochlorines and pyrethroids was observed in populations of both An. gambiae s.l and An. funestus. Other similar findings were reported from different parts of Africa (Aïzoun et al., 2014; Clarkson et al., 2021; Dabiré et al., 2008). This cross-resistance is largely attributed to several genetic and biochemical mechanisms such as kdr mutation, which reduced the sensitivity of the mosquitoes to both organochlorines and pyrethroids. These mutations alter the target site, making it less likely for the insecticides to bind effectively. It may also enhance the activity of detoxifying enzymes such as cytochrome P450 monooxygenases, esterases, and glutathione S-transferases (GSTs), which can degrade or sequester insecticides before they reach their target sites. These enzymes can be upregulated through gene amplification or increased gene expression. Some detoxification enzymes that evolved to break down organochlorines may also act on pyrethroids, contributing to cross-resistance (Brengues et al., 2003; Moyes et al., 2021; Strode et al., 2014). Similarly, after 2016, populations of An. gambiae s.l. and An. funestus exhibited notable cross-resistance between organophosphates and carbamates. This finding was also supported by different studies conducted in different parts of Africa (Menze et al., 2016; Mugenzi et al., 2023; Odero et al., 2024). This resistance could be primarily due to an increased level of certain detoxification enzymes and as a result of the upregulation of genes that allow these mosquitoes to metabolize these insecticides. This knowledge is pivotal for shaping current vector control strategies and emphasizes the need for ongoing surveillance and adaptive insecticide resistance management to combat malaria effectively.
This study provides a comprehensive overview of the available evidence on the insecticide susceptibility status of the major malaria vectors in Africa. By collecting data from multiple studies conducted across different regions of Africa, this study provides insights into insecticide susceptibility patterns to create a good picture of insecticide resistance in the major malaria vectors in the region beyond individual study sites or populations. However, the variable quality of the data across studies may affect the overall reliability of the conclusions. In addition, there might be limitations in accessing relevant data, especially from unpublished documents, ongoing research, or studies conducted in languages other than English, which could impact the comprehensiveness of the study.
Currently, An. gambiae s.l. and An. funestus are susceptible to pirimiphos-methyl, clothianidin, chlorfenapyr, and broflanilide and showed high resistance to all four insecticide classes (pyrethroid, carbamate, organochlorine, and most of the organophosphate group). From 2011 to 2015, a high frequency of resistance was documented in the major African malaria vectors. In addition, the combination of pyrethroids with synergists and chlorfenapyr improved the efficacy of some of these insecticides. Synergists are compounds that can enhance the insecticidal activity of insecticides by inhibiting the enzymes responsible for detoxification of insecticides in insects. For example, synergists such as piperonyl butoxide (PBO), S,S,S-tributylphosphorotrithioate, and diethyl maleate are strong inhibitors of cytochrome P450 monooxygenases, esterases, and GSTs, respectively.
Moreover, the use of insecticides in agriculture has historically contributed to cross-resistance in malaria vectors. For example, pyrethroids were commonly used in agriculture for pest control and public health due to their effectiveness against agricultural pests and in mosquito control, respectively. However, overreliance on a single class of insecticides (pyrethroids) triggered the emergence of resistance (Demissew et al., 2022; Hien et al., 2017). Hence, malaria-endemic countries should implement and promote IVM, including biological control methods, and also develop and implement insecticide resistance management strategies to mitigate or avert the impact of insecticide resistance in malaria control and elimination.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
AM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. DY: Supervision, Writing – review & editing. AS: Supervision, Visualization, Writing – review & editing. GA: Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. The publication fee was covered by the Department of Agricultural, Environmental and Food Sciences, University of Molise, Campobasso, Italy.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmala.2025.1478249/full#supplementary-material
ITNs, insecticide-treated bed nets; IRS, indoor residual spray; IVM, integrated vector management; KDT, knockdown time; WHO, World Health Organization.
Accrombessi M., Cook J., Dangbenon E., Yovogan B., Akpovi H., Sovi A., et al. (2023). Efficacy of pyriproxyfen-pyrethroid long-lasting insecticidal nets (LLINs) and chlorfenapyr-pyrethroid LLINs compared with pyrethroid-only LLINs for malaria control in Benin: a cluster-randomised, superiority trial. Lancet 401, 435–446. doi: 10.1016/S0140-6736(22)02319-4
Agossa F. R., Aïkpon R., Azondékon R., Govoetchan R., Padonou G. G., Oussou O., et al. (2014). Efficacy of various insecticides recommended for indoor residual spraying: pirimiphos methyl, potential alternative to bendiocarb for pyrethroid resistance management in Benin, West Africa. Trans. R. Soc. Trop. Med. Hygiene 108, 84–91. doi: 10.1093/trstmh/trt117
Aïkpon R., Agossa F., Ossè R., Oussou O., Aïzoun N., Oké-Agbo F., et al. (2013). Bendiocarb resistance in Anopheles Gambiae sl populations from Atacora department in Benin, West Africa: a threat for malaria vector control. Parasites Vectors 6, 192. doi: 10.1186/1756-3305-6-192
Aïzoun N., Azondekon R., Aïkpon R., Anagonou R., Gnanguenon V., Akogbéto M. (2014). Dynamics of insecticide resistance and exploring biochemical mechanisms involved in pyrethroids and dichlorodiphenyltrichloroethane (DDT) cross-resistance in Anopheles Gambiae sl populations from Benin, West Africa. J. Cell. Anim. Biol. 8, 41–50. doi: 10.5897/JCAB2014.0406
Baffour-Awuah S., Annan A. A., Maiga-Ascofare O., Dieudonné S. D., Adjei-Kusi P., Owusu-Dabo E., et al. (2016). Insecticide resistance in malaria vectors in Kumasi, Ghana. Parasites Vectors 9, 1–8. doi: 10.1186/s13071-016-1923-5
Baleta A. (2009). Insecticide resistance threatens malaria control in Africa. Lancet 374, 1581–1582. doi: 10.1016/S0140-6736(09)61933-4
Bass C., Nikou D., Donnelly M. J., Williamson M. S., Ranson H., Ball A., et al. (2007). Detection of knockdown resistance (kdr) mutations in Anopheles Gambiae: a comparison of two new high-throughput assays with existing methods. Malaria J. 6, 1–6. doi: 10.1186/1475-2875-6-111
Berry-Cabán C. S. (2011). DDT and silent spring: fifty years after. J. Mil. Vet. Hlth 19, 19–24. doi: 11.2021-55939783/JMVHVol19No4
Brengues C., Hawkes N. J., Chandre F., McCarroll L., Duchon S., Guillet P., et al. (2003). Pyrethroid and DDT cross-resistance in Aedes aEgypti is correlated with novel mutations in the voltage-gated sodium channel gene. Med. Veterinary Entomology 17, 87–94. doi: 10.1046/j.1365-2915.2003.00412.x
Brooke B. D., Kloke G., Hunt R. H., Koekemoer L. L., Tem E. A., Taylor M. E., et al. (2001). Bioassay and biochemical analyses of insecticide resistance in southern African Anopheles funestus (Diptera: Culicidae). Bull. entomological Res. 91, 265–272. doi: 10.1079/BER2001108
Brown A. W. (1958). The insecticide-resistance problem: a review of developments in 1956 and 1957. Bull. World Health Organ. 18, 309.
Carnevale P., Robert V., Snow R., Curtis C., Richard A., Boudin C., et al. (1991). The impact of impregnated mosquito nets on prevalence and morbidity related to malaria in sub-Saharan africa. Ann. Soc. Belg Med. Trop. 71 Suppl 1, 127–150.
Cisse M., Keita C., Dicko A., Dengela D., Coleman J., Lucas B., et al. (2015). Characterizing the insecticide resistance of Anopheles Gambiae in Mali. Malaria J. 14, 1–10. doi: 10.1186/s12936-015-0847-4
Clarkson C. S., Miles A., Harding N. J., O’Reilly A. O., Weetman D., Kwiatkowski D., et al. (2021). The genetic architecture of target-site resistance to pyrethroid insecticides in the African malaria vectors Anopheles Gambiae and Anopheles coluzzii. Mol. Ecol. 30, 5303–5317. doi: 10.1111/mec.v30.21
Coetzee M., Koekemoer L. L. (2013). Molecular systematics and insecticide resistance in the major African malaria vector Anopheles funestus. Annu. Rev. entomology 58, 393–412. doi: 10.1146/annurev-ento-120811-153628
Corbel V., Kont M. D., Ahumada M. L., Andréo L., Bayili B., Bayili K., et al. (2023). A new WHO bottle bioassay method to assess the susceptibility of mosquito vectors to public health insecticides: results from a WHO-coordinated multi-centre study. Parasites Vectors 16, 21. doi: 10.1186/s13071-022-05554-7
Dabiré K. R., Diabaté A., Agostinho F., Alves F., Manga L., Faye O., et al. (2008). Distribution of the members of Anopheles Gambiae and pyrethroid knock-down resistance gene (kdr) in Guinea-Bissau, West Africa. Bull. Soc. Pathol. Exot. 101 (2), 119-123.
Dadzie S., Appawu M. A., Kerah-Hinzoumbe C., Akogbeto M. C., Adimazoya M., Israel D. K., et al. (2016). Species composition and insecticide resistance status of Anopheles Gambiae (sl)(Culicidae) in Kome, southern Chad and the implications for malaria control. Parasites Vectors 9, 1–8. doi: 10.1186/s13071-016-1758-0
Davidson G. (1963). DDT-resistance and dieldrin-resistance in Anopheles quadrimaculatus. Bull. World Health Organ. 29, 177–184.
Demissew A., Animut A., Kibret S., Tsegaye A., Hawaria D., Degefa T., et al. (2022). Evidence of pyrethroid resistance in Anopheles amharicus and Anopheles arabiensis from Arjo-Didessa irrigation scheme, Ethiopia. PloS One 17, e0261713. doi: 10.1371/journal.pone.0261713
Dengela D., Seyoum A., Lucas B., Johns B., George K., Belemvire A., et al. (2018). Multi-country assessment of residual bio-efficacy of insecticides used for indoor residual spraying in malaria control on different surface types: results from program monitoring in 17 PMI/USAID-supported IRS countries. Parasites Vectors 11, 1–4. doi: 10.1186/s13071-017-2608-4
EPA, US (1975). DDT Regulatory History: A Brief Survey (to 1975). (Environmental Protection Agency). Available online at: https://archive.epa.gov/epa/aboutepa/ddt-regulatory-history-brief-survey-1975.html.
Hakizimana E., Karema C., Munyakanage D., Iranzi G., Githure J., Tongren J. E., et al. (2016). Susceptibility of Anopheles Gambiae to insecticides used for malaria vector control in Rwanda. Malaria J. 15, 1–1. doi: 10.1186/s12936-016-1618-6
Hancock P. A., Hendriks C. J., Tangena J. A., Gibson H., Hemingway J., Coleman M., et al. (2020). Mapping trends in insecticide resistance phenotypes in African malaria vectors. PloS Biol. 18, e3000633. doi: 10.1371/journal.pbio.3000633
Hancock P. A., Wiebe A., Gleave K. A., Bhatt S., Cameron E., Trett A., et al. (2018). Associated patterns of insecticide resistance in field populations of malaria vectors across Africa. Proc. Natl. Acad. Sci. 115, 5938–5943. doi: 10.1073/pnas.1801826115
Hayes W. J., Laws E. R. (1991). Handbook of Pesticide Toxicology. 1 ed Vol. 2 (New York, NY: Academic Press, Inc).
Hien A. S., Soma D. D., Hema O., Bayili B., Namountougou M., Gnankiné O., et al. (2017). Evidence that agricultural use of pesticides selects pyrethroid resistance within Anopheles Gambiae s. l. populations from cotton growing areas in Burkina Faso, West Africa. PloS One 12, e0173098. doi: 10.1371/journal.pone.0173098
Huho B., Briët O., Seyoum A., Sikaala C., Bayoh N., Gimnig J., et al. (2013). Consistently high estimates for the proportion of human exposure to malaria vector populations occurring indoors in rural Africa. Int. J. Epidemiol. 42, 235–247. doi: 10.1093/ije/dys214
Ibrahim H., Kheir R., Helmi S., Lewis J., Crane M. (1998). Effects of organophosphorus, carbamate, pyrethroid and organochlorine pesticides, and a heavy metal on survival and cholinesterase activity of Chironomus riparius Meigen. Bull. Environ. Contamination Toxicol. 60, 448–455. doi: 10.1007/s001289900646
Juache-Villagrana A. E., Pando-Robles V., Garcia-Luna S. M., Ponce-Garcia G., Fernandez-Salas I., Lopez-Monroy B., et al. (2022). Assessing the impact of insecticide resistance on vector competence: a review. Insects 13, 377. doi: 10.3390/insects13040377
Kafy H. T., Ismail B. A., Mnzava A. P., Lines J., Abdin M. S. E., Eltaher J. S., et al. (2017). Impact of insecticide resistance in Anopheles arabiensis on malaria incidence and prevalence in Sudan and the costs of mitigation. Proc. Natl. Acad. Sci. 114, E11267–E11275. doi: 10.1073/pnas.1713814114
Kerah-Hinzoumbé C., Péka M., Nwane P., Donan-Gouni I., Etang J., Samè-Ekobo A., et al. (2008). Insecticide resistance in Anopheles Gambiae from south-western Chad, Central Africa. Malaria J. 7, 1–9. doi: 10.1186/1475-2875-7-192
Kinfe E., Irish S., Hailemariam A., Wuletaw Y., AbateTemesgen S., Tekie H. (2021). Susceptibility of Anopheles Gambiae s.l., and Anopheles funestus s.l. to seven insecticides in southern Ethiopia. Ethiopia J. Public Health Nutr. 4, 154–159. doi: 10.1371/journal.pone.0173098
Kolawole E. O., Ayeni E. T., Abolade S. A., Ugwu S. E., Awoyinka T. B., Ofeh A. S., et al. (2022). Malaria endemicity in Sub-Saharan Africa: Past and present issues in public health. Microbes Infect. Diseases. 4 (1), 242–251. doi: 10.21608/mid.2022.150194.1346
Mabaso M. L. H., Sharp B., Lengeler C. (2004). Historical review of malarial control in southern African with emphasis on the use of in door residual house-spraying. Trop. Med. Int. Health 9, 846–856. doi: 10.1111/j.1365-3156.2004.01263.x
Martinez-Torres D., Chandre F., Williamson M. S., Darriet F., Berge J. B., Devonshire A. L., et al. (1998). Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles Gambiae ss. Insect Mol. Biol. 7, 174–184. doi: 10.1046/j.1365-2583.1998.72062.x
Matowo N. S., Martin J., Kulkarni M. A., Mosha J. F., Lukole E., Isaya G., et al. (2021). An increasing role of pyrethroid-resistant Anopheles funestus in malaria transmission in the Lake Zone, Tanzania. Sci. Rep. 11, 13457. doi: 10.1038/s41598-021-92741-8
Mbabazi R., Maredia K., El-Sayed B. B., Babumba A. K., Savadogo M., Akinbo O. (2021). “Integrated Management of Malaria Vectors in Africa,” in Genetically Modified and other Innovative Vector Control Technologies (Springer), 163–197. doi: 10.1007/978-981-16-2964-8_9
Mekuriaw W., Yewhalaw D., Dugassa S., Taffese H., Bashaye S., Nigatu W., et al. (2020). Distribution and trends of insecticide resistance in malaria vectors in Ethiopia, (1986-2017): a review. Ethiopian J. Public Health Nutr. 03, 51–61. Available online at: https://ejphn.ephi.gov.et/index.php/ejphn/article/view/139 (Accessed March 20, 2025).
Menze B. D., Riveron J. M., Ibrahim S. S., Irving H., Antonio-Nkondjio C., Awono-Ambene P. H., et al. (2016). Multiple insecticide resistance in the malaria vector Anopheles funestus from Northern Cameroon is mediated by metabolic resistance alongside potential target site insensitivity mutations. PloS One 11, e0163261. doi: 10.1371/journal.pone.0163261
Mosha J. F., Kulkarni M. A., Lukole E., Matowo N. S., Pitt C., Messenger L. A., et al. (2022). Effectiveness and cost-effectiveness against malaria of three types of dual-active-ingredient long-lasting insecticidal nets (LLINs) compared with pyrethroid-only LLINs in Tanzania: a four-arm, cluster-randomised trial. Lancet 399, 1227–1241. doi: 10.1016/S0140-6736(21)02499-5
Moyes C. L., Athinya D. K., Seethaler T., Battle K. E., Sinka M., Hadi M. P., et al. (2020). Evaluating insecticide resistance across African districts to aid malaria control decisions. Proc. Natl. Acad. Sci. 117, 22042–22050. doi: 10.1073/pnas.2006781117
Moyes C. L., Lees R. S., Yunta C., Walker K. J., Hemmings K., Oladepo F., et al. (2021). Assessing cross-resistance within the pyrethroids in terms of their interactions with key cytochrome P450 enzymes and resistance in vector populations. Parasites Vectors 14, 1–13. doi: 10.1186/s13071-021-04609-5
Msugupakulya B. J., Kaindoa E. W., Ngowo H. S., Kihonda J. M., Kahamba N. F., Msaky D. S., et al. (2020). Preferred resting surfaces of dominant malaria vectors inside different house types in rural south-eastern Tanzania. Malaria J. 19, 1–15. doi: 10.1186/s12936-020-3108-0
Mugenzi L. M., Akosah-Brempong G., Tchouakui M., Menze B. D., Tekoh T. A., Tchoupo M., et al. (2022). Escalating pyrethroid resistance in two major malaria vectors Anopheles funestus and Anopheles Gambiae (sl) in Atatam, Southern Ghana. BMC Infect. Dis. 22, 799. doi: 10.1186/s12879-022-07795-4
Mugenzi L. M. J., Tekoh T. A., Ibrahim S. S., Muhammad A., Kouamo M., Wondji M. J., et al. (2023). The duplicated P450s CYP6P9a/b drive carbamates and pyrethroids cross-resistance in the major African malaria vector Anopheles funestus. PloS Genet. 19, e1010678. doi: 10.1371/journal.pgen.1010678
Mzilahowa T., Chiumia M., Mbewe R. B., Uzalili V. T., Luka-Banda M., Kutengule A., et al. (2016). Increasing insecticide resistance in Anopheles funestus and Anopheles arabiensis in Malawi 2011–2015. Malaria J. 15, 1–5. doi: 10.1186/s12936-016-1610-1
Namountougou M., Soma D. D., Kientega M., Balboné M., Kaboré D. P., Drabo S. F., et al. (2019). Insecticide resistance mechanisms in Anopheles Gambiae complex populations from Burkina Faso, West Africa. Acta Tropica 197, 105054. doi: 10.1016/j.actatropica.2019.105054
Ngwej L. M., Hattingh I., Mlambo G., Mashat E. M., Kashala J. C., Malonga F. K., et al. (2019). Indoor residual spray bio-efficacy and residual activity of a clothianidin-based formulation (SumiShield® 50WG) provides long persistence on various wall surfaces for malaria control in the Democratic Republic of the Congo. Malaria J. 18, 1–8. doi: 10.1186/s12936-019-2710-5
Odero J. O., Nambunga I. H., Masalu J. P., Mkandawile G., Bwanary H., Hape E. E., et al. (2024). Genetic markers associated with the widespread insecticide resistance in malaria vector Anopheles funestus populations across Tanzania. Parasites Vectors 17, 230. doi: 10.1186/s13071-024-06315-4
Olé Sangba M. L., Deketramete T., Wango S. P., Kazanji M., Akogbeto M., Ndiath M. O. (2016). Insecticide resistance status of the Anopheles funestus population in Central African Republic: a challenge in the war. Parasites Vectors 9, 1–8. doi: 10.1186/s13071-016-1510-9
Olé Sangba M. L., Sidick A., Govoetchan R., Dide-Agossou C., Ossè R. A., Akogbeto M., et al. (2017). Evidence of multiple insecticide resistance mechanisms in Anopheles Gambiae populations in Bangui, Central African Republic. Parasites Vectors 10, 1–10. doi: 10.1186/s13071-016-1965-8
Ondeto B. M., Nyundo C., Kamau L., Muriu S. M., Mwangangi J. M., Njagi K., et al. (2017). Current status of insecticide resistance among malaria vectors in Kenya. Parasites Vectors 10, 1–13. doi: 10.1186/s13071-017-2361-8
Ongono J. S., Béranger R., Baghdadli A., Mortamais M. (2020). Pesticides used in Europe and autism spectrum disorder risk: can novel exposure hypotheses be formulated beyond organophosphates, organochlorines, pyrethroids and carbamates?-A systematic review. Environ. Res. 187, 109646. doi: 10.1016/j.envres.2020.109646
Osman S. O. S., Toto T. H., Abdalmajed M. A., Jihad A., Azrag S. R. (2019). Monitoring of insecticide resistance of anopheles arabiensis patton to DDT 4%, deltamethrin 0.05%, permethrin 0.75% And bendiocarb 0.1% In river nile state, Sudan. J. Public Health 5, 555656. doi: 10.519080/JOJPH.552019.555605.555656
Pwalia R., Joannides J., Iddrisu A., Addae C., Acquah-Baidoo D., Obuobi D., et al. (2019). High insecticide resistance intensity of Anopheles Gambiae (sl) and low efficacy of pyrethroid LLINs in Accra, Ghana. Parasites Vectors 12, 1–9. doi: 10.1186/s13071-019-3556-y
Quilty J. R., Cattle S. R. (2011). Use and understanding of organic amendments in Australian agriculture: a review. Soil Res. 49, 1–26. doi: 10.1071/SR10059
Raghavendra K., Barik T. K., Reddy B. P., Sharma P., Dash A. P. (2011). Malaria vector control: from past to future. Parasitol. Res. 108, 757–779. doi: 10.1007/s00436-010-2232-0
Ramphul U., Boase T., Bass C., Okedi L. M., Donnelly M. J., Müller P. (2009). Insecticide resistance and its association with target-site mutations in natural populations of Anopheles Gambiae from eastern Uganda. Trans. R. Soc. Trop. Med. Hygiene 103, 1121–1126. doi: 10.1016/j.trstmh.2009.02.014
Ranson H., Lissenden N. (2016). Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 32, 187–196. doi: 10.1016/j.pt.2015.11.010
Riveron J. M., Watsenga F., Irving H., Irish S. R., Wondji C. S. (2018). High Plasmodium infection rate and reduced bed net efficacy in multiple insecticide-resistant malaria vectors in Kinshasa, Democratic Republic of Congo. J. Infect. Dis. 217, 320–328. doi: 10.1093/infdis/jix570
Roark R. C. (1938). Synthetic organic compounds used as insecticides. Can. Entomologist 70, 248–253. doi: 10.4039/Ent70248-12
Sinka M. E. (2013). “Global distribution of the dominant vector species of malaria,” in Anopheles mosquitoes-New insights into malaria vectors (IntechOpen). doi: 10.5772/54163
Sinka M. E., Bangs M. J., Manguin S., Coetzee M., Mbogo C. M., Hemingway J., et al. (2010). The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasites Vectors 3, 1–34. doi: 10.1186/1756-3305-3-117
Sinka M. E., Bangs M. J., Manguin S., Rubio-Palis Y., Chareonviriyaphap T., Coetzee M., et al. (2012). A global map of dominant malaria vectors. Parasites Vectors 5, 1–11. doi: 10.1186/1756-3305-5-69
Sinka M. E., Pironon S., Massey N. C., Longbottom J., Hemingway J., Moyes C. L., et al. (2020). A new malaria vector in Africa: Predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc. Natl. Acad. Sci. 117, 24900–24908. doi: 10.1073/pnas.2003976117
Slater L., Ashraf S., Zahid O., Ali Q., Oneeb M., Akbar M. H., et al. (2022). Current methods for the detection of Plasmodium parasite species infecting humans. Curr. Res. Parasitol. Vector-Borne Dis., 100086. doi: 10.1016/j.crpvbd.2022.100086
Snetselaar J., Rowland M. W., Manunda B. J., Kisengwa E. M., Small G. J., Malone D. J., et al. (2021). Efficacy of indoor residual spraying with broflanilide (TENEBENAL), a novel meta-diamide insecticide, against pyrethroid-resistant anopheline vectors in northern Tanzania: An experimental hut trial. PloS One 16, e0248026. doi: 10.1371/journal.pone.0248026
Sougoufara S., Doucouré S., Sembéne P. M., Harry M., Sokhna C. (2017). Challenges for malaria vector control in sub-Saharan Africa: resistance and behavioral adaptations in Anopheles populations. J. Vector Borne Dis. 54, 4. doi: 10.4103/0972-9062.203156
Sougoufara S., Harry M., Doucouré S., Sembène P. M., Sokhna C. (2016). Shift in species composition in the Anopheles Gambiae complex after implementation of long-lasting insecticidal nets in Dielmo, Senegal. Med. veterinary entomology 30, 365–368. doi: 10.1111/mve.12171
Sougoufara S., Ottih E. C., Tripet F. (2020). The need for new vector control approaches targeting outdoor biting Anopheline malaria vector communities. Parasites Vectors 13 (1), 295. doi: 10.1186/s13071-020-04170-7
Sreehari U., Raghavendra K., Tiwari S. N., Sreedharan S., Ghosh S. K., Valecha N. (2018). Small-scale (Phase II) evaluation of the efficacy and residual activity of SumiShield® 50 WG (clothianidin 50%, w/w) for indoor residual spraying in comparison to deltamethrin, bendiocarb and pirimiphos-methyl for malaria vector control in Karnataka state, India. J. Vector Borne Dis. 55 (2), 122–129. doi: 10.4103/0972-9062.242559
Strode C., Donegan S., Garner P., Enayati A. A., Hemingway J. (2014). The impact of pyrethroid resistance on the efficacy of insecticide-treated bed nets against African anopheline mosquitoes: systematic review and meta-analysis. PloS Med. 11, e1001619. doi: 10.1371/journal.pmed.1001619
Tungu P. K., Rowland M. W., Messenger L. A., Small G. J., Bradley J., Snetselaar J., et al. (2022). Large-scale (Phase III) evaluation of broflanilide 50WP (VECTRON™ T500) for indoor residual spraying for malaria vector control in Northeast Tanzania: study protocol for a two-arm, non-inferiority, cluster-randomised community trial. BMC Infect. Dis. 22, 171. doi: 10.1186/s12879-022-07138-3
USAID (2022). 16TH ANNUAL REPORT TO CONGRESS (CDC), 95. Available online at: https://stacks.cdc.gov/view/cdc/116690.
Utzinger J., Tozan Y., Singer B. H. (2001). Efficacy and cost-effectiveness of environmental management for malaria control. Trop. Med. Int. Health 6, 677–687. doi: 10.1046/j.1365-3156.2001.00769.x
van de Straat B., Russell T. L., Staunton K. M., Sinka M. E., Burkot T. R. (2021). A global assessment of surveillance methods for dominant malaria vectors. Sci. Rep. 11, 1–13. doi: 10.1038/s41598-021-94656-w
Verhaeghen K., Van Bortel W., Roelants P., Backeljau T., Coosemans M. (2006). Detection of the East and West African kdr mutation in Anopheles Gambiae and Anopheles arabiensis from Uganda using a new assay based on FRET/Melt Curve analysis. Malaria J. 5, 1–9. doi: 10.1186/1475-2875-5-16
Vythilingam I., Chiang G. L., Amatachaya C. (1992). Adulticidal effect of cyfluthrin against mosquitos of public health importance in Malaysia. Southeast Asian J. Trop. Med. Public Health 23, 111–115.
Weedall G. D., Riveron J. M., Hearn J., Irving H., Kamdem C., Fouet C., et al. (2020). An Africa-wide genomic evolution of insecticide resistance in the malaria vector Anopheles funestus involves selective sweeps, copy number variations, gene conversion and transposons. PloS Genet. 16, e1008822. doi: 10.1371/journal.pgen.1008822
WHO (2022a). Standard operating procedure for testing insecticide susceptibility of adult mosquitoes in WHO tube tests (Geneva: World Health Organization).
Williams J., Flood L., Praulins G., Ingham V. A., Morgan J., Lees R. S., et al. (2019). Characterisation of Anopheles strains used for laboratory screening of new vector control products. Parasites Vectors 12, 1–4. doi: 10.1186/s13071-019-3774-3
Wondji C. S., Dabire R. K., Tukur Z., Irving H., Djouaka R., Morgan J. C. (2011). Identification and distribution of a GABA receptor mutation conferring dieldrin resistance in the malaria vector Anopheles funestus in Africa. Insect Biochem. Mol. Biol. 41, 484–491. doi: 10.1016/j.ibmb.2011.03.012
Yadouleton A. W., Asidi A., Djouaka R. F., Braïma J., Agossou C. D., Akogbeto M. C. (2009). Development of vegetable farming: a cause of the emergence of insecticide resistance in populations of Anopheles Gambiae in urban areas of Benin. Malaria J. 8, 1–8. doi: 10.1186/1475-2875-8-103
Yadouleton A. W., Padonou G., Asidi A., Moiroux N., Bio-Banganna S., Corbel V., et al. (2010). Insecticide resistance status in Anopheles Gambiae in southern Benin. Malaria J. 9, 1–6. doi: 10.1186/1475-2875-9-83
Yewhalaw D., Balkew M., Shililu J., Suleman S., Getachew A., Ashenbo G., et al. (2017). Determination of the residual efcacy of carbamate and organophosphate insecticides used for indoor residual spraying for malaria control in Ethiopia. Malaria Journa 16, 471. doi: 10.1186/s12936-017-2122-3
Yewhalaw D., Kweka E. J. (2016). Insecticide resistance in East Africa -history, distribution and drawbacks on malaria vectors and disease control. Insecticides resistance 39, 189–215. doi: 10.5772/61570
Zacharia J. T. (2011). Identity, physical and chemical properties of pesticides. Pesticides modern world-trends pesticides Anal. 21, 1–18.
Zahar A. R. (1984). Vector control operations in the African context. Bull. World Health Organ. 62, 89–100.
Zeng X., Zhang Y., Kwong J. S. W., Zhang C., Li S., Sun F., et al. (2015). The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J. Evidence-Based Med. 8, 2–10. doi: 10.1111/jebm.2015.8.issue-1
Keywords: malaria, susceptibility status, insecticide resistance, Anopheles gambiae s.l, Anopheles funestus, Africa
Citation: Minwuyelet A, Yewhalaw D, Sciarretta A and Atenafu G (2025) Evaluating insecticide susceptibility in major African malaria vectors: a meta-analysis and systematic review. Front. Malar. 3:1478249. doi: 10.3389/fmala.2025.1478249
Received: 09 August 2024; Accepted: 07 March 2025;
Published: 08 April 2025.
Edited by:
Annette Elizabeth Kaiser, University of Duisburg-Essen, GermanyReviewed by:
Bhavna Gupta, Vector Control Research Centre (ICMR), IndiaCopyright © 2025 Minwuyelet, Yewhalaw, Sciarretta and Atenafu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Awoke Minwuyelet, YXdva2VtaW53dXllbGV0NUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.