- 1Clinical Trial Unit, Oxford University Clinical Research Unit Indonesia, Jakarta, Indonesia
- 2Infectious Diseases and Immunology Research Center, Indonesia Medical Education and Research Institute, Jakarta, Indonesia
- 3Department of Infectious Diseases, Exeins Health Initiative, Jakarta, Indonesia
- 4Eijkman Research Center for Molecular Biology, National Research and Innovation Agency, Cibinong, Indonesia
- 5Treatment Center, Puskesmas Weoe, East Nusa Tenggara, Weoe, Indonesia
- 6Population Health and Immunity Division, Walter and Eliza Hall Institute of Medical Research, Melbourne, VIC, Australia
- 7Department of Medical Biology, University of Melbourne, Melbourne, VIC, Australia
- 8Center for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom
- 9Vector Borne Diseases and Tropical Public Health Division, Burnet Institute, Melbourne, VIC, Australia
- 10Department of Parasitology, Medical Faculty, Universitas Indonesia, Jakarta, Indonesia
Introduction: In areas where malaria transmission has been successfully reduced, surveillance based solely on clinical cases becomes increasingly challenging. Antibodies generated by the host in response to malaria infections may persist in the circulation for several months or longer. We assessed a serological surveillance tool to measure malaria transmission in eastern Indonesia where reported cases have been recently declining.
Methods: In June 2021, we conducted a cross-sectional survey of elementary schoolchildren aged 5 to 14 years residing in six villages in an endemic area of West Timor, Indonesia. Annual Parasite Incidence (API, cases/1,000 residents/year) of these villages ranged from 0.0 to 4.1 in 2021. Finger-prick plasma samples were tested using a multiplexed Luminex MAGPIX® bead array system to measure IgG antibodies against a panel of 8 Plasmodium vivax antigens. Using a random forest classification algorithm, individuals with predicted exposure to P. vivax in the prior 9 months were identified.
Results: 15 of 398 (4%) schoolchildren were seropositive for recent P. vivax exposure. Remarkably, 87% (13/15) of seropositive children were from one village, the one with the highest API (4.1). In contrast, one seropositive child was from a village with an API of 1.3, and another from a village with an API of 0.0.
Conclusion: Our serological survey data confirms the reported malaria cases from PHC in the villages with likely ongoing transmission. Malaria programs may consider Lamea as the target for intervention.
1 Introduction
The global malaria elimination campaign has successfully reduced malaria transmission in many parts of the world (World Health Organization, 2021). A large proportion of areas formerly designated as meso-high endemic have shifted to a low endemicity level (Sitohang et al., 2018). The most common cause of human malaria, Plasmodium falciparum, has declined in prevalence over the past decades, attributable to effective treatment and escalated control efforts (World Health Organization, 2021). However, this trend has been less pronounced for the second major species, Plasmodium vivax (Battle et al., 2019; Alemayehu et al., 2021; Angrisano and Robinson, 2022). P. vivax has thus reportedly become the major species in many countries transitioning from medium-high to low endemicity (Quah et al., 2019; Price et al., 2020; Angrisano and Robinson, 2022).
Conventional surveillance methods involving case reporting in low transmission settings are challenging (referred to as passive case detection, PCD) (Edwards et al., 2021; Ochwedo et al., 2021; Tayipto et al., 2022). As the number of cases become sparse and spatially heterogenous, parasitological and entomological surveys become operationally and logistically costly and inefficient while, at the same time, resources are limited and should be used in the most efficient way (Greenhouse et al., 2019). Furthermore, PCD provides an incomplete picture due to large proportions of asymptomatic infections, including hidden liver-stage parasites (hypnozoites) in the case of P. vivax (Tadesse et al., 2018; Koepfli et al., 2021; Tayipto et al., 2022). These asymptomatic and latent reservoirs may silently contribute to ongoing transmission.
Diagnosis of P. vivax is more challenging due to lower parasitemia levels and the presence of dormant parasites in the liver (Angrisano and Robinson, 2022). As with other pathogens, an infection by P. vivax initiates the production of antibodies in response to the invading organism (Longley et al., 2017). Longley et al. (2017) assessed antibodies to 307 proteins of P. vivax origin, such as merozoite surface protein 1 and the circumsporozoite protein. Through a down selection pipeline and extensive validation in three geographic regions, they identified 8 antigen-specific antibodies as indicative of recent P. vivax infection within the prior 9 months (Longley et al., 2020). These individuals are likely to harbor hidden liver-stage hypnozoites, due to the biology of relapse timing (White and Imwong, 2012). Relapse not only causes morbidity but also maintain transmission (Bantuchai et al., 2022). Even low-density asymptomatic infections harbor sexual stage parasites and can contribute to onward transmission (Slater et al., 2019; Almeida et al., 2021), and it is now well acknowledged that the hidden hypnozoite reservoir is the dominant (>80%) source of acute blood-stage infections in P. vivax-endemic communities (Robinson et al., 2015). These blood-stage parasitemia stemming from relapsing hypnozoites may serve as infectious reservoirs and predict the probable continuous transmission (Bantuchai et al., 2022). Thus, the identification of individuals with recent exposure of P. vivax can offer valuable insights into understanding the malaria burden, especially in regions with low endemicity.
Serological survey may support the surveillance system for monitoring and planning of malaria program. This study aims to measure seroprevalence in one endemic area in eastern Indonesia which very recently demonstrated declining transmission through health center clinical case data.
2 Materials and methods
2.1 Study area
The study was conducted in Malaka district, West Timor, Indonesia (Figure 1). In the study area, malaria transmission varies significantly, with the highest endemicity observed in a region served by two primary health centers (PHC), Weoe and Alkani, located in the Wewiku subdistrict. The area has low endemicity as shown by the annual parasite index (API) per 1,000 population, a commonly used transmission indicator. API is measured by calculating the number of new microscopically/RDT-confirmed malaria cases reported by PHC in a year, divided by the total population number within the catchment area, multiplied by 1,000. The API in 2021 was 1.5 and 2.10 in Weoe and Alkani, respectively (District Health Officer, personal communication).
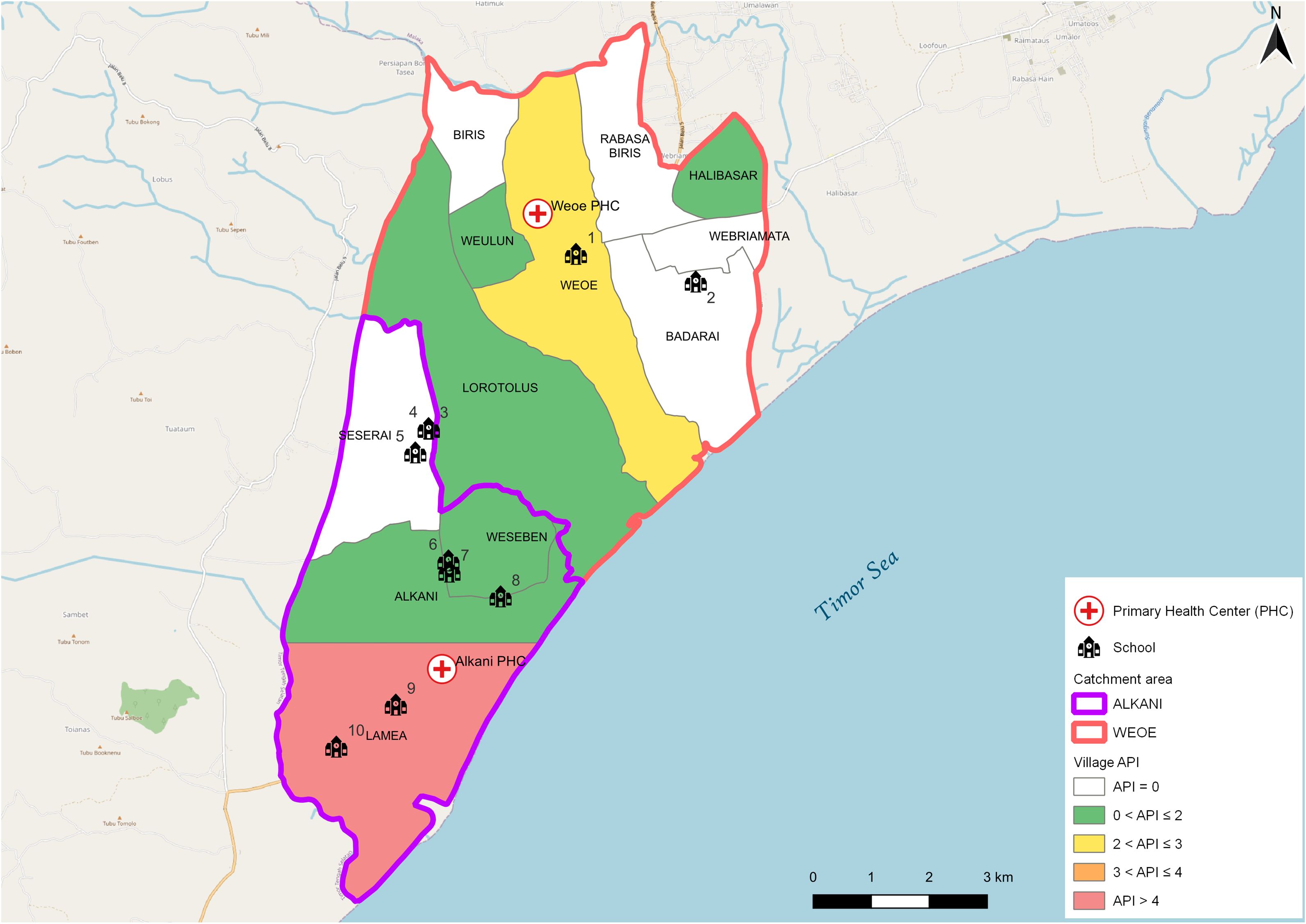
Figure 1 Map of the study area. API varied from 0.0 to 4.1 in the study area (Weoe and Alkani PHC). Numbers represent the identity of schools sampled in this survey.
2.2 Sample collection
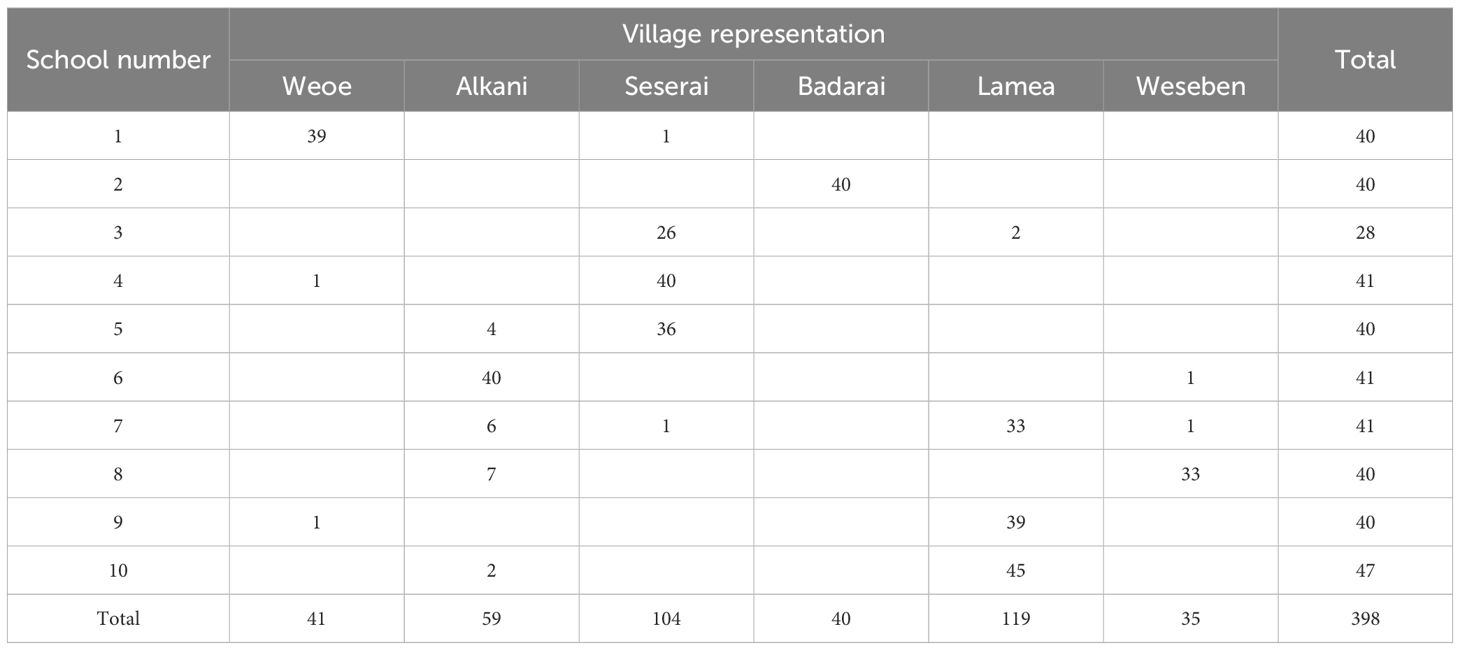
This study utilized a purposive sampling technique to select schools. The criteria for selection were the location of the schools in the endemic villages with the highest number of malaria cases in the last two years as reported by both PHCs. Ten of the total 24 elementary schools were selected and visited for blood collection during 16–26 June 2021 (Figure 1, Table 1). School-age children are typically engaged in limited travel compared to adults. Thus, they may serve as an ideal representative to assess local transmission in the area.
At each school, forty children from first to fifth grade were invited to participate in the survey. This number was chosen to fulfill the sample size of 400, assuming seropositive rate of 20%, design effect of 1.5, precision of 0.05, and loss of 8% of subjects. This sample size represented 50% of the total number of children at each school. Since each endemic village consisted of several sub-villages, the selection process, conducted by the teachers, was designed to ensure that each sub-village within the school’s catchment area was adequately represented. However, one school (no. 3) could only provide 28 children due to their few numbers of students. In contrast, other schools could provide 41 (nos. 4, 6, 7) and 47 (no. 10) schoolchildren. Finger-prick blood samples (400 µL) were collected from each subject into microtainer EDTA tubes and stored temporarily in a cooler box for later plasma separation in the field laboratory. Sex, age, and sub-village of residence were recorded from each participant.
2.3 Laboratory procedures
In the field laboratory, whole blood was centrifugated at 5,000 rpm for 10 minutes to separate plasma. Plasma was shipped to Jakarta by cold chain (2–8 °C) and stored temporarily at −80°C prior to the serological assay. We specifically utilized a multiplexed antibody assay against 8 P. vivax targets, where the combined serological signature has been previously validated to inform on recent exposure to P. vivax parasites in the prior 9 months (Longley et al., 2020).
IgG antibodies against the panel of 8 P. vivax antigens were measured using a multiplexed Luminex MAGPIX® bead array system as previously described (Longley et al., 2020; Mazhari et al., 2020). The eight P. vivax antigens were: PVX_099980 (merozoite surface protein 1–19, MSP1–19), PVX_096995 (Pv-fam-a), PVX_112670 (Pv-fam-a), PVX_087885 (rhoptry associated membrane antigen, RAMA), PVX_097625 (MSP8), PVX_097720 (MSP3.10), PVX_094255 (reticulocyte binding protein 2b, RBP2b) and KMZ83376.1 (erythrocyte binding protein region II, EBPII) (Supplementary Table 1). In this study, magnetic beads were used (Mazhari et al., 2020). Plasma samples were assayed at a 1/100 dilution along with blank, positive, and negative controls. A 2-fold serial dilution from 1/50 to 1/25,600 of the positive control (hyperimmune plasma pool from Papua New Guinea) was used on each plate to generate a standard curve and allow plate-plate standardization. Negative controls were from individuals from Jakarta, Indonesia, that had no recent history of malaria. The median fluorescent intensity (MFI) values from the MAGPIX instrument were converted to arbitrary relative antibody units (RAU) using the standard curve data in a five-parameter logistic function (Longley et al., 2020).
2.4 Data analysis
Data analysis and presentation were performed in R (version 3.5.3) and Microsoft Excel 2019. A Random Forest classification algorithm was used to predict which individuals had exposure to P. vivax in the prior 9 months (Chotirat et al., 2021). The algorithm used the IgG antibody data generated against the 8 P. vivax antigens as described above. The algorithm was trained with 4 existing datasets: Thailand (n=826), Brazil (n=925), the Solomon Islands (n=754) and negative non-endemic controls (n=274) (Longley et al., 2020). Different diagnostic targets along the receiver operator characteristic curve of the algorithm could be selected and we aimed for a balanced target of 79% specificity and 79% sensitivity. Of note, the algorithm was trained using data generated with non-magnetic beads and the BioPlex-200 platform whilst the current antibody data set was generated using magnetic beads and the MAGPIX platform. Prior research has demonstrated moderate-strong correlations when comparing these two data types generated in malaria-endemic areas (Mazhari et al., 2020). The classification algorithm was accessed through a RShiny App available at: https://gitlab.pasteur.fr/tobadia/pvserotat-rshiny-app. API during 2019–2021 was calculated based on digital malaria information system (e-SISMAL).
2.5 Ethical consideration
This study was approved by Ethical committee of Medical Faculty, University of Indonesia (KET-1173/UN2.F1/ETIK/PPM.00.02/2019), Walter and Eliza Hall Institute of Medical Research Human Research Ethics Committee (19–17), and Oxford Tropical Research Ethics Committee (OxTREC) (57–19). Parental informed consent, and assent for those aged 12 year or more, were obtained from all study subjects prior to blood sampling.
3 Results
3.1 Demography
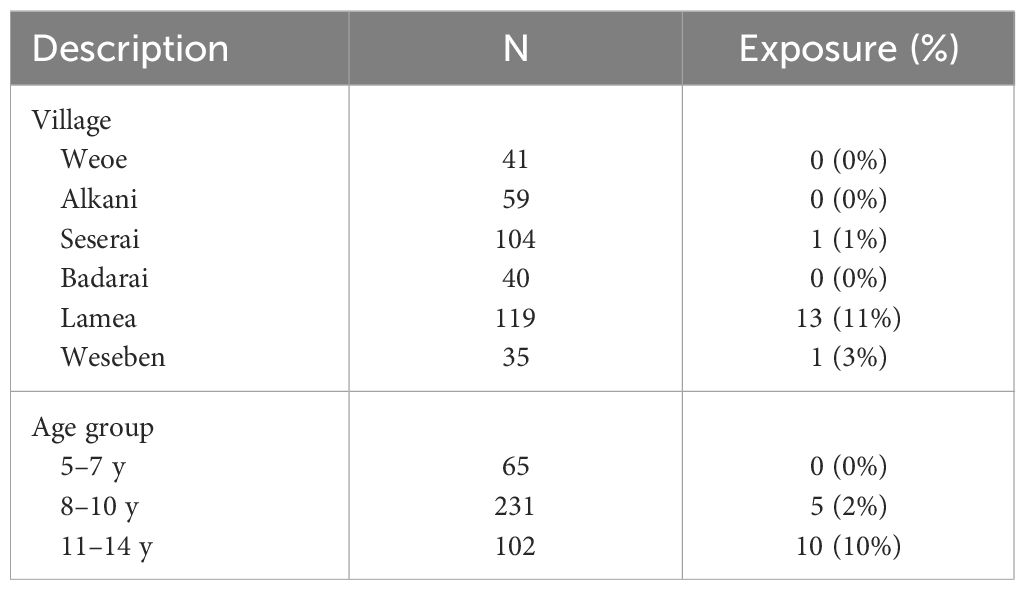
A total of 398 schoolchildren participated in the survey (Table 1). Males and females were represented equally (200/198). More than half the schoolchildren were 8–10 years of age (n=231), followed by 11–14 years (n=102), and 5–7 years (n=65). The median age was 9 years (range 5–14 years). These subjects domiciled in the six endemic villages, majority in Lamea (119/398 = 30%) and Seserai (104/398 = 26%) (Table 1).
3.2 Predicted recent P. vivax exposure
Total IgG antibody levels against the 8 P. vivax antigens assayed were used in a Random Forest classification algorithm to classify each child as recently exposed to P. vivax in the prior 9 months or not (binary outcome). Higher antibody levels were demonstrated in the seropositive subjects, particularly against RBP2b, MSP1–19 and Pv-fam-a, in which distinct separation between the two groups was observed (Supplementary Figure 1).
The overall predicted exposure status was 4% (15/398). A highly disproportionate result was observed, with 87% (13/15) of exposure-classified subjects found in Lamea, whereas one was from Seserai, and another one from Weseben (Table 2). Furthermore, a higher exposure status was observed in the group of 11–14 years old (Table 2). In Lamea, a majority (69%, 9/13) of the seropositive children were aged 11–14 years, even though this age group constituted only 24% (28/119) of the total participants in the village. In contrast, only 31% (4/13) of the seropositive children were aged 8–10 years, despite this age group comprising 59% (70/119) of the total participants in the village (Supplementary Table 2).
These sero-positivity patterns very closely reflect recent trends in malaria transmission in Wewiku subdistrict. Previously a meso-endemic region with API of 124 per 1,000 population in 2012 and community prevalence of approximately 28% by PCR in 2013 (Sutanto et al., 2018), a significant decrease in malaria cases has been reported in the last three years. At Weoe PHC, API decreased from 14.50 in 2019 to 0.83 in 2020, and 1.5 in 2021. Meanwhile, Alkani PHC which initially had a higher API, also reported a notable decrease from 61.00 in 2019 to 5.78 in 2020, and further to 2.10 in 2021 (District Health Officer, personal communication). Children and adolescent were the most affected group by malaria in both PHCs in 2019 (< 20 years: 68%, Supplementary Figure 2).
In 2019, the majority (67%) of the 472 local malaria cases were reported from Lamea and Weoe village, followed by Alkani, Seserai, Weseben, and Badarai (Figure 2A) with Lamea having by far the highest malaria incidence among those six malarious villages (Figure 2B, PHC data, personal comm.). While in 2020 and 2021 Lamea and Weoe accounted for a similar number of malaria case (65%and 67%), with the majority of cases still in the 0–19 age group (60% and 53%), the relative burden of cases shifted significantly to more populous Weoe in 2021 (70% vs 23% of cases from both villages, p = 0.002).
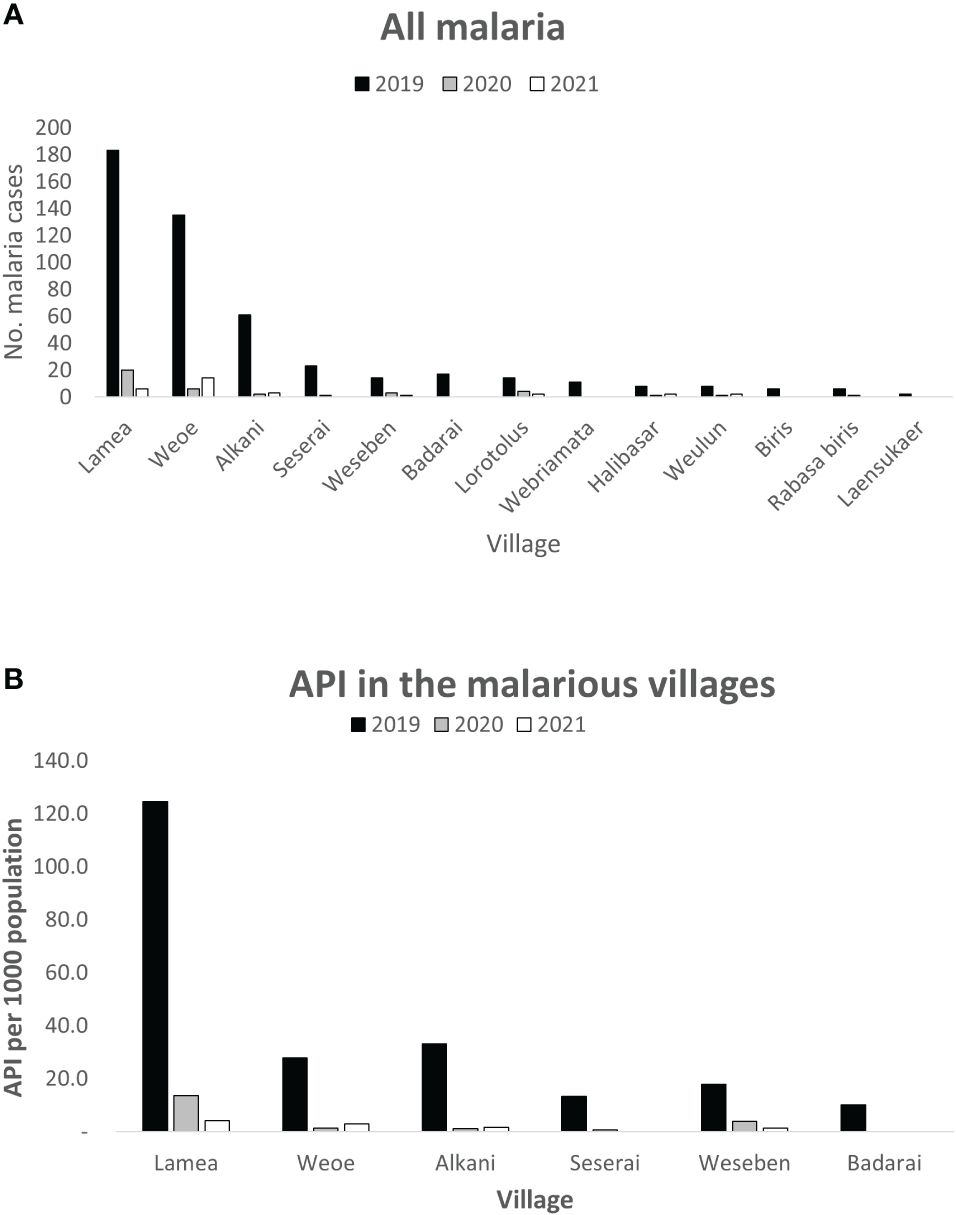
Figure 2 Distribution of reported malaria cases by village in Wewiku subdistrict during 2019–2021. Lamea and Weoe were shown to be the major contributors of all malaria cases in the area (A). Lamea was also depicted to have the highest annual parasite incidence per 1,000 population (B).
4 Discussion
We successfully surveyed schoolchildren using a serological marker of recent exposure to P. vivax and identified a lingering focus of transmission in three villages. Serology predicted low overall levels of recent exposure to P. vivax (4% positivity). This number was relatively similar with the reported API in the area (1.5 and 2.1 per 1,000 individuals in Weoe and Alkani PHC, respectively).
A study in Thailand also reported areas with active transmission in an overall low endemic region with the highest predicted recent exposure by serology in the areas corresponding with higher PCR prevalence in the area (seropositivity: 15.1% and 12.5% vs. PCR: 3.82% and 3.12% in Bongti and Suan Phueng, respectively) (Chotirat et al., 2021). Another study conducted in Peru also identified areas with active transmission (Iquitos and Mazan), with predicted recent P. vivax exposure levels by serology of 56.5% and 38.2%, compared to prevalence of 5.3% and 2.7% by PCR (Rosado et al., 2022).
As malaria levels near pre-elimination, spatial clustering of cases even among proximal villages is becoming pronounced. This allows malaria control programs to focus their resources on areas of confirmed residual transmission. Our serological data indicate that recent exposure to P. vivax was concentrated in Lamea village where schoolchildren contributed 87% of all subjects classified as recently exposed to P. vivax. This finding is well aligned with PHC data which reported the highest incidence of clinical malaria cases in Lamea. As levels of transmission and thus the number of malaria cases decrease, these geographical patterns of local transmission become much harder to identify from PHCdata (e-SISMAL).
Serological surveys of recent malaria exposure in primary school children can provide accurate information that can supplement routine case surveillance. Given school is compulsory in Indonesia and primary school aged children travel less than adults, they provide an excellent population to confirm the continued presence of ongoing local transmission. As our serological exposure markers can detect an exposure to a P. vivax infection in the past 9 months with 80% sensitivity and 80% specificity (Longley et al., 2020), the presence of seropositive schoolchildren is thus a clear indication of ongoing local transmission.
A potential limitation of our study was that the Random Forest classification algorithm was trained on prior datasets generated using a non-magnetic bead assay, whilst the current data was generated using an updated version of the lab assay using magnetic beads as the scaffold. Data using these two different assay set ups are well correlated (Mazhari et al., 2020), but possibly slight variations may be reported when the algorithm is re-trained on magnetic-bead data. Moreover, as the seroprevalence was much lower than the initial assumption (4% vs. 20%), the power was less than expected for comparison between schools. Nevertheless, this study demonstrated the concurrence between serological results and the village-level API in the remaining high-burden villages in the study area.
Serological exposure surveys can therefore provide malaria program managers with important actionable data on local malaria transmission patterns.
5 Conclusions
We have demonstrated that a serological survey deploying multiplexed antibody detection of recent P. vivax exposure can be used to identify areas with ongoing residual transmission where further attention and focal interventions are warranted. These findings can be utilized as supplemental data for malaria programs to further strategy planning.
Data availability statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the privacy protection of the subjects presented here.
Ethics statement
The studies involving humans were approved by Ethical committee of Medical Faculty, University of Indonesia. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
AK: Investigation, Writing – original draft. RU: Formal analysis, Investigation, Writing – original draft. RN: Formal analysis, Supervision, Writing – review & editing. IE: Writing – review & editing. KL: Writing – original draft. VR: Writing – review & editing. RL: Formal analysis, Methodology, Validation, Writing – original draft. JB: Conceptualization, Methodology, Supervision, Writing – review & editing. LR: Conceptualization, Methodology, Supervision, Writing – review & editing. IS: Conceptualization, Methodology, Supervision, Writing – review & editing. IM: Conceptualization, Methodology, Supervision, Validation, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Fundings for the study was provided by Australian Government National Health and Medical Research Council (GNT1102297). We also acknowledge the Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS. RL, LR, and IM are supported by NHMRC Fellowships (GNT1173210, GNT2017630 and GNT1043345).
Acknowledgments
We would like to thank Ramin Mazhari (WEHI Australia) for providing coupled beads and training on the assay, Thomas Obadia, Michael White, and Narimane Nekkab (Institute Pasteur) for the development of SeroTAT algorithm. We also thank former regent of Malaka, dr. Stefanus Bria, for welcoming us to work in the area, former head of Investment and One Stop Integrated Service of Malaka, Pak Yanuarius Bria Seran, for his great support. Malaka District Health Officer (drg. Paskalia F. Halik, dr. Lina Sembiring, Bu Angelina, Pak Deni, Bu Okta) for providing us with support and assistance for this study. Our deep gratitude to Mama Sinta, kak Idhus, and all staff of Puskesmas Weoe, Pak Idhus, and all staff of Puskesmas Alkani for their warm welcome and great support in the field. We are indebted to school principal, teacher and staff of SD GMIT Weoe, SD Hanemasin, SD Klisuklor, SD Kuluoan, SD Laensukaer, SD Umatoos Fatuk, SD Weakar, SD Weakar 2, SD Weseben, and SD Wekbelar. We highly appreciated hard work of the field laboratory team of OUCRU ID (Anggia Sasmitha, Pujo Arum Ahmad) and Dedi Sudiana (OUCRU ID, field captain). Finally, this work is not possible without the participation of all study subjects and their parents.
Conflict of interest
RL and IM are inventors on filed patent PCT/US17/67926 on a system, method, apparatus, and diagnostic test for P. vivax.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmala.2024.1362585/full#supplementary-material
Supplementary Figure 1 | Antibody level against the 8 P. vivax antigens in school 9. In this school, 87% (13/15) of children were classified as recently exposed. Relative antibody units (RAU) were log10 transformed for each participant. Data are categorized by the Random Forest seropositivity results. The boxplots show the median IgG level per group with the whiskers indicating the median ± 1.5 IQR.
Supplementary Figure 2 | Malaria distribution by age group in Weoe PHC and Alkani PHC during 2019–2021.
References
Alemayehu G. S., Blackburn K., Lopez K., Cambel Dieng C., Lo E., Janies D., et al. (2021). Detection of high prevalence of Plasmodium falciparum histidine-rich protein 2/3 gene deletions in Assosa zone, Ethiopia: implication for malaria diagnosis. Malar J. 20, 109. doi: 10.1186/s12936-021-03629-x
Almeida G. G., Costa P. A. C., Araujo M. D. S., Gomes G. R., Carvalho A. F., Figueiredo M. M., et al. (2021). Asymptomatic Plasmodium vivax malaria in the Brazilian Amazon: Submicroscopic parasitemic blood infects Nyssorhynchus darlingi. PloS Negl. Trop. Dis. 15, e0009077. doi: 10.1371/journal.pntd.0009077
Angrisano F., Robinson L. J. (2022). Plasmodium vivax - How hidden reservoirs hinder global malaria elimination. Parasitol. Int. 87, 102526. doi: 10.1016/j.parint.2021.102526
Bantuchai S., Imad H., Nguitragool W. (2022). Plasmodium vivax gametocytes and transmission. Parasitol. Int. 87, 102497. doi: 10.1016/j.parint.2021.102497
Battle K. E., Lucas T. C. D., Nguyen M., Howes R. E., Nandi A. K., Twohig K. A., et al. (2019). Mapping the global endemicity and clinical burden of Plasmodium vivax, 2000–17: a spatial and temporal modelling study. Lancet 394, 332–343. doi: 10.1016/S0140-6736(19)31096-7
Chotirat S., Nekkab N., Kumpitak C., Hietanen J., White M. T., Kiattibutr K., et al. (2021). Application of 23 novel serological markers for identifying recent exposure to Plasmodium vivax parasites in an endemic population of western Thailand. Front. Microbiol. 12, 643501. doi: 10.3389/fmicb.2021.643501
Edwards H. M., Dixon R., Zegers de Beyl C., Celhay O., Rahman M., Myint Oo M., et al. (2021). Prevalence and seroprevalence of Plasmodium infection in Myanmar reveals highly heterogeneous transmission and a large hidden reservoir of infection. PloS One 16, e0252957. doi: 10.1371/journal.pone.0252957
Greenhouse B., Daily J., Guinovart C., Goncalves B., Beeson J., Bell D., et al. (2019). Priority use cases for antibody-detecting assays of recent malaria exposure as tools to achieve and sustain malaria elimination. Gates Open Res. 3, 131. doi: 10.12688/gatesopenres
Koepfli C., Nguitragool W., de Almeida A. C. G., Kuehn A., Waltmann A., Kattenberg E., et al. (2021). Identification of the asymptomatic Plasmodium falciparum and Plasmodium vivax gametocyte reservoir under different transmission intensities. PloS Negl. Trop. Dis. 15, e0009672. doi: 10.1371/journal.pntd.0009672
Longley R. J., White M. T., Takashima E., Morita M., Kanoi B. N., Li Wai Suen C. S. N., et al. (2017). Naturally acquired antibody responses to more than 300 Plasmodium vivax proteins in three geographic regions. PloS Negl. Trop. Dis. 11, e0005888. doi: 10.1371/journal.pntd.0005888
Longley R. J., White M. T., Takashima E., Brewster J., Morita M., Harbers M., et al. (2020). Development and validation of serological markers for detecting recent Plasmodium vivax infection. Nat. Med. 26, 741–749. doi: 10.1038/s41591-020-0841-4
Mazhari R., Brewster J., Fong R., Bourke C., Liu Z. S. J., Takashima E., et al. (2020). A comparison of non-magnetic and magnetic beads for measuring IgG antibodies against Plasmodium vivax antigens in a multiplexed bead-based assay using Luminex technology (Bio-Plex 200 or MAGPIX). PloS One 15, e0238010. doi: 10.1371/journal.pone.0238010
Ochwedo K. O., Omondi C. J., Magomere E. O., Olumeh J. O., Debrah I., Onyango S. A., et al. (2021). Hyper-prevalence of submicroscopic Plasmodium falciparum infections in a rural area of western Kenya with declining malaria cases. Malar J. 20, 472. doi: 10.1186/s12936-021-04012-6
Price R. N., Commons R. J., Battle K. E., Thriemer K., Mendis K. (2020). Plasmodium vivax in the era of the shrinking P. falciparum map. Trends Parasitol. 36, 560–570. doi: 10.1016/j.pt.2020.03.009
Quah Y. W., Waltmann A., Karl S., White M. T., Vahi V., Darcy A., et al. (2019). Molecular epidemiology of residual Plasmodium vivax transmission in a paediatric cohort in Solomon Islands. Malar J. 18, 106. doi: 10.1186/s12936-019-2727-9
Robinson L. J., Wampfler R., Betuela I., Karl S., White M. T., Li Wai Suen C. S., et al. (2015). Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model. PloS Med. 12, e1001891. doi: 10.1371/journal.pmed.1001891
Rosado J., Carrasco-Escobar G., Nolasco O., Garro K., Rodriguez-Ferruci H., Guzman-Guzman M., et al. (2022). Malaria transmission structure in the Peruvian Amazon through antibody signatures to Plasmodium vivax. PloS Negl. Trop. Dis. 16, e0010415. doi: 10.1371/journal.pntd.0010415
Sitohang V., Sariwati E., Fajariyani S. B., Hwang D., Kurnia B., Hapsari R. K., et al. (2018). Malaria elimination in Indonesia: halfway there. Lancet Glob Health 6, e604–e606. doi: 10.1016/S2214-109X(18)30198-0
Slater H. C., Ross A., Felger I., Hofmann N. E., Robinson L., Cook J., et al. (2019). The temporal dynamics and infectiousness of subpatent Plasmodium falciparum infections in relation to parasite density. Nat. Commun. 10, 1433. doi: 10.1038/s41467-019-09441-1
Sutanto I., Kosasih A., Elyazar I. R. F., Simanjuntak D. R., Larasati T. A., Dahlan M. S., et al. (2018). Negligible impact of mass screening and treatment on mesoendemic malaria transmission at west timor in Eastern Indonesia: A cluster-randomized trial. Clin. Infect. Dis. 67, 1364–1372. doi: 10.1093/cid/ciy231
Tadesse F. G., Slater H. C., Chali W., Teelen K., Lanke K., Belachew M., et al. (2018). The relative contribution of symptomatic and asymptomatic Plasmodium vivax and Plasmodium falciparum infections to the infectious reservoir in a low-endemic setting in Ethiopia. Clin. Infect. Dis. 66, 1883–1891. doi: 10.1093/cid/cix1123
Tayipto Y., Liu Z., Mueller I., Longley R. J. (2022). Serology for Plasmodium vivax surveillance: A novel approach to accelerate towards elimination. Parasitol. Int. 87, 102492. doi: 10.1016/j.parint.2021.102492
White N. J., Imwong M. (2012). “Chapter Two - Relapse,” in Advances in Parasitology. Eds. Hay S. I., Price R., Baird J. K. (London: Academic Press), 113–150.
Keywords: Plasmodium vivax, serological, transmission, school, surveillance
Citation: Kosasih A, Utami RAS, Noviyanti R, Elyazar IRF, Lestari KD, Raimanus VS, Longley RJ, Baird JK, Robinson LJ, Sutanto I and Mueller I (2024) Agreement between serological data on schoolchildren and the number of malaria cases in the remaining high-burden villages of Indonesia. Front. Malar. 2:1362585. doi: 10.3389/fmala.2024.1362585
Received: 28 December 2023; Accepted: 31 May 2024;
Published: 20 June 2024.
Edited by:
Andre Lin Ouedraogo, Bill and Melinda Gates Foundation, United StatesReviewed by:
Wendy Prudhomme O’Meara, Duke University, United StatesMelissa Chola Kapulu, KEMRI Wellcome Trust Research Programme, Kenya
Copyright © 2024 Kosasih, Utami, Noviyanti, Elyazar, Lestari, Raimanus, Longley, Baird, Robinson, Sutanto and Mueller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ivo Mueller, bXVlbGxlckB3ZWhpLmVkdS5hdQ==
†Present address: Rhea J. Longley, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
 Ayleen Kosasih
Ayleen Kosasih Retno Ayu Setya Utami
Retno Ayu Setya Utami Rintis Noviyanti
Rintis Noviyanti Iqbal R. F. Elyazar
Iqbal R. F. Elyazar Karina Dian Lestari1
Karina Dian Lestari1 Rhea J. Longley
Rhea J. Longley Ivo Mueller
Ivo Mueller