- Rheumatology Unit, Department of Medicine DIMED, University of Padua, Padua, Italy
Background: With the widespread availability of monoclonal antibodies targeting type 2 inflammation, managing pregnancies in patients with eosinophil-associated diseases, including eosinophilic granulomatosis with polyangiitis (EGPA), has become a crucial issue.
Methods: Starting from a two-case series of patients with EGPA, safely treated with anti-interleukin (IL)5/IL5R monoclonal antibodies during pregnancy, we conducted a comprehensive literature review to identify cases reporting the use of monoclonal antibodies for treating EGPA and other eosinophil-associated diseases in pregnant women.
Results: We present two cases of patients with ANCA-negative EGPA. The first case involves a 35-year-old patient with benralizumab, resulting in successful disease control and a healthy pregnancy despite a history of miscarriage and gestational diabetes. The second case describes a 35-year-old woman who continued mepolizumab during pregnancy, leading to a healthy infant despite two prior early miscarriages. A literature review of 22 papers, covering 97 patients using biologics during pregnancy found no reports specific to EGPA but documented safe outcomes with monoclonal antibodies like mepolizumab, benralizumab, and dupilumab in other eosinophil-associated disorders. These biologics were effective in managing symptoms and reducing the need for oral glucocorticoids, with no observed teratogenic effects. However, complications such as gestational diabetes and preterm births were noted, particularly with dupilumab. No adverse events or pregnancy complications directly attributable to the biological therapy were reported.
Conclusions: Uncontrolled disease during pregnancy significantly threatens pregnancy viability, while the use of monoclonal antibodies effectively manages maternal disease, reduces glucocorticoid use, and helps prevent complications, even though more data are needed to establish risks and benefits.
1 Introduction
Over the past two decades, a range of inflammatory diseases affecting multiple organ systems and characterized by elevated eosinophil counts have been described and characterized. These eosinophil-associated diseases include common conditions such as asthma, chronic rhinosinusitis with nasal polyps (CRSwNP), and atopic dermatitis (AD), less common eosinophilic gastrointestinal diseases, and rare conditions such as allergic bronchopulmonary aspergillosis (ABPA), eosinophilic granulomatosis with polyangiitis (EGPA), and hypereosinophilic syndromes (HES) (1).
EGPA is a rare, potentially life-threatening autoimmune vasculitis associated with antineutrophil cytoplasmic antibodies (ANCA). It is characterized by necrotizing vasculitis of small vessels, eosinophil-rich granulomatous inflammation, and elevated blood eosinophils. EGPA typically presents between the 5th and 6th decades of life but can also affect women of childbearing age (2). Treating EGPA is challenging due to its rarity, broad clinical manifestations, and similarities with other vasculitic or eosinophilic conditions. The primary treatment is systemic glucocorticoids (GCs), often combined with immunosuppressive agents depending on disease severity and the organs involved (3).
Monoclonal antibodies targeting type 2 inflammation have proven effective in treating eosinophil-associated diseases, especially when conventional treatments are ineffective or poorly tolerated. Initially approved for asthma, these therapies are now also approved for chronic spontaneous urticaria, AD, EGPA, CRSwNP, and HES. Currently, seven biologics, namely omalizumab, mepolizumab, reslizumab, benralizumab, dupilumab, tezepelumab, and tralokinumab, are approved for these conditions (4, 5). Nevertheless, the randomized clinical trials that led to Food and Drug Administration (FDA) approval frequently excluded pregnant women, thereby leaving the safety and efficacy of these biologics during pregnancy largely unknown.
Managing pregnancies in patients with eosinophil-associated diseases requires a careful risk-benefit assessment. This due to the potential exacerbation of eosinophilic and atopic conditions, which can lead to unfavorable pregnancy outcomes, and limited availability of drugs during this period (6–8).
In this paper, we present a two-case series of patients with EGPA who were safely treated with anti-interleukin (IL)5/IL5R monoclonal antibodies during pregnancy. Additionally, we provide a comprehensive review of the available English-language literature on the use of biologics targeting type 2 inflammation during pregnancy in eosinophil-associated disorders, focusing on their safety concerning maternal and fetal outcomes.
2 Methods
Starting from clinical cases of two patients with long-standing EGPA in regular follow-up at the Padua Vasculitis Center, successfully treated with anti-IL5/R during pregnancy, we conducted an extensive literature search in MEDLINE via PubMed for original articles published up to July 15, 2024, reporting on the use of monoclonal antibodies to treat EGPA and other eosinophil-associated diseases in pregnant women. The search utilized the following MeSH terms: “eosinophilic granulomatosis with polyangiitis”, “EGPA”, “Churg-Strauss syndrome”, “hypereosinophilic syndrome”, “HES”, “severe asthma”, “chronic sinusitis with nasal polyps”, “atopic dermatitis”, “eosinophilic esophagitis”, “Benralizumab”, “Dupilumab”, “Mepolizumab”, “Reslizumab”, “Tezepelumab”, “Tralokinumab”, “pregnancy”, “post-partum”, and “breastfeeding”.
Omalizumab was excluded from the review due to established safety data in pregnancy from the Xolair Pregnancy Registry (EXPECT) study on severe asthma (9). The EXPECT study, the largest prospective study on omalizumab use during pregnancy, found similar rates of live births and major congenital malformations in 230 pregnant women with asthma exposed to omalizumab compared to a matched external cohort.
For each publication, we focused on clinical findings, treatment management, and maternal and fetal outcomes. We excluded studies where biologics were used after delivery to treat relapses or worsening of the diseases, as well as studies where biologics were discontinued before conception or where the treatment involved the father instead of the mother.
Statistical analysis was performed using the free software Jamovi (version 1.6.15). Medians and interquartile range were calculated for continuous variables, while percentages and relative frequency distributions were used for categorical variables. Analyses were conducted only on subjects with available data.
All patients signed informed consent forms, and this study was conducted in accordance with the Ethical Principles for Medical Research outlined in the Declaration of Helsinki.
3 Results
3.1 Case description
3.1.1 Case 1
The first case involves a 35-years-old patient diagnosed with ANCA-negative EGPA in 2018, fulfilling the 2022 ACR/EULAR classification criteria for EGPA (10). At the time of diagnosis, she presented with hypereosinophilia (blood eosinophil count of 15,000/mmc), pulmonary infiltrates, systemic symptoms, and skin purpura, along with a history of severe asthma and chronic sinusitis with nasal polyposis. Initially, she was treated with GCs, methotrexate up to 15 mg/week, followed by mepolizumab 100 mg/4 weeks (as the 300 mg/4 weeks dose was not approved at that time), but asthma control was not achieved. Subsequently, she was administered benralizumab 30 mg every 8 weeks for a period of two years in order to control her uncontrolled asthma. This resulted in the successful cessation of oral GCs after six years of continuous treatment, and the achievement of disease remission. The patient had no history of smoking, was overweight with a body mass index (BMI) of 36, and tested positive for lupus anticoagulant. While on benralizumab, she experienced a miscarriage at 7 weeks of gestation. However, she subsequently became pregnant again and continued benralizumab treatment alongside antiplatelet therapy. Benralizumab was discontinued at the end of the second trimester, and the patient refrained from further benralizumab treatment throughout breastfeeding. The pregnancy was complicated by gestational diabetes, which required insulin treatment, and ended in a cesarean delivery at 40 weeks of gestation. No vasculitic relapses, asthma exacerbations, or increases in eosinophil levels were observed. The infant was born healthy. During breastfeeding, the mother received a short course of oral GCs due to an asthma exacerbation accompanied by a transient increase in blood eosinophils (2,300/mmc) and C-reactive protein (17 mg/L).
3.1.2 Case 2
The second case involves a 36-years-old patient diagnosed with ANCA-negative EGPA, fulfilling the 2022 ACR/EULAR classification criteria for EGPA (10). She had a history of adult-onset asthma, chronic rhinosinusitis with nasal polyps, systemic symptoms, myocarditis and serositis. Initially, she was treated with high-dose GCs combined with cyclophosphamide, followed by azathioprine and oral GCs for 6 years Due to uncontrolled asthma and persistent eosinophilia (blood eosinophil count above 1,000/mmc), she was switched to mepolizumab 300 mg every 4 weeks. The patient was a former smoker and had previously undergone a cesarean delivery in a prior pregnancy. While receiving mepolizumab, the patient experienced two early spontaneous miscarriages at 6 weeks of gestation. However, during a subsequent pregnancy, which occurred one year after starting the biologic, she continued mepolizumab until the end of the second trimester (25 weeks of gestation). The pregnancy proceeded without complications, resulting in the birth of a healthy infant via cesarean delivery at 38 weeks of gestation. Throughout her pregnancy, the patient did not experience any adverse events, hypereosinophilia, or disease relapse. However, during the postpartum period, her asthma symptoms worsened and were not adequately managed by combination inhalers. These symptoms were effectively controlled with the resumption of mepolizumab at a dosage of 300 mg every GC weeks.
Clinical features, blood tests and patient-reported outcome measures of the two EGPA cases during conception, pregnancy and post-partum are listed in Table 1.
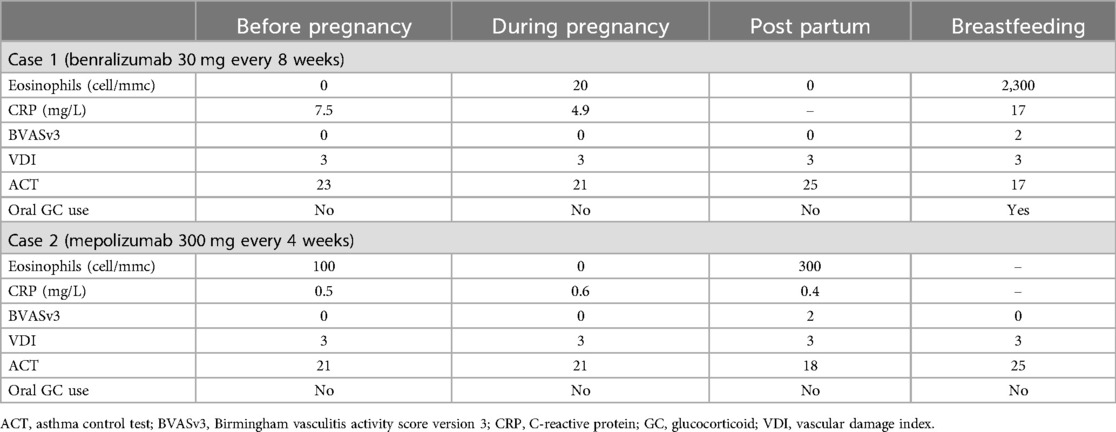
Table 1. Blood tests, disease activity and damage scores of the two patients treated with anti-IL5/5R monoclonal antibodies at different stages of pregnancy.
3.2 Literature review
A total of 22 papers documenting the use of biologics in eosinophil-associated diseases during pregnancy, encompassing 97 patients, were retrieved from the published literature (11–32). These include mostly case reports, along with four case series (11, 15, 25, 30), one longitudinal study (27) and one retrospective cohort study (32).
No reports were found for EGPA patients treated with monoclonal antibodies targeting type 2 inflammation during pregnancy. Monoclonal antibodies were used, continued or started when disease control was lost before or during pregnancy and other therapeutic options were unavailable. Most patients had only partial responses to standard therapies or encountered issues with immunosuppressants (20) or infections (16, 25). Table 2 provides a summary of maternal and neonatal outcomes following exposure to biologic therapies at different stages of pregnancy. Table 3 presents a summary of all cases retrieved from the literature review, including our two EGPA cases, detailing demographic and clinical features, timing of biologic exposure, and maternal and neonatal outcomes in patients with eosinophil-associated diseases treated with biologic therapies.
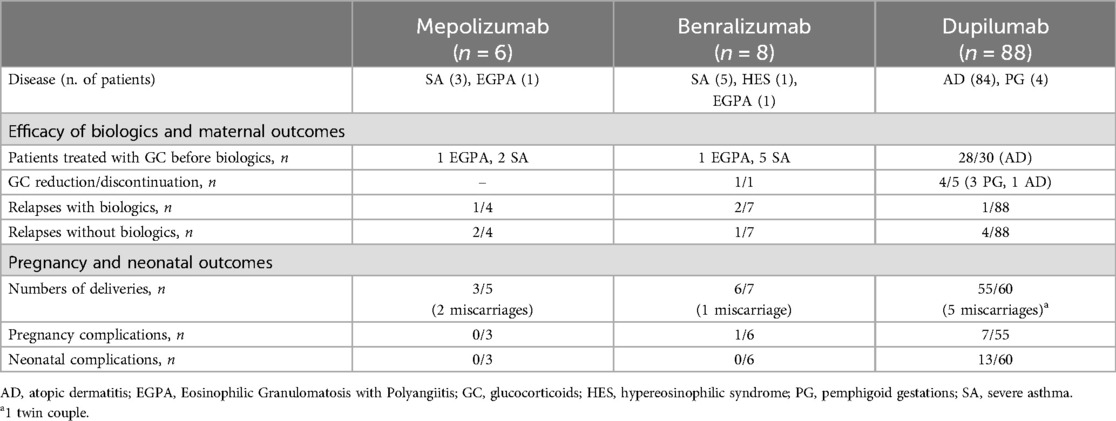
Table 2. Maternal and neonatal outcomes following exposure to mepolizumab, benralizumab, and dupilumab during different stages of pregnancy.
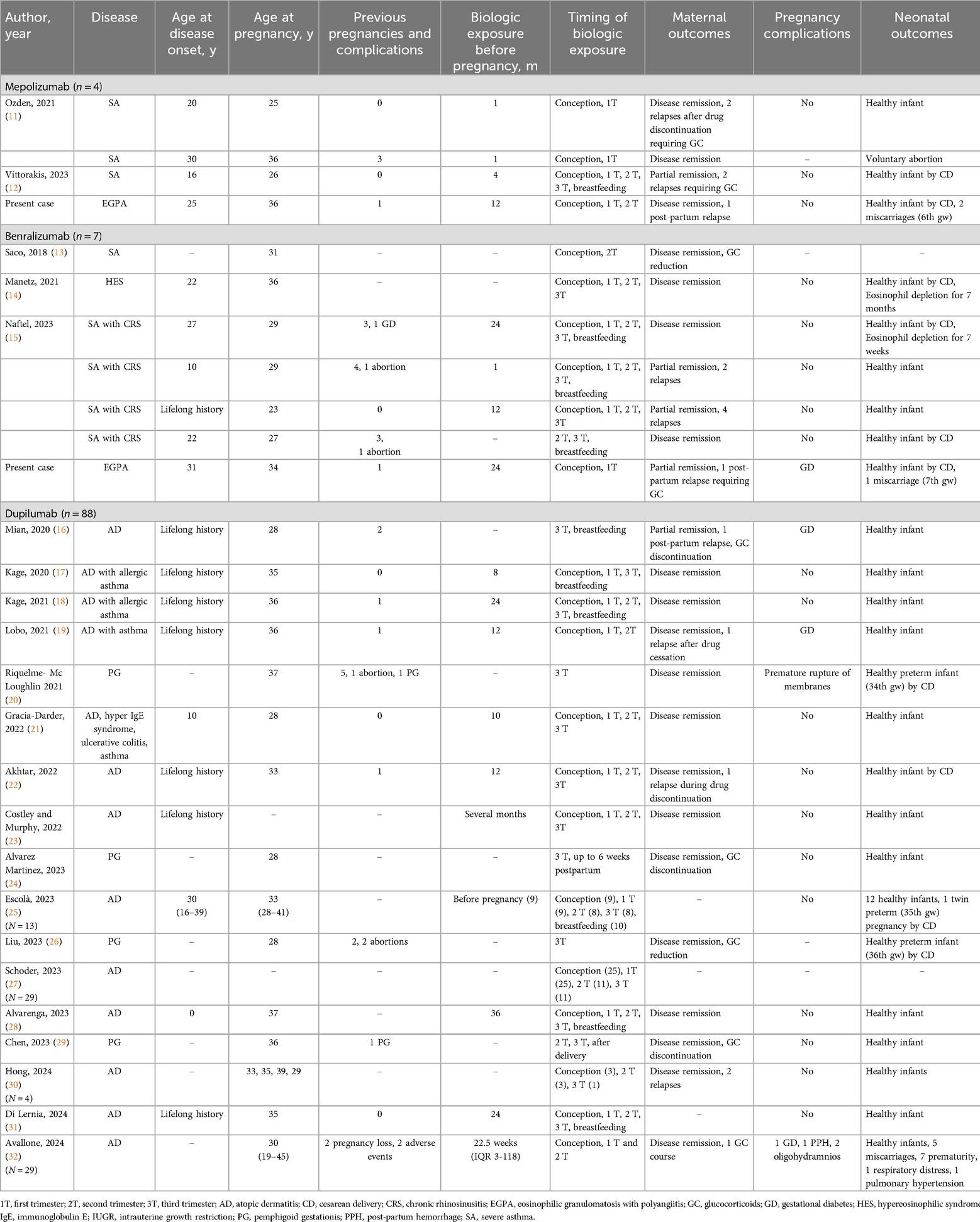
Table 3. Demographic and clinical features, timing of biologic exposure, maternal and neonatal outcomes in patients with eosinophil-associated diseases treated with biologic therapies.
The median duration of therapies before conception could not be calculated due to insufficient data, and information on the duration of treatment in terms of gestational weeks was often unavailable. However, Figure 1 illustrates the proportion of patients exposed to mepolizumab, benralizumab, and dupilumab during various stages of pregnancy, including conception, the first, second, and third trimesters, and breastfeeding.

Figure 1. Proportion of patients receiving mepolizumab, benralizumab and dupilumab across different stages of pregnancy and lactation.
Globally, no teratogenic effects were observed, and all infants were healthy at birth and during the observation period.
3.2.1 Mepolizumab
A total of three pregnant patients with chronic asthma were treated with mepolizumab 100 mg every 4 weeks (11, 12). All patients were exposed to the drug at conception and during the first trimester (11, 12). Only one patient continued treatment into the third trimester and during breastfeeding (12). One pregnancy was voluntarily terminated during the first trimester due to concerns about drug's potential unknown effects (11). No adverse events or pregnancy complications were reported, except for the voluntary abortion mentioned above (11). The efficacy of the drug in controlling the disease exhibited considerable variability. One patient experienced a relapse after discontinuing the drug in the second trimester (11), while another patient achieved only partial asthma control, with two relapses during treatment (12). Two patients had previously received oral GCs but were not using them at the time of conception. Both patients who experienced relapses required a short course of oral GCs (11, 12). No further details on the timing and dosage are of oral GCs available in the published literature.
3.2.2 Benralizumab
Six pregnant patients, comprising five patients with chronic asthma (13, 15) and one with HES (14), were treated with benralizumab 30 mg every 8 weeks. Five patients were exposed to the drug at conception (13–15), and four continued into the first trimester (14, 15). Six were exposed to the drug during the second trimester (13–15). Five patients continued treatment through the third trimester (14, 15), and three infants were breastfed while their mothers were receiving benralizumab (15). Two patients experienced asthma attacks during treatment, but other risk factors, such as smoking habits and poor adherence to topical treatment, were also reported (15). No pregnancy complications occurred. Blood eosinophils were measured in two infants and were undetectable for several weeks, with no observed developmental or immune system issues (14, 15). Five patients had previously received oral GCs (13–15). Only one patient was using GCs when starting benralizumab and was able to reduce the dosage during the pregnancy (13).
3.2.3 Dupilumab
A total of 88 pregnant patients were identified, including 84 patients with AD (16–19, 21–23, 25, 27, 28, 30–32) and four with pemphigoid gestationis (PG) (20, 24, 26, 29) treated with dupilumab. Among these, 74 out of 84 patients with AD (17–19, 21–23, 25, 27, 28, 30–32) were exposed to the drug at conception, while none of the patients with PG were exposed at conception. In the first trimester, 74 patients continued treatment (17–19, 21–23, 25, 27, 28, 31, 32), decreasing to 30 in the second trimester (17–19, 21–23, 25, 27–31) and 31 in the third trimester (16–18, 20–31). 15 infants were breastfed while their mothers were on dupilumab (16–18, 25, 28, 31).
Dupilumab generally controlled disease activity, though one patient (1.1%) with AD experienced a flare during treatment and shortly after the delivery (16). Additionally, four (4.5%) patients with AD relapsed after discontinuing dupilumab, with relapses occurring in both the first (30) and third trimesters (19, 22, 30). All patients with PG achieved disease control and remission (20, 24, 26, 29).
Pregnancy complications were reported, including three cases of gestational diabetes (16, 19, 32), two cases of oligohydramnios (32), one case of postpartum hemorrhage (32), five miscarriages (32) in AD, and one case of premature rupture of membranes in PG (20). Of the three cases of gestational diabetes, two occurred in patients receiving GC treatment, and one occurred in a patient not treated with GC. Additionally, there were 11 preterm births (20, 25, 26, 32), one infant with respiratory distress and one infant with pulmonary hypertension (32). Most pregnancy and neonatal complications occurred in cases where dupilumab was discontinued before the third trimester. Of the 7 pregnancy complications, 5 occurred in these cases. Similarly, 9 out of 11 neonatal complications (after excluding 2 cases with missing data from a total of 13) were associated with early discontinuation of dupilumab. Despite these complications, no teratogenic effects were observed, and all infants were healthy at birth and during the observation period.
Regarding GCs, 28 out of 30 patients had previously received GC treatment, while no data on prior GC use were available for the remaining 58 patients. Of the 28 patients, only five were undergoing GC therapy at the start of dupilumab treatment (16, 20, 24, 26, 29). Four of these patients were able to reduce or discontinue GCs during pregnancy (16, 24, 26, 29). Finally, one patient who had not previously been treated with GCs required a short course of GCs to control AD symptoms (32).
3.2.4 Reslizumab, tezepelumab and tralokinumab
There are no published human data on the use of reslizumab, tezepelumab, or tralokinumab during pregnancy, making the potential risks to maternal and fetal outcomes unknown. Animal studies in mice, rabbits, and monkeys exposed to these drugs at higher doses have shown no evidence of embryo-fetal developmental harm (33–35). However, the absence of human data leaves the safety of these treatments during pregnancy uncertain.
4 Discussion
To the best of our knowledge, the clinical cases described in this paper are the first published reports of managing EGPA in pregnancy with benralizumab or mepolizumab. Both patients had relapsing, refractory EGPA and responded well to biologic therapy, without the use of oral glucocorticoids during pregnancy. There were no adverse events or relapses, although the treatments were stopped in the third trimester. Both pregnancies ended in cesarean sections with healthy neonates. While both patients experienced miscarriages during treatment, a direct link to the biologics could not be established due to the presence of additional risk factors such as advanced maternal age and autoimmune diseases. Moreover, in the patient treated with benralizumab, the complication of gestational diabetes was associated with other risk factors, such as maternal age and overweight. In both of our patients, other potential predictors of miscarriage were excluded.
The patients presented in this paper underscore the challenges of managing pregnancy in patients with EGPA and eosinophil-associated diseases. A major concern is the limited availability of safe medications during this period. Most immunosuppressive drugs, except azathioprine and cyclosporine, are contraindicated during pregnancy (36). Additionally, glucocorticoids, a cornerstone of treatment for eosinophil-mediated diseases, carry gestational risks, including gestational diabetes, stillbirth, preterm birth, low birth weight, and cesarean delivery (37). Optimizing medical management for these patients is crucial, but current data and guidelines are insufficient for routine clinical practice.
The widespread use of monoclonal antibodies targeting type 2 inflammation in eosinophil-associated conditions raises concerns about their safety during pregnancy, given the limited data available. Pregnant or breastfeeding women, or those planning conception, are typically excluded from clinical trials, leaving safety assessments reliant on case reports, animal studies, small retrospective studies, or pregnancy registries, which may be biased and lack consistent medication adherence.
Current literature indicates that many patients discontinue monoclonal antibody therapy in the third trimester due to concerns about transplacental transfer and persistence in breast milk. Immunoglobulins are transported across the placenta in a linear fashion as pregnancy progresses (38), aligning with the development of the fetal Fc receptor for immunoglobulin G (IgG) (39–41). While IgG can be transferred through breast milk, its secretion and absorption are limited to the first few days of life and in preterm infants with immature gastrointestinal tracts (42, 43). Preclinical studies indicate that benralizumab and mepolizumab cross the placenta in the third trimester, with mepolizumab present in breast milk at less than 0.5% of maternal serum concentration (44).
No adverse fetal effects have been observed with any of the treatments. In cases where benralizumab and mepolizumab were continued throughout pregnancy, no complications were reported, and full-term healthy infants were delivered. The absence of eosinophils in two infants exposed to benralizumab until birth was not linked to developmental or immune issues, although long-term effects remain unclear (14, 15). Eosinophil suppression in mice also shows no associated developmental problems (45). Data on dupilumab suggests that complications mainly occur when the drug is discontinued before the third trimester, though most infants were born healthy. Additionally, there are concerns that inhibiting IL-13, which promotes mucus-secreting goblet cell proliferation, might impact fetal conjunctival development (27, 46–48), particularly before the third trimester when these cells mature (49, 50). However, no infants exposed to dupilumab exhibited any eye-related developmental issues. Moreover, aside from a single reported case of benralizumab-induced eosinophil depletion in an infant, no safety concerns have been identified in breastfed infants. Overall, the available data suggest that biologics are unlikely to have a significant impact on pregnancy or fetal outcomes. However, it is crucial to highlight that data on long-term risks and the potential effects of these drugs on fetal and neonatal development remain uncertain, as follow-up was not reported in many cases.
During pregnancy, the immune system shifts from cell-mediated immunity (type 1 inflammation) to humoral immunity (type 2 inflammation) to maintain immunotolerance and prevent fetal rejection (51). However, this immunological shift can also trigger, exacerbate, or worsen underlying autoimmune or eosinophilic conditions. Some observational studies have explored the outcomes of patients affected with systemic vasculitis during pregnancy (36, 52), with reports of EGPA onset, miscarriage or vasculitis flare, particularly in the first and second trimesters and postpartum (6, 46, 47). Active EGPA is associated with a range of pregnancy complications, including preeclampsia (rare), fetal loss (10%–15%), therapeutic abortion (5%–10%), preterm deliveries (10%–40%), cesarean deliveries (15%–40%), intrauterine growth restriction (10%–25%) and low birth weight infants (10%–30%) (7, 46). Asthma attacks and AD flares also tend to increase during pregnancy (48, 53). Uncontrolled asthma is linked to adverse pregnancy outcomes, including gestational hypertension, diabetes, preeclampsia, premature rupture of membranes, ante- or postpartum hemorrhage, as well as neonatal complications like preterm birth, low birth weight, neonatal death, and congenital malformations (53, 54). Premature rupture of membranes and preterm labor are also complications associated with PG (55), while gestational diabetes is common, particularly with comorbidities and advanced maternal age (56). Although AD itself is not a direct risk factor for adverse pregnancy outcomes, uncontrolled AD can lead to complications like eczema herpeticum, bacterial infections, and reduced quality of life (57–59). In the literature, relapses of asthma and AD have been reported in both treated patients, with some confounding factors as mentioned above, and in untreated patients, particularly after the discontinuation of biologics. However, the use of glucocorticoids during pregnancy, known to carry risks for both mother and infant, has significantly decreased with the introduction of biologics, leading to improved management of the underlying disease.
These data underscore the ongoing risk of disease relapse after biologics discontinuation or following delivery due to immunological shifts. Consequently, uncontrolled disease during pregnancy significantly threatens pregnancy viability, while the use of monoclonal antibodies effectively manages maternal disease, reduces glucocorticoid use, and helps prevent complications. Given that, the 2020 European Respiratory Society/Thoracic Society of Australia and New Zealand (ERS/TSANZ) consider omalizumab probably safe, while mepolizumab, benralizumab, reslizumab, and dupilumab possibly acceptable if conventional therapies fail (60).
We must acknowledge several limitations, primarily related to data heterogeneity, incomplete reporting of maternal and infant outcomes, and the lack of a control group. Additionally, safety information for benralizumab, mepolizumab, and dupilumab in EGPA patients must be inferred from studies in other conditions, thus preventing definitive conclusions. Moreover, the conclusions of this study are constrained by the unavailability of some key predictors, such as maternal age, underlying medical conditions, comorbidities and concomitant drug treatments, which were not reported in the analyzed papers.
In conclusion, our cases, along with the literature review, are encouraging and suggest favorable outcome for monoclonal antibodies targeting type 2 inflammation during pregnancy and breastfeeding. However, more data are needed to establish risks and benefits. These preliminary results can help prevent adverse outcomes that may arise when effective biologic therapy is discontinued due to uncertainty about the medication's effects during pregnancy. They may also aid in the risk-benefit assessment, supporting the decision to continue therapy in patients who become pregnant unintentionally, based on their individual risk profiles.
Currently, observational studies and industry-sponsored registries evaluating pregnancy outcomes are accumulating evidence for dupilumab in AD and asthma (61–64), mepolizumab in asthma (65, 66) and benralizumab in asthma (67). Although no specific registries exist for monoclonal antibodies in EGPA, the Vasculitis Pregnancy Registry (VPREG) covers all vasculitides (68). Longitudinal studies with adequate follow-up and comparison cohorts are necessary due to the limited number of current cases.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FD: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, Resources, Visualization. LI: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing, Resources, Visualization. AC: Investigation, Supervision, Writing – review & editing. AD: Supervision, Validation, Visualization, Writing – review & editing, Funding acquisition. RP: Data curation, Supervision, Validation, Writing – original draft, Writing – review & editing, Funding acquisition, Methodology, Resources, Visualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Open Access funding provided by Università degli Studi di Padova | University of Padua, Open Science Committee.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kang N, Kim TB. Eosinophilic-associated disease overlap: what do we know about it? Allergy Asthma Immunol Res. (2023) 15(5):539–42. doi: 10.4168/aair.2023.15.5.539
2. Kitching AR, Anders HJ, Basu N, Brouwer E, Gordon J, Jayne DR, et al. ANCA-associated vasculitis. Nat Rev Dis Primer. (2020) 6(1):71. doi: 10.1038/s41572-020-0204-y
3. Hellmich B, Sanchez-Alamo B, Schirmer JH, Berti A, Blockmans D, Cid MC, et al. Recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann Rheum Dis. (2024) 83(1):30–47. doi: 10.1136/ard-2022-223764
4. Quirce S, Cosío BG, España A, Blanco R, Mullol J, Santander C, et al. Management of eosinophil-associated inflammatory diseases: the importance of a multidisciplinary approach. Front Immunol. (2023) 14:1192284. doi: 10.3389/fimmu.2023.1192284
5. Sitek AN, Li JT, Pongdee T. Risks and safety of biologics: a practical guide for allergists. World Allergy Organ J. (2023) 16(1):100737. doi: 10.1016/j.waojou.2022.100737
6. Vernon-Elliot J, Prasad JD, Bonney A. Critical deterioration of chronic eosinophilic pneumonia during pregnancy. BMJ Case Rep CP. (2024) 17(2):e259019. doi: 10.1136/bcr-2023-259019
7. Gatto M, Iaccarino L, Canova M, Zen M, Nalotto L, Ramonda R, et al. Pregnancy and vasculitis: a systematic review of the literature. Autoimmun Rev. (2012) 11(6–7):A447–59. doi: 10.1016/j.autrev.2011.11.019
8. Murphy VE, Gibson PG, Schatz M. Managing asthma during pregnancy and the postpartum period. J Allergy Clin Immunol Pract. (2023) 11(12):3585–94. doi: 10.1016/j.jaip.2023.07.020
9. Namazy J, Cabana MD, Scheuerle AE, Thorp JMJ, Chen H, Carrigan G, et al. The Xolair pregnancy registry (EXPECT): the safety of omalizumab use during pregnancy. J Allergy Clin Immunol. (2015) 135(2):407–12. doi: 10.1016/j.jaci.2014.08.025
10. Grayson PC, Ponte C, Suppiah R, Robson JC, Craven A, Judge A, et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for eosinophilic granulomatosis with polyangiitis. Arthritis Rheumatol. (2022) 74(3):386–92. doi: 10.1002/art.41982
11. Ozden G, Pınar Deniz P. May mepolizumab used in asthma correct subfertility? Ann Med. (2021) 53(1):456–8. doi: 10.1080/07853890.2021.1900591
12. Vittorakis SK, Giannakopoulou G, Samitas K, Zervas E. Successful and safe treatment of severe steroid depended eosinophilic asthma with mepolizumab in a woman during pregnancy. Respir Med Case Rep. (2023) 41:101785. doi: 10.1016/j.rmcr.2022.101785
13. Saco T, Tabatabaian F. Breathing for two: a case of severe eosinophilic asthma during pregnancy treated with benralizumab. Ann Allergy Asthma Immunol. (2018) 121(5):S92. doi: 10.1016/j.anai.2018.09.300
14. Manetz S, Maric I, Brown T, Kuang FL, Wetzler L, Battisto E, et al. Successful pregnancy in the setting of eosinophil depletion by benralizumab. J Allergy Clin Immunol Pract. (2021) 9(3):1405–1407.e3. doi: 10.1016/j.jaip.2020.11.060
15. Naftel J, Eames C, Kerley S, Whitfield C, Rayala-Montaniel E, Cook P, et al. Benralizumab treatment of severe asthma in pregnancy: a case series. J Allergy Clin Immunol Pract. (2023) 11(9):2919–21. doi: 10.1016/j.jaip.2023.06.061
16. Mian M, Dunlap R, Simpson E. Dupilumab for the treatment of severe atopic dermatitis in a pregnant patient: a case report. JAAD Case Rep. (2020) 6(10):1051–2. doi: 10.1016/j.jdcr.2020.08.001
17. Kage P, Simon JC, Treudler R. A case of atopic eczema treated safely with dupilumab during pregnancy and lactation. J Eur Acad Dermatol Venereol JEADV. (2020) 34(6):e256–7. doi: 10.1111/jdv.16235
18. Kage P, Simon JC, Treudler R. Case of atopic eczema treated with dupilumab throughout conception, pregnancy, and lactation. J Dermatol. (2021) 48(10):E484–5. doi: 10.1111/1346-8138.16033
19. Lobo Y, Lee RC, Spelman L. Atopic dermatitis treated safely with dupilumab during pregnancy: a case report and review of the literature. Case Rep Dermatol. (2021) 13(2):248–56. doi: 10.1159/000515246
20. Riquelme-Mc Loughlin C, Mascaró JM. Treatment of pemphigoid gestationis with dupilumab. Clin Exp Dermatol. (2021) 46(8):1578–9. doi: 10.1111/ced.14765
21. Gracia-Darder I, Pons De Ves J, Reyero Cortina M, Martín-Santiago A. Patient with atopic dermatitis, hyper IgE syndrome and ulcerative colitis, treated successfully with dupilumab during pregnancy. Dermatol Ther. (2022) 35(2):e15237. doi: 10.1111/dth.15237
22. Akhtar NH, Khosravi-Hafshejani T, Akhtar D, Dhadwal G, Kanani A. The use of dupilumab in severe atopic dermatitis during pregnancy: a case report. Allergy Asthma Clin Immunol Off J Can Soc Allergy Clin Immunol. (2022) 18(1):9. doi: 10.1186/s13223-022-00650-w
23. Costley M, Murphy B. Severe atopic dermatitis treated successfully with dupilumab throughout pregnancy. Clin Exp Dermatol. (2022) 47(5):960–1. doi: 10.1111/ced.15049
24. Alvarez Martinez D, Russo G, Fontao L, Laffitte E. Successful therapy of pemphigoid gestationis with dupilumab-A new case. J Eur Acad Dermatol Venereol JEADV. (2023) 37(6):e752–3. doi: 10.1111/jdv.18911
25. Escolà H, Figueras-Nart I, Bonfill-Orti M, Coll Puigserver N, Martin-Santiago A, Rodríguez Serna M, et al. Dupilumab for atopic dermatitis during pregnancy and breastfeeding: clinical experience in 13 patients. J Eur Acad Dermatol Venereol. (2023) 37(9):e1156–60. doi: 10.1111/jdv.19165
26. Liu Y, Yuan J, Xia Y, Du X, Geng S. A case of pemphigoid gestationis successfully treated with dupilumab. J Eur Acad Dermatol Venereol JEADV. (2023) 37(9):e1164–5. doi: 10.1111/jdv.19171
27. Schoder K, Zhu Y, Schneeweiss S, Merola J, Savage TJ, Gibbs LR, et al. Use of systemic immunomodulating medications in pregnant women with atopic dermatitis: a nationwide US study. J Am Acad Dermatol. (2023) 89(1):178–81. doi: 10.1016/j.jaad.2023.02.047
28. Alvarenga JM, Maria Lé A, Torres T. Dupilumab for atopic dermatitis during pregnancy and breastfeeding: a case report. Actas Dermosifiliogr. (2023) S0001-7310(23):00817–7. doi: 10.1016/j.ad.2023.10.005
29. Chen RE, Yokoyama CC, Anadkat MJ. Pemphigoid gestationis treated with dupilumab. JAAD Case Rep. (2023) 41:10–2. doi: 10.1016/j.jdcr.2023.08.013
30. Hong N, Park SY, Kook HD, Lee DH, Jung HJ, Park MY, et al. Atopic dermatitis treated safely with dupilumab during pregnancy and lactation: a case series of four patients. Australas J Dermatol. (2024) 65(4):e100–3. doi: 10.1111/ajd.14255
31. Di Lernia V, Peccerillo F. Long-Term follow-up of dupilumab treatment during conception, pregnancy and lactation. Indian J Dermatol. (2024) 69(2):193–5. doi: 10.4103/ijd.ijd_447_23
32. Avallone G, Cavallo F, Tancredi A, Maronese CA, Bertello M, Fraghì A, et al. Association between maternal dupilumab exposure and pregnancy outcomes in patients with moderate-to-severe atopic dermatitis: a nationwide retrospective cohort study. J Eur Acad Dermatol Venereol JEADV. (2024) 38(9):1799–808. doi: 10.1111/jdv.19794
33. Hom S, Pisano M. Reslizumab (Cinqair): an interleukin-5 antagonist for severe asthma of the eosinophilic phenotype. P T Peer-Rev J Formul Manag. (2017) 42(9):564–8.
34. U.S. Food and Drug Administration. Highlights of prescribing information. Tezepelumab. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761224s001lbl.pdf (accessed August 05, 2024).
35. U.S. Food and Drug Administration. Highlights of prescribing information. Tralokinumab. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761180Orig1s000lbl.pdf (accessed August 05, 2024).
36. Andreoli L, Gerardi MC, Gerosa M, Rozza D, Crisafulli F, Erra R, et al. Management of pregnancy in autoimmune rheumatic diseases: maternal disease course, gestational and neonatal outcomes and use of medications in the prospectiveItalian P-RHEUM.it study. RMD Open. (2024) 10(2):e004091. doi: 10.1136/rmdopen-2024-004091
37. Perlow JH, Montgomery D, Morgan MA, Towers CV, Pronto M. Severity of asthma and perinatal outcome. Am J Obstet Gynecol. (1992) 167(4, Part 1):963–7. doi: 10.1016/S0002-9378(12)80020-2
38. van Gendt J, Emaus R, Visschedijk MC, Touw DJ, Bouwknegt DG, de Leeuw K, et al. Pharmacokinetics of monoclonal antibodies throughout pregnancy: a systematic literature review. Clin Pharmacokinet. (2024) 63(5):589–622. doi: 10.1007/s40262-024-01370-7
39. Koren G, Ornoy A. The role of the placenta in drug transport and fetal drug exposure. Expert Rev Clin Pharmacol. (2018) 11(4):373–85. doi: 10.1080/17512433.2018.1425615
40. Porter ML, Lockwood SJ, Kimball AB. Update on biologic safety for patients with psoriasis during pregnancy. Int J Womens Dermatol. (2017) 3(1):21–5. doi: 10.1016/j.ijwd.2016.12.003
41. Chambers CD, Johnson DL. Emerging data on the use of anti-tumor necrosis factor-alpha medications in pregnancy. Birt Defects Res A Clin Mol Teratol. (2012) 94(8):607–11. doi: 10.1002/bdra.23033
42. Beltagy A, Aghamajidi A, Trespidi L, Ossola W, Meroni PL. Biologics during pregnancy and breastfeeding among women with rheumatic diseases: safety clinical evidence on the road. Front Pharmacol. (2021) 12:621247. doi: 10.3389/fphar.2021.621247
43. Prabhu SS, Suvarna P. Biological agents in pregnancy and lactation—a rational approach. J Skin Sex Transm Dis. (2019) 1(2):54–60. doi: 10.25259/JSSTD_19_2019
44. Ramos C L, Namazy J. Monoclonal antibodies (biologics) for allergic rhinitis, asthma, and atopic dermatitis during pregnancy and lactation. Immunol Allergy Clin North Am. (2023) 43(1):187–97. doi: 10.1016/j.iac.2022.07.001
45. Chu VT, Fröhlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. (2011) 12(2):151–9. doi: 10.1038/ni.1981
46. Ross C, D’Souza R, Pagnoux C. Pregnancy outcomes in systemic vasculitides. Curr Rheumatol Rep. (2020) 22(10):63. doi: 10.1007/s11926-020-00940-5
47. Ohta R, Fujimori T, Sano C. Eosinophilic granulomatosis with polyangiitis gradually worsening with consecutive pregnancies: a case report. Cureus (2024) 16(2):e54832. doi: 10.7759/cureus.54832
48. Koutroulis I, Papoutsis J, Kroumpouzos G. Atopic dermatitis in pregnancy: current status and challenges. Obstet Gynecol Surv. (2011) 66(10):654–63. doi: 10.1097/OGX.0b013e31823a0908
49. Tukler Henriksson J, Coursey TG, Corry DB, De Paiva CS, Pflugfelder SC. IL-13 Stimulates proliferation and expression of mucin and immunomodulatory genes in cultured conjunctival goblet cells. Invest Ophthalmol Vis Sci. (2015) 56(8):4186–97. doi: 10.1167/iovs.14-15496
50. Bakker DS, Ariens LFM, van Luijk C, van der Schaft J, Thijs JL, Schuttelaar MLA, et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. (2019) 180(5):1248–9. doi: 10.1111/bjd.17538
51. Wang W, Sung N, Gilman-Sachs A, Kwak-Kim J. T helper (th) cell profiles in pregnancy and recurrent pregnancy losses: Th1/Th2/Th9/Th17/Th22/tfh cells. Front Immunol. (2020) 11:2025. doi: 10.3389/fimmu.2020.02025
52. Fredi M, Lazzaroni MG, Tani C, Ramoni V, Gerosa M, Inverardi F, et al. Systemic vasculitis and pregnancy: a multicenter study on maternal and neonatal outcome of 65 prospectively followed pregnancies. Autoimmun Rev. (2015) 14(8):686–91. doi: 10.1016/j.autrev.2015.03.009
53. Murphy V, Namazy J, Powell H, Schatz M, Chambers C, Attia J, et al. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG Int J Obstet Gynaecol. (2011) 118(11):1314–23. doi: 10.1111/j.1471-0528.2011.03055.x
54. Murphy V, Wang G, Namazy J, Powell H, Gibson P, Chambers C, et al. The risk of congenital malformations, perinatal mortality and neonatal hospitalisation among pregnant women with asthma: a systematic review and meta-analysis. BJOG Int J Obstet Gynaecol. (2013) 120(7):812–22. doi: 10.1111/1471-0528.12224
55. Abdelhafez MMA, Ahmed KAM, Daud MNBM, Jeffree MS, Kadir F, Baharuddin DMP, et al. Pemphigoid gestationis and adverse pregnancy outcomes: a literature review. J Gynecol Obstet Hum Reprod. (2022) 51(5):102370. doi: 10.1016/j.jogoh.2022.102370
56. Sweeting A, Hannah W, Backman H, Catalano P, Feghali M, Herman WH, et al. Epidemiology and management of gestational diabetes. Lancet Lond Engl. (2024) 404(10448):175–92. doi: 10.1016/S0140-6736(24)00825-0
57. Wollenberg A, Zoch C, Wetzel S, Plewig G, Przybilla B. Predisposing factors and clinical features of eczema herpeticum: a retrospective analysis of 100 cases. J Am Acad Dermatol. (2003) 49(2):198–205. doi: 10.1067/S0190-9622(03)00896-X
58. Babalola O, Strober BE. Treatment of atopic dermatitis in pregnancy. Dermatol Ther. (2013) 26(4):293–301. doi: 10.1111/dth.12074
59. Mawhirt SL, Fonacier L. Atopic dermatitis and allergic contact dermatitis in pregnancy. In: Namazy JA, Schatz M, editors. Asthma, Allergic and Immunologic Diseases During Pregnancy: A Guide to Management. Cham: Springer International Publishing (2019). p. 101–21. Available online at: doi: 10.1007/978-3-030-03395-8_7
60. Middleton PG, Gade EJ, Aguilera C, MacKillop L, Button BM, Coleman C, et al. ERS/TSANZ task force statement on the management of reproduction and pregnancy in women with airways diseases. Eur Respir J. (2020) 55(2):1901208. doi: 10.1183/13993003.01208-2019
61. An Observational Retrospective Cohort Study Being Conducted in Women With Atopic Dermatitis (AD). Available online at: https://clinicaltrials.gov/study/NCT03936335 (accessed August 05, 2024).
62. Post-Authorization Safety Study to Monitor Pregnancy and Infant Outcomes Following Administration of Dupilumab During Planned or Unexpected Pregnancy in North America. (2021). Available online at: https://catalogues.ema.europa.eu/node/2826/administrative-details (accessed August 05, 2024).
63. Mother to Baby. Available online at: https://mothertobaby.org/ongoing-study/dupixent-dupilumab/ (accessed August 05, 2024).
64. Mother To Baby | Fact Sheets. Dupilumab (Dupixent®) 2022 October. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK582685/ (accessed August 05, 2024).
65. Mother To Baby | Fact Sheets. Mepolizumab (Nucala®) 2022 July. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK582824/ (accessed August 05, 2024).
66. The Mepolizumab Pregnancy Exposure Study: a VAMPSS post marketing surveillance study of Mepolizumab safety in pregnancy (200870 NPSS (Nucala Pregnancy Surveillance Study)). Available online at: https://catalogues.ema.europa.eu/node/2613/administrative-details (accessed August 05, 2024).
67. Mother To Baby | Fact Sheets. Benralizumab (Fasenra®) 2022 November Available online at: https://www.ncbi.nlm.nih.gov/books/NBK582599/ (accessed August 05, 2024).
68. Vasculitis Pregnancy Registry (VPREG). Available online at: https://www.vasculitisfoundation.org/treatments-research/vasculitis-pregnancy-registry/ (accessed August 05, 2024).
Keywords: EGPA, pregnancy, mepolizumab, dupilumab, benralizumab, vasculitis, conception, breastfeeding
Citation: Davanzo F, Iorio L, Calligaro A, Doria A and Padoan R (2024) Monoclonal antibodies targeting type 2 inflammation in eosinophil-associated diseases during pregnancy: insights from two eosinophilic granulomatosis with polyangiitis cases and a comprehensive literature review. Front. Lupus 2:1479884. doi: 10.3389/flupu.2024.1479884
Received: 12 August 2024; Accepted: 21 October 2024;
Published: 5 November 2024.
Edited by:
Angela Tincani, Rheumatology Unit ASST-Spedali Civili and University of Brescia, ItalyReviewed by:
Giuseppe Barilaro, Hospital Clinic of Barcelona, SpainPeter Villiger, University of Bern, Switzerland
Copyright: © 2024 Davanzo, Iorio, Calligaro, Doria and Padoan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Doria, YWRvcmlhQHVuaXBkLml0
 Federica Davanzo
Federica Davanzo Luca Iorio
Luca Iorio Antonia Calligaro
Antonia Calligaro Andrea Doria
Andrea Doria Roberto Padoan
Roberto Padoan