- 1Division of Rheumatology and Systemic Inflammatory Diseases, III Department of Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
- 2Department of Rheumatology, Medical Faculty of Heinrich-Heine-University, University Hospital Düsseldorf, Heinrich-Heine-Heine University, Düsseldorf, Germany
Systemic Lupus Erythematosus (SLE) and Antiphospholipid Syndrome (APS) have a substantial impact on pregnancy outcomes and require meticulous planning and management. This article explores the complex interrelationships between SLE, APS, and pregnancy and provides an overview of the associated risks and predictors. The crucial role of pre-conception counselling, risk stratification and tailored treatment plans is highlighted, accompanied by a suggested practical approach. Recent advancements in therapeutic approaches and emerging research on promising targeted interventions indicate the potential for enhanced maternal and fetal outcomes.
1 Introduction
Systemic Lupus Erythematosus (SLE) primarily affects women of childbearing age. With ongoing advancements in treatment, patients may anticipate attaining a near-normal quality of life, wherein family planning may assume a pivotal role. Among rheumatic diseases, SLE has a distinctive interaction with pregnancy, as both can exert a detrimental influence on one another. This is particularly relevant for the approximately 40% of SLE patients who present with antiphospholipid antibodies (aPL) or secondary Antiphospholipid Syndrome (APS) (1). APS is characterized by the occurrence of thrombosis and/or obstetric complications in the presence of aPL, including anticardiolipin antibodies (aCL), anti-b2 glycoprotein-I antibodies (ab2GPI), and lupus anticoagulant (LA) (2, 3).
For women with SLE and APS who wish to conceive, pregnancy counselling is of fundamental importance. It includes stratification and adjustment of risk factors, optimization of medication and individualized planning of antenatal care. This article outlines pregnancy planning considerations for patients with SLE and APS, drawing on current research and clinical guidelines.
2 Pregnancy outcomes in SLE and APS
The prognosis for women with SLE who wish to become pregnant has improved markedly in recent decades, with notable declines in both maternal and fetal mortality. However, the incidence of maternal and fetal morbidity rates remains higher than in the general population, carrying an increased risk of pre-eclampsia, miscarriage, fetal loss, stillbirth, preterm birth, intrauterine growth restriction (IUGR), and small for gestational age (SGA) infants (4, 5). Furthermore, there is a considerable risk of SLE flares during pregnancy and, to a lesser extent, postpartum (6). Consequently, all pregnancies in SLE are considered high-risk pregnancies, though the level of risk can vary depending on disease severity, current activity and additional risk factors, both related to and independent of SLE (7). A detailed discussion of this topic will be presented subsequently.
One of the most important additional risk factors is the presence of aPL. These antibodies are associated with an elevated risk of adverse pregnancy outcomes (APOs), including pre-eclampsia, thromboembolism, early and late pregnancy loss, placental insufficiency, IUGR, preterm delivery, and perinatal mortality (8). Depending on the data source and the population included, the live birth rate in women with untreated APS is around 50%, while it can be increased as high as 75%–85% with appropriate treatment according to recent guidelines (9, 10).
3 Predicting and modifying pregnancy outcomes
Given the complex nature of SLE, considerable effort has been made to identify reliable risk factors for individuals at increased risk prior to or during the early stages of gestation. The PROMISSE study marked a significant advancement in this field of research. The study provided baseline predictors of APOs in the 1st trimester, including the presence of LA (OR = 8.32), antihypertensive use (OR = 7.05), high disease activity with a PGA >1 (OR = 4.02), while non-Hispanic white ethnicity was identified as a protective factor (OR = 0.45). Among women without baseline risk factors, the APO rate was 7.8%, compared to 58% in those who were LA positive or non-white/Hispanic and undergoing antihypertensive treatment, which led to a fetal/neonatal mortality rate of 22% due to complications of prematurity (11). Other studies have shown that existing or previous lupus nephritis is a significant risk factor for unfavorable maternal and fetal outcomes (12, 13).
The PROMISSE study, in conjunction with other research, has demonstrated a correlation between reduced complement levels in the pre-conception or early gestational period and an increased risk of disease flares, pre-eclampsia, preterm delivery, and IUGR (14). This warrants close monitoring of complement dynamics for early detection of disease flares (15).
Disease activity of SLE at the time of conception is the single most important predictor for maternal and fetal morbidity, and it is modifiable. It is well established that achieving a low disease activity state (LDAS) or remission is crucial when planning a pregnancy (7). Active disease within 6–12 months before conception is associated with a two-fold increased risk of flares and maternal and fetal complications (16, 17). In recent years, new definitions of and a particular focus on remission and LDAS in SLE have emerged, raising the question of whether these states affect the outcome of our patients differently (18). Two international studies concluded that remission (as defined by DORIS) at conception is a stronger protective factor against relapse and complications during pregnancy than LDAS, with fewer relapses occurring in the 2 years postpartum (19, 20).
Hydroxychloroquine (HCQ) is another well-supported protective factor, with extensive empirical evidence for its ability to reduce the likelihood of both disease flares and APOs. Emerging evidence indicates that HCQ may be effective in preventing pre-eclampsia (21–23). In addition, HCQ is being investigated for its likely beneficial effect in refractory APS, which will be addressed in a later section.
This growing body of evidence highlights the importance of prenatal planning and the pursuit of disease remission before and throughout pregnancy in patients with SLE. Increasingly precise predictors of individual patient trajectories at the outset of pregnancy planning enable targeted interventions and closer monitoring for those at higher risk. At the same time, this facilitates the efficient distribution of healthcare resources, avoiding superfluous and potentially tedious diagnostic procedures for patients at relatively lower risk.
4 Pregnancy planning in SLE and APS
It is of utmost importance to inquire about the patient's perceptions of family planning at the appropriate time and in a repetitive manner. This is a crucial step in addressing several pivotal issues along the patient`s journey, even before the actual planning of the pregnancy. Several important topics must be addressed:
1. Contraception: When pregnancy is either undesired or must be postponed due to active disease or the need for teratogenic medication, it is essential to evaluate the most reliable and suitable contraceptive methods. Changes in medication provide an excellent opportunity for contraceptive counselling, a need underscored by the continued underuse of effective contraception in SLE patients (24, 25).
2. Fertility: Advances in SLE management, such as the use of gonadotropin-releasing hormone analogues (GnRHa), the Euro-Lupus regimen, and new therapeutic options, have mitigated infertility risks due to the gonadotoxic effects of cyclophosphamide (26). However, treatment can still indirectly impact fertility by delaying pregnancy to a time when ovarian reserve is declining. In both scenarios, fertility preservation options should be discussed, and referral to a gynecologist may be necessary.
3. Pregnancy: When pregnancy is desired, a comprehensive conception plan must be developed. From the patient's perspective, one of the primary objectives is to acquire knowledge on this special topic and to build their individual multidisciplinary network. Ideally, patients should be aware that pregnancy in these conditions will require foresight planning and in some cases a modification in medication or other measures, which may result in further postponement of their plans. This approach helps to avoid unplanned pregnancies and unfavorable maternal and fetal outcomes.
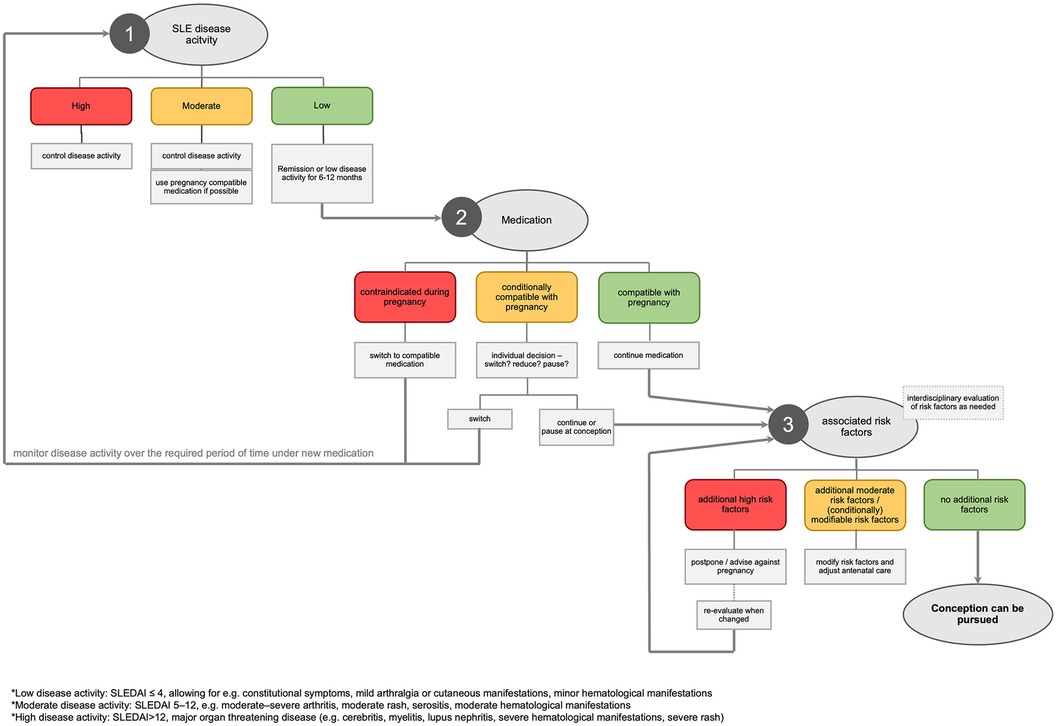
Effective pregnancy planning for women with SLE and APS necessitates consideration at multiple levels. An algorithmic approach, as illustrated in Figure 1 and outlined in the following section, can help to address these issues systematically and ensure thoroughness.
4.1 Step 1: evaluation of SLE disease activity
It is recommended that SLE should be in remission or at least in LDAS for 6 to 12 months prior to conception (7, 27). This range results from the varying severity of the disease. If the assessment reveals high disease activity, SLE must be treated following established guidelines and pregnancy must be postponed. In case of moderate disease activity, the objective of optimal disease control must take precedence over the possibility of conception. However, the rheumatologist can often use medication that is already compatible with pregnancy.
4.2 Step 2: medication management
All medication taken by the woman must be reviewed for safety during pregnancy. Compatible drugs should be continued throughout pregnancy with risks weighed against benefits for both mother and child.
Given its numerous beneficial effects on disease control and complications, it is recommended that HCQ may be continued (or started). Other disease-modifying anti-rheumatic drugs (DMARDs) with a favorable safety profile during pregnancy include azathioprine, cyclosporine and tacrolimus. While prednisolone is inactivated in the placenta and can be applied during pregnancy, prolonged use of doses above 7.5 mg/day has been associated with an increased risk of gestational diabetes, SGA babies, and a shorter gestational age (28).
There is growing evidence supporting the use of biologics such as belimumab and rituximab during pregnancy when clinically indicated. However, this is not yet sufficient to justify a general recommendation, and any decision to use these drugs during pregnancy must be made on a case-by-case basis. In approximately 500 pregnancies involving belimumab and approximately 300 pregnancies involving rituximab, respectively, no evidence of a teratogenic effect has been identified (29–32). A similar amount of data is available on ocrelizumab, a B-cell inhibitor that targets CD20 (32). The use of rituximab in the 2nd or 3rd trimester has been associated with the potential for transient B-cell depletion in the neonate in small case series (33). It is therefore recommended to avoid live vaccinations of infants if the mother has been treated from the 2nd trimester onwards with one of these biologics.
For drugs with known teratogenic effects (namely cyclophosphamide, mycophenolate mofetil and methotrexate), discontinuation before conception is advised, with the usual recommendation being that compatible alternatives be employed. The effectiveness and tolerability of the new treatment should be assessed for at least 3–6 months before attempting pregnancy.
Angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers are often used in the management of lupus nephritis or hypertension. Exposure during the 2nd and 3rd trimesters can result in inadequate blood supply to the placenta, leading to fetal hypotension, renal impairment, and oligohydramnios (34, 35). In line with current recommendations, the most practical approach is to cease these medications at the time of a positive pregnancy test and, if necessary, employ safe alternatives, such as methyldopa (36). SGLT2 inhibitors are increasingly used due to their nephroprotective features. in vitro studies suggest placental transfer and the available data on SGLT2 inhibitors in human pregnancy is very scarce and insufficient to allow the formulation of any recommendations regarding their use (37, 38).
Phenprocoumon and Warfarin are associated with an increased risk of major malformations if the unborn child is exposed at 9th gestational week (GW) or later and is replaced by therapeutic low-molecular-weight heparin (LMWH) (39). In clinical practice, this change is justifiable in the case of a positive pregnancy test up to the 6th week of pregnancy if a regular menstrual cycle, comprehensive information and adherence are given.
Nonsteroidal anti-inflammatory drugs (NSAIDs) can be used until the 28th week at the lowest effective dose, when risk of premature closure of the ductus arteriosus botalli increases, but their use should be restricted from the 20th week due to potential renal dysfunction and oligohydramnios (40). In contrast, low-dose acetylsalicylic acid (LDA) is safe and demonstrated reduction of pre-eclampsia risk if started before 16th GW (41). Hence, it is recommended for all women with SLE, particularly if additional risk factors are present (e.g., extreme age, arterial hypertension, pre-existing renal disease or aPL).
4.3 Step 3: assessment of associated risk factors
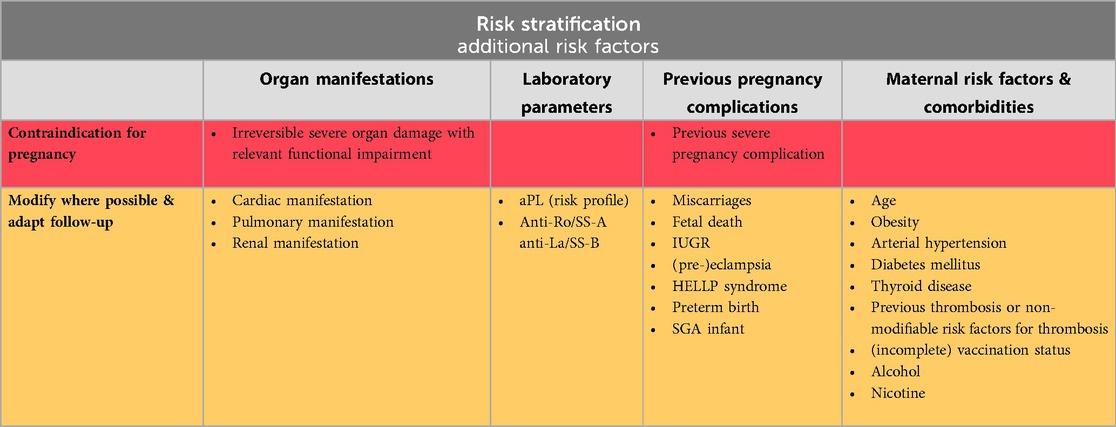
Although often overlooked, the assessment of associated risk factors is an element as important as the evaluation of disease activity and the adjustment of medication. These can be broadly divided into three categories: Factors directly related with SLE (severe organ manifestations or damage, and some autoantibodies that pose additional risk), maternal risk factors and comorbidities, and previous pregnancy complications. Table 1 provides a comprehensive overview of the key considerations.
Basic measures include advising all women planning a pregnancy to take daily folic acid and ensure adequate vitamin D intake, particularly if heparin or glucocorticoids (GCs) are employed. It is also highly recommended that vaccination status is up to date.
While most additional risk factors can be managed effectively, there are instances where pregnancy is contraindicated. This is particularly true in cases of severe cardiac, pulmonary, or renal impairment, or when previous severe pregnancy complications occurred despite appropriate treatment (27).
4.4 Digression: neonatal lupus
The presence of anti-Ro/SS-A and anti-La/SS-B antibodies can lead to neonatal lupus, which manifests in two forms that differ in timing and severity. About 10% of neonates may experience transient, self-limiting postnatal symptoms, such as annular erythematous lesions, asymptomatic liver involvement, mild hepatosplenomegaly, and cytopenia. More concerning is the risk o congenital heart block (CHB), which may develop between the 18th and 26th GW in 1%–2% of cases, with a recurrence risk of approximately 17% in subsequent pregnancies (42–44). For women with a previously affected fetus, weekly fetal echocardiograms are recommended from 16th GW to monitor for CHB. In women with no prior history of CHB, the optimal frequency of monitoring is debated, with suggestions ranging from biweekly checks to checks during routine obstetric visits, largely due to the fact that there is no effective treatment if CHB is detected (45). Fluorinated GCs and IVIG have shown no better efficacy than a wait-and-see strategy (46, 47). HCQ is currently the only medication proven to reduce the risk of CHB when started before conception or early in pregnancy (48, 49).
4.5 Digression: aPL and APS
According to EULAR and ACR recommendations, women with SLE and high-risk aPL profile (LA positive or double/triple positive for aPL) without previous thrombotic or obstetric manifestations should receive LDA during pregnancy. If a history of obstetric APS is present, LDA should be initiated prior to conception and supplemented with prophylactic LMWH as soon as the pregnancy test is positive until 6–12 weeks postpartum. In the case of thrombotic APS, LDA should be started upon pregnancy in addition to therapeutic LMWH. In refractory cases despite these measurements, escalation from prophylactic to therapeutic LMWH or addition of HCQ or low-dose prednisolone can be considered on a case-by-case basis (27, 50).
4.6 Final remarks: individualized risk stratification and tailored treatment plan
Considering disease activity, medication and additional risk factors, an individualized risk stratification is carried out step by step with a special attention to modifiable risks. Based on this assessment, patient and physician can collaboratively set up a tailored treatment plan with necessary measures before and during pregnancy. This also sets the starting point to build a multidisciplinary team with obstetricians and others to monitor and manage the pregnancy closely. Of course, frequent adjustments to the treatment plan might be made as pregnancy progresses.
5 Discussion
Pregnancy in women with SLE and APS requires meticulous planning and management to mitigate risks and improve outcomes. By leveraging current knowledge and available treatments, rheumatologists can support these patients in achieving successful pregnancies. However, major challenges remain, and ongoing research is promising to address these.
5.1 Neonatal lupus
Up until this point in time, it has not been possible to provide women with anti-Ro/SS-A and anti-La/SS-B antibodies with a more accurate estimation of the actual risk of their offspring developing CHB than the figures previously mentioned. This impairs optimized and cost-effective monitoring and therapeutic approaches. The STOP BLOQ study examines a multi-step approach to address various obstacles simultaneously. The initial findings of this ongoing study appear to corroborate the hypothesis that elevated anti-Ro/SS-A titers are associated with an increased prevalence of CHB, and that low titers may possess a potential negative predictive value. Moreover, the authors present compelling evidence for the efficacy of home monitoring conducted by expectant women for the prompt identification of newly emerging CHB (51). The hypothesis that this early detection of CHB in a population at higher risk provides a window of opportunity for therapeutic intervention is now being investigated.
5.2 Therapeutic options in APS
The protective role of HCQ in the context of pregnancy is well established in SLE. However, its potential benefit in the context of APS is less clear. Retrospective cohort studies suggest that the addition of HCQ to standard treatment in refractory obstetric APS is associated with fewer miscarriages, a higher live birth rate and a lower prevalence of pre-eclampsia and IUGR (52, 53).
However, the validity of these studies is limited due to their retrospective nature, heterogeneous groups and small cohort size. Given the potential benefit of this long-known, pregnancy-compatible and well-tolerated substance in refractory high-risk pregnancies, there is an urgent need for more reliable data. Accordingly, the HYPATIA study, a prospective, randomized, controlled trial designed to address this question, is highly commended.
Another promising avenue for improving pregnancy outcomes of women with APS is the IMPACT study. The authors have previously identified TNF-α as a critical downstream effector of abnormal placental development in APS, which can lead to fetal damage, pre-eclampsia and placental insufficiency (54). They are now investigating the potential protective effect of certolizumab in relation to APOs associated with poor placentation. Preliminary results indicate safety even with respect to the development of anti-dsDNA-antibodies or signs of SLE (55).
5.3 Future directions
These studies, along with other ambitious and outstanding projects, will enhance our understanding and broaden our diagnostic and therapeutic armamentarium. This will inform updates to the guidelines and eventually improve the care and counselling provided to women with SLE and APS.
Author contributions
IH: Visualization, Writing – original draft. RF-B: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
I would like to thank the scientific committee of the RheumaPreg 2023 to invite me for an oral presentation on this topic. Furthermore, I extend my gratitude to Martin Krusche for fine-granular discussions, mental support and creating capacities for the realization of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ruiz-Irastorza G, Egurbide MV, Ugalde J, Aguirre C. High impact of antiphospholipid syndrome on irreversible organ damage and survival of patients with systemic lupus erythematosus. Arch Intern Med. (2004) 164(1):77–82. doi: 10.1001/archinte.164.1.77
2. Barbhaiya M, Zuily S, Naden R, Hendry A, Manneville F, Amigo MC, et al. 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Ann Rheum Dis. (2023) 75(10):1687–702. doi: 10.1002/art.42624
3. Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. (2002) 46(4):1019–27. doi: 10.1002/art.10187
4. Mehta B, Jannat-Khah D, Glaser KK, Luo Y, Sammaritano LR, Branch DW, et al. Fetal and maternal morbidity in pregnant patients with lupus: a 10-year US nationwide analysis. RMD Open. (2023) 9(1):e002752. doi: 10.1136/rmdopen-2022-002752
5. He WR, Wei H. Maternal and fetal complications associated with systemic lupus erythematosus. Medicine (Baltimore). (2020) 99(16):e19797. doi: 10.1097/MD.0000000000019797
6. Skorpen CG, Lydersen S, Gilboe I, Skomsvoll JF, Salvesen KÅ, Palm Ø, et al. Disease activity during pregnancy and the first year postpartum in women with systemic lupus erythematosus. Arthritis Care Res (Hoboken). (2017) 69(8):1201–8. doi: 10.1002/acr.23102
7. Andreoli L, Bertsias GK, Agmon-Levin N, Brown S, Cervera R, Costedoat-Chalumeau N, et al. EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis. (2017) 76(3):476–85. doi: 10.1136/annrheumdis-2016-209770
8. Saccone G, Berghella V, Maruotti GM, Ghi T, Rizzo G, Simonazzi G, et al. Antiphospholipid antibody profile based obstetric outcomes of primary antiphospholipid syndrome: the PREGNANTS study. Am J Obstet Gynecol. (2017) 216(5):525.e1–12. doi: 10.1016/j.ajog.2017.01.026
9. Alijotas-Reig J, Esteve-Valverde E, Ferrer-Oliveras R, Sáez-Comet L, Lefkou E, Mekinian A, et al. The European registry on obstetric antiphospholipid syndrome (EUROAPS): a survey of 1000 consecutive cases. Autoimmun Rev. (2019) 18(4):406–14. doi: 10.1016/j.autrev.2018.12.006
10. Bouvier S, Cochery-Nouvellon E, Lavigne-Lissalde G, Mercier E, Marchetti T, Balducchi JP, et al. Comparative incidence of pregnancy outcomes in treated obstetric antiphospholipid syndrome: the NOH-APS observational study. Blood. (2014) 123(3):404–13. doi: 10.1182/blood-2013-08-522623
11. Buyon JP, Kim MY, Guerra MM, Laskin CA, Petri M, Lockshin MD, et al. Predictors of pregnancy outcomes in patients with lupus: a cohort study. Ann Intern Med. (2015) 163(3):153–63. doi: 10.7326/M14-2235
12. Smyth A, Oliveira GHM, Lahr BD, Bailey KR, Norby SM, Garovic VD. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol. (2010) 5(11):2060–8. doi: 10.2215/CJN.00240110
13. Saavedra MA, Cruz-Reyes C, Vera-Lastra O, Romero GT, Cruz-Cruz P, Arias-Flores R, et al. Impact of previous lupus nephritis on maternal and fetal outcomes during pregnancy. Clin Rheumatol. (2012) 31(5):813–9. doi: 10.1007/s10067-012-1941-4
14. Radin M, Cecchi I, Crisafulli F, Klumb EM, de Jesús GR, Lacerda MI, et al. Complement levels during the first trimester predict disease flare and adverse pregnancy outcomes in systemic lupus erythematosus: a network meta-analysis on 532 pregnancies. Autoimmun Rev. (2023) 22(12):103467. doi: 10.1016/j.autrev.2023.103467
15. Crisafulli F, Andreoli L, Zucchi D, Reggia R, Gerardi MC, Lini D, et al. Variations of C3 and C4 before and during pregnancy in systemic lupus erythematosus: association with disease flares and obstetric outcomes. J Rheumatol. (2023) 50(10):1296–301. doi: 10.3899/jrheum.2022-1135
16. Yang H, Liu H, Xu D, Zhao L, Wang Q, Leng X, et al. Pregnancy-Related systemic lupus erythematosus: clinical features, outcome and risk factors of disease flares — a case control study. PLoS One. (2014) 9(8):e104375. doi: 10.1371/journal.pone.0104375
17. Kwok LW, Tam LS, Zhu T, Leung YY, Li E. Predictors of maternal and fetal outcomes in pregnancies of patients with systemic lupus erythematosus. Lupus. (2011) 20(8):829–36. doi: 10.1177/0961203310397967
18. Van Vollenhoven R, Voskuyl A, Bertsias G, Aranow C, Aringer M, Arnaud L, et al. A framework for remission in SLE: consensus findings from a large international task force on definitions of remission in SLE (DORIS). Ann Rheum Dis. (2017) 76(3):554–61. doi: 10.1136/annrheumdis-2016-209519
19. Tani C, Zucchi D, Haase I, Larosa M, Crisafulli F, Strigini FAL, et al. Are remission and low disease activity state ideal targets for pregnancy planning in systemic lupus erythematosus? A multicentre study. Rheumatology (Oxford). (2021) 60(12):5610–9. doi: 10.1093/rheumatology/keab155
20. Radin M, Schreiber K, Cecchi I, Signorelli F, De Jesús G, Aso K, et al. Disease activity at conception predicts lupus flare up to two years after birth: a multicentre long term follow-up study. Semin Arthritis Rheum. (2022) 57(152113). doi: 10.1016/j.semarthrit.2022.152113
21. Do SC, Rizk NM, Druzin ML, Simard JF. Does hydroxychloroquine protect against preeclampsia and preterm delivery in systemic lupus erythematosus pregnancies? Am J Perinatol. (2020) 37(09):873–80. doi: 10.1055/s-0039-3402752
22. Seo MR, Chae J, Kim YM, Cha HS, Choi SJ, Oh S, et al. Hydroxychloroquine treatment during pregnancy in lupus patients is associated with lower risk of preeclampsia. Lupus. (2019) 28(6):722–30. doi: 10.1177/0961203319843343
23. Haase I, Chehab G, Sander O, Schneider M, Fischer-Betz R. Ab0341 SLE pregnancies at high risk for pre-eclampsia benefit most from combination of low dose aspirin and hydroxychloroquine. Ann Rheum Dis. (2021) 80(Suppl 1):1195–6. doi: 10.1136/annrheumdis-2021-eular.3839
24. Schwarz EB, Manzi S. Risk of unintended pregnancy among women with systemic lupus erythematosus. Arthritis Rheum. (2008) 59(6):863–6. doi: 10.1002/art.23712
25. Yazdany J, Trupin L, Kaiser R, Schmajuk G, Gillis JZ, Chakravarty E, et al. Contraceptive counseling and use among women with systemic lupus erythematosus: a gap in health care quality? Arthritis Care Res (Hoboken). (2011) 63(3):358–65. doi: 10.1002/acr.20402
26. Tamirou F, Husson SN, Gruson D, Debiève F, Lauwerys BR, Houssiau FA. Brief report: the euro-lupus low-dose intravenous cyclophosphamide regimen does not impact the ovarian reserve, as measured by Serum levels of anti–müllerian hormone. Arthritis Rheumatol. (2017) 69(6):1267–71. doi: 10.1002/art.40079
27. Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse MEB, Lockshin MD, et al. 2020 American College of rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Care Res (Hoboken). (2020) 72(4):461–88. doi: 10.1002/art.41191
28. De Man YA, Hazes JMW, Van Der Heide H, Willemsen SP, De Groot CJM, Steegers EAP, et al. Association of higher rheumatoid arthritis disease activity during pregnancy with lower birth weight: results of a national prospective study. Arthritis Rheum. (2009) 60(11):3196–206. doi: 10.1002/art.24914
29. Juliao P, Wurst K, Pimenta JM, Gemzoe K, Landy H, Moody MA, et al. Belimumab use during pregnancy: interim results of the belimumab pregnancy registry. Birth Defects Res. (2023) 115(2):188–204. doi: 10.1002/bdr2.2091
30. Petri M, Landy H, Clowse MEB, Gemzoe K, Khamashta M, Kurtinecz M, et al. Belimumab use during pregnancy: a summary of birth defects and pregnancy loss from belimumab clinical trials, a pregnancy registry and postmarketing reports. Ann Rheum Dis. (2023) 82(2):217–25. doi: 10.1136/ard-2022-222505
31. Russell MD, Dey M, Flint J, Davie P, Allen A, Crossley A, et al. British Society for rheumatology guideline on prescribing drugs in pregnancy and breastfeeding: immunomodulatory anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford). (2023) 62(4):e48–88. doi: 10.1093/rheumatology/keac551
32. Dobson R, Rog D, Ovadia C, Murray K, Hughes S, Ford HL, et al. Anti-CD20 therapies in pregnancy and breast feeding: a review and ABN guidelines. Pract Neurol. (2023) 23(1):6–14. doi: 10.1136/pn-2022-003426
33. Schwake C, Steinle J, Thiel S, Timmesfeld N, Haben S, Ayzenberg I, et al. Neonatal B-cell levels and infant health in newborns potentially exposed to anti-CD20 monoclonal antibodies during pregnancy or lactation. Neurol Neuroimmunol Neuroinflamm. (2024) 11(4):e200264. doi: 10.1212/NXI.0000000000200264
34. Burrows RF, Burrows EA. Assessing the teratogenic potential of angiotensin-converting enzyme inhibitors in pregnancy. Aust NZ J Obst Gynaeco. (1998) 38(3):306–11. doi: 10.1111/j.1479-828X.1998.tb03072.x
35. Bar J, Hod M, Merlob P. Angiotensin converting enzyme inhibitors use in the first trimester of pregnancy. Int J Risk Safety Med. (1997) 10(1):23–6. doi: 10.3233/JRS-1997-10102
36. Schreiber K, Frishman M, Russell MD, Dey M, Flint J, Allen A, et al. British Society for rheumatology guideline on prescribing drugs in pregnancy and breastfeeding: comorbidity medications used in rheumatology practice. Rheumatology (Oxford). (2023) 62(4):e89–104. doi: 10.1093/rheumatology/keac552
37. Muller DRP, Stenvers DJ, Malekzadeh A, Holleman F, Painter RC, Siegelaar SE. Effects of GLP-1 agonists and SGLT2 inhibitors during pregnancy and lactation on offspring outcomes: a systematic review of the evidence. Front Endocrinol. (2023) 14(1215356):1–9. doi: 10.3389/fendo.2023.1215356
38. Kuoni S, Steiner R, Saleh L, Lehmann R, Ochsenbein-Kölble N, Simões-Wüst AP. Safety assessment of the SGLT2 inhibitors empagliflozin, dapagliflozin and canagliflozin during pregnancy: an ex vivo human placenta perfusion and in vitro study. Biomed Pharmacother. (2024) 171(116177):1–8. doi: 10.1016/j.biopha.2024.116177
39. Schaefer C, Hannemann D, Meister R, Eléfant E, Paulus W, Vial T, et al. Vitamin K antagonists and pregnancy outcome: a multi-centre prospective study. Thromb Haemost. (2006) 95(06):949–57. doi: 10.1160/TH06-02-0108
40. Dathe K, Hultzsch S, Pritchard LW, Schaefer C. Risk estimation of fetal adverse effects after short-term second trimester exposure to non-steroidal anti-inflammatory drugs: a literature review. Eur J Clin Pharmacol. (2019) 75(10):1347–53. doi: 10.1007/s00228-019-02712-2
41. Poon LC, Wright D, Rolnik DL, Syngelaki A, Delgado JL, Tsokaki T, et al. Aspirin for evidence-based preeclampsia prevention trial: effect of aspirin in prevention of preterm preeclampsia in subgroups of women according to their characteristics and medical and obstetrical history. Am J Obstet Gynecol. (2017) 217(5):585.e1–585.e5. doi: 10.1016/j.ajog.2017.07.038
42. Brito-Zerón P, Izmirly PM, Ramos-Casals M, Buyon JP, Khamashta MA. The clinical spectrum of autoimmune congenital heart block. Nat Rev Rheumatol. (2015) 11(5):301–12. doi: 10.1038/nrrheum.2015.29
43. Maldini C, Otaduy C, Mollerach FB, Scolnik M, Virasoro BM, Pisoni C, et al. 271 frequency of neonatal lupus in reference centers in the management of pregnancy and autoimmune diseases. Lupus Sci Med. (2019) 6. doi: 10.1136/lupus-2019-lsm.271
44. Llanos C, Izmirly PM, Katholi M, Clancy RM, Friedman DM, Kim MY, et al. Recurrence rates of cardiac manifestations associated with neonatal lupus and maternal/fetal risk factors. Arthritis Rheum. (2009) 60(10):3091–7. doi: 10.1002/art.24768
45. Costedoat-Chalumeau N, Morel N, Fischer-Betz R, Levesque K, Maltret A, Khamashta M, et al. Routine repeated echocardiographic monitoring of fetuses exposed to maternal anti-SSA antibodies: time to question the dogma. Lancet Rheumatol. (2019) 1(3):e187–93. doi: 10.1016/S2665-9913(19)30069-4
46. Hoxha A, Mattia E, Zanetti A, Carrara G, Morel N, Costedoat-Chalumeau N, et al. Fluorinated steroids are not superior to any treatment to ameliorate the outcome of autoimmune mediated congenital heart block: a systematic review of the literature and meta-analysis. Clin Exp Rheumatol. (2020) 38(4):783–91.32573408
47. Fischer-Betz R, Specker C. Pregnancy in systemic lupus erythematosus and antiphospholipid syndrome. Best Pract Res Clin Rheumatol. (2017) 31(3):397–414. doi: 10.1016/j.berh.2017.09.011
48. Barsalou J, Costedoat-Chalumeau N, Berhanu A, Fors-Nieves C, Shah U, Brown P, et al. Effect of in utero hydroxychloroquine exposure on the development of cutaneous neonatal lupus erythematosus. Ann Rheum Dis. (2018) 77(12):1742–9. doi: 10.1136/annrheumdis-2018-213718
49. Izmirly P, Kim M, Friedman DM, Costedoat-Chalumeau N, Clancy R, Copel JA, et al. Hydroxychloroquine to prevent recurrent congenital heart block in fetuses of anti-SSA/ro-positive mothers. J Am Coll Cardiol. (2020) 76(3):292–302. doi: 10.1016/j.jacc.2020.05.045
50. Tektonidou MG, Andreoli L, Limper M, Amoura Z, Cervera R, Costedoat-Chalumeau N, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. (2019) 78(10):1296–304. doi: 10.1136/annrheumdis-2019-215213
51. Buyon JP, Masson M, Izmirly CG, Phoon C, Acherman R, Sinkovskaya E, et al. Prospective evaluation of high titer autoantibodies and fetal home monitoring in the detection of atrioventricular block among anti-SSA/Ro pregnancies. Arthritis Rheumatol. (2024) 76(3):411–20. doi: 10.1002/art.42733
52. Mekinian A, Lazzaroni MG, Kuzenko A, Alijotas-Reig J, Ruffatti A, Levy P, et al. The efficacy of hydroxychloroquine for obstetrical outcome in anti-phospholipid syndrome: data from a European multicenter retrospective study. Autoimmun Rev. (2015) 14(6):498–502. doi: 10.1016/j.autrev.2015.01.012
53. Sciascia S, Hunt BJ, Talavera-Garcia E, Lliso G, Khamashta MA, Cuadrado MJ. The impact of hydroxychloroquine treatment on pregnancy outcome in women with antiphospholipid antibodies. Am J Obstet Gynecol. (2016) 214(2):273.e1–8. doi: 10.1016/j.ajog.2015.09.078
54. Berman J, Girardi G, Salmon JE. TNF-α is a critical effector and a target for therapy in antiphospholipid antibody-induced pregnancy loss. J Immunol. (2005) 174(1):485–90. doi: 10.4049/jimmunol.174.1.485
55. Salmon JE, Guerra M, Kim M, Ware Branch D. 1201 IMPACT study: preliminary results of a trial with a biologic to prevent preeclampsia in women with antiphospholipid syndrome. In: Lupus Nephritis. Lupus Foundation of America (2022). p. A84–5. Available online at: https://lupus.bmj.com/lookup/doi/10.1136/lupus-2022-lupus21century.83 (cited August 12, 2024).
Keywords: Systemic Lupus Erythematosus, Antiphospholipid Syndrome, pregnancy, pregnancy counselling, obstetric outcomes
Citation: Haase I and Fischer-Betz R (2024) Pregnancy planning in lupus and APS patients. Front. Lupus 2:1479881. doi: 10.3389/flupu.2024.1479881
Received: 12 August 2024; Accepted: 24 September 2024;
Published: 18 October 2024.
Edited by:
Laura Andreoli, University of Brescia, ItalyReviewed by:
Valentina Canti, Vita-Salute San Raffaele University, ItalyDina Zucchi, University of Pisa, Italy
Copyright: © 2024 Haase and Fischer-Betz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isabell Haase, aS5oYWFzZUB1a2UuZGU=
 Isabell Haase
Isabell Haase Rebecca Fischer-Betz2
Rebecca Fischer-Betz2