- Department of Rheumatology, Institute of Medicine, University of Tsukuba, Ibaraki, Japan
Systemic lupus erythematosus (SLE) presents unique challenges in pregnancy management due to the increased risk of pregnancy-related complications and potential for disease flare during pregnancy. In all SLE pregnancies, low-dose aspirin (LDA) is recommended to reduce the risk of preeclampsia, a significant pregnancy complication, despite limited evidence specifically targeting this population. This study aimed to evaluate the efficacy of LDA in improving pregnancy outcomes among patients with SLE and to explore the optimal dosage and timing of LDA administration. We conducted a retrospective single-center study including 75 pregnancies, the majority of which were planned except for three unplanned cases. Adverse pregnancy outcomes (APOs) were observed in 32 pregnancies (42.6%), with low birth weight being the most frequent (n = 25, 33.3%), followed by preeclampsia (n = 16, 21.3%). In our study with a limited sample size, no significant differences in APOs were found between the LDA-prescribed and non-prescribed groups. However, within the LDA prescribed group, earlier initiation before 6 weeks of gestation, was associated with significantly higher birth weights (p = 0.01) and lower rates of early onset preeclampsia (p = 0.04) compared to later administration. Additionally, a daily 100 mg dose was more beneficial than an 80 mg dose in improving birth weight (p = 0.002) and reducing the frequency of APOs (p = 0.01). Our study highlights the necessity of assessing individual risk when prescribing LDA in lupus pregnancies and the potential benefits of early initiation and optimal dosing of LDA in improving pregnancy outcomes.
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease that predominantly affects females of childbearing age (1). Family planning is a crucial consideration for all individuals, and those with SLE should have access to the same reproductive options as the general population. However, pregnancy in patients with SLE requires special attention due to the increased risk of pregnancy-related complications, such as preeclampsia, preterm delivery, and other adverse pregnancy outcomes (APOs), as well as the risk of disease flare (2–4). Preeclampsia is a particularly significant concern in pregnant patients with SLE, not only because of the higher risk relative to healthy individuals but also due to the difficulty in distinguishing it from flare of lupus nephritis, a manifestation of disease activity. The clinical features of these conditions can be very similar, yet they require distinct therapeutic approaches (5). Previous studies have shown that patients with lupus have a higher risk for preeclampsia, complicating in 9%–23% of pregnancies (2, 6). Additionally, the presence of active lupus nephritis further increases the risk of developing preeclampsia (7).
Given the significance of preeclampsia in pregnant patients with SLE, the prescription of low-dose aspirin (LDA) as a preventive measure is conditionally recommended for all patients with SLE, as well as for all antiphospholipid antibody (aPL) positive patients, according to the 2020 American College of Rheumatology (ACR) guideline for the management of reproductive health in rheumatic and musculoskeletal diseases (RMD) (8). This prophylactic measure is recommended to commence in the first trimester with 81 or 100 mg of daily LDA. However, the recommendation acknowledges that the quality of evidence is “very low” especially among patients with negative aPL tests, as there are no prospective studies specifically evaluating the impact of LDA therapy on pregnancy risks in patients with SLE, and the existing data are all observational (8–10). Thus, it remains uncertain whether all patients with lupus are at an elevated risk for pregnancy complications, particularly those without established risk factors such as concomitant lupus nephritis, aPL positivity, and active serological and clinical disease status (4, 11). In line with this, the European Congress of Rheumatology (EULAR) recommendations for women's health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with SLE and/or antiphospholipid syndrome (APS) advocate the use of LDA in patients with SLE who are at higher risk for preeclampsia, including those with nephritis or positive aPL (12). Adding to the inconclusive evidence on the LDA use on lupus, with slight variations among clinical guidelines, the optimal dosage and initiation timing of LDA remains unclear.
In this study, we aim to address 2 key points that arise from the limited existing evidence. First, we investigate whether LDA impacts pregnancy outcomes in patients with lupus, considering the presence of aPL positivity. Second, we seek to determine the optimal dose and initiation timing of LDA in pregnancies in patients with SLE.
Methods
This is a retrospective single center study conducted at University of Tsukuba Hospital. We included 75 pregnancies in patients with SLE who were followed at the hospital from January 2017 to January 2022. All patients fulfilled the 2019 EULAR/ACR classification criteria for SLE (13). All the clinical, serological, and pregnancy outcome data were collected from the medical records. Diagnosis of APS followed the revised Sapporo classification criteria (14) and retrospectively confirmed fulfilling 2023 ACR/EULAR classification criteria (15). Antiphospholipid antibody positivity was defined in accordance with the 2023 classification criteria, using ELISA by LSI Medience, Japan. Non-criteria antiphospholipid antibody positivity was defined as either low-titer positivity observed in one or multiple examinations or as a non-persistent single positivity (detected only once across multiple examinations conducted at least 12 weeks apart). To maintain consistency and reliability, the aPL profile was determined exclusively on the tests conducted in our hospital.
APOs included early spontaneous abortion before the 10th week of gestation, preeclampsia (early-onset: before 34 weeks of gestation, late-onset: at or after 34 weeks of gestation), preterm delivery before 37 weeks of gestation, low birth weight (less than 2,500 g), and intrauterine fetal death after 12 weeks of gestation. We analyzed pregnancy outcomes considering 2 groups: those with APS diagnosis and criteria aPL positivity, and those without, to minimize the potential interference of aPL positivity on pregnancy outcomes, given the significant impact of aPL positivity and APS diagnosis on pregnancy outcomes in patients with lupus (4, 16–18).
Statistical analysis was conducted using GraphPad Prism version 10.0 (GraphPad Software, San Diego, CA, USA). Continuous variables were described using median and interquartile range (IQR), while categorical variables were presented as frequencies and percentages. For comparing continuous variables between 2 groups, Mann-Whitney U-test was employed, due to the small sample size. Categorical variables were analyzed using Fisher's Exact test.
Approval for this study was obtained from the Clinical Research Ethics Review Committee, University of Tsukuba Hospital (approval number: H29-154). With the approval of the Clinical Research Ethics Review Committee at the University of Tsukuba Hospital, the requirement for written informed consent was waived using the opt-out method on the website (https://tsukubarheumatology.jp/), due to the retrospective and observational design of the study, which utilized only clinical data obtained through daily clinical practice.
Results
Characteristics of the study population
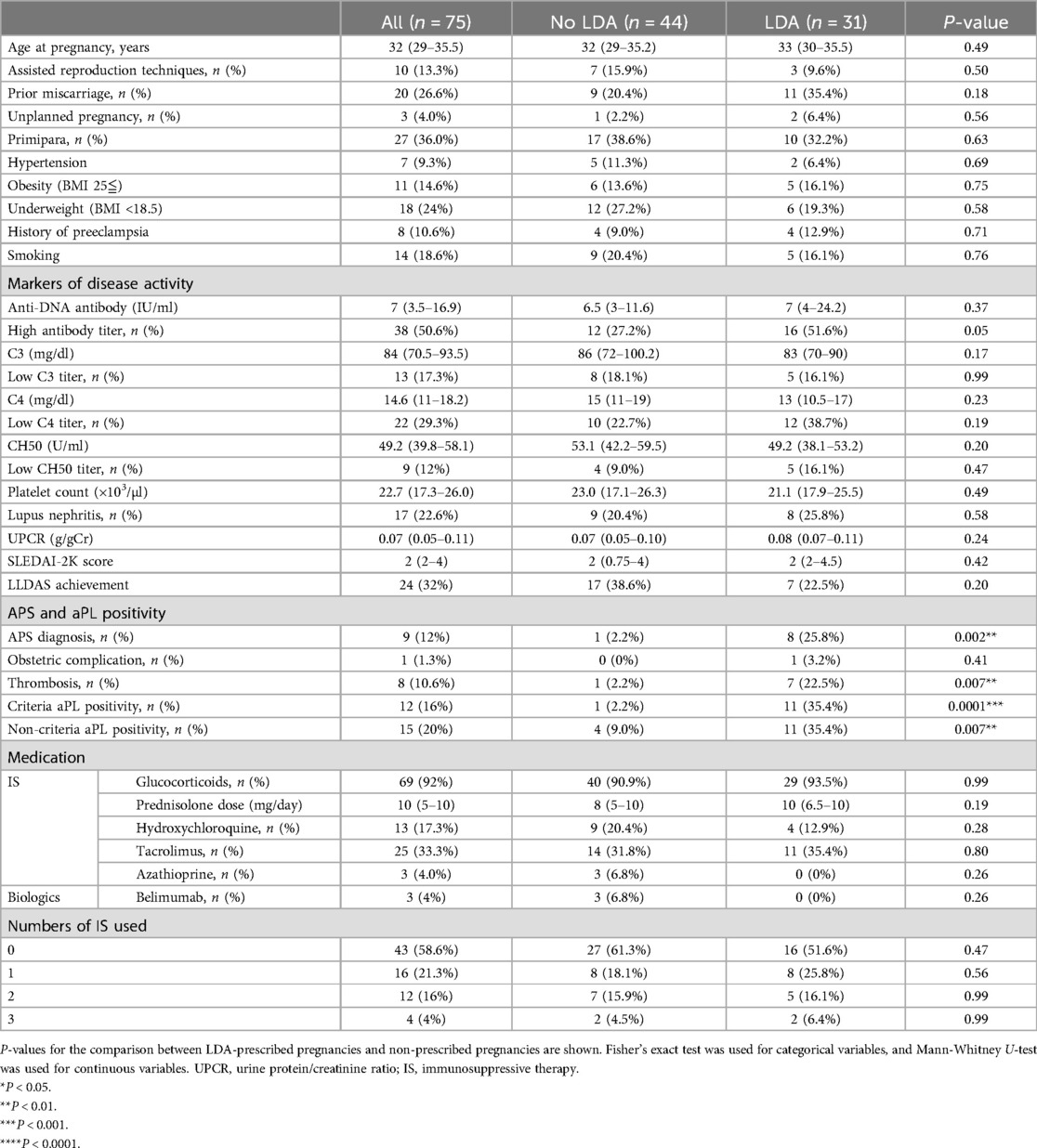
The study included 75 pregnancies from 57 Japanese women. The characteristics of the study population are shown in Table 1. The median age at conception was 32 years (IQR 29–35.5), and 10 pregnancies (13.3%) were supported by assisted reproduction therapy clinics. Among these pregnancies, 20 (26.6%) involved prior miscarriages, 8 (10.6%) had a history of preeclampsia, and 27 (36.0%) were primipara. Comorbidity risk factors included hypertension in 7 pregnancies (9.3%), obesity in 11 (14.6%), and smoking in 14 pregnancies (18.6%). In our study population, underweight (BMI <18.5) was more frequently observed than obesity. Serological activity of lupus was assessed by measuring the median anti-DNA antibody titer, which was 7 IU/ml, (IQR 3.5–16.9), the C3 level, which was 84 mg/dl (IQR 70.5–93.5), the C4 level, which was 14.65 mg/dl (IQR 11–18.25), and the CH50 level, which was 49.2 U/ml (39.85–58.17). The median platelet count was 22.7 × 103/µl (IQR 17.3–26). SLEDAI-2 K score, reflecting overall disease activity, was 2 (IQR 2–4). These results reflect that most patients conceived under well-controlled disease. However, despite stable disease activity according to SLEDAI-2 K scores and serological findings, 68% of the pregnancies were not in LLDAS remission status, primarily due to the prescribed dose of prednisolone. Most of the pregnancies were planned, except for three, necessitating treatment change when the physician became aware that the patients were pregnant. In one of these cases, the patient was exposed to mycophenolate mofetil, which was discontinued immediately upon the physician's recognition of the pregnancy. A history of lupus nephritis was present in 17 pregnancies (16%), with a median urine protein to creatinine ratio of 0.07 g/gCr (IQR 0.05–0.11). At conception, most cases of lupus nephritis were in remission, with urine protein levels below 0.5 g/gCr, except for two cases of active nephritis, both of which were in unplanned pregnancies.
Regarding medication use, glucocorticoids were prescribed in 69 pregnancies (92.0%), with a median dosage of 10 mg/day (IQR 5–10) as prednisolone equivalent. A total of 62.6% of the pregnancies were treated with more than 7.5 mg/day, which accounted for 92.1% of the cases for not achieving LLDAS status. Hydroxychloroquine was used in 13 pregnancies (17.3%). Other immunosuppressants used included tacrolimus in 25 pregnancies (33.3%), azathioprine in 3 pregnancies (4.0%), and belimumab in 3 pregnancies (4.0%). Immunosuppressants, in addition to glucocorticoids, were used in 31 pregnancies (41.3%), with 1 immunosuppressant used in 16 pregnancies (21.3%), 2 in 12 pregnancies (16%), and 3 in 3 pregnancies (4%). All immunosuppressants were initiated before conception.
APS diagnosis was made in 9 pregnancies (12%), with previous thrombotic events in 8 patients (10.6%), and obstetric complications in 1 patient (1.3%). Among the 8 patients with a history of thrombotic events, 4 pregnancies were managed with anticoagulation during pregnancy. Criteria aPL positivity was observed in 12 pregnancies (16%), while non-criteria aPL (not persistent or low titer) was observed in 15 pregnancies (20%). For the determination of the non-criteria aPL profile, one case was examined nine times, two cases were examined eight times, three cases were examined six times, two cases were examined five times, one case was examined four times, two cases were examined three times, tree cases were examined twice, and one case was examined only once. Among aPL-positive pregnancies, triple aPL positivity was significantly more frequent in pregnancies with criteria-positive aPL, while single aPL positivity was more common in those with non-criteria aPL (Supplementary Table S1). Additionally, LAC positivity was significantly higher in pregnancies with criteria-positive aPL compared to those with non-criteria aPL (p = 0.0004, Supplementary Table S1).
LDA as a prophylactic treatment during pregnancies was prescribed in 31 pregnancies (41.3%), with a dose of 81 mg in 14 pregnancies (18.6%), and 100 mg in 17 pregnancies (22.6%). Among the pregnancies prescribed with LDA, it was initiated before 6 weeks of gestation in 19 pregnancies (61.2%), with the majority (16 pregnancies) starting even before conceiving. Later initiation timing varied between 10 and 16 weeks of gestation. No significant differences were observed between the LDA and non-LDA prescribed groups in terms of markers of SLE activity, frequency of renal disease, or medication use. However, there was a significant difference in the prevalence of aPL positivity and APS diagnosis. Among the LDA prescribed group, there was a significantly higher prevalence of APS diagnosis and positivity for both criteria and non-criteria aPLs (APS: 1/44, 2.2% vs. 8/31, 25.8%, p = 0.002; criteria aPL: 1/44, 2.2% vs. 11/31, 35.4%, p = 0.0001; non-criteria aPL: 4/44, 9.0% vs. 11/31, 35.4%, p = 0.007), particularly in those with a history of thrombotic events (1/44, 2.2% vs. 7/31, 22.5%, p = 0.007). Interestingly, when analyzing the basic characteristics at conception, excluding pregnancies with lupus nephritis, which is one of the main factors related to APOs (4), no significant difference was observed in the prevalence of non-criteria aPL positivity between the LDA-prescribed and non-prescribed groups (Supplementary Table S2). Notable variability exists in both dosage and timing of LDA initiation, particularly among those commenced after 6 weeks of gestation.
Pregnancy outcome
Overall, APOs occurred in 32 pregnancies (42.6% of all pregnancies) as shown in Table 2. The most common APO observed was low birth weight, occurring in 25 pregnancies (33.3%), followed by preeclampsia in 16 pregnancies (21.3%), and preterm delivery in 13 pregnancies (17.3%). Disease flare of SLE, necessitating treatment changes, occurred in 8 pregnancies (10.6%). Among these disease flares, the recurrence of cutaneous symptoms was the most frequent manifestation, observed in 4 cases, followed by nephritis in 2 cases, worsening serological markers in 1 case, and onset of thrombotic microangiopathy (TMA) in 1 case. There was no significant difference in the frequency of total APOs (18/44, 40.9% vs. 14/31, 45.1%, p = 0.81) or observed disease flare between pregnancies that were not prescribed LDA and those that were. When analyzing the use of LDA in relation to APOs across all patients, including those with APS or of the aPL positive criteria, no significant differences in pregnancy outcomes related to LDA use were found. To account for the potential effect of APS/aPL positivity, we analyzed the total APOs after excluding 15 of those patients. Again, no significant difference was found between the LDA-prescribed and non-prescribed patients (17/42, 40.4% vs. 8/18, 44.4%, p = 0.77). While APS diagnosis and criteria aPL are associated with APOs, non-criteria aPL, especially low titer aPL, is also reported to be related to APOs (19). Accordingly, we compared APOs between patients with APS or criteria aPL positivity, those with non-criteria aPL positivity, and those with no aPL positivity (Supplementary Table S3). The results indicated that spontaneous abortion prior to 10 weeks was significantly higher in pregnancies with non-criteria aPL positivity (p = 0.03). In summary, our study, with its small sample size and primarily planned pregnancies, did not reveal a significant difference in APOs among all patients with lupus, including those with APS/criteria aPL, nor among patients with lupus without APS/criteria aPL between the LDA-prescribed and non-prescribed groups. Additionally, a more detailed analysis of the aPL profile suggested a potential contribution of non-criteria aPL to early spontaneous abortion.
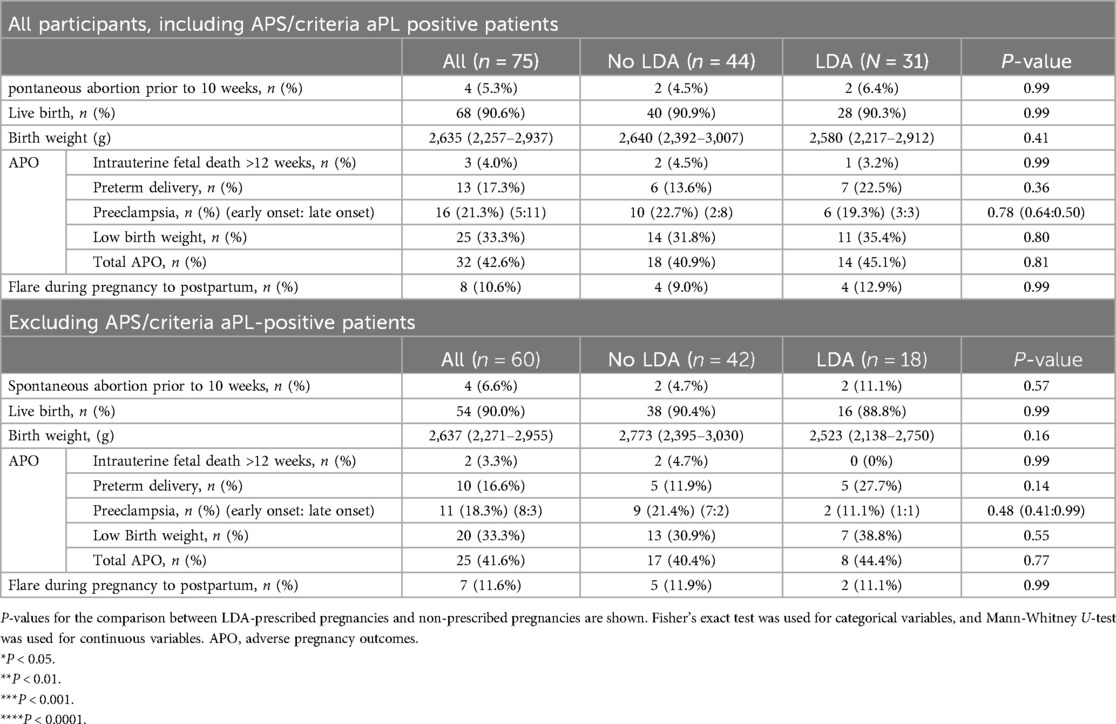
Table 2. Pregnancy outcomes among all patients with SLE and those without APS nor criteria aPL-positive patients.
Initiation timing and dosage use of LDA
Although we did not find any difference in the occurrence of APOs based on LDA usage, the beneficial role of LDA in reducing the risk of preeclampsia is well established among high-risk pregnancies, not specifically but including all SLE pregnancies (20, 21). Therefore, we further analyzed the APOs within the LDA-prescribed group to determine if the initiation timing or the dosage of LDA was associated with the occurrence of APOs.
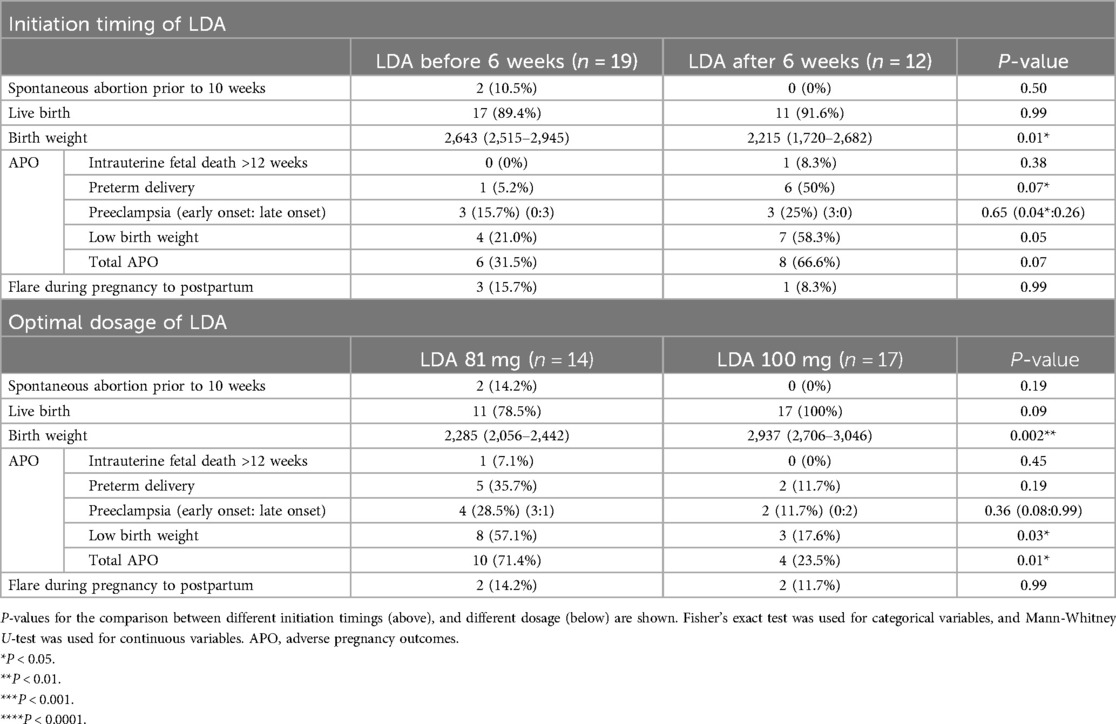
First, we analyzed the initiation timing of LDA and its relationship to APOs by dividing the patients into 2 groups: those who initiated LDA within 6 weeks of gestation, approximately when pregnancy is recognized or prior to conception, and those who started after 6 weeks (Table 3). We observed a significantly higher birth weight in the group that started LDA before 6 weeks compared to those who started after 6 weeks (2,643 g vs. 2,215 g, p = 0.01). Additionally, while the overall incidence of preeclampsia did not differ significantly between the 2 groups, early onset preeclampsia was significantly lower in the group that initiated LDA before 6 weeks compared to those who started after 6 weeks (0/19, 0% vs. 3/12, 25%, p = 0.04). Other outcomes, including the frequency of live birth, preterm delivery, low birth weight, and total APOs, did not differ significantly between the 2 groups.
Next, we examined the relationship between LDA dosage and APOs. We observed that in the group prescribed 100 mg of LDA, birth weight was significantly higher compared to the 81 mg group (2,285 g vs. 2,937 g, p = 0.002). Consistent with this finding, the incidence of low birth weight was significantly lower in the 100 mg LDA group compared to the 81 mg group (8/14, 57.1% vs. 3/17, 17.6%, p = 0.03). There were no significant differences in the occurrence of preeclampsia, including subtypes of preeclampsia, or intrauterine fetal death between the 2 dosage groups. However, the overall frequency of APOs was significantly lower in the 100 mg group compared to the 81 mg group (10/14, 71.4% vs. 4/17, 23.5%, p = 0.01) driven by a non-significant but consistently lower frequency of each individual APO analyzed. When comparing the initiation of LDA before 6 weeks of gestation with a 100 mg dosage to other usages, we observed significantly better birth weight (2,930 g vs. 2,315 g, p = 0.001), with less low birth weight (1/11, 9% vs. 10/20, 50%, p = 0.04), and less preterm delivery (0, 0% vs. 7, 35%, p = 0.03) (Supplementary Table S4).
In conclusion, our findings suggest that for pregnancies in women with lupus who are prescribed LDA, initiating this prophylactic treatment before 6 weeks of gestation may be more beneficial than the later initiation. Furthermore, a dosage of 100 mg appears to offer advantages over 81 mg in improving pregnancy outcomes.
Discussion
In this study, our primary aim was to elucidate the beneficial effect of LDA in pregnancies among patients with lupus, the majority of whom conceived without active organ involvement. Additionally, we aimed to investigate the optimal initiation timing and dosage of LDA. Our findings indicated no significant difference in the frequency of APOs among lupus pregnancies, regardless of APS diagnosis or the criteria aPL positivity. However, among lupus pregnancies prescribed LDA, earlier initiation and a dosage exceeding 81 mg appeared to be beneficial in mitigating the risk of APOs.
Behind our aim lies an ongoing discussion on whether all lupus pregnancies should receive prophylactic LDA therapy, irrespective of individual risk factors such as disease activity, lupus nephritis involvement, aPL profile, and other concomitant risk factors (4, 11). Due to our small sample size, our results did not provide clear evidence of LDA benefits or identify specific risk factors associated with LDA efficacy. Consistent with our findings, previous multicenter prospective cohort data (4), which evaluated APO risks in patients with stable lupus, found no difference in APOs irrespective of LDASA use, consistent with our findings. Similarly, another multicenter retrospective study reported comparable results among patients with SLE without high-risk factors such as lupus nephritis and aPL positivity (11). Our findings align with these studies, highlighting the heterogeneity among lupus pregnancies and the need for risk stratification to determine the necessity of LDA. This underscores the importance of disease control at conception and the necessity for risk stratification when planning pregnancies in patients with SLE, in addition to the use of LDA (8, 12).
Another important point regarding LDA requirements is the substantial disparity between LDA prescription rates and guideline recommendations (8, 12). In our study, the prescription rate was 39.6%, which is below half of the recommended level. Similarly, an international cohort study reported LDA usage at 25%, regardless of aPL status (22). While we did not conclusively demonstrate the benefits of LDA prescription among SLE pregnancies with stable disease activity, the incidence of APOs, notably preeclampsia (21.3% overall; 18.3% without APS/aPL positivity), exceeded the prevalence reported in the general population (1%–5.6%) (5, 23). This highlights the necessity for LDA administration in specific patients with SLE during pregnancy. In addition to the gap between recommendations and actual LDA prescription rates, the use of hydroxychloroquine, also recommended for management pregnancies in lupus (8), was notably low in our study (17.3%). Similar to this percentage, previous multicenter cohort data of SLE patients from Japan reported a low hydroxychloroquine prescription rate of 18.3% (24). A study investigating the reasons for this low prescription rate in Japan, compared to other countries, pointed to the influence of the 1974 withdrawal of chloroquine from the Japanese market due to cases of retinopathy (25). Given the accumulating evidence of the safety and efficacy of hydroxychloroquine in lupus pregnancies (26) both physicians and patients need to be better informed to facilitate its use among women of childbearing age.
Additionally, in our data, APS diagnosis and aPL positivity were the primary reasons for prescribing LDA in lupus patients, while non-criteria aPL positivity played a lesser role in decisions regarding LDA use in pregnancies without lupus nephritis. Given the established inclusion of LDA in standard APS management during pregnancy (8, 12), these findings are unsurprising. However, in non-criteria aPL positive patients, our results suggest that, besides aPL positivity, multiple risk factors, particularly concomitant lupus nephritis, may influence physician's decision to prescribe LDA in lupus pregnancies. Until we can accurately stratify which subset of pregnant patients with lupus truly require LDA, we believe there are significant benefits of LDA use during pregnancy based on risk-benefit consideration and cost-effectiveness (8), which warrant further investigation.
The second point highlighted by our data is the discussion regarding the optimal timing for initiating LDA and determining its dosage. Despite LDA being recommended for all SLE pregnancies by clinical guidelines such as those from The American College of Obstetricians and Gynecologists (ACOG) (27), National Institute for Health and Care Excellence (NICE) (28), International Society for the Study of Hypertension in Pregnancy (ISSHP) (29), and ACR (8), guidance on dosage and initiation timing vary slightly among guidelines. The ACOG suggests LDA 81 or 100 mg before the first trimester (27), a dosage the same as ACR, which recommends LDASA 81 or 100 mg starting from 12 to 16 weeks of gestation (8). On the other hand, NICE recommends 75 mg–150 mg from 12 weeks (28), and ISSHP advises 75–162 mg before 16 weeks (29) (Supplementary Table S5). Due to uncertainties regarding the optimal dosage of LDA, a meta-analysis comparing aspirin doses of 75–81 mg vs. 150–162 mg has been conducted, indicating that higher doses are significantly associated with a reduction in preeclampsia risk compared to lower doses (30). However, the scarcity of high-quality evidence highlights the challenges faced in conducting cohort studies and clinical trials among pregnant patients with rheumatic diseases (31). Therefore, we advocate for further research that includes diverse racial and ethnic populations to elucidate the optimal LDA dosage and its appropriate application in SLE pregnancies.
Not only the dosage of LDA but also the timing of its initiation remains a question. Clinical guidelines generally recommend initiating LDA between 12 and 16 weeks of gestation (8, 12, 27–29). However, our data indicated significant improvements in birth weight and a reduction in the frequency of early-onset preeclampsia among pregnancies that began LDA before 6 weeks of gestation compared to those with later initiation. Considering the distinct pathophysiology of early-onset preeclampsia in contrast to late-onset preeclampsia, where early-onset preeclampsia is primarily due to defective placentation, and placental development begins as early as the end of third week post-fertilization (23, 32), it may be reasonable to initiate preventative therapy early in high-risk pregnancies related to placentation issues. This, however, requires further investigation. Furthermore, the importance of preconception care among lupus pregnancies, as well as planned pregnancies (8, 12), potentially facilitates early LDA initiation and improves compliance.
This study has several limitations. First, the data only includes Japanese patients. Racial disparities are one of the important factors that influence disease activity among patients with lupus and pregnancy outcomes (1, 27). Although we highlighted several important points, further studies employing more diverse populations are necessary. Second, the study's small sample size and its single-center, retrospective design limits its generalizability. The small sample size made it challenging to accurately adjust for individual risk factors across multiple pregnancies included in the study. For instance, multiple occurrences of preeclampsia in the same patient may have contributed to the high rate of preeclampsia observed in our data. Additionally, although numerous known risk factors are associated with APOs in lupus pregnancies, we were unable to account for them through multivariable analysis due to the limited sample size. Instead, we performed analyses excluding the most significant factors, such as lupus nephritis, APS, and criteria aPL positivity. Consequently, the results should be interpreted with caution due to the small sample size available for comparison. Furthermore, our study lacked specific criteria for physician's decisions regarding LDA prescription and dosage. Beyond APS and criteria aPL positivity, the reasons for LDA prescription were unclear, along with the low prescription rates. These real-world practice conditions, including low hydroxychloroquine prescription rates, and insufficient steroid tapering, may have also biased the results.
Despite these substantial limitations, which challenge the generalizability of our findings, we believe our study addresses a critical and realistic issue in lupus pregnancy management. To the best of our knowledge, this is the first report assessing the optimal dose and initiation timing for managing lupus pregnancies. Larger studies are needed to validate these findings, which we believe will be meaningful in optimizing care and enhancing outcomes for lupus pregnancy.
In summary, our findings highlight 2 key points. First, the beneficial effects of LDA on all pregnancies among patients with lupus were not conclusively identified, suggesting the critical need for individualized risk stratification. Second, while further investigation is warranted, our data suggest that exploring the optimal dosage and timing of LDA administration may enhance pregnancy outcomes in patients with SLE. Future research should therefore focus on refining these variables to inform more personalized treatment strategies and improve management approaches for pregnancies in patients with SLE.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Clinical Research Ethics Review Committee, University of Tsukuba Hospital, (approval number: H29-154). The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because because of the retrospective and observational design that used only clinical data obtained through daily clinical practice.
Author contributions
SA: Writing – review & editing, Writing – original draft, Visualization, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal Analysis, Data curation, Conceptualization. HT: Writing – review & editing, Supervision, Funding acquisition. MY: Writing – review & editing, Data curation. AO: Writing – review & editing, Data curation. AK: Writing – review & editing, Data curation. HM: Writing – review & editing, Data curation. HA: Writing – review & editing, Data curation. YK: Writing – review & editing, Data curation. IM: Writing – review & editing, Supervision, Funding acquisition, Data curation.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported in part by Health and Labour Sciences Research Grants for research on intractable diseases (Research Team for Autoimmune diseases, Program Grant Number 23FC1017) from the Ministry of Health, Labour and Welfare (MHLW) of Japan. This work was also supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology and Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 23K19583, 24K19232.
Acknowledgment
The authors thank Thomas Mayers for revising the English of our manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/flupu.2024.1470870/full#supplementary-material
References
1. Hoi A, Igel T, Mok CC, Arnaud L. Systemic lupus erythematosus. Lancet. (2024) 403(10441):2326–38. doi: 10.1016/S0140-6736(24)00398-2
2. Clowse ME, Jamison M, Myers E, James AH. A national study of the complications of lupus in pregnancy. Am J Obstet Gynecol. (2008) 199(2):127.e1–6. doi: 10.1016/j.ajog.2008.03.012
3. Mehta B, Jannat-Khah D, Glaser KK, Luo Y, Sammaritano LR, Branch DW, et al. Fetal and maternal morbidity in pregnant patients with lupus: a 10-year US nationwide analysis. RMD Open. (2023) 9(1):e002752. doi: 10.1136/rmdopen-2022-002752
4. Buyon JP, Kim MY, Guerra MM, Laskin CA, Petri M, Lockshin MD, et al. Predictors of pregnancy outcomes in patients with lupus: a cohort study. Ann Intern Med. (2015) 163(3):153–63. doi: 10.7326/M14-2235
5. Tarter L, Bermas BL. Expert perspective on a clinical challenge: lupus and pregnancy. Arthritis Rheumatol. (2024) 76(3):321–31. doi: 10.1002/art.42756
6. Chen YJ, Chang JC, Lai EL, Liao TL, Chen HH, Hung WT, et al. Maternal and perinatal outcomes of pregnancies in systemic lupus erythematosus: a nationwide population-based study. Semin Arthritis Rheum. (2020) 50(3):451–7. doi: 10.1016/j.semarthrit.2020.01.014
7. Yang H, Liu H, Xu D, Zhao L, Wang Q, Leng X, et al. Pregnancy-related systemic lupus erythematosus: clinical features, outcome and risk factors of disease flares—a case control study. PLoS One. (2014) 9(8):e104375. doi: 10.1371/journal.pone.0104375
8. Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse MEB, Lockshin MD, et al. 2020 American college of rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Rheumatol. (2020) 72(4):529–56. doi: 10.1002/art.41191
9. Imbasciati E, Tincani A, Gregorini G, Doria A, Moroni G, Cabiddu G, et al. Pregnancy in women with pre-existing lupus nephritis: predictors of fetal and maternal outcome. Nephrol Dial Transplant. (2009) 24:519–25. doi: 10.1093/ndt/gfn348
10. Duley L, Meher S, Hunter KE, Seidler AL, Askie LM. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. (2019) 10:CD004659. doi: 10.1002/14651858.CD004659.pub3
11. Tani C, Zucchi D, Haase I, Gerosa M, Larosa M, Cavagna L, et al. Impact of low-dose acetylsalicylic acid on pregnancy outcome in systemic lupus erythematosus: results from a multicentre study. Lupus Sci Med. (2022) 9(1):e000714. doi: 10.1136/lupus-2022-000714
12. Andreoli L, Bertsias GK, Agmon-Levin N, Brown S, Cervera R, Costedoat-Chalumeau N, et al. EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis. (2017) 76(3):476–85. doi: 10.1136/annrheumdis-2016-209770
13. Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European league against rheumatism/American college of rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. (2019) 71(9):1400–12. doi: 10.1002/art.40930
14. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. (2006) 4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x
15. Barbhaiya M, Zuily S, Naden R, Hendry A, Manneville F, Amigo MC, et al. ACR/EULAR APS classification criteria collaborators. 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Ann Rheum Dis. (2023) 82(10):1258–70. doi: 10.1136/ard-2023-224609
16. Venturelli V, Abrantes AM, Rahman A, Isenberg DA. The impact of antiphospholipid antibodies/antiphospholipid syndrome on systemic lupus erythematosus. Rheumatology(Oxford. (2024) 63(SI):SI72–85. doi: 10.1093/rheumatology/kead618
17. Larosa M, Le Guern V, Guettrot-Imbert G, Morel N, Abisror N, Morati-Hafsaoui C, et al. Evaluation of lupus anticoagulant, damage, and remission as predictors of pregnancy complications in systemic lupus erythematosus: the French GR2 study. Rheumatology (Oxford). (2022) 61(9):3657–66. doi: 10.1093/rheumatology/keab943
18. Huang J, Zhu Q, Wang B, Wang H, Xie Z, Zhu X, et al. Antiphospholipid antibodies and the risk of adverse pregnancy outcomes in patients with systemic lupus erythematosus: a systemic review and meta-analysis. Expert Rev Clin Immunol. (2024) 20(7):793–801. doi: 10.1080/1744666X.2024.2324005
19. Pregnolato F, Gerosa M, Raimondo MG, Comerio C, Bartoli F, Lonati PA, et al. EUREKA algorithm predicts obstetric risk and response to treatment in women with different subsets of anti-phospholipid antibodies. Rheumatology (Oxford). (2021) 60(3):1114–24. doi: 10.1093/rheumatology/keaa203
20. Rolnik DL, Wright D, Poon LC, O'Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. (2017) 377(7):613–22. doi: 10.1056/NEJMoa1704559
21. Henderson JT, Vesco KK, Senger CA, Thomas RG, Redmond N. Aspirin use to prevent preeclampsia and related morbidity and mortality: updated evidence report and systematic review for the US preventive services task force. JAMA. (2021) 326(12):1192–206. doi: 10.1001/jama.2021.8551
22. Mendel A, Bernatsky SB, Hanly JG, Urowitz MB, Clarke AE, Romero-Diaz J, et al. Low aspirin use and high prevalence of pre-eclampsia risk factors among pregnant women in a multinational SLE inception cohort. Ann Rheum Dis. (2019) 78(7):1010–2. doi: 10.1136/annrheumdis-2018-214434
23. Dimitriadis E, Rolnik DL, Zhou W, Estrada-Gutierrez G, Koga K, Francisco RPV, et al. Pre-eclampsia. Nat Rev Dis Primers. (2023) 9(1):8. doi: 10.1038/s41572-023-00417-6
24. Saito M, Yajima N, Yanai R, Tsubokura Y, Ichinose K, Yoshimi R, et al. Prevalence and treatment conditions for hypertension and dyslipidaemia complicated with systemic lupus erythematosus: a multi-centre cross-sectional study. Lupus. (2021) 30(7):1146–53. doi: 10.1177/09612033211006790
25. Manabe A, Sada RM, Miyake H, Akebo H, Tsugihashi Y, Hatta K. An observational study to identify causative factors for not using hydroxychloroquine in systemic lupus erythematosus. Sci Rep. (2024) 14(1):7750. doi: 10.1038/s41598-024-58463-3
26. Schreiber K, Giles I, Costedoat-Chalumeau N, Nelson-Piercy C, Dolhain RJ, Mosca M, et al. Global comment on the use of hydroxychloroquine during the periconception period and pregnancy in women with autoimmune diseases. Lancet Rheumatol. (2023) 5(9):e501–6. doi: 10.1016/S2665-9913(23)00215-1
27. ACOG committee opinion No.743: low-dose aspirin use during pregnancy. Obstet Gynecol. (2018) 132(1):e44–52. doi: 10.1097/AOG.0000000000002708
28. Hypertension in Pregnancy: Diagnosis and Management. London: National Institute for Health and Care Excellence (NICE) (2019).
29. Magee LA, Brown MA, Hall DR, Gupte S, Hennessy A, Karumanchi SA, et al. The 2021 international society for the study of hypertension in pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. (2022) 27:148–69. doi: 10.1016/j.preghy.2021.09.008
30. Ghesquiere L, Guerby P, Marchant I, Kumar N, Zare M, Foisy MA, et al. Comparing aspirin 75–81 mg vs 150–162 mg for prevention of preterm preeclampsia: systematic review and meta-analysis. Am J Obstet Gynecol MFM. (2023) 5(7):101000. doi: 10.1016/j.ajogmf.2023.101000
31. Schreiber K, Graversgaard C, Hunt BJ, Wason JMS, Costedoat-Chalumeau N, Aguilera S, et al. Challenges of designing and conducting cohort studies and clinical trials in populations of pregnant people. Lancet Rheumatol. (2024) 6:e560–72. doi: 10.1016/S2665-9913(24)00118-8.38876128
Keywords: pregnancy, adverse pregnancy outcome, low-dose aspirin, systemic lupus erythematosus, antiphospholipid antibody
Citation: Abe S, Tsuboi H, Yagishita M, Ohyama A, Kitada A, Miki H, Asashima H, Kondo Y and Matsumoto I (2024) Low-dose aspirin in systemic lupus erythematosus pregnancy: impact on pregnancy outcomes and optimal management. Front. Lupus 2:1470870. doi: 10.3389/flupu.2024.1470870
Received: 26 July 2024; Accepted: 16 September 2024;
Published: 26 September 2024.
Edited by:
Guilherme Ramires De Jesús, Rio de Janeiro State University, BrazilReviewed by:
Amanda Eudy, Duke University, United StatesGerard Espinosa, Hospital Clinic of Barcelona, Spain
Copyright: © 2024 Abe, Tsuboi, Yagishita, Ohyama, Kitada, Miki, Asashima, Kondo and Matsumoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroto Tsuboi, aGlyb3RvLXRzdWJvaUBtZC50c3VrdWJhLmFjLmpw
 Saori Abe
Saori Abe Hiroto Tsuboi
Hiroto Tsuboi Mizuki Yagishita
Mizuki Yagishita Haruka Miki
Haruka Miki Yuya Kondo
Yuya Kondo Isao Matsumoto
Isao Matsumoto