- 1Division of Rheumatology and Clinical Immunology, School of Medicine, University of Pittsburgh, Pittsburgh, PA, United States
- 2Division of Rheumatic Diseases, University of Texas Southwestern Medical Center, Dallas, TX, United States
- 3Divisions of Rheumatology, Pediatric Rheumatology, University of Chicago Medicine, Chicago, IL, United States
- 4Division of Rheumatology, Weill Cornell Medicine, Cornell University, New York, NY, United States
- 5Division of Rheumatology, School of Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, United States
Pregnant women with rheumatic and musculoskeletal diseases (RMDs) have a higher risk of adverse pregnancy and perinatal outcomes compared to those without RMDs. Although evidence-based guidelines have been developed for the reproductive health care and management of these individuals, multiple areas of uncertainty exist around the diagnosis and treatment of pregnant patients with confirmed or suspected RMDs. We present a series of outpatient cases that address areas of uncertainty in the field of reproductive rheumatology. Expert opinions were elicited from rheumatologists who have expertise in the reproductive health of individuals with RMDs to build new understanding around diagnosis or treatment approaches. The cases focused on the interpretation of antiphospholipid antibodies in various clinical scenarios, diagnosis and management of nephrotic-range proteinuria during pregnancy, and the use of tumor necrosis factor inhibitors during pregnancy. Our objective was not to replace existing guidelines and classification criteria but rather to provide a range of expert opinions that rheumatologists might consider when tailoring treatment and care for patients, particularly in challenging situations with limited data.
Introduction
While many pregnant individuals with rheumatic and musculoskeletal diseases (RMDs) experience safe and healthy pregnancies, they have a higher risk of experiencing adverse pregnancy outcomes than individuals without RMDs, including preeclampsia, preterm birth, and maternal and fetal mortality (1). To enhance reproductive outcomes among individuals with RMDs and to standardize safe and effective treatment approaches during pregnancy, the European League Against Rheumatism (EULAR), the British Society for Rheumatology (BSR), and the American College of Rheumatology (ACR) have developed evidence-based guidelines for reproductive health (2–4). The ACR and EULAR have also codeveloped classification criteria for systemic lupus erythematosus (SLE) and antiphospholipid syndrome (APS), both of which are associated with high risk of pregnancy morbidity. APS in particular may co-occur with and complicate multiple RMDs (5, 6). In addition, the United States Preventive Services Task Force (USPSTF) has published a guideline for the use of low-dose aspirin to prevent preeclampsia in people with risk factors such as systemic lupus erythematosus and antiphospholipid syndrome (7).
However, guidelines and classification criteria do not—and cannot—address all of the real-world clinical scenarios that affect the care of pregnant individuals with RMDs. In clinical practice, rheumatologists may be confronted by challenging scenarios in the “gray zone” of management, without high-quality evidence or guidelines to support their medical decision-making. In this study, we present several cases from the outpatient clinical setting that address areas of uncertainty in the field of reproductive rheumatology. Rheumatologists with expertise in reproductive rheumatology served as clinical consultants and provided feedback about how they might approach the cases in their own clinical practices. Our objective was not to reach a consensus on the diagnosis or management of each case but to present different expert opinions that an outpatient rheumatologist might consider when developing their own diagnostic or treatment plans for pregnant patients with RMDs.
Materials and methods
The University of Pittsburgh Institutional Review Board deemed this study as exempt. Four rheumatologists were recruited via referral sampling based on their expertise in reproductive rheumatology, including clinical care and research related to pregnancy in the RMDs (LS, BB, CE, JZ); participation as co-investigators in the multi-site Maternal Autoimmune Disease Research Alliance (MADRA) Registry (LS, BB, CE, JZ); co-authorship of the 2020 ACR Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases, including service as guidelines chair (LS, BB); and dual certification in both pediatric and adult rheumatology (CE). These experts practice in academic rheumatology centers representing the northeast, southern, western, and midwestern regions of the United States.
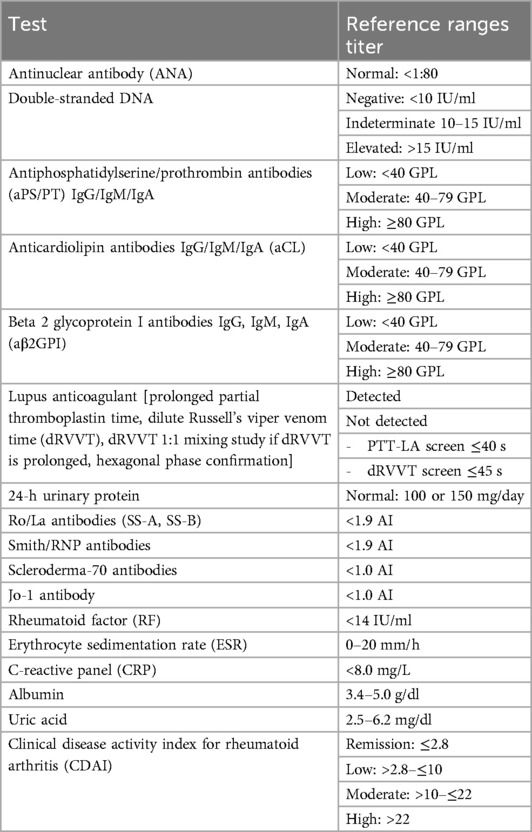
Cases and clinical questions were informed by (1) the PI's (MBT) clinical practice, a specialized reproductive health and pregnancy-focused rheumatology clinic in the UPMC healthcare system (Pittsburgh, PA); (2) case presentations at the 12th International Conference on Reproduction, Pregnancy, and Rheumatic Diseases and other regional and national meetings; and (3) feedback from co-authors and other rheumatology colleagues. Cases were edited for simplicity and clarity, with the goal of highlighting one or two key topics of clinical uncertainty for discussion. The cases were circulated to the experts for review prior to a virtual group session. During the session, the PI presented each case as well as a series of clinical questions about diagnosis, management, or treatment. Twenty minutes of discussion were maximally allocated to each case. The session concluded with a discussion of other “gray zone” areas in the outpatient management of patients with RMDs. Cases, clinical questions, and expert opinions are presented in the Results section. Reference ranges are presented in Table 1.
Results
Case 1: does this pregnant patient have a high risk of obstetric antiphospholipid syndrome (OAPS) and require anticoagulation?
Interpreting antiphospholipid antibodies of unclear significance in a pregnant patient with recurrent pregnancy loss
CC: E.G. is a 25-year-old female, currently pregnant at 8 weeks of gestation (G3P020), who has a history of recurrent pregnancy loss. She has been healthy and is prescribed only a prenatal vitamin. She is referred to you because of abnormal labs ordered by her obstetrician:
• Antinuclear antibody (ANA): 1:80 speckled pattern
• Anticardiolipin antibody (aCL) IgM: 24.4 GPL
Obstetric History
• G3P0020
○ Three total pregnancies
▪ 0 term births, 0 preterm births, 2 miscarriages, 0 living children
▪ Currently pregnant
• Pregnancy 1: anembryonic pregnancy
• Pregnancy 2: intrauterine fetal demise at 25 weeks of gestation
○ Cause of death unknown
○ Pathology done outside the hospital: hemorrhagic findings were observed in the fetal lung, but no additional findings were reported.
Maternal Labs
• ANA 1:80 speckled
• Double-stranded (dsDNA) negative
• Extractable nuclear antigens negative
○ Ro/La (SSA/B)
○ Smith/RNP antibodies
○ SCL-70
○ JO-1
• Complements normal
• Antiphospholipid syndrome (APS) workup conducted 12 weeks apart:
○ aCL IgM 24.4 GPL, then 25.7 GPL 12 weeks later
▪ aCL IgG and IgA negative
○ Lupus anticoagulant (LAC) negative
○ Beta-2-glycoprotein I (B2GPI) antibodies negative
Review of Systems and Physical Exam
• Feels well, but tearful and anxious about losing another pregnancy
• Vitals and physical exam normal
Update
• After the initial visit, a physician colleague orders antiphosphatidylserine antibodies with the following results, which remain positive on serial testing. You are asked to comment on these findings and the patient's risk of OAPS.
○ Antiphosphatiydylserine IgG > 150 GPL
○ Antiphosphatidylserine IgM > 150 GPL
In this case, the patient, who was pregnant, had previously experienced two recent and consecutive pregnancy losses—one pre-fetal loss (<10 weeks) and one fetal death at 25 weeks of gestation—without pathologic evidence of placental insufficiency. The patient had persistently positive anticardiolipin IgM antibodies at low titers. However, the patient also had high-titer antiphosphatidylserine (aPS) antibodies, which are not currently included in the classification criteria for antiphospholipid syndrome (APS). The clinical question was whether this patient should be treated for APS during the current pregnancy to prevent another pregnancy loss.
Antiphospholipid syndrome (APS) is an autoimmune condition that is associated with the presence of antiphospholipid antibodies [aPL, i.e., lupus anticoagulant (LAC) and/or anticardiolipin (aCL) or anti-beta-2-glycoprotein I IgG/IgM antibodies (aβ2GPI)] and evidence of thrombosis across the vasculature, including placental insufficiency and complement activation (8, 9). As detailed in the 2023 ACR/EULAR antiphospholipid syndrome (APS) classification criteria, pregnancy morbidity can be a manifestation of obstetric APS (OAPS), with clinical features that include pre-fetal death before 10 weeks of gestation, fetal death between 10 and 15 weeks or 16 and 34 weeks of gestation without preeclampsia or placental insufficiency, or preeclampsia or placental insufficiency with or without severe features and with or without fetal death (5). Points are assigned to each clinical and laboratory domain, and at least three points from each clinical or laboratory domain—with a total score of at least 6—are required to meet the classification criteria for APS.
Among the laboratory tests in the APS classification criteria, LAC explains most of the thrombotic risk attributed to antiphospholipid antibodies (10–13), and even if treated, people with positive vs. negative LAC have a 30% greater likelihood of adverse pregnancy outcomes (14). Other serologies associated with APS include positive aCL or anti-beta-2-glycoprotein I antibodies (5, 13). aCL or aβ2GPI IgG antibodies may be associated with pregnancy losses irrespective of titer; in contrast, the clinical significance of isolated aCL and aβ2GPI IgM isotypes is unclear and has not consistently been demonstrated to increase the risk of adverse pregnancy outcomes, particularly when in low or medium titers (<80 GPL) (14–17).
While not included in the current ACR/EULAR classification criteria for APS, antiphosphatidylserine/prothrombin (aPS/PT) antibodies are antiphospholipid antibodies that have been evaluated for potential significance in the diagnosis of APS (18). aPS/PT antibodies are detected by solid-phase assays and include antibodies to phosphatidylserine–prothrombin and prothrombin alone. These antibodies are less sensitive to anticoagulation and acute phase proteins than the LAC, although newer laboratory approaches can remove anticoagulants from plasma and increase the reliability of the LAC (19). Conflicting estimates of the sensitivity and specificity of aPS/PT antibodies have been reported in the literature, and while some prospective studies demonstrate an association of aPS/PT with thrombosis, small cohort sizes and restriction to single centers have limited the generalizability of findings (18, 19). In a communication from the International Society on Thrombosis and Haemostasis Scientific and Standardization Committee, aPS/PT antibodies were not felt to provide additional value beyond LAC in the diagnosis of thrombotic APS but were felt to potentially add value to the diagnosis of thrombotic APS as compared to aCL and antibodies—particularly when present in high titers (19).
Antiphosphatidylserine/prothrombin antibodies may also add value to the diagnosis of OAPS. In a retrospective study of 653 controls and patients with OAPS and thrombotic APS, aPS/PT IgG or IgM were present in 40.5% and 32.1% of patients with OAPS, respectively (19). In regression analyses, OAPS was significantly associated with aPS/PT IgG and/or IgM, even after the addition of aCL IgG, aCL IgM, aβ2GPI IgG, or aβ2GPI IgM to models. However, when adjusting for the presence of LAC, the associations between aPS/PT IgG or IgM and OAPS became insignificant. These data suggested a possible added benefit of aPS/PT IgG and IgM in the workup of OAPS—particularly if LAC is negative. The associations of aPS/PT antibodies with APS were also evaluated in a study of Chinese patients with OAPS, APS, and seronegative APS, many of whom had concomitant autoimmune or connective tissue diseases (20). The study reported that aPS/PT antibodies were detected in approximately 50% of seronegative APS patients, and over 90% of patients with LAC positivity also had positive aPS/PT IgG and IgM. In addition, aPS/PT IgG and IgM were strongly associated with fetal loss (OR 10.41, 95% CI: 5.47–21.63). These data cumulatively suggest that aPS/PT IgG and IgM antibodies are associated with adverse pregnancy outcomes; are correlated with LAC, which is the most robust test at present for diagnosing APS; and may potentially be useful in cases in which some index of suspicion for APS exists but other aPLs are negative or inconclusive.
Classification criteria are developed, in part, to standardize a patient population for research studies (21); thus, the ACR/EULAR criteria may have limited utility in the diagnosis of APS in real-world clinical practice. While many individuals affected by pregnancy loss may not meet the classification criteria for OAPS, other tests in the “gray zone” of diagnosis may help to support a clinical diagnosis of OAPS, which, if treated, can increase the likelihood of improved pregnancy outcomes (13). Recurrent and/or late-stage pregnancy losses can be devastating to patients and families, particularly when there is no clear etiology of fetal death. We sought to understand if and how reproductive rheumatologists used low-titer aCL and high-titer aPS/PT antibodies to evaluate a person with recurrent fetal loss.
Expert opinion
How would you approach determining if antiphospholipid syndrome had been a factor in the patient's two pregnancy losses?
Experts did not attribute the clinicopathologic finding of pulmonary hemorrhage on prior fetal autopsy to the patient's antiphospholipid antibodies. However, experts overwhelmingly felt that a review of the placental pathology of the prior pregnancy would have been important in determining if the antiphospholipid syndrome could have contributed to the earlier pregnancy losses. Experts recommended requesting a second histologic opinion from an academic-affiliated pathologist, as the pathologic review of the current case was limited. If no lesions characteristic of APS were observed in the placental pathology, experts felt that the likelihood of APS complicating the prior pregnancy was low despite the presence of high-titer aPS/PT antibodies.
Would you recommend anticoagulation to a pregnant person with recurrent, low-titer antiphospholipid antibodies and recurrent pregnancy loss?
Experts did not feel the patient's low-titer aCL IgM was a clinically significant finding and would not have recommend anticoagulation based on this individual result.
Do you check antiphosphatidylserine/prothrombin antibodies and in what contexts?
Most experts would consider checking aPS/PT antibodies in a patient with recurrent pregnancy losses with placental pathology suggestive of vascular insufficiency or thrombosis and who otherwise had negative lupus anticoagulant and subthreshold negative aCL and aβ2GPI. One expert routinely checked for antichromatin antibody levels and aPS/PT antibodies among patients whom they assessed for connective tissue disease and recurrent pregnancy losses. However, other experts rarely tested for aPS/PT antibodies as part of their approach for determining the etiology of pregnancy loss, unless clinical suspicion for APS was already high.
How would you treat this patient?
All experts would recommend low-dose aspirin (LDASA) at 12 weeks of gestation for primary prevention of preeclampsia for this patient with recurrent pregnancy loss and high-titer aPS/PT antibodies. Most experts did not feel that this patient required low-molecular-weight heparin (LMWH) based on her serologic profile and in the absence of compelling evidence of placental insufficiency. However, given the patient's devastating prior pregnancy outcomes, two experts felt that prophylactic-dose LMWH could have been considered and recommended a shared decision-making conversation with the patient to discuss the potential risks and benefits of treatment. Experts would not have recommended anticoagulation if the patient had high-titer aPS/PT antibodies without a history of recurrent fetal losses or other clinical evidence of APS.
Case 2: did this person have obstetric antiphospholipid syndrome and require anticoagulation in a future pregnancy?
Treatment decisions for a patient with positive antiphospholipid antibodies and a history of abnormal placental pathology who is planning for a future pregnancy
CC: D.C. is a 39-year-old, non-pregnant (G1P1) female, referred by her obstetrician for pregnancy planning. She was diagnosed with undifferentiated connective tissue disease (UCTD) by another rheumatologist due to sicca, fatigue, myalgias, arthralgias, and the following labs:
• ANA 1:1,280 speckled pattern; aβ2GPI antibody IgM, 59.7 GPL; aCL IgM, 25.8 GPL
• Negative or normal extractable nuclear antigens, rheumatoid factor (RF), complements, serum protein electrophoresis (SPEP), kappa lambda light chains, complete blood count (CBC), complete metabolic pattern (CMP), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP)
• Normal eye exam (negative Schirmer/dry eye testing with ophthalmologist)
• Deferred minor salivary gland biopsy, sudomotor testing
• Multiple medication intolerances, could not tolerate brand or generic hydroxychloroquine
Obstetric History
• G1P1001
• 1 term birth, 0 preterm births, 0 abortions, 1 living child delivered 1 year ago
• Prior pregnancy: Healthy term pregnancy. Spontaneous vaginal delivery at 38 weeks
• Birthweight (21st growth percentile), normal APGAR. The child is healthy.
• Pathology: Obtained as the placenta appeared small in size. Features of maternal malperfusion were observed, with a hypoplastic placenta in the <3rd percentile of size, hypermature chronic villi, increased syncytial knots, delayed maturation, and involving up to 20% of villi examined. Narrow inserting three-vessel umbilical cord. No thrombi or infarcts were observed.
Maternal Labs
• ANA 1:1,280 speckled
• dsDNA negative
• Extractable nuclear antigens negative
○ Ro/La (SSA/B)
○ Smith/RNP
○ SCL-70
○ JO-1
• CBC, CMP, complements, RF, ESR, CRP, SPEP negative or normal
• Anti-beta-2-glycoprotein I (aβ2GPI) IgM 60.0 GPL on two occasions 12 weeks apart
○ aβ2GPI IgG, IgA negative
• Anticardiolipin (ACL) IgM 26.0 GPL on two occasions 12 weeks apart
○ ACL IgG and IgA negative on two occasions 12 weeks apart
• Lupus anticoagulant (LAC) negative on two occasions 12 weeks apart
Review of Systems and Physical Exam
• Multiple complaints on review of systems, including dysesthesias, fatigue, cognitive dysfunction, arthralgias, myalgias, hair shedding, and migraines
• Vitals and physical exam normal. Sensitive to light touch throughout the exam.
Update
• Another clinician rechecks antiphospholipid antibodies. Anti-beta-2-glycoprotein I and anticardiolipin antibodies are now negative. Testing was done in the same laboratory network but at a different laboratory than the prior testing. LAC remains negative.
In this “gray zone” case, the patient had a presumed diagnosis of undifferentiated connective tissue disease (UCTD) and was planning for a future pregnancy in the context of a prior term pregnancy with normal-weight neonate and placental findings suggestive of malperfusion or insufficiency. The patient also tested positive for moderate-titer aβ2GPI IgM antibodies and low-titer aCL IgM antibodies on two of three occasions.
UCTD is a condition in which a person has symptoms and/or laboratory tests that are suggestive of but do not meet the threshold for diagnosis of a specific connective tissue disease (CTD) such as systemic lupus erythematosus (SLE) or Sjogren's disease (22). UCTD may be associated with abnormal serologic findings, and in one review, 10%–24.8% of individuals with UCTDs have aPLs (23). Up to 30% of patients with UCTD, over time, will develop a systemic CTD, including diseases such as systemic lupus erythematosus, which has the potential to worsen during pregnancy (24).
As described in Case 1, placental insufficiency is included as a clinical domain in the 2023 ACR/EULAR antiphospholipid syndrome classification criteria and defined as estimated fetal weight of less than the 10th percentile for gestational age or postnatal birth weight less than the 10th percentile for gestational age in the absence of genetic conditions or fetal–neonatal syndromes associated with growth restriction. In addition, one or more of the following features are required: (1) fetal hypoxemia: abnormal or non-reassuring fetal surveillance tests or abnormal Doppler flow velocimetry waveform analysis; (2) severe intrauterine fetal growth restriction, defined as estimated fetal or postnatal birth weight of <3rd percentile for gestational age; (3) oligohydramnios; (4) maternal vascular malperfusion on placental histology: placental thrombosis/infarction, inadequate remodeling of the uterine spiral arteries, decreased vascular synctitial membranes, increased syncytial knots, or decidual inflammation.
Not all placental lesions are specific for APS. As indicated in the ACR/EULAR APS criteria, maternal vascular malperfusion on placental histology or small placenta size is insufficiently specific to add to the classification criteria for APS. Up to 50% of all healthy pregnancies demonstrate some evidence of vascular malperfusion, with increased incidence in the general population among pregnant people of advanced maternal age (age equal to or greater than 35, such as this patient) and/or who have obesity (25, 26).
EULAR recommends preconception or first-trimester use of LDASA for asymptomatic aPL carriers, people with SLE without prior thrombotic APS or OAPS, and females with OAPS history irrespective of pregnancy status (27). Among pregnant individuals with positive aPLs, the ACR Reproductive Health Guideline conditionally recommends treatment with LDASA starting in the first trimester if no prior thromboembolism or obstetric APS but does not advocate for the use of anticoagulation (2). UCTD alone is not an indication for anticoagulation during pregnancy, although an SLE diagnosis warrants treatment with LDASA starting around 12–16 weeks for preeclampsia risk reduction (2, 7, 28).
In contrast, the ACR Reproductive Health Guideline does advocate for treatment with prophylactic-dose LMWH and LDASA among people with obstetric APS and treatment with therapeutic-dose LMWH or unfractionated heparin (UFH) and LDASA for thrombotic APS (2). LMWH and UFH are associated with relatively few health risks to the pregnant person who requires anticoagulation and do not cross the placenta (29). UFH is associated with fewer bleeding episodes, a longer half-life, lower risk of heparin-induced thrombocytopenia, and less loss of bone mineral density than fractionated heparin. Enoxaparin, a type of LMWH, is associated with lower bleeding rates than full-strength aspirin, and bleeding rates are similar between enoxaparin users and untreated controls (30, 31).
In this case, while the patient had a prior healthy pregnancy outcome, OAPS was considered given positive antiphospholipid antibodies on two occasions and abnormal placental findings. However, subsequent antiphospholipid testing was negative. We sought to understand how experts would make decisions around the patient's diagnosis and need for anticoagulation for a future pregnancy.
Expert opinion
How would you characterize this person's risk for adverse pregnancy outcome based on their possible UCTD diagnosis?
Most of the experts felt that a diagnosis of UCTD could provide more support for a diagnosis of APS; however, they felt that this patient's symptoms could also have been explained by fibromyalgia or centralized pain syndrome. Experts felt that the diagnostic testing for Sjogren's disease was incomplete. While the patient's ophthalmologic testing had not demonstrated dry eye, two experts advocated for minor salivary gland biopsy. One expert felt that if a diagnosis of Sjogren's disease was established, the addition of therapeutic-dose hydroxychloroquine could have been used to enhance pregnancy outcomes and perhaps provide a weak antithrombotic benefit to the patient given her positive aPLs. Other experts felt less confident that pregnant patients with Sjogren's disease benefitted from hydroxychloroquine during pregnancy, unless they had high-titer Ro or La antibodies; hydroxychloroquine has been suggested to reduce the incidence of congenital heart block as part of the neonatal lupus syndrome (2, 32). Experts would not have been more likely to provide anticoagulation to this patient even if Sjogren's disease were diagnosed, although several experts felt more confident that the aPLs could be clinically significant in that context.
Did this patient have obstetric APS in her first pregnancy?
Given the limited associations between aCL and aβ2GPI IgMs and adverse obstetric outcomes, experts felt that placental features of the prior pregnancy would be important in assessing the possibility of OAPS in this patient and guiding future treatment decisions. As placentas with evidence of malperfusion or hypoplasia are fairly common, even among healthy pregnancies, the pathology was not clearly suggestive of APS; however, several experts suggested that fetal growth restriction or small for gestational age birthweight (defined as birthweight <10th percentile for gestational age (33)) during the prior pregnancy would have strengthened their recommendations for anticoagulation during a subsequent pregnancy.
How would you treat this patient in a future pregnancy?
Given the positive aPLs on two of three occasions and non-specific evidence of placental malperfusion in the G1 pregnancy, all experts felt that LDASA was indicated in a subsequent pregnancy. The experts all agreed that the patient did not require anticoagulation while she was not pregnant. However, in the context of persistently elevated aPLs, experts felt that LMWH and LDASA could be appropriate treatments during a subsequent pregnancy, particularly if she was found to have a CTD with the completion of the diagnostic workup.
However, in the presence of the positive ANA, fibromyalgia, and variably elevated aPLs without objective evidence of a specific CTD—the current presentation of the patient— treatment recommendations varied between experts. Several experts did not feel that the combination of LMWH and LDASA was indicated for a subsequent pregnancy, whereas other experts felt that patient preference could guide the decision-making and anticoagulation would be reasonable if desired by the patient. Experts mentioned that other consultants, including hematologists and obstetrician-gynecologists, could also help the patient ascertain the risks and benefits of treatment or non-treatment. In this case, the patient had poor tolerance of conventional medications and generally preferred a non-pharmacologic approach.
How do you make treatment decisions when a person has had contradictory tests for antiphospholipid antibodies?
In this case, aβ2GPI and aCL antibodies were positive on two separate occasions 12 weeks apart but were negative when tested several weeks later. The negative test diminished most of the experts’ interest in treating the patient with LMWH in a subsequent pregnancy. Experts described a common challenge in the diagnosis of APS as the requirement of serial testing over at least a 12-week period, and if conducted in different laboratories, could yield variable and contradictory results due to differences in the calibrations and standards across assays. Most experts therefore advised patients to receive repeat aPL testing in the same clinical laboratory if possible.
Case 3: does this pregnant patient have lupus nephritis?
Making treatment decisions for a pregnant patient with nephrotic-range proteinuria without a renal biopsy
CC: R.L. is a 32-year-old female, currently pregnant at 18 weeks of gestation (G3P1011), who was incidentally found by her obstetrician to have proteinuria of around 3 g per day. Nephrology was consulted and ordered low-dose aspirin; however, they refused renal biopsy as they felt she was a high risk for poor postprocedural outcomes. Rheumatology was consulted to evaluate for lupus nephritis due to the following results:
• ANA 1:1,280–1:5,120 titers with cytoplasmic patterns
• Negative renal ultrasound with dopplers, no thrombus observed
Obstetric History
• G3P1011
• 1 term birth, 0 preterm births, 1 miscarriage, 1 living child
• Prior pregnancy loss: 25 weeks of gestation in 2018. Placental/fetal pathology not obtained.
• Subsequent pregnancy was complicated by gestational hypertension, but the infant was full term and normal weight. The child is healthy.
• Currently pregnant at 18 weeks of gestation.
Maternal Labs
• ANA 1:1,280–5,120 cytoplasmic pattern
• dsDNA 14 IU/ml (intermediate finding) on two occasions, negative (<10) 6 weeks later
• Remaining extractable nuclear antigens negative
○ Ro/La (SSA/B)
○ Smith/RNP
○ SCL-70
• Complements normal
• CBC normal
• Creatinine 0.4–0.6 mg/dl
• Albumin 3.0 mg/dl
• Uric acid 4.9 mg/dl
• Anti-phospholipase A2 receptor (PLA2R) antibody negative
• Antiphospholipid syndrome workup:
○ Lupus anticoagulant positive on 2 occasions
○ Anticardiolipin IgM GPL 22.5, negative on 1 occasion
○ Anti-beta-2-glycoprotein I IgM GPL 24.2, negative on 1 occasion
• Urine white blood cells and red blood cells within normal limits
Review of Systems and Physical Exam
• Feels well, no complaints
• Blood pressure 101/71, other vitals and physical exam normal with exception of body mass index of 50
Update
Proteinuria
− 16 weeks of gestation: 3,017 mg (24 h urine collection)
− 19 weeks of gestation: 2,890 mg (24 h urine collection))
− 22 weeks of gestation: 6,030 mg (24 h urine collection)
In this case, the pregnant patient, who was healthy except for an elevated BMI of 45, was incidentally found to have nephrotic-range proteinuria; subsequent workup revealed high-titer ANA, weakly positive double-stranded DNA antibodies, and persistently positive lupus anticoagulant. She had a prior pregnancy that ended in fetal death at 25 weeks of gestation, with no placental pathology or fetal autopsy performed. The clinical question was if the nephrotic-range proteinuria in this case was indicative of lupus nephritis (LN); at the patient's institution, renal biopsy was considered too high-risk to perform given her stage of pregnancy and elevated BMI.
Mild proteinuria is common in normal pregnancy and can rise from a healthy pre-pregnancy range of 0–150 mg/dl prior to 300 mg/dl during pregnancy; the highest levels of urinary protein are generally observed in the second and third trimesters. Urinary protein levels greater than 300 mg/day increase suspicion of glomerular disease (34). Preeclampsia, a hypertensive disorder of pregnancy that is associated with sustained blood pressure elevation, is a common cause of nephrotic-range proteinuria after 20 weeks of gestation (35). At any stage of pregnancy, however, proteinuria can also be secondary to focal segmental glomerulosclerosis, minimal change disease, diabetes, APS nephropathy, or systemic lupus erythematosus, among other rare diagnoses such as amyloidosis (34).
The gold standard for urinary protein quantification is the 24 h urine collection, although the protein/creatinine ratio is highly correlated with 24 h protein quantification. Nephrotic-range proteinuria includes proteinuria of greater or equal to 3.5 g/day over 24 h, hypoalbuminemia (less than 3.5 g/dl), and peripheral edema. Serum albumin concentration also decreases during pregnancy; thus some studies suggest using different cutoffs for hypoalbuminemia depending on trimester, i.e., albumin <3.1 g/dl in the first trimester, <2.6 g/dl in the second trimester, and <2.3 g/dl in the third trimester (34, 36). Hypoalbuminemia is particularly important when considering risks associated with nephrotic syndrome, such as venous thromboembolism, which can occur among up to 40% of patients with nephrotic syndrome—particularly those with membranous glomerulonephritis. Thrombotic risk appears to increase with decreasing serum albumin, particularly in individuals with serum albumin less than 2.5 g/dl (37).
Renal biopsy is a critical part of the workup of nephrotic-range proteinuria, particularly in cases with diagnostic uncertainty (38). Renal biopsy is overall a safe procedure but can be associated with microhematuria and perirenal hematoma, and the incidence of postprocedural complications ranges from 2% to 6.7% (38). Generally, renal biopsy can be safely performed on the pregnant patient prior to 25 weeks of gestation (39); however, gestational limits around renal biopsies may differ across institutions, particularly those with limited expertise in the procedure.
In this case, the rheumatology service was asked to comment on the possibility of SLE and lupus nephritis. New SLE can arise during pregnancy. Several small studies suggest that new-onset SLE tends to occur during the first and second trimesters, and LN is more commonly seen in new-onset SLE during pregnancy than SLE diagnosed in non-pregnant individuals (40). However, SLE can be challenging to diagnose during pregnancy. Complements C3 and C4 rise by 10%–50% during normal pregnancy, which can mask hypocomplementemia secondary to immune activation; erythrocyte sedimentation rate can increase by 30%–70%; mild dilutional anemia and thrombocytopenia in the range of 100–150,000/microliter are common, and mild proteinuria can be physiologic (41). Certain subtypes of LN can also be difficult to diagnose—for example, membranous glomerulonephritis is associated with nephrotic-range proteinuria, but many patients lack the systemic symptoms associated with SLE (e.g., fevers, rash, serositis, inflammatory arthritis), and renal pathology is often needed to confirm the diagnosis (42, 43).
Pregnant individuals with SLE have at least a twofold higher risk of preeclampsia than other pregnant individuals (35)—particularly individuals who have lupus nephritis—however, LN and preeclampsia can be difficult to differentiate from each other. Preeclampsia and LN can also present concurrently in the same patient—leading to greater complexity in diagnosis (41, 44). Both can be associated with hypertension, progressive renal insufficiency, hemolysis, and thrombocytopenia (41). However, as preeclampsia is a manifestation of placental insufficiency, urgent delivery of the fetus may be indicated, whereas in LN, delivery is not an approach to treatment. Uric acid levels can help to differentiate LN from preeclampsia; uric acid levels may be high in preeclampsia but are generally normal in LN (44). The ratio of the serum soluble fms-like tyrosine kinase 1 (sFLt-1) to placental growth factor (PlGF), which is not yet widely used in the United States, is an angiogenic marker that has the potential to differentiate between preeclampsia—in which the ratio is elevated—and other etiologies of hypertensive disorders of pregnancy or LN (45).
The limited availability of effective and pregnancy-compatible treatments can undermine LN management for the pregnant patient (2). As described in the ACR and EULAR guidelines for reproductive health management, mycophenolate mofetil or mycophenolic acid and cyclophosphamide—the first-line drugs for severe LN—are known teratogens (cyclophosphamide can be used in later stages of pregnancy for severe and organ-threatening disease) (2). LN treatments that are compatible with pregnancy have some limitations. High-dose steroids, typically recommended for severe LN, are immunosuppressive and, at doses of prednisone higher than 20 mg daily, can enter the fetal circulation, which may potentially lead to neonatal adrenal suppression. Hydroxychloroquine, while safe at all stages of pregnancy and not immunosuppressive, is an ineffective monotherapy for LN. Azathioprine is a second-line treatment for LN that is safe for the fetus at all stages of pregnancy but, in inflammatory bowel disease, has been associated with a rare but elevated risk of intrahepatic cholestasis of pregnancy that might warrant its discontinuation if bile acid and liver function tests are elevated; this complication has rarely been reported in patients with SLE, but requires future study (46). Calcineurin inhibitors can be used to reduce proteinuria, but their efficacy as stand-alone agents in LN or in SLE is unclear based on a paucity of data in diverse cohorts (6). Rituximab may be used in organ-threatening renal disease, but administration in the second or third trimester has been associated with reversible but months-long CD19+ B-cell depletion in the neonate (2, 47). Thus, advancing treatment in cases of diagnostic uncertainty given the potential side effects of treatment and unclear clinical outcomes can be challenging during pregnancy.
Experts were asked about how they might manage the asymptomatic pregnant patient with high-titer ANA, low or indeterminate-range dsDNA, positive lupus anticoagulant, and nephrotic-range proteinuria without renal pathology.
Expert opinion
What are your leading differential diagnoses?
Experts’ leading differential diagnoses included lupus nephritis, membranous glomerulonephritis, focal segmental glomerulosclerosis (obesity as a risk factor), ANCA vasculitis, and thrombotic microangiopathy related to APS. Experts felt it would be helpful to ascertain if the patient had proteinuria prior to the pregnancy; if not, they felt that this might increase suspicion of new-onset lupus nephritis and APS nephropathy, both of which can develop in pregnancy. Gestational cutoffs for renal biopsy varied across experts’ institutions, and across institutions, experts reported variable levels of enthusiasm among renal and interventional colleagues in facilitating renal biopsies. Overall, experts felt this case would be difficult to diagnose accurately without a renal tissue sample; however, they felt that the management of nephrotic-range proteinuria was straightforward.
How did laboratory and ultrasound testing assist with refining the differential diagnosis without biopsy, and would any additional labs be helpful?
One expert recommended checking anti-chromatin antibody level, and if positive, would consider treating as LN. PLA2R antibody, which is highly specific for idiopathic membranous nephropathy, was negative in this case, which was helpful in reducing suspicion of membranous nephropathy. Ultrasound with Doppler was normal in this case, reducing suspicion of renal vein thrombosis from APS. C1q antibodies, if orderable at the institution, could have increased suspicion for proliferative lupus nephritis.
Would you recommend any treatment to this person?
All experts recommended starting prednisone (corticosteroid) at doses between 20 mg and 60 mg daily for nephrotic-range proteinuria; one expert indicated that they would provide pulse dose steroids over a 3 day period and then transition to calcineurin inhibitors. Most experts advocated for the consideration of calcineurin inhibitors to treat proteinuria. One expert recommended checking for antichromatin antibody; if positive, they would treat with azathioprine for presumed lupus nephritis. Experts felt that hydroxychloroquine was a medication with a low risk of side effects and could be added to the treatment as lupus nephritis was on the differential. Azathioprine was considered reasonable if the suspicion of lupus nephritis was moderate or high, but experts were unsure that it would be indicated for the other diagnostic considerations.
One expert indicated that most people with proteinuria over three grams daily should receive anticoagulation with low-molecular-weight during pregnancy, no matter the etiology, due to increased thrombotic risk. Another expert indicated that if a patient's albumin level was less than 2 mg/dl in the context of nephrotic-range proteinuria, they recommended anticoagulation during pregnancy. These recommendations are consistent with published guidance (48). The patient's persistently positive lupus anticoagulant augmented experts’ strong recommendation for treatment-dose anticoagulation during pregnancy.
Case 4: should this pregnant patient with rheumatoid arthritis switch from adalimumab to certolizumab pegol for safety reasons?
Treatment decision-making around tumor necrosis factor inhibitors during pregnancy
CC: M.R. is a 25-year-old female, currently pregnant at 16 weeks of gestation (G1P0), with a history of seropositive rheumatoid arthritis (RA). She is prescribed adalimumab. Her sister-in-law, an internist, reviewed the ACR Reproductive Health Guideline and recommended switching to certolizumab from adalimumab based on the recommendations. M.R. seeks your opinion.
Past Medical History
• Diagnosed at age 20 with severe synovitis of the MCPs, PIPs, and wrists. No erosions on radiographs. She is otherwise healthy.
• Treatment history: Failed methotrexate, leflunomide. Transitioned to adalimumab 2 years ago.
• Current medications: Adalimumab taken subcutaneously every 2 weeks. Prenatal vitamin.
Review of Systems and Physical Exam
• Pain of 2 out of 10 in severity, which is stable. Forty-five minutes of morning stiffness, also stable.
• Swelling/tenderness of right metacarpophalangeal joints 2 and 3.
○ Clinical disease activity index (CDAI): 6 (low disease activity)
In this real-world case, the pregnant patient has seropositive rheumatoid arthritis (RA) treated with adalimumab. She is experiencing low but persistent disease activity. She is questioning if she should be switched to certolizumab for safety reasons now that she is pregnant.
RA disease activity improves for 40%–90% of patients during pregnancy; however, only a minority of patients experience remission, approximately 20% of women experience severe or worsening RA over pregnancy, and treatment is often needed through pregnancy to preserve functional status (49, 50). Pregnancy-compatible treatments for RA include hydroxychloroquine, sulfasalazine, prednisone, and tumor necrosis factor inhibitors (TNFi) (2). NSAIDs can be safely used before 20 weeks of gestation as per the Food and Drug Administration guidelines (51). In addition, while RA symptoms may improve during pregnancy, RA patients still have a higher risk of preeclampsia, preterm growth, and fetal growth restriction than pregnant individuals without RA (52). Treatment during pregnancy may facilitate better pregnancy outcomes among patients with RA; for example, in one study, treatment with TNFi during pregnancy was associated with increased birth weight of infants born to patients with well-controlled RA (53).
TNFi are immunosuppressive medications that increase the risk of infection in adult patients (54). TNFi can be detected in umbilical cord blood, increasing concern for neonatal immunosuppression and potential risk of infection (55). Multiple studies have not found significant associations between fetal exposure to TNFi and serious neonatal infections (56, 57). However, to reduce potential infection risk, the ACR Reproductive Health Guideline conditionally recommends discontinuing the TNFis adalimumab, infliximab, golimumab, and etanercept in the third trimester of pregnancy or several half-lives prior to delivery, as these medications pass into the fetal circulation in the late stages of pregnancy (2).
In contrast to other TNFi, certolizumab pegol, a PEGylated TNFi, has very minimal or no active placental transfer because of its molecular structure; thus, the developing fetus is unlikely to be exposed to the treatment during pregnancy (58, 59). Thus, certolizumab does not need to be discontinued during any stage of pregnancy to reduce the risk of neonatal immunosuppression (2). Given a case of fatal disseminated tuberculosis in a neonate who was exposed to infliximab during pregnancy and received Bacillus Calmette-Guerin (BCG) vaccination at 3 months of age, certolizumab is favored above the other TNFis in countries in which TB is endemic and/or BCG vaccination is administered within 6 months of age (60).
Because the studies of certolizumab pegol in pregnancy and lactation are arguably more robust than other pregnancy studies of TNFi, and because it does not pass into the placental circulation, certolizumab is strongly recommended in the ACR Reproductive Health Guideline (2). In contrast, adalimumab, infliximab, golimumab, and etanercept—other TNFis that are not PEGylated and can cross into the placental circulation—are conditionally recommended during pregnancy (2).
Given these recommendations, some clinicians choose to switch RA patients using adalimumab, infliximab, golimumab, and etanercept to certolizumab during pregnancy—as was recommended in this case—to optimize safety in the pregnant patient. However, no available data assess the pregnancy and perinatal outcomes of people who switch to certolizumab from another TNFi. The risk of RA flare during a switch in treatment could augment the risk of adverse pregnancy and perinatal outcomes, including maternal pain and loss of function. Insurance coverage may also be a barrier to switching TNFi to certolizumab. We queried experts about how they might advise a pregnant patient with mildly persistent RA about TNFi treatment selection and management.
Expert opinion
Would you advise switching this patient from adalimumab to certolizumab from a safety perspective?
All experts would continue adalimumab in this patient with persistent RA without switching to certolizumab during pregnancy. Experts felt that TNFis were safe during all stages of pregnancy from the perspective of fetal development. Experts felt the risk of disease flare is moderate when switching TNFis during pregnancy, which could increase the risk of preterm birth and preeclampsia. Experts felt that ideally, patient-clinician discussions around treatment should occur prior to pregnancy in the case of a planned or anticipated pregnancy; switching treatments during pregnancy was not ideal given the risk of precipitating a disease flare.
How would you advise the patient to manage her treatment in the third trimester if she continues to have disease activity?
All experts agreed that the patient could safely continue adalimumab during all trimesters of pregnancy and should hold the adalimumab at 32 weeks of gestation only if the disease was well-controlled. For patients with persistent RA, most experts felt that patients could either stop the adalimumab at 36 weeks of gestation or, if the disease continued to be persistent and limiting, would continue through all stages of pregnancy. All experts felt that the poor outcomes associated with active RA were of greater concern than the theoretical risk of neonatal immunosuppression. Experts indicated that publications about the infection risk in neonates who have been exposed to a TNFi in the third trimester have been reassuring all experts felt that if the patient had ongoing disease activity, the patient should be treated in the third trimester and beyond without treatment modification.
Experts varied in their recommendations if a theoretical cesarean operation was planned for this patient with persistent RA activity, given the potential for maternal infection or impaired wound healing. One expert recommended continuing the patient's adalimumab until 36 weeks of gestation if a planned surgery (e.g., cesarean section) was scheduled at around 37 or 38 weeks. Most experts felt that missing one or two doses of TNFi would not significantly affect the patient's disease activity, and she could resume her treatment immediately after delivery. Two experts also indicated that most patients at their institutions were advised by obstetrician-gynecologists to hold their TNFi for 3 weeks prior to delivery.
Summary
While available guidelines and classification criteria have supported effective diagnosis and treatment of autoimmune conditions during pregnancy, we highlight several of the many “gray zones” that exist in the management of pregnant patients with RMD. Pregnant patients who are severely ill may receive aggressive treatments that are in the grey zone, and such management might be easily justified in the service of preserving maternal and/or fetal life. However, we felt that there is a significant benefit in highlighting outpatient cases with diagnostic or treatment uncertainty, as rheumatologists are more likely to encounter and care for patients in non-critical clinical scenarios.
Some rheumatologists might feel uncomfortable advancing treatments for pregnant people who appear clinically or medically stable. If so, this would be understandable; pregnant individuals have been largely excluded from randomized clinical trials of treatments due to ethical concerns, so clinicians have been left in the position of having to manage the pregnancies of people with known or suspected autoimmune diseases with limited and sometimes poor-quality evidence to support their decision-making. Rheumatologists may also feel concerned about exposing pregnant patients to medications that have side effects or have questionable safety during pregnancy. The inability at some institutions to advance diagnostic testing during pregnancy, such as renal biopsy (e.g., Case 3), may further undermine some rheumatologists’ comfort level in advancing treatments that may immunosuppress the patient and/or are associated with side effects. Ultimately, some rheumatologists may feel that advancing treatments for the pregnant patient in the context of diagnostic uncertainty and without critical illness, might undermine their oath of primum non nocere—to first do no harm.
However, non-action may also not serve the patient and cause harm. An important ethical consideration is that treatment during pregnancy may be necessary to prevent organ failure or severe maternal morbidity or mortality, even when limited diagnostic data are available to guide medical decision-making. In addition, failure to treat or to advance care may lead to adverse outcomes that eventually threaten maternal and fetal health, even if the pregnant patient appears immediately stable.
Robust data are urgently needed to inform medical decision-making for pregnant individuals with RMDs. In addition, shared medical decision-making between patient and physician is essential in developing a treatment plan in the gray zone of management. In this context, clinicians share the potential risks and benefits of various approaches and provide their interpretation of a clinical case. Some patients may feel inclined toward treatment whereas others may not. In these cases, the clinician's ideal role is to facilitate the patients’ goals-concordant care if clinically reasonable and support them throughout their pregnancy experience along with other medical specialists (61). Even in the case of an adverse outcome, the patient will have participated in the treatment decisions and had the opportunity to express their goals and preferences for care.
In addition to the four cases discussed herein, experts felt that discussion of other “gray zone” areas in outpatient management should involve international reproductive rheumatology colleagues to ascertain regional differences in approaches, share experiential knowledge, and build new understanding and shared strategies in treating patients in the grey zone of management. The International Conference on Reproductive, Pregnancy, and Rheumatic Diseases (also known as Rheumapreg) provides one important venue for international collaborations. Our reproductive rheumatology experts were particularly interested in future discussions about placental pathology interpretation continuation or discontinuation of IL-1, IL-6, IL-17 and IL12/23 inhibitors in spondyloarthritis, belimumab usage during lupus pregnancy, and consideration of low-dose aspirin for all pregnancies of patients with RMDs.
Experts also advocated for multidisciplinary collaborations when assessing gray zone cases. For example, placental pathologies that might seem alarming to the rheumatologist might be less concerning to a maternal–fetal medicine specialist; conversely, serologic profiles that appear alarming to non-rheumatologists might be less concerning to the rheumatologist (e.g., an isolated positive ANA). Hematologists might expand a thrombotic workup to include genetic thrombophilia tests, some of which may be fairly unfamiliar to rheumatologists but may inform assessment of thrombotic risk and anticoagulation decisions. Multidisciplinary collaboration can help to more accurately evaluate clinical scenarios as well as the risks and benefits of different diagnostic and treatment approaches. In addition, a cohesive message shared by the multidisciplinary team may help to provide confidence and reassurance to the pregnant individual and family.
The expert opinions shared herein are not meant to be interpreted as evidence-based guidelines or criteria and might differ from the perspectives and opinions of other experts in the field. However, this manuscript serves to provide rheumatologists with points of consideration as they approach the outpatient care of pregnant patients. Ultimately, shared decision-making and multidisciplinary collaboration between the pregnant patient and the clinician team are essential for advancing person-centered, preference-concordant health care in the reproductive rheumatology context.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the University of Pittsburgh Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because we adapted case reports to have different patient initials, ages, lab values, and other clinical features. These cases are not recognizable, but the principles in management are emphasized. In addition, we created two de novo cases that were informed by questions provided at meetings.
Author contributions
IM: Conceptualization, Investigation, Project administration, Writing – original draft, Writing – review & editing. BB: Writing – original draft, Writing – review & editing. CE: Writing – original draft, Writing – review & editing. LS: Writing – original draft, Writing – review & editing. JZ: Writing – original draft, Writing – review & editing. MB: Conceptualization, Data curation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The authors declare no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Birru Talabi M, Clowse MEB, Schwarz EB, Callegari LS, Moreland L, Borrero S. Family planning counseling for women with rheumatic diseases. Arthritis Care Res (Hoboken). (2018) 70(2):169–74. doi: 10.1002/acr.23267
2. Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse MEB, Lockshin MD, et al. 2020 American College of Rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Rheumatol. (2020) 72(4):529–56. doi: 10.1002/art.41191
3. Andreoli L, Bertsias GK, Agmon-Levin N, Brown S, Cervera R, Costedoat-Chalumeau N, et al. EULAR Recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis. (2017) 76(3):476–85. doi: 10.1136/annrheumdis-2016-209770
4. Russell MD, Dey M, Flint J, Davie P, Allen A, Crossley A, et al. British Society for Rheumatology guideline on prescribing drugs in pregnancy and breastfeeding: immunomodulatory anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford). (2023) 62(4):e48–88. doi: 10.1093/rheumatology/keac551
5. Barbhaiya M, Zuily S, Naden R, Hendry A, Manneville F, Amigo MC, et al. 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Ann Rheum Dis. (2023) 82(10):1258–70. doi: 10.1136/ard-2023-224609
6. Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. (2019) 71(9):1400–12. doi: 10.1002/art.40930
7. US Preventive Services Task Force. Aspirin use to prevent preeclampsia and related morbidity and mortality: US preventive services task force recommendation statement. JAMA. (2021) 326(12):1186–91. doi: 10.1001/jama.2021.14781
8. Schreiber K, Sciascia S, de Groot PG, Devreese K, Jacobsen S, Ruiz-Irastorza G, et al. Antiphospholipid syndrome. Nat Rev Dis Primers. (2018) 4:18005. doi: 10.1038/nrdp.2018.5
9. Schreiber K, Hunt BJ. Managing antiphospholipid syndrome in pregnancy. Thromb Res. (2019) 181(Suppl 1):S41–6. doi: 10.1016/S0049-3848(19)30366-4
10. Yelnik CM, Laskin CA, Porter TF, Branch DW, Buyon JP, Guerra MM, et al. Lupus anticoagulant is the main predictor of adverse pregnancy outcomes in aPL-positive patients: validation of PROMISSE study results. Lupus Sci Med. (2016) 3(1):e000131. doi: 10.1136/lupus-2015-000131
11. Bertolaccini ML, Amengual O, Andreoli L, Atsumi T, Chighizola CB, Forastiero R, et al. 14th International Congress on Antiphospholipid Antibodies task force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmun Rev. (2014) 13(9):917–30. doi: 10.1016/j.autrev.2014.05.001
12. Erton ZB, Sevim E, de Jesus GR, Cervera R, Ji L, Pengo V, et al. Pregnancy outcomes in antiphospholipid antibody positive patients: prospective results from the Antiphospholipid Syndrome Alliance for Clinical Trials and International Networking (APS ACTION) clinical database and repository (‘registry’). Lupus Sci Med. (2022) 9(1). doi: 10.1136/lupus-2021-000633
13. Lockshin MD, Kim M, Laskin CA, Guerra M, Branch DW, Merrill J, et al. Prediction of adverse pregnancy outcome by the presence of lupus anticoagulant, but not anticardiolipin antibody, in patients with antiphospholipid antibodies. Arthritis Rheum. (2012) 64(7):2311–8. doi: 10.1002/art.34402
14. Branch DW, Lim MY. How I diagnose and treat antiphospholipid syndrome in pregnancy. Blood. (2024) 143(9):757–68. doi: 10.1182/blood.2023020727
15. Lynch A, Marlar R, Murphy J, Davila G, Santos M, Rutledge J, et al. Antiphospholipid antibodies in predicting adverse pregnancy outcome. A prospective study. Ann Intern Med. (1994) 120(6):470–5. doi: 10.7326/0003-4819-120-6-199403150-00004
16. Opatrny L, David M, Kahn SR, Shrier I, Rey E. Association between antiphospholipid antibodies and recurrent fetal loss in women without autoimmune disease: a metaanalysis. J Rheumatol. (2006) 33(11):2214–21.
17. Antovic A, Sennstrom M, Bremme K, Svenungsson E. Obstetric antiphospholipid syndrome. Lupus Sci Med. (2018) 5(1):e000197. doi: 10.1136/lupus-2016-000197
18. Khogeer H, Alfattani A, Al Kaff M, Al Shehri T, Khojah O, Owaidah T. Antiphosphatidylserine antibodies as diagnostic indicators of antiphospholipid syndrome. Lupus. (2015) 24(2):186–90. doi: 10.1177/0961203314552462
19. Vandevelde A, Chayoua W, de Laat B, Moore GW, Musial J, Zuily S, et al. Added value of antiphosphatidylserine/prothrombin antibodies in the workup of thrombotic antiphospholipid syndrome: communication from the ISTH SSC subcommittee on lupus anticoagulant/antiphospholipid antibodies. J Thromb Haemost. (2022) 20(9):2136–50. doi: 10.1111/jth.15785
20. Zhang X, Liu Z, Guo F, Wang Q, Bai W, Zhao A. Antiphosphatidylserine/prothrombin antibodies (aPS/PT) and risk of obstetric anti-phospholipid syndrome. Am J Reprod Immunol. (2023) 89(6):e13621. doi: 10.1111/aji.13621
21. Aggarwal R, Ringold S, Khanna D, Neogi T, Johnson SR, Miller A, et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res (Hoboken). (2015) 67(7):891–7. doi: 10.1002/acr.22583
22. Antunes M, Scire CA, Talarico R, Alexander T, Avcin T, Belocchi C, et al. Undifferentiated connective tissue disease: state of the art on clinical practice guidelines. RMD Open. (2018) 4(Suppl 1):e000786. doi: 10.1136/rmdopen-2018-000786
23. Hasbani G E, Viola M, Sciascia S, Taher AT, Uthman I. Antiphospholipid antibodies in inflammatory and autoimmune rheumatic and musculoskeletal diseases beyond lupus: a systematic review of the available evidence. Rheumatol Ther. (2021) 8(1):81–94. doi: 10.1007/s40744-020-00273-w
24. Sciascia S, Roccatello D, Radin M, Parodis I, Yazdany J, Pons-Estel G, et al. Differentiating between UCTD and early-stage SLE: from definitions to clinical approach. Nat Rev Rheumatol. (2022) 18(1):9–21. doi: 10.1038/s41584-021-00710-2
25. Romero R, Kim YM, Pacora P, Kim CJ, Benshalom-Tirosh N, Jaiman S, et al. The frequency and type of placental histologic lesions in term pregnancies with normal outcome. J Perinat Med. (2018) 46(6):613–30. doi: 10.1515/jpm-2018-0055
26. Torous VF, Roberts DJ. Placentas from women of advanced maternal age. Arch Pathol Lab Med. (2020) 144(10):1254–61. doi: 10.5858/arpa.2019-0481-OA
27. Tektonidou MG, Andreoli L, Limper M, Amoura Z, Cervera R, Costedoat-Chalumeau N, et al. EULAR Recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. (2019) 78(10):1296–304. doi: 10.1136/annrheumdis-2019-215213
29. Greer IA, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood. (2005) 106(2):401–7. doi: 10.1182/blood-2005-02-0626
30. (ACOG) ACoOaG. ACOG Practice Bulletin No. 196: Thromboembolism in Pregnancy. In., vol. 132: e1-e17.
31. Jacobson B, Rambiritch V, Paek D, Sayre T, Naidoo P, Shan J, et al. Safety and efficacy of enoxaparin in pregnancy: a systematic review and meta-analysis. Adv Ther. (2020) 37(1):27–40. doi: 10.1007/s12325-019-01124-z
32. Izmirly PM, Costedoat-Chalumeau N, Pisoni CN, Khamashta MA, Kim MY, Saxena A, et al. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation. (2012) 126(1):76–82. doi: 10.1161/CIRCULATIONAHA.111.089268
33. Osuchukwu OO, Reed DJ. Small for gestational age (Archived). In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing (2024).
34. Fakhouri F, Schwotzer N, Cabiddu G, Barratt J, Legardeur H, Garovic V, et al. Glomerular diseases in pregnancy: pragmatic recommendations for clinical management. Kidney Int. (2023) 103(2):264–81. doi: 10.1016/j.kint.2022.10.029
35. Dong Y, Yuan F, Dai Z, Wang Z, Zhu Y, Wang B. Preeclampsia in systemic lupus erythematosus pregnancy: a systematic review and meta-analysis. Clin Rheumatol. (2020) 39(2):319–25. doi: 10.1007/s10067-019-04823-8
36. Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol. (2009) 114(6):1326–31. doi: 10.1097/AOG.0b013e3181c2bde8
37. Lin R, McDonald G, Jolly T, Batten A, Chacko B. A systematic review of prophylactic anticoagulation in nephrotic syndrome. Kidney Int Rep. (2020) 5(4):435–47. doi: 10.1016/j.ekir.2019.12.001
38. Hull KL, Adenwalla SF, Topham P, Graham-Brown MP. Indications and considerations for kidney biopsy: an overview of clinical considerations for the non-specialist. Clin Med (Lond). (2022) 22(1):34–40. doi: 10.7861/clinmed.2021-0472
39. Luciano RL, Moeckel GW. Update on the native kidney biopsy: core curriculum 2019. Am J Kidney Dis. (2019) 73(3):404–15. doi: 10.1053/j.ajkd.2018.10.011
40. Zhao C, Zhao J, Huang Y, Wang Z, Wang H, Zhang H, et al. New-onset systemic lupus erythematosus during pregnancy. Clin Rheumatol. (2013) 32(6):815–22. doi: 10.1007/s10067-013-2180-z
41. Dao KH, Bermas BL. Systemic lupus erythematosus management in pregnancy. Int J Womens Health. (2022) 14:199–211. doi: 10.2147/IJWH.S282604
42. Mok CC. Membranous nephropathy in systemic lupus erythematosus: a therapeutic enigma. Nat Rev Nephrol. (2009) 5(4):212–20. doi: 10.1038/nrneph.2009.14
43. Moroni G, Calatroni M, Donato B, Ponticelli C. Kidney biopsy in pregnant women with glomerular diseases: focus on lupus nephritis. J Clin Med. (2023) 12(5). doi: 10.3390/jcm12051834
44. Tan B, So PN, Krishnan A, Carriazo S, Bahamonde JR, Lamech TM, et al. Approach to pregnancy in patients with lupus nephritis. Kidney Med. (2023) 5(11):100724. doi: 10.1016/j.xkme.2023.100724
45. Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennstrom M, et al. Predictive value of the sFlt-1:plGF ratio in women with suspected preeclampsia. N Engl J Med. (2016) 374(1):13–22. doi: 10.1056/NEJMoa1414838
46. FDA. FDA alerts Health Care Professionals of Pregnancy Problems Associated with Thiopurines. Silver Spring, MD: Food and Drug Administration (2024). Available online at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-alerts-health-care-professionals-pregnancy-problems-associated-thiopurines (Accessed September 1, 2024).
47. Schwake C, Steinle J, Thiel S, Timmesfeld N, Haben S, Ayzenberg I, et al. Neonatal B-cell levels and infant health in newborns potentially exposed to anti-CD20 monoclonal antibodies during pregnancy or lactation. Neurol Neuroimmunol Neuroinflamm. (2024) 11(4):e200264. doi: 10.1212/NXI.0000000000200264
48. Wiles K, Chappell L, Clark K, Elman L, Hall M, Lightstone L, et al. Clinical practice guideline on pregnancy and renal disease. BMC Nephrol. (2019) 20(1):401. doi: 10.1186/s12882-019-1560-2
49. Jethwa H, Lam S, Smith C, Giles I. Does rheumatoid arthritis really improve during pregnancy? A systematic review and metaanalysis. J Rheumatol. (2019) 46(3):245–50. doi: 10.3899/jrheum.180226
50. Hunt N, Talabi MB. Family planning and rheumatoid arthritis. Curr Rheumatol Rep. (2019) 21(5):16. doi: 10.1007/s11926-019-0816-y
51. FDA. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): Drug Safety Communication—Avoid Use of NSAIDs in Pregnancy at 20 Weeks or Later. Silver Spring, MD: Food and Drug Administration (2020). Available online at: https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-recommends-avoiding-use-nsaids-pregnancy-20-weeks-or-later-because-they-can-result-low-amniotic#:~:text=On%20October%2015%2C%202020%2C%20FDA,problems%20in%20an%20unborn%20baby (Accessed September 1, 2024).
52. Smeele HTW, Dolhain R. Current perspectives on fertility, pregnancy and childbirth in patients with rheumatoid arthritis. Semin Arthritis Rheum. (2019) 49(3S):S32–5. doi: 10.1016/j.semarthrit.2019.09.010
53. Smeele HTW, Roder E, Mulders A, Steegers EAP, Dolhain R. Tumour necrosis factor inhibitor use during pregnancy is associated with increased birth weight of rheumatoid arthritis patients’ offspring. Ann Rheum Dis. (2022) 81(10):1367–73. doi: 10.1136/ard-2022-222679
54. He B, Li Y, Luo WW, Cheng X, Xiang HR, Zhang QZ, et al. The risk of adverse effects of TNF-alpha inhibitors in patients with rheumatoid arthritis: a network meta-analysis. Front Immunol. (2022) 13:814429. doi: 10.3389/fimmu.2022.814429
55. Ghalandari N, Kemper E, Crijns IH, Wolbink G, Rispens T, Smeele HT, et al. Analysing cord blood levels of TNF inhibitors to validate the EULAR points to consider for TNF inhibitor use during pregnancy. Ann Rheum Dis. (2022) 81(3):402–5. doi: 10.1136/annrheumdis-2021-221036
56. Luu M, Benzenine E, Doret M, Michiels C, Barkun A, Degand T, et al. Continuous anti-TNFalpha use throughout pregnancy: possible complications for the mother but not for the fetus. A retrospective cohort on the French national health insurance database (EVASION). Am J Gastroenterol. (2018) 113(11):1669–77. doi: 10.1038/s41395-018-0176-7
57. Mahadevan U, Long MD, Kane SV, Roy A, Dubinsky MC, Sands BE, et al. Pregnancy and neonatal outcomes after fetal exposure to biologics and thiopurines among women with inflammatory bowel disease. Gastroenterology. (2021) 160(4):1131–9. doi: 10.1053/j.gastro.2020.11.038
58. Clowse M, Fischer-Betz R, Nelson-Piercy C, Scheuerle AE, Stephan B, Dubinsky M, et al. Pharmacovigilance pregnancy data in a large population of patients with chronic inflammatory disease exposed to certolizumab pegol. Ther Adv Musculoskelet Dis. (2022) 14:1759720X221087650. doi: 10.1177/1759720X221087650
59. Clowse MEB, Scheuerle AE, Chambers C, Afzali A, Kimball AB, Cush JJ, et al. Pregnancy outcomes after exposure to certolizumab pegol: updated results from a pharmacovigilance safety database. Arthritis Rheumatol. (2018) 70(9):1399–407. doi: 10.1002/art.40508
60. Cheent K, Nolan J, Shariq S, Kiho L, Pal A, Arnold J. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. (2010) 4(5):603–5. doi: 10.1016/j.crohns.2010.05.001
Keywords: reproductive health, women’s health, lupus, antiphospholipid antibodies, treatment selection
Citation: Mani I, Bermas B, Edens C, Sammaritano L, Zell J and Birru Talabi M (2024) Challenging cases in rheumatic disease pregnancy: management perspectives from reproductive rheumatologists. Front. Lupus 2:1455456. doi: 10.3389/flupu.2024.1455456
Received: 26 June 2024; Accepted: 15 August 2024;
Published: 23 September 2024.
Edited by:
Laura Andreoli, University of Brescia, ItalyReviewed by:
Anca Bobirca, Carol Davila University of Medicine and Pharmacy, RomaniaFrancesca Crisafulli, University of Brescia, Italy
Copyright: © 2024 Mani, Bermas, Edens, Sammaritano, Zell and Birru Talabi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mehret Birru Talabi, bXNiOTBAcGl0dC5lZHU=
 Iswariya Mani
Iswariya Mani Bonnie Bermas
Bonnie Bermas Cuoghi Edens3
Cuoghi Edens3 Mehret Birru Talabi
Mehret Birru Talabi