- School of Clinical Sciences at Monash Health, Monash University, Melbourne, VIC, Australia
Systemic lupus erythematosus (SLE, lupus) is a chronic autoimmune disease characterised by a heterogeneous clinical presentation and complex underlying immunologic dysfunction. This poses a significant challenge to the accurate assessment of disease activity, which is central to both clinical management and research in SLE. This review aims to describe common barriers to accurately measuring disease activity in SLE and different approaches to disease activity assessment. We will cover the evaluation of disease activity in clinical practice and discuss the role of widely used and emerging disease activity instruments in both clinical and research contexts, including measures of flare, treat-to-target disease states and clinical trial endpoints.
Introduction to SLE and disease activity
SLE is a multisystem disease associated with substantial individual and public health burden. The global prevalence approximates 1 in 2000 (1). SLE is most frequently diagnosed in women aged 15–44 years (2) and is more common in patients of non-Caucasian ancestry (2, 3). The pathophysiology of SLE is incompletely understood, but numerous genetic and environmental risk factors have been identified. Immunological dysfunction, including autoantibody production, culminates in tissue inflammation across a range of organ systems, leading to the diverse manifestations that characterise the disease (4).
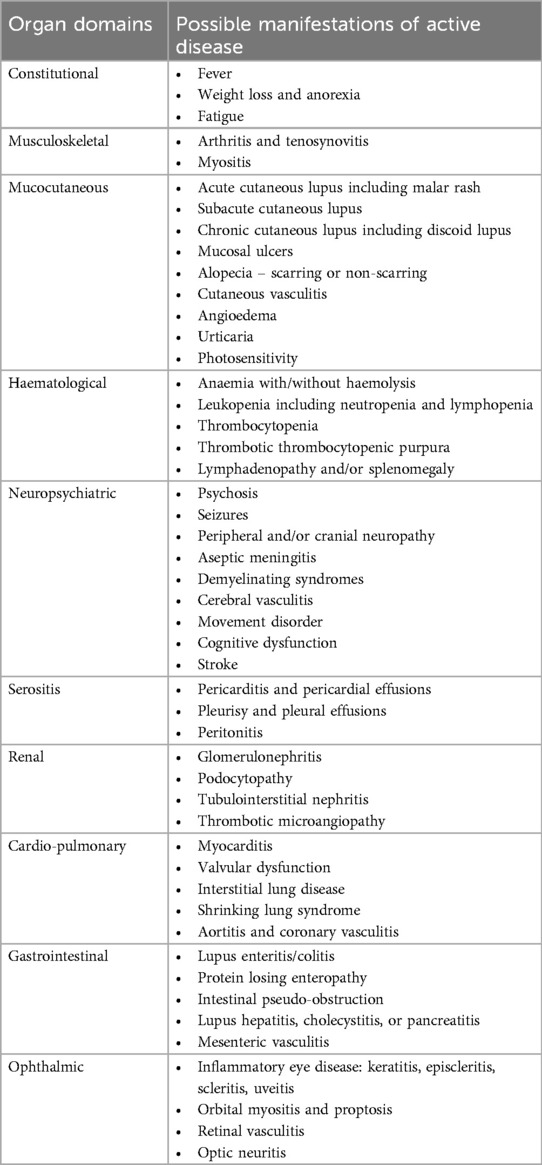
The diagnosis of SLE is based on a combination of consistent clinical features and characteristic immunologic findings, such as anti-nuclear (ANA), anti-double stranded DNA (dsDNA) and anti-Smith antibodies, and/or low complement levels. Mucocutaneous and musculoskeletal manifestations affect up to 90% of SLE patients, while lupus nephritis, haematologic disorders, constitutional symptoms and serositis also occur frequently (5). Less common features include neuropsychiatric, cardio-pulmonary, ophthalmic and gastrointestinal involvement, although rates of different organ system involvement may vary between populations including ethnic groups (6). Organ involvement can occur in innumerable combinations and range in severity from mild and readily treatable to organ and/or life-threatening. While SLE is most commonly a relapsing-remitting disease with unpredictable fluctuations in disease activity, persistently active disease or more prolonged periods of remission also occur in some patients (7). It is thus extraordinarily difficult to predict the clinical presentation, time course and disease outcomes in any individual patient.
Just as there is no single diagnostic test for SLE, management is also tailored to the individual according to specific disease manifestations, comorbidities, and patient preferences (8). Non-pharmacological strategies, antimalarials, traditional immunosuppressive drugs and newer biologic agents all have a role in aiming for “remission of disease symptoms, prevention of damage accrual and minimisation of drug side effects, as well as improvement of quality of life” (9). Unfortunately, current therapeutic strategies frequently fall short of achieving these desired treatment goals, and SLE continues to be associated with significant adverse health outcomes, including irreversible organ damage, premature mortality, and reduced health-related quality of life (HRQoL) (10). Understanding and accurately assessing disease activity in SLE is essential to bridging these unmet needs; both clinically to characterise an individual patient's disease status and tailor treatment, and in research to improve our understanding of the efficacy of new treatments and the best way to utilise different therapeutic strategies to optimise patient health outcomes.
Disease activity in SLE
Disease activity refers to inflammatory disease manifestations that are potentially reversible with immunomodulatory treatments. Assessing disease activity is vital due to the association between high disease activity or flare and adverse health outcomes, including irreversible end-organ damage, mortality, and reduced quality of life (11, 12). Conversely, beneficial effects of achieving low disease activity states and remission are well established (13, 14).
Assessing disease activity in SLE is challenging. Activity in SLE can be in a single organ or occur across multiple domains in various combinations, and factors like damage, comorbidities and treatment effects can all confound the assessment of disease activity. Unlike diseases such as hypertension or diabetes where there are biomarkers that allow for direct monitoring of disease status (i.e., blood pressure, or blood glucose levels), in SLE there is no single biomarker that is sufficient for quantifying disease activity. Thus, clinical assessment of disease activity requires a nuanced approach that is individualised based on each unique patient and their disease context. In research, where standardised measurement of disease activity is required, we rely on human-constructed outcome measures to quantify and score disease activity levels and changes over time. Both these approaches will be discussed in the sections below.
Clinical assessment of disease activity
The clinical assessment of disease activity relies on a combination of patient-reported symptoms, physical examination findings, and results of laboratory tests and other investigations, which may vary depending on the individual patient and their specific combination of disease features. Disease activity should be assessed at each clinical visit, with frequency according to clinical need.
Disease activity assessment relies on the clinician evaluating the breadth of inflammatory activity across the vast number of potential manifestations (Table 1), as well as the severity of individual features, which may not be concordant across all affected domains. The history and examination are the foundation of this assessment, screening for symptoms and signs of possible disease activity. More detailed evaluation of identified abnormalities should consider attribution to active SLE (as opposed to other causes), and indicators of severity and impact on the patient. For example, clinical evaluation of joint pain in a patient with SLE would include differentiating inflammatory from non-inflammatory joint pain, examining for joint line tenderness and/or swelling, documenting the number, distribution, and severity of joint involvement, and considering the impact on patient function and quality of life.
For some manifestations the history and examination alone may provide sufficient information and clarity about disease activity, but in other cases additional investigations are necessary. Most notably, manifestations such as renal or haematological activity do not always present with overt symptoms but may still be prognostically significant (15, 16). Therefore, routine assessment of disease activity should include a full blood count, renal function, and urinary evaluation for protein (e.g., spot urine protein-creatinine ratio) and sediment. In addition to these organ-based investigations, other laboratory markers of disease activity frequently tested are anti-dsDNA antibody titres (may be elevated in active disease), complements (C3 and/or C4 may be low in active disease) and acute phase reactants (may be elevated in active disease). Anti-dsDNA and complement abnormalities are described in up to 90% of SLE patients, particularly those with lupus nephritis (17, 18). While a rise in anti-dsDNA titre and/or decline in complement levels may predict subsequent disease flares, this is not absolute, and thus is generally not sufficient to escalate treatment (19, 20). Serological abnormalities may also persist despite therapy and clinical remission in a subset of patients (21). Other antibodies associated with SLE, such as ANA or anti-Smith antibodies, while useful for classification and diagnosis, have no role in the monitoring of disease activity. In contrast, acute phase reactants such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) can be helpful but are non-specific in nature. ESR is elevated in up to half of SLE patients and may fluctuate with flares and responses to therapy (22, 23). CRP is less traditionally considered a biomarker of SLE activity, and significant elevations should raise suspicion of concurrent infection, especially if discordant with ESR (24). Increases are nonetheless observed in a significant proportion of patients with active disease, and associations have been identified with serositis and musculoskeletal activity in particular (25).
While some patients present with clear-cut features of disease activity that warrant escalation in immunosuppression, for others, even with a thorough clinical assessment and supporting investigations, the attribution of abnormalities to disease activity can remain uncertain. A common challenge is distinguishing active disease from symptoms and abnormalities of other causes, such as irreversible organ damage, medication effects or concurrent medical issues. Some of the frequently encountered abnormalities detected in SLE patients, examples of causes to consider other than active disease, and potential investigative approaches are outlined in Table 2. It is also important to be aware of common non-inflammatory SLE symptoms, sometimes referred to as “type 2” activity, including fatigue, brain fog, and mood disturbance, which have a marked impact on quality of life but are often discordant with inflammatory symptoms and respond poorly to immunosuppression (26, 27). It is therefore vital to distinguish these symptoms from traditional disease activity to avoid unnecessary immunosuppression and facilitate directed investigation and treatment. Clearly, untangling the multifactorial nature of many SLE features can pose a great challenge, even for experienced clinicians, and it is important to keep an open mind about the aetiology of identified abnormalities, particularly if responses to immunomodulatory treatment are not as expected.

Table 2. Examples of how to approach the assessment and attribution of common SLE symptoms and abnormalities to active disease.
The role of disease activity measures
In part due to the complexity of assessing disease activity in SLE, many instruments have been developed to quantify disease activity and facilitate standardised measurement. While many were developed primarily for research purposes, some may also have utility in clinical practice. Notably, recent SLE guidelines suggest using a validated instrument as part of disease activity assessment at each visit (28). As well as providing a measure of disease activity at a particular point in time, the scores generated by a disease activity measure may also be used to define levels of disease activity that have clinical meaning, such as treat-to-target endpoints like low disease activity or remission, or quantify changes in disease activity over time, such as when defining a flare or improvement in response to therapy.
The most common and widely used SLE disease activity measures are summarised in Table 3. This list is not exhaustive, and the sheer number of available measures highlights that no single instrument is fit for all purposes. Most disease activity measures are clinician-reported, as they require judgement of whether to attribute abnormalities to active inflammation. In contrast, patient-reported outcomes are ideally placed to understand symptom burden (including type 2 symptoms) and HRQoL, but are not necessarily specific to disease activity, as they will also capture the effects of factors such as damage, comorbidities, and impact of chronic illness (29).
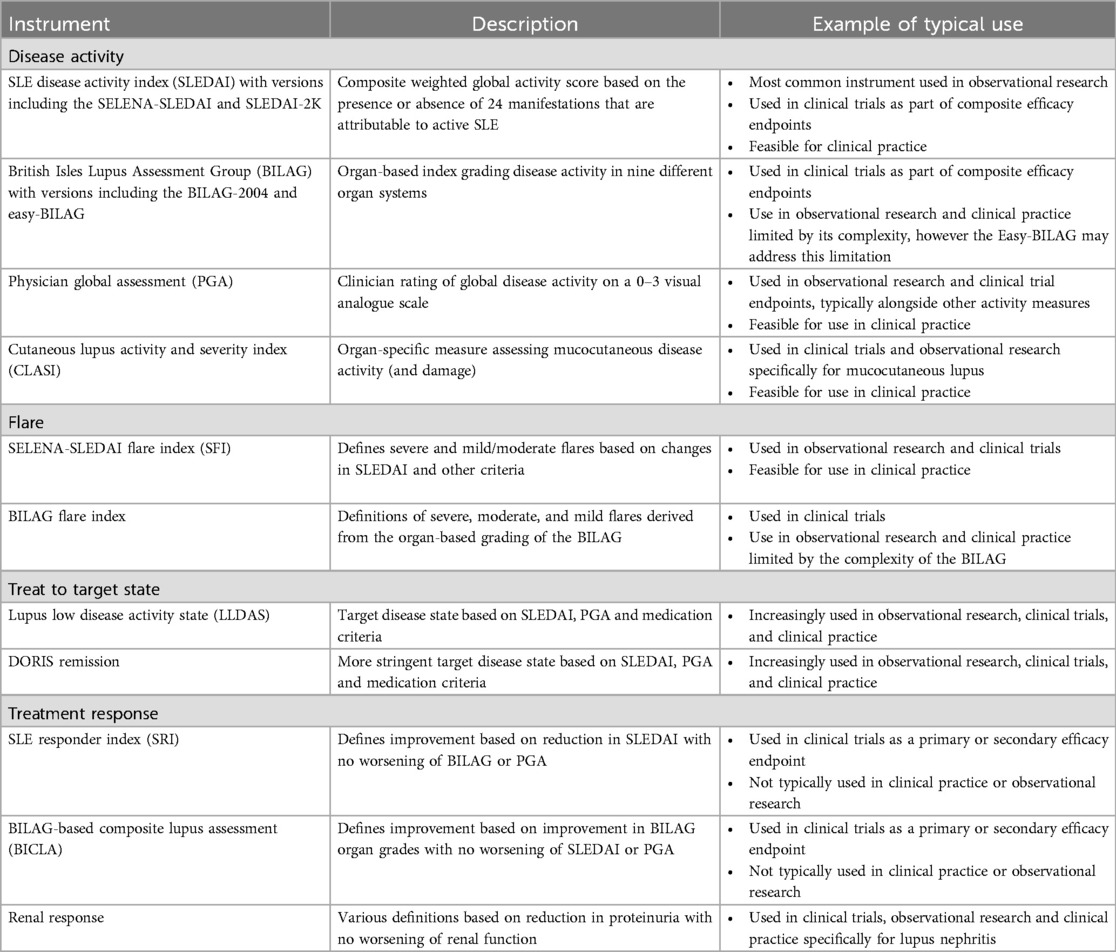
Table 3. Widely used outcome measures for evaluating disease activity and related concepts in SLE, including examples of their typical use in research and clinical settings.
Systemic lupus erythematosus disease activity index (SLEDAI)
The Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) (30) is the most widely used disease activity instrument in SLE. The SLEDAI-2000 (SLEDAI-2K) (31) and Safety of Estrogens in Lupus Erythematosus National Assessment-SLEDAI (SELENA-SLEDAI) (32) are the versions in most common use. The SLEDAI is a composite global disease activity measure consisting of 24 items covering 9 organ systems, of which 16 are clinical and 8 are laboratory variables. Haemolytic anaemia and gastrointestinal lupus are not included. Each item is graded as present or absent, typically based on the preceding 30 days (33). The SLEDAI has weightings for each item between 1 and 8, and the total score is calculated by adding the scores for all items present. This means the same total score can reflect very different patterns of disease activity between patients. Active disease has been defined as a SLEDAI ≥3–4 (34, 35) but higher cut-offs (e.g., SLEDAI ≥6) are typically required for clinical trial entry, and a definition of a high disease activity state has been proposed as SLEDAI-2K ≥10 (36). The SLEDAI has established reliability and validity (37) and predicts important patient outcomes such as damage and mortality (38). It is also simple to use and is feasible in a clinical setting. The most significant limitation is its inability to capture partial improvement or deterioration, or grade severity of a particular manifestation due to the binary nature of scoring (38, 39). For example, presence of any “inflammatory type rash” scores 2 points on the SLEDAI. If the rash were to worsen, or significantly improve but not resolve, the SLEDAI would still score 2 points for rash, even though clinically there has been a change. Caution must therefore be applied when using the SLEDAI to monitor changes in disease activity over time. Another consideration when using the SLEDAI (and other outcome measures incorporating the SLEDAI as a component) is that the measure includes serological markers such as anti-dsDNA and complements, which are not readily accessible in all settings. A modified version of the SLEDAI excluding these serological parameters has previously been reported and validated (37). Although disease activity scores and thresholds using this modified version cannot be considered interchangeable with the original SLEDAI, from a clinical perspective the modified SLEDAI is likely to be sufficient, given it is the clinical features of SLE activity that should primarily inform decision-making.
British isles lupus assessment group (BILAG)
The British Isles Lupus Assessment Group (BILAG) was first developed in 1988 (40) but the 2004 revision (BILAG-2004) (41) is the version in most common use. Unlike the SLEDAI it is not a composite score, but rather a set of organ-based scales grading 9 organ systems from A to E, where A represents major activity, B intermediate activity, C mild activity, D previous involvement but currently inactive, and E no previous activity. These grades are assigned based on an algorithm that integrates 97 disease activity items scored by the clinician as one of not present, improving, the same, worse, or new. Thus, it is a transitional index based on the previous assessment. The BILAG is extremely comprehensive, covering almost all active lupus manifestations. It is well-validated (41), reliable (42) and has superior responsiveness to the SLEDAI (43) despite its use of discrete categories of activity. The main barrier to widespread adoption of the BILAG is feasibility, due to its large number of items, complex scoring algorithm and requirement for formal training for optimal performance (39). The recent development of the Easy-BILAG (44) attempts to address these concerns by offering a more transparent and user-friendly format to improve feasibility and accuracy, whilst maintaining full fidelity to the BILAG-2004. The Easy-BILAG is therefore proposed as the recommended format for scoring the BILAG-2004 index in routine clinical care.
Physician global assessment (PGA)
The Physician Global Assessment (PGA) (45, 46) is a global score of disease activity considering all manifestations, in the opinion of the clinician, assessed on a visual analogue scale. It shows strong concordance with other validated disease activity measures and is sensitive to change, however its reliability is variable (47). This has led to recent efforts to standardise the PGA instrument for use in SLE (48), and the current recommended format is a 0–3 scale where 0 corresponds with no activity, 0.5–1 mild activity and >2–3 severe activity. Typically, the PGA is used in combination with other measures like the SLEDAI to ensure manifestations that are not specifically measured are still captured.
Organ-specific disease activity measures
Traditional SLE disease activity measures such as the SLEDAI and BILAG have focussed on capturing breadth of activity across the heterogeneity and scope of SLE. In contrast, organ-specific measures provide more comprehensive evaluation of a limited set of disease features. This allows for more detailed quantification of activity and change over time. The most common example currently used in SLE is the Cutaneous Lupus Activity and Severity Index (CLASI) which produces a score for mucocutaneous lupus based on items assessing rash, mucosal ulcers, and alopecia (49).
Flare
Measures of flare are predominantly used in research settings. The SELENA-SLEDAI Flare Index (SFI) (50) defines mild/moderate or severe flares based on either an increase in SELENA-SLEDAI score, specific manifestations that are new or worse, defined changes in medications, or an increase in PGA score. It has been shown to perform well for severe flares but is less reliable for mild to moderate flares (51). Concerns about the SFI include outdated medication criteria that do not incorporate newer treatment options or current steroid dosing practices, and contention about how specific manifestations are used to define mild/moderate vs. severe flares. As an alternative, the BILAG can also be used to define flares based on the A-E grading of each organ system. A severe flare is defined as one or more new A grades, a moderate flare as two or more new B grades, and a mild flare as one new B grade (52).
Treat-to-target endpoints
Pre-defined disease activity states such as “remission” and “low disease activity” represent treat-to-target endpoints, whereby therapy is adjusted with the aim of achieving and maintaining a disease state that is associated with clinical benefit. A consensus definition for remission in SLE has been defined by the Definition of Remission in SLE (DORIS) Taskforce, as a clinical SLEDAI-2K = 0, PGA <0.5, and prednisolone ≤5 mg daily with immunosuppression and antimalarials allowed (53). While remission is considered a gold standard treatment target, attainment may not be feasible for many patients (54), and low disease activity represents a less stringent alternative that still confers significant prognostic benefit. The Lupus Low Disease Activity State (LLDAS) is the best validated and most widely adopted of the various low disease activity definitions. LLDAS is defined as SLEDAI-2K ≤4 with no activity in major organ systems, no new lupus activity, PGA ≤1, prednisolone ≤7.5 mg daily and standard maintenance doses of immunosuppression (55). Attainment of LLDAS has been shown to protect against damage accrual, flare, and mortality as well as being associated with improved HRQoL (56–58). While prospective treat-to-target trials are yet to be completed, these target disease activity states have been incorporated into the most recent SLE management guidelines (28) and have been adopted as secondary endpoints in recent SLE clinical trials (59–61).
Clinical trial endpoints: SRI and BICLA
The SLE Responder Index (SRI) (62) and the BILAG-based Composite Lupus Assessment (BICLA) (63) are the most frequently used primary efficacy endpoints in SLE clinical trials. Both the SRI and BICLA combine the SLEDAI, BILAG and PGA assessments. When defining response with the SRI, improvement is determined by the SLEDAI where a reduction of 4 or more points is usually required (the SRI-4). In contrast the BICLA uses the BILAG to define improvement, based on an improvement in all organ systems graded BILAG A and B at the start of the trial. The other two outcome measures (BILAG and PGA for the SRI, and SLEDAI and PGA for the BICLA) ensure concurrent worsening of disease manifestations are not missed by the instrument primarily defining improvement. While these trial endpoints have led to successful drug registrations including belimumab (64) and most recently anifrolumab (65), the SRI and BICLA have well-recognised limitations, and their inconsistent performance across a number of late phase clinical trial programs in SLE are well-documented (66, 67). For example, the SRI and BICLA were developed as adaptations of pre-existing disease activity measures that were not designed for the clinical trial setting, meaning their included items and weightings are not specifically validated for the purpose of defining response in this context (66). They had limited validation prior to widespread adoption, in the absence of suitable alternatives, and can be quite complex and obscure to interpret (66). The reliance on crossing arbitrary binary or discrete thresholds when defining response is particularly problematic. For example, 4 points are scored for arthritis in the SLEDAI-2K, defined as signs of inflammation in 2 or more joints. Thus an SRI-4 response could be achieved if a patient had a joint count that reduced from 2 to 1, which may be of questionable clinical significance. Conversely, a patient whose joint count improved from 20 to 4 would still score 4 points for arthritis on the SLEDAI, and this improvement would not contribute towards being classified as an SRI-4 responder. Such limitations must therefore be considered in the interpretation of clinical trial results based on current efficacy endpoints.
Emerging disease activity measures
Several novel measures of disease activity are also at various stages of development and validation, and although promising, are yet to be widely adopted or replace legacy measures. The SLE Disease Activity Score or SLE-DAS (68) is a global activity score modelled on the DAS-28 used in rheumatoid arthritis. It is a weighted score incorporating 17 items, and several studies have been performed aiming to validate the measure and its scoring thresholds (69–71). The Lupus Foundation of America Rapid Evaluation of Activity in Lupus is another recently developed disease activity measure comprising a set of visual analogue scales rating activity for each major organ system separately (72). It is simple to use, sensitive to change and has a corresponding patient-reported outcome measure that can be deployed alongside the clinician-reported scales (73).
The limitations of current SLE clinical trial endpoints have also led to efforts to develop new approaches to treatment response measurement. The Lupus Multivariable Outcome Score (LuMOS) is a data driven response index developed from the belimumab trial dataset based on six variables including the SELENA-SLEDAI, components of the BILAG, serological markers and prednisolone dose (74). Most recently, the Treatment Response Measure for SLE (TRM-SLE) project has been launched, with a novel outcome measure under development focussing on detailed assessment of activity and response in a select set of domains, specifically tailored for the context of lupus clinical trials (75).
Conclusion
In summary, the clinician impression of overall disease activity in SLE is derived from a comprehensive history and examination supplemented by laboratory and other investigations. This includes identifying what combination of symptoms and other abnormalities represent active inflammation in an individual patient at a particular point in time, characterising their severity and impact, and excluding confounding factors such as damage and comorbidities. Clinical evaluation can be supported by employing validated disease activity measures; however, an understanding of the characteristics and context-specific limitations of different instruments is essential to their appropriate use and interpretation. While assessment of disease activity represents just one component of the broader evaluation and management of patients with SLE, it is essential for directing appropriate clinical decision-making and advancing through research our understanding of the disease and its effective treatment.
Author contributions
AL: Writing – review & editing, Writing – original draft. AW: Writing – review & editing, Writing – original draft. KC: Writing – review & editing, Writing – original draft, Supervision, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jingru T, Dingyao Z, Xu Y, Yaqing H, Qianjin L. Global epidemiology of systemic lupus erythematosus: a comprehensive systematic analysis and modelling study. Ann Rheum Dis. (2023) 82(3):351. doi: 10.1136/ard-2022-223035
2. Rees F, Doherty M, Grainge MJ, Lanyon P, Zhang W. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology. (2017) 56(11):1945–61. doi: 10.1093/rheumatology/kex260
3. Barber MRW, Drenkard C, Falasinnu T, Hoi A, Mak A, Kow NY, et al. Global epidemiology of systemic lupus erythematosus. Nat Rev Rheumatol. (2021) 17(9):515–32. doi: 10.1038/s41584-021-00668-1
4. Dörner T, Furie R. Novel paradigms in systemic lupus erythematosus. Lancet. (2019) 393(10188):2344–58. doi: 10.1016/S0140-6736(19)30546-X
5. Fanouriakis A, Tziolos N, Bertsias G, Boumpas DT. Update οn the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis. (2021) 80(1):14–25. doi: 10.1136/annrheumdis-2020-218272
6. Aguirre A, Izadi Z, Trupin L, Barbour KE, Greenlund KJ, Katz P, et al. Race, ethnicity, and disparities in the risk of end-organ lupus manifestations following a systemic lupus erythematosus diagnosis in a multiethnic cohort. Arthritis Care Res (Hoboken). (2023) 75(1):34–43. doi: 10.1002/acr.24892
7. Tselios K, Gladman DD, Touma Z, Su J, Anderson N, Urowitz MB. Disease course patterns in systemic lupus erythematosus. Lupus. (2018) 28(1):114–22. doi: 10.1177/0961203318817132
8. Durcan L, O'Dwyer T, Petri M. Management strategies and future directions for systemic lupus erythematosus in adults. Lancet. (2019) 393(10188):2332–43. doi: 10.1016/S0140-6736(19)30237-5
9. Antonis F, Myrto K, Alessia A, Martin A, Ingeborg B, John NB, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. (2019) 78(6):736. doi: 10.1136/annrheumdis-2019-215089
10. Singh RR, Yen EY. SLE mortality remains disproportionately high, despite improvements over the last decade. Lupus. (2018) 27(10):1577–81. doi: 10.1177/0961203318786436
11. García EG, Gil JJF, Cortes JI, Sanjuan FMO, Verdejo IC, Ivorra JAR. The impact of disease activity on health-related quality of life in patients with systemic lupus erythematosus. Med Clin (Barc). (2023) 160(10):428–33. doi: 10.1016/j.medcli.2022.11.019
12. Lopez R, Davidson JE, Beeby MD, Egger PJ, Isenberg DA. Lupus disease activity and the risk of subsequent organ damage and mortality in a large lupus cohort. Rheumatology (Oxford). (2012) 51(3):491–8. doi: 10.1093/rheumatology/ker368
13. Shi Y, Li M, Liu L, Wang Z, Wang Y, Zhao J, et al. Relationship between disease activity, organ damage and health-related quality of life in patients with systemic lupus erythematosus: a systemic review and meta-analysis. Autoimmun Rev. (2021) 20(1):102691. doi: 10.1016/j.autrev.2020.102691
14. Ronald FV, Marta M, George B, David I, Annegret K, Kirsten L, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis. (2014) 73(6):958. doi: 10.1136/annrheumdis-2013-205139
15. Gasparotto M, Gatto M, Binda V, Doria A, Moroni G. Lupus nephritis: clinical presentations and outcomes in the 21st century. Rheumatology. (2020) 59(Supplement_5):v39–51. doi: 10.1093/rheumatology/keaa381
16. Velo-García A, Castro SG, Isenberg DA. The diagnosis and management of the haematologic manifestations of lupus. J Autoimmun. (2016) 74:139–60. doi: 10.1016/j.jaut.2016.07.001
17. Conti F, Ceccarelli F, Perricone C, Massaro L, Marocchi E, Miranda F, et al. Systemic lupus erythematosus with and without anti-dsDNA antibodies: analysis from a large monocentric cohort. Mediators Inflamm. (2015) 2015:328078. doi: 10.1155/2015/328078
18. Mosca M, Chimenti D, Pratesi F, Baldini C, Anzilotti C, Bombardieri S, et al. Prevalence and clinico-serological correlations of anti-alpha-enolase, anti-C1q, and anti-dsDNA antibodies in patients with systemic lupus erythematosus. J Rheumatol. (2006) 33(4):695–7.16583471
19. Yeo AL, Kandane-Rathnayake R, Koelmeyer R, Golder V, Louthrenoo W, Chen YH, et al. SMART-SLE: serology monitoring and repeat testing in systemic lupus erythematosus-an analysis of anti-double-stranded DNA monitoring. Rheumatology (Oxford). (2024) 63(2):525–33. doi: 10.1093/rheumatology/kead231
20. Swaak AJ, Groenwold J, Bronsveld W. Predictive value of complement profiles and anti-dsDNA in systemic lupus erythematosus. Ann Rheum Dis. (1986) 45(5):359–66. doi: 10.1136/ard.45.5.359
21. LeBlanc BAW, Gladman DD, Urowitz MB. Serologically active clinically quiescent systemic lupus erythematosus–predictors of clinical flares. J Rheumatol. (1994) 21(12):2239–41.7699623
22. Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M, Vidal X, Mitjavila F, Castro Salomó A, et al. Enteric-coated mycophenolate sodium versus azathioprine in patients with active systemic lupus erythematosus: a randomised clinical trial. Ann Rheum Dis. (2017) 76(9):1575–82. doi: 10.1136/annrheumdis-2016-210882
23. Dima A, Opris D, Jurcut C, Baicus C. Is there still a place for erythrocyte sedimentation rate and C-reactive protein in systemic lupus erythematosus? Lupus. (2016) 25(11):1173–9. doi: 10.1177/0961203316651742
24. Littlejohn E, Marder W, Lewis E, Francis S, Jackish J, McCune WJ, et al. The ratio of erythrocyte sedimentation rate to C-reactive protein is useful in distinguishing infection from flare in systemic lupus erythematosus patients presenting with fever. Lupus. (2018) 27(7):1123–9. doi: 10.1177/0961203318763732
25. Gaitonde S, Samols D, Kushner I. C-reactive protein and systemic lupus erythematosus. Arthritis Rheum. (2008) 59(12):1814–20. doi: 10.1002/art.24316
26. Escoda T, Jourde-Chiche N, Granel B, Cornec D, Chiche L. Complex relationships between inflammatory manifestations/type 1 and type 2 symptoms in systemic lupus erythematosus: a narrative literature review. Lupus. (2023) 32(8):942–51. doi: 10.1177/09612033231179773
27. Eudy AM, Rogers JL, Corneli A, McKenna K, Maheswaranathan M, Pisetsky DS, et al. Intermittent and persistent type 2 lupus: patient perspectives on two distinct patterns of type 2 SLE symptoms. Lupus Sci Med. (2022) 9(1). doi: 10.1136/lupus-2022-000705
28. Antonis F, Myrto K, Jeanette A, Martin A, Laurent A, Sang-Cheol B, et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann Rheum Dis. (2024) 83(1):15. doi: 10.1136/ard-2023-224762
29. Mahieu M, Yount S, Ramsey-Goldman R. Patient-reported outcomes in systemic lupus erythematosus. Rheum Dis Clin North Am. (2016) 42(2):253–63. doi: 10.1016/j.rdc.2016.01.001
30. Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The committee on prognosis studies in SLE. Arthritis Rheum. (1992) 35(6):630–40. doi: 10.1002/art.1780350606
31. Dafna DG, Dominique I, Murray BU. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. (2002) 29(2):288.11838846
32. Buyon JP, Petri MA, Kim MY, Kalunian KC, Grossman J, Hahn BH, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. (2005) 142(12_Part_1):953–62. doi: 10.7326/0003-4819-142-12_Part_1-200506210-00004
33. Touma Z, Urowitz M, Gladman D. SLEDAI-2K for a 30-day window. Lupus. (2010) 19(1):49–51. doi: 10.1177/0961203309346505
34. Gladman DD, Urowitz MB, Kagal A, Hallett D. Accurately describing changes in disease activity in systemic lupus erythematosus. J Rheumatol. (2000) 27(2):377–9.10685800
35. Yee CS, Farewell VT, Isenberg DA, Griffiths B, Teh LS, Bruce IN, et al. The use of systemic lupus erythematosus disease activity index-2000 to define active disease and minimal clinically meaningful change based on data from a large cohort of systemic lupus erythematosus patients. Rheumatology (Oxford). (2011) 50(5):982–8. doi: 10.1093/rheumatology/keq376
36. Koelmeyer R, Nim HT, Nikpour M, Sun YB, Kao A, Guenther O, et al. High disease activity status suggests more severe disease and damage accrual in systemic lupus erythematosus. Lupus Sci Med. (2020) 7(1). doi: 10.1136/lupus-2019-000372
37. Uribe AG, Vilá LM, McGwin G Jr, Sanchez ML, Reveille JD, Alarcón GS. The systemic lupus activity measure-revised, the Mexican systemic lupus erythematosus disease activity Index (SLEDAI), and a modified SLEDAI-2K are adequate instruments to measure disease activity in systemic lupus erythematosus. J Rheumatol. (2004) 31(10):1934–40.15468356
38. Arora S, Isenberg DA, Castrejon I. Measures of adult systemic lupus erythematosus: disease activity and damage. Arthritis Care Res (Hoboken). (2020) 72(S10):27–46. doi: 10.1002/acr.24221
39. Mikdashi J, Nived O. Measuring disease activity in adults with systemic lupus erythematosus: the challenges of administrative burden and responsiveness to patient concerns in clinical research. Arthritis Res Ther. (2015) 17(1):183. doi: 10.1186/s13075-015-0702-6
40. Symmons DP, Coppock JS, Bacon PA, Bresnihan B, Isenberg DA, Maddison P, et al. Development and assessment of a computerized index of clinical disease activity in systemic lupus erythematosus. Members of the British isles lupus assessment group (BILAG). Q J Med. (1988) 69(259):927–37. doi: 10.1093/oxfordjournals.qjmed.a068256
41. Yee CS, Farewell V, Isenberg DA, Rahman A, Teh LS, Griffiths B, et al. British Isles lupus assessment group 2004 index is valid for assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum. (2007) 56(12):4113–9. doi: 10.1002/art.23130
42. Yee CS, Farewell V, Isenberg DA, Prabu A, Sokoll K, Teh LS, et al. Revised British isles lupus assessment group 2004 index: a reliable tool for assessment of systemic lupus erythematosus activity. Arthritis Rheum. (2006) 54(10):3300–5. doi: 10.1002/art.22162
43. Yee CS, Farewell V, Isenberg DA, Griffiths B, Teh LS, Bruce IN, et al. The BILAG-2004 index is sensitive to change for assessment of SLE disease activity. Rheumatology (Oxford). (2009) 48(6):691–5. doi: 10.1093/rheumatology/kep064
44. Carter LM, Gordon C, Yee CS, Bruce I, Isenberg D, Skeoch S, et al. Easy-BILAG: a new tool for simplified recording of SLE disease activity using BILAG-2004 index. Rheumatology (Oxford). (2022) 61(10):4006–15. doi: 10.1093/rheumatology/keab883
45. Liang MH, Socher SA, Larson MG, Schur PH. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum. (1989) 32(9):1107–18. doi: 10.1002/anr.1780320909
46. Petri M, Genovese M, Engle E, Hochberg M. Definition, incidence, and clinical description of flare in systemic lupus erythematosus. A prospective cohort study. Arthritis Rheum. (1991) 34(8):937–44. doi: 10.1002/art.1780340802
47. Chessa E, Piga M, Floris A, Devilliers H, Cauli A, Arnaud L. Use of physician global assessment in systemic lupus erythematosus: a systematic review of its psychometric properties. Rheumatology. (2020) 59(12):3622–32. doi: 10.1093/rheumatology/keaa383
48. Piga M, Chessa E, Morand EF, Ugarte-Gil MF, Tektonidou M, van Vollenhoven R, et al. Physician global assessment international standardisation COnsensus in systemic lupus erythematosus: the PISCOS study. Lancet Rheumatol. (2022) 4(6):e441–e9. doi: 10.1016/S2665-9913(22)00107-2
49. Albrecht J, Taylor L, Berlin JA, Dulay S, Ang G, Fakharzadeh S, et al. The CLASI (cutaneous lupus erythematosus disease area and severity index): an outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol. (2005) 125(5):889–94. doi: 10.1111/j.0022-202X.2005.23889.x
50. Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. (2005) 353(24):2550–8. doi: 10.1056/NEJMoa051135
51. Isenberg D, Sturgess J, Allen E, Aranow C, Askanase A, Sang-Cheol B, et al. Study of flare assessment in systemic lupus erythematosus based on paper patients. Arthritis Care Res (Hoboken). (2018) 70(1):98–103. doi: 10.1002/acr.23252
52. Ehrenstein MR, Conroy SE, Heath J, Latchman DS, Isenberg DA. The occurrence, nature and distribution of flares in a cohort of patients with systemic lupus erythematosus: a rheumatological view. Br J Rheumatol. (1995) 34(3):257–60. doi: 10.1093/rheumatology/34.3.257
53. Vollenhoven RF, Bertsias G, Doria A, Isenberg D, Morand E, Petri MA, et al. 2021 DORIS definition of remission in SLE: final recommendations from an international task force. Lupus Sci Med. (2021) 8(1):e000538. doi: 10.1136/lupus-2021-000538
54. Theresa RW, Laurence SM, Michelle P. Remission in systemic lupus erythematosus: durable remission is rare. Ann Rheum Dis. (2017) 76(3):547. doi: 10.1136/annrheumdis-2016-209489
55. Franklyn K, Lau CS, Navarra SV, Louthrenoo W, Lateef A, Hamijoyo L, et al. Definition and initial validation of a lupus low disease activity state (LLDAS). Ann Rheum Dis. (2016) 75(9):1615–21. doi: 10.1136/annrheumdis-2015-207726
56. Golder V, Kandane-Rathnayake R, Hoi AY, Huq M, Louthrenoo W, An Y, et al. Association of the lupus low disease activity state (LLDAS) with health-related quality of life in a multinational prospective study. Arthritis Res Ther. (2017) 19(1):62. doi: 10.1186/s13075-017-1256-6
57. Golder V, Kandane-Rathnayake R, Huq M, Nim HT, Louthrenoo W, Luo SF, et al. Lupus low disease activity state as a treatment endpoint for systemic lupus erythematosus: a prospective validation study. Lancet Rheumatol. (2019) 1(2):e95–e102. doi: 10.1016/S2665-9913(19)30037-2
58. Kandane-Rathnayake R, Golder V, Louthrenoo W, Chen YH, Cho J, Lateef A, et al. Lupus low disease activity state and remission and risk of mortality in patients with systemic lupus erythematosus: a prospective, multinational, longitudinal cohort study. Lancet Rheumatol. (2022) 4(12):e822–e30. doi: 10.1016/S2665-9913(22)00304-6
59. Morand EF, Isenberg DA, Wallace DJ, Kao AH, Vazquez-Mateo C, Chang P, et al. Attainment of treat-to-target endpoints in SLE patients with high disease activity in the atacicept phase 2b ADDRESS II study. Rheumatology (Oxford). (2020) 59(10):2930–8. doi: 10.1093/rheumatology/keaa029
60. Morand EF, Trasieva T, Berglind A, Illei GG, Tummala R. Lupus low disease activity state (LLDAS) attainment discriminates responders in a systemic lupus erythematosus trial: post-hoc analysis of the phase IIb MUSE trial of anifrolumab. Ann Rheum Dis. (2018) 77(5):706–13. doi: 10.1136/annrheumdis-2017-212504
61. Oon S, Huq M, Golder V, Ong PX, Morand EF, Nikpour M. Lupus low disease activity state (LLDAS) discriminates responders in the BLISS-52 and BLISS-76 phase III trials of belimumab in systemic lupus erythematosus. Ann Rheum Dis. (2019) 78(5):629–33. doi: 10.1136/annrheumdis-2018-214427
62. Luijten KMAC, Tekstra J, Bijlsma JWJ, Bijl M. The systemic lupus erythematosus responder index (SRI); A new SLE disease activity assessment. Autoimmun Rev. (2012) 11(5):326–9. doi: 10.1016/j.autrev.2011.06.011
63. Wallace DJ, Kalunian K, Petri MA, Strand V, Houssiau FA, Pike M, et al. Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann Rheum Dis. (2014) 73(1):183–90. doi: 10.1136/annrheumdis-2012-202760
64. Dubey AK, Handu SS, Dubey S, Sharma P, Sharma KK, Ahmed QM. Belimumab: first targeted biological treatment for systemic lupus erythematosus. J Pharmacol Pharmacother. (2011) 2(4):317–9. doi: 10.4103/0976-500X.85930
65. AstraZeneca. Saphnelo (anifrolumab) approved in the US for moderate to severe systemic lupus erythematosus United Kingdom: AstraZeneca (2024). Available from: Available online at: https://www.astrazeneca.com/media-centre/press-releases/2021/saphnelo-approved-in-the-us-for-sle.html# (updated 02/08/2021).
66. Connelly K, Golder V, Kandane-Rathnayake R, Morand EF. Clinician-reported outcome measures in lupus trials: a problem worth solving. Lancet Rheumatol. (2021) 3(8):e595–603. doi: 10.1016/S2665-9913(21)00119-3
67. Dolgin E. Lupus in crisis: as failures pile up, clinicians call for new tools. Nat Biotechnol. (2019) 37(1):7–8. doi: 10.1038/nbt0119-7
68. Diogo J, Ana M, Carla H, Margherita Z, Maddalena L, Luca I, et al. Derivation and validation of the SLE disease activity score (SLE-DAS): a new SLE continuous measure with high sensitivity for changes in disease activity. Ann Rheum Dis. (2019) 78(3):365. doi: 10.1136/annrheumdis-2018-214502
69. Diogo J, Maddalena L, Carla H, Ana M, Margherita Z, Paulo T, et al. Systemic lupus erythematosus disease activity score (SLE-DAS) enables accurate and user-friendly definitions of clinical remission and categories of disease activity. Ann Rheum Dis. (2021) 80(12):1568. doi: 10.1136/annrheumdis-2021-220363
70. Mathew A, Chengappa KG, Shah S, Negi VS. SLE-DAS: ready for routine use? Ann Rheum Dis. (2020) 79(9):e116. doi: 10.1136/annrheumdis-2019-215704
71. Jesus D, Henriques C, Matos A, Doria A, Inês LS. Systemic lupus erythematosus disease activity score remission and low disease activity states discriminate drug from placebo and better health-related quality of life. Arthritis Care Res (Hoboken) (2024) 76(6):788–95. doi: 10.1002/acr.25305
72. Askanase A, Li X, Pong A, Shum K, Kamp S, Carthen F, et al. Preliminary test of the LFA rapid evaluation of activity in lupus (LFA-REAL): an efficient outcome measure correlates with validated instruments. Lupus Sci Med. (2015) 2(1):e000075. doi: 10.1136/lupus-2014-000075
73. Askanase AD, Tang W, Zuraw Q, Gordon R, Brotherton B, Merrill JT. Evaluation of the LFA-REAL clinician-reported outcome (ClinRO) and patient-reported outcome (PRO): prespecified analysis of the phase III ustekinumab trial in patients with SLE. Lupus Sci Med. (2023) 10(1):e000875. doi: 10.1136/lupus-2022-000875
74. Abrahamowicz M, Esdaile JM, Ramsey-Goldman R, Simon LS, Strand V, Lipsky PE. Development and validation of a novel evidence-based lupus multivariable outcome score for clinical trials. Arthritis Rheumatol. (2018) 70(9):1450–8. doi: 10.1002/art.40522
Keywords: SLE, lupus, systemic lupus erythematosus, outcome measure, disease activity
Citation: Lin A, Wakhlu A and Connelly K (2024) Disease activity assessment in systemic lupus erythematosus. Front. Lupus 2:1442013. doi: 10.3389/flupu.2024.1442013
Received: 1 June 2024; Accepted: 6 August 2024;
Published: 21 August 2024.
Edited by:
Chak Lau, The University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Maria Leandro, University College London Hospitals NHS Foundation Trust, United KingdomCindy Hilary Flower, The University of the West Indies, Barbados
Copyright: © 2024 Lin, Wakhlu and Connelly. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kathryn Connelly, a2F0aHJ5bi5jb25uZWxseUBtb25hc2guZWR1
 Angela Lin
Angela Lin Ambika Wakhlu
Ambika Wakhlu Kathryn Connelly
Kathryn Connelly