
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Lupus , 04 April 2024
Sec. Reproductive Issues in Lupus
Volume 2 - 2024 | https://doi.org/10.3389/flupu.2024.1375857
This article is part of the Research Topic Reproductive Issues In Lupus, Antiphospholipid Syndrome And Other Autoimmune Rheumatic Diseases: Highlights From RheumaPreg2023 View all 14 articles
Objectives: There is insufficient knowledge about pregnancy outcomes in women with juvenile idiopathic arthritis (JIA). Our objective was to explore a possible association of inflammatory active JIA and pregnancy outcomes, including preterm birth, preeclampsia, gestational hypertension, and offspring gestational weight.
Methods: We linked data from the Norwegian nationwide observational register RevNatus with data from the Medical Birth Registry of Norway (MBRN) for the period 2010 to 2019. Singleton births in women with JIA (n = 181) included in RevNatus were cases. After excluding births in mothers with rheumatic inflammatory diseases, the remaining singleton births registered in MBRN, served as population controls (n = 575 798).
Results: Preterm birth was more frequent in women with active JIA (17.6%) and of equivalent frequency in women with inactive JIA (3.1%), compared to population controls (4.9%). Preeclampsia had similar rates in women with JIA and population controls while gestational hypertension was more frequent in women with active JIA (7.2%) and inactive JIA (6.9%) compared to population controls (1.7%). Abnormal fetal growth occurred in similar rates in women with JIA and population controls.
Conclusion: Having active JIA in pregnancy increased the risk for preterm birth (risk difference 12.7, 95% CI 4.7 to 25.3) and gestational hypertension (risk difference 6.2, 95% CI 1.4 to 16.8). There was no increased risk for preeclampsia or abnormal fetal growth compared to population controls.
Juvenile idiopathic arthritis (JIA) is the most frequent chronic rheumatic disease in childhood, emerging before 16 years of age (1). It has a heterogenous clinical presentation divided into seven subgroups and affects more girls than boys. A Nordic study reported an incidence of 15 per 100,000 children per year with a median age at onset of 5.5 years (2). Persistent active disease was reported in 53% in early adulthood, on or off medication (3).
Preterm birth is defined as a live birth before 37 weeks of gestation and occurs in approximately 10% of births globally and in close to 6% of births in the Nordic countries (4). Preterm births may be spontaneous or initiated (5). Risk factors for spontaneous preterm birth include higher maternal age, obesity, smoking, maternal stress, earlier preterm birth and intra-amniotic infection or inflammation (6). The decision for initiating preterm birth through induction of labor or caesarean section may be due to one or several factors including maternal comorbidities, obstetric history, and psychosocial factors. In high-income countries most children born preterm reach adulthood. A concern is the association of early adulthood mortality with both early and late preterm birth for cardiovascular and other diseases (4).
Preeclampsia is a hypertensive disorder of pregnancy affecting 2% to 8% of pregnant women around the world and is the cause of substantial maternal and perinatal morbidity and mortality (7). The definition of preeclampsia has changed the last years. Traditionally new-onset hypertension and proteinuria after gestational week 20 were both compulsory findings, while it has later been defined as new onset preeclampsia-associated signs also in the absence of proteinuria (7, 8). In Norway, the prevalence has decreased over two decades, to 2.7% in 2015–2018. A gradual increase in labor induction and aspirin use may have altered the prevalence (9). The revised two-stage placental model of preeclampsia suggest that both early- (<34 gestational weeks) and late-onset preeclampsia (≥34 gestational weeks) result from placental syncytiotrophoblast stress eventually leading to the clinical stage including new-onset hypertension, renal or other organ dysfunction as well as growth restriction of the fetus (8). It incorporates known risk factors for preeclampsia like higher maternal age, nulliparity, diabetes, chronic hypertension, obesity, assisted reproduction, twin pregnancy and some chronic autoimmune diseases that may increase the risk for or accelerate the development of the clinical stage (8).
Abnormal fetal growth resulting in small for gestational age (SGA) is mostly a concern in early-onset preeclampsia. Both SGA and large for gestational age (LGA) are being discussed as risk factors for the growing child and adult life (10). There is increasing evidence that preterm birth, preeclampsia, and abnormal fetal growth increase the risk for maternal cardiovascular disease later in life (11).
Literature on pregnancy outcomes in women with JIA is limited. Three recent European studies (12–14), three American studies (15–17) and one Australian study (18) reported increased risk for preterm birth in women with JIA. Three of the studies also found an increased risk for preeclampsia (14, 15, 18), but only one study found an increased risk for SGA (14). Active disease, medication use or the disease itself are potential causes of the increased risk for pregnancy complications. In one study preterm births occurred in women with disease flares, as defined by increased clinical disease activity prompting intensified therapy (13). Another study reported disease activity based on a patient activity scale (17), and did not find an association with disease activity. The other studies did not have disease activity assessments during pregnancy.
In this study we aimed to explore the possible associations of active disease with preterm birth, preeclampsia, gestational hypertension, and offspring birth weight in women with JIA.
Data from the RevNatus register and the Medical Birth Registry of Norway (MBRN) were linked in this population-based cohort.
RevNatus is a Norwegian nationwide medical quality register operated by The Norwegian National Advisory Unit on Pregnancy and Rheumatic Diseases (NKSR). Women with inflammatory rheumatic diseases are prospectively followed from the time of planning a pregnancy until one year after delivery. Female patients 16 years or older with a rheumatic diagnosis are eligible for inclusion before or during pregnancy. They are followed at the local outpatient rheumatology clinics before pregnancy, in every trimester during pregnancy and three times in the year after birth. Demographic variables, disease activity, medication, laboratory status, obstetric history, pregnancy outcome, self-reported health status and breastfeeding are recorded.
MBRN is a mandatory national health registry. It records information about maternal health preconception and during pregnancy, and complications in the mother and child in the course of pregnancy and birth. Inflammatory rheumatic disease in the mother are coded in MBRN according to the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) (19). Data is accessible about two years after registration.
Singleton births recorded in MBRN 2010 to 2019 qualified for inclusion in the current study.
A written informed consent is required before inclusion in RevNatus. The registry was approved by the Regional Committee for Medical and Health Research Ethics (REK) Mid Norway in 2006. The present study was approved by REK Mid Norway in June 2019 (2019/779/REK Midt) and July 2020 (minor change). Access to data from MBRN was granted in June 2020 (MBRN assignment PDB 2804). Two patient representatives contributed to the outline, development and dissemination plans of the project.
Patient group variables retrieved from RevNatus contained educational status and disease-specific information encompassing disease activity assessment and use of medication. Maternal variables included age, parity, smoking, body mass index (BMI), diabetes, chronic hypertension and assisted reproductive technology (ART) as well as the outcomes preeclampsia, gestational hypertension, preterm birth and z-score for birth weight in the current pregnancy. Variables were obtained from MBRN.
The juvenile arthritis disease activity score (JADAS) could not be utilized, as it is not validated in adults with JIA. We used the Disease Activity Score with CRP (DAS28-CRP-3).The assessment is a composite score including the total tender and swollen joints among 28 joints and CRP (20). It is used in RA and other arthritis and has been validated for use in pregnant women with RA (21). The European alliance of associations for rheumatology (EULAR) has defined the four disease categories remission (<2,6), low disease activity (≥2.6 but ≤3.2), moderate disease activity (>3.2 but ≤5.1) and high disease activity (>5,1) (22). Inactive JIA was defined as DAS28-CRP-3 < 2.6 in 2nd or 3rd trimester and active JIA as DAS28-CRP-3 ≥ 2.6 in 2nd or 3rd trimester.
Preterm birth was defined as birth <37 weeks of gestation, with early preterm birth <34 gestational weeks and late preterm birth ≥34 weeks and <37 gestational weeks.
Preeclampsia was defined according to the MBRN definition at the time the data were collected as new onset blood pressure elevation ≥140/90 mmHg and proteinuria after gestational week 20. Gestational hypertension was defined as new onset blood pressure elevation ≥140/90 mmHg after gestational week 20 without proteinuria.
Z-score for birthweight was based on birth weight, gestational age, and sex. Small for gestational age (SGA) was defined as fetal weight <10th percentile and <2.5th percentile. Large for gestational age (LGA) was defined as fetal weight >90th percentile and >97.5th percentile.
We reported descriptive statistics for the inactive JIA group, active JIA group and population controls as well as disease related characteristics of the inactive JIA group and active JIA group. Pairwise group comparisons of the inactive JIA group with population controls, the active JIA group with the population controls and the active JIA group with the inactive JIA group were performed using independent samples T-test for continuous variables and the Pearson chi squared test, the Fisher's exact test or the unconditional z-pooled test (23) for dichotomous variables.
Proportions and risk differences for the main outcomes preterm birth, late preterm birth, preeclampsia, gestational hypertension, SGA and LGA were calculated comparing the inactive JIA group and active JIA group one at a time with population controls. We calculated 95% confidence intervals (CI) for risk differences using Newcombes method (24). Two-sided p < 0.05 were considered to represent statistical significance, and 95% confidence intervals (CI) are reported where relevant. The statistical analyses were performed using IBM SPSS Statistics for Windows, version 28.0.1, STATA MP 17, and https://www4.stat.ncsu.edu/∼boos/exact/.
There were 196 singleton births in women with JIA registered in RevNatus and MBRN during 2010 to 2019. Disease activity assessment in 3rd trimester was available in 160/196 cases. We added cases with disease activity assessment in 2nd trimester (21/36), while 15 cases did not have data. The resulting 181/196 cases (92.3%) with reported disease activity in 2nd or 3rd trimester (n = 181) were included in this study. Most of the women had inactive JIA, 130/181 (71.8%), while 51/181 (28.2%) had active JIA. After excluding singleton births in mothers with rheumatic inflammatory diseases according to the ICD-10 codes lined out in Supplementary Table S1, the remaining singleton births registered in MBRN during this decade (n = 575,798) served as controls.
Table 1 outlines characteristics known to influence the occurrence of preterm birth, preeclampsia, gestational hypertension, and abnormal fetal growth. Women with inactive JIA were younger and had a higher proportion of nulliparous women compared to population controls. Women with active JIA had a higher proportion of obese women compared to population controls. There were no significant differences when comparing women with inactive JIA and women with active JIA to population controls concerning maternal age >35 years, smoking, diabetes, kidney disease, chronic hypertension, or ART.
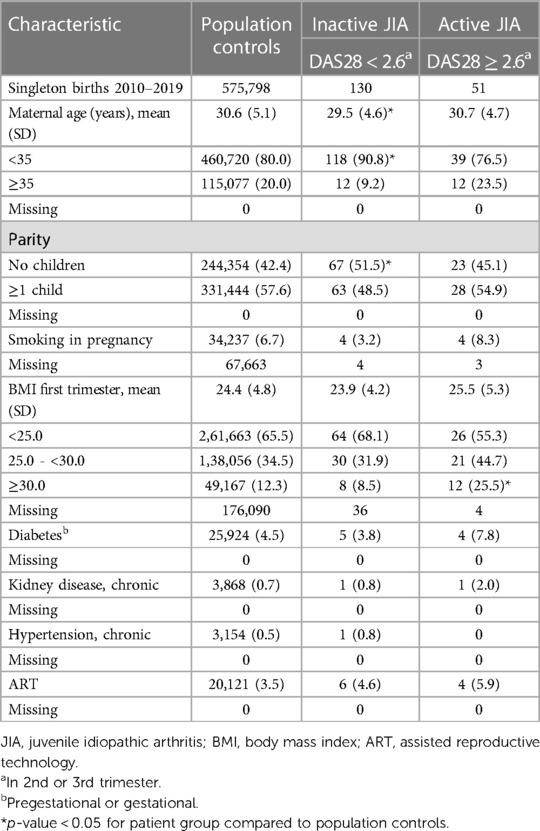
Table 1. Characteristics of controls and patient groups, reported as n (%) unless specified as mean (SD).
In Table 2 disease characteristics in the two disease activity groups are described. Two thirds of the women with JIA had a high educational level. The majority were diagnosed fulfilling the International League Against Rheumatism (ILAR) classification criteria for JIA (1). Women with active JIA had longer disease duration, had more commonly erosive disease and were more often RF or CCP IgG positive compared to women with inactive JIA. A higher proportion of women with active JIA used prednisolone in pregnancy, while the use of the disease modifying medications hydroxychloroquine (HCQ), sulfasalazine (SSZ) and tumor necrosis factor inhibitors (TNFi's) were more frequent in women with inactive JIA. Most women on TNFi's stopped during 1st trimester (data not shown). Approximately half of the women with JIA irrespective of disease activity status used methotrexate and/or a TNFi before pregnancy.
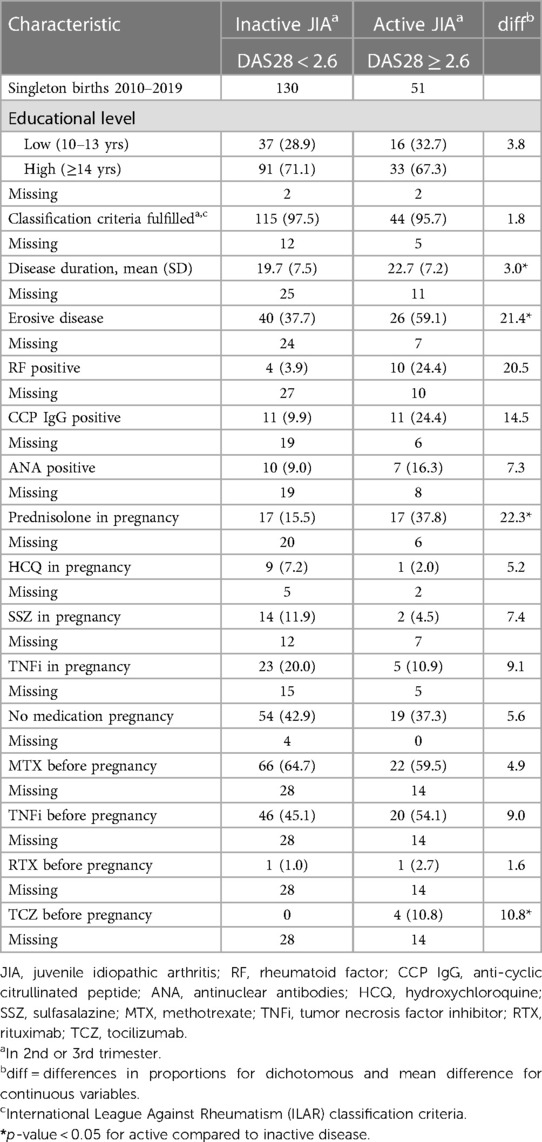
Table 2. Clinical characteristics of patient groups, reported as n (%) unless specified as mean (SD).
Two women with inactive JIA reported use of methotrexate in 1st trimester. In the group of women with active JIA one reported use of rituximab and one use of tocilizumab in 1st trimester (data not shown).
Proportions and risk differences of the outcomes preterm birth, preeclampsia and gestational hypertension are presented in Table 3.
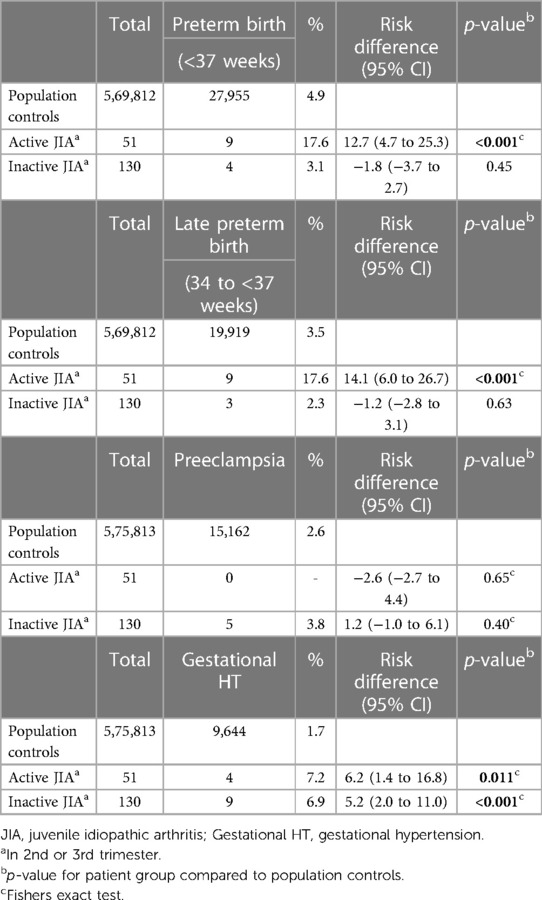
Table 3. Preterm birth, preeclampsia and gestational hypertension, expressed as proportions and risk differences.
Preterm birth occurred more frequently in active JIA (9/51, 17.6%) than in population controls (27,955/569,812, 4.9%), with a risk difference of 12.7%. They were all late preterm. Women with inactive JIA did not have an increased risk of preterm birth compared with population controls. One early preterm birth in week 27 occurred in a woman with inactive JIA and preeclampsia (data not shown).
There was not an increased risk for preeclampsia in women with active or inactive JIA compared with population controls. Gestational hypertension occurred more frequently in active JIA (4/51, 7.2%) and inactive JIA (9/130, 6.9%) compared to population controls (9,644/575,813, 1.7%), with a risk difference of 6.2% and 5.2%, respectively.
Table 4 shows SGA and LGA, looking both at the 10 percentile/90 percentile (z-score −1.28 to 1.28, <−1.3 and >1.3) and the 2.5 percentile/97.5 percentile (z-score −1.96 to 1.96, <−2.0 and >2.0). There were no differences in offspring of women with active or inactive JIA compared to offspring of population controls.
In the present study we found an increased risk for preterm birth in women with JIA. This is in accordance with earlier studies (12–18). More importantly, we found that active JIA increases the risk for preterm birth, whereas inactive JIA does not.
Women with JIA had stable low disease activity or inactive disease in pregnancy. This has also been demonstrated in other cohorts of pregnant women with JIA the last decades (12, 13, 25–27). A possible association with active JIA in pregnancy has been investigated in two earlier studies, with conflicting results (13, 17). One study found no association between preterm birth and active disease. The authors argue that the disease activity assessment was limited as it did not include joint count and CRP and may have underestimated disease activity (17). Our findings are in alignment with a small study of 22 pregnancies (13) and provides evidence to the notion that disease activity matters.
Inactive disease was not found to be associated with preterm birth. The women with inactive JIA had a lower proportion of preterm birth compared to population controls. This might be due to tight control with a focus on disease specific as well as other risk factors and is reassuring, underpinning the importance of “treating to target” also in pregnancy, aiming for inactive disease (28).
All preterm births in women with active JIA in our study were late preterm (pregnancy weeks 34–36). This has also been reported in recent previous studies (13, 15, 17). This finding may be related to better disease control and less inflammation. In cohorts from earlier time-periods preterm birth was seen both early (<34 weeks) and late (34–36 weeks) (14), indicating less controlled disease.
Preterm birth can be divided into phenotypes such as preterm prelabor rupture of membranes (PPROM), medically indicated and spontaneous preterm birth. We did not have information about these phenotypes in our study, but one previous study found increased risk of late (pregnancy weeks 32–36) PPROM and medically indicated preterm birth in women with JIA, while spontaneous preterm birth occurred more frequently both early (pregnancy weeks 20–31) and late preterm (15). Inflammation is a key factor for both PPROM and spontaneous preterm birth (6) suggesting that inflammatory active disease in the mother can be a relevant risk factor.
We found no cases of preeclampsia in women with active JIA. However, we found two cases of gestational hypertension that might have resulted in medically indicated preterm birth. Cesarean section was not performed in these two cases, and we do not know whether they were induced or not. However, five of the remaining seven preterm births in women with active JIA had emergency cesarean section of unknown reason. In Norway active JIA may be an indication for induction of labor, and very rarely for elective cesarean section. Induction of labor due to active JIA will usually be done at gestational weeks 39–40.
Women with active JIA had a higher proportion of obesity than population controls. However, there were no preterm births in obese women with active JIA (data not shown).
In two studies, prednisolone use has been discussed as a possible marker of disease activity and a risk factor for preterm birth (12, 17). In our study, a higher proportion of women with active JIA using prednisolone in pregnancy had preterm birth compared to non-users, though not of statistical significance (p = 0.053). The proportions were similar for women with inactive JIA irrespective of prednisolone use, see online Supplementary Table S2. This indicates that active disease is a risk factor, irrespective of prednisolone use.
There are conflicting results concerning the risk of preeclampsia in women with JIA. A large Swedish prospective study found a strong association between JIA persisting into adulthood and preeclampsia (14), arguing that medication use and active disease were important factors, although there was no available information on these factors in the study. Two other studies have reported increased risk (15, 18). However, these data were from the era before the guidelines of tight follow up and rigorous treatment in pregnancy were implemented, with a higher proportion of women with active disease during pregnancy. Studies with no increased risk of preeclampsia (12, 13, 17) have data from a later time span with better disease control. Our patients with active JIA had low disease activity, favoring less complications.
In Norway, the prevalence of preeclampsia has gradually decreased the last 20 years (9). In 2020 preeclampsia occurred in 2.6%, with only 0.9% being preterm (29). The last maternal death due to preeclampsia was reported in 2012 (9). This is in contrast to many other countries including USA, where gestational hypertension is increasing, and maternal mortality has increased with 50% during the same time period (30). The decline in preeclampsia in Norway has happened despite a parallel increased proportion of women with risk factors for preeclampsia such as advanced age, nulliparity and use of ART (9). Autoimmune disease is another risk factor considered in the risk assessment early in pregnancy (9). Tighter follow up of patients with risk factors, and treatment with aspirin and/or labor induction when indicated, may explain low rates of preeclampsia in women with JIA.
In our study gestational hypertension was more frequent in active JIA than population controls. Two of four pregnancies with gestational hypertension in active JIA ended up as late preterm birth. A theoretical interpretation that has been discussed in a two-stage placental model of preeclampsia is that preterm birth might prevent clinical manifestations of preeclampsia to evolve (8). There was an increased risk of gestational hypertension also in women with inactive JIA. A higher proportion of nulliparous women in this group may have contributed to the increased risk.
We did not find an increased risk for SGA in offspring of our patients compared to offspring of population controls. Remaus et al. found such an association (14), probably due to less controlled disease. Our findings are reassuring and in line with the other findings of late preterm birth and no increased risk of preeclampsia.
A high proportion of women with JIA reported treatment with methotrexate before pregnancy, indicating the need for disease specific medication. The proportion of women on TNFi's preconception was also high. However, the proportion of women on pregnancy compatible medication during pregnancy was much lower, indicating undertreatment. One reason might be little documentation during the first years of the study on the use of TNFi's. A tight follow up and proper information may potentially improve outcomes by suppressing disease.
There are several risk factors for hypertensive disorders, preterm birth and abnormal fetal weight. In this study the exposure was JIA, and we did not find it relevant to adjust for the risk factors presented in Table 1, as we do not consider these variables as confounders. Risk assessment including these risk factors is performed early in pregnancy (29). In women with active JIA, obesity was more common than in population controls. Smoking was reported in small numbers in all groups. Active disease, overweight and smoking are all modifiable risk factors that should be taken into account in the preconception planning and counselling.
A major strength of this study is the disease activity assessment during pregnancy. Further, the prospective follow up, a large patient group and the linkage of two registries improves the quality.
A limitation is that there are no validated disease activity assessments for pregnant women with JIA. DAS28-CRP-3 is considered to be reliable for assessing disease activity in JIA (31). However, it does not evaluate ancles, toes and jaw, joints that may be affected in JIA. We may therefore have underestimated disease activity in some patients. We used disease activity assessment only in the second part of pregnancy and do not know how active disease early in pregnancy might impact on the evolvement of hypertensive disorders later in pregnancy. Another limitation is that we did not have access to the subtypes of JIA. Potentially, such information could shed light on possible differences in disease characteristics, disease activity, general risk factors and pregnancy complications between subtypes.
In this prospective cohort of women with JIA, active disease in the second part of pregnancy increased the risk for late preterm birth and gestational hypertension. Preeclampsia and SGA in offspring occurred in similar rates as population controls. Tight control targeting inactive disease is advocated.
The datasets presented in this article are not readily available. The data cannot be shared publicly due to the requirements of the involved register holders and the general data protection regulation, to protect the privacy of individuals. Requests to access the datasets should be directed to https://www.fhi.no/en/.
The studies involving humans were approved by the Regional Committee for Medical and Health Research Ethics (REK) Mid Norway. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
CG: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. SL: Conceptualization, Formal Analysis, Methodology, Writing – review & editing. KS: Conceptualization, Writing – review & editing, Supervision. MW: Conceptualization, Supervision, Visualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by The Norwegian Women's Public Health Association (grant number 70076). They were not involved in the collection, analysis, or interpretation of the data, nor the writing or submission for publication.
The authors thank the Medical Birth Registry of Norway (MBRN) for providing data and the Norwegian Women's Public Health Association for funding the project. We thank Hege S. Koksvik and Bente Jakobsen at The Norwegian National Advisory Unit on Pregnancy and Rheumatic Diseases for facilitating the access to RevNatus data. We also thank the participating departments of rheumatology at the following hospitals for including patients in RevNatus: Betanien Hospital, Skien; Diakonhjemmet Hospital, Oslo; Haugesund Sanitetsforenings Rheumatism Hospital, Haugesund; Haukeland University Hospital, Bergen; Helse Førde, Førde Hospital, Førde; Helse Møre og Romsdal, Ålesund hospital, Ålesund; Lillehammer Hospital for Rheumatic Diseases, Lillehammer; Nordland Hospital, Bodø; St. Olavs hospital Trondheim University Hospital, Trondheim; Sørlandet Hospital Kristiansand, Kristiansand; University Hospital of North Norway, Tromsø; Vestre Viken Hospital, Drammen; Østfold Hospital, Moss; Helgelandssykehuset, Mo i Rana, Levanger Hospital, Levanger.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/flupu.2024.1375857/full#supplementary-material
1. Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. (2004) 31(2):390–2.14760812
2. Nordal E, Zak M, Aalto K, Berntson L, Fasth A, Herlin T, et al. Ongoing disease activity and changing categories in a long-term nordic cohort study of juvenile idiopathic arthritis. Arthritis Rheum. (2011) 63(9):2809–18. doi: 10.1002/art.30426
3. Glerup M, Rypdal V, Arnstad ED, Ekelund M, Peltoniemi S, Aalto K, et al. Long-term outcomes in juvenile idiopathic arthritis: eighteen years of follow-up in the population-based nordic juvenile idiopathic arthritis cohort. Arthritis Care Res (Hoboken). (2020) 72(4):507–16. doi: 10.1002/acr.23853
4. Risnes K, Bilsteen JF, Brown P, Pulakka A, Andersen AN, Opdahl S, et al. Mortality among young adults born preterm and early term in 4 nordic nations. JAMA Netw Open. (2021) 4(1):e2032779. doi: 10.1001/jamanetworkopen.2020.32779
5. Walani SR. Global burden of preterm birth. Int J Gynaecol Obstet. (2020) 150(1):31–3. doi: 10.1002/ijgo.13195
6. Cobo T, Kacerovsky M, Jacobsson B. Risk factors for spontaneous preterm delivery. Int J Gynaecol Obstet. (2020) 150(1):17–23. doi: 10.1002/ijgo.13184
7. Ives CW, Sinkey R, Rajapreyar I, Tita ATN, Oparil S. Preeclampsia-pathophysiology and clinical presentations: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 76(14):1690–702. doi: 10.1016/j.jacc.2020.08.014
8. Staff AC. The two-stage placental model of preeclampsia: an update. J Reprod Immunol. (2019) 134-135:1–10. doi: 10.1016/j.jri.2019.07.004
9. Sole KB, Staff AC, Räisänen S, Laine K. Substantial decrease in preeclampsia prevalence and risk over two decades: a population-based study of 1,153,227 deliveries in Norway. Pregnancy Hypertens. (2022) 28:21–7. doi: 10.1016/j.preghy.2022.02.001
10. Damhuis SE, Ganzevoort W, Gordijn SJ. Abnormal fetal growth: small for gestational age, fetal growth restriction, large for gestational age: definitions and epidemiology. Obstet Gynecol Clin North Am. (2021) 48(2):267–79. doi: 10.1016/j.ogc.2021.02.002
11. Seid AK, Morken NH, Klungsøyr K, Kvalvik LG, Sorbye LM, Vatten LJ, et al. Pregnancy complications in last pregnancy and mothers’ long-term cardiovascular mortality: does the relation differ from that of complications in first pregnancy? A population-based study. BMC Womens Health. (2023) 23(1):355. doi: 10.1186/s12905-023-02503-z
12. Drechsel P, Stüdemann K, Niewerth M, Horneff G, Fischer-Betz R, Seipelt E, et al. Pregnancy outcomes in DMARD-exposed patients with juvenile idiopathic arthritis-results from a JIA biologic registry. Rheumatology (Oxford). (2020) 59(3):603–12. doi: 10.1093/rheumatology/kez309
13. García-Fernández A, Gerardi MC, Crisafulli F, Filippini M, Fredi M, Gorla R, et al. Disease course and obstetric outcomes of pregnancies in juvenile idiopathic arthritis: are there any differences among disease subtypes? A single-centre retrospective study of prospectively followed pregnancies in a dedicated pregnancy clinic. Clin Rheumatol. (2021) 40(1):239–44. doi: 10.1007/s10067-020-05404-w
14. Remaeus K, Johansson K, Askling J, Stephansson O. Juvenile onset arthritis and pregnancy outcome: a population-based cohort study. Ann Rheum Dis. (2017) 76(11):1809–14. doi: 10.1136/annrheumdis-2016-210879
15. Kolstad KD, Mayo JA, Chung L, Chaichian Y, Kelly VM, Druzin M, et al. Preterm birth phenotypes in women with autoimmune rheumatic diseases: a population-based cohort study. BJOG. (2020) 127(1):70–8. doi: 10.1111/1471-0528.15970
16. Mohamed MA, Goldman C, El-Dib M, Aly H. Maternal juvenile rheumatoid arthritis may be associated with preterm birth but not poor fetal growth. J Perinatol. (2016) 36(4):268–71. doi: 10.1038/jp.2015.193
17. Smith CJF, Förger F, Bandoli G, Chambers CD. Factors associated with preterm delivery among women with rheumatoid arthritis and women with juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). (2019) 71(8):1019–27. doi: 10.1002/acr.23730
18. Chen JS, Ford JB, Roberts CL, Simpson JM, March LM. Pregnancy outcomes in women with juvenile idiopathic arthritis: a population-based study. Rheumatology (Oxford). (2013) 52(6):1119–25. doi: 10.1093/rheumatology/kes428
19. Irgens LM. The medical birth registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand. (2000) 79(6):435–9. doi: 10.1034/j.1600-0412.2000.079006435.x
20. Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. (1995) 38(1):44–8. doi: 10.1002/art.1780380107
21. de Man YA, Hazes JM, van de Geijn FE, Krommenhoek C, Dolhain RJ. Measuring disease activity and functionality during pregnancy in patients with rheumatoid arthritis. Arthritis Rheum. (2007) 57(5):716–22. doi: 10.1002/art.22773
22. van Gestel AM, Prevoo ML, van ‘t Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European league against rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American college of rheumatology and the world health organization/international league against rheumatism criteria. Arthritis Rheum. (1996) 39(1):34–40. doi: 10.1002/art.1780390105
23. Lydersen S, Langaas M, Bakke O. The exact unconditional z-pooled test for equality of two binomial probabilities: optimal choice of the berger and boos confidence coefficient. J Stat Comput Simul. (2012) 82(9):1311–6. doi: 10.1080/00949655.2011.579969
24. Fagerland MW, Lydersen S, Laake P. Recommended confidence intervals for two independent binomial proportions. Stat Methods Med Res. (2015) 24(2):224–54. doi: 10.1177/0962280211415469
25. Ursin K, Lydersen S, Skomsvoll JF, Wallenius M. Disease activity of juvenile idiopathic arthritis during and after pregnancy: a prospective multicenter study. J Rheumatol. (2017) 45(2):257–65. doi: 10.3899/jrheum.161410
26. Gerosa M, Chighizola CB, Pregnolato F, Pontikaki I, Luppino AF, Argolini LM, et al. Pregnancy in juvenile idiopathic arthritis: maternal and foetal outcome, and impact on disease activity. Ther Adv Musculoskelet Dis. (2022) 14:1759720X221080375. doi: 10.1177/1759720X221080375
27. Förger F, Bandoli G, Luo Y, Robinson L, Johnson DL, Chambers CD. No association of discontinuing tumor necrosis factor inhibitors before gestational week twenty in well-controlled rheumatoid arthritis and juvenile idiopathic arthritis with a disease worsening in late pregnancy. Arthritis Rheumatol. (2019) 71(6):901–7. doi: 10.1002/art.40821
28. Gotestam Skorpen C, Hoeltzenbein M, Tincani A, Fischer-Betz R, Elefant E, Chambers C, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. (2016) 75(5):795–810. doi: 10.1136/annrheumdis-2015-208840
29. Sverre JM, Smedslund G, Stoinska-Schneider AK. First-Trimester Screening for Preeclampsia with the Use of an Algorithm: A Health Technology Assessment. Oslo, Norway: Norwegian Institute of Public Health (2022).
30. Kassebaum N. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet. (2016) 388(10053):1775–812. doi: 10.1016/S0140-6736(16)31470-2
Keywords: juvenile idiopathic arthritis, inflammation, pregnancy and rheumatic disease, epidemiology, women's health
Citation: Götestam Skorpen C, Lydersen S, Salvesen KÅ and Wallenius M (2024) Preterm birth, preeclampsia, gestational hypertension and offspring birth weight in women with active juvenile idiopathic arthritis and healthy controls. Front. Lupus 2:1375857. doi: 10.3389/flupu.2024.1375857
Received: 24 January 2024; Accepted: 21 March 2024;
Published: 4 April 2024.
Edited by:
Laura Andreoli, University of Brescia, ItalyReviewed by:
Maria Chiara Gerardi, Niguarda Ca 'Granda Hospital, Italy© 2024 Götestam Skorpen, Lydersen, Salvesen and Wallenius. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carina Götestam Skorpen Y2FyaW5hLnNrb3JwZW5AbnRudS5ubw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.