- Rheumatology Section, Internal Medicine Department, Hamad Medical Corporation, Doha, Qatar
Introduction: The presence of anti-Ro/SSA antibodies is associated with an increased risk of adverse maternal and neonatal outcomes in patients with autoimmune rheumatic diseases. We evaluated the rate of adverse maternal and neonatal outcomes in a cohort of pregnant women with positive anti-Ro/SSA antibodies in Qatar and explored the significance of neonatal anti-Ro/SSA antibodies.
Methods: This retrospective observational study was conducted at the largest tertiary institute in Qatar between July 2016 and January 2021. The study included pregnant women with confirmed anti- Ro/SSA positivity who were consistently followed to evaluate maternal and fetal complications.
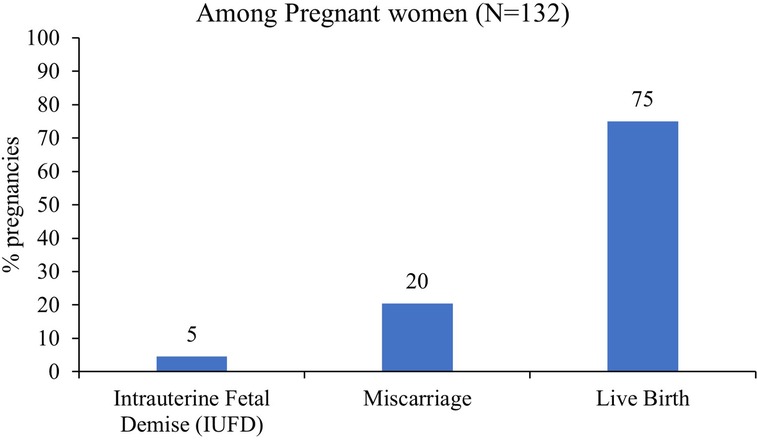
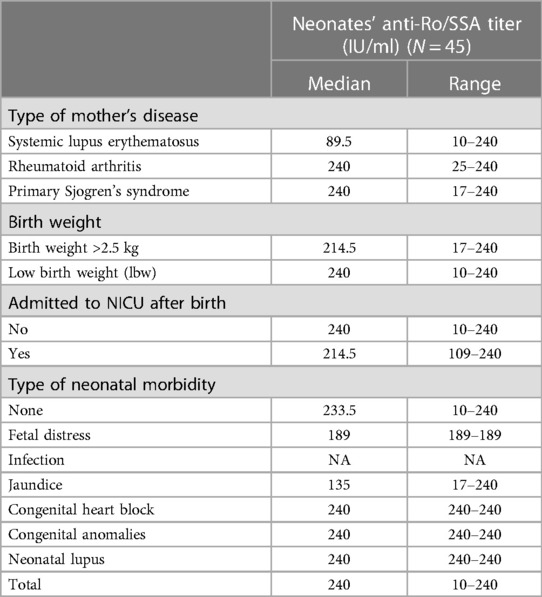
Results: One-hundred-thirty-two pregnancies from 79 women were included in the analysis. Anti-Ro/SSA positivity was observed in all pregnancies, whereas anti-LA/SSB positivity only in 23.5% of pregnancies. Of the 132 pregnancies, 99 (75%), 27 (20%), and 6 (4.6%) resulted in live birth, miscarriage, and intrauterine fetal demise (IUFD), respectively. Among the 99 live births, serology testing for anti-Ro/SSA was performed on 84 neonates, of which only 45 were positive. Neonates born to mothers with primary SS and rheumatoid arthritis (RA) had higher median antibody titers (240 IU/ml) than those born to mothers with systemic lupus erythematosus (SLE) (89.5 IU/ml)). The rheumatic diseases (SS, RA, or SLE) showed no significant correlation with adverse pregnancy and fetal outcomes. Congenital heart block (CHB) was recorded in only two infants (2%), and one infant had neonatal lupus (1%). Interestingly, CHB was only observed in previously asymptomatic women who were subsequently diagnosed with SS. Most women (85.9%) were treated with hydroxychloroquine throughout pregnancy. These women had lower rates of miscarriage and neither of their infants presented with CHB.
Conclusion: Miscarriage is the most common adverse outcome reported in this cohort. The incidence of CHB was among asymptomatic carriers only. Nevertheless, hydroxychloroquine use seems to lower the vulnerability to these adverse events. However, these findings need to be validated in larger controlled cohorts. This study is one of few to report results on neonatal anti-Ro/SSA antibody testing.
1 Introduction
Antibodies targeting specific cellular proteins in human connective tissues are valuable diagnostic and prognostic markers of numerous autoimmune diseases (1). However, antibodies do not cause direct harm. A classic example of autoantibodies playing a direct role in disease pathogenesis is the anti-Ro/SSA antibodies (2). Although these antibodies are prevalent in the general population, they are not capable of causing disease in most healthy individuals. Their significance becomes evident during pregnancy, as they can cross the placenta and potentially affect the fetal heart (3) and other tissues such as the skin, liver, and hematopoietic system (4).
These antibodies are associated with the development of congenital heart block (CHB) and neonatal lupus syndrome, while these conditions are both rare and transient (2, 5). Early detection of these complications can aid in the management of first- and second-degree heart blocks. Regular surveillance of the fetal heart rate is considered the standard of care in specialized medical centers. In cases of a complete heart block, a high risk of intrauterine fetal demise (IUFD) and neonatal death exists. Dexamethasone and intravenous immunoglobulin (IVIG) are among the drugs commonly used in the management of these complications (6, 7). Consequently, these fetuses are often delivered prematurely, while the placement of permanent pacemakers is required to ensure adequate cardiac function (8, 9)
Hydroxychloroquine is commonly used to treat autoimmune diseases. Many studies support its benefits in preventing CHB recurrence in infants of women positive for anti-Ro/SSA antibody. However, there is not yet enough evidence for the use of hydroxychloroquine as a primary prevention of CHB (5).
Other obstetric complications such as pre-eclampsia, miscarriage, and preterm labor are frequently observed in patients with autoimmune diseases. Limited data is available regarding the association between these complications and anti-Ro/SSA positivity (5, 10). However, advancements in treatment strategies in the field of rheumatology allow many women with autoimmune diseases to conceive (11).
Research on obstetric and fetal complications in patients with anti-Ro/SSA positivity is of interest in optimizing patient care and improving pregnancy outcomes. However, current evidence regarding this subject is scarce. To address this knowledge gap, we aimed to describe the obstetric and fetal complications in patients with anti-Ro/SSA positivity in relation to their underlying diseases and anti-rheumatic medications in a cohort of pregnant women from Qatar.
2 Materials and methods
2.1 Study design and participants
This national, observational, retrospective study included pregnant women who were followed-up at a specialized pregnancy and rheumatic disease clinic using a standardized approach and management, located at the Hamad Medical Corporation, the largest tertiary institute in Qatar. Pregnancy and rheumatic disease clinics have been well established at Hamad General Hospital since 2005 and are jointly managed by rheumatology experts in collaboration with high-risk obstetric and fetal medicine clinics.
To mitigate potential selection bias inherent in retrospective studies, the patients list was reviewed between July 2016 and January 2021, and each consecutive patient was screened for eligibility. The inclusion criterion for the study was any pregnant woman with anti-Ro/SSA positivity and those who were actively monitored at the pregnancy and rheumatic disease clinic and had documented pregnancy and neonatal outcomes. Pregnant women with anti-Ro/SSA positivity diagnosed according to the American College of Rheumatology classification criteria were categorized based on their underlying disease a s SS, SLE, or RA. Notably, if the same woman had more than one pregnancy within the specified period, each pregnancy was considered as a separate case.
2.2 Sample size
The cohort size was based on the number of pregnancies identified during the study period, because the presence of anti-Ro/SSA during pregnancy is rare in the general population. The total number of pregnancies included in this study was 132, which is consistent with the numbers typically reported in other specialized centers worldwide during the same time period. As this study was retrospective and exploratory in nature, it aimed to generate a hypothesis; thus, formal sample size calculation was not implemented.
2.3 Data collection
Retrospective data were collected from the electronic records of pregnant women who visited the clinic between July 2016 and December 2021. Baseline characteristics such as patients' demographics, underlying rheumatic disease type, pregnancy complications, medication during pregnancy, and autoimmune serology profiles, including anti-Ro/SSA and anti LA/SSB titer, were obtained. The outcome measures included maternal, fetal and neonatal outcomes and correlation between antibody titers and medication during pregnancy. Maternal adverse events included pre-eclampsia (new-onset hypertension with systolic and/or diastolic blood pressure greater than or equal to 140 mmHg and 90 mmHg, respectively, after 20 weeks of gestation with proteinuria and/or end-organ dysfunction), eclampsia (defined as new-onset generalized tonic-clonic seizures in a woman with pre-eclampsia), and gestational diabetes (impaired glucose tolerance test after 20 weeks of gestation). Fetal outcomes included live birth, IUFD (IUFD that occurs at or greater than 20 weeks of gestation or at a birth weight greater than or equal to 350 g), miscarriage (spontaneous pregnancy loss at any time from conception until 24 weeks of gestation), preterm delivery (delivery of a live birth before 37 weeks of gestation), term delivery (labor of a live birth at or after 37 weeks of gestation), and low birth weight (birth weight less than 2.5 kg). Neonatal morbidities included any illness requiring treatment, intervention, or monitoring during theneonatal period. Particular attention was paid to CHB and neonatal lupus (NL).
2.4 Statistical analysis
Continuous and categorical data were analyzed using descriptive statistics, including frequencies and percentages. The Shapiro-Wilk test was used to assess data normality. Sub-group comparisons were conducted with a type-I error risk of 5%. The student's t-test was used for normally distributed variables, whereas the Wilcoxon/Mann-Whitney test was used for non-normally distributed quantitative variables. For categorical variables, the chi-square test or Fisher's exact test was used. The results were analyzed using the STATA 13 software (Stata Statistical Software: Release 13. College Station, TX: StataCorp LLC.), and a two-tailed p-value of <0.05 was considered statistically significant.
2.5 Ethical considerations
This study adhered to the principles of the Declaration of Helsinki and Good Clinical Practice and the laws and regulations of the Ministry of Public Health, Qatar. The study was approved by the Institutional Review Board of the Medical Research Centre at Hamad General Hospital (MRC 01- 21-130). Due to its retrospective nature, the study was exempted from obtaining informed consent.
3 Results
3.1 Characteristics of the participants
One-hundred-thirty-two pregnancies from 79 women were included in the analysis. The median age at disease diagnosis was 29 years, whereas the median age at pregnancy was 32 years in the selected cohort (Table 1). In this study, participants had a maximum of four pregnancies (an average of two pregnancies following disease diagnosis. Given that Anti-Ro/SSA positivity was a requirement for inclusion, in all 132 pregnancies results were positive. However, LA-positivity was observed only in 31 (23.5%) pregnant women. In the selected samples, the median Ro/SSA antibody titer was 240 IU/ml), ranging from 9.4 to 282 IU/ml), and the median LA antibody titer was 84 IU/ml), ranging from 9.7 to 320 IU/ml). Among the included women, 64 (48.5%) had primary SS, 11 (8.3%) RA, and 57 (43.1%) SLE. Overall, 68% of the participants were from Arab nations and 32% were from other nations in Europe, America, and Asia. There were 15 maternal adverse events, including pre-eclampsia/eclampsia (4.6%) and gestational diabetes (6.9%). A significant proportion of women received folic acid during pregnancy (93.8%), followed by hydroxychloroquine (85.9%), and vitamin D (82.0%). Of the 132 pregnancies, 99 (75%) resulted in live births, 27 (20%) ended in miscarriages, and 6 (4.6%) resulted in IUFD (Figure 1).
3.2 Fetal outcomes and neonatal morbidity
Of the 99 live births, 82 (75%) were full term and 17 (16.5%) preterm births (Table 2). Among the live births, 56 (56.7%) were delivered vaginally, 35 (35.1%) were via cesarean section, and 8 (8.3%) required assisted delivery. Most babies had a birth weight of 2.5 kg or above, with only 12 babies (12.4%) classified as low birth weight (<2.5 kg). A total of 23 pregnancies presented at least one neonatal morbidity. Among these cases, three (3.1%) had fetal distress, one (1%) infection, 12 (12.4%) jaundice, and four (4.1%) congenital anomalies. Additionally, two live births developed CHB and one had neonatal lupus.
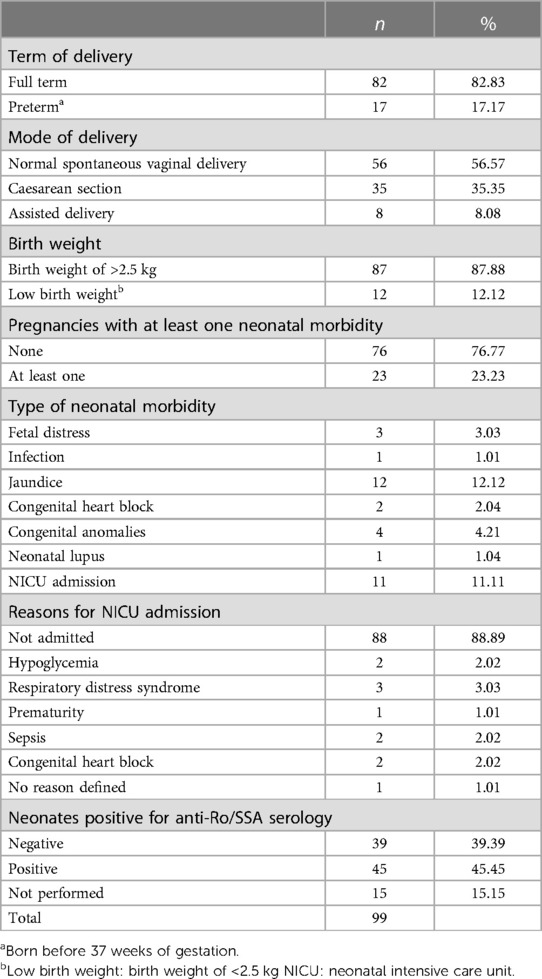
Table 2. Obstetric outcomes and neonatal morbidity among pregnant women with anti-Ro/SSA antibody positivity.
Eleven infants required admission to the neonatal intensive care unit (NICU): two for hypoglycemia, three for respiratory distress syndrome, two for sepsis, and two for CHB.
A total of 45 newborns were tested positive for anti-Ro/SSA antibodies. The anti-Ro/SSA titers in newborns ranged from 10 to 240 IU/ml) (median, 240 IU/ml); Table 3). When examining the neonates' anti-Ro/SSA titers based on the mothers' underlying diseases, we found that neonates born to mothers with primary SS and RA had a higher median titer of 240 IU/ml) than those born to mothers with SLE (89.5 IU/ml)).
3.3 Case presentation of congenital heart block and neonatal lupus
CHB was diagnosed in two neonates of two previously asymptomatic mothers who were found to have an anti-Ro/SSA titer >240 IU/ml) upon investigation of abnormal fetal heart rate (at 26 weeks of gestation). Subsequently, both mothers were diagnosed with primary SS during the first year of postpartum follow-up.
The first woman, who also had gestational diabetes, delivered a fetus weighing 3 kg with a complete heart block via assisted delivery at 37 weeks. In addition to hydroxychloroquine administration at 26 weeks of gestation, no additional treatment was provided during pregnancy. The neonate required NICU admission for 4 days. Although bradycardia persisted throughout infancy, permanent pacemaker insertion was not required. Unfortunately, the anti-Ro/SSA antibody testing was not performed at birth.
The second woman, who had an additional comorbidity of hypothyroidism, received betamethasone and hydroxychloroquine following the diagnosis of congenital heart block in her neonate at 26 weeks of gestation. Despite treatment, the complete heart block in the fetus was not reversed. She delivered via cesarean section at 38 weeks of age and the newborn weighed 2.8 kg. The neonate was admitted to the NICU for 1 week and required permanent pacemaker insertion. The infant tested positive for Ro antibodies with a neonatal anti-Ro/SSA titer of 240 IU/ml). Interestingly, each of these mothers had another pregnancy documented during the study period, and, while on hydroxychloroquine, they delivered healthy babies without a CHB. Furthermore, another newborn was diagnosed with neonatal lupus, while the sole manifestation was a skin rash without organ involvement. The rash resolved spontaneously. In this case, the mother of the baby was diagnosed with primary SS and presented an anti-Ro/SSA titer of 240 IU/ml). The delivery mode was a cesarean section, and the neonate weighed 3.5 kg. The newborn infant was tested positive for anti-Ro/SSA antibodies, with a titer of 240 IU/ml). The mother received hydroxychloroquine throughout pregnancy, while the neonate was not admitted to the NICU.
3.4 Adverse obstetric outcomes and neonatal morbidity in different disease subgroups
The adverse outcomes, delivery characteristics, and neonatal morbiditywere compared between mothers diagnosed with SLE, RA, and SS (Table 4). The analysis did not reveal any significant association between the mother's primary disease and pregnancy outcomes, including IUFD, miscarriage, live births as well as pregnancy-related complications, mode of delivery, neonatal birth weight, and neonatal morbidity. However, a significant association was found between the type of maternal disease and the neonates who were tested positive for anti-Ro/SSA antibodies. The incidence of preterm birth, lower birth weight, neonatal morbidity, and admission to the NICU after birth was higher among neonates with a positive anti-Ro/SSA serology than among those in which Ro/SSA antibodies were not detected (Table 5). However, this association was not statistically significant.
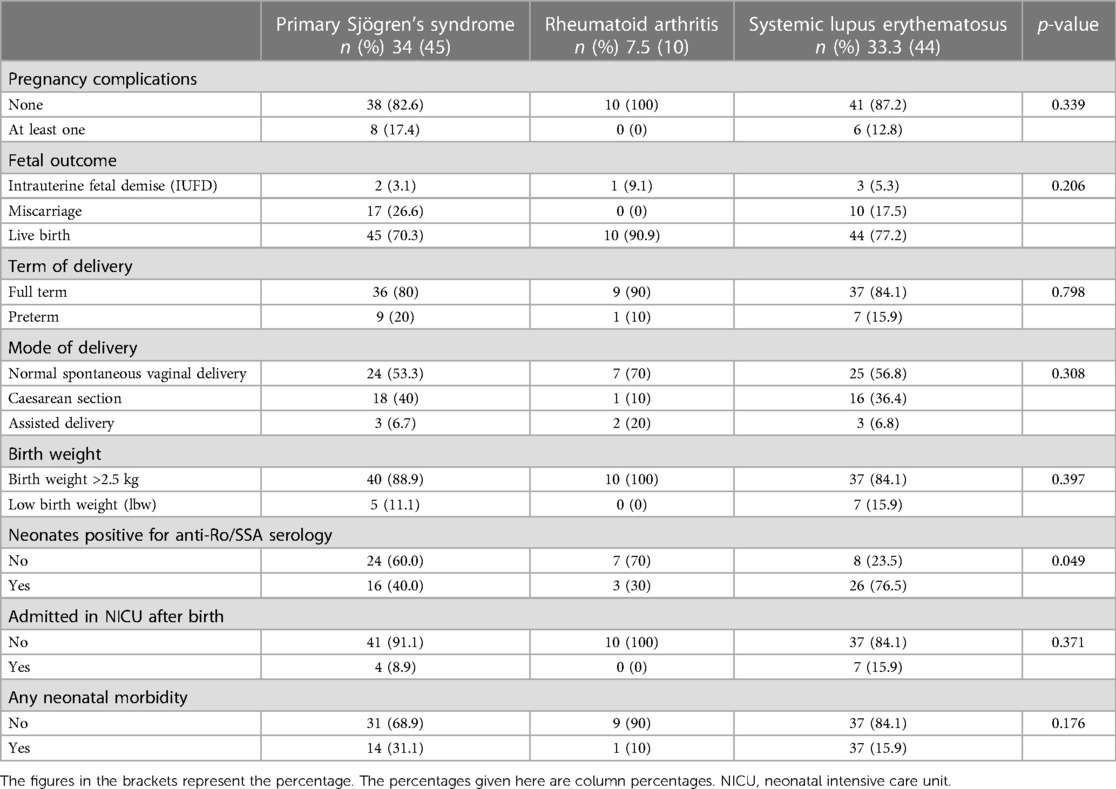
Table 4. Association between the type of rheumatic disease among pregnant women and pregnancy outcomes, complications, and neonatal morbidities.
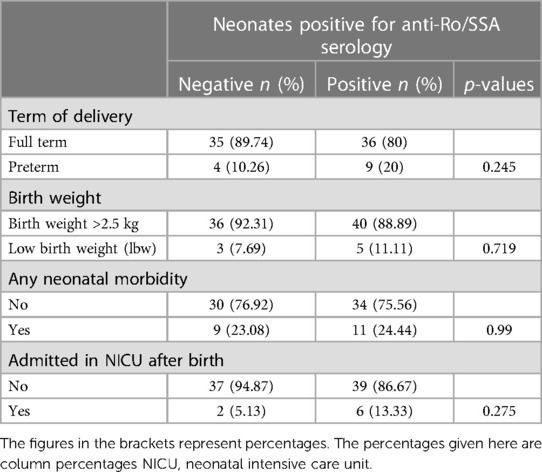
Table 5. Association between neonates’ positivity for anti-Ro/SSA serology and term of delivery, birth weight, neonatal morbidities, and NICU admission.
3.5 Adverse obstetric outcomes and morbidities in different treatment subgroups
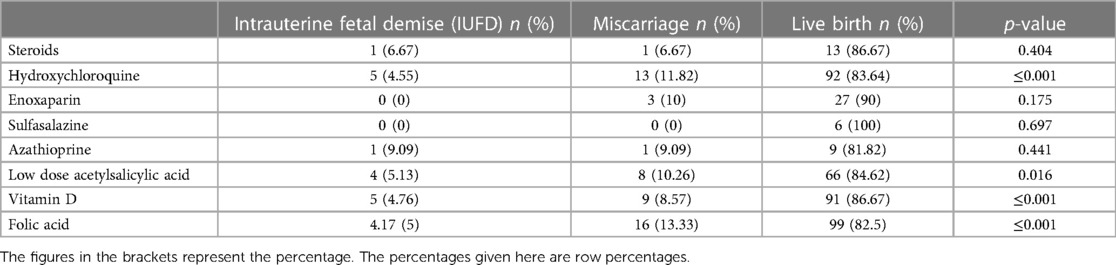
Pregnancy outcomes were also examined based on the medications prescribed for the mothers during pregnancy (Table 6). The study found no correlation between sulfasalazine, azathioprine, steroids, and enoxaparin sodium use and any pregnancy outcome. However, significantly lower miscarriage rates (p < 0.05) were observed in patients who received hydroxychloroquine (11.8%), low-dose acetylsalicylic acid (10.3%), vitamin D (8.6%), or folic acid (13.3%) than in those who did not receive any treatment. The results of the multivariate logistic regression analysis, which aimed to identify the risk factors for miscarriage (Table 7), indicated that women in which hydroxychloroquine (OR = 0.11) or Vitamin D (OR = 0.07) was administered exhibited significantly reduced odds of miscarriage.
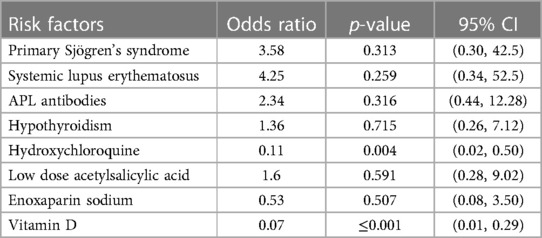
Table 7. Logistic regression analysis of associated risk factors for miscarriage among pregnant women with auto- immune disease.
4 Discussion
We aimed to provide a comprehensive analysis of the profile of pregnant women who were tested positive for anti-Ro/SSA antibodies and investigated the outcomes of their pregnancies. By describing the specific characteristics of this group, this study aimed to shed light on the potential implications and effects of anti-Ro/SSA antibodies during pregnancy. Additionally, this study examined the various pregnancy outcomes experienced by these women, offering valuable insights into the associated risks and challenges they may face during gestation. We aimed to demonstrate the existence of a relationship between anti-Ro/SSA antibodies and pregnancy outcomes; thus, affecting, potentially, future treatment approaches in this unique population.
Anti-Ro/SSA is a specific type of antinuclear antibody often associated with various conditions, including primary SS, SLE, and RA (12). In this study cohort, most women with anti-Ro/SSA antibodies had primary SS, followed by those with SLE.
Autoimmune diseases influence pregnancy outcomes, increasing the risk of miscarriages and premature labor (13, 14). These effects may be attributed to the potential impact of therapy on the fetus and the passage of autoantibodies through the placenta (15, 16). Consistent with findings of other studies, in the present study, we observed that of the total pregnancies, 20% and 5% resulted in miscarriage and IUFD, respectively, while 17% of 99 live births were preterm newborns. The rate of prematurity in this study is comparable with that reported in previous studies, suggesting that patients with autoimmune diseases have an increased risk of preterm birth (17, 18). Moreover, 12% of the newborn in this cohort had low birth weights, whereas this percentage was higher among those born to a mother with SLE, similar to that reported in the literature (5, 19). Maternal antibodies have been linked to mid-pregnancy miscarriages and may be associated with placental blood vessel thrombosis, although this association has not been confirmed definitively (20). Additionally, in 19% of all pregnancies, an overlap of anti-Ro/SSA and antiphospholipid (APL) antibodies was observed. APL antibody testing is routinely performed in patients with a history of miscarriage or in those with SLE. Although APL antibody positivity was not significantly associated with increased risk of miscarriage in this cohort, it may represent a confounding factor for the increased risk of pregnancy-related morbidity.
After the 12th week of pregnancy, anti-Ro/SSA autoantibodies can cross the placenta and damage the developing fetal tissue, leading to CHB and transient neonatal lupus (21). However, other genetic components may also contribute to the development of these conditions (21, 22). In this cohort, two neonates had CHB (2%) and one neonatal lupus (1%). The current findings indicated an incidence of 2% for CHB in neonates born to anti-Ro/SSA positive women, similar to that reported in previous studies in different settings (22, 23). Notably, CHB was only observed in previously asymptomatic women within this cohort, a trend that was also noted by Fredi et al. (24) in a recent study in which these women were referred to as asymptomatic carriers. Hydroxychloroquine, an antimalarial medication, is widely used during pregnancy because of its favorable safety profile. This drug reduces neonatal morbidity in women with SLE by significantly decreasing the rate of premature birth and intrauterine growth restriction (25). In this study, women who received hydroxychloroquine had lower rates of miscarriage. Moreover, hydroxychloroquine is prescribed for secondary prevention of CHB in women with anti-Ro/SSA positivity (9). Although promising, its role in the primary prevention of CHB is yet to be defined. IVIG was proposed as a potential therapeutic agent in the management of second-degree congenital heart block, however its administration at replacement doses did not prevent the recurrence of CHB in mothers who had a previous child with CHB (6, 22). None of the cases in our study received IVIG. Treatment with dexamethasone may be beneficial in the early stages of fetal CHB but does not seem to improve established third-degree heart block (26).
Unfortunately, there is no universally accepted protocol for the prevention of CHB and the associated mortality. Katsafourou et al. suggested that women with autoimmune diseases should receive proper counseling, multidisciplinary surveillance, and individualized antenatal care (14). Therefore, it's recommended for these pregnancies to be managed with serial surveillance using fetal echocardiography between 16 and 18 weeks of gestation until week 26 (27).
Furthermore, vitamin D and folic acid supplementation were associated with improved birth outcomes.
When assessing all live births, 45% of the neonates were found seropositive for anti-Ro/SSA antibodies. The average anti-Ro/SSA titer in neonates was higher among those who were born to mothers with primary SS, had a low birth weight and required admission to the NICU. The presence of anti-Ro/SSA autoantibodies is a defining feature of neonatal lupus. Several clinical manifestations, such as CHB, cutaneous lupus, hepatobiliary disease, hematological manifestations, and central nervous system diseases, may occur in these infants (25). Zuppa et al. (28) suggested that neonates must be monitored for 6–9 months, as 10% of the infants in their study had anti-Ro/SSA persistence in the blood even at 9 months. The highest incidence of hematological and hepatobiliary neonatal lupus features was observed at 3 months of age. However, available literature on anti-Ro/SSA antibody testing in neonates remains limited.
4.1 Limitations and strengths
Although not all associations mentioned in this study reached statistical significance, valuable insights into the risks associated with pregnancy and neonatal outcomes among women with anti-Ro/SSA positivity are provided. Furthermore, this is the first study to shed light on autoimmune diseases in pregnant women in this geographical region. It is also a rare report documenting anti-Ro/SSA titers in neonates. As the study was retrospective and exploratory in nature, formal sample size calculation and power analysis were not conducted. Therefore, the results should be perceived as laying the groundwork for more comprehensive future investigations in this area.
5 Conclusion
Although an elevated rate of miscarriages, cesarean delivery, and preterm birth was noted in this cohort of women with anti-Ro/SSA positivity, the study was not controlled to draw solid conclusions. It's important to consider other factors that may influence this outcome. Therefore, larger, controlled studies are required to validate this risk. Notably, hydroxychloroquine use was associated with a lower risk of miscarriage and no incidence of CHB. Finally, the significance of neonatal anti-Ro/SSA positivity remains unclear. In this study, the median titer was higher in neonates born to mothers with primary SS; however, further research is needed to explore the significance and potential implications of neonatal anti-Ro/SSA antibody testing.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by this study adhered to the principles of the Declaration of Helsinki and Good Clinical Practice and within the laws and regulations of the Ministry of Public Health, Qatar. The study was approved by the Institutional Review Board of the Medical Research Centre at Hamad General Hospital (MRC 01-21-130). Due to its retrospective nature, the study was exempt from obtaining informed consent. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this was a retrospective data obtained from the electronic health records and did not involve patient interaction.
Author contributions
SA: Conceptualization, Data curation, Formal Analysis, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ES: Conceptualization, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. NH: Investigation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank Mrs. Hadeel Ashour, Research Co-Ordinator, Department of Rheumatology, Hamad Medical Corporation, for enabling the data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
SS, Sjögren's syndrome; SLE, systemic lupus erythematosus; IUFD, intrauterine fetal death; RA, rheumatoid arthritis; CHB, congenital heart block; NICU, neonatal intensive care unit; APL, antiphospholipid.
References
1. Didier K, Bolko L, Giusti D, Toquet S, Robbins A, Antonicelli F, et al. Autoantibodies associated with connective tissue diseases: what meaning for clinicians? Front Immunol. (2018) 9:541. doi: 10.3389/fimmu.2018.00541
2. Wainwright B, Bhan R, Trad C, Cohen R, Saxena A, Buyon J, et al. Autoimmune-mediated congenital heart block. Best Pract Res Clin Obstet Gynaecol. (2020) 64:41–51. doi: 10.1016/j.bpobgyn.2019.09.001
3. Brucato A, Cimaz R, Caporali R, Ramoni V, Buyon J. Pregnancy outcomes in patients with autoimmune diseases and anti-Ro/SSA antibodies. Clin Rev Allergy Immunol. (2011) 40(1):27–4. doi: 10.1007/s12016-009-8190-6
4. Izmirly PM, Costedoat-Chalumeau N, Pisoni CN, Khamashta MA, Kim MY, Saxena A, et al. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation. (2019) 139(2):271–3. doi: 10.1161/circulationaha.111.089268
5. Martínez-Sánchez N, Pérez-Pinto S, Robles-Marhuenda Á, Arnalich-Fernández F, Martín Cameán M, Hueso Zalvide E, et al. Obstetric and perinatal outcome in anti-Ro/SSA-positive pregnant women: a prospective cohort study. Immunol Res. (2017) 65(2):487–94. doi: 10.1007/s12026-016-8888-5
6. Ruffatti A, Cerutti A, Favaro M, Del Ross T, Calligaro A, Hoxha A, et al. Plasmapheresis, intravenous immunoglobulins and bethametasone—a combined protocol to treat autoimmune congenital heart block: a prospective cohort study. Clin Exp Rheumatol. (2016) 34(4):706–13.27385463
7. Hansahiranwadee W. Diagnosis and management of fetal autoimmune atrioventricular block. Int J Womens Health. (2020) 12:633–9. doi: 10.2147/IJWH.S257407
8. Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M, et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. (1998) 31(7):1658–66. doi: 10.1016/S0735-1097(98)00161-2
9. Izmirly P, Kim M, Friedman Deborah M, Costedoat-Chalumeau N, Clancy R, Copel Joshua A, et al. Hydroxychloroquine to prevent recurrent congenital heart block in fetuses of anti-SSA/Ro- positive mothers. J Am Coll Cardiol. (2020) 76(3):292–302. doi: 10.1016/j.jacc.2020.05.045
10. Skog A, Lagnefeldt L, Conner P, Wahren-Herlenius M, Sonesson S-E. Outcome in 212 anti- Ro/SSA positive pregnancies and population-based incidence of congenital heart block. Acta Obstet Gynecol Scand. (2016) 95(1):98–105. doi: 10.1111/aogs.12785
11. Merz WM, Fischer-Betz R, Hellwig K, Lamprecht G, Gembruch U. Pregnancy and autoimmune disease. Dtsch Arztebl Int. (2022) 119(9):145–56. doi: 10.3238/arztebl.m2021.0353
12. Yoshimi R, Ueda A, Ozato K, Ishigatsubo Y. Clinical and pathological roles of Ro/SSA autoantibody system. Clin Dev Immunol. (2012) 2012:606195. doi: 10.1155/2012/606195
13. Gleicher N, Weghofer A, Barad DH. Cutting edge assessment of the impact of autoimmunity on female reproductive success. J Autoimmun. (2012) 38(2–3):J74–80. doi: 10.1016/j.jaut.2011.05.016
14. Katsafourou P, Shillito J, Gooi J, Martin M. Pregnancy outcome in anti ro positive women—outcomes of a targeted approach. Arch Dis Child Fetal Neonatal Ed. (2011) 96(Suppl 1):Fa136. doi: 10.1136/archdischild.2011.300157.44
15. Østensen M, Khamashta M, Lockshin M, Parke A, Brucato A, Carp H, et al. Anti-inflammatory and immunosuppressive drugs and reproduction. Arthritis Res Ther. (2006) 8(3):209. doi: 10.1186/ar1957
16. Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. (2012) 2012:985646. doi: 10.1155/2012/985646
17. Kronemyer B. Preterm birth phenotypes in women with autoimmune disease. Contemp OB/GYN J. (2019) 64(11):70–8. doi: 10.1111/1471-0528.15970
18. Priori R, Gattamelata A, Modesti M, Colafrancesco S, Frisenda S, Minniti A, et al. Outcome of pregnancy in Italian patients with primary Sjögren syndrome. J Rheumatol. (2013) 40(7):1143–7. doi: 10.3899/jrheum.121518
19. De Carolis S, Salvi S, Botta A, Garofalo S, Garufi C, Ferrazzani S, et al. The impact of primary Sjogren’s syndrome on pregnancy outcome: our series and review of the literature. Autoimmun Rev. (2014) 13(2):103–7. doi: 10.1016/j.autrev.2013.09.003
20. Buyon JP. Systemic lupus erythematosus and the maternal-fetal dyad. Baillieres Clin Rheumatol. (1990) 4(1):85–103. doi: 10.1016/S0950-3579(05)80245-6
21. Zuppa AA, Fracchiolla A, Cota F, Gallini F, Savarese I, D’Andrea V, et al. Infants born to mothers with anti-SSA/Ro autoantibodies: neonatal outcome and follow-up. Clin Pediatr (Phila). (2008) 47(3):231–6. doi: 10.1177/0009922807307264
22. Friedman DM, Llanos C, Izmirly PM, Brock B, Byron J, Copel J, et al. Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block: results of a multicenter, prospective, open-label clinical trial. Arthritis Rheum. (2010) 62(4):1138–46. doi: 10.1002/art.27308
23. De Carolis S, Salvi S, Botta A, Santucci S, Martino C, Garofalo S, et al. Which intrauterine treatment for autoimmune congenital heart block? Open Autoimmun J. (2010) 2:1–10. doi: 10.2174/1876894601002010001
24. Fredi M, Argolini LM, Angeli F, Trespidi L, Ramoni V, Zatti S, et al. Anti-SSA/Ro positivity and congenital heart block: obstetric and foetal outcome in a cohort of anti-SSA/ro positive pregnant patients with and without autoimmune diseases. Clin Exp Rheumatol. (2023) 41(3):685–93. doi: 10.55563/clinexprheumatol/2ju0yv
25. Leroux M, Desveaux C, Parcevaux M, Julliac B, Gouyon JB, Dallay D, et al. Impact of hydroxychloroquine on preterm delivery and intrauterine growth restriction in pregnant women with systemic lupus erythematosus: a descriptive cohort study. Lupus. (2015) 24(13):1384–91. doi: 10.1177/0961203315591027
26. Kapur A, Dey M, Tangri M, Bandhu HC. Maternal anti-ro/SSA and anti-la/SSB antibodies and fetal congenital heart block. J Obstet Gynaecol India. (2015) 65:193–5. doi: 10.1007/s13224-014-0608-2
27. Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse MEB, Lockshin MD, et al. 2020 American college of rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Care Res (Hoboken). (2020) 72(4):461–88. doi: 10.1002/acr.24130
Keywords: anti-RO/SSA positivity, neonatal lupus, Sjögren’s syndrome, rheumatoid arthritis, congenital heart block, lupus
Citation: Al Emadi S, Satti E and Hadwan N (2024) Insights into maternal and neonatal anti-Ro/SSA antibodies: implications on pregnancy and neonatal health. Front. Lupus 2:1358121. doi: 10.3389/flupu.2024.1358121
Received: 19 December 2023; Accepted: 10 May 2024;
Published: 30 May 2024.
Edited by:
Laura Andreoli, University of Brescia, ItalyReviewed by:
Micaela Fredi, University of Brescia, ItalyAriela Hoxha, University Hospital of Padua, Italy
© 2024 Al Emadi, Satti and Hadwan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samar Al Emadi, c2FsZW1hZGlAaGFtYWQucWE=
 Samar Al Emadi
Samar Al Emadi Eman Satti
Eman Satti Nawal Hadwan
Nawal Hadwan