
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Lupus, 26 September 2023
Sec. Disease Mechanisms in Lupus
Volume 1 - 2023 | https://doi.org/10.3389/flupu.2023.1197309
This article is part of the Research TopicShowcase of Key Advances and Challenges in LupusView all 8 articles
Objective: Premature atherosclerosis is associated with systemic lupus erythematosus (SLE). We have previously shown an association of anti-Ro60/La/Ro52 with antioxidized low-density lipoprotein (LDL) in SLE. Here, we hypothesized that carotid intima–media thickening (CIMT) would be associated with antioxidized LDL (anti-oxLDL)/antilipoprotein lipase (ALPL) in a specific SLE autoantibody subset (anti-Ro60 positive, anti-RNP positive, anti-SmRNP positive, or extractable nuclear antigen antibody negative).
Methods: We carried out a case-control study (one time-point testing) of CIMT, ALPL, anti-oxLDL, anti-low density lipoprotein (ALDL), and anti-LDL in 114 SLE patients and 117 age/sex-matched controls. The levels of total cholesterol, LDL, high-density lipoprotein (HDL), triglycerides, and HDL-Trig were also measured. A student's t-test was used for statistical analysis.
Results: Interestingly, the level of CIMT was highest in the SLE subset with anti-Ro60 (23/114). CIMT and anti-oxLDL were statistically significantly elevated in the anti-Ro60 SLE subset (1.3 ± 1.66, p < 0.01; 0.26 ± 0.16, p < 0.002, respectively) compared with controls (0.54 ± 1.26; 0.165 ± 0.13, respectively), but not anti-LPL/anti-LDL. CIMT was significantly elevated (0.9 ± 1.71; p < 0.05) in the SLE subset without antiextractable nuclear antigen (ENA) (63/114) compared with controls. The other antibodies in this subset were not statistically different from other SLE subsets or controls. Only antioxLDL was significantly elevated (0.29 ± 0.27; p < 0.005) in the SLE subset with anti-RNP (14/114) compared with controls, while none were elevated in the anti-SmRNP subset (6/114). We did not find any significant differences in lipids between the various SLE subsets.
Conclusion: CIMT segregates in anti-Ro and ENA negative groups either with or without anti-oxLDL. It will be clinically important if cardiovascular events are augmented in the SLE anti-Ro subset having elevated antioxidized LDL antibodies.
Premature atherosclerosis is an important late complication of systemic lupus erythematosus (SLE), while also being an important issue in patients with early SLE. There is an increased prevalence of atherosclerotic plaque formation in SLE subjects, in addition to an elevated risk of cardiovascular disease (CVD) (1–6). Non-traditional risk factors such as cytokines, chemokines, and autoantibodies, in addition to traditional risk factors, contribute to the development of CVD (7–9). Of late, it has become evident that atherosclerosis and its corollary, CVD, are inflammatory ailments and that the immune system influences the development of disease (10). It is, therefore, of interest to investigate the elevated risk of CVD in SLE since immune mechanisms in human atherosclerosis could be elucidated. Autoantibodies targeting oxidized low-density lipoprotein (LDL), cardiolipin, and β2 glycoprotein 1 are associated with SLE and antiphospholipid syndrome–related vasculopathies. Immunochemical epitopes found on oxidized LDL are seen in atherosclerotic lesions (11–13). Antibodies to oxidized LDL are increased in atherosclerosis and are also found in SLE, diabetes, hypertension, and pre-eclampsia (11, 12, 14, 15).
Autoantibodies in SLE target a 60,000 molecular-weight protein (Ro60 or SS-A) or a 48,000 molecular-weight La (SSB) autoantigen of the Ro RNP particle, associated non-covalently with one or more of four short uridine-rich human cytoplasmic RNAs (hY RNAs). Up to 50% of SLE subjects have anti-Ro60. Anti-La occurs in considerably fewer SLE subjects. SLE autoantibodies also target the autoantigen Ro52 (also known as TRIM21) (16–18).
Sm and nuclear ribonucleoprotein (nRNP) antigens are also commonly targeted in SLE. These proteins are involved in the splicing of pre-mRNA in association with U small nuclear RNAs. Anti-Sm autoantibodies are found in the sera of roughly 20%–25% of all SLE subjects. These antibodies form part of the criteria for SLE classification and are highly specific for SLE (19).
It is important to note that mortality from lupus manifestations has diminished due to better treatment modalities. However, deaths caused by CVD from atherosclerosis in SLE have not. In fact, CVD is responsible for more than one-third of all deaths in SLE subjects (20, 21).
Carotid intima–media thickening (CIMT), measured by an ultrasound of the carotid arteries, is a valuable predictor of CVD and is associated with the clinical risk of angina and myocardial infarction (22). Ultrasound has been of great use in detecting atherosclerotic plaque and also in measuring carotid artery intima–media thickness (IMT) (23). Owing to ease of visualization and reproducibility, IMT is preferably measured in the common carotid artery. Furthermore, internal carotid artery measurement has been successfully used to detect and measure carotid plaque in subjects with atherosclerosis/cardiovascular-related conditions (24, 25).
Previously, we investigated the extent of coronary risk caused by antilipoprotein lipase and antioxidized LDL in the context of CIMT in SLE subjects and normal controls (15). The study found that antilipoprotein lipase (anti-LPL) was associated with oxidatively modified LDL, production of antioxidized LDL antibodies, CIMT, and coronary risk in some SLE patients (15, 26).
Based on our recent findings, which demonstrated an elevated susceptibility of SLE subjects with anti-Ro 60, La, and Ro 52 antibodies to develop antioxidized LDL (ox-LDL) antibodies (26), we formulated the hypothesis that atherosclerotic plaque would be associated with antioxidized LDL (anti-oxLDL)/antilipoprotein lipase (ALPL) in a specific SLE autoantibody subset (anti-Ro60 positive, anti-RNP positive, anti-SmRNP positive, or extractable nuclear antigen antibody negative).
Data collected from an earlier study (15) were analyzed after obtaining study approval from the Institutional Review Board of the Oklahoma Medical Research Foundation. This study used 114 SLE subjects (104 women and 10 men) and 117 age/sex-matched controls. None of the subjects had Sjögren's syndrome. The subjects were not on any lipid-lowering medication. The study subjects met the SLE 1982 revised classification criteria of the American College of Rheumatology. The OMRF Clinical Immunology Laboratory, a CLIA-approved facility, carried out serological studies. Antinuclear antibody (ANA) was tested by indirect immunofluorescence using a HEp-2 substrate. Antidouble-stranded DNA was determined by Crithidia lucilliae immunofluorescence and autoantibodies to extractable nuclear antigens by double immunodiffusion. Normal controls were selected to match the SLE subjects for sex and age. None of the controls were taking any lipid-lowering medications. The study was approved by the Oklahoma Medical Research Foundation Institutional Review Board.
All assays were carried out in the previous study and reported (15) and are briefly described here.
The CIMT procedure was performed by vascular technicians using the Accuson Sequoia Ultrasound Imager (Siemens Medical Solutions USA, Inc., Malvern, USA) at the University of Oklahoma Medical Center, Oklahoma City. The Sequoia uses 6L3 and 8L3 transducers that provide a linear array format with expanded MultiHertz™ multiple frequency imaging.
The FDA-approved CIMT test is done by rubbing the ultrasound probe (transponder) over each side of the subject's neck to obtain measurements of the thickness of the common carotid artery walls. It is also possible to obtain information regarding the presence of occlusion from these scans, but the instrument does not distinguish between hard and soft plaques. The CIMT values correlate well with coronary artery findings (96% correlation) (27–29).
The IMT of the common carotid artery was measured in millimeters (15). The study group was provided with a duplex carotid screen (both arteries) using Doppler sonography. The distance between two echogenic lines corresponding to the lumen–intima interface and the media–adventitia interface of the carotid arterial wall visualized on B-mode vascular ultrasound gives the observed CIMT values (29, 30). The atherosclerotic CIMT score is expressed as a sum of the values determined in both arteries. Only subjects with a value entry in the ultrasound test were taken into account for statistical analysis (15).
The assays were performed as in the previous study (15). Lipoprotein lipase, oxidized LDL, or LDL was coated onto ELISA plates, and blocked with milk, and subject sera were added at 100-fold dilution and incubated overnight. The plates were washed and incubated with an antihuman IgG alkaline phosphatase conjugate, followed by a substrate and O.D. read at 405 nm.
Cholesterol and triglyceride determination, and high-density lipoprotein (HDL)/LDL isolation were performed as described (15).
Values are presented as mean ± standard deviation (SD). Statistical analysis was carried out using Student's t-test, with p < 0.05 considered statistically significant.
Double immunodiffusion studies showed that 63 SLE subjects (55.26%) did not have autoantibodies against extractable nuclear antigen (anti-ENA), 14 (12.2%) had antibodies against ribonucleoprotein (anti-RNP), 23 (20.18%) had anti-Ro60, 6 (5.26%) had anti-SmRNP, four (3.5%) had unidentified precipitin lines, and four (3.5%) had miscellaneous antibodies. CIMT scores were highest in the SLE subset with anti-Ro60 autoantibodies compared with all other lupus subsets and normal controls (Table 1, Figure 1). While elevated compared with other SLE subsets, the CIMT values were not statistically different. However, CIMT in this subset was statistically different from CIMT in the normal controls (p < 0.01) (Table 1, Figure 1). Antibodies against oxidized LDL were also elevated in this subset compared with other SLE subsets. However, antioxidized LDL antibodies in this subset were significant only when compared with normal controls (p < 0.002) (Table 1, Figure 2).

Table 1. Antibodies to low-density lipoprotein (LDL), oxidized LDL (oxLDL) and lipoprotein lipase LPL and SLE disease activity index (SLEDAI), carotid intima–media thickening (CIMT) in subsets of lupus patients and controls.

Figure 1. Carotid intima–media thickening (CIMT) in SLE autoantibody subsets and normal controls. CIMT was used to determine intimal thickening scores expressed on a scale of 0 to 10. SLE subjects were divided into subsets based on their autoantibody profile as determined by immunodiffusion studies and analyzed for CIMT scores. CIMT is given in millimeters. *p < 0.01 compared with control. **p < 0.05 compared with control.

Figure 2. Antioxidized LDL antibodies in SLE autoantibody subsets and normal controls. Antibodies against oxidized LDL were determined as mentioned in “Subjects and methods.” SLE subjects were divided into subsets based on their autoantibody profile determined by immunodiffusion studies and analyzed for antioxidized LDL antibodies. *p < 0.005 compared with control. **p < 0.002 compared with control.
CIMT was significantly elevated in the SLE subset with autoantibodies against ENA compared with normal controls (p < 0.05). There were no differences in the levels of antibodies against oxidized LDL, LPL, and LDL in this and other subsets of SLE, nor in normal controls (Tables 1, 2, Figures 1–3).

Table 2. Total cholesterol, total triglycerides, LDL cholesterol, and HDL cholesterol in subsets of lupus patients and normal controls.
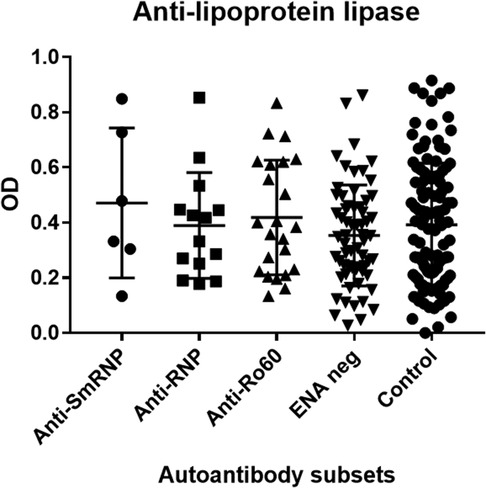
Figure 3. Antilipoprotein lipase antibodies in SLE autoantibody subsets and normal controls. Antibodies against antilipoprotein lipase were determined as mentioned in “Subjects and methods.” SLE subjects were divided into subsets based on their autoantibody profile determined by immunodiffusion studies and analyzed for antilipoprotein lipase antibodies. OD, optical density.
The SLE subset with anti-RNP antibodies had significantly higher levels of antioxidized LDL antibodies (p < 0.005) compared with the control group but not within other SLE subsets (Table 1, Figure 2). CIMT and antibodies against LPL or LDL were not different in this subset compared with the control group and other SLE subsets (Table 1, Figure 1). The behavior of the anti-SmRNP SLE subset was not significantly different from that of normal controls and other SLE subsets with respect to CIMT in addition to antibodies directed against oxidized LDL, LDL, or LPL (Table 1, Figures 1–3). HDL-cholesterol, LDL-cholesterol, and triglyceride levels were not statistically different within the various subsets of SLE (Table 2).
There was no significant difference in CIMT when antioxLDL-positive/ALPL-positive or antioxLDL-negative/ALPL-negative SLE subjects were compared with either antioxLDL-positive/ALPL-positive or antioxLDL-negative/ALPL-negative normal controls.
Of the 23 subjects with anti-Ro60 autoantibodies, three had an unidentified line in the double immunodiffusion assay, while seven others had anti-La antibodies. One out of 14 subjects with anti-RNP also had an unidentified line in the double immunodiffusion assay. Therefore, in terms of the analysis carried out in this study, none of the antibodies reported here were counted multiple times in the CIMT analysis.
The observation that (a) antioxidized LDL and CIMT are significantly increased in SLE subjects with anti-Ro60 autoantibodies and (b) CIMT is significantly elevated in ENA-negative SLE subjects compared with normal controls appears to be the most interesting result obtained from this study.
Studies have shown that SLE subjects have a nine- to 50-fold risk for myocardial infarction compared with the general population (31, 32). While traditional risk factors contribute to CVD in SLE, non-traditional risk factors play an important part (33, 34). Contrary to what is seen in the general population, young subjects with premenopausal lupus have more commonly premature CVD (1). Atherosclerosis in SLE is linked to inflammation (35). SLE subjects suffering from a cardiovascular event are more likely to be diagnosed with lupus at an older age, to have a longer duration of lupus, to have a longer period of corticosteroid use, to be hypercholesterolemic, and to be postmenopausal than SLE subjects without any cardiovascular event (31, 33).
That immune dysregulation typical of lupus is important for CIMT progression and vascular complications is borne out by the observation that a higher damage index score and less aggressive immunosuppression are associated with an increased CVD (36–38). Both the innate and adaptive immune systems that trigger the inflammatory state of lupus may also be associated with the development and progression of CVD (39). The results of two earlier studies from our group and the data from this study support an important role for autoantibodies as a potentially increased risk for atherosclerosis in SLE.
Our study looked at the association of autoantibodies targeting RNP, SmRNP, Ro, La, oxidized LDL, LDL, or lipoprotein lipase with carotid intima–media thickening in SLE and control subjects. We found that the level of CIMT was the highest in the group with anti-Ro. This group also had statistically significant high levels of antibodies targeting oxidized LDL.
Studies have shown that the measurement of CIMT by ultrasound of the carotid arteries is a useful predictor of coronary artery disease associated with coronary disease events such as angina and myocardial infarction (22, 40). Carotid Doppler ultrasonography of the carotid artery, which is widely used to measure the intima–media thickness, has served well as a biomarker for atherosclerosis and plaque characterization (41). CIMT, a well-validated index for the detection of early-stage atherosclerosis, is associated with CVD, cerebrovascular disease, and peripheral vascular disease (42, 43). An atherosclerotic plaque is defined as either a focal wall thickening at least 50% larger than the surrounding vessel wall or a distinct intimal thickening of more than 1 mm that protrudes into the lumen and is separate from the adjacent boundary (44).
Carotid artery atherosclerosis is assumed to progress below the intimal layer in the subintima. In contrast, carotid plaque originates in the intimal layer and embodies the atherosclerotic process itself (45–47). A CIMT value of more than 1.5 mm or a focal intimal medial thickening of more than 50% of the surrounding area is a frequently reported threshold value to define diffuse plaque (48, 49). However, confusion arises as ultrasound resolution currently permits the visualization of discrete protuberant plaque lesions smaller than this threshold value. Also, different studies have even reported varying CIMT thresholds for plaque. One study defined plaque as focal thickening of the intima-media larger than 1 mm that was at least twice as thick as the neighboring normal CIMT, thus giving different definitions of plaque ranging from 0.5 to >1.5 mm (50). A different study then defined plaque as CIMT >1.2 mm (51). The European Mannheim consensus, however, defined plaque as a focal thickening that intrudes into the lumen by 0.5 mm or by 50% of the neighboring intimal–medial thickness or where CIMT is greater than 1.5 mm (52).
We know that patients with anti-Ro and anti-La have highly statistically significant elevations of antioxidized LDL and antiphospholipid antibodies (26). We studied antioxLDL antibodies in an SLE patient over a period of 137 months. We found that the level of antioxLDL was very high when we began studying this SLE patient, and it stayed highly elevated for almost the entire study period (23). We also found that rabbits immunized with Ro60 or Ro peptides develop an SLE-like disease with high levels of anti-Ro60 and intermolecular epitope spreading to La, oxidized LDL, and phospholipids (manuscript in preparation). However, the exact mechanism by which CIMT and antibodies against oxidized LDL arise in SLE subjects with anti-Ro is not known.
Ultraviolet irradiation or caloric stress may induce an increased expression of autoantigens. The ultraviolet-irradiated skin is better targeted by anti-Ro antibodies under experimental conditions. This manifestation has been appreciated in the skin of subjects with subacute cutaneous lupus erythematosus. In these subjects, the availability of autoantigens increases after sun exposure and such a factor can induce in situ formation of the Ro/anti-Ro immune complex (53). Ultraviolet exposure has been shown to increase free radical release by skin cells (54). Increased production of reactive oxygen species by this process or by the depletion of antioxidants or antioxidant enzymes by autoantibodies (55, 56) may increase oxidative stress, which may lead to LDL oxidation. Antilipoprotein lipase autoantibodies found in SLE are believed to allow LDL to persist in the circulation by hindering lipid transport further downstream (15). Therefore, LDL becomes an ideal candidate for oxidative modification. LDL modified in this fashion behaves like a neoantigen, allowing the host to see it as a non-self-antigen and inducing the host to make antibodies against such antigens (57, 58). Free radicals and antibodies to oxidized LDL have been implicated in the atherosclerosis found in SLE (59).
Oxidized LDL has been shown to be complex with plasma β2-glycoprotein I (β2GPI) and become autoantigenic, eliciting the production of specific antiphospholipid antibodies (60). We recently reported significantly elevated IgG antiphospholipid levels that correlated with antioxidized LDL in SLE (26). In an interesting twist to the investigative process, the question now being asked is whether atherosclerosis is an autoimmune disease. Elevated levels of oxLDL/β2GPI were first observed in SLE and antiphospholipid syndrome, and subsequently in coronary heart disease and type 2 diabetes mellitus. When subjects with chronic coronary heart disease were studied prospectively over a 2-year period, the early plasma concentrations of oxLDL/β2GPI were found to correlate with the number and severity of cardiovascular events (60). Recent work shows an association between anti-Ro and low IgM antiphosphoryl choline antibodies in SLE patients. Low IgM antiphosphoryl choline is associated with CVD in subjects who do not have antiphospholipid antibodies (61).
The associations of antioxLDL with the anti-Ro60, anti-RNP, or anti-SmRNP subsets did not show significant differences. Likewise, the association between anti-LPL and the various autoantibody subsets was not significant. It is difficult to determine whether this is a truly negative association caused by the relatively small sample size of the anti-Ro60, anti-RNP, or anti-SmRNP cohorts. However, we are confident that there is a real negative association of antioxLDL or anti-LPL with the anti-ENA subsets because of the sample size of this subset.
We acknowledge several limitations of this study, such as the absence of data on traditional risk factors such as smoking, arterial hypertension, and obesity, in addition to organ lesions such as lupus nephritis.
It would be of great interest to investigate whether subjects with subacute cutaneous lupus erythematosus have increased CIMT and elevated levels of antibodies against oxidized LDL and lipoprotein lipase since they do not have much systemic inflammation. Furthermore, exploring the relationship between autoantibody subsets in SLE and cardiovascular events would be extremely interesting. It is possible that cardiovascular events are augmented in an SLE subset with anti-Ro and elevated levels of antioxidized LDL antibodies, which could have significant clinical consequences.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Institutional Review Board, Oklahoma Medical Research Foundation, Oklahoma City. The studies were conducted in accordance with local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
This work was conceptualized, analyzed, and written by BK and RS. Data collection was done by JF. Data analysis and editing were performed by SN. All authors contributed to the article and approved the submitted version.
This work was supported by Grant # ARO53483, Grant # GM104938 and Grant # A1082714 from NIH to RHS.
The authors are grateful to Morris Reichlin and Marianne Reichlin, Oklahoma Medical Research Foundation, Oklahoma City, United States, for their contributions to this study. Sadly, Reichlin, who spearheaded the original study (Ref # 15), passed away in 2018, and his wife, Marianne Reichlin, passed away in 2022. She assisted with some of the work described in the original study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author RS declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum. (1999) 42:338–46. doi: 10.1002/1529-0131(199902)42:2%3C338::AID-ANR17%3E3.0.CO;2-U
2. Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. (2001) 44:2331–7. doi: 10.1002/1529-0131(200110)44:10%3C2331::AID-ART395%3E3.0.CO;2-I
3. Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. (2006) 54:2550–7. doi: 10.1002/art.21955
4. D'Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet. (2007) 369:587–96. doi: 10.1016/s0140-6736(07)60279-7
5. Hurst C, Soto M, Vina ER, Rodgers KE. Renin-angiotensin system-modifying antihypertensive drugs can reduce the risk of cardiovascular complications in lupus: a retrospective cohort study. Am J Med. (2023) 136:284–93.e4. doi: 10.1016/j.amjmed.2022.11.016
6. Sagheer S, Deka P, Pathak D, Khan U, Zaidi SH, Akhlaq A, et al. Clinical outcomes of acute myocardial infarction hospitalizations with systemic lupus erythematosus: an analysis of nationwide readmissions database. Curr Probl Cardiol. (2022) 47:101086. doi: 10.1016/j.cpcardiol.2021.101086
7. Soltesz P, Kerekes G, Der H, Szucs G, Szanto S, Kiss E, et al. Comparative assessment of vascular function in autoimmune rheumatic diseases: considerations of prevention and treatment. Autoimmun Rev. (2011) 10:416–25. doi: 10.1016/j.autrev.2011.01.004
8. Svenungsson E, Jensen-Urstad K, Heimburger M, Silveira A, Hamsten A, de Faire U, et al. Risk factors for cardiovascular disease in systemic lupus erythematosus. Circulation. (2001) 104:1887–93. doi: 10.1161/hc4101.097518
9. Richter P, Cardoneanu A, Rezus C, Burlui AM, Rezus E. Non-traditional pro-inflammatory and pro-atherosclerotic risk factors related to systemic lupus erythematosus. Int J Mol Sci. (2022) 23(20):23–41. doi: 10.3390/ijms232012604
10. Frostegard J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. (2013) 11:117. doi: 10.1186/1741-7015-11-117
11. Sherer Y, Zinger H, Shoenfeld Y. Atherosclerosis in systemic lupus erythematosus. Autoimmunity. (2010) 43:98–102. doi: 10.3109/08916930903374527
12. Freire de Carvalho J, Sherer Y, Shoenfeld Y. The fine-tuning of anti-oxidized low-density lipoprotein antibodies in cardiovascular disease and thrombosis. Thromb Haemost. (2007) 98:1157–9. doi: 10.1160/TH07-11-0652
13. Liu Y, Yu X, Zhang W, Zhang X, Wang M, Ji F. Mechanistic insight into premature atherosclerosis and cardiovascular complications in systemic lupus erythematosus. J Autoimmun. (2022) 132:102863. doi: 10.1016/j.jaut.2022.102863
14. Charach G, Rabinovich A, Argov O, Weintraub M, Charach L, Ayzenberg O, et al. Anti-oxidized low-density lipoprotein antibodies in chronic heart failure. World J Cardiol. (2012) 4:302–8. doi: 10.4330/wjc.v4.i11.302
15. Fesmire J, Wolfson-Reichlin M, Reichlin M. Effects of autoimmune antibodies anti-lipoprotein lipase, anti-low density lipoprotein, and anti-oxidized low density lipoprotein on lipid metabolism and atherosclerosis in systemic lupus erythematosus. Rev Bras Reumatol. (2010) 50:539–51. doi: 10.1590/S0482-50042010000500007
16. Kurien BT, Chambers TL, Thomas PY, Frank MB, Scofield RH. Autoantibody to the leucine zipper region of 52 kDa Ro/SSA binds native 60 kDa Ro/SSA: identification of a tertiary epitope with components from 60 kDa Ro/SSA and 52 kDa Ro/SSA. Scand J Immunol. (2001) 53:268–76. doi: 10.1046/j.1365-3083.2001.00870.x
17. Szczerba BM, Kaplonek P, Wolska N, Podsiadlowska A, Rybakowska PD, Dey P, et al. Interaction between innate immunity and Ro52-induced antibody causes Sjogren’s syndrome-like disorder in mice. Ann Rheum Dis. (2016) 75:617–22. doi: 10.1136/annrheumdis-2014-206297
18. Robbins A, Hentzien M, Toquet S, Didier K, Servettaz A, Pham BN, et al. Diagnostic utility of separate anti-Ro60 and anti-Ro52/TRIM21 antibody detection in autoimmune diseases. Front Immunol. (2019) 10:444. doi: 10.3389/fimmu.2019.00444
19. Kurien BT, Scofield RH. Autoantibody determination in the diagnosis of systemic lupus erythematosus. Scand J Immunol. (2006) 64:227–35. doi: 10.1111/j.1365-3083.2006.01819.x
20. Reiss AB, Jacob B, Ahmed S, Carsons SE, DeLeon J. Understanding accelerated atherosclerosis in systemic lupus erythematosus: toward better treatment and prevention. Inflammation. (2021) 44:1663–82. doi: 10.1007/s10753-021-01455-6
21. Barbhaiya M, Feldman CH, Guan H, Gómez-Puerta JA, Fischer MA, Solomon DH, et al. Race/ethnicity and cardiovascular events among patients with systemic lupus erythematosus. Arthritis Rheumatol. (2017) 69:1823–31. doi: 10.1002/art.40174
22. Cicorella N, Zanolla L, Franceschini L, Cacici G, De Cristan B, Arieti M, et al. Usefulness of ultrasonographic markers of carotid atherosclerosis (intima-media thickness, unstable carotid plaques and severe carotid stenosis) for predicting presence and extent of coronary artery disease. J Cardiovasc Med (Hagerstown). (2009) 10:906–12. doi: 10.2459/JCM.0b013e32832e62fd
23. de Groot E, van Leuven SI, Duivenvoorden R, Meuwese MC, Akdim F, Bots ML, et al. Measurement of carotid intima-media thickness to assess progression and regression of atherosclerosis. Nat Clin Pract Cardiovasc Med. (2008) 5:280–8. doi: 10.1038/ncpcardio1163
24. Polak JF, Pencina MJ, Pencina KM, O'Donnell CJ, Wolf PA, D'Agostino RB Sr. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. (2011) 365:213–21. doi: 10.1056/NEJMoa1012592
25. Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. (2012) 220:128–33. doi: 10.1016/j.atherosclerosis.2011.06.044
26. Kurien BT, Fesmire J, Anderson CJ, Scofield RH. Anti-Ro and concomitant anti-la autoantibodies strongly associated with anti-oxLDL or anti-phospholipid antibody in systemic lupus erythematosus. J Clin Rheumatol. (2016) 22:418–25. doi: 10.1097/rhu.0000000000000429
27. Providers PH. Carotid Artery Intimal Thickness Test (CIMT) (2023).[AQ: Please provide complete details for reference [27].]
28. Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. (2008) 21:93–111; quiz 189–190. doi: 10.1016/j.echo.2007.11.011
29. Kasliwal RR, Bansal M, Desai D, Sharma M. Carotid intima-media thickness: current evidence, practices, and Indian experience. Indian J Endocrinol Metab. (2014) 18:13–22. doi: 10.4103/2230-8210.126522
30. Ho SS. Current status of carotid ultrasound in atherosclerosis. Quant Imaging Med Surg. (2016) 6:285–96. doi: 10.21037/qims.2016.05.03
31. Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA Jr., Jansen-McWilliams L, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham study. Am J Epidemiol. (1997) 145:408–15. doi: 10.1093/oxfordjournals.aje.a009122
32. Vavlukis M, Pop-Gjorcevab D, Poposka L, Sandevska E, Kedev S. Myocardial infarction in systemic lupus erythematosus—the sex-specific risk profile. Curr Pharm Des. (2021) 27:3221–8. doi: 10.2174/1381612826666201210110809
33. Lloyd-Jones DM. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation. (2010) 121:1768–77. doi: 10.1161/circulationaha.109.849166
34. Benvenuti F, Gatto M, Larosa M, Iaccarino L, Punzi L, Doria A. Cardiovascular risk factors, burden of disease and preventive strategies in patients with systemic lupus erythematosus: a literature review. Expert Opin Drug Saf. (2015) 14:1373–85. doi: 10.1517/14740338.2015.1073259
35. Jha SB, Rivera AP, Flores Monar GV, Islam H, Puttagunta SM, Islam R, et al. Systemic lupus erythematosus and cardiovascular disease. Cureus. (2022) 14:e22027. doi: 10.7759/cureus.22027
36. Barsalou J, Bradley TJ, Tyrrell PN, Slorach C, Ng LW, Levy DM, et al. Impact of disease duration on vascular surrogates of early atherosclerosis in childhood-onset systemic lupus erythematosus. Arthritis Rheumatol. (2016) 68:237–46. doi: 10.1002/art.39423
37. Roman MJ, Shanker BA, Davis A, Lockshin MD, Sammaritano L, Simantov R, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. (2003) 349:2399–406. doi: 10.1056/NEJMoa035471
38. Sandevska E, Gjorcheva DP, Vavlukis M, Sandevski A, Kafedziska I, Krstik-Damjanovska L, et al. Myocardial perfusion abnormalities in young and premenopausal women with systemic lupus erythematosus, detected with 99MTC MIBI myocardial perfusion scintigraphy—prevalence and correlation with proatherogenic factors. Pril (Makedon Akad Nauk Umet Odd Med Nauki). (2018) 39:79–92. doi: 10.2478/prilozi-2018-0045
39. Merched AJ, Daret D, Li L, Franzl N, Sauvage-Merched M. Specific autoantigens in experimental autoimmunity-associated atherosclerosis. FASEB J. (2016) 30:2123–34. doi: 10.1096/fj.201500131
40. Johnsen SH, Mathiesen EB. Carotid plaque compared with intima-media thickness as a predictor of coronary and cerebrovascular disease. Curr Cardiol Rep. (2009) 11:21–7. doi: 10.1007/s11886-009-0004-1
41. Lee W. General principles of carotid Doppler ultrasonography. Ultrasonography. (2014) 33:11–7. doi: 10.14366/usg.13018
42. Craven TE, Ryu JE, Espeland MA, Kahl FR, McKinney WM, Toole JF, et al. Evaluation of the associations between carotid artery atherosclerosis and coronary artery stenosis. A case-control study. Circulation. (1990) 82:1230–42. doi: 10.1161/01.cir.82.4.1230
43. Kablak-Ziembicka A, Tracz W, Przewlocki T, Pieniazek P, Sokolowski A, Konieczynska M. Association of increased carotid intima-media thickness with the extent of coronary artery disease. Heart. (2004) 90:1286–90. doi: 10.1136/hrt.2003.025080
44. Sobchak C, Akhtari S, Harvey P, Gladman D, Chandran V, Cook R, et al. Value of carotid ultrasound in cardiovascular risk stratification in patients with psoriatic disease. Arthritis Rheumatol. (2019) 71:1651–9. doi: 10.1002/art.40925
45. Johri AM, Nambi V, Naqvi TZ, Feinstein SB, Kim ESH, Park MM, et al. Recommendations for the assessment of carotid arterial plaque by ultrasound for the characterization of atherosclerosis and evaluation of cardiovascular risk: from the American Society of Echocardiography. J Am Soc Echocardiogr. (2020) 33:917–33. doi: 10.1016/j.echo.2020.04.021
46. Spence JD. Carotid plaque measurement is superior to IMT invited editorial comment on: carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis-Yoichi Inaba, M.D., Jennifer A. Chen M.D., Steven R. Bergmann M.D., Ph.D. Atherosclerosis. (2012) 220:34–5. doi: 10.1016/j.atherosclerosis.2011.07.006
47. To T-D, Naqvi TZ. Intima-Media thickness and carotid plaques in cardiovascular risk assessment. In: Ultrasound and carotid bifurcation atherosclerosis. Nicolaides A, Beach KW, Kyriacou E, Pattichis CS, editors. London: Springer (2012). p. 397–418. doi: 10.1007/978-1-84882-688-5_23
48. Darabian S, Hormuz M, Latif MA, Pahlevan S, Budoff MJ. The role of carotid intimal thickness testing and risk prediction in the development of coronary atherosclerosis. Curr Atheroscler Rep. (2013) 15:306. doi: 10.1007/s11883-012-0306-4
49. Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). an update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. (2012) 34:290–6. doi: 10.1159/000343145
50. Barnett PA, Spence JD, Manuck SB, Jennings JR. Psychological stress and the progression of carotid artery disease. J Hypertens. (1997) 15:49–55. doi: 10.1097/00004872-199715010-00004
51. Handa N, Matsumoto M, Maeda H, Hougaku H, Ogawa S, Fukunaga R, et al. Ultrasonic evaluation of early carotid atherosclerosis. Stroke. (1990) 21:1567–72. doi: 10.1161/01.str.21.11.1567
52. Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness consensus (2004–2006). an update on behalf of the advisory board of the 3rd and 4th watching the risk symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. (2007) 23:75–80. doi: 10.1159/000097034
53. Herrera-Esparza R, Villalobos R, Bollain YGJJ, Ramirez-Sandoval R, Sanchez-Rodriguez SH, Pacheco-Tovar G, et al. Apoptosis and redistribution of the Ro autoantigen in Balb/c mouse like in subacute cutaneous lupus erythematosus. Clin Dev Immunol. (2006) 13:163–6. doi: 10.1080/17402520600876796
54. Huang CH, Li HJ, Wu NL, Hsiao CY, Lin CN, Chang HH, et al. Photoprotective effects of cycloheterophyllin against UVA-induced damage and oxidative stress in human dermal fibroblasts. PLoS One. (2016) 11:e0161767. doi: 10.1371/journal.pone.0161767
55. Kurien BT, Scofield RH. Free radical mediated peroxidative damage in systemic lupus erythematosus. Life Sci. (2003) 73:1655–66. doi: 10.1016/S0024-3205(03)00475-2
56. Kurien BT, Scofield RH. Lipid peroxidation in systemic lupus erythematosus. Indian J Exp Biol. (2006) 44:349–56.16708886
57. Scofield RH, Kurien BT, Ganick S, McClain MT, Pye Q, James JA, et al. Modification of lupus-associated 60-kDa Ro protein with the lipid oxidation product 4-hydroxy-2-nonenal increases antigenicity and facilitates epitope spreading. Free Radic Biol Med. (2005) 38:719–28. doi: 10.1016/j.freeradbiomed.2004.11.001
58. Kurien BT, Scofield RH. Autoimmunity and oxidatively modified autoantigens. Autoimmun Rev. (2008) 7:567–73. doi: 10.1016/j.autrev.2008.04.019
59. Singh RB, Mengi SA, Xu YJ, Arneja AS, Dhalla NS. Pathogenesis of atherosclerosis: a multifactorial process. Exp Clin Cardiol. (2002) 7:40–53.19644578
60. Kraml P. OxLDL/β2-glycoprotein I complex as a pro-atherogenic autoantigen. Is atherosclerosis an autoimmune disease? Vnitr Lek. (2016) 62:48–51.26967237
Keywords: oxidized LDL, antioxLDL, atherosclerosis, plaque, SLE, autoantibody subset, carotid intima-media thickening
Citation: Kurien BT, Fesmire J, Nath SK and Scofield RH (2023) Increased carotid intima–media thickening and antioxidized low-density lipoprotein in an anti-Ro60 SLE autoantibody subset. Front. Lupus 1:1197309. doi: 10.3389/flupu.2023.1197309
Received: 30 March 2023; Accepted: 8 September 2023;
Published: 26 September 2023.
Edited by:
Andras Perl, Upstate Medical University, United StatesReviewed by:
Elena Gerasimova, V.A. Nasonova Research Institute of Rheumatology, Russia© 2023 Kurien, Fesmire, Nath and Scofield. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: R. Hal Scofield SGFsLVNjb2ZpZWxkQG9tcmYub3Voc2MuZWR1
†Deceased
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.