- Animal Free Research UK, London, United Kingdom
Introduction: There have been relatively few attempts to quantitatively assess if, and in which areas, the use of non-animal methods (NAMs) is increasing in biomedical research and importantly, how this compares to the use of live animals.
Methods: We conducted a bibliometric analysis of the relative publication of papers reporting the use of NAMs-only compared to those reporting the use of animals, even if they also reported the use of NAMs, over the period 2003 to 2022 across seven research areas (breast cancer, lung disease, blood cancer, heart disease, neurodegenerative diseases, diabetes and toxicology) and five regions (USA, China, France, Germany, United Kingdom).
Results: We found that the relative number of publications of research using NAMs-only has been higher than animal-based research for the last 20 years for all research areas and is growing. Research areas differed in their relative publication of NAMs-only based work, with breast cancer and lung disease having consistently the highest ratio of NAMs-only to animal-based publications and heart disease, diabetes and toxicology showing the greatest change over the time period. A key period of change was 2016–18. By 2022 the UK had the highest NAMs-only to animal-based research ratio than any other country for five of the seven research areas and China the lowest for six, accounting for publication rate. Tissue and in silico-based methods were the most common of all NAMs-only publications; lab-on-a-chip and stem cell models are increasing in their use but at much lower levels and rate of increase.
Conclusion: We found that proportionately the reliance on animals in these research areas is decreasing, which will be encouraging to those that support the replacement of animal experiments.
1 Introduction
The development of non-animal methods (NAMs) such as cell and tissue-based systems (in vitro methods) and computer-based technologies (in silico methods) in medical research has been increasing at a rapid pace in recent years (Heath et al., 2016). [The term NAM is now more commonly used to describe New Approach Methodologies (see Stucki et al., 2022), essentially meaning the same thing, but since the definition does not always preclude any animal use, for clarity we are using NAM to mean non-animal methods.] Despite advances in the complexity and sophistication of these methods over the past two decades there has been limited quantitative analysis of the actual uptake of NAMs in the scientific community. This is surprising given its potential impact on the quality of medical science as well as on animal use, which are both pressing issues within academia and pharma (Hartung, 2017), politically (European Parliament, 2021) and with the public (Ormandy and Schuppli, 2014). There has also been little evidence in the peer-reviewed literature as to the extent to which the implementation of these new technologies has increased. Whilst there has been some exploration of the trends in the uptake of NAMs (Goh et al., 2015; Tian et al., 2020), this is limited to specific research areas and technologies.
Tian et al., 2020 performed a bibliometric analysis demonstrating that the number of publications using in vitro techniques has increased exponentially across science in the last 20 years and was still increasing. The authors reported a significant upturn occurring around 2012. However, they did not look at other, more advanced NAMs technologies, restricting their search to “single-cell analysis” (undefined), the scope was kept broad to any entry in the database they used (Web of Science) and animal use was not analysed. Carvalho, C. et al., 2019; 2020 analysed citation rates for papers reporting the use in vitro, in silico and in vivo methods and found that clinical researchers were citing non-animal methods more than animal-based methods. However, they looked at major depressive disorder only and did not specifically look at publication rates.
EURL ECVAM (the European Union’s Reference Laboratory and Centre for the Validation of Alternative Methods) has recently commissioned seven studies to collate the types of NAMs being employed in seven disease areas; breast cancer, respiratory tract diseases, immuno-oncology including blood cancer, auto-immune diseases, cardiovascular diseases, neurodegenerative diseases and immunogenicity testing for advanced therapy medicinal products (see https://joint-research-centre.ec.europa.eu/eu-reference-laboratory-alternatives-animal-testing-eurl-ecvam/biomedical-research/breast-cancer_en) These technical reports list and describe the technologies and do report increased publication but over a relatively short time frame (typically 2013–2019) without a comparison to uptake in animal use across time.
The pharmaceutical industry has examined the change in the use of in vitro methods in their sector. Goh et al., 2015 asked members of the Association of the British Pharmaceutical Industry (ABPI) to go back through their records and count the number of compounds screened using non-animal techniques. The authors reported an exponential increase in the use of in vitro methods between 1980 and 2013 with a large step up occurring from 2000 to 2005. However, this survey covered only safety pharmacology, absorption, distribution, metabolism and excretion (ADME) and toxicity studies. The use of in silico methods was only briefly mentioned.
So, to date there has not been any direct comparison between the publication of papers reporting the use of NAMs and papers reporting the use of animals across a range of disease research areas. This would be useful to those interested in whether the use of NAMs is correlating with a decrease in the use of live animals. The absolute numbers of animals used in research and testing at least in Europe has been fairly static over the last 20–30 years (Daneschian et al., 2015; Taylor and Rego Alvarez, 2019; Busquet et al., 2020), but it is not known if relatively speaking actual use may be decreasing, for example, because more science is being done (see NC3Rs, 2010). It is important to look at trends in the uptake of NAMs across research areas and regions for several reasons. It provides useful intelligence to NAMs developers and funders on whether the methods they are producing are in fact being used, and importantly, in which areas. Institutions investing in NAMs with a view to accelerating a reduction in animal use via replacement are also keen to see if they are being effective and in the research areas in which they focus. A closer evaluation of the relative uptake of NAMs could also provide intelligence for policymakers to help guide future funding decisions and strategy.
This project was commissioned by Animal Free Research UK in order to help answer some of these questions. The purpose of this project was to conduct a bibliometric analysis to assess the relative publication rate of papers using NAMs technologies compared to those using animals, across a range of research areas and regions of the world between the period 2003 to 2022. Specific aims of the project were to establish:
1. If the number of publications using NAMs is increasing and if this is being mirrored by a decrease in the number of animal-only based publications
2. Which research areas are using NAMs more than others
3. Which regions are publishing more NAMs-only based research
4. The relative use of more advanced NAMs across the research areas.
2 Methods and data
2.1 Literature search
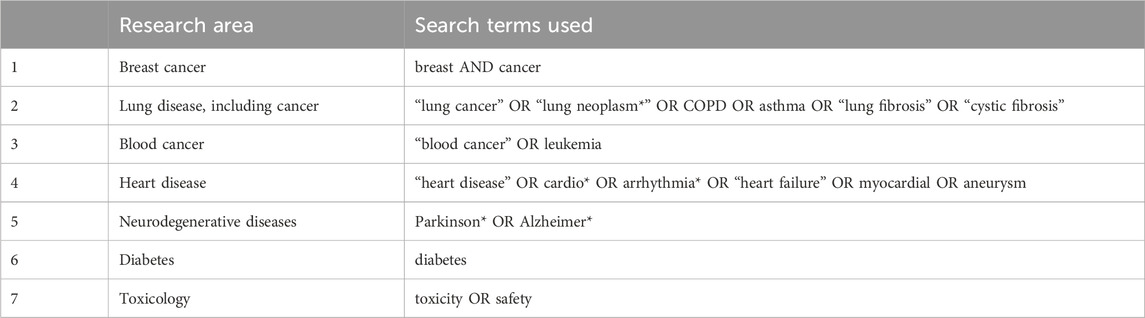
The database PubMed https://pubmed.ncbi.nlm.nih.gov/ was used as this is freely available and still one of the main repositories of publications for biomedical science. The inclusion and exclusion search terms used in the EURL ECVAM reports described above were used and then adapted based on Carvalho et al., 2020. See Table 1 for the search terms used.

Table 1. The search terms used to identify NAMs and animal-based papers and how the search was built using Boolean operators.
To identify suitable NAMs terms the EURL ECVAM report on breast cancer was used as the starting point as this had the most extensive list of terms. Pilot searches confirmed that this extended list produced much higher numbers of relevant papers rather than just using broad terms such as “in vitro” or MeSH terms such as “in vitro techniques”. Additional terms and combinations of terms were piloted to ensure that the largest number of relevant papers would be identified using the fewest number of terms. For example, the terms microfluidic* OR microphys* OR chip* were the most effective at identifying the largest number of relevant papers related to lab-on-a-chip and similar devices. The * truncation tool was used to further limit the number of words by allowing the identification of all words starting with the same first few letters (e.g., “organ*” would identify “organ”, “organs”, “organoids”, etc.).
For the animal search terms it was evident that various combinations were needed, i.e., both nonhuman primate and non-human primate and rats and rat (PubMed does not allow the use of the * for words of less than four letters). The search was performed identically for all research areas even though some animal species are used in some areas more than others, e.g., rabbits are used in cardiovascular research more frequently than monkeys. This extension of the search terms also made the scope of the animal search comparable to the NAMs search.
Some of the most common publication types such as “Review” or “Clinical Trial” were excluded to decrease the proportion of the results that would not be primary research or would be clinical research. For the purposes of this review, human-based, i.e., clinical research, as a NAM was not considered.
Based on the literature described above and the general presumption that the last 20 years has seen the largest change in the use of NAMs the date range was set to 2003 to 2022 inclusive, as at the time of the search (November 2023) 2022 was the last complete year for publications.
The results were not screened, i.e., there were no further inclusion or exclusion criteria applied and no removals were made.
To obtain papers reporting the use of NAMs in isolation (i.e., no animal-based work in the same publication) search 1 was conducted (see Table 2.). To obtain papers reporting the use of NAMs in conjunction with animal studies, search 2 was conducted. Finally, to obtain papers reporting the use of animals only, search 3 was conducted.
An example search sentence for NAMs-only papers for breast cancer research with a United Kingdom affiliation would be:
(NAMs terms [Title/Abstract]) NOT (Animal terms [Title/Abstract]) AND (breast cancer terms [Title/Abstract]) AND (Region term [Affiliation]) NOT (publication terms [Publication Type])
The actual search sentence:
(“model*” [Title/Abstract] OR “assay*” [Title/Abstract] OR “in vitro” [Title/Abstract] OR “cultur*” [Title/Abstract] OR “cell*” [Title/Abstract] OR “tissue*” [Title/Abstract] OR “organ*” [Title/Abstract] OR “spheroid*” [Title/Abstract] OR “3D” [Title/Abstract] OR “microfluidic*” [Title/Abstract] OR “microphys*” [Title/Abstract] OR “chip*” [Title/Abstract] OR “stem cell*” [Title/Abstract] OR “in silico” [Title/Abstract] OR “simulation*” [Title/Abstract] OR “algorithm*” [Title/Abstract] OR “mathematic*” [Title/Abstract] OR “comput*” [Title/Abstract]) AND (“mouse” [Title/Abstract] OR “murine” [Title/Abstract] OR “mice” [Title/Abstract] OR “rat” [Title/Abstract] OR “rats” [Title/Abstract] OR “monkey” [Title/Abstract] OR “nonhuman primate*” [Title/Abstract] OR “non human primate*” [Title/Abstract] OR “macaque*” [Title/Abstract] OR “marmoset*” [Title/Abstract] OR “rabbit*” [Title/Abstract] OR “guinea pig*” [Title/Abstract] OR “dog” [Title/Abstract] OR “dogs” [Title/Abstract] OR “animal model*” [Title/Abstract] OR “transgenic model*” [Title/Abstract] OR “in-vivo” [Title/Abstract]) AND (“Breast" [Title/Abstract] AND “cancer" [Title/Abstract]) AND (“United Kingdom” [Affiliation]) NOT (“case reports” [Publication Type] OR “clinical study” [Publication Type] OR “clinical trial” [Publication Type] OR “comment” [Publication Type] OR “review” [Publication Type])
2.2 Research areas
Six biomedical research areas, breast cancer, lung disease, including lung cancer, blood cancer, heart disease, neurodegenerative diseases and diabetes were initially chosen because Animal Free Research UK has current research projects in these areas. Four had also been covered by the EURL ECVAM reports. However, they also constitute some of the major areas of research focus more generally. Toxicology was also included because it has historically received a lot of attention by NAMs developers. The search terms for the research areas were based, where possible, on the first level terminology used in the EURL ECVAM reports and were kept deliberately simple, see Table 3. In our search, neurodegenerative diseases was limited to Alzheimer’s and Parkinson’s disease only and heart disease did not include broader cardiovascular issues such as stroke. Pilot searches again ensured that the maximum number of relevant papers were being returned with the most efficient use of search terms.
2.3 Regions
It was decided to compare the relative publication of NAMs based work for each of the research areas from the United States, China, France, Germany and the United Kingdom. The United States and China are countries with a major science interest. Within Europe, France, Germany and the United Kingdom have the largest economies; together these three countries are also the top three users of animals in research and testing in Europe based both on publications (Taylor and Rego Alvarez, 2019a) and numbers of animals used (Taylor and Rego Alvarez, 2019b). The name of the country was added into the search under the Affiliation (see Table 1). The other countries were not excluded from each search, i.e., when searching for United Kingdom publications the United States, China, France and Germany were not excluded, to allow for collaborative work to be included.
2.4 Advanced NAMs
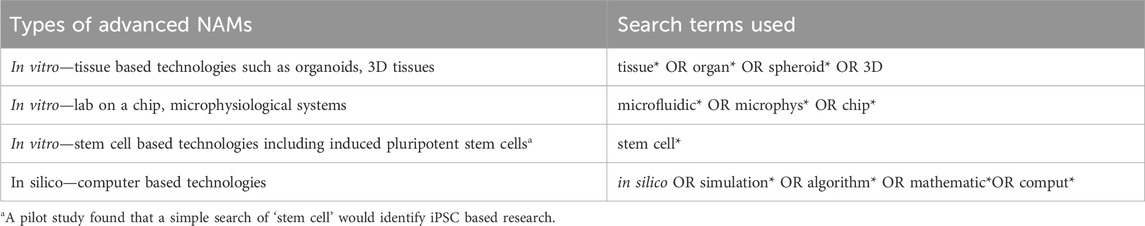
To assess the uptake of more advanced NAMs the key words already in the NAMs search terms were used to separately identify publications reporting the use of; tissue, lab-on-a-chip, stem cell and in silico-based technologies (see Table 4 for search terms used). These were considered examples of more advanced technologies than single-cell based studies. Tissue based methods encompassed organoids and 3D cultures; lab-on-a-chip included organ-on-a-chip and other microfluidic chamber based methods. The same search sentence as in search 1 (see Table 2) was conducted, only substituting the terms in Table 4. for the NAMs terms. So, for example, to identify lab-on-a-chip research (with no animal or animal tissue use) the search sentence used in search 1 was used, substituting the general NAMs terms with the terms that we had found would retrieve most lab-on-a-chip papers; microfluidic* OR microphys* OR chip*.
2.5 Analysis
The publication numbers for each of the three searches (NAMs-only, NAMs and animals (i.e., both) and animal-only) for each research area was plotted between 2003–2022. The publication numbers for each of the four advanced NAMs between 2003–2022 was also plotted.
It has long been standard scientific practice to conduct in vitro work and then confirm these results in animals. Our interest was in those researchers that felt that reporting NAMs-only based work was of sufficient scientific importance to be reported alone. To control for differences in publication rate between the research areas and regions a ratio of NAMs-only to animal-based work was used to compare the trend in the publication of NAMs-only work fairly. For this, the number of papers reporting the use of NAMs-only (search 1 in Table 2) was divided by the number of papers reporting the use of animals (search 2 plus search 3). Papers reporting the use of animals and NAMs, i.e., search 2, were considered animal-based work even though they also used NAMs.
To control for publication rates between the research areas, the proportion of the number of advanced NAMs publications (the total of tissue, lab-on-a-chip, stem cell and in silico) out of all NAMs-only publications (i.e., search 1) was compared between the research areas.
3 Results
3.1 Is the publication of NAMs-based research increasing?
For the combined total publications across the seven research areas, the reporting of research using NAMs-only has consistently grown over the last 20 years (Figure 1). This growth is greater than that for work using both NAMs and animals, such that by 2022 the number of publications of NAMs-only work was proportionately greater than work involving animals. In 2022 there were 102,379 publications in total retrieved that had used NAMs-only, 33,637 that had used both NAMs and animals and 4,151 that had used animals only. There appears to have been little change in the publication rate of research using animals only over the last 20 years and the number of publications is significantly less than that using NAMs only.
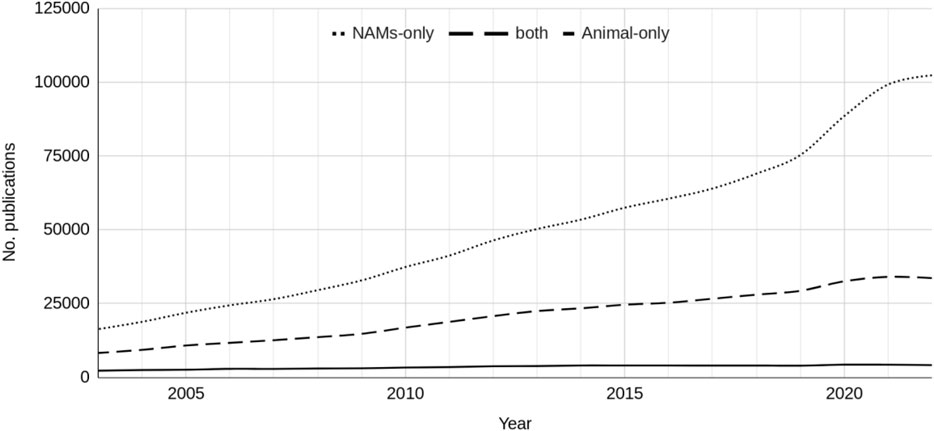
Figure 1. The total number of publications from the seven research areas that reported the use of NAMs-only, NAMs and animals and animals-only, between 2003 and 2022.
The ratio of NAMs-only based research publications to animal-based publications, which controls for publication rate, has seen a steady increase between 2003 and 2022 (Figure 2). The ratio is about 1.5 for 2003, equating to three NAMs publications for every two that use animals. By 2022 this had nearly doubled to 2.7, approaching three NAMs publications for every one using animals. There was a slight steepening of the curve around 2017.
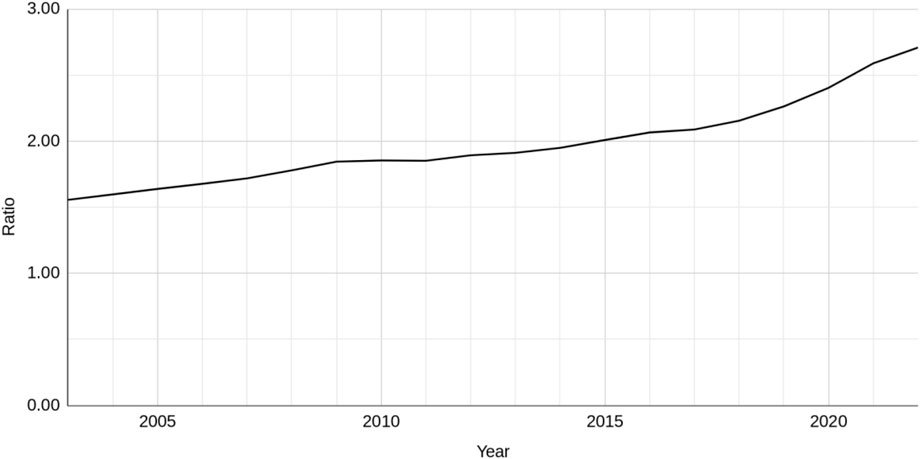
Figure 2. The ratio of NAMs-only to animal-based publications across all seven research areas between 2003 and 2022.
3.2 Which research areas are seeing the greatest uptake of NAMs?
In 2022 there were 16,467 publications in total retrieved on lung disease, 14,371 on breast cancer, 32,553 on heart disease, 5,679 on blood cancer, 19,146 on diabetes, 40,802 on toxicology and 11,149 on neurodegenerative diseases. The change in use of NAM and animal-based work in each of the seven research areas separately reflects the overall picture (Figure 3). There is a consistent pattern across all the research areas indicating an increasing divergence of publication of NAMs-only work away from animal-only work and work that used both NAMs and animals over the last 20 years. By 2022, for all research areas, the number of publications of NAMs-only work was proportionately greater than work involving animals. Whilst there was also growth in the number of publications that used NAMs and animals over the time period, this growth was much less steep. The growth in the number of publications of animal-only based work was dwarfed by the other two types of publications. For most research areas, with the exception of neurodegenerative diseases, the number of papers reporting the use of animal-only appears to have not increased over the last 20 years at all. There seemed to be a plateauing effect in the number of publications, of any type, in 2022 for many of the research areas.
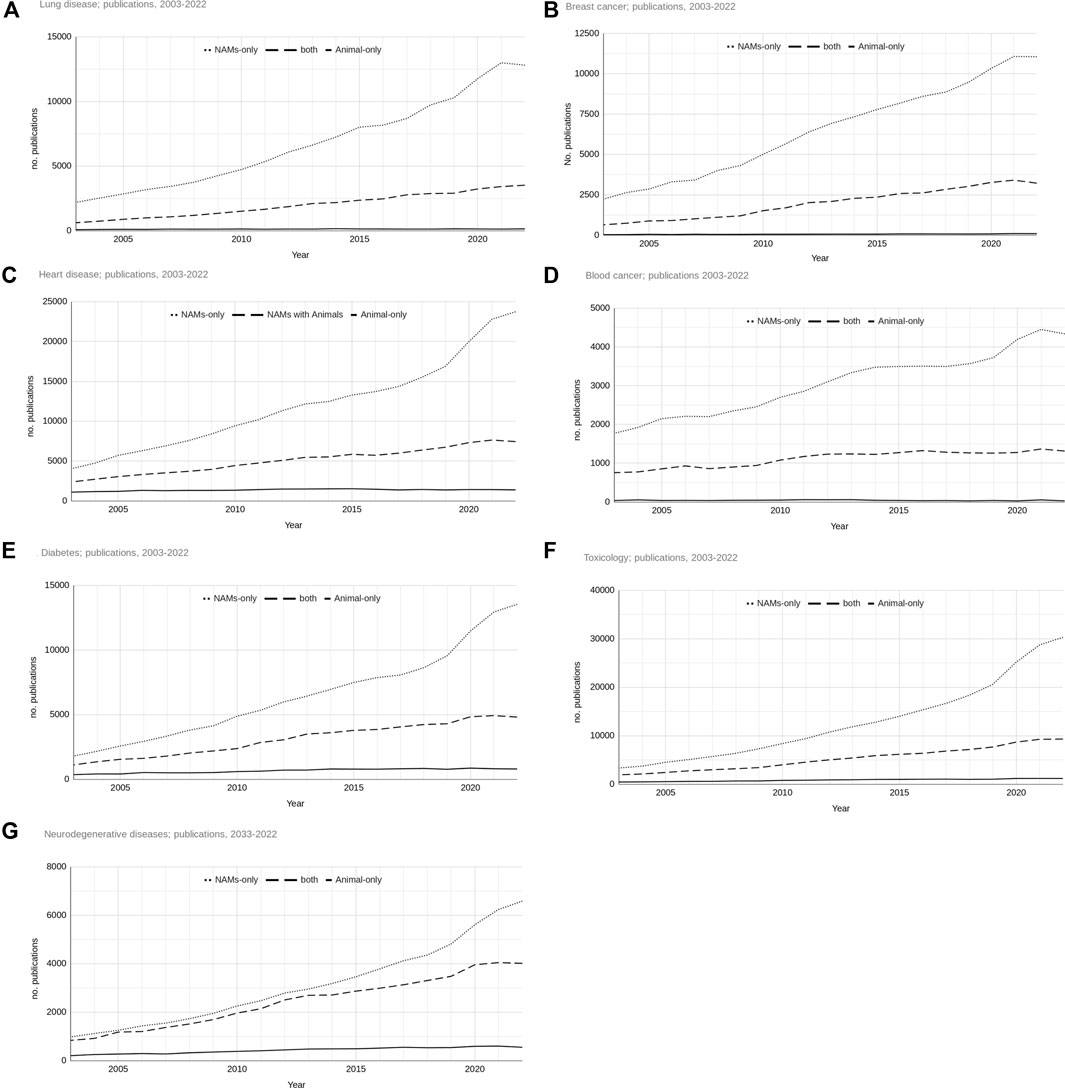
Figure 3. Total numbers of publications for NAMs-only, NAMs and animal and animal-only based research from 2003 to 2022 for the seven research areas ((A) - lung disease, (B) - breast cancer, (C) - heart disease, (D) - blood cancer, (E) - diabetes, (F) - toxicology, (G) - neurodegenerative diseases).
Controlling for publication by looking at the ratio of NAMs-only to animal-based publications enables the rate of change between the research areas, to be determined more accurately (Figure 4). Except for breast cancer research, all of these ratios show an increase over the last 20 years. Breast cancer has consistently had the highest ratio, around 3:1, which has not changed over the time period. Lung disease has also consistently had a ratio of around 3:1 but this has increased slightly in 2017 to 3.5:1 by 2022. Blood cancer also had a relatively high ratio of above 2:1 in 2003 and this has also increased to just over 3.1 by 2022.
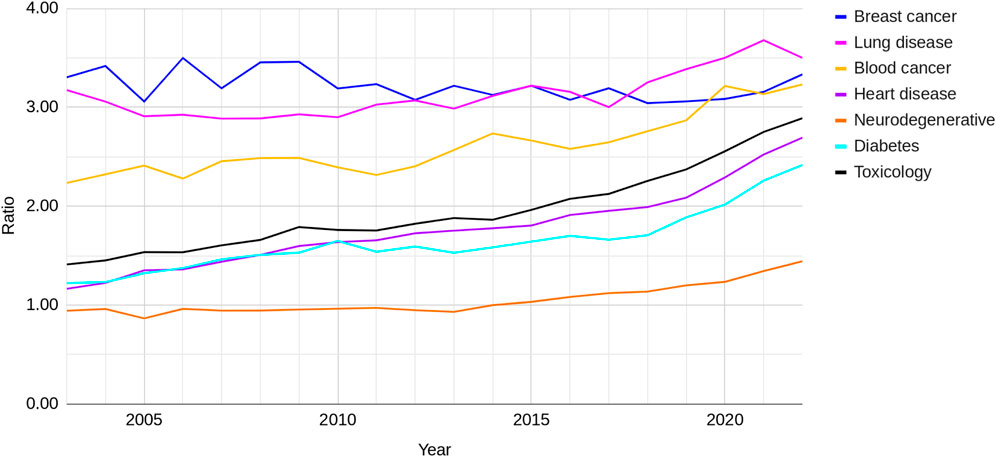
Figure 4. The ratio of NAMs-only to animal-based publications for seven research areas between 2003 and 2022.
The other research areas started at a ratio of around 1:1 in 2003 and, except for neurodegenerative diseases, have shown the steepest rise in the divergence away from animal-based publications. Heart disease and diabetes have shown the greatest change, with ratios increasing from just above 1:1 in 2003 to around 2.5:1 in 2022. The ratio for toxicology increased from around 1.5:1 to nearly 3:1. There was a distinctive increase in the rate of change of the ratio for lung disease, blood cancer, diabetes, heart disease and toxicology around 2016–2018.
The ratio for neurodegenerative diseases also began at about 1:1 in 2003 and a divergence from animal-based research was not seen until around 2016. By 2020 the rate of change seems to be comparable to the other diseases. Compared to the other research areas, neurodegenerative disease research still has a comparable number of papers that use NAMs-only to those that use animals.
3.3 Which regions are publishing more NAMs-only based research
To control for publication rate differences between the regions (China constituted 51%, United States 27%, Germany 9%, the United Kingdom 8% and France 5% of the total publications retrieved in 2022) we compared the ratio of NAMs-only to animal-based publications between the regions. For the combined total across the seven research areas the United Kingdom has consistently had the highest ratio of NAMs-only to animal-based publications compared to the United States, China, France and Germany (Figure 5). The ratio for the United Kingdom was 2.5:1 in 2003 and by 2022 was close to 4.5:1.
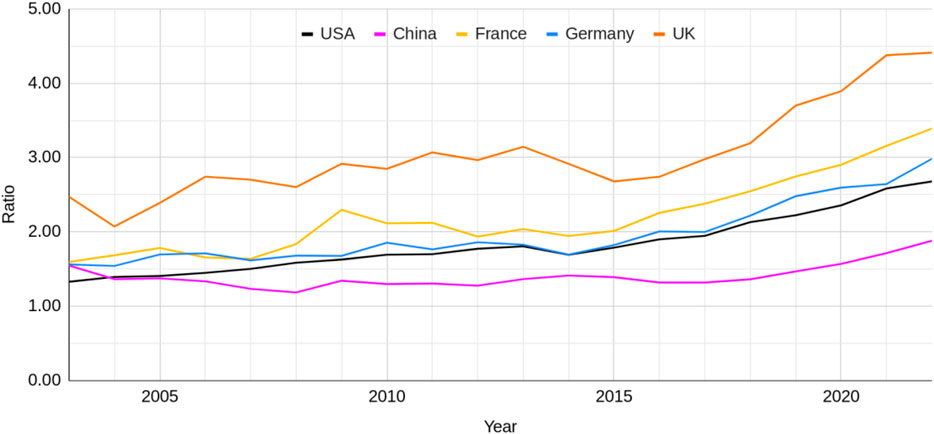
Figure 5. The ratio of NAMs-only to animal-based publications across all seven research areas from the USA, China, France, Germany and the United Kingdom between 2003 and 2022.
All geographical regions, however, have increased in their ratios over the time period. The ratios for all but the United Kingdom were around 1.5:1 in 2003. The trajectory for the United States, France and Germany was quite similar, with a clear increase in the rate of change around 2015 and a final ratio in 2022 around 3:1. The United Kingdom and China also showed an increase in their rate of change around this time. China has consistently had the lowest NAMs-only to animal-based research ratio than any other country and its rate of change has been smaller than the other countries.
Figure 6 shows the ratio of NAMs-only to animal-based research publications between 2003 and 2022 for the United States, China, France, Germany and the United Kingdom for the seven research areas separately. The trendline only is given for visual clarity as there was some fluctuations for each region over the years. Over the last 20 years the United Kingdom has consistently had a higher NAMs-only to animal-based research ratio than any other country for breast cancer, lung disease, heart disease, neurodegenerative diseases, diabetes and toxicology. By 2022 the United Kingdom had the highest NAMs-only to animal-based research ratio than any other country for lung disease, heart disease, neurodegenerative diseases, diabetes and toxicology. By 2022 France had the highest NAMs-only to animal-based research ratio than any other country for breast cancer and blood cancer.
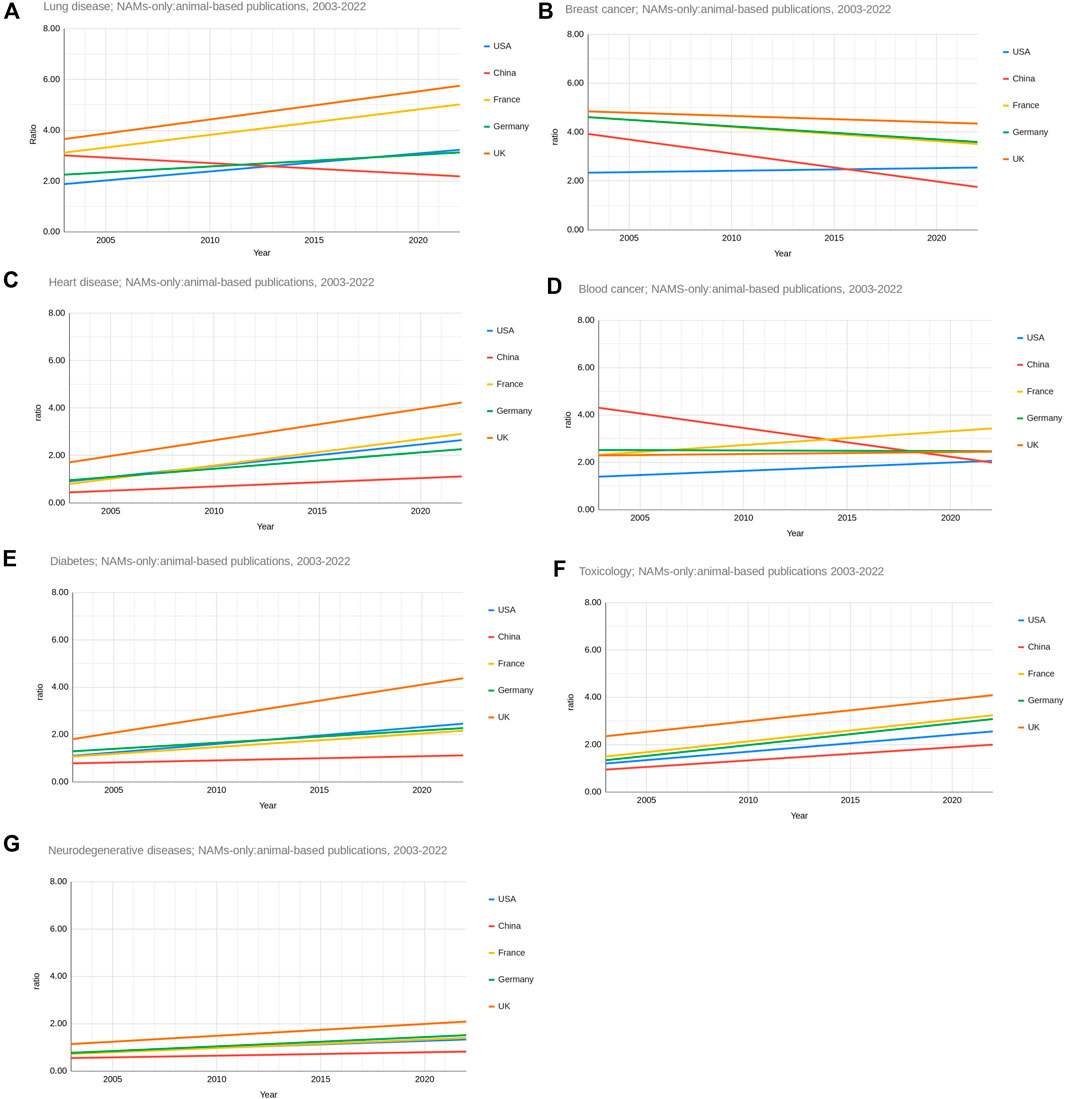
Figure 6. Ratio of NAMs only:animal-based publications from United States, China, France, Germany and the United Kingdom from 2003 to 2022 for the seven research areas ((A) - lung disease, (B) - breast cancer, (C) - heart disease, (D) - blood cancer, (E) - diabetes, (F) - toxicology, (G) - neurodegenerative diseases). Trendlines only are shown.
The ratios of NAMs-only to animal-based research publications for breast cancer, blood cancer and neurodegenerative diseases for each country have not increased significantly over the last 20 years. China is notable in that its ratio of NAMs-only to animal-based research appears to have decreased over this period for breast and blood cancer research.
3.4 What is the comparative use of more advanced NAMs across the research areas?
Across the combined research areas, the use of non-animal tissue and in silico models has dominated the literature compared to lab-on-a-chip and stem cell research (Figure 7). The reporting of the use of these two model types has increased exponentially from very low levels during the last 20 years. There appears to have been an increase in the growth rate around 2019. The number of publications reporting the use of lab-on-a-chip and stem cell models has also increased but not to the scale of the other two advanced NAM types. In 2022 across the combined research areas there were 26,910 publications reporting the use of tissue methods, 19,039 in silico models, 2,992 stem cells and 716 lab-on-a-chip methods.
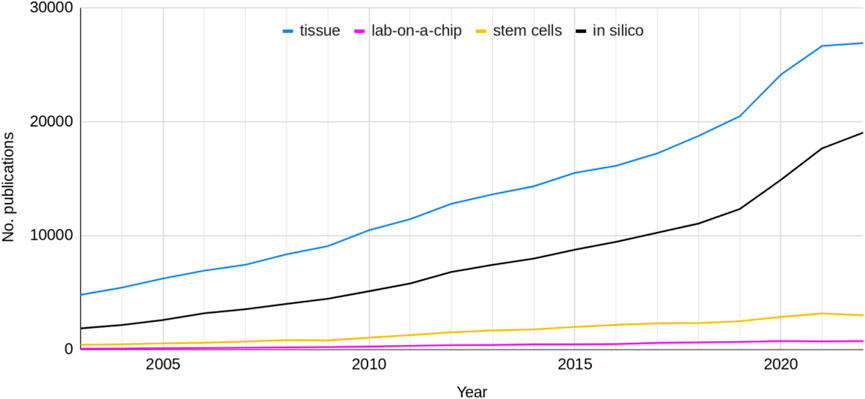
Figure 7. The total number of publications from the seven research areas reporting the use of tissue, lab-on-a-chip, stem cell and in silico models, between 2003 and 2022.
The pattern in the use of advanced NAMs over the last 20 years was similar for many of the research areas (Figure 8). Except for blood cancer research, tissue-based methods and in silico methods dominated the number of publications. For all research areas there has been a steady increase in the use of these methods over the last 20 years. For blood cancer, the use of stem cells seems to have started to dominate the other methods from around 2006. For breast cancer and toxicology there is a larger difference between the number of tissue and in silico-based papers, with the tissue-based papers dominating. For neurodegenerative diseases, although generally similar to the other research areas, there was a crossover between tissue and in silico methods in 2013 when the use of in silico methods began to rise more steeply than tissue-based methods and by 2022 was dominating.
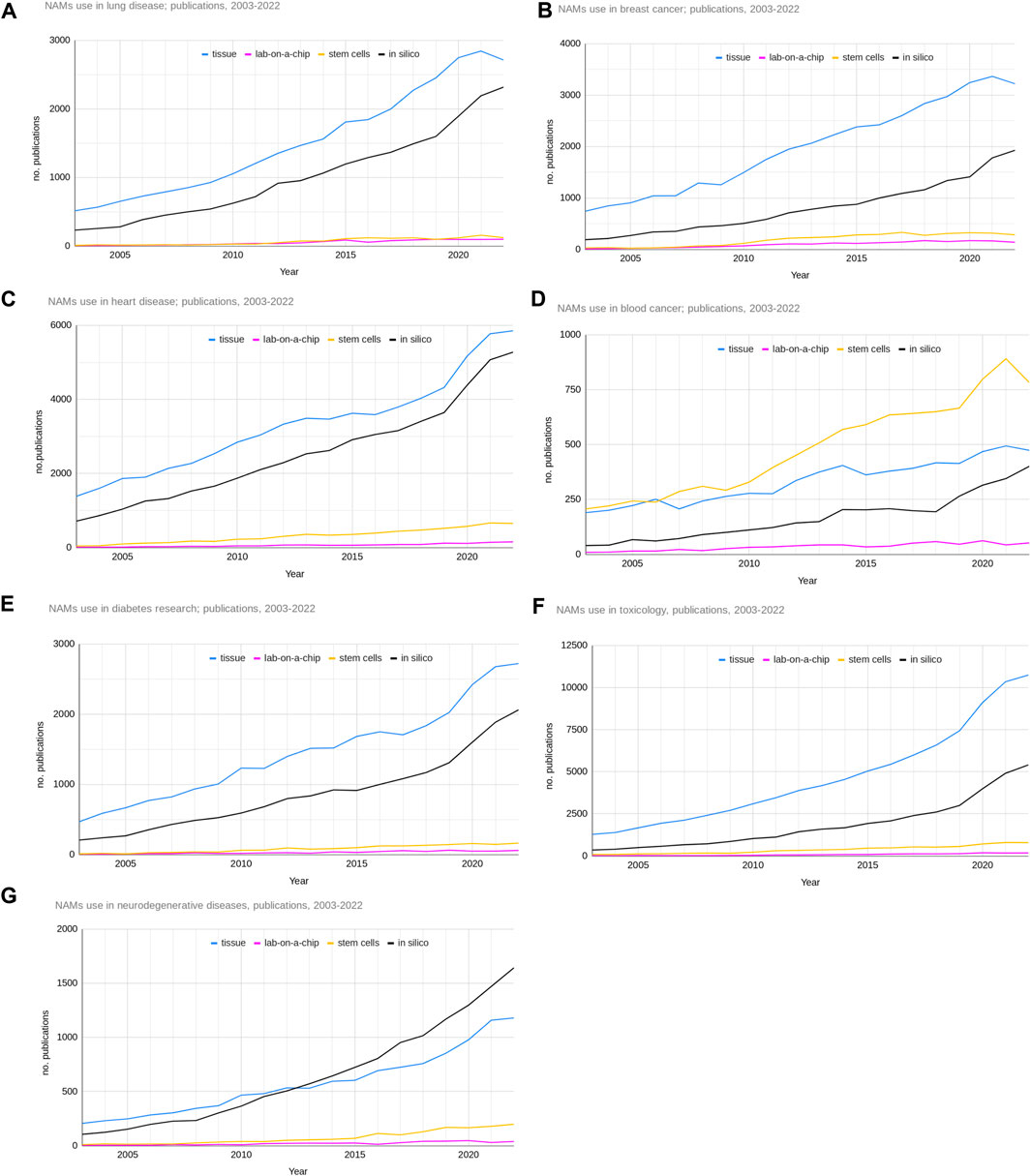
Figure 8. Total numbers of publications of advanced NAMs based research from 2003 to 2022 for the seven research areas ((A) - lung disease, (B) - breast cancer, (C) - heart disease, (D) - blood cancer, (E) - diabetes, (F) - toxicology, (G) - neurodegenerative diseases).
The number of publications reporting the use of lab-on-a-chip methods is dwarfed by the number reporting the use of tissue and in silico methods, but it is clear from Figure 8. that their use is increasing and for most research areas this appears to have been over the last 10 years. By 2022 the research areas with the greatest proportion of lab-on-a-chip use out of the advanced NAMs were blood cancer (3% lab-on-a-chip), breast cancer (2.5%) and lung disease, including cancer (2%) (data not shown).
The change in the proportion of advanced NAMs publications (the total of tissue, lab-on-a-chip, stem cell and in silico) out of all NAMs-only publications between 2003 and 2022 for all research areas is shown in Figure 9. There has been relatively little change over the last 20 years with the exception of breast cancer, neurodegenerative diseases and most notably blood cancer, which had the lowest proportion of advanced NAMs out of the seven disease areas in 2003 and has seen the largest increase. Toxicology has consistently had the higher proportion of advanced NAMs out of all NAMs-only publications over time. In 2022, 55% of all NAMS-only papers in toxicology were reporting the use of more advanced NAMs. The proportion of advanced NAMs use in the other research areas by 2022 was around 40%, heart disease and diabetes around 50%.
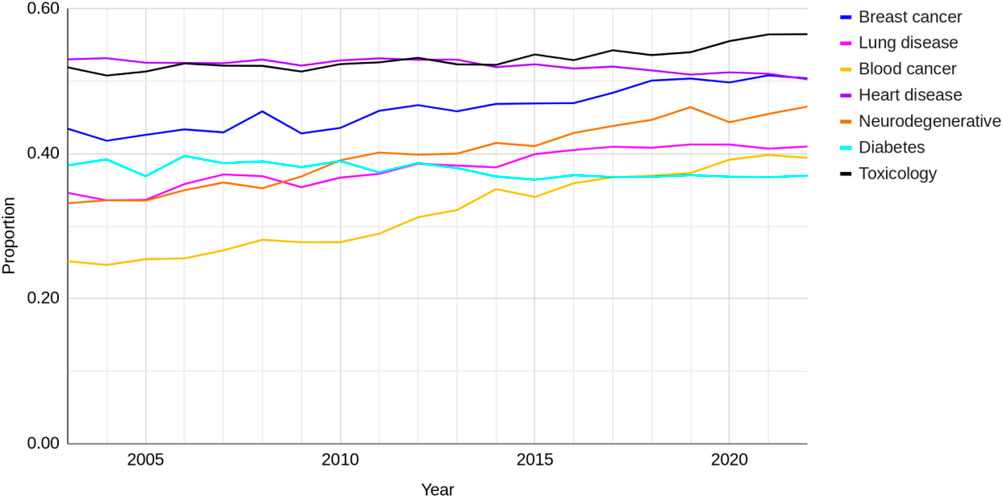
Figure 9. The proportion of advanced NAMs (total) out of all NAMs-only publications for seven research areas between 2003 and 2022.
It is notable that neurodegenerative disease has a higher proportion of advanced NAMs use compared to lung disease, blood cancer and diabetes, despite it having a lower ratio of NAMs-only to animal-based papers of all the research areas.
4 Discussion
4.1 Is the publication of NAMs-based research increasing?
The number of publications of research using NAMs-only has been higher than animal-based research for the last 20 years for all research areas. Until around 2019 the growth was linear but since then appears to be exponential.
In their survey of single-cell publications (undefined) Tian et al., 2020 also found that in vitro use had expanded exponentially across science in the last 20 years and was still increasing. Interestingly, they also noticed a significant upturn occurring about 2012, which is a little earlier to the findings in this study. Tian et al. found that the United States was the largest publisher of single-cell studies (40%), followed by China (14%), Germany (12%), Japan (9%) and the United Kingdom (7%). Tian et al. however did not control for overall publication rate or for animal use within the NAMs papers. We also found that China (51%) and the United States (27%) dominated the publication numbers overall but once we had controlled for this, China and to a lesser extent, the United States, compared poorly to France, Germany and the United Kingdom in their relative publication of NAMs-only based work. The current study adds an extra layer of insights because, for the first time, it compares the relative publication rate of NAMs-only work between countries, accounting for differences in scientific output and publication rate overall.
A pharmaceutical industry paper (Goh et al., 2015) reported a growth spurt in the use of NAMs around 2000–2005 and based on our study, this also seems to be when the use of NAMs-only for many research areas started to diverge from animal-based work. A divergence of NAMs-based research from animal-based research was observed in our study in the early 2000s with another spike in publication growth around 2016–18. Why this has occurred cannot be determined from this piece of work but there have been several regulatory drivers that could have instigated this change, at least in Europe, exactly around this time (Taylor and Alvarez, 2020). Most notable of these were the implementation of the cosmetics animal testing bans in 2003 and the negotiations and subsequent creation of the REACH legislation, which began in 2001. Both of these put pressure on the cosmetics and chemicals industries to develop alternative methods, which was supported by the European Commision with significant funding (EC, 2013; 2018). In the case of the cosmetics testing bans that was because animal testing would no longer be permitted from 2013. In the case of REACH, the incentive was to develop alternative methods in order to prevent the use of several million animals that would otherwise be used in the new requirements for testing (van der Jagt et al., 2004). It is interesting that the spike in NAMs publications corresponded with the last REACH deadline for the registration of chemicals in 2018. The fact that toxicology has seen the one of the largest increases in the dominance of the reporting of NAMs-only work between 2003 and 2022 and is the largest user of more advanced NAMs would also support the suggestion that regulatory drivers have played a role in encouraging the development of NAMs.
The number of papers reporting the use of both NAMs and animals has also increased over this time period but not as much as NAMs-only publications and the absolute number of papers reporting the use of animals only has remained relatively stable. This means that proportionately the reliance on animals is decreasing because the number of publications overall has increased.
4.2 Which research areas are seeing the greatest uptake of NAMs?
Breast cancer and lung disease have consistently had the highest ratio of NAMs-only to animal-based publications over the last 20 years, approximately three times as many NAMs-only based publications compared to animal-based ones. This ratio has not changed significantly over this time compared to the other research areas. Based on the trajectory of the curve NAMs-only publications are likely to have dominated these research areas for some time prior to 2003. This might be expected as cancer research in particular has had a long history of using in vitro methods to culture and examine cancer cells (lung cancer was included in the search for lung disease research). The first immortalised human cell line (HeLa) was created in 1951 and one of, if not the first, paper to discuss in vitro spheroid models for cancer research was published in 1971 (Sutherland et al., 1971).
The divergence away from the publication of animal-based research appears to have occurred closer to 2003 for all other research areas with the exception of neurodegenerative diseases. Heart disease and diabetes have shown the greatest change, with ratios increasing from just above 1:1 in 2003 to around 2.5:1 in 2022. Toxicology increased its NAMs-only to animal-based ratio from around 1.5:1 to nearly 3:1. There was a distinctive increase in the rate of change of the ratio for lung disease, blood cancer, diabetes, heart disease and toxicology around 2016–2018. It is possible that the high failure rate of drugs through clinical trials, particularly for these disease areas (94.7% cancer, 94.1% neurology, 95.2% cardiovascular and 92.5% for respiratory diseases, all below the mean for “all indications”, see Thomas et al., 2021) has caused for more focus on NAMs.
Compared to the other research areas, neurodegenerative diseases has had a noticeably lower ratio of NAMs-only to animal-based publications over the last 20 years. In this research area NAMs-only publications began to be more numerous than animal-based work from 2016. Why this should be is interesting; perhaps traditionally this field has been more animal-based because it is harder–or perceived to be harder–o mimic the disease in vitro or in silico? A recent workshop looking at the use of NAMs noted that this field may be particularly “risk averse” to novel concepts (Cassotta et al., 2022).
4.3 Which regions are publishing more NAMs only based research?
For the last 20 years the United Kingdom has consistently had a higher NAMs-only to animal-based research ratio than any other country for six out of the seven research areas we looked at and it is increasing. Whilst the United Kingdom has a strong science tradition it may not necessarily be expected to have performed better than the US or France or Germany, who also have strong science economies, nevertheless this seems to be the case. The United Kingdom has, however traditionally had a greater concern for animal welfare; for example, their legislation governing the use of animals in experiments was the first in the world (Cruelty to Animals Act 1876) and organisations funding the development of alternative methods such as Animal Free Research UK have been around since the 1970s. Taylor (2014) identified from a survey of EU member states in 2013 that the United Kingdom was the most generous provider of funding to alternative methods, although the amounts were still reported to be comparatively low, a tiny percentage of overall R&D budgets.
It is of concern and also a surprise given their strong science traditions, that China and to a lesser extent the United States, are performing less well compared to the European countries in their relative publication of NAMs-only based research. In some research areas China may be comparably publishing the least proportion of NAMs-only research out of all the regions over time. The fact that this pattern was observed in the United States and China - where there have been no corresponding regulatory drivers to influence change in research patterns towards NAMs (Taylor and Alvarez, 2020) - would indeed support the hypothesis that these regulatory drivers have played a part in accelerating the change in Europe. Regulatory change may therefore be needed in order to facilitate changes in research focus (Taylor, 2019). More change from the United States may be seen in coming years if the FDA Modernization Act 2.0 (Zushin et al., 2023), which encourages drug developers to submit NAMs-based data in support of their applications, is capitalised upon.
4.4 What is the comparative use of more advanced NAMs across the research areas?
We compared the publication rate between four (non-mutually exclusive) types of “advanced” NAMs; tissue based (including organoids and 3D models), stem cells, lab-on-a-chip (including organ-on-a-chip and other microphysiological devices) and in silico models. It was perhaps surprising to find, given the overall exponential increase in the use of NAMs, that there has not been an exponential rise in the use of lab-on-a-chip or stem cells over the last 20 years. In fact, there has been a rise in their use, but it is dwarfed by the growth in the use of tissue and in silico methods. The search was perhaps a little unfair given that the number of methods falling within the scope of tissue and in silico methodologies would be much larger than lab-on-a-chip, such that one would might expect them to dominate. Nonetheless the growth in lab-on-a-chip research is visible, particularly over the last 10 years and, out of the fields we looked at appears to be greatest in cancer research. The number of publications retrieved however, precluded us looking at this in more depth. Lab-on-a-chip methods require extensive engineering and construction and are not yet particularly high throughput. There is still a focus on standardisation and validation of these devices which may generate fewer publications (Zuang and Dura, 2022).
It is interesting that by 2022 neurodegenerative diseases used proportionally more advanced NAMs than lung disease, blood cancer and diabetes out of its NAMs-only use. This might support the hypothesis that neurodegenerative diseases has had to wait for more advanced NAMs methods to become available before it can begin to use them in isolation and this would explain its relatively slower start compared to other diseases that are more easily modeled in vitro or in silico.
Whilst for most of the research areas, of the advanced NAMs, tissue-based and in silico methods were more popular, for blood cancer it was stem cells. It is possible that the search strategy was inadvertently picking up the use of stem cells as a therapeutic since this is a key research area for blood cancer.
Toxicology seems to be doing the best in terms of using more advanced NAMs out of all its NAMS-only work and this has been increasing steadily since about 2014. Toxicology has received a lot of attention in terms of the use of animals and has been a particular focus of the regulatory drivers discussed in Taylor and Alvarez (2020) that have played a significant role in encouraging the use of NAMs in general as well as the development of more advanced NAMs.
4.5 Limitations of the study
As can be seen from Figure 1., by 2022 roughly a quarter of publications were those that used both NAMs and animals. For the purposes of this study these were considered animal-based work. This approach therefore underestimates the proportion of NAMs-based research being conducted. Furthermore, due to the search strategy, there was a small, but real risk that key NAMs-only papers such as validation studies in which the results of in vitro methods are compared with in vivo results would fall into the animal-based category. On balance, however, the importance of looking at genuinely animal-free research which did not need any comparison with animal experiments was greater than the risk that some key papers would fall under the group considered NAMs and animal-based work. It is possible that a proportion of this NAMs-only work will indeed be followed up by animal-based work published in a separate paper. If this was the case though a corresponding increase in the number of animal-only papers over time, perhaps on a lower level, would be expected, but this was not observed.
Other countries were not excluded within a specific country search, i.e., when searching for United Kingdom publications, the United States, China, France and Germany were not excluded. This exclusion would have removed any collaborative work between countries which is likely to be significant. There are therefore very likely to be duplicates in the search results, i.e., a collaboration between United Kingdom and United States scientists would come up in both the United States and the United Kingdom searches. Similarly, for the advanced NAMs searches other technologies were not excluded from each search so there is likely to be duplicates within each, i.e., a paper reporting the use of stem cells in a 3D culture would appear in both the stem cells and the tissue searches. It was felt that this was unavoidable.
Finally, the search terms used were based on those used by EURL ECVAM, the European Commission’s Centre for the Validation of Alternative Methods in their reports on the use of NAMs in several key research areas. We supplemented the search terms they used and conducted pilot searches to ensure that the maximum number of relevant papers were being identified with the most efficient use of search terms. It is, however, possible that some types of NAMs and some relevant topics within the research areas we looked at are missing.
4.6 Implications for NAMs based research and animal use
There was a consistent pattern across all the research areas that not only was the number of publications of NAMs-only work far greater than that of animal-only work and work that used both NAMs and animals but that this divergence was accelerating. This should be cause for celebration and encouragement to those who support the use of NAMs for both scientific and moral reasons. The trend is moving swiftly in the right direction. There was some evidence of plateauing in 2022 for both NAMs and animal-based research across all the research areas. There has been a notable impact of the COVID-19 pandemic on research (see Gao et al., 2021) but most papers investigating this phenomenon looked at publication rate very soon after the pandemic, i.e., in 2020 or 2021 (e.g., Abramo et al., 2022). In our study a decrease in publication rate in 2020 and 2021 was not seen so it may be that the effects of the pandemic on research output are only now being seen. This is quite likely given that research can take one to 2 years to be published and that the impact on bench-based research would have been in 2020. Alternatively, an artifact of PubMed could account for this plateau, i.e., a delay in the uploading of publications such that a search in early 2024 still does not give a complete picture for 2022. This might explain the findings in relation to the early effects of the pandemic; researchers thought they were seeing a decrease in publication rate but may have been simply searching the databases preemptively.
The findings in this study may come as a surprise to those who perceive that animal-only research dominates biomedical research. However, it might have been more reasonably assumed that the number of papers reporting both the use of NAMs and animals would be the greater, if not the dominant, publication form. This is because it has been standard scientific practice to conduct in vitro work and then confirm these results in animals. Indeed, NAMs researchers have recently begun expressing concern that they are being asked to replicate their in vitro results with an animal experiment in order to achieve publication, something that they increasingly feel is scientifically unjustified (European Parliament, 2021; Krebs et al., 2023). In the first survey of its kind, Krebs et al. (2023) found that approximately half of a small survey of respondents said they had been asked by journal editors and reviewers for animal data to support their results generated using NAMs before publication would be accepted.
Conversely, it may have always been assumed that NAMs-only methods would dominate the publication space since these are typically the methods that are used first in any project, not just to avoid testing on animals, but because some of them can be cheaper and quicker. However, unless there has been a decrease in the translation rate between in vitro and in vivo methods - which is entirely possible, especially given the reproducibility crisis (Baker, 2016) - one would expect to see a corresponding (if lower) rise in the publications of animal-only work as the in vitro work is followed up in vivo. This was not observed. Furthermore, the increase in the rate of change in the ratio of NAMs-only to animal-based publications seen for many of the research areas around 2016–18 would suggest that something else is happening other than simply that “more research” is being done. It is of course difficult to know from this data alone what these influences are.
This study appears to show that there has been little change in the absolute number of publications that use animals-only over the last 20 years. This is supported by the fact that there has also been little change in the actual numbers of animals used the last 20 years (Daneschian et al., 2015; Taylor and Rego Alvarez, 2019; Busquet et al., 2020). However, research overall, mostly involving NAMs only, is increasing exponentially, so dependence on animals is–in relative terms–decreasing. This suggests that there are still researchers that are dependent on animals who need to be reached (see section on “entrenchment” in Taylor, 2019). Efforts such as the USA’s National Institutes of Health Complement Animal Research in Experimentation (NIH, 2024) program to accelerate the development and use of NAMs more widely is an example of the kind of initiatives that will help.
We would be particularly keen to hear the views of NAMs researchers on these results and what they mean to them.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
KT: Formal Analysis, Investigation, Methodology, Writing–original draft, Writing–review and editing. SM: Project administration, Writing–review and editing. JB: Conceptualization, Methodology, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The work was commissioned and funded by Animal Free Research UK.
Acknowledgments
The authors are grateful for the comments on the manuscript from Oscar Lavery from the Advanced Bioengineering Group at the University of Glasgow.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1Note for the animal-only search you need to switch the construction around, i.e,
2A pilot study found that a simple search of “stem cell” would identify iPSC based research
References
Abramo, G., D’Angelo, C. A., and Mele, I. (2022). Impact of Covid-19 on research output by gender across countries. Scientometrics 127, 6811–6826. doi:10.1007/s11192-021-04245-x
Baker, M. (2016). 1,500 scientists lift the lid on reproducibility. Nature 533, 452–454. doi:10.1038/533452a
Busquet, F., Kleensang, A., Rovida, C., Herrmann, K., Leist, M., and Hartung, T. (2020). New European Union statistics on laboratory animal use – what really counts!. ALTEX 37, 167–186. doi:10.14573/altex.2003241
Carvalho, C., Varela, S. A., Bastos, L. F., Orfão, I., Beja, V., Sapage, M., et al. (2019). The relevance of in silico, in vitro and non-human primate based approaches to clinical research on major depressive disorder. Altern. Laboratory Animals 47, 128–139. doi:10.1177/0261192919885578
Carvalho, C., Varela, S. A. M., Marques, T. A., Knight, A., and Vicente, L. (2020). Are in vitro and in silico approaches used appropriately for animal-based major depressive disorder research? PLoS ONE 15 (6), e0233954. doi:10.1371/journal.pone.0233954
Cassotta, M., Geerts, H., Harbom, L., Outeiro, T. F., Pediaditakis, I., Reiner, O., et al. (2022). The future of Parkinson’s disease research: a new paradigm of human-specific investigation is necessary… and possible. ALTEX 39, 694–709. doi:10.14573/altex.2203161
Daneshian, M., Busquet, F., Hartung, T., and Leist, M. (2015). Animal use for science in Europe. ALTEX 32, 261–274. doi:10.14573/altex.1509081
European Commission (2013). Report from the commission to the European parliament, the council, the European economic and social committee and the committee of the regions in accordance with Article 117(4) REACH and Article 46(2) CLP, and a review of certain elements of REACH in line with Articles 75(2), 138(3) and 138(6) of REACH, SWD(2013) 25 Final. Brussels, Belgium: European Commission. .
European Commission (2018). Commission staff working document accompanying the document communication from the commission to the European parliament, the council and the European economic and social committee. Commission General Report on the Operation of REACH and Review of Certain Elements. Brussels, 5.3.2018. SWD(2018) 58 final. PART 1/7 (Conclusions and Actions). Brussels, Belgium: European Commission.
European Parliament (2021). Report of the Conference Towards the Replacement of Animals for Scientific Purposes. Brussels: Publications Office of the European Union.
European Parliament (2021). Resolution of 16 September 2021 on plans and actions to accelerate the transition to innovation without the use of animals in research, regulatory testing and education (2021/2784(RSP)).
Gao, J., Yin, Y., Myers, K. R., Lakhani, K. R., and Wang, D. (2021). Potentially long-lasting effects of the pandemic on scientists. Nat. Commun. 12, 6188. doi:10.1038/s41467-021-26428-z
Goh, J.-Y., Weaver, R. J., Dixon, L., Platt, N. J., and Roberts, R. A. (2015). Development and use of in vitro alternatives to animal testing by the pharmaceutical industry 1980–2013. Toxicol. Res. 4, 1297–1307. doi:10.1039/c5tx00123d
Hartung, T. (2017). Opinion versus evidence for the need to move away from animal testing. ALTEX 34, 193–200. doi:10.14573/altex.1703291
Heath, J. R., Ribas, A., and Mischel, P. S. (2016). Single-cell analysis tools for drug discovery and development. Nat. Rev. Drug Discov. 15, 204–216. doi:10.1038/nrd.2015.16
Krebs, C. E., Camp, C., Constantino, H., Courtot, L., Kavanagh, O., McCarthy, J., et al. (2023a). Author guide for addressing animal methods bias in publishing. Adv. Sci. 10 (30), e2303226. doi:10.1002/advs.202303226
Krebs, C. E., Lam, A., McCarthy, J., Constantino, H., and Sullivan, K. (2023b). A survey to assess animal methods bias in scientific publishing. ALTEX 40, 665–676. doi:10.14573/altex.2210212
National Institutes of Health (2024). Complement animal research in experimentation (Complement-ARIE) program. Available at: https://commonfund.nih.gov/complementarie (Accessed April 12, 2024).
NC3Rs (2010). Evaluating progress in the 3Rs: the NC3rs framework. Available at: https://nc3rs.org.uk/sites/default/files/2021-09/Evaluating_progress_in_the_3Rs-vthe_NC3Rs_framework.pdf.
Ormandy, E. H., and Schuppli, C. A. (2014). Public attitudes toward animal research: a review. Animals 4, 391–408. doi:10.3390/ani4030391
Sachs, N., de Ligt, J., Kopper, O., Gogola, E., Bounova, G., Weeber, F., et al. (2018). A living bio bank of breast cancer organoids captures disease heterogeneity. Cell. 172, 373–386.e10. doi:10.1016/j.cell.2017.11.010
Stucki, A. O., Barton-Maclaren, T. S., Bhuller, Y., Henriquez, J. E., Henry, T. R., Hirn, C., et al. (2022). Use of new approach methodologies (NAMs) to meet regulatory requirements for the assessment of industrial chemicals and pesticides for effects on human health. Front. Toxicol. 4 (4), 964553. doi:10.3389/ftox.2022.964553
Sutherland, R. M., McCredie, J. A., and Inch, R. (1971). Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J. Natl. Cancer Inst. 46, 113–120.
Taylor, K. (2014). EU Member State government contribution to alternative methods. ALTEX 31, 215–218. doi:10.14573/altex.1401061
Taylor, K. (2019). “Chapter 24: recent developments in alternatives to animal testing,” in Animal experimentation: working towards a paradigm change. Human-animal studies: Vol. 22. Editors K. Herman, and K. Jayne (Brill publishers), 585–609. doi:10.1163/9789004391192_025
Taylor, K., and Alvarez, L. R. (2020). Regulatory drivers in the last 20 years towards the use of in silico techniques as replacements to animal testing for cosmetic-related substances. Comput. Toxicol. 13, 100112. doi:10.1016/j.comtox.2019.100112
Taylor, K., and Rego Alvarez, L. (2019a). A summary of EU national statistical reports of animal experiments in 2014-2016. ALTEX 36, 314–319. doi:10.14573/altex.1812211
Taylor, K., and Rego Alvarez, L. R. (2019b). An estimate of the number of animals used for scientific purposes worldwide in 2015. Altern. Laboratory Animals 47, 196–213. doi:10.1177/0261192919899853
Thomas, D. W., Chancellor, D., Micklus, A., Lafever, S., Hay, M., Chaudhury, S., et al. (2021). Clinical development success rates and contributing factors 2011-2020. Biotechnology Innovation Organization, Informa Pharma Intelligence and Quantitative Life Sciences. Available at: https://go.bio.org/rs/490-EHZ-999/images/ClinicalDevelopmentSuccessRates2011_2020.pdf.
Tian, H., Liu, H., Zhu, Y., Xing, D., and Wang, B. (2020). The trends of single-cell analysis: a global study. Biomed. Res. Int. 2020, 7425397. doi:10.1155/2020/7425397
van der Jagt, S., Munn, J., Tørsløv, J., and de Bruijn, J. (2004). Alternative Approaches Can Reduce the Use of Test Animals under REACH. Addendum to the report “Assessment of additional testing needs under REACH. Effects of (Q)SARS, risk based testing and voluntary industry initiatives”. Ispra, Italy: European Commission, Directorate General, Joint Research Centre Institute for Health and Consumer Protection.
Zuang, V., Dura, A., Ahs Lopez, E., Barroso, J., Batista Leite, S., Berggren, E., et al. (2022) Non-animal methods in science and regulation – EURL ECVAM status report 2021, EUR 30960 EN, Publications Office of the European Union, Luxembourg, ISBN 978-92-76-46511-9, doi:10.2760/93290.JRC127780
Keywords: non-animal methods, animal testing, bibliometric analysis, new approach methodologies, lab-on-a-chip, organoid, stem cell, in silico approaches
Citation: Taylor K, Modi S and Bailey J (2024) An analysis of trends in the use of animal and non-animal methods in biomedical research and toxicology publications. Front. Lab. Chip. Technol. 3:1426895. doi: 10.3389/frlct.2024.1426895
Received: 02 May 2024; Accepted: 15 July 2024;
Published: 19 August 2024.
Edited by:
Slawomir Jakiela, Warsaw University of Life Sciences, PolandReviewed by:
Mario Rothbauer, Medical University of Vienna, AustriaIlona Grabowska-Jadach, Warsaw University of Technology, Poland
Copyright © 2024 Taylor, Modi and Bailey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephanie Modi, c3RlcGhhbmllQGFuaW1hbGZyZWVyZXNlYXJjaHVrLm9yZw==
†Present address: Jarrod Bailey, Physicians Committee for Responsible Medicine (PCRM), Washington, DC, United States.
 Katy Taylor
Katy Taylor Stephanie Modi
Stephanie Modi Jarrod Bailey
Jarrod Bailey