- 1Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States
- 2San Francisco Veterans Affairs Medical Center, School of Medicine, University of California, San Francisco, San Francisco, CA, United States
- 3Department of Psychiatry, San Francisco Veterans Affairs Medical Center, University of California, San Francisco, San Francisco, CA, United States
- 4Department of Neurology, San Francisco Veterans Affairs Medical Center, University of California, San Francisco, San Francisco, CA, United States
- 5Department of Medicine, San Francisco Veterans Affairs Medical Center, University of California, San Francisco, San Francisco, CA, United States
Background: Brain aneurysms represent a significant cause of hemorrhagic stroke. Prior research has demonstrated links between stress and stroke, including brain aneurysms. We aimed to determine relationships between select psychiatric disorders and aneurysms and aneurysmal SAH.
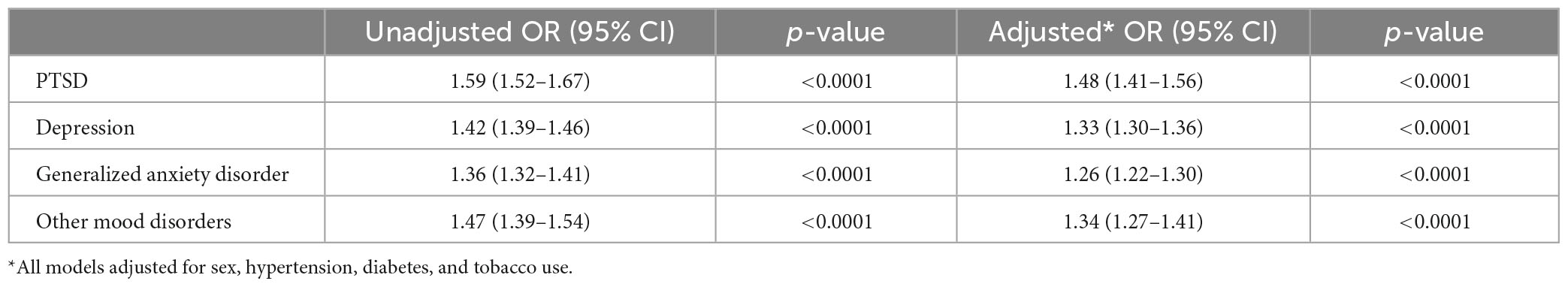
Methods: We performed retrospective, case-control study of a National Veterans Affairs population with two experimental groups (aneurysm-only and aneurysmal SAH) and 10-fold controls per group matched by age, date, and clinical data source. The studied the presence of 4 psychiatric disorders: Posttraumatic stress disorder (PTSD), major depressive disorder (MDD), generalized anxiety disorder (GAD), and other mood disorders. Our main outcomes Unadjusted and multivariable adjusted ORs of PTSD, MDD, GAD, and mood disorders within aneurysm-only and aSAH groups.
Results: In 6,320,789 US Veterans who were enrolled for at least 5 years in Medicare and/or the Veterans Health Administration, we identified 35,094 cases of aneurysm without SAH and 5,749 cases of aneurysm with SAH between 1/2005 and 12/2019. In analyses adjusted for sex, hypertension, and tobacco use, patients with aneurysm were more likely than matched controls to have a history of PTSD (OR 1.48), MDD (OR 1.33), GAD (OR 1.26), and other mood disorders (OR 1.34) (all p-values < 0.0001). Similarly, patients with aSAH were more likely than controls to have a history of PTSD (OR 1.35), MDD (OR 1.38), GAD (OR 1.18), and other mood disorders (OR 1.30) (all p-values < 0.0001).
Conclusion: The study, the largest of its kind, further suggests links between psychiatric disorders and stroke. This is important as patients with aneurysms are not routinely screened for such psychiatric risk factors. Additional research on this topic could lead to novel strategies to improve stroke prevention.
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) carries significant morbidity and is fatal in approximately 30% of cases (Korja et al., 2016; Korja and Kaprio, 2016). Brain aneurysms are relatively common with an estimated prevalence of 1%–3% depending on the adult population (Vlak et al., 2011; Imaizumi et al., 2018). Aneurysm size and genetic factors have been shown to play a role in rupture while several other factors, including smoking and hypertension, also influence risk and have informed models of stroke. Identification and management of such underlying modifiable risk factors and exposures have led to a global decline of aSAH (Etminan et al., 2019). Within this same 20-year interval (1993 – 2015), no significant change in the annual incidence of aSAH has occurred within the US (Golnari et al., 2020) despite an increase in the elective treatment of aneurysms overall. As such, the neurovascular community continues to search for other patient characteristics, such as female sex, biomarkers (e.g., MR-based aneurysmal wall enhancement) and modifiable risk factors (e.g., drug use), to more reliably differentiate at-risk subgroups as effectively as aneurysmal size.
The role of psychosocial stress in stroke is well established, and neuropsychiatric disorders, including major depressive disorder and schizophrenia, are considered significant risk factors for adverse clinical events (Piepoli et al., 2016; Kivimäki and Steptoe, 2018; Levine et al., 2021). Mechanisms through which mental health disturbances increase stroke risk have not, however, been fully elucidated. Evidence indicates roles for refractory hypertension, inflammation, autonomic nervous system disturbance, and/or endocrine dysfunction (Grippo and Johnson, 2009; Calvillo et al., 2019; Levine et al., 2021). Despite sharing pathophysiological mechanisms (e.g., atherosclerosis) and risk factors (e.g., hypertension) with myocardial infarction and ischemic stroke, a connection between aneurysms, aSAH and psychiatric disorders and environmental stressors has yet to be well-established. Significant financial and domestic stress has been associated with aneurysm presence and rupture, although these findings need validation in larger samples (de Wilde et al., 2019). Recent work has also demonstrated a possible interaction between PTSD, among other psychiatric disorders, and hemorrhagic stroke in young Veterans (Gaffey et al., 2021). After adjusting for demographics, health behaviors, traditional cardiovascular risk factors, and psychiatric comorbidities, PTSD proved non-influential in risk modeling. However, this study had a relatively small percentage of subarachnoid hemorrhage patients and did not address aneurysms directly.
Given the need for better risk stratification of patients with unruptured aneurysms and an incomplete understanding of the interaction between aneurysms, aSAH, and psychiatric disorders, we performed a retrospective case-control study of a large United States Veteran population to examine psychological conditions and their correlations with aneurysms and aSAH.
Methods
Sources of data
We utilized the STROBE checklist for case-control studies for manuscript development (STROBE, 2023) and adhered to observation case-control study guidelines. We used data from the Veterans Health Administration (VA) Corporate Data Warehouse (CDW). The CDW contains information on VA inpatient and outpatient visits and associated clinical diagnoses and procedures, information on non-VA visits reimbursed by VA, vital signs, VA pharmacy records, laboratory data, and other patient-level variables. We also used Medicare data from the Centers for Medicare and Medicaid Services (CMS) for all Veterans enrolled in or eligible for VHA healthcare and linked these data to VA data.
Participants
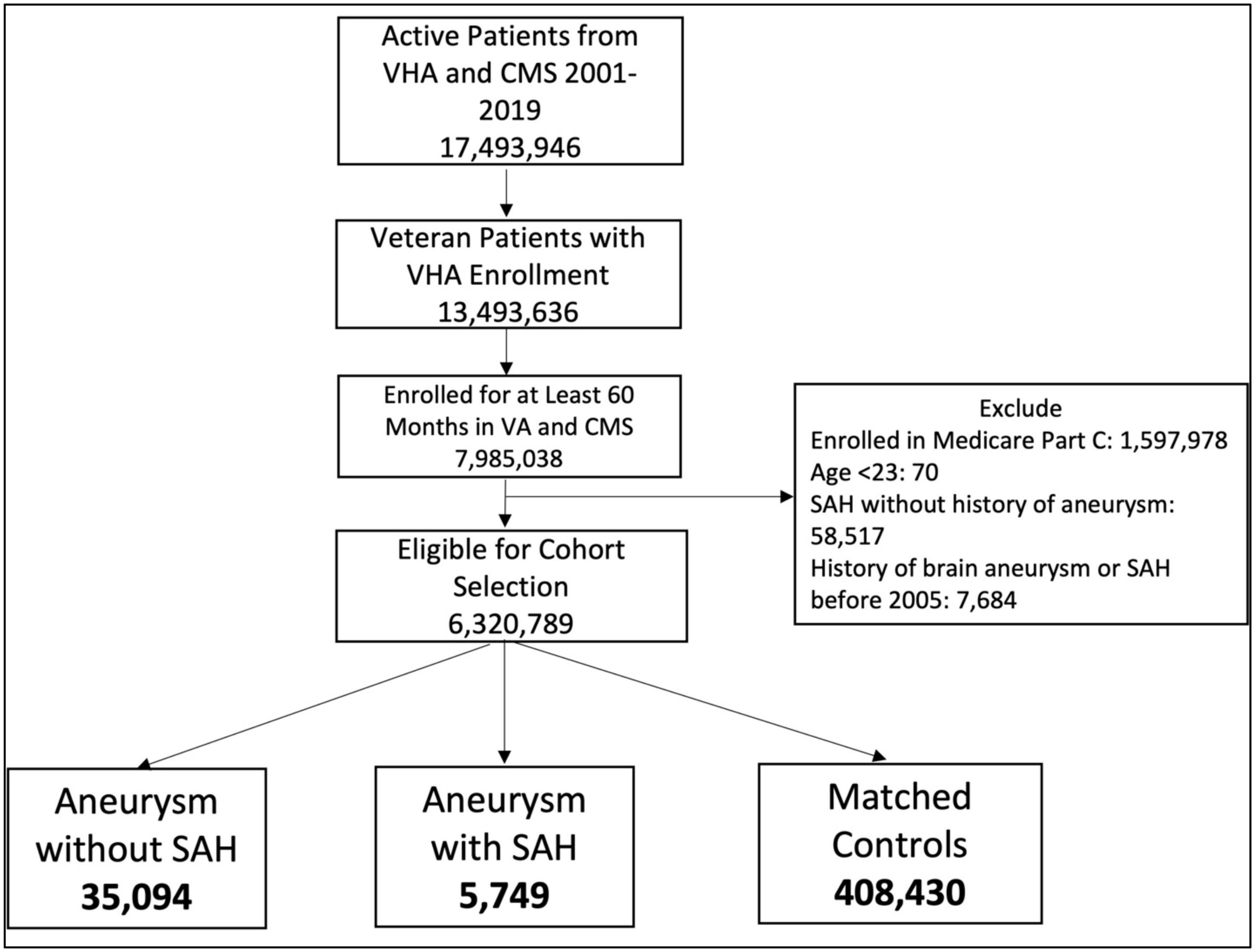
We examined records from 2000 to 2019 to identify patients eligible for VA care who were enrolled in VHA or Medicare for at least 60 months and had at least 1 VA visit during that time period (Figure 1). Patients enrolled in Medicare Part C at any time during the study period were excluded from the analysis because we only had data available for Medicare Parts A and B. We used ICD and CPT codes (Supplementary Table 1) to identify two case groups: (1) Brain aneurysm only and (2) Brain aneurysm plus non-traumatic SAH. Patients needed 1 ICD code from inpatient visits, 1 procedure code, or 2 outpatient codes qualify. For aneurysm we used codes ICD-9 437.3, ICD-10 I67.1, and CPT codes 61697, 61698, 61700, 61702, 61624. For non-traumatic SAH we used codes ICD-9 430 and ICD-10 I60. We considered the index date to be the date of the first qualifying aneurysm CPT or ICD code. We then randomly selected 10 controls per case from remaining patients who did not have any aneurysm or SAH codes but had a clinical visit on that index date. Cases and controls were matched by age, date of diagnosis (case diagnosis matched to a clinical visit date for controls), and site of index visit (VA vs. CMS). We required that the mental health diagnoses occur in the 5-year period before the index date, i.e., as it relates to the medical record, the diagnosis of a mental health condition preceded the diagnosis of aneurysm without or aneurysm with SAH. After applying selection criteria, 6,320,789 patients were eligible for inclusion. The case and control selection and matching process yielded 35,094 aneurysm-only and 5,749 aSAH patients and 10x matched control patients for each of these groups (Figure 1).
This study was approved by the University of California, San Francisco Human Research Protection Program and the San Francisco Veterans Affairs Healthcare System Research and Development Committee. As the study involved secondary analysis of healthcare records, no participant contact was involved, and informed consent was not required.
Variables
We evaluated the 5-year period prior to the index date to establish diagnoses of several mental health conditions using ICD codes: PTSD, major depressive disorder (MDD), generalized anxiety disorder (GAD), and other mood disorders (Supplementary Table 1). We collected additional demographic information (age and gender) and used the same prior 5-year period to assess diagnoses of common comorbidities and stroke risk factors (hypertension, diabetes, and tobacco use).
Statistical analyses
Differences in patient characteristics between cases and controls were compared using the Chi-square test for categorical variables and Wilcoxon rank-sum test for continuous variables. Univariable logistic regression models were constructed for each mental health predictor with the outcome of brain aneurysm. Analogous models were constructed with the outcome of aSAH. Subsequent models included all psychiatric factors simultaneously to examine the independent associations of the mental health variables with the outcomes. Multivariable models adjusted for sex, hypertension, diabetes, and tobacco use. All analyses were performed with SAS Enterprise Guide version 8.3 (SAS Institute, Cary, NC).
Results
Patients with Aneurysm-only were older than those with aSAH (70.8 years vs 68.1 years). Female sex, hypertension, and tobacco use were more common in both aneurysmal groups relative to controls (Tables 1, 2, p-value < 0.0001).
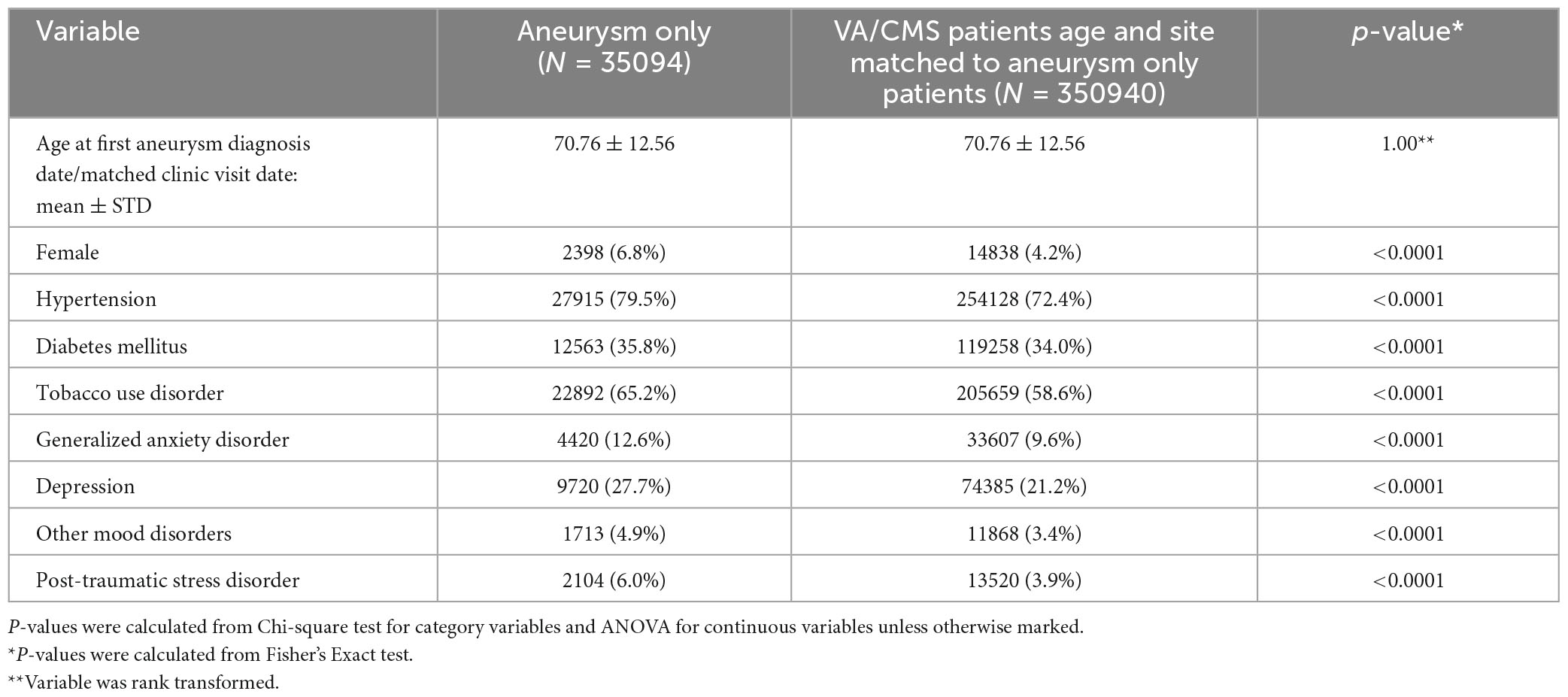
Table 1. Characteristics of participants with aneurysm only versus controls matched for age, date, and site of diagnosis (VA or CMS).
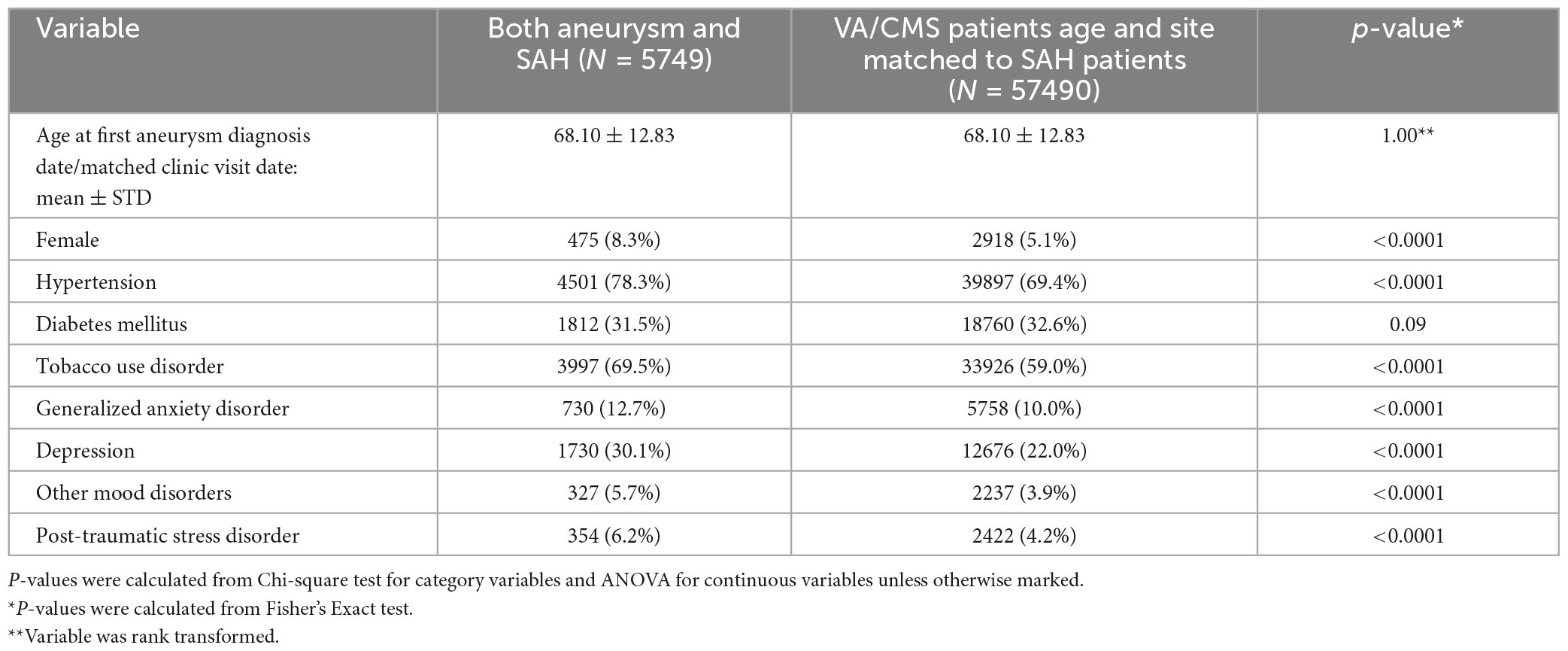
Table 2. Characteristics of participants with aneurysm and SAH vs. controls matched for age, date, and site of diagnosis (VA or CMS).
In univariate analysis, all psychiatric disorders were more common in patients with Aneurysm-only (Table 1) and aSAH (Table 2) than in controls. In analyses adjusted for hypertension, tobacco use, and sex, patients with Aneurysm-only were more likely than age- and site-matched controls to have a history of PTSD (OR 1.48), MDD (OR 1.33), GAD (OR 1.26), and other mood disorders (OR 1.34) (Table 3; all p-values < 0.0001). Similarly, patients with aSAH were more likely than age- and site-matched controls to have a history of PTSD (OR 1.35), MDD (OR 1.38), GAD (OR 1.18), and other mood disorders (OR 1.30) (Table 4). In secondary multivariable models incorporating all psychiatric disorders simultaneously, all psychiatric variables remained independently associated with Aneurysm-only (Supplementary Table 2), but only MDD and other mood disorders remained independently associated with aSAH (Supplementary Table 3).
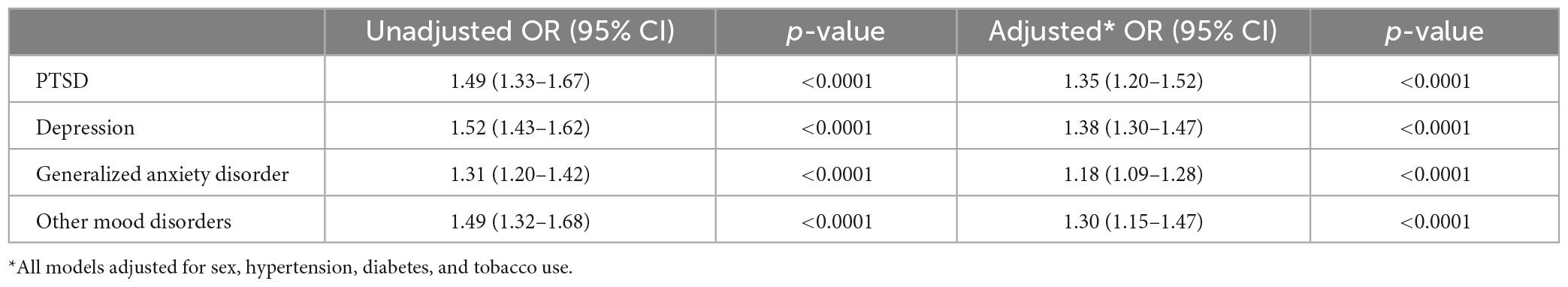
Table 4. Unadjusted and adjusted associations of mental health conditions aneurysm with subarachnoid hemorrhage.
Discussion
The study represents the largest examination of the intersection of psychiatric disorders and brain aneurysms and aSAH. The results expand upon prior work implicating hypertension, smoking, and female sex as aneurysm risk factors (Zuurbier et al., 2022). In multivariable modeling, PTSD, MDD, GAD, and other mood disorders were all significantly associated with aneurysms. Associations with psychiatric disorders were generally less pronounced within the aSAH group relative to the aneurysm-only group. This latter result may reflect under-recording of psychiatric disorders in non-VA healthcare facilities as most ruptured aneurysms are managed outside the VA system. Post-traumatic stress disorder and MDD had the strongest correlations with aneurysm or aSAH, respectively. This is important to the stroke community as most patients with aneurysms are not screened for PTSD or MDD signs and symptoms as part of aneurysmal workup. Further, therapies, pharmacological or behavioral, dedicated to the treatment of such disorders, are not typically considered a stroke risk reduction strategy.
As noted, several studies have suggested a role for psychiatric disturbance in stroke (Liu et al., 2021; Perkins et al., 2021) one of which found an association of aneurysms with hemorrhagic stroke, though it was not specific to SAH (de Wilde et al., 2019). In comparison to the study by Gaffey et al. (2021), the current study included a significantly older population (∼70 years vs. 30 years) with a greater prevalence of hypertension (∼80% vs. 13%). Such age discrepancies may have contributed to differences in the fraction of psychiatric diagnoses, with GAD (∼12% vs. 16%) and PTSD (∼6% vs. 28%) being more common relative to the current work and MDD (∼30 vs. 10%) being less so. Importantly, of hemorrhagic stroke subtypes, only 104 patients (25%) were SAH in nature and thus conclusions from this prior study regarding aneurysmal pathophysiology were more limited.
Mechanisms
Several factors have been proposed as mechanisms through which mental health disorders could affect aneurysm growth and rupture, including hypertension, inflammation, oxidative stress, and immunoregulatory dysfunction. These mechanisms, whether independently or synergistically, can have a negative effect on the molecular-cellular environment of aneurysms, eventually leading to rupture. Hypertension, a consistent risk factor for aneurysms may result from stress-induced hypothalamic-pituitary axis dysfunction. This increases the secretion of glucocorticoids and circulating catecholamines, which over time leads to vascular smooth muscle cell modulation and endothelial damage (Everson-Rose et al., 2014).
Neuropsychiatric disorders have shown association with elevated inflammation, as demonstrated by increases in C-reactive protein, interleukins, and fibrinogen (Everson-Rose et al., 2014; Beurel et al., 2020). Aneurysmal pathophysiological modeling suggests following an initial insult to the endothelium due to hemodynamic wall stress, upregulation of the inflammatory pathways may initiate a feed-forward injury cycle. The chronic translocation of leukocytes through the endothelium can accelerate degradation of the vessel wall, making the aneurysms more prone to rupture. Levels of proteins such as vascular adhesion molecule 1 and von Willebrand factor, which are important in the leukocyte translocation pathway, have been shown to be higher in individuals with depression (Lopez-Vilchez et al., 2016). Additionally, TNF-a, a cytokine implicated in aneurysm pathogenesis (Starke et al., 2014), has been shown to be elevated in patients with PTSD and MDD. TNF-a upregulates signaling pathways that lead to apoptosis and cellular degradation, such as the expression of calcium channels, Toll-like receptors, and matrix metalloproteinases. TNF-a and interleukin 1 signal downstream pro-inflammatory cytokines and interleukins while MMPs increase vascular permeability, leading to hemorrhage (Liu et al., 2022). Such neurovascular immunological dysfunction may be mitigated in part through flow- and pressure-dependent triggers, though the upstream genetics and may offer opportunities to modify risk either through medical and/or lifestyle changes.
Beyond these inflammatory and immunoregulatory alterations, environmental, occupational and psychosocial stress can increase reactive oxygen species (ROS) and cause vascular dysfunction (Loria et al., 2011). In mouse models, cigarette smoke exposure is increases ROS and NOX1 which precipitates upregulation of proinflammatory/matrix remodeling genes, leading to vascular smooth muscle cell modulation and aneurysm formation and rupture (Laaksamo et al., 2013). Increased oxidative stress and ROS production also affects the renin-angiotensin system and glucocorticoid pathways with downstream effects on endothelial cell function (Griessenauer et al., 2018). Moreover, the upregulation of free radicals, superoxide dismutase and glutathione in the setting of occupational and domestic stress is also associated with MDD (van Sloten et al., 2014). Though no studies have specifically addressed these mechanisms in patients with aneurysms, studies of patients with cardiovascular risk factors have demonstrated stress reduction can reduce oxidative stress and improve markers of endothelial function (Said and El-Gohary, 2016).
Lastly, patients with psychiatric disorders may disproportionately use and/or abuse substances modifying stroke risk. Chronic alcohol use has been implicated as a risk factor for hypertension and stroke (Smyth et al., 2022) and intensity of alcohol use has also been shown to demonstrate a dose-dependent relationship to aneurysm rupture (Can et al., 2018a). Heroin and methamphetamine use have also been implicated in case series of aSAH patients (Can et al., 2018b; Swor et al., 2019). It is possible that pharmacological interventions for psychiatric disorders may also affect stroke risk. There is controversy about how selective serotonin reuptake inhibitors may affect stroke with or without other drug modifiers (Trajkova et al., 2019; Gaffey et al., 2021). More comprehensive, prospective studies will be needed to elucidate these potentially confounding relationships.
Limitations
As this is an observational study, we cannot prove that psychiatric disorders caused aneurysms or aSAH. We relied on medical records to establish diagnoses of variables and outcomes, and this could be subject to misclassification bias. Our findings may also be influenced by ascertainment bias if patients diagnosed with mental health disorders were more likely to seek care (e.g., undergo brain imaging) and have aneurysms diagnosed. However, aneurysm rupture would likely lead to symptoms that prompted treatment-seeking independent of prior mental health and treatment status. It is encouraging that established risk factors such as hypertension, female sex, and tobacco use were also associated with aneurysmal diagnoses suggesting the results might more accurately reflect reality. Finally, though we attempted to match or control for important factors that are associated with mental health disorders and could impact aneurysm and aSAH risk, residual confounding remains a concern.
Data availability statement
The data presented in this article are not readily available because the VA does not permit sharing. Requests to access the datasets should be directed to DC, ZGFuaWVsLmNvb2tlQHVjc2YuZWR1.
Ethics statement
The studies involving human participants were reviewed and approved by the UCSF IRB. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
DC initiated and led the research team in carrying out the project. All authors contributed to the research and manuscript preparation and review.
Funding
This study was funded by the VA Health Services Research and Development (HSR&D) Service, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004), and the San Francisco VA Health Care System Measurement Science QUERI (Project Number QUE 20-009).
Acknowledgments
We wish to recognize the veterans themselves as well as the clinical and research staff at the SFVAMC.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnint.2023.1207610/full#supplementary-material
References
Beurel, E., Toups, M., and Nemeroff, C. (2020). The bidirectional relationship of depression and inflammation: Double trouble. Neuron 107, 234–256. doi: 10.1016/j.neuron.2020.06.002
Calvillo, L., Gironacci, M., Crotti, L., Meroni, P., and Parati, G. (2019). Neuroimmune crosstalk in the pathophysiology of hypertension. Nat. Rev. Cardiol. 16, 476–490. doi: 10.1038/s41569-019-0178-1
Can, A., Castro, V., Ozdemir, Y., Dagen, S., Dligach, D., Finan, S., et al. (2018a). Alcohol consumption and aneurysmal subarachnoid hemorrhage. Transl. Stroke Res. 9, 13–19. doi: 10.1007/s12975-017-0557-z
Can, A., Castro, V., Ozdemir, Y., Dagen, S., Dligach, D., Finan, S., et al. (2018b). Heroin use is associated with ruptured saccular aneurysms. Transl. Stroke Res. 9, 340–346. doi: 10.1007/s12975-017-0582-y
de Wilde, A., Greebe, P., Rinkel, G., and Algra, A. (2019). Stress in patients with (un)ruptured intracranial aneurysms vs population-based controls. Neurosurgery 84, 1065–1071. doi: 10.1093/neuros/nyy143
Etminan, N., Chang, H., Hackenberg, K., de Rooij, N., Vergouwen, M., Rinkel, G., et al. (2019). Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: A systematic review and meta-analysis. JAMA Neurol. 76, 588–597. doi: 10.1001/jamaneurol.2019.0006
Everson-Rose, S., Roetker, N., Lutsey, P., Kershaw, K., Longstreth, W. Jr., Sacco, R., et al. (2014). Chronic stress, depressive symptoms, anger, hostility, and risk of stroke and transient ischemic attack in the multi-ethnic study of atherosclerosis. Stroke 45, 2318–2323. doi: 10.1161/STROKEAHA.114.004815
Gaffey, A., Rosman, L., Burg, M., Haskell, S., Brandt, C., Skanderson, M., et al. (2021). Posttraumatic stress disorder, antidepressant use, and hemorrhagic stroke in young men and women: A 13-year cohort study. Stroke 52, 121–129. doi: 10.1161/STROKEAHA.120.030379
Golnari, P., Nazari, P., Garcia, R., Weiss, H., Shaibani, A., Hurley, M., et al. (2020). Volumes, outcomes, and complications after surgical versus endovascular treatment of aneurysms in the United States (1993-2015): Continued evolution versus steady-state after more than 2 decades of practice. J. Neurosurg. 134, 848–861. doi: 10.3171/2019.12.JNS192755
Griessenauer, C., Tubbs, R., Foreman, P., Chua, M., Vyas, N., Lipsky, R., et al. (2018). Association of renin-angiotensin system genetic polymorphisms and aneurysmal subarachnoid hemorrhage. J. Neurosurg. 128, 86–93. doi: 10.3171/2016.9.JNS161593
Grippo, A., and Johnson, A. (2009). Stress, depression and cardiovascular dysregulation: A review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress 12, 1–21. doi: 10.1080/10253890802046281
Imaizumi, Y., Mizutani, T., Shimizu, K., Sato, Y., and Taguchi, J. (2018). Detection rates and sites of unruptured intracranial aneurysms according to sex and age: An analysis of MR angiography-based brain examinations of 4070 healthy Japanese adults. J. Neurosurg. 130, 573–578. doi: 10.3171/2017.9.JNS171191
Kivimäki, M., and Steptoe, A. (2018). Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol. 15, 215–229. doi: 10.1038/nrcardio.2017.189
Korja, M., and Kaprio, J. (2016). Controversies in epidemiology of intracranial aneurysms and SAH. Nat. Rev. Neurol. 12, 50–55. doi: 10.1038/nrneurol.2015.228
Korja, M., Lehto, H., Juvela, S., and Kaprio, J. (2016). Incidence of subarachnoid hemorrhage is decreasing together with decreasing smoking rates. Neurology 87, 1118–1123. doi: 10.1212/WNL.0000000000003091
Laaksamo, E., Tulamo, R., Liiman, A., Baumann, M., Friedlander, R., Hernesniemi, J., et al. (2013). Oxidative stress is associated with cell death, wall degradation, and increased risk of rupture of the intracranial aneurysm wall. Neurosurgery 72, 109–117. doi: 10.1227/NEU.0b013e3182770e8c
Levine, G., Cohen, B., Commodore-Mensah, Y., Fleury, J., Huffman, J. C., Khalid, U., et al. (2021). Psychological health, well-being, and the mind-heart-body connection: A scientific statement from the American heart association. Circulation 143, e763–e783. doi: 10.1161/CIR.0000000000000947
Liu, Q., Zhang, Y., Yang, J., Yang, Y., Li, M., Chen, S., et al. (2022). The relationship of morphological-hemodynamic characteristics, inflammation, and remodeling of aneurysm wall in unruptured intracranial aneurysms. Transl. Stroke Res. 13, 88–99. doi: 10.1007/s12975-021-00917-1
Liu, Y., Cheng, P., Liu, N., Li, B., Ma, Y., Zuo, W., et al. (2021). Neuroticism increases the risk of stroke: Mendelian randomization study. Stroke 52, e742–e743. doi: 10.1161/STROKEAHA.121.036131
Lopez-Vilchez, I., Diaz-Ricart, M., Navarro, V., Torramade, S., Zamorano-Leon, J., Lopez-Farre, A., et al. (2016). Endothelial damage in major depression patients is modulated by SSRI treatment, as demonstrated by circulating biomarkers and an in vitro cell model. Transl. Psychiatry 6:e886. doi: 10.1038/tp.2016.156
Loria, A., Kang, K., Pollock, D., and Pollock, J. (2011). Early life stress enhances angiotensin II-mediated vasoconstriction by reduced endothelial nitric oxide buffering capacity. Hypertension 58, 619–626. doi: 10.1161/HYPERTENSIONAHA.110.168674
Perkins, J., Wilkins, S., Kamran, S., and Shuaib, A. (2021). Post-traumatic stress disorder and its association with stroke and stroke risk factors: A literature review. Neurobiol. Stress 14:100332. doi: 10.1016/j.ynstr.2021.100332
Piepoli, M., Hoes, A., Agewall, S., Albus, C., Brotons, C., Catapano, A., et al. (2016). 2016 European guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 252, 207–274. doi: 10.1016/j.atherosclerosis.2016.05.037
Said, M., and El-Gohary, O. (2016). Effect of noise stress on cardiovascular system in adult male albino rat: Implication of stress hormones, endothelial dysfunction and oxidative stress. Gen. Physiol. Biophys. 35, 371–377. doi: 10.4149/gpb_2016003
Smyth, A., O’Donnell, M., Rangarajan, S., Hankey, G., Oveisgharan, S., Canavan, M., et al. (2022). Alcohol intake as a risk factor for acute stroke: The INTERSTROKE study. Neurology 100, e142–e153. doi: 10.1212/WNL.0000000000201388
Starke, R., Chalouhi, N., Jabbour, P., Tjoumakaris, S., Gonzalez, L., Rosenwasser, R., et al. (2014). Critical role of TNF-α in cerebral aneurysm formation and progression to rupture. J Neuroinflammation 11:77. doi: 10.1186/1742-2094-11-77
STROBE. (2023). STROBE statement—checklist of items that should be included in reports of case-control studies. Available online at: https://www.strobe-statement.org/checklists/ (accessed February 22, 2023).
Swor, D., Maas, M., Walia, S., Bissig, D. P., Liotta, E. M., Naidech, A. M., et al. (2019). Clinical characteristics and outcomes of methamphetamine-associated intracerebral hemorrhage. Neurology 93, e1–e7. doi: 10.1212/WNL.0000000000007666
Trajkova, S., d’Errico, A., Soffietti, R., Sacerdote, C., and Ricceri, F. (2019). Use of antidepressants and risk of incident stroke: A systematic review and meta-analysis. Neuroepidemiology 53, 142–151. doi: 10.1159/000500686
van Sloten, T., Schram, M., Adriaanse, M., Dekker, J., Nijpels, G., Teerlink, T., et al. (2014). Endothelial dysfunction is associated with a greater depressive symptom score in a general elderly population: The Hoorn study. Psychol. Med. 44, 1403–1416. doi: 10.1017/S0033291713002043
Vlak, M., Algra, A., Brandenburg, R., and Rinkel, G. (2011). Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 10, 626–636. doi: 10.1016/S1474-4422(11)70109-0
Keywords: aneurysm, stroke, posttraumatic stress disorder (PTSD), depression, generalized anxiety disorder
Citation: Cooke DL, Shen H, Duvvuri M, Thompson D, Neylan T, Wolfe W, Hetts S, Ovbiagele B, Whooley M and Cohen B (2023) Association of select psychiatric disorders with incident brain aneurysm and subarachnoid hemorrhage among veterans. Front. Integr. Neurosci. 17:1207610. doi: 10.3389/fnint.2023.1207610
Received: 17 April 2023; Accepted: 19 July 2023;
Published: 02 August 2023.
Edited by:
Masashi Tanaka, Health Science University, JapanReviewed by:
Chao Yang, Wuhan University, ChinaRene Viso, Sanatorio Nuestra Señora Del Rosario, Argentina
Copyright © 2023 Cooke, Shen, Duvvuri, Thompson, Neylan, Wolfe, Hetts, Ovbiagele, Whooley and Cohen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel L. Cooke, RGFuaWVsLmNvb2tlQHVjc2YuZWR1
 Daniel L. Cooke
Daniel L. Cooke Hui Shen2
Hui Shen2 Thomas Neylan
Thomas Neylan Steven Hetts
Steven Hetts Beth Cohen
Beth Cohen