
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Integr. Neurosci. , 18 October 2018
Volume 12 - 2018 | https://doi.org/10.3389/fnint.2018.00051
This article is part of the Research Topic Cannabinoid Therapeutics: What's Hot View all 24 articles
Neurological therapeutics have been hampered by its inability to advance beyond symptomatic treatment of neurodegenerative disorders into the realm of actual palliation, arrest or reversal of the attendant pathological processes. While cannabis-based medicines have demonstrated safety, efficacy and consistency sufficient for regulatory approval in spasticity in multiple sclerosis (MS), and in Dravet and Lennox-Gastaut Syndromes (LGS), many therapeutic challenges remain. This review will examine the intriguing promise that recent discoveries regarding cannabis-based medicines offer to neurological therapeutics by incorporating the neutral phytocannabinoids tetrahydrocannabinol (THC), cannabidiol (CBD), their acidic precursors, tetrahydrocannabinolic acid (THCA) and cannabidiolic acid (CBDA), and cannabis terpenoids in the putative treatment of five syndromes, currently labeled recalcitrant to therapeutic success, and wherein improved pharmacological intervention is required: intractable epilepsy, brain tumors, Parkinson disease (PD), Alzheimer disease (AD) and traumatic brain injury (TBI)/chronic traumatic encephalopathy (CTE). Current basic science and clinical investigations support the safety and efficacy of such interventions in treatment of these currently intractable conditions, that in some cases share pathological processes, and the plausibility of interventions that harness endocannabinoid mechanisms, whether mediated via direct activity on CB1 and CB2 (tetrahydrocannabinol, THC, caryophyllene), peroxisome proliferator-activated receptor-gamma (PPARγ; THCA), 5-HT1A (CBD, CBDA) or even nutritional approaches utilizing prebiotics and probiotics. The inherent polypharmaceutical properties of cannabis botanicals offer distinct advantages over the current single-target pharmaceutical model and portend to revolutionize neurological treatment into a new reality of effective interventional and even preventative treatment.
Cannabis burst across the Western medicine horizon after its introduction by William O’Shaughnessy in 1838 (O’Shaughnessy, 1838–1840; Russo, 2017b), who described remarkable successes in treating epilepsy, rheumatic pains, and even universally fatal tetanus with the “new” drug. Cannabis, or “Indian hemp,” was rapidly adopted by European physicians noting benefits on migraine by Clendinning in England (Clendinning, 1843; Russo, 2001) and neuropathic pain, including trigeminal neuralgia by Donovan in Ireland (Donovan, 1845; Russo, 2017b). These developments did not escape notice of the giants of neurology on both sides of the Atlantic, who similarly adopted its use in these indications: Silas Weir Mitchell, Seguin, Gowers and Osler (Mitchell, 1874; Seguin, 1877; Gowers, 1888; Osler and McCrae, 1915). While medicinal cannabis suffered a period of obscurity and quiescence, mainly attributable to quality control issues and political barriers, modern data on migraine (Russo, 2004, 2016b; Rhyne et al., 2016) and neuropathic pain, whether central or peripheral support its common application by affected patients (Rog et al., 2005; Nurmikko et al., 2007; Russo and Hohmann, 2013; Serpell et al., 2014), additionally supported by the National Academies of Science, Engineering and Medicine (National Academies of Sciences Engineering and Medicine (U.S.). Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda, 2017).
It has been noted for some time that muscle tone on the central level is mediated by the endocannabinoid system (Baker et al., 2003), but some additional years were necessary to bring this “aspirin of the 21st century” through Phase I–III Randomized Clinical Trials (RCTs; Novotna et al., 2011) and post-marketing assessment to demonstrate its safety, efficacy and consistency (Rekand, 2014; Fife et al., 2015; Maccarrone et al., 2017). That preparation, nabiximols (US Adopted Name; Sativex®) has currently attained regulatory approval in 30 countries for spasticity associated with multiple sclerosis (MS), and in Canada for central neuropathic pain in MS (Rog et al., 2005), and for opioid-resistant cancer pain (Johnson et al., 2010). Recent surveys find usage rates for cannabis of 20%–60% among MS patients (Rudroff and Honce, 2017). An earlier attempt to demonstrate neuroprotection in head trauma after intravenous administration of single doses of the non-intoxicating cannabinoid analog, dexanabinol, failed (Maas et al., 2006), but hope remains for other preparations in stroke and other brain insults (Latorre and Schmidt, 2015; Russo, 2015; Pacher et al., 2018). Table 1 summarizes the current status of cannabis-based drugs in neurological conditions not discussed at length herein, including sleep disturbance (Russo et al., 2007; Babson et al., 2017), glaucoma (Merritt et al., 1980), lower urinary tract symptoms (LUTS; Brady et al., 2004; Kavia et al., 2010), social anxiety (Bergamaschi et al., 2011), Tourette syndrome (Müller-Vahl et al., 2002, 2003) and schizophrenia (Leweke et al., 2012; McGuire et al., 2018). This Perspective article will rather focus on several neurological syndromes that overlap in their pathophysiology or have yet to receive concerted attention in clinical trials of cannabis-based medicines.
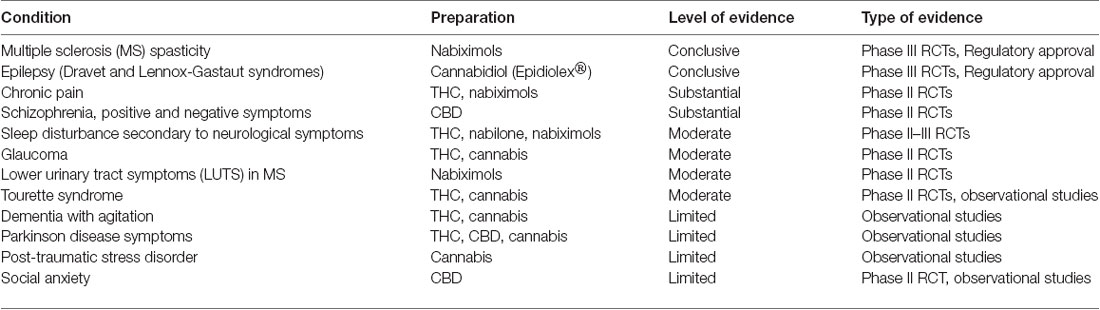
Table 1. Neurological conditions for which cannabis-based treatments have been employed (revised, reformatted and supplemented from MacCallum and Russo, 2018).
This author has previously addressed the pathophysiology of migraine (Sarchielli et al., 2007), post-traumatic stress (Hill et al., 2013), Parkinson disease (PD; Pisani et al., 2005) and other conditions as putative clinical endocannabinoid deficiency disorders wherein disturbances in endocannabinoid tone have been demonstrated objectively (Russo, 2004, 2016b).
Various synthetic fatty acid amidohydrolase (FAAH) inhibitors have been investigated for neurological therapeutics (Nozaki et al., 2015), but none have advanced to Phase III clinical trials. This is a mechanism of action seemingly shared with cannabidiol (Bisogno et al., 2001).
After elucidation of phytocannabinoid structures in the 1960s, their pharmacology was slowly revealed (reviewed by Cascio and Pertwee, 2014; Pertwee and Cascio, 2014; Russo and Marcu, 2017; Figure 1). Various components were tested for anticonvulsant activities with findings of ED50 in mice of 80 mg/kg for tetrahydrocannabinol (THC), 120 mg/kg for cannabidiol (CBD) and 200 mg/kg for tetrahydrocannabinolic acid A (THCA-A), the carboxylic acid precursor to THC found in raw cannabis flowers (Karler and Turkanis, 1979). Although dose-response was tested, it is unclear that very low doses were assessed and given the biphasic tendencies of cannabinoids, it is possible that positive lower dose effects may have remained unnoticed. CBD was considered an excellent candidate for development based on its lack of untoward psychoactive sequelae. However, little work was done until a series of small human trials in Brazil in following decades (reviewed by Russo, 2017a).
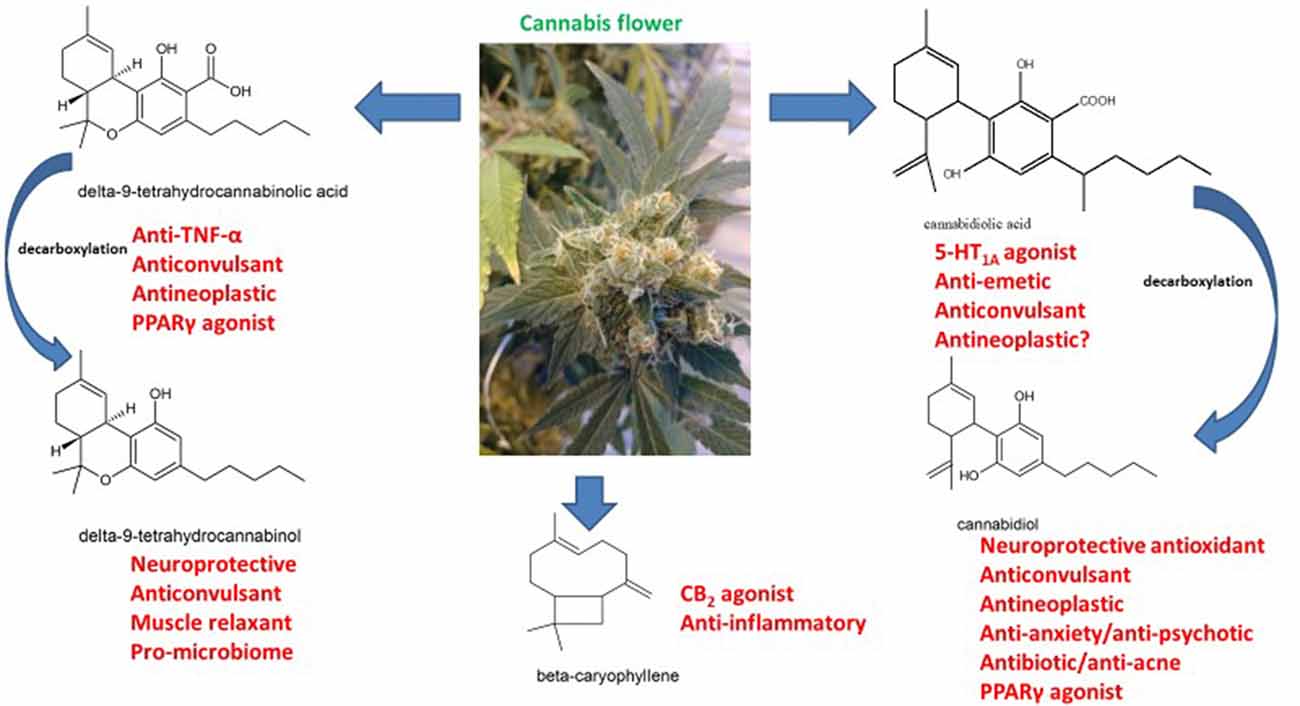
Figure 1. The pharmacology of phytocannabinoids pertinent to treatment of neurodegenerative disorders (molecular structures drawn by ER with ACD/ChemSketch 2015.2.5).
Subsequent investigation demonstrated that seizure threshold is mediated by the endocannabinoid system (Wallace et al., 2003), and that THC produced a 100% reduction in seizures, whereas phenobarbital and diphenylhydantoin did not. Additionally, animal studies demonstrated both acute increases in endocannabinoid production and a long-term up-regulation of CB1 production as apparent compensatory effects counteracting glutamate excitotoxicity, and that anticonvulsant effect was present at sub-sedating levels.
Sporadic case reports of successful utilization of THC in seizures associated with severe neurological conditions in children in Germany followed (Lorenz, 2004; Gottschling, 2011), but the prime focus returned to CBD due to strong anticonvulsant results in laboratory investigation (Jones et al., 2010), which led directly to a pharmaceutical development program. The lay public quickly became aware of these developments, with promotion of the concept by Project CBD1 and publicity associated with the case of Charlotte Figi and significant improvement in seizures associated with Dravet syndrome, as portrayed on the Weeds documentary on Cable News Network (Maa and Figi, 2014). Positive survey results (Porter and Jacobson, 2013) were tempered, however, by studies suggesting strong ascertainment bias in parental reporting of seizure frequency: response rate for families moving to the state of Colorado for cannabidiol treatment was 47% vs. only 22% for those already living there, and were three-fold higher for those reporting >50% response (Press et al., 2015). More careful observational studies with a standardized cannabidiol oral extract with THC removed (Epidiolex®) provided more compelling results (Devinsky et al., 2016) with a 55% median reduction in seizures in Dravet and Lennox-Gastaut Syndrome (LGS) patients at high dose. Subsequent Phase III results in Dravet syndrome at CBD 20 mg/kg/d showed strong statistical significance in seizure frequency and Caregiver Global Impression of Change (Devinsky et al., 2017). More recent studies have bolstered evidence for safety and efficacy of the preparation in both conditions (Devinsky et al., 2018; Thiele et al., 2018). As a result, it received US Food and Drug Administration approval in June 2018.
Interestingly, extensive observations from other practitioners (Russo et al., 2015) seemed to indicate similar therapeutic successes with much lower doses of CBD when utilized in cannabis-based preparations with small concomitant amounts of THC, THCA and linalool, a terpenoid component of cannabis (Russo, 2017a; Sulak et al., 2017; Pamplona et al., 2018). Selective cannabis breeding via Mendelian techniques raises the possibility of producing chemovars with multiple anticonvulsant components that may produce synergistic benefits (Lewis et al., 2018). THCA is an intriguing issue, in that there is debate about whether it harbors CB1 activity, or rather is due to spontaneous decarboxylation to THC (McPartland et al., 2017; Figure 1). Cannabidiolic acid (CBDA) was also recently reported to demonstrate anticonvulsant activity (Bonni Goldstein, personal communication), possibly attributable to its serotonergic activity (Bagdy et al., 2007), in that CBDA demonstrates 100-fold greater affinity for the 5-HT1A receptor (Bolognini et al., 2013) as compared to CBD (Russo et al., 2005).
Strong scientific evidence of cytotoxic benefit of phytocannabinoids has been available since 1975 (Munson et al., 1975) and highlighted three decades later (Ligresti et al., 2006), but the historical record suggests ancient use by Egyptian Copts (THC and/or THCA; Reymond, 1976; Russo, 2007) with similar claims by Renaissance herbalists in Europe (CBD and/or CBDA; Russo, 2007). Brain tumors are the subject of an excellent current review (Dumitru et al., 2018). To summarize available research, specific pro-apoptotic activity of THC in C6 glioma was reported (Sánchez et al., 1998), and shrinkage of in situ human glioma cell line tumors was observed with CBD (Massi et al., 2004). Intra-tumoral THC administration in glioblastoma multiforme (GBM) produced slight life prolongation over expectations in nine human patients (Guzmán et al., 2006). Case reports from Canada documented total regression of residua in two pilocytic astrocytomata in children after smoked cannabis (Foroughi et al., 2011). Careful laboratory analysis has established synergistic benefits of combinations of THC, CBD and standard chemotherapy with temozolomide on glioma (Torres et al., 2011). Clinical application of the concept has been reported online in a Phase II randomized controlled trial (RCT) of 21 patients with recurrent GBM on temozolomide plus nabiximols up to 12 sprays per day (32.4 mg THC plus 30 mg CBD plus terpenoids) vs. placebo with an 83% 1-year survival vs. 53% in controls (p = 0.042) and survival exceeding 550 days vs. 369 for controls, and only two withdrawals in each group due to adverse events (AEs)2.
Such encouraging results are supplemented by a recent report that THCA is a peroxisome proliferator-activated receptor-gamma (PPARγ) agonist (IC50 = 470 nM, Ki = 209 nM) > CBGA (517.7 nM) and ≫ than CBDA, CBD or THC (Nadal et al., 2017). THCA improved neuronal viability in an animal model of Huntington disease (HD), and decreased striatal neurodegeneration (blocked by PPARγ antagonist), and it was suggested as a therapeutic agent in HD. This finding, however, has much larger implications and could explain claims of therapeutic efficacy in epilepsy noted above (Sun et al., 2008), as well tumors, and perhaps even in major depression (Colle et al., 2017a,b). In contrast to other neutral cannabinoids and terpenoids, THCA is reported not to cross the blood-brain barrier (BBB), but if true, that hindrance may not be applicable in the context of chronic epilepsy (Oby and Janigro, 2006), or in brain tumors wherein that barrier is compromised.
As reviewed (Elrod and Sun, 2008), PPARs are ligand-binding transcription factors on nuclear membranes that affect adipogenesis, apoptosis and many other functions. PPARγ stimulation may kill cancer cells without toxicity to normal cells, such as astrocytes, and their effects are additive with other cytotoxic agents. Butyrate and capsaicin may be natural ligands. PPARγ has been identified in many cancers including those affecting the brain, where it regulates target gene transcription (Shen et al., 2016), and its activation inhibits tumor cell growth. These authors suggested that PPARγ agonist may prove useful in treating brain tumors, and may extend as well to “benign” lesions, such as meningioma, wherein pioglitazone demonstrated activity (Gehring et al., 2011; Shen et al., 2016).
Thus, a Type II cannabis preparation, with equal THC and CBD concentration, combining THC, CBD, THCA and even CBDA along with cytotoxic terpenoids such as limonene may prove extremely useful in cancer treatment (Lewis et al., 2018).
As early as 1888, Gowers noted benefits of “Indian hemp” on a parkinsonian syndrome (Gowers, 1888; Russo, 2007). Because of the density of cannabinoid receptors in basal ganglia, PD has been an area of active research, but with mixed results therapeutically. An oral THC:CBD extract showed no significant benefits on dyskinesia or other signs in 17 patients (Carroll et al., 2004), but CBD was helpful in five PD patients with psychosis (Zuardi et al., 2009) and 21 patients with more general symptoms (Chagas et al., 2014b) and more specifically on rapid eye movement sleep disorder in four patients (Chagas et al., 2014a). An observational study showed 22/28 patients tolerated smoked cannabis (presumably THC-predominant) and showed acute benefits on tremor, rigidity and bradykinesia (Lotan et al., 2014). Five of nine patients using cannabis reported great improvement, particularly on mood and sleep (Finseth et al., 2015).
A carefully crafted survey of 339 Czech patients using oral cannabis leaves reported significant alleviations of multiple symptoms (Venderová et al., 2004), particularly those using the treatment for three or more months, with improvement in general function (p < 0.001), resting tremor (p < 0.01), bradykinesia (p < 0.01), and rigidity (p < 0.01) with few side effects.
Whereas PD is commonly attributed to cell loss in the substantia nigra, with chronicity, widespread pathology is the norm. In common with Alzheimer disease (AD), tau proteins that regulate microtubule assembly, cytoskeletal integrity and axonal transport in neurons develop neurofibrillary tangles (Lei et al., 2010). Interestingly, nabiximols reduced such tangles in parkin-null human tau-expressing mice with improvement in dopamine metabolism, glial function and oxidative stress, as well as reducing anxiety and self-injury (Casarejos et al., 2013).
Recent reviews (Aso and Ferrer, 2014; Ahmed et al., 2015) have nicely summarized the pathophysiology of AD: a neurodegenerative disease with senile plaques formed of fibrillar β-amyloid (Aβ) from cleavage of the Aβ precursor protein (APP) by β- and γ-secretases and by presence of neurofibrillary tangles composed of hyper-phosphorylated and nitrated tau protein. The latter precedes Aβ deposition in sporadic cases. Once the process begins, deterioration is inexorable. Additional pathology includes functional mitochondrial defects, increased reactive oxygen species (ROS) and reactive nitrogen species (RNS), and failure of enzymes involved in energy production that, in turn, produces nerve cell exhaustion. Eventually, synapses and dendritic branching fail, with consequent progressive neuronal wastage. Dementia and cognitive decline develop, and no treatment arrests the process. Intervention must begin at an early preclinical stage to have any hope of success. Endocannabinoid function modulates the primary pathological processes of AD during the silent phase of neurodegeneration: protein misfolding, neuroinflammation, excitotoxicity, mitochondrial dysfunction and oxidative stress. CB2 levels increase in AD especially in microglia around senile plaques, and its stimulation stimulates Aβ removal by macrophages.
The epidemiology of AD is fascinating (Mayeux and Stern, 2012). North America and Western Europe have highest rates (6.4% and 5.4% at age 60), then Latin America (4.9%), and China (4%; ascertainment bias vs. mirroring economic development and Western diet?). Prevalence is lower for Africans in homelands, as opposed to higher rates in the Western European and American diaspora. Head trauma increases Aβ deposition and neuronal tau expression, and diabetes, obesity, trans-fats and head trauma all increase AD risk. Mediterranean diet (increased monounsaturated olive oil, and omega-3 from fish), education and physical activity reduce it.
No current pharmacotherapy is approved for agitation in AD. Commonly used anti-psychotics, antidepressants, anxiolytics and hypnotics are often associated with increased mortality in demented patients (Kales et al., 2007), with an FDA “Black Box Warning.” Four acetylcholinesterase inhibitors are approved in the USA to improve memory: galantamine, donepezil, tacrine and rivastigmine. None show strong evidence of efficacy and are of limited benefit on a temporary basis. Various NMDA receptor antagonists in development have proven largely ineffective on disease progression or have proven toxic. In contrast, treatment with cannabinoids appears both more promising and benign. As demonstrated in 1998 (Hampson et al., 1998), and the subject of USA patent US09674028, CBD is a neuroprotective antioxidant, more potent than ascorbate or tocopherol, that works on the same NMDA target without attendant toxicity. Subsequently (Iuvone et al., 2004), CBD inhibited Aβ plaque formation, prevented ROS production and peroxidation of lipids in PC12 cells exposed to Aβ, limited neuronal apoptosis from caspase 3 reduction, and counteracted increases in intracellular Ca++ from Aβ. In an in vivo model (Esposito et al., 2006), CBD was anti-inflammatory via reduction in inducible nitric oxide synthase (iNOS) and IL-1β expression and release. It also inhibited tau protein hyper-phosphorylation in Aβ-stimulated PC12 neurons. Subsequently, it was shown that CBD’s MOA seemed to be selectively mediated via PPARγ (Esposito et al., 2011): dose dependently antagonizing pro-inflammatory NO, tumor necrosis factor-alpha (TNF-α), and IL-1β. That effect was blocked by GW9662 (PPARγ antagonist), reducing reactive gliosis via selective PPARγ-related NFκB inhibition. Both AEA and CBD promoted neurogenesis after Aβ exposure.
In addition to its neuroprotective antioxidant effects (Iuvone et al., 2004), THC competitively inhibited acetylcholinesterase, increasing levels, and prevented Aβ aggregation via binding to the enzyme in a critical region affecting amyloid production (Eubanks et al., 2006).
On the clinical side, various trials of THC in AD have produced positive results. In 1997 (Volicer et al., 1997), in 15 institutionalized dementia patients refusing nutrition, an RCT 6-week crossover trial of THC (Marinol®) 2.5 mg twice daily led to increased body-mass index (BMI), with decreased Cohen-Mansfield Agitation Inventory (CMAI) scores, improved negative affect scores, and a notable carry-over effect when THC was administered first. In 2006 (Walther et al., 2006), an open-label 2-week study of five AD and one vascular dementia patient taking THC 2.5 mg at 19:00 h showed benefit noted on nocturnal motor activity, agitation, appetite, and irritability with no AEs. A 2015 study (van den Elsen et al., 2015) failed, however: an RCT in 50 demented patients with neuropsychiatric symptoms received 1.5 mg THC vs. placebo thrice daily for 3 weeks with no benefit noted to THC. A total lack of AEs indicated to the even the authors that the administered dosage was inadequate and that higher doses might be required.
Initial trials of herbal cannabis for AD have begun sporadically, with a more focused effort in a California nursing home (Hergenrather, 2017). Patients were treated with a variety of preparations: THC-predominant (2.5–30 mg/dose), CBD predominant, and THCA, mainly in tinctures and confections. Marked benefit was reported on neuroleptic drug sparing, decreased agitation, increased appetite, aggression, sleep quality, objective mood, nursing care demands, self-mutilation and pain control.
Based on its pharmacology (Russo and Marcu, 2017), cannabis components may provide myriad benefits on target symptoms in this complex disorder:
• Agitation: THC, CBD, linalool
• Anxiety: CBD, THC (low dose), linalool
• Psychosis: CBD
• Insomnia/Restlessness: THC, linalool
• Anorexia: THC
• Aggression: THC, CBD, linalool
• Depression: THC, limonene, CBD
• Pain: THC, CBD
• Memory: alpha-pinene (Russo, 2011; Russo and Marcu, 2017) + THC
• Neuroprotection: CBD, THC
• Reduced Aβ plaque formation: THC, CBD, THCA
Thus, an extract of a Type II chemovar of cannabis (THC/CBD) with a sufficient pinene fraction would seem to be an excellent candidate for clinical trials (Lewis et al., 2018).
The neuroprotective antioxidant effects of the cannabinoids (Hampson et al., 1998) are particularly relevant in their ability to counteract “glutamate excitoxicity,” which leads to neuronal demise after traumatic brain injury (TBI). Anecdotally, cannabis, particularly chemovars combining THC and CBD, have been extremely helpful in treatment of chronic traumatic encephalopathy (CTE) symptoms: headache, nausea, insomnia, dizziness, agitation, substance abuse, and psychotic symptoms. CTE, previously known as dementia pugilistica, or “punch-drunk syndrome” has garnered a great deal of attention due to its apparent frequency among long-term players of American football but including victims of repetitive head injury from causes as diverse as other contact sports, warfare and even “heading” in soccer. A recent study (Mez et al., 2017) showed 87% of autopsied American football players demonstrated CTE with tau aggregates in neurons and astrocytes, neurofibrillary tangles in superficial cortical layers and hippocampus, α-synuclein and Aβ deposition. Microglia were present early in the course, whose premonitory symptoms include dementia, personality change, rage, and attention problems. Ninety-six percent demonstrated a degenerative course. Heretofore, this has been considered a post-mortem pathological diagnosis, but two current studies support the ability for pre-mortem identification. CCL11 protein is a chemokine associated with cognitive decline and enhances microglial production of ROS and excitotoxic cell death. CSF examination in CTE patients were elevated compared to controls and AD patients (p = 0.028), and correlated to years of football played (p = 0.04; Cherry et al., 2017), indicating CCL11 may be a premortem biomarker for the syndrome. Additionally, PET imaging binding levels in a CTE patient before death correlated with postmortem tau deposition (p = 0.02). The greatest tau concentration was observed in parasagittal and paraventricular cortical and brainstem areas (Omalu et al., 2018), allowing pre-mortem diagnosis and distinction from AD. Neuroprotective benefits of phytocannabinoids, particularly CBD, further outlined below, provide support for trials of these agents in post-traumatic syndrome and CTE prevention.
Human gut harbors 100 trillion micro-organisms at a concentration of 1012 bacteria/ml, and exceeding the human genome 100-fold (Musso et al., 2010). This is termed the microbiome. Obese humans have lower Bacteroidetes and higher Firmicutes counts. Recent review (Clarke et al., 2012; Russo, 2016b) supports the efficacy of probiotics (supplemental beneficial gut lactic acid bacteria) in treating irritable bowel syndrome without AEs. Microbiota regulate 5-HT1A, BDNF and NMDA expression (Sampson et al., 2016), and experimental transplantation of the microbiome of Parkinson patients to mice was demonstrated to increase their motor deficits, supporting the finding of a pro-inflammatory dysbiosis (microbiome imbalance) in that disorder (Keshavarzian et al., 2015).
Another recent review elucidates additional findings of pertinence to the current discussion (He and Shi, 2017). The combination of prebiotics (dietary fiber that serves as bacterial feedstock, reviewed by Russo, 2016a), and deficient in modern Western diets (Calame et al., 2008; Slavin, 2013) and probiotics may be termed, “synbiotics.” Translocation of bacterial fragments produces “metabolic endotoxemia” from bacterial lipopolysaccharides (LPS). Probiotics may help control PPARγ, “the master regulator of adipogenesis” and TNF-α in inflammation. Additional research supports that prebiotic galacto-oligosaccharides (as from beans) decrease TNF-α, and interleukin production (He and Shi, 2017). GPR41 and GPR43 are orphan receptors for short-chain fatty acids (SCFA) that can increase release of 5-HT and other factors. Additionally, prebiotics change microbiota to reduce adipogenesis and stabilize the gut barrier. Furthermore, CB2 levels correlate to Lactobacillus concentrations and negatively with potentially pathogenic Clostridium species.
Other experiments relate the microbiome to the ECS. A direct effect of Lactobacillus acidophilus NCFM strain via oral administration to induce CNR2 (gene encoding the CB2 receptor) mRNA expression above that of resting human HT-29 epithelial cells (p < 0.01) was demonstrated. An enhancement of morphine antinociceptive effect in rats (p < 0.001) was also demonstrated which was inhibited by administration of the CB2 antagonist, AM-630 (p < 0.001; Rousseaux et al., 2007). Additionally, THC altered the microbiome balance in obese DIO mice affecting the Firmicutes: Bacteroidetes ratio (p = 0.021). Furthermore, THC prevented weight gain despite a high-fat diet (Cluny et al., 2015). This explains, perhaps, how the stereotype of the “skinny hippie” is more accurate than that of the lazy, obese “stoner.”
Additional dietary factors include the function of bitter taste receptors (Tepper et al., 2014), present not only on the tongue, but in the gut, and hypothalamus (Herrera Moro Chao et al., 2016), wherein interaction with ECS appetite mechanisms seem to be operative.
Diet is also a key factor in acne vulgaris, whose pathophysiology and epidemiology are surprisingly relevant to this discussion. Acne was not observed in Inuit populations living a traditional lifestyle over 30 years, but became common with adoption of a Western diet and lifestyle (Cordain et al., 2002). Similarly, no acne was observed in Papua New Guinea or Paraguay among traditional indigenous peoples. Neither population demonstrated markers of insulin resistance, nor leptin elevations. The author then suggests that in many respects, the epidemiology of acne parallels that of AD. The relationship becomes more salient in light of recent findings (Emery et al., 2017) demonstrating that neuroinflammation is a stimulus to AD development and is triggered by infectious insults. Additionally, AD brains demonstrated 5–10× greater bacterial loads, especially with Actinobacteria, particularly Propionibacterium acnes, a gram-positive an aerobic resident of skin, mouth and gut and pathological agent of acne. P. acnes has been cultured from AD brains, can grow there, and stimulate alpha synuclein fibrillar formation in PD, amyloid fibrillization in AD, and biofilm formation, which is opposed by cannabinoids, and cannabis terpenoids limonene, alpha-pinene (Soni et al., 2015; Subramenium et al., 2015; Russo and Marcu, 2017).
An additional parallel pertains to the TRPV4 receptor (Zhang et al., 2013). TRPV4 is expressed in cerebral endothelial cells where it mediates Ca++ and influx acetylcholine-induced dilation. Cerebral hypoperfusion with impaired vessel dilation is a pathogenetic factor in AD. That function is impaired in a mouse model of AD and is sensitive to oxidative stress from Aβ, which is alleviated by antioxidants. The authors suggested TRPV4 as a target for AD treatment.
Cannabidiol, in addition to its anti-inflammatory and bacteriostatic effects, is a TRPV4 agonist that works as a sebostatic agent in acne (Oláh et al., 2014), while cannabis terpenoids limonene, linalool potently inhibited P. acnes and consequent TNF-α production (Kim et al., 2008). Alpha-pinene was also a potent inhibitor of the bacterium (Raman et al., 1995; reviewed by Russo, 2011).
The importance of these relationships becomes apparent as efforts are made to integrate disparate threads (Bowe and Logan, 2011). Mental health impairment scores in acne patients surprisingly exceed those with epilepsy and diabetes. Oral probiotics regulate inflammatory cytokines in skin. Intestinal microbiota, skin inflammation and psychiatric symptoms are thus intertwined in a “gut-brain-skin axis.” The author posits that acne-induced processes could also affect PD, AD and CTE pathophysiology (Figure 2).
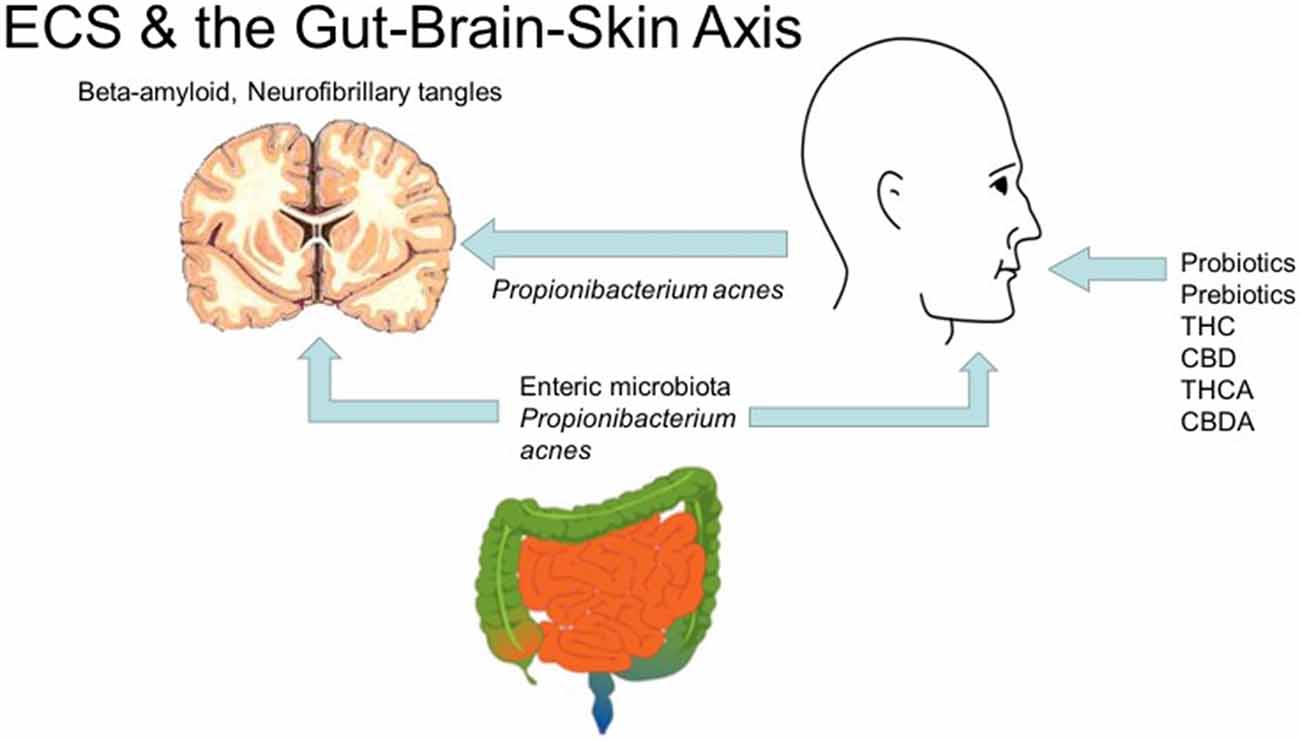
Figure 2. Cannabis, the endocannabinoid system and the gut-brain-skin axis (diagrams of brain, gut by Mikael Hagstrom, face by Mouagip, all public domain).
It is the opinion of many that neurology is facing therapeutic brick walls. The current single target receptor model of pharmacotherapy has not proven universally salutary in the face of complex neurodegenerative diseases. Rather, reconsideration must be given to an older proven model of botanical synergy that may enable polytherapy in single preparations (Russo, 2011; Brodie et al., 2015; Russo and Marcu, 2017; Lewis et al., 2018). Such approaches, combined with nutritional and lifestyle management may make neurology a more preventative and therapeutic specialty, rather than merely diagnostic, and provide better treatment for epilepsy, tumors, AD, PD and TBI/CTE. Suggested strategies include:
• Aerobic activity (Raichlen et al., 2012; Schenkman et al., 2018)
• Education as a lifestyle
• Anti-inflammatory, prebiotic and probiotic diet emphasizing saturated and monounsaturated and omega-3 EFAs, bioflavonoids (berries), fermented foods, protein and minimizing carbohydrates (Fallon and Enig, 1999; Perlmutter and Loberg, 2015)
• Supplementation with cannabis extracts providing THC, CBD, THCA, CBDA, caryophyllene and other select terpenoids (Figure 1; Russo and Marcu, 2017; Lewis et al., 2018).
Legitimate concerns surround the psychoactive sequelae of THC, but as amply demonstrated by the nabiximols RCTs and supported by mitigating effects of cannabidiol and cannabis terpenoids (Russo, 2011; Russo and Marcu, 2017; Lewis et al., 2018; MacCallum and Russo, 2018), cannabis-based drugs portend to provide future safe and effective treatments for heretofore recalcitrant neurological conditions.
The author confirms being the sole contributor to this work and has approved it for publication.
This study was performed without outside funding.
ER is Director of Research and Development for the International Cannabis and Cannabinoids Institute (ICCI), Prague, Czechia.
The assistance of the Inter-Library Loan staff of Mansfield Library of the University of Montana in providing research materials is greatly appreciated.
Ahmed, A., van der Marck, M. A., van den Elsen, G., and Olde Rikkert, M. (2015). Cannabinoids in late-onset Alzheimer’s disease. Clin. Pharmacol. Ther. 97, 597–606. doi: 10.1002/cpt.117
Aso, E., and Ferrer, I. (2014). Cannabinoids for treatment of Alzheimer’s disease: moving toward the clinic. Front. Pharmacol. 5:37. doi: 10.3389/fphar.2014.00037
Babson, K. A., Sottile, J., and Morabito, D. (2017). Cannabis, cannabinoids, and sleep: a review of the literature. Curr. Psychiatry Rep. 19:23. doi: 10.1007/s11920-017-0775-9
Bagdy, G., Kecskemeti, V., Riba, P., and Jakus, R. (2007). Serotonin and epilepsy. J. Neurochem. 100, 857–873. doi: 10.1111/j.1471-4159.2006.04277.x
Baker, D., Pryce, G., Giovannoni, G., and Thompson, A. J. (2003). The therapeutic potential of cannabis. Lancet Neurol. 2, 291–298. doi: 10.1016/s1474-4422(03)00381-8
Bergamaschi, M. M., Queiroz, R. H., Chagas, M. H., de Oliveira, D. C., De Martinis, B. S., Kapczinski, F., et al. (2011). Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology 36, 1219–1226. doi: 10.1038/npp.2011.6
Bisogno, T., Hanus, L., De Petrocellis, L., Tchilibon, S., Ponde, D. E., Brandi, I., et al. (2001). Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 134, 845–852. doi: 10.1038/sj.bjp.0704327
Bolognini, D., Rock, E. M., Cluny, N. L., Cascio, M. G., Limebeer, C. L., Duncan, M., et al. (2013). Cannabidiolic acid prevents vomiting in Suncus murinus and nausea-induced behaviour in rats by enhancing 5-HT1A receptor activation. Br. J. Pharmacol. 168, 1456–1470. doi: 10.1111/bph.12043
Bowe, W. P., and Logan, A. C. (2011). Acne vulgaris, probiotics and the gut-brain-skin axis—back to the future? Gut Pathog. 3:1. doi: 10.1186/1757-4749-3-1
Brady, C. M., DasGupta, R., Dalton, C., Wiseman, O. J., Berkley, K. J., and Fowler, C. J. (2004). An open-label pilot study of cannabis-based extracts for bladder dysfunction in advanced multiple sclerosis. Mult. Scler. 10, 425–433. doi: 10.1191/1352458504ms1063oa
Brodie, J. S., Di Marzo, V., and Guy, G. W. (2015). Polypharmacology shakes hands with complex aetiopathology. Trends Pharmacol. Sci. 36, 802–821. doi: 10.1016/j.tips.2015.08.010
Calame, W., Weseler, A. R., Viebke, C., Flynn, C., and Siemensma, A. D. (2008). Gum arabic establishes prebiotic functionality in healthy human volunteers in a dose-dependent manner. Br. J. Nutr. 100, 1269–1275. doi: 10.1017/S0007114508981447
Carroll, C. B., Bain, P. G., Teare, L., Liu, X., Joint, C., Wroath, C., et al. (2004). Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology 63, 1245–1250. doi: 10.1212/01.wnl.0000140288.48796.8e
Casarejos, M. J., Perucho, J., Gomez, A., Muñoz, M. P., Fernandez-Estevez, M., Sagredo, O., et al. (2013). Natural cannabinoids improve dopamine neurotransmission and tau and amyloid pathology in a mouse model of tauopathy. J. Alzheimers Dis. 35, 525–539. doi: 10.3233/jad-130050
Cascio, M. G., and Pertwee, R. G. (2014). “Known pharmacological actions of nine nonpsychotropic phytocannabinoids,” in Handbook of Cannabis, ed. R. G. Pertwee (Oxford: Oxford Unversity Press), 137–156.
Chagas, M. H., Eckeli, A. L., Zuardi, A. W., Pena-Pereira, M. A., Sobreira-Neto, M. A., Sobreira, E. T., et al. (2014a). Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson’s disease patients: a case series. J. Clin. Pharm. Ther. 39, 564–566. doi: 10.1111/jcpt.12179
Chagas, M. H., Zuardi, A. W., Tumas, V., Pena-Pereira, M. A., Sobreira, E. T., Bergamaschi, M. M., et al. (2014b). Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J. Psychopharmacol. 28, 1088–1098. doi: 10.1177/0269881114550355
Cherry, J. D., Stein, T. D., Tripodis, Y., Alvarez, V. E., Huber, B. R., Au, R., et al. (2017). CCL11 is increased in the CNS in chronic traumatic encephalopathy but not in Alzheimer’s disease. PLoS One 12:e0185541. doi: 10.1371/journal.pone.0185541
Clarke, G., Cryan, J. F., Dinan, T. G., and Quigley, E. M. (2012). Review article: probiotics for the treatment of irritable bowel syndrome—focus on lactic acid bacteria. Aliment. Pharmacol. Ther. 35, 403–413. doi: 10.1111/j.1365-2036.2011.04965.x
Clendinning, J. (1843). Observation on the medicinal properties of Cannabis sativa of India. Med. Chir. Trans. 26, 188–210. doi: 10.1177/095952874302600116
Cluny, N. L., Keenan, C. M., Reimer, R. A., Le Foll, B., and Sharkey, K. A. (2015). Prevention of diet-induced obesity effects on body weight and gut microbiota in mice treated chronically with Δ9-tetrahydrocannabinol. PLoS One 10:e0144270. doi: 10.1371/journal.pone.0144270
Colle, R., de Larminat, D., Rotenberg, S., Hozer, F., Hardy, P., Verstuyft, C., et al. (2017a). Pioglitazone could induce remission in major depression: a meta-analysis. Neuropsychiatr. Dis. Treat. 13, 9–16. doi: 10.2147/ndt.s121149
Colle, R., de Larminat, D., Rotenberg, S., Hozer, F., Hardy, P., Verstuyft, C., et al. (2017b). PPAR-γ agonists for the treatment of major depression: a review. Pharmacopsychiatry 50, 49–55. doi: 10.1055/s-0042-120120
Cordain, L., Lindeberg, S., Hurtado, M., Hill, K., Eaton, S. B., and Brand-Miller, J. (2002). Acne vulgaris: a disease of Western civilization. Arch. Dermatol. 138, 1584–1590. doi: 10.1001/archderm.138.12.1584
Devinsky, O., Cross, J. H., Laux, L., Marsh, E., Miller, I., Nabbout, R., et al. (2017). Trial of cannabidiol for drug-resistant seizures in the dravet syndrome. N. Engl. J. Med. 376, 2011–2020. doi: 10.1056/NEJMoa1611618
Devinsky, O., Marsh, E., Friedman, D., Thiele, E., Laux, L., Sullivan, J., et al. (2016). Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 15, 270–278. doi: 10.1016/s1474-4422(15)00379-8
Devinsky, O., Patel, A. D., Thiele, E. A., Wong, M. H., Appleton, R., Harden, C. L., et al. (2018). Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology 90, e1204–e1211. doi: 10.1212/wnl.0000000000005254
Donovan, M. (1845). On the physical and medicinal qualities of Indian hemp (Cannabis indica); with observations on the best mode of administration and cases illustrative of its powers. Dubl. J. Med. Sci. 26, 368–402. doi: 10.1007/bf02971741
Dumitru, C. A., Sandalcioglu, I. E., and Karsak, M. (2018). Cannabinoids in glioblastoma therapy: new applications for old drugs. Front. Mol. Neurosci. 11:159. doi: 10.3389/fnmol.2018.00159
Elrod, H. A., and Sun, S. Y. (2008). PPARγ and apoptosis in cancer. PPAR Res. 2008:704165. doi: 10.1155/2008/704165
Emery, D. C., Shoemark, D. K., Batstone, T. E., Waterfall, C. M., Coghill, J. A., Cerajewska, T. L., et al. (2017). 16S rRNA next generation sequencing analysis shows bacteria in Alzheimer’s post-mortem brain. Front. Aging Neurosci. 9:195. doi: 10.3389/fnagi.2017.00195
Esposito, G., De Filippis, D., Maiuri, M. C., De Stefano, D., Carnuccio, R., and Iuvone, T. (2006). Cannabidiol inhibits inducible nitric oxide synthase protein expression and nitric oxide production in β-amyloid stimulated PC12 neurons through p38 MAP kinase and NF-κB involvement. Neurosci. Lett. 399, 91–95. doi: 10.1016/j.neulet.2006.01.047
Esposito, G., Scuderi, C., Valenza, M., Togna, G. I., Latina, V., De Filippis, D., et al. (2011). Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS One 6:e28668. doi: 10.1371/journal.pone.0028668
Eubanks, L. M., Rogers, C. J., Beuscher, A. E. IV., Koob, G. F., Olson, A. J., Dickerson, T. J., et al. (2006). A molecular link between the active component of marijuana and Alzheimer’s disease pathology. Mol. Pharm. 3, 773–777. doi: 10.1021/mp060066m
Fallon, S., and Enig, M. C. (1999). Nourishing Traditions: The Cookbook That Challenges Politically Correct Nutrtition and the Diet Dictocrats. Washington, DC: New Trends Publishing.
Fife, T. D., Moawad, H., Moschonas, C., Shepard, K., and Hammond, N. (2015). Clinical perspectives on medical marijuana (cannabis) for neurologic disorders. Neurol. Clin. Pract. 5, 344–351. doi: 10.1212/cpj.0000000000000162
Finseth, T. A., Hedeman, J. L., Brown, R. P. II., Johnson, K. I., Binder, M. S., and Kluger, B. M. (2015). Self-reported efficacy of cannabis and other complementary medicine modalities by Parkinson’s disease patients in colorado. Evid. Based Complement. Alternat. Med. 2015:874849. doi: 10.1155/2015/874849
Foroughi, M., Hendson, G., Sargent, M. A., and Steinbok, P. (2011). Spontaneous regression of septum pellucidum/forniceal pilocytic astrocytomas—possible role of Cannabis inhalation. Childs. Nerv. Syst. 27, 671–679. doi: 10.1007/s00381-011-1410-4
Gehring, S., Tapia-Pérez, J. H., Kirches, E., Firsching, R., Keilhoff, G., Schneider, T., et al. (2011). Cytotoxic effects of statins and thiazolidinediones on meningioma cells. J. Neurooncol. 102, 383–393. doi: 10.1007/s11060-010-0351-1
Gottschling, S. (2011). Cannbinoide bei kindern. gute erfahrungen bei schmerzen, spastik und in der onkologie. Ange. Schmerzth. Palliat. 4, 55–57. doi: 10.1007/bf03359593
Gowers, W. R. (1888). A Manual of Diseases of the Nervous System. Philadelphia, PA: P. Blakiston Son & Co.
Guzmán, M., Duarte, M. J., Blázquez, C., Ravina, J., Rosa, M. C., Galve-Roperh, I., et al. (2006). A pilot clinical study of Δ9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br. J. Cancer 95, 197–203. doi: 10.1038/sj.bjc.6603236
Hampson, A. J., Grimaldi, M., Axelrod, J., and Wink, D. (1998). Cannabidiol and (−)Δ9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. U S A 95, 8268–8273. doi: 10.1073/pnas.95.14.8268
He, M., and Shi, B. (2017). Gut microbiota as a potential target of metabolic syndrome: the role of probiotics and prebiotics. Cell Biosci. 7:54. doi: 10.1186/s13578-017-0183-1
Herrera Moro Chao, D., Argmann, C., Van Eijk, M., Boot, R. G., Ottenhoff, R., Van Roomen, C., et al. (2016). Impact of obesity on taste receptor expression in extra-oral tissues: emphasis on hypothalamus and brainstem. Sci. Rep. 6:29094. doi: 10.1038/srep29094
Hill, M. N., Bierer, L. M., Makotkine, I., Golier, J. A., Galea, S., McEwen, B. S., et al. (2013). Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology 38, 2952–2961. doi: 10.1016/j.psyneuen.2013.08.004
Iuvone, T., Esposito, G., Esposito, R., Santamaria, R., Di Rosa, M., and Izzo, A. A. (2004). Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on β-amyloid-induced toxicity in PC12 cells. J. Neurochem. 89, 134–141. doi: 10.1111/j.1471-4159.2003.02327.x
Johnson, J. R., Burnell-Nugent, M., Lossignol, D., Ganae-Motan, E. D., Potts, R., and Fallon, M. T. (2010). Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J. Pain Symptom Manage. 39, 167–179. doi: 10.1016/j.jpainsymman.2009.06.008
Jones, N. A., Hill, A. J., Smith, I., Bevan, S. A., Williams, C. M., Whalley, B. J., et al. (2010). Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J. Pharmacol. Exp. Ther. 332, 569–577. doi: 10.1124/jpet.109.159145
Kales, H. C., Valenstein, M., Kim, H. M., McCarthy, J. F., Ganoczy, D., Cunningham, F., et al. (2007). Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am. J. Psychiatry 164, 1568–1576; quiz 1623. doi: 10.1176/appi.ajp.2007.06101710
Karler, R., and Turkanis, S. A. (1979). “Cannabis and epilepsy,” in Marihuana Biological Effects: Analysis, Metabolism, Cellular Responses, Reproduction and Brain, eds G. G. Nahas and W. D. M. Paton (Oxford, UK: Pergamon Press), 619–641.
Kavia, R., De Ridder, D., Constantinescu, C., Stott, C., and Fowler, C. (2010). Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Mult. Scler. 16, 1349–1359. doi: 10.1177/1352458510378020
Keshavarzian, A., Green, S. J., Engen, P. A., Voigt, R. M., Naqib, A., Forsyth, C. B., et al. (2015). Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 30, 1351–1360. doi: 10.1002/mds.26307
Kim, S. S., Baik, J. S., Oh, T. H., Yoon, W. J., Lee, N. H., and Hyun, C. G. (2008). Biological activities of Korean Citrus obovoides and Citrus natsudaidai essential oils against acne-inducing bacteria. Biosci. Biotechnol. Biochem. 72, 2507–2513. doi: 10.1271/bbb.70388
Latorre, J. G., and Schmidt, E. B. (2015). Cannabis, cannabinoids, and cerebral metabolism: potential applications in stroke and disorders of the central nervous system. Curr. Cardiol. Rep. 17:627. doi: 10.1007/s11886-015-0627-3
Lei, P., Ayton, S., Finkelstein, D. I., Adlard, P. A., Masters, C. L., and Bush, A. I. (2010). Tau protein: relevance to Parkinson’s disease. Int. J. Biochem. Cell Biol. 42, 1775–1778. doi: 10.1016/j.biocel.2010.07.016
Leweke, F. M., Piomelli, D., Pahlisch, F., Muhl, D., Gerth, C. W., Hoyer, C., et al. (2012). Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2:e94. doi: 10.1038/tp.2012.15
Lewis, M. A., Russo, E. B., and Smith, K. M. (2018). Pharmacological foundations of cannabis chemovars. Planta Med. 84, 225–233. doi: 10.1055/s-0043-122240
Ligresti, A., Moriello, A. S., Starowicz, K., Matias, I., Pisanti, S., De Petrocellis, L., et al. (2006). Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharmacol. Exp. Ther. 318, 1375–1387. doi: 10.1124/jpet.106.105247
Lorenz, R. (2004). On the application of cannabis in paediatrics and epileptology. Neuro Endocrinol. Lett. 25, 40–44.
Lotan, I., Treves, T. A., Roditi, Y., and Djaldetti, R. (2014). Cannabis (medical marijuana) treatment for motor and non-motor symptoms of Parkinson disease: an open-label observational study. Clin. Neuropharmacol. 37, 41–44. doi: 10.1097/wnf.0000000000000016
Maa, E., and Figi, P. (2014). The case for medical marijuana in epilepsy. Epilepsia 55, 783–786. doi: 10.1111/epi.12610
Maas, A. I., Murray, G., Henney, H. III., Kassem, N., Legrand, V., Mangelus, M., et al. (2006). Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol. 5, 38–45. doi: 10.1016/s1474-4422(05)70253-2
MacCallum, C. A., and Russo, E. B. (2018). Practical considerations in medical cannabis administration and dosing. Eur. J. Intern. Med. 49, 12–19. doi: 10.1016/j.ejim.2018.01.004
Maccarrone, M., Maldonado, R., Casas, M., Henze, T., and Centonze, D. (2017). Cannabinoids therapeutic use: what is our current understanding following the introduction of THC, THC:CBD oromucosal spray and others? Expert Rev. Clin. Pharmacol. 10, 443–455. doi: 10.1080/17512433.2017.1292849
Massi, P., Vaccani, A., Ceruti, S., Colombo, A., Abbracchio, M. P., and Parolaro, D. (2004). Antitumor effects of cannabidiol, a nonpsychoactive cannabinoid, on human glioma cell lines. J. Pharmacol. Exp. Ther. 308, 838–845. doi: 10.1124/jpet.103.061002
Mayeux, R., and Stern, Y. (2012). Epidemiology of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2:a006239. doi: 10.1101/cshperspect.a006239
McGuire, P., Robson, P., Cubala, W. J., Vasile, D., Morrison, P. D., Barron, R., et al. (2018). Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am. J. Psychiatry 175, 225–231. doi: 10.1176/appi.ajp.2017.17030325
McPartland, J. M., MacDonald, C., Young, M., Grant, P. S., Furkert, D. P., and Glass, M. (2017). Affinity and efficacy studies of tetrahydrocannabinolic acid A at cannabinoid receptor types one and two. Cannabis Cannabinoid Res. 2, 87–95. doi: 10.1089/can.2016.0032
Merritt, J. C., Crawford, W. J., Alexander, P. C., Anduze, A. L., and Gelbart, S. S. (1980). Effect of marihuana on intraocular and blood pressure in glaucoma. Ophthalmology 87, 222–228. doi: 10.1016/s0161-6420(80)35258-5
Mez, J., Daneshvar, D. H., Kiernan, P. T., Abdolmohammadi, B., Alvarez, V. E., Huber, B. R., et al. (2017). Clinicopathological evaluation of chronic traumatic encephalopathy in players of american football. JAMA 318, 360–370. doi: 10.1001/jama.2017.8334
Mitchell, S. W. (1874). Headaches, from heat-stroke, from fevers, after meningitis, from over use of brain, from eye strain. Med. Surg. Rep. 31, 67–70.
Müller-Vahl, K. R., Schneider, U., and Emrich, H. M. (2002). Combined treatment of Tourette syndrome with Δ-9-THC and dopamine receptor agonists. J. Cannab. Thera. 2, 145–154. doi: 10.1300/j175v02n03_10
Müller-Vahl, K. R., Schneider, U., Prevedel, H., Theloe, K., Kolbe, H., Daldrup, T., et al. (2003). Δ9-tetrahydrocannabinol (THC) is effective in the treatment of tics in tourette syndrome: a 6-week randomized trial. J. Clin. Psychiatry 64, 459–465. doi: 10.4088/jcp.v64n0417
Munson, A. E., Harris, L. S., Friedman, M. A., Dewey, W. L., and Carchman, R. A. (1975). Antineoplastic activity of cannabinoids. J. Natl. Cancer Inst. 55, 597–602.
Musso, G., Gambino, R., and Cassader, M. (2010). Gut microbiota as a regulator of energy homeostasis and ectopic fat deposition: mechanisms and implications for metabolic disorders. Curr. Opin. Lipidol. 21, 76–83. doi: 10.1097/mol.0b013e3283347ebb
Nadal, X., Del Río, C., Casano, S., Palomares, B., Ferreiro-Vera, C., Navarrete, C., et al. (2017). Tetrahydrocannabinolic acid is a potent PPARγ agonist with neuroprotective activity. Br. J. Pharmacol. 174, 4263–4276. doi: 10.1111/bph.14019
National Academies of Sciences Engineering and Medicine (U.S.). Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. (2017). The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press.
Novotna, A., Mares, J., Ratcliffe, S., Novakova, I., Vachova, M., Zapletalova, O., et al. (2011). A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex®), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur. J. Neurol. 18, 1122–1131. doi: 10.1111/j.1468-1331.2010.03328.x
Nozaki, C., Markert, A., and Zimmer, A. (2015). Inhibition of FAAH reduces nitroglycerin-induced migraine-like pain and trigeminal neuronal hyperactivity in mice. Eur. Neuropsychopharmacol. 25, 1388–1396. doi: 10.1016/j.euroneuro.2015.04.001
Nurmikko, T. J., Serpell, M. G., Hoggart, B., Toomey, P. J., Morlion, B. J., and Haines, D. (2007). Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain 133, 210–220. doi: 10.1016/j.pain.2007.08.028
Oby, E., and Janigro, D. (2006). The blood-brain barrier and epilepsy. Epilepsia 47, 1761–1774. doi: 10.1111/j.1528-1167.2006.00817.x
Oláh, A., Tóth, B. I., Borbirò, I., Sugawara, K., Szöllõsi, A. G., Czifra, G., et al. (2014). Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J. Clin. Invest. 124, 3713–3724. doi: 10.1172/jci64628
Omalu, B., Small, G. W., Bailes, J., Ercoli, L. M., Merrill, D. A., Wong, K. P., et al. (2018). Postmortem autopsy-confirmation of antemortem [F-18]FDDNP-PET scans in a football player with chronic traumatic encephalopathy. Neurosurgery 82, 237–246. doi: 10.1093/neuros/nyx536
O’Shaughnessy, W. B. (1838–1840). On the preparations of the Indian hemp, or gunjah (Cannabis indica); Their effects on the animal system in health and their utility in the treatment of tetanus and other convulsive diseases. Trans. Med. Phys. Soc. Bengal 71–102, 421–461.
Osler, W., and McCrae, T. (1915). The Principles and Practice of Medicine. New York, NY London: Appleton and Company.
Pacher, P., Steffens, S., Haskó, G., Schindler, T. H., and Kunos, G. (2018). Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly. Nat. Rev. Cardiol. 15, 151–166. doi: 10.1038/nrcardio.2017.130
Pamplona, F. A., da Silva, L. R., and Coan, A. C. (2018). Potential clinical benefits of CBD-rich Cannabis extracts over purified CBD in treatment-resistant epilepsy: Observational data meta-analysis. Front. Neurol. 9:759. doi: 10.3389/fneur.2018.00759
Perlmutter, F., and Loberg, K. (2015). Brain Maker. The Power of Gut Microbes to Heal and Protect Your Brain—For Life. New York, NY: Little, Brown and Co.
Pertwee, R. G., and Cascio, M. G. (2014). “Known pharmacological actions of Δ-9-tetrahydrocannabinol and of four other chemical constituents that activate cannabinoid receptors,” in Handbook of Cannabis, ed. R. G. Pertwee (Oxford: Oxford University Press), 115–136.
Pisani, A., Fezza, F., Galati, S., Battista, N., Napolitano, S., Finazzi-Agro, A., et al. (2005). High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson’s disease patients. Ann. Neurol. 57, 777–779. doi: 10.1002/ana.20462
Porter, B. E., and Jacobson, C. (2013). Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav. 29, 574–577. doi: 10.1016/j.yebeh.2013.08.037
Press, C. A., Knupp, K. G., and Chapman, K. E. (2015). Parental reporting of response to oral cannabis extracts for treatment of refractory epilepsy. Epilepsy Behav. 45, 49–52. doi: 10.1016/j.yebeh.2015.02.043
Raichlen, D. A., Foster, A. D., Gerdeman, G. L., Seillier, A., and Giuffrida, A. (2012). Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the ‘runner’s high’. J. Exp. Biol. 215, 1331–1336. doi: 10.1242/jeb.063677
Raman, A., Weir, U., and Bloomfield, S. F. (1995). Antimicrobial effects of tea-tree oil and its major components on Staphylococcus aureus, Staph. epidermidis and Propionibacterium acnes. Lett. Appl. Microbiol. 21, 242–245. doi: 10.1111/j.1472-765x.1995.tb01051.x
Rekand, T. (2014). THC:CBD spray and MS spasticity symptoms: data from latest studies. Eur. Neurol. 71, 4–9. doi: 10.1159/000357742
Reymond, E. A. E. E. (1976). From the Contents of the Libraries of the Suchos Temple in the Fayyum, Part I, A Medical Book From Crocodilopolis. Papyrus Vindobonensis D. 6257. Vienna, Austria: Österreichische Nationalbibliothek.
Rhyne, D. N., Anderson, S. L., Gedde, M., and Borgelt, L. M. (2016). Effects of medical marijuana on migraine headache frequency in an adult population. Pharmacotherapy 36, 505–510. doi: 10.1002/phar.1673
Rog, D. J., Nurmiko, T., Friede, T., and Young, C. (2005). Randomized controlled trial of cannabis based medicine in central neuropathic pain due to multiple sclerosis. Neurology 65, 812–819. doi: 10.1212/01.wnl.0000176753.45410.8b
Rousseaux, C., Thuru, X., Gelot, A., Barnich, N., Neut, C., Dubuquoy, L., et al. (2007). Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat. Med. 13, 35–37. doi: 10.1038/nm1521
Rudroff, T., and Honce, J. M. (2017). Cannabis and multiple sclerosis-the way forward. Front. Neurol. 8:299. doi: 10.3389/fneur.2017.00299
Russo, E. B. (2001). Hemp for headache: an in-depth historical and scientific review of cannabis in migraine treatment. J. Canna. Thera. 1, 21–92. doi: 10.1300/j175v01n02_04
Russo, E. B. (2004). Clinical endocannabinoid deficiency (CECD): can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? Neuro Endocrinol. Lett. 25, 31–39.
Russo, E. B. (2007). History of cannabis and its preparations in saga, science and sobriquet. Chem. Biodivers 4, 2624–2648. doi: 10.1002/cbdv.200790144
Russo, E. B. (2011). Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 163, 1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x
Russo, E. B. (2015). Synthetic and natural cannabinoids: the cardiovascular risk. Br. J. Cardiol. 22, 7–9.
Russo, E. B. (2016a). Beyond cannabis: plants and the endocannabinoid system. Trends Pharmacol. Sci. 37, 594–605. doi: 10.1016/j.tips.2016.04.005
Russo, E. B. (2016b). Clinical endocannabinoid deficiency reconsidered: current research supports the theory in migraine, fibromyalgia, irritable bowel, and other treatment-resistant syndromes. Cannabis Cannabinoid Res. 1, 154–165. doi: 10.1089/can.2016.0009
Russo, E. B. (2017a). Cannabis and epilepsy: an ancient treatment returns to the fore. Epilepsy Behav. 70, 292–297. doi: 10.1016/j.yebeh.2016.09.040
Russo, E. B. (2017b). “History of cannabis as medicine: nineteenth century irish physicians and correlations of their observations to modern research,” in Cannabis Sativa L.: Botany and Biotechnology, eds S. Chanda, H. Lata and M. Elsohly (Switzerland: Springer International Publishing), 63–78.
Russo, E. B., Burnett, A., Hall, B., and Parker, K. K. (2005). Agonistic properties of cannabidiol at 5-HT-1a receptors. Neurochem. Res. 30, 1037–1043. doi: 10.1007/s11064-005-6978-1
Russo, E. B., Guy, G. W., and Robson, P. J. (2007). Cannabis, pain, and sleep: lessons from therapeutic clinical trials of Sativex, a cannabis-based medicine. Chem. Biodivers 4, 1729–1743. doi: 10.1002/cbdv.200790150
Russo, E. B., and Hohmann, A. G. (2013). “Role of cannabinoids in pain management,” in Comprehensive Treatment of Chronic Pain by Medical, Interventional and Behavioral Approaches, eds T. Deer and V. Gordin (New York, NY: Springer), 181–197.
Russo, E. B., and Marcu, J. (2017). Cannabis pharmacology: the usual suspects and a few promising leads. Adv. Pharmacol. 80, 67–134. doi: 10.1016/bs.apha.2017.03.004
Russo, E. B., Mead, A. P., and Sulak, D. (2015). Current status and future of cannabis research. Clin. Res. 58–63. doi: 10.14524/CR-15-0004
Sampson, T. R., Debelius, J. W., Thron, T., Janssen, S., Shastri, G. G., Ilhan, Z. E., et al. (2016). Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469.e12–1480.e12. doi: 10.1016/j.cell.2016.11.018
Sánchez, C., Galve-Roperh, I., Canova, C., Brachet, P., and Guzmán, M. (1998). Δ9-tetrahydrocannabinol induces apoptosis in C6 glioma cells. FEBS Lett. 436, 6–10. doi: 10.1016/s0014-5793(98)01085-0
Sarchielli, P., Pini, L. A., Coppola, F., Rossi, C., Baldi, A., Mancini, M. L., et al. (2007). Endocannabinoids in chronic migraine: CSF findings suggest a system failure. Neuropsychopharmacology 32, 1384–1390. doi: 10.1038/sj.npp.1301320
Schenkman, M., Moore, C. G., Kohrt, W. M., Hall, D. A., Delitto, A., Comella, C. L., et al. (2018). Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: a phase 2 randomized clinical trial. JAMA Neurol. 75, 219–226. doi: 10.1001/jamaneurol.2017.3517
Serpell, M., Ratcliffe, S., Hovorka, J., Schofield, M., Taylor, L., Lauder, H., et al. (2014). A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur. J. Pain 18, 999–1012. doi: 10.1002/j.1532-2149.2013.00445.x
Shen, Y., Lu, Y., Yu, F., Zhu, C., Wang, H., and Wang, J. (2016). Peroxisome proliferator-activated receptor-γ and its ligands in the treatment of tumors in the nervous system. Curr. Stem Cell Res. Ther. 11, 208–215. doi: 10.2174/1574888X10666150728122034
Slavin, J. (2013). Fiber and prebiotics: mechanisms and health benefits. Nutrients 5, 1417–1435. doi: 10.3390/nu5041417
Soni, D., Smoum, R., Breuer, A., Mechoulam, R., and Steinberg, D. (2015). Effect of the synthetic cannabinoid HU-210 on quorum sensing and on the production of quorum sensing-mediated virulence factors by Vibrio harveyi. BMC Microbiol. 15:159. doi: 10.1186/s12866-015-0499-0
Subramenium, G. A., Vijayakumar, K., and Pandian, S. K. (2015). Limonene inhibits streptococcal biofilm formation by targeting surface-associated virulence factors. J. Med. Microbiol. 64, 879–890. doi: 10.1099/jmm.0.000105
Sulak, D., Saneto, R., and Goldstein, B. (2017). The current status of artisanal cannabis for the treatment of epilepsy in the United States. Epilepsy Behav. 70, 328–333. doi: 10.1016/j.yebeh.2016.12.032
Sun, H., Huang, Y., Yu, X., Li, Y., Yang, J., Li, R., et al. (2008). Peroxisome proliferator-activated receptor γ agonist, rosiglitazone, suppresses CD40 expression and attenuates inflammatory responses after lithium pilocarpine-induced status epilepticus in rats. Int. J. Dev. Neurosci. 26, 505–515. doi: 10.1016/j.ijdevneu.2008.01.009
Tepper, B. J., Banni, S., Melis, M., Crnjar, R., and Tomassini Barbarossa, I. (2014). Genetic sensitivity to the bitter taste of 6-n-propylthiouracil (PROP) and its association with physiological mechanisms controlling body mass index (BMI). Nutrients 6, 3363–3381. doi: 10.3390/nu6093363
Thiele, E. A., Marsh, E. D., French, J. A., Mazurkiewicz-Beldzinska, M., Benbadis, S. R., Joshi, C., et al. (2018). Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 391, 1085–1096. doi: 10.1016/S0140-6736(18)30136-3
Torres, S., Lorente, M., Rodríguez-Fornés, F., Hernández-Tiedra, S., Salazar, M., García-Taboada, E., et al. (2011). A combined preclinical therapy of cannabinoids and temozolomide against glioma. Mol. Cancer Ther. 10, 90–103. doi: 10.1158/1535-7163.mct-10-0688
van den Elsen, G. A., Ahmed, A. I., Verkes, R. J., Kramers, C., Feuth, T., Rosenberg, P. B., et al. (2015). Tetrahydrocannabinol for neuropsychiatric symptoms in dementia: a randomized controlled trial. Neurology 84, 2338–2346. doi: 10.1212/WNL.0000000000001675
Venderová, K., Ruzicka, E., Vorísek, V., and Visnovský, P. (2004). Survey on cannabis use in Parkinson’s disease: subjective improvement of motor symptoms. Mov. Disord. 19, 1102–1106. doi: 10.1002/mds.20111
Volicer, L., Stelly, M., Morris, J., McLaughlin, J., and Volicer, B. J. (1997). Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 12, 913–919. doi: 10.1002/(sici)1099-1166(199709)12:9<913::aid-gps663>3.3.co;2-4
Wallace, M. J., Blair, R. E., Falenski, K. W., Martin, B. R., and DeLorenzo, R. J. (2003). The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J. Pharmacol. Exp. Ther. 307, 129–137. doi: 10.1124/jpet.103.051920
Walther, S., Mahlberg, R., Eichmann, U., and Kunz, D. (2006). Δ-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology 185, 524–528. doi: 10.1007/s00213-006-0343-1
Zhang, L., Papadopoulos, P., and Hamel, E. (2013). Endothelial TRPV4 channels mediate dilation of cerebral arteries: impairment and recovery in cerebrovascular pathologies related to Alzheimer’s disease. Br. J. Pharmacol. 170, 661–670. doi: 10.1111/bph.12315
Keywords: cannabis, pain, brain tumor, epilepsy, Alzheimer disease, Parkinson disease, traumatic brain injury, microbiome
Citation: Russo EB (2018) Cannabis Therapeutics and the Future of Neurology. Front. Integr. Neurosci. 12:51. doi: 10.3389/fnint.2018.00051
Received: 26 July 2018; Accepted: 01 October 2018;
Published: 18 October 2018.
Edited by:
Fabricio A. Pamplona, Entourage Phytolab, BrazilReviewed by:
Kirsten R. Müller-Vahl, Hannover Medical School, GermanyCopyright © 2018 Russo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ethan B. Russo, ZXRoYW4ucnVzc29AaWNjaS5zY2llbmNl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.