- Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences (RAS), Pushchino, Russia
Alzheimer’s disease (AD) and temporal lobe epilepsy (TLE) are the most common forms of neurodegenerative disorders characterized by the loss of cells and progressive irreversible alteration of cognitive functions, such as attention and memory. AD may be an important cause of epilepsy in the elderly. Early diagnosis of diseases is very important for their successful treatment. Many efforts have been done for defining new biomarkers of these diseases. Significant advances have been made in the searching of some AD and TLE reliable biomarkers, including cerebrospinal fluid and plasma measurements and glucose positron emission tomography. However, there is a great need for the biomarkers that would reflect changes of brain activity within few milliseconds to obtain information about cognitive disturbances. Successful early detection of AD and TLE requires specific biomarkers capable of distinguishing individuals with the progressing disease from ones with other pathologies that affect cognition. In this article, we review recent evidence suggesting that magnetoencephalographic recordings and coherent analysis coupled with behavioral evaluation can be a promising approach to an early detection of AD and TLE.
Highlights
- Data reviewed include the results of clinical and experimental studies.
- Theta and gamma rhythms are disturbed in epilepsy and AD.
- Common and different behavioral and oscillatory features of pathologies are compared.
- Coherent analysis can be useful for an early diagnostics of diseases.
Introduction
It is known that theta and gamma oscillations are closely related to cognitive processes. The theta rhythm (4–12 Hz) is prominent oscillations recorded in the hippocampus and surrounding limbic structures during exploration and REM sleep (Vanderwolf, 1969; Bland, 1986; Buzsáki, 2002). Theta oscillations have also been registered in the neocortex where they are particularly pronounced in the frontal midline (Klimesch et al., 1997; Kahana et al., 1999; Jensen and Tesche, 2002; Canolty et al., 2006; Guderian et al., 2009), and in the subcortical areas (Paré et al., 2002; Magill et al., 2006; Nerad and McNaughton, 2006; DeCoteau et al., 2007; Kabanova et al., 2011). The theta rhythm is important in the formation and retrieval of episodic and spatial memory (Hasselmo, 2005). The gamma rhythm (25–100 Hz) usually co-occurs with theta rhythm in the hippocampus (Bragin et al., 1995; Strogatz, 2003; Montgomery et al., 2008). In the neocortex, gamma oscillations were identified in the frontal and parietal areas (Bouyer et al., 1981; Benchenane et al., 2010). The gamma rhythm is considered to play a role in attention (Fries, 2009; Jutras et al., 2009; Buzsáki and Wang, 2010) and in the maintenance of relevant information in memory (Sauseng et al., 2009; Sridharan and Knudsen, 2015). Evidence accumulates indicating that the coupling between the phase of slow oscillations (in particular, theta) and the amplitude of fast oscillations (gamma) may be involved in information processing (Tort et al., 2009; Canolty and Knight, 2010; Lisman and Jensen, 2013).
A crucial component of the neural processing underlying cognition is communication between selective brain structures (Livanov et al., 1977; Engel et al., 2001; Vinogradova, 2001; Igarashi et al., 2014). Mounting evidence points to brain rhythms as a fundamental mechanism of dynamical coupling between brain areas; this is proved by task- and state-dependent changes in the coherence of local field potentials (LFPs; Fell et al., 2001; Varela et al., 2001; Buzsáki, 2004; Womelsdorf et al., 2006; Sauseng et al., 2008; Takehara-Nishiuchi and McNaughton, 2008; Astasheva et al., 2016; Bott et al., 2016; Vinck et al., 2016) and cross-correlated unit activity (Tabuchi et al., 2000; Engel et al., 2001; Igarashi et al., 2014). Synchronized activities of brain areas exert strong effects on their ability to interact with each other (Womelsdorf et al., 2006), and provide a mechanism for the formation of cell ensembles and their coordination by linking the activity of multiple neurons (Harris et al., 2003; Colgin and Moser, 2010; Buzsáki, 2010; Buzsáki and Watson, 2012; Igarashi et al., 2014). Besides, the oscillations can be considered as rhythmic changes in neuronal excitability (Volgushev et al., 1998; Fries, 2005).
The hypothesis “communication through coherence” by Fries (2005) is now widely accepted (Jensen et al., 2007; Mitchell et al., 2008; Colgin et al., 2009; Rutishauser et al., 2010; Wang, 2010; Colgin, 2011, 2015; Igarashi et al., 2014; Astasheva et al., 2016). This hypothesis assumes that anatomic communications can become effective or inefficient owing to presence or lack of rhythmic synchronization (Fries, 2005; Bastos et al., 2015).
It is known that communication between selective brain structures as well as oscillatory activity in them can violate in neurological and psychiatric disorders (Bakker et al., 2012; Buzsáki and Watson, 2012; Froriep et al., 2012; Kirihara et al., 2012; Inostroza et al., 2013; Laurent et al., 2015). However, there is much to be learned and discussed. Despite decades of research, the disturbances in the rhythm coherence underlying pathologies, such as temporal lobe epilepsy (TLE) and Alzheimer’s disease (AD) remain poorly understood.
This review article summarizes the data on the alterations of the theta and gamma coherence based on examples from TLE and AD. In addition, we analyzed the information on some similarities and differences in these disorders, mainly in the disturbances of specific types of memory, theta and gamma rhythms and their coherence. These analyses may shed light on plausible links between neural damage and rhythmic disturbances in these diseases and help to design new approaches to early diagnostics of pathologies.
Coherence of the Theta and Gamma Rhythms
The brain cortex generates great number of oscillations at different frequencies. Low-frequency brain rhythms are dynamically involved across distributed brain regions by sensory signals or cognitive tasks; at the same time, high-frequency brain activity reflects local cortical processing (Canolty and Knight, 2010). External or internal events can lead to the synchronization of rhythms and thus form a more complex functional phenomenon known as phase coherence or phase coupling (Fell et al., 2008; Cavanagh et al., 2009; Canolty and Knight, 2010). The standard phase coherence reveals the relative constancy of the phase difference between two oscillations of the same frequency, i.e., within-frequency synchrony (Rodriguez et al., 1999; Hurtado et al., 2004). It was shown that phase coupling reflects various cognitive processes in humans (Canolty et al., 2006; Axmacher et al., 2010), monkeys (Canolty and Knight, 2010), rats (Montgomery and Buzsáki, 2007; Tort et al., 2008, 2009; Nácher et al., 2013) and mice (Wulff et al., 2009). The within-frequency phase coupling between oscillations in different brain areas (see Figure 1) was studied extensively because of its proposed role in the regulation of inter-area communications (Womelsdorf et al., 2006; Gregoriou et al., 2009; Siegel et al., 2009). Similarly to within-frequency synchrony, the cross-frequency phase–phase coupling, may serve as a mechanism for regulation of communications between different spatiotemporal scales (Palva et al., 2005, 2010; Holz et al., 2010). Besides, the correlation between the amplitude envelopes of two brain waves at different frequencies, called cross-frequency amplitude–amplitude coupling, is also an oscillatory characteristic (Shirvalkar et al., 2010; Tanninen et al., 2017). The amplitude–amplitude cross-frequency coupling was observed by some authors (Friston, 1997; Palva et al., 2010; Shirvalkar et al., 2010), but despite correlations with behavior, its functional role remains unclear.
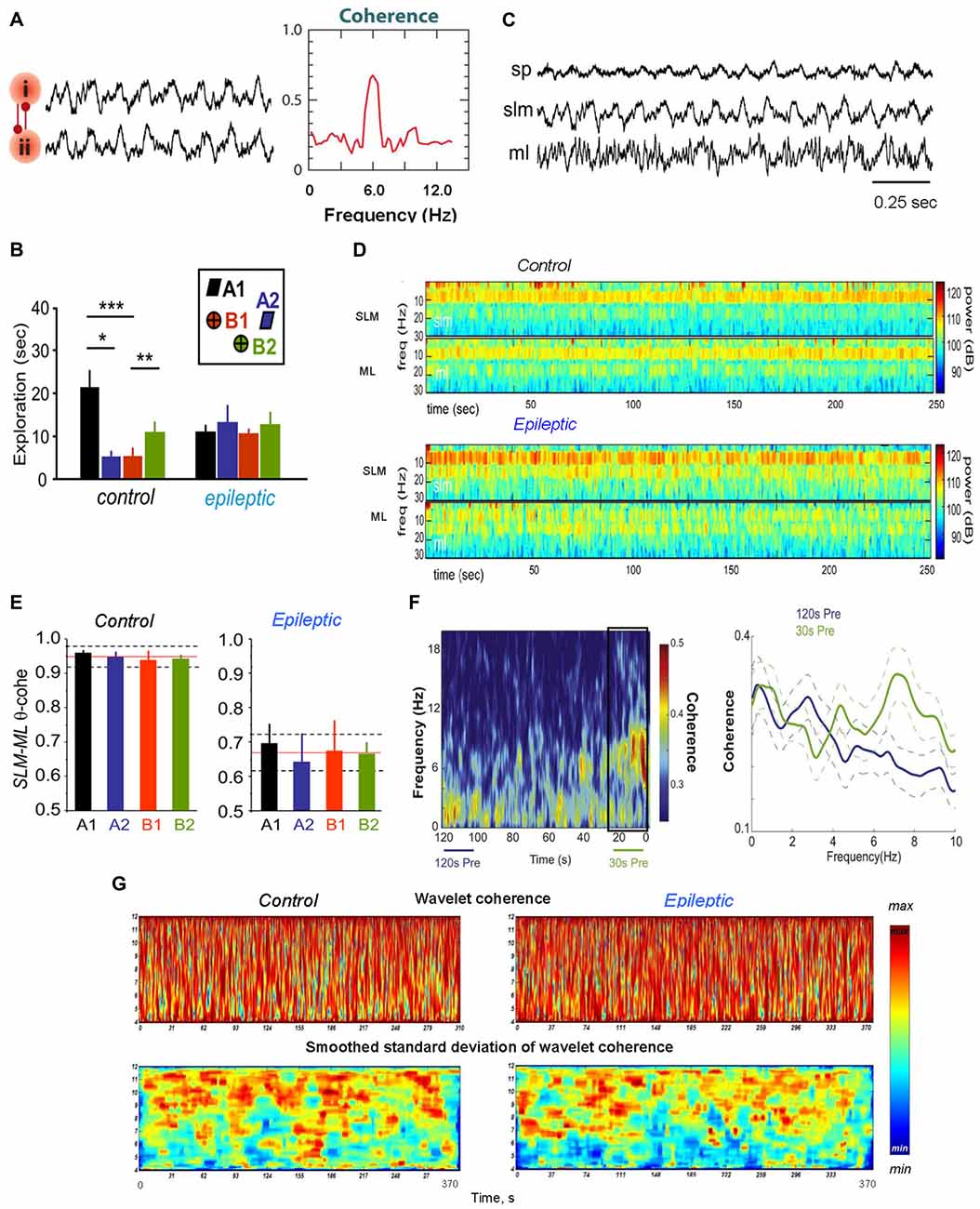
Figure 1. Theta coherence between brain areas changes during epileptogenesis. (A) Phase–phase coupling of theta oscillations between two brain areas (i) and (ii). To the left: synthetic data used for theta rhythm illustration. To the right: coherence spectrum (or phase-specific measures) between two signals can determine the strength of theta phase coupling. (B) Behavioral data for rats during the performance the episodic-like memory task. Distribution of exploratory times per object in the test phase for the control and epileptic groups; *p < 0.05, **p < 0.01, ***p < 0.005. The inset represents the object configuration in the task. (C) Representative hippocampal activity of an epileptic rat recorded in the stratum pyramidale (SP), lacunosum moleculare (SLM) and moleculare (ML) during walking. (D) Specific alterations in hippocampal theta activity in temporal lobe epilepsy (TLE) brain during object exploration in the episodic-like memory task; the time–frequency power spectrum of hippocampal field potentials in the SLM and the ML layers is shown for the 1–30 Hz frequency band. (E) Theta coherence between hippocampal SLM–ML layers during exploration of each individual object in the episodic-like memory task; the mean values of theta coherence per object within the mean (red line) and standard deviation (discontinuous line) for the whole session in the control and epileptic animals are shown. (F) Theta coherence between the hippocampus and medial prefrontal cortex (mPFC) increases pre-ictally. To the left: a mean coherogram (coherence vs. time, 0–20 Hz) of 120 s and 30 s pre-ictal local field potential (LFP) segments from the hippocampus and mPFC (30 s pre-ictal segment is designated by a black rectangle). To the right: mean ± standard error of the mean (SEM; solid ± dashed lines) coherence of 120 s (blue lines) and 30 s (green lines) recordings before seizures. (G) Representative wavelet coherograms and smoothed standard deviation of wavelet coherence of LFPs recorded in the hippocampus and medial septal-diagonal band (MSDB) in healthy (left) and epileptic animals. Adapted with permission from Buzsáki and Watson (2012) (A), Inostroza et al. (2013) (B–E), Broggini et al. (2016) (F) and Kabanova et al. (2011) (G).
The phase coupling between theta and gamma oscillations, namely, the phase–amplitude cross-frequency coupling (phase–amplitude CFC) or “nested” oscillations (Buzsáki et al., 1983, 2003; Soltesz and Deschênes, 1993; Bragin et al., 1995; Lisman and Idiart, 1995; Mormann et al., 2005; Canolty et al., 2006; Sirota et al., 2008; Tort et al., 2008, 2009; Tort et al., 2010; Sauseng et al., 2009; Wulff et al., 2009; Scheffer-Teixeira et al., 2012; Schomburg et al., 2014) and the phase–phase CFC (or “n:m phase-locking”) in which several gamma cycles are entrained within one cycle of theta (Tass et al., 1998; Belluscio et al., 2012; Zheng and Zhang, 2013; Xu et al., 2015; Zheng et al., 2016) are the most studied phenomena of phase coherence. The phase–amplitude CFC describes the dependence between the phase of the low-frequency rhythm and the amplitude of the high-frequency oscillations (Canolty and Knight, 2010; see Figure 2). Thus, it reflects the interrelations between local microscale (Colgin et al., 2009; Quilichini et al., 2010) and system-level macroscale neuronal networks (Lisman and Idiart, 1995; Canolty and Knight, 2010; Szczepanski et al., 2014). This is probably the most prominent “law” underlying the hierarchy of the system of brain oscillators, when the phase of slower oscillations modulates the amplitude of a faster rhythm (or rhythms; Bragin et al., 1995; Buzsáki, 2006; Buzsáki and Mizuseki, 2014). Thus, phase–amplitude CFC can be used as an index of cortical excitability and network interactions (Knight, 2007; Haider and McCormick, 2009; Voytek et al., 2013). In non-epileptogenic hippocampi of neurosurgical patients and in a healthy brain of rodents, the degree of theta–gamma phase–amplitude coupling increases with learning (Tort et al., 2008, 2009; Lega et al., 2016). In the hippocampus, gamma and theta oscillations normally show a marked phase–amplitude CFC considered to be central to hippocampal functions (Tort et al., 2008, 2009; Newman et al., 2013). Thus, during spatial learning, the strength of hippocampal theta–gamma coupling usually directly correlated with the increase in correct performance of a cognitive task (Tort et al., 2009; Figure 2C).
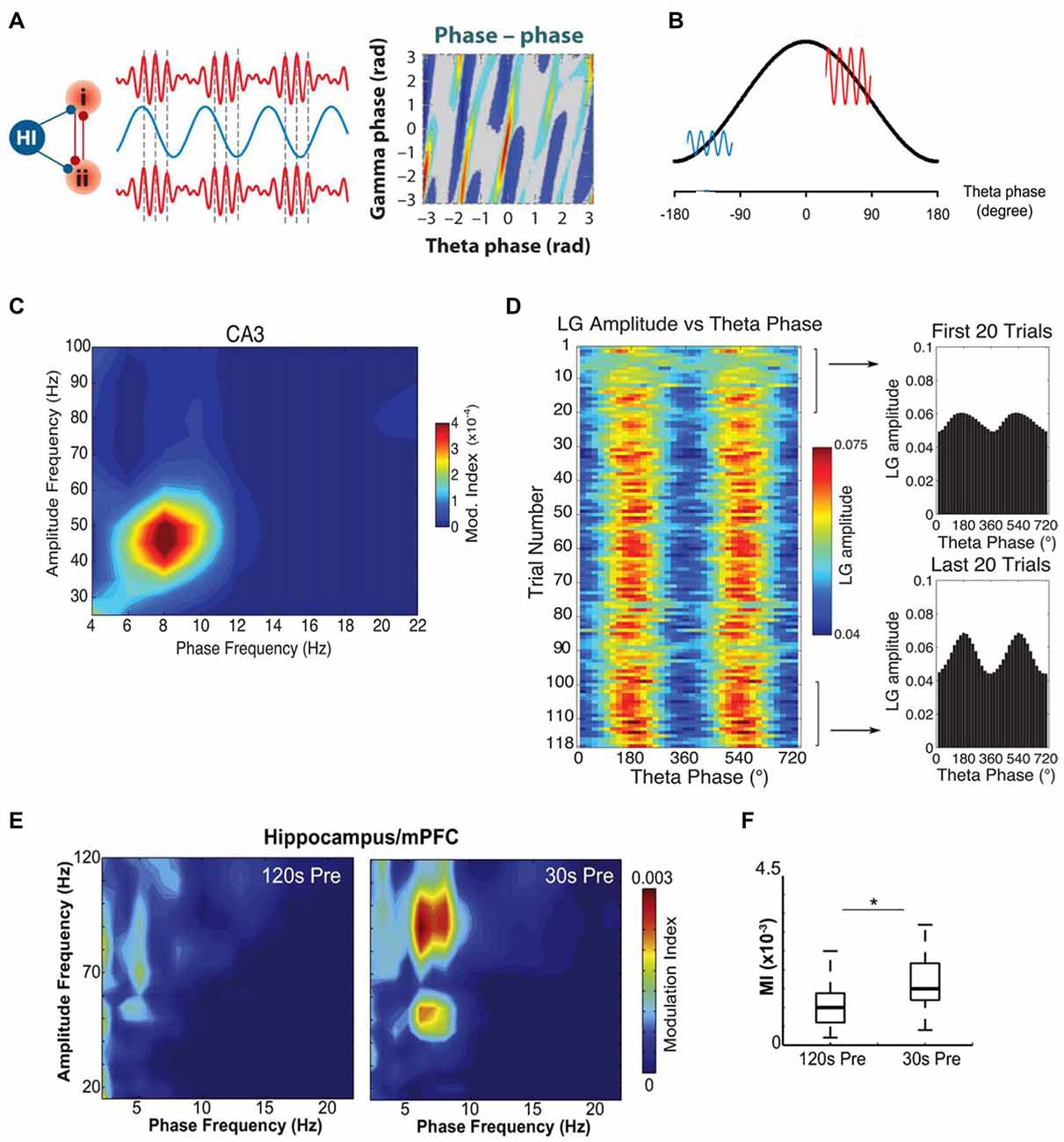
Figure 2. Theta–gamma cross-frequency coupling (CFC) and its alteration in a rat model of TLE. (A) Schematic illustration of cross-frequency phase-phase coupling. Phases of theta and gamma oscillations are correlated, as shown (to the right) by the phase-phase plot of the two frequencies; (i) and (ii)—different brain areas, Hi—hippocampus. (B) A heuristic model of cross-frequency phase–amplitude coupling. Gamma oscillations are large (red line) in the excitatory phase of theta wave (black line) and small (blue line) in the inhibitory phase of theta wave. (C) The theta phase modulates the low-frequency gamma (LG) amplitude. A phase–amplitude comodulogram computed for LFP of the hippocampal CA3 field recorded at SP during execution of spatial task is shown. (D) Theta modulation of the LG amplitude in the CA3 region during context exploration increases with learning. Color scale representation of the mean LG amplitude as a function of the theta phase for each trial in the session (left). The mean LG amplitude per theta phase averaged over the first and last 20 trials is also shown (right). (E) Example of comodulation maps of hippocampal theta phase modulating mPFC gamma oscillation amplitude 120 s and 30 s before seizure onset. (F) Box plot showing mean hippocampal theta/mPFC gamma modulation index (MI), 120 s and 30 s before seizure onset; *p < 0.001. Adapted with permission from Buzsáki and Watson (2012) (A), Kirihara et al. (2012) (B), Tort et al. (2009) (C,D) and Broggini et al. (2016) (E,F).
At the same time, phase–phase CFC provides, as believed, a physiological mechanism for the linkage of the activity generated at significantly different rates. Since gamma oscillations are faster than theta ones, numerous cycles of gamma arise during a single cycle of theta (Figures 2A,B). The phenomenon of phase–phase theta–gamma coupling means that gamma waves always begin at the same phase of theta waves. Phase–phase CFC was hypothesized to take part in cognitive processes, such as attention and memory (Lisman and Idiart, 1995; Schack and Weiss, 2005; Sauseng et al., 2009; Holz et al., 2010; Fell and Axmacher, 2011). An influential model in which theta and gamma oscillations would interact to produce a neural code (“7 ± 2 short-term memories”) has been proposed a decade ago (Lisman and Idiart, 1995); later it was improved, but the essence of this theta–gamma coding model was remained (Jensen and Lisman, 2005; Lisman, 2005; Lisman and Buzsáki, 2008; Lisman and Jensen, 2013). Latest findings show that this mechanism indeed is used by the hippocampus (Belluscio et al., 2012; Zheng and Zhang, 2013; Xu et al., 2015; Zheng et al., 2016; but see Scheffer-Teixeira and Tort, 2016). It is assumed that the temporal coordination of neuronal spikes by phase–phase theta–gamma coupling may improve transferring information as well as spike timing-dependent plasticity (Markram et al., 1997; Fell and Axmacher, 2011). Desynchronization of these rhythms could be altered in certain neurodegenerative pathologies.
Coherence Bias in TLE and AD
Alterations in Rhythm Coherence in the Epileptic Brain
Epilepsy, a disorder associated with increased network excitability and neuron loss, is usually accompanied by rewiring in the brain (for review see Morimoto et al., 2004). TLE is the most common and pharmacologically resistant type of adult focal epilepsy. In patients with TLE, a selective and marked degradation of episodic (autobiographic) memory was shown, in which specific memory items are placed within temporal context during encoding and retrieval (Dupont et al., 2000). Animals with TLE also exhibited a highly specific impairment of the episodic-like memory while preserving other forms of hippocampal-dependent memories (Burgess et al., 2002; Helmstaedter, 2002; Tulving, 2002; Chauvière et al., 2009).
Hippocampal Network
The analysis of hippocampal LFPs in neurosurgical patients during the execution of episodic memory tasks revealed a sharp increase of gamma oscillations before successful item encoding in non-epileptogenic hippocampi. At the same time, the epileptogenic hippocampi exhibited a significant decrease in the gamma band power, which predicts successful item encoding (Lega et al., 2012, 2015). Thus, typical changes in the gamma band power during this process are reversed for human epileptogenic hippocampus (Lega et al., 2015). Besides, it was shown in the TLE model (Inostroza et al., 2013) that kainate-treated rats with deficit of episodic-like memory exhibited reduction of hippocampal theta power and coherence along the CA1–dentate axis. In TLE animals, decreased theta coherence in the LFP signals was concentrated between the hippocampal stratum lacunosum-moleculare (SLM) and ML of dentate gyrus (DG; Figures 1D,E). Inostroza et al. (2013) believe that these data point to discoordination of hippocampal inputs from layers III and II of the entorhinal cortex (EC) and from the contralateral hippocampus as a possible cause for dysfunction of episodic-like memory in TLE animals.
Hippocampal–Entorhinal Cortex Network
It is known that a crucial mechanism of episodic memory is the coherence of neuronal activity in the hippocampal–entorhinal circuit; this mechanism is usually impaired in TLE (Helmstaedter, 2002). An alteration of theta coherence between the EC and the DG was revealed in behaving kainate-injected epileptic mice during the interictal phase (Froriep et al., 2012). Indeed, in epileptic mice, the theta activity in the EC was delayed with respect to that of the DG, while the theta activity in healthy animals was synchronized between EC and DG, demonstrating the within-frequency phase coupling. On the basis of a computational neural mass model, the authors suggested that hippocampal cell loss destroyed the coupling of the subnetworks, which induced the EC–DG shift (Froriep et al., 2012).
In experiments with healthy rats, the inputs from the medial and lateral EC (via temporoammonic and perforant inputs) evoked a firing of hippocampal neurons, which reflects an integrated representation of spatial and temporal information (O’Keefe and Nadel, 1978; Komorowski et al., 2009; Mankin et al., 2012; Kraus et al., 2013; Kitamura et al., 2014) as well as new experience (Frank et al., 2000; Wood et al., 2000). This neuronal coding is precisely organized within a time scale, which is controlled by ongoing oscillations, especially by the hippocampal theta and gamma rhythms (Bland and Oddie, 2001; Hasselmo et al., 2002; Huxter et al., 2008; Mizuseki et al., 2009; Easton et al., 2012; Buzsáki and Moser, 2013; Lisman and Jensen, 2013). A careful measurement of the proximodistal coherence of the theta activity in the dorsal hippocampus of normal and epileptic animals showed that healthy rats exhibited a stronger coordination between the temporoammonic and perforant entorhinal inputs near CA3 field (at proximal locations), while epileptic rats showed stronger coordination near subiculum (at distal locations; Laurent et al., 2015). This opposing trend in epileptic rats was associated with the connectivity constraint, which accompanies cell death in the hippocampus. Laurent et al. (2015) also discovered that the “appropriate timing between entorhinal inputs arriving over several theta cycles at the proximal and distal ends of the dorsal hippocampus was impaired in epileptic rats.” It is important that “the computational reconstruction of LFP signals predicted that timing variability has a major impact on repairing theta coherence.” Thus, the proximodistal organization of entorhinal inputs plays an important role in temporal lobe physiology, and this organization alters during TLE (Laurent et al., 2015).
Hippocampal–Medial Prefrontal Cortex Network
As was mentioned above, experiments with healthy animals showed that theta and gamma oscillations are usually present and work in synchrony in the hippocampus and medial prefrontal cortex (mPFC) during the performance of cognitive tasks (Tort et al., 2008; Benchenane et al., 2010). Hippocampal theta oscillations are normally coupled to mPFC theta waves (Benchenane et al., 2010) and modulate hippocampal and mPFC gamma oscillations during cognitive behavior (Jones and Wilson, 2005; Siapas et al., 2005; Tort et al., 2008). In a TLE model generated by perforant path stimulation, abnormal changes in the hippocampal−mPFC circuit were observed during the recording of mPFC and hippocampal LFPs in rats with spontaneous recurrent seizures (Broggini et al., 2016). Broggini et al. (2016) showed that recurrent seizures weaken hippocampal theta rhythm while the hippocampal and mPFC theta coherence increases during a period preceding the onset of seizures (Figure 1F). Simultaneously with the increase in theta synchrony a stronger coupling between hippocampal theta and mPFC gamma oscillations was observed (Figures 2E,F). Using the Granger causality, it was shown that the increase in hippocampus–mPFC synchrony in the preictal phase was provoked by hippocampal networks. The data indicate that the increase in hippocampal—mPFC coherence may predict the seizure onset (Broggini et al., 2016). Besides, the too strong coupling of hippocampal theta and mPFC gamma oscillations may induce abnormal plasticity in mPFC communications (Zheng and Zhang, 2015), which can be a reason of changes observed in mPFC cells (Tang and Loke, 2010).
Hippocampal–Septal Network
The registration of LFPs in the hippocampus and medial septal-diagonal band (MSDB) complex of rats and guinea pigs revealed that normally theta oscillations were relatively synchronous in these brain regions (Nerad and McNaughton, 2006; Astasheva and Kichigina, 2009; Kabanova et al., 2011). Usually theta power in the MSDB was smaller compared to that in the hippocampus, but the frequency of theta oscillations, although it did not coincide in these structures, did not differ significantly. The theta coherence between the hippocampus and MSDB was relatively high: a phase analysis revealed no clear unidirectional shifts (<10 ms) in the hippocampal and MSDB theta phases in healthy animals (Nerad and McNaughton, 2006; Kabanova, 2011; Kabanova et al., 2011). In chronic epileptic animals, a significant decrease of the theta power was revealed in the hippocampus (Arabadzisz et al., 2005; Colom et al., 2006; Dugladze et al., 2007; Astasheva and Kichigina, 2009; Marcelin et al., 2009) and MSDB (Sinelnikova, 2012). In addition, in a pilocarpine rat model of TLE, a dysfunctional and uncoupled septohippocampal network was revealed (García-Hernández et al., 2010). However, in the perforant path kindling model of TLE some increase in synchronization between hippocampus and MSDB within the theta band was observed in waking guinea pigs during epileptogenesis (Figure 1G; Kabanova et al., 2011). Besides, in this model of TLE, a dramatic increase of the theta oscillations simultaneously in the rabbit hippocampus and MSDB before (within 20 s) the seizures was observed (Kitchigina and Butuzova, 2009). This phenomenon reminds the events in the hippocampal–mPFC network over time prior to seizure onset in rats in the same model of TLE (Broggini et al., 2016). Interestingly, in a perforant path kindling model of TLE in guinea pigs, the interactions between the hippocampus and MSDB changed for opposite during epileptogenesis: at the beginning of kindling, the MSDB was ahead in the theta phase, but after formation of the pathological focus, MSDB lagged the hippocampus (Kabanova et al., 2011). In addition, the relationships between rhythmic bursts of septal neurons and the phases of the hippocampal theta waves during spontaneous seizures in rabbits with TLE model could reverse to almost opposite comparative to interictal ones (Kitchigina and Butuzova, 2009); i.e., these relationships were not constants.
It was shown in earlier experiments that the natural theta rhythm evoked, e.g., by sensory stimuli prevents seizure onset under the influence of epileptogenic factors (Miller et al., 1994; Colom et al., 2006; Kitchigina and Butuzova, 2009). At the same time, excessive theta synchrony leads to the generation of epileptiform activity (Kitchigina and Butuzova, 2009). Thus, for the prevention of seizure development, a strong control of the level and pattern of the hippocampus—MSDB theta synchronization is necessary.
Alterations in the Rhythm Coherence in Alzheimer’s Disease and in the AD Models
Disturbances of Theta and Gamma Rhythms in Brain With AD Pathology
AD is a progressive neurodegenerative disease associated with an irreversible deterioration of cognitive functions, especially memory. Although the etiology of AD remains unknown and now there is no reliable treatment, a consensus has emerged early in this century on the amyloid hypothesis (Selkoe, 2000; Palop and Mucke, 2010), which posits that the amyloid β (Aβ) peptide, a major constituent of amyloid plaques, is mostly responsible for the alteration of cognitive functions (Francis et al., 2010; Palop and Mucke, 2010). In the last years, however, this hypothesis was challenged: a potential role of an impairment of metabolism of amyloid precursor protein (APP) and its progress through tau pathology were considered in the etiology of AD (for review, see Kametani and Hasegawa, 2018). Moreover, the recent data of experiments with wild-type and APP/PS1 transgenic mice indicate that amyloid plaques can possess capacity for binding additional Aβ (Gureviciene et al., 2017).
Various forms of memory are disturbed in AD (Didic et al., 2011). It has been assumed that navigation deficits can help to separate individuals at higher risk of developing AD from patients with other neurodegenerative diseases (Lithfous et al., 2013). As it was revealed in some works, AD patients, as opposed to healthy age-matched control subjects, exhibit an increase in the relative power of slow oscillations (in particular, theta rhythm) and a decrease in the relative power of fast oscillations (gamma rhythm; Adler et al., 2003; Herrmann and Demiralp, 2005; van der Hiele et al., 2007; Czigler et al., 2008; Moretti et al., 2010). On the contrary, in other works, an increased gamma rhythm power and the lack of theta increase in AD patients were reported (Caravaglios et al., 2010; Wang et al., 2017). Some authors noted that changes in EEG of resting AD patients might not be specific, and various types of dementia can also exhibit similar network disturbances (Herrmann and Demiralp, 2005). Besides, contrary to the data on AD patients, a decrease of both theta and gamma bands was revealed in Tg5xFAD mice, a transgenic mouse model of AD; in this case, the decrease preceded alterations in learning performances in spatial task (Schneider et al., 2014). In addition, transgenic APP23 mice, another mouse model of AD, demonstrated the compromised spectral contributions of hippocampal theta and gamma oscillations, compared to non-transgenic controls: a markedly lower spectral power of theta oscillations (~10 Hz) and a higher power of gamma oscillations (25–50 Hz; Ittner et al., 2014), changes opposite to those in AD patients. Hence, a decrease or an increase in theta and gamma oscillations power per se may not be specific for this pathology (Herrmann and Demiralp, 2005).
Alterations in Theta–Gamma Coherence Are Indicative for Brain With AD Pathology
Probably, most convincing evidence of rhythm disturbances in a pathological AD brain is alterations in the theta–gamma CFC. Thus, in humans with AD, an enhanced CFC between the gamma and low-frequency bands (in particular, theta) compared to healthy control was revealed (Wang et al., 2017). During performance of working memory tasks, evidence for a relationship between altered theta-gamma coupling and working memory deficits in individuals with AD was obtained (Goodman et al., 2018).
In the AD model (adult APP23 transgenic free-roaming mice), an impairment of cross-frequency gamma amplitude modulation by hippocampal theta rhythm was observed (Ittner et al., 2014; Figure 3). It is important that these changes were observed before the onset of Aβ plaque pathology. Moreover, it was shown on TgCRND8 mice that a significant proportion of 1-month-old animals exhibited marked alterations in the theta–gamma coupling in the output region of the hippocampus, the subiculum. This uncoupling of rhythms arises before any histopathological abnormalities such as the presence of amyloid plaques (Goutagny et al., 2013). In addition, it was shown that 1-month-old TgCRND8 mice expressed extremely low levels of Aβ compared to controls. Goutagny et al. (2013) suggested that in animals (TgCRND8 mice) disturbed theta–gamma CFC in the subiculum may be the earliest detectable AD-related biomarker. This is in contrast with the existing hypothesis, which states that the beginning of hippocampal network alterations and memory deficits in animal models of AD are caused by the overproduction of soluble Aβ (Francis et al., 2010; Palop and Mucke, 2010; Scott et al., 2012).
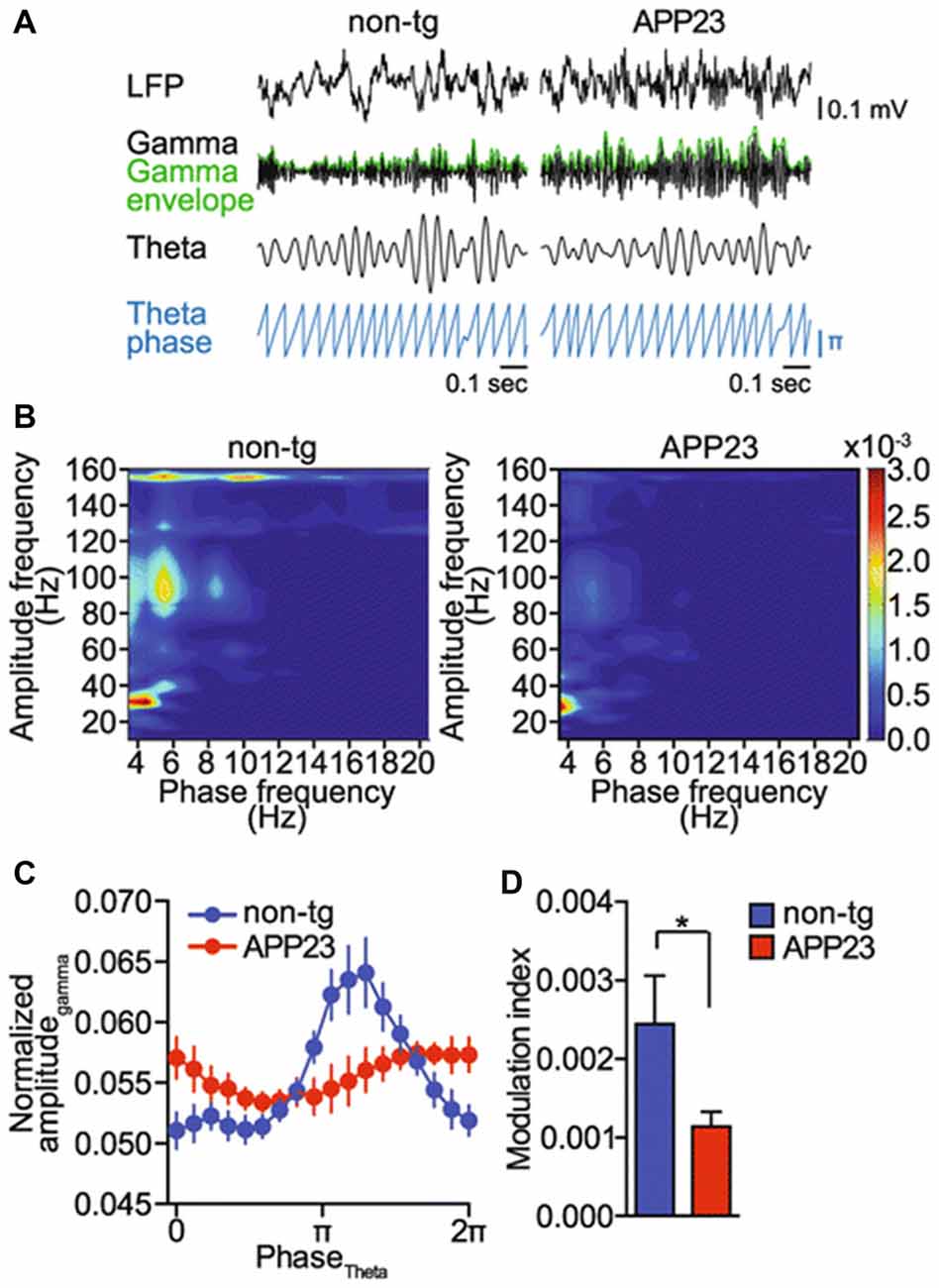
Figure 3. Gamma amplitude modulation by theta phase is impaired in a mouse model of Alzheimer’s disease (AD; amyloid precursor protein 23, APP23 mice). (A) Raw EEG (LFP), band pass filtered signals for theta (4–12 Hz) and gamma (25–100 Hz) oscillations, gamma amplitude envelope (green) and theta phase in APP23 and non-transgenic (non-tg) mice (blue). Representative signals from five animals per genotype are shown. (B) Representative phase–amplitude comodulograms computed for hippocampal LFPs recorded in non-tg and APP23 mice. (C) Phase–amplitude plot computed for hippocampal LFPs recorded in non-tg and APP23 mice (means ± SEM). (D) MI computed for the phase–amplitude distributions shown in (C); *p < 0.05. Adapted from Ittner et al. (2014; Open Access).
Interestingly, though APP is supposed to be critically involved in the pathophysiology of AD, APP-deficient mice exhibit cognitive deficits (Seabrook et al., 1999; Senechal et al., 2008); this confirms that APP plays an important role in the functioning of neurons in the healthy brain. Recently, strongly diminished theta–gamma coupling in LFPs from the dorsal hippocampus and parietal cortex was revealed in APP knockout mice. Besides, cross-regional hippocampal–prefrontal CFC was largely disrupted in these knockout mice (Zhang et al., 2016). This effect may be of importance for the origination of cognitive deficits in APP-deficient animals. Thus, APP is important for the interaction of rhythms of different frequencies. The facts mentioned above possibly indicate, that very thin frontier between functioning of APP in the healthy and pathological brains exists.
Quite recently, it has been tested whether a preclinical AD pathologic feature, tau aggregation in the EC, can disrupt the coordination of LFPs between its two efferent regions, the hippocampus and prelimbic mPFC (Figure 4; Tanninen et al., 2017). Tanninen and colleagues revealed strengthened phase–phase and amplitude–amplitude couplings of theta and gamma oscillations in these two regions during associative learning in healthy rats (the rats underwent trace eyeblink conditioning and were learned to associate two stimuli separated by a short time interval). In tau-expressing rats, the hippocampus and PFC showed a significant attenuation of stimulus-evoked theta oscillations. In addition, despite normal memory acquisition, the learning-related oscillatory coupling between the hippocampus and the PFC in these rats was diminished; at the same time, the entorhinal tau overexpression enhanced the stimulus-evoked theta–gamma phase–amplitude coupling within the mPFC (Figure 4). The authors suggested that the tau aggregation in the EC caused aberrant long-range circuit activity during associative learning, indicating the disturbances in neural oscillations of preclinical AD stages (Tanninen et al., 2017).
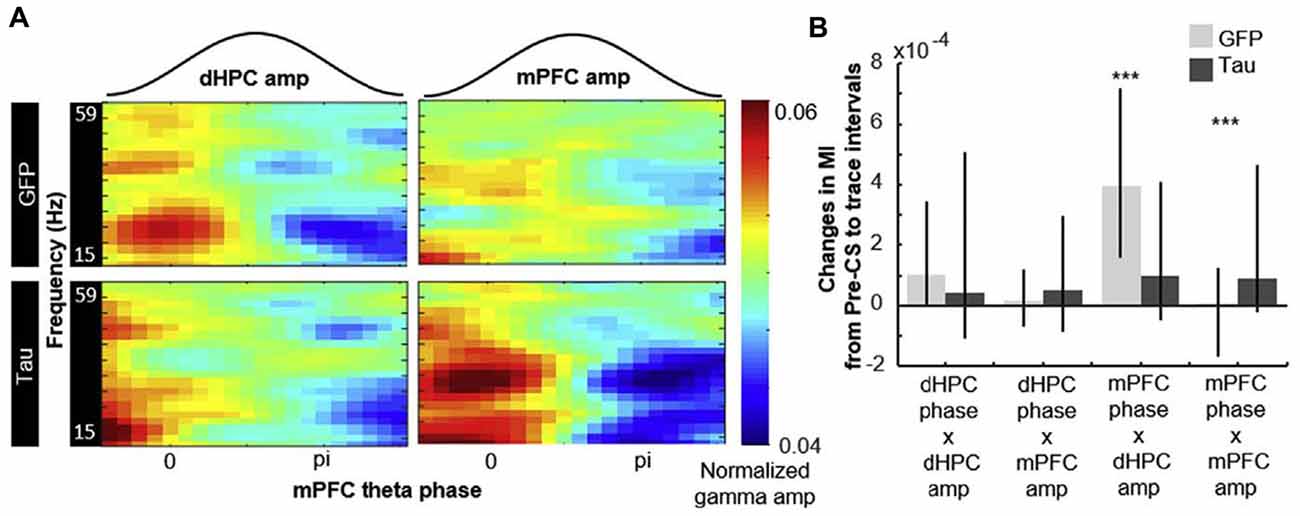
Figure 4. Entorhinal tau overexpression affects phase–amplitude hippocampal—prefrontal CFC. The degree of modulation of gamma amplitude by mPFC theta phase quantified with the MI. (A) Representative examples of normalized hippocampal (to the left) and prefrontal (to the right) gamma amplitude aligned to concurrent prefrontal theta phase. (B) Changes in the MI before and after the CS (median ± 25th and 75th percentile). Compared against GFP-expressing rats (light gray), the change in MI of prefrontal theta-hippocampal gamma coupling was smaller while that of prefrontal theta-prefrontal gamma coupling was larger in tau-expressing rats (dark gray); ***p < 0.001. Abbreviations: CS, conditioned stimulus; GFP, green fluorescent protein. Adapted with permission from Tanninen et al. (2017).
The tau aggregation has been also described in epileptic patients and animals with marked cognitive disturbances (Thom et al., 2011; Tai et al., 2017, 2018). Though the data about rhythmic abnormalities are absent in the works mentioned, these disorder can be expected to occur.
Electro-Clinical Data for Patients With “Epileptic Prodromal AD”
A similarity in the alterations of oscillatory activity in the AD/TLE brain (in particular, disturbances in theta-gamma coherence in hippocampal-cortical networks) suggests that these diseases have some common properties and, probably, at least partially similar mechanisms of their development.
The potential relation between TLE and AD has been supported by experimental and clinical data. Thus, aging is a common and well-established risk factor for epilepsy and AD (Armon et al., 2000; Maguire and Frith, 2003; Amatniek et al., 2006; Bernardi et al., 2010; Born et al., 2014; Chan et al., 2015). Besides, AD may be an important cause of epileptic disorders, as shown in elderly humans (Armon et al., 2000; Bird et al., 2005; Bernardi et al., 2010; Palop and Mucke, 2016) and in animals with AD models (Bezzina et al., 2015; Chan et al., 2015). Patients with AD have a 5- to 10-fold increased risk of the development of seizures or other forms of epileptiform activity (Amatniek et al., 2006). Although seizure pathology was previously believed to be secondary to AD, it was found that neuronal activity can regulate regional vulnerability to Aβ (Palop et al., 2007; Palop and Mucke, 2010; Bero et al., 2011); in particular, enhanced neuronal excitability can increase Aβ generation (Cirrito et al., 2008). Moreover, disturbed activity may contribute to the development of cognitive violations: epileptiform and rhythmic abnormalities in the temporal regions (in particular in the hippocampus) can cause amnestic disorders, which were reduced by antiepileptic drug treatment (Gallassi, 2006; Bakker et al., 2012). In patients with seizures in combination with AD, a case series from California with so called “vu/déjà vu” phenomena was described (Vossel et al., 2013), while another series from France (Cretin et al., 2016) had some cases that were termed “epileptic prodromal AD.” The authors believed that there is an epileptic version of AD, which usually starts with seizures as an initial symptom followed by cognitive deficit. Similar signs of cognitive and behavioral impairments in TLE and AD have been recently described by Chin and Scharfman (2013).
Many clinical evidence indicates an increased comorbidity of seizure pathology in AD: it is becoming clear that AD is associated with neuronal hyperexcitability as well as network hypersynchronicity, which is the main reasons of epilepsy development (Eichler and Meier, 2008; Noebels, 2011; Saito et al., 2012; Varga et al., 2014). Indeed, epileptic prodromal AD patients suffer from seizures sometimes even before developing clear cognitive disorders. The epileptiform activity may manifest itself in the early stages of AD more often than was previously proposed. Thus, seizures in patients with AD and amnestic mild cognitive impairment are associated with an earlier appearance of cognitive decline (Amatniek et al., 2006; Scarmeas et al., 2009; Irizarry et al., 2012; Vossel et al., 2013). In the study of Sarkis and colleagues, the authors describe patients with recurrent medically refractory epileptic auras, which ultimately lead to the disease diagnosed as AD (Sarkis et al., 2017).
At the same time, neurodegenerative processes peculiar to dementia can play a central role in the development of epilepsy in the patients predisposed to cognitive deficit. Adult-onset epilepsy of unknown cause could thus represent a risk factor for the ongoing neurodegenerative damage, even when epileptic manifestations and clinically recognized dementia are separated by long time (Cretin et al., 2016; Sarkis et al., 2017).
The facts of cognitive impairment in animals with epileptiform activity were derived from different studies devoted to the role of the tau peptide (Roberson et al., 2011) or APP overexpression in mouse models of AD (Born et al., 2014). Genetic suppression of the APP level resulted in a normalization of EEG activity (Born et al., 2014) as well as the tau reduction was beneficial for animals in multiple models of AD (Roberson et al., 2007, 2011; Gómez de Barreda et al., 2010; Ittner et al., 2010). Unfortunately, this is only true in animal models of the disease, not in AD patients.
In the hippocampus, one of the main foci of cell death in TLE and AD brains, the network hypersynchronicity and epileptiform activity can be the result of formation of extensive aberrant neuronal connections. This aberrant remodeling was revealed in epileptic rats and in APP transgenic mice (Harris et al., 2003; Palop et al., 2007; Minkeviciene et al., 2009; Palop and Mucke, 2010; Vogt et al., 2011). The aberrant reconstruction can be a cause of alterations in the oscillatory activity and rhythm coherence in brains with TLE and AD pathologies.
Conclusion
It is known that the main problem in diagnosis of neurodegenerative diseases is the detection of neuronal abnormalities at early stages of their development. At present significant achievements have been made in the development of methods for the detection of some biomarkers of AD and TLE, including cerebrospinal fluid and plasma measurements and glucose positron emission tomography (Shiihara et al., 2006; Scholl-Bürgi et al., 2008; Mattsson et al., 2009; Shaw et al., 2009; Visser et al., 2009; Blennow et al., 2007; Jack et al., 2011; van Karnebeek et al., 2012). However, there is an urgent need for biomarkers that would reflect changes in brain functioning within few milliseconds to obtain information about the progressing cognitive deficiency (Yener and Basar, 2013; Wang and Meng, 2016). The application of magnetoencephalography in combination with the coherent analysis, in particular during cognitive loading, is a promising approach to early diagnosis of these diseases. Thus, the specific disturbances in interactions of theta–gamma oscillations in hippocampal, hippocampal–entorhinal, hippocampal–prefrontal and hippocampal–septal networks were revealed in the epileptic brain. In the AD models, marked changes were observed in the theta–gamma coupling in the subiculum, an output region of the hippocampus. In addition, a decreased theta–gamma coupling between the hippocampus and the parietal cortex as well as between the hippocampus and the PFC was also shown.
At present, the methods for detection of theta–gamma coherence during cognitive loading are still not absolutely perfect. Thus, changes in theta–gamma coupling may simply reflect memory-related increases in gamma power and phase synchrony (Montgomery and Buzsáki, 2007). Novel approaches (in particular, optogenetics) should allow one to alter the relationship between gamma power and theta phase without affecting theta/gamma rhythms themselves (Colgin, 2015). This manipulation would enable one to directly determine how coupling between theta and gamma oscillations affects neuronal activity and memory operations in the brain. New approaches would help to elaborate precise early biomarkers for the diagnosis of AD and TLE. The advances of coherence methods in the detection of rhythm violation will help to deepen our understanding of the mechanisms of disturbances in theta–gamma relationship in the AD/TLE brain. Possibly, in future, specific disturbances in theta–gamma coherence will serve as markers of particular cell damage and will allow one to direct therapeutic influences to certain neural loci at early stages of the development of the disease.
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Funding
This work was supported by the Russian Foundation for Basic Research (grant numbers 15-04-05463, 17-44-500312, 18-015-00157).
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The author is grateful to SV. Sidorova and IE. Mysin for comments and help in preparing the manuscript.
References
Adler, G., Brassen, S., and Jajcevic, A. (2003). EEG coherence in Alzheimer’s dementia. J. Neural Transm. 110, 1051–1058. doi: 10.1007/s00702-003-0024-8
Amatniek, J. C., Hauser, W. A., DelCastillo-Castaneda, C., Jacobs, D. M., Marder, K., Bell, K., et al. (2006). Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia 47, 867–872. doi: 10.1111/j.1528-1167.2006.00554.x
Arabadzisz, D., Antal, K., Parpan, F., Emri, Z., and Fritschy, J.-M. (2005). Epileptogenesis and chronic seizures in a mouse model of temporal lobe epilepsy are associated with distinct EEG patterns and selective neurochemical alterations in the contralateral hippocampus. Exp. Neurol. 194, 76–90. doi: 10.1016/j.expneurol.2005.01.029
Armon, C., Peterson, G. W., and Liwnicz, B. H. (2000). Alzheimer’s disease underlies some cases of complex partial status epilepticus. J. Clin. Neurophysiol. 17, 511–518. doi: 10.1097/00004691-200009000-00011
Astasheva, E., Kitchigina, V., and Astashev, M. (2016). Analysis of oscillations in the brain during sensory stimulation: cross-frequency relations. Lect. Notes Comput. Sci. 9719, 673–680. doi: 10.1007/978-3-319-40663-3_77
Astasheva, E. V., and Kichigina, V. F. (2009). Activation of the glutamatergic system of the medial septal region accelerates epileptogenesis. Zh. Vyssh. Nerv. Deiat. Im. I P Pavlova 59, 743–749. doi: 10.1007/s11055-011-9418-5
Axmacher, N., Henseler, M. M., Jensen, O., Weinreich, I., Elger, C. E., and Fell, J. (2010). Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc. Natl. Acad. Sci. U S A 107, 3228–3233. doi: 10.1073/pnas.0911531107
Bakker, A., Krauss, G. L., Albert, M. S., Speck, C. L., Jones, L. R., Stark, C. E., et al. (2012). Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 74, 467–474. doi: 10.1016/j.neuron.2012.03.023
Bastos, A. M., Vezoli, J., and Fries, P. (2015). Communication through coherence with inter-areal delays. Curr. Opin. Neurobiol. 31, 173–180. doi: 10.1016/j.conb.2014.11.001
Belluscio, M. A., Mizuseki, K., Schmidt, R., Kempter, R., and Buzsáki, G. (2012). Cross-frequency phase-phase coupling between θ and γ oscillations in the hippocampus. J. Neurosci. 32, 423–435. doi: 10.1523/JNEUROSCI.4122-11.2012
Benchenane, K., Peyrache, A., Khamassi, M., Tierney, P. L., Gioanni, Y., Battaglia, F. P., et al. (2010). Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron 66, 921–936. doi: 10.1016/j.neuron.2010.05.013
Bernardi, S., Scaldaferri, N., Vanacore, N., Trebbastoni, A., Francia, A., D’Amico, A., et al. (2010). Seizures in Alzheimer’s disease: a retrospective study of a cohort of outpatients. Epileptic Disord. 12, 16–21. doi: 10.1684/epd.2010.0290
Bero, A. W., Yan, P., Roh, J. H., Cirrito, J. R., Stewart, F. R., Raichle, M. E., et al. (2011). Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat. Neurosci. 14, 750–756. doi: 10.1038/nn.280
Bezzina, C., Verret, L., Juan, C., Remaud, J., Halley, H., Rampon, C., et al. (2015). Early onset of hypersynchronous network activity and expression of a marker of chronic seizures in the Tg2576 mouse model of Alzheimer’s disease. PLoS One 10:e0119910. doi: 10.1371/journal.pone.0119910
Bird, S. B., Orr, P. G., Mazzola, J. L., Brush, D. E., and Boyer, E. W. (2005). Levofloxacin-related seizure activity in a patient with Alzheimer’s disease: assessment of potential risk factors. J. Clin. Psychopharmacol. 25, 287–288. doi: 10.1097/01.jcp.0000162811.15066.8e
Bland, B. H. (1986). The physiology and pharmacology of hippocampal formation theta rhythms. Prog. Neurobiol. 26, 1–54. doi: 10.1016/0301-0082(86)90019-5
Bland, B. H., and Oddie, S. D. (2001). Theta band oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorimotor integration. Behav. Brain Res. 127, 119–136. doi: 10.1016/s0166-4328(01)00358-8
Blennow, K., Zetterberg, H., Minthon, L., Lannfelt, L., Strid, S., Annas, P., et al. (2007). Longitudinal stability of CSF biomarkers in Alzheimer’s disease. Neurosci. Lett. 419, 18–22. doi: 10.1016/j.neulet.2007.03.064
Born, H. A., Kim, J. Y., Savjani, R. R., Das, P., Dabaghian, Y. A., Guo, Q., et al. (2014). Genetic suppression of transgenic APP rescues hypersynchronous network activity in a mouse model of Alzheimer’s disease. J. Neurosci. 34, 3826–3840. doi: 10.1523/JNEUROSCI.5171-13.2014
Bott, J. B., Muller, M. A., Jackson, J., Aubert, J., Cassel, J. C., Mathis, C., et al. (2016). Spatial reference memory is associated with modulation of theta-gamma coupling in the dentate gyrus. Cereb. Cortex 26, 3744–3753. doi: 10.1093/cercor/bhv177
Bouyer, J., Montaron, M., and Rougeul, A. (1981). Fast fronto-parietal rhythms during combined focused attentive behaviour and immobility in cat: cortical and thalamic localizations. Electroencephalogr. Clin. Neurophysiol. 51, 244–252. doi: 10.1016/0013-4694(81)90138-3
Bragin, A., Jandó, G., Nádasdy, Z., Hetke, J., Wise, K., and Buzsáki, G. (1995). γ (40–100 Hz) oscillation in the hippocampus of the behaving rat. J. Neurosci. 15, 47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995
Broggini, A. C. S., Esteves, I. M., Romcy-Pereira, R. N., Leite, J. P., and Leão, R. N. (2016). Pre-ictal increase in theta synchrony between the hippocampus and prefrontal cortex in a rat model of temporal lobe epilepsy. Exp. Neurol. 279, 232–242. doi: 10.1016/j.expneurol.2016.03.007
Burgess, N., Maguire, E., and O’Keefe, J. (2002). The human hippocampus and spatial and episodic memory. Neuron 35, 625–641. doi: 10.1016/s0896-6273(02)00830-9
Buzsáki, G. (2002). Theta oscillations in the hippocampus. Neuron 33, 325–340. doi: 10.1016/s0896-6273(02)00586-x
Buzsáki, G. (2004). Large-scale recording of neuronal ensembles. Nat. Neurosci. 7, 446–451. doi: 10.1038/nn1233
Buzsáki, G. (2010). Neural syntax: cell assemblies, synapsembles, and readers. Neuron 68, 362–385. doi: 10.1016/j.neuron.2010.09.023
Buzsáki, G., Buhl, D. L., Harris, K. D., Csicsvari, J., Czéh, B., and Morozov, A. (2003). Hippocampal network patterns of activity in the mouse. Neuroscience 116, 201–211. doi: 10.1016/s0306-4522(02)00669-3
Buzsáki, G., Leung, L. W., and Vanderwolf, C. H. (1983). Cellular bases of hippocampal EEG in the behaving rat. Brain Res. Rev. 287, 139–171. doi: 10.1016/0165-0173(83)90037-1
Buzsáki, G., and Mizuseki, K. (2014). The log-dynamic brain: how skewed distributions affect network operations. Nat. Rev. Neurosci. 15, 264–278. doi: 10.1038/nrn3687
Buzsáki, G., and Moser, E. I. (2013). Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 16, 130–138. doi: 10.1038/nn.3304
Buzsáki, G., and Wang, X. J. (2010). Mechanisms of γ oscillations. Annu. Rev. Neurosci. 35, 203–225. doi: 10.1146/annurev-neuro-062111-150444
Buzsáki, G., and Watson, B. O. (2012). Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin. Neurosci. 14, 345–367.
Canolty, R., Edwards, E., Dalal, S., Soltani, M., Nagarajan, S., Kirsch, H., et al. (2006). High γ power is phase-locked to theta oscillations in human neocortex. Science 313, 1626–1628. doi: 10.1126/science.1128115
Canolty, R. T., and Knight, R. T. (2010). The functional role of cross-frequency coupling. Trends Cogn. Sci. 14, 506–515. doi: 10.1016/j.tics.2010.09.001
Caravaglios, G., Castro, G., Costanzo, E., Di Maria, G., Mancuso, D., and Muscoso, E. G. (2010). Theta power responses in mild Alzheimer’s disease during an auditory oddball paradigm: lack of theta enhancement during stimulus processing. J. Neural Transm. 117, 1195–1208. doi: 10.1007/s00702-010-0488-2
Cavanagh, J. F., Cohen, M. X., and Allen, J. J. (2009). Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J. Neurosci. 29, 98–105. doi: 10.1523/JNEUROSCI.4137-08.2009
Chan, J., Jones, N. C., Bush, A. I., O’Brien, T. J., and Kwan, P. (2015). A mouse model of Alzheimer’s disease displays increased susceptibility to kindling and seizure-associated death. Epilepsia 56, e73–e77. doi: 10.1111/epi.12993
Chauvière, L., Rafrafi, N., Thinus-Blanc, C., Bartolomei, F., Esclapez, M., and Bernard, C. (2009). Early deficits in spatial memory and theta rhythm in experimental temporal lobe epilepsy. J. Neurosci. 29, 5402–5410. doi: 10.1523/JNEUROSCI.4699-08.2009
Chin, J., and Scharfman, H. E. (2013). Shared cognitive and behavioral impairments in epilepsy and Alzheimer’s disease and potential underlying mechanisms. Epilepsy Behav. 26, 343–351. doi: 10.1016/j.yebeh.2012.11.040
Cirrito, J. R., Kang, J.-E., Lee, J., Stewart, F. R., Verges, D. K., Silverio, L., et al. (2008). Endocytosis is required for synaptic activity-dependent release of amyloid-β in vivo. Neuron 58, 42–51. doi: 10.1016/j.neuron.2008.02.003
Colgin, L. L. (2011). Oscillations and hippocampal-prefrontal synchrony. Curr. Opin. Neurobiol. 21, 467–474. doi: 10.1016/j.conb.2011.04.006
Colgin, L. L. (2015). Theta-γ coupling in the entorhinal-hippocampal system. Curr. Opin. Neurobiol. 31, 45–50. doi: 10.1016/j.conb.2014.08.001
Colgin, L. L., Denninger, T., Fyhn, M., Hafting, T., Bonnevie, T., Jensen, O., et al. (2009). Frequency of γ oscillations routes flow of information in the hippocampus. Nature 462, 353–357. doi: 10.1038/nature08573
Colgin, L. L., and Moser, E. I. (2010). γ oscillations in the hippocampus. Physiology 25, 319–329. doi: 10.1152/physiol.00021.2010
Colom, L. V., Garcia-Hernandez, A., Castaceda, M. T., Perez-Cordova, M. G., and Garrido-Sanabria, E. R. (2006). Septohippocampal networks in chronically epileptic rats: potential antiepileptic effects of theta rhythm generation. J. Neurophysiol. 95, 3645–3653. doi: 10.1152/jn.00040.2006
Cretin, B., Sellal, F., Philippi, N., Bousiges, O., Di Bitonto, L., Martin-Hunyadi, C., et al. (2016). Epileptic prodromal Alzheimer’s disease, a retrospective study of 13 new cases: expanding the spectrum of Alzheimer’s disease to an epileptic variant? J. Alzheimers Dis. 52, 1125–1133. doi: 10.3233/JAD-150096
Czigler, B., Csikos, D., Hidasi, Z., Anna Gaál, Z., Csibri, E., Kiss, E., et al. (2008). Quantitative EEG in early Alzheimer’s disease patients—power spectrum and complexity features. Int. J. Psychophysiol. 68, 75–80. doi: 10.1016/j.ijpsycho.2007.11.002
DeCoteau, W. E., Thorn, C., Gibson, D. J., Courtemanche, R., Mitra, P., Kubota, Y., et al. (2007). Learning-related coordination of striatal and hippocampal theta rhythms during acquisition of a procedural maze task. Proc. Natl. Acad. Sci. U S A 104, 5644–5649. doi: 10.1073/pnas.0700818104
Didic, M., Barbeau, E. J., Felician, O., Tramoni, E., Guedj, E., Poncet, M., et al. (2011). Which memory system is impaired first in Alzheimer’s disease? J. Alzheimers Dis. 27, 11–22. doi: 10.3233/JAD-2011-110557
Dugladze, T., Vida, I., Tort, A. B., Gross, A., Otahal, J., Heinemann, U., et al. (2007). Impaired hippocampal rhythmogenesis in a mouse model of mesial temporal lobe epilepsy. Proc. Natl. Acad. Sci. U S A 104, 17530–17535. doi: 10.1073/pnas.0708301104
Dupont, S., Van de Moortele, P. F., Samson, S., Hasboun, D., Poline, J., Adam, C., et al. (2000). Episodic memory in left temporal lobe epilepsy: a functional MRI study. Brain 123, 1722–1732. doi: 10.1093/brain/123.8.1722
Easton, A., Douchamps, V., Eacott, M., and Lever, C. (2012). A specific role for septohippocampal acetylcholine in memory? Neuropsychologia 50, 3156–3168. doi: 10.1016/j.neuropsychologia.2012.07.022
Eichler, S. A., and Meier, J. C. (2008). E-I balance and human diseases—from molecules to networking. Front. Mol. Neurosci. 1:2. doi: 10.3389/neuro.02.002.2008
Engel, A., Fries, P., and Singer, W. (2001). Dynamic predictions: oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2, 704–716. doi: 10.1038/35094565
Fell, J., and Axmacher, N. (2011). The role of phase synchronization in memory processes. Nat. Rev. Neurosci. 12, 105–118. doi: 10.1038/nrn2979
Fell, J., Klaver, P., Lehnertz, K., Grunwald, T., Schaller, C., Elger, C. E., et al. (2001). Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nat. Neurosci. 4, 1259–1264. doi: 10.1038/nn759
Fell, J., Ludowig, E., Rosburg, T., Axmacher, N., and Elger, C. E. (2008). Phase-locking within human mediotemporal lobe predicts memory formation. Neuroimage 43, 410–419. doi: 10.1016/j.neuroimage.2008.07.021
Francis, B. M., Kim, J., Barakat, M. E., Fraenkl, S., Yucel, Y. H., Peng, S., et al. (2010). Object recognition memory and BDNF expression are reduced in young TgCRND8 mice. Neurobiol. Aging 33, 555–563. doi: 10.1016/j.neurobiolaging.2010.04.003
Frank, L. M., Brown, E. N., and Wilson, M. (2000). Trajectory encoding in the hippocampus and entorhinal cortex. Neuron 27, 169–178. doi: 10.1016/s0896-6273(00)00018-0
Fries, P. (2005). A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. 9, 474–480. doi: 10.1016/j.tics.2005.08.011
Fries, P. (2009). Neuronal γ-band synchronization as a fundamental process in cortical computation. Annu. Rev. Neurosci. 32, 209–224. doi: 10.1146/annurev.neuro.051508.135603
Froriep, U. P., Kumar, A., Cosandier-Rimélé, D., Häussler, U., Kilias, A., Haas, C. A., et al. (2012). Altered theta coupling between medial entorhinal cortex and dentate gyrus in temporal lobe epilepsy. Epilepsia 53, 1937–1947. doi: 10.1111/j.1528-1167.2012.03662.x
Gallassi, R. (2006). Epileptic amnesic syndrome: an update and further considerations. Epilepsia 47, 103–105. doi: 10.1111/j.1528-1167.2006.00704.x
García-Hernández, A., Bland, B. H., Facelli, J. C., and Colom, L. V. (2010). Septohippocampal networks in chronic epilepsy. Exp. Neurol. 222, 86–92. doi: 10.1016/j.expneurol.2009.12.010
Gómez de Barreda, E., Pérez, M., Gómez Ramos, P., de Cristobal, J., Martín-Maestro, P., Morán, A., et al. (2010). Tau-knockout mice show reduced GSK3-induced hippocampal degeneration and learning deficits. Neurobiol. Dis. 37, 622–629. doi: 10.1016/j.nbd.2009.11.017
Goodman, M. S., Kumar, S., Zomorrodi, R., Ghazala, Z., Cheam, A. S. M., Barr, M. S., et al. (2018). Theta-γ coupling and working memory in Alzheimer’s dementia and mild cognitive impairment. Front. Aging Neurosci. 10:101. doi: 10.3389/fnagi.2018.00101
Goutagny, R., Gu, N., Cavanagh, C., Jackson, J., Chabot, J.-G., Quirion, R., et al. (2013). Alterations in hippocampal network oscillations and theta-γ coupling arise before Aβ overproduction in a mouse model of Alzheimer’s disease. Eur. J. Neurosci. 37, 1896–1902. doi: 10.1111/ejn.12233
Gregoriou, G. G., Gotts, S. J., Zhou, H., and Desimone, R. (2009). High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science 324, 1207–1210. doi: 10.1126/science.1171402
Guderian, S., Schott, B. H., Richardson-Klavehn, A., and Düzel, E. (2009). Medial temporal theta state before an event predicts episodic encoding success in humans. Proc. Natl. Acad. Sci. U S A 106, 5365–5370. doi: 10.1073/pnas.0900289106
Gureviciene, I., Gurevicius, K., Mugantseva, E., Kislin, M., Khiroug, L., and Tanila, H. (2017). Amyloid plaques show binding capacity of exogenous injected amyloid-β. J. Alzheimers Dis. 55, 147–157. doi: 10.3233/JAD-160453
Haider, B., and McCormick, D. (2009). Rapid neocortical dynamics: cellular and network mechanisms. Neuron 62, 171–189. doi: 10.1016/j.neuron.2009.04.008
Harris, K. D., Csicsvari, J., Hirase, H., Dragoi, G., and Buzsáki, G. (2003). Organization of cell assemblies in the hippocampus. Nature 424, 552–556. doi: 10.1038/nature01834
Hasselmo, M. E. (2005). What is the function of hippocampal theta rhythm?—Linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus 15, 936–949. doi: 10.1002/hipo.20116
Hasselmo, M. E., Bodelόn, C., and Wyble, B. P. (2002). A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 14, 793–817. doi: 10.1162/089976602317318965
Helmstaedter, C. (2002). Effects of chronic epilepsy on declarative memory systems. Prog. Brain Res. 135, 439–453. doi: 10.1016/s0079-6123(02)35041-6
Herrmann, C. S., and Demiralp, T. (2005). Human EEG γ oscillations in neuropsychiatric disorders. Clin. Neurophysiol. 116, 2719–2733. doi: 10.1016/j.clinph.2005.07.007
Holz, E. M., Glennon, M., Prendergast, K., and Sauseng, P. (2010). Theta-γ phase synchronization during memory matching in visual working memory. Neuroimage 52, 326–335. doi: 10.1016/j.neuroimage.2010.04.003
Hurtado, J. M., Rubchinsky, L. L., and Sigvardt, K. A. (2004). Statistical method for detection of phase-locking episodes in neural oscillations. J. Neurophysiol. 91, 1883–1898. doi: 10.1152/jn.00853.2003
Huxter, J. R., Senior, T. J., Allen, K., and Csicsvari, J. (2008). Theta phase-specific codes for two-dimensional position, trajectory and heading in the hippocampus. Nat. Neurosci. 11, 587–594. doi: 10.1038/nn.2106
Igarashi, K. M., Lu, L., Colgin, L. L., Moser, M. B., and Moser, E. I. (2014). Coordination of entorhinal-hippocampal ensemble activity during associative learning. Nature 510, 143–147. doi: 10.1038/nature13162
Inostroza, M., Brotons-Mas, J. R., Laurent, F., Cid, E., and de la Prida, L. M. (2013). Specific impairment of “what-where-when” episodic-like memory in experimental models of temporal lobe epilepsy. J. Neurosci. 33, 17749–17762. doi: 10.1523/JNEUROSCI.0957-13.2013
Irizarry, M. C., Jin, S., He, F., Emond, J. A., Raman, R., Thomas, R. G., et al. (2012). Incidence of new-onset seizures in mild to moderate Alzheimer disease. Arch. Neurol. 69, 368–372. doi: 10.1001/archneurol.2011.830
Ittner, A. A., Gladbach, A., Bertz, J., Suh, L. S., and Ittner, L. M. (2014). p38 MAP kinase-mediated NMDA receptor-dependent suppression of hippocampal hypersynchronicity in a mouse model of Alzheimer’s disease. Acta Neuropathol. Commun. 2:149. doi: 10.1186/s40478-014-0149-z
Ittner, L. M., Ke, Y. D., Delerue, F., Bi, M., Gladbach, A., van Eersel, J., et al. (2010). Dendritic function of tau mediates amyloid-β toxicity in Alzheimer’s disease mouse models. Cell 142, 387–397. doi: 10.1016/j.cell.2010.06.036
Jack, C. R. Jr., Vemuri, P., Wiste, H. J., Weigand, S. D., Aisen, P. S., Trojanowski, J. Q., et al. (2011). Evidence for ordering of Alzheimer disease biomarkers. Arch. Neurol. 68, 1526–1535. doi: 10.1001/archneurol.2011.183
Jensen, O., Kaiser, J., and Lachaux, J. P. (2007). Human γ-frequency oscillations associated with attention and memory. Trends Neurosci. 30, 317–324. doi: 10.1016/j.tins.2007.05.001
Jensen, O., and Lisman, J. E. (2005). Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends Neurosci. 28, 67–72. doi: 10.1016/j.tins.2004.12.001
Jensen, O., and Tesche, C. (2002). Frontal theta activity in humans increases with memory load in a working memory task. Eur. J. Neurosci. 15, 1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x
Jones, M. W., and Wilson, M. A. (2005). Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 3:e402. doi: 10.1371/journal.pbio.0030402
Jutras, M. J., Fries, P., and Buffalo, E. A. (2009). γ-band synchronization in the macaque hippocampus and memory formation. J. Neurosci. 29, 12521–12531. doi: 10.1523/JNEUROSCI.0640-09.2009
Kabanova, I. V. (2011). Coherence and Phase Analysis of Theta Oscillations in the Septohippocampal System During Epileptogenesis. Thesis for Master in Biological Science (in Russian). Printing house “11-th Format”, Moscow: Moscow Institute of Physics and Technology (State University).
Kabanova, I. V., Sinel’nikova, V. V., Popova, I. I. U., Kichigina, V. F., and Aliev, R. R. (2011). Coherence and phase analysis of theta-oscillations in the septohippocampal system during seizures. Zh. Vyssh. Nerv. Deiat. Im. I P Pavlova 61, 763–768.
Kahana, M. J., Sekuler, R., Caplan, J. B., Kirschen, M., and Madsen, J. R. (1999). Human theta oscillations exhibit task dependence during virtual maze navigation. Nature 399, 781–784. doi: 10.1038/21645
Kametani, F., and Hasegawa, M. (2018). Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease. Front. Neurosci. 12:25. doi: 10.3389/fnins.2018.00025
Kirihara, K., Rissling, A. J., Swerdlow, N. R., Braff, D. L., and Light, G. A. (2012). Hierarchical organization of γ and theta oscillatory dynamics in schizophrenia. Biol. Psychiatry 71, 873–880. doi: 10.1016/j.biopsych.2012.01.016
Kitamura, T., Pignatelli, M., Suh, J., Kohara, K., Yoshiki, A., Abe, K., et al. (2014). Island cells control temporal association memory. Science 343, 896–901. doi: 10.1126/science.1244634
Kitchigina, V. F., and Butuzova, M. V. (2009). Theta activity of septal neurons during different epileptic phases: the same frequency but different significance? Exp. Neurol. 216, 449–458. doi: 10.1016/j.expneurol.2009.01.001
Klimesch, W., Doppelmayr, M., Schimke, H., Ripper, B. (1997). Theta synchronization and α desynchronization in a memory task. Psychophysiology 34, 169–176. doi: 10.1111/j.1469-8986.1997.tb02128.x
Knight, R. T. (2007). Neural networks debunk phrenology. Science 316, 1578–1579. doi: 10.1126/science.1144677
Komorowski, R. W., Manns, J. R., and Eichenbaum, H. (2009). Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens where. J. Neurosci. 29, 9918–9929. doi: 10.1523/JNEUROSCI.1378-09.2009
Kraus, B. J., Robinson, R. J. II, White, J. A., Eichenbaum, H., and Hasselmo, M. E. (2013). Hippocampal “time cells”: time versus path integration. Neuron 78, 1090–1101. doi: 10.1016/j.neuron.2013.04.015
Laurent, F., Brotons-Mas, J. R., Cid, E., Lopez-Pigozzi, D., Valero, M., Gal, B., et al. (2015). Proximodistal structure of theta coordination in the dorsal hippocampus of epileptic rats. J. Neurosci. 35, 4760–4775. doi: 10.1523/jneurosci.4297-14.2015
Lega, B., Burke, J., Jacobs, J., and Kahana, M. J. (2016). Slow-theta-to-γ phase-amplitude coupling in human hippocampus supports the formation of new episodic memories. Cereb. Cortex 26, 268–278. doi: 10.1093/cercor/bhu232
Lega, B., Dionisio, S., Bingaman, W., Najm, I., and Gonzalez-Martinez, J. (2015). The γ band effect for episodic memory encoding is absent in epileptogenic hippocampi. Clin. Neurophysiol. 126, 866–872. doi: 10.1016/j.clinph.2014.07.035
Lega, B., Jacobs, J., and Kahana, M. (2012). Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus 22, 748–761. doi: 10.1002/hipo.20937
Lisman, J. (2005). The theta/γ discrete phase code occuring during the hippocampal phase precession may be a more general brain coding scheme. Hippocampus 15, 913–922. doi: 10.1002/hipo.20121
Lisman, J., and Buzsáki, G. (2008). A neural coding scheme formed by the combined function of γ and theta oscillations. Schizophr. Bull. 34, 974–980. doi: 10.1093/schbul/sbn060
Lisman, J. E., and Idiart, M. A. (1995). Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science 267, 1512–1515. doi: 10.1126/science.7878473
Lisman, J. E., and Jensen, O. (2013). The theta-γ neural code. Neuron 77, 1002–1016. doi: 10.1016/j.neuron.2013.03.007
Lithfous, S., Dufour, A., and Després, O. (2013). Spatial navigation in normal aging and the prodromal stage of Alzheimer’s disease: insights from imaging and behavioral studies. Ageing Res.Rev. 12, 201–213. doi: 10.1016/j.arr.2012.04.007
Livanov, M. N., Krylov, V. Y. U., Ostrjakova, T. V., and Shulgina, G. I. (1977). Slow field potential oscillations as one of the basic mechanisms of integrative activity of neurons [proceedings]. Act. Nerv. Super. (Praha) 19, 43–44.
Magill, P. J., Sharott, A., Bolam, J. P., and Brown, P. (2006). Delayed synchronization of activity in cortex and subthalamic nucleus following cortical stimulation in the rat. J. Physiol. 574, 929–946. doi: 10.1113/jphysiol.2006.110379
Maguire, E. A., and Frith, C. D. (2003). Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain 126, 1511–1523. doi: 10.1093/brain/awg157
Mankin, E. A., Sparks, F. T., Slayyeh, B., Sutherland, R. J., Leutgeb, S., and Leutgeb, J. K. (2012). Neuronal code for extended time in the hippocampus. Proc. Natl. Acad. Sci. U S A 109, 19462–19467. doi: 10.1073/pnas.1214107109
Marcelin, B., Chauvière, L., Becker, A., Migliore, M., Esclapez, M., and Bernard, C. (2009). h channel-dependent deficit of theta oscillation resonance and phase shift in temporal lobe epilepsy. Neurobiol. Dis. 33, 436–447. doi: 10.1016/j.nbd.2008.11.019
Markram, H., Lübke, J., Frotscher, M., and Sakmann, B. (1997). Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275, 213–215. doi: 10.1126/science.275.5297.213
Mattsson, N., Zetterberg, H., Hansson, O., Andreasen, N., Parnetti, L., Jonsson, M., et al. (2009). CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 302, 385–393. doi: 10.1001/jama.2009.1064
Miller, J. W., Turner, G. M., and Gray, B. C. (1994). Anticonvulsant effects of the experimental induction of hippocampal theta activity. Epilepsy Res. 18, 195–204. doi: 10.1016/0920-1211(94)90040-x
Minkeviciene, R., Rheims, S., Dobszay, M. B., Zilberter, M., Hartikainen, J., Fülöp, L., et al. (2009). Amyloid β-induced neuronal hyperexcitability triggers progressive epilepsy. J. Neurosci. 29, 3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009
Mitchell, D. J., McNaughton, N., Flanagan, D., and Kirk, I. J. (2008). Frontal-midline theta from the perspective of hippocampal “theta”. Prog. Neurobiol. 86, 156–185. doi: 10.1016/j.pneurobio.2008.09.005
Mizuseki, K., Sirota, A., Pastalkova, E., and Buzsáki, G. (2009). Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron 64, 267–280. doi: 10.1016/j.neuron.2009.08.037
Montgomery, S. M., and Buzsáki, G. (2007). γ oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc. Natl. Acad. Sci. U S A 104, 14495–14500. doi: 10.1073/pnas.0701826104
Montgomery, S. M., Sirota, A., and Buzsáki, G. (2008). Theta and γ coordination of hippocampal networks during waking and rapid eye movement sleep. J. Neurosci. 28, 6731–6741. doi: 10.1523/jneurosci.1227-08.2008
Moretti, D. V., Pievani, M., Geroldi, C., Binetti, G., Zanetti, O., Rossini, P. M., et al. (2010). EEG markers discriminate among different subgroup of patients with mild cognitive impairment. Am. J. Alzheimers Dis. Other Demen. 25, 58–73. doi: 10.1177/1533317508329814
Morimoto, K., Fahnestock, M., and Racine, R. J. (2004). Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog. Neurobiol. 73, 1–60. doi: 10.1016/j.pneurobio.2004.03.009
Mormann, F., Fell, J., Axmacher, N., Weber, B., Lehnertz, K., Elger, C. E., et al. (2005). Phase/amplitude reset and theta-γ interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus 15, 890–900. doi: 10.1002/hipo.20117
Nácher, V., Ledberg, A., Deco, G., and Romo, R. (2013). Coherent delta-band oscillations between cortical areas correlate with decision making. Proc. Natl. Acad. Sci. U S A 110, 15085–15090. doi: 10.1073/pnas.1314681110
Nerad, L., and McNaughton, N. (2006). The septal EEG suggests a distributed organization of the pacemaker of hippocampal theta in the rat. Eur. J. Neurosci. 24, 155–166. doi: 10.1111/j.1460-9568.2006.04902.x
Newman, E. L., Gillet, S. N., Climer, J. R., and Hasselmo, M. E. (2013). Cholinergic blockade reduces theta-γ phase amplitude coupling and speed modulation of theta frequency consistent with behavioral effects on encoding. J. Neurosci. 33, 19635–19646. doi: 10.1523/jneurosci.2586-13.2013
Noebels, J. (2011). A perfect storm: converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia 52, 39–46. doi: 10.1111/j.1528-1167.2010.02909.x
O’Keefe, J., and Nadel, L. (1978). The Hippocampus as a Cognitive Map. Oxford University Press: Oxford University Press.
Palop, J. J., Chin, J., Roberson, E. D., Wang, J., Thwin, M. T., Bien-Ly, N., et al. (2007). Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55, 697–711. doi: 10.1016/j.neuron.2007.07.025
Palop, J. J., and Mucke, L. (2010). Amyloid-β-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat. Neurosci. 13, 812–818. doi: 10.1038/nn.2583
Palop, J. J., and Mucke, L. (2016). Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 17, 777–792. doi: 10.1038/nrn.2016.141
Palva, J. M., Monto, S., Kulashekhar, S., and Palva, S. (2010). Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proc. Natl. Acad. Sci. U S A 107, 7580–7585. doi: 10.1073/pnas.0913113107
Palva, J. M., Palva, S., and Kaila, K. (2005). Phase synchrony among neuronal oscillations in the human cortex. J. Neurosci. 25, 3962–3972. doi: 10.1523/jneurosci.4250-04.2005
Paré, D., Collins, D. R., and Pelletier, J. G. (2002). Amygdala oscillations and the consolidation of emotional memories. Trends Cogn. Sci. 6, 306–314. doi: 10.1016/s1364-6613(02)01924-1
Quilichini, P., Sirota, A., and Buzsáki, G. (2010). Intrinsic circuit organization and theta-γ oscillation dynamics in the entorhinal cortex of the rat. J. Neurosci. 30, 11128–11142. doi: 10.1523/jneurosci.1327-10.2010
Roberson, E. D., Hope, O. A., Martin, R. C., and Schmidt, D. (2011). Geriatric epilepsy: research and clinical directions for the future. Epilepsy Behav. 22, 103–111. doi: 10.1016/j.yebeh.2011.04.005
Roberson, E. D., Scearce-Levie, K., Palop, J. J., Yan, F., Cheng, I. H., Wu, T., et al. (2007). Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science 316, 750–754. doi: 10.1126/science.1141736
Rodriguez, E., George, N., Lachaux, J. P., Martinerie, J., Renault, B., and Varela, F. J. (1999). Perception’s shadow: long-distance synchronization of human brain activity. Nature 397, 430–433. doi: 10.1038/17120
Rutishauser, U., Ross, I. B., Mamelak, A. N., and Schuman, E. M. (2010). Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature 464, 903–907. doi: 10.1038/nature08860
Saito, Y., Inoue, T., Zhu, G., Kimura, N., Okada, M., Nishimura, M., et al. (2012). Hyperpolarization-activated cyclic nucleotide gated channels: a potential molecular link between epileptic seizures and Aβ generation in Alzheimer’s disease. Mol. Neurodegener. 7:50. doi: 10.1186/1750-1326-7-50
Sarkis, R. A., Kim, C., Willment, K. C., Gale, S. A., and Dworetzky, B. A. (2017). Recurrent epileptic auras as a presenting symptom of Alzheimer’s disease. Front. Neurol. 8:360. doi: 10.3389/fneur.2017.00360
Sauseng, P., Klimesch, W., Gruber, W. R., and Birbaumer, N. (2008). Cross-frequency phase synchronization: a brain mechanism of memory matching and attention. Neuroimage 40, 308–317. doi: 10.1016/j.neuroimage.2007.11.032
Sauseng, P., Klimesch, W., Heise, K. F., Gruber, W. R., Holz, E., Karim, A. A., et al. (2009). Brain oscillatory substrates of visual short-term memory capacity. Curr. Biol. 19, 1846–1852. doi: 10.1016/j.cub.2009.08.062
Scarmeas, N., Honig, L. S., Choi, H., Cantero, J., Brandt, J., Blacker, D., et al. (2009). Seizures in Alzheimer disease: who, when, and how common? Arch. Neurol. 66, 992–997. doi: 10.1001/archneurol.2009.130
Schack, B., and Weiss, S. (2005). Quantification of phase synchronization phenomena and their importance for verbal memory processes. Biol. Cybern. 92, 275–287. doi: 10.1007/s00422-005-0555-1
Scheffer-Teixeira, R., Belchior, H., Caixeta, F. V., Souza, B. C., Ribeiro, S., and Tort, A. B. (2012). Theta phase modulates multiple layer-specific oscillations in the CA1 region. Cereb. Cortex 22, 2404–2414. doi: 10.1093/cercor/bhr319
Scheffer-Teixeira, R., and Tort, A. B. (2016). On cross-frequency phase-phase coupling between theta and gamma oscillations in the hippocampus. Elife 5:e20515. doi: 10.7554/eLife.20515
Schneider, F., Baldauf, K., Wetzel, W., and Reymann, K. G. (2014). Behavioral and EEG changes in male 5xFAD mice. Physiol. Behav. 135, 25–33. doi: 10.1016/j.physbeh.2014.05.041
Scholl-Bürgi, S., Haberlandt, E., Heinz-Erian, P., Deisenhammer, F., Albrecht, U., Sigl, S. B., et al. (2008). Amino acid cerebrospinal fluid/plasma ratios in children: influence of age, gender, and antiepileptic medication. Pediatrics 121, e920–e926. doi: 10.1542/peds.2007-1631
Scott, L., Feng, J., Kiss, T., Needle, E., Atchison, K., Kawabe, T. T., et al. (2012). Age-dependent disruption in hippocampal theta oscillation in amyloid-β overproducing transgenic mice. Neurobiol. Aging 33, 1481.e13–1481.e23. doi: 10.1016/j.neurobiolaging.2011.12.010
Schomburg, E. W., Fernández-Ruiz, A., Mizuseki, K., Bereányi, A., Anastassiou, C. A., Koch, C., et al. (2014). Theta phase segregation of input-specific gamma patterns in entorhinal-hippocampal networks. Neuron 84, 470–485. doi: 10.1016/j.neuron.2014.08.051
Seabrook, G. R., Smith, D. W., Bowery, B. J., Easter, A., Reynolds, T., Fitzjohn, S. M., et al. (1999). Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology 38, 349–359. doi: 10.1016/s0028-3908(98)00204-4
Selkoe, D. J. (2000). Toward a comprehensive theory for Alzheimer’s disease. Hypothesis: Alzheimer’s disease is caused by the cerebral accumulation and cytotoxicity of amyloid β-protein. Ann. N. Y. Acad. Sci. 924, 17–25. doi: 10.1111/j.1749-6632.2000.tb05554.x
Senechal, Y., Kelly, P. H., and Dev, K. K. (2008). Amyloid precursor protein knockout mice show age-dependent deficits in passive avoidance learning. Behav. Brain Res. 186, 126–132. doi: 10.1016/j.bbr.2007.08.003
Shaw, L. M., Vanderstichele, H., Knapik-Czajka, M., Clark, C. M., Aisen, P. S., Petersen, R. C., et al. (2009). Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 110, 403–413. doi: 10.1002/ana.21610
Shiihara, T., Kato, M., Ichiyama, T., Takahashi, Y., Tanuma, N., Miyata, R., et al. (2006). Acute encephalopathy with refractory status epilepticus: bilateral mesial temporal and claustral lesions, associated with a peripheral marker of oxidative DNA damage. J. Neurol. Sci. 250, 159–161. doi: 10.1016/j.jns.2006.07.002
Shirvalkar, P. R., Rapp, P. R., and Shapiro, M. L. (2010). Bidirectional changes to hippocampal theta-γ comodulation predict memory for recent spatial episodes. Proc. Natl. Acad. Sci. U S A 107, 7054–7059. doi: 10.1073/pnas.0911184107
Siapas, A. G., Lubenov, E. V., and Wilson, M. A. (2005). Prefrontal phase locking to hippocampal theta oscillations. Neuron 46, 141–151. doi: 10.1016/j.neuron.2005.02.028
Siegel, M., Warden, M. R., and Miller, E. K. (2009). Phase-dependent neuronal coding of objects in short-term memory. Proc. Natl. Acad. Sci. U S A 106, 21341–21346. doi: 10.1073/pnas.0908193106
Sinelnikova, V. V. (2012). Thesis for Doctorate in Biological Science (in Russian). Printing house “11-th Format”, Moscow: Institute of Theoretical and Experimental Biophysics Russian Academia of Sciences (in Russian).
Sirota, A., Montgomery, S., Fujisawa, S., Isomura, Y., Zugaro, M., and Buzsáki, G. (2008). Entrainment of neocortical neurons and γ oscillations by the hippocampal theta rhythm. Neuron 60, 683–697. doi: 10.1016/j.neuron.2008.09.014
Soltesz, I., and Deschênes, M. (1993). Low- and high-frequency membrane potential oscillations during theta activity in CA1 and CA3 pyramidal neurons of the rat hippocampus under ketamine-xylazine anesthesia. J. Neurophysiol. 70, 97–116. doi: 10.1152/jn.1993.70.1.97
Sridharan, D., and Knudsen, E. I. (2015). γ oscillations in the midbrain spatial attention network: linking circuits to function. Curr. Opin. Neurobiol. 31, 189–198. doi: 10.1016/j.conb.2014.11.006
Strogatz, S. H. (2003). Nonlinear Dynamics and Chaos: With Applications to Physics, Biology, Chemistry and Engineering. Cambridge, MA: Perseus Books Publishing.
Szczepanski, S. M., Crone, N. E., Kuperman, R. A., Auguste, K. I., Parvizi, J., and Knight, R. T. (2014). Dynamic changes in phase-amplitude coupling facilitate spatial attention control in fronto-parietal cortex. PLoS Biol. 12:e1001936. doi: 10.1371/journal.pbio.1001936
Tabuchi, E. T., Mulder, A. B., and Wiener, S. I. (2000). Position and behavioral modulation of synchronization of hippocampal and accumbens neuronal discharges in freely moving rats. Hippocampus 10, 717–728. doi: 10.1002/1098-1063(2000)10:6<717::aid-hipo1009>3.0.co;2-3
Tai, Y.-H., Lin, Y.-Y., Wang, K.-C., Chang, C.-L., Chen, R.-Y., Wu, C.-C., et al. (2018). Curcuminoid submicron particle ameliorates cognitive deficits and decreases amyloid pathology in Alzheimer’s disease mouse model. Oncotarget 9, 10681–10697. doi: 10.18632/oncotarget.24369
Tai, T. Y., Warner, L. N., Jones, T. D., Jung, S., Concepcion, F. A., Skyrud, D. W., et al. (2017). Antiepileptic action of c-Jun N-terminal kinase (JNK) inhibition in an animal model of temporal lobe epilepsy. Neuroscience 349, 35–47. doi: 10.1016/j.neuroscience.2017.02.024
Takehara-Nishiuchi, K., and McNaughton, B. L. (2008). Spontaneous changes of neocortical code for associative memory during consolidation. Science 322, 960–963. doi: 10.1126/science.1161299
Tang, F. R., and Loke, W. K. (2010). Cyto-, axo- and dendro-architectonic changes of neurons in the limbic system in the mouse pilocarpine model of temporal lobe epilepsy. Epilepsy Res. 89, 43–51. doi: 10.1016/j.eplepsyres.2009.10.015
Tanninen, S. E., Nouriziabari, B., Morrissey, M. D., Bakir, R., Dayton, R. D., Klein, R. L., et al. (2017). Entorhinal tau pathology disrupts hippocampal-prefrontal oscillatory coupling during associative learning. Neurobiol. Aging 58, 151–162. doi: 10.1016/j.neurobiolaging.2017.06.024
Tass, P., Rosenblum, M. G., Weule, J., Kurths, J., Pikovsky, A., Volkmann, J., et al. (1998). Detection of n:m phase locking from noisy data: application to magnetoencephalography. Phys. Rev. Lett. 81, 3291– 3294. doi: 10.1103/physrevlett.81.3291
Thom, M., Liu, J. Y. W., Thompson, P., Phadke, R., Narkiewicz, M., Martinian, L., et al. (2011). Neurofibrillary tangle pathology and Braak staging in chronic epilepsy in relation to traumatic brain injury and hippocampal sclerosis: a post-mortem study. Brain 134, 2969–2981. doi: 10.1093/brain/awr209
Tort, A. B. L., Komorowski, R. W., Eichenbaum, H., and Kopell, N. (2010). Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J. Neurophysiol. 104, 1195–1210. doi: 10.1152/jn.00106.2010
Tort, A. B. L., Komorowski, R. W., Manns, J. R., Kopell, N. J., and Eichenbaum, H. (2009). Theta-γ coupling increases during the learning of item-context associations. Proc. Natl. Acad. Sci. U S A 106, 20942–20947. doi: 10.1073/pnas.0911331106
Tort, A. B. L., Kramer, M. A., Thorn, C., Gibson, D. J., Kubota, Y., Graybiel, A. M., et al. (2008). Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc. Natl. Acad. Sci. U S A 105, 20517–20522. doi: 10.1073/pnas.0810524105
Tulving, E. (2002). Episodic memory: from mind to brain. Annu. Rev. Psychol. 53, 1–25. doi: 10.1146/annurev.psych.53.100901.135114
van der Hiele, K., Vein, A. A., van der Welle, A., van der Grond, J., Westendorp, R. G., Bollen, E. L., et al. (2007). EEG and MRI correlates of mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 28, 1322–1329. doi: 10.1016/j.neurobiolaging.2006.06.006
van Karnebeek, C. D., Hartmann, H., Jaggumantri, S., Bok, L. A., Cheng, B., Connolly, M., et al. (2012). Lysine restricted diet for pyridoxine-dependent epilepsy: first evidence and future trials. Mol. Genet. Metab. 107, 335–344. doi: 10.1016/j.ymgme.2012.09.006
Vanderwolf, C. (1969). Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr. Clin. Neurophysiol. 26, 407–418. doi: 10.1016/0013-4694(69)90092-3
Varela, F., Lachaux, J., Rodriguez, E., and Martinerie, J. (2001). The brainweb: phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2, 229–239. doi: 10.1038/35067550
Varga, E., Juhász, G., Bozsó, Z., Penke, B., Fülöp, L., and Szegedi, V. (2014). Aβ1-42 enhances neuronal excitability in the CA1 via NR2B subunit-containing NMDA receptors. Neural Plast. 2014:584314. doi: 10.1155/2014/584314
Vinck, M., Bos, J. J., Van Mourik-Donga, L. A., Oplaat, K. T., Klein, G. A., Jackson, J. C., et al. (2016). Cell-type and state-dependent synchronization among rodent somatosensory, visual, perirhinal cortex, and hippocampus CA1. Front. Syst. Neurosci. 29:187. doi: 10.3389/fnsys.2015.00187
Vinogradova, O. S. (2001). Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus 11, 578–598. doi: 10.1002/hipo.1073
Visser, P. J., Verhey, F., Knol, D. L., Scheltens, P., Wahlund, L. O., Freund-Levi, Y., et al. (2009). Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. J. Alzheimers Dis. 8, 619–627. doi: 10.1016/S1474-4422(09)70139-5
Vogt, D. L., Thomas, D., Galvan, V., Bredesen, D. E., Lamb, B. T., and Pimplikar, S. W. (2011). Abnormal neuronal networks and seizure susceptibility in mice overexpressing the APP intracellular domain. Neurobiol. Aging 32, 1725–1729. doi: 10.1016/j.neurobiolaging.2009.09.002
Volgushev, M., Chistiakova, M., and Singer, W. (1998). Modification of discharge patterns of neocortical neurons by induced oscillations of the membrane potential. Neuroscience 83, 15–25. doi: 10.1016/s0306-4522(97)00380-1
Vossel, K. A., Beagle, A. J., Rabinovici, G. D., Shu, H., Lee, S. E., Naasan, G., et al. (2013). Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol. 70, 1158–1166. doi: 10.1001/jamaneurol.2013.136
Voytek, B., D’Esposito, M., Crone, N., and Knight, R. T. (2013). A method for event-related phase/amplitude coupling. Neuroimage 64, 416–424. doi: 10.1016/j.neuroimage.2012.09.023
Wang, X. J. (2010). Neurophysiological and computational principles of cortical rhythms in cognition. Physiol. Rev. 90, 1195–1268. doi: 10.1152/physrev.00035.2008
Wang, J., Fang, Y., Wang, X., Yang, H., Yu, X., and Wang, H. (2017). Enhanced γ activity and cross-frequency interaction of resting-state electroencephalographic oscillations in patients with Alzheimer’s disease. Front. Aging Neurosci. 9:243. doi: 10.3389/fnagi.2017.00243
Wang, B., and Meng, L. (2016). Functional brain network alterations in epilepsy: a magnetoencephalography study. Epilepsy Res. 126, 62–69. doi: 10.1016/j.eplepsyres.2016.06.014
Womelsdorf, T., Fries, P., Mitra, P. P., and Desimone, R. (2006). γ-band synchronization in visual cortex predicts speed of change detection. Nature 439, 733–736. doi: 10.1038/nature04258
Wood, E. R., Dudchenko, P. A., Robitsek, R. J., and Eichenbaum, H. (2000). Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron 27, 623–633. doi: 10.1016/s0896-6273(00)00071-4
Wulff, P., Ponomarenko, A. A., Bartos, M., Korotkova, T. M., Fuchs, E. C., Bähner, F., et al. (2009). Hippocampal theta rhythm and its coupling with γ oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc. Natl. Acad. Sci. U S A 106, 3561–3566. doi: 10.1073/pnas.0813176106
Xu, X., Liu, C., Li, Z., and Zhang, T. (2015). Effects of hydrogen sulfide on modulation of theta-γ coupling in hippocampus in vascular dementia rats. Brain Topogr. 28, 879–894. doi: 10.1007/s10548-015-0430-x
Yener, G. G., and Basar, E. (2013). Biomarkers in Alzheimer’s disease with a special emphasis on event-related oscillatory responses. Suppl. Clin. Neurophysiol. 62, 237–273. doi: 10.1016/b978-0-7020-5307-8.00020-x
Zhang, X., Zhong, W., Brankačk, J., Sascha, W., Weyer, U., Müller, C., et al. (2016). Impaired theta-γ coupling in APP-deficient mice. Sci. Rep. 6:21948. doi: 10.1038/srep21948
Zheng, C., Bieri, K. W., Hsiao, Y. T., and Colgin, L. L. (2016). Spatial sequence coding differs during slow and fast γ rhythms in the hippocampus. Neuron 89, 398–408. doi: 10.1016/j.neuron.2015.12.005
Zheng, C., and Zhang, T. (2013). Alteration of phase-phase coupling between theta and γ rhythms in a depression-model of rats. Cogn. Neurodyn. 7, 167–172. doi: 10.1007/s11571-012-9225-x
Keywords: Alzheimer’s disease, temporal lobe epilepsy, memory, electroencephalography, oscillatory activity, coherent analysis, early diagnostics
Citation: Kitchigina VF (2018) Alterations of Coherent Theta and Gamma Network Oscillations as an Early Biomarker of Temporal Lobe Epilepsy and Alzheimer’s Disease. Front. Integr. Neurosci. 12:36. doi: 10.3389/fnint.2018.00036
Received: 25 February 2018; Accepted: 30 July 2018;
Published: 27 August 2018.
Edited by:
Olivier A. Coubard, CNS-Fed, FranceReviewed by:
Benjamin Cretin, Hôpitaux Universitaires de Strasbourg, FranceVinay V. Parikh, Temple University, United States
Copyright © 2018 Kitchigina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valentina F. Kitchigina, dmtpdGNoaWdpbmFAZ21haWwuY29t
 Valentina F. Kitchigina
Valentina F. Kitchigina