
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Integr. Neurosci. , 23 May 2018
Volume 12 - 2018 | https://doi.org/10.3389/fnint.2018.00018
Chronic pain can result from many pain syndromes including complex regional pain syndrome (CRPS), phantom limb pain and chronic low back pain, among others. On a molecular level, chronic pain syndromes arise from hypersensitization within the dorsal horn of the spinal cord, a process known as central sensitization. Central sensitization involves an upregulation of ionotropic and metabotropic glutamate receptors (mGluRs) similar to that of long-term potentiation (LTP). Regions of the brain in which LTP occurs, such as the amygdala and hippocampus, are implicated in fear- and memory-related brain circuity. Chronic pain dramatically influences patient quality of life. Individuals with chronic pain may develop pain-related anxiety and pain-related fear. The syndrome also alters functional connectivity in the default-mode network (DMN) and salience network. On a cellular/molecular level, central sensitization may be reversed through degradative glutamate receptor pathways. This, however, rarely happens. Instead, cortical brain regions may serve in a top-down regulatory capacity for the maintenance or alleviation of pain. Specifically, the medial prefrontal cortex (mPFC), which plays a critical role in fear-related brain circuits, the DMN, and salience network may be the driving forces in this process. On a cellular level, the mPFC may form new neural circuits through LTP that may cause extinction of pre-existing pain pathways found within fear-related brain circuits, the DMN, and salience network. In order to promote new LTP connections between the mPFC and other key brain structures, such as the amygdala and insula, we propose a holistic rehabilitation program including cognitive behavioral therapy (CBT) and revolving around: (1) cognitive reappraisals; (2) mindfulness meditation; and (3) functional rehabilitation. Unlike current medical interventions focusing upon pain-relieving medications, we do not believe that chronic pain treatment should focus on reversing the effects of central sensitization. Instead, we propose here that it is critical to focus on non-invasive efforts to promote new neural circuits originating from the mPFC.
Recent advances in brain imaging techniques have made it possible to devise explanatory mechanisms for the development and maintenance of chronic pain. Amongst many other medical achievements, magnetic resonance imaging (MRI) is used to visualize the anatomical integrity of the spine and its associated structures. In particular, MRI has become an imaging technique used to verify clinical presentations for disc herniation. Despite this advance in the ability to visualize nervous system structures with high clarity, questions remain as to whether MRI findings coincide with clinical pain pathology and whether MRI has value in the diagnosis and therapeutic management of chronic pain (Davis et al., 2017). For example, early work in asymptomatic individuals used MRI to examine the lumbar spine (Jensen et al., 1994). Despite having no back pain, approximately 50% of the subjects showed bulges in at least one disc while roughly 25% had disc protrusions. This study demonstrated that structural abnormalities may, in fact, be present in the absence of pain symptoms. What accounts for such discrepancies between anatomical findings vs. clinical presentations? Why do some individuals with similar MRI findings report pain while others do not?
This article reviews and examines the physiological and psychological mechanisms underlying the development of chronic pain. We first describe neuronal alterations from the peripheral nervous system to the central nervous system, addressing both the spinal cord and higher cognitive brain regions. In addition, we offer a holistic mind-body approach to treating chronic pain, and end with a hypothesis to guide future investigation.
The neural correlates of chronic pain are highly complex, involving multiple structures and molecular and cellular changes within the central and peripheral nervous system. Prior work suggests that the neural correlates underlying chronic pain may be explained through the mechanism of central sensitization, referring to hyper-sensitization of the central nervous system in response to both noxious and innocuous stimuli that can result in pain (Latremoliere and Woolf, 2009; Woolf, 2011). When central sensitization takes place, prolonged peripheral nociceptive input results in the excitatory release of chemicals, triggering a transduction cascade. Multiple protein kinases phosphorylate the three ionotropic glutamate receptors, N-methyl-D-aspartate receptors (NMDAR), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR) and kainite receptors, thereby increasing receptor activity and density. This process results in excitatory postsynaptic potentials reaching the dorsal horn of the spinal cord (Ballantyne, 2006; Ultenius et al., 2006; Latremoliere and Woolf, 2009). Phosphorylation of AMPAR facilitates AMPAR insertion in the synapse (Esteban et al., 2003; Galan et al., 2004). In addition to the contribution of the three ionotropic glutamate receptors, metabotropic glutamate receptors (mGluRs) contribute to both presynaptic and postsynaptic neuronal excitability (Anwyl, 2009; Niswender and Conn, 2010), often through the activation of second messenger pathways. Recent evidence also indicates that increased neuronal excitability in the dorsal horn is attributed to GABA disinhibition through the potassium-chloride cotransporter type 2, KCC2 (Kahle et al., 2014). In this process, decreased KCC2 function leads to an increase in intracellular Cl− concentration, shifting the reversal potential of Cl− channels in GABAA receptors beyond the threshold for action potential generation. Postsynaptic neurons thus become depolarized (rather than hyperpolarized) and the resulting decrease in the overall inhibitory function of GABAA receptors facilitates central sensitization (Price et al., 2009; Kahle et al., 2014).
Another mechanism for neuronal excitability is through the action of G protein-coupled receptors (GPCRs), a diverse family of cell receptor proteins that are dispersed throughout the peripheral and central nervous system. GPCRs are located on the plasma membranes and nerve terminals of sensory neurons along nociceptive pain pathways (Pan et al., 2008). Nociceptor hyperexcitability, which is believed to contribute to the transition from acute to chronic pain, is associated with a change in GPCR signaling (Dina et al., 2009). Moreover, long-term synaptic remodeling results from the dynamic processes of protein synthesis and degradation (Alvarez-Castelao and Schuman, 2015). Theoretically, maladaptive nociceptive input mediated by GPCR signaling could normalize over time as a result of protein degradation and synthesis, thereby providing hope that chronic pain symptoms may abate. Currently, however, it is difficult to quantify GPCR turnover rate (Ross, 2014), and thus, the possibility that rapid turnover of sensory receptors could potentially reverse chronic pain symptoms requires further investigation.
Persistent pain sensations are not only modulated by neurons but also by glial cells, specifically astrocytes and microglia. Increased astroglial activity increases the release of excitatory neurochemicals, such as proinflammatory cytokines and precursors to glutamate (Broer et al., 2004; Guo et al., 2007; Milligan and Watkins, 2009; Chiang et al., 2011), and can affect chronic pain by its influence on nociceptive input and the recycling of glutamate (Guo et al., 2007; Milligan and Watkins, 2009). By converting extracellular glutamate into glutamine, astroglial cells provide presynaptic neurons with the raw ingredients to continue producing glutamate, an excitatory neurotransmitter (Broer et al., 2004; Chiang et al., 2011). In addition, astroglial activity produces pro-inflammatory cytokines which, in turn, increase nociceptor activity (Zhang and An, 2007; Uçeyler et al., 2009). Microglial cell activation also mediates neuronal excitability by the reversal of the inhibitory effect of GABA. Following neuronal injury, activated microglia release brain-derived neurotrophic factor which downregulates KCC2 in the dorsal horn, thereby facilitating the process of central sensitization through GABA excitation, as described above (Coull et al., 2005; Latremoliere and Woolf, 2009; Price et al., 2009; Taves et al., 2013). Further research into the excitatory role of glial cells may provide very useful information for the treatment of chronic pain syndromes. Unfortunately, glial cells are distributed throughout the central nervous system and serve many functions. As a result, treatment targeting glial cells has a high probability of causing adverse side effects.
Regardless of glial cell activation and recycling of glutamate via the glutamate-glutamine shuttle, ionotropic and mGluRs undergo degradative pathways. Research has shown that degradative pathways exist for NMDAR (Scott et al., 2004; Piguel et al., 2014), AMPAR (Ehlers, 2000; Barry and Ziff, 2002), kainate receptors (Martin and Henley, 2004; Lerma and Marques, 2013) and mGluRs (Latremoliere and Woolf, 2009; Klein et al., 2015). Hence, critical receptors involved in central sensitization undergo degradation (see Figure 1). Theoretically, then, biological mechanisms already exist that can reverse central sensitization via glutamate receptor degradation. If receptor degradation were the primary mechanism by which central sensitization were reversed, chronic pain should abate with time. Yet this is not always the case. One can argue, though, that increased astroglial activity can upregulate ionotropic or mGluRs and nociceptors (see Figure 1), canceling out any putative effect of glutamate receptor degradation. This is a valid point that will require further research in the future. Given that central sensitization does not simply reverse with time, it appears that astroglial activity facilitates central sensitization at a greater rate than receptor degradation reverses the process of central sensitization (Figure 1).
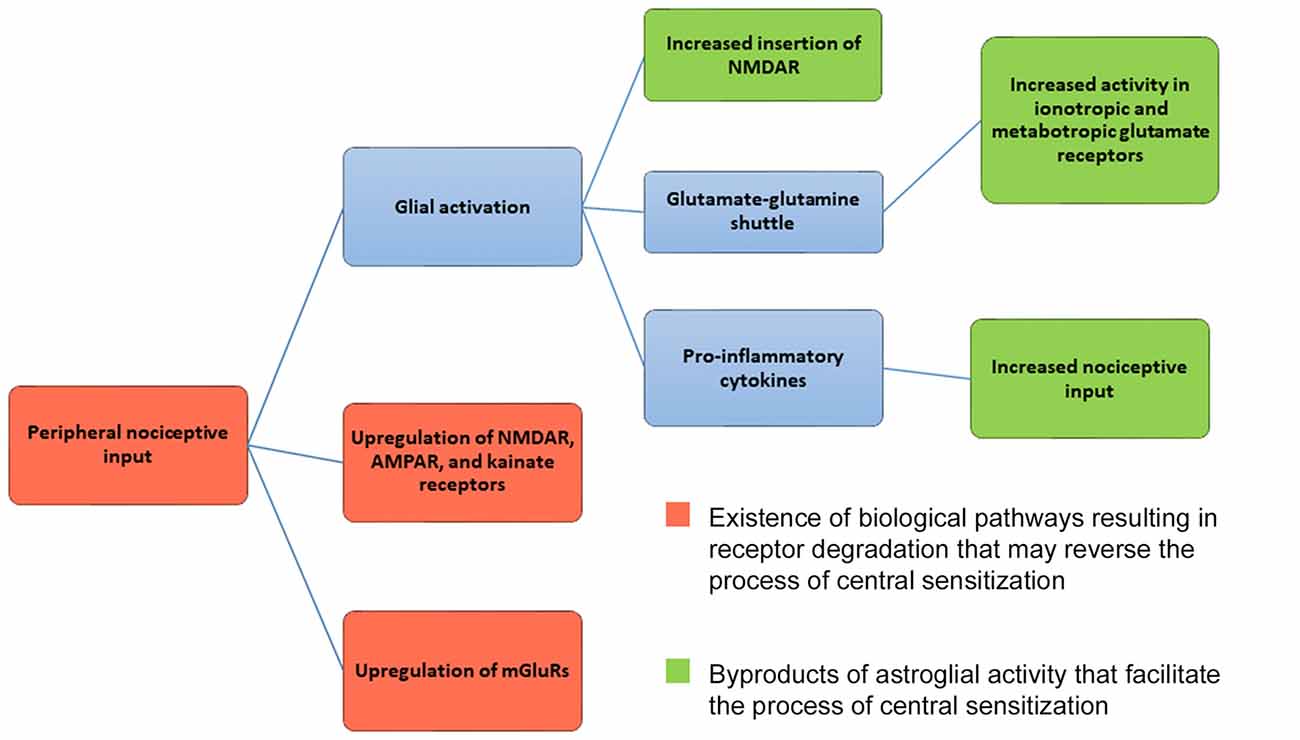
Figure 1. Mechanism for central sensitization. Central sensitization (central nervous system hypersensitivity) is initiated from the upregulation of ionotropic glutamate receptors (NMDAR, AMPAR, kainite receptors) and metabotropic glutamate receptors (mGluRs) in the presence of peripheral nociceptive input. As a result, neurons in the dorsal horn of the spinal cord and central nervous system respond to nociceptive input at lower thresholds, with new enlarged receptor fields, and undergo increased rates of spontaneous firing. Glial activation can further maintain the mechanisms underlying central sensitization by increasing NMDAR and AMPAR insertion in postsynaptic membranes. Glial cells release pro-inflammatory cytokines, serving as further nociceptive input. Astroglial cells also help to maintain glutamate levels via the glutamate-glutamine shuttle, which can influence both ionotropic and mGluR activity. As shown in red, ionotropic, metabotropic receptors and nociceptors are capable of being degraded. Given that degradative pathways exist, the process of central sensitization can be reversed. Activity by astroglial cells, however, may mitigate the effects of receptor degradation by upregulating and facilitating the process of central sensitization. Given that central sensitization does not reverse itself with time, it seems that astroglial activity overpowers the existence of degradative receptor pathways.
Evidence suggests that central sensitization is characterized by heterosynaptic facilitation (Ji et al., 2003; Malenka and Bear, 2004; Rygh et al., 2005; Latremoliere and Woolf, 2009; Galvan et al., 2011). Central sensitization is distinct from the phenomenon known as windup, which involves homosynaptic facilitation (Latremoliere and Woolf, 2009; Woolf, 2011). Windup refers to the increased magnitude of incoming C-fiber signals at the dorsal neurons due to the increased frequency of C-fiber activation (Li et al., 1999; Campbell and Meyer, 2006), and is associated with primary hyperalgesia (increased pain at the site of injury; Ikeda et al., 2006; Sandkühler, 2007; Latremoliere and Woolf, 2009). When central sensitization has taken place, neurons at the dorsal horn respond at a lower threshold to peripheral inputs, have increased receptive fields, and have increased rates of spontaneous firing. These structural and functional alterations help to explain why patients with chronic pain experience allodynia (pain response in the absence of a painful stimulus) and secondary hyperalgesia (pain outside the site of injury; Campbell and Meyer, 2006; Latremoliere and Woolf, 2009; Woolf, 2011).
This process appears very similar to the molecular and cellular changes that occur during long-term potentiation (LTP) in cortical and subcortical brain regions (Ji et al., 2003; Latremoliere and Woolf, 2009). Considering the strong parallels between central sensitization and LTP, it is reasonable to infer that similar (although perhaps not identical) processes are occurring in spinal structures and in cortical/subcortical regions that drive the move from acute to chronic pain. Interestingly, LTP in both the amygdala (Dityatev and Bolshakov, 2005; Sigurdsson et al., 2007) and hippocampus (Whitlock et al., 2006) has long been associated with fear conditioning. Moreover, chronic pain and fear conditioning are intricately connected (Turk and Wilson, 2010; Elsenbruch and Wolf, 2015), suggesting that spinal pain-related circuits and cortical/subcortical fear circuits may undergo some of the same neural alterations (or at the very least, similar parallel processes) during the transition from acute to chronic pain. Thus, we will now describe some of the cellular and behavioral mechanisms in fear conditioning, which will assist with understanding how to better treat chronic pain.
Pain-related fear promotes maladaptive cognitive and behavioral responses (Vlaeyen and Linton, 2000; Turk and Wilson, 2010; Parr et al., 2012; Elsenbruch and Wolf, 2015; Vlaeyen, 2015), best characterized by a fear-avoidance model (Leeuw et al., 2007). In this model, pain perception begins with catastrophization, the belief that pain symptoms are indicative of a far worse injury, in turn resulting in pain-related fear and pain-related anxiety. Importantly, pain-related fear and anxiety are not synonymous and appear to be two distinct phenomena, as fear-induced defensive responses and anxiety-related responses are mediated by different neural circuits (LeDoux, 2015). We argue that pain-related fear can stem directly from threat perception; if an individual views an ongoing activity or present object as threatening, that individual will actively attempt to escape from that situation. Pain-related anxiety, on the other hand, can result from the anticipation of pain; if anticipating future painful events, an individual will actively avoid that event or behavior. Through both escape and avoidance, then, chronic pain patients are likely to not use the painful body area and perhaps avoid activity altogether.
How do chronic pain patients acquire pain-related fear and then in turn pain-related anxiety? These processes appear to be the direct result of fear conditioning and avoidance learning. Pavlovian fear conditioning occurs due to the association between a previously neutral stimulus and an aversive stimulus that leads to an unpleasant response (Milad and Quirk, 2012; Elsenbruch and Wolf, 2015). In the case of conditioned fear of pain, the conditioned stimulus (CS) may include both interoceptive conditioning (IC) and functional movement (De Peuter et al., 2011). Interoception, as used here, does not only refer to sensation within the viscera, but also to somatosensory and nociceptive sensation. Because pain acts as an aversive stimulus, pain-related fear can be acquired through Pavlovian fear conditioning. When this is the case, an individual with chronic pain can reverse the process through extinction of the conditioned fear (Quirk et al., 2010; Milad and Quirk, 2012). In order for fear extinction to take place, two events must take place simultaneously or in very close temporal proximity: (1) the fear association must be active while; (2) a new non-painful stimulus is introduced (Quirk et al., 2010). Importantly, extinction is not synonymous with forgetting. Rather, extinction occurs when a new association either inhibits the original fear association or alters the affective and mnemonic properties of that association (Bouton, 2004; Craske et al., 2008; Radulovic and Tronson, 2010; De Peuter et al., 2011; Kattoor et al., 2013).
Fear-associated behaviors, however, do not only arise through Pavlovian conditioning, but also through operant conditioning (Bouton and Todd, 2014; Trask and Bouton, 2014). According to the two-factor theory and related models of maladaptive fear-related behaviors, Pavlovian conditioning facilitates fear acquisition while operant conditioning results in negative reinforcement that maintains avoidance and escape behaviors over time (Krypotos et al., 2015). In standard fear-avoidance models (Leeuw et al., 2007), initial escape behaviors are negatively reinforced due to the immediate reduction of pain. Avoidance behaviors then become associated with lack of pain altogether, and this continuation of negative reinforcement prolongs the appearance of those behaviors. Of particular relevance for our theory, avoidance learning may have a greater and more enduring effect of overall threat reduction when compared with extinction (Boeke et al., 2017), and negatively reinforced escape behaviors can persist even when the initial classically conditioned CS-US pairing is extinguished (Krypotos et al., 2015). It is also possible that the persistence of an avoidance behavior can prevent the acquisition of knowledge that a CS no longer signals the presence of a noxious US, thus preventing extinction of the CS-US bond. By actively controlling when and how to use a painful body area, chronic pain patients may be inadvertently driving inherent mechanisms of threat reduction and continuing with a pattern of learned avoidance behavior that may no longer be necessary.
Three primary brain structures have been associated with the fear conditioning process: the medial prefrontal cortex (mPFC), the amygdala and the hippocampus (Phelps et al., 2004; Phelps and LeDoux, 2005; Milad et al., 2007; Herry et al., 2010; Pape and Pare, 2010). How do we know that extinction does not erase fear memories within these structures and that alternate associations are learned during extinction? Behavioral studies have shown that fear associations return after fear extinction has taken place (Morris and Bouton, 2007; Milad and Quirk, 2012), suggesting that fear memories are not erased but rather are inhibited or altered by new memories and associations. Further, it is unlikely that the cellular and synaptic changes associated with fear extinction occur as a result of long-term depression (LTD), as prefrontal LTD results in increased fear recovery after extinction (Herry and Garcia, 2002; Courtin et al., 2013). Therefore, it appears as if the primary driving mechanism for the extinction of conditioned fear is the establishment of new synaptic connections through LTP, leading to formation of new associations that dampen the effects of the original fear memory.
As mentioned above, central sensitization is a critical factor in the development of chronic pain syndromes, including (but not limited to) phantom limb pain, complex regional pain syndrome (CRPS), musculoskeletal pain and post-surgical pain (Woolf, 2011). In order for central sensitization to be reversed, there must be a downregulation of ionotropic/mGluRs and nociceptors. However, if pain relief follows a similar mechanism to the extinction of conditioned fear, then central sensitization may not need to be reversed in order for an individual to experience pain resolution. Similar to the extinction of conditioned fear, it may be possible to bypass a necessary reversal of central sensitization by establishing new neural circuits that inhibit well-established pain associations. Are these new inhibitory associations formed exclusively between the mPFC, amygdala and hippocampus as is seen in fear conditioning? There is abundant evidence that the anterior cingulate cortex (ACC) and the insula are strongly involved in the perception and integration of various pain signals (Tracey and Mantyh, 2007; Lee and Tracey, 2010; Wiech et al., 2010; Segerdahl et al., 2015). Perhaps these regions are also critically involved in the abatement of chronic pain. Future studies should address these critical questions. Moreover, it is conceivable that the default mode network (DMN), described below, may play a key mediating role in pain processing in chronic pain syndromes.
Research designs utilizing functional MRI (fMRI) to examine the functional neural correlates of chronic pain can be categorized into three primary groups: (1) pain-induction trials; (2) resting state, in which no active cognitive or sensorimotor task is performed; and (3) active tasks that require perceptual, sensorimotor, or other cognitive and behavioral processes. The latter category may also include simple motor tasks, such as finger tapping, to serve as control or comparison conditions. During a resting state, researchers hope to identify underlying brain mechanisms that persist in chronic pain patients even after a painful stimulus is removed. The brain regions showing increased activation during wakeful rest, when compared with task-active states, have been described as the brain’s DMN (Raichle et al., 2001).
Researchers frequently investigate the DMN using three processes that can be compared to one another: (1) task-free or task-independent trials in which the subject is not assigned any stimulus or task; (2) simple sensorimotor and cognitive tasks; and (3) complex sensorimotor and cognitive tasks. By comparing either task-free designs or simple sensorimotor tasks to complex cognitive tasks, researchers can examine how brain regions in the DMN change from a resting state to a task-activated state.
Structures associated with the DMN include the mPFC, medial temporal lobes (including the hippocampus) and posterior cingulate cortex (PCC; Buckner et al., 2008; Harrison et al., 2008; Greicius et al., 2009; Sheline et al., 2009). Increased DMN activity is associated with self-referential thoughts, future planning, and autobiographical memory (Gusnard et al., 2001; Buckner et al., 2008; Peeters et al., 2015). Interestingly, chronic pain is associated with dysregulation within the DMN (Baliki et al., 2008, 2014; Otti et al., 2013), and this dysregulation may help to explain the disease processes of chronic pain syndromes.
The medial frontal lobe, including the ACC, serves many different roles including error detection (Holroyd et al., 2002), conflict monitoring (Botvinick et al., 2004), and other aspects of executive functioning and cognitive control (Ridderinkhof et al., 2004; Posner et al., 2007; Alexander and Brown, 2010). It is also implicated in the storage of fear memories and retrieval of those memories (Quinn et al., 2008). Functional subregions within mPFC include its dorsal (dmPFC) and ventral (vmPFC) components. The dmPFC appears to mediate action-related activity, in part by exerting top-down control on the motor cortex and inhibiting motor output (Narayanan and Laubach, 2006). The vmPFC is heavily implicated in emotional regulation (Euston et al., 2012). Together, these regions play a critical role in both the DMN and salience network, and individual differences in autonomic reactivity may correspond to functional connectivity of mPFC to other brain areas (Jennings et al., 2016). The ACC, although not part of the DMN, is activated along with other areas of mPFC during fear appraisal, but not necessarily during fear learning (Maier et al., 2012), and is an integral structure of the salience network, along with the insula (Sridharan et al., 2008; Bonnelle et al., 2012). In the case of acute nociceptive input, the anterior insula has been shown to integrate sensory information to help determine whether or not a stimulus is painful (Wiech et al., 2010), while the contralateral dorsal posterior insula has been associated with tracking the intensity of an applied noxious stimulus (Segerdahl et al., 2015). The conversion of acute pain-induced fear associations into long-lasting memories is associated with changes in functional connectivity of mPFC that are also related to self-reported anxiety levels (Tseng et al., 2017). It is important to note that the dorsolateral prefrontal cortex (dlPFC)—long known for its role in working memory, attention and inhibition—is also critical for emotional information processing (Etkin et al., 2006; Shafritz et al., 2006; Urry et al., 2006) and pain perception (Lorenz et al., 2003; Brighina et al., 2011).
If vmPFC communicates with the ACC, a key structure in the salience network during fear appraisal, then vmPFC may be able to downregulate salient pain signals. Studies have shown that pain-related fear correlates positively with pain intensity and disability (Crombez et al., 1999; Al-Obaidi et al., 2005; Gheldof et al., 2006). In addition, individuals who claim to have a higher pain-sensitivity level display increased activation in regions of PFC and ACC in response to acute pain compared with individuals with low pain sensitivity (Coghill et al., 2003). Furthermore, acceptance-based therapies have the potential to alter activation of PFC in response to pain (Jensen et al., 2012) and to alter functional connectivity between PFC and emotion-processing regions (Young et al., 2017). Importantly, therapies emphasizing acceptance of pain are associated with clinically-meaningful symptom reduction (Vowles et al., 2007a,b; Vowles and McCracken, 2008). It is not unreasonable to suggest that pain intensity and disability in chronic pain patients can be greatly reduced through top-down control of the mPFC on the DMN, salience network, and fear association network. Thus, chronic pain patients may have the ability to alter both brain activity and connectivity by altering thoughts related to pain perception, as described in a later section of this article.
There has been much speculation as to the significance and implications of DMN activity. Mason et al. (2007) believe that activity within the DMN during task-free (stimulus-independent) thoughts is a reflection of mind wandering. Others have countered that it is impossible to decipher whether resting-state brain activity is due to stimulus-independent thoughts (mind-wandering) or due to stimulus-dependent thoughts (Gilbert et al., 2007), particularly when DMN activation is observed when a simple task is compared with a more cognitively demanding task (McKiernan et al., 2003). In order to determine the difference between mind-wandering and either IC or hyperfixation/hypervilance (two examples of stimulus-dependent thoughts), researchers can use fMRI to assess chronic pain patients while at rest and while the patients actively monitor their symptoms, perhaps by alternating blocks of rest with active pain monitoring. Such a study will help to differentiate between stimulus-independent thoughts and pain stimulus-dependent thoughts. At the moment, we can hypothesize that dysregulation of the DMN in chronic pain results from stimulus-dependent thoughts focused around IC and pain perception.
Thus far, we have highlighted the critical importance of the mPFC in the development and maintenance of chronic pain. Research on chronic pain patients, however, has revealed altered activity and cortical reorganization within other brain regions. Before we examine specific chronic pain syndromes, we must first take into consideration two confounding factors when interpreting neuroimaging studies of chronic pain. First, for pain studies that require the application of noxious stimuli, one must discern whether the fMRI results are due to somatosensory sensation or due to pain sensation. Moulton and associates (Moulton et al., 2012) found that BOLD responses might not necessarily reflect pain but rather somatosensory input and integration, because activation of somatosensory and motor cortices correlated more with heat intensity than with heat-induced pain. On the other hand, fMRI can be used to discern thermal induced pain from heat sensation and social pain (Wager et al., 2013). It is important to note that in this study, the specificity and sensitivity between thermal-induced pain and social pain was markedly lower than the specificity and sensitivity between thermal induced pain and heat sensation. Thus, when examining fMRI results, it may be harder to differentiate physical pain from emotional pain. This issue highlights the intricate relationship between physiological pain and pain perception, including social aspects of pain.
Second, individuals suffering from chronic pain have usually undergone numerous interventional treatments and have unique personal experiences owing to the specific features of their chronic pain syndrome. Both of these factors have the potential to serve as major methodological confounds and/or contribute to individual differences. Further, cognitions and emotions clearly play a significant role in chronic pain, and daily mood may affect BOLD activation when using fMRI. As a result, no two chronic pain experiences are identical and large variances from individual differences are the norm, thereby providing further difficulty in dissecting the neural mechanisms of chronic pain.
The brainstem contains a collection of structures responsible for a descending pain modulation system, as structures within this region can produce analgesic effects and monitor nociceptive communication. Specifically, the periaqueductal gray-rostral ventromedial medulla (PAG-RVM) system allows for the bidirectional control of pronociception (nociceptive facilitation) and antinociception (nociceptive inhibition; Tracey and Mantyh, 2007; Heinricher et al., 2009). Chronic pain may arise from a dysregulation of nociceptive pathways, in particular an enhancement of pronociception or a reduction of antinociception (Heinricher et al., 2009). Within the RVM there are three types of neurons: ON, OFF and NEUTRAL cells. Evidence suggests that ON cells promote pronociception while OFF cells facilitate antinociception (Kincaid et al., 2006; Heinricher et al., 2009; Ossipov et al., 2010; Staud, 2013). Additionally, the dorsal reticular nucleus (DRt) and ventrolateral medulla (VLM) play a critical role in descending nociceptive control with the Drt facilitating nociception and the VLM inhibiting nociception (Heinricher et al., 2009). Although the PAG-RVM system combined with the Drt and VLM contribute to the development of chronic pain, these structures are both directly and indirectly modulated by the top-down influence of cortical and subcortical structures within the salience and fear networks, such as mPFC, ACC, amygdala, insula and hypothalamus (Tracey and Mantyh, 2007; Heinricher et al., 2009; Lee and Tracey, 2010). Thus, it appears that pain perception in general is modulated by higher cortical brain regions, which in turn can modify descending nociceptive pathways.
As mentioned previously, chronic pain can result from a variety of pain-related syndromes. Below, we provide neuroimaging and treatment results for a few of these syndromes, focusing upon the similarities and distinctions among the syndromes that have the potential to inform models of the brain mechanisms of chronic pain. Theoretically, these mechanisms may exert a top-down influence on the nociceptive modulation system in cases of dysregulation within the brainstem. Additional research must be conducted investigating the role of cognitions and emotions as they relate to cortical/subcortical regulation of brainstem structures, and fMRI is well-suited for that level of investigation. Such research may provide, for example, brain imaging evidence of the therapeutic effects of cognitive and acceptance based therapies in chronic pain management.
Phantom limb pain is described as pain occurring in a lost or amputated limb due to referred sensation from neighboring bodily regions (Nikolajsen and Jensen, 2001). Symptoms of phantom limb pain include but are not limited to burning, itching, tingling, electrical sensations, cramping, and muscle spasms (Flor, 2002). The neural mechanisms resulting in phantom limb pain mirror that of central sensitization (Woolf et al., 1992; Subedi and Grossberg, 2011; Woolf, 2011). CRPS, previously known as reflex sympathetic dystrophy (RSD), is a pain disorder characterized by allodynia, swelling, dysautonomia and temperature changes that cannot be attributed to another physiological disorder (Jänig and Baron, 2002; Marinus et al., 2011). Traumatic injury has been described as one of the leading causal factors resulting in CRPS, occurring in 30%–77% of presenting patients (McBride and Atkins, 2005). These symptoms, however, may spread from one part of the body, such as the right upper arm, to another distal extremity like the lower left leg. Similar to phantom limb pain, CRPS appears to be facilitated by the process of central sensitization (Bruehl, 2010; Woolf, 2011).
Theoretically, the symptoms of phantom limb pain and CRPS should reverse through NMDAR antagonists. If central sensitization facilitates both phantom limb pain and CRPS, downregulation of NMDAR should drastically reduce symptoms. NMDAR antagonists, however, have variable effects on patients with phantom limb pain (Nikolajsen et al., 1996; Huse et al., 2001; Maier et al., 2003; Robinson et al., 2004; Schley et al., 2007) and CRPS (Koffler et al., 2007; Schwartzman et al., 2009; Pickering and McCabe, 2014). In addition, sympathetic blocks that provide a local anesthetic to block incoming pain signals are not a universally successful treatment for CRPS (Cepeda et al., 2002, 2005; Meier et al., 2009; Stanton et al., 2013).
What accounts for the maintenance of both chronic pain disorders, considering that pharmacologic intervention is of limited value? Although the primary culprit may be faulty communication between mPFC and limbic structures, other brain regions have been implicated in both pain syndromes. Phantom limb pain is associated with cortical reorganization within the de-afferated primary motor cortex and S1 (Birbaumer et al., 1997; Lotze et al., 2001; MacIver et al., 2008). Similar to phantom limb pain, CRPS is associated with cortical reorganization of the primary motor cortex (Maihofner et al., 2007; Kirveskari et al., 2010; Pleger et al., 2014) and S1 (Maihofner et al., 2003; Pleger et al., 2014), although some research suggests this is not always the case (Di Pietro et al., 2013a,b). Regardless of cortical changes in S1, there is reason to believe that such alterations do not produce pain (Gustin et al., 2012). Knecht et al. (1998) reported that cortical reorganization normally resulting from amputation of a limb does not necessarily correspond to changes in phantom limb pain perception over time. In the study, over a 4-week period, the overall extent of cortical reorganization and number of sites associated with mislocalization of phantom limb pain remained constant. Mislocalization refers to changes in the location of painful areas of the body after the application of non-noxious stimuli (touch, vibration and heat) and painful stimuli. To the surprise of the researchers, the topography of mislocalized pain sensations had changed in every subject despite no significant changes in cortical reorganization. The results of this study suggest that pain sensation and perception are highly malleable and may not be causally related to cortical reorganization.
Adult-onset CRPS is clinically distinct from childhood-onset CRPS. Unlike in adult-onset CRPS, children with CRPS usually experience full resolution of pain symptoms (Low et al., 2007; Linnman et al., 2013; Weissmann and Uziel, 2016). One study examining childhood-onset CRPS found altered functional connectivity in five key brain structures: amygdala, ACC, caudate, post-central gyrus, and putamen (Linnman et al., 2013). Interestingly, the researchers observed that CRPS children with complete pain resolution still exhibited altered functional connectivity in these brain structures. Similar to cortical reorganization, then, altered functional connectivity and pain perception are not causally connected. Unfortunately, their analysis did not examine functional connectivity of mPFC, a region that would be of interest for pain perception and its relation to conditioned fear. Future studies should examine structural and functional connectivity between mPFC and limbic regions to address the question of whether pain perception in CRPS is related to neural circuitry of fear conditioning, and is perhaps a conditioned phenomenon.
Mirror therapy has been shown to be an effective therapeutic modality for phantom limb pain (Chan et al., 2007; Finn et al., 2017), while motor imagery therapy has been documented to be an effective treatment for CRPS (Moseley, 2004b; Bowering et al., 2013). Treatments targeting motor movement may help to regulate altered cortical representations of the affected limb in the primary motor cortex and S1. In addition, such therapeutic modalities may be effective through changes in proprioceptive representations of limb positioning and body movement within the dorsal visual processing stream, or “where” visual pathway (Preissler et al., 2013). In addition to changes within primary motor cortex, S1, and posterior parietal areas, mirror and motor imagery therapy may also facilitate the growth of new neural circuits that inhibit previously established fear-associated connections. From a classical conditioning standpoint, these therapies may lead to the formation of new CS-US pairings, in which the proprioceptive feedback resulting from the therapy (the CS) is associated with the pain-free state (the US). The CR, then, would be the relief state resulting from being pain free. Through classical conditioning, additional proprioceptive representations can serve as neutral stimuli to become associated with a pain-free state, thereby inhibiting the original pain associations.
As with phantom limb pain and CRPS, chronic back pain (CBP) is associated with central sensitization (Woolf, 2011; Roussel et al., 2013). In addition, CBP results in structural and functional changes in attentional, emotional, and default mode networks (Apkarian et al., 2004; Baliki et al., 2006, 2008; Tagliazucchi et al., 2010; Seminowicz et al., 2011; Hashmi et al., 2013; Zhang et al., 2014). Specifically, CBP is associated with a 5%–11% reduction in overall cortical volume, with symptom duration correlated with amount of gray matter loss (Apkarian et al., 2004). In addition, reduced gray matter density in dlPFC correlates with increased pain severity, while increased dlPFC thickness following surgery or other intervention procedures correlates with a reduction in reported pain (Apkarian et al., 2004; Seminowicz et al., 2011). These studies, however, did not differentiate the temporal divide between pain resolution and increase in dlPFC thickness. Thus, one cannot conclude whether symptoms decreased as a result of increased cortical gray matter density or whether patients achieved pain-free states prior to cortical reorganization. Similar results have been found in hip osteoarthritis (coxarthrosis; Rodriguez-Raecke et al., 2013), such that pain resolution preceded increased dlPFC thickness. Therefore, the relationship between dlPFC thickness and chronic pain may be merely correlational, or perhaps, pain resolution leads to alterations in cortical circuitry.
Interestingly, Hashmi and associates (Hashmi et al., 2013) noted a functional difference between CBP and acute back pain, classified as back pain occurring for less than 3 months. While experiencing increased levels of pain, subjects with acute back pain consistently showed increased activations in the ACC and insula, two key structures within the salience network. Individuals with CBP, however, showed increased activations in the mPFC and amygdala. The results of this study highlight the role of ACC and insula in initial pain detection. In another study, patients with CBP exhibited increased pain levels due to thermal stimulation, and their reported pain levels were positively associated with activation in insular cortex (Baliki et al., 2006). Spontaneous pain in these patients, however, was correlated with activation in mPFC. Thus, in CBP, pain perception in the absence of an overt threatening or noxious stimulus seems to be facilitated through mPFC. It is possible that when acute pain becomes chronic, the mPFC and fear associated structures may begin to play an important mediating role in the perception of pain. Further, dysregulation within this region may help to explain the altered dynamics within DMN observed in patients with CBP (Baliki et al., 2006, 2008; Hashmi et al., 2013).
Why do some individuals present with back pain while others with similar physiological findings do not? Perhaps subjects who do not report back pain are less likely to form pain-related fear whenever a back condition occurs. Alternatively, perhaps pain-free individuals with back bulges do not experience pain because the brain does not detect the disc bulge as being a threat. In a standard fear-avoidance model, pain-related fear involves threat perception (Leeuw et al., 2007). Perhaps the asymptomatic subjects with disc bulges have little to no pain-related fear, which explains their lack of symptoms. This model, however, is speculative and future research is necessary to provide further support for the idea that pain-related fear avoidance is causally associated with verbal report of pain symptoms.
Motor control therapy has been found to be a successful form of treatment for CBP (Macedo et al., 2012; Saragiotto et al., 2016). Similar to therapies for phantom limb pain and CRPS, motor control therapy may help to form new CS-US relationships that may inhibit or alter pre-existing pain and fear associations. Support for this theory comes from prior work showing that in conjunction with motor control therapy, pain physiology education courses can help decrease pain scores in CBP (Moseley, 2004a; Moseley and Butler, 2015). How can these education courses help to inhibit pain and fear associations? From a cognitive-learning perspective, maladaptive thoughts such as, “something must be seriously wrong with me, this pain is indicative of something worse,” may become the CR in response to pain. As discussed earlier, cognitive representations of pain may also serve as an antecedent CS leading to the CR of increased anxiety. Through cognitive remediation, an individual can reframe these thoughts in a more adaptive manner: “I am in pain right now, but from a physiological perspective, I am not in any life-threatening danger.” This cognitive reappraisal may then reduce pain-related fear and pain-related anxiety by preventing negative reinforcement, which in turn will reduce avoidance behaviors.
The molecular mechanisms underlying central sensitization involve glutamate and non-glutamate receptors (Figure 1), and are similar in nature to those of LTP in fear-related circuits. Currently, treatments seeking to alter the molecular mechanisms of central sensitization, such as NMDAR antagonists and nociceptive input, are not universally successful in treating different chronic pain syndromes. Similarly, previously observed cortical and functional alterations have not been causally related to chronic pain. Further, pain resolution in chronic pain syndromes may occur even if cortical and functional alterations are still apparent. Rather than focus on altered cortical thickness or connectivity, we suggest a greater emphasis be placed upon how structures communicate with one another, particularly how the mPFC communicates with other brain structures in the salience network, fear network and the DMN. As demonstrated in patients with CBP, the dlPFC plays a regulatory role in pain perception and may provide inhibitory pathways that suppress pain and fear circuits. However, given the mPFC’s connection to the DMN and fear circuit, along with its projections to the salience network, this brain structure may be of greater importance for pain modulation. Recent pre-clinical evidence indicating that vmPFC activity is required for the effectiveness of extinction-based therapies (Fucich et al., 2018) buttresses the importance of this structure in therapeutic success.
Therefore, to more successfully treat chronic pain conditions, we propose an alternative approach to psychotropic agents that downregulate glutamate and non-glutamate receptors. Instead of attempting to reverse central sensitization occurring at the level of the spinal cord, we suggest the use of a cognitive behavioral program that will foster the development of new synaptic connections between the mPFC and other structures that will, in turn, inhibit LTP within pain and fear circuits (Figure 2). We further propose that the most effective form of chronic pain rehabilitation is through a combination of cognitive reappraisals, mindfulness meditation, and functional rehabilitation (Figure 2). As previously mentioned in the discussion of CBP, cognitive reappraisals may help to reduce pain-related fear and anxiety, which may then reduce avoidance behaviors (see Table 1 for a list of cognitive reappraisals). Indirect evidence exists indicating that cognitive reappraisal can modify central sensitization processes, as cognitive behavioral therapy (CBT) emphasizing pain regulation strategies has been shown to reduce secondary hyperalgesia (Salomons et al., 2014). Evidence also indicates that therapy grounded in cognitive reappraisal and the reduction of catastrophic thoughts increases functional brain connectivity between S1 and anterior insula (Lazaridou et al., 2017) and between the DMN and executive control networks (Kucyi et al., 2016). CBT has also been shown to normalize aberrant functional connectivity in frontal-parietal attentional circuitry in chronic pain patients, with higher connectivity associated with greater reduction in pain intensity ratings (Yoshino et al., 2018). Similarly, in chronic pain patients, CBT has been shown to increase gray matter volume in lateral PFC, the pregenual region of ACC, posterior parietal cortex and somatosensory cortex, accompanied by a reduction in catastrophizing (Seminowicz et al., 2013). Thus, cognitive therapies for pain management have the potential to alter neural circuits related to attention, emotion, and the integration of pain signals. Hence, we hypothesize that cognitive reappraisals may promote the reduction of pain and the extinction of conditioned pain-related fear through top-down regulation of brain regions involved in emotion and sensory processing. This process may be mediated by a general reduction in the negative affective states associated with pain perception.
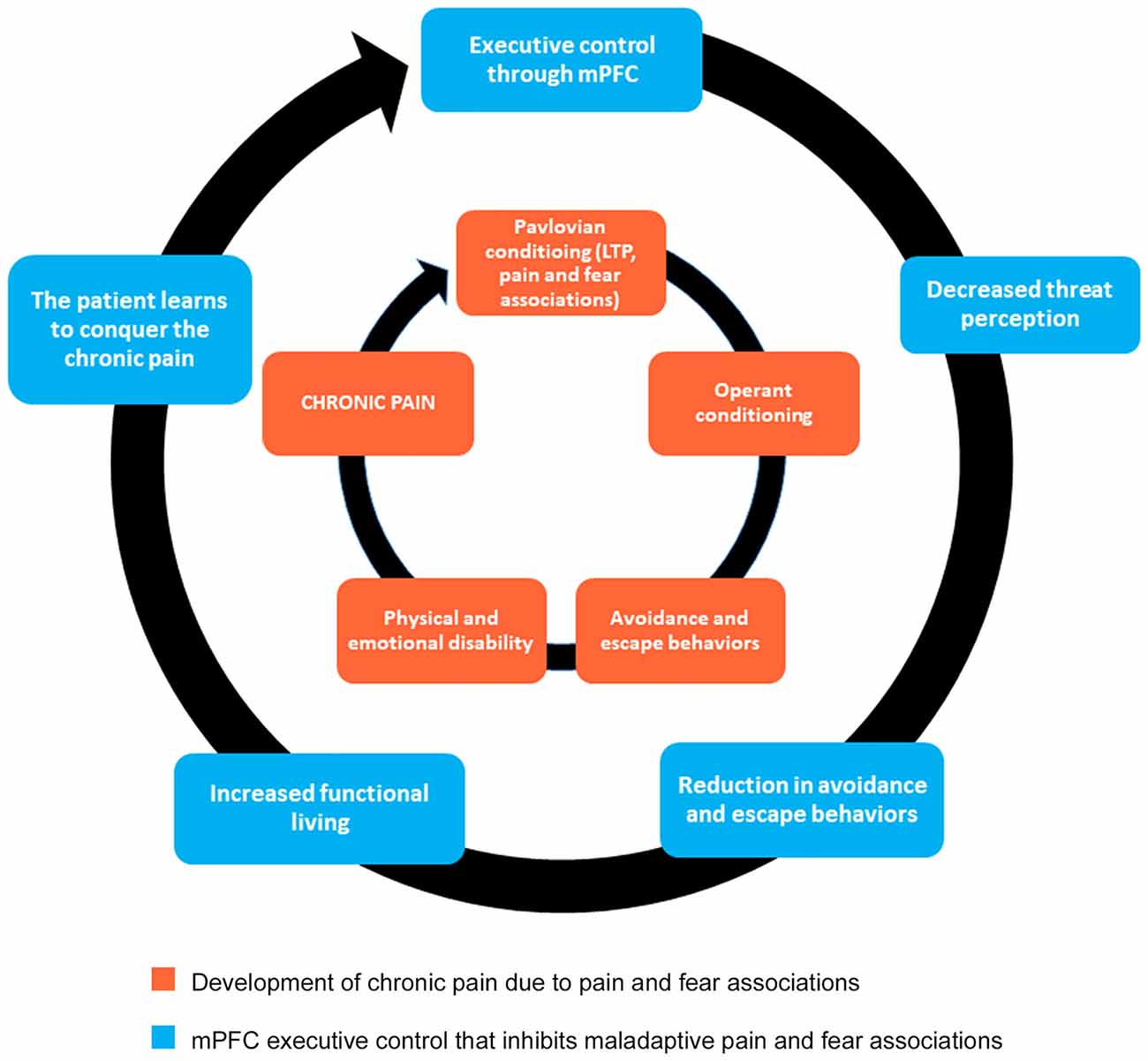
Figure 2. Mind-body approach to healing that promotes executive control originating from mPFC. The inner circle indicates the behavioral mechanisms underlying chronic pain. This approach to healing, grounded in cognitive reappraisal, mindfulness meditation, and functional rehabilitation, will promote new synaptic connections necessary for fear extinction (outer circle). Notice that the inner circle does not go away. Instead, by strengthening the components of the outer circle, the mPFC can exercise executive control by inhibiting maladaptive pathways (inner circle). As a result, chronic pain patients learn how to better cope with pain, in essence giving them the power to conquer the debilitating nature of their pain.
Unlike cognitive reappraisals, mindfulness meditation focuses on present moment awareness without becoming emotionally involved or overtaken with sensations or thoughts (Kabat-Zinn et al., 1985). Studies have demonstrated small to moderate treatment effects for both mindfulness meditation and mindfulness-based stress reduction (MBSR) for chronic pain and related conditions (Crowe et al., 2016; Hilton et al., 2017), and it appears as if change in mindfulness scores mediates better clinical outcomes (Alsubaie et al., 2017). Neuroimaging evidence indicates that reductions in pain severity accompanying MBSR for chronic pain are associated with increased functional connectivity between anterior insula and ACC (Su et al., 2016). Similarly, among mindfulness meditators, reductions in pain unpleasantness ratings accompanying a noxious stimulus have been associated with decreased activation in lateral PFC and increased activation in posterior insula (Gard et al., 2012). These authors also observed an increase in ACC activation during the anticipation of a painful stimulus accompanied by a reduction in anticipatory anxiety among the mindfulness meditators, but not controls. Further, in a direct comparison of the neural mechanisms of mindfulness to those of “sham” mindfulness, Zeidan and associates (Zeidan et al., 2015) observed that reduced pain intensity and unpleasantness ratings to a painful stimulus were associated with increased activation in subgenual ACC, orbitofrontal cortex and anterior insula during mindfulness, but not sham mindfulness. Additional studies have shown that mindfulness meditation is associated with reduction in anxiety in both clinical (Marchand, 2012) and nonclinical (Zeidan et al., 2014) populations, and compared with non-meditating subjects, mindfulness meditation increases co-activation of the mPFC and ACC (Brown and Jones, 2010). As previously mentioned, this co-activation is associated with fear appraisal (Maier et al., 2012).
Increased co-activation of mPFC and ACC in mindfulness meditators may be indicative of the mPFC’s top-down influence, or executive control, on the salience network. Further, if the salience network is involved in threat perception, the mPFC can deem the sensation of pain as non-threatening, which helps to explain pain reduction in mindfulness meditation (Grant and Rainville, 2009; Zeidan et al., 2011). By acting on the mPFC, mindfulness meditation can also help to normalize dysregulation within the DMN (Brewer et al., 2011). Importantly, evidence suggests that mindfulness meditation results in pain reduction through its effects on pain circuits rather than through placebo effects (Zeidan et al., 2015). Recent evidence also suggests that sleep deprivation may increase pain sensitivity and reduce the effectiveness of pain-reducing medication (Alexandre et al., 2017). Therefore, methods that help promote relaxation and enhance sleep quality may be useful additions to a mindfulness meditation or MBSR program for the treatment of chronic pain.
Lastly, functional rehabilitation aims to increase patient range of motion and functional movements in daily living, such as walking, squatting and bending over. Similar to mirror therapy in phantom limb pain, motor imagery therapy in CRPS, and motor control in CBP, functional rehabilitation will also form new CS-US relationships which may then be generalized to other stimuli. We emphasize here that functional rehabilitation does not promote mind over body. Therefore, if functional movement exacerbates the pain symptoms, a chronic pain patient should not try to push through the pain in that moment. Instead, chronic pain patients should carefully assess for pain by trying different functional movements and slowly increase movement or range of motion when pain is not prohibitive (Ambrose and Golightly, 2015).
Because complete pain reduction is not always feasible, it is critical that the chronic pain patient does not use pain resolution as a barometer for success, as continued pain and fear associations may become barriers to success. Instead, increased functionality in daily living should become the barometer. Hence, the goal of our suggested approach to treating chronic pain is not to eliminate the pain entirely, but to conquer the maladaptive cognitive appraisals and the established neural associations in fear circuitry that make it difficult to function. Such a comprehensive treatment plan should lead to increased success rates for chronic pain interventions. When necessary and where indicated, medication management should accompany these therapeutic programs. However, a combination of the three non-pharmacological therapies should mitigate the need for pharmacological intervention, and perhaps, eliminate it altogether over time.
In conclusion, we offer the hypothesis that the most effective and beneficial treatment program for long-term management of chronic pain requires three unique aspects of therapy: (1) cognitive reappraisals; (2) mindfulness meditation; and (3) functional rehabilitation. We further hypothesize that a multimodal treatment program will lead to increased connectivity between mPFC and other cortical/subcortical regions, such as insula and amygdala, compared with any one of these therapies alone. To test this hypothesis, we suggest the implementation of an 8- to 12-week four-arm randomized clinical trial for patients experiencing chronic pain. To attain sufficient power for detecting differences among groups, 120 patients should be randomized to one of the following conditions: CBT alone, mindfulness meditation alone, functional rehabilitation alone, or combined multimodal therapy including CBT, mindfulness and functional rehabilitation. The randomization process should lead to approximately 30 patients per group, which is an adequate sample size even with the likelihood of patient drop-out and issues with image acquisition, such as participant motion or other artifact.
For CBT, we recommend a program consisting of weekly 45-min sessions comprised of: psychoeducation regarding the learned mechanisms of avoidance, cognitive restructuring through reappraisal, attention diversion and self-regulatory skills. Although prior studies have utilized a group approach to CBT (Seminowicz et al., 2013; Yoshino et al., 2018), we recommend individually-tailored CBT, if feasible, to maximize effectiveness. For mindfulness, we recommend a modified protocol based upon prior work (Zeidan et al., 2011, 2015). Weekly 45-min sessions with the therapist should include a 30-min mindfulness program comprised of: following the breath, progressive body scan and nonjudgmental awareness of thoughts. The therapist conducting these sessions should intentionally instruct the participant to become aware of body sensations, teaching the participants to allow these sensations to arise without judgment or emotional reaction. For functional rehabilitation, we suggest twice weekly 45-min sessions encompassing a program of walking, strength training, and stretching exercises (Lee and Kang, 2016), including walking outdoors or on a treadmill, depending on feasibility. Because it would not be feasible to incorporate full versions of these different therapies into a program for the combined therapy group, the multimodal treatment program should include weekly 90-min sessions incorporating abbreviated aspects of each of the three individual therapies. For each session, we recommend 30 min of CBT, followed by a 20 min mindfulness session, and then 40 min of functional rehabilitation.
To examine putative changes in functional brain connectivity, along with regional brain activity associated with monitoring painful sensations, study participants should be scanned twice: once prior to the initiation of assigned therapy and once following the conclusion of the intervention. Scanning protocol should include a 5-min “eyes-closed” resting state fMRI series, followed by a simple block-design fMRI procedure that includes alternating blocks of maintaining fixation on a central fixation cross and actively monitoring potentially painful body sensations. Changes in structural connectivity can be assessed by including a diffusion tensor imaging (DTI) scanning series, if available.
We acknowledge the ambitious nature of this proposed clinical trial, requiring many hours of therapeutic services and a total of 240 MRI scanning sessions. Therefore, we recommend a multi-site approach to this intensive investigation. Despite the likelihood that such a study might take a few years to complete, the knowledge gained through this study will have lasting impacts on treatment recommendations for chronic pain.
JG conceived the primary hypothesis put forward in this article. Both JG and KS contributed to searching prior literature, and both authors contributed to the writing and editing of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Brian Hainline, MD and Caron Hunter, LMT for providing the inspiration for a biopsychosocial approach to the neural mechanisms of chronic pain, and for discussing the importance of a holistic approach to the treatment of chronic pain that extends beyond alleviation of symptoms. The authors would also like to thank Erin Ward-Ciesielski, PhD for helpful comments regarding theoretical viewpoints mentioned in this article.
Alexander, W. H., and Brown, J. W. (2010). Computational models of performance monitoring and cognitive control. Top. Cogn. Sci. 2, 658–677. doi: 10.1111/j.1756-8765.2010.01085.x
Alexandre, C., Latremoliere, A., Ferreira, A., Miracca, G., Yamamoto, M., Scammell, T. E., et al. (2017). Decreased alertness due to sleep loss increases pain sensitivity in mice. Nat. Med. 23, 768–774. doi: 10.1038/nm.4329
Al-Obaidi, S. M., Beattie, P., Al-Zoabi, B., and Al-Wekeel, S. (2005). The relationship of anticipated pain and fear avoidance beliefs to outcome in patients with chronic low back pain who are not receiving workers: compensation. Spine 30, 1051–1057. doi: 10.1097/01.brs.0000160848.94706.83
Alsubaie, M., Abbott, R., Dunn, B., Dickens, C., Keil, T. F., Henley, W., et al. (2017). Mechanisms of action in mindfulness-based cognitive therapy (MBCT) and mindfulness-based stress reduction (MBSR) in people with physical and/or psychological conditions: a systematic review. Int. J. Geriatr. Psychiatry 55, 74–91. doi: 10.1016/j.cpr.2017.04.008
Alvarez-Castelao, B., and Schuman, E. M. (2015). The regulation of synaptic protein turnover. J. Biol. Chem. 290, 28623–28630. doi: 10.1074/jbc.R115.657130
Ambrose, K. R., and Golightly, Y. M. (2015). Physical exercise as non-pharmacological treatment of chronic pain: why and when. Best Pract. Res. Clin. Rheumatol. 29, 120–130. doi: 10.1016/j.berh.2015.04.022
Anwyl, R. (2009). Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology 56, 735–740. doi: 10.1016/j.neuropharm.2009.01.002
Apkarian, A. V., Sosa, Y., Sonty, S., Levy, R. M., Harden, R. N., Parrish, T. B., et al. (2004). Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J. Neurosci. 24, 10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004
Baliki, M. N., Chialvo, D. R., Geha, P. Y., Levy, R. M., Harden, R. N., Parrish, T. B., et al. (2006). Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J. Neurosci. 26, 12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006
Baliki, M. N., Geha, P. Y., Apkarian, A. V., and Chialvo, D. R. (2008). Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J. Neurosci. 28, 1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008
Baliki, M. N., Mansour, A. R., Baria, A. T., and Apkarian, A. V. (2014). Functional reorganization of the default mode network across chronic pain conditions. PLoS One 9:e106133. doi: 10.1371/journal.pone.0106133
Ballantyne, J. C. (2006). The Massachusetts General Hospital Handbook of Pain Management. 3rd Edn. Philadelphia, PA: Lippincott, WIlliams and Wilkins.
Barry, M. F., and Ziff, E. B. (2002). Receptor trafficking and the plasticity of excitatory synapses. Curr. Opin. Neurobiol. 12, 279–286. doi: 10.1016/s0959-4388(02)00329-x
Birbaumer, N., Lutzenberger, W., Montoya, P., Larbig, W., Unertl, K., Topfner, S., et al. (1997). Effects of regional anesthesia on phantom limb pain are mirrored in changes in cortical reorganization. J. Neurosci. 17, 5503–5508. doi: 10.1523/JNEUROSCI.17-14-05503.1997
Boeke, E. A., Moscarello, J. M., LeDoux, J. E., Phelps, E. A., and Hartley, C. A. (2017). Active avoidance: neural mechanisms and attenuation of pavlovian conditioned responding. J. Neurosci. 37, 4808–4818. doi: 10.1523/JNEUROSCI.3261-16.2017
Bonnelle, V., Ham, T. E., Leech, R., Kinnunen, K. M., Mehta, M. A., Greenwood, R. J., et al. (2012). Salience network integrity predicts default mode network function after traumatic brain injury. Proc. Natl. Acad. Sci. U S A 109, 4690–4695. doi: 10.1073/pnas.1113455109
Botvinick, M. M., Cohen, J. D., and Carter, C. S. (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn. Sci. 8, 539–546. doi: 10.1016/j.tics.2004.10.003
Bouton, M. E. (2004). Context and behavioral processes in extinction. Learn. Mem. 11, 485–494. doi: 10.1101/lm.78804
Bouton, M. E., and Todd, T. P. (2014). A fundamental role for context in instrumental learning and extinction. Behav. Processes 104, 13–19. doi: 10.1016/j.beproc.2014.02.012
Bowering, K. J., O’Connell, N. E., Tabor, A., Catley, M. J., Leake, H. B., Moseley, G. L., et al. (2013). The effects of graded motor imagery and its components on chronic pain: a systematic review and meta-analysis. J. Pain 14, 3–13. doi: 10.1016/j.jpain.2012.09.007
Brewer, J. A., Worhunsky, P. D., Gray, J. R., Tang, Y. Y., Weber, J., and Kober, H. (2011). Meditation experience is associated with differences in default mode network activity and connectivity. Proc. Natl. Acad. Sci. U S A 108, 20254–20259. doi: 10.1073/pnas.1112029108
Brighina, F., De Tommaso, M., Giglia, F., Scalia, S., Cosentino, G., Puma, A., et al. (2011). Modulation of pain perception by transcranial magnetic stimulation of left prefrontal cortex. J. Headache Pain 12, 185–191. doi: 10.1007/s10194-011-0322-8
Broer, A., Deitmer, J. W., and Broer, S. (2004). Astroglial glutamine transport by system N is upregulated by glutamate. Glia 48, 298–310. doi: 10.1002/glia.20081
Brown, C. A., and Jones, A. K. (2010). Meditation experience predicts less negative appraisal of pain: electrophysiological evidence for the involvement of anticipatory neural responses. Pain 150, 428–438. doi: 10.1016/j.pain.2010.04.017
Bruehl, S. (2010). An update on the pathophysiology of complex regional pain syndrome. Anesthesiology 113, 713–725. doi: 10.1097/ALN.0b013e3181e3db38
Buckner, R. L., Andrews-Hanna, J. R., and Schacter, D. L. (2008). The brain’s default network: anatomy, function, and relevance to disease. Ann. N Y Acad. Sci. 1124, 1–38. doi: 10.1196/annals.1440.011
Campbell, J. N., and Meyer, R. A. (2006). Mechanisms of neuropathic pain. Neuron 52, 77–92. doi: 10.1016/j.neuron.2006.09.021
Cepeda, M. S., Carr, D. B., and Lau, J. (2005). Local anesthetic sympathetic blockade for complex regional pain syndrome. Cochrane Database Syst. Rev. 4:CD004598. doi: 10.1002/14651858.cd004598
Cepeda, M. S., Lau, J., and Carr, D. B. (2002). Defining the therapeutic role of local anesthetic sympathetic blockade in complex regional pain syndrome: a narrative and systematic review. Clin J. Pain 18, 216–233. doi: 10.1097/00002508-200207000-00002
Chan, B. L., Witt, R., Charrow, A. P., Magee, A., Howard, R., Pasquina, P. F., et al. (2007). Mirror therapy for phantom limb pain. N. Engl. J. Med. 357, 2206–2207. doi: 10.1056/NEJMc071927
Chiang, C. Y., Dostrovsky, J. O., Iwata, K., and Sessle, B. J. (2011). Role of glia in orofacial pain. Neuroscientist 17, 303–320. doi: 10.1177/1073858410386801
Coghill, R. C., McHaffie, J. G., and Yen, Y. F. (2003). Neural correlates of interindividual differences in the subjective experience of pain. Proc. Natl. Acad. Sci. U S A 100, 8538–8542. doi: 10.1073/pnas.1430684100
Coull, J. A., Beggs, S., Boudreau, D., Boivin, D., Tsuda, M., Inoue, K., et al. (2005). BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021. doi: 10.1038/nature04223
Courtin, J., Bienvenu, T. C., Einarsson, E. Ö., and Herry, C. (2013). Medial prefrontal cortex neuronal circuits in fear behavior. Neuroscience 240, 219–242. doi: 10.1016/j.neuroscience.2013.03.001
Craske, M. G., Kircanski, K., Zelikowsky, M., Mystkowski, J., Chowdhury, N., and Baker, A. (2008). Optimizing inhibitory learning during exposure therapy. Behav. Res. Ther. 46, 5–27. doi: 10.1016/j.brat.2007.10.003
Crombez, G., Vlaeyen, J. W., Heuts, P. H., and Lysens, R. (1999). Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain 80, 329–339. doi: 10.1016/s0304-3959(98)00229-2
Crowe, M., Jordan, J., Burrell, B., Jones, V., Gillon, D., and Harris, S. (2016). Mindfulness-based stress reduction for long-term physical conditions: a systematic review. Aust. N Z J. Psychiatry 50, 21–32. doi: 10.1177/0004867415607984
Davis, K. D., Flor, H., Greely, H. T., Iannetti, G. D., Mackey, S., Ploner, M., et al. (2017). Brain imaging tests for chronic pain: medical, legal and ethical issues and recommendations. Nat. Rev. Neurol. 13, 624–638. doi: 10.1038/nrneurol.2017.122
De Peuter, S., Van Diest, I., Vansteenwegen, D., Van den Bergh, O., and Vlaeyen, J. W. (2011). Understanding fear of pain in chronic pain: interoceptive fear conditioning as a novel approach. Eur. J. Pain 15, 889–894. doi: 10.1016/j.ejpain.2011.03.002
Di Pietro, F., McAuley, J. H., Parkitny, L., Lotze, M., Wand, B. M., Moseley, G. L., et al. (2013a). Primary motor cortex function in complex regional pain syndrome: a systematic review and meta-analysis. J. Pain 14, 1270–1288. doi: 10.1016/j.jpain.2013.07.004
Di Pietro, F., McAuley, J. H., Parkitny, L., Lotze, M., Wand, B. M., Moseley, G. L., et al. (2013b). Primary somatosensory cortex function in complex regional pain syndrome: a systematic review and meta-analysis. J. Pain 14, 1001–1018. doi: 10.1016/j.jpain.2013.04.001
Dina, O. A., Khasar, S. G., Gear, R. W., and Levine, J. D. (2009). Activation of Gi induces mechanical hyperalgesia poststress or inflammation. Neuroscience 160, 501–507. doi: 10.1016/j.neuroscience.2009.03.001
Dityatev, A. E., and Bolshakov, V. Y. (2005). Amygdala, long-term potentiation, and fear conditioning. Neuroscientist 11, 75–88. doi: 10.1177/1073858404270857
Ehlers, M. D. (2000). Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron 28, 511–525. doi: 10.1016/s0896-6273(00)00129-x
Elsenbruch, S., and Wolf, O. T. (2015). Could stress contribute to pain-related fear in chronic pain? Front. Behav. Neurosci. 9:340. doi: 10.3389/fnbeh.2015.00340
Esteban, J. A., Shi, S. H., Wilson, C., Nuriya, M., Huganir, R. L., and Malinow, R. (2003). PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat. Neurosci. 6, 136–143. doi: 10.1038/nn997
Etkin, A., Egner, T., Peraza, D. M., Kandel, E. R., and Hirsch, J. (2006). Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51, 871–882. doi: 10.1016/j.neuron.2006.07.029
Euston, D. R., Gruber, A. J., and McNaughton, B. L. (2012). The role of medial prefrontal cortex in memory and decision making. Neuron 76, 1057–1070. doi: 10.1016/j.neuron.2012.12.002
Finn, S. B., Perry, B. N., Clasing, J. E., Walters, L. S., Jarzombek, S. L., Curran, S., et al. (2017). A randomized, controlled trial of mirror therapy for upper extremity phantom limb pain in male amputees. Front. Neurol. 8:267. doi: 10.3389/fneur.2017.00267
Flor, H. (2002). Phantom-limb pain: characteristics, causes, and treatment. Lancet Neurol. 1, 182–189. doi: 10.1016/s1474-4422(02)00074-1
Fucich, E. A., Paredes, D., Saunders, M. O., and Morilak, D. A. (2018). Activity in the ventral medial prefrontal cortex is necessary for the therapeutic effects of extinction in rats. J. Neurosci. 38, 1408–1417. doi: 10.1523/JNEUROSCI.0635-17.2017
Galan, A., Laird, J. M., and Cervero, F. (2004). In vivo recruitment by painful stimuli of AMPA receptor subunits to the plasma membrane of spinal cord neurons. Pain 112, 315–323. doi: 10.1016/j.pain.2004.09.011
Galvan, E. J., Cosgrove, K. E., and Barrionuevo, G. (2011). Multiple forms of long-term synaptic plasticity at hippocampal mossy fiber synapses on interneurons. Neuropharmacology 60, 740–747. doi: 10.1016/j.neuropharm.2010.11.008
Gard, T., Holzel, B. K., Sack, A. T., Hempel, H., Lazar, S. W., Vaitl, D., et al. (2012). Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cereb. Cortex 22, 2692–2702. doi: 10.1093/cercor/bhr352
Gheldof, E. L., Vinck, J., Van den Bussche, E., Vlaeyen, J. W., Hidding, A., and Crombez, G. (2006). Pain and pain-related fear are associated with functional and social disability in an occupational setting: evidence of mediation by pain-related fear. Eur. J. Pain 10, 513–525. doi: 10.1016/j.ejpain.2005.07.005
Gilbert, S. J., Dumontheil, I., Simons, J. S., Frith, C. D., and Burgess, P. W. (2007). Comment on “Wandering minds: the default network and stimulus-independent thought”. Science 317:43; author reply 43. doi: 10.1126/science.1140801
Grant, J. A., and Rainville, P. (2009). Pain sensitivity and analgesic effects of mindful states in Zen meditators: a cross-sectional study. Psychosom. Med. 71, 106–114. doi: 10.1097/PSY.0b013e31818f52ee
Greicius, M. D., Supekar, K., Menon, V., and Dougherty, R. F. (2009). Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex 19, 72–78. doi: 10.1093/cercor/bhn059
Guo, W., Wang, H., Watanabe, M., Shimizu, K., Zou, S., LaGraize, S. C., et al. (2007). Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J. Neurosci. 27, 6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007
Gusnard, D. A., Akbudak, E., Shulman, G. L., and Raichle, M. E. (2001). Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. U S A 98, 4259–4264. doi: 10.1073/pnas.071043098
Gustin, S. M., Peck, C. C., Cheney, L. B., Macey, P. M., Murray, G. M., and Henderson, L. A. (2012). Pain and plasticity: is chronic pain always associated with somatosensory cortex activity and reorganization? J. Neurosci. 32, 14874–14884. doi: 10.1523/JNEUROSCI.1733-12.2012
Harrison, B. J., Pujol, J., López-Solà, M., Hernández-Ribas, R., Deus, J., Ortiz, H., et al. (2008). Consistency and functional specialization in the default mode brain network. Proc. Natl. Acad. Sci. U S A 105, 9781–9786. doi: 10.1073/pnas.0711791105
Hashmi, J. A., Baliki, M. N., Huang, L., Baria, A. T., Torbey, S., Hermann, K. M., et al. (2013). Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 136, 2751–2768. doi: 10.1093/brain/awt211
Heinricher, M. M., Tavares, I., Leith, J. L., and Lumb, B. M. (2009). Descending control of nociception: specificity, recruitment and plasticity. Brain Res. Rev. 60, 214–225. doi: 10.1016/j.brainresrev.2008.12.009
Herry, C., Ferraguti, F., Singewald, N., Letzkus, J. J., Ehrlich, I., and Luthi, A. (2010). Neuronal circuits of fear extinction. Eur. J. Neurosci. 31, 599–612. doi: 10.1111/j.1460-9568.2010.07101.x
Herry, C., and Garcia, R. (2002). Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J. Neurosci. 22, 577–583. doi: 10.1523/JNEUROSCI.22-02-00577.2002
Hilton, L., Hempel, S., Ewing, B. A., Apaydin, E., Xenakis, L., Newberry, S., et al. (2017). Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann. Behav. Med. 51, 199–213. doi: 10.1007/s12160-016-9844-2
Holroyd, C. B., Coles, M. G., and Nieuwenhuis, S. (2002). Medial prefrontal cortex and error potentials. Science 296, 1610–1611; author reply 1610–1611. doi: 10.1126/science.296.5573.1610
Huse, E., Larbig, W., Flor, H., and Birbaumer, N. (2001). The effect of opioids on phantom limb pain and cortical reorganization. Pain 90, 47–55. doi: 10.1016/s0304-3959(00)00385-7
Ikeda, H., Stark, J., Fischer, H., Wagner, M., Drdla, R., Jäger, T., et al. (2006). Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science 312, 1659–1662. doi: 10.1126/science.1127233
Jänig, W., and Baron, R. (2002). Complex regional pain syndrome is a disease of the central nervous system. Clin. Auton. Res. 12, 150–164. doi: 10.1007/s10286-002-0022-1
Jennings, J. R., Sheu, L. K., Kuan, D. C., Manuck, S. B., and Gianaros, P. J. (2016). Resting state connectivity of the medial prefrontal cortex covaries with individual differences in high-frequency heart rate variability. Psychophysiology 53, 444–454. doi: 10.1111/psyp.12586
Jensen, M. C., Brant-Zawadzki, M. N., Obuchowski, N., Modic, M. T., Malkasian, D., and Ross, J. S. (1994). Magnetic resonance imaging of the lumbar spine in people without back pain. N. Engl. J. Med. 331, 69–73. doi: 10.1056/nejm199407143310201
Jensen, K. B., Kosek, E., Wicksell, R., Kemani, M., Olsson, G., Merle, J. V., et al. (2012). Cognitive Behavioral Therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain 153, 1495–1503. doi: 10.1016/j.pain.2012.04.010
Ji, R. R., Kohno, T., Moore, K. A., and Woolf, C. J. (2003). Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 26, 696–705. doi: 10.1016/j.tins.2003.09.017
Kabat-Zinn, J., Lipworth, L., and Burney, R. (1985). The clinical use of mindfulness meditation for the self-regulation of chronic pain. J. Behav. Med. 8, 163–190. doi: 10.1007/bf00845519
Kahle, K. T., Khanna, A., Clapham, D. E., and Woolf, C. J. (2014). Therapeutic restoration of spinal inhibition via druggable enhancement of potassium-chloride cotransporter KCC2-mediated chloride extrusion in peripheral neuropathic pain. JAMA Neurol. 71, 640–645. doi: 10.1001/jamaneurol.2014.21
Kattoor, J., Gizewski, E. R., Kotsis, V., Benson, S., Gramsch, C., Theysohn, N., et al. (2013). Fear conditioning in an abdominal pain model: neural responses during associative learning and extinction in healthy subjects. PLoS One 8:e51149. doi: 10.1371/journal.pone.0051149
Kincaid, W., Neubert, M. J., Xu, M., Kim, C. J., and Heinricher, M. M. (2006). Role for medullary pain facilitating neurons in secondary thermal hyperalgesia. J. Neurophysiol. 95, 33–41. doi: 10.1152/jn.00449.2005
Kirveskari, E., Vartiainen, N. V., Gockel, M., and Forss, N. (2010). Motor cortex dysfunction in complex regional pain syndrome. Clin. Neurophysiol. 121, 1085–1091. doi: 10.1016/j.clinph.2010.01.032
Klein, M. E., Castillo, P. E., and Jordan, B. A. (2015). Coordination between translation and degradation regulates inducibility of mGluR-LTD. Cell Rep. 9, 1459–1466. doi: 10.1016/j.celrep.2015.02.020
Knecht, S., Henningsen, H., Höhling, C., Elbert, T., Flor, H., Pantev, C., et al. (1998). Plasticity of plasticity? Changes in the pattern of perceptual correlates of reorganization after amputation. Brain 121, 717–724. doi: 10.1093/brain/121.4.717
Koffler, S. P., Hampstead, B. M., Irani, F., Tinker, J., Kiefer, R. T., Rohr, P., et al. (2007). The neurocognitive effects of 5 day anesthetic ketamine for the treatment of refractory complex regional pain syndrome. Arch. Clin. Neuropsychol. 22, 719–729. doi: 10.1016/j.acn.2007.05.005
Krypotos, A. M., Effting, M., Kindt, M., and Beckers, T. (2015). Avoidance learning: a review of theoretical models and recent developments. Front. Behav. Neurosci. 9:189. doi: 10.3389/fnbeh.2015.00189
Kucyi, A., Salomons, T. V., and Davis, K. D. (2016). Cognitive behavioral training reverses the effect of pain exposure on brain network activity. Pain 157, 1895–1904. doi: 10.1097/j.pain.0000000000000592
Latremoliere, A., and Woolf, C. J. (2009). Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J. Pain 10, 895–926. doi: 10.1016/j.jpain.2009.06.012
Lazaridou, A., Kim, J., Cahalan, C. M., Loggia, M. L., Franceschelli, O., Berna, C., et al. (2017). Effects of cognitive-behavioral therapy (CBT) on brain connectivity supporting catastrophizing in fibromyalgia. Clin. J. Pain 33, 215–221. doi: 10.1097/AJP.0000000000000422
LeDoux, J. E. (2015). Anxious: Using the Brain to Understand Treat Fear and Anxiety. New York, NY: Penguin Books.
Lee, J. S., and Kang, S. J. (2016). The effects of strength exercise and walking on lumbar function, pain level, and body composition in chronic back pain patients. J. Exerc. Rehabil. 12, 463–470. doi: 10.12965/jer.1632650.325
Lee, M. C., and Tracey, I. (2010). Unravelling the mystery of pain, suffering, and relief with brain imaging. Curr. Pain Headache Rep. 14, 124–131. doi: 10.1007/s11916-010-0103-0
Leeuw, M., Goossens, M. E., Linton, S. J., Crombez, G., Boersma, K., and Vlaeyen, J. W. (2007). The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J. Behav. Med. 30, 77–94. doi: 10.1007/s10865-006-9085-0
Lerma, J., and Marques, J. M. (2013). Kainate receptors in health and disease. Neuron 80, 292–311. doi: 10.1016/j.neuron.2013.09.045
Li, J., Simone, D. A., and Larson, A. A. (1999). Windup leads to characteristics of central sensitization. Pain 79, 75–82. doi: 10.1016/s0304-3959(98)00154-7
Linnman, C., Becerra, L., Lebel, A., Berde, C., Grant, P. E., and Borsook, D. (2013). Transient and persistent pain induced connectivity alterations in pediatric complex regional pain syndrome. PLoS One 8:e57205. doi: 10.1371/journal.pone.0057205
Lorenz, J., Minoshima, S., and Casey, K. L. (2003). Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 126, 1079–1091. doi: 10.1093/brain/awg102
Lotze, M., Flor, H., Grodd, W., Larbig, W., and Birbaumer, N. (2001). Phantom movements and pain. An fMRI study in upper limb amputees. Brain 124, 2268–2277. doi: 10.1093/brain/124.11.2268
Low, A. K., Ward, K., and Wines, A. P. (2007). Pediatric complex regional pain syndrome. J. Pediatr. Orthop. 27, 567–572. doi: 10.1097/BPO.0b013e318070cc4d
Macedo, L. G., Latimer, J., Maher, C. G., Hodges, P. W., McAuley, J. H., Nicholas, M. K., et al. (2012). Effect of motor control exercises versus graded activity in patients with chronic nonspecific low back pain: a randomized controlled trial. Phys. Ther. 92, 363–377. doi: 10.2522/ptj.20110290
MacIver, K., Lloyd, D. M., Kelly, S., Roberts, N., and Nurmikko, T. (2008). Phantom limb pain, cortical reorganization and the therapeutic effect of mental imagery. Brain 131, 2181–2191. doi: 10.1093/brain/awn124
Maier, C., Dertwinkel, R., Mansourian, N., Hosbach, I., Schwenkreis, P., Senne, I., et al. (2003). Efficacy of the NMDA-receptor antagonist memantine in patients with chronic phantom limb pain—results of a randomized double-blinded, placebo-controlled trial. Pain 103, 277–283. doi: 10.1016/s0304-3959(02)00456-6
Maier, S., Szalkowski, A., Kamphausen, S., Perlov, E., Feige, B., Blechert, J., et al. (2012). Clarifying the role of the rostral dmPFC/dACC in fear/anxiety: learning, appraisal or expression? PLoS One 7:e50120. doi: 10.1371/journal.pone.0050120
Maihofner, C., Baron, R., DeCol, R., Binder, A., Birklein, F., Deuschl, G., et al. (2007). The motor system shows adaptive changes in complex regional pain syndrome. Brain 130, 2671–2687. doi: 10.1093/brain/awm131
Maihofner, C., Handwerker, H. O., Neundorfer, B., and Birklein, F. (2003). Patterns of cortical reorganization in complex regional pain syndrome. Neurology 61, 1707–1715. doi: 10.1212/01.WNL.0000098939.02752.8e
Malenka, R. C., and Bear, M. F. (2004). LTP and LTD: an embarrassment of riches. Neuron 44, 5–21. doi: 10.1016/j.neuron.2004.09.012
Marchand, W. R. (2012). Mindfulness-based stress reduction, mindfulness-based cognitive therapy and Zen meditation for depression, anxiety, pain, and psychological distress. J. Psychiatr. Pract. 18, 233–252. doi: 10.1097/01.pra.0000416014.53215.86
Marinus, J., Moseley, G. L., Birklein, F., Baron, R., Maihöfner, C., Kingery, W. S., et al. (2011). Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol. 10, 637–648. doi: 10.1016/S1474-4422(11)70106-5
Martin, S., and Henley, J. M. (2004). Activity-dependent endocytic sorting of kainate receptors to recycling or degradation pathways. EMBO J. 23, 4749–4759. doi: 10.1038/sj.emboj.7600483
Mason, M. F., Norton, M. I., Van Horn, J. D., Wegner, D. M., Grafton, S. T., and Macrae, C. N. (2007). Wandering minds: the default network and stimulus-independent thought. Science 315, 393–395. doi: 10.1126/science.1131295
McBride, A., and Atkins, R. (2005). Complex regional pain syndrome. Curr. Orthopaedics 19, 155–165. doi: 10.1016/j.cuor.2005.02.011
McKiernan, K. A., Kaufman, J. N., Kucera-Thompson, J., and Binder, J. R. (2003). A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J. Cogn. Neurosci. 15, 394–408. doi: 10.1162/089892903321593117
Meier, P. M., Zurakowski, D., Berde, C. B., and Sethna, N. F. (2009). Lumbar sympathetic blockade in children with complex regional pain syndromes: a double blind placebo-controlled crossover trial. Anesthesiology 111, 372–380. doi: 10.1097/ALN.0b013e3181aaea90
Milad, M. R., and Quirk, G. J. (2012). Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 63, 129–151. doi: 10.1146/annurev.psych.121208.131631
Milad, M. R., Wright, C. I., Orr, S. P., Pitman, R. K., Quirk, G. J., and Rauch, S. L. (2007). Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatry 62, 446–454. doi: 10.1016/j.biopsych.2006.10.011
Milligan, E. D., and Watkins, L. R. (2009). Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 10, 23–36. doi: 10.1038/nrn2533
Morris, R. W., and Bouton, M. E. (2007). The effect of yohimbine on the extinction of conditioned fear: a role for context. Behav. Neurosci. 121, 501–514. doi: 10.1037/0735-7044.121.3.501
Moseley, G. L. (2004a). Evidence for a direct relationship between cognitive and physical change during an education intervention in people with chronic low back pain. Eur. J. Pain 8, 39–45. doi: 10.1016/s1090-3801(03)00063-6
Moseley, G. L. (2004b). Graded motor imagery is effective for long-standing complex regional pain syndrome: a randomised controlled trial. Pain 108, 192–198. doi: 10.1016/j.pain.2004.01.006
Moseley, G. L., and Butler, D. S. (2015). Fifteen years of explaining pain: the past, present, and future. J. Pain 16, 807–813. doi: 10.1016/j.jpain.2015.05.005
Moulton, E. A., Pendse, G., Becerra, L. R., and Borsook, D. (2012). BOLD responses in somatosensory cortices better reflect heat sensation than pain. J. Neurosci. 32, 6024–6031. doi: 10.1523/JNEUROSCI.0006-12.2012
Narayanan, N. S., and Laubach, M. (2006). Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron 52, 921–931. doi: 10.1016/j.neuron.2006.10.021
Nikolajsen, L., Hansen, C. L., Nielsen, J., Keller, J., Arendt-Nielsen, L., and Jensen, T. S. (1996). The effect of ketamine on phantom pain: a central neuropathic disorder maintained by peripheral input. Pain 67, 69–77. doi: 10.1016/0304-3959(96)03080-1
Nikolajsen, L., and Jensen, T. S. (2001). Phantom limb pain. Br. J. Anaesth. 87, 107–116. doi: 10.1093/bja/87.1.107
Niswender, C. M., and Conn, P. J. (2010). Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 50, 295–322. doi: 10.1146/annurev.pharmtox.011008.145533
Ossipov, M. H., Dussor, G. O., and Porreca, F. (2010). Central modulation of pain. J. Clin. Invest. 120, 3779–3787. doi: 10.1172/JCI43766
Otti, A., Guendel, H., Wohlschläger, A., Zimmer, C., and Noll-Hussong, M. (2013). Frequency shifts in the anterior default mode network and the salience network in chronic pain disorder. BMC Psychiatry 13:84. doi: 10.1186/1471-244X-13-84
Pan, H. L., Wu, Z. Z., Zhou, H. Y., Chen, S. R., Zhang, H. M., and Li, D. P. (2008). Modulation of pain transmission by G-protein-coupled receptors. Pharmacol. Ther. 117, 141–161. doi: 10.1016/j.pharmthera.2007.09.003
Pape, H. C., and Pare, D. (2010). Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 90, 419–463. doi: 10.1152/physrev.00037.2009
Parr, J. J., Borsa, P. A., Fillingim, R. B., Tillman, M. D., Manini, T. M., Gregory, C. M., et al. (2012). Pain-related fear and catastrophizing predict pain intensity and disability independently using an induced muscle injury model. J. Pain 13, 370–378. doi: 10.1016/j.jpain.2011.12.011
Peeters, S. C., van de Ven, V., Gronenschild, E. H., Patel, A. X., Habets, P., Goebel, R., et al. (2015). Default mode network connectivity as a function of familial and environmental risk for psychotic disorder. PLoS One 10:e0120030. doi: 10.1371/journal.pone.0120030
Phelps, E. A., Delgado, M. R., Nearing, K. I., and LeDoux, J. E. (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43, 897–905. doi: 10.1016/j.neuron.2004.08.042
Phelps, E. A., and LeDoux, J. E. (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48, 175–187. doi: 10.1016/j.neuron.2005.09.025
Pickering, A. E., and McCabe, C. S. (2014). Prolonged ketamine infusion as a therapy for complex regional pain syndrome: synergism with antagonism? Br. J. Clin. Pharmacol. 77, 233–238. doi: 10.1111/bcp.12157
Piguel, N. H., Fievre, S., Blanc, J. M., Carta, M., Moreau, M. M., Moutin, E., et al. (2014). Scribble1/AP2 complex coordinates NMDA receptor endocytic recycling. Cell Rep. 9, 712–727. doi: 10.1016/j.celrep.2014.09.017
Pleger, B., Draganski, B., Schwenkreis, P., Lenz, M., Nicolas, V., Maier, C., et al. (2014). Complex regional pain syndrome type I affects brain structure in prefrontal and motor cortex. PLoS One 9:e85372. doi: 10.1371/journal.pone.0085372
Posner, M. I., Rothbart, M. K., Sheese, B. E., and Tang, Y. (2007). The anterior cingulate gyrus and the mechanism of self-regulation. Cogn. Affect. Behav. Neurosci. 7, 391–395. doi: 10.3758/cabn.7.4.391
Preissler, S., Feiler, J., Dietrich, C., Hofmann, G. O., Miltner, W. H., and Weiss, T. (2013). Gray matter changes following limb amputation with high and low intensities of phantom limb pain. Cereb. Cortex 23, 1038–1048. doi: 10.1093/cercor/bhs063
Price, T. J., Cervero, F., Gold, M. S., Hammond, D. L., and Prescott, S. A. (2009). Chloride regulation in the pain pathway. Brain Res. Rev. 60, 149–170. doi: 10.1016/j.brainresrev.2008.12.015
Quinn, J. J., Ma, Q. D., Tinsley, M. R., Koch, C., and Fanselow, M. S. (2008). Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learn. Mem. 15, 368–372. doi: 10.1101/lm.813608
Quirk, G. J., Pare, D., Richardson, R., Herry, C., Monfils, M. H., Schiller, D., et al. (2010). Erasing fear memories with extinction training. J. Neurosci. 30, 14993–14997. doi: 10.1523/JNEUROSCI.4268-10.2010
Radulovic, J., and Tronson, N. C. (2010). Molecular specificity of multiple hippocampal processes governing fear extinction. Rev. Neurosci. 21, 1–17. doi: 10.1515/revneuro.2010.21.1.1
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., and Shulman, G. L. (2001). A default mode of brain function. Proc. Natl. Acad. Sci. U S A 98, 676–682. doi: 10.1073/pnas.98.2.676
Ridderinkhof, K. R., Ullsperger, M., Crone, E. A., and Nieuwenhuis, S. (2004). The role of the medial frontal cortex in cognitive control. Science 306, 443–447. doi: 10.1126/science.1100301
Robinson, L. R., Czerniecki, J. M., Ehde, D. M., Edwards, W. T., Judish, D. A., Goldberg, M. L., et al. (2004). Trial of amitriptyline for relief of pain in amputees: results of a randomized controlled study. Arch. Phys. Med. Rehabil. 85, 1–6. doi: 10.1016/S0003-9993(03)00476-3
Rodriguez-Raecke, R., Niemeier, A., Ihle, K., Ruether, W., and May, A. (2013). Structural brain changes in chronic pain reflect probably neither damage nor atrophy. PLoS One 8:e54475. doi: 10.1371/journal.pone.0054475
Ross, E. M. (2014). G Protein-coupled receptors: multi-turnover GDP/GTP exchange catalysis on heterotrimeric G proteins. Cell. Logist. 4:e29391. doi: 10.4161/cl.29391
Roussel, N. A., Nijs, J., Meeus, M., Mylius, V., Fayt, C., and Oostendorp, R. (2013). Central sensitization and altered central pain processing in chronic low back pain: fact or myth? Clin. J. Pain 29, 625–638. doi: 10.1097/ajp.0b013e31826f9a71
Rygh, L. J., Svendsen, F., Fiskå, A., Haugan, F., Hole, K., and Tjølsen, A. (2005). Long-term potentiation in spinal nociceptive systems—how acute pain may become chronic. Psychoneuroendocrinology 30, 959–964. doi: 10.1016/j.psyneuen.2005.04.007
Salomons, T. V., Moayedi, M., Erpelding, N., and Davis, K. D. (2014). A brief cognitive-behavioural intervention for pain reduces secondary hyperalgesia. Pain 155, 1446–1452. doi: 10.1016/j.pain.2014.02.012
Sandkühler, J. (2007). Understanding LTP in pain pathways. Mol. Pain 3:9. doi: 10.1186/1744-8069-3-9
Saragiotto, B. T., Maher, C. G., Yamato, T. P., Costa, L. O., Costa, L. C., Ostelo, R. W., et al. (2016). Motor control exercise for nonspecific low back pain: a cochrane review. Spine 41, 1284–1295. doi: 10.1097/BRS.0000000000001645
Schley, M., Topfner, S., Wiech, K., Schaller, H. E., Konrad, C. J., Schmelz, M., et al. (2007). Continuous brachial plexus blockade in combination with the NMDA receptor antagonist memantine prevents phantom pain in acute traumatic upper limb amputees. Eur. J. Pain 11, 299–308. doi: 10.1016/j.ejpain.2006.03.003
Schwartzman, R. J., Alexander, G. M., Grothusen, J. R., Paylor, T., Reichenberger, E., and Perreault, M. (2009). Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double-blind placebo controlled study. Pain 147, 107–115. doi: 10.1016/j.pain.2009.08.015
Scott, D. B., Michailidis, I., Mu, Y., Logothetis, D., and Ehlers, M. D. (2004). Endocytosis and degradative sorting of NMDA receptors by conserved membrane-proximal signals. J. Neurosci. 24, 7096–7109. doi: 10.1523/JNEUROSCI.0780-04.2004
Segerdahl, A. R., Mezue, M., Okell, T. W., Farrar, J. T., and Tracey, I. (2015). The dorsal posterior insula subserves a fundamental role in human pain. Nat. Neurosci. 18, 499–500. doi: 10.1038/nn.3969
Seminowicz, D. A., Shpaner, M., Keaser, M. L., Krauthamer, G. M., Mantegna, J., Dumas, J. A., et al. (2013). Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J. Pain 14, 1573–1584. doi: 10.1016/j.jpain.2013.07.020
Seminowicz, D. A., Wideman, T. H., Naso, L., Hatami-Khoroushahi, Z., Fallatah, S., Ware, M. A., et al. (2011). Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J. Neurosci. 31, 7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011
Shafritz, K. M., Collins, S. H., and Blumberg, H. P. (2006). The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. Neuroimage 31, 468–475. doi: 10.1016/j.neuroimage.2005.11.053
Sheline, Y. I., Barch, D. M., Price, J. L., Rundle, M. M., Vaishnavi, S. N., Snyder, A. Z., et al. (2009). The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. U S A 106, 1942–1947. doi: 10.1073/pnas.0812686106
Sigurdsson, T., Doyère, V., Cain, C. K., and LeDoux, J. E. (2007). Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology 52, 215–227. doi: 10.1016/j.neuropharm.2006.06.022
Sridharan, D., Levitin, D. J., and Menon, V. (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U S A 105, 12569–12574. doi: 10.1073/pnas.0800005105
Stanton, T. R., Wand, B. M., Carr, D. B., Birklein, F., Wasner, G. L., and O’Connell, N. E. (2013). Local anaesthetic sympathetic blockade for complex regional pain syndrome. Cochrane Database Syst. Rev. 8:CD004598. doi: 10.1002/14651858.CD004598.pub3
Staud, R. (2013). The important role of CNS facilitation and inhibition for chronic pain. Int. J. Clin. Rheumtol. 8, 639–646. doi: 10.2217/ijr.13.57
Su, I. W., Wu, F. W., Liang, K. C., Cheng, K. Y., Hsieh, S. T., Sun, W. Z., et al. (2016). Pain perception can be modulated by mindfulness training: a resting-state fMRI study. Front. Hum. Neurosci. 10:570. doi: 10.3389/fnhum.2016.00570
Subedi, B., and Grossberg, G. T. (2011). Phantom limb pain: mechanisms and treatment approaches. Pain Res. Treat. 2011:864605. doi: 10.1155/2011/864605
Tagliazucchi, E., Balenzuela, P., Fraiman, D., and Chialvo, D. R. (2010). Brain resting state is disrupted in chronic back pain patients. Neurosci. Lett. 485, 26–31. doi: 10.1016/j.neulet.2010.08.053
Taves, S., Berta, T., Chen, G., and Ji, R. R. (2013). Microglia and spinal cord synaptic plasticity in persistent pain. Neural Plast. 2013:753656. doi: 10.1155/2013/753656
Tracey, I., and Mantyh, P. W. (2007). The cerebral signature for pain perception and its modulation. Neuron 55, 377–391. doi: 10.1016/j.neuron.2007.07.012
Trask, S., and Bouton, M. E. (2014). Contextual control of operant behavior: evidence for hierarchical associations in instrumental learning. Learn. Behav. 42, 281–288. doi: 10.3758/s13420-014-0145-y
Tseng, M. T., Kong, Y., Eippert, F., and Tracey, I. (2017). Determining the neural substrate for encoding a memory of human pain and the influence of anxiety. J. Neurosci. 37, 11806–11817. doi: 10.1523/JNEUROSCI.0750-17.2017
Turk, D. C., and Wilson, H. D. (2010). Fear of pain as a prognostic factor in chronic pain: conceptual models, assessment, and treatment implications. Curr. Pain Headache Rep. 14, 88–95. doi: 10.1007/s11916-010-0094-x
Uçeyler, N., Schäfers, M., and Sommer, C. (2009). Mode of action of cytokines on nociceptive neurons. Exp. Brain Res. 196, 67–78. doi: 10.1007/s00221-009-1755-z
Ultenius, C., Linderoth, B., Meyerson, B. A., and Wallin, J. (2006). Spinal NMDA receptor phosphorylation correlates with the presence of neuropathic signs following peripheral nerve injury in the rat. Neurosci. Lett. 399, 85–90. doi: 10.1016/j.neulet.2006.01.018
Urry, H. L., van Reekum, C. M., Johnstone, T., Kalin, N. H., Thurow, M. E., Schaefer, H. S., et al. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J. Neurosci. 26, 4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006
Vlaeyen, J. W. (2015). Learning to predict and control harmful events: chronic pain and conditioning. Pain 156, S86–S93. doi: 10.1097/j.pain.0000000000000107
Vlaeyen, J. W., and Linton, S. J. (2000). Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain 85, 317–332. doi: 10.1016/s0304-3959(99)00242-0
Vowles, K. E., and McCracken, L. M. (2008). Acceptance and values-based action in chronic pain: a study of treatment effectiveness and process. J. Consult. Clin. Psychol. 76, 397–407. doi: 10.1037/0022-006x.76.3.397
Vowles, K. E., McCracken, L. M., and Eccleston, C. (2007a). Processes of change in treatment for chronic pain: the contributions of pain, acceptance and catastrophizing. Eur. J. Pain 11, 779–787. doi: 10.1016/j.ejpain.2006.12.007
Vowles, K. E., McNeil, D. W., Gross, R. T., McDaniel, M. L., Mouse, A., Bates, M., et al. (2007b). Effects of pain acceptance and pain control strategies on physical impairment in individuals with chronic low back pain. Behav. Ther. 38, 412–425. doi: 10.1016/j.beth.2007.02.001
Wager, T. D., Atlas, L. Y., Lindquist, M. A., Roy, M., Woo, C. W., and Kross, E. (2013). An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 368, 1388–1397. doi: 10.1056/NEJMoa1204471
Weissmann, R., and Uziel, Y. (2016). Pediatric complex regional pain syndrome: a review. Pediatr. Rheumatol. Online J. 14:29. doi: 10.1186/s12969-016-0090-8
Whitlock, J. R., Heynen, A. J., Shuler, M. G., and Bear, M. F. (2006). Learning induces long-term potentiation in the hippocampus. Science 313, 1093–1097. doi: 10.1126/science.1128134
Wiech, K., Lin, C. S., Brodersen, K. H., Bingel, U., Ploner, M., and Tracey, I. (2010). Anterior insula integrates information about salience into perceptual decisions about pain. J. Neurosci. 30, 16324–16331. doi: 10.1523/JNEUROSCI.2087-10.2010
Woolf, C. J. (2011). Central sensitization: implications for the diagnosis and treatment of pain. Pain 152, S2–S15. doi: 10.1016/j.pain.2010.09.030
Woolf, C. J., Shortland, P., and Coggeshall, R. E. (1992). Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature 355, 75–78. doi: 10.1038/355075a0
Yoshino, A., Okamoto, Y., Okada, G., Takamura, M., Ichikawa, N., Shibasaki, C., et al. (2018). Changes in resting-state brain networks after cognitive-behavioral therapy for chronic pain. Psychol. Med. 48, 1148–1156. doi: 10.1017/s0033291717002598
Young, K. S., Burklund, L. J., Torre, J. B., Saxbe, D., Lieberman, M. D., and Craske, M. G. (2017). Treatment for social anxiety disorder alters functional connectivity in emotion regulation neural circuitry. Psychiatry Res. 261, 44–51. doi: 10.1016/j.pscychresns.2017.01.005
Zeidan, F., Emerson, N. M., Farris, S. R., Ray, J. N., Jung, Y., McHaffie, J. G., et al. (2015). Mindfulness meditation-based pain relief employs different neural mechanisms than placebo and sham mindfulness meditation-induced analgesia. J. Neurosci. 35, 15307–15325. doi: 10.1523/JNEUROSCI.2542-15.2015
Zeidan, F., Martucci, K. T., Kraft, R. A., Gordon, N. S., McHaffie, J. G., and Coghill, R. C. (2011). Brain mechanisms supporting the modulation of pain by mindfulness meditation. J. Neurosci. 31, 5540–5548. doi: 10.1523/JNEUROSCI.5791-10.2011
Zeidan, F., Martucci, K. T., Kraft, R. A., McHaffie, J. G., and Coghill, R. C. (2014). Neural correlates of mindfulness meditation-related anxiety relief. Soc. Cogn. Affect. Neurosci. 9, 751–759. doi: 10.1093/scan/nst041
Zhang, J. M., and An, J. (2007). Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 45, 27–37. doi: 10.1097/AIA.0b013e318034194e
Keywords: chronic pain, prefrontal cortex, associative learning, cognitive behavioral therapy, mindfulness meditation, functional recovery, review of literature
Citation: Greenwald JD and Shafritz KM (2018) An Integrative Neuroscience Framework for the Treatment of Chronic Pain: From Cellular Alterations to Behavior. Front. Integr. Neurosci. 12:18. doi: 10.3389/fnint.2018.00018
Received: 21 February 2018; Accepted: 04 May 2018;
Published: 23 May 2018.
Edited by:
Elizabeth B. Torres, Rutgers University, The State University of New Jersey, United StatesReviewed by:
Isaura Tavares, Faculty of Medicine, University of Porto, PortugalCopyright © 2018 Greenwald and Shafritz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keith M. Shafritz, a2VpdGguc2hhZnJpdHpAaG9mc3RyYS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.