
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Integr. Neurosci. , 08 January 2016
Volume 9 - 2015 | https://doi.org/10.3389/fnint.2015.00067
This article is part of the Research Topic All 3 types of glial cells are important for memory formation View all 16 articles
A close interrelation between anxiety and memory was first suggested by Kalueff and Nutt (1996), reviewing effects of γ-aminobutyrate (GABA) on both conditions. Wall and Messier (2000) showed subsequently that pretreatment with an opioid kappa receptor antagonist was anxiogenic and disrupted working memory. Additional papers supporting interactions between anxiety and memory were cited by Kalueff (2007). They included demonstration of memory improvement by serotonin, whereas a decreased ability to increase serotonin is a model of anxiety.
Serotonin acts on many different receptors. The present paper specifically deals with the 5-HT2B receptor, which is expressed in human brain (Schmuck et al., 1994; Bonhaus et al., 1995). Its mRNA expression is two times higher in freshly isolated (Lovatt et al., 2007) mouse astrocytes than in neurons (Li et al., 2012). It is necessary for consolidation of one-trial aversive learning in day-old chickens (Gibbs and Hertz, 2014) at an early stage of memory consolidation. It is also required for the therapeutic effect of serotonin-specific reuptake inhibitors (SSRIs) in major depression (Diaz et al., 2012; Li et al., 2012; Hertz et al., 2015b), a disease often accompanied by anxiety. The 5-HT2B receptor in cultured astrocytes is stimulated by fluoxetine (Li et al., 2008: Qiao et al., 2015) and all other SSRIs (Zhang et al., 2010). Chronic treatment of mice with fluoxetine for 14 days up-regulates the astrocytic, but not the neuronal 5-HT2B receptor, although this receptor is expressed in both cell types (Li et al., 2012; Hertz et al., 2015b). Decrease of its astrocytic gene expression parallels development of a depressive phenotype in a mouse model of Parkinson's disease (Zhang et al., 2015), and Pitychoutis et al. (2015) reported schizophrenia-like symptoms in mice lacking the 5-HT2B receptor gene or treated with a receptor inhibitor. Schizophrenia is often associated with depressed mood (Fortunati et al., 2015).
Inhibition of learning by a 5-HT2B∕C receptor antagonist (SB221284) and equipotent rescue of impaired learning by the 5-HT2B receptor agonists fluoxetine and paroxetine (Gibbs and Hertz, 2014) injected intracerebrally at specific times shows the importance of this receptor for establishment of memory soon after training. The similar potency of the two drugs is important, because they have widely different affinities for SERT (Wong and Bymaster, 1995) whereas all SSRIs have similar affinity for the 5-HT2B receptor (Zhang et al., 2010). Another SSRI, citalopram, counteracts spatial memory deficits (Ren et al., 2015). Mice lacking the 5-HT2B receptor gene show learning disabilities (Pitychoutis et al., 2015).
Fluoxetine increases free cytosolic Ca2+ ([Ca2+]i) and stimulates glycogenolysis (Chen et al., 1995) with similar potency by stimulation of 5-HT2B receptors (Kong et al., 2002; Figure 1A). [Ca2+]i regulates many astrocytic functions, including gliotransmission and glycogenolysis (Gucek et al., 2012; Hertz et al., 2015a). Inhibition of glycogenolysis with DAB (1,4-dideoxy-1,4-imino-D-arabinitol) prevents 5-HT2B-receptor-mediated memory enhancement by serotonin or fluoxetine during the early part of memory formation after one-trial aversive learning in the day-old chicken, a precocious animal (Gibbs and Hertz, 2014). In brain both glycogen and its degrading enzyme glycogen phosphorylase are virtually confined to astrocytes (Ibrahim, 1975; Pfeiffer-Guglielmi et al., 2003). Induction of glycogenolysis by fluoxetine occurs both in our cultured astrocytes, differentiated by dibutyryl cyclic AMP and in astrocytes grown in the absence of this agent (Allaman et al., 2011). The association with increased [Ca2+]i (Chen et al., 1995) is important because increased [Ca2+]i is a requirement for stimulation of glycogenolysis in astrocytes (Xu et al., 2014a; Hertz et al., 2015a) as in muscle (Ozawa, 2011). In rat brain 5-HT2 receptor stimulation similarly induces glycogenolysis (Darvesh and Gudelsky, 2003). The enhanced glycogenolysis is accompanied by an increased lactate release (Allaman et al., 2011). This might affect neurons either by use of lactate as an additional metabolic fuel, as suggested by Suzuki et al. (2011) and Newman et al. (2011), or by lactate signaling (Tang et al., 2014; Bergersen, 2015). The signaling mechanism established by Tang et al. (2014) is, like memory (Gibbs et al., 2006; Newman et al., 2011; Suzuki et al., 2011; Gibbs and Hutchinson, 2012; Duran et al., 2013), glycogenolysis-dependent, and its signaling is specifically directed to neurons releasing noradrenaline. Noradrenaline has effects on both neurons and astrocytes (O'Donnell et al., 2012).
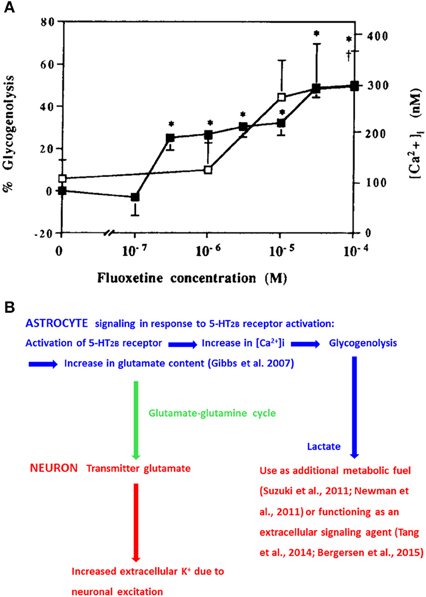
Figure 1. (A) Effects of fluoxetine concentrations between 100 nM and 100 μM on glycogenolysis (filled squares and left ordinate) and [Ca2+]i (open squares and right ordinate) in well differentiated cultures of mouse astrocytes. Values significantly different from baseline are indicated by * for glycogenolysis and by † for [Ca2+]i (From Chen et al., 1995). (B) Chart showing 5-HT2B receptor-mediated effects on [Ca2+]i, glycogenolysis, and glutamate content in astrocytes (blue) and effects of glutamate transfer to neurons (green arrow) and of glycogenolysis-evoked release of lactate on neurons (red). These effects are acutely important for learning, and drug-induced chronic effects of 5-HT2B receptor stimulation have therapeutic effect, also on impaired memory, in major depression (fluoxetine) and in schizophrenia (clozapine). However, as discussed in “Concluding remarks” in these situations it appears that it is a decreased effect on the receptor or on [Ca2+]ithat is therapeutically effective.
Glycogenolysis is also required for formation of glutamate, and its metabolite GABA (Figure 1B) in the brain in vivo (Gibbs et al., 2007, 2008) at a time when glutamate production must be evoked by 5-HT2B stimulation (Gibbs and Hertz, 2014). It also increases uptake of glutamate into cultured astrocytes and neurons as well as release of lactate from astrocytes (Sickmann et al., 2009). Glutamate is synthesized intracerebrally from glucose. This can only occur in astrocytes, because neurons lack an enzyme, pyruvate carboxylase, needed for its synthesis (reviewed by Gibbs et al., 2008; Hertz, 2013). Glutamate is subsequently converted to glutamine and carried to neurons (green arrow in Figure 1B) in an extremely active glutamine-glutamate/GABA cycle, which also returns released transmitter glutamate to neurons after its initial astrocytic accumulation (reviewed by Hertz, 2013; Hertz and Rothman, in press). The importance of glutamate receptor activity for memory is beyond doubt (Riedel et al., 2003), and interruption of the glutamine-glutamate/GABA cycle by inhibition of either glutamine synthetase (Kant et al., 2014) or astrocytic glutamate uptake (Gibbs et al., 2004) abolishes learning. GABA is also important for learning (Kalueff and Nutt, 1996; Gibbs and Bowser, 2009), and besides its neuronal effects stimulates glycogenolysis in cultured astrocytes and brain slices (Xu et al., 2014a).
A major role of glutamate (Figure 1B) is stimulation of postsynaptic glutamate receptors, leading to increases in brain metabolism (Howarth et al., 2012) and in extracellular K+ concentration (Hertz et al., 2015c and references therein). Cellular re-accumulation of K+ includes an initial uptake mediated by the astrocytic Na+,K+-ATPase (MacAulay and Zeuthen, 2012; Hertz et al., 2015c), release of astrocytically accumulated K+ by Kir4.1 channels (Bay and Butt, 2012) and neuronal reuptake. The astrocytic Na+,K+-ATPase is important for learning (Moseley et al., 2007; Schaefer et al., 2011; Hertz et al., 2013; Tadi et al., 2015). Extracellular K+ concentrations high enough to stimulate the Na+, K+, 2 Cl− co-transporter NKCC1 (>10–12 mM) also causes release of gliotransmitters (Song et al., 2014; Xu et al., 2014b; Liu et al., 2015). Both astrocytic release of glutamate (Lee et al., 2014) and ATP (Gibbs et al., 2011; Stehberg et al., 2012) are crucial for learning. Facilitation of learning by K+-mediated depolarization of oligodendrocytes and increased myelination at high extracellular K+ concentration (Roitbak, 1984) and attributed to increased ability of the myelinated axon to carry out rapid impulse conduction has recently been confirmed and characterized by Yamazaki et al. (2014).
Fluoxetine is better known for its antidepressant effect, which in contrast to the acute stimulation of the 5-HT2B receptor during learning takes several weeks to materialize. During this time many changes occur in gene expression and editing, as shown in mice chronically treated with fluoxetine. Studies in neuronal and astrocytic cell fractions freshly obtained from these mice (Lovatt et al., 2007) showed that most of these alterations occurred in astrocytes, although some neuronal changes also took place (Li et al., 2012; Peng et al., 2014; Hertz et al., 2015b). This finding suggests that astrocytes play a major role in the antidepressant effects of SSRIs (Li et al., 2012; Hertz et al., 2015b), a conclusion in agreement with results by many other authors (e.g., Ongür et al., 1998; Kugaya and Sanacora, 2005; Ongür et al., 2007; Valentine and Sanacora, 2009; Rajkowska and Stockmeier, 2013; Rajkowska et al., 2013; Bernstein et al., 2015; Hertz et al., 2015b and references therein). It is especially interesting that Bechtholt-Gompf et al. (2010) found that blockade of astrocytic glutamate uptake in rats induces signs of anhedonia (a component of depression that is easily measurable in animals) and impaired spatial memory.
Some of the editing changes reduced normally occurring effects of transmitters. Li et al. (2011) showed that in astrocyte cultures treated for sufficient length of time with fluoxetine, the effects on [Ca2+]i by acute administration of several transmitters or ryanodine receptor agonists are reduced or abolished. On the other hand, the effect of an increased extracellular concentration of K+ was increased. Thus, chronic treatment with an SSRI diminishes or alters some of the normal responses of the 5-HT2B receptor to stimulation. This might partly be explained by inhibition of capacitative Ca2+ entry, mediated by glycogenolysis-dependent (Müller et al., 2014) TRPC1 channels, which causes depletion of Ca2+ stores. Due to this inhibition refilling of depleted Ca2+ stores by addition of 2 mM CaCl2 to the medium was greatly reduced (Li et al., 2011). All effects of chronic fluoxetine administration could be replicated by TRPC1 channel antibody. However, the expression of Cav1.2, a gene of an L-channel for Ca2+ which is stimulated by elevations in extracellular K+ concentrations of at least 10 mM is increased (Du et al., 2014), probably explaining the enhanced K+ effect on [Ca2+]i described above. The 5-HT2B receptor itself is also edited by chronic fluoxetine treatment, rapidly reducing the effects of its stimulation of the IP3 receptor (Peng et al., 2014). Since chronic SSRI treatment improves memory in depressed patients (Table in Krysta et al., 2015), inhibition of glutamate-induced increase in astrocytic [Ca2+]i and thus in release of gliotransmitter glutamate (Peng et al., 2012) has no deleterious effect on learning, at least not when combined with [Ca2+]i increase by elevation of the extracellular K+ concentration. In this connection it seems of considerable interest that Medina et al. (2015) described down-regulation of mRNA expression of glutamate transporters, K+ channels and gap junction proteins in hippocampus of patients having suffered from major depression. Most of these genes are selectively expressed in astrocytes. Abnormalities of Na+,K+-ATPase function in depressed patients have been described by De Lores Arnaiz and Ordieres (2014)
Schizophrenia is treatable both by the dopamine antagonist haloperidol and atypical antipsychotics like clozapine, which is an antagonist at the 5-HT2B receptor in the fundus of the stomach (Villazón et al., 2003). Again, acute stimulation of the 5-HT2B receptor is likely to increase [Ca2+]i, glycogenolysis and glutamate formation (Figure 1B). An increase in [Ca2+]i by stimulation of astrocytic dopamine receptors is reduced by exposure to clozapine (Reuss and Unsicker, 2001), and this seems also to be the case after clozapine activation of 5-HT2B receptors. A resulting reduced production of glutamate (Figure 1B) in mice lacking 5-HT2B receptors may explain a decreased content of glutamate in some brain areas (Pitychoutis et al., 2015), which may contribute to the impairment of learning.
Activation of the astrocytic 5-HT2B receptor stimulates an increase in [Ca2+]i, glycogenolysis, glutamate formation, and the effect of glutamate on extracellular K+, all of which are involved in learning (Figure 1B). However, Sibille et al. (2015) found that acute inhibition of Ca2+ signaling in astrocytes by [Ca2+]i chelation potentiates excitatory synaptic transmission. This apparent contradiction may be explained by the complexity of astrocytic [Ca2+]i regulation (Volterra et al., 2014). An important difference between Gibbs and Hertz (2014) and Sibille et al. (2015) is that the latter authors elicited astrocytic increase in [Ca2+]i in response to adjacent neuronal activity during GABAA receptor inhibition, whereas the former described transmitter-induced, glycogenolytic (and thus Ca2+-dependent) effects on learning without GABAA receptor inhibition.
Drugs used for treatment of symptoms of major depression (fluoxetine) and of schizophrenia (clozapine), which included memory impairment, interfered with 5-HT2B receptor-activated functions, but in different manners: the SSRI fluoxetine edited and thereby reduced some normal effects of this receptor, whereas clozapine caused a decrease in [Ca2+]i. This effect is consistent with the enhancement of excitatory synaptic transmission described by Sibille et al. (2015). Disposition to both major depression and schizophrenia is probably inborn, and perhaps these patients display quantitative and/or qualitative abnormalities in 5-HT2B-mediated signaling, which might also affect learning processes. In agreement with this notion 5-HT2B receptors play a major role during brain development (Lauder et al., 2000).
All authors planned or carried out reviewed experiments. YC and LH wrote the paper and MEG edited it.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Allaman, I., Fiumelli, H., Magistretti, P. J., and Martin, J. L. (2011). Fluoxetine regulates the expression of neurotrophic/growth factors and glucose metabolism in astrocytes. Psychopharmacology (Berl) 216, 75–84. doi: 10.1007/s00213-011-2190-y
Bay, V., and Butt, A. M. (2012). Relationship between glial potassium regulation and axon excitability: a role for glial Kir4.1 channels. Glia 60, 651–660. doi: 10.1002/glia.22299
Bechtholt-Gompf, A. J., Walther, H. V., Adams, M. A., Carlezon, W. A. Jr., Ongür, D., and Cohen, B. M. (2010). Blockade of astrocytic glutamate uptake in rats induces signs of anhedonia and impaired spatial memory. Neuropsychopharmacology 35, 2049–2059. doi: 10.1038/npp.2010.74
Bergersen, L. H. (2015). Lactate transport and signaling in the brain: potential therapeutic targets and roles in body-brain interaction. J. Cereb. Blood Flow Metab. 35, 176–185. doi: 10.1038/jcbfm.2014.206
Bernstein, H. G., Meyer-Lotz, G., Dobrowolny, H., Bannier, J., Steiner, J., Walter, M., et al. (2015). Reduced density of glutamine synthetase immunoreactive astrocytes in different cortical areas in major depression but not in bipolar I disorder. Front. Cell. Neurosci. 9:273. doi: 10.3389/fncel.2015.00273
Bonhaus, D. W., Bach, C., Desouza, A., Salazar, F. H., Matsuoka, B. D., Zuppan, P., et al. (1995). The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Br. J. Pharmacol. 115, 622–628. doi: 10.1111/j.1476-5381.1995.tb14977.x
Chen, Y., Peng, L., Zhang, X., Stolzenburg, J. U., and Hertz, L. (1995). Further evidence that fluoxetine interacts with a 5-HT2C receptor in glial cells. Brain Res. Bull. 38, 153–159. doi: 10.1016/0361-9230(95)00082-P
Darvesh, A. S., and Gudelsky, G. A. (2003). Activation of 5-HT2 receptors induces glycogenolysis in the rat brain. Eur. J. Pharmacol. 464, 135–140. doi: 10.1016/S0014-2999(03)01432-8
De Lores Arnaiz, G. R., and Ordieres, M. G. (2014). Brain Na(+), K(+)-ATPase activity in aging and disease. Int. J. Biomed. Sci. 10, 85–102.
Diaz, S. L., Doly, S., Narboux-Nême, N., Fernández, S., Mazot, P., Banas, S. M., et al. (2012). 5-HT(2B) receptors are required for serotonin-selective antidepressant actions. Mol. Psychiatry 17, 154–163. doi: 10.1038/mp.2011.159
Du, T., Liang, C., Li, B., Hertz, L., and Peng, L. (2014). Chronic fluoxetine administration increases expression of the L-channel gene Cav1.2 in astrocytes from the brain of treated mice and in culture and augments K(+)-induced increase in [Ca(2+)]i. Cell Calcium 55, 166–174. doi: 10.1016/j.ceca.2014.01.002
Duran, J., Saez, I., Gruart, A., Guinovart, J. J., and Delgado-García, J. M. (2013). Impairment in long-term memory formation and learning-dependent synaptic plasticity in mice lacking glycogen synthase in the brain. J. Cereb. Blood Flow Metab. 33, 550–556. doi: 10.1038/jcbfm.2012.200
Fortunati, R., Ossola, P., Camerlengo, A., Bettini, E., De Panfilis, C., Tonna, M., et al. (2015). Anhedonia in schizophrenia: the role of subjective experiences. Compr. Psychiatry 62, 152–160. doi: 10.1016/j.comppsych.2015.07.011
Gibbs, M. E., Anderson, D. G., and Hertz, L. (2006). Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia 54, 214–222. doi: 10.1002/glia.20377
Gibbs, M. E., and Bowser, D. N. (2009). Astrocytes and interneurons in memory processing in the chick hippocampus: roles for G-coupled protein receptors, GABA(B) and mGluR1. Neurochem. Res. 34, 1712–1720. doi: 10.1007/s11064-009-9980-1
Gibbs, M. E., and Hertz, L. (2014). Serotonin mediation of early memory formation via 5-HT2B receptor-induced glycogenolysis in the day-old chick. Front. Pharmacol. 5:54 doi: 10.3389/fphar.2014.00054
Gibbs, M. E., Hertz, L., and Ng, K. T. (2004). Inhibition of short-term memory formation in the chick by blockade of extracellular glutamate uptake. Neurobiol. Learn. Mem. 81, 115–119. doi: 10.1016/j.nlm.2003.10.002
Gibbs, M. E., Hutchinson, D., and Hertz, L. (2008). Astrocytic involvement in learning and memory consolidation. Neurosci. Biobehav. Rev. 32, 927–944. doi: 10.1016/j.neubiorev.2008.02.001
Gibbs, M. E., and Hutchinson, D. S. (2012). Rapid turnover of glycogen in memory formation. Neurochem. Res. 37, 2456–2463. doi: 10.1007/s11064-012-0805-2
Gibbs, M. E., Lloyd, H. G., Santa, T., and Hertz, L. (2007). Glycogen is a preferred glutamate precursor during learning in 1-day-old chick: biochemical and behavioral evidence. J. Neurosci. Res. 85, 3326–3333. doi: 10.1002/jnr.21307
Gibbs, M. E., Shleper, M., Mustafa, T., Burnstock, G., and Bowser, D. N. (2011). ATP derived from astrocytes modulates memory in the chick. Neuron Glia Biol. 7, 177–186. doi: 10.1017/S1740925X12000117
Gucek, A., Vardjan, N., and Zorec, R. (2012). Exocytosis in astrocytes: transmitter release and membrane signal regulation. Neurochem. Res. 37, 2351–2363. doi: 10.1007/s11064-012-0773-6
Hertz, L. (2013). The Glutamate-Glutamine (GABA) Cycle: importance of late postnatal development and potential reciprocal interactions between biosynthesis and degradation. Front. Endocrinol. (Lausanne) 4:59. doi: 10.3389/fendo.2013.00059
Hertz, L., Gerkau, N. J., Xu, J., Durry, S., Song, D., Rose, C. R., et al. (2015c). Roles of astrocytic Na(+),K(+)-ATPase and glycogenolysis for K(+) homeostasis in mammalian brain. J. Neurosci. Res. 93, 1019–1030. doi: 10.1002/jnr.23499
Hertz, L., and Rothman, D. L. (in press). “Glucose, lactate, β-hydroxybutyrate, acetate, GABA, succinate as substrates for synthesis of glutamate GABA in the glutamine-glutamate/GABA cycle,” in Advances in Neurobiology, eds U. Sonnewald A. Schousboe (New York, NY: Springer).
Hertz, L., Rothman, D. L., Li, B., and Peng, L. (2015b). Chronic SSRI stimulation of astrocytic 5-HT2B receptors change multiple gene expressions/editings and metabolism of glutamate, glucose and glycogen: a potential paradigm shift. Front. Behav. Neurosci. 9:25. doi: 10.3389/fnbeh.2015.00025
Hertz, L., Xu, J., Song, D., Du, T., Li, B., Yan, E., et al. (2015a). Astrocytic glycogenolysis: mechanisms and functions. Metab. Brain Dis. 30, 317–333. doi: 10.1007/s11011-014-9536-1
Hertz, L., Xu, J., Song, D., Du, T., Yan, E., and Peng, L. (2013). Brain glycogenolysis, adrenoceptors, pyruvate carboxylase, Na(+),K(+)-ATPase and Marie E. Gibbs' pioneering learning studies. Front. Integr. Neurosci. 7:20. doi: 10.3389/fnint.2013.00020
Howarth, C., Gleeson, P., and Attwell, D. (2012). Updated energy budgets for neural computation in the neocortex and cerebellum. J. Cereb. Blood Flow Metab. 32, 1222–1232. doi: 10.1038/jcbfm.2012.35
Ibrahim, M. Z. (1975). Glycogen and its related enzymes of metabolism in the central nervous system. Adv. Anat. Embryol. Cell Biol. 52, 3–89. doi: 10.1007/978-3-642-86875-7
Kalueff, A., and Nutt, D. J. (1996). Role of GABA in memory and anxiety. Depress. Anxiety 4, 100–110.
Kalueff, A. V. (2007). Neurobiology of memory and anxiety: from genes to behavior. Neural Plast. 2007:78171. doi: 10.1155/2007/78171
Kant, D., Tripathi, S. S., Qureshi, M. F., Tripathi, S. II, Pandey, S., Singh, G., et al. (2014). The effect of glial glutamine synthetase inhibition on recognition and temporal memories in the rat. Neurosci. Lett. 560, 98–102. doi: 10.1016/j.neulet.2013.12.033
Kong, E. K., Peng, L., Chen, Y., Yu, A. C., and Hertz, L. (2002). Up-regulation of 5-HT2B receptor density and receptor-mediated glycogenolysis in mouse astrocytes by long-term fluoxetine administration. Neurochem. Res. 27, 113–120. doi: 10.1023/A:1014862808126
Krysta, K., Krzystanek, M., Janas-Kozik, M., Klasik, A., and Krupka-Matuszczyk, I. (2015). Impact of pharmacological and psychological treatment methods of depressive and anxiety disorders on cognitive functioning. J. Neural Transm. 122(Suppl. 1), 101–110. doi: 10.1007/s00702-014-1282-3
Kugaya, A., and Sanacora, G. (2005). Beyond monoamines: glutamatergic function in mood disorders. CNS Spectr. 10, 808–819.
Lauder, J. M., Wilkie, M. B., Wu, C., and Singh, S. (2000). Expression of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors in the mouse embryo. Int. J. Dev. Neurosci. 18, 653–662. doi: 10.1016/S0736-5748(00)00032-0
Lee, H. S., Ghetti, A., Pinto-Duarte, A., Wang, X., Dziewczapolski, G., Galimi, F., et al. (2014). Astrocytes contribute to gamma oscillations and recognition memory. Proc. Natl. Acad. Sci. U.S.A. 111, E3343–E3352. doi: 10.1073/pnas.1410893111
Li, B., Dong, L., Fu, H., Wang, B., Hertz, L., and Peng, L. (2011). Effects of chronic treatment with fluoxetine on receptor-stimulated increase of [Ca2+]i in astrocytes mimic those of acute inhibition of TRPC1 channel activity. Cell Calcium 50, 42–53. doi: 10.1016/j.ceca.2011.05.001
Li, B., Dong, L., Wang, B., Cai, L., Jiang, N., and Peng, L. (2012). Cell type-specific gene expression and editing responses to chronic fluoxetine treatment in the in vivo mouse brain and their relevance for stress-induced anhedonia. Neurochem. Res. 37, 2480–2495. doi: 10.1007/s11064-012-0814-1
Li, B., Zhang, S., Zhang, H., Nu, W., Cai, L., Hertz, L., et al. (2008). Fluoxetine-mediated 5-HT2B receptor stimulation in astrocytes causes EGF receptor transactivation and ERK phosphorylation. Psychopharmacology (Berl) 201, 443–458. doi: 10.1007/s00213-008-1306-5
Liu, Z., Song, D., Yan, E., Verkhratsky, A., and Peng, L. (2015). Chronic treatment with anti-bipolar drugs suppresses glutamate release from astroglial cultures. Amino Acids 47, 1045–1051. doi: 10.1007/s00726-015-1936-y
Lovatt, D., Sonnewald, U., Waagepetersen, H. S., Schousboe, A., He, W., Lin, J. H., et al. (2007). The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J. Neurosci. 27, 12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007
MacAulay, N., and Zeuthen, T. (2012). Glial K(+) clearance and cell swelling: key roles for cotransporters and pumps. Neurochem. Res. 37, 2299–2309. doi: 10.1007/s11064-012-0731-3
Medina, A., Watson, S. J., Bunney, W. Jr., Myers, R. M., Schatzberg, A., Barchas, J., et al. (2015). Evidence for alterations of the glial syncytial function in major depressive disorder. J. Psychiatr. Res. 72, 15–21. doi: 10.1016/j.jpsychires.2015.10.010
Moseley, A. E., Williams, M. T., Schaefer, T. L., Bohanan, C. S., Neumann, J. C., Behbehani, M. M., et al. (2007). Deficiency in Na,K-ATPase alpha isoform genes alters spatial learning, motor activity, and anxiety in mice. J. Neurosci. 27, 616–626. doi: 10.1523/JNEUROSCI.4464-06.2007
Müller, M. S., Fox, R., Schousboe, A., Waagepetersen, H. S., and Bak, L. K. (2014). Astrocyte glycogenolysis is triggered by store-operated calcium entry and provides metabolic energy for cellular calcium homeostasis. Glia 62, 526–534. doi: 10.1002/glia.22623
Newman, L. A., Korol, D. L., and Gold, P. E. (2011). Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS ONE 6:e28427. doi: 10.1371/journal.pone.0028427
O'Donnell, J., Zeppenfeld, D., Mcconnell, E., Pena, S., and Nedergaard, M. (2012). Norepinephrine: a neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem. Res. 37, 2496–2512. doi: 10.1007/s11064-012-0818-x
Ongür, D., Drevets, W. C., and Price, J. L. (1998). Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc. Natl. Acad. Sci. U.S.A. 95, 13290–13295. doi: 10.1073/pnas.95.22.13290
Ongür, D., Pohlman, J., Dow, A. L., Eisch, A. J., Edwin, F., Heckers, S., et al. (2007). Electroconvulsive seizures stimulate glial proliferation and reduce expression of Sprouty2 within the prefrontal cortex of rats. Biol. Psychiatry 62, 505–512. doi: 10.1016/j.biopsych.2006.11.014
Ozawa, E. (2011). Regulation of phosphorylase kinase by low concentrations of Ca ions upon muscle contraction: the connection between metabolism and muscle contraction and the connection between muscle physiology and Ca-dependent signal transduction. Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci. 87, 486–508. doi: 10.2183/pjab.87.486
Peng, L., Gu, L., Li, B., and Hertz, L. (2014). Fluoxetine and all other SSRIs are 5-HT2B agonists - importance for their therapeutic effects. Curr. Neuropharmacol. 12, 365–379. doi: 10.2174/1570159X12666140828221720
Peng, L., Li, B., Du, T., Wang, F., and Hertz, L. (2012). Does conventional anti-bipolar and antidepressant drug therapy reduce NMDA-mediated neuronal excitation by downregulating astrocytic GluK2 function? Pharmacol. Biochem. Behav. 100, 712–725. doi: 10.1016/j.pbb.2011.03.021
Pfeiffer-Guglielmi, B., Fleckenstein, B., Jung, G., and Hamprecht, B. (2003). Immunocytochemical localization of glycogen phosphorylase isozymes in rat nervous tissues by using isozyme-specific antibodies. J. Neurochem. 85, 73–81. doi: 10.1046/j.1471-4159.2003.01644.x
Pitychoutis, P. M., Belmer, A., Moutkine, I., Adrien, J., and Maroteaux, L. (2015). Mice lacking the serotonin Htr2B receptor gene present an antipsychotic-sensitive schizophrenic-like phenotype. Neuropsychopharmacology 40, 2764–2773. doi: 10.1038/npp.2015.126
Qiao, J., Wang, J., Wang, H., Zhang, Y., Zhu, S., Adilijiang, A., et al. (2015). Regulation of astrocyte pathology by fluoxetine prevents the deterioration of Alzheimer phenotypes in an APP/PS1 mouse model. Glia. doi: 10.1002/glia.22926. [Epub ahead of print].
Rajkowska, G., Hughes, J., Stockmeier, C. A., Javier Miguel-Hidalgo, J., and Maciag, D. (2013). Coverage of blood vessels by astrocytic endfeet is reduced in major depressive disorder. Biol. Psychiatry 73, 613–621. doi: 10.1016/j.biopsych.2012.09.024
Rajkowska, G., and Stockmeier, C. A. (2013). Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr. Drug Targets 14, 1225–1236. doi: 10.2174/13894501113149990156
Ren, Q. G., Gong, W. G., Wang, Y. J., Zhou, Q. D., and Zhang, Z. J. (2015). Citalopram attenuates tau hyperphosphorylation and spatial memory deficit induced by social isolation rearing in middle-aged rats. J. Mol. Neurosci. 56, 145–153. doi: 10.1007/s12031-014-0475-4
Reuss, B., and Unsicker, K. (2001). Atypical neuroleptic drugs downregulate dopamine sensitivity in rat cortical and striatal astrocytes. Mol. Cell. Neurosci. 18, 197–209. doi: 10.1006/mcne.2001.1017
Riedel, G., Platt, B., and Micheau, J. (2003). Glutamate receptor function in learning and memory. Behav. Brain Res. 140, 1–47. doi: 10.1016/S0166-4328(02)00272-3
Roitbak, A. I. (1984). Neuroglia: Eigenschaften, Funktionen, Bedeutung. Jena: Gustav Fischer Verlag.
Schaefer, T. L., Lingrel, J. B., Moseley, A. E., Vorhees, C. V., and Williams, M. T. (2011). Targeted mutations in the Na,K-ATPase alpha 2 isoform confer ouabain resistance and result in abnormal behavior in mice. Synapse 65, 520–531. doi: 10.1002/syn.20870
Schmuck, K., Ullmer, C., Engels, P., and Lübbert, H. (1994). Cloning and functional characterization of the human 5-HT2B serotonin receptor. FEBS Lett. 342, 85–90. doi: 10.1016/0014-5793(94)80590-3
Sibille, J., Zapata, J., Teillon, J., and Rouach, N. (2015). Astroglial calcium signaling displays short-term plasticity and adjusts synaptic efficacy. Front. Cell. Neurosci. 9:189. doi: 10.3389/fncel.2015.00189
Sickmann, H. M., Walls, A. B., Schousboe, A., Bouman, S. D., and Waagepetersen, H. S. (2009). Functional significance of brain glycogen in sustaining glutamatergic neurotransmission. J. Neurochem. 109(Suppl. 1), 80–86. doi: 10.1111/j.1471-4159.2009.05915.x
Song, D., Xu, J., Bai, Q., Cai, L., Hertz, L., and Peng, L. (2014). Role of the intracellular nucleoside transporter ENT3 in transmitter and high K+ stimulation of astrocytic ATP release investigated using siRNA against ENT3. ASN Neuro 6:1759091414543439. doi: 10.1177/1759091414543439
Stehberg, J., Moraga-Amaro, R., Salazar, C., Becerra, A., Echeverrìa, C., Orellana, J. A., et al. (2012). Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. FASEB J. 26, 3649–3657. doi: 10.1096/fj.11-198416
Suzuki, A., Stern, S. A., Bozdagi, O., Huntley, G. W., Walker, R. H., Magistretti, P. J., et al. (2011). Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144, 810–823. doi: 10.1016/j.cell.2011.02.018
Tadi, M., Allaman, I., Lengacher, S., Grenningloh, G., and Magistretti, P. J. (2015). Learning-induced gene expression in the hippocampus reveals a role of neuron -astrocyte metabolic coupling in long term memory. PLoS ONE 10:e0141568. doi: 10.1371/journal.pone.0141568
Tang, F., Lane, S., Korsak, A., Paton, J. F., Gourine, A. V., Kasparov, S., et al. (2014). Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat. Commun. 5, 3284. doi: 10.1038/ncomms4284
Valentine, G. W., and Sanacora, G. (2009). Targeting glial physiology and glutamate cycling in the treatment of depression. Biochem. Pharmacol. 78, 431–439. doi: 10.1016/j.bcp.2009.04.008
Villazón, M., Enguix, M. J., Tristan, H., Honrubia, M. A., Brea, J., Maayani, S., et al. (2003). Different pharmacological properties of two equipotent antagonists (clozapine and rauwolscine) for 5-HT2B receptors in rat stomach fundus. Biochem. Pharmacol. 66, 927–937. doi: 10.1016/S0006-2952(03)00426-X
Volterra, A., Liaudet, N., and Savtchouk, I. (2014). Astrocyte Ca(2)(+) signalling: an unexpected complexity. Nat. Rev. Neurosci. 15, 327–335. doi: 10.1038/nrn3725
Wall, P. M., and Messier, C. (2000). Concurrent modulation of anxiety and memory. Behav. Brain Res. 109, 229–241. doi: 10.1016/S0166-4328(99)00177-1
Wong, D. T., and Bymaster, F. P. (1995). Development of antidepressant drugs. fluoxetine (Prozac) and other selective serotonin uptake inhibitors. Adv. Exp. Med. Biol. 363, 77–95. doi: 10.1007/978-1-4615-1857-0_11
Xu, J., Song, D., Bai, Q., Cai, L., Hertz, L., and Peng, L. (2014a). Basic mechanism leading to stimulation of glycogenolysis by isoproterenol, EGF, elevated extracellular K+ concentrations, or GABA. Neurochem. Res. 39, 661–667. doi: 10.1007/s11064-014-1244-z
Xu, J., Song, D., Bai, Q., Zhou, L., Cai, L., Hertz, L., et al. (2014b). Role of glycogenolysis in stimulation of ATP release from cultured mouse astrocytes by transmitters and high K+ concentrations. ASN Neuro 6:e00132. doi: 10.1042/AN20130040
Yamazaki, Y., Fujiwara, H., Kaneko, K., Hozumi, Y., Xu, M., Ikenaka, K., et al. (2014). Short- and long-term functional plasticity of white matter induced by oligodendrocyte depolarization in the hippocampus. Glia 62, 1299–1312. doi: 10.1002/glia.22681
Zhang, S., Li, B., Lovatt, D., Xu, J., Song, D., Goldman, S. A., et al. (2010). 5-HT2B receptors are expressed on astrocytes from brain and in culture and are a chronic target for all five conventional ‘serotonin-specific reuptake inhibitors’. Neuron Glia Biol. 6, 113–125. doi: 10.1017/S1740925X10000141
Keywords: astrocytes, calcium, clozapine, fluoxetine, memory, psychiatric disorder
Citation: Chen Y, Du T, Peng L, Gibbs ME and Hertz L (2016) Sequential Astrocytic 5-HT2B Receptor Stimulation, [Ca2+]i Regulation, Glycogenolysis, Glutamate Synthesis, and K+ Homeostasis are Similar but Not Identical in Learning and Mood Regulation. Front. Integr. Neurosci. 9:67. doi: 10.3389/fnint.2015.00067
Received: 06 October 2015; Accepted: 14 December 2015;
Published: 08 January 2016.
Edited by:
Sidney A. Simon, Duke University, USAReviewed by:
Alberto Granato, Catholic University, ItalyCopyright © 2016 Chen, Du, Peng, Gibbs and Hertz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leif Hertz, bGhlcnR6NTM4QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.