
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Integr. Neurosci., 15 May 2012
Volume 6 - 2012 | https://doi.org/10.3389/fnint.2012.00022
This article is part of the Research TopicCognitive Functions of the Posterior Parietal CortexView all 14 articles
When several different objects are presented, visual objects are perceived correctly only if their features are identified and then bound together. Illusory-conjunction errors result when an object is correctly identified but is combined incorrectly. The parietal cortex (PC) has been shown repeatedly to play an important role in feature binding. The present study builds on a series of recent studies that have made use of visual search paradigms to elucidate the neural system involved in feature binding. This experiment attempts to define the role the PC plays in binding the properties of a visual object that varies on the features of color and size in rats. Rats with PC lesions or control surgery were exposed to three blocks of 20 trials administered over a 1-week period, with each block containing 10-one feature and 10-two feature trials. The target object consisted of one color object (e.g., black and white) and one size object (e.g., short and tall). Of the 10 one feature trials, five of the trials were tailored specifically for size discrimination and five for color discrimination. In the two-feature condition, the animal was required to locate the targeted object among four objects with two objects differing in size and two objects differing in color. The results showed that the PC lesioned compared to control rats had difficulty in learning the one and two features components of the task and the rats also performed more poorly on the one vs. two feature components of the task. Based on a subsequent error analysis for color and size, the results showed a significant increase in illusory conjunction errors for the PC lesioned rats relative to controls for color and relative to color discrimination, suggesting that the PC may support feature binding as it relates to color. There was an increase in illusory conjunctions errors for both the PC lesioned and control animals for size, but this appeared to be due to highly variable performance with size discrimination. Overall these results suggest that the PC rats display performance errors that appear to be consistent with the notion of illusory conjunction errors.
It has been suggested that the parietal cortex (PC) may play an important role in binding features of objects, objects, and places, as well as egocentric and allocentric spatial processing. There are data with rodents that support a role for the PC in cross-modal, as well as egocentric and allocentric spatial processing (Long et al., 1998; Rogers and Kesner, 2007), but there are no data that have assessed the role of the PC in rodents on binding of object features. Treisman (1998) suggested that the binding of different features of objects may involve using spatial attention to locations to aid in the selection of various features that are currently active in the same location, while suppressing features from other locations to prevent erroneous binding. Furthermore, the PC may play a very important role in ensuring that illusory conjunction errors do not appear in a variety of tasks including search tasks. Thus, the PC may be directly involved in perceptual binding between, for example, a shape and a color or a shape and a size requiring spatial attention. Support for this idea comes from the performance of patient RM with bilateral PC damage, who had difficulty in tasks requiring binding shape and color or shape and size. When shown two different colored letters, RM made many errors in the form of illusory conjunctions combining the shape of one letter with the color of the other (Friedman-Hill et al., 1995). Similarly, in a visual search task requiring the detection of a target based on the conjunction of two features, RM made many errors, but RM had no difficulty in detecting a target based on one feature (Robertson et al., 1997). The study was designed to develop an animal model of feature binding and determine whether PC lesions relative to sham lesions in rats result in the production of illusory conjunction errors using a visual search paradigm similar to the (Robertson et al., 1997) study with objects that varied either only on features of color or size (one feature) or the combination of color and size (two features).
Eleven male Long–Evans rats initially weighing ∼350 g were used as subjects. At the beginning of the study, all rats were food-deprived to 80% of their free-feed weight and allowed access to water ad libitum. The rats were housed independently in standard plastic rodent cages and maintained on a 12-h light/dark cycle. All testing was conducted in the light portion of the light/dark cycle.
A white cheese board served as the testing apparatus for the experiment. The surface of the apparatus stood 65 cm above the floor, was 119 cm in diameter, and was 3.5 cm in thickness. One-hundred and seventy-seven food wells (2.5 cm in diameter and 1.5 cm in depth) were drilled into surface of the round board in evenly spaced parallel rows and columns, which were 5 cm apart. The apparatus was kept in a well-lit room with no windows; one door, a chair, a small table, and posters on the walls served as distal spatial cues. A black start box (24 cm long, 15 cm wide, and 17 cm high) was constructed to house the rat between trials. The black box was positioned on top of the round board perpendicular to the rows and parallel to the columns with the posterior edge of the box at the edge of the cheeseboard. The box had a hinged top for easily transferring animals into and out of the box. The front of the box had a guillotine door that could only be raised and lowered by the experimenter. Stimuli were three-dimensional wooden block objects 2 cm in diameter that differed from each other in both color (black or white) and size (4 or 6 cm in height).
During the first week of training, rats were handled 15 min daily. During the second week of training, rats were introduced to the apparatus. Rats were given 15 min to explore the white cheese board. Froot Loops (Kellogg, Battle Creek, MI) were randomly distributed over the maze to induce exploration.
Rats were anesthetized with pentobarbital (Nembutal; 60 mg/kg i.p.). Each rat was placed in a stereotaxic apparatus (David Kopf Instruments) with an isothermal heating pad to maintain body temperature at 37°C. With its head level, the scalp was incised and retracted to expose bregma and lambda and positioned them in the same horizontal plane. PC lesions were made via aspiration. The lesions were 1 mm posterior to bregma to 4.5 mm posterior to bregma, 2 mm lateral to midline to approximately 1 mm above the rhinal sulcus in the medial-lateral plane, and 2 mm ventral to dura. Control lesions underwent the same procedure as the PC lesioned rats, except that no tissue was removed. Following surgery, the incisions were sutured and the rats were allowed to recover for one week before experimentation. They also received Children's Tylenol in water as an analgesic. All animal care and experimental procedures conformed to the National Institutes of Health and Institution for Animal Care and Use Committee guidelines for proper care and use of experimental animals.
Three blocks of 20 trials were administered over a 1-week period, and each block contained 10-one feature and 10-two feature trials. The target object consisted of one color object (e.g., black and white) or one size object (e.g., short and tall). Of the 10-one feature trials, five of the trials were tailored specifically for size discrimination and five for object discrimination. In the one-feature condition the subject was required to locate the targeted object among four other objects that differed in either color or height, i.e., if the target object was a small black block, then four small white blocks for the color condition, and four tall black blocks for the size condition would surround the object. In the two-feature condition, the animal was required to locate the targeted object among four objects with two objects differing in size and two objects differing in color. For both the one- and two-feature conditions, the target object for each animal was randomly predetermined and remained consistent throughout the experiment, whereas placing of the other objects varied on each trial. The rule to be learned in order to obtain a food reward was to discriminate between the size and colored objects in order to displace the targeted object. For each trial, the randomly targeted object covered a baited food-well in one of five randomly assigned spatial locations. The inter-trial interval was 30 s. The number of errors for each trial was recorded and the food reward was Froot Loops breakfast cereal (Kellogg's).
At the end of the experiments, each rat was given a lethal intraperitoneal injection of sodium pentobarbital. The rat was perfused intracardially with 10% (wt/vol) formalin in 0.1 M phosphate buffer. The brain was then removed and stored in 30% (vol/vol) sucrose-formalin for one week. Transverse sections (24 μm) were cut with a cryostat through the lesioned area and stained with cresyl violet.
The PC lesions extended from 1 mm posterior to bregma to 4.5 mm posterior to bregma, and 2 mm lateral to midline to approximately 1 mm above the rhinal sulcus in the medial-lateral plane (Figure 1). There was some sparing of the PPC at the ventrolateral aspect adjacent to the temporal association cortex (TeA) as well as some sparing between 1 and 2 mm lateral to midline. The PC lesions generally did not result in damage to the dorsal or ventral hippocampus, fimbria/fornix, or temporal cortices.
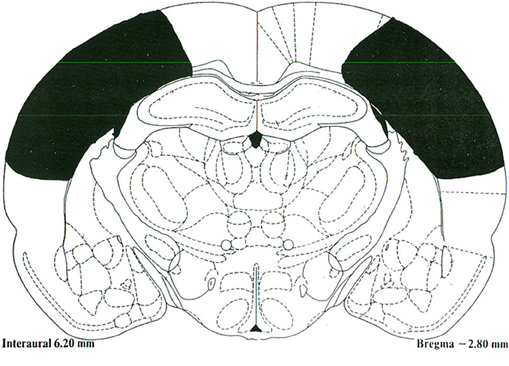
Figure 1. A schematic representative lesion of the posterior parietal cortex projected unto a stereotaxic map of the rat brain [Paxinos and Watson (1997)].The Rat Brain: In Stereotaxic Coordinates. San Diego, CA: Academic Press.
For all three analyses a repeated measures ANOVA with groups (control and PC) as the between variable and trials (blocks 1, 2, and 3) as well as features (one and two) as the within variables was used. When applicable a Neman–Keuls paired comparison test was used. Even though rats could make multiple errors, the acquisition data were analyzed based only on whether the first response was an error or was correct and was displayed as mean percent correct. In contrast, for the size and color search analysis all errors were counted and were displayed as mean total for color or size errors.
The results are shown in Figure 2 and indicate that for the control rats the mean percent correct performance improved across blocks of trials for both the one- and two-feature condition, but for the PC lesioned rats there was better performance for the one compared to the two-feature condition, but little improvement across blocks of trials. The analysis revealed a significant group effect [F(1, 10) = 8.26, p = 0.016], a significant blocks of trials effect [F(2, 20) = 5.67, p = 0.011], and a significant feature effect [F(1, 10) = 16.53, p = 0.002], but no significant interactions. These data suggest that PC rats make many errors resulting in impaired performance especially for both the one- and two-feature condition suggesting that they are susceptible to discrimination problems as well as the making of illusory conjunction errors.
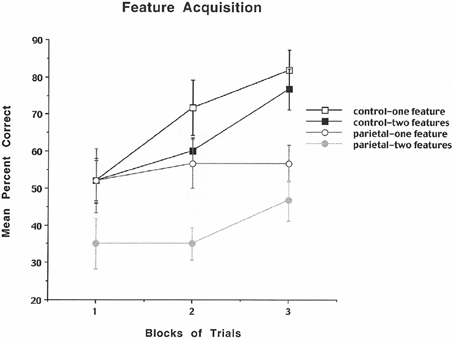
Figure 2. Mean number of search errors for one or two features for control and parietal cortex lesioned rats as a function of blocks of trials. Each block consisted of 20 trials.
To analyze further whether the errors were either based on problems with size or color discrimination, the data were analyzed in terms of mean total number of color or size errors across blocks of trials for the one- and two-feature conditions. The results for mean number of errors for color are shown in Figure 3 and indicate that for the control rats the mean total number of errors decreased across blocks of trials for both the one- and two-feature condition. For the first block the PC lesioned rats displayed a high mean total number of errors for the two-feature condition relative to the one-feature condition and for the one- and two-feature conditions for the control group. For the third block of trials the PC lesioned rats displayed a high mean total number of errors in both the one- and two-feature conditions relative to the control one- and two-feature conditions. The analysis revealed a significant group effect [F(1, 10) = 25.4, p < 0.0005], a significant blocks of trials effect [F(2, 20) = 10.14, p = 0.0009], a significant feature effect [F(1, 10) = 5.1, p = 0.047], and a significant interaction between groups, blocks of trials, and features [F(2, 20) = 4.3, p = 0.028]. A subsequent Newman–Keuls test for the interaction effect revealed that for the first block the PC lesioned rats displayed a significantly higher mean total number of errors for the two-feature condition relative to the one-feature condition and for the one- and two-feature conditions for the control group (p < 0.01). For the third block of trials the PC lesioned rats displayed a significantly higher mean total number of errors in both the one- and two-feature conditions relative to the control one- and two-feature conditions (p < 0.05). The results for color errors indicate that PC lesioned rats relative to controls made only a few errors in detecting the one feature component of the task, but they made many errors throughout all three blocks of trials for the two-feature condition suggesting the appearance of illusory conjunction errors.
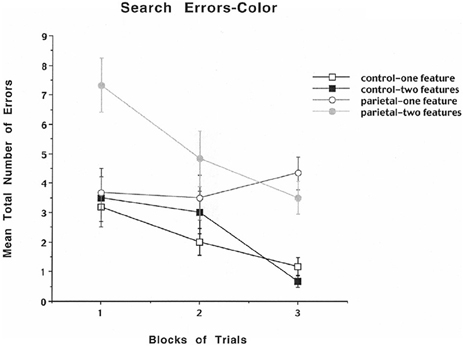
Figure 3. Mean number of search errors for one or two features for object color for control and parietal cortex lesioned rats as a function of blocks of trials. Each block consisted of 20 trials.
The results for mean number of errors for size are shown in Figure 4 and indicate that there are no obvious differences between the PC and control groups for either one or two features in part due to the variability in the results. A similar repeated measures ANOVA that was used to analyze search errors for color was used for search errors for size. The analysis revealed that there were no significant differences. Even though there was an increase in illusory conjunctions errors for both the PC lesion and control animals for size, this increase was not significant which is likely due to enhanced variability in performing the size discrimination.
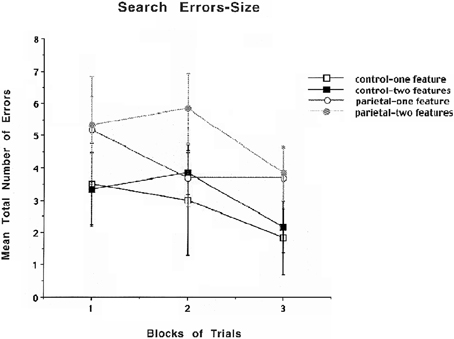
Figure 4. Mean number of search errors for one or two features for object size for control and parietal cortex lesioned rats as a function of blocks of trials. Each block consisted of 20 trials.
The data show that the control animals displayed a small but not significant increase in errors for the two compared to the one-feature condition. It is assumed by Treisman's (1998) feature integration theory that the two-feature condition is more difficult than the one-feature condition requiring the recruitment of attentional processes, so there might be a possibility that the task for control rats was not difficult enough and thus requiring minimal recruitment of attentional processes. Even though the control rats did not differ significantly in terms of the one- vs. two-feature condition for shapes or color, there are also data with humans showing that using shapes and color that parallel the findings with rats in that there was no significant difference in latency to respond to the one compared to the two-feature condition (Shafritz et al., 2002). The data also show that PC lesions in rats appear to disrupt acquisition of the task, which could be due to the difficulty in discriminating the features of the task, but in the first two blocks of trials, the PC lesioned rats do not show a deficit for the one-feature condition, but show a clear deficit for the two-feature condition suggesting that the PC may indeed be involved in feature binding as reflected by illusory conjunction errors. The data with PC lesions in rats parallel the findings with PC in humans in that a bilateral parietal damaged patient made consistent illusory conjunction errors in a visual search task based on the conjunction of two features of an object (Robertson et al., 1997).
The results also show a significant increase in illusory conjunction errors for the PC lesioned rats relative to controls for color and relative to color discrimination, suggesting that the PC may support feature binding as it relates to color. The lack of a significant effect for size is likely due to the size difference of 2 cm that was used in this experiment, especially because in more recent research findings, it can be shown that rats in an exploratory-based paradigm detect a novelty change in size only when the size differs in 6 or 8 cm, but not 2 or 4 cm (unpublished observations). Even though the control rats appeared to have more difficulty with shapes compared to color, the previously mentioned study (Shafritz et al., 2002) reported that the participants were also less accurate with shapes compared to color, which is consistent with the rat data. Thus, it appears that the PC in rats supports the binding of visual features within objects or landmarks, a process which has been assumed to be mediated by spatial attention. One should also note that there is the possibility that the PC rats are performing a single feature match in the two-feature condition.
One additional role for the rodent PC could be to bind across modalities to maintain the association between landmark and spatial location information. In other words, the PC may not be involved in memory for a single landmark or a single spatial location, but rather in the processing that assigns a specific landmark to a specific spatial location. To test this hypothesis, rats with small lesions of the PC were tested in an object/spatial location paired-associate task that required concurrent memory for both object and spatial location information. In addition, memory for a landmark only or a spatial location only information was also assessed. The results indicated that small lesions of the PC as defined by Reep et al. (1994) and larger PC lesions disrupted learning of the object-place paired-associate task, but did not disrupt the learning of a spatial or object discrimination (Long et al., 1998). The deficit in the paired associate task (which requires memory for both landmark and spatial location information), in the absence of deficits in either the landmark or the spatial location only memory, supports the idea that the PC is involved in the memory for the binding of landmark and spatial location information. Even though there are many studies in humans that report on the role of PC in processing of objects or spatial locations, there are not many articles that have dealt with the binding of objects and locations. One study (van Asselen et al., 2009) examined a population of stroke patients with varying degrees of PC damage. The results showed that in a combined object-place task, there was an impairment that was primarily due to damage in the left posterior PC. Thus, there appear to be some parallels in the binding function between locations and landmarks in rats and humans.
Another role for the rodent PC could be to bind egocentric and allocentric information in long-term memory comes from a study by Rogers and Kesner (2007). They trained rats in two versions of a modified Hebb–Williams maze to test the role of the PC in processing egocentric and allocentric information during acquisition and retention. In the first version, unlike traditional Hebb–Williams mazes, the maze was made of 1.3 cm Plexiglas, measuring 25 cm in height with a 7.5 cm strip, also painted black, placed on the bottom of the barriers. This spatial arrangement allowed the rat to use extra maze cues. Extra maze cues included two posters, a map, and a hanging doll. Given that this maze allowed for the use of extra maze cues, learning might be primarily based on allocentric cues, so they labeled this task an allocentric task. The second maze used in these experiments was the same modified Hebb–Williams maze mentioned above; however, the walls were 50.8 cm high, made of 0.6 cm red Plexiglas. The apparatus was kept in a well-lit room with no windows or extramaze cues. This maze is assumed to be learned primarily on the basis of egocentric and local topological cues, because the walls were raised, made opaque, and there were few, if any, extra maze cues. They labeled this task as an egocentric task. Bilateral lesions were made to PC before maze testing (acquisition) or after maze testing (retention). The results indicated that lesions of the PC impaired egocentric maze acquisition, but the animals had no difficulty in learning the allocentric version of the maze task. Similar deficits following PC lesions were reported by Boyd and Thomas (1977) during acquisition of the standard Hebb–Williams maze, which did not give the rats an opportunity to use extra maze cues. During retention, lesions of the PC produced a significant impairment on both maze versions, suggesting that the PC may be combining both egocentric and allocentric information during normal learning of the maze, but after a PC lesion the combined information may not be available to the animal. These results suggest that long-term retention of spatial information requires that the PC binds egocentric and allocentric information.
Thus, it appears that the PC in rats may play an important role in binding features of objects, cross-modal (objects and spatial locations), as well as egocentric and allocentric spatial processing.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
I would like to thank Kristen Larsen for running the experiment. The research was supported by Grant sponsor: National Institutes of Health; Grant number: 5R01MH065314-02.
Boyd, M. G., and Thomas, R. K. (1977). Posterior association cortex lesions in rats: mazes, pattern discrimination and reversal learning. Physiol. Psychol. 5, 455–461.
Friedman-Hill, S., Robertson, L., and Treisman, A. (1995). Parietal contributions to visual feature binding: evidence from a patient with bilateral lesions. Science 269, 853–855.
Long, J. M., Mellem, J. E., and Kesner, R. P. (1998). The effects of parietal cortex lesions on an object/spatial location paired-associate task in rats. Psychobiology 26, 128–133.
Paxinos, G., and Watson, C. (1997). The Rat Brain: In Stereotaxic Coordinates. San Diego, CA: Academic Press.
Reep, R. L., Chandler, H. C., King, V., and Corwin, J. V. (1994). Rat posterior parietal cortex: topography of cortico-cortical and thalamic connections. Exp. Brain Res. 100, 67–84.
Robertson, L., Treisman, A., Friedman-Hill, S., and Grabowecky, M. (1997). The interaction of spatial and object pathways: evidence from Balint's syndrome. J. Cogn. Neurosci. 9, 295–317.
Rogers, J. L., and Kesner, R. P. (2007). Hippocampal-parietal cortex interactions: evidence from a disconnection study in the rat. Behav. Brain Res. 179, 19–27.
Shafritz, K. H., Gore, J. C., and Marois, R. (2002). The role of the parietal cortex in visual feature binding. Proc. Natl. Acad. Sci. U.S.A. 99, 10917–10922.
Treisman, A. (1998). Feature binding, attention and object perception. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353, 1295–1306.
Keywords: illusory conjunctions, parietal cortex, rats
Citation: Kesner RP (2012) Parietal lesions produce illusory conjunction errors in rats. Front. Integr. Neurosci. 6:22. doi: 10.3389/fnint.2012.00022
Received: 16 December 2011; Accepted: 03 May 2012;
Published online: 15 May 2012.
Edited by:
David J. Bucci, Dartmouth College, USAReviewed by:
Kevin Pang, VA Medical Center - New Jersey Health Care System, USACopyright: © 2012 Kesner. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Raymond P. Kesner, Department of Psychology, University of Utah, 380 S. 1530 E. Rm. 502, Salt Lake City, UT 84112, USA. e-mail:cmF5Lmtlc25lckBwc3ljaC51dGFoLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.