
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Insect Sci. , 06 March 2025
Sec. Invasive Insect Species
Volume 5 - 2025 | https://doi.org/10.3389/finsc.2025.1558242
This study use landmark based geometric morphometrics (GM) of the head and the thorax on eight species of thrips of the species-rich genus Thrips. Among the selected species, four were classified as common and not significant, while four were identified as quarantine-significant and agriculturally important in the USA. The results indicate the potential for using both sets of landmarks, which, in some cases, were complementary. When one set did not reveal significant differences in shape, the other provided valuable insights. The geometric morphometric analysis of the selected landmarks revealed statistically significant differences in head morphology and the configuration of setal insertion points on the mesothorax and metathorax. Principal component analysis (PCA) served as the primary method to examine the ordinal distribution of the eight species within the morphospace. The analysis highlighted T. australis and T. angusticeps as the most morphologically distinct species in terms of head shape, while T. nigropilosus, T. obscuratus, and T. hawaiiensis exhibited the greatest divergence in thoracic morphology. The results further demonstrate the potential of geometric morphometric (GM) methods for identifying taxa that are challenging to distinguish using traditional taxonomy based on external morphology. This is particularly relevant for morphologically conservative taxa, such as thrips with minimal or no wing venation (a feature often used in GM studies of winged insects), species complexes (e.g., T. hawaiiensis and related species examined in this study), and taxa exhibiting morphological similarity due to convergent evolution associated with shared ecological niches.
Thysanoptera (commonly named thrips) comprises more than 7000 described species. Currently, nearly 850 genera are accepted (including 65 genera of fossils), the suprageneric classification remains in flux, and the evolutionary relationships among groups are still obscure, resulting in nearly half of the recognized genera being monotypic/monobasic (there are almost 440 genera with only one species). In addition, around 230 genera include no more than five species, and only ten genera include more than 100 species, in which the genus Thrips is included (1, 2).
The suborder Terebrantia comprises eight extant families, the largest of which is Thripidae, which includes more than 2000 species in nearly 290 genera (1). The family Thripidae has been generally divided into four subfamilies (Dendrothripinae, Sericothripinae, Panchaetothripinae, and Thripinae), of which Thripinae is the largest, with more than 1800 species and over 200 genera (3). According to Masumoto and Okajima (4), even though the suprageneric relationships within the subfamily Thripinae are unclear, some monophyletic groups are recognized (5–7) one of which is the genus Thrips, which are among the most important agricultural pests globally because of the damage inflicted by their oviposition, feeding, and their ability to transmit plant viruses (8).
With over 280 species of Thrips worldwide, many of which are common pests and vectors of viruses, the need for accurate identification is not just critical but crucial. However, their identification can be challenging due to the lack of comprehensive information about the biology, distribution, and variation within and between species. This difficulty in identification can have severe consequences in the regular trade or agricultural commodities, particularly in the USA, where the most intercepted group of thrips belong to this genus (9). Thrips species have successfully colonized a wide range of natural and non-natural habitats, demonstrating remarkable ecological adaptability. Their presence ranges from forests and grasslands to agricultural and urban landscapes, where they occupy diverse niches including foliage, flowers, bark, and leaf litter (10). However, collecting specimens for identification poses significant challenges due to their small size, rapid dispersal, and tendency to occupy hidden microhabitats. These difficulties highlight the need for targeted sampling techniques, such as suction traps, sticky cards, and Berlese-Tullgren funnels, which vary in efficiency depending on species habitat and behavior (10). Different methodologies have been used to identify Thrips species morphologically, focusing on analyzing phenotypic traits to distinguish complexes of cryptic species (11). A comparison of traditional morphological methods and modern approaches including geometric morphometrics, molecular techniques, and biochemical analyses has enhanced the recognition of Thrips species (11, 12). This comparison highlights the strengths and limitations of each method, emphasizing how geometric morphometrics complements traditional techniques by quantifying subtle morphological differences that are difficult to discern visually. Musa et al. (13) use traditional morphometrics to differentiate between subspecies of Thrips tabaci based on various body traits. GM methods have been used across multiple insect taxa, including beetles. For example, Cáceres et al. (14) applied these techniques alongside traditional taxonomic methods to redefine the generic limits of Syndesini beetles by analyzing key morphological traits, such as mandibles and pronotal tubercles. Their study provided statistical support for distinguishing genera and clarified biogeographic patterns associated with Gondwanan vicariance. Additionally, GM tools have been employed to identify developmental instability by examining organismal symmetry, which can reflect the effects of various types of stress (15).
In this study, we explore the potential of geometric morphometrics as a complementary tool for species identification within the genus Thrips. By integrating this approach with traditional taxonomy based on external morphology, we aim to facilitate a more rapid and cost-effective method for distinguishing species of quarantine significance from those frequently intercepted but of lesser concern.
For this study, eight commonly intercepted species of the genus Thrips at U.S. ports of entry were selected. Half of these species are considered of quarantine significance (not present, or with limited distribution and under eradication), while the other half are classified as not quarantine-significant (already present in the continental USA). All specimens analyzed were slide-mounted adult females with high-resolution images. These images were obtained from USDA-APHIS-PPQ, where USDA specialists previously identified the specimens and included them in the ImageID database. Additionally, other specialists in the group verified that the few images sourced from other websites were correctly identified, with USDA specialists reviewing and confirming their accuracy (Table 1).
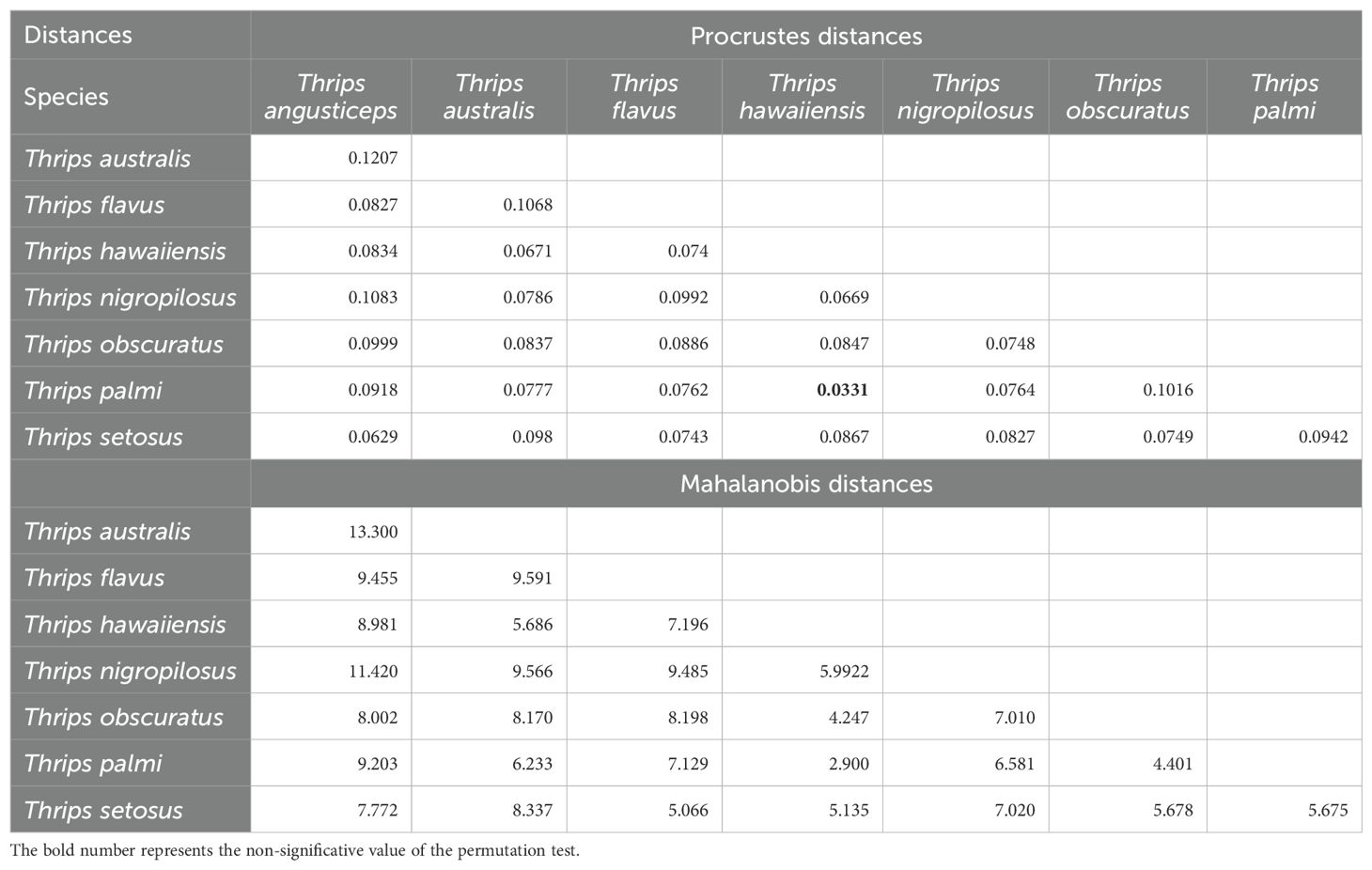
Table 1. Morphometric Procrustes and Mahalanobis distances of head shape between Thrips species, with respective permutation comparison p-values.
Fifty-eight and fifty specimens were used for the head and thorax, respectively; images were processed using Photoshop vs 26.0 (2025, Adobe Creative Cloud), cropped to the target tagma, and enhanced through higher contrast and sharpening. Landmarks placing were processed using the software TPS Dig2 v2.17 (16). For the head, 11 landmarks were digitized as illustrated in Figure 1A, and for the thorax, we used the distribution of 10 setae in the meso and metanotum (Figure 1B). The cartesian coordinates from the landmarks were processed using a Procrustes fit analysis in the software MorphoJ 1.07a which standardized the samples by removing the effects of size, position, and rotation (17). Head and thorax shape variation were analyzed using principal component analysis (PCA) based on the covariance matrix of individual shapes. An average shape covariance matrix was computed to identify species-specific shape characteristics, followed by its corresponding PCA (18, 19). These analyses were employed to provide a clear visualization of the morphospace. Differences between groups were evaluated using a permutation test with 10,000 iterations, incorporating Mahalanobis and Procrustes distances. Procrustes distances measure the absolute magnitude of shape deviations from the centroid size, while Mahalanobis distances account for variance and indicate how distinct an individual is relative to others in the sample (20, 21). Together, these metrics summarize overall patterns of similarity and highlight shape differences among species (22, 23). All the morphometrics analyses were performed using the package geomorph (24) and ggplot2 (25) in software R and MorphoJ 1.07a (26).
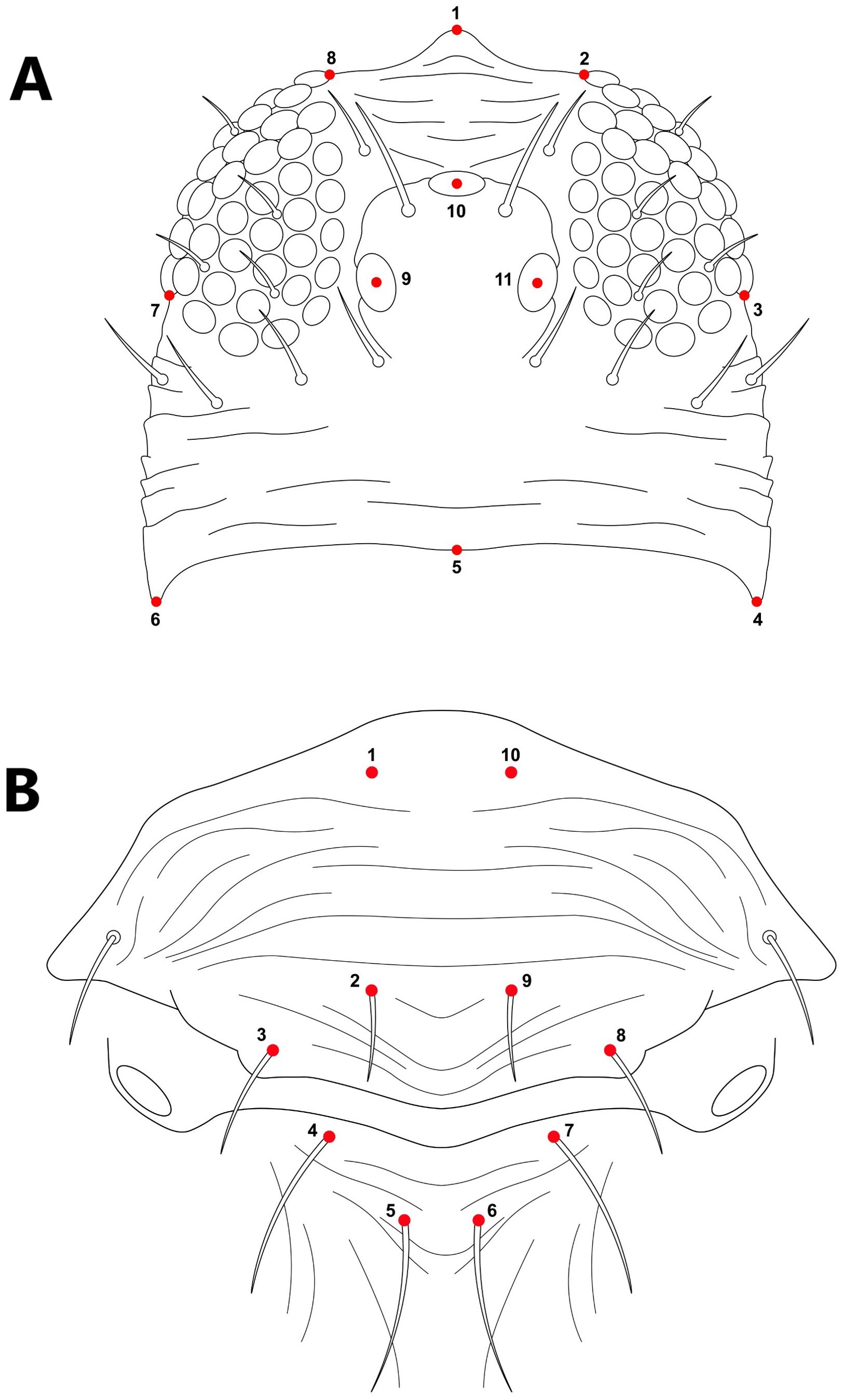
Figure 1. Landmark representation of (A) head morphology with 11 landmarks and (B) the thorax with 10 landmarks around their setae in the mesonotum and metanotum.
A PCA of the covariance matrix showed that a particular head shape is directly related to each species. The first three PCs accounted for over 73% (PC1 = 33.07%; PC2 = 25.94%; PC3 = 14.02%) of the total head shape variation. The PCA reveals a clustering of individuals, primarily driven by variance along PC2. The extremes of the vertical axis are notably characterized by specimens of T. angusticeps and T. australis. In the central region of the morphospace, overlapping groups were observed, including T. hawaiiensis and T. palmi, as well as T. nigropilosus and T. obscuratus (Figure 2A). The PCA illustrates the average head shape of each species, showing clear differentiation at the two extremes of the PCA, with T. australis and T. angusticeps. These species exhibit a flattened head shape characterized by opposing vectorial movements of landmarks #1 and #5 (head height) and #4 and #8 (head width). Similar shapes were observed among T. flavus, T. setosus, and T. angusticeps, located in the mid-left of the morphospace. Meanwhile, T. palmi, T. australis, and T. hawaiiensis occupied the lower-right extreme, generally displaying elongated, semi-oval shapes (Figure 3A). ANOVA analyses revealed no significant differences in size across the species (centroid size: F = 0.99, p = 0.4480), but significant differences in shape (Procrustes distances: F = 7.89, p < 0.0001).
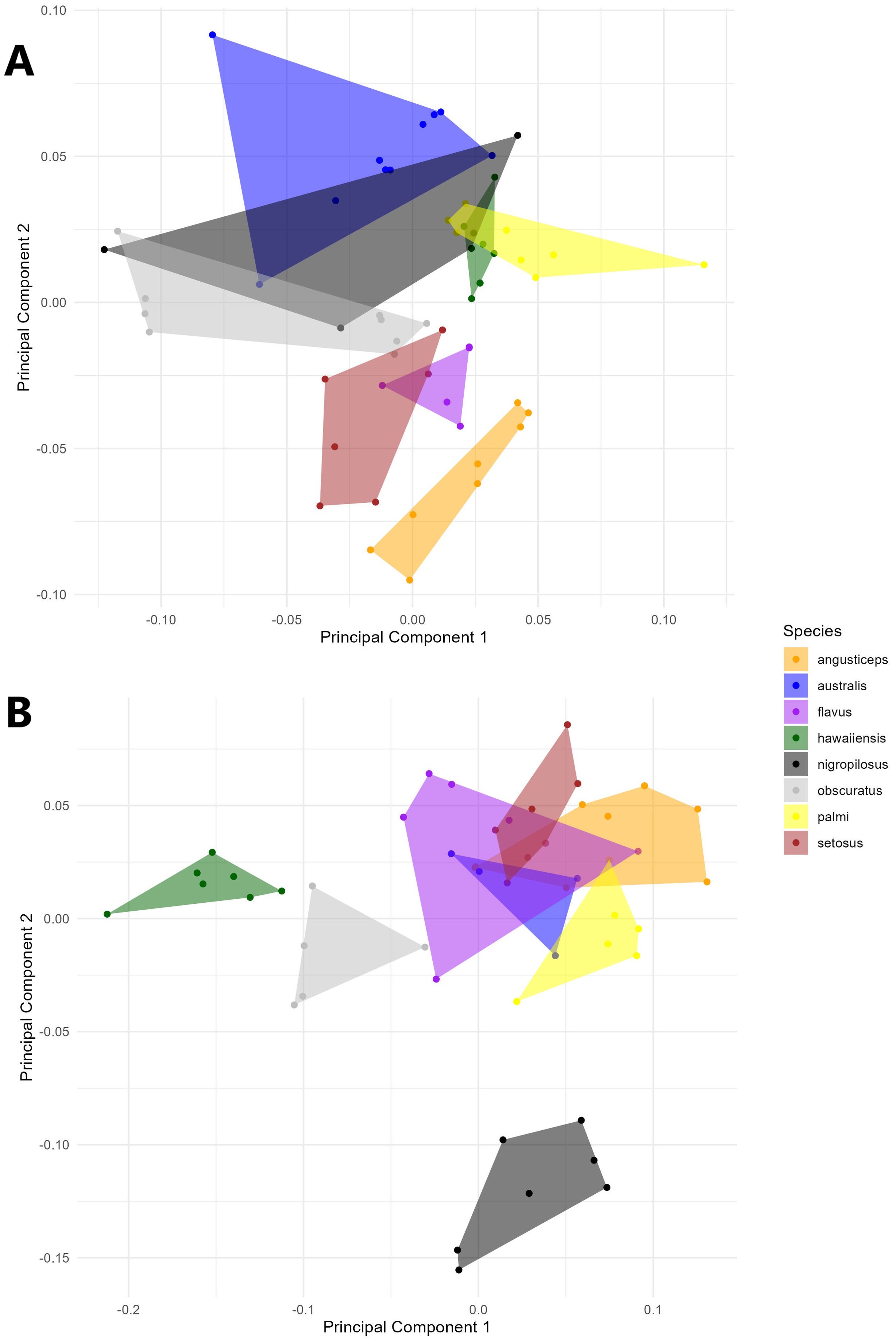
Figure 2. Principal component analysis of the Thrips species every color represent a single specie for (A) the head and (B) the thorax.
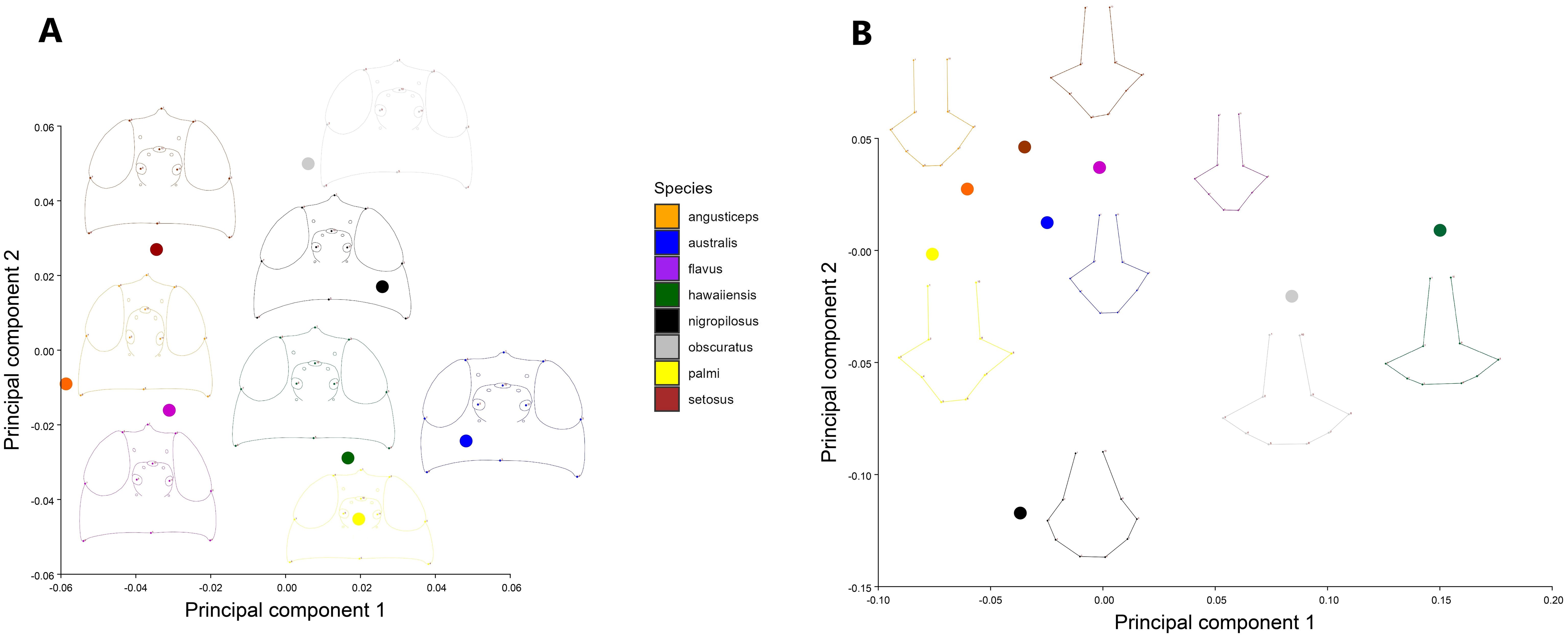
Figure 3. Principal component analysis of the average shape for (A) the head and (B) the thorax. The points on the morphospace represent the average shape for each species of Thrips.
A violin plot of centroid size distribution showed that four species exhibited greater variance in head size, with T. angusticeps displaying both the largest size and the highest variance. In contrast, T. palmi, T. australis, and T. flavus had smaller sizes and lower variance. Notably, T. setosus showed minimal variance in head size (Figure 4A).
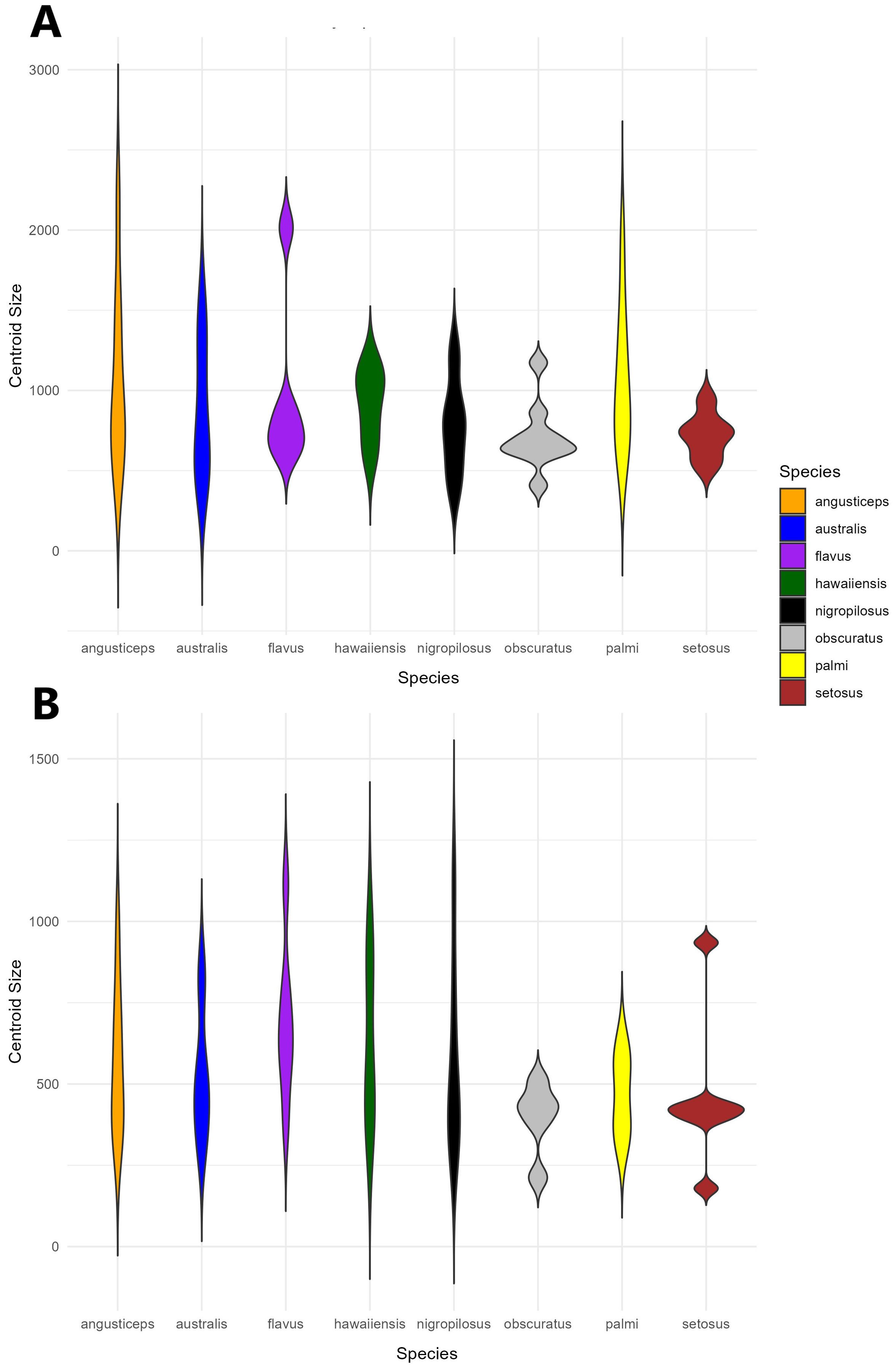
Figure 4. Violin plot showing the distribution of centroid size by species for (A) head shape and (B) thorax shape. The width of the violin indicates a higher density of individuals.
Shape variance was statistically significant between species, as determined by permutation tests of morphological distances (Table 1). The largest Procrustes and Mahalanobis distances were observed between T. angusticeps and T. australis, indicating the greatest differences in head shape. In contrast, the shortest distances, reflecting the most similar head shapes, were between T. hawaiiensis and T. palmi. When analyzing Procrustes distances, T. australis exhibited the most distinct head shape, significantly differing (p < 0.0001) from three other species. Similarly, T. angusticeps showed significant differences (p < 0.0001) with two other species.
A PCA of the covariance matrix of thorax shape revealed that meso- and metathoracic setae insertions are distinctly associated with each Thrips species. The first three principal components (PCs) accounted for over 70% of the variation in thorax shape (PC1 = 39.59%; PC2 = 18.94%; PC3 = 12.22%). The PCA shows clustering of individuals primarily driven by variance along PC1, in contrast to the head shape, where variance was predominantly explained by PC2. Despite the higher percentage of variance explained, the overall shape variation in the thorax was less pronounced compared to the head, with overlapping species groups observed in the upper-right corner of the morphospace. However, certain species, such as T. hawaiiensis and T. obscuratus, showed more apparent separation. Notably, the most significant variance along PC2 was attributed to specimens of T. nigropilosus (Figure 2B).
The average shape PCA reveals a similar overall pattern among specimens, with T. nigropilosus exhibiting the most disparate shape. In this species, the distribution of setae forms an oval pattern, with a smaller and flattened thorax. In contrast, the shape of the cluster of specimens varies depending on the position of the setae, resulting in more elongated thoraxes in T. angusticeps and T. setosus. T. palmi displays an arrow-shaped elongation, while T. obscuratus and T. hawaiiensis exhibit wider, more horizontally elongated thoraxes, contributing to greater disparity in these species (Figure 3B). ANOVA analyses revealed no significant differences in size across the species (centroid size: F = 1.75, p = 0.1224), but significant differences in shape (Procrustes distances: F = 11.47, p < 0.0001).
A violin plot of centroid size distribution indicated that five species exhibited greater variance in thorax size, with T. nigropilosus displaying both the largest size and the highest variance. In contrast, T. obscuratus and T. palmi showed smaller sizes with lower variance. Similar to the head, T. setosus exhibited minimal variance, although its sizes were distributed into three distinct groups (Figures 4A, B).
Finally, shape variance was found to be statistically significant among species, as determined by permutation tests of morphological distances (Table 2). The largest Mahalanobis and Procrustes distances, reflecting the greatest differences in the shape formed by thoracic setal insertions, were observed between T. angusticeps and T. nigropilosus, and between T. hawaiiensis and T. palmi, respectively. In contrast, the shortest distances, indicating the most similar shapes formed by thoracic setal insertions, were found between T. flavus and T. setosus. When analyzing Procrustes distances, T. angusticeps exhibited the most distinct shape, as it significantly differed (p < 0.0001) from four other Thrips species.
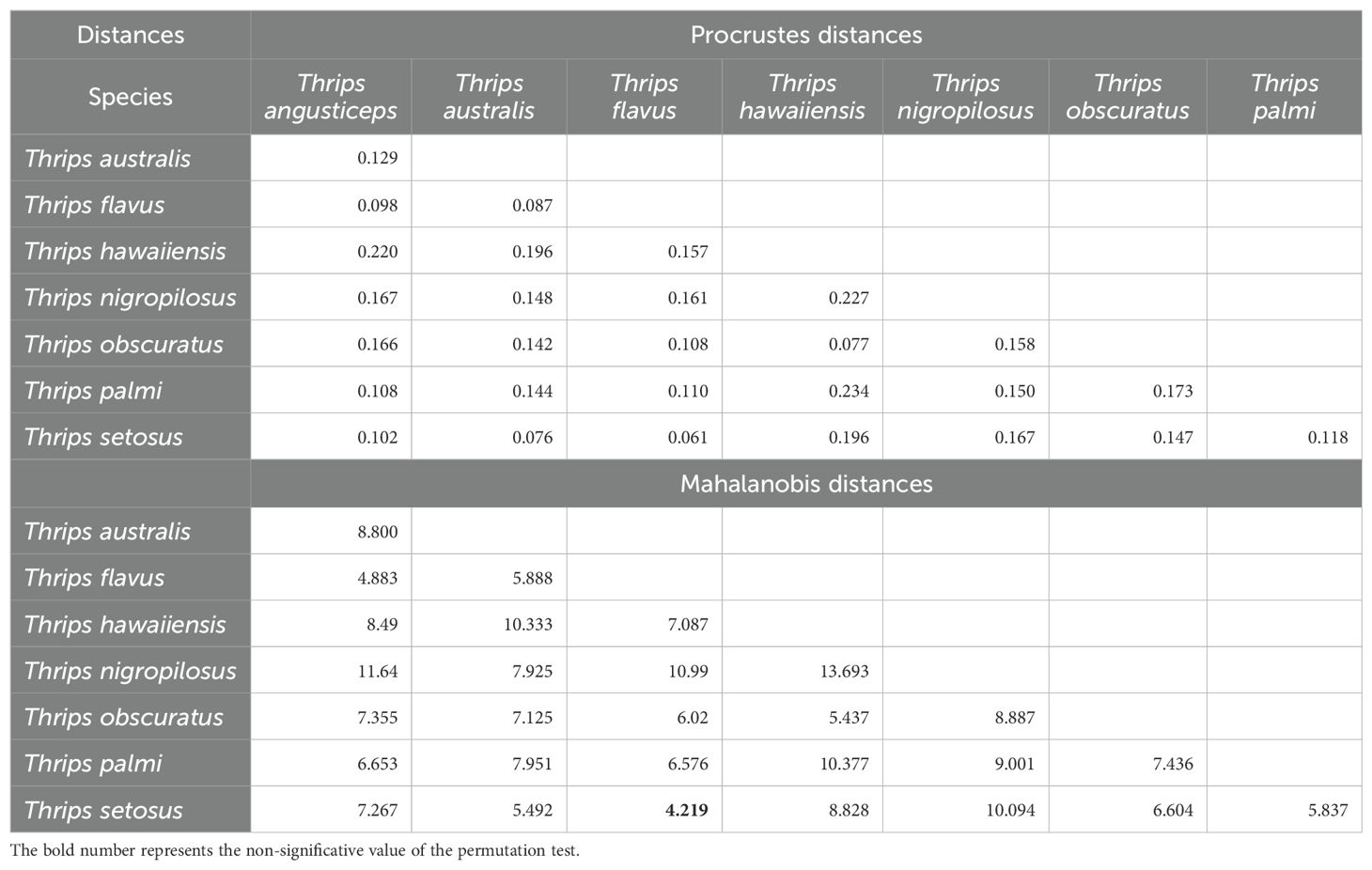
Table 2. Morphometric Procrustes and Mahalanobis Distances of thorax shape between Thrips species, with respective permutation comparison p-values.
The findings of this study clearly demonstrate the presence of significant interspecific differences within the genus Thrips, particularly in the morphological configurations of the head and the insertion patterns of mesothoracic and metathoracic setae. Geometric morphometrics, renowned for its precision in detecting subtle shape variations, provides a valuable tool for examining morphological differentiation among species. Unlike genetic markers, which often require more gradual genotypic changes to reveal population structure, geometric morphometrics can rapidly detect phenotypic modification, offering a practical advantage for monitoring and assessing morphological diversity. In the context of Thrips species differentiation, analyzing thorax and head shapes through geometric morphometrics enhances the resolution of species identification, especially when traditional taxonomic traits are less distinct or when closely related species exhibit convergence due to ecological or evolutionary pressures. There are very few works using the study of shape to separate among genera or species of thrips; most recently, a published work by Smith-Pardo (27) found that there were statistically significant differences among the pronotal shapes of phytophagous genera of thrips in the subfamily Phlaeothripinae; before that, there was only the work by Dos Santos et al. (28) which used geometric morphometrics to separate Gynaikothrips uzeli (Zimmerman) from Gynaikothrips ficorum (Marchal) as well as their sexes; other works involved traditional morphometry (size and proportions) in distinguishing among some species of thrips.
Although the results shown here only present a limited number of species and specimens in the same genus of thrips, they provide good evidence that geometric morphometrics can be used to discriminate among closely related species in the genus, which can sometimes be challenging to identify, in particular when such groups are highly diverse and speciose, as in the genus Thrips (4). Our findings highlight the utility of geometric morphometrics in disentangling complex morphological relationships between species, even when size differences are not significant. The clear shape differentiation revealed by the morphometrics distances, along the patterns captured in morphospace through PCA, point out the utility of GM for species identification. This precision is critical not only for distinguishing morphologically similar taxa but also for understanding their morphological traits’ ecological and evolutionary implications (29–31). By providing a robust framework for analyzing subtle but meaningful variations, geometric morphometrics advances species-level taxonomy and the broader study of morphological adaptations in response to ecological pressures (32). Our results highlight the utility of landmark-based analyses and the importance of employing complementary sets of landmarks to achieve robust species-level differentiation. In cases where one set of landmarks is insufficient to reveal statistical differences, another may provide the necessary resolution. For instance, T. palmi (a quarantine-significant species) and T. hawaiiensis (a common, non-quarantine-significant species in the USA) exhibit no statistically significant differences in head shape but show significant differences in the shape formed by the insertion of mesothoracic and metathoracic setae. Conversely, other species pairs demonstrate the reverse pattern, with significant differences in head shape but not in the thoracic setal insertion patterns. These findings underscore the complementary nature of multiple landmark sets in capturing species-specific morphological variations, enhancing the accuracy and reliability of geometric morphometric analyses.
As demonstrated in Thrips species and other taxa, landmark-based analyses provide a robust framework for distinguishing closely related species (30, 33–36). These methods enable the quantification of shape variations, as highlighted in studies of Nyctelia beetles, where cryptic species designations were challenged by detecting subtle morphological differences that traditional approaches had overlooked (30). Similarly, geometric morphometrics have revealed that complementary landmark sets, such as those used for head and thoracic morphology in Thrips, can uncover significant interspecific differences, even when individual sets alone are insufficient. This capability underscores the potential of geometric morphometrics not only in resolving taxonomic challenges but also in refining the concept of cryptic species by addressing methodological limitations. Furthermore, as recently discussed by Smith-Pardo et al. (in press), even in cases where discrete external morphological characters are commonly used in traditional taxonomy, these may not be as helpful for taxa where there is extensive convergence or constraint due to sharing highly similar ecological habits or life history strategies, such as in the case of phytophagous thrips that feed on leaves and flowers, in this case, GM can be helpful when dealing with complex taxonomic problems associated with similar, external morphological characters.
It is also important to highlight that the application of GM enhances scientific rigor in describing critical aspects of phenotypic dimension, as emphasized by Viscosi and Cardini (37). Finally, geometric morphometrics serves as a powerful tool for the identification of species within closely related groups based on external morphology. Moreover, its application in distinguishing quarantine-significant species from other morphologically similar taxa offers a fast, economical, and reliable method for pest identification. By integrating advanced analytical techniques like geometric morphometrics into traditional taxonomic frameworks, we can bridge the gap between morphology and genetics, paving the way for more precise and efficient species identification.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The manuscript presents research on animals that do not require ethical approval for their study.
AS-P: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LP: Formal analysis, Investigation, Methodology, Visualization, Writing – review & editing. HB: Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding was received from the USDA-Animal and Plant Health Inspection Service (APHIS), Plant Protection and Quarantine (PPQ), Science and Technology (S&T); from grant ANID/ANILLO/ATE230025; and from the ANID through Convocatoria Nacional Subvención a Instalación en la Academia Convocatoria Año 2021, Grant SA77210040.
AS-P thanks the USDA-Animal and Plant Health Inspection Service (APHIS), Plant Protection and Quarantine (PPQ), Science and Technology (S&T) for the continuous support of his research on the morphometrics of insects of agricultural importance; HB thanks the funding of grant ANID/ANILLO/ATE230025; LP acknowledges financial support from the ANID.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
The findings and conclusions in this publication have not been formally disseminated by the U.S. Department of Agriculture and should not be construed to represent any Agency determination or policy. Mention of trade names or commercial products in this publication is solely to provide specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mound LA. So many thrips-so few tospoviruses. In: Thrips and tospoviruses: proceedings of the 7th international symposium on thysanoptera. Australian National Insect Collection, Canberra (2002). p. 15–8.
2. Mound LA, Hastenpflug-Vesmanis A. All genera of the world: order Thysanoptera (Animalia: Arthropoda: Insecta). Megataxa. (2021) 6:2–69-62–69. doi: 10.11646/megataxa.6.1.2
3. Zhang S, Mound L, Feng J. Morphological phylogeny of thripidae (Thysanoptera: terebrantia). Invertebrate Systematics. (2019) 33:671–96. doi: 10.1071/IS19001
4. Masumoto M, Okajima S. Review of the genus Thrips and related genera (Thysanoptera, Thripidae) from Japan. Zootaxa. (2013) 3678:1–65. doi: 10.11646/zootaxa.3678.1.1
5. Masumoto M, Okajima S. Trichromothrips Priesner (Thysanoptera, Thripidae) of Japan and Taiwan, with descriptions of four new species and a review of the Trichromothrips group of genera. Zootaxa. (2005) 1082:1–27-21–27. doi: 10.11646/zootaxa.1082.1.1
6. Masumoto M, Okajima S. A revision of and key to the world species of Mycterothrips Trybom (Thysanoptera, Thripidae). Zootaxa. (2006) 1261:1–90-91–90. doi: 10.11646/zootaxa.1261.1.1
7. Masumoto M, Okajima S. The genus Scirtothrips Shull (Insecta, Thysanoptera, Thripidae) and three related genera in Japan. Zootaxa. (2007) 1552:1–33-31–33. doi: 10.11646/zootaxa.1552.1.1
8. Stuart RR, Gao Y-L, Lei Z-R. Thrips: pests of concern to China and the United States. Agric Sci China. (2011) 10:867–92. doi: 10.1016/S1671-2927(11)60073-4
9. Nickle DA. Commonly intercepted thrips at US ports-of-entry from Africa, Europe, and the Mediterranean. IV. Miscellaneous thripine genera excluding Frankliniella, Iridothrips, and Thrips (Thysanoptera: Thripidae). Proc Entomological Soc Washington. (2009) 111:215–38. doi: 10.4289/0013-8797-111.1.215
10. Marullo R, Bonsignore CP, Vono G. Thrips: a review of sampling methods in relation to their habitats. Bull Insectol. (2021) 74:241–51.
11. Palmer J, Wetton M. A morphometric analysis of the Thrips hawaiiensis (Morgan) species-group (Thysanoptera: Thripidae). Bull Entomological Res. (1987) 77:397–406. doi: 10.1017/S000748530001186X
12. Mehle N, Trdan S. Traditional and modern methods for the identification of thrips (Thysanoptera) species. J Pest Sci. (2012) 85:179–90. doi: 10.1007/s10340-012-0423-4
13. Musa S, Ladányi M, Varela RCL, Fail J. A morphometric analysis of Thrips tabaci Lindeman species complex (Thysanoptera: Thripidae). Arthropod Structure Dev. (2023) 72:101228. doi: 10.1016/j.asd.2022.101228
14. Cáceres JSD, Pérez LM, Grossi PC, Benitez H. Defining generic limits in Syndesini MacLeay 1819 (Coleoptera: Lucanidae: Syndesinae) through taxonomy and geometric morphometrics. Zoologischer Anzeiger. (2023) 305:28–41. doi: 10.1016/j.jcz.2023.05.002
15. Benítez HA, Lemic D, Villalobos-Leiva A, Bažok R, Órdenes-Claveria R, Pajač Živković I, et al. Breaking symmetry: fluctuating asymmetry and geometric morphometrics as tools for evaluating developmental instability under diverse agroecosystems. Symmetry. (2020) 12:1789. doi: 10.3390/sym12111789
17. Rohlf FJ, Slice D. Extensions of the Procustes methods for the optimal superimposition of landmarks. Systematic Zoology. (1990) 39:40–59. doi: 10.2307/2992207
19. Lemic D, Benítez HA, Bažok R. Intercontinental effect on sexual shape dimorphism and allometric relationships in the beetle pest Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae). Zoologischer Anzeiger - A J Comp Zoology. (2014) 253:203–6. doi: 10.1016/j.jcz.2014.01.001
20. Klingenberg CP, Monteiro LR. Distances and directions in multidimensional shape spaces: Implications for morphometric applications. Systematic Biol. (2005) 54:678–88. doi: 10.1080/10635150590947258
21. Klingenberg CP. Size, shape, and form: concepts of allometry in geometric morphometrics. Dev Genes Evol. (2016) 226:113–37. doi: 10.1007/s00427-016-0539-2
22. Albrecht GH. Multivariate analysis and the study of form, with special reference to canonical variate analysis. Am Zoologist. (1980) 20:679–93. doi: 10.1093/icb/20.4.679
23. Pretorius E, Scholtz C. Geometric morphometrics and the analysis of higher taxa: a case study based on the metendosternite of the Scarabaeoidea (Coleoptera). Biol J Linn Soc. (2001) 74:35–50. doi: 10.1006/bijl.2001.0568
24. Adams DC, Otárola-Castillo E. Geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods Ecol Evol. (2013) 4:393–99. doi: 10.1111/mee3.2013.4.issue-4
25. Wickham H. ggplot2. Wiley Interdiscip reviews: Comput Stat. (2011) 3:180–5. doi: 10.1002/wics.v3.2
26. Klingenberg CP. MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour. (2011) 11:353–7. doi: 10.1111/j.1755-0998.2010.02924.x
27. Smith-Pardo AH. Differences in pronotum shape in Phlaeothripinae thrips (Thysanoptera) of quarantine importance. Academia Biol. (2024) 2:1–8. doi: 10.20935/AcadBiol7310
28. Dos Santos PP, Junior JCS, Nunes LA. Differences in wings may be sufficient to separate the sexes and two species of Gynaikothrips Zimmermann (Thysanoptera: Phlaeothripidae)? EntomoBrasilis. (2022) 15:e992–2. doi: 10.12741/ebrasilis.v15.e992
29. Benítez HA, Lemic D, Bazok R, Gallardo-Araya CM, Mikac KM. Evolutionary directional asymmetry and shape variation in Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae): an example using hind wings. Biol J Linn Soc. (2014) 111:110–8. doi: 10.1111/bij.2014.111.issue-1
30. Zúniga-Reinoso Á, Benítez HA. The overrated use of the morphological cryptic species concept: An example with Nyctelia darkbeetles (Coleoptera: Tenebrionidae) using geometric morphometrics. Zoologischer Anzeiger-A J Comp Zoology. (2015) 255:47–53. doi: 10.1016/j.jcz.2015.01.004
31. Nuñez-Vallecillo M, Rivera A, Górski K, Brante A, Benítez HA. Ecomorphological analyses reveal impact of land-based stressors on stock structure of two commercially important fish species (Lutjanus synagris and Haemulon plumierii) in the Caribbean. Fisheries Res. (2021) 234:105812. doi: 10.1016/j.fishres.2020.105812
32. Adams DC, Rohlf FJ, Slice DE. A field comes of age: geometric morphometrics in the 21st century. Hystrix-Italian J Mammalogy. (2013) 24:7–14. doi: 10.4404/hystrix-24.1-6283
33. Gérard M, Martinet B, Dehon M, Rasmont P, Williams PH, Michez D. The utility of wing morphometrics for assigning type specimens to cryptic bumblebee species. Systematic Entomology. (2020) 45:849–56. doi: 10.1111/syen.12430
34. Chatpiyaphat K, Sumruayphol S, Dujardin JP, Samung Y, Phayakkaphon A, Cui L, et al. Geometric morphometrics to distinguish the cryptic species Anopheles minimus and An. harrisoni in malaria hot spot villages, western Thailand. Med veterinary entomology. (2021) 35:293–301. doi: 10.1111/mve.12493
35. Karanovic T. Using landmark-based geometric morphometrics for holotype selection in cryptic species: A case study of Western Australian Halicyclops (Copepoda, Cyclopoida). Crustaceana. (2022) 95:631–66. doi: 10.1163/15685403-bja10211
36. Sagastume-Espinoza KO, Simmons LW, Harvey MS. Use of geometric morphometrics to distinguish trapdoor spider morphotypes (Mygalomorphae: Anamidae: Proshermacha): a useful tool for mygalomorph taxonomy. J Arachnology. (2024) 52:31–40. doi: 10.1636/JoA-S-22-033
Keywords: quarantine significant, pests, taxonomy, morphometrics, shape variation, phenotype
Citation: Smith-Pardo AH, Pérez LM and Benítez HA (2025) Unlocking species identity: geometric morphometrics of head and thorax shapes in invasive and non-invasive quarantine-significant thrips (Thysanoptera: Terebrantia). Front. Insect Sci. 5:1558242. doi: 10.3389/finsc.2025.1558242
Received: 09 January 2025; Accepted: 03 February 2025;
Published: 06 March 2025.
Edited by:
Francesco Nugnes, National Research Council (CNR), Portici, ItalyReviewed by:
Carmelo Peter Bonsignore, Mediterranea University of Reggio Calabria, ItalyCopyright © 2025 Smith-Pardo, Pérez and Benítez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Allan H. Smith-Pardo, YWxsYW4uaC5zbWl0aC1wYXJkb0B1c2RhLmdvdg==; Hugo A. Benítez, aGJlbml0ZXpAdWNtLmNs
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.