
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Insect Sci., 11 March 2025
Sec. Insect Molecular Genetics
Volume 5 - 2025 | https://doi.org/10.3389/finsc.2025.1547963
This article is part of the Research TopicUnravelling Insect Vector Diversity: Genetic And Phenotypic Insights From The Global SouthView all articles
Introduction: The genus Panstrongylus is one of the most important within the subfamily Triatominae, which includes vectors of Trypanosoma cruzi, the etiological agent of Chagas disease (CD). In particular, Panstrongylus chinai and P. howardi have drawn attention for their role in disease transmission. These species exhibit notable ecological and morphological differences. Previous studies have investigated aspects such as morphometry, cytogenetics, and ecological niches, including experimental crosses between these species that resulted in viable F1 hybrids. However, no F2 generation was produced, as the eggs laid were empty and failed to hatch, limiting the study to F1 hybrids.
Methods: We analyzed wing morphometric traits (size and shape) from 262 individuals, including P. chinai, P. howardi, and their hybrids, using geometric morphometry techniques. This study aimed to build upon previous findings by analyzing the wing morphometric and environmental adaptations of P. chinai, P. howardi, and their hybrids (♀P. howardi × ♂P. chinai) to determine whether the hybrids exhibited similarities in wing size and shape, regardless of maternal or paternal phenotype.
Results: Differences in centroid size were observed between the parental species, with P. howardi having a larger size, but no significant differences were found among the hybrids. Females showed greater shape similarity between P. howardi and the ♀P. chinai × ♂P. howardi hybrids, while males showed similarity among hybrids. Discriminant analysis was more effective for distinguishing parental groups than with hybrids. The K-means algorithm successfully classified the parental species and hybrid groups, although with low assignment percentages and a different number of groups than expected.
Discussion: The smaller wing size in hybrid offspring may indicate lower fitness, potentially due to genetic effects or reduced viability. Geometric morphometry effectively distinguishes parental species from hybrids, supporting previous research in Triatominae. The study suggests that environmental and reproductive pressures may influence these species and explores the dispersive capabilities of triatomines, contributing to the understanding of hybridization processes.
Chagas disease is a neglected tropical disease caused by the protozoan Trypanosoma cruzi and is transmitted mainly through the feces of triatomine bugs. It affects six to seven million people (1) and poses significant health challenges, particularly in Central and South America (2). In Ecuador, approximately 230,000 people are infected, and 6.2 million people are at risk of infection (3).
The subfamily Triatominae comprises more than 155 species (4–9). In Ecuador, 16 species have been reported across 18 of the 24 provinces (10). The main vectors in the country are Rhodnius ecuadoriensis Lent & Leon, 1958, and Triatoma dimidiata (Latreille, 1811) though most studies have focused on the former rather than the latter (11–16). However, the epidemiological importance of several species considered secondary vectors has increased due to their fast expansion into human-inhabited areas (17) and changing climate conditions (18, 19). The Panstrongylus genus is becoming more significant in Chagas disease (CD) transmission. Key characteristics of some species within this genus include: (i) a short to medium life cycle (149.5 to 531 days), (ii) prolonged contact with the host and a quick response to their presence, (iii) the capacity to consume large volumes of blood, (iv) frequent defecation while feeding, and (v) high rates of T. cruzi infection (20). Six species of this genus have also been reported in Ecuador: Panstrongylus chinai (Del Ponte, 1929), P. howardi (Neiva, 1911), P. geniculatus (Latreille, 1811), P. lignarius/P. herreri (Walker, 1873), and P. rufotuberculatus (Champion, 1899) (10, 14, 21).
Panstrongylus chinai is primarily found in domiciliary habitats (22) in Loja and El Oro provinces (16, 20, 23), while P. howardi is endemic to the province of Manabí, typically inhabiting peridomiciliary habitats (21, 24) and occasionally occurring in sylvatic environments (25). The importance of these species as vectors is highlighted by their increasing spread and adaptation to human-inhabited areas.
Regarding its morphology, P. chinai can be completely black and/or brown. Its scutellar process is elongated, distally sharpened, and subcylindrical. The body length ranges from 22 to 27 mm, the pronotum width from 5.5 to 7 mm, and the abdomen can be up to 12 mm wide (26). In contrast, P. howardi displays a brown-orangish coloration, has a second rostral segment longer than the first, and lacks denticles on its femur. Its body length ranges from 25 to 29 mm, the pronotum width from 6 to 6.5 mm, and the abdomen width from 8.5 to 11 mm (26). Despite their differences, these species are closely related, and hybridization has been observed in experimental crosses (14). To investigate this, multiple methods were employed, including antennal morphology, geometric morphometrics of the head, wings, and eggs, cytogenetic analysis, ecological niche modeling, and experimental crosses. The crosses produced viable F1 hybrids with a P. howardi-like coloration but a P. chinai-like body size. However, further crosses between F1 hybrids resulted in no copulation or viable offspring. Hybridization, which generates genetic variation and novel traits, may underlie these findings. The sterility or nonviability of F1 hybrids could result from genetic incompatibilities, loss of local adaptations, or disruption of co-adapted genes (27).
Geometric morphometrics is a method that quantifies morphological variation and was crucial in this study, allowing for the analysis of traits influenced by both genetic and environmental factors (28, 29). In insect studies, it is important to recognize that adult morphological markers are fixed, reflecting both life history and environmental adaptations. The environment in which an insect develops can lead to variations in its phenotype, behavior, physiology, and morphology, affecting its dispersal potential and influencing factors such as habitat selection, host preference, and vector capacity (30, 31). In triatomines, geometric morphometrics has been applied to various taxonomic issues, including habitat markers, population structure, domiciliation, flight characteristics, and the effects of insecticide exposure and resistance (12, 14, 32–39). It has also been particularly useful in studying hybrids to explore speciation hypotheses, identify closely related species, and analyze morphological traits and morphometric changes associated with dispersal (9, 40–42).
Geometric morphometrics is a powerful tool for studying the phenotypic traits of triatomines, including wing morphology. This method allows for the quantification of morphological variation and provides insights into ecological adaptations, dispersal potential, and vector capacity. Furthermore, it has been vital in differentiating medically important species in Ecuador, such as R. ecuadoriensis (12), P. rufotuberculatus (43), P. chinai, and T. carrioni (Larrousse 1926) (44). This study aims to characterize the populations of P. chinai, P. howardi, and their experimental hybrids by comparing wing morphometric traits and exploring their potential environmental adaptations. The hybrids, which exhibit P. howardi-like coloration and P. chinai-like body size, are expected to have wing traits similar to P. howardi due to their dominant phenotype, along with intermediate wing characteristics between the two parental species. Additionally, hybrids may display phenotypic traits aligning with one of the parental species. This research highlights the morphological variability in hybrids and examines how phenotypic inheritance and sexual variation may influence their characteristics.
The Panstrongylus chinai and P. howardi individuals (both parental) used in this study were collected from two regions of Ecuador—the Southern Andean region and the Central Coastal region—specifically from five rural communities in Loja province (Bellamaria [−4.194885, −79.610214, and 1,106 m above the sea level {masl}]; Guara [− 4.25007, − 79.57979, and 1,081 masl]; Huayco [−4.091017, −79.322817, and 1449 masl]; Vega del Carmen [−4.11105, −79.7074, and 1,612 masl]; and Tacoranga [−4.106107, −79.590123, and 1,141 masl]) and one rural community of the Manabí province (Bejuco [−0.949592, −80.3365, and 383 masl]). These individuals were collected under permits No. 002-07-IC-FAU-DNBAPVS/MA, No. 006-RM-DPM-MA, No. 006-IC-FLO-DPL-MA, No. 008-RM-DPM-MA, No. 008-IC-INSEC-DPL-MA, No. 010-IC-FAU-DNBAPVS/MA, and No. 016-07 IC-FAU-DNBAPVS/MA, all granted by the Ministry of Environment of Ecuador.
Loja province features a rugged, hilly landscape with altitudes ranging from 700 to 3,700 m. Average temperatures range from 15.4°C to 26.6°C, with annual rainfall varying from 0.2 to 200 mm (45). The province plays a significant economic role, with 61% of its land dedicated to agriculture and livestock, producing crops such as corn, sugarcane, coffee, and rice (46). Traditional homes are constructed with adobe walls, dirt floors, and ceramic tile roofs to help regulate temperature (47).
Manabí province, characterized by flatter terrain and a 350-km coastal expanse, has elevations reaching up to 500 m. Its average temperature ranges from 20.3°C to 26.6°C, with rainfall varying from 10 to 5,000 mm (45). The province plays a key role in Ecuador’s economy, with 84% of its land used for sugarcane, plantain, and oil palm cultivation (46). Traditional homes are constructed using bamboo (caña guadúa) for walls and floors, with roofs made of palm fronds or, occasionally, zinc (24).
While both regions’ housing styles are adapted to local climates and lifestyles, they do not meet health or technical standards, increasing the risk of triatomine infestation and T. cruzi infection (47).
The collected specimens were transported under mobilization permit number MAE-DNB-CM2015-0030 to the Insectary of the Center for Research for Health in Latin America (CISeAL). The identification of developmental stages and the sex of adult triatomines was performed using a dichotomous key by Lent and Wygodzinsky (4).
These individuals were maintained in an incubator equipped with a dual-chamber system, replicating the original microhabitat temperature and humidity conditions of each province (Manabí: 27°C ± 5°C, 75% ± 5% RH; Loja: 24°C ± 6°C, 70% ± 5% RH) with a 12-h photoperiod in both chambers, as described in Villacís et al. (11) and Santillán-Guayasamín et al. (48) at the Insectary of the CISeAL. For the experimental crosses and the production of hybrids, individuals from the Manabí and Loja communities were used, as described in Villacís et al. (14). Fifth-instar nymphs (NV) of P. chinai and P. howardi were separated to obtain virgin females for crossing. After emerging as adults, males and females were placed in plastic vials with filter paper to facilitate movement and humidity control. Biweekly blood meals were provided using immobilized pigeons, following protocol 15-H-034, approved by the American Association for Laboratory Animal Science - IACUC. Nine interspecific crosses were performed: five with one female of P. chinai and two males of P. howardi (♀Pc × ♂Ph) and four with one female of P. howardi and two males of P. chinai (♀Ph × ♂Pc). These pairings were based on the availability of insects to ensure copulation. After F1 adults emerged, nine couples were formed to attempt F2 offspring. However, no copulation occurred between F1 hybrids, and the eggs laid were empty and did not hatch, so only F1 hybrids were used (14).
For morphometric analysis, we extracted the wings of 262 individuals as follows: P. chinai (38♀ and 53♂), P. howardi (36♀ and 47♂), and 88 hybrids between these two species. All analyses were conducted using only the right wing of male and female individuals from each group.
Body size measurements (from the clypeus to the genitalia) were taken for 30 adult individuals from each group using a digital caliper (Didimatic Caliper, Model CD-6”C, Mitutoyo Corporation, Kawasaki, Japan): Panstrongylus chinai (15♀ and 15♂), P. howardi (15♀ and 15♂), and hybrids (15♀ and 15♂). Each measurement was conducted once, with no replicates.
Each wing was extracted, placed on a slide, and covered with a drop of Hoyer’s medium. A cover slip was added to evenly distribute the medium, and the slide was left to dry for a week until it solidified. Hoyer’s medium was chosen for its ability to clean the tissue, enhance visibility due to its high refractive index, and preserve the structure for up to 20 years (49). Afterward, a digital image of each wing was captured using an OLYMPUS SZX7 stereoscope and a camera (OLYMPUS® CMOS 10.6 MP Microscope Digital Camera, Model SC100, Olympus Corporation, Tokyo, Japan). Nine type I landmarks were used for morphometric analyses (Figure 1). Given that some wings from the three groups, particularly those from P. howardi, were highly transparent, Adobe Lightroom (Adobe Photoshop Lightroom Classic CC v.7.5, Adobe Inc, San Jose, CA, USA) was used to adjust the lighting and enhance the visibility of the landmark locations.
Before marking landmarks and conducting morphometric analyses, repeatability was tested to assess the accuracy of landmark digitization. Fifteen wing images from P. howardi, P. chinai, and hybrids of both sexes were randomly selected and digitized twice by the same user. The repeatability index was calculated using Procrustes analysis of variance (ANOVA) to evaluate measurement error. The statistical method used by the XY Online Morphometrics (XYOM) software for shape repeatability estimation followed a model II one-way ANOVA for repeated measurements (50) and Procrustes ANOVA (51).
To obtain shape variables and centroid sizes, generalized Procrustes analyses were performed for each sex using ANOVA (52). Both size and shape variables enabled the subsequent analyses detailed below.
The global size of the wings was computed as centroid size (CS), defined as the square root of the sum of the squared distances between the center of the landmark configuration and each individual landmark (53). Wing sizes (CS) were compared between groups (P. chinai and P. howardi and hybrids ♀Pc × ♂Ph and ♀Ph × ♂Pc) for each sex using a one-way ANOVA.
The comparison of shapes between hybrids and parental specimens for each sex was conducted using principal component analysis (PCA) of the Procrustes residuals and discriminant function analysis (DFA). The statistical significance of the analyses on wing size and shape was assessed using a nonparametric permutation test (5,000 runs).
The classification, based on the cross-check test using Mahalanobis Distances, indicates the percentage of individuals correctly assigned to each group (reclassification) (54).
The metric disparity (MD) index quantifies the variance in wing shape. This index provides a single value that measures the variability in a sample’s shape and is calculated based on the Euclidean distances of each configuration relative to the consensus (55). The significance of potential differences in metric disparity between groups was assessed using bootstrap-based statistical methods and the F-test for equality of variances.
Size and shape variables were analyzed separately, and their possible relationship, or allometry, was measured using linear regression techniques. Centroid size was regressed against the first two principal components of shape (PC1, PC2) using the XYOM software, which is freely available online (http://xyom.io54).
We use unsupervised analysis techniques to evaluate the assignment of hybrid individuals concerning their parental species. The classification was performed using covariance-based PCA and the K-means classification algorithm. PCA is a tool that enhances the visualizations of multidimensional data by removing correlations between variables, which is a common feature of morphometric traits. By creating new uncorrelated variables, PCA graphically illustrates the primary trends structuring the data. The K-means algorithm, a blind classification technique, identifies potential natural groupings within the data without relying on predefined labels (56). The analysis primarily relied on inter-individual distances, specifically the Euclidean distance calculated from the raw variables. The predefined number of groups (K) was always set to four, corresponding to the two parental species and the two hybrid groups, each derived from a mother of a different species. The group composition determined by the K-means algorithm was compared with the pre-established classification, enabling the calculation of the percentage of correct classification. The algorithm was initiated using the “naive sharding centroid” method, and the optimal number of clusters was determined using the “elbow graph” method, which analyzes the average distance to the centroid as a function of K. For each sex, the K-means algorithm was applied to both shape and size variables.
The collection of landmarks and statistical analyses was conducted using several modules of the XYOM platform, a free online program that enables users to follow the entire geometric morphometrics analysis workflow with ease (http://xyom.io54). It is user-friendly and does not require installation, configuration, or manual updates. Since it is a cloud-based platform, a Google Drive account is necessary to store all the information related to the landmark digitization process. XYOM also uses Plotly to enhance the editing and visualization of its various statistical outputs. Users can download the results and combine multiple output files to create new datasets for further analysis. This feature is particularly useful when working with large datasets (54).
Morphometry measures and describes the body morphology of organisms, providing quantitative data on their environmental interactions. Recent advancements have made morphometric analyses more visual and less labor-intensive. Chagas disease vectors, or “kissing bugs”, belonging to the Triatominae subfamily, are widely studied for their morphological plasticity. Research has focused on aspects such as domesticity, food preferences, dispersal, insecticide resistance, speciation processes, and taxonomy (40, 57). In addition, morphological stasis in Triatoma brasiliensis brasiliensis, as observed through geometric morphometrics by Paschoaletto et al. (58), highlights its adaptability to climate changes and global invasiveness. These findings offer valuable insights into the evolutionary patterns of wing morphology in Triatominae as disease vectors.
Geometric morphometrics, based on the quantitative analysis of triatomine wing venation characters, has proven to be a reliable tool for identifying triatomines, particularly when distinguishing morphologically similar species (sibling species or hybrids). Our results support the findings of Villacís et al. (14) and Barnabé et al. (59), confirming that P. chinai and P. howardi should not be synonymized and that these taxa represent two valid yet closely related species.
This is the first study on wing geometric morphometrics in hybrid individuals of the genus Panstrongylus. Hybridization events are usually considered an evolutionary dead end because they frequently result in nonviable or infertile offspring (60, 61). Nevertheless, an alternative approach suggests that natural hybridization generates novel combinations of genes and alleles, fostering favorable genetic conditions for rapid and significant evolutionary changes (40, 62).
Our findings indicate that the hybrids exhibit distinct morphometric characteristics in their wings compared to their parental species. Additionally, the level of phenotypic similarity between the hybrids and their parental species—P. chinai or P. howardi—depends on the sex combination of the crossings.
The body size of the hybrids, whether produced from ♀P. chinai × ♂P. howardi (♀Pc × ♂Ph) or ♀P. howardi × ♂P. chinai (♀Ph × ♂Pc), was consistently smaller than that of the parental species, P. chinai and P. howardi. Specifically, the body length of P. chinai males and females averaged 23.19 mm ± 0.91 mm and 23.86 mm ± 1.48 mm, respectively, whereas P. howardi had larger individuals, with averages of 26.02 mm ± 1.28 mm in males and 27.02 mm ± 1.66 mm in females. The hybrids, on the other hand, showed smaller sizes (♂Pc × ♂Ph: 22.75 mm ± 1.1 mm in males and 23.41 mm ± 0.8 mm in females; ♀Ph × ♂Pc: 22.46 mm ± 0.27 mm in males and 22.61 mm ± 0.5 mm in females). However, no significant size differences were detected between the two hybrid groups (Figure 2). This smaller body size in hybrids may suggest a potential decrease in overall fitness, aligning with previous findings in other species where hybrid offspring tend to exhibit reduced sizes. According to Dujardin et al. (63), laboratory-reared triatomines often experience a significant reduction in size over successive generations, likely due to a higher survival rate of smaller individuals under such conditions. The inclusion of additional generations, influenced by environmental changes (such as laboratory conditions), may impose a cost on body size (64). Notably, body size is not always related to the centroid size of the structure under study (65).
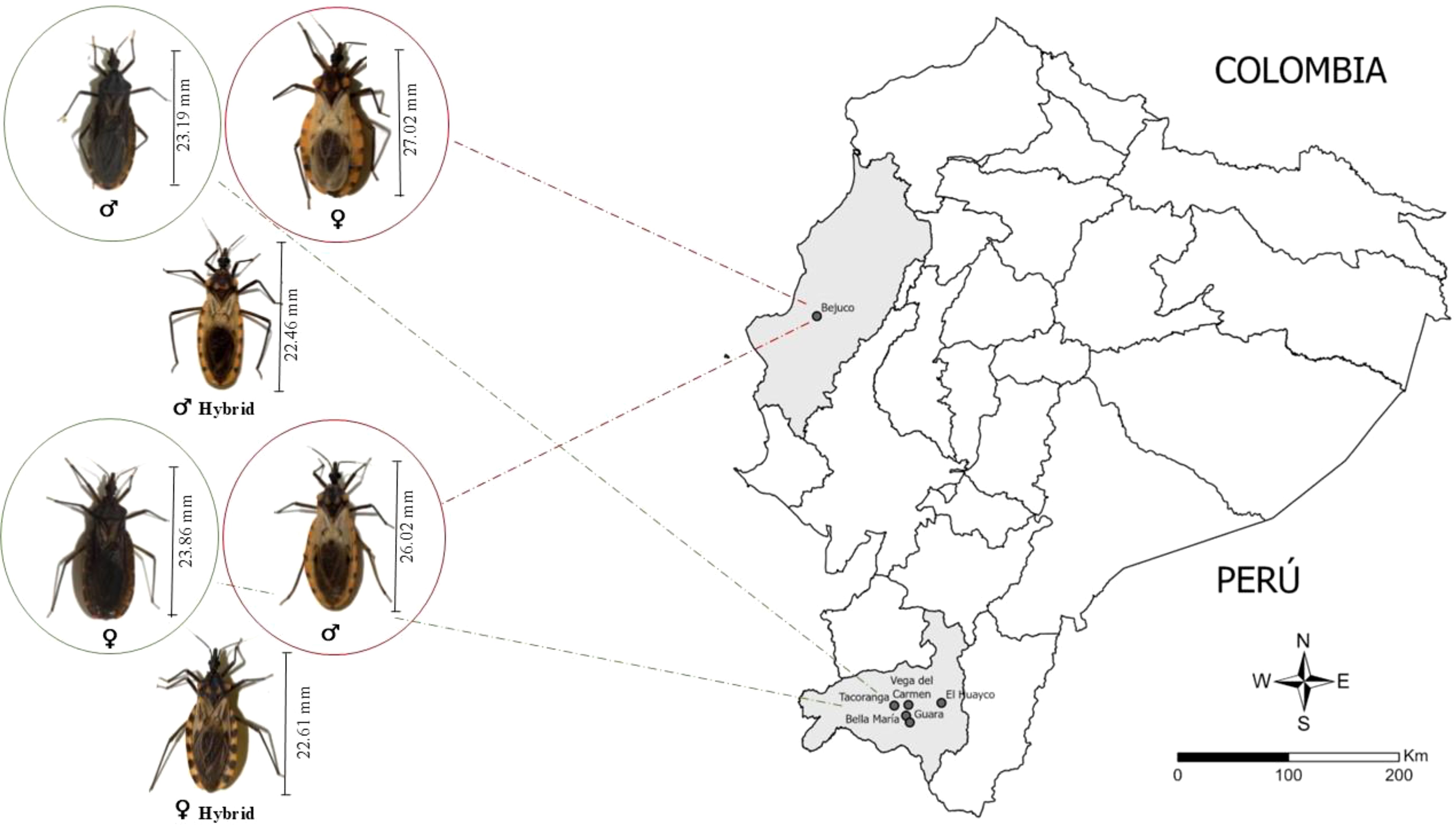
Figure 2. The map of Ecuador shows Manabí and Loja provinces in light gray, with dark grey points indicating the communities where the parental species (Panstrongylus chinai and P. howardi) were collected. In addition, the figure presents body length measurements of the parental species (Panstrongylus chinai and P. howardi) and their hybrids.
Fifteen images (right wings of P. howardi, P. chinai, and hybrid individuals of both sexes) were randomly chosen and digitized twice by the same user. The quality of landmark digitization in our sets showed a repeatability score of 98% for centroid size and 81.9% for shape. Wing size, measured by centroid size, showed notable differences between the parental species, with P. chinai having significantly smaller wings (p < 0.001) (12.93 mm ± 0.62 mm in females and 11.70 mm ± 0.81 mm in males) compared to P. howardi (14.49 mm ± 0.95 mm in females and 14.27 mm ± 0.69 mm in males). However, no significant differences were found in centroid size between the two hybrid groups. Both hybrid groups differed significantly from P. howardi, while only the ♀Pc × ♂Ph hybrid females differed from P. chinai (p < 0.01) (Figures 3A, B). This indicates that size differences in wings between the parental species do not extend to the hybrids, which do not show heterosis in this trait. Hybrid phenotypic traits are typically expected to be intermediate between those of their parental species. For example, hybrids of Triatoma sherlocki Papa, Jurberg, Carcavallo, Cerqueira, Barata, 2002, and T. juazeirensis Costa & Felix, 2007, showed intermediate sizes, which may enhance their fitness, particularly for dispersal (41). However, in our study, the hybrids exhibited smaller wing sizes, possibly due to lower fitness or dominant genetic effects. Similarly, hybrids between Drosophila buzzatii and D. koepferae displayed a range of sizes—larger, smaller, and intermediate—indicating that genetic factors such as dominance (66) and epistasis can influence size variability in hybrids (67). Since centroid size is a trait strongly influenced by environmental factors (63), we cannot ignore the potential impact of parental origin (natural conditions) in relation to the hybrid offspring raised in the laboratory (laboratory conditions).
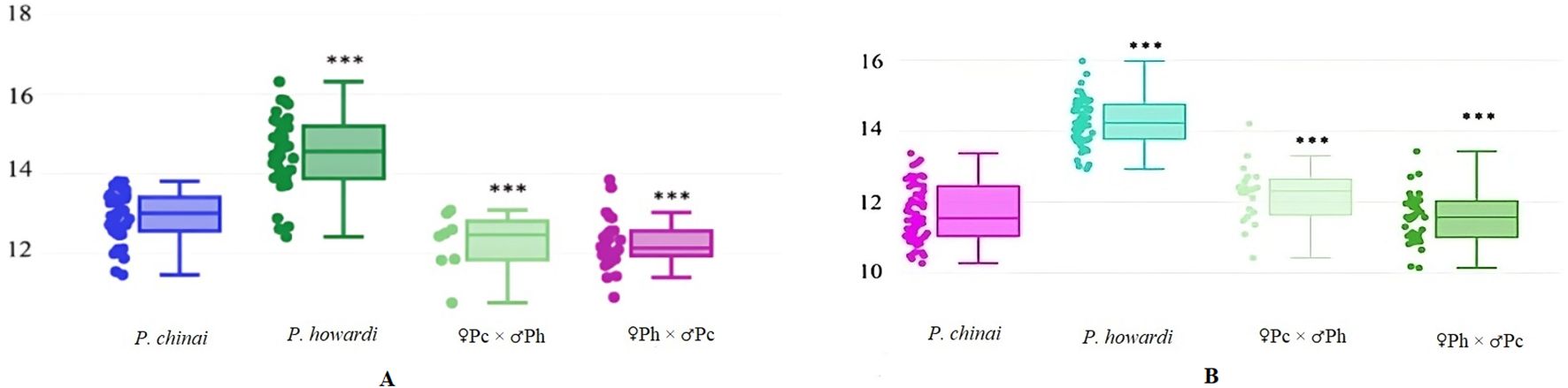
Figure 3. The figure displays the centroid size of the wings of P. chinai, P. howardi, and their hybrids. The line inside each box represents the median, while the bars indicate the maximum and minimum values. (A) Female hybrid from the ♀Ph × ♂Pc. (B) Male P. chinai showing the lower fence. In both females and males, asterisks denote significant differences between P. howardi and P. chinai, as well as between P. howardi and hybrids (♀Pc × ♂Ph and ♀Ph × ♂Pc crosses). ***p < 0.001. IQR, interquartile range.
The allometric effect was low for both sexes in the hybrids: in females, it was 15.7% and 5.6% for the first two factors, while in males, it was 12.5% and 9%, respectively. In addition, we compared the variance of shape, also referred to as MD, between the parental species and the hybrids, and no significant differences in metric disparity (MD = 0.00094) were observed. However, differences in wing morphology were detected, with hybrid males from ♀Pc × ♂Ph exhibiting the lowest variability (MD = 0.00058) (Table 1). Females of the ♀Pc × ♂Ph hybrid group exhibited thinner wings compared to their parental counterparts, potentially reflecting different flight strategies. Previous studies have suggested that varying wing morphotypes may influence flight behavior (37, 68). The observed shape differences, especially in hybrids, could have functional implications, possibly affecting flight capabilities and dispersal behavior. Similarly to what is observed in Apis mellifera, we can infer that differences in wing conformation in hybrids may impact and potentially alter their aerodynamics (69, 70).
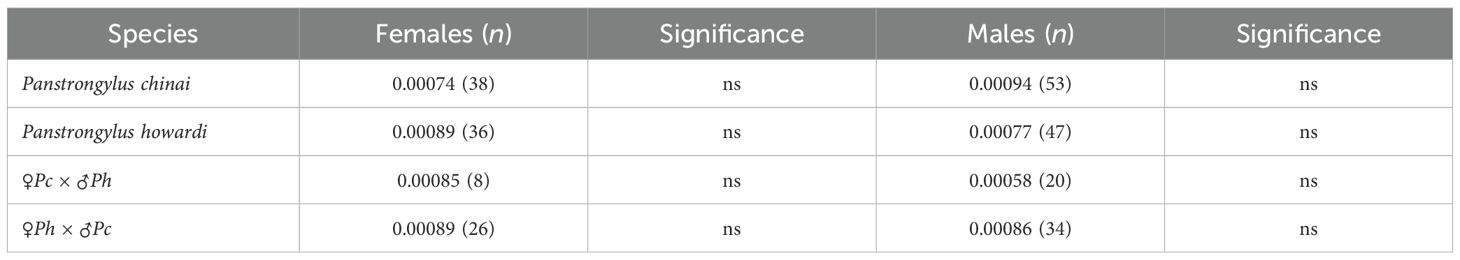
Table 1. The metric disparity (MD) of wing shape in the parental species (P. chinai and P. howardi) and hybrid groups.
The average objects (based on the residual coordinates) showed shape changes between P. chinai and P. howardi (parental), and the hybrids (♀Pc × ♂Ph and ♀Ph × ♂Pc), specifically related to the length of the membranous part of the wing and the width of the coriaceous part. In females, the primary difference was located at landmark 6 (the crossing between the radial and subcostal veins), while in males, it was at landmark 3 (the crossing between the middle radial and ulnar veins). Moreover, in females and males, landmark 9 (the crossing between the cubital and postcubital veins) exhibited notable differences, particularly between P. chinai and ♀Ph × ♂Pc in males, and P. howardi and ♀Ph × ♂Pc in females. In the latter case, this hybrid (♀Ph × ♂Pc) displayed a longer and narrower wing (Figure 4).

Figure 4. The figure presents the average wing shapes based on residual coordinates, illustrating the differences between females and males of the parental species (P. chinai and P. howardi) and the hybrid groups. (A) Females; (B) males.
For the classification: (i) The smallest Euclidean distance was observed between P. howardi and the ♀Pc × ♂Ph in females (0.01444), while in males, the smallest Euclidean distance was between the two hybrid groups (0.00954). This suggests that hybrid males may exhibit phenotypic characteristics that blur the boundaries between parental species and their hybrids (Table 2). This result highlights a trend where hybrids could potentially be considered a single phenotypic group, particularly when classified based on wing size and shape. (ii) The DA (validated reclassification) produced better results when classification was based on parental species rather than the hybrids, with scores generally higher for males (75%, 87%, 45%, 74%) than for females (74%, 64%, 38%, 58%). (iii) The K-means algorithm for wing shape successfully classified the parental species and hybrid groups, identifying an optimal k-value of 5 for females and 4 for males. While the K-means algorithm for wing size classified the groups with an optimal k = 3 in both females and males, conformation provided better classification, whereas size failed to distinguish between the two hybrid groups (k = 3, with parental species and a single hybrid group). This limitation may be due to the initial samples (parental) originating from different locations, leading to a higher-than-expected k. A similar situation was observed in Hernández et al. (37), where the lower k-value could be attributed to the uniform conditions in laboratory breeding, which tend to minimize potential differences.
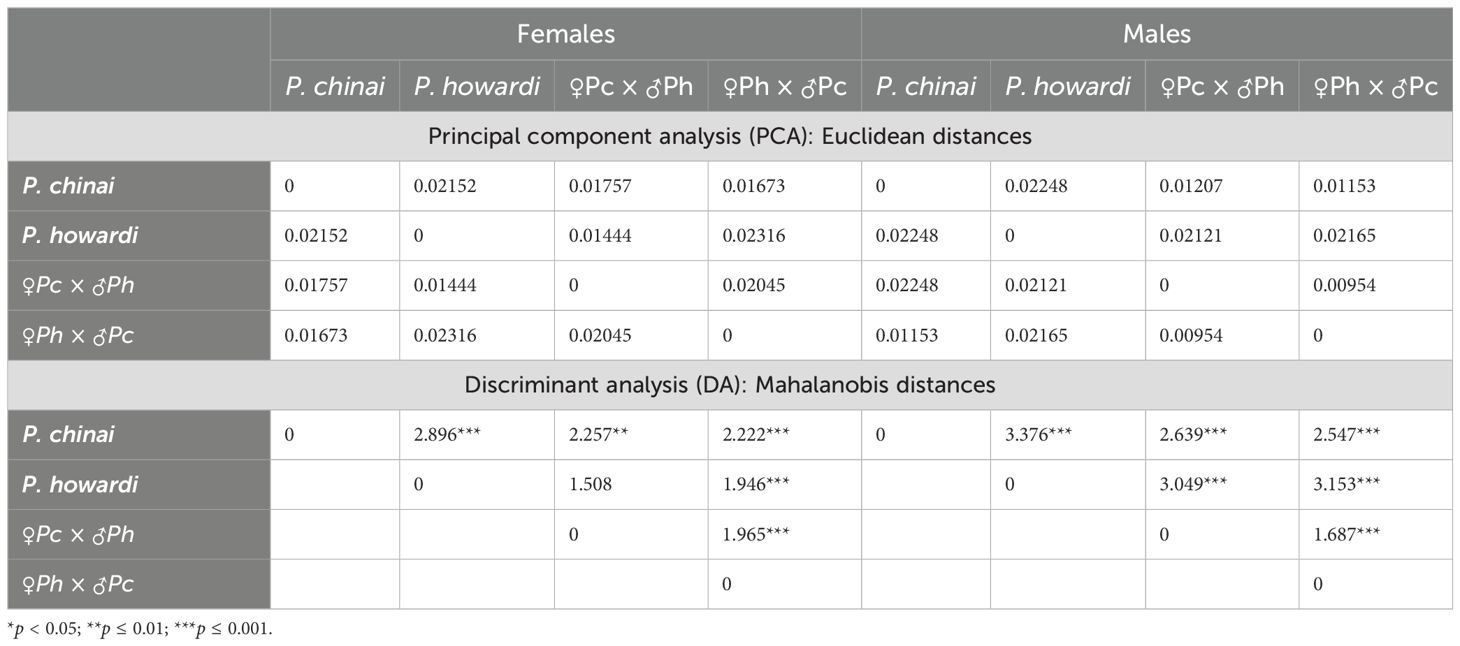
Table 2. Discriminant analysis (Mahalanobis distances) and principal component analysis (Euclidean distances) were performed between the parental species (P. chinai and P. howardi) and hybrid groups.
When analyzing the morphological similarities of the groups using Euclidean and Mahalanobis distances, the highest similarity is observed between each parent and the hybrid in which that parent is the male in the cross. Specifically, P. chinai shows greater similarity with the hybrid ♀Ph × ♂Pc, while P. howardi is more similar to the hybrid ♀Pc × ♂Ph in both females and males. This raises the question: Does the male exhibit greater phenotypic dominance in wing conformation?
According to Vicente et al. (42), the hybrids present intermediate patterns, which could provide greater fitness than their parental species in: (i) the invasion process (domiciliary and peridomiciliary), (ii) shorter life cycles, (iii) feeding and defecation patterns (transmission dynamics of T. cruzi). These patterns may be similar to or indicate a higher susceptibility to T. cruzi infection than their parental species under laboratory conditions (71), highlighting the possible vector competence of these insects for Chagas disease. While P. chinai is distributed in the Loja and El Oro provinces (14, 23), P. howardi is restricted to Manabí province (21). Despite search efforts, no hybrid individuals have been found in nature between these regions, even after conducting predictive models through ecological niche modeling (14). In addition, for P. chinai, P. howardi, and their hybrids (♀Pc × ♂Ph and ♀Ph × ♂Pc crosses), no significant differences were observed in the preoviposition period or the average number of eggs (14). However, P. chinai could be considered a univoltine species, as its complete life cycle takes 371.4 days ± 22.3 days (20), whereas P. howardi completes its life cycle in 186.9 days ± 12.2 days (Castillo et al., unpublished data), nearly half the duration of P. chinai. In the transmission dynamics of T. cruzi, P. chinai presented a natural infection rate of 14% (23), while P. howardi had a rate of 53.2% (21). No information on the life cycle or infection index of the hybrids has been reported so far. Nevertheless, anthropization and climate change can impact the incidence, seasonal transmission, and geographic distribution of vectors and the diseases they transmit (72–74). Changes to the ecological landscape pose an epidemiological risk for the emergence of endemic diseases and may facilitate the convergence of different species, leading to the formation of natural hybrids.
Both size and shape contribute to understanding the similarities and differences between groups. While size is a useful characteristic for discrimination, shape offers greater resolution and is less influenced by environmental variation (75). Panstrongylus chinai displays a notably dark coloration, allowing it to hide in dark places such as under beds and holes within adobe walls inside domiciles. It is also commonly found in peridomiciliary habitats, including chicken coops, as well as pigeon and rat nests (23). On the other hand, P. howardi exhibits a distinct orangish coloration that enables it to camouflage effectively among bricks (21). However, hybrids (♀Pc × ♂Ph and ♀Ph × ♂Pc crosses) of both sexes generally displayed a coloration characteristic of the P. howardi phenotype. In addition, P. chinai exhibited the highest variability in CS and shape, which may be attributed to the geographical range where the specimens were collected (five communities in Loja province at altitudes ranging from 700 to 1,400 masl). Furthermore, abiotic and biotic conditions of breeding habitats can influence the wing geometry through carry-over effects from the immature to the adult stage. Individuals from a single sampling location would likely reduce shape variation within each species, potentially leading to an overestimation of actual interspecific variation.
This study found that the greatest Euclidean distance (indicating the most dissimilar phenotype) was between P. howardi and the ♀Ph × ♂Pc hybrid. This suggests that P. chinai exerts greater phenotypic influence when the male is the parental specimen. Despite this, DA based on parental species generally outperformed hybrid-based classification, with males showing better classification results than females. Overall, the hybrids’ size and shape tended to resemble P. chinai, though this was not always the case.
Considering these species as “secondary”—P. chinai in Loja and P. howardi in Manabí—we recommend further expanding knowledge of the genus Panstrongylus in Ecuador and Latin America. Geometric morphometrics has proven to be a highly useful and cost-effective technique for identifying sibling species and hybrids. Further research should explore additional morphological traits (i.e., head, pronotum, and antennal phenotype) and molecular markers, incorporating integrative approaches (7, 8, 14) to elucidate the evolutionary and ecological dynamics of triatomine hybridization (76, 77).
In summary, our study confirms the utility of geometric morphometrics in distinguishing P. chinai, P. howardi, and their hybrid offspring. The hybrids tend to display traits more closely related to one of the parental species, but differences in wing size and shape suggest potential shifts in flight behavior and overall fitness. Hybridization may not always confer evolutionary benefits; therefore, its ecological implications in this genus warrant further investigation, particularly regarding its role in vector-borne disease transmission. Future research should explore additional morphological, behavioral, and molecular traits to fully understand the dynamics of hybridization and its impact on the fitness and spread of these species.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
ÁL: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Writing – original draft. MH: Formal analysis, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. CY: Formal analysis, Investigation, Resources, Writing – review & editing. MG: Funding acquisition, Writing – review & editing. AV: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding was provided by Pontificia Universidad Católica del Ecuador (K13063 and K13023); the National Institutes of Health, Fogarty International Center, Global Infectious Disease Training Grant (1D43TW008261-01A1); the National Institute of Allergy and Infectious Diseases, Division of Microbiology and Infectious Diseases, Academic Research Enhancement Award (1R15AI077896-01); Children’s Heartlink; and the European Commission Framework Programme 7 Project “Comparative epidemiology of genetic lineages of Trypanosoma cruzi” ChagasEpiNet, Contract No. 223034. María Laura Hernández is member of the CONICET Researcher’s Career (Argentina).
We acknowledge the inhabitants of the visited communities and the personnel of the Ministry of Health who participated in the collection of the triatomines. Moreover, we thank Santiago Cadena for maintaining the triatomine colonies in the insectary of the CISeAL. Technical assistance was provided by Ignacio Pacheco. We also thank Ana Laura Carbajal de la Fuente and the staff of UnOVE-CeNDIE (ANLIS-Malbrán) for their collaboration.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pan American Health Organization (PAHO). Chagas disease (2024). Available online at: https://www.paho.org/en/topics/chagas-disease (Accessed October 03, 2024).
2. WHO. Chagas disease (also known as American trypanosomiasis) (2024). Available online at: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (Accessed September 18, 2024).
3. Dumonteil E, Herrera C, Martini L, Grijalva MJ, Guevara AG, Costales JA, et al. Chagas disease has not been controlled in Ecuador. PLoS One. (2016) 11:e0158145. doi: 10.1371/journal.pone.0158145
4. Lent H, Wygodzinsky P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas’ disease. Bull Am Mus. Nat His. (1979) 163:366–82. Available online at: https://www.cabidigitallibrary.org/doi/full/10.5555/19790567716.
5. Schofield CJ, Galvão C. Classification, evolution, and species groups within the Triatominae. Acta Trop. (2009) 110:88–100. doi: 10.1016/j.actatropica.2009.01.010
6. Justi SA, Galvão C. The evolutionary origin of diversity in Chagas disease vectors. Trends Parasitol. (2017) 33:42–52. doi: 10.1016/j.pt.2016.11.002
7. Alevi KCC, de Oliveira J, da Silva Rocha D, Galvão C. Trends in taxonomy of Chagas disease vectors (Hemiptera, Reduviidae, Triatominae): from Linnaean to integrative taxonomy. Pathogens. (2021) 10:1627. doi: 10.3390/pathogens10121627
8. Costa J, Dale C, Galvão C, Almeida CE, Dujardin JP. Do the new triatomine species pose new challenges or strategies for monitoring Chagas disease? An overview from 1979-2021. Memórias do Instituto Oswaldo Cruz. (2021) 116:e210015. doi: 10.1590/0074-02760210015
9. Téllez-Rendón J, Esteban L, Rengifo-Correa L, Díaz-Albiter H, Huerta H, Dale C. Triatoma yelapensis sp. nov. (Hemiptera: Reduviidae) from Mexico, with a key of Triatoma species recorded in Mexico. Insects. (2023) 14:331. doi: 10.3390/insects14040331
10. Abad-Franch F, Paucar CA, Carpio CC, Cuba Cuba CA, Aguilar VHM, Miles MA, et al. Biogeography of triatominae (Hemiptera: Reduviidae) in Ecuador: implications for the design of control strategies. Mem Inst Oswaldo Cruz. (2001) 96:611–20. doi: 10.1590/S0074-02762001000500004
11. Villacís AG, Arcos-Terán L, Grijalva MJ. Life cycle, feeding and defecation patterns of Rhodnius Ecuadoriensis (Lent & León 1958) (Hemiptera: Reduviidae: Triatominae) under laboratory conditions. Mem Inst Oswaldo Cruz. (2008) 103:690–5. doi: 10.1590/s0074-02762008000700011
12. Villacís AG, Grijalva MJ, Catalá SS. Phenotypic variability of Rhodnius Ecuadoriensis populations at the Ecuadorian central and southern Andean region. J Med Entomol. (2010) 47:1034–43. doi: 10.1603/me10053
13. Villacís AG, Marcet PL, Yumiseva CA, Dotson EM, Tibayrenc M, Brenière SF, et al. Pioneer study of population genetics of Rhodnius Ecuadoriensis (Hemiptera: Reduviidae) from the central coastand southern Andean regions of Ecuador. Infect Genet Evol. (2017) 53:116–27. doi: 10.1016/j.meegid.2017.05.019
14. Villacís AG, Dujardin JP, Panzera F, Yumiseva CA, Pita S, Santillán-Guayasamín S, et al. Chagas vectors Panstrongylus Chinai (Del Ponte 1929) and Panstrongylus howardi (Neiva 1911): chromatic forms or true species? Parasitol vectors. (2020) 13:1–21. doi: 10.1186/s13071-020-04097-z
15. Grijalva MJ, Villacís AG. Presence of Rhodnius Ecuadoriensis in sylvatic habitats in the southern highlands (Loja Province) of Ecuador. J Med Entomol. (2009) 46:708–11. doi: 10.1603/033.046.0339
16. Grijalva MJ, Suarez-Davalos V, Villacís AG, Ocaña-Mayorga S, Dangles O. Ecological factors related to the widespread distribution of sylvatic Rhodnius Ecuadoriensis populations in southern Ecuador. Parasitol Vectors. (2012) 5:1–10. doi: 10.1186/1756-3305-5-17
17. Santos CMD, Jurberg J, Galvão C, Rocha DDS, Fernandez JIR. Estudo morfométrico do gênero Panstrongylus Berg 1879 (Hemiptera, Reduviidae, Triatominae). Mem Inst Oswaldo Cruz. (2003) 98:939–44. doi: 10.1590/s0074-02762003000700014
18. Garrido R, Bacigalupo A, Peña-Gómez F, Bustamante RO, Cattan PE, Gorla DE, et al. Potential impact of climate change on the geographical distribution of two wild vectors of Chagas disease in Chile: Mepraia spinolai and Mepraia gajardoi. Parasitol Vectors. (2019) 12:1–16. doi: 10.1186/s13071-019-3744-9
19. Vivas RJ, García JE, Guhl F, Hernández C, Velásquez N, Ramírez JD, et al. Systematic review on the biology, ecology, genetic diversity and parasite transmission potential of Panstrongylus geniculatus (Latreille 1811) in Latin America. Mem Inst Oswaldo Cruz. (2021) 116:e200528. doi: 10.1590/0074-02760200528
20. Mosquera KD, Villacís AG, Grijalva MJ. Life cycle, feeding, and defecation patterns of Panstrongylus Chinai (Hemiptera: Reduviidae: Triatominae) under laboratory conditions. J Med Entomol. (2016) 53:776–81. doi: 10.1093/jme/tjw027
21. Villacís AG, Ocaña-Mayorga S, Lascano MS, Yumiseva CA, Baus EG, Grijalva MJ. Abundance, natural infection with trypanosomes, and food source of an endemic species of triatomine, Panstrongylus howardi (Neiva 1911), on the Ecuadorian Central Coast. Am J Trop Med Hyg. (2015) 92:187. doi: 10.4269/ajtmh.14-0250
22. Patterson JS, Barbosa SE, Feliciangeli MD. On the genus Panstrongylus Berg 1879: evolution, ecology and epidemiological significance. Acta Trop. (2009) 110:187–99. doi: 10.1016/j.actatropica.2008.09.008
23. Grijalva MJ, Villacís AG, Ocaña-Mayorga S, Yumiseva CA, Moncayo AL, Baus EG. Comprehensive survey of domiciliary triatomine species capable of transmitting Chagas disease in southern Ecuador. PLoS Negl Trop Dis. (2015) 9:e0004142. doi: 10.1371/journal.pntd.0004142
24. Grijalva MJ, Villacís AG, Moncayo AL, Ocaña-Mayorga S, Yumiseva CA, Baus EG. Distribution of triatomine species in domestic and peridomestic environments in central coastal Ecuador. PLoS Negl Trop Dis. (2017) 11:e0005970. doi: 10.1371/journal.pntd.0005970
25. Suarez-Davalos V, Dangles O, Villacis AG, Grijalva MJ. Microdistribution of sylvatic triatomine populations in central-coastal Ecuador. J Med Entomol. (2010) 47:80–8. doi: 10.1093/jmedent/47.1.80
26. Carcavallo RU, Jurberg J, Lent H, Lent H. Phylogeny of the triatominae. In: Carcavallo RU, Girón IG, Jurberg J, editors. Part A. General approach, vol. 3 . Atlas of Chagas Disease Vectors in the Americas, Editora Fiocruz, Rio de Janeiro (1999). p. 925–980.
27. Fournier D, Aron S. Hybridization and invasiveness in social insects—The good, the bad and the hybrid. Curr Opin Insect Sci. (2021) 46:1–9. doi: 10.1016/j.cois.2020.12.004
28. Rohlf FJ, Marcus LF. A revolution morphometrics. Trends Ecol Evol. (1993) 8:129–32. doi: 10.1016/0169-5347(93)90024-j
29. Stanton DE, Reeb C. Morphogeometric approaches to non-vascular plants. Front Plant Sci. (2016) 7:916. doi: 10.3389/fpls.2016.00916
30. Benard MF, McCauley SJ. Integrating across life-history stages: consequences of natal habitat effects on dispersal. Am Nat. (2008) 171:553–67. doi: 10.1086/587072
31. Clobert J, Le Galliard JF, Cote J, Meylan S, Massot M. Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol Lett. (2009) 12:197–209. doi: 10.1111/j.1461-0248.2008.01267.x
32. Abrahan L, Hernández L, Gorla D, Catalá S. Phenotypic diversity of Triatoma infestans at the microgeographic level in the Gran Chaco of Argentina and the Andean valleys of Bolivia. J Med Entomol. (2008) 45:660–6. doi: 10.1093/jmedent/45.4.660
33. Hernández L, Abrahan L, Moreno M, Gorla D, Catalá S. Phenotypic variability associated to genomic changes in the main vector of Chagas disease in the southern cone of South America. Acta Trop. (2008) 106:60–7. doi: 10.1016/j.actatropica.2008.01.006
34. Hernández ML, Abrahan LB, Dujardin JP, Gorla DE, Catalá SS. Phenotypic variability and population structure of peridomestic Triatoma infestans in rural areas of the arid Chaco (Western Argentina): spatial influence of macro-and microhabitats. Vector Borne Zoonotic Dis. (2011) 11:503–13. doi: 10.1089/vbz.2009.0253
35. Hernández ML, Dujardin JP, Gorla DE, Catalá SS. Potential sources of Triatoma infestans reinfesting peridomiciles identified by morphological characterization in Los Llanos, La Rioja, Argentina. Mem Inst Oswaldo Cruz. (2013) 108:91–7. doi: 10.1590/s0074-02762013000100015
36. Hernández ML, Espinoza J, Gomez M, Gorla D. Morphological changes associated with brachypterous Triatoma guasayana (Hemiptera, Reduviidae) and their relationship with flight. Int J Trop Insect Sci. (2020) 40:413–21. doi: 10.1007/s42690-019-00092-9
37. Hernández ML, Dujardin JP, Villacís AG, Yumiseva CA, Remón C, Mougabure-Cueto G. Resistance to deltamethrin in Triatoma infestans (Hemiptera: Reduviidae): Does it influence the phenotype of antennae, wings, and heads? Acta Trop. (2023) 245:106976. doi: 10.1016/j.actatropica.2023.106976
38. Nattero J, Piccinali RV, Gaspe MS, Gürtler RE. Fluctuating asymmetry and exposure to pyrethroid insecticides in Triatoma infestans populations in northeastern Argentina. Infect Genet Evol. (2019) 74:103925. doi: 10.1016/j.meegid.2019.103925
39. Gigena GV, Rodríguez CS, Fiad FG, Hernández ML, Carbajal-de-la-Fuente AL, Piccinali RV, et al. Phenotypic variability in traits related to flight dispersal in the wing dimorphic species Triatoma guasayana. Parasitol Vectors. (2023) 16:8. doi: 10.1186/s13071-022-05570-7
40. Costa J, Peterson AT, Dujardin JP. Morphological evidence suggests homoploid hybridization as a possible mode of speciation in the Triatominae (Hemiptera, Heteroptera, Reduviidae). Infect Genet Evol. (2009) 9:263–70. doi: 10.1016/j.meegid.2008.12.005
41. Almeida CE, Oliveira HL, Correia N, Dornak LL, Gumiel M, Neiva VL, et al. Dispersion capacity of Triatoma sherlocki, Triatoma juazeirensis and laboratory-bred hybrids. Acta Trop. (2012) 122:71–9. doi: 10.1016/j.actatropica.2011.12.001
42. Vicente RD, Madeira FF, Borsatto KC, Garcia ACC, Cristal DC, Delgado LMG, et al. Morphological, cytological and molecular studies and feeding and defecation pattern of hybrids from experimental crosses between Triatoma sordida and T. rosai (Hemiptera, Triatominae). Pathogens. (2022) 11:1302. doi: 10.3390/pathogens11111302
43. Pita S, Gómez-Palacio A, Lorite P, Dujardin JP, Chavez T, Villacís AG, et al. Multidisciplinary approach detects speciation within the kissing bug Panstrongylus rufotuberculatus populations (Hemiptera, Heteroptera, Reduviidae). Mem Inst Oswaldo Cruz. (2022) 116:e210259. doi: 10.1590/0074-02760210259
44. Santillán-Guayasamín S, Villacís AG, Grijalva MJ, Dujardin JP. The modern morphometric approach to identify eggs of Triatominae. Parasitol vectors. (2017) 10:1–10. doi: 10.1186/s13071-017-1982-2
45. Instituto Nacional de Meteorología e Hidrología (INAMHI). Boletín meteorológico. Ecuador: INAMHI (2023). Available at: https://www.inamhi.gob.ec/pronostico/cwrf/2023/Boletin_CWRF.pdf (Accessed September 22, 2024).
46. Instituto Nacional de Estadística y Censos (INEC). Resultados del Censo 2010 de Poblacion y Vivienda en el Ecuador (2023). Available online at: https://www.censoEcuador.gob.ec/ (Accessed September 18, 2024).
47. Nieto-Sanchez C, Baus EG, Guerrero D, Grijalva MJ. Positive deviance study to inform a Chagas disease control program in southern Ecuador. Mem Inst Oswaldo Cruz. (2015) 110:299–309. doi: 10.1590/0074-02760140472
48. Santillán-Guayasamín S, Villacís AG, Grijalva MJ, Dujardin JP. Triatominae: does the shape change of non-viable eggs compromise species recognition? Parasitol vectors. (2018) 11:1–12. doi: 10.1186/s13071-018-3104-1
49. Stern DL, Sucena E. Rapid mounting of adult Drosophila structures in Hoyer’s medium. Cold Spring Harb. Protoc. (2012) 1):pdb–prot067371. doi: 10.1101/pdb.prot067371
50. Arnqvist, Martensson. Measurement error in geometric morphometrics: empirical strategies to assess and reduce its impact on measures of shape. Acta Zool Acad Sci Hung. (1998) 44:73–96. Available online at: https://arnqvist.org/az1998.pdf.
51. Goodall C. Procrustes methods in the statistical analysis of shape. J R Stat Soc: Ser B (Methodological). (1991) 53:285–321. doi: 10.1111/j.2517-6161.1991.tb01825.x
52. Rohlf FJ. Shape statistics: Procrustes superimpositions and tangent spaces. J Classif. (1999) 16:197–223. doi: 10.1007/s003579900054
53. Bookstein FL. Morphometric Tools for Landmark Data: Geometry and Biology. (1992) Cambridge University Press.
54. Dujardin S, Dujardin JP. Geometric morphometrics in the cloud. Infect Genet Evol. (2019) 70:189–96. doi: 10.1016/j.meegid.2019.02.018
55. Zelditch ML, Swiderski DL, Sheets HD. Geometric Morphometrics for Biologists: A Primer. 2a ed. Academic Press (2014) Elsevier´s Science and Tecnology Rights Department in Oxford, UK. Available at: https://books.google.at/books?id=5DLZ4lALRTEC (Accessed October 16, 2024).
56. Pérez-Ortega J, Nely-Almanza-Ortega N, Vega-Villalobos A, Pazos-Rangel R, Zavala-Díaz C, Martínez-Rebollar A. The K-means algorithm evolution. In: Sud K, Erdogmus P, Kadry S, editors. Introduction to Data Science and Machine Learning. London: IntechOpen (2020).
57. Dujardin JP. The body of Chagas disease vectors. Pathogens. (2025) 14:98. doi: 10.3390/pathogens14010098
58. Paschoaletto L, Dale C, Lima-Neiva V, Carbajal-De-La-Fuente AL, de Oliveira J, Benítez HA, et al. Morphological stasis in time? a Triatoma brasiliensis brasiliensis study using geometric morphometrics in the long run. Animals. (2022) 12(11):1362. doi: 10.3390/ani12111362
59. Barnabé C, Grijalva MJ, Santillán-Guayasamín S, Yumiseva CA, Waleckx E, Brenière SF, et al. Genetic data support speciation between Panstrongylus howardi and Panstrongylus Chinai, vectors of Chagas disease in Ecuador. Infect Genet Evol. (2020) 78:104103. doi: 10.1016/j.meegid.2019.104103
61. Pérez R, Hernández M, Quintero O, Scvortzoff E, Canale D, Mendez L, et al. Cytogenetic analysis of experimental hybrids in species of Triatominae (Hemiptera-Reduviidae). Genetica. (2005) 125:261–70. doi: 10.1007/s10709-005-0369-z
62. Arnold ML. Natural hybridization and evolution. Oxford, United Kingdom: Oxford University Press (1997).
63. Dujardin JP, Steindel M, Chavez T, Machane M, Schofield CJ. Changes in the sexual dimorphism of Triatominae in the transition from natural to artificial habitats. Mem Inst Oswaldo Cruz. (1999) 94:565–9. doi: 10.1590/s0074-02761999000400024
64. Levy RC, Kozak GM, Wadsworth CB, Coates BS, Dopman EB. Explaining the sawtooth: latitudinal periodicity in a circadian gene correlates with shifts in generation number. J Evol Biol. (2015) 28:40–53. doi: 10.1111/jeb.12562
65. Dujardin J-P. Modern morphometrics of arthropods: A phenotypic approach to species recognition and population structure Infect. In: Genet. Evol. Amsterdam: Elsevier (2024). p. 385–425.
66. Orr HA. Fitness and its role in evolutionary genetics. Nat Rev Genet. (2009) 10:531–9. doi: 10.1038/nrg2603
67. Carreira VP, Soto IM, Fanara JJ, Hasson E. A study of wing morphology and fluctuating asymmetry in interspecific hybrids between Drosophila buzzatii and D. koepferae. Genetica. (2008) 133:1–11. doi: 10.1007/s10709-007-9176-z
68. Fiad FG, Cardozo M, Rodríguez CS, Hernández ML, Crocco LB, Gorla DE. Ecomorphological variation of the Triatoma guasayana wing shape in semi-arid Chaco region. Acta Trop. (2022) 232:106488. doi: 10.1016/j.actatropica.2022.106488
69. Schneider SS, Leamy LJ, Lewis LA, DeGrandi-Hoffman G. The influence of hybridization between African and European honeybees, Apis mellifera, on asymmetries in wing size and shape. Evolution. (2003) 57:2350–64. doi: 10.1111/j.0014-3820.2003.tb00247.x
70. Hepburn HR, Radloff SE, Fuchs S. Flight machinery dimensions of honeybees, Apis mellifera. J Comp Physiol B. (1999) 169:107–12. doi: 10.1007/s003600050200
71. Correia N, Paschoaletto L, Reigada C, Gonçalves TCM, Moreira CJDC, Costa J. Experimental Hybrids of the Triatoma brasiliensis Species Complex show higher susceptibility to the Trypanosoma cruzi infection than their Parentals. Microorganisms. (2023) 11:2850. doi: 10.3390/microorganisms11122850
72. Costa J, Dornak LL, Almeida CE, Peterson AT. Distributional potential of the Triatoma brasiliensis species complex at present and under scenarios of future climate conditions. Parasites Vectors. (2014) 7:1–10. doi: 10.1186/1756-3305-7-238
73. Ceccarelli S, Rabinovich JE. Global climate change effects on Venezuela’s vulnerability to chagas disease is linked to the geographic distribution of five triatomine species. J Med Entomology. (2015) 52:1333–43. doi: 10.1093/jme/tjv119
74. Ravazi A, Oliveira JD, Madeira FF, Nunes GM, Reis Y, Oliveira ABBD, et al. Climate and environmental changes and their potential effects on the dynamics of Chagas Disease: Hybridization in Rhodniini (Hemiptera, Triatominae). Insects. (2023) 14:378. doi: 10.3390/insects14040378
75. Garros C, Dujardin JP. Genetic and phenetic approaches to Anopheles systematics. Anopheles mosquitoes: new insights into malaria vectors. Rijeka: InTech (2013) p. 81–105.
76. Gurgel-Gonçalves R, Ferreira JBC, Rosa AF, Bar ME, Galvão C. Geometric morphometrics and ecological niche modelling for delimitation of near-sibling triatomine species. Med Veterinary Entomology. (2011) 25:84–93. doi: 10.1111/j.1365-2915.2010.00920.x
Keywords: Ecuador, Chagas disease, hybrids, morphometrics, triatomines
Citation: Lara Á, Hernández ML, Yumiseva CA, Grijalva MJ and Villacís AG (2025) Hybrids versus parental species: insights from wing phenotype similarities and differences in triatomine insects Front. Insect Sci. 5:1547963. doi: 10.3389/finsc.2025.1547963
Received: 19 December 2024; Accepted: 18 February 2025;
Published: 11 March 2025.
Edited by:
Carlos Eduardo Almeida, Federal University of Bahia (UFBA), BrazilReviewed by:
Steve B. S. Baleba, International Centre of Insect Physiology and Ecology (ICIPE), KenyaCopyright © 2025 Lara, Hernández, Yumiseva, Grijalva and Villacís. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anita G. Villacís, YWd2aWxsYWNpc0BwdWNlLmVkdS5lYw==; María Laura Hernández, bWFyaWFsYXVyYS5oZXJuYW5kZXpAZ21haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.