
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Insect Sci., 02 July 2024
Sec. Insect Physiology
Volume 4 - 2024 | https://doi.org/10.3389/finsc.2024.1430422
Introduction: Control of the mosquito Aedes albopictus is confounded by its behavior due to females preferring to oviposition in small natural and artificial containers that are often difficult to remove or treat with insecticides. Autodissemination strategies utilizing highly potent insect growth regulators (IGRs) have emerged as promising tools for the control of this container-inhabiting species. The intended goal of autodissemination approaches is to use mosquitoes to self-deliver an IGR to these cryptic oviposition locations. Previous studies have focused on the efficacy of these approaches to impact natural populations, but little focus has been placed on the impacts on mosquitoes when exposed to non-lethal doses of IGRs similar to the levels they would be exposed to with autodissemination approaches.
Methods: In this study, the impact of non-lethal doses of pyriproxyfen (PPF) on the reproductive fitness of Ae. albopictus was investigated. Female and male Ae. albopictus mosquitoes were exposed to non-lethal doses of PPF and their fecundity and fertility were measured. To examine the impact of non-lethal doses of PPF, the expression of the ecdysone-regulated genes USP, HR3, and Vg, which are involved in vitellogenesis, was determined.
Results: Our results demonstrated a significant reduction in female fecundity and in the blood feeding and egg hatching rates upon exposure to non-lethal doses of PPF. Oocyte development was also delayed in PPF-treated females. Furthermore, exposure to non-lethal doses of PPF altered the expression of the genes involved in vitellogenesis, indicating disruption of hormonal regulation. Interestingly, PPF exposure also reduced the sperm production in males, suggesting a potential semi-sterilization effect.
Discussion: These findings suggest that non-lethal doses of PPF could enhance the efficacy of autodissemination approaches by impacting the reproductive fitness of both males and females. However, further research is needed to validate these laboratory findings in field settings and to assess their practical implications for vector control strategies.
Aedes albopictus is a vector of dengue, chikungunya, and Zika viruses. Unfortunately, there are no vaccines and therapeutics available to limit the transmission of the aforementioned viruses; therefore, vector control remains one of the only effective measures to limit transmission (1). Ae. albopictus is considered a container-inhabiting species, preferring to oviposit in natural and artificial containers found to be associated with human activity. Locating and treating these often abundant and cryptic habitats can present a challenge for mosquito control professionals (2–4). Hence, there is a definitive need for novel approaches to supplement the traditional vector control methods for the effective control of Ae. albopictus and other container-inhabiting species.
Autodissemination strategies have been suggested as a potential novel method for the control of Ae. albopictus (3, 5–13). Two approaches have been successful in reducing natural populations of Aedes spp. The first is autodissemination augmented by males (ADAM), which utilizes laboratory- or factory-reared male mosquitoes that are treated with an insect growth regulator (IGR) and released into the natural environment, where they can directly deliver an IGR to cryptic larval habitats or indirectly by copulating with naturally occurring females (14, 15). The second is autodissemination stations, which rely on attracting females to a station wherein they are then exposed to non-lethal doses of an IGR and leave the station to disseminate the IGR to cryptic larval habitats (5, 7, 10, 11, 13, 16–18).
While autodissemination approaches have been demonstrated to impact mosquito populations, there may also be unintended effects on the male and female mosquitoes exposed to IGRs associated with the application of these approaches. One commonly used IGR that has been used for autodissemination approaches is pyriproxyfen (PPF). PPF is a juvenile hormone analog (JHA). JHAs act similarly to a natural juvenile hormone (JH) in insects. In general, JH and 20-hydroxyecdysone (20E) are two hormones that regulate the developmental, physiological, and reproductive processes in insects. JH interferes with the metamorphosis of the immature stage to the adult stage by retaining the juvenile characteristics in insects, whereas 20E elicits metamorphosis. The antagonistic action of JH and 20E plays an important role in inhibiting important physiological processes, including reproduction and development (19). JH production promotes the primary follicle cells in the ovary to start developing approximately 3 days after eclosion until the production of 20E after a blood meal, which results in oogenesis. In Aedes species, 20E plays a major role in upregulating the key genes, such as EcR (ecdysone receptor), HR3 (hormone receptor), USP (ultraspiracle), and Vg (vitellogenin), that are involved in oogenesis and vitellogenesis. Previous research has demonstrated that exposure to IGRs can disrupt the antagonistic action of these hormones impacting mosquito reproduction, resulting in other unintended physiological effects. The topical application of methoprene, another JHA, downregulated the expression of the ecdysone-regulated genes USP, HR3, and Vg, which are involved in 20E action and oogenesis (20). Furthermore, exposure to PPF has been demonstrated to result in sterilization and fertility effects in adult male and female Anopheles arabiensis, Anopheles gambiae, Aedes aegypti, and Culex quinquefasciatus (12, 21–24). In addition, exposure to non-lethal doses of PPF as larvae has been previously demonstrated to increase the susceptibility of Ae. aegypti to dengue virus (25).
In this work, experiments were conducted to examine whether non-lethal doses of PPF will reduce the fertility and fecundity of male and female Ae. albopictus, respectively. Specifically, these experiments examined the effects of non-lethal doses of PPF on the fecundity and the blood feeding and egg hatching rates of PPF-treated Ae. albopictus females and the fertility of males. The results are discussed in the context of the unintended impacts of these non-lethal doses of PPF on male and female mosquitoes associated with the use of autodissemination approaches for mosquito control. The results highlight the potential benefits of non-lethal doses to the effectiveness of autodissemination approaches for mosquito control.
The Ae. albopictus used in the experiments were from a colony established from eggs collected in Lubbock, TX, 33.5846° N, 101.8456° W, and were reared and maintained for ~20 generations in laboratory conditions prior to starting the experiments. The mosquitoes were maintained at 28 ± 2°C temperature and 80% ± 5% relative humidity (RH) in a 16:8 light/dark cycle. The eggs were hatched in a 1:1 solution of deionized water and fermented liver powder (0.6 g/L) (MP Biomedicals, Santa Ana, CA, USA). Larvae were fed a 60-g/L bovine liver powder slurry ad libitum. Pupae were collected and placed in 24.5-cm × 24.5-cm × 24.5-cm BugDorm-4S2222 insect rearing cages (MegaView Science Co., Taichung, Taiwan) and provided with a 10% sucrose solution.
For the experiments, Esteem 35WP (Valent Biosciences, Libertyville, IL, USA) was used to treat mosquitoes. Esteem 35WP is formulated with 35% PPF as the active ingredient and was mixed with an inert fluorescent powder (Yu Mingjie pigments, Longdong, Shenzhen, China) to dilute the initial formulations for different adult doses (0.015, 0.03, 0.06, and 0.12 ng). As an untreated control for the PPF dust formulation, the mosquitoes were dusted with only the fluorescent powder, with no Esteem. A total of 30 newly emerged females were treated with each different concentration using a handheld bellow duster (Harris Manufacturing Co. LLC, Cartersville, NC, USA) in a cardboard mailing tube (63.5 mm wide and 20.3 cm long), capped on both ends with No-See-Um netting (Equinox, Williamsport, PA, USA). The mosquitoes were transferred into the rearing cages, with the females fed bovine blood with Na-citrate using an artificial blood feeder and a sausage casing membrane once per week for 3 weeks. A 140-mL Souffle cup (Pactiv, Lake Forest, IL, USA) containing 100 mL deionized water lined with a seed germination paper (Anchor Paper Company, St. Paul, MN, USA) was placed in each cage as an oviposition container. The egg papers were collected for three gonotrophic cycles and the number of eggs counted. The number of blood-fed females was recorded for each cycle. The egg hatching rates were determined for only the first gonotrophic cycle by calculating the mean of three replicate patch counts of approximately 370–1,800 hatched and unhatched eggs. Each treatment consisted of three replicates.
To visualize the impact of PPF on oocyte development, Ae. albopictus females were treated with 0.12, 0.06, 0.03, and 0.015 ng PPF approximately 24 h post-emergence. The treated females were blood-fed 48 h post-exposure and separated into different cages. A total of 10 females from each treatment were collected at 48 and 72 h post-blood feeding. Subsequently, the female mosquitoes were dissected and the ovaries extracted on a small drop of 1× phosphate-buffered saline (PBS). The extracted ovaries were immediately visualized using a Leica S9i stereomicroscope (Leica Microsystems Inc., Buffalo Grove, IL, US), and images were taken using an integrated camera at ×40 magnification.
A total of five females were treated with a single dose of PPF at different concentrations (0.015, 0.03, 0.06, and 0.12 ng) and were collected 48 h after blood feeding for each treatment. Each treatment consisted of three replicates. The total RNA was extracted using the Qiagen RNeasy kit following the manufacturer’s instructions (Qiagen, Hilden, Germany). cDNA synthesis was performed using the LunaScript®RT SuperMix Kit (#E3010; New England Biolabs, Ipswich, MA, USA) from 1 μg of extracted RNA following the manufacturer’s instructions. The quantitative reverse transcription PCR (qRT-PCR) of the target genes and all the reactions were performed in a 20-μL total reaction volume containing 1 μL cDNA with 1.6 μL primer mix with 10 mM each of a forward and a reverse primer and 10 μL 2× PowerUp™ SYBR™ Green Master Mix (Applied Biosystems, Foster City, CA, USA). All of the samples were analyzed using a qPCR master mix on an ABI 7300 Real-Time qPCR System (Applied Biosystems, Foster City, CA, USA). The temperature profile of the qRT-PCR consisted of 10 min at 95°C, 35 cycles of 30 s at 95°C, 30 s at 54°C, and 1 min at 72°C, followed by a 10-min extension step at 72°C. The relative expression levels of EcR, HR3, USP, and Vg were normalized to that of the ribosomal protein S7 (RPS7) using the 2−ΔΔCt method. The primers for the specific genes are listed in Supplementary Table S1. Each gene was amplified for four biological and two technical replicates at each time point.
Sperm quantification was performed as described in Ponlawat and Harrington (26). In brief, Ae. albopictus males were collected <5–6 h post-emergence and were treated topically with 0.03, 0.06, 0.12 ng of Esteem. After approximately 24 h, 10 mosquitoes were selected, and the testes were dissected in 50 μL PBS using a Leica S9i stereomicroscope (Leica Microsystems Inc., Deerfield, IL, US). Each pool of 10 males was replicated three times. A total of 10 dissected testes were then combined with 150 μL of PBS for a total volume of 200 μL. The testes were gently sheared using a dissection probe, and the sample was mixed well with the P10 pipettor to dislodge any sperm clumps. Eight 5-μL aliquots from the total volume were added to a new microscope slide. Each separate aliquot from the replicate pools was then air-dried for 1–2 h and then fixed with 70% ethanol. Immediately thereafter, the slides were stained with 1 μg/mL DAPI for 10–15 min and the excess stain washed off. The total number of sperm was observed using a Leica fluorescence microscope at ×200 magnification and was counted using ImageJ.
The fecundity, egg hatching, and sperm count data were examined for equality of variance and normal distribution to meet the assumptions of a parametric test. A general linear model with a normal distribution was used to examine for differences in the female fecundity and the number of blood-fed females for the different PPF treatments and gonotrophic cycles. The differences in female fecundity and blood-fed females for each treatment within each gonotrophic cycle were examined using t-tests. A repeated measures ANOVA was used to determine differences in the total egg number produced for each cage replicate when combining the data from all three gonotrophic cycles. The egg hatching rates of the females from all three gonotrophic cycles were compared by performing an arcsine square root transformation of the proportion of eggs hatched and an ANOVA. ANOVA was also used to compare the sperm production of males after PPF exposure. Pairwise comparisons of the sperm counts between treatments were performed using t-tests. Differences in the gene expression were examined using the non-parametric Kruskal–Wallis multiple comparisons followed by pairwise Wilcoxon tests between the PPF treatments for each gene. All statistical analyses were performed and the graphs designed using JMP Pro 16.
Non-lethal doses of PPF were demonstrated to affect the fecundity of Ae. albopictus females over the three gonotrophic cycles (χ2 = 10.7, DF = 3, p = 0.01) (Figure 1A). When the effects of PPF dose were examined, a reduction in fecundity was observed (χ2 = 3.6, DF = 1, p = 0.05), but not for each gonotrophic cycle (χ2 = 2.4, DF = 1, p = 0.12) (Figure 1A). The effect of the interaction of gonotrophic cycle and PPF dose was also associated with a reduction in the fecundity of Ae. albopictus females (χ2 = 5.6, DF = 1, p = 0.01) (Figure 1A). Non-lethal doses of PPF were also demonstrated to impact the number of Ae. albopictus females that blood fed (χ2 = 11.4, DF = 3, p = 0.0096) (Figure 1B). Examination of the effects of PPF dose revealed an effect of a reduction in the number of females that took a blood meal (χ2 = 6.6, DF = 1, p = 0.01) and over each gonotrophic cycle (χ2 = 5.4, DF = 1, p = 0.01) (Figure 1B). The effect of the interaction of gonotrophic cycle and PPF dose was not associated with a reduction in the number of females that blood fed (χ2 = 0.14, DF = 1, p = 0.69) (Figure 1B). When combining the egg production over all three gonotrophic cycles, a significant effect of PPF treatment on female fecundity was also observed, where PPF-treated females produced fewer eggs (repeated measures ANOVA: F = 6.1, DF = 1, p = 0.03) (Figure 1C). The data also suggest that, when females were treated with PPF, they produced eggs with significantly lower hatching rates than the untreated females (ANOVA: F = 3.9, DF = 4, p = 0.04) (Figure 1D).
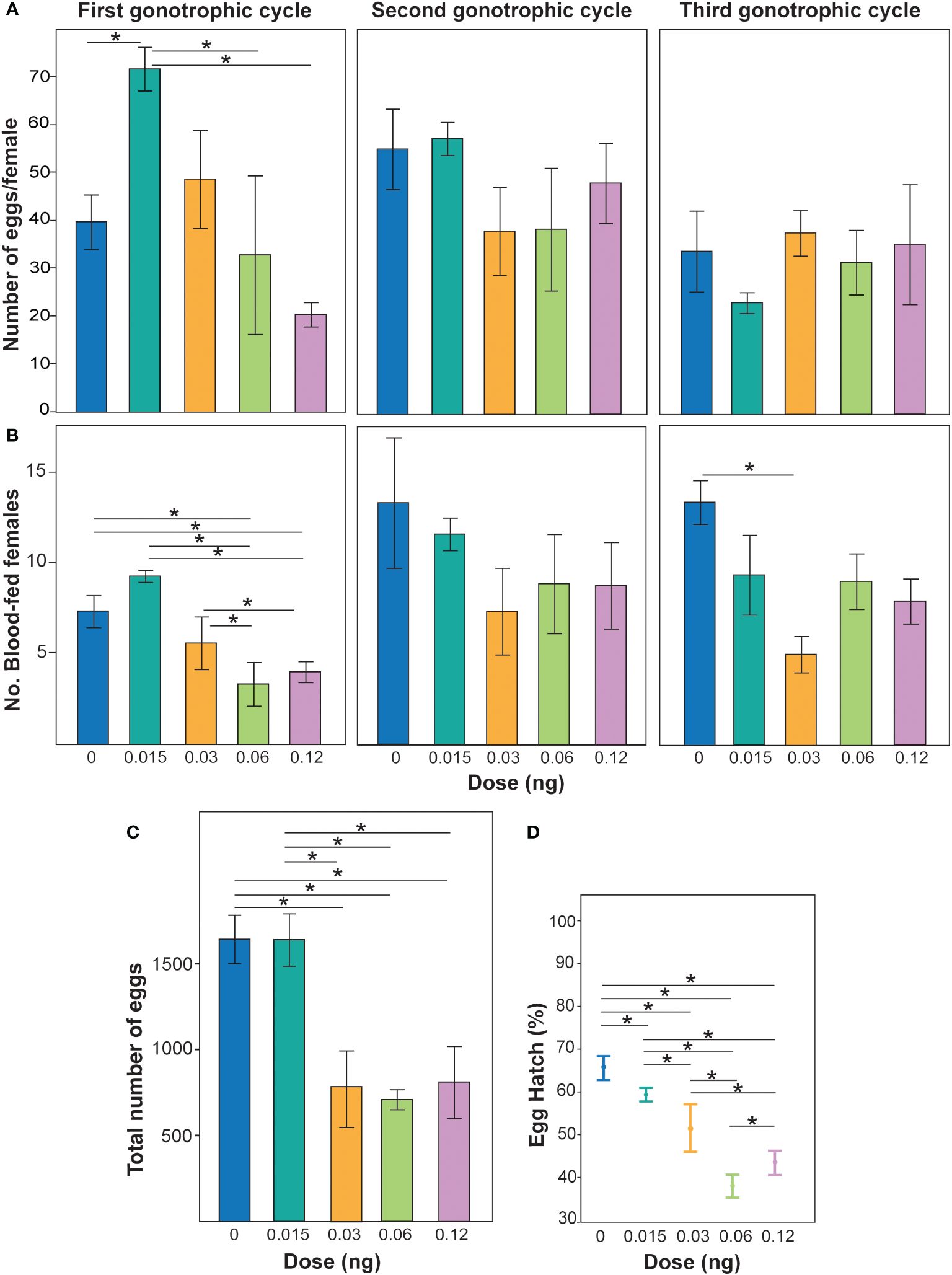
Figure 1 (A) Effect of non-lethal doses of pyriproxyfen (PPF) on Aedes albopictus fecundity. Different colored bars represent the mean ± SEM number of eggs laid per female treated with different concentrations of PPF and untreated females. Asterisks and lines above each bar represent significant differences determined using t-tests (*p < 0.05). (B) Number of blood-fed females post-PPF treatment compared with untreated females over three gonotrophic cycles. Different colored bars represent the mean ± SEM number of females seeking blood post-treatment with different PPF concentrations and those with no treatment. Asterisks with lines above each bar represent significant differences between treatments determined using pairwise Wilcoxon test (*p < 0.05). (C) Total fecundity of females when combining all eggs from blood females over the three gonotrophic cycles and replicates. Different colored bars represent the mean ± SEM number of eggs in replicate treatments. Asterisks and lines above each bar represent significant differences determined using different t-tests (*p < 0.05). (D) Percentage of egg hatching collected from females from the first gonotrophic cycle. Data are represented as the mean of three patch counts ± SEM. Asterisks with lines in each mean represent significant differences between treatments according to pairwise t-tests (*p < 0.05).
PPF exposure was also observed to affect ovarian maturation and oocyte development. Images of the dissected ovaries demonstrated the effect of increasing PPF exposure rates on adult females, showing a delay in ovarian maturation and oocyte development compared with the ovaries of the control mosquitoes (Figure 2A).
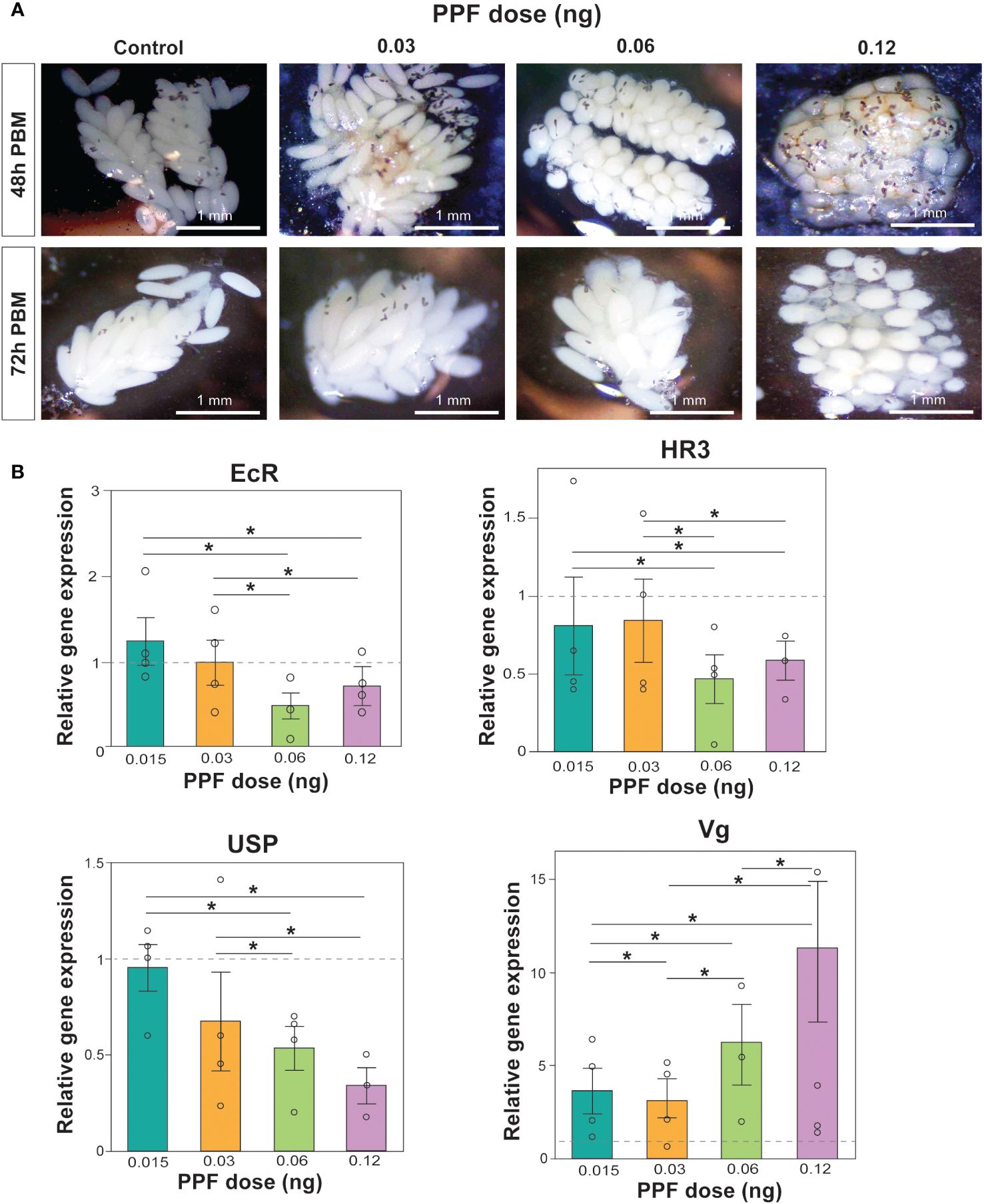
Figure 2 (A) Effects of pyriproxyfen (PPF) on Aedes albopictus follicle morphology and oocyte development. Labels on the left of the images refer to the number of hours post-blood meal, while the labels on top of the figure refer to the amount of PPF exposure. (B) Effects of PPF treatment on the expression of the 20-hydroxyecdysone (20E)-responsive genes after PPF exposure 48 h after a blood meal. Data are presented as the mean ± SEM of four independent replicates. Paired Wilcoxon tests (Bonferroni corrected: p < 0.02) were used to determine differences among treatments for each gene. Significant differences between treatments are represented by asterisks and lines above bars.
As an additional support for the impact of PPF on the egg production and the hatching rates, the relative expression levels of the 20E-regulated genes involved in vitellogenesis were measured using qRT-PCR. Comparison of the relative expression of EcR (Kruskal–Wallis test: χ2 = 5.6, DF = 4, p = 0.23), HR3 (Kruskal–Wallis test: χ2 = 4.4, DF = 4, p = 0.35), USP (Kruskal–Wallis test: χ2 = 9.4, DF = 4, p = 0.052), and Vg (Kruskal–Wallis test: χ2 = 8.6, DF = 4, p = 0.07) showed that the overall gene expression was not impacted by the non-lethal doses of PPF (Figure 2B). While there were no significant differences in the overall gene expression, a downregulation of EcR, HR3, and USP and an upregulation of Vg were observed when comparing the effects between different doses of PPF 48 h post-blood feeding (Figure 2B).
Using the previously described methodology, the sperm production of Ae. albopictus males was determined by diluting the pooled male testes dissections (Figure 3A) into quantifiable amounts that could be counted on microscope slides (Figure 3B). Comparison of all treatments showed no effect of PPF on the fertility and sperm production of Ae. albopictus males (ANOVA: F = 2.1, DF = 3, p = 0.1) (Figure 3). However, in the pairwise post-hoc comparisons, the mean number of sperm produced by males (mean ± SE = 268.6 ± 43) in the 0.12-ng treatment dose was significantly lower than that of untreated control males (mean ± SE = 387.9 ± 42) and the 0.03-ng treatment dose (mean ± SE = 435.25 ± 24.2) (Figure 3C).
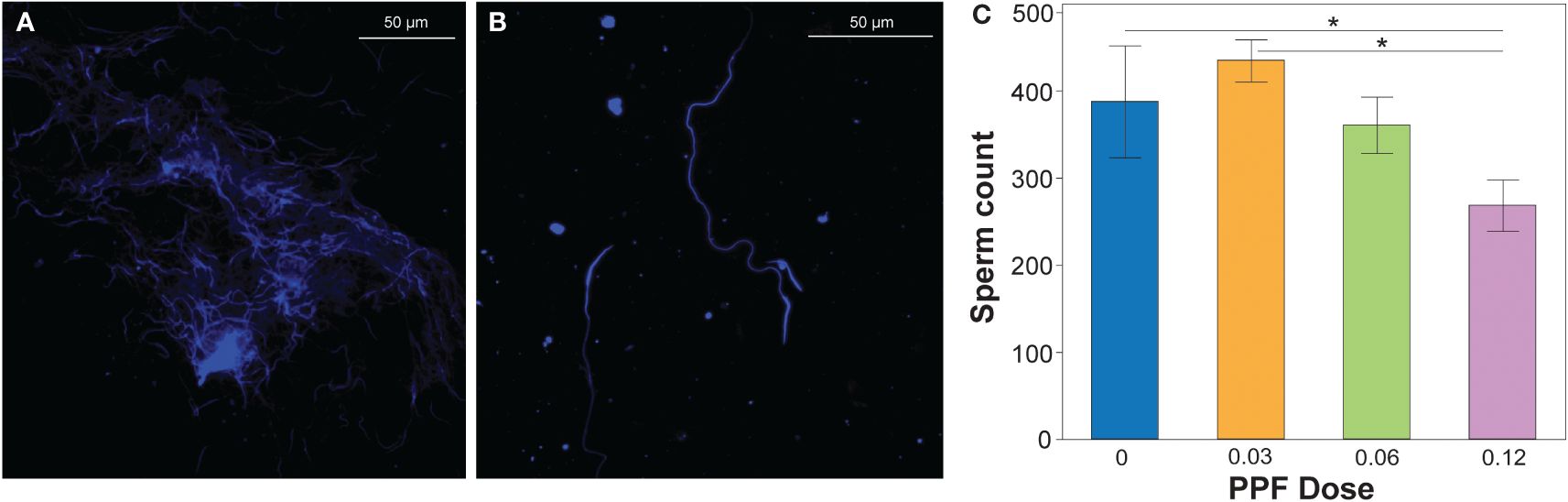
Figure 3 (A) DAPI-stained sperm extracted from the dissected testes of an undiluted sample. (B) Sperm extracted from the dissected testes of a diluted sample. The number of sperm was counted from the diluted sperm extractions, which were used as a proxy for production by males. (C) Effect of pyriproxyfen (PPF) on the sperm production of Aedes albopictus males. Paired t-tests (Bonferroni corrected: p < 0.02) were used to examine the effect of PPF dose on sperm production in males. Significant differences are indicated by a line and asterisk above the sperm counts for the different treatments.
The results presented here suggest that non-lethal doses of PPF impact the reproductive fitness of Ae. albopictus females. The impact of PPF was observed to be more pronounced in the first gonotrophic cycle than the second and third cycles. This reduction in egg production in the first gonotrophic cycle could be the result of the topical application of PPF. Specifically, PPF could be falling or rubbed off the scales of adult females as they age, consequently decreasing the impact on the fecundity in each subsequent gonotrophic cycle. The observed results are similar to those of earlier studies that reported a reduction in fecundity due to PPF exposure in Ae. aegypti, An. arabiensis, An. gambiae, and C. quinquefasciatus (23, 24, 27–29). In addition, this work revealed a non-significant concentration-dependent decline in the number of females that blood fed. The responses of Ae. albopictus females to PPF could be due to an undetermined behavioral effect on the blood seeking ability. This is not surprising considering the impacts JH and 20E have on the physiology of insects. It appears likely that, when the JH titers are high in female mosquitoes, they would not be host seeking for a blood meal. This is in accordance with a study reporting that the injection of methoprene and JH III resulted in a lower proportion of females choosing a blood meal over honey compared with the untreated adult female mosquitoes (30).
The observed PPF dose-dependent decline in the egg hatching rates suggests the indirect impact of PPF on the production of viable eggs. Previous studies have demonstrated that, when An. gambiae came in contact with the PPF-treated bed nets, ovary development was delayed and females had reduced fecundity (24, 31). JH also affects adult insects by controlling different physiological functions, including vitellogenin, an egg yolk precursor of protein production and synthesis in Tribolium castaneum males (32). The JH titers are higher during the previtellogenic stage or 48 h post-eclosion, and the levels drop during blood feeding when the ecdysteroid and Vg synthesis begin (33). In the present study, the observed impacts to follicle development post-blood feeding and the reproductive impacts of the non-lethal doses of PPF are expected to be related to the 20E-responsive gene cascade and the associated genes involved in vitellogenesis. These studies demonstrated a non-significant downregulation in EcR, HR3, and USP, which are involved in Vg synthesis, possibly due to the inhibition of 20E action by PPF treatment in adult females. Conversely, we observed an increase in the mRNA levels of Vg in the PPF-exposed female mosquitoes during the previtellogenic stage, which is responsible for egg yolk protein synthesis in females. These results are similar to those of previous studies that reported an increase in the level of Vg in PPF-treated mosquitoes 24 h after a blood meal (20, 34). Conversely, the action of two hormones, JH and 20E, regulate the reproductive maturation in mosquitoes; in this study, the expression levels of the 20E-responsive genes in PPF-treated mosquitoes were significantly lower than those of untreated mosquitoes. This disruption in the normal hormonal responses due to exposure to non-lethal doses of PPF appeared to impact oocyte development and to reduce the potential for viable progeny of the Ae. albopictus females.
Previous studies have also focused mainly on the effects of PPF on the fitness of female mosquitoes. In this study, the effect of PPF on sperm production in male mosquitoes was also investigated. A reduction in sperm production in the PPF-treated mosquitoes, when treated with 0.12 ng of PPF, was observed compared with the untreated control. Fertility reduction in the male mosquito population could subsequently impact the viable hatching embryos in females if they were to mate with PPF-exposed males. These results are similar to those of a previous study that observed a significant reduction in sperm production in the PPF-treated laboratory-reared population compared with field populations in Thailand (35). The PPF-induced reduction in male fertility could be interpreted as a semi-sterilization effect. In this work, the males were exposed to 0.12 ng of PPF, the highest dose. Previous work demonstrated that males associated with the use of ADAM approaches were exposed to up to 5 ng of PPF, suggesting that an increased dose of PPF would possibly have an additive effect on lowering the fertility of males (36). This result could be beneficial to increasing the success of autodissemination approaches, particularly ADAM approaches, by inducing a sterilization effect if PPF-treated males were to mate with naturally occurring females. Subsequently, this will impact the population growth rates, in addition to the effects on larvae associated with the dissemination of PPF to larval habitats, and increase the efficacy of autodissemination approaches.
The observed impacts of non-lethal doses of PPF on the reproductive fitness of females and males are a potential benefit to autodissemination approaches. When females and males are exposed to low doses of PPF in autodissemination stations, when males are dosed with PPF as part of an ADAM approach, and when females are indirectly exposed via copulation attempts, these non-lethal impacts could improve the success of these approaches. For example, in either of the aforementioned approaches, females that produce fewer eggs and hatching eggs and males that are semi-sterilized by PPF could have an unintended impact on population growth and consequently have an impact on population control. However, it remains to be seen whether these results observed in laboratory studies can be translated to field studies and measurable impacts as part of autodissemination control approaches. More studies are needed to determine the transfer of PPF to mosquitoes in the field and its persistence on exposed mosquitoes in more natural conditions.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
SK: Data curation, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. CB: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was funded by an American Mosquito Control Association (AMCA) research funds grant (#2020–02) (https://www.mosquito.org/) awarded to CB and SK. The funders had no role in the study design, data collection, analysis, or preparation and publication of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/finsc.2024.1430422/full#supplementary-material
1. Wilson AL, Courtenay O, Kelly-Hope LA, Scott TW, Takken W, Torr SJ, et al. The importance of vector control for the control and elimination of vector-borne diseases. PloS Negl Trop Dis. (2020) 14:e0007831. doi: 10.1371/journal.pntd.0007831
2. Champion SR, Vitek CJ. Aedes aEgypti and Aedes albopictus Habitat Preferences in South Texas, USA. Environ Health Insights. (2014) 8:35–42. doi: 10.4137/EHI.S16004
3. Chandel K, Suman DS, Wang Y, Unlu I, Williges E, Williams GM, et al. Targeting a hidden enemy: pyriproxyfen autodissemination strategy for the control of the container mosquito aedes albopictus in cryptic habitats. PloS Negl Trop Dis. (2016) 10:e0005235. doi: 10.1371/journal.pntd.0005235
4. Gonzalez PV, Gonzalez Audino PA, Masuh HM. Oviposition Behavior in Aedes aEgypti and Aedes albopictus (Diptera: Culicidae) in Response to the Presence of Heterospecific and Conspecific Larvae. J Med Entomol. (2016) 53:268–72. doi: 10.1093/jme/tjv189
5. Gaugler R, Suman D, Wang Y. An autodissemination station for the transfer of an insect growth regulator to mosquito oviposition sites. Med Vet Entomol. (2012) 26:37–45. doi: 10.1111/j.1365-2915.2011.00970.x
6. Suman DS, Farajollahi A, Healy S, Williams GM, Wang Y, Schoeler G, et al. Point-source and area-wide field studies of pyriproxyfen autodissemination against urban container-inhabiting mosquitoes. Acta Trop. (2014) 135:96–103. doi: 10.1016/j.actatropica.2014.03.026
7. Wang Y, Suman DS, Bertrand J, Dong L, Gaugler R. Dual-treatment autodissemination station with enhanced transfer of an insect growth regulator to mosquito oviposition sites. Pest Manag Sci. (2014) 70:1299–304. doi: 10.1002/ps.3702
8. Bibbs CS, Anderson C, Xue RD. Autodissemination of insect growth regulator, methoprene, with two formulations against aedes albopictus. J Am Mosq Control Assoc. (2016) 32:247–50. doi: 10.2987/15-6532.1
9. Lloyd AM, Farooq M, Estep AS, Xue RD, Kline DL. Evaluation of Pyriproxyfen Dissemination via Aedes albopictus From a Point-Source Larvicide Application in Northeast Florida. J Am Mosq Control Assoc. (2017) 33:151–5. doi: 10.2987/14-6459.1
10. Unlu I, Suman DS, Wang Y, Klingler K, Faraji A, Gaugler R. Effectiveness of autodissemination stations containing pyriproxyfen in reducing immature Aedes albopictus populations. Parasit Vectors. (2017) 10:139. doi: 10.1186/s13071-017-2034-7
11. Suman DS, Wang Y, Faraji A, Williams GM, Williges E, Gaugler R. Seasonal field efficacy of pyriproxyfen autodissemination stations against container-inhabiting mosquito Aedes albopictus under different habitat conditions. Pest Manag Sci. (2018) 74:885–95. doi: 10.1002/ps.4780
12. Kunambi HJ, Ngowo H, Ali A, Urio N, Ngonzi AJ, Mwalugelo YA, et al. Sterilized Anopheles funestus can autodisseminate sufficient pyriproxyfen to the breeding habitat under semi-field settings. Malar J. (2023) 22:280. doi: 10.1186/s12936-023-04699-9
13. Ligsay AD, Regencia ZJG, Tambio KJM, Aytona MJM, Generale AJA, Alejandro GJD, et al. Efficacy assessment of autodissemination using pyriproxyfen-treated ovitraps in the reduction of dengue incidence in Paranaque City, Philippines: A spatial analysis. Trop Med Infect Dis. (2023) 8:66. doi: 10.3390/tropicalmed8010066
14. Mains JW, Brelsfoard CL, Dobson SL. Male mosquitoes as vehicles for insecticide. PloS Negl Trop Dis. (2015) 9:e0003406. doi: 10.1371/journal.pntd.0003406
15. Brelsfoard CL, Mains JW, Mulligan S, Cornel A, Holeman J, Kluh S, et al. Aedes aEgypti males as vehicles for insecticide delivery. Insects. (2019) 10. doi: 10.3390/insects10080230
16. Lwetoijera D, Harris C, Kiware S, Dongus S, Devine GJ, Mccall PJ, et al. Effective autodissemination of pyriproxyfen to breeding sites by the exophilic malaria vector Anopheles arabiensis in semi-field settings in Tanzania. Malar J. (2014) 13:161. doi: 10.1186/1475-2875-13-161
17. Kartzinel MA, Alto BW, Deblasio MW 2nd. Burkett-Cadena NDTesting of Visual and Chemical Attractants in Correlation with the Development and Field Evaluation of an Autodissemination Station for the Suppression of Aedes aEgypti and Aedes albopictus in Florida. J Am Mosq Control Assoc. (2016) 32:194–202. doi: 10.2987/16-6555.1
18. Thammavong P, Boyer S, Luangamath P, Phommavanh N, Vungkyly V, Nilaxay S, et al. Small-scale field assessment against the dengue vector Aedes aEgypti using the auto-dissemination approach in an urban area of Vientiane, Lao PDR. PloS One. (2022) 17:e0270987. doi: 10.1371/journal.pone.0270987
19. Liu S, Li K, Gao Y, Liu X, Chen W, Ge W, et al. Antagonistic actions of juvenile hormone and 20-hydroxyecdysone within the ring gland determine developmental transitions in Drosophila. Proc Natl Acad Sci U.S.A. (2018) 115:139–44. doi: 10.1073/pnas.1716897115
20. Bai H, Gelman DB, Palli SR. Mode of action of methoprene in affecting female reproduction in the African malaria mosquito, Anopheles Gambiae. Pest Manage Sci. (2010) 66:936–43. doi: 10.1002/ps.v66:9
21. Iwanaga K, Kanda T. The effects of a juvenile hormone active oxime ether compound on the metamorphosis and reproduction of an anopheline vector, anopheles balabacensis (Diptera: culicidae). Appl Entomol Zool. (1988) 23:186–93. doi: 10.1303/aez.23.186
22. Suman DS, Wang Y, Dong L, Gaugler R. Effects of larval habitat substrate on pyriproxyfen efficacy against aedes albopictus (Diptera: culicidae). J Med Entomol. (2013) 50:1261–6. doi: 10.1603/ME13068
23. Mbare O, Lindsay SW, Fillinger U. Pyriproxyfen for mosquito control: female sterilization or horizontal transfer to oviposition substrates by Anopheles Gambiae sensu stricto and Culex quinquefasciatus. Parasit Vectors. (2014) 7:280. doi: 10.1186/1756-3305-7-280
24. Myers A, Fagbohoun J, Houetohossou G, Ndombidje B, Govoetchan R, Todjinou D, et al. Identifying suitable methods for evaluating the sterilizing effects of pyriproxyfen on adult malaria vectors: a comparison of the oviposition and ovary dissection methods. Malar J. (2024) 23:164. doi: 10.1186/s12936-024-04983-2
25. Aldridge RL, Alto BW, Roxanne Connelly C, Okech B, Siegfried B, Eastmond BH, et al. Does prior exposure to larvicides influence dengue virus susceptibility in Aedes aEgypti (Diptera: Culicidae)? J Med Entomol. (2024) 61:166–74. doi: 10.1093/jme/tjad137
26. Ponlawat A, Harrington LC. Age and body size influence male sperm capacity of the dengue vector aedes aEgypti (Diptera: culicidae). J Med Entomol. (2007) 44:422–6. doi: 10.1093/jmedent/44.3.422
27. Harris C, Lwetoijera DW, Dongus S, Matowo NS, Lorenz LM, Devine GJ, et al. Sterilising effects of pyriproxyfen on Anopheles arabiensis and its potential use in malaria control. Parasit Vectors. (2013) 6:144. doi: 10.1186/1756-3305-6-144
28. Jaffer A, Protopopoff N, Mosha FW, Malone D, Rowland MW, Oxborough RM. Evaluating the sterilizing effect of pyriproxyfen treated mosquito nets against Anopheles Gambiae at different blood-feeding intervals. Acta Trop. (2015) 150:131–5. doi: 10.1016/j.actatropica.2015.07.011
29. Yadav K, Dhiman S, Acharya BN, Ghorpade RR, Sukumaran D. Pyriproxyfen treated surface exposure exhibits reproductive disruption in dengue vector Aedes aEgypti. PloS Negl Trop Dis. (2019) 13:e0007842. doi: 10.1371/journal.pntd.0007842
30. Hancock RG, Foster WA. Exogenous juvenile hormone and methoprene, but not male accessory gland substances or ovariectomy, affect the blood/nectar choice of female Culex nigripalpus mosquitoes. Med Vet Entomol. (2000) 14:376–82. doi: 10.1046/j.1365-2915.2000.00253.x
31. Koama B, Namountougou M, Sanou R, Ndo S, Ouattara A, Dabiré RK, et al. The sterilizing effect of pyriproxyfen on the malaria vector Anopheles Gambiae: physiological impact on ovaries development. Malaria J. (2015) 14:101. doi: 10.1186/s12936-015-0609-3
32. Parthasarathy R, Tan A, Sun Z, Chen Z, Rankin M, Palli SR. Juvenile hormone regulation of male accessory gland activity in the red flour beetle, Tribolium castaneum. Mech Dev. (2009) 126:563–79. doi: 10.1016/j.mod.2009.03.005
33. Raikhel AS, Kokoza VA, Zhu J, Martin D, Wang S-F, Li C, et al. Molecular biology of mosquito vitellogenesis: from basic studies to genetic engineering of antipathogen immunity. Insect Biochem Mol Biol. (2002) 32:1275–86. doi: 10.1016/S0965-1748(02)00090-5
34. Ahmed TH, Saunders TR, Mullins D, Rahman MZ, Zhu J. Molecular action of pyriproxyfen: Role of the Methoprene-tolerant protein in the pyriproxyfen-induced sterilization of adult female mosquitoes. PloS Negl Trop Dis. (2020) 14:e0008669. doi: 10.1371/journal.pntd.0008669
35. Fansiri T, Pongsiri A, Khongtak P, Nitatsukprasert C, Chittham W, Jaichapor B, et al. The impact of insect growth regulators on adult emergence inhibition and the fitness of Aedes aEgypti field populations in Thailand. Acta Tropica. (2022) 236:106695. doi: 10.1016/j.actatropica.2022.106695
Keywords: non-lethal insecticides, pyriproxyfen, autodissemination, reproductive fitness, vector control
Citation: Kancharlapalli SJ and Brelsfoard CL (2024) The impact of non-lethal doses of pyriproxyfen on male and female Aedes albopictus reproductive fitness. Front. Insect Sci. 4:1430422. doi: 10.3389/finsc.2024.1430422
Received: 09 May 2024; Accepted: 13 June 2024;
Published: 02 July 2024.
Edited by:
Sengodan Karthi, Manonmaniam Sundaranar University, IndiaReviewed by:
Ranganathan Muthusamy, Adhiyamaan College of Engineering, IndiaCopyright © 2024 Kancharlapalli and Brelsfoard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Corey L. Brelsfoard, Y29yZXkuYnJlbHNmb2FyZEB0dHUuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.