
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Insect Sci. , 23 August 2023
Sec. Insect Physiology
Volume 3 - 2023 | https://doi.org/10.3389/finsc.2023.1197945
This article is part of the Research Topic Rising Stars in Insect Physiology View all 11 articles
G protein-coupled receptors (GPCRs) control numerous physiological processes in insects, including reproduction. While many GPCRs have known ligands, orphan GPCRs do not have identified ligands in which they bind. Advances in genomic sequencing and phylogenetics provide the ability to compare orphan receptor protein sequences to sequences of characterized GPCRs, and thus gain a better understanding of the potential functions of orphan GPCRs. Our study sought to investigate the functions of two orphan GPCRs, AAEL003647 and AAEL019988, in the yellow fever mosquito, Aedes aegypti. From our phylogenetic investigation, we found that AAEL003647 is orthologous to the SIFamide-2/SMYamide receptor. We also found that AAEL019988 is orthologous to the Trapped in endoderm (Tre1) receptor of Drosophila melanogaster. Next, we conducted a tissue-specific expression analysis and found that both receptors had highest expression in the ovaries, suggesting they may be important for reproduction. We then used RNA interference (RNAi) to knock down both genes and found a significant reduction in the number of eggs laid per individual female mosquito, suggesting both receptors are important for Ae. aegypti reproduction.
Mosquitoes are a persistent threat to global health due to their ability to transmit pathogens among vertebrate hosts through blood feeding, which is required for many mosquito species to produce eggs. The events beginning with blood meal digestion and ultimately leading to egg production are coordinated by several reproductive hormones, including insulin-like peptide 3 (ILP3) and ovary ecdysteroidogenic hormone (OEH), which are released shortly after a blood meal is consumed (1–3). Release of ILP3 from brain neurosecretory cells stimulates blood meal digestion, and ILP3 and OEH both stimulate secretion of 20-hydroxyecdysone (20E) from the ovaries (1–4). After 20E is released into the hemolymph, expression of yolk protein precursors (YPP) in the fat body is induced, initiating the production of yolk proteins, including vitellogenin, which are subsequently transported to the ovaries and packaged into oocytes resulting in egg formation (5, 6).
Hormone signaling pathways have been exploited to control insect populations. Insect chemical growth regulators (IGRs), such as 20E antagonists, target insect hormonal pathways and have been utilized to control insect disease vectors (7, 8). IGRs are attractive control measures due to their selective toxicity against insects and decreased rate of insecticide resistance developed against them relative to traditional pesticides (9, 10). IGR targets such as, JH and 20E and their receptors, are widely conserved in insects increasing the chances of negative effects on non-target species (7, 8, 11–13). An attractive alternative to IGRs that act on JH or 20E are compounds that selectively target hormones or hormone receptors that are not widely conserved across all insect groups. G protein-coupled receptors (GPCRs) and their ligands may present taxa-specific targets, as insect genomes often encode unique GPCRs, including many that bind peptide hormones that regulate important aspects of insect physiology (14–16).
Hormone-binding GPCRs are essential in modulating insect physiology, including in metabolism (17, 18), reproduction (19), behavior (20), immunity (21), and embryonic development (22), as they transduce systemic hormonal signals into target cells. In addition to modulating a diverse number of functions in insects, GPCRs are the largest class of receptor and bind a variety of ligands, including neurotransmitters (23) and peptide hormones (24, 25). Peptide hormones govern many physiological functions in insects including feeding (26–29), mating behavior (30), development (31–33), metabolism (1, 18, 34–36), immunity (37), diuresis (38–40), and reproduction (1, 19, 41). While the ligands of many GPCRs have been identified, even well-studied organisms still encode GPCRs whose ligands are unknown.
Comparative genomics and phylogenetic analyses are useful tools in the identification of ligands of former orphan receptors (3, 19). Phylogenetic placement of orphan receptors, such as in the case of the OEH receptor of Aedes aegypti mosquitoes, can provide insights into potential ligands. A Venus flytrap domain-containing receptor tyrosine kinase was found to be closely related to the mosquito insulin receptor, and also displayed the same species distribution pattern as neuroparsin peptide hormones including OEH. Subsequent biochemical and molecular studies determined that the gene in question was an OEH receptor (3). Tissue-specific expression patterns are also useful in determining the functional roles and ligands of hormone receptors. We identified that the neuropeptide CNMa and its receptor, CNMaR, which were first identified in Drosophila melanogaster, were specifically expressed in Ae. aegypti ovaries and hypothesized that it was likely important for reproduction (3, 19, 42). In Culicidae, the CNMa receptor underwent gene duplication, resulting in two receptors, CNMaR-1a and CNMaR-1b, which both actively bind CNMa in vitro (19). In Ae. aegypti, CNMa and CNMaR-1b are highly expressed in female ovaries and modulate the production of eggs (19, 43).
We chose to examine two orphan GPCRs of Aedes aegypti, AAEL003647 and AAEL019988. These orphan GPCRs were chosen for further investigation based on their expression in female reproductive tissues following a blood meal (43), suggesting a potential role in the modulation of reproductive physiology. We built phylogenetic trees to identify closely related receptors and provide insight into possible functions of the receptors. To understand the tissue tropism and temporal distribution of AAEL003647 and AAEL019988, we conducted a detailed expression analysis of both GPCRs in juvenile and adult mosquitoes. Using RNAi, we then investigated the functional consequences of silencing the GPCRs on fecundity. These results shed new light on the role of these orphan GPCRs on the reproductive physiology of Ae. aegypti mosquitoes.
UGAL strain Aedes aegypti were used for all experiments. Mosquito colonies were maintained at 27°C on a 16:8h L:D cycle. Larvae were fed Cichlid Gold fish pellets (Hikari, USA, Hayward, CA), and adult mosquitoes were fed an 8% sucrose solution until 2 days post-emergence. Adult females were fed defibrinated rabbit blood (Hemostat Laboratories, Dixon, CA, USA) by an artificial feeding apparatus warmed to 37°C.
Putative AAEL003647 and AAEL019988 orthologs were identified using OrthoDB (44). Taxa were chosen to represent all possible insect orders with available genome sequences (Tables S1, S2). Protein sequences were aligned using hmmalign as implemented in HMMER (45) with the –trim option. Gaps in alignments were manually removed, and trimmed alignments were used to construct maximum likelihood phylogenies using PhyML (46) using the options “-d aa -m LG -f e -o tl -b -2”. FigTree version 1.4.4 was used for visualization of trees and trees were rooted on the midpoint. Accessions of included sequences are given in file S1.
Eight to ten-day old, non-blood fed mated females were collected and dissected into head, gut, fat body, abdominal carcass (“pelt”), and clean ovaries without bursa or accessory glands in sterile, nuclease-free, Aedes saline. Additional ovary samples were collected from females at 2-hour intervals post-feeding (pbf) until 12 hours, then at 24, 48, and 72 hours pbf. Four or more tissue samples were collected for each tissue and time point. After collection, tissue samples were stored at -80°C prior to RNA extraction. Tissue samples were thawed on ice and homogenized with a rotor pestle. Total RNA was isolated from homogenized tissues using the RNeasy Mini kit (Qiagen, Venlo, The Netherlands) according to manufacturer instructions. DNA was removed from each RNA sample using the Turbo DNA-free kit (Ambion, Austin, TX, USA). One hundred nanograms of RNA was used as input to synthesize cDNA using the iScript cDNA synthesis kit (BioRad, Hercules, CA, USA). cDNA templates were used for quantitative real-time PCR, with the Quantifast SYBR Green PCR kit (Qiagen) and gene specific primers (Table S3). Standard curves for each gene were generated by cloning qPCR products into the pSCA vector with the Strataclone PCR cloning kit (Agilent, Santa Clara, CA, USA), isolating plasmid DNA using the GeneJET Plasmid Miniprep Kit (Thermo Scientific, Vilnius, Lithuania), and preparing plasmid standards to a known copy number. Expression levels of ribosomal protein S7 were used as a housekeeping gene to normalize transcript abundance.
A 400-500 bp region of each gene was chosen as a target for dsRNA synthesis for AAEL003647 and AAEL019988, subsequently referred to as ds3647 and ds19988, respectively. Primers including the T7 promoter sequence were used to amplify each target using cDNA synthesized from RNA isolated from whole body, non-blood fed females (Table S3). PCR products were cloned into the pSCA vector and plasmid DNA was extracted using methods listed above. Plasmid DNA from each target and an EGPF control were used as the templates for dsRNA synthesis. dsRNA was synthesized using the MEGAscript RNAi kit (Ambion, Vilnius, Lithuania), according to manufacturer instructions. Following dsRNA synthesis, dsRNA was precipitated in ethanol and resuspended in Aedes saline to a concentration of 2µg/µL.
Newly emerged (≤ 1d post eclosion) mated females were injected with 2 µg ds3647, ds19988, or dsEGFP. To validate receptor knockdown, whole body females were collected 7 days post-injection. qPCR was used to validate knockdown of each gene using the methods detailed above. Females were blood fed three days post-injection and separated into individual egg laying chambers consisting of a damp paper towel in a plastic cup with a lid and a dental wick with 8% sucrose solution, for yolk deposition and fecundity bioassays. For yolk deposition bioassays, females were collected at 24, 48, and 72 hours PBF. Ovaries were dissected and yolk deposition per oocyte was measured along the anterior-posterior axis using an ocular micrometer. Five oocytes were measured and averaged per female, and 5 females were used per time point and treatment. Egg laying was measured by providing females with a wet paper towel at 72 h post blood feeding to stimulate egg deposition. Females were given 48 h to deposit eggs. After 48 h hours, the number of eggs laid per individual female was counted. Another cohort of knockdown females were allowed to lay eggs then dissected and the number of retained, mature oocytes were counted. Eggs that were laid were separated by parent and allowed to hatch, and the proportion of hatched versus unhatched eggs was recorded for each treatment.
Our phylogenetic analysis included diverse insect species to identify the closest receptor relatives across both holometabolous and hemimetabolous insects. Our results for AAEL003647 indicate this receptor groups in a strongly supported clade of receptors that are distinct from, but sister to, the SIFamide receptors (Figure 1). These receptors are found in the genomes of culicids as well as cockroaches (Periplaneta americana and Blattella germanica), termites (Zootermopsis nevadensis). This robustly supported sister clade to SIFamide receptors suggest an ancient split between SIFamide receptors and the orthologs of AAEL003647 which predates the split of hemi- and holometabolous insects. Orthologs of AAEL003647 appear to have been lost in many lineages. No orthologs were found in lepidopteran, coleopteran or hymenopteran genomes. In contrast, most sequenced hemipteran genomes contained orthologs, several of which have subsequently duplicated. In the order Diptera, AAEL003647 orthologs were found in most nematoceran genomes, but absent from many available brachyceran genomes, including sequences from all members of the genus Drosophila. This loss was not complete in Brachycera, as Rhagoletis zephyria and Hermetia illucens both encode AAEL003647 orthologs in their genomes. Within the Culicidae, each species examined has a single ortholog of AAEL003647 with the notable exception of Anopheles maculatus, which has five orthologous sequences in OrthoDB (44). Two sequences were identified as orthologs of the SIFamide receptor (AMAM023590 and AMAM011260), and two orthologs identified as orthologs of AAEL003647 (AMAM023042 and AMAM009506). All of these sequences are lacking the complete 7 transmembrane region of canonical GPCRs and it seems likely that these sequences do not reflect true orthologs but rather annotation artefacts, potentially fragments of a single ortholog to the SIFamide receptor and AAEL003647. An additional duplication in Anopheles maculatus groups with the SIFamide-like receptor of Thrips palmi. Further investigation of this ortholog suggests that it is unique to An. maculatus, and that its grouping with non-mosquito sequences is likely an artifact of the alignment. Improved sequencing of the An. maculatus genome will likely resolve this in the future.
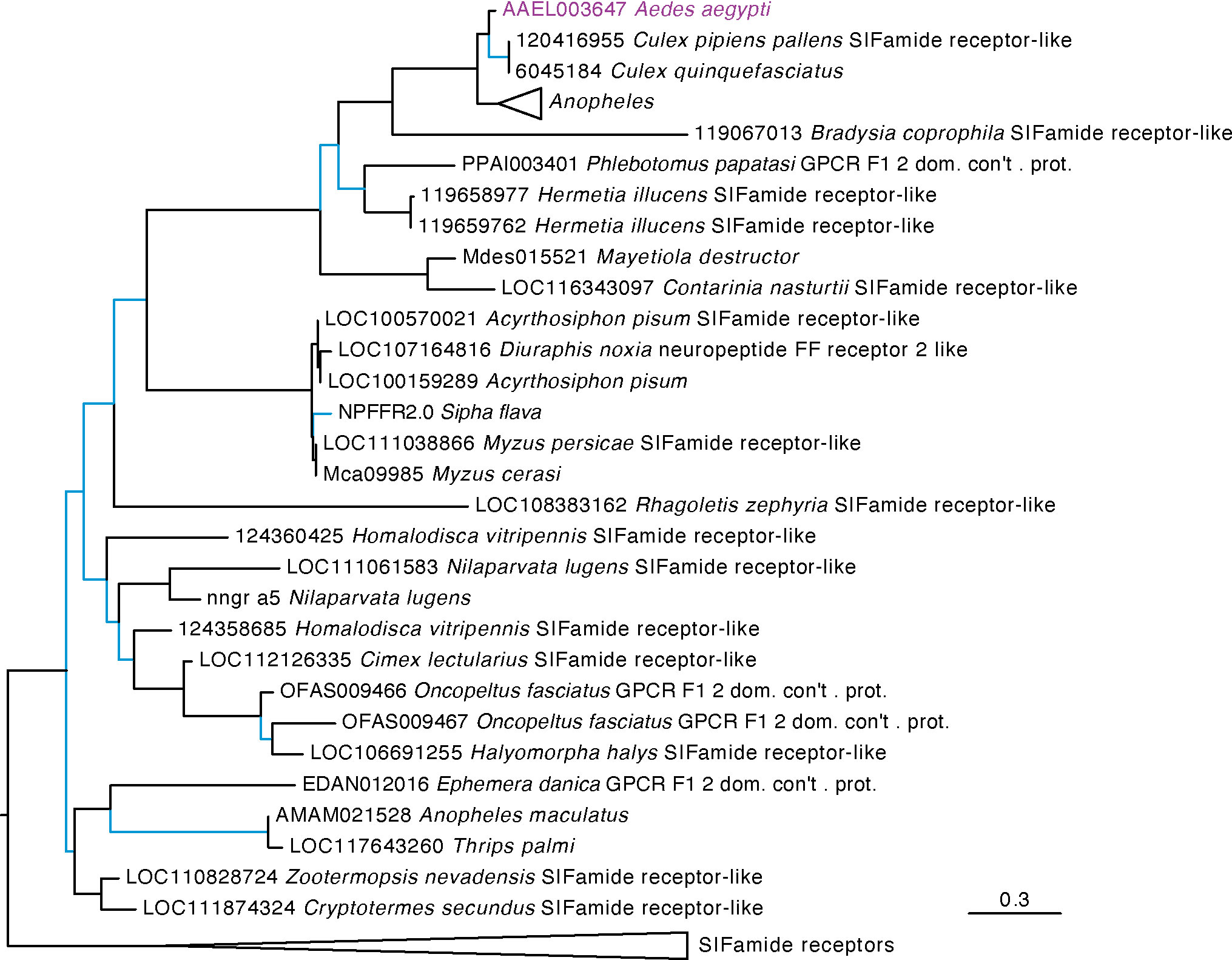
Figure 1 Maximum likelihood tree of AAEL003647 and its orthologs in other insects. Orthologs of AAEL003647 have been lost in many brachyceran taxa, including members of the genus Drosophila. AAEL003647 is most closely related to the SIFamide receptor. Sequences were downloaded from OrthoDB and aligned against a 7 transmembrane GPCR model (7tm-1.hmm) in hmmalign. Trees were built in PhyML. Support values are aLRT SH-like, and branches with support values < 0.95 are colored light blue. F1 2 domain containing protein is abbreviated as “F1 2 dom. con’t. prot.” Due to space constraints, orthologs of AAEL003647 in Anopheles species and SIFamide receptor sequences were collapsed. A full tree containing the Anopheles taxa is available in Supplementary Figure S1.
Our analysis identified AAEL019988 as an ortholog of the D. melanogaster trapped in endoderm (tre1) GPCR with strong support (Figure 2). Tre1 appears to be highly conserved among holometabolous insects but is absent from many hemimetabolous lineages. Only the orders Blattodea, Odonata, Thysanoptera, and Grylloblattidae encode orthologs. The sister group to this clade includes both the GPCRs Moody and Moody-like, which are known to be important to blood-brain barrier in Drosophila melanogaster (47).
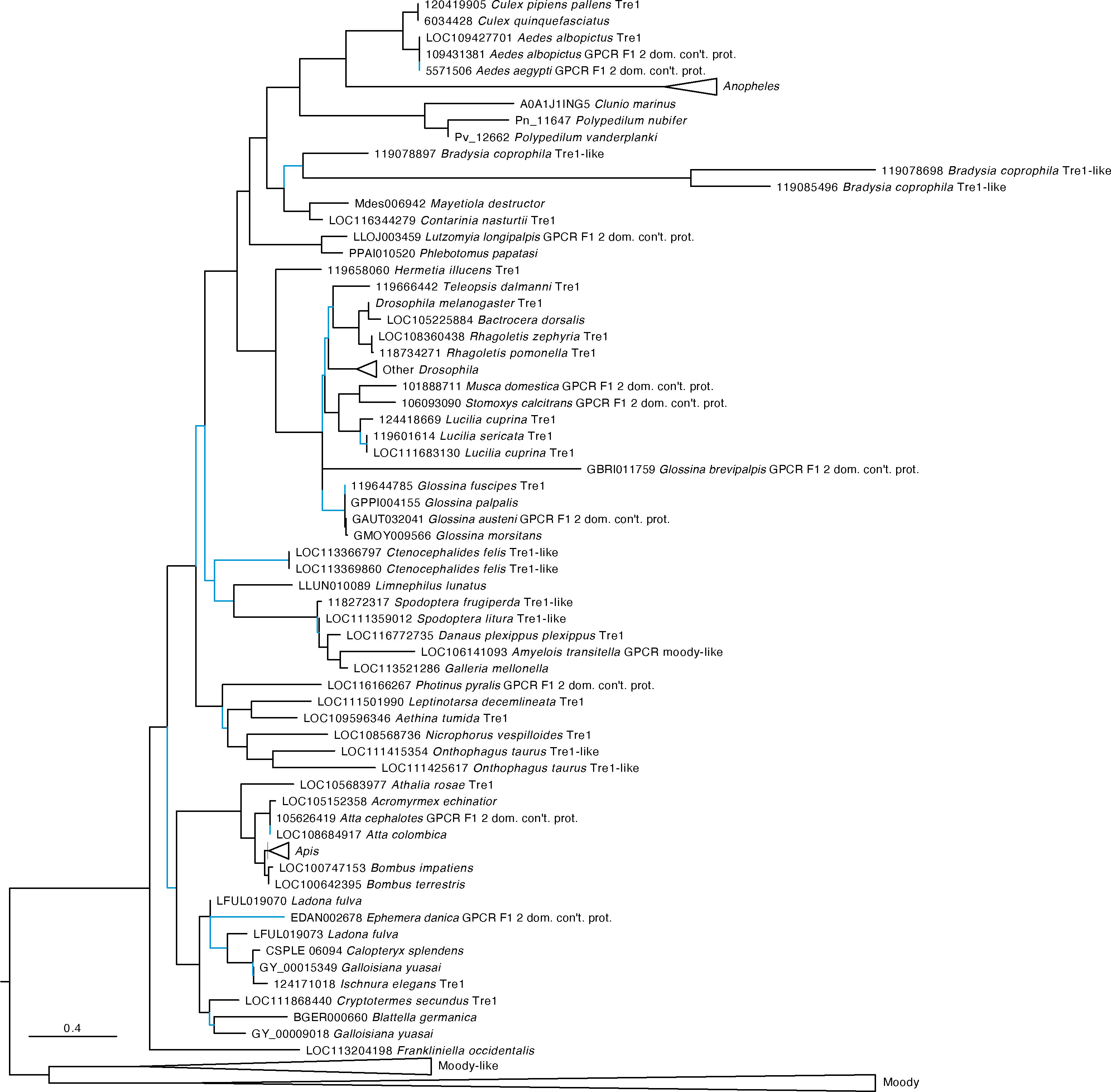
Figure 2 Maximum likelihood tree of AAEL019988 and its orthologs in other insects. AAEL019988 is absent in most but not all hemimetabolous insects and is conserved in most holometabolous lineages. The tree was rooted at the midpoint which formed two major clades, the orthologs of Trapped in endoderm 1 (tre1) and the orthologs of moody and moody-like. Sequences were downloaded from OrthoDB and aligned against a 7 transmembrane GPCR model (7tm-1.hmm) in hmmalign. Trees were built in PhyML. F1 2 domain containing protein is abbreviated as “F1 2 dom. con’t. prot.” Support values are aLRT SH-like and branches with low support (< 0.95) are highlighted in blue. Due to space constraints, sequences from Anopheles, Drosophila, and Apis species, as well as Moody and Moody-like sequences, were collapsed. A full tree with the expanded AAEL019988 orthologs is shown in Figure S2.
We investigated expression patterns of AAEL003647 and AAEL019988 among life stages, sexes, and tissues. Expression of AAEL003647 was highest in females relative to males and immature stages (one-way ANOVA, p < 0.0001) (Figure 3A). Expression of AAEL019988 was higher in adult females relative to 1st, 3rd, 5th instar larval, and pupal stage mosquitoes (one-way ANOVA, p < 0.05). There was no significant difference in expression between females and males (Figure 3B). We next examined tissue tropism of the receptors in females. The highest expression of AAEL003647 and AAEL019988 was observed in the ovaries (Figures 4A, B). We next measured receptor expression across a time series following a blood meal. Our results demonstrate that expression of AAEL003647 was highest in non-blood fed, 2h, 4h, and 6h pbf female ovaries (Figure 4C). Expression of AAEL019988 was highest in NBF ovaries (Figure 4D).
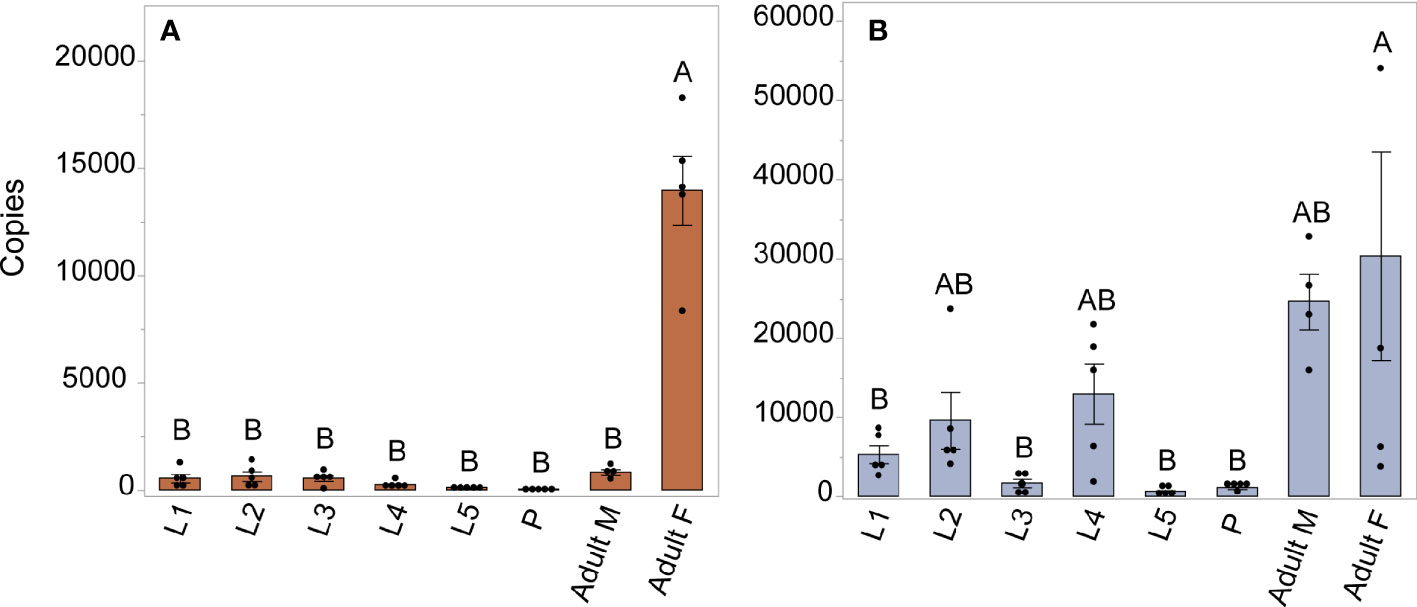
Figure 3 Expression profile of AAEL003647 and AAEL019988 in whole bodies of mosquitoes across life stages and sexes. The x-axis represents the number of copies of AAEL003647 and AAEL019988 per 100ng of RNA. (A) Expression of AAEL003647 is significantly higher in adult females (one-way ANOVA, p < 0.0001). (B) Expression of AAEL019988 was also significantly higher in adult females relative to 1st, 3rd, and 5th stage larvae and pupae (one-way ANOVA, p < 0.05). Treatments connected by the same letter are not significantly different (p > 0.05, one-way ANOVA). The letters above each bar/box in these figures indicate statistical significance. Different letters indicate statistical significance, and connected letters indicate no statistical significance.
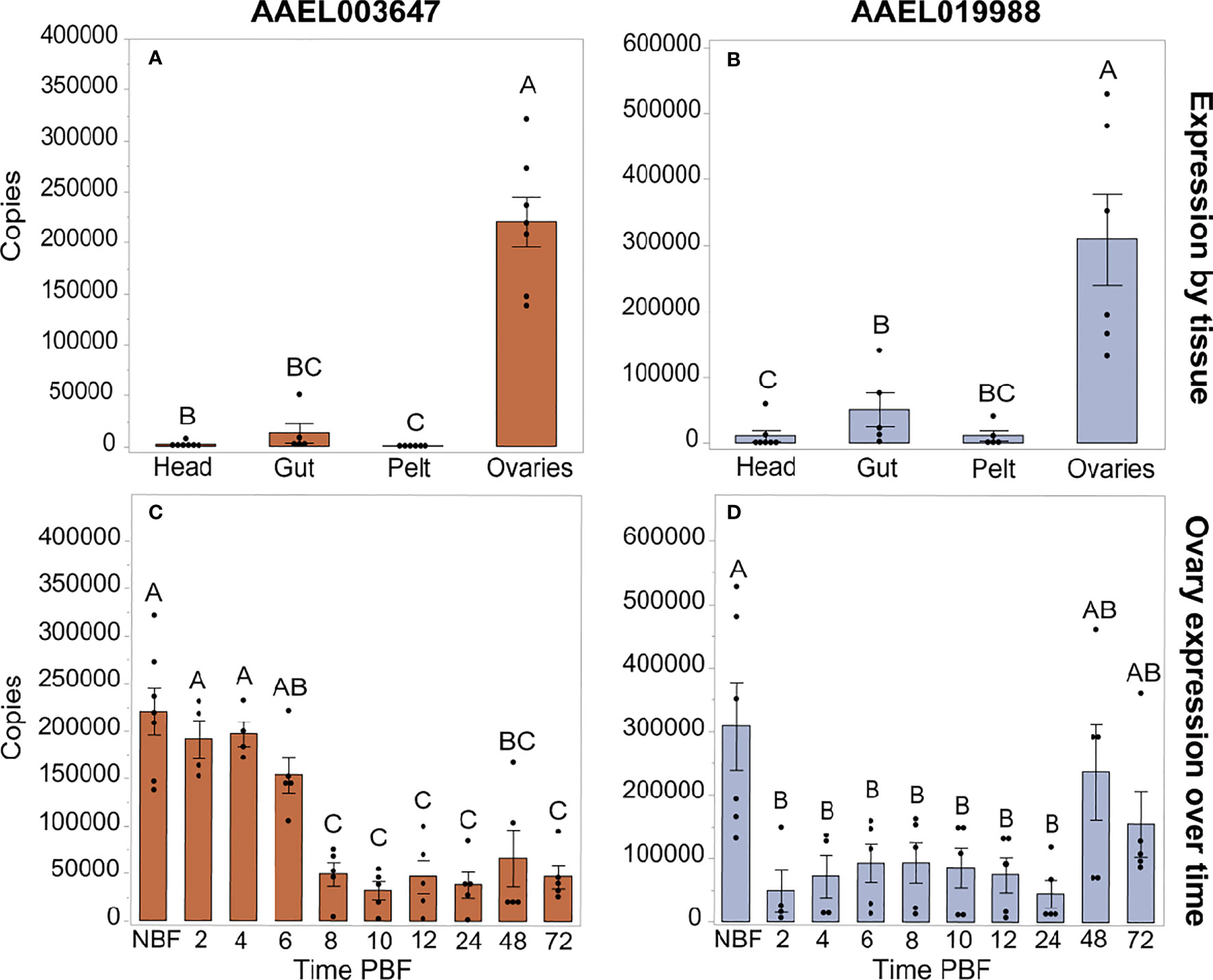
Figure 4 Expression profiles of AAEL003647 and AAEL019988 in NBF Ae. aegypti tissues (A, B) and in whole bodies following a blood meal (C, D). Expression of AAEL003647 and AAEL019988 is highest in the ovaries for (A) AAEL003647 (one-way ANOVA, p ≤ 0.003) and (B) AAEL019988 (one-way ANOVA, p ≤ 0.0092). (C) Expression of AAEL003647 is significantly higher in the ovaries of NBF, 2h, 4h, and 6h pbf females (one-way ANOVA, p < 0.05). (D) Expression of AAEL019988 is significantly higher in the ovaries of NBF females (one-way ANOVA, p < 0.05). The letters above each bar/box in these figures indicate statistical significance. Different letters indicate statistical significance, and connected letters indicate no statistical significance.
The peaks of expression prior to feeding and nearing the time of oviposition informed our hypothesis that AAEL003647 and AAEL019988 may be important in regulation of egg production and/or oviposition. To understand the effects of both orphan GPCRs on oviposition, we injected newly eclosed female mosquitoes with 2 µg of ds3647, ds19988, or dsEGFP. For each receptor, we were able to achieve an 85% whole body transcript knockdown (one-way ANOVA, p < 0.0163, p < 0.0163, respectively; Figures 5A, B). Following dsRNA injection, females were allowed to mate and were fed 3 days post-injection. After feeding, females were separated into individual enclosures for oviposition assays. We found that ds3647 and ds19988 injected females laid significantly fewer eggs than dsEGFP injected females (one-way ANOVA, p = 0.0184, p = 0.0393, respectively; Figure 5C).
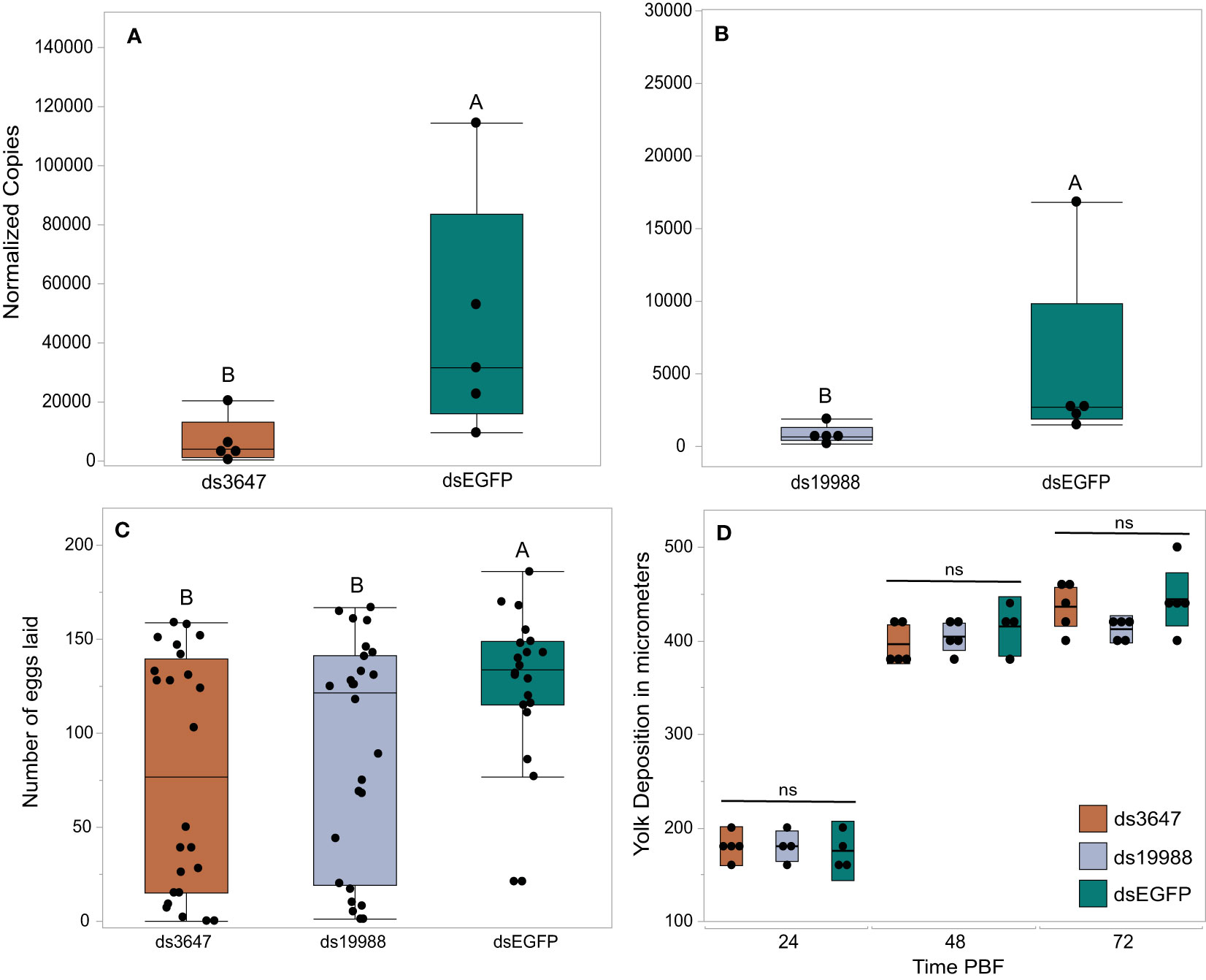
Figure 5 RNAi knockdowns (A, B), oviposition bioassays (C), and yolk deposition (D). (A, B) Receptor knockdown validation. We achieved an 85% whole body transcript knockdown for AAEL003647 (A) (Wilcoxon rank-sum test, p = 0.0163) and AAEL019988 (B) (Wilcoxon rank-sum test, p = 0.0163). The x-axis represents the number of copies of AAEL003647 and AAEL019988 per 100ng of RNA. Transcripts were normalized by ribosomal S7 expression. (C) Knockdown of AAEL003647 and AAEL019988 resulted in a significant decrease in the number of eggs laid relative to dsEGFP controls (Wilcoxon rank-sum test, p = 0.0184, p = 0.0393, respectively). (D) Knockdown of AAEL003647 and AAEL019988 had no effect on yolk uptake (Wilcoxon rank-sum test, p > 0.05). ns = not significant, indicating average yolk length among each experimental treatment is not statistically significantly different. The letters above each bar/box in these figures indicate statistical significance. Different letters indicate statistical significance, and connected letters indicate no statistical significance.
The observed reduction in egg laying by mosquitoes treated with ds3647 or ds19988 could be due to a disruption of egg maturation or egg laying. To disentangle this, we examined whether yolk deposition of ds3647 and ds19988 injected females was impaired, which would suggest that the receptors are important in post-vitellogenic egg development. We injected newly eclosed females with dsEGFP, ds3647 or ds19988, fed females a blood meal at 3 days post injection, and dissected ovaries from blood fed females at 24, 48, and 72h pbf. Following dissection, we measured the packaged yolk in per individual oocyte with an ocular micrometer. We found no significant difference among oocyte yolk lengths in ds3647, ds19988, or dsEGFP injected females (one-way ANOVA, p > 0.05; Figure 5D), suggesting that the receptors mediate physiological events after egg maturation. We then examined the effect of receptor knockdown on the egg retention and egg hatching. Knockdown of AAEL003647 did not result in retained eggs in females, but AAEL019988 knockdown mosquitoes retained more mature oocytes than dsEGFP controls (Figure 6A, p = 0.0476; Wilcoxon rank-sum test). Of eggs that were laid, there was no difference in the proportion of eggs that hatched, suggesting that knockdown of the receptors does not interfere with fertilization (Figure 6B).
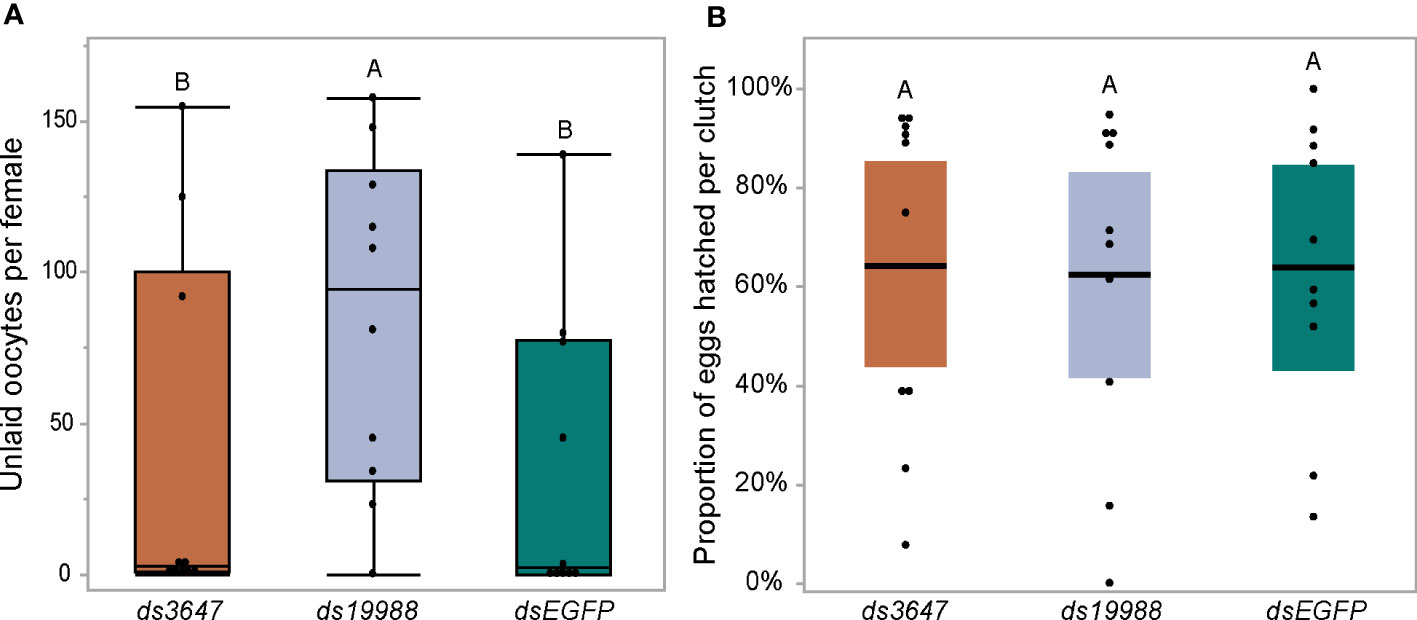
Figure 6 Effect of RNAi knockdown of AAEL003647 and AAEL019988 on egg retention (A) and egg hatching (B) of Ae. aegypti. Knockdown females were blood fed then allowed to lay eggs in individual cups. Females were then dissected and the number of unlaid, retained eggs were counted. Eggs were then allowed to hatch under standard conditions and successfully hatched larvae were counted. dsAAEL019988 females retained significantly more eggs than controls (p = 0.0476; Wilcoxon rank-sum test) while there was no significant difference between AAEL003647 knockdowns and controls. The letters above each bar/box in these figures indicate statistical significance. Different letters indicate statistical significance, and connected letters indicate no statistical significance.
Our phylogenetic analysis identified that ancestor of SIFaR underwent gene duplication early in arthropod evolution. This paralog is retained in several arthropod lineages including members of the Culicidae, Ae. aegypti (AAEL003647) and Anopheles gambiae (AGAP003335). The SIFamide receptor binds the peptide hormone SIFamide, which is localized to neurosecretory cells in the insect brain and central nervous system (29, 48, 49, 50). SIFamide is conserved among hemimetabolous and holometabolous insects and acts as a neurohormone to modulate appetitive behavior (28), feeding (29), heart contractions (29), and mating behavior (30). The phylogenetic relationships of insect SIFaR receptors indicate an ancient divergence early in arthropod evolution, as evidenced by the presence of two receptor genes in diverse insect species including aphids, cockroaches, and mosquitoes. Veenstra recently identified a novel peptide hormone, SMYamide, in the genome of the American cockroach Periplaneta americana (48). Phylogenetic analysis of the novel peptide revealed that it was sister to the P. americana SIFamide peptide, and though binding assays were not performed, the results suggest that SMYamide likely binds the protein encoded by the SIFaR-2 gene of P. americana. Our expanded phylogenetic analysis indicates that the P. americana SIFaR-2 is an ortholog of AAEL003647, though we could not identify an ortholog of SMYamide in the Ae. aegypti genome. Future binding studies of AAEL003647 will focus on determining if the receptor binds SIFamide, a distant ortholog of SMYamide, or a novel peptide hormone.
The Drosophila melanogaster orphan GPCR, Trapped in Endoderm 1 (Tre1), was identified as an ortholog of AAEL019988 in our phylogenetic analysis. Tre1 is essential for the transepithelial migration of germ cells through the posterior midgut during embryogenesis (51–55). Tre1 is also important for the initiation of courtship behavior D. melanogaster (56). The role of Tre1 in germ cell migration and in courtship may have led to the co-option of this signaling system to regulate reproduction in Ae. aegypti. Interestingly, Tre1 is absent in most hemimetabolous insects.
Our expression profiles of AAEL003647 and AAEL019988 indicate that transcript abundance of both receptors is highest in adult females’ ovaries, suggesting potential roles in egg production. To determine the potential roles of each orphan receptor in female reproductive physiology, we carried out a series of knockdown experiments which resulted in fecundity reduction in ds3647- and ds19988-injected females. Subsequently, we found that knockdown of both orphan receptors did not affect the amount of yolk packaged into oocytes, suggesting limited interactions with ILP3 and OEH, which are reproductive hormones that are known to modulate oogenesis (1–3). These results point to a role in oviposition rather than egg production.
The role of the SIFamide, a sister clade to AAEL003647, provides potential clues towards the mechanism of this receptor and its as-yet unknown ligand. SIFamide has been implicated in modulation of feeding and mating behavior in Drosophila (28, 29). SIFamidergic neurons are activated during starving conditions and are inhibited by the myosin inhibitory peptide (MIP) which modulates satiation (28). This SIFa/MIP neuropathway governs feeding behavior in Drosophila, but also directly affects mating behavior (28, 29). SIFa acts on fruitless in Drosophila, which modulates courtship behavior; upon inhibition of SIFaR, male flies exhibited bisexual mating behaviors (30). Although AAEL003647 and SIFaR belong to phylogenetically sister clades, it does not guarantee functional similarity. However, there is a possibility these receptors share similar functions, including modulation of oviposition by interaction with MIP.
AAEL019988 is an ortholog of Tre1, which in Drosophila regulates mating behavior. Luu et al., 2016 found that some fruitless expressing neurons also expressed Tre1, and that male and female flies exhibited expression of Tre1 in a sexually dimorphic fashion (56). Female Tre1 expression was induced in males by generating transgenic males expressing the female Tre1 splice form, traf. This Tre1 “feminization” in males resulted in latency in initiation of courtship behavior and complete absence of courtship initiation behavior in some males. However, there was no significant effect of Tre1 feminization on the number of offspring per Tre1 mutant male that mated with a female (56). We found that knockdown of AAEL019988 disrupts egg laying but not egg development, suggesting that it may have evolved alternative functions including but not limited to mating behaviors in Ae. aegypti. Future studies of AAEL003647 and AAEL019988 will examine the impacts of these orphan receptors on feeding and mating behavior, including through interactions with fruitless in Ae. aegypti.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Conceptualization: KV. Methodology: NK-S and KV. Experimentation: NK-S, KS, LA, and KV. Writing—original draft preparation: NK-S. Writing—review and editing: KV, NK-S, KS, and LA. Project administration: KV. All authors have read and agreed to the published version of the manuscript.
This research was grant funded by the National Institutes for Allergy and Infectious Diseases of the National Institutes of Health awarded to KV (Award #K22AI127849).
The authors would like to express their gratitude to Jena Johnson, Logan Harrell, Lilith South, and Severen Brown for their maintenance of the mosquito colony used for this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author KV declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/finsc.2023.1197945/full#supplementary-material
1. Brown MR, Clark KD, Gulia M, Zhao Z, Garczynski SF, Crim JW, et al. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc Natl Acad Sci (2008) 105(15):5716–21. doi: 10.1073/pnas.0800478105
2. Dhara A, Eum JH, Robertson A, Gulia-Nuss M, Vogel KJ, Clarke KD, et al. Ovary ecdysteroidogenic hormone functions independently of the insulin receptor in the yellow fever mosquito, Aedes aegypti. Insect Biochem Mol Biol (2013) 43(12):1100–8. doi: 10.1016/j.ibmb.2013.09.004
3. Vogel KJ, Brown MR, Strand MR. Ovary ecdysteroidogenic hormone requires a receptor tyrosine kinase to activate egg formation in the mosquito Aedes aegypti. Proc Natl Acad Sci (2015) 112(16):5057–62. doi: 10.1073/pnas.1501814112
4. Roy SG, Hansen IA, Raikhel AS. Effect of insulin and 20-hydroxyecdysone in the fat body of the yellow fever mosquito, Aedes aegypti. Insect Biochem Mol Biol (2007) 37(12):1317–26. doi: 10.1016/j.ibmb.2007.08.004
5. Hansen IA, Attardo GM, Rodriguez SD, Drake LL. Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Front Physiol (2014) 5:103. doi: 10.3389/fphys.2014.00103
6. Matsumoto S, Brown MR, Suzuki A, Lea AO. Isolation and characterization of ovarian ecdysteroidogenic hormones from the mosquito, Aedes aegypti. Insect Biochem (1989) 19(7):651–6. doi: 10.1016/0020-1790(89)90100-5
7. Siddall JB. Insect growth regulators and insect control: a critical appraisal. Environ Health Perspectives. (1976) 14:119–26. doi: 10.1289/ehp.7614119
8. Ekoka E, Maharaj S, Nardini L, Dahan-Moss Y, Koekemoer LL. 20-Hydroxyecdysone (20E) signaling as a promising target for the chemical control of malaria vectors. Parasit Vectors. (2021) 14(1):86. doi: 10.1186/s13071-020-04558-5
9. Hafez AM, Abbas N. Insecticide resistance to insect growth regulators, avermectins, spinosyns and diamides in Culex quinquefasciatus in Saudi Arabia. Parasites Vectors. (2021) 14(1):558. doi: 10.1186/s13071-021-05068-8
10. Santos VSV, Limongi JE, Pereira BB. Association of low concentrations of pyriproxyfen and spinosad as an environment-friendly strategy to rationalize Aedes aegypti control programs. Chemosphere (2020) 247:125795. doi: 10.1016/j.chemosphere.2019.125795
11. El-Sheikh ESA, Kamita SG, Hammock BD. Effects of juvenile hormone (JH) analog insecticides on larval development and JH esterase activity in two spodopterans. Pesticide Biochem Physiol (2016) 128:30–6. doi: 10.1016/j.pestbp.2015.10.008
12. Mulla MS. The future of insect growth regulators in vector control. J Am Mosq Control Assoc (1995) 11(2 Pt 2):269–73.
13. Subramanian S, Shankarganesh K. Insect hormones (as pesticides). In: Ecofriendly pest management for food security. Academic Press, Elsevier (2016). p. 613–50. doi: 10.1016/B978-0-12-803265-7.00020-8
14. Meyer JM, Ejendal KFK, Avramova LV, Garland-Kuntz EE, Giraldo-Calderón GI, Brust TF, et al. A “Genome-to-Lead” approach for Insecticide discovery: pharmacological characterization and screening of Aedes aegypti D1-like dopamine receptors. PloS Negl Trop Diseases. (2012) 6(1):e1478. doi: 10.1371/journal.pntd.0001478
15. Hill CA, Meyer JM, Ejendal KFK, Echeverry DF, Lang EG, Avramova LV, et al. Re-invigorating the insecticide discovery pipeline for vector control: GPCRs as targets for the identification of next gen insecticides. Pesticide Biochem Physiol (2013) 106(3):141–8. doi: 10.1016/j.pestbp.2013.02.008
16. Audsley N, Down RE. G protein coupled receptors as targets for next generation pesticides. Insect Biochem Mol Biol (2015) 67:27–37. doi: 10.1016/j.ibmb.2015.07.014
17. Kohyama-Koganeya A, Kim YJ, Miura M, Hirabayashi YA. Drosophila orphan G protein-coupled receptor BOSS functions as a glucose-responding receptor: loss of boss causes abnormal energy metabolism. Proc Natl Acad Sci (2008) 105(40):15328–33. doi: 10.1073/pnas.0807833105
18. Alves-Bezerra M, De Paula IF, Medina JM, Silva-Oliveira G, Medeiros JS, Gäde G, et al. Adipokinetic hormone receptor gene identification and its role in triacylglycerol metabolism in the blood-sucking insect Rhodnius prolixus. Insect Biochem Mol Biol (2016) 69:51–60. doi: 10.1016/j.ibmb.2015.06.013
19. Keyes-Scott NI, Lajevardi A, Swade KR, Brown MR, Paluzzi JP, Vogel KJ. The peptide hormone CNMa influences egg production in the mosquito Aedes aegypti. Insects (2022) 13(3):230. doi: 10.3390/insects13030230
20. Petruccelli E, Lark A, Mrkvicka JA, Kitamoto T. Significance of DopEcR, a G-protein coupled dopamine/ecdysteroid receptor, in physiological and behavioral response to stressors. J Neurogenetics. (2020) 34(1):55–68. doi: 10.1080/01677063.2019.1710144
21. Thuma L, Carter D, Weavers H, Martin P. Drosophila immune cells extravasate from vessels to wounds using Tre1 GPCR and Rho signaling. J Cell Biol (2018) 217(9):3045–56. doi: 10.1083/jcb.201801013
22. Benton MA, Frey N, Nunes da Fonseca R, von Levetzow C, Stappert D, Hakeemi MS, et al. Fog signaling has diverse roles in epithelial morphogenesis in insects. Elife (2019) 8:e47346. doi: 10.7554/eLife.47346
23. Kastner KW, Shoue DA, Estiu GL, Wolford J, Fuerst MF, Markley LD, et al. Characterization of the Anopheles Gambiae octopamine receptor and discovery of potential agonists and antagonists using a combined computational-experimental approach. Malaria J (2014) 13(1):434. doi: 10.1186/1475-2875-13-434
24. Bainton RJ, Tsai LTY, Schwabe T, DeSalvo M, Gaul U, Heberlein U. moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila. Cell (2005) 123(1):145–56. doi: 10.1016/j.cell.2005.07.029
25. Wu K, Li S, Wang J, Ni Y, Huang W, Liu Q, et al. Peptide hormones in the insect midgut. Front Physiol (2020) 11:191. doi: 10.3389/fphys.2020.00191
26. Bloom M, Lange AB, Orchard I. Identification, functional characterization, and pharmacological analysis of two sulfakinin receptors in the medically-important insect Rhodnius prolixus. Sci Rep (2019) 9(1):13437. doi: 10.1038/s41598-019-49790-x
27. Aguilar R, Maestro JL, Vilaplana L, Chiva C, Andreu D, Bellés X. Identification of leucomyosuppressin in the German cockroach, Blattella germanica, as an inhibitor of food intake. Regul Peptides. (2004) 119(1-2):105–12. doi: 10.1016/j.regpep.2004.01.005
28. Martelli C, Pech U, Kobbenbring S, Pauls D, Bahl B, Sommer MV, et al. SIFamide translates hunger signals into appetitive and feeding behavior in Drosophila. Cell Rep (2017) 20(2):464–78. doi: 10.1016/j.celrep.2017.06.043
29. Ayub M, Hermiz M, Lange AB, Orchard I. SIFamide influences ieeding in the Chagas disease vector, Rhodnius prolixus. Front Neuroscicience. (2020) 14:134. doi: 10.3389/fnins.2020.00134
30. Terhzaz S, Rosay P, Goodwin SF, Veenstra JA. The neuropeptide SIFamide modulates sexual behavior in Drosophila. Biochem Biophysics Res Commun (2007) 352(2):305–10. doi: 10.1016/j.bbrc.2006.11.030
31. Yamanaka N, Roller L, Žitňan D, Satake H, Mizoguchi A, Kataoka H, et al. Bombyx orcokinins are brain-gut peptides involved in the neuronal regulation of ecdysteroidogenesis. J Comp Neurology. (2011) 519(2):238–46. doi: 10.1002/cne.22517
32. Sterkel M, Oliveira PL, Urlaub H, Hernandez-Martinez S, Rivera-Pomar R, Ons S. OKB, a novel family of brain-gut neuropeptides from insects. Insect Biochem Mol Biol (2012) 42(7):466–73. doi: 10.1016/j.ibmb.2012.03.003
33. Wulff JP, Sierra I, Sterkel M, Holtof M, Van Wielendaele P, Francini F, et al. Orcokinin neuropeptides regulate ecdysis in the hemimetabolous insect Rhodnius prolixus. Insect Biochem Mol Biol (2017) 81:91–102. doi: 10.1016/j.ibmb.2017.01.003
34. Zandawala M, Hamoudi Z, Lange AB, Orchard I. Adipokinetic hormone signalling system in the Chagas disease vector, Rhodnius prolixus. Insect Mol Biol (2015) 24(2):264–76. doi: 10.1111/imb.12157
35. Nässel DR, Vanden Broeck J. Insulin/IGF signaling in Drosophila and other insects: factors that regulate production, release and post-release action of the insulin-like peptides. Cell Mol Life Sci (2016) 73(2):271–90. doi: 10.1007/s00018-015-2063-3
36. Defferrari MS, Orchard I, Lange AB. An insulin-like growth factor in Rhodnius prolixus is involved in post-feeding nutrient balance and growth. Front Neurosci (2016) 10:566. doi: 10.3389/fnins.2016.00566
37. Kim JK, Han SH, Kim CH, Jo YH, Futahashi R, Kikuchi Y, et al. Molting-associated suppression of symbiont population and up-regulation of antimicrobial activity in the midgut symbiotic organ of the Riptortus–Burkholderia symbiosis. Devlopmental Comp Immunol (2014) 43(1):10–4. doi: 10.1016/j.dci.2013.10.010
38. Te Brugge V, Paluzzi JP, Schooley DA, Orchard I. Identification of the elusive peptidergic diuretic hormone in the blood-feeding bug Rhodnius prolixus : a CRF-related peptide. J Exp Biol (2011) 214(3):371–81. doi: 10.1242/jeb.046292
39. Cannell E, Dornan AJ, Halberg KA, Terhzaz S, Dow JAT, Davies SA. The corticotropin-releasing factor-like diuretic hormone 44 (DH 44) and kinin neuropeptides modulate desiccation and starvation tolerance in Drosophila melanogaster. Peptides (NY). (2016) 80:96–107. doi: 10.1016/j.peptides.2016.02.004
40. Capriotti N, Ianowski JP, Gioino P, Ons S. The neuropeptide CCHamide 2 regulates diuresis in the Chagas’ disease vector Rhodnius prolixus. J Exp Biol (2019) 222(10):jeb203000. doi: 10.1242/jeb.203000
41. Silva-Oliveira G, De Paula IF, Medina JM, Alves-Bezerra M, Gondim KC. Insulin receptor deficiency reduces lipid synthesis and reproductive function in the insect Rhodnius prolixus. Biochim Biophys Acta (BBA) - Mol Cell Biol Lipids. (2021) 1866(2):158851. doi: 10.1016/j.bbalip.2020.158851
42. Vogel KJ, Brown MR, Strand MR. Phylogenetic investigation of peptide hormone and growth factor receptors in five dipteran genomes. Front Endocrinol (Lausanne). (2013) 4. doi: 10.3389/fendo.2013.00193
43. Akbari OS, Antoshechkin I, Amrhein H, Williams B, Diloreto R, Sandler J, et al. The developmental transcriptome of the mosquito Aedes aegypti, an invasive species and major arbovirus vector. G3: Genes|Genomes|Genetics. (2013) 3:1493–509. doi: 10.1534/g3.113.006742
44. Kuznetsov D, Tegenfeldt F, Manni M, Seppey M, Berkeley M, Kriventseva EV, et al. OrthoDB v11: annotation of orthologs in the widest sampling of organismal diversity. Nucleic Acids Res (2023) 51(D1):D445–51. doi: 10.1093/nar/gkac998
45. Eddy SR. Accelerated profile HMM searches. PloS Comput Biol (2011) 7:e1002195. doi: 10.1371/journal.pcbi.1002195
46. Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3. 0. Syst Biol (2010) 59(3):307–21. doi: 10.1093/sysbio/syq010
47. Hatan M, Shinder V, Israeli D, Schnorrer F, Volk T. The Drosophila blood brain barrier is maintained by GPCR-dependent dynamic actin structures. J Cell Biol (2011) 192(2):307–19. doi: 10.1083/jcb.201007095
48. Veenstra JA. The neuropeptide SMYamide, a SIFamide paralog, is expressed by salivary gland innervating neurons in the American cockroach and likely functions as a hormone. Peptides (NY). (2021) 136:170466. doi: 10.1016/j.peptides.2020.170466
49. Sellami A, Veenstra JA. SIFamide acts on fruitless neurons to modulate sexual behavior in Drosophila melanogaster. Peptides (NY). (2015) 74:50–6. doi: 10.1016/j.peptides.2015.10.003
50. Siju KP, Reifenrath A, Scheiblich H, Neupert S, Predel R, Hansson BS, et al. Neuropeptides in the antennal lobe of the yellow fever mosquito, Aedes aegypti. J Comp Neurology. (2014) 522(3):592–608. doi: 10.1002/cne.23434
51. Kunwar PS, Sano H, Renault AD, Barbosa V, Fuse N, Lehmann R. Tre1 GPCR initiates germ cell transepithelial migration by regulating Drosophila melanogaster E-cadherin. J Cell Biol (2008) 183(1):157–68. doi: 10.1083/jcb.200807049
52. Ma T, Matsuoka S, Drummond-Barbosa D. RNAi-based screens uncover a potential new role for the orphan neuropeptide receptor Moody in Drosophila female germline stem cell maintenance. PloS One (2020) 15(12):e0243756. doi: 10.1371/journal.pone.0243756
53. LeBlanc MG, Lehmann R. Domain-specific control of germ cell polarity and migration by multifunction Tre1 GPCR. J Cell Biol (2017) 216(9):2945–58. doi: 10.1083/jcb.201612053
54. Kunwar PS, Starz-Gaiano M, Bainton RJ, Heberlein U, Lehmann R. Tre1, a G protein-coupled receptor, directs transepithelial migration of Drosophila germ cells. PloS Bioogy. (2003) 1(3):e80. doi: 10.1371/journal.pbio.0000080
55. Kamps AR, Pruitt MM, Herriges JC, Coffman CR. An evolutionarily conserved arginine is essential for tre1 G protein-coupled receptor function during germ cell migration in Drosophila melanogaster. PloS One (2010) 5(7):e11839. doi: 10.1371/journal.pone.0011839
Keywords: insect physiology, GPCR, reproduction, insect endocrinology, vector biology
Citation: Keyes-Scott NI, Swade KR, Allen LR and Vogel KJ (2023) RNAi-mediated knockdown of two orphan G protein-coupled receptors reduces fecundity in the yellow fever mosquito Aedes aegypti. Front. Insect Sci. 3:1197945. doi: 10.3389/finsc.2023.1197945
Received: 31 March 2023; Accepted: 19 July 2023;
Published: 23 August 2023.
Edited by:
Nicholas Teets, University of Kentucky, United StatesReviewed by:
Yoonseong Park, Kansas State University, United StatesCopyright © 2023 Keyes-Scott, Swade, Allen and Vogel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kevin J. Vogel, a2p2b2dlbEB1Z2EuZWR1
†Present address: Kyle R. Swade, Department of Molecular and Cellular Biology, Kennesaw State University, Kennesaw, GA, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.