- 1Department of Biological Sciences, University of Cape Town, Cape Town, South Africa
- 2School of Life Sciences, University of KwaZulu-Natal, Pietermaritzburg, South Africa
- 3Research and Exhibitions Department, Iziko Museums of South Africa, Cape Town, South Africa
Anoplolepis gracilipes is an invasive species that is a major threat to native ecosystems worldwide. It has been listed as one of the top 100 worst invasive species in the world and is well known for its negative impact on native arthropods and some vertebrates. This study aimed to confirm the presence or absence of A. gracilipes in some major South African harbours. We did so by surveying four harbours in the Western Cape and KwaZulu-Natal provinces, using pitfall trapping, yellow pan traps, and baiting. In addition, ant collections from Iziko Museums of South Africa (Cape Town, South Africa), University of KwaZulu-Natal (Pietermaritzburg campus, South Africa), Iimbovane Outreach Project (Stellenbosch University, South Africa), and AfriBugs CC (Pretoria, South Africa) were examined for specimens of A. gracilipes. The invasive species A. gracilipes was not detected from any of the sampled harbours during this study, nor in the main ant collections in South Africa. The only, and potentially erroneous published record of A. gracilipes in South Africa, is from Durban harbour and subsequent possibly erroneous citizen science observations are from other coastal sites such as Gansbaai, Knysna, Table Bay, and Kalk Bay. This is a positive outcome for conservation authorities as this species is highly invasive and, if introduced, will likely outcompete native fauna and result in ecosystem collapse. Although A. gracilipes was not detected in the samples from this study, early detection and eradication of this species should be prioritised. This can be achieved through existing pest monitoring programs at harbours, and continued border biosecurity measures.
1 Introduction
Anoplolepis gracilipes (1) is a well-known, widely distributed invasive species and has spread globally through human-mediated pathways (2). It has been recorded across the pacific tropics, from India to China, Japan, Australia, Chile, Mexico and California. Although the origin of this species is unknown (3), ecological niche modelling to reconstruct the ancestral distribution range suggests the origin of A. gracilipes might have been South Asia (4). Based on its current known distribution, A. gracilipes prefers warm and humid areas (3–5). It is known to thrive in highly disturbed habitats and areas with intermediate human activity. However, this species also inhabits undisturbed areas such as natural forests (2). The main introduction pathway of A. gracilipes is at ports and harbours as stowaways in containers and from transporting bulk materials (6, 7).
Anoplolepis gracilipes has been listed as one of the 100 worst invasive alien species in the world by the International Union for Conservation of Nature (IUCN) through its Invasive Species Specialist Group (ISSG) and Global Invasive species database (7). In South Africa, it is listed under NEMBA category 2b and South African Biodiversity Act, 2004 (Act No.10 of 2004). Species in this category must be controlled as no risk assessment has been done, nor is the distribution of the species known (8). Previous studies have investigated the spatial distribution patterns and population structure of A. gracilipes (4). This species has a high density of ground foraging workers and is numerically and behaviourally dominant (2). Its dominance facilitates its success in out-competing native ant species (9). The most severe ecological consequences of A. gracilipes include the displacement of native ants and other species of vertebrates and invertebrates (4, 9). This species also alters natural ecosystems’ structure, composition, and function (2). For example, on Christmas Island, A. gracilipes rapidly eliminated keystone species such as the red land crab (10), which caused major irreversible ecosystem disruption and made way for secondary invasions (11). The red land crab plays an important role in Christmas Island’s Forest ecosystem by facilitating litter breakdown and influencing forest composition by eating leaves and seedlings of rainforest trees (7). Regions predicted to be highly susceptible to A. gracilipes invasion include Asia, Australia, Africa, and South America (4). These areas should particularly focus on preventing the introduction of this invasive species.
Various records of A. gracilipes have been reported in South Africa (12, 13; https://www.inaturalist.org/observations/1160269; https://antmaps.org/?mode=species&species=Anoplolepis.gracilipes). The first published record of A. gracilipes was from Durban harbour (12). Other observations for this species are from Gansbaai, Knysna, Table Bay and Kalk Bay (Supplementary Figure 1) (13; https://www.inaturalist.org/observations/1160269; https://antmaps.org/?mode=species&species=Anoplolepis.gracilipes). Slingsby (13) suggested that this species has expanded its historical distribution into new areas of the Western Cape, which is alarming given the negative impact this species has on other species worldwide. However, no pictures or specimens are available for these records. This study aimed to verify the existing distributional records for the invasive species, A. gracilipes in South Africa and monitor its main introduction pathway (harbours). This was achieved by not only sampling ants at different harbours in South Africa but focusing on areas where this species was previously recorded and also examining ant museum collections in South Africa.
2 Methods and materials
2.1 Study area
Harbours are usually the first detection site of A. gracilipes in other regions (14). This study was conducted at four different harbours in South Africa, namely: Kalk Bay (34.1293° S, 18.4493° E) and V&A Waterfront (33.9050° S, 18.4204° E) in the Western Cape Province, and Durban (29.8723° S, 31.0249° E) and Richards Bay harbours (28.8000° S, 32.0833° E) in KwaZulu-Natal Province. Sampling sites were chosen based on previously recorded sites (Durban: 12; Gansbaai, Knysna, Table Bay, and Kalk Bay: 13; Kalk Bay: https://www.inaturalist.org/observations/1160269). The other records of A. gracilipes included Gansbaai and Knysna (15), but due to logistical constraints, these sites could not be sampled. Nevertheless, the four harbours sampled were representative of the major harbours and most confirmed records of this species in South Africa to date.
At each harbour, at least two sites surrounding the harbour were sampled. Where possible, these sites were replicated and sites with vegetation were selected as most ants need soil for nesting (16). These included: two sites in Kalk Bay (inside harbour and outside harbour); two sites in V&A Waterfront (Transnet building and helicopter pad) (Figure 1); five sites in Durban (Bayhead heritage site, Island and Channel View Park, South beach, Royal Natal view park and Umhlanga rocks) and four sites in Richards Bay (Port of Richards Bay, Pelican Island, Palm beach and Alkantstrand beach) (Figure 2). The study sites were characterized by different habitat types, such as grasslands, mangroves, sand dunes, and rocky shores (17). These site types were chosen as these spanned the range of habitat types available across the harbours (17).
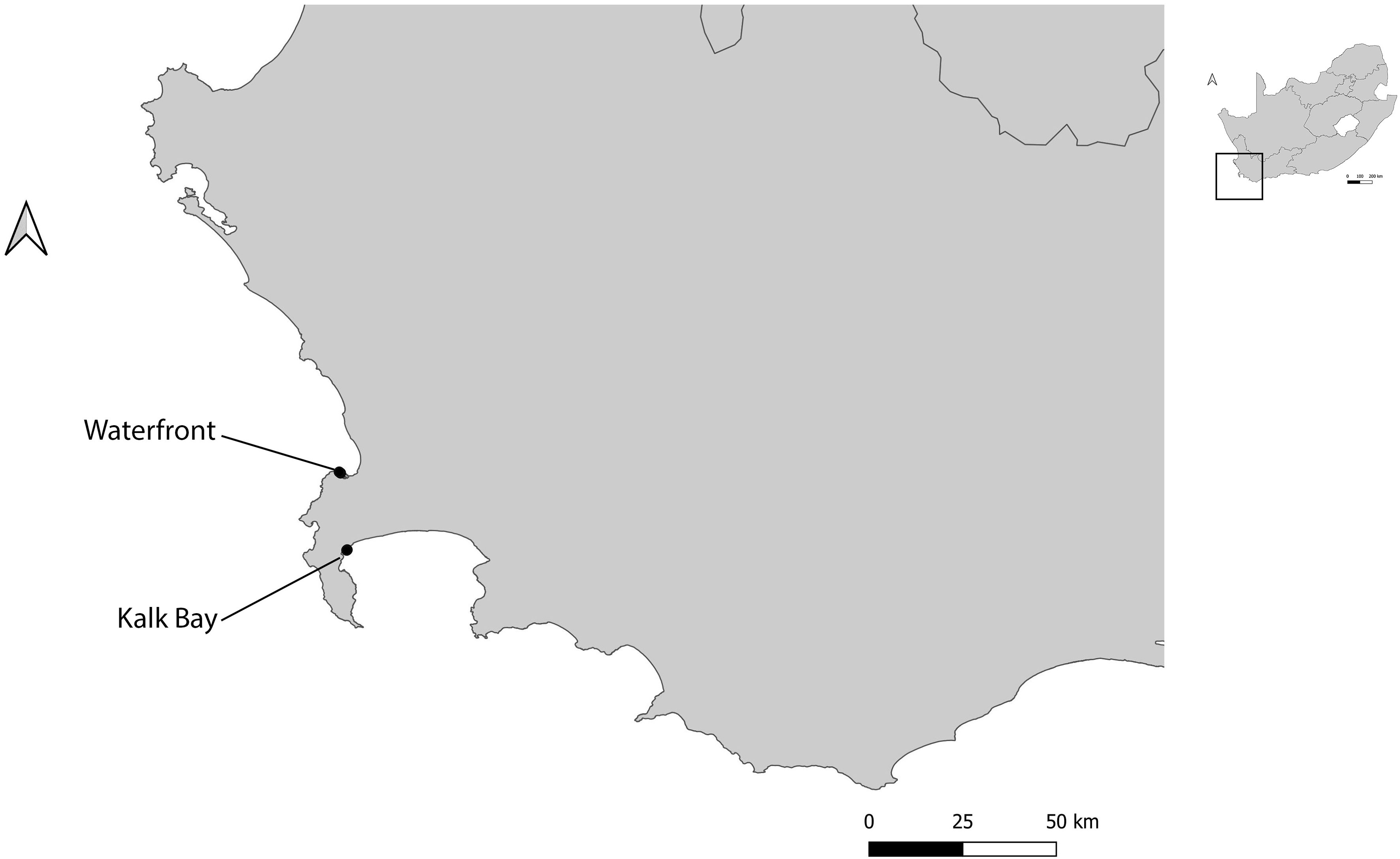
Figure 1 Location of different study sites used for ant sampling in two harbours (Kalk Bay and V&A Waterfront), Western Cape, South Africa.
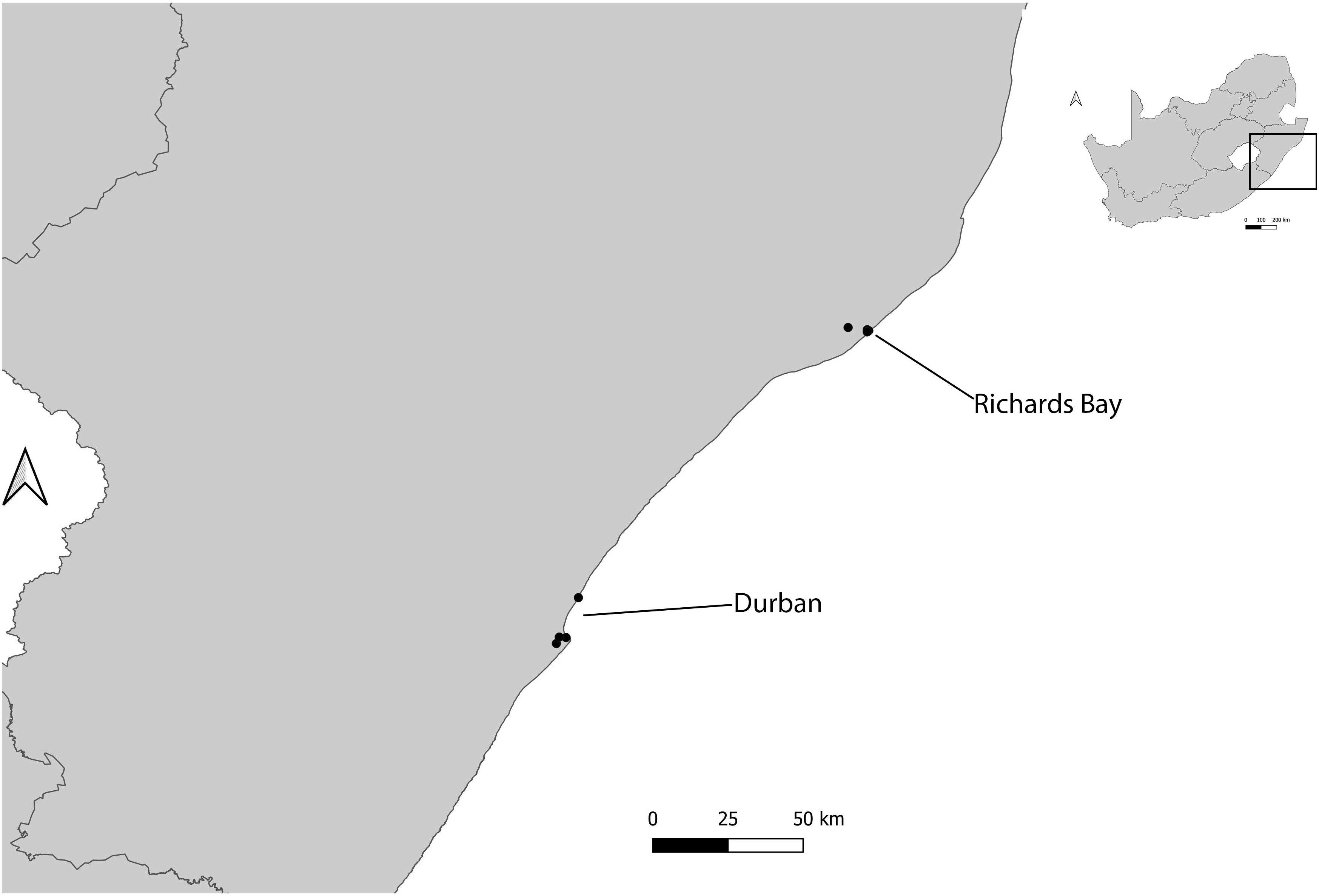
Figure 2 Location of different study sites used for ant sampling in two harbours (Durban and Richards Bay), KwaZulu-Natal, South Africa.
2.2 Ant sampling and species identification
A combination of collection techniques was employed to collect all ant species present at each harbour. Ants were sampled using standardized pitfall trapping (18–20), yellow pan traps, and baiting. Where possible, sampling was done every two weeks for three months between June and September 2021. At each site, 10 pitfall traps were laid out in a sample grid (2 x 5) with 10 m spacing between traps. This resulted in 310 samples (Supplementary Table 2). Pitfall traps were half-filled with 100% propylene glycol that neither repels nor attracts insects (21) and were left open in the field for three days and three nights. In areas with concrete where pitfall traps could not be used, yellow pan traps were used with the same layout method as pitfall traps as an additional method to cover the whole area of the harbours (Supplementary Table 2). Yellow pan traps have also been used in other studies to sample ants (22–24).
In addition to these trapping methods, the baiting method was used across all study sites after the pitfall traps and yellow pan traps had been taken out. At the same sites as the pitfall traps and yellow pan traps, two card papers, one with tuna and one with peanut butter mixed with jam (used as attractants for ants), were laid out across all sites for one hour. These baits were found to be effective in attracting ants (25). All ants found at bait traps were collected with an aspirator and placed in vials containing 96% ethanol. Samples were processed and identified to genus in the laboratory using available keys (26) and AntWeb (www.antweb.org). Specimens were stored in 96% ethanol and voucher species were mounted. Where possible, species were confirmed using the Iziko Museums of South Africa’s reference ant collection. Those that could not be identified to species level were assigned as morphospecies. The second and third authors of the current study (ant experts) also confirmed the identifications. All specimens were labelled, catalogued and entered into the Iziko Museum of South Africa’s database (Specify 6 V6.7.01). The ant collection at Iziko Museums of South Africa (Cape Town, South Africa), University of KwaZulu-Natal (Pietermaritzburg campus, South Africa), Iimbovane Outreach Project (Stellenbosch University, South Africa), and AfriBugs CC (Pretoria, South Africa) were examined for specimens of A. gracilipes. This species is recognised by its monomorphic and remarkably long and slender yellow-brownish body of 4-5mm with a dark abdomen (2, 27), and its extremely long legs and antennae, with scapes longer than the body (27, 28).
2.3 Data analysis
Species accumulation curves were made using the specaccum() function in the vegan package in R V4.1.2 (29) to ensure sampling was done at an adequate level to capture resident biodiversity within a defined margin of error.
3 Results
In total, 10,041 specimens were sampled, comprising 66 species from 27 genera and five subfamilies (Supplementary Table 1). Myrmicinae was the most diverse and abundant subfamily with 10 genera, 45 species and 77% of the total abundance, followed by Formicinae with eight genera, 12 species and 18% of the total abundance. Dorylinae was the least diverse with two genera, two species and 0.5% of the total abundance. The most specious genera were Tetramorium (18 species), Pheidole (eight species) and Monomorium (five species). Genus Pheidole was most abundant in KwaZulu-Natal harbours, while Tetramorium was the most species-rich genus. The most abundant species in the Western Cape harbours were Lepisiota capensis and Linepithema humile, while Tetramorium was the most species-rich genus. The invasive species A. gracilipes was not detected from any of the sampled harbours during this study, nor in the main ant collections in South Africa. However, in the Western Cape harbours, we collected a lot of Linepithema humile which is a major problem in the Western Cape province and none in the KwaZulu-Natal harbours (Supplementary Table 1).
The four accumulation curves from various harbours show that the increase in sampling sites resulted in an increased number of species (Supplementary Figure 2). Most of the accumulation curves reached or nearly reached a horizontal asymptote indicating sufficient sampling, although the Richards Bay curve indicated that more sampling might be needed at this site in the future.
4 Discussion
In this study, four harbours were sampled in the Western Cape and KwaZulu-Natal provinces in South Africa, including the exact locations where A. gracilipes was previously recorded. However, no specimens of this species were found. Although the species could have been accidentally introduced to South Africa at ports and harbours, no specimens, images, or drawings exist of the records of A. gracilipes from South Africa. Therefore, the specimens determined as A. gracilipes could have been misidentified. Although other species of Anoplolepis were found in harbours and could have been misidentified as A. gracilipes, A. gracilipes (Formicinae) is morphologically more similar to the minor workers of Camponotus maculatus (Formicinae) and to some species of Leptomyrmex (Dolichoderinae) than to other Anoplolepis species. These ant genera have long limbs and a similar-sized, slender body. However, A. gracilipes and C. maculatus can be distinguished from Leptomyrmex by the presence of an acidopore, which is often easily overlooked. The photo of A. gracilipes on iNaturalist is presented by a drawing and not the actual specimen collected (Supplementary Figure 1). A character like an acidopore is not clearly visible without magnification, thus the identification remains questionable. Anoplolepis gracilipes and C. maculatus can be separated based on the number of antennal segments. In A. gracilipes, antennae has 11 segments, whereas in C. maculatus has 12 segments. These two species can also be separated based on the shape of the mesosoma.
The characters that separate A. gracilipes from other species in the genus Anoplolepis include their monomorphic (26) and remarkably long and slender yellow-brownish body of 4-5mm with a dark abdomen (2). The legs and antennae are extremely long, with scapes longer than the body (27, 28). In Kalk Bay harbour, where A. gracilipes was recorded in 2012 (P. Slingsby, https://www.inaturalist.org/observations/1160269; Supplementary Figure 1), the alien invasive Linepithema humile (Argentine ant) was found to be the dominant ant species across all Western Cape province harbours in this study, occurring in almost all pitfall traps (Supplementary Table 1).
Since the detection of A. gracilipes at Kalk Bay in 2012, the site was transformed into a parking area (P. Slingsby, https://www.inaturalist.org/observations/1160269; Supplementary Figure 3), and this species was not found again. This could have been a result of A. gracilipes not being able to compete with the abundant L. humile (Argentine ants) present at this site (13). Linepithema humile is one of the most widespread ant invasive species in South Africa that has successfully invaded at least six of the nine provinces in South Africa (15, 30). This species is also listed as one of the world’s 100 worst invaders (7, 31). It is well known for its aggressiveness and displacement of native invertebrates and small vertebrates (2, 32, 33). Anoplolepis gracilipes has eliminated the red land crab, a keystone species, in parts of the island resulting in significant ecosystem disruption in Christmas Island (11). On other islands such as Seychelles, when Anoplolepis gracilipes occurred in high abundance, it took over the nests, preventing scooty terns from nesting, thus leading to the death of chicks (11). Several native ant species have been successfully displaced by L. humile, disrupting plant-ant mutualism (34, 35). Thus, if there was a small population of A. gracilipes present at this site, it could have potentially been displaced by L. humile. Moreover, the latest study by Lee and Scotty Yang (2) suggested that the South African population of this species has been eradicated.
Once alien species are established, their management is costly and is considerably more than the prevention of new invasive species (36). The measures taken to prevent losses or enable restoration of ecosystem services in an invaded area can be very costly. For example, an estimated US$300 billion per year is spent as a result of invasive species in the United States, British Isles, Australia, South Africa, India, and Brazil alone (8). In the vineyards in Western Cape, chemical stem barriers were effective in most ant pests (37). However, chemical stem barriers are ineffective in controlling species of the genus Anoplolepis (38). Although most chemicals are registered for use in controlling invasive species, the negative impact on other organisms caused by the use of these chemicals far exceeds their cause of action (39). Therefore, the early detection of invasive species is critical to increasing the chances of successful management (36). This is especially prudent given the impact A. gracilipes had on the wine and grape industry in countries in Central America (40). The Western Cape wine industry plays a huge role in the country’s economy, with a contribution of about R31 billion to the gross domestic product and more than 160 000 employment opportunities, which is 57% and 62% contribution to the country’s total wine industry contribution, respectively (https://www.wosa.co.za/The-Industry/Statistics/World-Statistics/). This is because the wine industry of South Africa is more concentrated in the Western Cape (https://www.wosa.co.za/The-Industry/Statistics/World-Statistics/). However, ants such as L. humile and some species of Anoplolepis are a major problem in vineyards of the Western Cape province (38).
Ants, in particular A. gracilipes, feed on honeydew produced by aphids and other scale insects, thereby protecting them from infestation promoted by a build-up of honeydew and protecting aphids from predators (41). Through the consumption of honeydew by ants, the survival of honeydew-producing pests is increased (42). This increases the damaging effects on crops through pest outbreaks in agroecosystem (42). Furthermore, in the Cape Floristic Region, most fynbos plants are dispersed by ants (31). Therefore, the presence of A. gracilipes may also negatively impact fynbos seed dispersal. Despite the absence of this species in this study, prevention of the introduction of A. gracilipes in South Africa should be prioritized. The economic and ecological impacts of this species can be reduced through quarantine programs in susceptible areas (3).
Ants are sensitive to changing climatic conditions such as temperature, water stress, and wind (43). However, invasive species are known to have broader tolerances to warming and drying conditions than indigenous species, as found for other soil-dwelling invertebrates (44). The foraging activity of A. gracilipes is largely affected by ambient temperature, with the highest activity levels at 26°C and 30°C (45). This species prefers moist tropical lowlands. However, there is still potential for possible invasion in arid regions, mainly because this species can still thrive in urban and irrigated areas (3). This is therefore why ongoing monitoring should be done throughout southern Africa. This can easily be accomplished through existing pest monitoring programs at harbours, and through standard border control monitoring. Monitoring greenhouses and plant nurseries is recommended as alien ants can be introduced in greenhouses, for example through soil movement in potted plants (46).
Conclusions
Although A. gracilipes was not detected in the samples from this study, ongoing monitoring is essential to ensure the early detection and eradication of this species. Additional taxonomic information should be provided to persons at ports of entry. Other global monitoring programs should include mesic tropical, subtropical, and warm temperate mainland islands that are most susceptible to being invaded by A. gracilipes (47). In addition, the use of citizen science platforms like iNaturalist is a useful research tool for the early detection of severe pests such as A. gracilipes. However, it is recommended that records from sources such as iNaturalist and antmaps (https://antmaps.org/; https://www.inaturalist.org/) need to be carefully verified before being included in species lists. For future studies, more sampling should be done along provincial borders, including the Eastern Cape borders and neighbouring countries to South Africa (Namibia and Mozambique), which were not sampled in this study.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
All authors contributed to the study’s conception and design. Field sampling was performed by AN, TM, and CJ-S. Laboratory analyses and statistical analysis were performed by AN. The first draft of the manuscript was written by AN, and all authors commented on previous versions. All authors read and approved the final manuscript.
Funding
This study was funded by the NRF Foundational Biodiversity Information Programme grant number 129110.
Acknowledgments
We are grateful to Iziko Museums of South Africa (Cape Town, South Africa) for providing us with full access to their resources and to work with their ant collection and accessioning the collected material. V&A Waterfront, Kalk Bay, Durban, and Richards Bay harbour gave permission to sample. We thank the Iimbovane outreach project (Stellenbosch University, South Africa), Afribugs CC (Pretoria, South Africa), and the University of KwaZulu-Natal (Pietermaritzburg campus, South Africa) for loaning us their material. We thank everyone involved in field and lab assistance, especially Lindiwe Khoza and Nokubonga Thabethe.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/finsc.2023.1176810/full#supplementary-material
References
1. Smith F. Catalogue of the hymenopterous insects collected at Sarawak, borneo; mount ophir, malacca; and at Singapore, by a. r. wallace. [part]. J Proc Linn Soc London Zool (1857) 2:42–88. doi: 10.1111/j.1096-3642.1857.tb01759.x
2. Lee CY, Scotty Yang CC. Biology, ecology, and management of the invasive longlegged ant, Anoplolepis gracilipes. Annu Rev Entomol (2022) 67:43–63. doi: 10.1146/annurev-ento-033121-102332
3. Wetterer JK. Worldwide distribution and potential spread of the long-legged ant, Anoplolepis gracilipes (Hymenoptera: Formicidae). Sociobiology (2005) 45(1):77–97.
4. Chen Y. Global potential distribution of an invasive species, the yellow crazy ant (Anoplolepis gracilipes) under climate change. Integr Zool (2008) 3(3):166–75. doi: 10.1111/j.1749-4877.2008.00095.x
5. Li HM, Xiao H, Peng H, Han HX, Xue DY. Potential global range expansion of a new invasive species, the erythrina gall wasp, Quadrastichus erythrinae Kim. Raffles Bull Zool (2006) 54:229–34.
6. Abbott K, Harris R, Lester P. Invasive ant risk assessment: anoplolepis gracilipes. Wellington, New Zealand: Landcare Research contract report for Biosecurity New Zealand, Ministry of Agriculture and Forestry (2005).
7. Lowe S, Browne M, Boudjelas S, De Poorter M. 100 of the world’s worst invasive alien species a selection from the global invasive species database. Published by The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN (2000). 12pp.
8. van Wilgen BW, Measey J, Richardson DM, Wilson JR, Zengeya TA. Biological invasions in south Africa: an overview. Biol Invasions South Afr (2020) 1:3–31. doi: 10.1007/978-3-030-32394-3_1
9. Drescher J, Feldhaar H, Blüthgen N. Interspecific aggression and resource monopolization of the invasive ant Anoplolepis gracilipes in Malaysian Borneo. Biotropica (2011) 43(1):93–9. doi: 10.1111/j.1744-7429.2010.00662.x
10. O’Dowd DJ, Green PT, Lake PS. Invasional meltdown on an ‘oceanic’ island. Ecol Lett (2003) 6:812–7. doi: 10.1046/j.1461-0248.2003.00512.x
11. Abbott KL. Spatial dynamics of supercolonies of the invasive yellow crazy ant, Anoplolepis gracilipes, on Christmas island, Indian ocean. Diversity Distributions (2006) 12(1):101–10. doi: 10.1111/j.1366-9516.2006.00193.x
12. Prins AJ, Robertson HG, Prins A. Pest ants in urban and agricultural. applied myrmecology: a world perspective 25. vander meer RK. 2019. Applied Myrmecology: a World Perspective. CRC Press (1990).
14. Suhr EL, O’Dowd DJ, Suarez AV, Cassey P, Wittmann TA, Ross JV, et al. Ant interceptions reveal roles of transport and commodity in identifying biosecurity risk pathways into Australia. NeoBiota (2019) 53:1–24. doi: 10.3897/neobiota.53.39463
15. Mothapo NP, Wossler TC. Resource competition assays between the African big-headed ant, Pheidole megacephala and the invasive Argentine ant, Linepthima humile: mechanisms of inter-specific displacement. Ecol Entomol (2014) 39:501–10. doi: 10.1111/een.12126
16. Underwood EC, Fisher BL. The role of ants in conservation monitoring: if, when, and how. Biol Conserv (2006) 132(2):166–82. doi: 10.1016/j.biocon.2006.03.022
17. Rutherford MC, Mucina L, Powrie LW. Biomes and bioregions of southern Africa. In: The vegetation of south Africa, Lesotho, and Swaziland. (2006) 19:30–51.
18. Parr CL, Chown SL. Inventory and bioindicator sampling: testing pitfall and winkler methods with ants in a south African savanna. J Insect Conserv (2001) 5:27–36. doi: 10.1023/A:1011311418962
19. Ivanov K, Keiper J. Ant (Hymenoptera: Formicidae) diversity and community composition along sharp urban forest edges. Biodivers Conserv (2010) 19:3917–33. doi: 10.1007/s10531-010-9937-3
20. Munyai TC, Foord SH. Ants on a mountain: spatial, environmental and habitat associations along an altitudinal transect in a centre of endemism. J Insect Conserv (2012) 16(5):677–95. doi: 10.1007/s10841-011-9449-9
21. Munyai TC, Foord SH. An inventory of epigeal ants of the western soutpansberg mountain range, south Africa. Koedoe (2015) 57:1–12. doi: 10.4102/koedoe.v57i1.1244
22. Fisher BL. Diversity patterns of ants (Hymenoptera: Formicidae) along an elevational gradient on monts doudou in southwestern Gabon. California Acad Sci Memoir (2004) 28:269–86.
24. Haneda NF, Sajap AS, Hussin MZ. A study of two ant (Hymenoptera: Formicidae) sampling methods in tropical rain forest. J. Appl. Sci. (2005) 5:1732–1734.
25. Nyamukondiwa C, Addison P, Shelly T. Food preference and foraging activity of ants: recommendations for field applications of low-toxicity baits. J Insect Sci (2014) 14(1):48.
26. Fisher BL, Bolton B. Ants of Africa and Madagascar: a guide to the genera. Berkeley: Univ of California Press (2016).
27. Sarnat E. PIAkey: identification guide to ants of the pacific islands. edition 2.0, lucid v. 3.4. Davis: USDA/APHIS/PPQ Center for Plant Health Science and Technology and University of California (2008).
28. Taylor. The ants of (sub-Saharan) Africa (2015). Available at: http://antsofafrica.org/ (Accessed 02 September 2021).
29. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (2017). Available at: https://www.R-project.org.
30. Luruli N. Distribution of the Argentine ant linepithema humile (Mayr) in South Africa. Cape Town, South Africa: Stellenbosch University (2007).
31. Nyamukondiwa C. Assessment of toxic baits for the control of ants (Hymenoptera: Formicidae) in south African vineyards. PhD dissertation. Stellenbosch University (2008).
32. Rowles AD, O’Dowd DJ. Interference competition by Argentine ants displaces native ants: implications for biotic resistance to invasion. Biol Invasions (2007) 9:73–85. doi: 10.1007/s10530-006-9009-5
33. Suarez AV, Bolger DT, Case TJ. (1998) Effects of fragmentation and invasion on native ant communities in coastal southern California. Ecology. (2008) 79(6):2041–2056.
34. Bond W, Slingby P. Collapse of an ant-plant mutualism: the Argentine ant (Iridomyrmex humilis) and myrmecochorous proteaceae. Ecology (1984) 35(4):1031–7. doi: 10.2307/1938311
35. Devenish AJM, Gomez C, Bridle JR, Newton RJ, Sumner S. Invasive ants take and squander native seeds: implications for native plant communities. Biol Invasions (2019) 21:451–66. doi: 10.1007/s10530-018-1829-6
36. Reed RN, Hopken MW, Steen DA, Falk BG, Piaggio AJ. Integrating early detection with DNA barcoding: species identification of a nonnative monitor lizard (Squamata: Varanidae) carcass in mississippi, U.S.A. Manage Biol Invasions (2016) 7(2):193–7. doi: 10.3391/mbi.2016.7.2.07
37. Addison A. Chemical stem barriers for the control of ants (Hymenoptera: formicidae) in vineyards. South Afr. J. Enology Viticulture (2002) 23(1):1–8.
38. Addison P, Samways MJ. A survey of ants (Hymenoptera: Formicidae) that forage in the Western cape province, south Africa. Afr Entomol (2000) 8:251–60.
39. Walton VM. Development of and integrated pest management system for vine mealybug (Planococcus ficus) (Signoret). vineyards in the Western Cape Province, South Africa: Stellenbosch University (2003).
40. Hiller T, Haelewaters D. A case of silent invasion: citizen science confirms the presence of Harmonia axyridis (Coleoptera, Coccinellidae) in central America. PloS One (2019) 14(7):e0220082. doi: 10.1371/journal.pone.0220082
41. Novgorodova TA, Ryabinin AS. Ant–aphid relations in the south of Western Siberia (Hymenoptera: Formicidae; Hemiptera: Aphididae). Arthropod-Plant Interact (2018) 12(3):369–76. doi: 10.1007/s11829-017-9584-7
42. Styrsky JD, Eubanks MD. Ecological consequences of interactions between ants and honeydew producing insects. Proc R Soc Biol Sci Ser (2007) 274:151–64. doi: 10.1098/rspb.2006.3701
43. Porter SD, Tschinkel WR. Fire ant thermal preferences: behavioral control of growth and metabolism. Behav Ecol Sociobiol (1993) 32(5):321–9. doi: 10.1007/BF00183787
44. Janion-Scheepers C, Phillips L, Sgrò CM, Duffy GA, Hallas R, Chown SL. Basal resistance enhances warming tolerance of alien over indigenous species across latitude. Proc Natl Acad Sci (2018) 115(1):145–50. doi: 10.1073/pnas.1715598115
45. Chong KF, Lee CY. Influences of temperature, relative humidity, and light intensity on the foraging activity of field populations of the longlegged ant, Anoplolepis gracilipes (Hymenoptera: Formicidae). Sociobiology (2009) 54(2):531.
46. Blatrix R, Colin T, Wegnez P, Galkowski C, Geniez P. Introduced ants (Hymenoptera: Formicidae) of mainland France and Belgium, with a focus on greenhouses. Annales la Société Entomol France (N.S.) (2018) 54(4):293–308. doi: 10.1080/00379271.2018.1490927
47. McGeoch M. Invasive insects-risks and pathways project (2019). Available at: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Invasive+Insects-Risks+and+Pathways+project+&btnG.
Keywords: Anoplolepis gracilipes, biosecurity, early detection, introduction pathway, invasive species, South Africa
Citation: Ndaba A, Munyai TC, Mbanyana N, van Noort S and Janion-Scheepers C (2023) Now you see me, now you don’t: verifying the absence of alien invasive yellow crazy ant Anoplolepis gracilipes in South Africa. Front. Insect Sci. 3:1176810. doi: 10.3389/finsc.2023.1176810
Received: 28 February 2023; Accepted: 22 May 2023;
Published: 07 June 2023.
Edited by:
Marion Javal, Université de Montpellier, FranceReviewed by:
Jerome Gippet, Université de Lausanne, SwitzerlandEmma J. Hudgins, Carleton University, Canada
Copyright © 2023 Ndaba, Munyai, Mbanyana, van Noort and Janion-Scheepers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abusisiwe Ndaba, bmRiYWJ1MDAxQG15dWN0LmFjLnph
 Abusisiwe Ndaba
Abusisiwe Ndaba Thinandavha Caswell Munyai
Thinandavha Caswell Munyai Nokuthula Mbanyana
Nokuthula Mbanyana Simon van Noort
Simon van Noort Charlene Janion-Scheepers
Charlene Janion-Scheepers