- 1College of Animal Science, Guizhou University, Guiyang, China
- 2Key Laboratory of Animal Genetics, Breeding & Reproduction in the Plateau Mountainous Region, Ministry of Education, Guizhou University, Guiyang, Guizhou, China
The aim of this study was to investigate the effect of distillery wastewater (DWW) on the nutrient composition, fermentation quality and microbial community of Sorghum propinquum silage during the micro-permeation of air. S. propinquum without (CK) or with L. buchneri, (LAB), distillery wastewater yellow serofluid (Y) and distillery spent wash (S) was ensiled for 60 days, and then subjected to a micro-permeation stability of air test for 6 days. After 60 days of storage, treatments with DWW and LAB decreased the loss of DM, inhibited the degradation of protein and reduced the production of ammonia nitrogen in silage relative to the control. In particular, S. propinquum silage treated with yellow serofluid sustained higher levels. Moreover, the addition of DWW resulted in higher levels of acetic and propionic acid than the other treatments. During the micro-permeation of air, the addition of DWW was effective in inhibiting the reduction of lactic acid content, and unique genera Roseburia and Faecalibacterium, which are beneficial for livestock production, discovered in DWW-treated S. propinquum silage. In conclusion, the addition of DWW was efficacious in improving the nutritional composition and microbial community of S. propinquum silage during the micro-permeation of air.
1 Introduction
Distillery industries are the second-largest wastewater generating agriculture-processing industries just next to paper pulp, with approximately 12–15 liters of wastewater generated per liter of ethanol production (Wagha and Nemadeb, 2018). DWW (mainly including yellow serofluid and distillery spent wash) is generated from ethanol production (Oosterkamp et al., 2019). The generation of DWW occurs in various stages of the white liquor production process (such as washing, fermentation and distillation) DWW is acidic, with a dark brown color and extremely high chemical oxygen demand, biological oxygen demand, heavy metals, endocrine-disrupting chemicals, as well as macroscopic and microscopic minerals (Chandra et al., 2018). DWW has many refractory organic and inorganic pollutants, which can cause serious environmental pollution and threaten the health of humans and animals (Tamanna and Mahmood, 2015). For example, the discharge of untreated/partially treated DWW can cause soil infertility and uncontrollable algae growth (Bharagava and Chandra, 2010).
In recent years, bioconversion of agro-industrial wastes into value-added products has become very important (Dashtban et al., 2009). Efforts are being made all over the world to convert it from “waste” to “new resource”. To date, there are many physicochemical and biological approaches to treat DWW. They focus on the production of various organic molecules from DWW, including organic acids, alcohols, polymers, biogas, proteins and fatty acids (Reis et al., 2019). This production approach is limited to pretreating DWW in anticipation of producing value-added products. However, this approach increases the cost of the pretreatment process and the conditions for generating the products appear to be harsh relative to the direct utilization of DWW. In the context of large-scale generation of DWW, this processing approach appears to be a drop in the bucket. Therefore, it is in line with the principle of cleaner production to explore the direct utilization of DWW to increase the utilization rate of DWW and reduce the environmental risk of DWW emissions. Notably, the maximum yield of Lactobacillus sake was 4.6 g/L on a basal medium with 75% shochu wastewater added (Yuliani et al., 2011). This implies that the addition of DWW contributes to the proliferation of lactic acid bacteria, which provides a prior condition for the direct utilization of DWW.
Silage refers to a storage technology or method, mainly lactic acid bacteria fermentation to convert water-soluble carbohydrates into organic acids (mainly lactic acid), which is used to lower the pH of silage to inhibit microbial activity and nutrient depletion. Since lactic acid bacteria in natural plants are constrained by environmental factors and thus low in content (Liao et al., 2023), it is difficult for lactic acid bacteria to rapidly form dominant flora during the fermentation process, and they are also unable to lower the pH in a short period, resulting in poor fermentation quality. Moreover, when there is a slight infiltration of air into the silage, the invasion of oxygen will activate the inhibited aerobic microorganisms, damaging silage quality. Therefore, how to raise the number of lactic acid bacteria during fermentation has attracted attention. DWW has a promoting effect on the proliferation of lactic acid bacteria, which implies that the addition of DWW to silage might provide favorable conditions for the propagation of lactic acid bacteria. However, there is little information on the effect of direct DWW addition on silage. In addition, the effects of DWW on silage microorganisms are unknown. Revealing these effects has important implications for the direct utilization of DWW with silage to reduce DWW emissions.
In this study, we investigated the potential value of distillery wastewater as a silage additive for the clean production of sorghum silage. Therefore, this study is useful for bioremediation of distillery residues as mentioned above and for cleaner production of livestock silage.
2 Materials and methods
2.1 Silage preparation
The DWW was taken from the Jinsha Huisha Distillery in Guizhou Province, China. The two types of DWW were yellow serofluid and distillery spent wash. Yellow serofluid is a yellow-brown pouring slurry formed at the bottom of the cellar during the fermentation process of Sorghum bicolor raw materials, and the distillery spent wash is a mixed slurry left at the bottom of the pot during the distillation procedure. The pH values of the yellow serofluid and spent wash were 4.45 and 3.94, respectively, both of which contained a large amount of Lactobacillus and Weissella that were beneficial for silage fermentation. Sorghum propinquum was taken from Tongzi County, Guizhou Province, southwestern China (28° 7’ N, 106° 34’ E), with an altitude of 1,219.15 meters on July 21, 2022. The growth period was the heading period. Fresh crops were chopped to approximately 2–3 cm with a laboratory-type chopper, ensiled and vacuumized in duplicate polyethylene bags (30 × 40 cm). Fill each polyethylene bag with approximately 0.6 kilograms of fresh chopped forage and store it at ambient temperature (<25°C). The silages were used to test aerobic stability after 60 days of ensiling. Fresh forages and silages during the micro-permeation of air were sampled for assay of chemical composition, fermentation profile, and microbial community (three repetitions per treatment). The following treatments were applied: (1) no additives (CK); (2) commercial LAB inoculant (LAB, L. buchneri, 106 cfu/g FM, supplied by Gaofuji Biotechnology Co., Ltd., Chengdu, China); (3) yellow serofluid additive (Y, treated at a rate of 4%); and (4) distillery spent wash additive (S, treated at a rate of 4%). All treatment groups of grass were completely mixed with additives.
2.2 Chemical analysis
Chemical analysis was determined in triplicate. The dry matter (DM) content of the fresh forages and silages after enzyme deactivation was measured by oven drying at 65°C for 48 hours. Crude protein (CP) was determined by the Dumas Nitrogen analyzer (model: N Pro) (Wiles et al., 1998). Determination of neutral detergent fiber (NDF) and acid detergent fiber (ADF) by a modified procedure using sulfite and amylase (Van Soest et al., 1991). The content of water-soluble carbohydrate (WSC) was measured by the anthrone reaction-rate method (Murphy, 1958). NH3-N was measured in the silages by the extraction of 20 g of frozen samples with 180 mL distilled water for 24 hours in cold storage (Weatherburn, 1967). The silage juice for the determination of pH value by desktop pH meter. Determination of lactic acid (LA), acetic acid (AA), propionic acid (PA) and butyric acid (BA) contents by Ultra-High Performance Liquid Chromatography (model: Vanquish Core) (Muck and Dickerson, 1988).
The ensiled forages with four treatments were mingled and put (unpackaged but compacted) in unpolluted buckets, made the air slightly infiltrate, and a thermocouple wire was slotted in the center of the silage stack. The thermocouple wires were bonded to a data logger, which logged the temperature every 10 min and then averaged the temperature every half hour. Corruption begins when the temperature in silage masses rises to 2°C higher than the ambient temperature.
2.3 Statistical analysis
Factorial analysis of variance was used to evaluate the effects of the micro-permeation time (D), treatment (T), and their interactions (D × T) on the silage parameters in the General Line Model of SPSS (SPSS 27.0 program SPSS Inc.). Differences were only considered significant when the probability level was below than 0.05 (P < 0.05).
3 Results and discussion
3.1 Chemical composition and bacterial genera of S. propinquum before ensilage
Table 1 shows the chemical composition and dominant bacterial genera of fresh S. propinquum and DWW prior to ensilage. The DM of S. propinquum was 31.46%, and the contents of NDF and ADF were 62.35% DM and 29.57% DM, separately. The content of CP in S. propinquum was 9.75% DM, and the content of WSC was 9.56% DM. Mostly, the WSC content of the fresh forage serves as a considerable supplement for anaerobic fermentation, and that a forage with over 5% DM WSC content tends to excellent fermentation performance (Cai et al., 1998). Therefore, the content of WSC in fresh S. propinquum was adequate for silage fermentation.
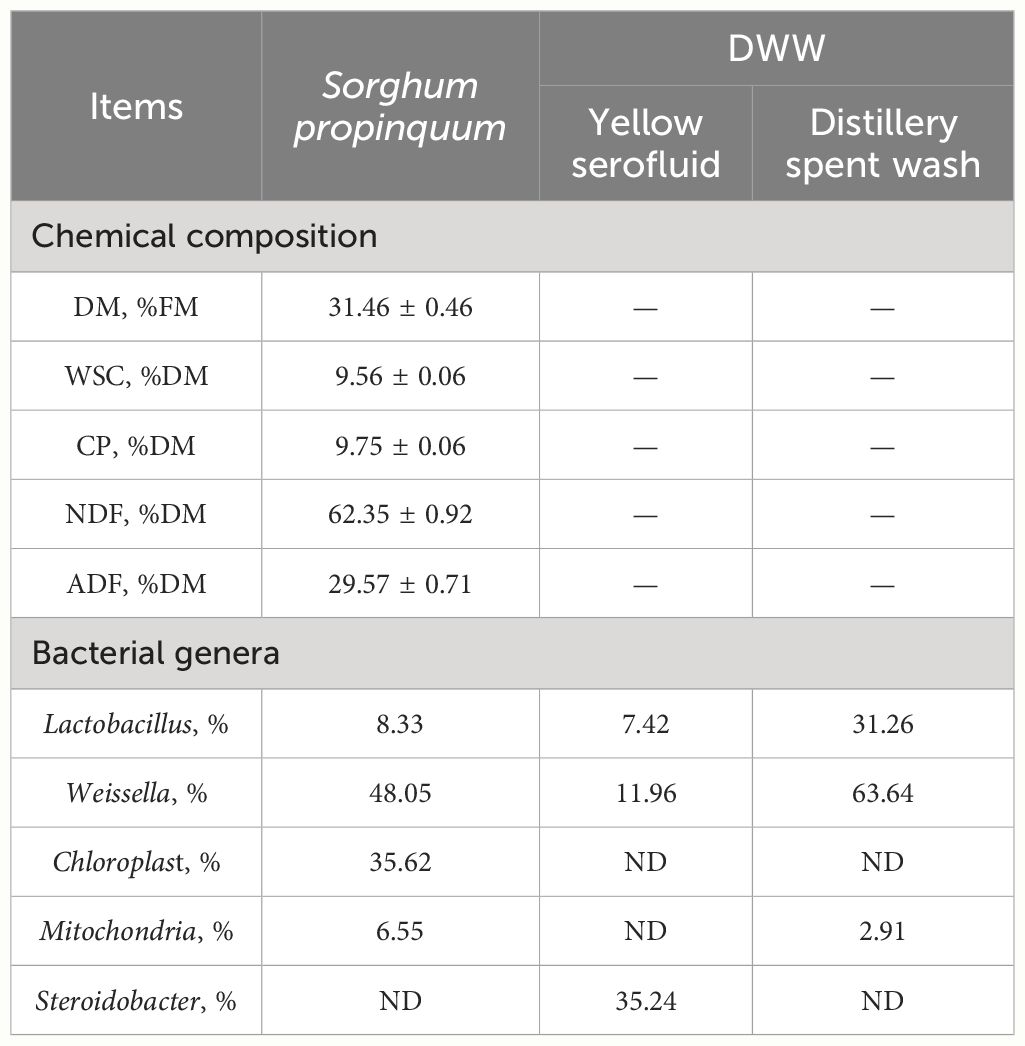
Table 1 Chemical composition and bacterial genera of fresh Sorghum propinquum and DWW (mean ± standard error).
The contents of Lactobacillus in S. propinquum, yellow serofluid and distillery spent wash were 8.33, 7.42 and 31.26%, and the contents of Weissella were 48.05, 11.96 and 63.64% respectively. The Chloroplast and Mitochondria contents in S. propinquum were 35.62% and 6.55% respectively. Moreover, yellow serofluid contained a large source of Steroidobacter (35.24%).
3.2 Chemical composition of S. propinquum after 60 days of ensiling and during the micro-permeation of air
The LAB- and DWW-treated S. propinquum silage had a higher DM content than CK after 60 days of ensiling (Table 2). The content of CP differed significantly among different treatments (P < 0.01). Y- and S-treated S. propinquum silage had increased CP content by 1.2 and 0.11%, respectively, but no additives and LAB inoculated S. propinquum silage had reduced it by 1.12 and 0.43%, respectively, after 60 days of anaerobic fermentation. This may be connected with the possession of considerable Lactobacillus and Weissella in the DWW. In this study, the S. propinquum silage with different treatments expressed no significant difference in WSC content. Moreover, fermented S. propinquum had further contents of NDF and ADF than fresh S. propinquum after 60 days of anaerobic storage, which perhaps due to the depletion of accessible nutrients generating a multiplication in NDF and ADF contents during the anaerobic fermentation (Li et al., 2022). Thus, we considered that the phenomenon discovered in this study probably owing to the treated LAB and DWW impacting on anaerobic fermentation, which reduces the loss of dry matter content in S. propinquum silage during the anaerobic fermentation.
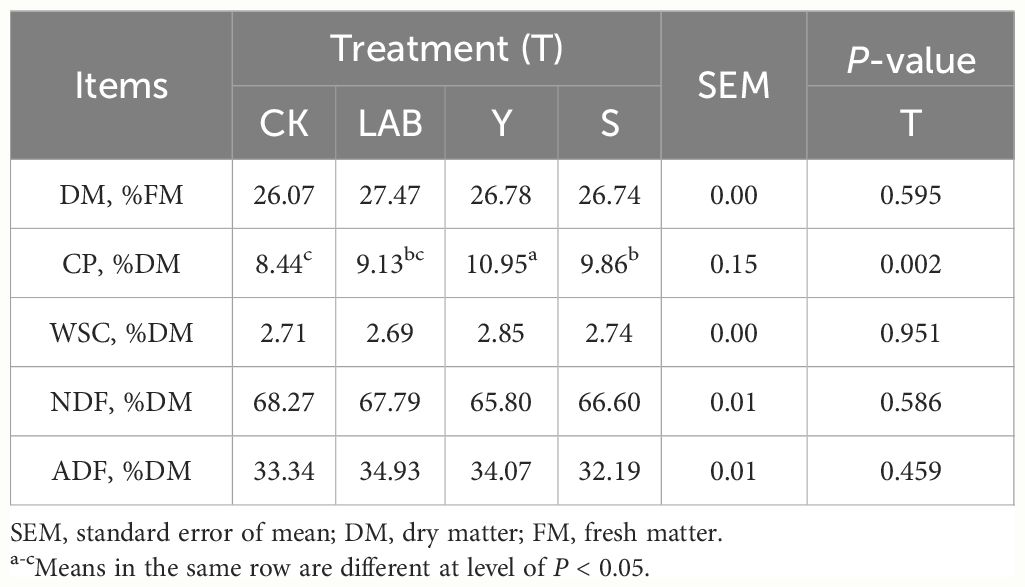
Table 2 Chemical composition of Sorghum propinquum silage treated without (CK) or with lactic acid bacteria (LAB), yellow serofluid (Y) or distillery spent wash (S).
During the micro-permeation of air, the time of all silages with different treatments at 2°C above the ambient temperature was equivalent (>192 h). LAB and DWW played important roles in improving the fermentation characteristics of S. propinquum silage after 60 days of ensiling and during the micro-permeation of air, as shown in Table 3. All silages with different treatments reached a relatively low pH value (< 4.70) after 60 days of fermentation. In this study, the various S. propinquum silage with different treatments expressed no significant difference in the pH value. Studies have verified that inoculation with Lactobacillus buchneri or Bacillus subtilis either improved or didn’t impact silage pH (Kung et al., 2003; Lara et al., 2016). The pH values of the various S. propinquum silage with different inoculants gradually increased, but they were all significantly below 5.0 during the micro-permeation of air. This is because the silage is opened in the air, the invasion of oxygen will activate the inhibited aerobic microorganisms, increasing the pH value. These aerobic microorganisms will affect the aerobic stability of silage (Tennant et al, 2017). The DWW and LAB additives influenced the DM parameter (P > 0.05), and the time of air micro-permeation also affected the parameter (P < 0.01). LAB- and DWW-treated S. propinquum silage had higher DM content than CK after 60 days of fermentation. LAB- and Y-treated S. propinquum silage and untreated S. propinquum silage on the sixth day of micro-permeation of air had significantly higher DM contents than those at silage opening after 60 days of ensiling. However, the content of DM in S-treated S. propinquum silage was reduced during the micro-permeation of air. The micro-exposure of silage to air can begin the aerobic spoilage process, resulting in pH increases and DM losses (Woolford, 1990). However, in this experiment, the various S. propinquum silage did not lead to DM losses. Studies have indicated that the DM content of S. propinquum silage increased during the aerobic exposure stage (de Oliveira et al., 2009), which is consistent with our research. As a byproduct of protein decomposition, the content of ammonia nitrogen is influenced by the activity of Clostridium and plant protease. In this study, the CK treatment was significantly higher than the LAB- and DWW-treated S. propinquum silage after 60 days of anaerobic fermentation. This change is due to the inhibition of protein-degrading microorganisms by the addition of LAB and DWW (Bai et al., 2020). However, with increasing micro-permeation time, the LAB- and DWW-treated treatments showed an increasing trend. This may be because when silage is exposed to air, aerobic microorganisms begin to multiply, and amino acids are decomposed into ammonia, hydrogen sulfide, and amines, increasing the degradation rate and leading to an increase in ammonium nitrogen content (Hashemzadeh-Cigari et al., 2014). Surprisingly, the ammonium nitrogen treated with CK showed a decreasing trend. As is well known, ammonium nitrogen is easily volatilized when exposed to air, so we believe that the factor reducing the CK treatment comes from the volatilization of ammonium nitrogen. Although aerobic microorganisms produce ammonia nitrogen during the micro-permeation of air, this production is minimal.
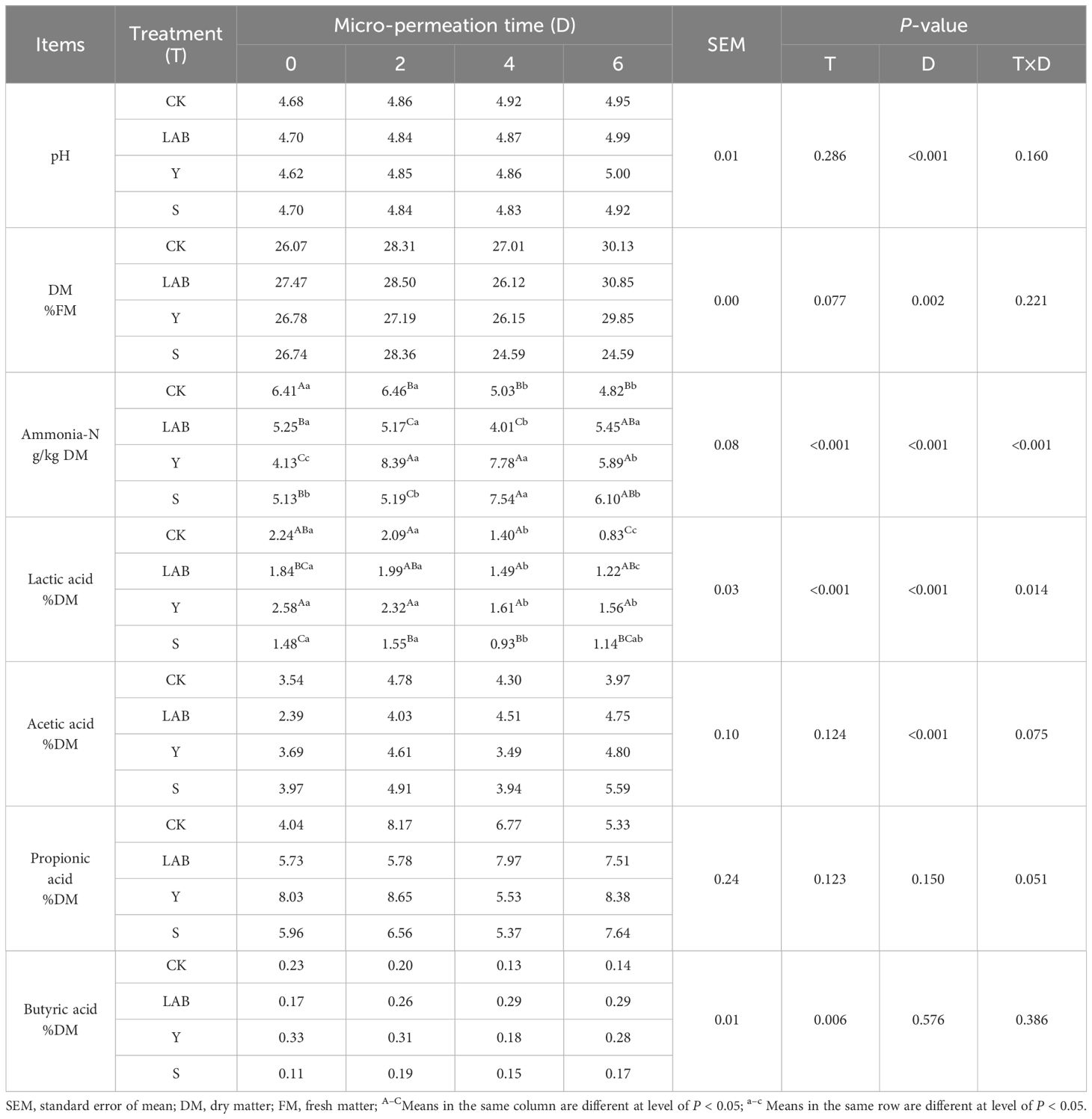
Table 3 Fermentation profiles of Sorghum propinquum silage treated without (CK) or with lactic acid bacteria (LAB), yellow serofluid (Y), or distillery spent wash (S).
The Y-treated S. propinquum silage had a higher lactic acid content than CK, which increased by 0.34% after 60 days of anaerobic fermentation, improving the fermentation profiles of silage. Y-, S- and LAB-treated Sorghum propinquum silage had higher lactic acid content than CK after 6 d of air micro-permeation, increasing by 0.73, 0.31 and 0.39%, respectively, which improves the air micro-permeation of silage. During the micro-permeation of air, all treatments showed a decrease in lactic acid, while acetic acid and propionic acid showed an increase. The reason may be that lactic acid is easily utilized by yeast during the micro-permeation of air, leading to secondary fermentation of silage. In addition, the heterotypic fermentation lactic acid bacteria attached to the silages themselves decompose lactic acid to produce acetic acid, which to some extent maintains a low pH level in S. propinquum silage (Romero et al., 2017). As acids with antibacterial properties, the increase in acetic acid and propionic acid content indicates an increase in aerobic stability of silage (Driehuis et al., 1999). In this experiment, after 6 days of air micro-permeation, the addition of DWW seemed to have the same effect as LAB, with similar levels of acetic acid and propionic acid (P > 0.05). This indicates that DWW seems to be a potential silage additive that is equivalent to L. buchneri in improving the aerobic stability of silage. The presence of butyric acid during the micro-permeation of air is common, due to the reproduction of butyric acid-producing bacteria caused by the micro-permeation of air. In summary, we believe that DWW has the same additive effect as L. buchneri as a silage additive. However, the underlying mechanism still needs further exploration.
3.3 Bacterial community after 60 days of ensiling and during the micro-permeation of air
To further explore the effect of DWW as an additive on microorganisms in anaerobic fermentation of S. propinquum silage, we analyzed the alpha diversity of S. propinquum silage during the micro-permeation of air in Table 4. We found that there was no significant interaction between days and treatment in the different treatments of DWW additives (S and Y), LAB, and CK. A significant effect was found only in the Pielou E index (P < 0.05). The Pielou E index represents the difference in quantity between different species in S. propinquum silage. As micro-permeation of air progressed, the Pielou E index values of LAB- and DWW-treated S. propinquum silage were higher than those of the CK treatment. This may be due to the addition of DWW and LAB causing an increase in some species, leading to an increase in diversity between species. In previous studies, it was also found that the addition of LAB resulted in an increase in species during the aerobic exposure (Bai et al., 2020). In addition, DWW does contain some acid-resistant microorganisms that are inhibited during the anaerobic fermentation, but this state is relieved when exposed to oxygen (micro-permeation of air) (Tripathi et al., 2022). At the beginning of air micro-permeation, the Pielou-e index values of the S and Y treatments were lower than that of LAB, confirming the abovementioned mechanism.
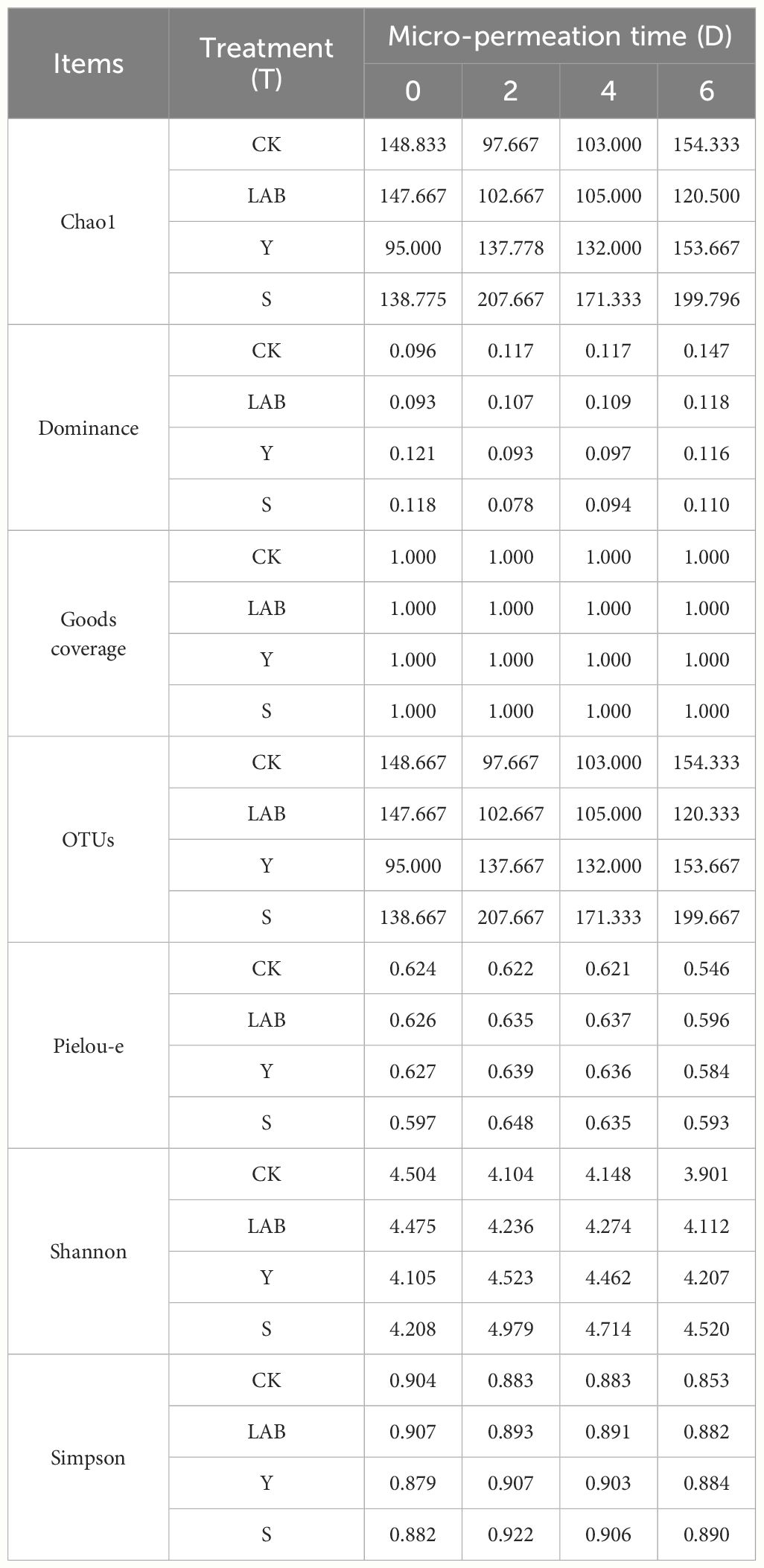
Table 4 Bacterial alpha diversity of Sorghum propinquum silage treated without (CK) or with lactic acid bacteria (LAB), yellow serofluid (Y), or distillery spent wash (S).
The diversity component analysis we used was principal coordinate analysis (PCoA), which extracted the most significant elements and constructions from multidimensional data through a battery of feature values and feature vector sorting. We used the Bray−Curtis distance for PCoA, and the principal coordinate combination with the highest contribution rate was selected for graphical revealing, as shown in Figure 1. If the sample distance was relatively close, it indicated that the species composition structure was similar. Hence, samples with extraordinary similarity in community structure tended to cluster together, while samples with significant community differences were farther apart. In this experiment, the distance between samples of the same treatment was very close, illustrating that the bacterial community composition structure was very similar and the excellent repeatability of this experiment. For different treated samples, the distance between them was relatively large, and the community composition varied greatly, which was due to the different additives used in our different treatments. From the figure, we can see that there are some distinctions in the bacterial community composition of different treatments, and there are also some distinctions in the bacterial community composition of each treatment when it is just micro-exposed to the air compared with the later period of air micro-permeation. This is because when silage is exposed to air, aerobic microorganisms begin to multiply (Hashemzadeh-Cigari et al., 2014).
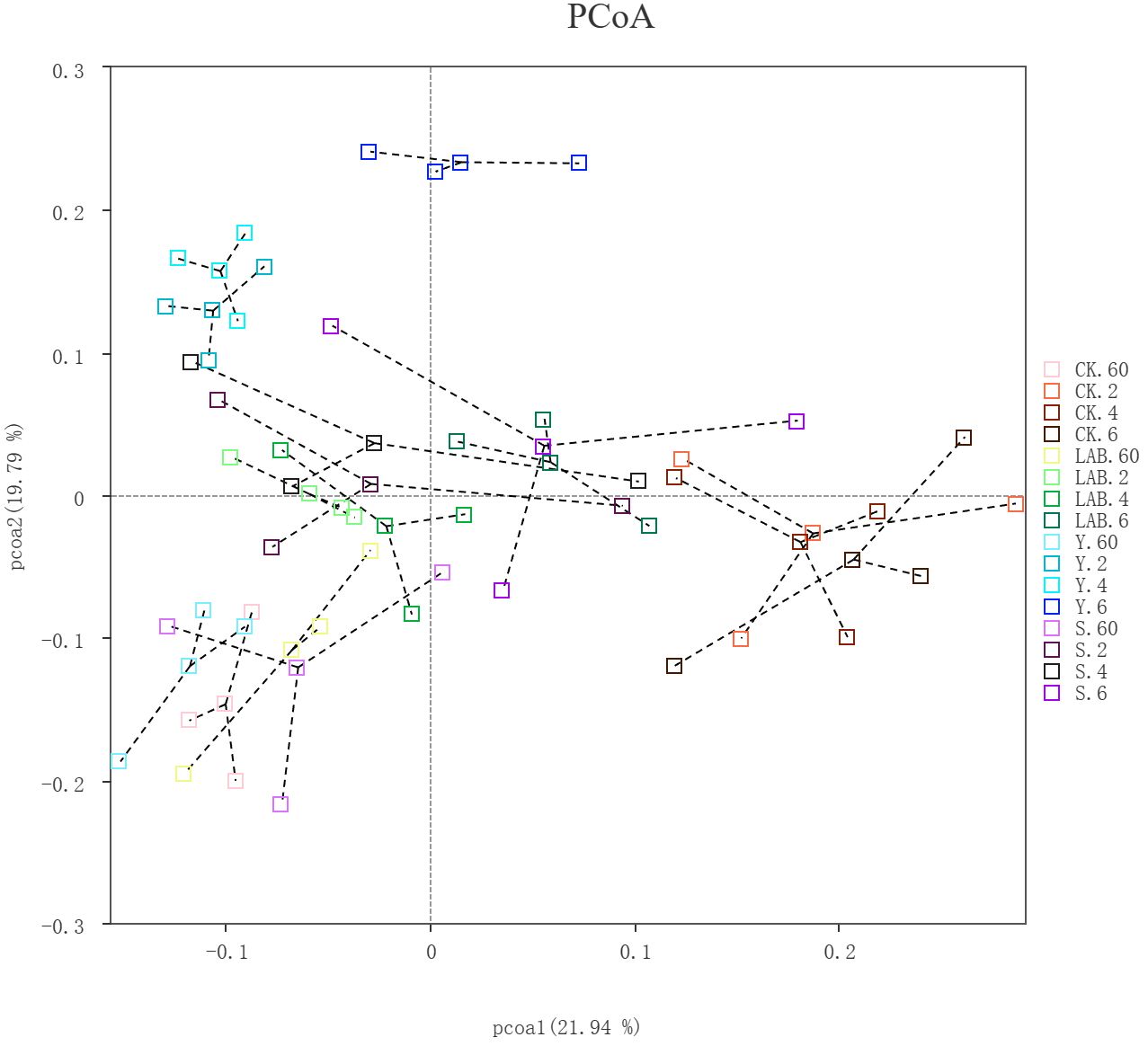
Figure 1 PCoA plot of the bacterial community structure of Sorghum propinquum silage treated without (CK) or with lactic acid bacteria (LAB), yellow serofluid (Y), or distillery spent wash (S).
Network cooccurrence at the genus level of S. propinquum silage treated with different additives is shown in Figure 2. We calculated the Spearman correlation index of all samples to obtain the species correlation coefficient matrix and set the filtering conditions as follows: (1) connections with correlation coefficients <0.6 were removed, (2) node self-connections were filtered out, and (3) connections with node abundance less than 0.005% were removed to obtain the network graph. The dominant genera of bacteria in all treatment groups were Lactobacillus and Weissella, which had a negative correlation. However, the genus-level structure of S. propinquum silage varied among different additive treatments. We found that Y-, S- and LAB-treated S. propinquum silage increased the negative correlation coefficient between Lactobacillus and other adverse bacteria and enhanced the negative associations within bacteria but reduced the negative correlation coefficient between Lactobacillus and Weissella.
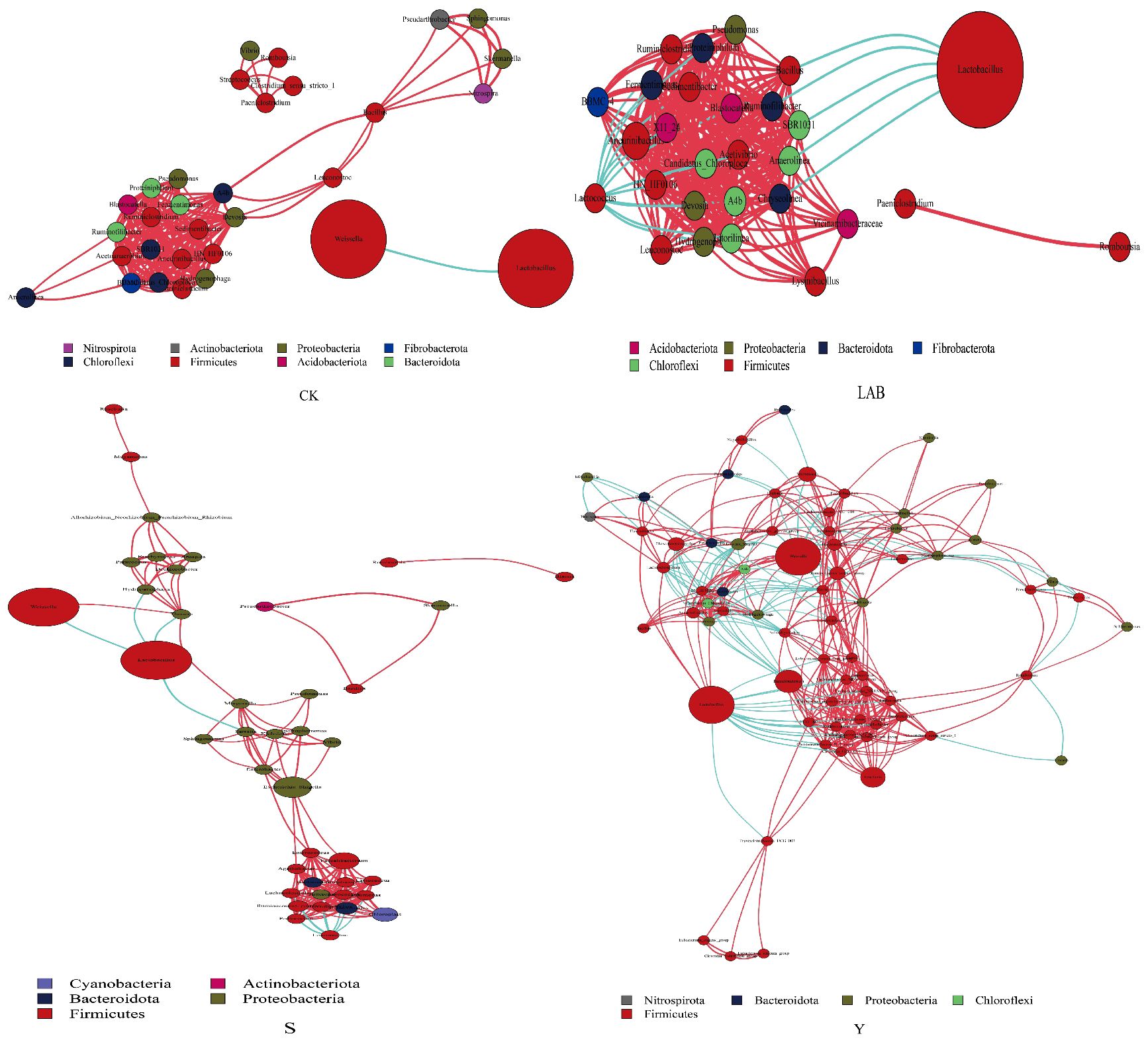
Figure 2 Network co-occurrence among genus-level of Sorghum propinquum silage treated without (CK) or with lactic acid bacteria (LAB), yellow serofluid (Y), or distillery spent wash (S). Different nodes represent different genus-level, node size represents the average relative abundance of genus, and nodes in the same phylum-level have the same color. The thickness of the connections between nodes is positively correlated with the absolute value of the correlation coefficient between species interactions, with red indicating a positive correlation and blue indicating a negative correlation.
This study ascertained phylum-, genus-, and species-level alterations in the microbial composition of S. propinquum silage and distinctions in bacterial taxa (Figure 3). The phylum-level bacterial composition of S. propinquum silage is displayed in Figure 3A. Compared with fresh forages, an increase in Firmicutes accompanied by a decrease in Proteobacteria became the only dominant phylum. During the fermentation period of silage, Proteobacteria gradually replaced Firmicutes. This is because Proteobacteria is the maximum phylum-level bacteria, commonly found in various raw silage materials (Du et al., 2021), and Firmicutes contains various lactic acid bacteria that are the principal participants in silage storage (Li et al., 2021). With increasing air micro-permeation time, Firmicutes decreased and Proteobacteria increased in the CK treatment group, but Firmicutes did not decrease in S. propinquum silage treated with LAB and DWW, which is owing to the inferior inhibition of aerobic micro-organisms in the CK treatments, resulting in a speedy growth in other phyla, which is consistent with the conclusion of Liu et al. (2022). The primary bacteria existed in the lactic acid fermentation of silage pertain to the genera Weissella, Lactobacillus, Leuconostoc, and Pedicoccus (Ni et al., 2018). The genus-level changes are shown in Figure 3B. There are high abundances of Weissella, Chloroplast and some Lactobacillus in fresh S. propinquum. After 60 days of fermentation of silage, Lactobacillus in S. propinquum silage becomes the dominant genus and contains many Weissella. There was no significant difference between the different treatments. During the micro-permeation of air, Lactobacillus and Weissella had almost no change and remained at a relatively high level, which also showed that the S. propinquum silage had good aerobic stability and did not start to decay after six days of air micro-permeation. In this experiment, we found that there are unique genera Roseburia and Faecalibacterium in the S. propinquum silage treated with DWW. The S. propinquum silage treated with Y contained a small amount of Roseburia compared with other treatments, and the S. propinquum silage treated with Y and S contained a small amount of Faecalibacterium compared with CK and LAB treatments. Roseburia and Faecalibacterium are bacteria that are beneficial for animal health. Dietary fibers were metabolized by Roseburia intestinalis and Faecalibacterium prausnitzii as primary short-chain fatty acid producers, supplying energy sources for enterocytes and attaining anti-inflammatory functions in the gut (Hiippala et al., 2018). Zhang et al. (2019) also confirmed stress-related microbial species and further tested the beneficial function of Roseburia, a butyrate-producing genus that was dramatically reduced in rats, on stress-induced visceral hypersensitivity. A study showed that colonization with Roseburia intestinalis reduces the levels of inflammation markers and atherosclerosis in a diet-dependent pattern and verified that the final product (butyrate) of bacterial metabolism guides these functions (Kasahara et al., 2018). There are findings that highlight that R. intestinalis is a main degrader of some dietary fiber and that this metabolic capacity could be utilized to selectively accelerate crucial members of the healthy microbiota using β-mannan-based therapeutic interventions (La Rosa et al., 2019). According to reports, in murine models, F. prausnitzii cells or their cell-free epipelagic can alleviate the severity of acute (Sokol et al., 2008), slow acting (Martín et al., 2014) and low-grade (Martín et al., 2015) chemical-induced inflammation. Therefore, when livestock are fed silage containing Roseburia and Faecalibacterium, it will have a good effect on the prevention of intestinal inflammation, which is no different from using DWW as a silage additive.
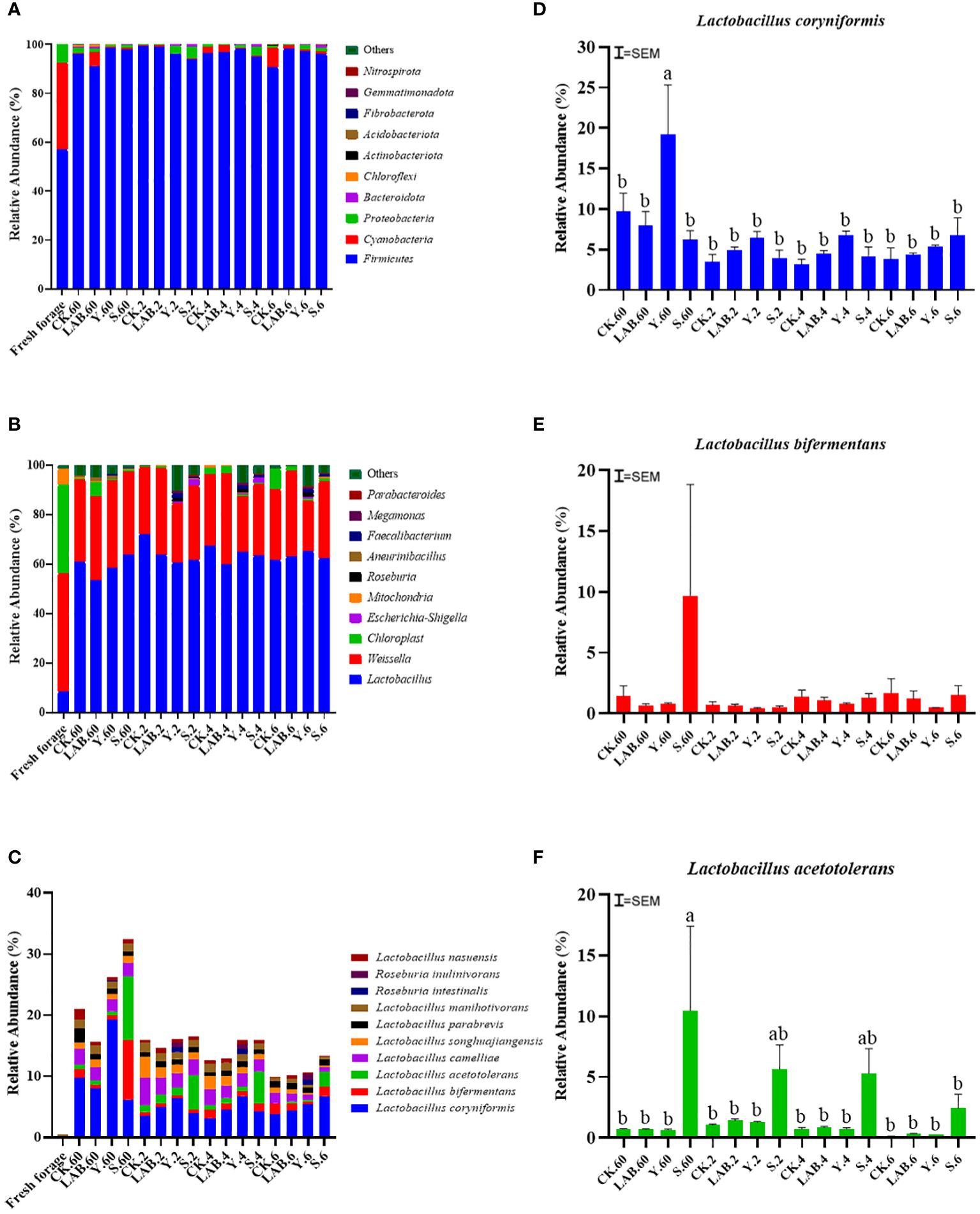
Figure 3 The relative abundance of phylum (A), genus (B) and species-level (C) including Lactobacillus coryniformis (D), Lactobacillus bifermentans (E) and Lactobacillus acetotolerans (F) changes of Sorghum propinquum silage treated without (CK) or with lactic acid bacteria (LAB), yellow serofluid (Y), or distillery spent wash (S) .a–b Means in the different columns are different at level of P < 0.05.
After 60 days of silage, the abundances of Lactobacillus coryniformis, Lactobacillus bifermentans, Lactobacillus acetotolerans and Lactobacillus camelliae in S. propinquum significantly increased, as shown in Figure 3C. Y-treated S. propinquum silage had higher Lactobacillus coryniformis content than the CK treatment, which increased by 9.51% (Figure 3D), and S-treated S. propinquum silage had higher Lactobacillus bifermentans and Lactobacillus acetotolerans content than the CK treatment, which increased by 8.27 and 9.75% (Figures 3E, F), respectively, after 60 days of anaerobic fermentation. During the micro-permeation of air, the content of Lactobacillus coryniformis in all treatments significantly decreased, but Y- and S-treated S. propinquum silage had higher Lactobacillus coryniformis content than the CK treatment from start to finish (increased by 1.57 and 2.93%). The contents of Lactobacillus bifermentans and Lactobacillus acetotolerans in the S treatment significantly decreased, but S-treated S. propinquum silage consistently had higher Lactobacillus bifermentans and Lactobacillus acetotolerans contents (increased by 8.26 and 9.75%) than the CK treatment silage. Therefore, we believe that DWW as a silage additive enhances the aerobic stability of S. propinquum.
A Spearman correlation between fermentation parameters and kinetics of the top 10 genera and species during the ensiling of S. propinquum was generated and illustrated in Figure 4. Lactobacillus played an important role in improving the lactic acid content and diminishing the pH value in subsequent anaerobic storage (Cai et al., 1998). Hence, our study demonstrated that lactic acid was positively correlated with certain Lactobacillus genera (L. coryniformis, L. camelliae, and L. songhuajiangensis; P < 0.05). Y-treated S. propinquum silage had a higher Lactobacillus coryniformis content than the CK treatment, which led to a higher capacity for acid production and anaerobic storage. We also found that ammonia-N was positively connected with the Roseburia and Faecalibacterium genera, which explains why the increase in ammonia-N in DWW-treated S. propinquum silage was higher than that in the CK treatment during the micro-permeation of air.
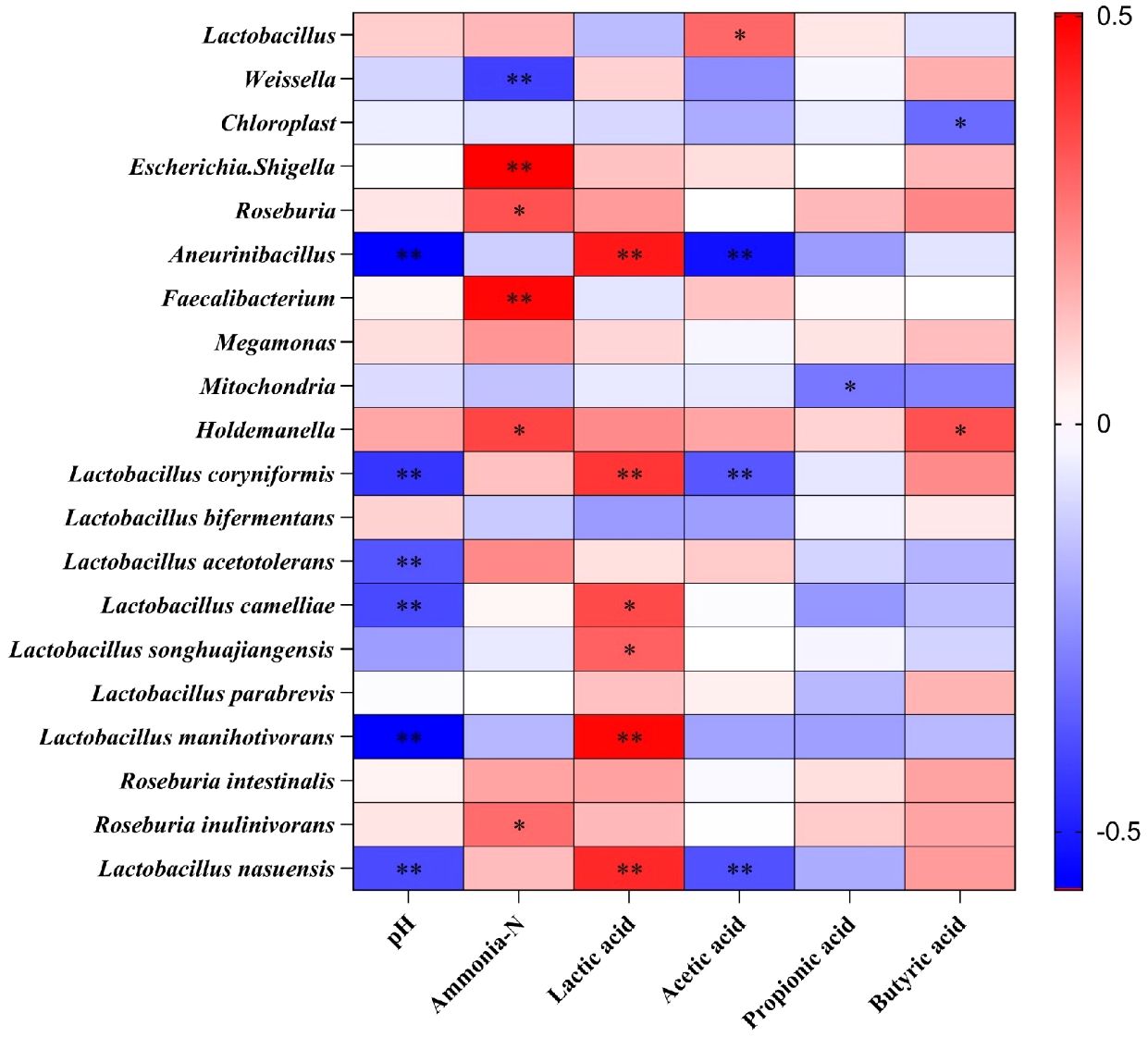
Figure 4 Spearman correlations between silage fermentation parameters and relative abundance of top 10 genus and species. *P < 0.05, **P < 0.01.
4 Conclusion
The microbial community of S. propinquum silage was altered by the addition of DWW, which was comparable to the L. buchneri inoculant in terms of improving fermentation quality and aerobic stability. In particular, S. propinquum silage treated with DWW maintained higher lactic acid levels during the micro-permeation of air. Hence, it is feasible to use DWW as a silage additive, and our research builds a fundamental basis for the treatment and utilization of DWW. On this basis, we should study the impact of adding a large amount of DWW on the quality of wrapped silage to solve industrial pollution in distilleries. However, the effect of ammoniacal nitrogen content on feed should be considered when pursuing DWW as a silage additive.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
XH: Writing – review & editing, Writing – original draft, Data curation. GL: Writing – review & editing, Formal analysis, Data curation. LL: Writing – review & editing, Software. CL: Writing – review & editing, Software. XT: Writing – review & editing, Investigation. CheC: Writing – review & editing, Software, Formal Analysis. MZ: Writing – review & editing, Investigation. PL: Writing – review & editing, Supervision, Resources, Project administration, Funding acquisition, Data curation. ChaC: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Funding acquisition, Data curation.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was supported by the National Key Research and Development Subject (2021YFD1300302).
Acknowledgments
We also thank Dr. Yixiao Xie, Dr. Hong Sun, Dr. Chunmei Wang, Dr. Yulong Zheng, Dr. Qiming Cheng and Master Qiang Yu from Guizhou university for their helps during experimental design and sample preparation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bai J., Xu D. M., Xie D. M., Wang M. S., Li Z. J., Guo X. S. (2020). Effects of antibacterial peptide-producing Bacillus subtilis and Lactobacillus buchneri on fermentation, aerobic stability, and microbial community of alfalfa silage. Bioresour. Technol. 315, 123881. doi: 10.1016/j.biortech.2020.123881
Bharagava R. N., Chandra R. (2010). Biodegradation of the major color containing compounds in distillery wastewater by an aerobic bacterial culture and characterization of their metabolites. Biodegradation. 21, 703–711. doi: 10.1007/s10532-010-9336-1
Cai Y., Benno Y., Ogawa M., Ohmomo S., Kumai S., Nakase T. (1998). Influence of Lactobacillus spp. from an inoculant and of Weissella and Leuconostoc spp. from forage crops on silages fermentation. Appl. Environ. Microbiol. 64, 2982–2987. doi: 10.1128/AEM.64.8.2982-2987.1998
Chandra R., Kumar V., Tripathi S., Sharma P. (2018). Heavy metal phytoextraction potential of native weeds and grasses from endocrine-disrupting chemicals rich complex distillery sludge and their histological observations during in-situ phytoremediation. Ecol. Eng. 111, 143–156. doi: 10.1016/j.ecoleng.2017.12.007
Dashtban M., Schraft H., Qin W. (2009). Fungal bioconversion of lignocellulosic residues; opportunities & Perspectives. Int. J. @ Biol. Sci. 5, 578–595. doi: 10.7150/ijbs.5.578
de Oliveira S. G., Berchielli T. T., Reis R. A., Vechetini M. E., Pedreira M. D. (2009). Fermentative characteristics and aerobic stability of sorghum silages containing different tannin levels. Anim. Feed. Sci. Tech. 154, 1–8. doi: 10.1016/j.anifeedsci.2009.07.003
Driehuis F., Elferink Oude S. J. W. H., Spoelstra S. F. (1999). Anaerobic lactic acid degradation during ensilages of whole crop maize inoculated with Lactobacillus buchneri inhibits yeast growth and improves aerobic stability. J. App. Microbiol. 87, 583–594. doi: 10.1046/j.1365-2672.1999.00856.x
Du Z., Sun L., Chen C., Lin J., Yang F., Cai Y. (2021). Exploring microbial community structure and metabolic gene clusters during silage fermentation of paper mulberry, a high-protein woody plant. Anim. Feed. Sci. Tech. 275, 114766. doi: 10.1016/j.anifeedsci.2020.114766
Hashemzadeh-Cigari F., Khorvash M., Ghorbani G. R., Ghasemi E., Taghizadeh A., Kargar S., et al. (2014). Interactive effects of molasses by homofermentative and heterofermentative inoculants on fermentation quality, nitrogen fractionation, nutritive value and aerobic stability of wilted alfalfa (Medicago sativa L) silages. J. Anim. Physiol. An. N. 98, 290–299. doi: 10.1111/jpn.12079
Hiippala K., Jouhten H., Ronkainen A., Hartikainen A., Kainulainen V., Jalanka J., et al. (2018). The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients. 10, 988. doi: 10.3390/nu10080988
Kasahara K., Krautkramer K. A., Org E., Romano K. A., Kerby R. L., Vivas E. I., et al. (2018). Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat. Microbiol. 3, 1461–1471. doi: 10.1038/s41564-018-0272-x
Kung L., Taylor C. C., Lynch M. P., Neylon J. M. (2003). The effect of treating alfalfa with lactobacillus buchneri 40788 on silage fermentation, aerobic stability, and nutritive value for lactating dairy cows1. J. Dairy. Sci. 86, 336–343. doi: 10.3168/jds.S0022-0302(03)73611-X
Lara E. C., Basso F. C., de Assis F. B., Souza F. A., Berchielli T. T., Reis R. A. (2016). Changes in the nutritive value and aerobic stability of corn silages inoculated with Bacillus subtilis alone or combined with Lactobacillus plantarum. Anim. Prod. Sci. 56, 1867–1874. doi: 10.1071/AN14686
La Rosa S. L., Leth M. L., Michalak L., Hansen M. E., Pudlo N. A., Glowacki R., et al. (2019). The human gut Firmicute Roseburia intestinalis is a primary degrader of dietary β-mannans. Nat. Commun. 10, 905. doi: 10.1038/s41467-019-08812-y
Li P., Zhao W., Yan L., Chen L., Chen Y., Gou W., et al. (2022). Inclusion of abandoned rhubarb stalk enhanced anaerobic fermentation of alfalfa on the Qinghai Tibetan Plateau. Bioresour. Technol. 347, 126347. doi: 10.1016/j.biortech.2021.126347
Li M., Zi X., Zhou H., Lv R., Tang J., Cai Y. (2021). Effect of lactic acid bacteria, molasses, and their combination on the fermentation quality and bacterial community of cassava foliage silage. Anim. Sci. J. 92, 13635. doi: 10.1111/asj.13635
Liao C., Na B., Tang X., Zhao M., Zhang C., Chen S., et al. (2023). Contribution of the bacterial community of poorly fermented oat silage to biogas emissions on the Qinghai Tibetan plateau. Sci. Total Environ. 897, 165336. doi: 10.1016/j.scitotenv.2023.165336
Liu Y., Li Y., Lu Q., Sun L., Du S., Liu T., et al. (2022). Effects of lactic acid bacteria additives on the quality, volatile chemicals and microbial community of leymus chinensis silage during aerobic exposure. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.938153
Martín R., Chain F., Miquel S., Lu J., Gratadoux J., Sokol H., et al. (2014). The commensal bacterium faecalibacterium prausnitzii is protective in DNBS-induced chronic moderate and severe colitis models. Inflamm. Bowel. Dis. 20, 417–430. doi: 10.1097/01.MIB.0000440815.76627.64
Martín R., Miquel S., Chain F., Natividad L. M., Jury J., Lu J., et al. (2015). Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 15, 67. doi: 10.1186/s12866-015-0400-1
Muck R. E., Dickerson J. T. (1988). Storage temperature effects on proteolysis in alfalfa silages. Trans. Am. Soc Agric. Biol. Eng. 31, 1005–1009. doi: 10.13031/2013.30813
Murphy R. P. (1958). A method for the extraction of plant samples and the determination of total soluble carbohydrates. J. Sci. Food Agric. 9, 714–717. doi: 10.1002/jsfa.2740091104
Ni K. K., Zhao J. Y., Zhu B. G., Su R. N., Pan Y., Ma J. K., et al. (2018). Assessing the fermentation quality and microbial community of the mixed silage of forage soybean with crop corn or sorghum. Bioresour. Technol. 265, 563–567. doi: 10.1016/j.biortech.2018.05.097
Oosterkamp M. J., Bauer S., Ibanez A. B., Mendez-Garcia C., Hong P. Y., Cann I., et al. (2019). Identification of methanogenesis and syntrophy as important microbial metabolic processes for optimal thermophilic anaerobic digestion of energy cane thin stillage. Bioresour. Technol. 7, 100254. doi: 10.1016/j.biteb.2019.100254
Reis C. E. R., Carvalho A. K. F., Bento H. B. S., de Castro H. F. (2019). Integration of microbial biodiesel and bioethanol industries through utilization of vinasse as substrate for oleaginous fungi. Bioresour. Technol. 6, 46–53. doi: 10.1016/j.biteb.2018.12.009
Romero J. J., Zhao Y., Balseca-Paredes M. A., Tiezzi F., Gutierrez-Rodriguez E., Castillo M. S. (2017). Laboratory silo type and inoculation effects on nutritional composition, fermentation, and bacterial and fungal communities of oat silages. J. Dairy. Sci. 100, 1812–1828. doi: 10.3168/jds.2016-11642
Sokol H., Pigneur B., Watterlot L., Lakhdari O., Bermúdez-Humarán L. G., Gratadoux J. J., et al. (2008). Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. U.S.A. 105, 16731–16736. doi: 10.1073/pnas.0804812105
Tamanna N., Mahmood N. (2015). Food processing and maillard reaction products: effect on human health and nutrition. Int. J. Food. Sci. Tech. 2015, 526762. doi: 10.1155/2015/526762
Tennant R. K., Sambles C. M., Diffey G. E., Moore K. A., Love J. (2017). Metagenomic analysis of silage. J. Vis. Exp. 13, 54936. doi: 10.3791/54936
Tripathi S., Purchase D., Al-Rashed S., Chandra R. (2022). Microbial community dynamics and their relationships with organic and metal pollutants of sugarcane molasses-based distillery wastewater sludge. Environ. pollut. 292, 118267. doi: 10.1016/j.envpol.2021.118267
Van Soest P. J., Robertson J. B., Lewis B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy. Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Wagha M. P., Nemadeb P. D. (2018). Biogas generation from distillery spent wash by using an OPUR western biotechnology process: a case study. Desalin. Water Treat. 118, 241–248. doi: 10.5004/dwt.2018.22404
Weatherburn M. W. (1967). Phenol-hypochlorite reaction for determinations of ammonia. Anal. Chem. 39, 971–974. doi: 10.1021/ac60252a045
Wiles P. G., Gray I. K., Kissling R. C., Delahanty C., Evers J., Greenwood K., et al. (1998). Routine analysis of proteins by Kjeldahl and dumas methods: Review and Interlaboratory study using dairy products. J. AOAC Int. 81, 620–632. doi: 10.1093/jaoac/81.3.620
Woolford M. K. (1990). The detrimental effects of air on silage. J. Appl. Bacteriol. 68, 101–116. doi: 10.1111/j.1365-2672.1990.tb02554.x
Yuliani E., Imai T., Teeka J., Tomita S., Suprayogi (2011). Exopolysaccharide production from sweet potato-shochu distillery wastewater by lactobacillus sakei CY1. Biotechnol. Biotec. Eq. 25, 2329–2333. doi: 10.5504/BBEQ.2011.0049
Keywords: distillery wastewater, silage additive, fermentation, chemical composition, microbial community, micro-permeation
Citation: Huang X, Lu G, Li L, Liao C, Tang X, Chen C, Zhang M, Li P and Chen C (2024) Effect of distillery wastewater on chemical composition and microbial community of Sorghum propinquum silage during micro-permeation of air. Front. Ind. Microbiol. 2:1409699. doi: 10.3389/finmi.2024.1409699
Received: 08 April 2024; Accepted: 07 June 2024;
Published: 02 July 2024.
Edited by:
Julián Carrillo-Reyes, National Autonomous University of Mexico, MexicoReviewed by:
Shuai Du, Inner Mongolia Agricultural University, ChinaYushan Jia, Inner Mongolia Agricultural University, China
Copyright © 2024 Huang, Lu, Li, Liao, Tang, Chen, Zhang, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Chen, Z3pneXhnYzM4NTUyMThAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Xiaokang Huang
Xiaokang Huang Guangrou Lu1†
Guangrou Lu1† Xiaolong Tang
Xiaolong Tang Ping Li
Ping Li