
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 01 April 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1580616
This article is part of the Research Topic Combination of photodynamic therapy and immunotherapy to overcome cancer resistance View all articles
 Dan Zou1†
Dan Zou1† Ying Yan1†
Ying Yan1† Yifan Li1†
Yifan Li1† Huanhuan Ma1†
Huanhuan Ma1† Yuping Bai2
Yuping Bai2 Xueyan Wang1
Xueyan Wang1 Bofang Wang1
Bofang Wang1 Yunpeng Wang1
Yunpeng Wang1 Jingwei Ma1
Jingwei Ma1 Hao Chen1,3,4,5*
Hao Chen1,3,4,5*Synchronous multiple primary esophageal cancer (SMPEC) is a rare and aggressive condition often accompanied by obstructive dysphagia, significantly impacting patients’ quality of life. Current treatments, including chemotherapy, radiotherapy, immunotherapy, and targeted therapy, are limited in providing immediate symptom relief. This case report describes a 64-year-old female with SMPEC and metastases to thoracic lymph nodes, the lesser curvature of the stomach, and the right adrenal gland, presenting with severe dysphagia (score 4 on the Japanese Dysphagia Severity Scale). To rapidly alleviate symptoms, she underwent simultaneous metal stent implantation and photodynamic therapy (PDT). She started a liquid diet on the second day after treatment and resumed a normal diet one week later. Subsequently, she underwent systemic chemotherapy, targeted therapy, and immunotherapy. By the third treatment cycle, primary and metastatic lesions significantly decreased, achieving a partial response (PR) with stable disease and progression-free survival (PFS) exceeded 12 months. This triple therapy approach—combining stent implantation, PDT, and systemic treatments—proved effective and safe for advanced SMPEC, not only providing immediate dysphagia relief and selective tumor destruction but also delaying disease progression and improving patient outcomes.
Esophageal cancer is a common malignant tumor of the digestive tract. According to the latest statistics from 2024, there were over 510,000 new cases globally in 2022, resulting in approximately 445,000 deaths (1). In China, the number of new cases reached 224,000 in 2022, accounting for a significant proportion of global cases (2). Early esophageal cancer often has no obvious symptoms. As a result, most patients are diagnosed at an advanced stage, leading to a poor prognosis and a five-year survival rate of less than 5% (3). Synchronous multiple primary esophageal cancer(SMPEC) is a rare and aggressive malignancy. It is characterized by the presence of two or more distinct cancerous lesions in different areas of the esophagus. These lesions may appear simultaneously or within a six-month time frame (4). The incidence of SMPEC ranges from 0.1% to 10.0% and treatments for SMPEC commonly include surgical resection, radiotherapy, and chemotherapy (5). However, there are no standardized guidelines that systematically elaborate on SMPEC. A retrospective study indicates that patients with SMPEC accompanied by distant metastases have a median survival time of only 8.3 months (6). Several studies have revealed that SMPEC is often an independent factor for poor prognosis, with the prognosis of such patients being even worse (4, 7, 8). For patients with this condition, more advanced multidisciplinary treatment regimens should be considered.
Photodynamic therapy (PDT) is a highly selective and minimally invasive treatment modality. It utilizes specific photosensitizers (PS) that selectively accumulate in abnormal and rapidly proliferating tissues. It operates by destroying the target tissue through the interaction of light sources and oxygen. Compared with traditional treatment methods, PDT has advantages such as minimal trauma, low toxicity, high selectivity, a wide range of applications, and a low likelihood of developing drug resistance. However, PDT also has certain limitations, such as phototoxicity, the risk of perforation, and restricted penetration depth, which may limit its effectiveness when used alone, particularly in deeper or more advanced tumors. Given these challenges, a combination therapy approach is often required to enhance the efficacy of PDT, expand its therapeutic scope, and achieve better disease control. This report describes an SMPEC that underwent PDT simultaneously with metal stent implantation, which killed the tumor and relieved the patient’s dysphagia. After receiving systemic therapy, including chemotherapy, targeted therapy, and immunotherapy, the patient’s dysphagia completely disappeared. The primary and metastatic lesions were assessed as having achieved partial response (PR) in the short term, with long-term disease stability. The patient maintained a good quality of life without experiencing intolerable adverse effects.
A 64-year-old female patient was admitted with the chief complaint of “difficulty in swallowing for one month”. One month before admission, she developed dysphagia without any obvious triggering factors, being able to ingest only small amounts of liquid food. She was accompanied by acid reflux but without nausea, vomiting, abdominal pain or bloating. She had not received any treatment at other hospitals. There was no family history of cancer, and the physical examination revealed no positive findings. Contrast-enhanced computed tomography (CT) scan showed uneven thickening of the esophageal wall, approximately 8 cm in length, with a maximal thickness of about 0.9 cm. Enlarged lymph nodes were observed around the lesion, the largest of which measured approximately 16×14 mm, highly suggestive of esophageal cancer with lymph node metastasis. A round nodular shadow measuring approximately 17×11 mm was observed on the lesser curvature side of the stomach, highly suggestive of a metastatic tumor. A low-density nodular shadow measuring 15 × 10 mm was detected in the right adrenal area, also highly suggestive of a metastatic tumor (Figures 1A–E). Gastroscopy revealed multiple large, irregular, raised lesions separately located approximately 17-19 cm, 26–32 cm, and 34-37 cm from the incisors. Biopsies were taken at each lesion. Lesions in the middle and lower esophagus occupied about three-quarters of the lumen, suggesting advanced esophageal cancer (Figures 1F–H). Histopathological examination indicated squamous cell carcinoma (Figures 1I, J). Hematological analysis revealed a hemoglobin level of 85 g/L. Liver function tests showed an albumin level of 29 g/L. Electrolyte panel indicated a potassium level of 3.2 mmol/L. Renal function tests, coagulation profile, tumor markers, and electrocardiogram demonstrated no significant abnormalities.
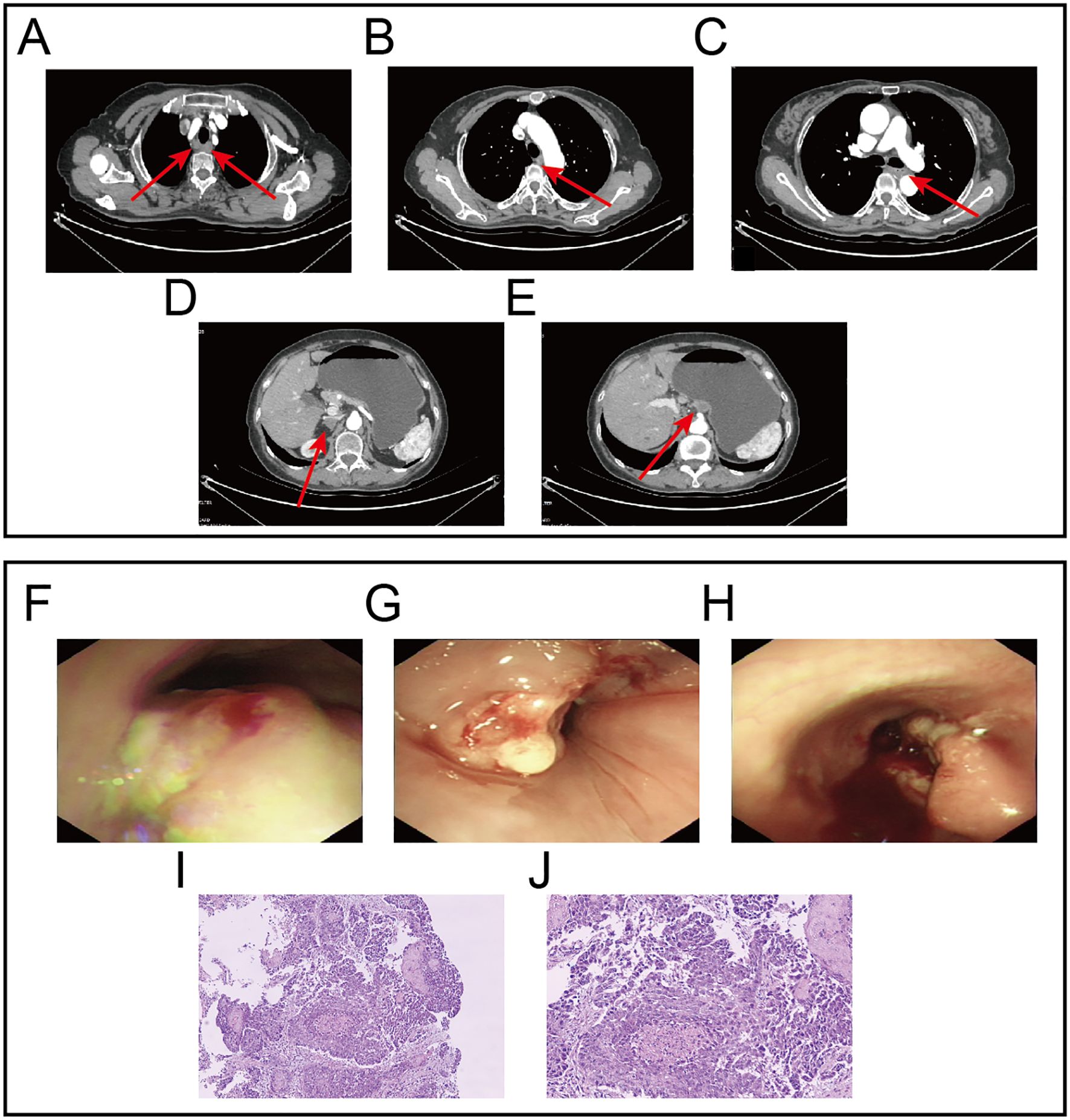
Figure 1. Gastroscopy images, Contrast-enhanced CT images and HE staining images at initial diagnosis. (A) Lesion in the upper segment of the esophagus and metastatic lymph nodes. (B) Lesion in the middle segment of the esophagus. (C) Lesion in the lower segment of the esophagus. (D) Metastatic lesion in the right adrenal gland. (E) Metastatic lesion on the lesser curvature side of the stomach.(F) Endoscopic findings revealed the first lesion located approximately 17-19 centimeters from the incisors. (G) Endoscopic findings revealed the second lesion located approximately 26-32 cm from the incisors. (H) Endoscopic findings revealed the third lesion located approximately 34-37 cm from the incisors. (I, J) Represent the HE staining results at 10x and 20x magnification, respectively.
Preliminary Diagnosis: The patient was diagnosed with synchronous multiple primary esophageal squamous cell carcinomas (cT3N2M1, Stage IVB); with local lymph node metastasis, a remote metastatic lesion on the lesser curvature side of the stomach, as well as metastasis in the right adrenal gland. Additionally, the patient was suffering from moderate anemia. The patient’s dysphagia score was 4, indicative of severe swallowing difficulty. Her ECOG performance status was 2, reflecting a moderate level of ambulatory and self-care ability, and her Karnofsky Performance Status (KPS) score was 60, suggesting a moderate level of functional impairment.
We conducted a multidisciplinary discussion for this SMPEC patient. To promptly alleviate dysphagia, our team performed endoscopic placement of metal stents and administered PDT. Systemic treatments including chemotherapy, targeted therapy, and immunotherapy were initiated to control disease progression. This comprehensive approach effectively relieved the patient’s symptoms and improved her quality of life.
Under intravenous anesthesia, an endoscopic procedure was carried out in accordance with the tumor emergency treatment. Due to severe esophageal stenosis, the endoscope was unable to advance through the narrowed segment, so a yellow zebra guidewire was inserted under endoscopic guidance into the stomach cavity under X-ray visualization. Following the guidewire, an intestinal metal stent (22×120 mm, WallFlex, Boston Scientific) was deployed into the middle and lower segments of the esophageal lesions to alleviate dysphagia. After the successful placement of the metal stent, an X-ray scan was conducted to confirm that the stent was properly expanded along the esophageal axis with normal positioning. It was ensured that both sides of the stent were about 1.5 cm above the upper and lower boundaries of the lesion. The upper and lower edges of the stent showed no significant compression or distortion (Figures 2A, B).
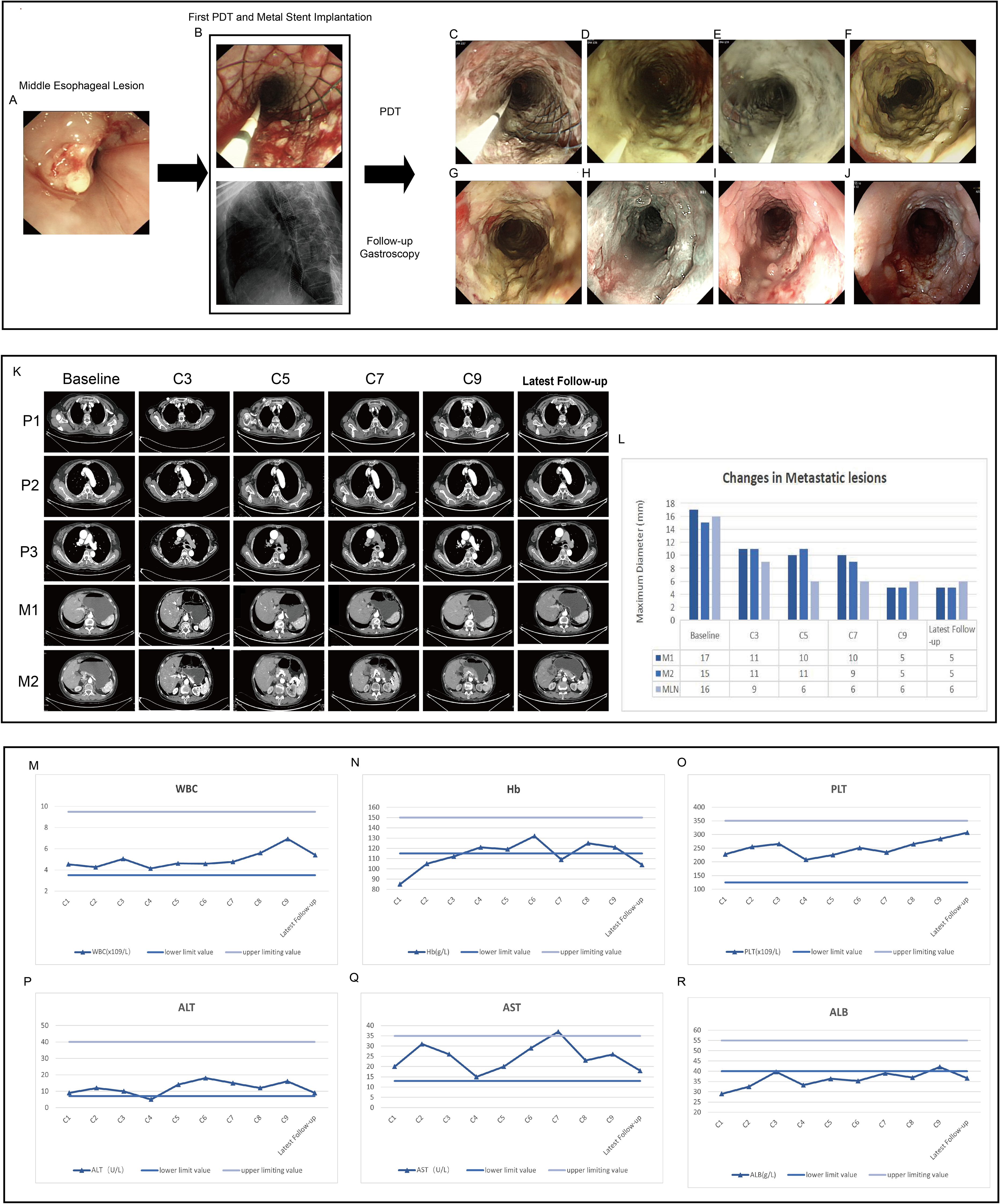
Figure 2. Endoscopic images, contrast-enhanced CT images and critical laboratory parameters before and after treatment. (A) Middle esophageal lesion prior to treatment. (B) Metal stent implantation and the first PDT to relieve obstruction at the middle segment lesion, intraoperative X-ray shows the stent is elongated in the middle, indicating that the stent is in place. (C–E) The second, third, and fourth PDT for the middle segment lesion of the esophageal cancer, respectively, and the mucosal tissue of the tumor gradually turns white and necrotic. (F–J) After one week, two months, five months, nine months and twelve months of treatment, lesion mucosa appeared white, necrotic, and detached, while partial esophageal mucosal roughness is observed. Post-treatment, the lumen remains patent. (K, L) Contrast-enhanced computed tomography demonstrated significant reduction in the size of the primary esophageal lesion and metastatic lesions after treatment. P1-P3 represent the primary lesions in the upper, middle, and lower segments of the esophagus, respectively. M1 indicates the metastatic lesion on the lesser curvature side of the stomach, and M2 indicates the metastatic lesion in the right adrenal gland. C3-C9 represent the re-examination every two cycles. MLN indicates the metastatic lymph nodes. (M–O) The changes in WBC, Hb, and PLT in the complete blood count during each treatment cycle. (P–R) The changes in ALT, AST, and ALB during each treatment cycle.
Forty-eight hours prior to the endoscopic procedure, the patient was transferred to the PDT ward and received an intravenous injection of Hematoporphyrin (Hiporfin, MaiLang Bio-Pharmaceutical Co., Ltd., China) at a dosage of 3 mg/kg. A cylindrical optical fiber (6 cm in length and 480 μm in diameter, SMA905 interface optical fiber system) and a semiconductor laser PDT device (PDT630II type, Guilin Xingda Photophysiotherapy Equipment Co., Ltd.) were used to introduce the fiber to the esophageal lesion site for the first PDT. Based on our team’s treatment experience and the energy calculation formula, which is as follows: E =(P_density × V) × t, where E represents the total energy (J), P_density represents the power density (W/cm3), V represents the tumor volume (cm³) and t represents the time (s). If the treatment is surface-based, the surface area rather than the volume, should be used. The lower segment was irradiated for 8 minutes at a power of 800 mW with an energy of 384 J, followed by the middle segment, which was irradiated for 10 minutes at a power of 800 mW with an energy of 480 J, and finally the upper segment, which was irradiated for 5 minutes at a power of 800 mW with an energy of 240 J. Based on our team’s treatment experience, we performed the second PDT the next day, with the same energy conditions as the first treatment. On the third and fourth days, we performed the third and fourth PDT, respectively, and adjusted the energy conditions to: each segment (lower, middle, and upper) was treated for 5 minutes with 800 mW power and 240 J energy (Table 1). During this process, the patient experienced no obvious discomfort, and the patient needed to avoid light for four weeks after PDT.
In addition to local treatments, systemic therapy is required to control the disease. The TX regimen is an effective chemotherapy protocol for esophageal cancer, widely used in late-stage patients who cannot undergo surgery. One week after the first PDT, the patient was administered the TX chemotherapy regimen: nab-paclitaxel (150 mg/m2, intravenous infusion on days 1 and 8); capecitabine (1000 mg/m2, taken orally from days 1 to 14); anlotinib (12 mg, taken orally from days 1 to 14), Tislelizumab (200 mg, intravenous infusion on day 1) constituting a 3-week cycle. Chemotherapy was continued until the completion of the 9 cycle, followed by maintenance targeted therapy and immunotherapy.
During the initial PDT session, a metal stent was implanted, and the patient underwent PDT for four consecutive days. Endoscopic examinations confirmed stent stability and showed progressive mucosal necrosis and sloughing(Figures 2A–E). One week post-PDT, significant necrotic tissue was observed (Figure 2F). Follow-ups at one, four, nine, and twelve months post-PDT showed tumor size reduction, new mucosal growth, and sustained esophageal patency (Figures 2G–J).
After completing the first treatment cycle, the patient’s dysphagia score showed a significant improvement, increasing by more than six points compared to the baseline. This score has remained at ≥9 since then, indicating a substantial alleviation of dysphagia symptoms.
The latest contrast-enhanced CT scan shows a significant reduction in the primary esophageal lesion. The paratracheal metastatic lymph node diameter decreased from 16 mm to 6 mm, the lesser curvature gastric metastasis from 17 mm to 5 mm, and the right adrenal metastasis from 15 mm to 5 mm (Figures 2K, L). According to iRECIST guidelines, the patient achieved a partial response (PR) for both primary and metastatic lesions.
Throughout the entire treatment course, the patient experienced only a mild decrease in albumin levels and moderate anemia. No intolerable bone marrow suppression, hepatic or renal dysfunction, hypertension, bleeding, or other common side effects associated with chemotherapy and targeted therapy were observed. No significant immune-related adverse effects were noted (Figures 2M–R). Additionally, no photosensitivity reactions, significant pain, bleeding, perforation, or infection were observed.
Currently, the patient remains under regular follow-up and continues to receive treatment and monitoring at our hospital. As of the latest follow-up (12 months post-treatment), the patient remains in stable condition with no evidence of disease progression, indicating a progression-free survival (PFS) of at least 12 months. No late complications, including perforation, severe stenosis, or long-term phototoxicity, have been observed. No late complications, such as perforation or severe stenosis, have been observed. The treatment course and follow-up timeline are illustrated in Figure 3A. Moreover, the patient remains alive, and overall survival (OS) has not yet been reached (NR), with ongoing follow-up being conducted to assess long-term outcomes.
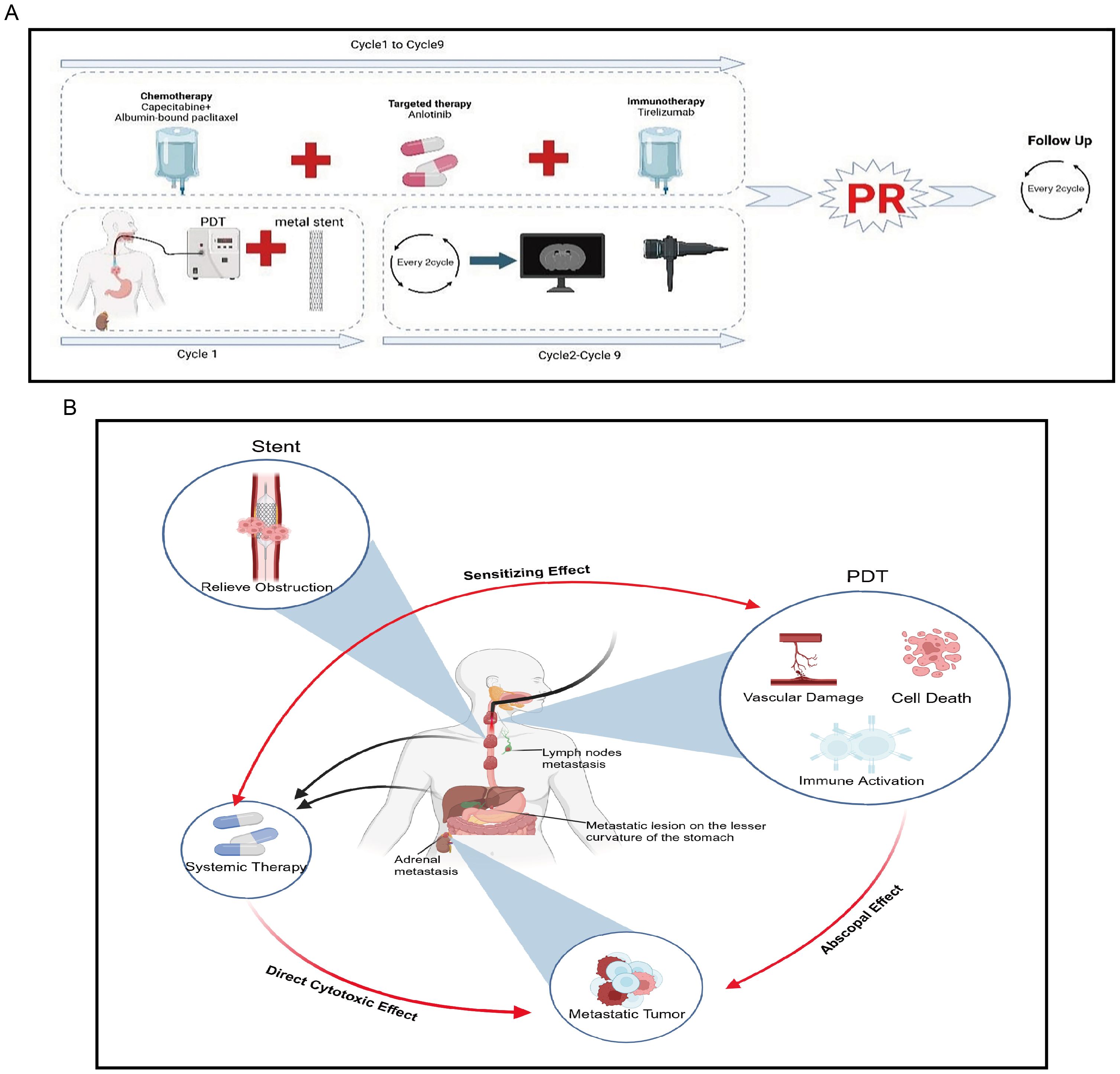
Figure 3. Overview of the patient’s treatment course reported in this study and brief mechanism of anti-tumor. (A) The treatment course. (B) Metal stents rapidly alleviate obstruction, PDT can exert local tumor-killing effects while generating “abscopal effects” and enhancing the therapeutic efficacy of systemic treatment.
The management of advanced esophageal cancer mainly involves palliative and multidisciplinary treatment strategies, with the primary aim of prolonging overall survival (OS), alleviating symptoms, and enhancing quality of life. Evidence from clinical studies highlights the limited efficacy of chemotherapy alone, with a reported median OS of only 9.1 months in patients with advanced esophageal squamous cell carcinoma (ESCC) (9). Doublet chemotherapy regimens based on 5-fluorouracil and platinum agents are recommended as first-line treatment options in various guidelines. Literature reports indicate that cisplatin combined with 5-FU as first-line chemotherapy has a response rate of approximately 30%, with a median OS of only 6.6 to 10.4 months (10–12). Recent advancements in targeted therapies and immunotherapies have significantly improved survival outcomes and quality of life in this patient population. A study suggests that apatinib monotherapy in patients with advanced esophageal cancer achieved a median PFS of 4.63 months and a median OS of 6.57 months (13). Multiple clinical studies have demonstrated that chemotherapy combined with immunotherapy significantly improves the prognosis of patients with advanced esophageal cancer (14–17). However, a considerable proportion of patients with advanced esophageal cancer still fail to benefit from immunotherapy. Although metal stent implantation is a key palliative treatment for relieving dysphagia and improving the quality of life in patients with advanced obstructive esophageal cancer, its use alone does not improve overall survival (18–20).
PDT has been applied alone or in combination with various solid tumors, showing significant efficacy, particularly in gastrointestinal tumors. Zeng R et al., in a retrospective study analyzing 32 patients with advanced obstructive esophageal cancer who underwent PDT, reported an effective rate of 78.1%, significantly relieving patients’ dysphagia symptoms (21). Yamashita et al. found that among 82 patients who received a first PDT, 27 underwent a second PDT. The local complete response (L-CR) rates for the first and second PDT were 63.0% and 40.7%, respectively. Therefore, a second PDT session proved to be an effective treatment method for local failure following the first PDT (21). A retrospective study involving 31 patients with esophageal cancer analyzed the outcomes after PDT. The OS for patients achieving complete response (CR) was 31.9 months, while those with PR had an OS of 28.2 months. The disease-free survival (DFS) for CR patients was reported to be 21.9 months. These findings suggest that PDT is a reasonable palliative treatment option for esophageal cancer (21). Our team previously reported a case of advanced recurrent esophageal cancer with dysphagia. This case highlights the potential of PDT as part of a combined therapeutic strategy, offering a novel approach for the management of advanced esophageal cancer (22). PDT is based on the interaction between PS, light of specific wavelengths, and oxygen, which generates reactive oxygen species (ROS) to destroy tumor or lesion tissues. In solid tumors, cancer cells have an increased uptake of LDL lipoproteins, and PS molecules have a high affinity for LDL, resulting in significantly higher accumulation of PS in cancer cells compared to normal cell (23, 24). The antitumor mechanisms of PDT mainly include three aspects:
1. Direct Killing of Tumor Cells: Multiple studies have found that PDT can induce tumor cell death, including apoptosis, necrosis, and autophagy (25–27).
2. Damage to Tumor Blood Vessels: Maas AL et al. found that the PS photofrin has an affinity for the collagen-containing vascular basement membrane, leading to tumor vascular damage in lung cancer mouse models after PDT (28). Multiple in vivo experiments have shown that PDT can cause damage to tumor vascular endothelium, slow down blood flow within tumor vessels, induce thrombosis, or significantly increase tumor vascular permeability, resulting in tumor vascular damage and ischemia (29).
3. Activation of Immune Responses Post-PDT: PDT induces immunogenic cell death in tumor cells, releasing tumor-associated antigens and activating the body’s antitumor immune responses (30, 31). Therefore, PDT not only directly kills tumor cells and destroys tumor blood vessels but also activates the body’s antitumor immunity, exerting comprehensive antitumor effects.
This represents the first report of applying PDT-based triple therapy to a rare case of an advanced SMPEC patient presenting with severe dysphagia. First, when combined with metal stents, PDT can rapidly alleviate dysphagia in patients, effectively eradicate tumors, and compensate for the limitations of other local therapies, such as the severe side effects of radiotherapy. Additionally, when used in combination with systemic therapy, PDT can enhance the therapeutic efficacy of both approaches, achieving an effect greater than the sum of its parts (1 + 1 + 1 + 1 + 1 > 5). Second, PDT can enhance the efficacy of systemic treatments. For example, PDT can damage tumor cell membranes, increasing membrane permeability, which enables chemotherapeutic drugs to enter the cells more easily and raises the intracellular concentration of the drugs (32). Studies have discovered that PDT synergizes with irinotecan to improve therapeutic outcomes in pancreatic cancer (33). PDT can also convert “cold tumors” into “hot tumors,” enhancing the efficacy of immune checkpoint inhibitors (34, 35). Our previous study demonstrated that PDT can reshape the tumor immune microenvironment in gastric cancer, leading to increased immune cell infiltration, enhanced immunotherapy efficacy, and prolonged survival in patients with advanced disease (34). Similarly, Tong Q et al. reported that combining Pheophorbide A-mediated PDT with αPD-L1 therapy upregulated intratumoral PD-L1 expression and promoted T cell infiltration, further enhancing the effectiveness of immunotherapy (36). Additionally, while exerting local anti-tumor effects, PDT can also kill metastatic lesions, producing an “abscopal effect”. PDT induces neutrophil infiltration into the tumor and activates tumor-specific cytotoxic CD8+ T cells, thereby stimulating anti-tumor immunity and killing tumor cells. The activity of these activated CD8+ T cells is not confined to the local tumor area but may also target distant sites such as metastases (37). A study using a colorectal cancer BALB/c mouse model found that PDT can recruit lymphocytes and inhibit the growth of distant, unirradiated metastatic lesions (38). Other studies have found that PDT induces the release of several immunostimulatory molecules, namely damage-associated molecular patterns (DAMPs), such as ATP, calreticulin, high-mobility group box 1 protein (HMGB1), heat shock proteins 70 and 90 (HSP 70 and 90), and cytokines/chemokines, thereby enhancing innate and adaptive immunity (39). Therefore, PDT not only directly kills tumor cells but also produces an “abscopal effect”, synergizing with chemotherapy, targeted therapy, and immunotherapy to enhance anti-tumor efficacy (Figure 3B).
Based on this case report, treating patients with SMPEC is highly challenging, and traditional monotherapies often fail to yield satisfactory outcomes. The comprehensive application of PDT combined with metal stent implantation, chemotherapy, targeted therapy, and immunotherapy provides an integrated approach of local control and systemic treatment for these patients, improving survival rates and quality of life. Nevertheless, this is a single-case report with limited generalizability. Moreover, the interactions between different drugs may involve multiple mechanisms, making combination therapy highly complex. Our team is currently conducting a single-center clinical trial to validate the efficacy, safety, and clinical applicability of this combination therapy (ChiCTR2200064280, ChiCTR2300076208). In the future, we aim to conduct large-scale, multicenter clinical trials to further confirm these findings.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Ethics Committee of The Second Hospital and Clinical Medical School, Lanzhou University, 2023A-472. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
DZ: Conceptualization, Writing – review & editing, Writing – original draft. YY: Writing – original draft. YL: Writing – original draft. HM: Writing – original draft. YB: Visualization, Writing – review & editing. XW: Visualization, Writing – review & editing. BW: Visualization, Writing – review & editing. YW: Investigation, Writing – review & editing. JM: Supervision, Writing – review & editing. HC: Conceptualization, Writing – review & editing, Writing – original draft.
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by National Natural Science Foundation of China (82473266, 82160129), Gansu Province Science and Technology Plan Project (24JRRA373), Key Project of Science and Technology in Gansu province (22ZD6FA054), Major scientific research project for scientific and technological innovation in the health industry of Gansu Province (GSWSZD-2024-17), General Project of Gansu Province Joint Research Fund (24JRRA929), Key Talents Project of Gansu Province (2019RCXM020), Gansu Province Postgraduate, Innovation Star Program (2025CXZX-115), Science and Technology Project of Chengguan District of Lanzhou City (2020SHFZ0039, 2020JSCX0073), Medical Research Innovation Ability Improvement Project of Lanzhou University (lzuyxcx-2022-160), Excellent Textbook Cultivation Project of Lanzhou University (lzuyxcx-2022-45), Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (CY2023-ZD-01, CY2023-QN-A01).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. (2024) 4:47–53. doi: 10.1016/j.jncc.2024.01.006
3. Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. (2022) 163:649–58.e2. doi: 10.1053/j.gastro.2022.05.054
4. Li M, Lin ZX. Characteristics and prognostic factors of synchronous multiple primary esophageal carcinoma: A report of 52 cases. Thorac Cancer. (2014) 5:25–30. doi: 10.1111/tca.2014.5.issue-1
5. Su J, Wei S, Li W, Chen H, Li L, Xu L, et al. Clinicopathological characteristics of synchronous multiple primary early esophageal cancer and risk factors for multiple lesions. Front Oncol. (2023) 13:1219451. doi: 10.3389/fonc.2023.1219451
6. Chen Z, Li S, He Z, Li G. Clinical analysis of 117 cases with synchronous multiple primary esophageal squamous cell carcinomas. Korean J Intern Med. (2021) 36:1356–64. doi: 10.3904/kjim.2017.280
7. Chen JY, Zhang SS, Fu XY, Wen J, Yang H, Zhang YJ, et al. The characteristics and prognostic significance of esophageal squamous cell carcinoma with synchronous multiple lesions: over 10-year experience. Esophagus. (2021) 18:851–60. doi: 10.1007/s10388-021-00856-8
8. Wang W, Liu X, Dang J, Li G. Survival and prognostic factors in patients with synchronous multiple primary esophageal squamous cell carcinoma receiving definitive radiotherapy: A propensity score-matched analysis. Front Oncol. (2023) 13:1132423. doi: 10.3389/fonc.2023.1132423
9. Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. (2022) 386:449–62. doi: 10.1056/NEJMoa2111380
10. Hayashi K, Ando N, Watanabe H, Ide H, Nagai K, Aoyama N, et al. Phase II evaluation of protracted infusion of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG) Trial (JCOG9407). Jpn J Clin Oncol. (2001) 31:419–23. doi: 10.1093/jjco/hye090
11. Hiramoto S, Kato K, Shoji H, Okita N, Takashima A, Honma Y, et al. A retrospective analysis of 5-fluorouracil plus cisplatin as first-line chemotherapy in the recent treatment strategy for patients with metastatic or recurrent esophageal squamous cell carcinoma. Int J Clin Oncol. (2018) 23:466–72. doi: 10.1007/s10147-018-1239-x
12. Ikeda G, Yamamoto S, Kato K. The safety of current treatment options for advanced esophageal cancer after first-line chemotherapy. Expert Opin Drug Saf. (2022) 21:55–65. doi: 10.1080/14740338.2021.1955100
13. Yanwei L, Feng H, Ren P, Yue J, Zhang W, Tang P, et al. Safety and efficacy of apatinib monotherapy for unresectable, metastatic esophageal cancer: A single-arm, open-label, phase II study. Oncologist. (2020) 25:e1464–e72. doi: 10.1634/theoncologist.2020-0310
14. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. (2021) 398:759–71. doi: 10.1016/S0140-6736(21)01234-4
15. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2019) 20:1506–17. doi: 10.1016/S1470-2045(19)30626-6
16. Shen L, Kato K, Kim SB, Ajani JA, Zhao K, He Z, et al. Tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma (RATIONALE-302): A randomized phase III study. J Clin Oncol. (2022) 40:3065–76. doi: 10.1200/JCO.21.01926
17. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. (2020) 21:832–42. doi: 10.1016/S1470-2045(20)30110-8
18. Lai A, Lipka S, Kumar A, Sethi S, Bromberg D, Li N, et al. Role of esophageal metal stents placement and combination therapy in inoperable esophageal carcinoma: A systematic review and meta-analysis. Dig Dis Sci. (2018) 63:1025–34. doi: 10.1007/s10620-018-4957-z
19. Kim KY, Tsauo J, Song HY, Kim PH, Park JH. Self-expandable metallic stent placement for the palliation of esophageal cancer. J Korean Med Sci. (2017) 32:1062–71. doi: 10.3346/jkms.2017.32.7.1062
20. Xu Z, Liu H, Li S, Han Z, Chen J, Liu X, et al. Palliative radiotherapy combined with stent insertion to relieve dysphagia in advanced esophageal carcinoma patients: A systematic review and meta-analysis. Front Oncol. (2022) 12:986828. doi: 10.3389/fonc.2022.986828
21. Yamashita H, Kadota T, Minamide T, Sunakawa H, Sato D, Takashima K, et al. Efficacy and safety of second photodynamic therapy for local failure after salvage photodynamic therapy for esophageal cancer. Dig Endosc. (2022) 34:488–96. doi: 10.1111/den.14072
22. Wang XY, Maswikiti EP, Zhu JY, Ma YL, Zheng P, Yu Y, et al. Photodynamic therapy combined with immunotherapy for an advanced esophageal cancer with an obstruction post metal stent implantation: A case report and literature review. Photodiagnosis Photodyn Ther. (2022) 37:102671. doi: 10.1016/j.pdpdt.2021.102671
23. Kwiatkowski S, Knap B, Przystupski D, Saczko J, Kędzierska E, Knap-Czop K, et al. Photodynamic therapy - mechanisms, photosensitizers and combinations. BioMed Pharmacother. (2018) 106:1098–107. doi: 10.1016/j.biopha.2018.07.049
24. Cruz PM, Mo H, McConathy WJ, Sabnis N, Lacko AG. The role of cholesterol metabolism and cholesterol transport in carcinogenesis: a review of scientific findings, relevant to future cancer therapeutics. Front Pharmacol. (2013) 4:119. doi: 10.3389/fphar.2013.00119
25. Greenwald BD. Photodynamic therapy for esophageal cancer. Update. Chest Surg Clin N Am. (2000) 10:625–37. doi: 10.1016/S1052-3359(25)00526-5
26. Buytaert E, Dewaele M, Agostinis P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim Biophys Acta. (2007) 1776:86–107. doi: 10.1016/j.bbcan.2007.07.001
27. He P, Zhang F, Xu B, Wang Y, Pu W, Wang H, et al. Research progress of potential factors influencing photodynamic therapy for gastrointestinal cancer. Photodiagnosis Photodyn Ther. (2023) 41:103271. doi: 10.1016/j.pdpdt.2022.103271
28. Maas AL, Carter SL, Wileyto EP, Miller J, Yuan M, Yu G, et al. Tumor vascular microenvironment determines responsiveness to photodynamic therapy. Cancer Res. (2012) 72:2079–88. doi: 10.1158/0008-5472.CAN-11-3744
29. Wang W, Moriyama LT, Bagnato VS. Photodynamic therapy induced vascular damage: an overview of experimental PDT. Laser Phys Letters. (2013) 10:023001. doi: 10.1088/1612-2011/10/2/023001
30. Garg AD, Nowis D, Golab J, Agostinis P. Photodynamic therapy: illuminating the road from cell death towards anti-tumour immunity. Apoptosis. (2010) 15:1050–71. doi: 10.1007/s10495-010-0479-7
31. Donohoe C, Senge MO, Arnaut LG, Gomes-da-Silva LC. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim Biophys Acta Rev Cancer. (2019) 1872:188308. doi: 10.1016/j.bbcan.2019.07.003
32. Chen B, Pogue BW, Luna JM, Hardman RL, Hoopes PJ, Hasan T. Tumor vascular permeabilization by vascular-targeting photosensitization: effects, mechanism, and therapeutic implications. Clin Cancer Res. (2006) 12:917–23. doi: 10.1158/1078-0432.CCR-05-1673
33. Huang HC, Mallidi S, Liu J, Chiang CT, Mai Z, Goldschmidt R, et al. Photodynamic therapy synergizes with irinotecan to overcome compensatory mechanisms and improve treatment outcomes in pancreatic cancer. Cancer Res. (2016) 76:1066–77. doi: 10.1158/0008-5472.CAN-15-0391
34. Yu Y, Xu B, Xiang L, Ding T, Wang N, Yu R, et al. Photodynamic therapy improves the outcome of immune checkpoint inhibitors via remodelling anti-tumour immunity in patients with gastric cancer. Gastric Cancer. (2023) 26:798–813. doi: 10.1007/s10120-023-01409-x
35. Gu B, Wang B, Li X, Feng Z, Ma C, Gao L, et al. Photodynamic therapy improves the clinical efficacy of advanced colorectal cancer and recruits immune cells into the tumor immune microenvironment. Front Immunol. (2022) 13:1050421. doi: 10.3389/fimmu.2022.1050421
36. Tong Q, Xu J, Wu A, Zhang C, Yang A, Zhang S, et al. Pheophorbide A-mediated photodynamic therapy potentiates checkpoint blockade therapy of tumor with low PD-L1 expression. Pharmaceutics. (2022) 14(11):2513. doi: 10.3390/pharmaceutics14112513
37. Kousis PC, Henderson BW, Maier PG, Gollnick SO. Photodynamic therapy enhancement of antitumor immunity is regulated by neutrophils. Cancer Res. (2007) 67:10501–10. doi: 10.1158/0008-5472.CAN-07-1778
38. Rocha LB, Gomes-da-Silva LC, Dąbrowski JM, Arnaut LG. Elimination of primary tumours and control of metastasis with rationally designed bacteriochlorin photodynamic therapy regimens. Eur J Cancer. (2015) 51:1822–30. doi: 10.1016/j.ejca.2015.06.002
Keywords: synchronous multiple primary esophageal cancer, photodynamic therapy, metal stent implantation, chemotherapy, targeted therapy, immunotherapy
Citation: Zou D, Yan Y, Li Y, Ma H, Bai Y, Wang X, Wang B, Wang Y, Ma J and Chen H (2025) Successful triple therapy for advanced synchronous multiple primary esophageal carcinoma with metal stenting, photodynamic and comprehensive systemic therapies—shining light on hope: a case report and literature review. Front. Immunol. 16:1580616. doi: 10.3389/fimmu.2025.1580616
Received: 20 February 2025; Accepted: 17 March 2025;
Published: 01 April 2025.
Edited by:
Kave Moloudi, University of Johannesburg, South AfricaReviewed by:
Alexander Chota, University of Johannesburg, South AfricaCopyright © 2025 Zou, Yan, Li, Ma, Bai, Wang, Wang, Wang, Ma and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Chen, ZXJ5X2NoZW5oQGx6dS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.