
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 14 April 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1576904
 Kai Shen1†
Kai Shen1† Yi Liao1†
Yi Liao1† Yang Dai1
Yang Dai1 Jie Ji1
Jie Ji1 Pu Kuang1
Pu Kuang1 Zhigang Liu1
Zhigang Liu1 Liping Xie1
Liping Xie1 Ting Niu1
Ting Niu1 Nenggang Jiang2*
Nenggang Jiang2* Hongbing Ma1*
Hongbing Ma1*Background: Aggressive natural killer (NK) cell leukemia (ANKL) is a rare NK cell neoplasm associated with Epstein-Barr virus (EBV) infection. Programmed cell death protein 1 (PD-1) blockade, which is successful in extranodal NK/T-cell lymphoma and EBV-related hemophagocytic lymphohistiocytosis, is considered to have a potential role in managing ANKL.
Objectives: This study aims to characterize ANKL clinically and evaluate the prognostic impact of anti-PD-1 antibody treatment.
Methods: We retrospectively analyzed the clinical characteristics and treatment regimens of ANKL patients from March 2009 to October 2023 in a single center. Data on clinical characteristics, treatment regimens and prognosis were collected from medical records. Overall survival (OS) of different risk groups was analyzed by Kaplan-Meier method. The least absolute shrinkage and selection operator (LASSO)-penalized Cox regression was used to identify the potential prognostic factors of ANKL.
Results: From March 2009 to October 2023 a total of 71 ANKL were retrieved with an OS of 2.0 months. Seven patients (9.9%) received PD-1 antibodies combined with various chemotherapies; thirty-five patients (49.3%) received asparaginase as part of chemotherapy; and eight patients (11.3%) received allogeneic HSCT after induction chemotherapy. Among patients who did not undergo allogeneic hematopoietic stem transplantation (HSCT), patients who received PD-1 antibodies as part of chemotherapy exhibited a superior OS than those without PD-1 antibodies (5.4 vs 1.6 months, p=0.035). The 1-year OS rate was 43% in the PD-1 subgroup compared with only 4% in the non-PD-1 subgroup. LASSO-Cox multivariate analysis revealed that PD-1 antibodies-containing regimens were associated with better survival (hazard ratio [HR]=0.349, 95% CI: 0.145~0.840, p=0.019). So was it with HSCT and asparaginase (HR=0.267, 95% CI=0.101~0.701 and HR=0.355, 95% CI=0.206~0.613, respectively).
Conclusion: ANKL still had a poor outcome in the past decade. Integration of anti-PD-1 antibody into chemotherapeutic therapy significantly improved the survival of ANKL. The prolonged survival attributed to PD-1 blockade could provide critical opportunities for patients awaiting HSCT.
Aggressive natural killer (NK) cell leukemia (ANKL) is a rare hematological malignancy characterized by the Epstein-Barr virus (EBV)-driven proliferation of mature natural killer (NK) cells (1). ANKL accounts for only 0.1% of lymphoid neoplasms and most frequently affects younger patients between the ages of 20 and 30 predominantly in Eastern Asian countries (2). ANKL has a fulminant clinical course, with a median overall survival (OS) of less than 2 months (3). Life-threatening complications such as hemophagocytic lymphohistocytosis (HLH), resulting in high fever, splenohepatomegly, disseminated intravascular coagulopathy (DIC) and multiple organ failure (4).
Unfortunately, early attempts utilizing chemotherapeutic regimens of non-Hodgkin lymphomas have all ended with a dismal outcome in ANKL (5). The only potential cure is induction with L-asparaginase-based chemotherapy followed with allogeneic hematopoietic stem transplant (HSCT) (6). However, most patients with ANKL will not have the chance of transplantation because of its aggressive course, limited response to the current treatment and the unavailability of matched donors. In recent years, targeted drugs such as programmed cell death protein 1 (PD-1) monoclonal antibodies have been used to treat mature NK/T cell lymphoma with remarkable success (7, 8). Our center first reported the successful treatment of relapsed/refractory EBV-associated HLH (EBV-HLH) with PD-1 antibodies in 2020 (9). Considering the close bond between EBV and ANKL, PD-1 antibodies have also been tested in ANKL patients and may prolong the survival in a limited number of patients (10). Since the clinical evidence regarding its management predominantly comes from case reports and small-sample retrospective studies, there is yet no consensus on the treatment of ANKL.
In this study, we retrospectively analyzed the clinical characteristics and treatment regimens of ANKL patients in our single center to explore the prognosis of ANKL patients in a real-world setting and the impact of PD-1 antibodies on prognosis.
This was a single-center retrospective analysis of ANKL patients who were diagnosed and treated at West China Hospital, Sichuan University (WCHSCU), from March 2009 to October 2023. The diagnosis of ANKL was adopted from the 2022 World Health Organization classification of lymphoid neoplasms (1), which included (1) the infiltration of atypical medium- to large-sized lymphocytes in the peripheral blood (PB), bone marrow (BM) or affected tissues; and (2) abnormal NK cells confirmed by flow cytometry (FCM) or immunohistochemistry (IHC). The exclusion criteria were as follows: (1) any other T or T-NK cell neoplasms confirmed by pathology, such as extranodal NK/T cell lymphoma (ENKTL), hepatosplenic and cutaneous γδT-cell lymphoma and peripheral T-cell lymphomas; (2) leukemic cells expressing clonal TCR confirmed by FCM or molecular pathological assays; and (3) pediatric patients aged younger than 14 years. Clinical data, including demographic information, clinical manifestations, laboratory parameters and outcomes, were collected from the electronic medical records. The follow-up period ended on December 14, 2023. This study was performed in accordance with the Declaration of Helsinki and approved by the Ethical Committee of WCHSCU.
All ANKL patients with HLH were initially treated with the HLH-1994 protocol which was the most used approach for HLH. Chemotherapy was administered once the diagnosis of ANKL was established. In addition to the standard LVD protocol (pegasparaginase + vincristine + dexamethasone), chemotherapeutic regimens such as anthracycline-based regimens (CHOP or CHOPE) and gemcitabine-based regimens (GED, GEMOX, GDP or GLIDE) could also be used with or without asparaginase. Two kinds of asparaginase, L-asparaginase and pegasparaginase, were both allowed. The choice of the chemotherapy protocol was determined at the discretion of the attending physicians based on their experience and each ANKL patient’s individual condition. PD-1 antibodies (either nivolumab or sintilimab) have been integrated into chemotherapy for certain EBV-infected ANKL patients. Generally, anti-PD-1 antibody with a dose of 200mg was given on day 1 of each chemotherapeutic cycle. HSCT was performed for eligible patients with controlled disease after chemotherapy once a matched or haplo-matched donor was available. For patients who responded to anti-PD-1 antibody -containing chemotherapy and did not undergo HSCT, maintenance with the administration of anti-PD-1 antibodies every 3 to 4 weeks similar to the maintenance therapy for Hodgkin lymphoma was continued until disease progression.
All the statistical analyzes were performed with R version 4.4.3 (The R Foundation for Statistical Computing) and SPSS version 29 (IBM Corp., Armonk, NY, USA). Continuous variables are expressed as the median (range). Categorical variables are presented as frequencies and percentages. The OS was defined as the time from admission to death or the last follow-up and was calculated via the Kaplan–Meier method. OS was compared between each risk group via the log-rank test. In the first-round multivariate analysis, variables with p values < 0.15 in the univariate analysis were firstly included using the forward stepwise Cox proportional hazard model. Secondly, a least absolute shrinkage and selection operator (LASSO) method was used to select and minimize prognostic variables using the R package “glmnet”. The LASSO-selected variables were further inputted into Cox analysis to determine the final results. A two-tailed p value < 0.05 was regarded as statistically significant.
A total of 71 patients diagnosed with ANKL were treated in our center from 2009 to 2023. Forty-one (57.7%) patients were male, and the male-to-female ratio was 4:3. The median age was 37 years (range, 14–65 years) (Figure 1). The most common presentations were fever (95.8%) and splenomegaly (78.9%). Fifty-five (77.5%) patients had HLH at diagnosis. Eleven (15.5%) patients manifested infectious mononucleosis (IM)-like symptoms (including fever, lymphocytosis or mononucleosis, lymphadenopathy, and hepatosplenomegaly) for more than 90 days prior to the fulminant course of ANKL, and they were categorized as having “subacute ANKL”. For all patients, the median time from onset to diagnosis was 46 days (range, 10–2425 days). Among them, eight patients were previously diagnosed with chronic EBV infection (CAEBV). The peripheral blood EBV-DNA load was assayed in 59 patients, and a positive quantification result was found in 56/59 (94.9%) patients.
Immunophenotyping via FCM was conducted on the BM aspirates of 58 patients. The median percentage of abnormal NK cells was 8.4% (range, 0.2–96%). The percentage of abnormal cells was no more than 28.5% in 75% of the assayed cases. Most patients were positive for CD2 (100%), CD56 (98%), CD38 (94%) and CD11c (75%) and negative for surface CD3 (0%), CD4 (2%), CD57 (4%) and CD8 (6%). Fifty-six percent of the patients were positive for CD7, and 39% were positive for CD16 (Figure 2). Eight patients (9.9%) received congenital HLH-related genetic tests, and four patients had positive heterozygous mutations involving the PRF, SLC7A7, LYST, and UNC13D genes. The baseline clinical and laboratory characteristics are summarized in Table 1.
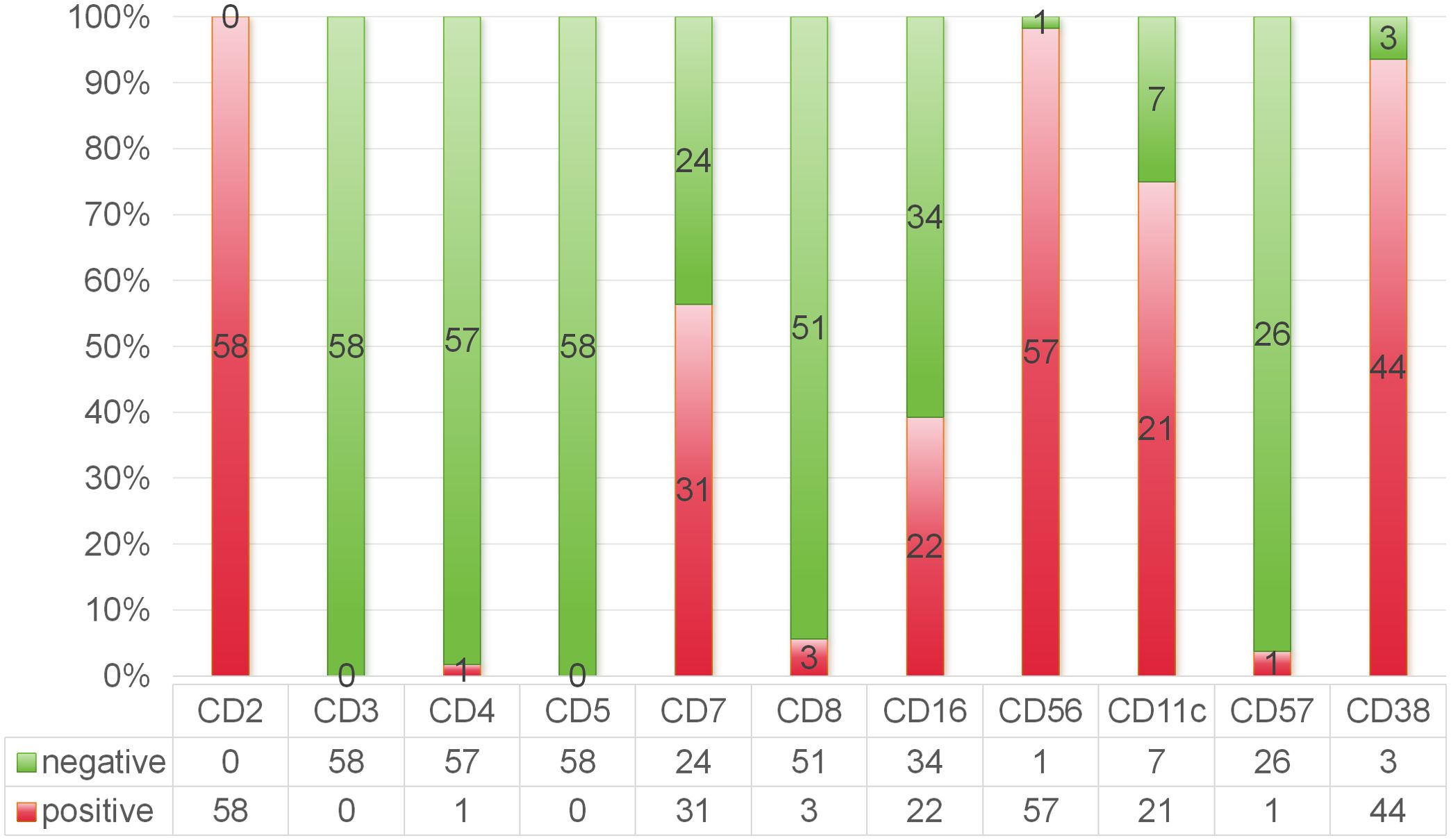
Figure 2. Immunophenotyping feature of ANKL by flow cytometry analysis of the bone marrow aspirates.
In terms of treatment, seven patients (9.9%) received PD-1 antibodies combined with various chemotherapies; thirty-five patients (49.3%) received asparaginase as part of chemotherapy; and eight patients (11.3%) received allogeneic HSCT after induction chemotherapy. None of the eight HSCT patients used PD-1 antibodies in the previous chemotherapy period prior to HSCT. The clinical details of the patients who received PD-1 therapy are summarized in Table 2. Notably, for patients presented with active HLH, ED regimen from HLH-1994 protocol was used to control the HLH prior to the initiation of anti-PD-1 antibody treatment.
At a median follow-up of 71.4 months (95% confidence interval [CI]: 15.73~127.07), the median OS of the total patients was 2.0 months (95% CI: 1.32~2.68) (Figure 3A). The 1-year OS rate was 15%. Patients who underwent HSCT had significantly longer OS than non-HSCT patients (11.7 vs 1.7 months, p=0.002) (Figure 3B). The 5-year OS rate of HSCT patients was 38%, whereas no patients without HSCT survived 5 years. As none of the eight HSCT patients received PD-1 antibody immunotherapy during induction chemotherapy, we further analyzed the impact of the PD-1 antibody on the survival of the non-HSCT group. Among the patients who did not undergo HSCT, patients who received PD-1 antibodies had a longer OS than did those who did not receive PD-1 antibodies (5.4 vs 1.6 months, p=0.035) (Figure 3C). The 1-year and 2-year OS rates for the PD-1 antibody subgroup were 43% and 26%, respectively, whereas the 1-year and 2-year OS rates for the non-PD-1 antibody subgroup were both as low as 4%. Similarly, non-HSCT patients treated with asparaginase-containing chemotherapy had longer OS than non-HSCT patients did (4.1 vs 1.1 months, p=0.002) (Figure 3D). The 1-year OS rate of the asparaginase subgroup was 12%, whereas for the non-asparaginase subgroup, the 1-year OS rate decreased to 6%.
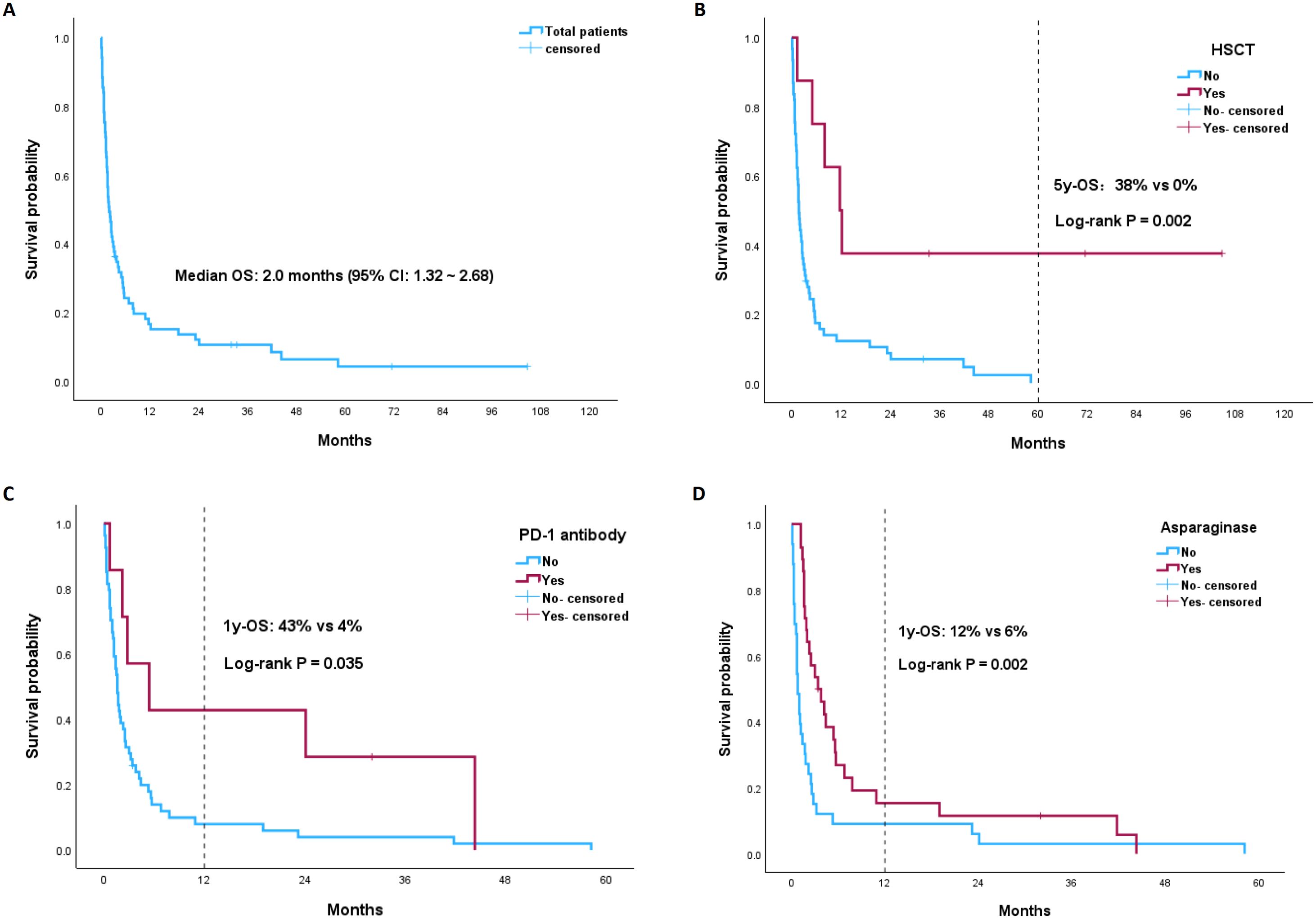
Figure 3. Kaplan-Meier (KM) plots of OS stratified by different risk groups: (A) the total ANKL patients; (B) HSCT vs non-HSCT group; (C) PD-1 antibody vs non-PD-1 antibody subgroup among the non-HSCT ANKL patients; (D) asparaginase vs non-asparaginase subgroup among the non-HSCT ANKL patients.
A variety of clinical features and therapeutic strategies as possible prognostic factors were evaluated by univariate analysis for all patients (Table 3). In addition to HSCT (p=0.002), asparaginase was also shown to be associated with better OS (p <0.001). PD-1 antibody, subacute clinical course and the presence of HLH marginally affected OS (p<0.15) and were included in the multivariate analysis. Multivariate analysis revealed that both HSCT and asparaginase were associated with better OS (hazard ratio [HR]=0.237, 95% CI=0.092~0.613 and HR=0.45, 95% CI=0.272~0.746, respectively). Moreover, PD-1 antibodies were also demonstrated to be valuable predictors of survival (HR=0.406, 95% CI: 0.172~0.958). The survival advantage of a subacute clinical course, which was not statistically significant according to univariate analysis, was revealed by multivariate analysis (HR=0.446, 95% CI: 0.215~0.923) (Table 4).
Considering the small sample number of the entire cohort and the limited number of PD-1 antibody subgroup, LASSO model was used and identified five potential prognostic factors, including splenomegaly, subacute clinical course, HSCT, PD-1 antibodies and asparaginase (Figure 4). Cox regression model was established based on all factors screened by LASSO regression even though hepatomegaly had a small coefficient of 0.054 close to zero. This new cox model still confirmed that PD-1 antibody was a significant prognostic factor for OS (HR=0.345, 95% CI: 0.145~0.840, p=0.019) (Table 5). So was it with HSCT and asparaginase (HR=0.267, 95% CI=0.101~0.701 and HR=0.355, 95% CI=0.206~0.613, respectively). A nomogram was developed based on the selected predictors to provide a visual tool for OS risk assessment (Supplementary Figure S1). The resulting nomogram showed excellent discrimination with the area under the curve (AUC) of 0.800, 0.845, and 0.849 for 1-, 2-, and 3-year survival, respectively (Supplementary Figure S2). Meanwhile, the 1- year and 2- year calibration curves demonstrated that this nomogram had good forecast precision (Supplementary Figure S3).
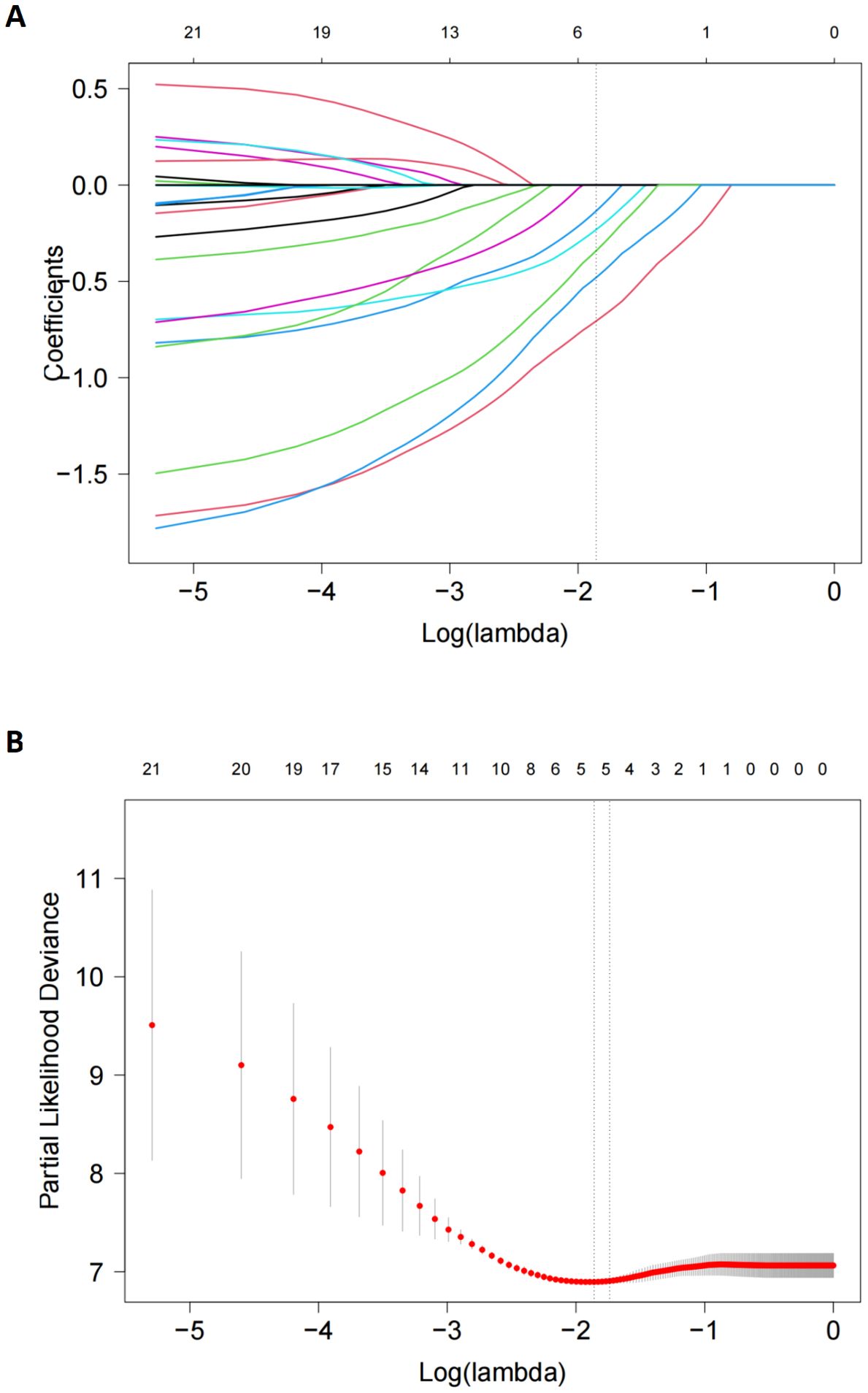
Figure 4. LASSO Cox regression model to select candidate variables associated with OS. (A) LASSO coefficient profiles of 21variables associated with OS. A dotted vertical line was drawn at the optimal λ value (0.176) chosen by 10-fold cross-validation. (B). Five risk factors selected using LASSO Cox regression analysis. The two dotted vertical lines were drawn at the optimal scores which resulted in 5 nonzero coefficients (hepatomegaly,-0.056; subacute clinical course, -0.165; HSCT, -0.646; PD-1 antibodies, -0.259; asparaginase, -0.408).
In our study we explored the clinical features and prognostic predicators of ANKL, a highly aggressive subtype of NK-cell neoplasm, in a real-world setting. A sample size of 71 in a single center which was accumulated in a span of more than 10 years, was considered large if the rarity of ANKL was taken into consideration. The median age was 37 years, and the incidence peak was around the 30s and 40s. Like the multicenter retrospective study of ANKL in the Chinese Han ethnicity by Tang et al (3), a subgroup of ANKL patients with a subacute clinical course was also demonstrated in our study, accounting for 15.5% of the total patients. Although this subtype resembled CAEBV-transformed ANKL in clinical presentation, they could be distinguished in onset age, clinical course, and prognosis. ANKL transforming from CAEBV could be younger and might have a slightly better outcome. The multivariate analysis in our study also revealed better survival in patients with this subacute subtype of ANKL.
FCM is a valuable tool for differentiation between ANKL and other NK-cell neoplasms. Typical ANKL cells are shown to have a prominent forward scatter, which is consistent with a larger cell size, and the expression of CD56 (CD56bright) (11). In a report with 12 European patients, neoplastic cells appeared to consistently express CD2 with CD16 and the presence of CD16 in ANKL patients could distinguish the disease from ENKTL, which was usually CD16 negative (12). Nevertheless, in our study, CD16 positivity was only 39%. Lower expression of CD16 on FCM seemed to be much more common in Chinese and Japanese ANKL patients. In a previous report of 47 Chinese ANKL patients, 91.9% had a CD56bright/CD16dim- and CD57-negative phenotype (13).The CD56bright/CD16dim group could also represent an earlier stage of differentiation of either neoplastic or nonneoplastic NK cells (14). Thus, the diagnosis of ANKL should be cautious when an atypical immunophenotype was revealed by FCM, especially for those with a suspicious background of EBV-HLH or CAEBV. Another diagnostic challenge is the common presence of only few neoplastic NK cells in the BM at the early stage. The median percentage of abnormal NK cells in our study was 8.4%, similar to a previous report in which the proportion of abnormal NK cells in the BM was less than 5% in half of the patients (15). Therefore, repeated BM aspirations are often needed for the diagnosis.
L-asparaginase disrupted the asparagine supply to tumor cells and induced the apoptosis of ANKL neoplastic cells (16). Several clinical studies have shown that L-asparaginase-based regimens are associated with survival of ANKL. The overall response rates for patients treated with L-asparaginase-containing regimens were between 40% and 70%, so that eligible patients can be followed with HSCT (17, 18). Nevertheless, early referral and the initiation of donor search are recommended, as outcomes are poor without HSCT. In our study, the 5-year OS rate of the HSCT group was 38%, whereas there was no long-term survivor in the non-HSCT group. Unfortunately, previous studies have shown that fewer than one-third of patients are able to undergo HSCT (6, 19, 20). Moreover, long-term outcomes of HSCT remained dismal internationally, with a median OS of less than 1 year, primarily due to high disease relapse rate within the first year after HSCT (6, 20, 21). In our study, the OS of the HSCT patients was 11.7 months, which was consistent with previous reports. Thus, there is a large unmet need for more effective therapeutic agents for relapsed or refractory (R/R) ANKL or for those who are unable to tolerate intensified chemotherapy.
Recent studies have identified 4 major dysregulated pathways involved in ANKL pathogenesis: epigenetic modulation, TP53 and DNA repair, JAK/STAT/MYC, and the PD-L1/PD-1 checkpoint (11, 22). Because of the similarity between ENKTL and ANKL, PD-1 blockade was considered to have a potential role in managing ANKL (23). They may be particularly promising for EBV-positive ANKLs. Early research revealed that PD-L1 expression on EBV-transformed cancer cells was mediated by latent membrane protein 1 which could play a vital role in immune evasion (24). A pathological study found that approximately one-third of the ANKL cases overexpressed PD-L1 (7). Our colleague Liu P et al (9) first tested the PD-1 inhibitor nivolumab in 7 R/R EBV-HLH patients, and 5/7 patients achieved complete clinical remission, with a median follow-up of 16 months. Nivolumab expanded PD-1-positive T cells reserve and normalized cytotoxic T cell function by promoting the expression of degranulation genes and costimulatory genes. This normalization of the cytotoxic activation process was closely correlated with EBV clearance in in HLH. Inspired by their success and considering the association between ANKL and EBV infection, we integrated PD-1 monoclonal antibodies as immunotherapy into chemotherapy regimens as both induction and maintenance therapies for ANKL.
For patients with flaring HLH at diagnosis, a three-step strategy (cooling, consolidation, and reconstruction) was first proposed for EBV-associated lymphoproliferative disorders by Swada et al (25). Similarly, after ANKL patient’s hyperinflammatory condition was alleviated (or cooled) by HLH-2004-like chemotherapy, PD-1 antibodies could be integrated into the ensuing intensified chemotherapy (consolidation), such as the GLIDE regimen, which was designed for NK/T cell lymphoma by our colleagues Ji J et al (26). It was noteworthy that immunotherapy like PD-1 blockade can lead to the overactivation of immune system, resulting in cytokine storm and HLH (27). Thus, the initiation of anti-PD-1 antibody treatment in ANKL should be cautious with preexisting HLH and “cooling” with etoposide-based chemotherapy was the key to avoid disastrous HLH flares which could be manifested as a sudden worsening of HLH-related symptoms. Meanwhile, serial monitoring of HLH parameters like serum ferritin and soluble IL-2 receptor is important in the early recognition of this immunotherapy-related complication. In our study, the PD-1 antibody subgroup of non-HSCT patients presented a superior OS than the PD-1 antibody subgroup of non-HSCT patients (5.4 vs 1.6 months, p=0.035). Even though PD-1 antibodies cannot eventually prevent relapses, survival was obviously prolonged, as 3 out of the 7 PD-1 antibody-treated patients survived for more than 12 months. We believe that the prolongation of survival time attributed to PD-1 blockade could provide transplantation-eligible patients with a valuable window to search for suitable donors and increase the opportunity for a peaceful transition to HSCT by increasing the response rate as part of frontline chemotherapy and reducing early relapse as maintenance. Thus, in the future, when we gain more experience in using immune checkpoint inhibitors for ANKL, more work could be done, such as devising prospective studies integrating both PD-1 inhibitors and asparaginase into frontline and bridging chemotherapy for transplant-eligible patients. Moreover, PD-1 inhibitors might be used after HSCT as maintenance therapy to prevent relapses.
This study had certain limitations. Firstly, as a retrospective study at a single center, some clinical details like the PD-L1 expression level on pathology and serial EBV-DNA monitoring data could not be as intact as those in prospective trials, which could limit the number of predictors in the multivariate analysis. Secondly, owing to the rarity of the disease and the real-world setting, the patients enrolled in our study had a prolonged time span. Thus, era effect might be a confounding factor as data completeness and the timeliness of diagnosis was improving over time. Thirdly, treatment regimens in our study could only be broadly categorized. This heterogeneity in treatment protocols could lead to certain biases. Moreover, the small number of patients in PD-1 antibodies subgroup might limit the statistical strength even though a LASSO-Cox model was used. Therefore, future research should focus on well-designed multi-center prospective trials incorporating mechanistic investigations to further validate the results of this study.
ANKL, a rare but extremely aggressive NK cell neoplasm, still had a poor outcome in the past decade according to our single-center experience. Asparaginase-containing chemotherapy followed by HSCT was the only possible cure for ANKL. Most importantly, the integration of an anti-PD-1 antibody into induction chemotherapy and maintenance therapy could significantly improve the survival of ANKL patients. The prolonged survival attributed to PD-1 blockade could help transplantation-eligible patients gain more time to search for suitable donors and increase the opportunity for a peaceful transition to HSCT. In the future, regimens containing novel therapeutic agents with potential efficacy, including PD-1 antibodies, should be validated by multi-center prospective trials.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of West China Hospital, Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because of its retrospective nature and no risk of leaking identifiable data for individual patients.
KS: Formal Analysis, Project administration, Writing – original draft, Writing – review & editing. YL: Formal Analysis, Project administration, Writing – original draft, Writing – review & editing. YD: Writing – original draft, Writing – review & editing. JJ: Writing – original draft, Writing – review & editing. PK: Writing – original draft, Writing – review & editing. ZL: Writing – original draft, Writing – review & editing. LX: Writing – original draft, Writing – review & editing. TN: Writing – original draft, Writing – review & editing. NJ: Conceptualization, Writing – original draft, Writing – review & editing. HM: Conceptualization, Project administration, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work is supported by the General Program of the Natural Science Foundation of Sichuan Provincial Department of Science and Technology (2025ZNSFSC0567)to Hongbing Ma.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer ZW declared a shared affiliation with the authors to the handling editor at time of review.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1576904/full#supplementary-material
1. Miranda RN, Amador C, Chan JKC, Guitart J, Rech KL, Medeiros LJ, et al. Fifth edition of the world health organization classification of tumors of the hematopoietic and lymphoid tissues: mature T-cell, NK-cell, and stroma-derived neoplasms of lymphoid tissues. Mod Pathol. (2024) 37:100512. doi: 10.1016/j.modpat.2024.100512
2. Zhang Y, Lee D, Gesiotto Q, Sokol L. Aggressive natural killer cell leukemia: diagnosis, treatment recommendations, and emerging therapies. Expert Rev Hematol. (2021) 14:731–40. doi: 10.1080/17474086.2021.1955345
3. Tang YT, Wang D, Luo H, Xiao M, Zhou HS, Liu D, et al. Aggressive NK-cell leukemia: clinical subtypes, molecular features, and treatment outcomes. Blood Cancer J. (2017) 7:660. doi: 10.1038/s41408-017-0021-z
5. El Hussein S, Medeiros LJ, Khoury JD. Aggressive NK cell leukemia: current state of the art. Cancers (Basel). (2020) 12:2900. doi: 10.3390/cancers12102900
6. Fujimoto A, Ishida F, Izutsu K, Yamasaki S, Chihara D, Suzumiya J, et al. Allogeneic stem cell transplantation for patients with aggressive NK-cell leukemia. Bone Marrow Transplant. (2021) 56:347–56. doi: 10.1038/s41409-020-01009-8
7. Kwong YL, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. (2017) 129:2437–42. doi: 10.1182/blood-2016-12-756841
8. He L, Chen N, Dai L, Peng X. Advances and challenges of immunotherapies in NK/T cell lymphomas. iScience. (2023) 26:108192. doi: 10.1016/j.isci.2023.108192
9. Liu P, Pan X, Chen C, Niu T, Shuai X, Wang J, et al. Nivolumab treatment of relapsed/refractory Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in adults. Blood. (2020) 135:826–33. doi: 10.1182/blood.2019003886
10. Liao Y, He HS, Wei YF, Shen DP, Ji XY, Huang C, et al. Clinical Characteristics of Aggressive NK-Cell Leukemia Zhongguo Shi Yan Xue Ye Xue Za Zhi. (2023) 31:1026–31. doi: 10.19746/j.cnki.issn.1009-2137.2023.04.015
11. El Hussein S, Patel KP, Fang H, Thakral B, Loghavi S, Kanagal-Shamanna R, et al. Genomic and immunophenotypic landscape of aggressive NK-cell leukemia. Am J Surg Pathol. (2020) 44:1235–43. doi: 10.1097/PAS.0000000000001518
12. Lima M, Spínola A, Fonseca S, Santos AH, Rodrigues J, Oliveira L, et al. Aggressive mature natural killer cell neoplasms: report on a series of 12 European patients with emphasis on flow cytometry based immunophenotype and DNA content of neoplastic natural killer cells. Leuk Lymphoma. (2015) 56:103–12. doi: 10.3109/10428194.2014.905772
13. Li Y, Wei J, Mao X, Gao Q, Liu L, Cheng P, et al. Flow cytometric immunophenotyping is sensitive for the early diagnosis of de novo aggressive natural killer cell leukemia (ANKL): A multicenter retrospective analysis. PloS One. (2016) 11:e0158827. doi: 10.1371/journal.pone.0158827
14. Luetke-Eversloh M, Killig M, Romagnani C. Signatures of human NK cell development and terminal differentiation. Front Immunol. (2013) 4:499. doi: 10.3389/fimmu.2013.00499
15. Li C, Tian Y, Wang J, Zhu L, Huang L, Wang N, et al. Abnormal immunophenotype provides a key diagnostic marker: a report of 29 cases of de novo aggressive natural killer cell leukemia. Transl Res. (2014) 163:565–77. doi: 10.1016/j.trsl.2014.01.010
16. Ando M, Sugimoto K, Kitoh T, Sasaki M, Mukai K, Ando J, et al. Selective apoptosis of natural killer-cell tumours by l-asparaginase. Br J Haematol. (2005) 130:860–8. doi: 10.1111/j.1365-2141
17. Ishida F, Ko YH, Kim WS, Suzumiya J, Isobe Y, Oshimi K, et al. Aggressive natural killer cell leukemia: therapeutic potential of L-asparaginase and allogeneic hematopoietic stem cell transplantation. Cancer Sci. (2012) 103:1079–83. doi: 10.1111/j.1349-7006.2012.02251.x
18. Jung KS, Cho SH, Kim SJ, Ko YH, Kang ES, Kim WS. L-asparaginase-based regimens followed by allogeneic hematopoietic stem cell transplantation improve outcomes in aggressive natural killer cell leukemia. J Hematol Oncol. (2016) 9:41. doi: 10.1186/s13045-016-0271-4
19. Ito T, Makishima H, Nakazawa H, Kobayashi H, Shimodaira S, Nakazawa Y, et al. Promising approach for aggressive NK cell leukaemia with allogeneic haematopoietic cell transplantation. Eur J Haematol. (2008) 81:107–11. doi: 10.1111/j.1600-0609.2008.01090.x
20. Jeong SH, Song HN, Park JS, Yang DH, Koh Y, Yoon SS, et al. Allogeneic stem cell transplantation for patients with natural killer/T cell lymphoid Malignancy: A multicenter analysis comparing upfront and salvage transplantation. Biol Blood Marrow Transplant. (2018) 24:2471–8. doi: 10.1016/j.bbmt.2018.07.034
21. Hamadani M, Kanate AS, DiGilio A, Ahn KW, Smith SM, Lee JW, et al. Allogeneic hematopoietic cell transplantation for aggressive NK cell leukemia. A center for international blood and marrow transplant research analysis. Biol Blood Marrow Transplant. (2017) 23:853–6. doi: 10.1016/j.bbmt.2017.01.082
22. Dufva O, Kankainen M, Kelkka T, Sekiguchi N, Awad SA, Eldfors S, et al. Aggressive natural killer-cell leukemia mutational landscape and drug profiling highlight JAKSTAT signaling as therapeutic target. Nat Commun. (2018) 9:1567. doi: 10.1038/s41467-018-03987-2
23. Merryman RW, Armand P, Wright KT, Rodig SJ. Checkpoint blockade in Hodgkin and non-Hodgkin lymphoma. Blood Adv. (2017) 1:2643–54. doi: 10.1182/bloodadvances.2017012534
24. Green MR, Rodig S, Juszczynski P, Ouyang J, Sinha P, O’Donnell E, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. (2012) 18:1611–8. doi: 10.1158/1078-0432.CCR-11-1942
25. Sawada A, Inoue M. Hematopoietic stem cell transplantation for the treatment of epstein–Barr virus-associated T- or NK-cell lymphoproliferative diseases and associated disorders. Front Pediatr. (2018) 6:334. doi: 10.3389/fped.2018.00334
26. Ji J, Liu T, Xiang B, Liu W, He C, Chen X, et al. A study of gemcitabine, l-asparaginase, ifosfamide, dexamethasone and etoposide chemotherapy for newly diagnosed stage IV, relapsed or refractory extranodal natural killer/T-cell lymphoma, nasal type. Leuk Lymphoma. (2014) 55:2955–7. doi: 10.3109/10428194.2014.907894
Keywords: aggressive natural killer cell leukemia, programmed cell death protein 1, immunotherapy, asparaginase, hematopoietic stem transplantation, prognosis
Citation: Shen K, Liao Y, Dai Y, Ji J, Kuang P, Liu Z, Xie L, Niu T, Jiang N and Ma H (2025) Integration of anti-PD-1 antibody into chemotherapeutic regimens improved the outcome of aggressive NK cell leukemia: a single-center retrospective real-world analysis. Front. Immunol. 16:1576904. doi: 10.3389/fimmu.2025.1576904
Received: 14 February 2025; Accepted: 27 March 2025;
Published: 14 April 2025.
Edited by:
Zong Sheng Guo, University at Buffalo, United StatesReviewed by:
Martín Manuel Ledesma, CONICET Institute of Experimental Medicine, National Academy of Medicine, (IMEX-ANM), ArgentinaCopyright © 2025 Shen, Liao, Dai, Ji, Kuang, Liu, Xie, Niu, Jiang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nenggang Jiang, ajc5MDExNEAxNjMuY29t; Hongbing Ma, aG9uZ2JpbmdtYUBmb3htYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.