
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol., 09 April 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1566805
This article is part of the Research TopicAntibody-drug Conjugates in Solid and Hematologic MalignanciesView all 3 articles
This article describes a 6-year-old patient diagnosed with “systemic mastocytosis with AML1::ETO+ AML”, he experience refractory disease during the course of treatment and salvage treatment was ineffective. The patient was administered gemtuzumab-ozogamicin therapy, resulting in rapid remission.
Systemic mastocytosis (SM) is a neoplasm characterized by the proliferation of mast cells (MCs) in extracutaneous organs. SM encompasses various entities, each with distinct clinical manifestations and prognostic outcomes (1). In the Mayo series, SM with associated hematologic neoplasm (SM-AHN) was the second most prevalent subgroup of SM, accounting for 40% of the cases (2). AHNs encompass a wide range of hematologic malignancies, including overlapping syndromes of MDS/MPN (such as CMML, which is the most common), as well as MDS, AML, and, less frequently, lymphomas and plasma cell neoplasms (3). KIT mutations are associated with enhanced proliferation of MCs and are frequently detectable in AML1::ETO+ AML, AML that occurs concomitantly often harbors the same KIT mutations to those found in neoplastic MCs, existing evidence suggests that leukemic blasts and MCs derive from a common malignant progenitor (4, 5). The prognosis for SM with AML1::ETO+ AML is extremely poor, posing significant therapeutic challenges (5–7). Aberrant MCs in SM display a distinct immune phenotype and express myeloid-associated antigens, indicating the potential feasibility of targeted therapy directed against specific cell surface markers. In this study, we present a case of a pediatric patient diagnosed with refractory SM with AML1::ETO+ AML, who experienced rapid remission after receiving gemtuzumab-ozogamicin (GO) therapy.
In August 2024, a pediatric patient sought medical attention at a local hospital, presenting with generalized petechiae lasting for over 20 days. Complete blood count revealed a white blood cell count of 16.3×10^9/L, a hemoglobin level of 76 g/L, and a platelet count of 12×10^9/L. Bone marrow smear analysis revealed that 53% of the cells were myeloblasts. Flow cytometry (FCM) of the bone marrow identified 32.15% of abnormal cells expressingCD117, CD34, HLA-DR, CD123, partially expressing CD19, and weakly expressing CD33, CD13, CD38, and MPO. These cells did not express TDT, cCD3, cCD79a, or other myeloid and lymphoid markers, they were identified as abnormal myeloid blasts. Furthermore, genetic sequencing identified the presence of the AML1::ETO fusion gene along with KIT N822K (VAF 9.19%) and KIT W577R (VAF 33.81%) somatic mutations. Chromosomal analysis resulted in a karyotype of 46,XY,del(7)(q32),t(8;21)(q22;q22.1)[1]/46,idem,del(9)(q12q21)[14].Based on the obtained findings, the patient was definitively diagnosed with AML.
The patient initially received the MAH regimen (mitoxantrone, cytarabine, and homoharringtonine) as induction therapy. Subsequent bone marrow morphology examinations revealed no response to the treatment. Following administration of the EA regimen (etoposide and cytarabine), bone marrow aspiration showed no signs of leukemic remission. Subsequently, the patient underwent chemotherapy with the CLAG regimen (claribine, cytarabine, and G-CSF).Unfortunately, peripheral blood examination showed no achievement of remission. The patient was then referred to our hospital for advanced treatment, where a comprehensive evaluation of disease condition was conducted.
Despite the patient’s persistent and severe neutropenia, bone marrow smears showed that primitive granulocytes comprised 10% of total cells, whereas mast cells were markedly increased, comprising 12% of the cells and exhibiting abnormal morphologies. Notably, clusters of abnormal mast cells were observed (Figure 1). FCM analysis of bone marrow samples revealed that 6.36% of nucleated cells were positive for CD34, CD117dim, CD13, CD33bri, CD38, HLA-DRdim, CD64dim, CD30, and CD25, and negative for CD7, CD11b, CD14, CD56, CD15, CD69, and CD16.These cells were characterized as malignant immature myeloid cells (Figure 2). Abnormal mast cells (Figure 2), which comprised 6.2% of nucleated cells, demonstrated increased SSC, enhanced CD117 and CD33 expression, decreased CD69 expression, abnormal CD64 and CD30 expression, and were negative for CD25, CD34, CD15, and CD16. The AML1::ETO transcript level had surged to 281.736%. Following the laboratory test results, the patient’s diagnosis was updated to SM with AML1::ETO+ AML.
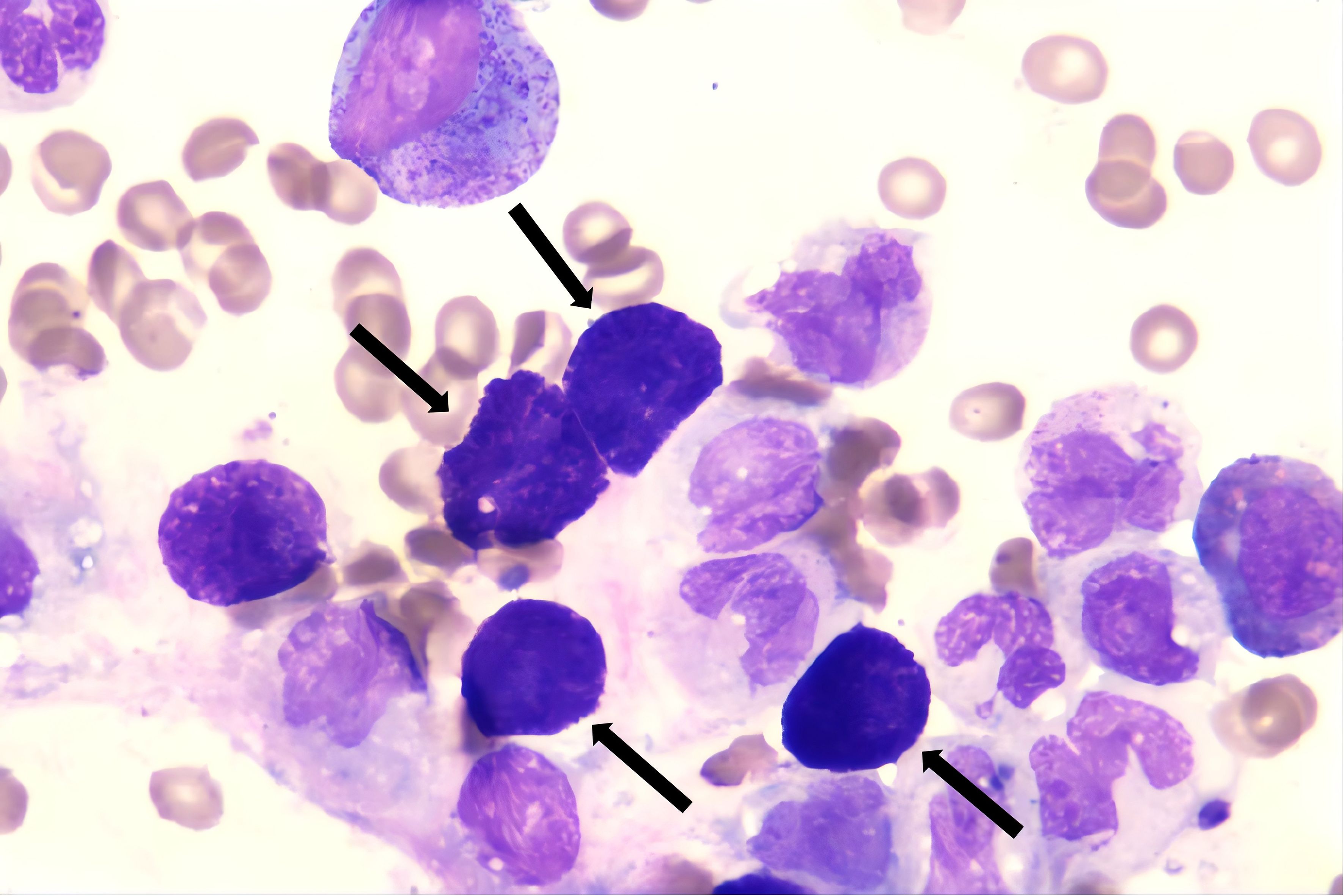
Figure 1. Mast cells (black arrow), present in clusters, are clearly visible on bone marrow smears. (Wright’s stain ×100).
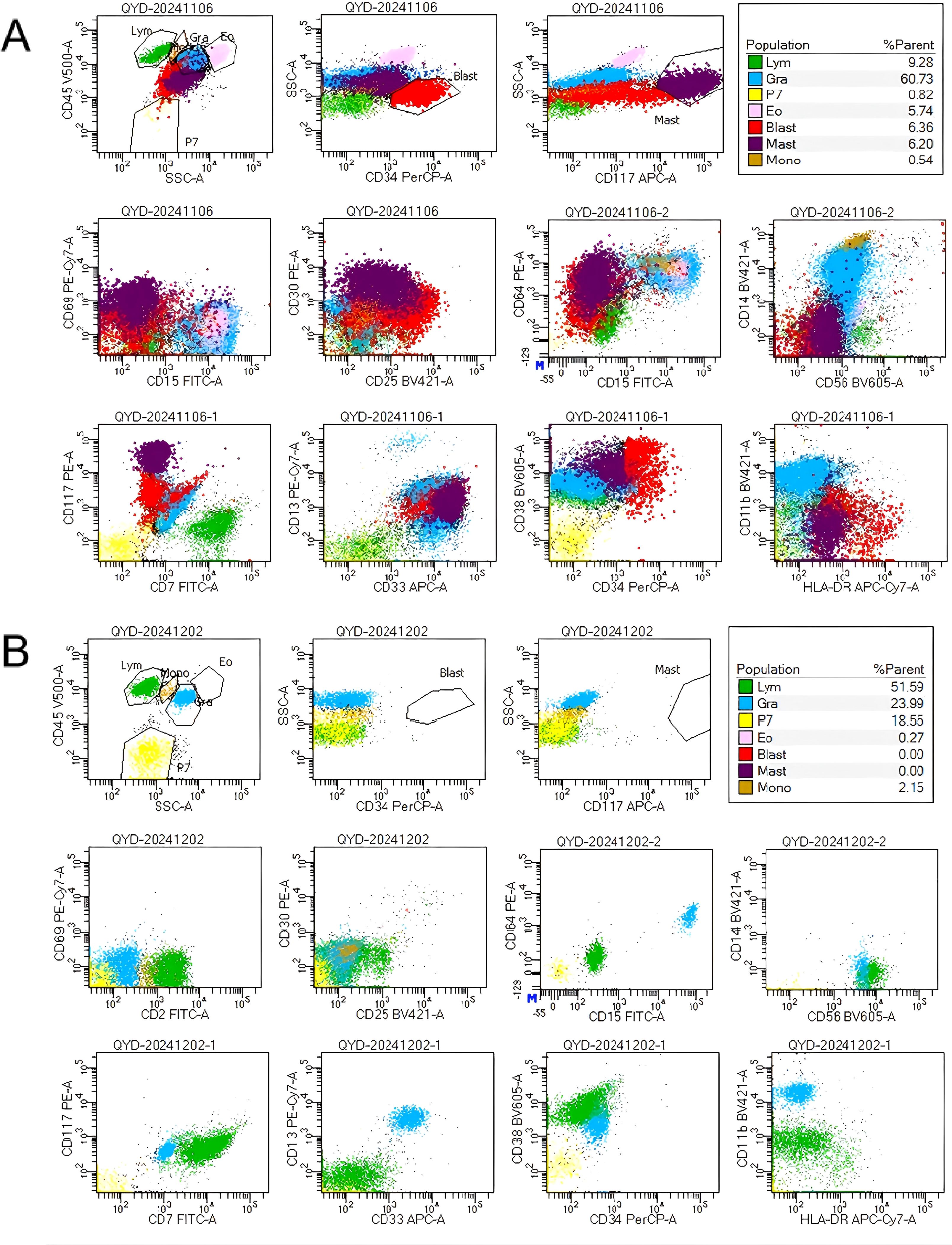
Figure 2. (A) Flow cytometry analysis of bone marrow identified two distinct abnormal cells: (1) Malignant immature myeloid cells, labeled as the ‘red group’, comprising 6.36% of nucleated cells, expressing CD34, CD117dim, CD13, CD33bri, CD38, HLA-DRdim, CD64dim, CD30, and CD25, and negative for CD7, CD11b, CD14, CD56, CD15, CD69.(2) Abnormal mast cells, designated the ‘purple group’, accounted for 6.2% of nucleated cells. These cells demonstrated increased SSC, enhanced CD117 and CD33 expression, decreased CD69 expression, abnormal CD64 and CD30 expression, and were negative for CD25, CD34, CD15. (B) After GO treatment, FCM of the bone marrow demonstrated the absence of leukemic cells and abnormal mast cells.
Due to the patient’s lack of response to three consecutive cycles of chemotherapy, further combination therapy was not pursued. The patient described herein possesses two mutations within the KIT gene: one in exon 11 and another in exon 17. Given that Avapritinib can inhibit mutations in both exons, it is an appropriate therapeutic option for this patient (8, 9). In real world practice, avapritinib demonstrates limited efficacy in treating KIT exon 11-mutated SM (10). Furthermore, considering the patient’s low white blood cell count, avapritinib was not administered to avert severe neutropenia and the risk of subsequent infections. Considering the robust expression of CD33 on both leukemic cells and abnormal mast cells in the patient, the administration of GO represents a logical therapeutic option. After thorough consultation and careful consideration, the patient’s parent provided both oral and written consent for the GO therapy. The patient subsequently received a weekly dosage of 3 mg/m²of GO. During treatment, only mild abdominal discomfort was observed, with no other treatment-related adverse reactions. Following two cycles of GO treatment, the patient underwent a bone marrow aspiration, revealing complete remission on the smear. FCM of the bone marrow demonstrated the absence of leukemic cells and abnormal mast cells, with a reduction in AML1::ETO gene quantitation to 0.372%. Given the patient’s favorable remission status, allogeneic hematopoietic stem cell transplantation (allo-HSCT) was promptly initiated.
On December 31, 2024, the patient underwent haploidentical allo-HSCT, neutrophils and platelets were implanted on days +12. At 3 weeks post-transplantation, the BM AML1::ETO transcript level was 0.002%. At 4 weeks post-transplantation, the patient exhibited abdominal distension and was subsequently diagnosed with sinusoidal obstruction syndrome (SOS). The condition improved after defibrotide treatment. At 6 weeks post-transplantation, the BM AML1::ETO transcript level was 0% (Figure 3).
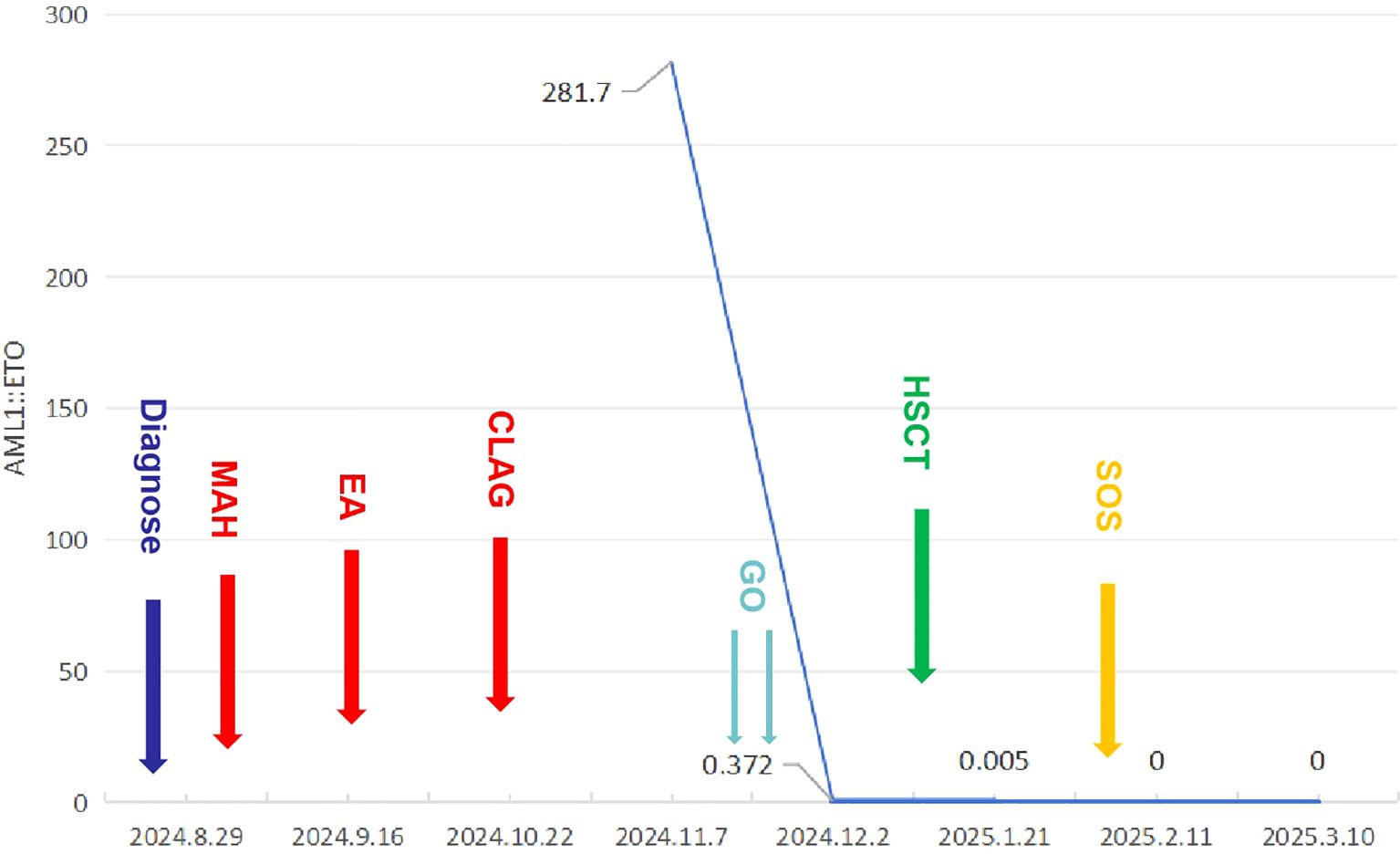
Figure 3. The patient’s complete treatment course, including variations in AML1::ETO transcript level throughout the therapeutic process.
Written informed consent for publication of this report and accompanying images was obtained from the parents.
AML with AML1-ETO fusion is generally associated with favorable outcomes due to high remission and survival rates. Approximately 25% of these cases harbor KIT mutations, which are linked to a poor prognosis (11, 12). A previous retrospective study demonstrated that 10% of AML1::ETO+ AML patients exhibited concurrent SM (13). A multicenter retrospective study, conducted in China from January 2009 to December 2022, identified 24 cases of SM with AML1::ETO+ AML across 16 centers and 212 cases of AML1::ETO+ AML with KIT mutations within the same timeframe (7). Based on the available data, we formulate a hypothesis that the coexistence of SM may contribute to some adverse effects of KIT on prognosis. Comprehensive screening is advisable for AML1::ETO+ AML patients with KIT mutations who demonstrate a suboptimal response to treatment, to ascertain the coexistence of SM. In cases of SM-AHN, abnormal mast cell infiltration can be masked by AHN cell infiltration. Consequently, we recommend repeating bone marrow aspiration and biopsy during the cytoreductive phase following chemotherapy, as this can improve the detection rate of SM, the rationale of this strategy is supported by our reported case.
SM with AML1::ETO+ AML often display a suboptimal response to standard induction chemotherapy, exhibiting frequent primary resistance to the treatment. Furthermore, aberrant MCs often persists even after achieving leukemia remission (6). The patient reported herein demonstrates primary drug resistance and failed to achieve remission despite undergoing salvage therapy with a cladribine-based regimen. Recently, small-molecule tyrosine kinase inhibitors (TKIs) targeting KIT have demonstrated promising clinical efficacy (1, 8, 14). Avapritinib has received FDA approval as a first-line treatment for adult patients with advanced SM, based on data from two clinical trials: EXPLORER (NCT02561988) (15) and PATHFINDER (NCT03580655) (16). However, considering the patient’s actual condition, avapritinib treatment is deemed unsuitable.
The abnormal mast cell immunophenotype is characterized by aberrant expression of CD25, CD2, or CD30. CD30, assessed using either flow cytometry or immunohistochemistry, is detected in 80% to 90% of SM cases (17). Therefore, targeting this antigen for the treatment of SM represents a viable strategy. A previous case series reported the clinical outcomes of four patients with CD30+ aggressive SM (ASM) or indolent SM who were treated with brentuximab vedotin (BV). Among the patients, two exhibited response. One patient demonstrated a durable response for over three years (18). In a subsequent Phase II clinical trial (NCT 01807598) (19), ten patients with CD30+ advanced SM, five of whom had SM-AHN, were enrolled. The results indicate that BV monotherapy is insufficient for the treatment of SM, especially in SM-AHN patients. Treating SM with AML1::ETO+ AML presents significant challenges, as it necessitates concurrent management of both disorders. In this scenario, CD30 may not serve as an optimal therapeutic target owing to its limited efficacy and the absence of expression on the surfaces of AML1::ETO+ AML cells. CD33 is expressed on early-committed myelomonocytic precursors and AML cells, but is absent on hematopoietic stem cells. Conversely, MCs exhibit high expression levels of CD33 on their surface membrane, irrespective of their maturation stage (20). Prior research has demonstrated that GO inhibits the proliferation and induces apoptosis in both normal and neoplastic MCs in vitro, GO neither induced secretion of histamine from MCs nor upregulated the anti-IgE-induced release of histamine in these cells (21). Presently, only one case report exist on the application of GO in the management of SM. Alvarez-Twose et al (22) reported a case of refractory mast cell leukemia negative for KIT mutations, which was successfully treated with monotherapy using GO, achieving sustained complete remission. Our successful case study, coupled with pertinent literature, has facilitated the progression of future clinical trials involving GO for the treatment of SM-AHN. Despite inherent limitations that may compromise the robustness of its conclusions, this case report still warrants recommending the therapeutic approach. The aim is to explore its potential benefits in patients with a poor prognosis.
Allo-HSCT significantly enhances the prognosis of patients with SM with AML1::ETO+ AML, warranting its strong recommendation for this patient population (7, 23). The reported patient achieved immunological remission and subsequently underwent allo-HSCT. Exposure to GO prior to transplantation was associated with an increased risk of developing specific transplant-related complications, notably SOS. Consequently, the implementation of more effective preventive measures for SOS is recommended. Allo-HSCT yields promising results in treating SM-AHN, with a 3-year overall survival rate of 74% (24); however, post-transplantation efficacy remains suboptimal for SM with AML1::ETO+ AML patients. The persistence of MCs in patients undergoing allo-HSCT constitutes a fascinating clinical observation, as post-transplantation relapse frequently leads to treatment failure (6).
A recent retrospective study has shown that the progression-free survival (PFS) following allo-HSCT for SM-AML is merely 0.7 years (25).The absence of a KIT D816V mutation, the presence of a complex karyotype, and the lack of pre-transplant TKI usage adversely affected PFS post allo-HSCT. Considering the patient’s currently favorable remission state, yet due to the presence of aforementioned risk factors, there is a high risk of disease relapse. Hence, initiating appropriate post-transplant maintenance therapy, such as TKIs, is a reasonable choice for treatment.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Ethics Committee of Beijing Lu Daopei Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
SX: Writing – original draft, Writing – review & editing. MC: Methodology, Software, Writing – review & editing. HS: Conceptualization, Methodology, Writing – review & editing. TW: Methodology, Writing – review & editing. LZ: Methodology, Writing – review & editing. XC: Investigation, Methodology, Project administration, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pardanani A. Systemic mastocytosis in adults: 2023 update on diagnosis, risk stratification and management. Am J Hematol. (2023) 98:1097–116. doi: 10.1002/ajh.26962
2. Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. (2009) 113:5727–36. doi: 10.1182/blood-2009-02-205237
3. Radia DH, Green A, Oni C, Moonim M. The clinical and pathological panoply of systemic mastocytosis. Br J Hematol. (2020) 188:623–40. doi: 10.1111/bjh.v188.5
4. Cornet E, Dumezy F, Roumier C, Lepelley P, Jouy N, Philippe N, et al. Involvement of a common progenitor cell in core binding factor acute myeloid leukemia associated with mastocytosis. Leuk Res. (2012) 36:1330–3. doi: 10.1016/j.leukres.2012.07.001
5. Xie W, Wang SA, Yin CC, Xu J, Li S, Bueso-Ramos CE, et al. Acute myeloid leukemia with t(8;21)(q22;q22.1)/RUNX1-RUNX1T1 and KIT Exon 8 mutation is associated with characteristic mastocytosis and dismal outcomes. Exp Mol Pathol. (2019) 108:131–6. doi: 10.1016/j.yexmp.2019.04.009
6. Pullarkat ST, Pullarkat V, Kroft SH, Wilson CS, Ahsanuddin AN, Mann KP, et al. Systemic mastocytosis associated with t(8;21)(q22;q22) acute myeloid leukemia. J Hematop. (2009) 2:27–33. doi: 10.1007/s12308-009-0023-2
7. Zhang Z, Yin J, Lian G, Bao X, Hu M, Liu Z, et al. A multicenter retrospective comparison between systemic mastocytosis with t(8;21) AML and KIT mutant t(8;21) AML. Blood Adv. (2024) 8:889–94. doi: 10.1182/bloodadvances.2023012006
8. Below S, Michaelis LC. Avapritinib in the treatment of systemic mastocytosis: an update. Curr Hematol Malig Rep. (2021) 16:464–72. doi: 10.1007/s11899-021-00650-4
9. Weisberg E, Meng C, Case AE, Sattler M, Tiv HL, Gokhale PC, et al. Comparison of effects of midostaurin, crenolanib, quizartinib, gilteritinib, sorafenib and BLU-285 on oncogenic mutants of KIT, CBL and FLT3 in hematological Malignancies. Br J Hematol. (2019) 187:488–501. doi: 10.1111/bjh.v187.4
10. Medawar G, Sakalabaktula K, Magri J, Rinker E, Baratam P. KIT V560D-mutated systemic mastocytosis associated with high-risk myelodysplastic syndrome: A unique case of systemic mastocytosis-associated hematologic neoplasm. Case Rep Hematol. (2024) 2024:4360304. doi: 10.1155/crh/4360304
11. Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. (2017) 129:424–47. doi: 10.1182/blood-2016-08-733196
12. Christen F, Hoyer K, Yoshida K, Hou HA, Waldhueter N, Heuser M, et al. Genomic landscape and clonal evolution of acute myeloid leukemia with t(8;21): an international study on 331 patients. Blood. (2019) 133:1140–51. doi: 10.1182/blood-2018-05-852822
13. Johnson RC, Savage NM, Chiang T, Gotlib JR, Cherry AM, Arber DA, et al. Hidden mastocytosis in acute myeloid leukemia with t(8;21)(q22;q22). Am J Clin Pathol. (2013) 140:525–35. doi: 10.1309/AJCP1Q0YSXEAHNKK
14. Xue S, Huang W, Liu F, Zhang Y, Hao Q, Cui B, et al. Rapid response to avapritinib of acute myeloid leukemia with t(8;21) and KIT mutation relapse post allo-HSCT. Leuk Lymph. (2022) 63:2247–50. doi: 10.1080/10428194.2022.2064994
15. DeAngelo DJ, Radia DH, George TI, Robinson WA, Quiery AT, Drummond MW, et al. Safety and efficacy of avapritinib in advanced systemic mastocytosis: the phase 1 EXPLORER trial. Nat Med. (2021) 27:2183–91. doi: 10.1038/s41591-021-01538-9
16. Gotlib J, Reiter A, Radia DH, Deininger MW, George TI, Panse J, et al. Efficacy and safety of avapritinib in advanced systemic mastocytosis: interim analysis of the phase 2 PATHFINDER trial. Nat Med. (2021) 27:2192–9. doi: 10.1038/s41591-021-01539-8
17. Nwogbo OV, Fang H, Wang W, Xu J, Miranda RN, Bose P, et al. Multicolor flow cytometric immunophenotyping is highly sensitive and specific in identifying aberrant mast cells in the diagnostic workup of systemic mastocytosis. Am J Clin Pathol. (2024) 161:598–608. doi: 10.1093/ajcp/aqad187
18. Borate U, Mehta A, Reddy V, Tsai M, Josephson N, Schnadig I. Treatment of CD30-positive systemic mastocytosis with brentuximab vedotin. Leuk Res. (2016) 44:25–31. doi: 10.1016/j.leukres.2016.02.010
19. Gotlib J, Baird JH, George TI, Langford C, Reyes I, Abuel J, et al. A phase 2 study of brentuximab vedotin in patients with CD30-positive advanced systemic mastocytosis. Blood Adv. (2019) 3:2264–71. doi: 10.1182/bloodadvances.2019000152
20. Valent P, Cerny-Reiterer S, Herrmann H, Mirkina I, George TI, Sotlar K, et al. Phenotypic heterogeneity, novel diagnostic markers, and target expression profiles in normal and neoplastic human mast cells. Best Pract Res Clin Hematol. (2010) 23:369–78. doi: 10.1016/j.beha.2010.07.003
21. Krauth MT, Bohm A, Agis H, Sonneck K, Samorapoompichit P, Florian S, et al. Effects of the CD33-targeted drug gemtuzumab ozogamicin (Mylotarg) on growth and mediator secretion in human mast cells and blood basophils. Exp Hematol. (2007) 35:108–16. doi: 10.1016/j.exphem.2006.09.008
22. Alvarez-Twose I, Martinez-Barranco P, Gotlib J, Gotlib J, García-Montero A, Morgado JM, Jara-Acevedo M, et al. Complete response to gemtuzumab ozogamicin in a patient with refractory mast cell leukemia. Leukemia. (2016) 30:1753–6. doi: 10.1038/leu.2016.30
23. McLornan DP, Czerw T, Damaj G, Ethell M, Gurnari C, Hernández-Boluda JC, et al. Allogeneic hematopoietic cell transplantation for advanced systemic mastocytosis: Best practice recommendations on behalf of the EBMT Practice Harmonization and Guidelines Committee. Leukemia. (2024) 38:699–711. doi: 10.1038/s41375-024-02182-1
24. Ustun C, Reiter A, Scott BL, Nakamura R, Damaj G, Kreil S, et al. Hematopoietic stem-cell transplantation for advanced systemic mastocytosis. J Clin Oncol. (2014) 32:3264–74. doi: 10.1200/JCO.2014.55.2018
Keywords: gemtuzumab-ozogamicin., systemic mastocytosis (SM), AML1::ETO, acute myeloid leukemia, systemic mastocytosis with an associated hematological neoplasm
Citation: Xue S, Chen M, Sun H-P, Wang T, Zhang L-N and Cao X-Y (2025) Case Report: Rapid response to gemtuzumab-ozogamicin in a pediatric patient with refractory systemic mastocytosis with AML1::ETO+ acute myeloid leukemia. Front. Immunol. 16:1566805. doi: 10.3389/fimmu.2025.1566805
Received: 25 January 2025; Accepted: 24 March 2025;
Published: 09 April 2025.
Edited by:
Khalil Saleh, Gustave Roussy Cancer Campus, FranceReviewed by:
Jing-dong Zhou, Jiangsu University Affiliated People’s Hospital, ChinaCopyright © 2025 Xue, Chen, Sun, Wang, Zhang and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xing-Yu Cao, Y2FveGluZ3l1MjAyMEBzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.