
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 17 April 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1566016
 Lingling Yin1,2,3†
Lingling Yin1,2,3† Bin Lv1,3†
Bin Lv1,3† Jiao Ge4†
Jiao Ge4† Yuekun Qi1,2,3
Yuekun Qi1,2,3 Jieyun Xia1,2,3
Jieyun Xia1,2,3 Sha Ma1,2,3
Sha Ma1,2,3 Ying Wang1,2,3
Ying Wang1,2,3 Yang Liu1,2,3
Yang Liu1,2,3 Dian Zhou1,2,3
Dian Zhou1,2,3 Jiang Cao1,2,3
Jiang Cao1,2,3 Zhiling Yan1,2,3
Zhiling Yan1,2,3 Kunming Qi1,2,3
Kunming Qi1,2,3 Wei Sang1,2,3
Wei Sang1,2,3 Depeng Li1,2,3
Depeng Li1,2,3 Hai Cheng1,2,3
Hai Cheng1,2,3 Wei Chen1,2,3
Wei Chen1,2,3 Kailin Xu1,2,3
Kailin Xu1,2,3 Weiying Gu4*
Weiying Gu4* Zhenyu Li1,2,3*
Zhenyu Li1,2,3* Feng Zhu1,2,3*
Feng Zhu1,2,3*Background: In recent years, chimeric antigen receptor (CAR)-T cell therapy has achieved tremendous efficacy in relapsed/refractory multiple myeloma (R/R MM). However, the impact of antibiotic (ATB) use on R/R MM patients treated with CAR-T is still not known. The aim of our study was to analyse the influence of ATB on the clinical outcomes of R/R MM patients treated with CAR-T cells.
Methods: In this retrospective study, 199 patients with R/R MM who received CAR-T cells between January 2018 and December 2023 were evaluated from two hospitals in China. They were stratified into ATB-group and No ATB-group according to whether ATB was administered in the 4 weeks before therapy. We mainly analyzed the efficacy, survival outcomes and cytotoxicity of CAR-T cell therapy in two groups of patients.
Result: In the ATB group (90 patients), the overall response rate (ORR) was 70% comparable to the No ATB group (109 patients: ORR, 81.7%; P = 0.054). The complete response rate (CRR) was 40%, which was significantly lower compared with No ATB group (CRR, 57.8%; P = 0.012). The median progression-free survival (PFS) was 6.7 months while the median overall survival (OS) was 21.9 months for the ATB group. The median PFS and OS for the No ATB group were 13.9 months and 36.1 months. There were significant differences in PFS (P = 0.007) and OS (P = 0.004) between the evaluated groups. Nonetheless, multivariate analysis found ATB use did not reduce the CRR (odds ratio [OR], 0.947; 95% confidence interval [CI], 0.251 to 3.565, P = 0.936). Besides, administration of ATB did not affect the PFS (hazard ratio [HR], 0.634; 95% CI, 0.28 to 1.436, P = 0.275) and OS (HR, 2.259; 95% CI, 0.755 to 6.762, P = 0.145) in R/R MM patients treated with CAR-T cells. Additionally, both groups of patients had similar incidences of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).
Conclusion: Our results point to a detrimental effect of ATB on treatment outcomes to CAR-T cell therapy. However, the use of ATB is not associated with the incidence of CRS or ICANS.
Chimeric antigen receptor (CAR)-T cell therapy represents a breakthrough in the treatment of relapsed/refractory multiple myeloma (R/R MM). Especially in recent years, it has shown unprecedented antitumor efficacy and stands at the novel forefront of current R/R MM therapy (1–3). Numerous studies have shown that the R/R MM patients treated with anti-B cell maturation antigen (anti-BCMA) CAR-T cells have overall response rates (ORR) ranging from 81% to 100% (4–8). The combination of anti-BCMA and anti-CD19 CAR-T cells has shown a manageable long-term safety profile, with an ORR as high as 92% and durable responses (9). G protein-coupled receptor, class C group 5 member D (GPRC5D), another promising target, has also demonstrated extremely high safety and efficacy (10–13). More recently, our center reported that anti-BCMA/GPRC5D bispecific CAR-T cells yielded 86% ORR and 62% complete response rate (CRR) with no fatal adverse events (14). However, despite the favorable outcomes, not all R/R MM patients respond to CAR-T therapy (15). Patients who do not respond to CAR-T treatment often experience disease progression and may even suffer severe CAR-T mediated adverse effects, such as cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity syndrome (ICANS) (16). Thus, the search for potential factors influencing its efficacy is urgent and extremely necessary for a more targeted selection of treatment populations in clinical practice.
Gut microbiota is increasingly considered an important factor associated with both tumor development and the effect of T cell-driven anticancer immunotherapy (17). Recently, growing evidence has indicated that gut microbiota signatures may be harnessed to predict therapeutic response or adverse effects in optimizing CAR-T cell therapy (18–21). Hu et al. observed significant differences in diversity and abundance of Bifidobacterium, Prevotella, Sutterella, and Collinsella between MM patients treated with second-generation BCMA CAR-T cells in complete remission (CR) and those in partial remission (PR) (21). In another recent study, Smith and colleagues found that higher abundance of Ruminococcus, Bacteroides and Faecalibacterium were associated with response to CD19 CAR-T cell therapy (22).
Antibiotic (ATB) therapy is commonly performed in clinic for R/R MM patients, who are more susceptible to infection because of hypogammaglobulinemia or treatment-related immune suppression. ATBs are potentially life-saving medicines, but they can impair the homeostasis of gut microbiota, resulting in decreased microbial abundance and diversity. Therefore, it is necessary to determine whether ATB use affects the efficacy of CAR-T treatment and the prognosis of R/R MM patients. Nowdays, the association between ATB use and the prognosis of cancer in CAR-T cell therapy remains controversial. Uribe-Herranz et al. found that mice receiving vancomycin in combination with CD19-directed CAR-T cell therapy showed increased tumor response and tumor-associated antigens (TAAs) cross-presentation compared with those of mice receiving CD19 CAR-T cell therapy alone in lymphoma murine model (23). Smith et al. conducted the first human study to investigate the influence of ATBs on the response and toxicity of anti-CD19 CAR-T cell therapy in patients with B cell malignancies. They demonstrated that exposure to ATBs in general and in particular to broad-spectrum anaerobe-targeting ones (piperacillin/tazobactam, meropenem and imipenem/cilastatin) within 4 weeks before therapy was associated with worse survival and increased neurotoxicity (22). In another recent study by Stein-Thoeringer et al., an association between exposure to ATBs prior to CD19 CAR-T cell infusion and increased incidences of cancer relapse/disease progression and worse overall survival (OS) in lymphoma patients was also observed (24). However, the impact of ATB on CAR-T cell efficacy in R/R MM has not been established.
Thus, in order to learn about the specific association between ATB use and CAR-T treatment of R/R MM patients and provide potential reference to clinic performance, we carried out a retrospective analysis to investigate the impact of ATB on outcomes of CAR-T cell therapy in R/R MM.
A retrospective cohort study was performed on R/R MM patients who received CAR-T cells at the Affiliated Hospital of Xuzhou Medical University and The First People’s Hospital of Changzhou during the period from January 2018 to December 2023 (ChiCTR1900026219, ChiCTR2000033194, ChiCTR2100048888). Patients age 18-75 years with good performance status (Karnofsky Performance score ≥50), a life expectancy of more than 12 weeks and adequate organ function. Patients with active infections, psychological or mental illnesses, severe allergies, or a history of severe allergies were excluded. The detailed inclusion and exclusion criteria could refer to our previous studies (10, 25). All patients were infused with BCMA- or GPRC5D-directed CAR-T cells. CAR structures were as described previously (10, 26, 27). Lymphodepletion regimen was cyclophosphamide (750 mg/m2/d, day -5) and fludarabine (30 mg/m2/d, days -5 to -3). All patients were followed-up until death or data lock (October 2024). The study was conducted with the approval from the ethics committee of the Affiliated Hospital of Xuzhou Medical University and The First People’s Hospital of Changzhou. Written informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Clinicopathologic characteristics of patients were collected at enrollment, including age, sex, MM type, International Staging System (ISS) stage, Karnofsky Performance score (KPS), tumor burden, prior treatment, cytogenetic abnormalities and extramedullary diseases (EMD). The class and indication of ATB treatment 4 weeks before CAR-T cell infusion were collected, if available. In order to avoid the influence of antiviral drugs on the results, patients with COVID-19 and other viral infections were not included in our study. Laboratory data was obtained by retrieving electronic medical records. Peripheral blood T cell counts (CD3, CD4, CD8) were assessed at leukapheresis. The levels of c-reactive protein (CRP), lactate dehydrogenase (LDH), beta-2 microglobulin (β2-MG), and erythrocyte sedimentation rate (ESR) were recorded at lymphodepletion.
The International Myeloma Working Group criteria was used to evaluate clinical response of patients with R/R MM (28). Progression-free survival (PFS) was calculated from CAR-T cell infusion to the date of disease progression or death. OS was defined as the time from CAR-T cell infusion to death from any cause. CRS or ICANS was graded according to the American Society for Transplantation and Cellular Therapy consensus grading system (29).
Descriptive statistics included medians (ranges) for all continuous variables and numbers (percentages) for categorical variables. The continuous variables were analyzed by the Mann-Whitney U test. χ2 or the Fisher’s exact test were used for categorical variables. Logistic regression was performed to estimate risk factors of CRR after CAR-T cell infusion. PFS and OS survival curves were estimated by the Kaplan-Meier method. Cox proportional hazards regression models were used for the analysis of clinical factors related to survival. Two-tailed P < 0.05 was considered statistically significant. All statistical analyses were performed using the IBM SPSS 27.0 software.
We identified 199 R/R MM patients treated with CAR-T cell during the study period. The median age was 59 years (range 29-74), and 53.8% of patients were male. Amongst this cohort, 90 (45.2%) patients received ATB within 4 weeks before CAR-T cell infusion and 109 (54.8%) patients did not. Respiratory tract infections were the most common indication for ATB prescriptions. By far, cephalosporin (CEP) was the most frequently administered ATB (63.3% of exposed patients), followed by 33.3% fluoroquinolone (FLU) (Figure 1, Table 1). The clinical characteristics of the patients are shown in Table 2. There was statistical difference between the two groups in terms of KPS (P = 0.033) and previous therapy lines (P = 0.014). Compared to the No ATB group, patients in the ATB group more frequently received a prior auto-hematopoietic stem cell transplantation (41.1% vs. 24.8%, P = 0.014). In the ATB group, the proportion of patients with EMD was significantly higher than that in the No ATB group (P = 0.002). Other baseline characteristics were not remarkably different between the two groups.
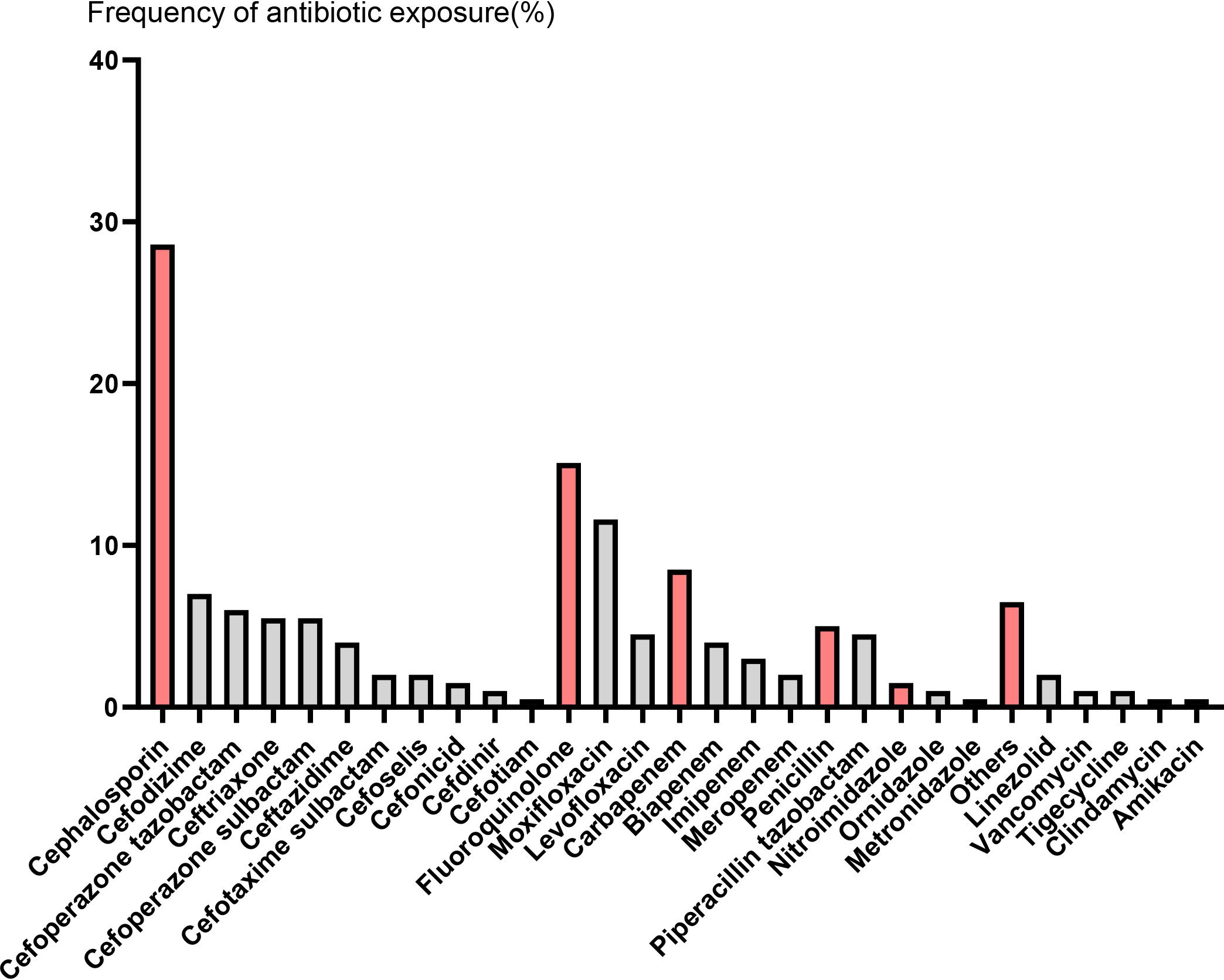
Figure 1. The frequency of antibiotic exposure in the four weeks prior to CAR-T cell infusion. Red columns represent different types of antibiotics, followed by gray columns that represent specific antibiotic names.
Patients achieved the best response within a median time of 1.8 months (range 0.4-7.3). In the ATB group, ORR was 70.0%, including 19 (21.1%) stringent complete response (sCR), 17 (18.9%) CR, 9 (10.0%) very good partial response (VGPR), and 18 (20.0%) PR. In the No ATB group, ORR was 81.7%, including 39 (35.8%) sCR, 24 (22.0%) CR, 14 (12.8%) VGPR, and 12 (11.0%) PR (Figure 2). The ues of ATB did not affect the ORR (P = 0.054). In contrast, difference in CRR between the ATB group and No ATB group was significant (P = 0.012) (Table 3). Owing to the varied effects of different ATB on the gut microbiota (30), we investigated whether different ATB could affect the efficacy of CAR-T therapy differently. We analyzed CEP and FLU for this purpose, which were selected as they were the most commonly used among patients, with CEP prescribed to 57 patients (28.6% of all patients) and FLU to 30 patients (15.1%), making them suitable for statistical analysis (Figure 1). Patients who received CEP had lower ORR (P = 0.005) and CRR (P = 0.009) compared to patients not receiving CEP. Likewise, patients received FLU had lower CRR (P = 0.002) than those who did not (Table 3).
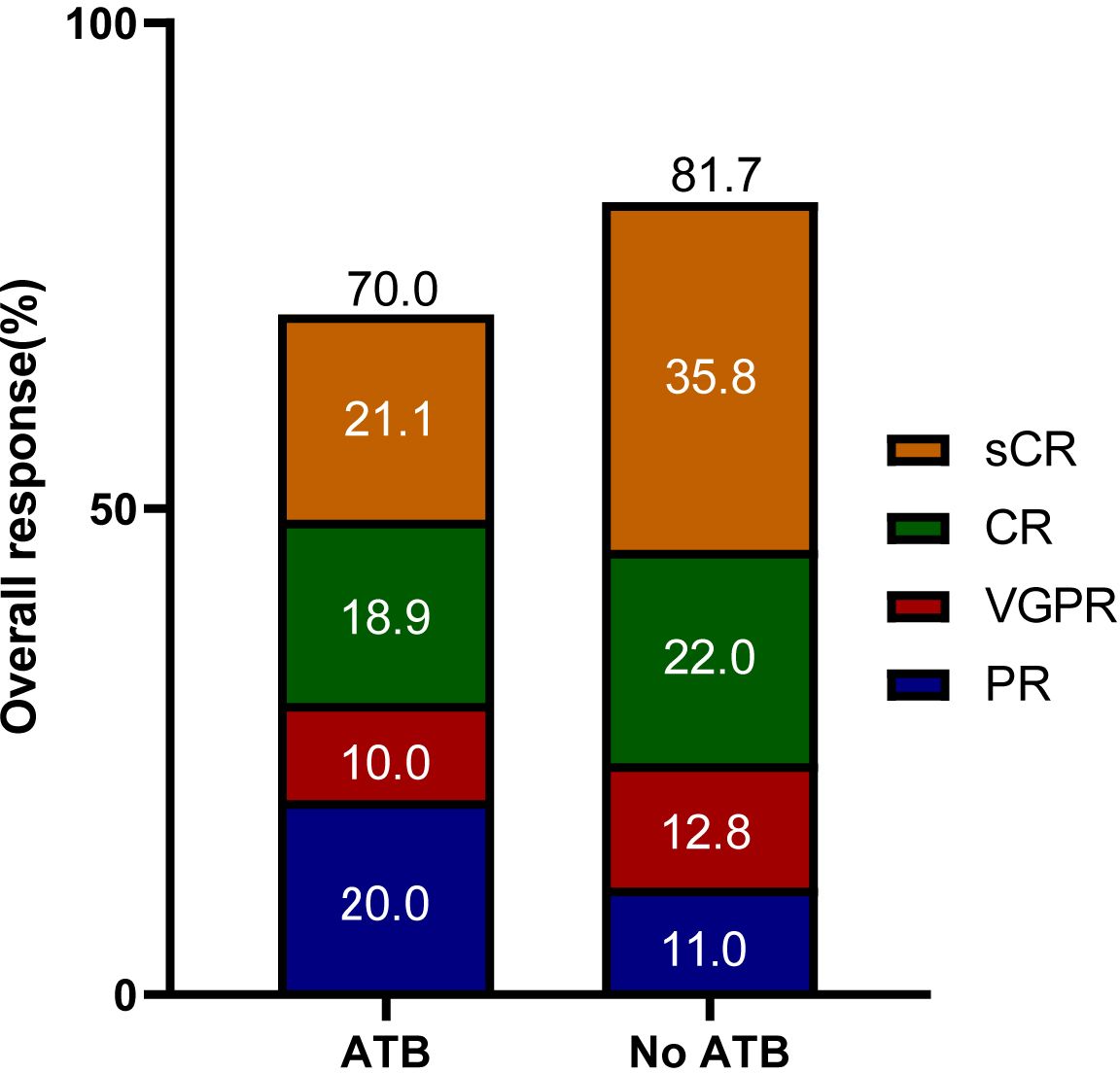
Figure 2. Overall responses of patients in the ATB group and No ATB group. sCR, stringent complete response; CR, complete response; PR, partial response; VGPR, very good partial response.
Considering the impact of ATB on CRR, we queried whether patients who were exposed to ATB were the ones with more advanced disease and disease-related comorbidities that led to ATB therapy. We further analyzed the clinical factors that might be associated with efficacy of CAR-T cell therapy in patients with R/R MM. Univariate analysis confirmed that ATB therapy was associated with poor response to CAR-T treatment but lost its association in multivariate analysis (Table 4). Multivariate analyses stratified by the types of ATB showed that CEP and FLU were not independent factors affecting CRR. In contrast, high-risk cytogenetics was an independent predictive factor for better CRR.
We next examined whether the use of ATB affected the PFS or OS in these patients. At a median follow-up of 16.9 months, the median PFS was only 6.7 months (95% CI, 5.055 to 8.345) and the median OS was only 21.9 months (95% CI, 13.664 to 30.136) in the ATB group, which were significantly shorter than those in the No ATB group (median PFS: 13.9 months, 95% CI, 9.63 to 18.17; median OS: 36.1 months, 95% CI, 27.226 to 44.974) (Figure 3A). Since CEP and FLU were commonly used, we also explored their effects on CAR-T cell immunotherapy long-term outcomes. Exposure to CEP during the 4 weeks preceding CAR-T cell infusion was associated with worse PFS (P = 0.008) and OS (P = 0.025) (Figure 3B). PFS was lower in patients treated with FLU (P = 0.023), with a trend toward a decreased rate in OS (P = 0.15) (Figure 3C).
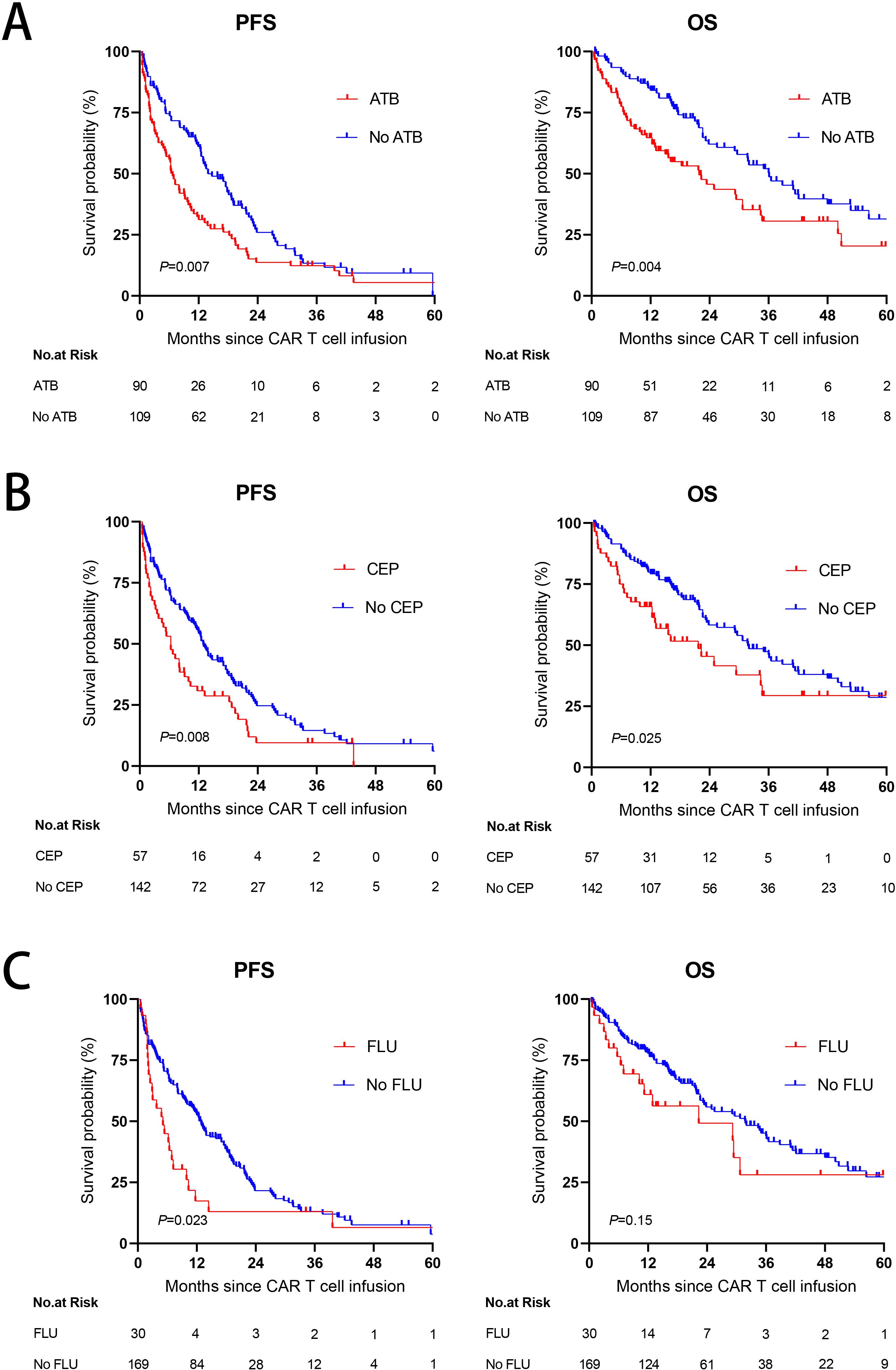
Figure 3. The impact of antibiotic use on PFS and OS. (A) Kaplan-Meier survival curves in R/R MM populations according to exposure to ATB within the 4 weeks before CAR-T cell infusion. (B, C) Kaplan-Meier curves of PFS and OS according to exposure to CEP (B) or FLU (C) within the 4 weeks before CAR-T cell infusion. P values were shown (log-rank test).
ATB-treated patients could feature more aggressive disease and/or deteriorated clinical status which may have a potential confounding effect on survival. To evaluate these possibilities, we investigated tumor burden and systemic inflammation in our patients prior to CAR-T cell therapy initiation. LDH or β2-MG as a surrogate marker for tumor burden had similar level between the ATB group and No ATB group (LDH: P = 0.378; β2-MG: P = 0.228). To eliminate the confounding effect of baseline inflammatory status on survival, we investigated differences in baseline inflammatory markers such as ESR and CRP. No associations were observed between ATB administration and ESR (P = 0.208), CRP (P = 0.064). In addition to tumor burden and physical status, the quality of autologous CAR-T cells also affects survival outcomes, which is heavily dependent on the quality of the T cells harvested from the patient. Intriguingly, patients receiving ATB displayed significantly lower peripheral CD4 T cell counts (P = 0.045) and a trend towards lower CD3 (P = 0.144) and CD8 T cells (P = 0.373) (Figure 4).
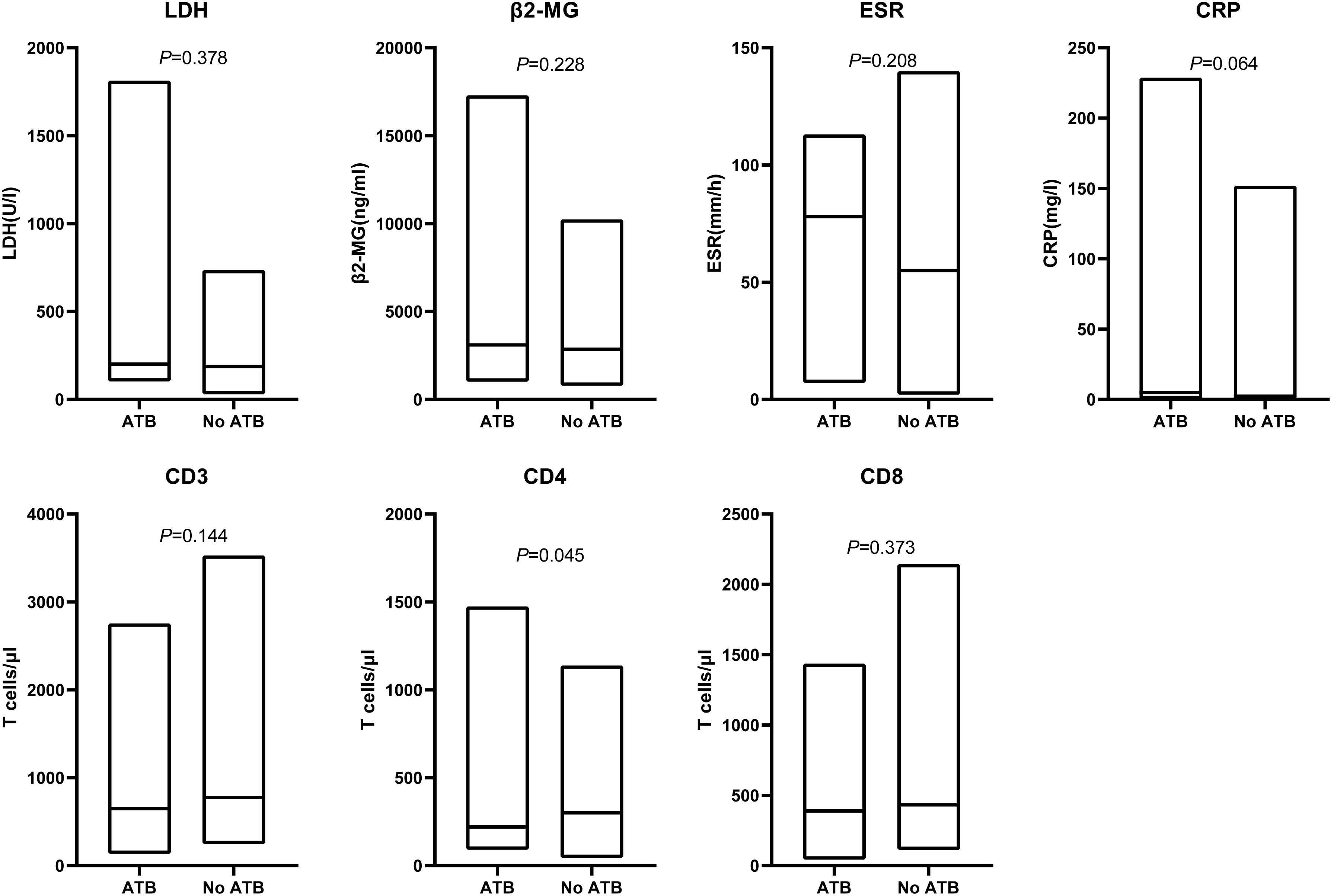
Figure 4. Associations of ATB use and tumor burden, systemic inflammation, peripheral blood T cell counts. Serum levels of LDH, β2-MG, ESR, CRP before lymphodepletion. Peripheral blood CD3, CD4 and CD8 T cell counts at the time point of leukapheresis. LDH, lactate dehydrogenase; β2-MG, beta-2 microglobulin; ESR, erythrocyte sedimentation rate;CRP, c-reactive protein. Line within the box plots indicates median, lower box bound indicates min, upper box bound indicates max. P values were calculated with the Mann–Whitney U test.
Finally, to further determine whether ATB was an independent prognostic factor for PFS and OS, we carried out univariate and multivariate analyses of the effect of ATB administration, taking into account classical prognostic factors relevant to R/R MM. The univariate analysis revealed that the use of any ATB or CEP was a risk factor affecting the PFS (ATB: HR 1.53, 95% CI, 1.121 to 2.087, P = 0.007; CEP: HR 1.571, 95% CI, 1.118 to 2.206, P = 0.009) and OS (ATB: HR 1.762, 95% CI, 1.197 to 2.595, P = 0.004; CEP: HR 1.617, 95% CI, 1.057 to 2.474, P = 0.027). However, multivariate analysis confirmed that ATB or CEP utilization had no independent adverse influence on PFS or OS. Univariate and multivariate analyses found that FLU also significantly affected the PFS (HR 4.185, 95% CI, 1.285 to 13.631, P = 0.018) of the patients, with no statistical significance in OS (HR 1.321, 95% CI, 0.334 to 5.227, P = 0.691) (Table 5).
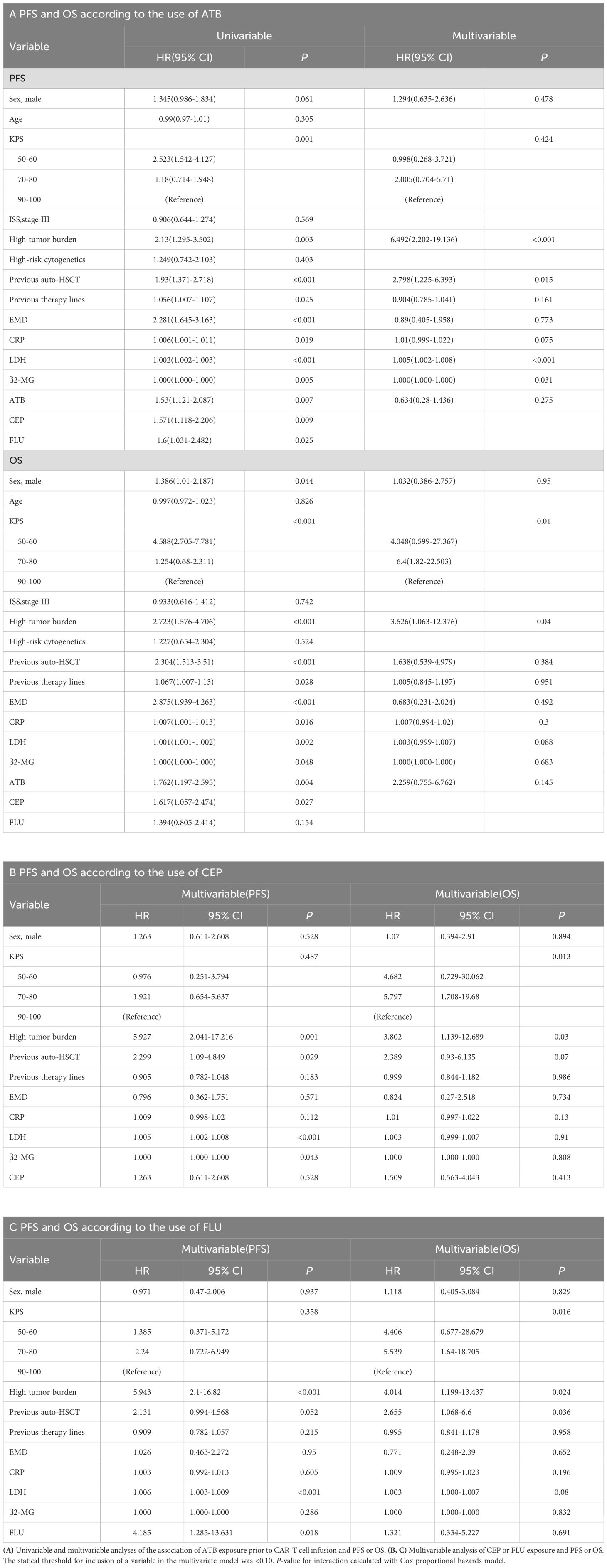
Table 5. Univariable and multivariable analyses of progression-free survival (PFS) and overall survival (OS).
Of all patients, 70.4% experienced CRS. Grade 3 or higher CRS, defined as severe CRS, occurred in 6 (3%) patients. Median time to onset of CRS was 8d (0-29 d), median duration of CRS was 4d (1-25d). In the ATB group, 36 (40%) patients had grade 1, 24 (26.7%) had grade 2, and 4 (4.4%) had grade≥ 3 CRS. In the No ATB group, 49 (45%) patients had grade 1, 25 (22.9%) had grade 2, and 2 (1.8%) had grade 3 CRS. ICANS occurred in 10 (5%) of 199 patients, and 4 (2%) patients had grade ≥3 ICANS. Median time to onset of ICANS was 10d (1-26 d), median duration of ICANS was 4d (1-13d). In the ATB group, 3 (3.3%) patients had grade 1, 1 (1.1%) had grade 2, and 1 (1.1%) had grade 4 ICANS. In the No ATB group, 2 (1.8%) patients had grade 1 and 3 (2.8%) had grade 3 ICANS. No significant difference was found in the incidence of CRS (P = 0.831) or ICANS (P = 0.757) between the two groups (Figure 5A). Similarly, we did stratified analyses of ATB classes, and the results were consistent (Figures 5B, C).
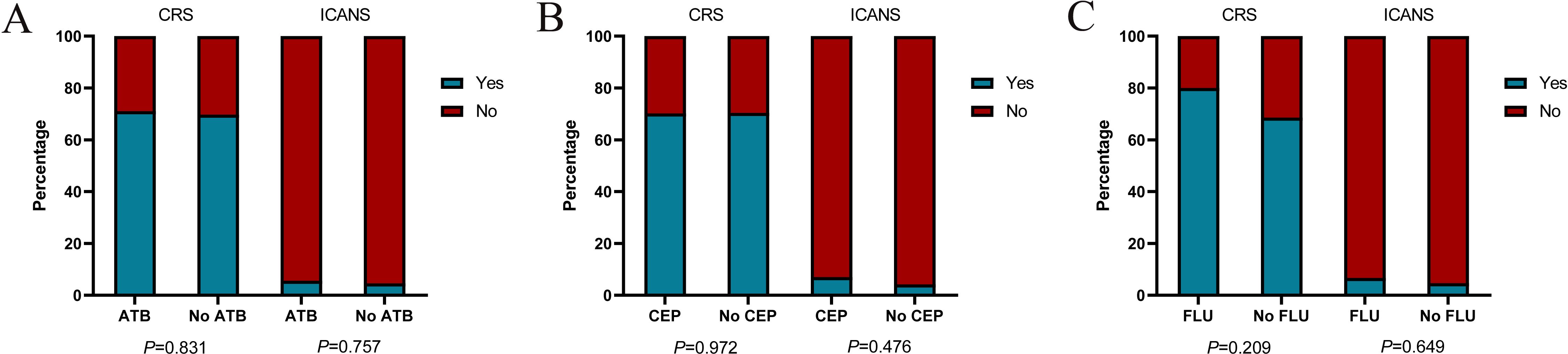
Figure 5. The incidences of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) in patients with CAR-T cell therapy. Histograms show the frequencies of CRS and ICANS according to exposure to any ATB (A), CEP (B), FLU (C) within the four weeks before CAR-T cell infusion. Blue (Yes) indicates the presence of CRS or ICANS of any grade, while red (No) indicates the absence of CRS or ICANS of any grade. P values were calculated using χ2 or Fisher’s exact test.
With the increased use of CAR-T in cancer therapeutics, tremendous effort has been made to search possible factors that influence its efficacy. Among the identified factors, increasing evidence has indicated a crucial role for the gut microbiota (18, 31). It is well known that ATB is the most common clinical cause of alterations in gut flora. There are theoretical concerns about whether the use of ATB will impair the efficiency of CAR-T cells. Currently, limited studies exploring the impact of ATB on clinical outcomes of CAR-T cell therapy are focused on lymphoma and acute lymphoblastic leukemia (ALL) (22, 24, 32), and no studies on MM have been conducted. To the best of our knowledge, this is the first retrospective study to investigate the relationship between ATB and CAR-T efficacy in the treatment of R/R MM.
In the present study, R/R MM patients who received ATB prior to CAR-T cell therapy had poor treatment response and worse long-term survival. Our results align with the previous cancer immunotherapy literature (33–37). In one study, patients with stage III and IV melanoma exposed to ATBs before initiation of immune checkpoint inhibitors (ICI) had statistically significantly worse OS than unexposed patients (38). Moreover, ATB exposure was associated with greater moderate to severe immune-mediated colitis. Similarly, in a study of 251 patients, of whom the 135 who received ATBs had lower response rates and shorter survival. Further research found that ATB exposure was associated with changes in certain cytokines and antibodies. In the lung cancer patients, they observed differences in interferon-gamma (IFN-γ), interleukin-8 (IL-8), and macrophage inflammatory proteins (39). In sum, ATB use is associated with poor therapeutic outcomes of immunotherapy. Our study also proved this view in CAR-T as a new immunotherapy method. In addition, Eng et al. (40) observed that ATB exposure, especially of FLU, prior to ICI therapy correlated with reduced OS. Similarly, Pederzoli et al. (41) linked FLU use to an increased recurrence rate. We evaluated the effects of CEP and FLU, the most commonly used ATBs in clinical hematology at our hospital, on CAR-T efficacy, indicating that FLU significantly and adversely affected the PFS but not OS. It may be attributed to our small sample size, which needs more studies for further verification.
Establishing a causal relationship between ATB administration and poorer prognosis in patients undergoing CAR-T cell therapy is challenging. Two scenarios were deduced for the impact of ATB on CAR-T efficacy. In the first scenario, ATB use, even in the short term, causes a loss of intestinal microbial diversity called dysbiosis, which can persist for up to several months after the ATB treatment (42, 43). Therefore, ATB-induced perturbation of the balanced microbiome would adversely impact CAR-T therapy efficacy. In the second scenario, patients with very low immunity might be more prone to infections, may be more often in need of ATB therapy. More and more studies showed that normal immune system further eliminate the residual tumor cells by the anti-tumor immunological reaction. Furthermore, immune failure in MM is the important factor for disease progress. Therefore, these individuals might inherently struggle to benefit from CAR-T cell therapy, potentially leading to reduced PFS and OS. In addition, those with cumulative ATB use could experience immunosuppression due to severe infections, which adversely affects the efficacy of CAR-T treatment. Thus, ATB treatment might simply reflect poor physical conditions rendering it a surrogate marker of dismal outcomes, without any relationship to its effects on the gut microbiome. Multivariate analysis correcting for ATB-independent markers of poor outcomes (such as age, sex, KPS, high-risk cytogenetics, prior lines of treatment, tumor burden, LDH, previous auto-HSCT, EMD, CRP and β2-MG) showed that ATB use was not independently associated with worse outcomes, suggesting that ATB indeed may not be an independent prognostic factor. Of course, validation of these findings in larger clinical studies will be needed.
CRS and ICANS are common side effects of CAR-T cell therapy. Another finding of our study was that ATB-exposed patients experienced similar rates of CRS and ICANS. Our results differ from prior findings in lymphoma (22, 24). It could be related to the profoundly different nature of MM compared to lymphoma. MM is characterized by an aggressive proliferation with the abnormal plasma cells being localized mostly in the bone marrow, while lymphoma cells are usually found in lymphoid organs. Secondly, the incidence of ICANS is relatively low due to the small sample size.
Notably, some inherent limitations do exist in our study. To begin with, this was a retrospective study, so unavoidable bias, confounding, and missing data would be anticipated. ATB use was entirely based on the patient’s electronic medical record and may not represent an accurate prescription of what the patient was currently taking. Conversely, the patient may have been prescribed but never taken antibiotic. In addition, the total number of patients analyzed was relatively small, which prevented us from conducting subgroup analyses based on the time of antibiotic used, route of administration, etc. Finally, we did not elucidate the mechanism by which ATB exert a detrimental effect on clinical outcomes. We speculate that ATB use causes dynamic changes in the gut microbiota that affect CAR-T efficacy.
An important future direction that would address our study’s limitations would be larger, multicenter studies with standardized prospective data collection. Further investigations should include the analysis of fecal microbiome so as to explore the mechanism of ATB affecting CAR-T treatment outcomes.
In conclusion, the use of ATB prior to CAR-T therapy affects clinical outcomes of R/R MM patients. Given the known overutilization of ATB in current society, clinicians should exercise caution in patients who are scheduled to receive CAR-T therapy.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics committee of the Affiliated Hospital of Xuzhou Medical University, The First People’s Hospital of Changzhou. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
LY: Writing – original draft, Writing – review & editing. BL: Data curation, Writing – review & editing. JG: Data curation, Writing – review & editing. KX: Supervision, Writing – review & editing. WG: Supervision, Writing – review & editing. ZL: Supervision, Validation, Writing – review & editing. FZ: Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (grant 81800159 and 82170187), Construction Project of High Level Hospital of Jiangsu Province (GSPJS202414, GSPJS202417, GSPJS202420), Jiangsu Provincial Medical Key Discipline (Laboratory) Cultivation Unit (JSDW202213).
We would like to thank all the patients and their families who participated in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bhatt P, Kloock C, Comenzo R. Relapsed/refractory multiple myeloma: A review of available therapies and clinical scenarios encountered in myeloma relapse. Curr Oncol. (2023) 30:2322–47. doi: 10.3390/curroncol30020179
2. Ding H, Wu Y. CAR-T therapy in relapsed refractory multiple myeloma. Curr Med Chem. (2024) 31:4362–82. doi: 10.2174/0109298673268932230920063933
3. Shi M, Wang J, Huang H, Liu D, Cheng H, Wang X, et al. Bispecific CAR T cell therapy targeting BCMA and CD19 in relapsed/refractory multiple myeloma: a phase I/II trial. Nat Commun. (2024) 15:3371. doi: 10.1038/s41467-024-47801-8
4. Brudno JN, Maric I, Hartman SD, Rose JJ, Wang M, Lam N, et al. T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol. (2018) 36:2267–80. doi: 10.1200/JCO.2018.77.8084
5. Wang D, Wang J, Hu G, Wang W, Xiao Y, Cai H, et al. (CT103A) in patients with relapsed/refractory multiple myeloma. Blood. (2021) 137:2890–901. doi: 10.1182/blood.2020008936
6. Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. (2021) 398:314–24. doi: 10.1016/S0140-6736(21)00933-8
7. Martin T, Usmani SZ, Berdeja JG, Agha M, Cohen AD, Hari P, et al. Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol. (2023) 41:1265–74. doi: 10.1200/JCO.22.00842
8. Liu Y, Jie X, Nian L, Wang Y, Wang C, Ma J, et al. A combination of pre-infusion serum ferritin, CRP and IL-6 predicts outcome in relapsed/refractory multiple myeloma patients treated with CAR-T cells. Front Immunol. (2023) 14:1169071. doi: 10.3389/fimmu.2023.1169071
9. Wang Y, Cao J, Gu W, Shi M, Lan J, Yan Z, et al. Long-term follow-up of combination of B-cell maturation antigen and CD19 chimeric antigen receptor T cells in multiple myeloma. J Clin Oncol. (2022) 40:2246–56. doi: 10.1200/JCO.21.01676
10. Xia J, Li H, Yan Z, Zhou D, Wang Y, Qi Y, et al. Anti-G protein-coupled receptor, class C group 5 member D chimeric antigen receptor T cells in patients with relapsed or refractory multiple myeloma: A single-arm, phase II Trial. J Clin Oncol. (2023) 41:2583–93. doi: 10.1200/JCO.22.01824
11. Mailankody S, Devlin SM, Landa J, Nath K, Diamonte C, Carstens EJ, et al. GPRC5D-targeted CAR T cells for myeloma. N Engl J Med. (2022) 387:1196–206. doi: 10.1056/NEJMoa2209900
12. Zhang M, Wei G, Zhou L, Zhou J, Chen S, Zhang W, et al. GPRC5D CAR T cells (OriCAR-017) in patients with relapsed or refractory multiple myeloma (POLARIS): a first-in-human, single-centre, single-arm, phase 1 trial. Lancet Haematol. (2023) 10:e107–e16. doi: 10.1016/S2352-3026(22)00372-6
13. Jurgens EM, Firestone RS, Chaudhari J, Hosszu K, Devlin SM, Shah UA, et al. Phase I trial of MCARH109, a G protein-coupled receptor class C group 5 member D (GPRC5D)-targeted chimeric antigen receptor T-cell therapy for multiple myeloma: an updated analysis. J Clin Oncol. (2024), JCO2401785. doi: 10.1200/JCO-24-01785
14. Zhou D, Sun Q, Xia J, Gu W, Qian J, Zhuang W, et al. Anti-BCMA/GPRC5D bispecific CAR T cells in patients with relapsed or refractory multiple myeloma: a single-arm, single-centre, phase 1 trial. Lancet Haematol. (2024) 11:e751–e60. doi: 10.1016/S2352-3026(24)00176-5
15. Sidana S, Patel KK, Peres LC, Bansal R, Kocoglu MH, Shune L, et al. Safety and efficacy of standard-of-care ciltacabtagene autoleucel for relapsed/refractory multiple myeloma. Blood. (2025) 145:85–97. doi: 10.1182/blood.2024025945
16. Nasiri F, Asaadi Y, Mirzadeh F, Abdolahi S, Molaei S, Gavgani SP, et al. Updates on CAR T cell therapy in multiple myeloma. biomark Res. (2024) 12:102. doi: 10.1186/s40364-024-00634-5
17. Schubert ML, Rohrbach R, Schmitt M, Stein-Thoeringer CK. The potential role of the intestinal micromilieu and individual microbes in the immunobiology of chimeric antigen receptor T-cell therapy. Front Immunol. (2021) 12:670286. doi: 10.3389/fimmu.2021.670286
18. Zhang PF, Xie D. Targeting the gut microbiota to enhance the antitumor efficacy and attenuate the toxicity of CAR-T cell therapy: a new hope? Front Immunol. (2024) 15:1362133. doi: 10.3389/fimmu.2024.1362133
19. Gabrielli G, Shouval R, Ghilardi G, van den Brink M, Ruella M. Harnessing the gut microbiota to potentiate the efficacy of CAR T cell therapy. Hemasphere. (2023) 7:e950. doi: 10.1097/HS9.0000000000000950
20. Asokan S, Cullin N, Stein-Thoeringer CK, Elinav E. CAR-T cell therapy and the gut microbiota. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15030794
21. Hu Y, Li J, Ni F, Yang Z, Gui X, Bao Z, et al. CAR-T cell therapy-related cytokine release syndrome and therapeutic response is modulated by the gut microbiome in hematologic Malignancies. Nat Commun. (2022) 13:5313. doi: 10.1038/s41467-022-32960-3
22. Smith M, Dai A, Ghilardi G, Amelsberg KV, Devlin SM, Pajarillo R, et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat Med. (2022) 28:713–23. doi: 10.1038/s41591-022-01702-9
23. Uribe-Herranz M, Beghi S, Ruella M, Parvathaneni K, Salaris S, Kostopoulos N, et al. Modulation of the gut microbiota engages antigen cross-presentation to enhance antitumor effects of CAR T cell immunotherapy. Mol Ther. (2023) 31:686–700. doi: 10.1016/j.ymthe.2023.01.012
24. Stein-Thoeringer CK, Saini NY, Zamir E, Blumenberg V, Schubert ML, Mor U, et al. A non-antibiotic-disrupted gut microbiome is associated with clinical responses to CD19-CAR-T cell cancer immunotherapy. Nat Med. (2023) 29:906–16. doi: 10.1038/s41591-023-02234-6
25. Wang Y, Li C, Xia J, Li P, Cao J, Pan B, et al. Humoral immune reconstitution after anti-BCMA CAR T-cell therapy in relapsed/refractory multiple myeloma. Blood Adv. (2021) 5:5290–9. doi: 10.1182/bloodadvances.2021004603
26. Yan Z, Cao J, Cheng H, Qiao J, Zhang H, Wang Y, et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: a single-arm, phase 2 trial. Lancet Haematol. (2019) 6:e521–e9. doi: 10.1016/S2352-3026(19)30115-2
27. Ma S, Li H, Zhou D, Zhang X, Shi M, Cao J, et al. Associations of granulocyte colony-stimulating factor with toxicities and efficacy of chimeric antigen receptor T-cell therapy in relapsed or refractory multiple myeloma. Cytotherapy. (2023) 25:653–8. doi: 10.1016/j.jcyt.2023.01.011
28. Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. (2016) 17:e328–e46. doi: 10.1016/S1470-2045(16)30206-6
29. Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. (2019) 25:625–38. doi: 10.1016/j.bbmt.2018.12.758
30. Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. (2019) 79:471–89. doi: 10.1016/j.jinf.2019.10.008
31. Hazra R, Chattopadhyay S, Mallick A, Gayen S, Roy S. Revealing the therapeutic properties of gut microbiota: transforming cancer immunotherapy from basic to clinical approaches. Med Oncol. (2024) 41:175. doi: 10.1007/s12032-024-02416-3
32. Prasad R, Rehman A, Rehman L, Darbaniyan F, Blumenberg V, Schubert ML, et al. Antibiotic-induced loss of gut microbiome metabolic output correlates with clinical responses to CAR T-cell therapy. Blood. (2024). doi: 10.1182/blood.2024025366
33. Zhou J, Huang G, Wong WC, Hu DH, Zhu JW, Li R, et al. The impact of antibiotic use on clinical features and survival outcomes of cancer patients treated with immune checkpoint inhibitors. Front Immunol. (2022) 13:968729. doi: 10.3389/fimmu.2022.968729
34. Wilson BE, Routy B, Nagrial A, Chin VT. The effect of antibiotics on clinical outcomes in immune-checkpoint blockade: a systematic review and meta-analysis of observational studies. Cancer Immunol Immunother. (2020) 69:343–54. doi: 10.1007/s00262-019-02453-2
35. Wu Q, Liu J, Wu S, Xie X. The impact of antibiotics on efficacy of immune checkpoint inhibitors in Malignancies: A study based on 44 cohorts. Int Immunopharmacol. (2021) 92:107303. doi: 10.1016/j.intimp.2020.107303
36. Alotaibi FM, Albalawi IAS, Anis AM, Alotaibi H, Khashwayn S, Alshammari K, et al. The impact of antibiotic use in gastrointestinal tumors treated with immune checkpoint inhibitors: systematic review and meta-analysis. Front Med (Lausanne). (2024) 11:1415093. doi: 10.3389/fmed.2024.1415093
37. Nie F, Guo J, Pan J, Guo Z, Wang C, Yan J, et al. Effects of antibiotics on the anti-tumor efficacy of immune checkpoint inhibitor therapy. Clin Transl Oncol. (2024). doi: 10.1007/s12094-024-03615-0
38. Mohiuddin JJ, Chu B, Facciabene A, Poirier K, Wang X, Doucette A, et al. Association of antibiotic exposure with survival and toxicity in patients with melanoma receiving immunotherapy. J Natl Cancer Inst. (2021) 113:162–70. doi: 10.1093/jnci/djaa057
39. von Itzstein MS, Gonugunta AS, Sheffield T, Homsi J, Dowell JE, Koh AY, et al. Association between antibiotic exposure and systemic immune parameters in cancer patients receiving checkpoint inhibitor therapy. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14051327
40. Eng L, Sutradhar R, Niu Y, Liu N, Liu Y, Kaliwal Y, et al. Impact of antibiotic exposure before immune checkpoint inhibitor treatment on overall survival in older adults with cancer: A population-based study. J Clin Oncol. (2023) 41:3122–34. doi: 10.1200/JCO.22.00074
41. Pederzoli F, Bandini M, Raggi D, Marandino L, Basile G, Alfano M, et al. Is there a detrimental effect of antibiotic therapy in patients with muscle-invasive bladder cancer treated with neoadjuvant pembrolizumab? Eur Urol. (2021) 80:319–22. doi: 10.1016/j.eururo.2021.05.018
42. Fishbein SRS, Mahmud B, Dantas G. Antibiotic perturbations to the gut microbiome. Nat Rev Microbiol. (2023) 21:772–88. doi: 10.1038/s41579-023-00933-y
Keywords: chimeric antigen receptor T cell, antibiotic, relapsed/refractory, multiple myeloma, outcome
Citation: Yin L, Lv B, Ge J, Qi Y, Xia J, Ma S, Wang Y, Liu Y, Zhou D, Cao J, Yan Z, Qi K, Sang W, Li D, Cheng H, Chen W, Xu K, Gu W, Li Z and Zhu F (2025) The impact of antibiotic use on outcomes of relapsed/refractory multiple myeloma patients treated with CAR-T therapy. Front. Immunol. 16:1566016. doi: 10.3389/fimmu.2025.1566016
Received: 24 January 2025; Accepted: 27 March 2025;
Published: 17 April 2025.
Edited by:
Claudine Kieda, Military Institute of Medicine (Poland), PolandReviewed by:
Pat Arndt, University of Minnesota Medical Center, United StatesCopyright © 2025 Yin, Lv, Ge, Qi, Xia, Ma, Wang, Liu, Zhou, Cao, Yan, Qi, Sang, Li, Cheng, Chen, Xu, Gu, Li and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiying Gu, Z3V3ZWl5aW5nMjAwMUAxNjMuY29t; Zhenyu Li, bGl6aGVueXVtZEAxNjMuY29t; Feng Zhu, ZnJhbmtmZW5nXzIwMDRAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.