- 1Acphazin Inc., Deerfield, IL, United States
- 2College of Science, Health, and Pharmacy, Roosevelt University, Schaumburg, IL, United States
Most early studies investigating the role of C-reactive protein (CRP) in tissue damage determined it supported pro-hemostatic and pro-inflammatory activities. However, these findings were not universal, as other data suggested CRP inhibited these same processes. A potential explanation for these disparate observations finally emerged with the recognition that CRP undergoes context-dependent conformational changes in vivo, and each of its three isoforms – pentameric CRP (pCRP), modified pentameric CRP (pCRP*), and monomeric CRP (mCRP) – have different effects. In this review, we consider this new paradigm and re-evaluate the role of CRP and its isoforms in the tissue repair process. Indeed, a growing body of evidence points toward the involvement of CRP not just in hemostasis and inflammation, but also in the resolution of inflammation and in tissue regeneration. Additionally, we briefly discuss the shortcomings of the currently available diagnostic tests for CRP and highlight the need for change in how CRP is currently utilized in clinical practice.
Introduction
The tissue repair process begins immediately after tissue damage and lasts for several weeks (1, 2). During this time, a series of biological processes occur that collectively staunch the injury (hemostasis) (3), stymie any invading pathogens (inflammation), (4), limit further damage (inflammation resolution and debris removal), (4, 5), and regenerate the tissue (angiogenesis, cellular proliferation, and tissue remodeling), (1, 4). While they overlap in practice, the various phases of the recovery process occur at roughly the following time frames: hemostasis, the first minutes to hours; inflammation, the first 72 hours; inflammation resolution, from 72 hours to ~1 week; and tissue regeneration and remodeling, ~1 week to ~1 month (1, 2).
For many years, C-Reactive Protein (CRP) was considered an important effector for only the earliest portions of the tissue repair response. This conclusion was driven by most biochemical and functional investigations of CRP determining that it potently supported pro-hemostatic and pro-inflammatory activities (6, 7). There was also a temporal logic to that argument, as plasma CRP concentrations increase up to 1000-fold during the pro-inflammatory phase and begin decreasing in tandem with the overall switch to inflammation resolution (8, 9). However, not all data were consistent with that interpretation. Some studies reported results in which CRP exhibited anti-inflammatory properties (10–15). Moreover, the 19-hour half-life of CRP means its levels are elevated above baseline even during the early tissue regeneration phase – a perplexing observation for something with strong pro-inflammatory potential (16). For a long time, these findings were difficult to reconcile and, to some extent, have limited the usefulness of CRP as a clinical tool and target.
Progress toward resolving these conflicting observations finally arrived with the recognition that CRP, in serum a very stable homo-pentameric macromolecule, undergoes conformational changes and dissociation at sites of inflammation in vivo (17). There had been in vitro observations to suggest a modified, monomeric version of CRP (mCRP) was the primary pro-inflammatory form of CRP (18–21), but evidence for the existence of mCRP in vivo had been difficult to obtain. The reasons for its delayed identification in vivo were multi-fold: for example, dissociation in vitro requires non-physiological amounts of heat, urea, or acidic environments (22–25); the exceptional insolubility of mCRP means it is only membrane-associated and/or -embedded in vivo (26–29); and, is inconsistently detectable on microvesicles in the serum of individuals without ongoing inflammatory disorders (27, 30–34). Nevertheless, improvements in techniques and reagents finally led to observations of pCRP dissociation in vivo in a rat model of myocardial infarction (17), its presence on circulating microvesicles in humans with inflammatory disorders (26, 27, 32–37), and its presence in human myocardial tissue and burn wounds (17, 38). A transitory intermediate form of CRP called pCRP* (pCRP star; also known as mCRPm) was identified shortly thereafter in which pCRP has undergone some conformational changes and exhibits some pro-inflammatory effector functions but has nevertheless not yet dissociated (39–42).
In this review, we distinguish between the three CRP isoforms and re-evaluate each of their potential roles in the tissue repair process. Specific isoforms of CRP will be described where possible, though many studies took place at a time where the need to differentiate the contributions of each isoform was not known or the ability to differentiate the isoforms was not readily possible. For these studies, the concentrations of CRP (low [i.e., non-saturating concentrations], pCRP*/mCRP; high, pCRP) and the time frame (≥0.5-2 hours, pCRP*/mCRP) in which results were observed provide potential ways to differentiate whether the reported effects were due to pCRP or pCRP*/mCRP (11, 39, 43). Nevertheless, there are inherent limitations to the discussion. Lastly, we briefly discuss the need for how CRP is used clinically to evolve in the wake of this new understanding of CRP bioactivity.
CRP isoforms and their bioavailability
Structure and general functions
Pentameric CRP is a compact, non-glycosylated, homo-polymeric molecule with a central void and radial symmetry (44). Each of the five monomers contains 206 amino acids and a single intramolecular disulfide bond, whereas the intermolecular interactions holding the pentamer together are non-covalent (44, 45). All monomers are oriented in the same direction, allowing pCRP to be conceptualized as two-sided (46). On one side is the binding face (or B-face), whose primary role is to bind phosphocholine (PC) (46–48). Though ubiquitously present, PC is normally buried within membranes and inaccessible to CRP. However, changes in membrane architecture due to lipid modification by enzymes (e.g., phospholipase A2) or reactive oxygen species (ROS) causes PC to ‘pop up’ and expose itself for CRP recognition (49–51). Upon exposure, it becomes a damage-associated molecular pattern (DAMP), an endogenous molecule containing a conserved motif the immune system utilizes to recognize abnormal situations and initiate an inflammatory response (52). Phosphocholine may also be found on Gram-positive bacterial cell walls (53), making it both a DAMP and pathogen-associated molecular pattern (PAMP; i.e., a conserved motif present on non-self organisms) (52). Interactions between CRP and PC are calcium-dependent and rely on CRP residues Phe-66 and Glu-81 (46). Notably, other DAMPs (e.g., oxidized low-density lipoprotein, histones, and fibronectin) and PAMPs (e.g., phosphoethanolamine [found on Gram-negative bacteria]) have also been identified as ligands for the CRP binding face (54–58).
On the reverse side of pCRP is the effector face, (also called the activating face or A-face), (46, 59). The most well-recognized binding partners for this half are the globular head of complement protein C1q and various Fc receptors (e.g., FcγRI [CD64], FcγRIIa [CD32a], FcγRIII [CD16], FcαRI [CD89]), (60, 61). Several other receptor binding partners have been suggested, including toll-like receptor 4 (TLR4), GPIbα, GPIIb/IIIa, CD31, CD36, integrin αvβ3, lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), and receptor activator of NF-κB ligand (62–70). While the binding site for C1q and the Fc receptors all overlap, the individual amino acids in CRP that facilitate binding to each ligand are distinct, even among the Fcγ receptors (FcγRs) (71). Importantly, three-dimensional models of the interaction between CRP and C1q suggest part of the interaction domain is inaccessible in the pentameric conformation (amino acids 199-206) (59). This implies pCRP is not inherently pro-inflammatory, instead requiring a conformational change into an alternative isoform for those activities to manifest. This is supported by the results of a clinical trial in which pCRP injected into healthy individuals did not elicit an inflammatory response (72). By extension, these results suggest environmental cues associated with ongoing inflammation are necessary to trigger conformational changes in pCRP, and the pro-inflammatory versions of CRP are amplifiers of inflammation rather than instigators. Unmodified pCRP may even have regulatory or anti-inflammatory activities, given in vitro observations of inhibitory effects on platelet, neutrophil, macrophage, dendritic cell (DC), and fibroblast activities in a dose-dependent manner (10–14, 73–78).
The pCRP* isoform is presumed to be an intermediate step between pCRP and its dissociation into mCRP (6, 39). Structurally, the pentameric assembly remains, but it has ‘relaxed’ sufficiently that the pro-inflammatory neoepitope (the aforementioned residues 199-206) is fully exposed (40, 41). At present, circumstances in vivo in which pCRP converts to pCRP* include ligand binding at regions of high membrane curvature and mildly acidic microenvironments such as those present at sites of inflammation (40, 79–82). Curved surfaces make PC more available, make hydrophobic regions of membrane lipids accessible, and expose binding sites on membrane-anchored proteins (81–83). Ultimately, the intermolecular interactions that hold pCRP subunits together undergo rearrangement resulting in exposure of the neoepitope (41). Alternatively (or additionally), acidic conditions can cause the protonation of histidine residues nearby the disulfide bonds within each CRP molecule (84). This alters the intramolecular hydrogen bonding network, causing structural changes in pCRP that again result in the exposure of the neoepitope.
Functionally, pCRP* stimulates the immune response by activating the classical complement pathway (39, 41). Interactions between CRP and C1q are primarily electrostatic in nature and demonstrate high avidity, making pCRP* the most potent CRP isoform at activating complement (41, 85). Of note, CRP-induced activation of the complement cascade biases it toward opsonization/phagocytosis as opposed to activation of the membrane attack complex (MAC)/cellular lysis (86, 87), thereby preventing excessive inflammation (87). Investigation of pCRP* activities beyond complement activation are limited due to its recent identification and the current limitations in experimentally distinguishing it from other isoforms. However, microvesicle-associated pCRP* can increase adhesion molecule expression on endothelial cells (41), and the overlap of the complement and FcγR binding sites implies pCRP* likely also stimulates FcγRs (71).
The terminal form of CRP is its monomeric form, mCRP. Dissolution of the pentamer occurs after newly exposed hydrophobic residues in pCRP* form interactions with the hydrophobic tails of lipids in membranes or with insoluble extracellular plaques in tissues (17, 22, 88). Thus, mCRP is found in vivo embedded within cellular membranes, associated with circulating microvesicles, or sequestered with insoluble components of the extracellular matrix (ECM) (17, 26–28, 38, 88, 89). The amino acids key to membrane-entry (residues 35-47, VCLHFYTELSSTR) preferentially interact with cholesterol, biasing mCRP membrane localization to lipid raft domains (28). Exposure of the cholesterol binding site is supported by reactive oxygen species (ROS) generated at sites of inflammation, presumably because the oxidative modifications to pCRP/pCRP* loosen its pentameric structure (79, 90). However, optimal exposure requires reduction of the intrachain disulfide bond, something it is primed to do in acidic conditions (79, 84, 89–91).
Pro-inflammatory activities have been described for mCRP in numerous settings and are discussed in detail in several recent reviews (6, 7, 92–99). In brief, mCRP promotes cellular chemotaxis and adhesion (14, 17, 18, 21, 68, 89, 100–105), augments platelet activation and aggregation (65, 70, 90, 106–108), and stimulates cytokine, ROS, and nitric oxide (NO) production (28, 38, 73, 79, 89, 102, 109–112). These effects are partially mediated through interactions with FcγRs, but not completely, as blockade of FcγRs does not completely abrogate the effects of mCRP (19–21, 113). Notably, mCRP potency is greater when the intramolecular disulfide bond in mCRP has been reduced (110). Monomeric CRP also retains the ability to interact with C1q and additionally interacts with negative regulators of complement activity (Factor H and C4-binding protein) (61, 114).
In summary, the long-appreciated role of CRP as an immune stimulant is now known to be attributable to pCRP* and mCRP, whereas pCRP is non- or anti-inflammatory. However, the bioactivities of CRP are not limited to impacting the inflammatory response. As we will shortly discuss, evidence has been accumulating to suggest CRP augments each additional phase of the tissue repair response: hemostasis, immune resolution, and tissue regeneration (Figure 1; Table 1).
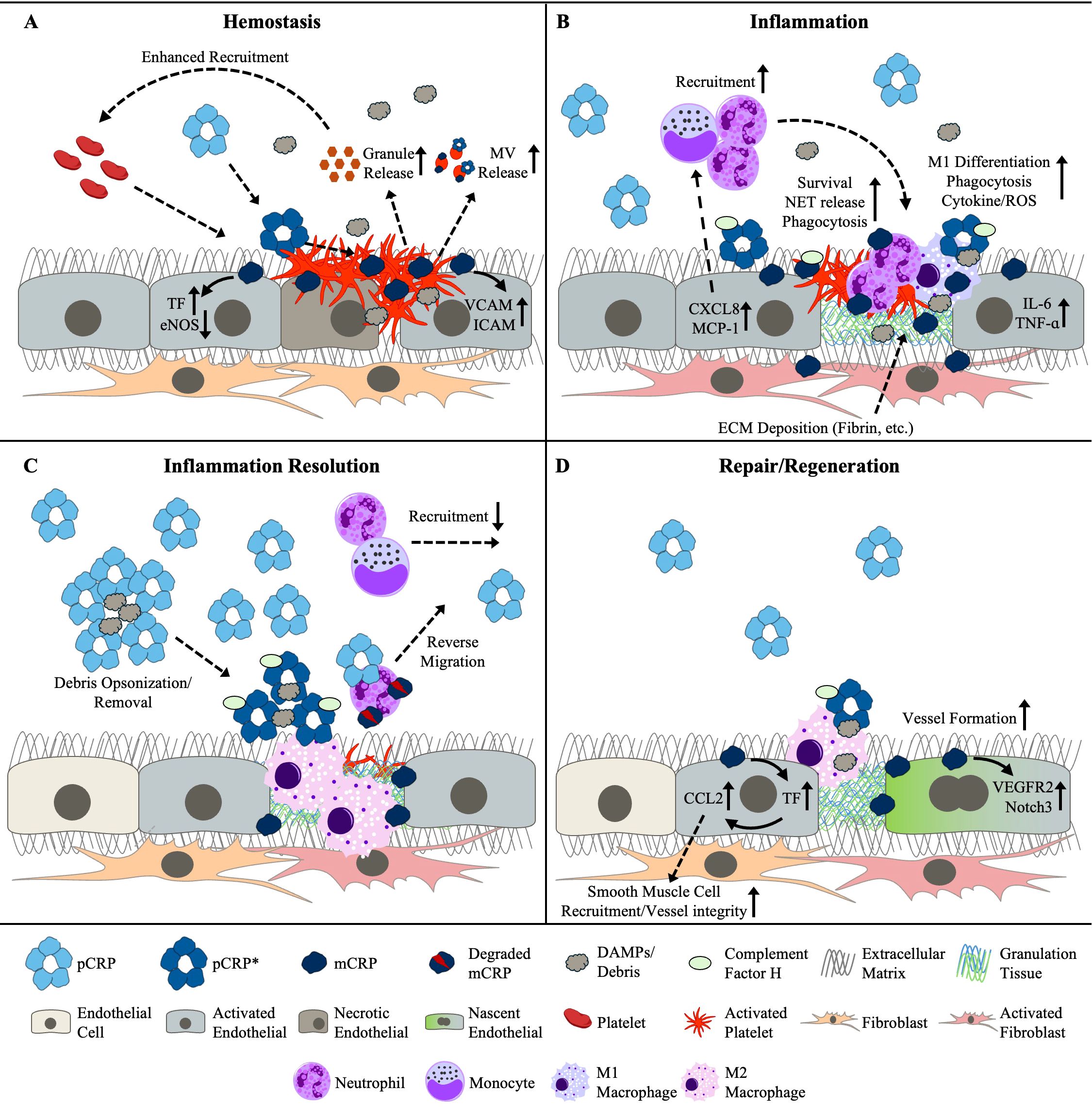
Figure 1. Reported and putative roles for the CRP isoforms on activities associated with (A) hemostasis, (B) inflammation, (C) the resolution of inflammation, and (D) tissue repair and regeneration. CRP, C-reactive protein; DAMPs, damage-associated molecular patterns ECM, extracellular matrix; eNOS, endothelial nitric oxide synthase; ICAM, intercellular adhesion molecule; IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein-1; mCRP, monomeric CRP; MV, microvesicle; NET, neutrophil extracellular trap; pCRP, pentameric CRP; pCRP*, pCRP star; ROS, reactive oxygen species; TF, tissue factor; TNF-α; tumor necrosis-factor-alpha; VCAM, vascular cell adhesion molecule; VEGFR2, vascular endothelial growth factor receptor 2.
CRP bioavailability
In the absence of ongoing inflammation, the steady-state concentration of pCRP in blood is <1 to 3 mg/L (115–117). Circulating pCRP is produced by hepatocytes, though extrahepatic macrophages, lymphocytes, endothelial cells, adipocytes, and smooth muscle cells can express CRP (98, 118). It is unknown if non-hepatic CRP is secreted as pCRP or acts as an autocrine factor. Information on the steady-state levels of pCRP* and mCRP in the blood is limited. The pro-inflammatory CRP isoforms have been detected on microvesicles, but most efforts to quantify pCRP*/mCRP concentrations in the serum of individuals without inflammatory disorders place its concentration from undetectable (<1) to 25 ng/mL (26, 27, 33–36, 119–123). Outside of the blood, immunohistochemical staining finds pCRP*/mCRP to be present in arterial plaques and areas surrounding recently damaged vascular tissue (89, 99, 103, 124–126).
During the early stages of an inflammatory response, hepatocytes respond to elevated levels of interleukin (IL-6) and IL-1β by releasing pre-existing stores of pCRP and dramatically increasing production of new pCRP (127–129). Serum concentrations of pCRP can rise to over 500 mg/L within the first 72 hours of the response (9, 130). Similarly, microvesicle-associated CRP levels significantly increase during acute inflammatory events (34, 120, 123). Both pCRP and mCRP levels remain elevated in chronic conditions (27, 31–33, 35–37, 119, 121), with multiple reports finding a direct correlation between mCRP levels and disease severity (31, 32, 35–37, 131). This was in contrast to pCRP concentrations, which were not consistently predictive. Relatedly, there is disagreement about whether a correlation exists between mCRP and pCRP concentrations. Among 12 studies reporting correlations included in this review, nine found a lack of significant correlation (27, 30–34, 36, 37, 120–122, 131, 132).
Once secreted, the half-life of pCRP is ~19 hours (16, 117). Its rate of disappearance is independent of its plasma concentration (16), making the measured pCRP concentrations a reflection of recent synthesis rates and not changes in consumption/excretion. Due to the large amount produced and its relatively slow half-life, it is common to see elevated concentrations of circulating pCRP for more than a week after an inciting inflammatory event (133). The rate at which pCRP converts to pCRP* in vivo and the length of time before pCRP* dissociates into mCRP are unknown. In vitro observations found the neoepitope could be detected 30 minutes after treating cells with pCRP and that evidence of pentamer dissociation appeared approximately 90 minutes later (39). This timeframe roughly agrees with a second study that reported the appearance of mCRP at approximately 2 hours post application of pCRP (43). Information on the half-life of mCRP in humans is unavailable, both in the circulation and in tissues. However, data from mouse models revealed mCRP could be detected in tissues for three times longer than pCRP in the blood (134).
CRP in tissue damage and repair
Hemostasis
When bleeding occurs, multiple intertwined processes are initiated to close the wounded blood vessel (3, 135, 136). One process begins when platelets adhere to collagen in the exposed ECM. Binding activates the platelets, which recruit additional platelets that together coalesce into a primary plug. Secretions from activated platelets also provide a means for the activation a second clotting process, the intrinsic coagulation cascade. Platelet-derived polyphosphates provide a binding surface for coagulation Factor XII. Binding activates Factor XII and, after several additional steps, culminates in the activation of Factor X. In a third process, the extrinsic coagulation pathway, circulating coagulation Factor VII complexes with Tissue Factor (TF) expressed on the surface of smooth muscle cells and fibroblasts and this complex also activates Factor X. Activated Factor X combines with and activates Factor V, forming prothrombinase; prothrombinase converts prothrombin into thrombin; thrombin converts fibrinogen into fibrin; and Factor XIII, also activated by thrombin, covalently crosslinks fibrin molecules together. This fibrin mesh combines with the platelet plug to form a stable patch over the wound and prevent further blood loss (3, 135).
Foremost among the ways CRP boosts hemostatic processes is by enhancing platelet activation and aggregation (65, 70, 90, 106–108, 112, 137, 138). Platelets provide optimal conditions for the conversion of pCRP into pCRP* and mCRP. More specifically, the membranes of activated platelets contain an abundance of oxidized phospholipids and undergo membrane ‘ruffling,’ thus providing both exposed PC and regions of increased membrane curvature (89, 136). Indeed, half of all platelet-derived microvesicles have neoepitope-expressing CRP associated with them in people with an acute inflammatory condition (34), suggesting a close relationship between the two effectors in vivo. Functionally, mCRP enhances signaling through the major platelet adhesion receptor GPIIb/IIIa and boosts the responsiveness of platelets to other stimuli, such as adenosine diphosphate, epinephrine, and thrombin (65, 70, 90, 106). Platelets stimulated with mCRP release more of their granules (65, 108), which contain a variety of pro-coagulation factors (e.g., Factor V, von Willebrand Factor, fibronectin) and pro-repair factors (e.g., platelet-derived growth factor [PDGF], insulin-like growth factor-1, transforming growth factor [TGF]-β) (136). Increased secretion of High Mobility Group Box 1 (HMGB1) by platelets stimulated with mCRP has also been reported, which has downstream effects on neutrophils (107). The platelet scavenger receptor CD36 and adhesion receptor GPIIb/IIIa have been implicated in mediating some of the effects mCRP exerts on platelets (65, 70). Additionally, we note that platelets express ample amounts of FcγRIIa and TLR4, and there is substantial overlap between the effects observed with mCRP and those with FcγRIIa and TLR4 stimulation (139, 140).
Platelet activities are also affected by interactions between CRP and endothelial cells. Like with platelets, CRP can bind and dissociate on the membranes of endothelial cells at sites of inflammation (21). Stimulation of endothelial cells with mCRP leads to the upregulation of vascular cell adhesion molecule (VCAM)-1 and intercellular adhesion molecule (ICAM-1) (21, 28, 105, 108, 141, 142), the latter of which is a ligand for platelet GPIIb/IIIa (136). Thus, mCRP reinforces a major GPIIb/IIIa adhesion and signaling axis for platelets from both ends. CRP also supports platelet adhesion by inhibiting the expression and activity of endothelial nitric oxide synthase (eNOS) in endothelial cells (143, 144). Under normal conditions, endothelial cells produce nitric oxide to prevent unnecessary platelet aggregation and degranulation (3). By inhibiting eNOS, mCRP facilitates platelet adhesion and aggregation.
While its effects are less direct, CRP also impacts the extrinsic and intrinsic coagulation cascades. Endothelial cells and smooth muscle cells exposed to CRP upregulate TF (43, 145–149), thereby boosting the extrinsic coagulation pathway. Support for the intrinsic pathway stems from CRP-mediated activation of neutrophils. Neutrophils that swarm to the injury site generate structures called Neutrophil Extracellular Traps (NETs) (150). While the primary role of NETs is the capture of pathogenic organisms and cellular debris, they include polyanions that can also activate Factor XII (151). Among its numerous effects on neutrophils, mCRP has recently been suggested to promote NET formation, though this may be through an indirect mechanism in which neutrophils increase NETs in response to the HMGB1 secreted by platelets (107, 152, 162).
Inflammation
The local immune response to tissue injury begins with the release of pro-inflammatory cytokines and DAMPs from damaged and dead cells (153–155). Nearby epithelial cells, endothelial cells, fibroblasts, mast cells, and tissue-resident macrophages respond to and amplify these signals to recruit nearby and circulating immune cells. For example, endothelial cells release IL-1β, IL-6, CXCL8 (i.e., IL-8), tumor necrosis factor (TNF)-α, and monocyte chemotactic protein (MCP)-1 to activate and attract immune cells and upregulate integrins like ICAM-1 and VCAM-1 to facilitate leukocyte adhesion to the site of damage (105, 108, 153–156). Aspects of the hemostasis response also contribute, with molecules such as thrombin stimulating cytokine secretion from local cells and platelets releasing pro-inflammatory chemokines and cytokines (136, 157).
A variety of innate immune cells, including neutrophils, monocytes, invariant NKT cells, mast cells, and plasmacytoid dendritic cells, respond to those pro-inflammatory cues and populate the wound site (154). Among them, neutrophils are the major effector for the first 24-48 hours post-injury, representing more than 50% of infiltrating leukocytes (4, 154). In general, their major activities are the secretion of antimicrobial substances (e.g., ROS) and the formation of NETs to capture and kill any potential pathogens. They also have a role as phagocytes, albeit only for smaller pieces of debris (150). After neutrophils, monocytes are the other major immune cell during the early response, peaking in number with a slight delay relative to neutrophils at approximately 72 hours post-injury (4). Responding monocytes initially differentiate into pro-inflammatory (i.e., M1) macrophages, release various pro-inflammatory cytokines and antimicrobial substances, and phagocytose pathogens, tissue debris, and apoptotic cells (4, 158). The specific contributions of the other cell types have been investigated more sparsely, though they are no less important to the timely repair of tissue damage (1).
The role of CRP in augmenting the acute inflammatory response is extensive and has been discussed at length by multiple other recent reviews (6, 7, 92–99). For brevity, we will briefly describe only a few key connections between CRP and neutrophils or monocytes/macrophages, and direct readers to the other reviews for more detailed information.
There is ample evidence linking CRP to enhanced neutrophil responses. First, CRP promotes neutrophil recruitment through its effects on endothelial cells and platelets. As described above, pCRP dissociates into mCRP on the surface of endothelial cells and promotes their activation. In doing so, mCRP boosts endothelial cell release of CXCL8 and upregulation of ICAM-1 (21, 28, 141, 159), a potent neutrophil chemoattractant and key ligand for neutrophil adhesion processes, respectively (4, 160). Similarly, CRP increases P-selection expression on platelets (65, 78, 138), which has a key role in neutrophil localization (21, 161). Neutrophils reciprocally upregulate CD11b/CD18 (Mac-1) after stimulation with mCRP (18, 162). In addition to its effects on neutrophil recruitment, stimulation of neutrophils with mCRP increases NO production, enhances phagocytosis of debris, delays their apoptosis, and has recently been demonstrated to be a potent inducer of NET formation (11, 19, 20, 107, 113, 152, 162). Some effects are downstream of interactions between mCRP and FcγRIIIb (19, 21), which is amply expressed on the neutrophil surface (163), whereas other effects may be downstream of FcαRI (164).
Monocyte and macrophage recruitment is similarly enhanced by CRP through the upregulation of MCP-1 expression in endothelial cells, and through the stimulation of receptors with which CRP is known to engage (21, 159, 165, 166), such as FcγRI, FcγRIIa, and toll-like receptor TLR4 (62, 63, 71, 167). Interactions between mCRP and monocyte (FcγRs) promote monocyte differentiation into M1 macrophages and contribute to the cellular metabolic reprogramming necessary for macrophages to perform their effector functions (17, 27, 111, 168–170). Cytokines released by monocytes/macrophages whose secretion has been augmented by mCRP include at least TNF-α, IL-1β, IL-6, and CXCL8 (63, 111, 112, 167, 169). Other effects of stimulating monocytes with mCRP include the upregulation of CD11b/CD18, increased NO and ROS production, and enhanced clearance of necrotic and apoptotic cells (14, 17, 73, 109, 111).
Inflammation resolution
While inflammation is necessary for the elimination of pathogens and clearance of cellular debris, prolonged inflammation will stymie reparative activities (4). To prevent this, there are numerous ‘built-in’ mechanisms to ensure a timely resolution to inflammatory processes. For example, NETs catch cytokines and chemokines produced during the initial response, which results in their degradation by NET-associated proteases and a reduction in further effector cell recruitment (171). Activated neutrophils recruit monocytes and macrophages (172), and those macrophages subsequently contribute to suppressing neutrophil responses through efferocytosis and promoting neutrophil reverse migration (173, 174). Efferocytosis simultaneously promotes the conversion of M1 macrophages to the pro-resolution M2 phenotype that produce anti-inflammatory factors such as TGF-β and IL-10 (175). Overall, by approximately 72 hours post-injury, the inflammatory response to tissue injury should be ending and a pro-repair microenvironment forming.
There is evidence to suggest CRP has its own negative feedback mechanism. As noted, circulating pCRP concentrations may increase up to 1000-fold in the first 72 hours of an inflammatory response (9, 130). Interestingly, several in vitro observations have found high concentrations of pCRP to cause the opposite effects of pCRP*/mCRP or outright suppressive activities (10–13, 73–75, 78, 170). For instance, elevated pCRP concentration may help abate inflammation by suppressing the differentiation of pro-inflammatory DCs and driving the formation of myeloid-derived suppressor cells and M2-type macrophages (13, 74, 75, 170, 176). Moreover, whereas lower concentrations of CRP promote neutrophil chemotaxis and adhesion, higher amounts inhibit those activities (11, 12, 78, 177). At least for neutrophils, mCRP and pCRP may bind different receptors (178), ostensibly providing a mechanistic basis for these opposing effects. Notably, whereas pCRP is generally resistant to proteolysis, mCRP is susceptible to degradation by neutrophil-associated proteases and those peptides demonstrate dominant negative-like activities in vitro (177, 179, 180). Thus, mCRP binding sites may not be re-exposed after degradation of mCRP, which would also shift the balance of CRP activities toward those mediated by pCRP. Such mechanisms may contribute to the enigmatic process of neutrophil reverse migration (173).
Elevated CRP concentrations may also help limit inflammation by reducing and/or obscuring DAMPs. For example, CRP neutralizes extracellular histones from inducing endothelial cell cytotoxicity by outcompeting cell-associated binding partners that facilitate histone uptake (54). Furthermore, CRP prevents the activation of endothelial cells and macrophages by modified lipids if allowed to complex with those lipids prior to being added to cell cultures, suggesting a potential competitive inhibitory effect when in excess (14, 77). Thus, we propose that upregulation of CRP may serve as a mechanism by which an inflammatory response is curtailed through use of CRP as an “antigen sink.” The role of CRP as an opsonin of cellular debris is arguably also an anti-inflammatory mechanism of action, given the interaction of CRP with inhibitors of the MAC results in the clearance of inflammatory materials without inciting an inflammatory response (86). Higher concentrations ostensibly facilitate greater clearance, especially as the peak of CRP concentrations coincide with the peak of monocyte/macrophage infiltration.
Altogether, these findings suggest the up to 1000-fold increase in CRP concentration seen during the first 72 hours of a response may constitute an anti-inflammatory process rather than one meant to amplify inflammation. These anti-inflammatory effects may be achieved through at least two mechanisms: saturation of mCRP binding followed by the initiation of alternative anti-inflammatory interactions, and hiding/eliminating DAMPs/PAMPs by acting as an antigen sink. Further research into the anti-inflammatory properties of CRP are needed, especially as there is some evidence suggesting additional feedback mechanisms. For example, stimulation of macrophages through FcγRI by CRP upregulates expression of the inhibitory liver X receptor (LXR) alpha and specific ligands may lead to differing pro- or anti-inflammatory effects (15, 170, 176).
Tissue regeneration and remodeling
The tissue regeneration and remodeling phase includes its own set of interdependent processes, which together account for the growth of new blood vessels (i.e., angiogenesis), the deposition of granulation tissue, the proliferation of parenchymal cells, and the remodeling of the tissue into a stable long-term structure (1, 4). Though conceptually these processes occur after hemostasis, inflammation, and inflammation resolution, considerable groundwork for them takes place during those earlier stages (4). For example, platelets secrete many pro-angiogenic factors (e.g., PDGF, vascular endothelial growth factor [VEGF], and TGF-β), stirring endothelial cells to proliferate and begin the formation of new capillaries (181). Proteases released by neutrophils free VEGF sequestered within the ECM and facilitate endothelial cell expansion into the wound (160). Histamine and trypase released by mast cells enhance fibroblast proliferation and the deposition of collagen (1, 4). Macrophages consume dead vasculature and secrete wound healing factors like arginase, TGF-β, VEGF, PDGF, and insulin-like growth factor (158). Indeed, there is an ever-growing list of interactions between the immune response to tissue damage and tissue regrowth.
Several observations now point toward mCRP being among the list of immune mediators to enhance tissue regeneration. Most of that evidence revolves around the effect of mCRP on neovascularization. For one, mCRP colocalizes with a marker of angiogenesis (i.e., endoglin [CD105]) in stroke patients (124, 182), suggesting a potential relationship in vivo. Results from in vitro wound healing assays support this, as aortic endothelial cells treated with mCRP exhibited greater vessel formation (124, 183). The upregulation of two critical receptors for vessel development, VEGF receptor 2 and Notch3, by endothelial cells after mCRP exposure offers a potential mechanism for this observation (183–185). Moreover, angiopoietins are upregulated downstream of Notch receptors and its production enhanced by hypoxia-inducible factor (HIF)-1α (184). CRP has also been shown to stimulate HIF-1α in a pro-angiogenic capacity (186), implying CRP both promotes Notch receptor expression and enhances signaling downstream of those receptors. There is also evidence to suggest mCRP contributes to both the formation and stability of neo-vessels by variably promoting or downregulating the expression of VE-cadherin and N-cadherin depending on co-stimulatory signals (187). The enhanced expression of TF by endothelial cells in the presence of mCRP adds another layer (43, 145–149), as TF activation increases endothelial cells secretion of CCL2, which recruits vascular smooth muscle cells that strengthen vessel integrity (43). Lastly, there are also indirect effects stemming from mCRP-mediated release of pro-angiogenic factors such as VEGF and PDGF (183, 186, 188).
The effect of CRP on other aspects of tissue regeneration and remodeling process has been investigated much more sparsely. There are likely effects on granulation tissue formation, since CRP has been reported to enhance the epithelial-to-mesenchymal transition (91). Conversely, other work has shown CRP can inhibit fibroblast migration (76), though this was again dose-dependent and so may represent another concentration-dependent negative feedback mechanism.
In the clinic
At present, the only diagnostic tests for CRP measures plasma concentrations of the pCRP isoform (27). Clinicians have traditionally used results of those tests to report the presence of inflammation if the levels are above 10 mg/L (roughly 3- to 10-fold above baseline). However, such individual measurements cannot discern whether the inflammation is due to a chronic ongoing inflammatory event or a recent acute inflammatory event that has concluded. And because the amount of pCRP made varies from event to event and person to person (189, 190), single measurements are also unable to discern how long ago or how severe such an acute event might have been. Therefore, given the currently available diagnostic tests, we encourage physicians to measure pCRP levels multiple times with the understanding that its concentration should halve approximately every 19 hours (16, 117, 189, 191), excluding any potential effects of changes in treatment regimen. This minor change could at least assist clinicians in diagnosing the nature of a condition as acute or chronic.
Regardless, the more significant benefit to clinical practice would be the development of a routine clinical method for determining the abundance of the pro-inflammatory CRP isoforms (i.e., pCRP* and mCRP), as advances in the CRP field over the previous decade have confirmed these to be the potentially immunopathogenic forms of CRP (6, 96, 99, 192). Unfortunately, both the standard and high-sensitivity tests for pCRP are unable to detect pCRP*/mCRP and, critically, most findings have found there is no definitive correlation between serum concentrations of pCRP and pCRP*/mCRP (27, 30–34, 36, 37, 120–122, 132). This means there is no concrete means of discerning the amount of potentially immunopathogenic CRP from current standard practices. Of note, this potential absence of a relationship between the different isoforms may also explain the lack of agreement among studies investigating whether baseline pCRP levels predict the incidence of various cardiovascular conditions (6). There are putative correlations between CRP-positive microparticles (which ostensibly represents ligand-bound, neoepitope-exposed CRP) and C1q-positive microparticles, suggesting there may yet be surrogate methodologies in the absence of direct mechanisms; but even these relationships may be condition-specific (119, 120). Ultimately, it is imperative that routine, standardized assays are developed for the specific detection of pCRP*/mCRP. Only then can the relationship between CRP and underlying inflammatory diseases be clearly elucidated.
Conclusion
The recognition that CRP undergoes context-dependent conformational changes in vivo has helped resolve long-standing contradictions in CRP research. Moreover, the distinct activities of pCRP, pCRP*, and mCRP have revealed the existence of a much more complex role for CRP in the biological response to tissue damage. Not only does CRP promote early hemostatic and inflammatory processes, but it also contributes to the resolution of inflammation and to angiogenesis. Moving forward, more efforts should be put toward defining the specific conditions in which each isoform is abundant, including considerations for factors such as the specific ligands available and cell receptor density. Toward that end, the development of standardized assays capable of detecting the pCRP* and mCRP isoforms is of paramount importance, as neither the general nor high-sensitivity CRP assays currently available have that ability. Such advances could also transform CRP from a general inflammatory marker into a more precise diagnostic tool, potentially enabling better monitoring of disease progression and therapeutic responses across a range of inflammatory conditions.
Author contributions
MP: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. PH: Resources, Supervision, Visualization, Writing – review & editing. IR: Resources, Supervision, Writing – review & editing. LP: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This submission was partially supported by a subcontract of an NIH RO1 grant awarded to Boston University Medical School Titled: Identification and characterization of the CD31-ApoE-mCRP pathway for Alzheimer’s disease in humans. Award Number: 1RF1AG075832-01A1.
Conflict of interest
LP is founder and shareholder of Acphazin, Inc. MP is an employee and shareholder of Acphazin, Inc. IR is an employee of Tabuk Pharmaceuticals.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: A cellular perspective. Physiol Rev. (2019) 99:665–706. doi: 10.1152/physrev.00067.2017
2. Almadani YH, Vorstenbosch J, Davison PG, Murphy AM. Wound healing: A comprehensive review. Semin Plast Surg. (2021) 35:141–4. doi: 10.1055/s-0041-1731791
3. Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. (2013) 93:327–58. doi: 10.1152/physrev.00016.2011
4. Soliman AM, Barreda DR. Acute inflammation in tissue healing. Int J Mol Sci. (2022) 24:641. doi: 10.3390/ijms24010641
5. Sugimoto MA, Sousa LP, Pinho V, Perretti M, Teixeira MM. Resolution of inflammation: what controls its onset? Front Immunol. (2016) 7:160. doi: 10.3389/fimmu.2016.00160
6. Dix C, Zeller J, Stevens H, Eisenhardt SU, Shing K, Nero TL, et al. C-reactive protein, immunothrombosis and venous thromboembolism. Front Immunol. (2022) 13:1002652. doi: 10.3389/fimmu.2022.1002652
7. Olson ME, Hornick MG, Stefanski A, Albanna HR, Gjoni A, Hall GD, et al. A biofunctional review of C-reactive protein (CRP) as a mediator of inflammatory and immune responses: differentiating pentameric and modified CRP isoform effects. Front Immunol. (2023) 14:1264383. doi: 10.3389/fimmu.2023.1264383
8. Muire PJ, Mangum LH, Wenke JC. Time course of immune response and immunomodulation during normal and delayed healing of musculoskeletal wounds. Front Immunol. (2020) 11:1056. doi: 10.3389/fimmu.2020.01056
9. Mantovani A, Garlanda C. Humoral innate immunity and acute-phase proteins. N Engl J Med. (2023) 388:439–52. doi: 10.1056/NEJMra2206346
10. Vigo C. Effect of C-reactive protein on platelet-activating factor-induced platelet aggregation and membrane stabilization. J Biol Chem. (1985) 260:3418–22. doi: 10.1016/S0021-9258(19)83638-4
11. Buchta R, Fridkin M, Pontet M, Contessi E, Scaggiante B, Romeo D. Modulation of human neutrophil function by C-reactive protein. Eur J Biochem. (1987) 163:141–6. doi: 10.1111/j.1432-1033.1987.tb10747.x
12. Tatsumi N, Hashimoto K, Okuda K, Kyougoku T. Neutrophil chemiluminescence induced by platelet activating factor and suppressed by C-reactive protein. Clin Chim Acta. (1988) 172:85–92. doi: 10.1016/0009-8981(88)90123-4
13. Svanberg C, Enocsson H, Govender M, Martinsson K, Potempa LA, Rajab IM, et al. Conformational state of C-reactive protein is critical for reducing immune complex-triggered type I interferon response: Implications for pathogenic mechanisms in autoimmune diseases imprinted by type I interferon gene dysregulation. J Autoimmun. (2023) 135:102998. doi: 10.1016/j.jaut.2023.102998
14. Eisenhardt SU, Starke J, Thiele JR, Murphy A, Bjorn Stark G, Bassler N, et al. Pentameric CRP attenuates inflammatory effects of mmLDL by inhibiting mmLDL–monocyte interactions. Atherosclerosis. (2012) 224:384–93. doi: 10.1016/j.atherosclerosis.2012.07.039
15. Hanriot D, Bello G, Ropars A, Seguin-Devaux C, Poitevin G, Grosjean S, et al. C-reactive protein induces pro- and anti-inflammatory effects, including activation of the liver X receptor alpha, on human monocytes. Thromb Haemost. (2008) 99:558–69. doi: 10.1160/TH07-06-0410
16. Vigushin DM, Pepys MB, Hawkins PN. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest. (1993) 91:1351–7. doi: 10.1172/JCI116336
17. Thiele JR, Habersberger J, Braig D, Schmidt Y, Goerendt K, Maurer V, et al. Dissociation of pentameric to monomeric C-reactive protein localizes and aggravates inflammation: in vivo proof of a powerful proinflammatory mechanism and a new anti-inflammatory strategy. Circulation. (2014) 130:35–50. doi: 10.1161/CIRCULATIONAHA.113.007124
18. Zouki C, Haas B, Chan JS, Potempa LA, Filep JG. Loss of pentameric symmetry of C-reactive protein is associated with promotion of neutrophil-endothelial cell adhesion. J Immunol. (2001) 167:5355–61. doi: 10.4049/jimmunol.167.9.5355
19. Khreiss T, Jozsef L, Hossain S, Chan JS, Potempa LA, Filep JG. Loss of pentameric symmetry of C-reactive protein is associated with delayed apoptosis of human neutrophils. J Biol Chem. (2002) 277:40775–81. doi: 10.1074/jbc.M205378200
20. Khreiss T, Jozsef L, Potempa LA, Filep JG. Loss of pentameric symmetry in C-reactive protein induces interleukin-8 secretion through peroxynitrite signaling in human neutrophils. Circ Res. (2005) 97:690–7. doi: 10.1161/01.RES.0000183881.11739.CB
21. Khreiss T, Jozsef L, Potempa LA, Filep JG. Conformational rearrangement in C-reactive protein is required for proinflammatory actions on human endothelial cells. Circulation. (2004) 109:2016–22. doi: 10.1161/01.CIR.0000125527.41598.68
22. Wu Y, Ji SR, Wang HW, Sui SF. Study of the spontaneous dissociation of rabbit C-reactive protein. Biochem (Mosc). (2002) 67:1377–82. doi: 10.1023/A:1021862027061
23. Taylor KE, van den Berg CW. Structural and functional comparison of native pentameric, denatured monomeric and biotinylated C-reactive protein. Immunology. (2007) 120:404–11. doi: 10.1111/j.1365-2567.2006.02516.x
24. Kresl JJ, Potempa LA, Anderson BE. Conversion of native oligomeric to a modified monomeric form of human C-reactive protein. Int J Biochem Cell Biol. (1998) 30:1415–26. doi: 10.1016/S1357-2725(98)00078-8
25. Potempa LA, Maldonado BA, Laurent P, Zemel ES, Gewurz H. Antigenic, electrophoretic and binding alterations of human C-reactive protein modified selectively in the absence of calcium. Mol Immunol. (1983) 20:1165–75. doi: 10.1016/0161-5890(83)90140-2
26. Habersberger J, Strang F, Scheichl A, Htun N, Bassler N, Merivirta RM, et al. Circulating microparticles generate and transport monomeric C-reactive protein in patients with myocardial infarction. Cardiovasc Res. (2012) 96:64–72. doi: 10.1093/cvr/cvs237
27. Crawford JR, Trial J, Nambi V, Hoogeveen RC, Taffet GE, Entman ML. Plasma levels of endothelial microparticles bearing monomeric C-reactive protein are increased in peripheral artery disease. J Cardiovasc Transl Res. (2016) 9:184–93. doi: 10.1007/s12265-016-9678-0
28. Ji SR, Ma L, Bai CJ, Shi JM, Li HY, Potempa LA, et al. Monomeric C-reactive protein activates endothelial cells via interaction with lipid raft microdomains. FASEB J. (2009) 23:1806–16. doi: 10.1096/fj.08-116962
29. Ji SR, Wu Y, Potempa LA, Qiu Q, Zhao J. Interactions of C-reactive protein with low-density lipoproteins: implications for an active role of modified C-reactive protein in atherosclerosis. Int J Biochem Cell Biol. (2006) 38:648–61. doi: 10.1016/j.biocel.2005.11.004
30. Melnikov I, Kozlov S, Saburova O, Zubkova E, Guseva O, Domogatsky S, et al. CRP is transported by monocytes and monocyte-derived exosomes in the blood of patients with coronary artery disease. Biomedicines. (2020) 8:435. doi: 10.3390/biomedicines8100435
31. Karlsson J, Wettero J, Potempa LA, Fernandez-Botran R, O’Neill Y, Wirestam L, et al. Extracellular vesicles opsonized by monomeric C-reactive protein (CRP) are accessible as autoantigens in patients with systemic lupus erythematosus and associate with autoantibodies against CRP. J Autoimmun. (2023) 139:103073. doi: 10.1016/j.jaut.2023.103073
32. Liang Y, Xu K, Liu W, Liu X, Yuan P, Xu P, et al. Monomeric C-reactive protein level is associated with osteoarthritis. Exp Ther Med. (2022) 23:277. doi: 10.3892/etm.2022.11206
33. Fujita C, Sakurai Y, Yasuda Y, Homma R, Huang CL, Fujita M. mCRP as a biomarker of adult-onset still’s disease: quantification of mCRP by ELISA. Front Immunol. (2022) 13:938173. doi: 10.3389/fimmu.2022.938173
34. Fendl B, Weiss R, Eichhorn T, Linsberger I, Afonyushkin T, Puhm F, et al. Extracellular vesicles are associated with C-reactive protein in sepsis. Sci Rep. (2021) 11:6996. doi: 10.1038/s41598-021-86489-4
35. Zhang L, Li HY, Li W, Shen ZY, Wang YD, Ji SR, et al. An ELISA assay for quantifying monomeric C-reactive protein in plasma. Front Immunol. (2018) 9:511. doi: 10.3389/fimmu.2018.00511
36. Giralt L, Figueras-Roca M, Eguileor BL, Romero B, Zarranz-Ventura J, Alforja S, et al. C-reactive protein-complement factor H axis as a biomarker of activity in early and intermediate age-related macular degeneration. Front Immunol. (2024) 15:1330913. doi: 10.3389/fimmu.2024.1330913
37. Munuswamy R, De Brandt J, Burtin C, Derave W, Aumann J, Spruit MA, et al. Monomeric CRP is elevated in patients with COPD compared to non-COPD control persons. J Inflammation Res. (2021) 14:4503–7. doi: 10.2147/JIR.S320659
38. Braig D, Kaiser B, Thiele JR, Bannasch H, Peter K, Stark GB, et al. A conformational change of C-reactive protein in burn wounds unmasks its proinflammatory properties. Int Immunol. (2014) 26:467–78. doi: 10.1093/intimm/dxu056
39. Ji SR, Wu Y, Zhu L, Potempa LA, Sheng FL, Lu W, et al. Cell membranes and liposomes dissociate C-reactive protein (CRP) to form a new, biologically active structural intermediate: mCRP(m). FASEB J. (2007) 21:284–94. doi: 10.1096/fj.06-6722com
40. Hammond DJ Jr., Singh SK, Thompson JA, Beeler BW, Rusinol AE, Pangburn MK, et al. Identification of acidic pH-dependent ligands of pentameric C-reactive protein. J Biol Chem. (2010) 285:36235–44. doi: 10.1074/jbc.M110.142026
41. Braig D, Nero TL, Koch HG, Kaiser B, Wang X, Thiele JR, et al. Transitional changes in the CRP structure lead to the exposure of proinflammatory binding sites. Nat Commun. (2017) 8:14188. doi: 10.1038/ncomms14188
42. Lv JM, Wang MY. In vitro generation and bioactivity evaluation of C-reactive protein intermediate. PloS One. (2018) 13:e0198375. doi: 10.1371/journal.pone.0198375
43. Pena E, de la Torre R, Arderiu G, Slevin M, Badimon L. mCRP triggers angiogenesis by inducing F3 transcription and TF signalling in microvascular endothelial cells. Thromb Haemost. (2017) 117:357–70. doi: 10.1160/TH16-07-0524
44. Shrive AK, Cheetham GM, Holden D, Myles DA, Turnell WG, Volanakis JE, et al. Three dimensional structure of human C-reactive protein. Nat Struct Biol. (1996) 3:346–54. doi: 10.1038/nsb0496-346
45. Lv JM, Lu SQ, Liu ZP, Zhang J, Gao BX, Yao ZY, et al. Conformational folding and disulfide bonding drive distinct stages of protein structure formation. Sci Rep. (2018) 8:1494. doi: 10.1038/s41598-018-20014-y
46. Thompson D, Pepys MB, Wood SP. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure. (1999) 7:169–77. doi: 10.1016/S0969-2126(99)80023-9
47. Agrawal A, Xu Y, Ansardi D, Macon KJ, Volanakis JE. Probing the phosphocholine-binding site of human C-reactive protein by site-directed mutagenesis. J Biol Chem. (1992) 267:25353–8. doi: 10.1016/S0021-9258(19)74047-2
48. Lee RT, Takagahara I, Lee YC. Mapping the binding areas of human C-reactive protein for phosphorylcholine and polycationic compounds. Relationship between the two types of binding sites. J Biol Chem. (2002) 277:225–32. doi: 10.1074/jbc.M106039200
49. Khan SA, Ilies MA. The phospholipase A2 superfamily: structure, isozymes, catalysis, physiologic and pathologic roles. Int J Mol Sci. (2023) 24:1353. doi: 10.3390/ijms24021353
50. Endale HT, Tesfaye W, Mengstie TA. ROS induced lipid peroxidation and their role in ferroptosis. Front Cell Dev Biol. (2023) 11:1226044. doi: 10.3389/fcell.2023.1226044
51. Rajab IM, Majerczyk D, Olson ME, Addams JMB, Choe ML, Nelson MS, et al. C-reactive protein in gallbladder diseases: diagnostic and therapeutic insights. Biophysics Rep. (2020) 6:49–67. doi: 10.1007/s41048-020-00108-9
52. Cicchinelli S, Pignataro G, Gemma S, Piccioni A, Picozzi D, Ojetti V, et al. PAMPs and DAMPs in sepsis: A review of their molecular features and potential clinical implications. Int J Mol Sci. (2024) 25:962. doi: 10.3390/ijms25020962
53. Zhang Y, Jen FE, Fox KL, Edwards JL, Jennings MP. The biosynthesis and role of phosphorylcholine in pathogenic and nonpathogenic bacteria. Trends Microbiol. (2023) 31:692–706. doi: 10.1016/j.tim.2023.01.006
54. Abrams ST, Zhang N, Dart C, Wang SS, Thachil J, Guan Y, et al. Human CRP defends against the toxicity of circulating histones. J Immunol. (2013) 191:2495–502. doi: 10.4049/jimmunol.1203181
55. Suresh MV, Singh SK, Agrawal A. Interaction of calcium-bound C-reactive protein with fibronectin is controlled by pH: in vivo implications. J Biol Chem. (2004) 279:52552–7. doi: 10.1074/jbc.M409054200
56. Chang MK, Binder CJ, Torzewski M, Witztum JL. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: Phosphorylcholine of oxidized phospholipids. Proc Natl Acad Sci U S A. (2002) 99:13043–8. doi: 10.1073/pnas.192399699
57. Schwalbe RA, Dahlback B, Coe JE, Nelsestuen GL. Pentraxin family of proteins interact specifically with phosphorylcholine and/or phosphorylethanolamine. Biochemistry. (1992) 31:4907–15. doi: 10.1021/bi00135a023
58. Salonen EM, Vartio T, Hedman K, Vaheri A. Binding of fibronectin by the acute phase reactant C-reactive protein. J Biol Chem. (1984) 259:1496–501. doi: 10.1016/S0021-9258(17)43435-1
59. Agrawal A, Shrive AK, Greenhough TJ, Volanakis JE. Topology and structure of the C1q-binding site on C-reactive protein. J Immunol. (2001) 166:3998–4004. doi: 10.4049/jimmunol.166.6.3998
60. Lu J, Mold C, Du Clos TW, Sun PD. Pentraxins and fc receptor-mediated immune responses. Front Immunol. (2018) 9:2607. doi: 10.3389/fimmu.2018.02607
61. Biro A, Rovo Z, Papp D, Cervenak L, Varga L, Fust G, et al. Studies on the interactions between C-reactive protein and complement proteins. Immunology. (2007) 121:40–50. doi: 10.1111/j.1365-2567.2007.02535.x
62. Liu N, Liu J, Ji Y, Lu P. Toll-like receptor 4 signaling mediates inflammatory activation induced by C-reactive protein in vascular smooth muscle cells. Cell Physiol Biochem. (2010) 25:467–76. doi: 10.1159/000303052
63. Liu N, Liu JT, Ji YY, Lu PP. C-reactive protein triggers inflammatory responses partly via TLR4/IRF3/NF-kappaB signaling pathway in rat vascular smooth muscle cells. Life Sci. (2010) 87:367–74. doi: 10.1016/j.lfs.2010.07.012
64. Boncler M, Rywaniak J, Szymanski J, Potempa LA, Rychlik B, Watala C. Modified C-reactive protein interacts with platelet glycoprotein Ibalpha. Pharmacol Rep. (2011) 63:464–75. doi: 10.1016/S1734-1140(11)70513-8
65. Molins B, Pena E, de la Torre R, Badimon L. Monomeric C-reactive protein is prothrombotic and dissociates from circulating pentameric C-reactive protein on adhered activated platelets under flow. Cardiovasc Res. (2011) 92:328–37. doi: 10.1093/cvr/cvr226
66. Jia ZK, Li HY, Liang YL, Potempa LA, Ji SR, Wu Y. Monomeric C-reactive protein binds and neutralizes receptor activator of NF-kappaB ligand-induced osteoclast differentiation. Front Immunol. (2018) 9:234. doi: 10.3389/fimmu.2018.00234
67. Zhang Z, Na H, Gan Q, Tao Q, Alekseyev Y, Hu J, et al. Monomeric C-reactive protein via endothelial CD31 for neurovascular inflammation in an ApoE genotype-dependent pattern: A risk factor for Alzheimer’s disease? Aging Cell. (2021) 20:e13501. doi: 10.1111/acel.v20.11
68. Fujita M, Takada YK, Izumiya Y, Takada Y. The binding of monomeric C-reactive protein (mCRP) to Integrins alphavbeta3 and alpha4beta1 is related to its pro-inflammatory action. PLoS One. (2014) 9:e93738. doi: 10.1371/journal.pone.0093738
69. Fujita Y, Kakino A, Nishimichi N, Yamaguchi S, Sato Y, Machida S, et al. Oxidized LDL receptor LOX-1 binds to C-reactive protein and mediates its vascular effects. Clin Chem. (2009) 55:285–94. doi: 10.1373/clinchem.2008.119750
70. de la Torre R, Pena E, Vilahur G, Slevin M, Badimon L. Monomerization of C-reactive protein requires glycoprotein IIb-IIIa activation: pentraxins and platelet deposition. J Thromb Haemost. (2013) 11:2048–58. doi: 10.1111/jth.12415
71. Bang R, Marnell L, Mold C, Stein MP, Clos KT, Chivington-Buck C, et al. Analysis of binding sites in human C-reactive protein for FcgammaRI, FcgammaRIIA, and C1q by site-directed mutagenesis. J Biol Chem. (2005) 280:25095–102. doi: 10.1074/jbc.M504782200
72. Lane T, Wassef N, Poole S, Mistry Y, Lachmann HJ, Gillmore JD, et al. Infusion of pharmaceutical-grade natural human C-reactive protein is not proinflammatory in healthy adult human volunteers. Circ Res. (2014) 114:672–6. doi: 10.1161/CIRCRESAHA.114.302770
73. Sproston NR, El Mohtadi M, Slevin M, Gilmore W, Ashworth JJ. The effect of C-reactive protein isoforms on nitric oxide production by U937 monocytes/macrophages. Front Immunol. (2018) 9:1500. doi: 10.3389/fimmu.2018.01500
74. Jimenez RV, Kuznetsova V, Connelly AN, Hel Z, Szalai AJ. C-reactive protein promotes the expansion of myeloid derived cells with suppressor functions. Front Immunol. (2019) 10:2183. doi: 10.3389/fimmu.2019.02183
75. Jimenez RV, Wright TT, Jones NR, Wu J, Gibson AW, Szalai AJ. C-reactive protein impairs dendritic cell development, maturation, and function: implications for peripheral tolerance. Front Immunol. (2018) 9:372. doi: 10.3389/fimmu.2018.00372
76. Kikuchi K, Kohyama T, Yamauchi Y, Kato J, Takami K, Okazaki H, et al. C-reactive protein modulates human lung fibroblast migration. Exp Lung Res. (2009) 35:48–58. doi: 10.1080/01902140802404138
77. Singh SK, Suresh MV, Prayther DC, Moorman JP, Rusinol AE, Agrawal A. C-reactive protein-bound enzymatically modified low-density lipoprotein does not transform macrophages into foam cells. J Immunol. (2008) 180:4316–22. doi: 10.4049/jimmunol.180.6.4316
78. Khreiss T, Jozsef L, Potempa LA, Filep JG. Opposing effects of C-reactive protein isoforms on shear-induced neutrophil-platelet adhesion and neutrophil aggregation in whole blood. Circulation. (2004) 110:2713–20. doi: 10.1161/01.CIR.0000146846.00816.DD
79. Li SL, Feng JR, Zhou HH, Zhang CM, Lv GB, Tan YB, et al. Acidic pH promotes oxidation-induced dissociation of C-reactive protein. Mol Immunol. (2018) 104:47–53. doi: 10.1016/j.molimm.2018.09.021
80. Erra Diaz F, Dantas E, Geffner J. Unravelling the interplay between extracellular acidosis and immune cells. Mediators Inflamm. (2018) 2018:1218297. doi: 10.1155/2018/1218297
81. Wang MS, Messersmith RE, Reed SM. Membrane curvature recognition by C-reactive protein using lipoprotein mimics. Soft Matter. (2012) 8:7909–18. doi: 10.1039/c2sm25779c
82. Alnaas AA, Moon CL, Alton M, Reed SM, Knowles MK. Conformational changes in C-reactive protein affect binding to curved membranes in a lipid bilayer model of the apoptotic cell surface. J Phys Chem B. (2017) 121:2631–9. doi: 10.1021/acs.jpcb.6b11505
83. Hatzakis NS, Bhatia VK, Larsen J, Madsen KL, Bolinger PY, Kunding AH, et al. How curved membranes recruit amphipathic helices and protein anchoring motifs. Nat Chem Biol. (2009) 5:835–41. doi: 10.1038/nchembio.213
84. Noone DP, van der Velden TT, Sharp TH. Cryo-electron microscopy and biochemical analysis offer insights into the effects of acidic pH, such as occur during acidosis, on the complement binding properties of C-reactive protein. Front Immunol. (2021) 12:757633. doi: 10.3389/fimmu.2021.757633
85. Noone DP, Isendoorn MME, Hamers S, Keizer ME, Wulffele J, van der Velden TT, et al. Structural basis for surface activation of the classical complement cascade by the short pentraxin C-reactive protein. Proc Natl Acad Sci U.S.A. (2024) 121:e2404542121. doi: 10.1073/pnas.2404542121
86. Gershov D, Kim S, Brot N, Elkon KB. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J Exp Med. (2000) 192:1353–64. doi: 10.1084/jem.192.9.1353
87. Haapasalo K, Meri S. Regulation of the complement system by pentraxins. Front Immunol. (2019) 10:1750. doi: 10.3389/fimmu.2019.01750
88. Strang F, Scheichl A, Chen YC, Wang X, Htun NM, Bassler N, et al. Amyloid plaques dissociate pentameric to monomeric C-reactive protein: a novel pathomechanism driving cortical inflammation in Alzheimer’s disease? Brain Pathol. (2012) 22:337–46. doi: 10.1111/j.1750-3639.2011.00539.x
89. Eisenhardt SU, Habersberger J, Murphy A, Chen YC, Woollard KJ, Bassler N, et al. Dissociation of pentameric to monomeric C-reactive protein on activated platelets localizes inflammation to atherosclerotic plaques. Circ Res. (2009) 105:128–37. doi: 10.1161/CIRCRESAHA.108.190611
90. Boncler M, Kehrel B, Szewczyk R, Stec-Martyna E, Bednarek R, Brodde M, et al. Oxidation of C-reactive protein by hypochlorous acid leads to the formation of potent platelet activator. Int J Biol Macromol. (2018) 107:2701–14. doi: 10.1016/j.ijbiomac.2017.10.159
91. Zhang L, Shen ZY, Wang K, Li W, Shi JM, Osoro EK, et al. C-reactive protein exacerbates epithelial-mesenchymal transition through Wnt/beta-catenin and ERK signaling in streptozocin-induced diabetic nephropathy. FASEB J. (2019) 33:6551–63. doi: 10.1096/fj.201801865RR
92. Luan YY, Yao YM. The clinical significance and potential role of C-reactive protein in chronic inflammatory and neurodegenerative diseases. Front Immunol. (2018) 9:1302. doi: 10.3389/fimmu.2018.01302
93. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. (2018) 9:754. doi: 10.3389/fimmu.2018.00754
94. McFadyen JD, Kiefer J, Braig D, Loseff-Silver J, Potempa LA, Eisenhardt SU, et al. Dissociation of C-reactive protein localizes and amplifies inflammation: evidence for a direct biological role of C-reactive protein and its conformational changes. Front Immunol. (2018) 9:1351. doi: 10.3389/fimmu.2018.01351
95. Yao Z, Zhang Y, Wu H. Regulation of C-reactive protein conformation in inflammation. Inflammation Res. (2019) 68:815–23. doi: 10.1007/s00011-019-01269-1
96. Potempa LA, Rajab IM, Olson ME, Hart PC. C-reactive protein and cancer: interpreting the differential bioactivities of its pentameric and monomeric, modified isoforms. Front Immunol. (2021) 12:744129. doi: 10.3389/fimmu.2021.744129
97. Rizo-Tellez SA, Sekheri M, Filep JG. C-reactive protein: a target for therapy to reduce inflammation. Front Immunol. (2023) 14:1237729. doi: 10.3389/fimmu.2023.1237729
98. Ji SR, Zhang SH, Chang Y, Li HY, Wang MY, Lv JM, et al. C-reactive protein: the most familiar stranger. J Immunol. (2023) 210:699–707. doi: 10.4049/jimmunol.2200831
99. Pastorello Y, Carare RO, Banescu C, Potempa L, Di Napoli M, Slevin M. Monomeric C-reactive protein: A novel biomarker predicting neurodegenerative disease and vascular dysfunction. Brain Pathol. (2023) 33:e13164. doi: 10.1111/bpa.13164
100. Li HY, Wang J, Meng F, Jia ZK, Su Y, Bai QF, et al. An intrinsically disordered motif mediates diverse actions of monomeric C-reactive protein. J Biol Chem. (2016) 291:8795–804. doi: 10.1074/jbc.M115.695023
101. Ullah N, Ma FR, Han J, Liu XL, Fu Y, Liu YT, et al. Monomeric C-reactive protein regulates fibronectin mediated monocyte adhesion. Mol Immunol. (2020) 117:122–30. doi: 10.1016/j.molimm.2019.10.013
102. Thiele JR, Zeller J, Kiefer J, Braig D, Kreuzaler S, Lenz Y, et al. A conformational change in C-reactive protein enhances leukocyte recruitment and reactive oxygen species generation in ischemia/reperfusion injury. Front Immunol. (2018) 9:675. doi: 10.3389/fimmu.2018.00675
103. Torzewski M, Rist C, Mortensen RF, Zwaka TP, Bienek M, Waltenberger J, et al. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. (2000) 20:2094–9. doi: 10.1161/01.ATV.20.9.2094
104. Fujita C, Sakurai Y, Yasuda Y, Takada Y, Huang CL, Fujita M. Anti-monomeric C-reactive protein antibody ameliorates arthritis and nephritis in mice. J Immunol. (2021) 207:1755–62. doi: 10.4049/jimmunol.2100349
105. Chirco KR, Whitmore SS, Wang K, Potempa LA, Halder JA, Stone EM, et al. Monomeric C-reactive protein and inflammation in age-related macular degeneration. J Pathol. (2016) 240:173–83. doi: 10.1002/path.4766
106. Miyazawa K, Kiyono S, Inoue K. Modulation of stimulus-dependent human platelet activation by C-reactive protein modified with active oxygen species. J Immunol. (1988) 141:570–4. doi: 10.4049/jimmunol.141.2.570
107. Xu PC, Lin S, Yang XW, Gu DM, Yan TK, Wei L, et al. C-reactive protein enhances activation of coagulation system and inflammatory response through dissociating into monomeric form in antineutrophil cytoplasmic antibody-associated vasculitis. BMC Immunol. (2015) 16:10. doi: 10.1186/s12865-015-0077-0
108. Zeller J, Loseff-Silver J, Khoshmanesh K, Baratchi S, Lai A, Nero TL, et al. Shear-sensing by C-reactive protein: linking aortic stenosis and inflammation. Circ Res. (2024) 135:1033–47. doi: 10.1161/CIRCRESAHA.124.324248
109. Zeller J, Bogner B, Kiefer J, Braig D, Winninger O, Fricke M, et al. CRP enhances the innate killing mechanisms phagocytosis and ROS formation in a conformation and complement-dependent manner. Front Immunol. (2021) 12:721887. doi: 10.3389/fimmu.2021.721887
110. Wang MY, Ji SR, Bai CJ, El Kebir D, Li HY, Shi JM, et al. A redox switch in C-reactive protein modulates activation of endothelial cells. FASEB J. (2011) 25:3186–96. doi: 10.1096/fj.11-182741
111. Pastorello Y, Manu D, Sawkulycz X, Caprio V, Banescu C, Dobreanu M, et al. mCRP-induced focal adhesion kinase-dependent monocyte aggregation and M1 polarization, which was partially blocked by the C10M inhibitor. Int J Mol Sci. (2024) 25:3097. doi: 10.3390/ijms25063097
112. Slevin M, Iemma RS, Zeinolabediny Y, Liu D, Ferris GR, Caprio V, et al. Acetylcholine inhibits monomeric C-reactive protein induced inflammation, endothelial cell adhesion, and platelet aggregation; A potential therapeutic? Front Immunol. (2018) 9:2124. doi: 10.3389/fimmu.2018.02124
113. Chi M, Tridandapani S, Zhong W, Coggeshall KM, Mortensen RF. C-reactive protein induces signaling through Fc gamma RIIa on HL-60 granulocytes. J Immunol. (2002) 168:1413–8. doi: 10.4049/jimmunol.168.3.1413
114. Mihlan M, Blom AM, Kupreishvili K, Lauer N, Stelzner K, Bergstrom F, et al. Monomeric C-reactive protein modulates classic complement activation on necrotic cells. FASEB J. (2011) 25:4198–210. doi: 10.1096/fj.11-186460
115. Carlson CS, Aldred SF, Lee PK, Tracy RP, Schwartz SM, Rieder M, et al. Polymorphisms within the C-reactive protein (CRP) promoter region are associated with plasma CRP levels. Am J Hum Genet. (2005) 77:64–77. doi: 10.1086/431366
116. Kardys I, de Maat MP, Uitterlinden AG, Hofman A, Witteman JC. C-reactive protein gene haplotypes and risk of coronary heart disease: the Rotterdam Study. Eur Heart J. (2006) 27:1331–7. doi: 10.1093/eurheartj/ehl018
117. Aziz N, Fahey JL, Detels R, Butch AW. Analytical performance of a highly sensitive C-reactive protein-based immunoassay and the effects of laboratory variables on levels of protein in blood. Clin Diagn Lab Immunol. (2003) 10:652–7. doi: 10.1128/CDLI.10.4.652-657.2003
118. Zeller J, Bogner B, McFadyen JD, Kiefer J, Braig D, Pietersz G, et al. Transitional changes in the structure of C-reactive protein create highly pro-inflammatory molecules: Therapeutic implications for cardiovascular diseases. Pharmacol Ther. (2022) 235:108165. doi: 10.1016/j.pharmthera.2022.108165
119. Biro E, Nieuwland R, Tak PP, Pronk LM, Schaap MC, Sturk A, et al. Activated complement components and complement activator molecules on the surface of cell-derived microparticles in patients with rheumatoid arthritis and healthy individuals. Ann Rheum Dis. (2007) 66:1085–92. doi: 10.1136/ard.2006.061309
120. van der Zee PM, Biro E, Trouw LA, Ko Y, de Winter RJ, Hack CE, et al. C-reactive protein in myocardial infarction binds to circulating microparticles but is not associated with complement activation. Clin Immunol. (2010) 135:490–5. doi: 10.1016/j.clim.2010.01.002
121. van Eijk IC, Tushuizen ME, Sturk A, Dijkmans BA, Boers M, Voskuyl AE, et al. Circulating microparticles remain associated with complement activation despite intensive anti-inflammatory therapy in early rheumatoid arthritis. Ann Rheum Dis. (2010) 69:1378–82. doi: 10.1136/ard.2009.118372
122. Karlsson J, Wettero J, Weiner M, Ronnelid J, Fernandez-Botran R, Sjowall C. Associations of C-reactive protein isoforms with systemic lupus erythematosus phenotypes and disease activity. Arthritis Res Ther. (2022) 24:139. doi: 10.1186/s13075-022-02831-9
123. Wang J, Tang B, Liu X, Wu X, Wang H, Xu D, et al. Increased monomeric CRP levels in acute myocardial infarction: a possible new and specific biomarker for diagnosis and severity assessment of disease. Atherosclerosis. (2015) 239:343–9. doi: 10.1016/j.atherosclerosis.2015.01.024
124. Slevin M, Matou-Nasri S, Turu M, Luque A, Rovira N, Badimon L, et al. Modified C-reactive protein is expressed by stroke neovessels and is a potent activator of angiogenesis in vitro. Brain Pathol. (2010) 20:151–65. doi: 10.1111/j.1750-3639.2008.00256.x
125. Torzewski J, Torzewski M, Bowyer DE, Frohlich M, Koenig W, Waltenberger J, et al. C-reactive protein frequently colocalizes with the terminal complement complex in the intima of early atherosclerotic lesions of human coronary arteries. Arterioscler Thromb Vasc Biol. (1998) 18:1386–92. doi: 10.1161/01.ATV.18.9.1386
126. Al-Baradie RS, Pu S, Liu D, Zeinolabediny Y, Ferris G, Sanfeli C, et al. Monomeric C-reactive protein localized in the cerebral tissue of damaged vascular brain regions is associated with neuro-inflammation and neurodegeneration-an immunohistochemical study. Front Immunol. (2021) 12:644213. doi: 10.3389/fimmu.2021.644213
127. Ngwa DN, Pathak A, Agrawal A. IL-6 regulates induction of C-reactive protein gene expression by activating STAT3 isoforms. Mol Immunol. (2022) 146:50–6. doi: 10.1016/j.molimm.2022.04.003
128. Kramer F, Torzewski J, Kamenz J, Veit K, Hombach V, Dedio J, et al. Interleukin-1beta stimulates acute phase response and C-reactive protein synthesis by inducing an NFkappaB- and C/EBPbeta-dependent autocrine interleukin-6 loop. Mol Immunol. (2008) 45:2678–89. doi: 10.1016/j.molimm.2007.12.017
129. Macintyre S, Samols D, Dailey P. Two carboxylesterases bind C-reactive protein within the endoplasmic reticulum and regulate its secretion during the acute phase response. J Biol Chem. (1994) 269:24496–503. doi: 10.1016/S0021-9258(19)51111-5
130. Vanderschueren S, Deeren D, Knockaert DC, Bobbaers H, Bossuyt X, Peetermans W. Extremely elevated C-reactive protein. Eur J Intern Med. (2006) 17:430–3. doi: 10.1016/j.ejim.2006.02.025
131. Molins B, Figueras-Roca M, Valero O, Llorenc V, Romero-Vazquez S, Sibila O, et al. C-reactive protein isoforms as prognostic markers of COVID-19 severity. Front Immunol. (2022) 13:1105343. doi: 10.3389/fimmu.2022.1105343
132. Williams RD, Moran JA, Fryer AA, Littlejohn JR, Williams HM, Greenhough TJ, et al. Monomeric C-reactive protein in serum with markedly elevated CRP levels shares common calcium-dependent ligand binding properties with an in vitro dissociated form of C-reactive protein. Front Immunol. (2020) 11:115. doi: 10.3389/fimmu.2020.00115
133. Santonocito C, De Loecker I, Donadello K, Moussa MD, Markowicz S, Gullo A, et al. C-reactive protein kinetics after major surgery. Anesth Analg. (2014) 119:624–9. doi: 10.1213/ANE.0000000000000263
134. Motie M, Schaul KW, Potempa LA. Biodistribution and clearance of 125I-labeled C-reactive protein and 125I-labeled modified C-reactive protein in CD-1 mice. Drug Metab Dispos. (1998) 26:977–81.
136. Scridon A. Platelets and their role in hemostasis and thrombosis-from physiology to pathophysiology and therapeutic implications. Int J Mol Sci. (2022) 23:12772. doi: 10.3390/ijms232112772
137. Potempa LA, Zeller JM, Fiedel BA, Kinoshita CM, Gewurz H. Stimulation of human neutrophils, monocytes, and platelets by modified C-reactive protein (CRP) expressing a neoantigenic specificity. Inflammation. (1988) 12:391–405. doi: 10.1007/BF00915774
138. Molins B, Pena E, Vilahur G, Mendieta C, Slevin M, Badimon L. C-reactive protein isoforms differ in their effects on thrombus growth. Arterioscler Thromb Vasc Biol. (2008) 28:2239–46. doi: 10.1161/ATVBAHA.108.174359
139. Qiao J, Al-Tamimi M, Baker RI, Andrews RK, Gardiner EE. The platelet Fc receptor, FcgammaRIIa. Immunol Rev. (2015) 268:241–52. doi: 10.1111/imr.2015.268.issue-1
140. Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. (2007) 13:463–9. doi: 10.1038/nm1565
141. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. (2000) 102:2165–8. doi: 10.1161/01.CIR.102.18.2165
142. Blann AD, Lip GY. Effects of C-reactive protein on the release of von Willebrand factor, E-selectin, thrombomodulin and intercellular adhesion molecule-1 from human umbilical vein endothelial cells. Blood Coagul Fibrinolysis. (2003) 14:335–40. doi: 10.1097/00001721-200306000-00003
143. Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. (2002) 106:1439–41. doi: 10.1161/01.CIR.0000033116.22237.F9
144. Singh U, Devaraj S, Vasquez-Vivar J, Jialal I. C-reactive protein decreases endothelial nitric oxide synthase activity via uncoupling. J Mol Cell Cardiol. (2007) 43:780–91. doi: 10.1016/j.yjmcc.2007.08.015
145. Cirillo P, Golino P, Calabro P, Cali G, Ragni M, De Rosa S, et al. C-reactive protein induces tissue factor expression and promotes smooth muscle and endothelial cell proliferation. Cardiovasc Res. (2005) 68:47–55. doi: 10.1016/j.cardiores.2005.05.010
146. Chen Y, Wang J, Yao Y, Yuan W, Kong M, Lin Y, et al. CRP regulates the expression and activity of tissue factor as well as tissue factor pathway inhibitor via NF-kappaB and ERK 1/2 MAPK pathway. FEBS Lett. (2009) 583:2811–8. doi: 10.1016/j.febslet.2009.07.037
147. Ji Y, Fish PM, Strawn TL, Lohman AW, Wu J, Szalai AJ, et al. C-reactive protein induces expression of tissue factor and plasminogen activator inhibitor-1 and promotes fibrin accumulation in vein grafts. J Thromb Haemost. (2014) 12:1667–77. doi: 10.1111/jth.12680
148. Li R, Ren M, Luo M, Chen N, Zhang Z, Luo B, et al. Monomeric C-reactive protein alters fibrin clot properties on endothelial cells. Thromb Res. (2012) 129:e251–6. doi: 10.1016/j.thromres.2012.03.014
149. Wu J, Stevenson MJ, Brown JM, Grunz EA, Strawn TL, Fay WP. C-reactive protein enhances tissue factor expression by vascular smooth muscle cells: mechanisms and in vivo significance. Arterioscler Thromb Vasc Biol. (2008) 28:698–704. doi: 10.1161/ATVBAHA.107.160903
150. Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. The neutrophil. Immunity. (2021) 54:1377–91. doi: 10.1016/j.immuni.2021.06.006
151. von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. (2012) 209:819–35. doi: 10.1084/jem.20112322
152. Zeller J, Cheung Tung Shing KS, Nero TL, McFadyen JD, Krippner G, Bogner B, et al. A novel phosphocholine-mimetic inhibits a pro-inflammatory conformational change in C-reactive protein. EMBO Mol Med. (2023) 15:e16236. doi: 10.15252/emmm.202216236
153. Huber-Lang M, Lambris JD, Ward PA. Innate immune responses to trauma. Nat Immunol. (2018) 19:327–41. doi: 10.1038/s41590-018-0064-8
154. Wang Z, Qi F, Luo H, Xu G, Wang D. Inflammatory microenvironment of skin wounds. Front Immunol. (2022) 13:789274. doi: 10.3389/fimmu.2022.789274
155. Johnson BZ, Stevenson AW, Prele CM, Fear MW, Wood FM. The role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines. (2020) 8:101. doi: 10.3390/biomedicines8050101
156. Li HY, Wang J, Wu YX, Zhang L, Liu ZP, Filep JG, et al. Topological localization of monomeric C-reactive protein determines proinflammatory endothelial cell responses. J Biol Chem. (2014) 289:14283–90. doi: 10.1074/jbc.M114.555318
157. Foley JH, Conway EM. Cross talk pathways between coagulation and inflammation. Circ Res. (2016) 118:1392–408. doi: 10.1161/CIRCRESAHA.116.306853
158. Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. (2016) 44:450–62. doi: 10.1016/j.immuni.2016.02.015
159. Wang Q, Zhu X, Xu Q, Ding X, Chen YE, Song Q. Effect of C-reactive protein on gene expression in vascular endothelial cells. Am J Physiol Heart Circ Physiol. (2005) 288:H1539–45. doi: 10.1152/ajpheart.00963.2004
160. Wang J. Neutrophils in tissue injury and repair. Cell Tissue Res. (2018) 371:531–9. doi: 10.1007/s00441-017-2785-7
161. Pitchford S, Pan D, Welch HC. Platelets in neutrophil recruitment to sites of inflammation. Curr Opin Hematol. (2017) 24:23–31. doi: 10.1097/MOH.0000000000000297
162. Karasu E, Halbgebauer R, Schutte L, Greven J, Blasius FM, Zeller J, et al. A conformational change of C-reactive protein drives neutrophil extracellular trap formation in inflammation. BMC Biol. (2025) 23:4. doi: 10.1186/s12915-024-02093-8
163. Wang Y, Jonsson F. Expression, role, and regulation of neutrophil fcgamma receptors. Front Immunol. (2019) 10:1958. doi: 10.3389/fimmu.2019.01958
164. Lu J, Marjon KD, Marnell LL, Wang R, Mold C, Du Clos TW, et al. Recognition and functional activation of the human IgA receptor (FcalphaRI) by C-reactive protein. Proc Natl Acad Sci U S A. (2011) 108:4974–9. doi: 10.1073/pnas.1018369108
165. Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcgamma receptors in dendritic cells and macrophages. Nat Rev Immunol. (2014) 14:94–108. doi: 10.1038/nri3582
166. Wei J, Zhang Y, Li H, Wang F, Yao S. Toll-like receptor 4: A potential therapeutic target for multiple human diseases. BioMed Pharmacother. (2023) 166:115338. doi: 10.1016/j.biopha.2023.115338
167. Liu N, Liu J, Ji Y, Lu P, Wang C, Guo F. C-reactive protein induces TNF-alpha secretion by p38 MAPK-TLR4 signal pathway in rat vascular smooth muscle cells. Inflammation. (2011) 34:283–90. doi: 10.1007/s10753-010-9234-z
168. Newling M, Sritharan L, van der Ham AJ, Hoepel W, Fiechter RH, de Boer L, et al. C-reactive protein promotes inflammation through fcgammaR-induced glycolytic reprogramming of human macrophages. J Immunol. (2019) 203:225–35. doi: 10.4049/jimmunol.1900172
169. Zha Z, Cheng Y, Cao L, Qian Y, Liu X, Guo Y, et al. Monomeric CRP aggravates myocardial injury after myocardial infarction by polarizing the macrophage to pro-inflammatory phenotype through JNK signaling pathway. J Inflammation Res. (2021) 14:7053–64. doi: 10.2147/JIR.S316816
170. Trial J, Cieslik KA, Entman ML. Phosphocholine-containing ligands direct CRP induction of M2 macrophage polarization independent of T cell polarization: Implication for chronic inflammatory states. Immun Inflammation Dis. (2016) 4:274–88. doi: 10.1002/iid3.2016.4.issue-3
171. Schauer C, Janko C, Munoz LE, Zhao Y, Kienhofer D, Frey B, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. (2014) 20:511–7. doi: 10.1038/nm.3547
172. Prame Kumar K, Nicholls AJ, Wong CHY. Partners in crime: neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. (2018) 371:551–65. doi: 10.1007/s00441-017-2753-2
173. Xu Q, Zhao W, Yan M, Mei H. Neutrophil reverse migration. J Inflammation (Lond). (2022) 19:22. doi: 10.1186/s12950-022-00320-z
174. Bratton DL, Henson PM. Neutrophil clearance: when the party is over, clean-up begins. Trends Immunol. (2011) 32:350–7. doi: 10.1016/j.it.2011.04.009
175. Elliott MR, Koster KM, Murphy PS. Efferocytosis signaling in the regulation of macrophage inflammatory responses. J Immunol. (2017) 198:1387–94. doi: 10.4049/jimmunol.1601520
176. Rodriguez W, Mold C, Kataranovski M, Hutt JA, Marnell LL, Verbeek JS, et al. C-reactive protein-mediated suppression of nephrotoxic nephritis: role of macrophages, complement, and Fcgamma receptors. J Immunol. (2007) 178:530–8. doi: 10.4049/jimmunol.178.1.530
177. Zouki C, Beauchamp M, Baron C, Filep JG. Prevention of in vitro neutrophil adhesion to endothelial cells through shedding of L-selectin by C-reactive protein and peptides derived from C-reactive protein. J Clin Invest. (1997) 100:522–9. doi: 10.1172/JCI119561
178. Heuertz RM, Schneider GP, Potempa LA, Webster RO. Native and modified C-reactive protein bind different receptors on human neutrophils. Int J Biochem Cell Biol. (2005) 37:320–35. doi: 10.1016/j.biocel.2004.07.002
179. Ying SC, Shephard E, de Beer FC, Siegel JN, Harris D, Gewurz BE, et al. Localization of sequence-determined neoepitopes and neutrophil digestion fragments of C-reactive protein utilizing monoclonal antibodies and synthetic peptides. Mol Immunol. (1992) 29:677–87. doi: 10.1016/0161-5890(92)90205-C
180. El Kebir D, Zhang Y, Potempa LA, Wu Y, Fournier A, Filep JG. C-reactive protein-derived peptide 201-206 inhibits neutrophil adhesion to endothelial cells and platelets through CD32. J Leukoc Biol. (2011) 90:1167–75. doi: 10.1189/jlb.0111032
181. Everts PA, Lana JF, Onishi K, Buford D, Peng J, Mahmood A, et al. Angiogenesis and tissue repair depend on platelet dosing and bioformulation strategies following orthobiological platelet-rich plasma procedures: A narrative review. Biomedicines. (2023) 11:1922. doi: 10.3390/biomedicines11071922
182. Slevin M, Matou S, Zeinolabediny Y, Corpas R, Weston R, Liu D, et al. Monomeric C-reactive protein–a key molecule driving development of Alzheimer’s disease associated with brain ischaemia? Sci Rep. (2015) 5:13281. doi: 10.1038/srep13281
183. Turu MM, Slevin M, Matou S, West D, Rodriguez C, Luque A, et al. C-reactive protein exerts angiogenic effects on vascular endothelial cells and modulates associated signalling pathways and gene expression. BMC Cell Biol. (2008) 9:47. doi: 10.1186/1471-2121-9-47
184. Guo M, Niu Y, Xie M, Liu X, Li X. Notch signaling, hypoxia, and cancer. Front Oncol. (2023) 13:1078768. doi: 10.3389/fonc.2023.1078768
185. Wang X, Bove AM, Simone G, Ma B. Molecular bases of VEGFR-2-mediated physiological function and pathological role. Front Cell Dev Biol. (2020) 8:599281. doi: 10.3389/fcell.2020.599281
186. Chen J, Gu Z, Wu M, Yang Y, Zhang J, Ou J, et al. C-reactive protein can upregulate VEGF expression to promote ADSC-induced angiogenesis by activating HIF-1alpha via CD64/PI3k/Akt and MAPK/ERK signaling pathways. Stem Cell Res Ther. (2016) 7:114. doi: 10.1186/s13287-016-0377-1
187. Boras E, Slevin M, Alexander MY, Aljohi A, Gilmore W, Ashworth J, et al. Monomeric C-reactive protein and Notch-3 co-operatively increase angiogenesis through PI3K signalling pathway. Cytokine. (2014) 69:165–79. doi: 10.1016/j.cyto.2014.05.027
188. Bello G, Cailotto F, Hanriot D, Kolopp-Sarda MN, Latger-Cannard V, Hess K, et al. C-reactive protein (CRP) increases VEGF-A expression in monocytic cells via a PI3-kinase and ERK 1/2 signaling dependent pathway. Atherosclerosis. (2008) 200:286–93. doi: 10.1016/j.atherosclerosis.2007.12.046
189. Cherny SS, Brzezinski RY, Wasserman A, Adler A, Berliner S, Nevo D, et al. Characterizing CRP dynamics during acute infections. Infection. (2024). doi: 10.1007/s15010-024-02422-7
190. Hage FG, Szalai AJ. C-reactive protein gene polymorphisms, C-reactive protein blood levels, and cardiovascular disease risk. J Am Coll Cardiol. (2007) 50:1115–22. doi: 10.1016/j.jacc.2007.06.012
191. Levinson T, Wasserman A. C-reactive protein velocity (CRPv) as a new biomarker for the early detection of acute infection/inflammation. Int J Mol Sci. (2022) 23:8100. doi: 10.3390/ijms23158100
192. Badimon L, Pena E, Arderiu G, Padro T, Slevin M, Vilahur G, et al. C-reactive protein in atherothrombosis and angiogenesis. Front Immunol. (2018) 9:430. doi: 10.3389/fimmu.2018.00430
193. Zhang Z, Lin W, Gan Q, Lei M, Gong B, Zhang C, et al. The influences of ApoE isoforms on endothelial adherens junctions and actin cytoskeleton responding to mCRP. Angiogenesis. (2024) 27:861–81. doi: 10.1007/s10456-024-09946-4
194. Verma S, Wang CH, Li SH, Dumont AS, Fedak PW, Badiwala MV, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. (2002) 106:913–9. doi: 10.1161/01.CIR.0000029802.88087.5E
195. Hattori Y, Matsumura M, Kasai K. Vascular smooth muscle cell activation by C-reactive protein. Cardiovasc Res. (2003) 58:186–95. doi: 10.1016/S0008-6363(02)00855-6
196. Cimmino G, Ragni M, Cirillo P, Petrillo G, Loffredo F, Chiariello M, et al. C-reactive protein induces expression of matrix metalloproteinase-9: a possible link between inflammation and plaque rupture. Int J Cardiol. (2013) 168:981–6. doi: 10.1016/j.ijcard.2012.10.040
197. Doronzo G, Russo I, Mattiello L, Trovati M, Anfossi G. C-reactive protein increases matrix metalloproteinase-2 expression and activity in cultured human vascular smooth muscle cells. J Lab Clin Med. (2005) 146:287–98. doi: 10.1016/j.lab.2005.07.010
198. Ho KJ, Owens CD, Longo T, Sui XX, Ifantides C, Conte MS. C-reactive protein and vein graft disease: evidence for a direct effect on smooth muscle cell phenotype via modulation of PDGF receptor-beta. Am J Physiol Heart Circ Physiol. (2008) 295:H1132–H40. doi: 10.1152/ajpheart.00079.2008
199. He W, Ren Y, Wang X, Chen Q, Ding S. C reactive protein and enzymatically modified LDL cooperatively promote dendritic cell-mediated T cell activation. Cardiovasc Pathol. (2017) 29:1–6. doi: 10.1016/j.carpath.2017.03.009
200. Van Vre EA, Bult H, Hoymans VY, Van Tendeloo VF, Vrints CJ, Bosmans JM. Human C-reactive protein activates monocyte-derived dendritic cells and induces dendritic cell-mediated T-cell activation. Arterioscler Thromb Vasc Biol. (2008) 28:511–8. doi: 10.1161/ATVBAHA.107.157016
201. Yoshida T, Ichikawa J, Giuroiu I, Laino AS, Hao Y, Krogsgaard M, et al. C reactive protein impairs adaptive immunity in immune cells of patients with melanoma. J Immunother Cancer. (2020) 8:e000234. doi: 10.1136/jitc-2019-000234
202. Tobiasova-Czetoova Z, Palmborg A, Lundqvist A, Karlsson G, Adamson L, Bartunkova J, et al. Effects of human plasma proteins on maturation of monocyte-derived dendritic cells. Immunol Lett. (2005) 100:113–9. doi: 10.1016/j.imlet.2005.03.009
203. Mold C, Clos TW. C-reactive protein inhibits plasmacytoid dendritic cell interferon responses to autoantibody immune complexes. Arthritis Rheumatol. (2013) 65:1891–901. doi: 10.1002/art.37968
204. Nazarov PG, Pronina AP. The influence of cholinergic agents on histamine release from HMC-1 human mast cell line stimulated with IgG, C-reactive protein and compound 48/80. Life Sci. (2012) 91:1053–7. doi: 10.1016/j.lfs.2012.08.004
205. Ruiz-Fernandez C, Ait Eldjoudi D, Gonzalez-Rodriguez M, Cordero Barreal A, Farrag Y, Garcia-Caballero L, et al. Monomeric CRP regulates inflammatory responses in human intervertebral disc cells. Bone Joint Res. (2023) 12:189–98. doi: 10.1302/2046-3758.123.BJR-2022-0223.R1
Keywords: pCRP, pCRP*, mCRP, hemostasis, inflammation resolution, tissue regeneration, therapeutic use
Citation: Potempa M, Hart PC, Rajab IM and Potempa LA (2025) Redefining CRP in tissue injury and repair: more than an acute pro-inflammatory mediator. Front. Immunol. 16:1564607. doi: 10.3389/fimmu.2025.1564607
Received: 21 January 2025; Accepted: 13 February 2025;
Published: 28 February 2025.
Edited by:
Yi Wu, Xi’an Jiaotong University, ChinaReviewed by:
Ivan Melnikov, Ministry of Health of the Russian Federation, RussiaToh Gang, St. Jude Children’s Research Hospital, United States
Copyright © 2025 Potempa, Hart, Rajab and Potempa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lawrence A. Potempa, bHBvdGVtcGEwMUByb29zZXZlbHQuZWR1
†Present address: Ibraheem M. Rajab, Tabuk Pharmaceuticals Manufacturing Company, Amman, Jordan
‡ORCID: Ibraheem M. Rajab, orcid.org/0000-0003-3751-0461
 Marc Potempa
Marc Potempa Peter C. Hart
Peter C. Hart Ibraheem M. Rajab
Ibraheem M. Rajab Lawrence A. Potempa
Lawrence A. Potempa