- 1Department of Neuroscience, Università Cattolica del Sacro Cuore, Rome, Italy
- 2UOC Neurophysiopathology, AORN Cardarelli, Naples, Italy
- 3Neuromuscular and Rare Disease Centre, Sant’Andrea Hospital, Rome, Italy
- 4Department of Neuroscience, Mental Health and Sensory Organs (NESMOS), Faculty of Medicine and Psychology, Sapienza University of Rome, Rome, Italy
- 5Department of General Thoracic Surgery, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy
- 6Neurology Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy
Introduction: Thymoma-associated myasthenia gravis (TAMG) accounts for 15–20% of all myasthenia gravis (MG) cases and is typically characterized by severe clinical manifestations and suboptimal response to conventional therapies. However, TAMG patients are underrepresented in clinical trials, leaving gaps in evidence for optimal treatment strategies. This study assessed the efficacy of complement inhibitors (CI) in TAMG population.
Methods: We retrospectively reviewed 23 TAMG patients who received CI, with a minimum follow-up of six months. Additionally, we randomly included 22 MG patients without thymoma, treated with CI, in the control group. Clinical outcomes were measured using Myasthenia Gravis-Activities of Daily Living (MG-ADL) and Quantitative Myasthenia Gravis (QMG) scores at baseline, three, and six months.
Results: Among the 23 TAMG patients, 21 initiated CI after thymectomy, with a median interval of eight years (IQR:2.5-15) post-surgery. Two patients achieved sufficient stabilization on CI to undergo thymectomy thereafter. The most frequent thymoma histological subtype was WHO type B2, detected in 43.5% of cases. Median MG-ADL score decreased from 11 (IQR:8-15) to 3 (IQR:2-5) and 4 (IQR:1-5) at three and six months, respectively (both p<0.001). Median QMG score decreased from 16 (IQR:14-22) to 10 (IQR: 5-11) at three and six months (both p<0.001). Prednisone dosage was tapered in 20 patients. No significant differences were observed between TAMG and MG patients without thymoma in MG-ADL, QMG and steroid reduction.
Conclusion: CI demonstrated significant improvements in MG-ADL and QMG scores, along with a steroid-sparing effect, suggesting its potential as an effective treatment for this challenging subpopulation.
1 Introduction
Thymoma-associated myasthenia gravis (TAMG) accounts for 15–20% of all myasthenia gravis (MG) cases and is typically associated with autoantibodies targeting the acetylcholine receptor (AChR-Abs) located at the postsynaptic membrane of the neuromuscular junction (1, 2). Thymomas are rare neoplasms originating from thymic epithelial cells, and their prognosis is unaffected by the concurrent presence of MG (3). Patients with TAMG typically present with generalized MG (gMG), characterized by muscle weakness affecting the limbs, neck and bulbar muscles (4–6).
Compared to other MG subtypes, TAMG is frequently more severe and associated with a higher risk of myasthenic crises and exacerbations (7, 8). These patients usually demonstrate a suboptimal response to standard treatments and have a poorer long-term prognosis, even after thymoma resection (9–11).
Conventional therapies for MG, including acetylcholinesterase inhibitors, corticosteroids and non-steroidal immunosuppressive therapies (ISTs), are effective for the majority of patients (12). However, approximately 10% of MG patients are classified as “refractory” to standard treatments, exhibiting an inadequate response to at least two ISTs other than corticosteroids (13–15). This finding underscores the urgent need for alternative therapeutic approaches.
Over the past five years, more targeted therapeutic strategies have been developed, such as eculizumab and ravulizumab — humanized monoclonal antibodies that inhibit the C5 protein. These therapies are currently approved for the treatment of acetylcholine receptor-positive generalized myasthenia gravis (AChR-gMG) (16–18) and have shown significant efficacy in symptom control. However, evidence supporting the use of complement inhibitors (CI) in TAMG remains limited, as these patients are frequently underrepresented or excluded from clinical trials, resulting in substantial gaps in treatment guidelines (19).
This study aims to report the real-world experience with CI therapy in patients with TAMG.
2 Methods
The study was approved by the Institutional Review Boards of the Fondazione Policlinico Universitario Agostino Gemelli IRCCS, the Cardarelli Hospital and the Sant’Andrea Hospital of Sapienza University (protocol ID 6743) and was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All involved patients provided informed consent for the use of their anonymized medical records for research purposes.
2.1 Study population
We designed a multicentre, retrospective study, collecting data from patients with TAMG who attended the MG outpatient clinics at Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Cardarelli Hospital and Sant’Andrea Hospital of Sapienza University, between June 2020 and November 2024.
Inclusion criteria were age over 18 years, a diagnosis of TAMG according to international guidelines (1, 20), and the initiation of treatment with either eculizumab or ravulizumab as part of clinical practice according to AIFA prescribing criteria, with a minimum follow-up of six months. Patients were treated with eculizumab if they had inadequate symptom control despite treatment with corticosteroids and at least two ISTs, with a Myasthenia Gravis-Activity Daily Living (MG-ADL) score of ≥6 and a Myasthenia Gravis Foundation of America (MGFA) classification of ≥III. Ravulizumab was prescribed for patients with an MG-ADL score of ≥6 and an MGFA classification of ≥IIb, who were unresponsive to at least one IST other than corticosteroids.
Data were collected from local databases and merged into a standardized database with predefined criteria for data categorization. We recorded demographics and clinical data, including age, sex, disease duration, MGFA clinical classification and Post Interventional Status (MGFA-PIS), thymoma histology and treatment, corticosteroid and ISTs dosages at baseline and during therapy, occurrence of myasthenic crises or exacerbations, MG-ADL and Quantitative Myasthenia Gravis (QMG) scores (21, 22).
2.2 Clinical outcomes analysis
Clinical outcomes were assessed using MG-ADL and QMG scores at baseline and at three, six and 12 months following the initiation of CI therapy. Responders were defined as patients achieving a reduction of at least 2 points in the MG-ADL score, and at least 3 points in the QMG score, compared to baseline. Moreover, we explored potential predictors of treatment response by adjusting outcome measures for baseline MG-ADL, baseline QMG, age, sex, and disease duration.
2.3 Steroid-tapering analysis
Steroid dosages were recorded at baseline and at three, six, and 12 months after CI therapy initiation. The percentage reduction in steroid doses from baseline at each time point were used to evaluate the steroid-sparing efficacy of CI therapy.
2.4 Control group of mg patients without thymoma
As a control group, we included randomly chosen patients with AChR-Abs-positive MG without thymoma, who were treated with CI according to international guidelines. These patients were recruited from the MG outpatient clinics at Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Cardarelli Hospital, and Sant’Andrea Hospital of Sapienza University between June 2020 and November 2024.
2.5 Statistical analysis
The Shapiro-Wilk test was employed to verify the Gaussian distribution of the data. Categorical variables were presented as absolute frequencies and percentages, whereas continuous variables were summarized with medians and interquartile ranges (IQR). Pairwise comparisons between baseline and different time points (3, 6, and 12 months) within each treatment group were performed using the Wilcoxon signed-rank test. The Mann-Whitney U test was employed to perform pairwise comparisons between TAMG and control groups at baseline and at different time points. Fisher’s exact test was used to compare categorical variables between TAMG and control groups at baseline. A p-value <0.05 was considered statistically significant.
3 Results
We retrospectively included 23 patients with TAMG in this study: 13 treated with eculizumab and 10 with ravulizumab. Ten patients were followed at the MG outpatient clinic of Fondazione Policlinico A. Gemelli IRCCS in Rome, eight at Cardarelli Hospital in Naples and five at Sant’Andrea Hospital of Sapienza University in Rome. All patients were AChR-Abs positive and previously naïve to CI therapy.
3.1 Patient population
Baseline demographic and clinical characteristics of the included patients are reported in Table 1; Figure 1A.
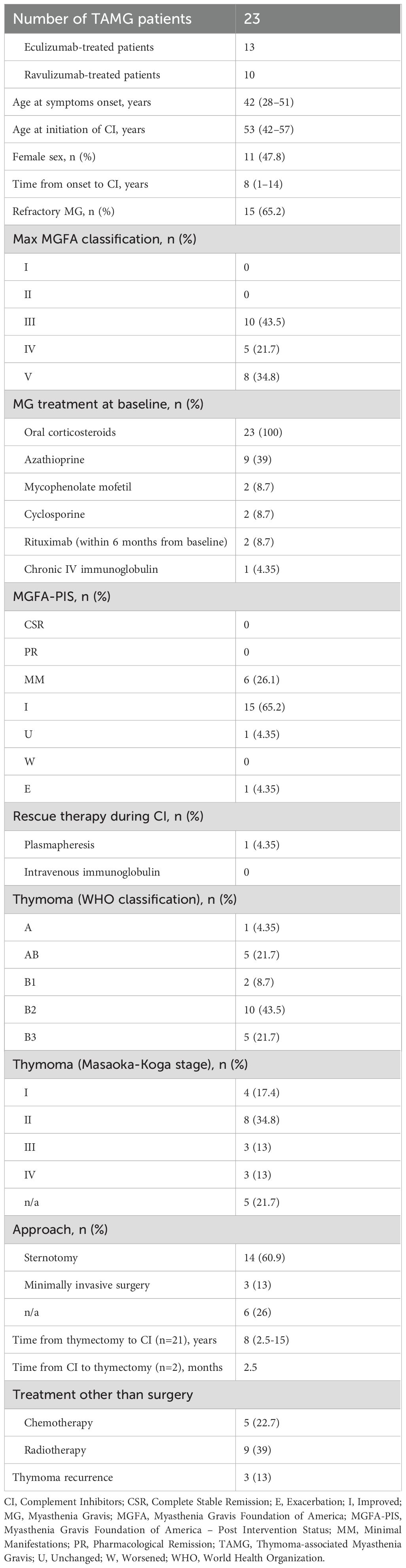
Table 1. Demographic and clinical characteristics of study population. Continuous and categorical variables are reported as median (IQR) and number (%).
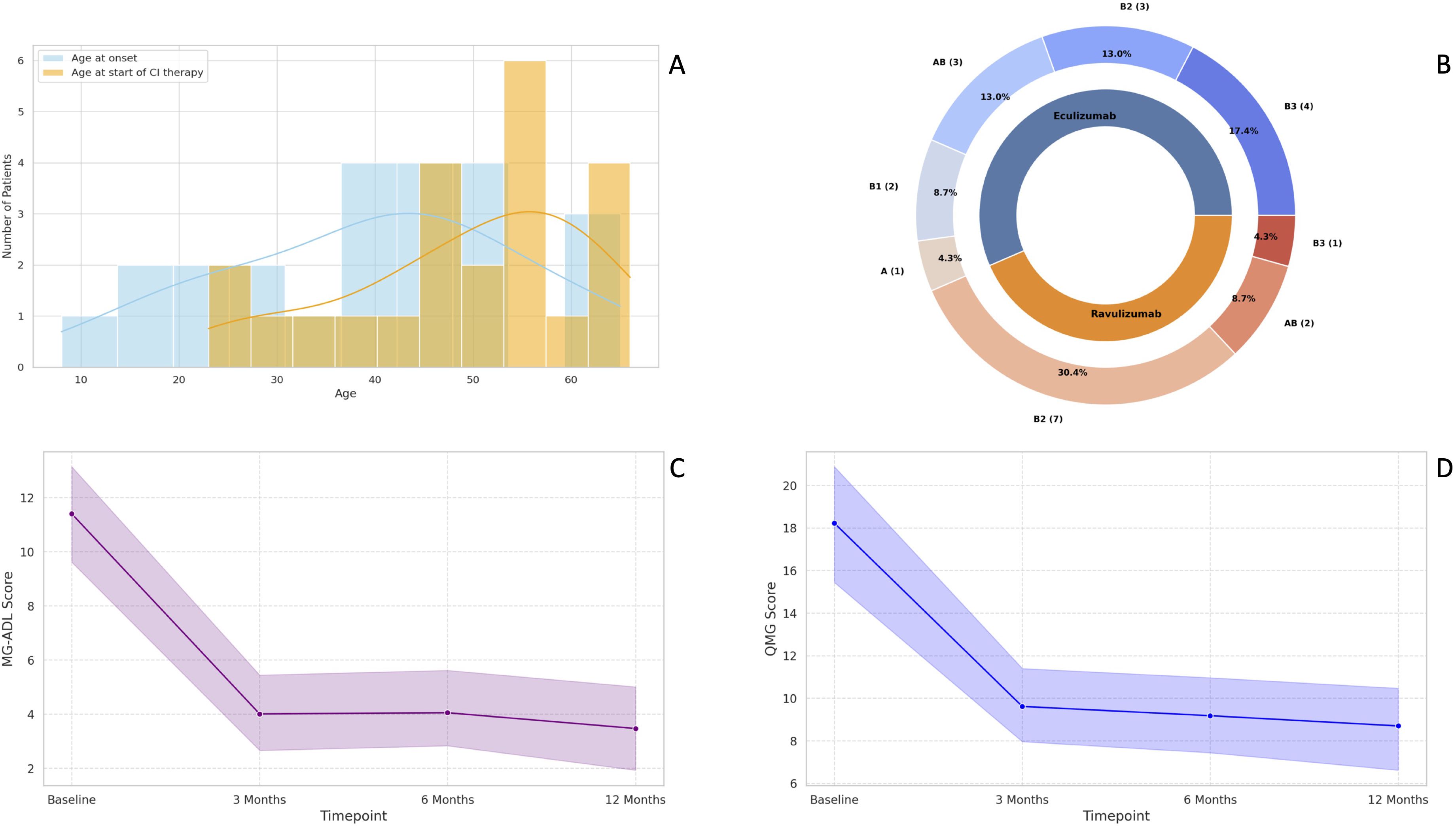
Figure 1. Baseline demographic characteristics of TAMG patients included in the study (A); Distribution of WHO thymoma histological subtypes in our cohort (B); Median change in MG-ADL scores at three, six- and 12-months follow-up (C); Median change in QMG scores at three, six- and 12-months follow-up (D). MG-ADL, Myasthenia Gravis-Activities of Daily Living; QMG, Quantitative Myasthenia Gravis; TAMG, Thymoma-associated Myasthenia Gravis; WHO, World Health Organization.
Patients had a median age of 53 years (IQR: 42-57) at baseline, 11 out of 23 (47.8%) were female, and all were Caucasians. All patients underwent a minimum six-months follow-up, while 14/23 (60.9%) had an available follow-up of 12 months.
Most patients (91.3%) started CI therapy after thymectomy, with a median interval of eight years (IQR: 2.5-15) after surgery. Conversely, two patients achieved sufficient clinical stabilization on CI to undergo thymectomy thereafter, with a median interval of 2.5 months.
The most common thymoma histological subtype was the WHO type B2, detected in 10 out of 23 patients (43.5%), followed by AB type and B3 type, identified in five patients each (21.74%) (Figure 1B). Only one patient had an ongoing thymoma recurrence at the time of CI therapy initiation.
Regarding safety, no infections were reported during treatment up to the last available follow-up.
3.2 MG-ADL
The median MG-ADL score significantly improved from 11 (IQR: 7-15) at baseline to 3 (IQR: 2-5) at three months and 4 (IQR: 1-5) at six months (both p < 0.001). At the six-month follow-up, 21 patients (91.3%) were classified as MG-ADL responders. Both non-responders were treated with ravulizumab and had thymoma histology classified as the WHO type B2. Neither received adjuvant therapy nor experienced thymoma recurrence. One of these two non-responders had a clinical exacerbation six months after initiating CI, requiring plasma exchange and an increase in daily steroid dosage (from 10 mg to 37.5 mg). At the 12-month follow-up, this patient showed significant improvement and was reclassified as a “late-responder” to CI therapy. However, the potential contribution of plasma exchange and increased steroid dosage to the observed improvement cannot be ruled out. Among the 14 patients with available 12-month follow-up data, the median MG-ADL score remained stable at 3 (IQR: 0–6) (Figure 1C).
3.3 QMG
The median QMG score improved from 16 (IQR: 14-22) to 10 (IQR: 7-14) at three months and remained stable at 10 (IQR: 5-11) at six months (both p < 0.001). After six months of CI therapy, 21 patients (91.3%) were classified as QMG responders. Among the two non-responders, one was treated with eculizumab and the other with ravulizumab. The eculizumab-treated non-responder had thymoma histology classified as the WHO AB subtype and was eventually reclassified as a “late-responder”, showing a 4-point improvement in QMG score after 12 months of CI therapy. The ravulizumab-treated non-responder had thymoma histology of the WHO B2 subtype, was treated with adjuvant chemotherapy and previously experienced thymoma recurrence. No further follow-up data were available for this patient, and a delayed response to CI therapy could not be ruled out. At 12 months, the median QMG score for the 14 TAMG patients with available follow-up clinical data further decreased to 8.5 (IQR: 5-10.5) (Figure 1D).
3.4 Predictors of treatment outcome
Our analysis showed that none of the examined covariates (baseline MG-ADL, baseline QMG, age, sex, and disease duration) significantly influenced changes in MG-ADL or QMG scores. Given the lack of significant associations, adjusted regression models did not provide additional explanatory value beyond unadjusted estimates.
3.5 Concomitant medications
At baseline, all patients were receiving corticosteroids, with a median daily dosage of 25 mg (IQR: 18.75-50). Moreover, 14 patients were on non-steroidal ISTs, including azathioprine (n=9), mycophenolate mofetil (n=2), cyclosporine (n=2), and rituximab within six months of CI initiation (n=2). The median treatment duration with non-steroidal ISTs before CI initiation was 8 years (IQR: 0-18). A total of 20 patients (87%) achieved a reduction in oral corticosteroid dosage. The percentage reduction in steroid dosage was 25.62% at three months (p < 0.001), increasing to 36.37% (p < 0.001) at six months after the first CI administration. Among the 14 patients with a 12-month follow-up, the median steroid dosage was 10 mg (IQR: 5-15.6). By the last available follow-up, three patients had discontinued steroid use entirely, two had withdrawn azathioprine, and one had discontinued cyclosporine. One patient initiated mycophenolate mofetil during CI therapy due to persistent symptom fluctuations, despite initial clinical improvement.
Among the two MG-ADL non-responders, one patient reduced the daily steroid dosage from 20 to 15 mg, while the other experienced a clinical deterioration, necessitating an increase in steroid dosage from 10 to 37.5 mg at 6 months, followed by a gradual taper to 25 mg at 12 months. Regarding QMG non-responders, one patient maintained a stable steroid daily dosage of 10 mg, while the other progressively reduced steroid use from 50 to 35 mg at 6 months, and further to 25 mg after one year, as he was reclassified as a QMG late responder to CI therapy.
3.6 MGFA-PIS
The MGFA-PIS (23) classification demonstrated significant improvement following CI therapy. At the last available follow-up, 15 patients (65.23%) were classified as having an “improved” status, while six patients (26.1%) achieved “minimal manifestation” status. One patient remained “unchanged” in MGFA-PIS, while another experienced a clinical exacerbation, requiring rescue therapy with plasma exchange and an increase in daily steroid dosage. No myasthenic crises were reported during the follow-up period.
3.7 Comparisons between TAMG patients and MG patients without thymoma
We retrospectively included 22 MG patients without thymoma in the control group, 19 treated with eculizumab and three with ravulizumab. Nine patients were followed at the MG outpatient clinic of Fondazione Policlinico A. Gemelli IRCCS in Rome, nine at Cardarelli Hospital in Naples and four at Sant’Andrea Hospital of Sapienza University in Rome. All were AChR-Abs positive and naïve to CI therapy.
The TAMG and control groups had comparable demographic and clinical characteristics at baseline, including sex, age at symptom onset, age at initiation of CI therapy, disease duration, disease severity (maximum MGFA score), median MG-ADL and QMG scores, median steroid dosage.
Pairwise comparisons using the Mann-Whitney U test revealed no statistically significant differences between the two groups in median MG-ADL score reduction (p = 0.41 at 3 months, p = 0.71 at 6 months, and p > 0.99 at 12 months), median QMG score reduction (p = 0.71 at 3 months, p = 0.58 at 6 months, and p = 0.81 at 12 months), and steroid tapering (p = 0.88 at 3 months, p = 0.87 at 6 months, and p = 0.99 at 12 months) at any time point.
At the six-month follow-up, three control patients (13.64%) were classified as non-responders based on both MG-ADL and QMG scores, while one was a non-responder for MG-ADL and another for QMG. Overall, the responder rate to CI therapy for MG patients without thymoma was 81.8% according to MG-ADL and to QMG scores (Figures 2A, B).
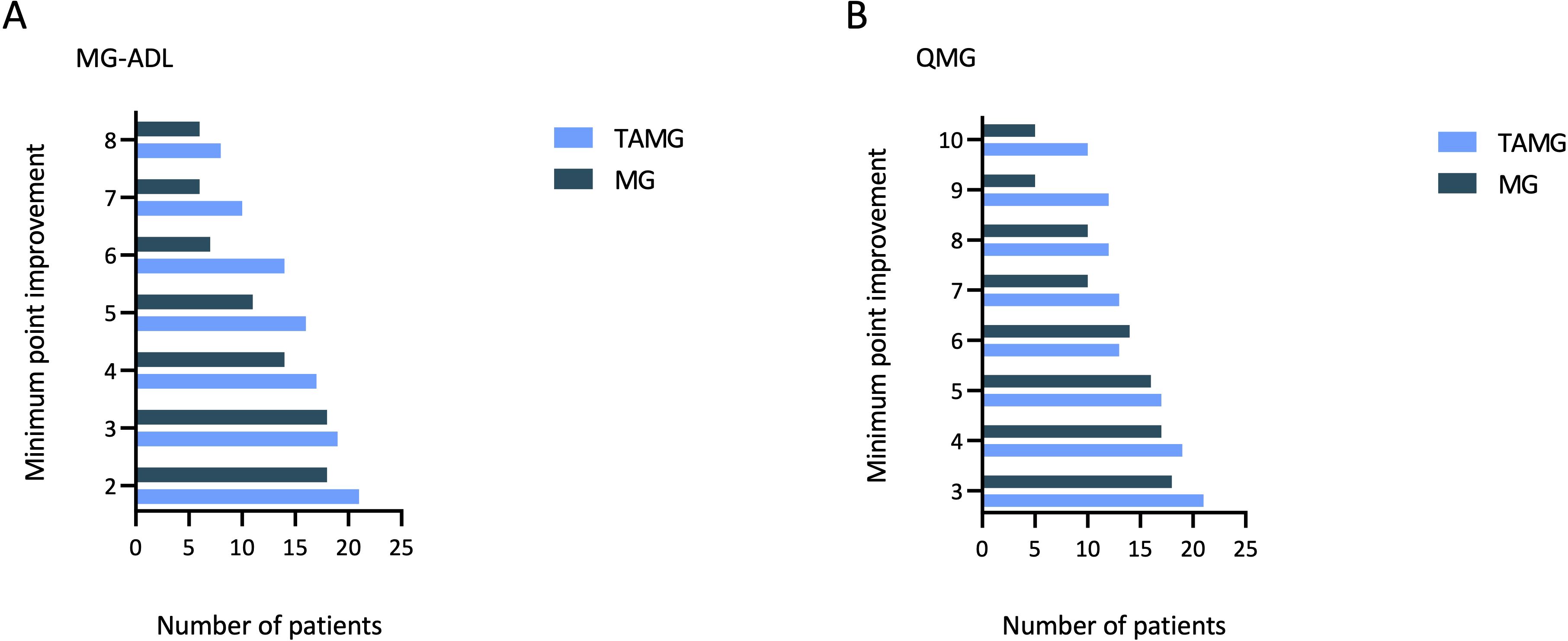
Figure 2. Bar chart for the distribution of minimal point improvement in MG-ADL score from baseline to 6-months follow-up for TAMG patients and MG patients without thymoma (A); Bar chart for the distribution of minimal point improvement in QMG score from baseline to 6-months follow-up for TAMG patients and MG patients without thymoma (B). MG-ADL, Myasthenia Gravis-Activities of Daily Living; QMG, Quantitative Myasthenia Gravis; TAMG, Thymoma-associated Myasthenia Gravis.
At the last available follow-up, the MGFA-PIS classification in the control group was as follows: 12 patients (55%) were classified as “improved”, four (18%) as “minimal manifestations”, and three (13.64%) as “unchanged”. One patient experienced a clinical exacerbation that required rescue therapy with two cycles of intravenous immunoglobulins and subsequently withdrew from CI therapy after six months of treatment. Additionally, “complete stable remission” and “pharmacological remission” were achieved by one patient each.
4 Discussion
In this real-world study, we evaluated the efficacy of CI therapy in patients with TAMG, a subgroup of MG characterized by challenging management and suboptimal response to conventional treatments. Our findings demonstrated a median reduction of 7 points in the MG-ADL score at six months, with a responder rate of 91.3%. Similarly, the QMG score showed a median improvement of 6 points at six months, with the same responder rate of 91.3%. Interestingly, the two MG-ADL non-responders were classified as QMG responders, and vice versa. These discrepancies likely reflect the differences in the assessment methods: MG-ADL reflects patient-reported symptoms over the previous week, while QMG is a physician-reported evaluation conducted at a single time point.
Among the four non-responders, three had thymoma histology classified as the WHO B2 subtype, which is not unexpected given its higher prevalence in our cohort. None of the non-responders experienced thymoma recurrence during treatment. While thymoma recurrence in TAMG is known to exacerbate clinical symptoms, its impact on CI therapy response remains unknown. Notably, in our cohort, one patient had an ongoing thymoma recurrence at CI initiation, and he was classified as a “responder” based on both MG-ADL and QMG scores.
The findings of this study align with the efficacy outcomes reported in the phase III REGAIN trial of eculizumab and the phase III CHAMPION MG trial of ravulizumab, as well as their respective open-label extension studies (16, 17, 24). While patients with a history of thymoma were excluded from REGAIN, they were eligible for CHAMPION MG if they had no active or untreated thymoma and no evidence of recurrence for at least five years prior to screening. Compared to these trials, our findings indicated a more robust and consistent response to CI therapy in TAMG patients, characterized by greater reductions in both MG-ADL and QMG scores. These results are consistent with Japanese post-market surveys and a recent American real-world study (25, 26). They also partially align with another Japanese real-world study, which reported milder improvements in this subgroup (27). Conversely, a recent German cohort study observed clinically meaningful responses to CI therapy in 61% of patients according to MG-ADL and 51% according to QMG. However, only 16% of the included patients had TAMG, and no specific analysis was conducted for this subgroup (28).
The notable improvements observed in our cohort may be partially explained by the high baseline disease burden, as reflected by elevated MG-ADL and QMG scores, which likely provided greater room for measurable clinical gains following treatment. To partially address this limitation, we compared the reductions in median MG-ADL and QMG scores, along with steroid tapering, between TAMG patients and a control group of MG patients without thymoma. No statistically significant differences were observed at any time point. Moreover, the marked clinical benefit in TAMG population were further supported by the MGFA-PIS classification, which demonstrated significant improvement at the last available follow-up for most patients. Only one non-responder experienced clinical exacerbation, requiring plasma exchange after six months, while no myasthenic crises were observed throughout the study period.
Another possible explanation for the increased efficacy of CI therapy observed in TAMG is that the greater disease severity and burden in this subgroup may be associated with a more pronounced complement activation and a higher degree of complement-mediated damage at the neuromuscular junction. However, further studies are needed to analyse the contribution of complement-mediated mechanisms in TAMG and to optimize targeted therapeutic strategies for these patients.
Most patients (91.3%) underwent thymectomy before CI therapy, raising the possibility that surgical intervention contributed to treatment outcomes. However, given the median interval of 8 years between thymectomy and CI therapy initiation, and the poor disease control at baseline, the direct role of thymectomy on disease stabilization remains unclear. Conversely, two patients underwent thymectomy after achieving sufficient clinical stabilization on CI therapy, with a median interval of 2.5 months between CI initiation and surgery. In these cases, thymectomy may have contributed to maintaining long-term clinical stability.
Steroid-tapering was achieved in the majority of TAMG patients, with many maintaining clinical stability or demonstrating improvement despite reductions in concomitant ISTs. These findings suggest the potential of CI therapy as a steroid-sparing option for this challenging MG subgroup, possibly mitigating the long-term side effects associated with prolonged corticosteroid use (29).
This study has several limitations, including a small sample size and a relatively short follow-up period, due to the recent approval of CI therapy. QMG assessments were performed by different raters, and changes in steroid dosages were not standardized but rather left to the discretion of the treating clinicians. Additionally, all patients were receiving corticosteroids at baseline, and 14 patients had been on ISTs for a median of 8 years before CI therapy initiation as an “add-on”, suggesting that long-term immunosuppression may have been contributed to the observed clinical improvements, especially considering its cumulative effects over time. Further research with larger patient cohorts and extended follow-up periods is needed to fully evaluate the efficacy of CI therapy and optimize its combination with standard immunosuppressive treatments in patients with TAMG, a subgroup that remains challenging and difficult-to-treat.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of the Fondazione Policlinico Universitario Agostino Gemelli IRCCS. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. CE: Investigation, Validation, Visualization, Writing – review & editing. LF: Investigation, Validation, Visualization, Writing – review & editing. SF: Investigation, Validation, Visualization, Writing – review & editing. ER: Investigation, Validation, Visualization, Writing – review & editing. FeH: Investigation, Validation, Visualization, Writing – review & editing. EM: Investigation, Validation, Visualization, Writing – review & editing. MM: Investigation, Validation, Visualization, Writing – review & editing. FrH: Investigation, Validation, Visualization, Writing – review & editing. RI: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work was funded by Italian Ministry of Health (grant GR-2019-12368727) and by the MGNet, a member of the Rare Disease Clinical Research Network (RDCRN) NIH U54 NS115054.
Conflict of interest
RI has received consultancy fees and speaker honoraria from Alexion, Argenx, UCB and Dianthus Therapeutics. LF served as a consultant/advisor for Alexion, Argenx, UCB and Dianthus.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren JJGM. Myasthenia gravis. Nat Rev Dis Primer. (2019) 5(1):30. doi: 10.1038/s41572-019-0079-y
2. Evoli A, Minisci C, Di Schino C, Marsili F, Punzi C, Batocchi AP, et al. Thymoma in patients with MG: characteristics and long-term outcome. Neurology. (2002) 59:1844–50. doi: 10.1212/01.wnl.0000032502.89361.0c
3. Zhao K, Liu Y, Jing M, Cai W, Jin J, Zhu Z, et al. Long-term prognosis in patients with thymoma combined with myasthenia gravis: a propensity score-matching analysis. Front Med. (2024) 11:1407830. doi: 10.3389/fmed.2024.1407830
4. Johnson SB, Eng TY, Giaccone G, Thomas CR. Thymoma: update for the new millennium. Oncologist. (2001) 6(3):239–46. doi: 10.1634/theoncologist.6-3-239
5. Raica M, Cimpean AM, Ribatti D. Myasthenia gravis and the thymus gland. A historical review. Clin Exp Med. (2008) 8(2):61–4. doi: 10.1007/s10238-008-0158-y
6. Fukuhara M, Higuchi M, Owada Y, Inoue T, Watanabe Y, Yamaura T, et al. Clinical and pathological aspects of microscopic thymoma with myasthenia gravis and review of published reports. J Thorac Dis. (2017) 9(6):1592–7. doi: 10.21037/jtd.2017.05.22
7. Akaishi T, Suzuki Y, Imai T, Tsuda E, Minami N, Nagane Y, et al. Response to treatment of myasthenia gravis according to clinical subtype. BMC Neurol. (2016) 16(1):225. doi: 10.1186/s12883-016-0756-3
8. Nelke C, Stascheit F, Eckert C, Pawlitzki M, Schroeter CB, Huntemann N. Independent risk factors for myasthenic crisis and disease exacerbation in a retrospective cohort of myasthenia gravis patients. J Neuroinflamm. (2022) 19(1):89. doi: 10.1186/s12974-022-02448-4
9. Álvarez-Velasco R, Gutiérrez-Gutiérrez G, Trujillo JC, Martínez E, Segovia S, Arribas-Velasco M, et al. Clinical characteristics and outcomes of thymoma-associated myasthenia gravis. Eur J Neurol. (2021) 28(6):2083–91. doi: 10.1111/ene.14820
10. Budde JM, Morris CD, Gal AA, Mansour KA, Miller JI. Predictors of outcome in thymectomy for myasthenia gravis. Ann Thorac Surg. (2001) 72:197–202. doi: 10.1016/S0003-4975(01)02678-9
11. Tian W, Yu H, Sun Y, He J, Wu Q, Ma C, et al. Thymoma negatively affects the neurological outcome of myasthenia gravis after thymectomy: a propensity score matching study. J Cardiothorac Surg. (2024) 19:37. doi: 10.1186/s13019-024-02511-6
12. Farmakidis C, Pasnoor M, Dimachkie MM, Barohn RJ. Treatment of myasthenia gravis. Neurol Clin. (2018) 36:311–37. doi: 10.1016/j.ncl.2018.01.011
13. Mantegazza R, Antozzi C. When myasthenia gravis is deemed refractory: clinical signposts and treatment strategies. Ther Adv Neurol Disord. (2018) 11:175628561774913. doi: 10.1177/1756285617749134
14. Schneider-Gold C, Hagenacker T, Melzer N, Ruck T. Understanding the burden of refractory myasthenia gravis. Ther Adv Neurol Disord. (2019) 12:1756286419832242. doi: 10.1177/1756286419832242
15. Tran C, Biswas A, Mendoza M, Katzberg H, Bril V, Barnett C. Performance of different criteria for refractory myasthenia gravis. Eur J Neurol. (2021) 28:1375–84. doi: 10.1111/ene.14675
16. Howard JF, Utsugisawa K, Benatar M, Murai H, Barohn RJ, Illa I, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. (2017) 16:976–86. doi: 10.1016/S1474-4422(17)30369-1
17. Vu T, Meisel A, Mantegazza R, Annane D, Katsuno M, Aguzzi R, et al. Terminal complement inhibitor ravulizumab in generalized myasthenia gravis. NEJM Evidence. (2022) 1:EVIDoa2100066. doi: 10.1056/EVIDoa2100066
18. Iorio R. Myasthenia gravis: the changing treatment landscape in the era of molecular therapies. Nat Rev Neurol. (2024) 20:84–98. doi: 10.1038/s41582-023-00916-w
19. Narayanaswami P, Sanders DB, Wolfe G, Benatar M, Cea G, Evoli A, et al. International consensus guidance for management of myasthenia gravis. Neurology. (2021) 96:114–22. doi: 10.1212/WNL.0000000000011124
20. Sanders DB, Wolfe GI, Benatar M, Evoli A, Gilhus NE, Illa I, et al. International consensus guidance for management of myasthenia gravis: Executive summary. Neurology. (2016) 87:419–25. doi: 10.1212/WNL.0000000000002790
21. Wolfe GI, Herbelin L, Nations SP, Foster B, Bryan WW, Barohn RJ. Myasthenia gravis activities of daily living profile. Neurology. (1999) 52:1487–9. doi: 10.1212/WNL.52.7.1487
22. Bedlack RS, Simel DL, Bosworth H, Samsa G, Tucker-Lipscomb B, Sanders DB. Quantitative myasthenia gravis score: assessment of responsiveness and longitudinal validity. Neurology. (2005) 64:1968–70. doi: 10.1212/01.WNL.0000163988.28892.79
23. Jaretzki A, Barohn RJ, Ernstoff RM, Kaminski HJ, Keesey JC, Penn AS, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology. (2000) 55:16–23. doi: 10.1212/WNL.55.1.16
24. Meisel A, Annane D, Vu T, Mantegazza R, Katsuno M, Aguzzi R, et al. Long-term efficacy and safety of ravulizumab in adults with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis: results from the phase 3 CHAMPION MG open-label extension. J Neurol. (2023) 270:3862–75. doi: 10.1007/s00415-023-11699-x
25. Tokuyasu D, Suzuki S, Uzawa A, Nagane Y, Masuda M, Konno S, et al. Real-world experience with eculizumab and switching to ravulizumab for generalized myasthenia gravis. Ann Clin Transl Neurol. (2024) 11:1338–46. doi: 10.1002/acn3.52051
26. Murai H, Suzuki S, Hasebe M, Fukamizu Y, Rodrigues E, Utsugisawa K. Safety and effectiveness of eculizumab in Japanese patients with generalized myasthenia gravis: interim analysis of post-marketing surveillance. Ther Adv Neurol Disord. (2021) 14:17562864211001995. doi: 10.1177/17562864211001995
27. Katyal N, Govindarajan R, Goyal N, Muley S, Muppidi S. Ravulizumab use for acetylcholine receptor-positive generalized myasthenia gravis in clinical practice. Front Neurol. (2024) 15:1378080. doi: 10.3389/fneur.2024.1378080
28. Huntemann N, Gerischer L, Herdick M, Nelke C, Stascheit F, Hoffmann S, et al. C5 complement inhibition versus FcRn modulation in generalised myasthenia gravis. J Neurol Neurosurg Psychiatry. (2025) 96(4):310–21. doi: 10.1136/jnnp-2024-334404
Keywords: thymoma, myasthenia gravis, complement inhibitors therapy, real-world, neuromuscular disease, neuroimmunology
Citation: Marini S, Erra C, Fionda L, Falso S, Rossini E, Habetswallner F, Meacci E, Marini M, Habetswallner F and Iorio R (2025) Complement inhibitor therapy in thymoma-associated myasthenia gravis: a real-world experience. Front. Immunol. 16:1562419. doi: 10.3389/fimmu.2025.1562419
Received: 17 January 2025; Accepted: 24 March 2025;
Published: 14 April 2025.
Edited by:
Sarah Hoffmann, Charité University Medicine Berlin, GermanyReviewed by:
Frauke Stascheit, Charité University Medicine Berlin, GermanySushan Luo, Fudan University, China
Shigeaki Suzuki, Keio University, Japan
Copyright © 2025 Marini, Erra, Fionda, Falso, Rossini, Habetswallner, Meacci, Marini, Habetswallner and Iorio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raffaele Iorio, cmFmZmFlbGUuaW9yaW9AcG9saWNsaW5pY29nZW1lbGxpLml0
 Sofia Marini
Sofia Marini Carmen Erra2
Carmen Erra2 Laura Fionda
Laura Fionda Elisa Meacci
Elisa Meacci Francesco Habetswallner
Francesco Habetswallner Raffaele Iorio
Raffaele Iorio