
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 08 April 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1560944
This article is part of the Research TopicInnovative Approaches to Cholangiocarcinoma: Diagnosis, Treatment, and Multidisciplinary CareView all 5 articles
Background: The Glasgow Prognostic Score (GPS) is a well-established prognostic indicator that effectively reflects the inflammatory, nutritional, and immune status of cancer patients. GPS has been shown to be associated with survival outcomes in many different cancers. However, its prognostic significance in biliary tract cancer (BTC) remains unclear. This meta-analysis aims to explore the prognostic value of GPS in BTC patients.
Methods: A systematic search was conducted in PubMed, Embase, and Web of Science to identify relevant studies. Survival data including overall survival (OS), disease-free survival (DFS) and recurrence-free survival (RFS) were the main observation indicators. Hazard ratios (HRs) with 95% confidence intervals (CIs) were extracted and pooled for meta-analysis.
Results: A total of 16 articles incorporating 1919 patients were included in the study. High GPS was associated with poor OS (HR:2.00, 95% CI:1.62-2.48) and DFS/RFS (HR:2.50, 95% CI:1.71-3.65). Subgroup analysis further confirmed the prognosis value of GPS in BTC patients.
Conclusions: GPS could serves as a valuable prognostic marker in BTC patients and may aid in risk stratification and treatment decision-making.
Cancers has exceeded other diseases as the leading threat to human health (1). Biliary tract cancer (BTC) is a highly malignant tumor characterized by an insidious onset and late-stage diagnosis. BTC comprises intrahepatic cholangiocarcinoma (iCCA), extrahepatic cholangiocarcinoma (eCCA), gallbladder cancer (GBC), and periampullary cancer (2). The pathogenesis and behavior of BTC vary across different regions, mainly affected by parasitic infection, chronic inflammation, cholelithiasis, viral infection, genetic factors and environmental factors (2, 3). In Southeast and East Asia, high prevalence of BTC is closely associated with bile duct parasites and chronic gallstone disease, whereas in Europe and North America, primary sclerosing cholangitis, inflammatory bowel disease and obesity are the primary risk factors (4). South America has a high incidence of GBC, while in Africa, schistosomiasis contributes significantly to BTC incidence (4). Notably, the global incidence of BTC is rising (5). Radical surgery remains the most important treatment method for BTC patients (6). Other therapies such as radiotherapy, chemotherapy and immunotherapy play critical roles in prolonging survival for BTC patients (7–12).
Cancer patients are often accompanied with malnutrition and systemic inflammation (13). The Glasgow Prognostic Score (GPS) has emerged as a novel prognostic tool, effectively reflecting the inflammatory and nutritional status of the cancer patients (14, 15). Several previous meta-analyses have demonstrated the significant prognostic significance of the GPS in urological and gynecologic cancers (16, 17). GPS and its modified counterparts, including the modified GPS (mGPS) were widely used inflammatory indices in clinical practice. A previous meta-analysis demonstrated the prognostic value of mGPS in BTC (18). Nevertheless, the prognostic value of GPS in BTC remained unclear. Several epidemiological studies found that GPS was associated with poor prognosis in BTC patients (19, 20). However, other studies suggested that GPS had no clear relationship with BTC (21, 22). To address this inconsistency, we conducted a comprehensive meta-analysis to evaluate the prognostic significance of GPS in BTC patients.
Three investigators conducted independently systematically searched the PubMed, Embase, and Web of Science databases for the related articles investigating the prognostic value of GPS in BTC. The search deadline was August 20, 2023. The search keywords were utilized: (bile duct adenoma OR bile duct neoplasms OR bile duct cancer OR bile duct tumor OR cholangiocarcinoma OR cholangiocellular carcinoma OR gallbladder cancer OR gallbladder carcinoma) AND (glasgow prognostic score OR GPS). There were no language restrictions. References of included studies were manually screened for additional relevant articles. We implemented this meta-analysis according to the PRISMA guidelines.
Inclusion criteria were as follows:(1) evaluated the correlation between GPS and the prognostic significance of BTC. (2) adequate data was utilized to analyze the hazard ratios (HRs) and 95% 95% confidence intervals (CIs). Exclusion standards were as follows: studies with inadequate data, duplicated data, letters, reviews or abstracts.
Three investigators independently extracted the following data: first author, publication year, study design, country, sample size and survival outcomes. Study quality was assessed using the Newcastle-Ottawa Scale (NOS) (23). If the study did not provide survival data directly, we utilized engage Digitizer version 4.1 to obtain survival data from the survival curve according to Tierney method (24).
HRs and corresponding 95% CIs were used to analyze the prognosis value of GPS in BTC. Heterogeneity was assessed using I2. We utilized a fixed-effects model if I2 <50%, and a random-effects model was employed if I2>50%. Subgroup analysis was performed to further test the prognostic value of GPS. Meta-regression was applied to search the source of heterogeneity. Sensitivity analysis was utilized to assess the stability of the outcomes. Begg’s test, Egger’s test and the trim-and-fill method were applied to evaluate the publication bias. All data analyses was performed using STATA 12.0 (STATA Corp., College Station, TX, USA).P <0.05 was statistically significant.
Through a systematic search, we initially identified 218 articles. After the deletion of 130 duplicated articles, 88 articles were retained. After screening titles and abstracts, 72 papers that did not meet the inclusion criteria were removed. Finally,16 articles were included in the meta-analysis (19–22, 25–36). The study selection process was illustrated in Figure 1.
A total of 16 retrospective studies comprising 1919 patients were enrolled in the meta-analysis. 8 studies were performed in Japan. 6 studies were from China. The other studies were from Italy and Sweden. The NOS scores of all incorporated studies were greater than 5. The characteristics of the included studies were displayed in Table 1.
16 studies assessed the association between high GPS and OS. Due to moderate heterogeneity (I2 = 54.3%), a random effects model was utilized. The pooled analysis revealed that high GPS was significantly correlated with worse OS (HR:2.00, 95% CI:1.62-2.48) (Figure 2).
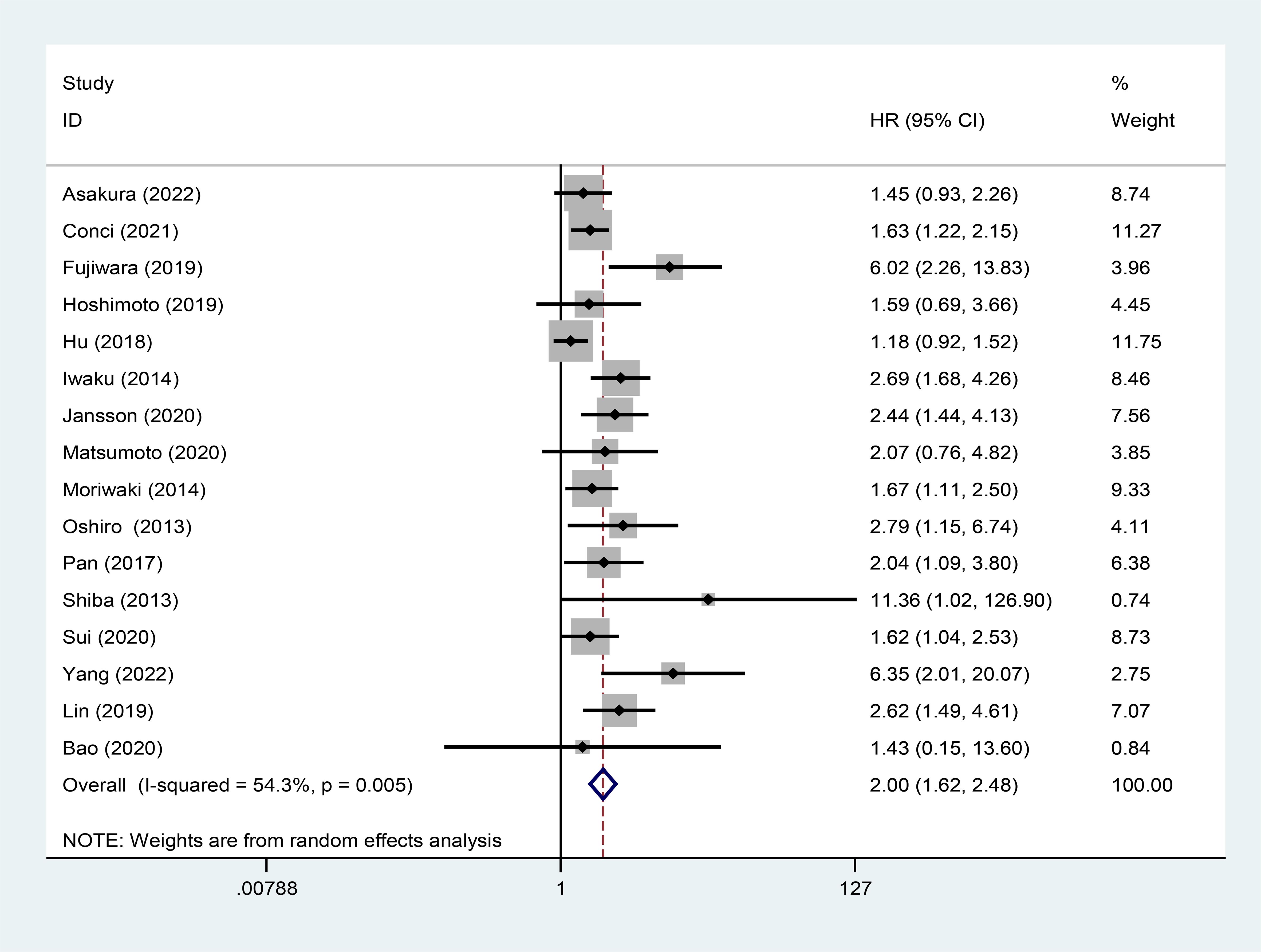
Figure 2. Forest plot of the relationship between high GPS and OS. GPS, glasgow prognostic score; OS, overall survival.
Subgroup analysis and meta-regression were performed based on country, treatment method, histological type and analysis type (Table 2). We discovered that high GPS had better prognostic value for iCCA and eCCA. As for the other subgroups, the results indicated that high GPS was a poor prognostic factor. Moreover, meta-regression suggested that histological type could be the source of heterogeneity.
5 studies displayed the relationship between high GPS and DFS/RFS. The meta-analysis showed that high GPS was associated with worse DFS/RFS (HR:2.50, 95% CI:1.71-3.65) (Figure 3).
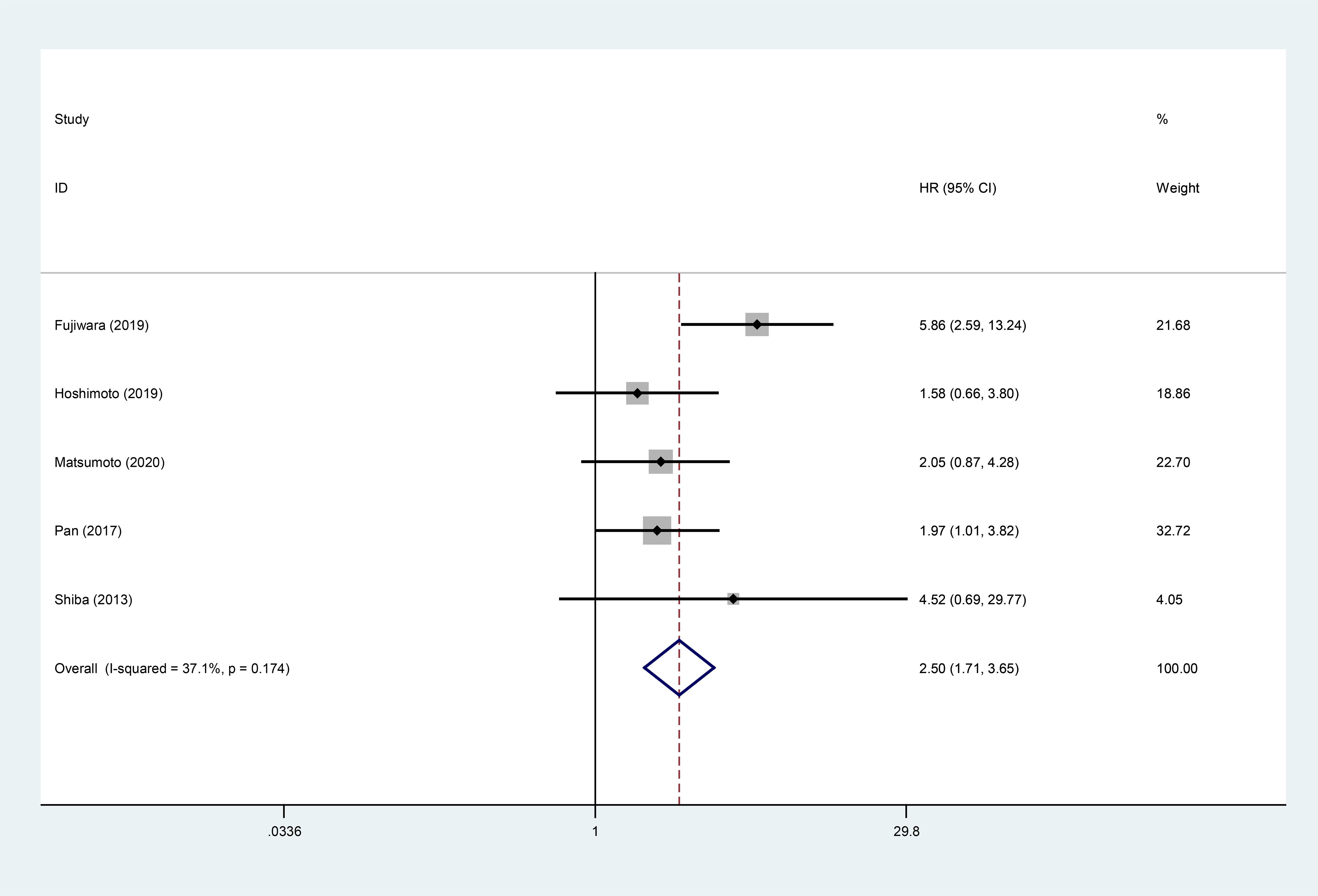
Figure 3. Forest plot of the relationship between high GPS and DFS/RFS. GPS, glasgow prognostic score; DFS/RFS, disease-free survival/recurrence-free survival.
Sensitivity analysis was used to detect the stability of the outcomes. The results were consistent with the comprehensive analysis, which confirmed that the results of meta-analysis were stable (Figures 4A, B). Begg’s and Egger’s tests were utilized to appraise publication bias. The p values of Begg’s and Egger’s tests for OS were 0.022 and 0.003 (Figure 5A), respectively. The trim-and-fill method demonstrated that the outcome for OS was not influenced by the bias (HR: 1.786, 95CI%:1.424-2.239) (Figure 5B). The p values of Begg’s and Egger’s tests for DFS/RFS values were 0.806 and 0.634 (Figure 5C), respectively. No publication bias was detected for DFS/RFS.
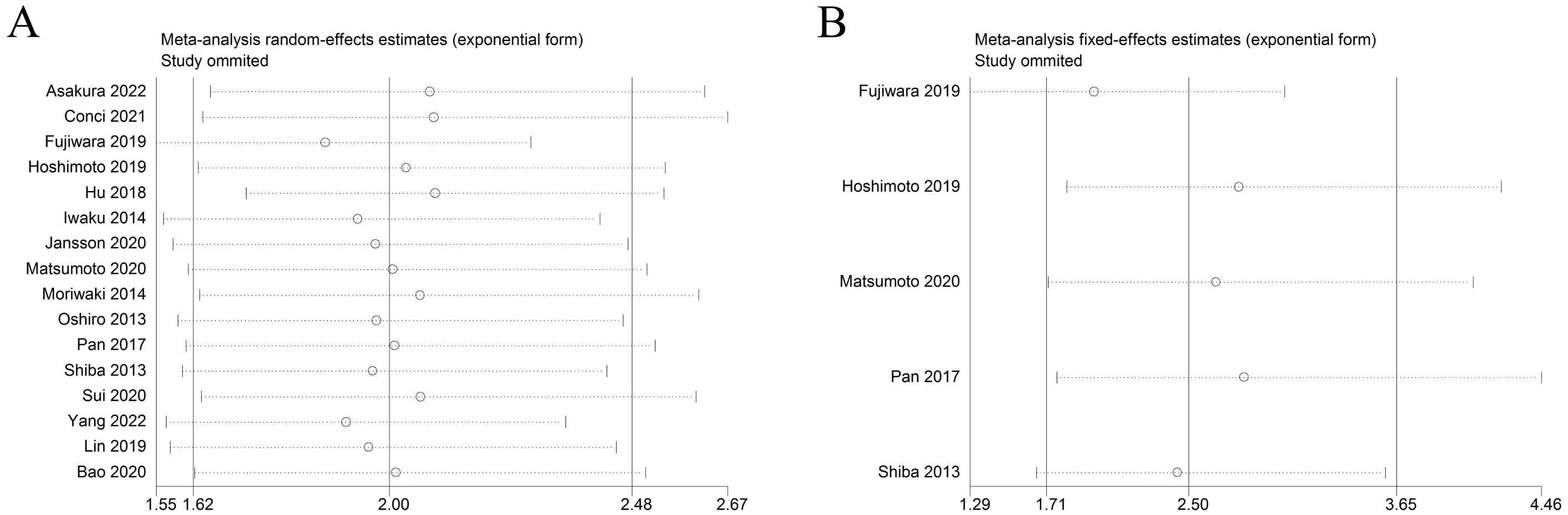
Figure 4. Sensitivity analysis. (A) sensitivity analysis for OS. (B) sensitivity analysis for DFS/RFS. GPS, glasgow prognostic score; OS, overall survival;DFS/RFS, disease-free survival/recurrence-free survival.
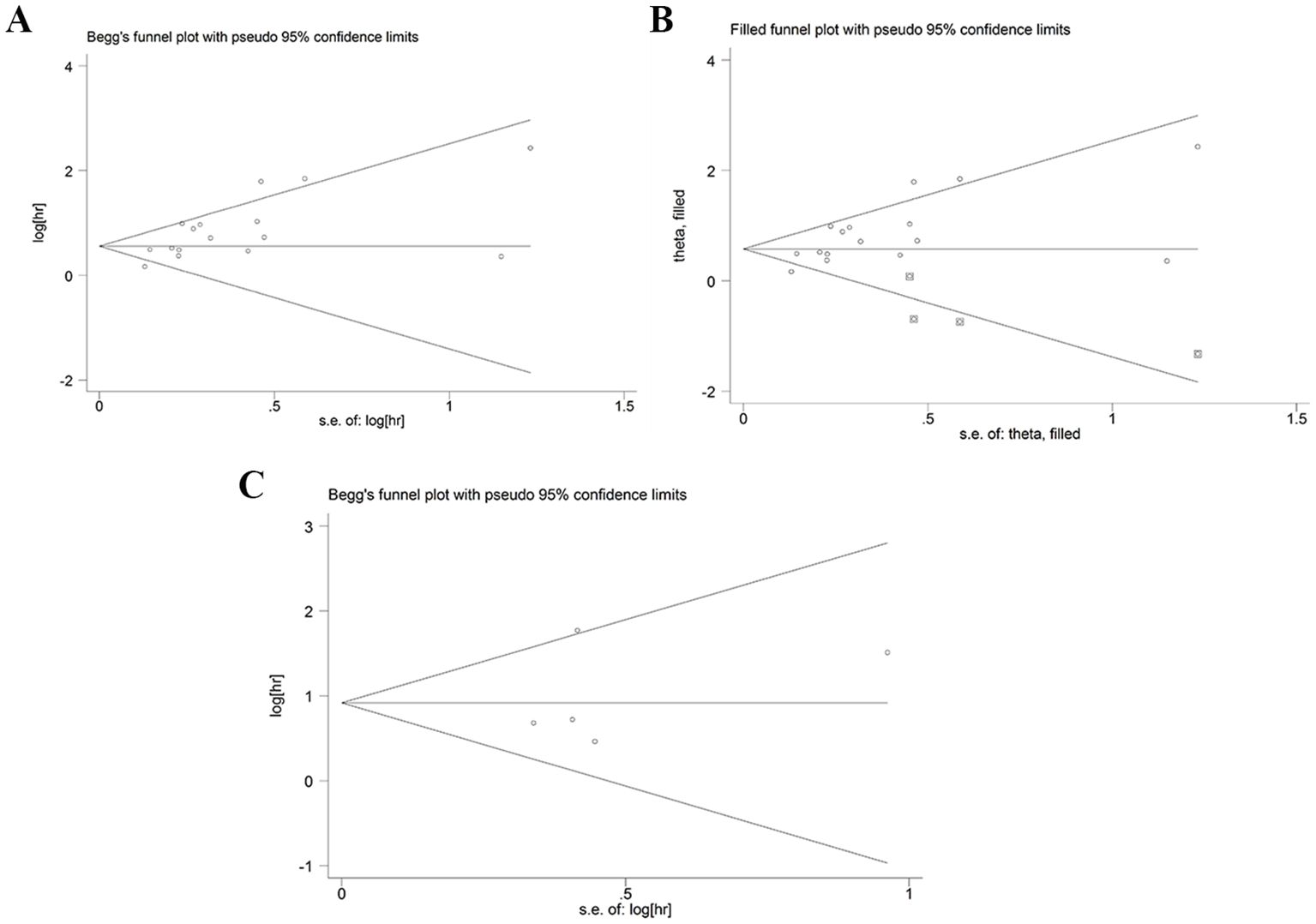
Figure 5. Publication bias. (A) publication bias for OS. (B) trim-and-fill method for OS. (C) publication bias for DFS/RFS. GPS, glasgow prognostic score; OS, overall survival; DFS/RFS, disease-free survival/recurrence-free survival.
Inflammation-based prognostic scores, such as the C-reactive protein-to-albumin ratio and systemic immune-inflammation index have been successfully used to predict the prognosis of BTC (37, 38). However, their widespread clinical application remains limited due to various constraints. Given the aggressive nature and poor prognosis of BTC, identifying simple and more effective prognostic indicators could significantly enhance the stratified management and treatment of BTC patients (39).
Forrest et al. firstly analyzed the prognosis value of GPS in lung cancer (40). Subsequently, the prognosis value of GPS was demonstrated in different cancers. However, the prognostic value of GPS in BTC has not been clarified. To our knowledge, our study was the first meta-analysis to discuss the prognostic impact of GPS in BTC patients. Our findings demonstrated that high GPS was significantly associated with worse OS and DFS/RFS in BTC. Subgroup analysis further confirmed the prognosis value of GPS in BTC patients. Additionally, sensitivity analysis and the trim-and-fill method proved the robustness and reliability of our results. The determination of the prognostic value of GPS in BCT would strongly support the practical application of GPS and its modified counterparts.
In terms of different regions, most of the studies included in this meta-analysis were conducted in Asia, with only one study focusing on Sweden. Therefore, the applicability of the conclusions to other regions or races remained to be further verified. In the current analysis, the majority of BTC patients had undergone surgical resection. The results showed that patients with high GPS had significantly worse OS. While early-stage BTC patients typically undergo surgery, many BTC patients were diagnosed at an advanced stage, precluding surgical intervention. Only three studies in our study evaluated the predictive value of advanced BTC in receiving palliative care. The potential predictive value of GPS in patients with advanced BTC remained to be further investigated. In addition, for BTC subtypes, we found that high GPS had better predictive value for iCCA and eCCA. However, GPS did not appear to have prognostic significance in GBC. Given that only one study focused on GBC, the finding may lack reliability, and more studies about the prognostic value of GPS in GBC were needed to further evaluate their relationship.
GPS is composed of serum C-reactive protein (CRP) and albumin. CRP is an acute-phase protein regulated by IL-6, IL-8, and tumor necrosis factor α (41). Elevated serum CRP levels indicated increased various inflammatory cytokines. Studies have shown that IL-6 could promote tumor proliferation, invasion and metastasis (42). In addition, CRP can suppress tumor lymphocyte activation and promote tumor immunosuppression (43). Studies have also demonstrated that CRP could directly promoted tumor cell proliferation, invasion and migration (44). Therefore, elevated CRP levels could indicate the significant systemic inflammatory response and impaired immune system. High CRP levels could be associated with adverse prognosis in different tumors (45).
Albumin is an important component of human plasma and can effectively reflect the nutritional and immune status of patients with cancer. Hypoproteinemia can lead to multiple immune cell dysfunction and subsequent immunosuppression (46, 47). Furthermore, hypoproteinemia could also promote the progression of cachexia in cancer patients (48). Studies have confirmed that albumin functions as an antitumor factor, directly inhibiting tumor cell proliferation (49). Moreover, albumin could inhibit inflammation by clearing reactive oxygen species and inhibiting oxidative stress (50). Accumulating evidences have suggested that hypoalbuminemia is a negative prognostic factor in various cancers (51–53).
Taken together, the high GPS, characterized by elevated CRP and low albumin levels, reflects significant systemic inflammation, malnutrition, and immune suppression. These fact may efficiently explain why high GPS was associated with poor prognosis in BTC patients.
Several limitations of our study should be acknowledged. Firstly, all articles were retrospective studies. Secondly, the survival data of 2 studies was obtained from the survival curves. They may not equate the actual value. Thirdly, publication bias was observed for OS. Fourthly, we did not evaluate the association between high GPS and clinicopathological characteristics due to lack of data. Finally, most of the studies were from Asia, which may affect the universality of the outcomes. More studies from different countries and regions were warranted.
Despite these shortcomings, our study also had merits. Firstly, the study was the first meta-analysis to explore the prognosis value of GPS in BTC. Secondly, sensitivity analysis confirmed the stability and reliability of our results. Thirdly, the trim-and-fill method verified that publication bias did not significantly affect our conclusions. Fourthly, subgroup analysis further supported the prognosis value of GPS. Finally, GPS can dynamically monitor the prognosis and treatment effect of BCT.
In conclusion, we found that high GPS predicted poor prognosis in patients with BTC. GPS can serve as a valuable prognostic marker for BTC, aiding in the identification of high-risk patients and facilitating personalized treatment strategies. Given the limitations of our study, well-designed, large-scale randomized controlled trials were needed to further validate our findings and explore the clinical utility of GPS and its modified counterparts in BTC.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
RL: Data curation, Formal analysis, Methodology, Writing – original draft. LW: Data curation, Formal analysis, Methodology, Writing – original draft. JYe: Data curation, Formal analysis, Methodology, Writing – original draft. XL: Data curation, Formal analysis, Methodology, Writing – original draft. WM: Supervision, Validation, Writing – original draft. XX: Project administration, Writing – review & editing. JYu: Conceptualization, Project administration, Writing – review & editing. WW: Conceptualization, Project administration, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Bridgewater JA, Goodman KA, Kalyan A, Mulcahy MF. Biliary tract cancer: epidemiology, radiotherapy, and molecular profiling. Am Soc Clin Oncol Educ book. Am Soc Clin Oncol. (2016) 35:e194–203. doi: 10.1200/edbk_160831
3. Mirallas O, López-Valbuena D, García-Illescas D, Fabregat-Franco C, Verdaguer H, Tabernero J, et al. Advances in the systemic treatment of therapeutic approaches in biliary tract cancer. ESMO Open. (2022) 7:100503. doi: 10.1016/j.esmoop.2022.100503
4. Marcano-Bonilla L, Mohamed EA, Mounajjed T, Roberts LR. Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. Chin Clin Oncol. (2016) 5:61. doi: 10.21037/cco.2016.10.09
5. Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet (London England). (2021) 397:428–44. doi: 10.1016/s0140-6736(21)00153-7
6. Marino D, Leone F, Cavalloni G, Cagnazzo C, Aglietta M. Biliary tract carcinomas: from chemotherapy to targeted therapy. Crit Rev Oncol Hematol. (2013) 85:136–48. doi: 10.1016/j.critrevonc.2012.06.006
7. Rizzo A, Brandi G. Neoadjuvant therapy for cholangiocarcinoma: A comprehensive literature review. Cancer Treat Res Commun. (2021) 27:100354. doi: 10.1016/j.ctarc.2021.100354
8. Mollica V, Rizzo A, Marchetti A, Tateo V, Tassinari E, Rosellini M, et al. The impact of ECOG performance status on efficacy of immunotherapy and immune-based combinations in cancer patients: the MOUSEION-06 study. Clin Exp Med. (2023) 23:5039–49. doi: 10.1007/s10238-023-01159-1
9. Rizzo A, Brandi G. Pitfalls, challenges, and updates in adjuvant systemic treatment for resected biliary tract cancer. Expert Rev Gastroenterol Hepatol. (2021) 15:547–54. doi: 10.1080/17474124.2021.1890031
10. Rizzo A, Ricci AD, Tober N, Nigro MC, Mosca M, Palloni A, et al. Second-line treatment in advanced biliary tract cancer: today and tomorrow. Anticancer Res. (2020) 40:3013–30. doi: 10.21873/anticanres.14282
11. Ricci AD, Rizzo A, Brandi G. Immunotherapy in biliary tract cancer: worthy of a second look. Cancer Control. (2020) 27:1073274820948047. doi: 10.1177/1073274820948047
12. Ricci AD, Rizzo A, Brandi G. The DNA damage repair (DDR) pathway in biliary tract cancer (BTC): a new Pandora’s box? ESMO Open. (2020) 5:e001042. doi: 10.1136/esmoopen-2020-001042
13. Chapek MA, Martindale RG. Nutrition in cancer therapy: Overview for the cancer patient. JPEN J Parenter Enteral Nutr. (2021) 45:33–40. doi: 10.1002/jpen.2259
14. Shimoda Y, Fujikawa H, Komori K, Watanabe H, Kano K, Yamada T, et al. Preoperative utility of the glasgow prognostic score on outcomes of patients with locally advanced gastric cancer. J Gastrointest Cancer. (2022) 53:265–71. doi: 10.1007/s12029-021-00584-3
15. Fan H, Shao ZY, Xiao YY, Xie ZH, Chen W, Xie H, et al. Comparison of the Glasgow Prognostic Score (GPS) and the modified Glasgow Prognostic Score (mGPS) in evaluating the prognosis of patients with operable and inoperable non-small cell lung cancer. J Cancer Res Clin Oncol. (2016) 142:1285–97. doi: 10.1007/s00432-015-2113-0
16. Nie D, Zhang L, Wang C, Guo Q, Mao X. A high Glasgow prognostic score (GPS) or modified Glasgow prognostic score (mGPS) predicts poor prognosis in gynecologic cancers: a systematic review and meta-analysis. Arch gynecol obstet. (2020) 301:1543–51. doi: 10.1007/s00404-020-05581-8
17. Qi F, Xu Y, Zheng Y, Li X, Gao Y. Pre-treatment Glasgow prognostic score and modified Glasgow prognostic score may be potential prognostic biomarkers in urological cancers: a systematic review and meta-analysis. Ann Trans Med. (2019) 7:531. doi: 10.21037/atm.2019.09.160
18. Zhou Y, Liu Z, Cheng Y, Li J, Fu W. Prognostic value of the modified Glasgow prognostic score in biliary tract cancer patients: a systematic review and meta-analysis. J Gastrointest Surg. (2024) 28:559–65. doi: 10.1016/j.gassur.2024.01.023
19. Fujiwara Y, Haruki K, Shiba H, Hamura R, Shirai Y, Furukawa K, et al. The comparison of inflammation-based prognostic scores in patients with extrahepatic bile duct cancer after pancreaticoduodenectomy. J Surg Res. (2019) 238:102–12. doi: 10.1016/j.jss.2019.01.033
20. Jansson H, Cornillet M, Björkström NK, Sturesson C, Sparrelid E. Prognostic value of preoperative inflammatory markers in resectable biliary tract cancer - Validation and comparison of the Glasgow Prognostic Score and Modified Glasgow Prognostic Score in a Western cohort. Eur J Surg Oncol. (2020) 46:804–10. doi: 10.1016/j.ejso.2019.12.008
21. Hoshimoto S, Hishinuma S, Shirakawa H, Tomikawa M, Ozawa I, Ogata Y. Association of preoperative platelet-to-lymphocyte ratio with poor outcome in patients with distal cholangiocarcinoma. Oncology. (2019) 96:290–8. doi: 10.1159/000499050
22. Hu HJ, Jin YW, Zhou RX, Ma WJ, Yang Q, Wang JK, et al. Clinical value of inflammation-based prognostic scores to predict the resectability of hyperbilirubinemia patients with potentially resectable hilar cholangiocarcinoma. J Gastrointest Surg (2019) 23:510–7. doi: 10.1007/s11605-018-3892-9
23. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
24. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
25. Asakura R, Yanagimoto H, Ajiki T, Tsugawa D, Mizumoto T, So S, et al. Prognostic impact of inflammation-based scores for extrahepatic cholangiocarcinoma. Digest surge. (2022) 39:65–74. doi: 10.1159/000521969
26. Bao Y, Yang J, Duan Y, Chen Y, Chen W, Sun D. The C-reactive protein to albumin ratio is an excellent prognostic predictor for gallbladder cancer. Biosci trends. (2021) 14:428–35. doi: 10.5582/bst.2020.03326
27. Conci S, Campagnaro T. Role of inflammatory and immune-nutritional prognostic markers in patients undergoing surgical resection for biliary tract cancers. Cancers (Basel) (2021) 13:359453. doi: 10.3390/cancers13143594
28. Iwaku A, Kinoshita A, Onoda H, Fushiya N, Nishino H, Matsushima M, et al. The Glasgow Prognostic Score accurately predicts survival in patients with biliary tract cancer not indicated for surgical resection. Med Oncol (Northwood London England). (2014) 31:787. doi: 10.1007/s12032-013-0787-1
29. Lin J, Fang T, Zhu M, Xu X, Zhang J, Zheng S, et al. Comparative performance of inflammation-based prognostic scores in patients operated for intrahepatic cholangiocarcinoma. Cancer Manage Res. (2019) 11:9107–19. doi: 10.2147/cmar.s198959
30. Matsumoto T, Itoh S. C-reactive protein : albumin ratio in patients with resectable intrahepatic cholangiocarcinoma. BJS Open (2020) 4:1146–52. doi: 10.1002/bjs5.50348
31. Moriwaki T, Ishige K, Araki M, Yoshida S, Nishi M, Sato M, et al. Glasgow Prognostic Score predicts poor prognosis among advanced biliary tract cancer patients with good performance status. Med Oncol (Northwood London England). (2014) 31:287. doi: 10.1007/s12032-014-0287-y
32. Oshiro Y, Sasaki R, Fukunaga K, Kondo T, Oda T, Takahashi H, et al. Inflammation-based prognostic score is a useful predictor of postoperative outcome in patients with extrahepatic cholangiocarcinoma. J Hepatobil Pancreat Sci. (2013) 20:389–95. doi: 10.1007/s00534-012-0550-6
33. Pan QX, Su ZJ, Zhang JH, Wang CR, Ke SY. Glasgow Prognostic Score predicts prognosis of intrahepatic cholangiocarcinoma. Mol Clin Oncol. (2017) 6:566–74. doi: 10.3892/mco.2017.1166
34. Shiba H, Misawa T, Fujiwara Y, Futagawa Y, Furukawa K, Haruki K, et al. Glasgow prognostic score predicts therapeutic outcome after pancreaticoduodenectomy for carcinoma of the ampulla of vater. Anticancer Res. (2013) 33:2715–21.
35. Sui K, Okabayashi T, Umeda Y, Oishi M, Kojima T, Sato D, et al. Prognostic utility of the glasgow prognostic score for the long-term outcomes after liver resection for intrahepatic cholangiocarcinoma: A multi-institutional study. World J surge. (2021) 45:279–90. doi: 10.1007/s00268-020-05797-4
36. Yang Z, Zhang D, Zeng H, Fu Y. Inflammation-based scores predict responses to PD-1 inhibitor treatment in intrahepatic cholangiocarcinoma. J Inflamm Res (2022) 15:5721–31. doi: 10.2147/jir.s385921
37. Dai M, Zhao X, Yu A, Zhao L, Kang Q, Yan S, et al. Prognostic and clinicopathological significance of C-reactive protein to albumin ratio in patients with bile duct cancer: A meta-analysis. Nutr Cancer. (2024) 76:914–926. doi: 10.1080/01635581.2022.2104876
38. Peng X, Wang X, Hua L, Yang R. Prognostic and clinical value of the systemic immune-inflammation index in biliary tract cancer: A meta-analysis. J Immunol Res (2022) 2022:6988489. doi: 10.1155/2022/6988489
39. Woods E, Le D, Jakka BK, Manne A. Changing landscape of systemic therapy in biliary tract cancer. Cancers (Basel) (2022) 14:2137. doi: 10.3390/cancers14092137
40. Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J cancer. (2004) 90:1704–6. doi: 10.1038/sj.bjc.6601789
41. Castell JV, Gómez-Lechón MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatol (Baltimore Md.). (1990) 12:1179–86. doi: 10.1002/hep.1840120517
42. Wehbe H, Henson R, Meng F, Mize-Berge J, Patel T. Interleukin-6 contributes to growth in cholangiocarcinoma cells by aberrant promoter methylation and gene expression. Cancer Res. (2006) 66:10517–24. doi: 10.1158/0008-5472.can-06-2130
43. Yoshida T, Ichikawa J, Giuroiu I, Laino AS, Hao Y, Krogsgaard M, et al. C reactive protein impairs adaptive immunity in immune cells of patients with melanoma. J Immunother Cancer. (2020) 8:e000234. doi: 10.1136/jitc-2019-000234
44. Du J, Hu W, Yang C, Wang Y, Wang X, Yang P. C-reactive protein is associated with the development of tongue squamous cell carcinoma. Acta Biochim Biophys Sin (Shanghai). (2018) 50:238–45. doi: 10.1093/abbs/gmy004
45. Zhu M, Ma Z, Zhang X, Hang D, Yin R, Feng J, et al. C-reactive protein and cancer risk: a pan-cancer study of prospective cohort and Mendelian randomization analysis. BMC Med (2022) 20:301. doi: 10.1186/s12916-022-02506-x
46. Jayarajan S, Daly JM. The relationships of nutrients, routes of delivery, and immunocompetence. Surg Clinics North America. (2011) 91:737–53. doi: 10.1016/j.suc.2011.04.004
47. McMillan DC, Watson WS, O’Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. (2001) 39:210–3. doi: 10.1207/S15327914nc392_8
48. Suzuki H, Asakawa A, Amitani H, Nakamura N, Inui A. Cancer cachexia–pathophysiology and management. J Gastroenterol. (2013) 48:574–94. doi: 10.1007/s00535-013-0787-0
49. Nojiri S, Joh T. Albumin suppresses human hepatocellular carcinoma proliferation and the cell cycle. Int J Mol Sci. (2014) 15:5163–74. doi: 10.3390/ijms15035163
50. Taverna M, Marie AL, Mira JP, Guidet B. Specific antioxidant properties of human serum albumin. Ann Intensive Care. (2013) 3:4. doi: 10.1186/2110-5820-3-4
51. Oñate-Ocaña LF, Aiello-Crocifoglio V, Gallardo-Rincón D, Herrera-Goepfert R, Brom-Valladares R, Carrillo JF, et al. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol. (2007) 14:381–9. doi: 10.1245/s10434-006-9093-x
52. Danan D, Shonka DC Jr., Selman Y, Chow Z, Smolkin ME, Jameson MJ. Prognostic value of albumin in patients with head and neck cancer. Laryngos. (2016) 126:1567–71. doi: 10.1002/lary.25877
Keywords: glasgow prognostic score, prognosis, biliary tract cancer, meta-analysis, survival
Citation: Liu R, Wang L, Ye J, Li X, Ma W, Xu X, Yu J and Wang W (2025) Preoperative glasgow prognostic score was an effective prognostic indicator in patients with biliary tract cancer. Front. Immunol. 16:1560944. doi: 10.3389/fimmu.2025.1560944
Received: 15 January 2025; Accepted: 14 March 2025;
Published: 08 April 2025.
Edited by:
Lorenzo Fornaro, Pisana University Hospital, ItalyReviewed by:
Giada Grelli, Pisana University Hospital, ItalyCopyright © 2025 Liu, Wang, Ye, Li, Ma, Xu, Yu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weixing Wang, d2FuZ3d4QHdodS5lZHUuY24=; Jia Yu, eW9nYXFxMTE2QHdodS5lZHUuY24=; Ximing Xu, RG9jdG9yeHUxMjBAYWxpeXVuLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.