- Department of Clinical Immunology of Xijing Hospital and Department of Cell Biology of National Translational Science Center for Molecular Medicine, Fourth Military Medical University, Xi’an, Shaanxi, China
Tissue resident regulatory T cells (tissue Tregs) are vital for maintaining immune homeostasis and controlling inflammation. They aid in repairing damaged tissues and influencing the progression of fibrosis. However, despite extensive research on how tissue Tregs interact with immune and non-immune cells during tissue repair, their pro- and anti-fibrotic effects in chronic tissue injury remain unclear. Understanding how tissue Tregs interact with various cell types, as well as their roles in chronic injury and fibrosis, is crucial for uncovering the mechanisms behind these conditions. In this review, we describe the roles of tissue Tregs in repair and fibrosis across different tissues and explore potential strategies for regulating tissue homeostasis. These insights hold promise for providing new perspectives and approaches for the treatment of irreversible fibrotic diseases.
1 Introduction
Organ damage and fibrosis are common pathological processes observed in clinical settings, which are typically a consequence of inadequate healing following tissue injury (1). Fibrosis, characterized by an excessive activation of fibroblasts and collagen deposition that can ultimately lead to organ dysfunction (2), is closely linked to chronic inflammation, frequently marked by abnormal immune or non-immune cell activation and cytokine dysregulation (3, 4). Thus, understanding the mechanisms underlying tissue repair and fibrosis is essential for developing effective treatment strategies.
Regulatory T cells (Tregs) are essential immune system components that primarily maintain immune tolerance and tissue homeostasis (5). Tregs exhibit specific phenotypes in different types of tissue, thereby enabling them to sense and respond to changes in the local microenvironment (6), modulate inflammatory responses (7), and maintain tissue homeostasis in situ (8). The Tregs comprising this specialized subset are referred to as tissue Tregs (9, 10). In addition to their well-established immunosuppressive functions, they have recently been found to play pivotal roles in regulating tissue damage and regeneration (11).
Given the diversity and complexity of the regulatory mechanisms that modulate tissue damage repair, Tregs can play dual roles in both tissue repair and fibrosis. While they can suppress inflammatory responses and promote tissue repair (12), under certain conditions, activated tissue Tregs may also facilitate the progression of fibrosis (13). For example, by secreting amphiregulin (AREG), these Tregs can stimulate the proliferation of fibroblasts, which has been shown to exacerbate fibrotic processes (14). Conversely, studies have demonstrated that the absence of tissue Tregs in models of chronic liver injury worsens liver fibrosis, underscoring their protective role in the fibrosis process (15). Consequently, further studies examining the mechanisms of action of Tregs with respect to different types of tissue damage and fibrosis could provide valuable insights for developing targeted therapies.
2 Tissue Tregs
2.1 Basic characteristics of tissue Tregs
In addition to the expression of classical markers, such as CD4, CD25, and transcription factor forkhead box protein 3 (Foxp3) (16), tissue Tregs are also characterized by the expression of other functional molecules, which may differ according to tissue type. These Tregs not only display T cell receptors (TCRs) that recognize unique antigens but also characterized by tissue-specific transcriptional expression in addition to Foxp3 (including the Il1rl1 gene encoding the IL-33 receptor ST2), which may make key contributions to their tissue-protective functions (17). The findings of recent research utilizing mouse single-cell sequencing data have indicated that tissue Tregs are characterized a common phenotype among different types of tissue, which features the prominent expression of markers typical of tissue-resident memory cells, such as CD69, CD103, CD11a, programmed cell death protein 1 (PD-1), and killer cell lectin-like receptor G1 (KLRG1) (18, 19). The expression of these activated functional molecules influences the residency of Tregs in tissues and facilitates their specific functions (20–22).
2.2 Mechanisms of tissue Tregs’ residency
The tissue homing ability of tissue Tregs is a fundamental aspect of their definition and function, influencing their distribution and roles across different tissues (23). The localization and homing of Tregs are influenced by various factors, include transcription factors, surface molecules, chemokines and their receptors, as well as lectins and their receptors. Additionally, numerous cytokines and signaling molecules in the tissue microenvironment play important roles in Tregs anchoring and homing (24–26).
2.2.1 Transcription factors
Among transcription factors, Hobit and Blimp-1 are central to the regulation of tissue residency of lymphocytes, including Tregs (24). KLF2 (Kruppel-like factor 2) regulates the migration patterns of naïve Tregs by modulating homeostatic and inflammatory homing receptors. In the absence of KLF2, Tregs cannot effectively migrate to secondary lymphoid organs (SLOs), and this reduction in migration can trigger autoimmune diseases, underscoring that SLOs are critical for maintaining peripheral tolerance. The severity of the disease correlates with impaired recruitment of Tregs to SLOs, while enhancing the entry of Tregs into SLOs can alleviate autoimmune conditions. Furthermore, stabilizing KLF2 expression within Tregs can enhance peripheral tolerance, underscoring the importance roles of KLF2 in regulating the trafficking of Tregs to SLOs (27). Other transcription factors also significant impact the localization and migration of Tregs. For instance, RUNX1 and BAF60b are associated with CCR9 expression on Tregs, thereby affecting their migration to inflamed tissues. The activity of RUNX1 is closely related to the function and homing capabilities of Tregs, while BAF60b inhibits the inflammatory process by regulating Treg migratory capacity. BAF60b functions as a transcriptional coactivator that interacts with RUNX1 to enhance CCR9 expression on Tregs, which in turn affects their ability to migrate to inflamed tissues (28). Furthermore, FOXO1 is another transcription factor that plays a significant role in regulating homing molecule expression in Tregs. Activation of FOXO1 can enhance Treg responses to chemokines, thereby improving their localization ability in specific microenvironments (29).
2.2.2 Chemokine receptors and adhesion molecules
The expression of specific chemokine receptors and adhesion molecules by Tregs allows them to respond to tissue chemokines, enabling precise migration and localization within those tissues (30). For instance, the expression of chemokine (C-C motif) receptor 4 (CCR4) enables Tregs to migrate to tissues such as the skin and lungs, where chemoattractant chemokine ligand (CCL) 17 and CCL22 are expressed (31, 32). CCR6 (33) and CCR10 (34) are highly expressed in intestine Tregs, promoting their homing and functional maintenance in the gut, Similarly, CCR9 binds to CCL25, facilitating the migration of Tregs to the intestine (35). G-protein-coupled receptor-15 (GPR15) also plays a critical role in regulating the homing of tissue Tregs to the colon (36). Mechanistically, the synergistic interaction between aryl hydrocarbon receptor (AhR) and Foxp3 enhances GPR15 expression in Tregs, whereas RORγt antagonizes AhR binding to the GPR15 site, thereby inhibiting GPR15 expression (37). Additionally, Tregs expressing high levels of CXCR4 preferentially home to the bone marrow, helping to alleviate inflammation (38). The expression of CCR5 is related to the migration potential of Tregs to inflammatory sites (39–41). Modulating the expression of these chemokines and activating their receptors can influence the homing and function of tissue Tregs, potentially providing therapeutic benefits in various immune-related diseases.
Adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1), also play a crucial role in the homing and tissue residency of Tregs (42). Lymphocyte Function-Associated Antigen-1 (LFA-1) is another key adhesion molecule that enhances Tregs adhesion to target cells or endothelial cells through interactions with ICAMs, thereby promoting their tissue-specific homing (43, 44). In mouse model, Tregs lacking LFA-1 exhibit significant homing defects, suggesting its indispensable role in Tregs migration (45). Furthermore, the α4β7 integrin expressed on Tregs facilitates their homing to the intestine by binding to mucosal addressin cell adhesion molecule-1 (MAdCAM-1) on intestinal endothelial cells (46). Layilin (LAYN), a C-type lectin-like receptor, is preferentially and highly expressed on activated Treg subsets in both healthy and diseased human skin. While LAYN expression on Tregs has minimal impact on the activation and in vitro suppressive capacity of Tregs, it exerts a cumulative anchoring effect on their dynamic movement in vivo. Specifically, LAYN promotes Tregs adhesion to the skin while restricting their suppressive capacity in the process (47).
2.2.3 Tissue microenvironment
The microenvironment of different tissues features unique cellular composition, cytokines, and metabolic characteristics, all of which significantly influence the homing and functional regulation of tissue Tregs. Tregs not only express T cell receptors (TCRs) that recognize specific tissue antigens but also respond specifically to factors released following tissue damage (20, 48). For example, IL-33, a cytokine from the IL-1 family, acts as an “alarm” molecule during inflammation and tissue injury. Produced by epithelial and endothelial cells, IL-33 promotes the migration of ST2-expressing Tregs into tissues to suppress local inflammation (49, 50). Similarly, IL-18 facilitates Tregs migration to the thymus via CCR6-CCL20 interaction (51), while IL-35 enhances Treg migration and suppressive functions by upregulating CCR5 expression (52). Furthermore, IL-2 is essential for Treg development, function, and homing to the gut, skin, and inflammatory sites (53, 54).
The metabolic environment within tissue also influences Tregs residence, proliferation, and maintenance. For example, in the atherosclerosis microenvironment, oxidized phospholipids impair Tregs function and homing (55). Retinoic acid (RA) enhances the expression of receptors that guide Tregs to the gut (56). Dietary components like L-tryptophan have been shown to regulate Tregs numbers by affecting the transcriptional level of GPR15, thereby influencing Treg homing and local immune homeostasis (57). Dopamine, a key regulator of leukocyte migration, affects immune cell migration based on precise local concentrations. Low dopamine levels preferentially activate high-affinity dopamine receptors DRD3 in Tregs, weakening their suppressive capacity and limiting their recruitment into the gut mucosa (58). These studies suggest that the chemical components and biological signals in the microenvironment not only affect Treg survival and function but also directly impact their homing mechanisms, highlighting the critical role of the microenvironment in regulating the behavior of tissue Tregs.
In summary, the homing mechanisms of Tregs are complex processes involving multiple factors and interactions, including the regulation of various signaling pathways and cytokines. These mechanisms not only affect the homing ability of Tregs but may also alter their functional characteristics, playing a significant regulatory role in various immune-related diseases.
2.3 Functions of tissue Tregs
Tissue Tregs have been shown to contribute to the maintenance of tissue health via well-established anti-inflammatory mechanisms (7), including the secretion of anti-inflammatory cytokines such as interleukin 10 (IL-10) (59) and transforming growth factor β (TGF-β) (60), which suppress the activity of effector T cells (Teffs). Additionally, Tregs can directly eliminated Teffs via the release of granzymes and perforin (61). They also compete with Teffs for interleukin 2 (IL-2) (62), thereby reducing both the responsiveness of target cells and the availability of IL-2, and also produce extracellular enzymes such as CD39 and CD73 (63) that promote adenosine production and interfere with Teffs metabolism. Moreover, these Tregs induce tolerance in dendritic cells via inhibitory receptors that include lymphocyte-associated protein 4 (CTLA-4) (64) and lymphocyte activation gene 3 (LAG3) (65), thereby further suppressing the activity of Teffs (66). Collectively, these mechanisms contribute to the key roles played by Tregs in maintaining immune homeostasis and preventing the occurrence of autoimmune diseases.
The findings of recent studies have revealed that in addition to their immunosuppressive effects, tissue Tregs are also characterized by non-immune regulatory functions (67), including a wide range of effects regarding tissue repair (68), angiogenesis (69), basal metabolism (70), and maintenance of the stem cell niche (71). Depending on the specific type of tissue or model, tissue Tregs can either promote or inhibit angiogenesis (72), and have been shown to contribute to the unique stem cell niche in the skin (73, 74), bone marrow (75), and gut (76), and during pregnancy, facilitate vascular remodeling in the uterus (77). Tissue Tregs are well characterized in adipose tissues, particularly visceral adipose tissue, in which they play key roles in regulating insulin sensitivity and supporting lipid metabolism (78), and their roles in tissue repair have also been extensively documented (79, 80), primarily in muscles (81), lungs (82), skin (83), and the central nervous system (84). Moreover, these Tregs have been established to secrete AREG (85) and keratinocyte growth factor (KGF) (86), which are essential for the induction of epithelial cell proliferation in the lungs (87) and skin (88), as well as in muscle-associated satellite cells (81).
Tissue Tregs demonstrate context-dependent functional duality in disease progression. While growing evidence highlights their beneficial role in suppressing inflammation and promoting tissue regeneration, emerging studies reveal their paradoxical capacity to drive fibrosis in specific pathological settings. Therefore, a comprehensive investigation into the mechanisms by which Tregs influence tissue repair and fibrosis is essential for understanding their dual roles in different pathological contexts and for providing new insights for clinical treatment.
3 Interactions between tissue Tregs and immune/non-immune cells in tissue repair and fibrosis
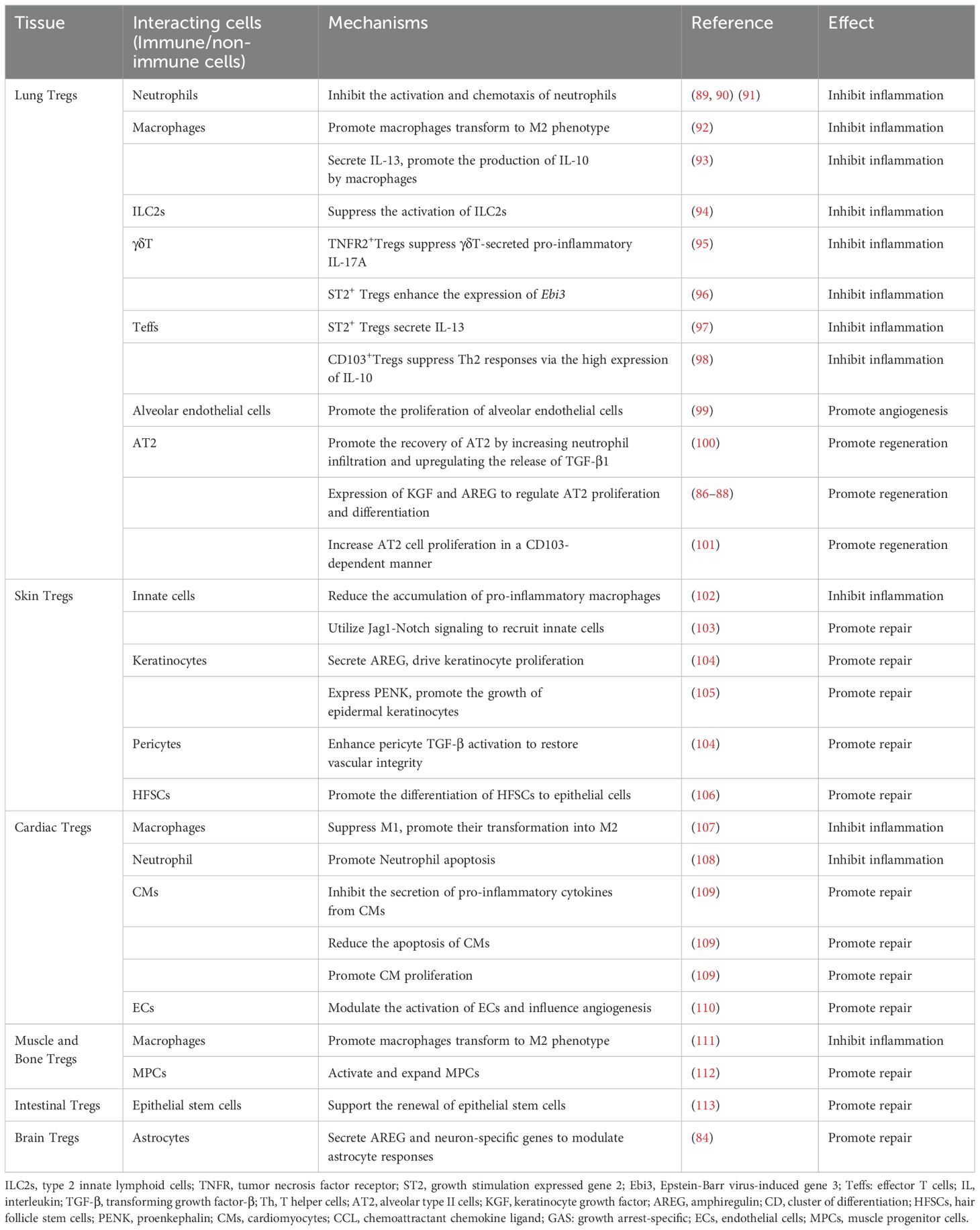
The regulation of tissue Tregs during tissue repair after acute injury and chronic inflammatory responses involves the intricate interplay of tissue Tregs with various immune and non-immune cells (Table 1).
3.1 In acute tissue injury
The immune response triggered by acute injury is complex and involves interactions among various immune cells. Following injury, damaged tissues release a variety of cytokines and chemokines that attract immune cells, such as neutrophils, macrophages, and T cells, to the site of injury (114). While these cells clear dead cells and pathogens, they also release pro-inflammatory factors to promote tissue repair. However, excessive inflammatory responses can lead to further tissue damage, thereby affecting the repair process. Animal models lacking Tregs exhibit excessive inflammatory responses and impaired tissue repair, suggesting that Tregs play a crucial role in modulating inflammation during this process (115, 116). They maintain immune homeostasis by suppressing excessive inflammation, which creates favorable conditions for tissue regeneration and repair (117–120). For instance, Tregs can resolve LPS-induced lung inflammation and promote tissue repair by modulating T helper (Th)1 and Th17 responses (120). In models of acute injury to mouse bone, muscle, and skin, local delivery of Tregs has been shown to promote tissue repair and regeneration by reducing the accumulation of neutrophils and cytotoxic T cells that produce pro-inflammatory cytokine IFN-γ (11). In turn, these responses facilitate the transition of monocytes/macrophages (Mo/MΦ) to an anti-inflammatory and pro-healing state, thereby accelerating wound healing (11). Moreover, in mice with corneal alkali burns, subconjunctival injection of Tregs has been shown to reduce excessive inflammation by producing IL-10 and TGF-β, while also improving corneal healing by increasing AREG levels and activating epidermal growth factor receptor (EGFR) (121).
3.2 In chronic inflammatory responses
In the context of chronic injury or the chronic phase following acute inflammation, tissue Tregs affect the inflammatory response by interacting with immune cells and also promote tissue repair by influencing local non-immune cells (122), such as parenchymal cells and stem cells. For example, Tregs stimulate the growth of alveolar type II (AT2) cells in damaged lung tissue, accelerating wound healing and tissue regeneration (123). Co-culture experiments demonstrate that Tregs directly enhance the proliferation of AT2 cells in a CD103-dependent manner, as CD103 binds to E-cadherin expressed by epithelial cells (124). Furthermore, in vivo depletion of Tregs in the mouse lung injury model not only reduced AT2 cells proliferation but also delayed the recovery of lung injury, and similar effects are observed when blocking CD103 (124). In addition, Tregs play a crucial role in promoting regeneration through modulation of tissue stem cells. These stem cells can be rapidly activated after tissue damage, migrate to the injury site, and repair the damaged tissue by differentiating into specific cell types. Studies suggest that Tregs enhance the differentiation and function of tissue stem cells by modulating local inflammatory responses, thereby improving the tissue repair efficiency (67). For instance, after skin damage, hair follicle stem cells are recruited to the damaged area and differentiate into epithelial cells to rebuild the skin barrier (125). In cardiovascular injury, Tregs have been found to promote the proliferation and differentiation of cardiac stem cells, thereby improving cardiac function (110).
Conversely, Tregs are also regulated by non-immune cells. Mesenchymal stem cells can activate Tregs through ICOS-ICOSL interactions, enabling Tregs to suppress the activity of ILC2s, which play a role in controlling type 2 immune responses mediated by the allergic cytokines IL-13, IL-5, and IL-9 (94). These mechanisms accordingly indicate that tissue Tregs not only play essential roles in immune regulation but, via their interactions with non-immune cells, also facilitate tissue repair and regeneration.
3.3 In fibrosis
As a consequence of defective repair, chronic inflammatory responses can lead to fibrosis (126–128). By suppressing inflammation and interacting with different types of non-immune cell, tissue Tregs can contribute to the regulation of fibrotic processes (Table 2), a key aspect of which is the functional regulation of fibroblasts, which play central roles in both wound healing and fibrosis. Research has shown that tissue Tregs promote fibroblasts proliferation and activation by secreting AREG (14, 149). Although this process is conducive to developing an extracellular matrix and tissue regeneration, the excessive proliferation and activation of fibroblasts often lead to tissue fibrosis. By suppressing inflammation and attenuating excessive fibroblast activity, tissue Tregs can contribute to preventing fibrosis and scar formation (150). These bidirectional interactions influence both the immune status of the local microenvironment and the overall quality of tissue healing and functional recovery. Consequently, studying the interactions between tissue Tregs and fibroblasts could provide valuable insights for the development of new treatment strategies designed to enhance tissue repair and prevent fibrosis.
4 The roles of tissue Tregs in tissue repair and fibrosis
In this section, we discussed the specific roles of tissue Tregs in tissue repair and fibrosis across various pathological contexts, focusing on tissues such as the lung, skin, bone, skeletal muscle, liver, heart, intestine, and brain.
4.1 Lung Tregs
4.1.1 The role of lung Tregs in acute lung injury
Inflammatory responses trigger the local infiltration of lung Tregs (151), which are instrumental in resolving lung inflammation and promoting tissue recovery in cases of acute respiratory infections and acute lung injury (Figure 1). In this regard, it has been established that the expression of CCR4 is vital for initiating the lung-specific recruitment of Tregs, and it has been demonstrated that a deficiency in CCR4 is associated with limited lung trafficking and an inability to suppress lung inflammation effectively (32). In the context of acute lung injury (ALI), the release of local inflammatory factors such as IL-6 and TNF-α can also promote the activation and proliferation of Tregs, thereby enhancing their infiltration into lung tissue (152). Subsequent to their recruitment or expansion in the lungs, Tregs contribute to maintaining homeostasis by interacting with different immune cell types (82, 92). In models of acute lung injury, the recruitment of diverse types of immune cells, including neutrophils and macrophages, along with inflammatory mediator release, leads to endothelial damage (153, 154). Tregs regulate immune responses and suppress inflammatory through various mechanisms. They inhibit the activation and chemotaxis of neutrophils by secreting TGF-β and IL-10, reducing their aggregation and activity at inflammatory sites (89). Furthermore, Tregs reduce neutrophil-mediated inflammation by downregulating pro-inflammatory cytokines such as IFN-γ. This mechanism has been validated in various inflammatory diseases, including ALI models, where Treg deficiency leads to excessive neutrophil activation and exacerbated inflammation (90). Tregs also promote the transformation of macrophages from M1 to M2 phenotype by enhancing IL-10 secretion, thereby alleviating inflammation in ALI (155, 156). Moreover, Tregs enhance macrophage anti-inflammatory functions via IL-13 secretion, which stimulates macrophages to produce IL-10. This IL-10 induces autocrine-paracrine signaling of Vav1 in macrophages and activates Rac1 to promote macrophage efferocytosis (153). Through these mechanisms, Tregs enhance the phagocytic function of macrophages, enhancing apoptotic cell clearance, and preventing necrosis and subsequent inflammation.
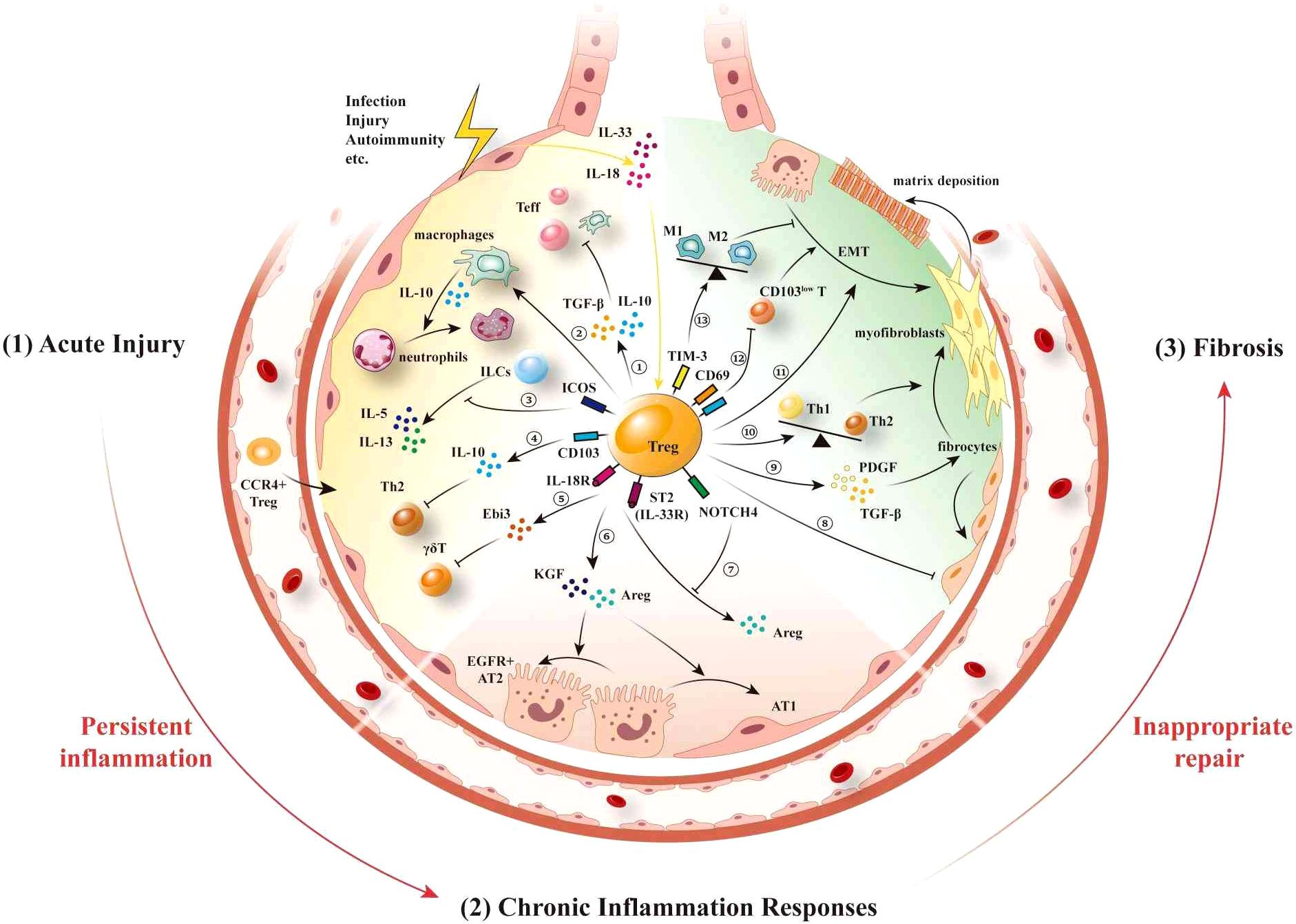
Figure 1. Lung Tregs interacts with other immune/non-immune cells following injury. After suffering injury, lung epithelial cells release the “alarmins” IL-18 and IL-33, thereby promoting the migration of inflammatory cells to the lungs, leading to pulmonary inflammation. Expression of CCR4 stimulates the recruitment of Tregs to the lungs (1). During the acute phase of injury, neutrophils and macrophages are initially recruited to participate in the acute inflammatory response, further causing damage to the lung epithelium. ① Lung Tregs secrete the inhibitory factors IL-10 and TGF-β to suppress the proliferation and activation of other immune cells. ② Lung Tregs promote neutrophil apoptosis mediated by macrophage, and ③ also suppress the activation of ILC2s by directly inducing ICOS, which inhibits the production of IL-5 and IL-13 from ILCs. ④ CD103+ Treg-expressed IL-10 suppresses Th2-type inflammatory responses. ⑤ IL-18 and IL-33 activate IL-18R and ST2 on Tregs, thereby inhibiting the function of γδT cells by via the secretion of Ebi3 (2). During the chronic inflammatory response phase, ⑥ ST2+ Tregs secrete AREG and KGF, thereby promoting the proliferation and differentiation of AT2 cells. ⑦ By expressing vimentin and NOTCH4, Tregs respectively inhibit the secretion of AREG mediated by the activation of IL-18R and ST2 (3). At the fibrotic stage, ⑧ lung Tregs can control the recruitment of fibroblasts and alleviate pulmonary fibrosis. ⑨ Tregs promote fibroblast proliferation and their transformation to myofibroblasts and matrix deposition by secreting PDGF and TGF-β. ⑩ Tregs promote Th2 polarization, which in turn promotes fibrosis. ⑪ Tregs promote EMT, whereas ⑫ CD69highCD103high Tregs in the lung inhibit fibrosis caused by CD103low resident memory T cells. ⑬ TIM-3+ Tregs contribute to reducing pneumonia and lung injury by regulating macrophage polarization. IL, interleukin; CCR, chemokine (C-C motif) receptor; TGF-β, transforming growth factor-β; ILC2, type 2 innate lymphoid cells; ICOS, inducing co-stimulation; ST2, growth stimulation expressed gene 2; Ebi3, Epstein-Barr virus-induced gene 3; Th, T helper cells; AREG, amphiregulin; KGF, keratinocyte growth factor; AT2, alveolar type II cells; PDGF, platelet-derived growth factor; EMT, epithelial-mesenchymal transition; CD, cluster of differentiation; TIM-3, mucin domain-containing protein 3.
Certain subsets of lung Tregs have been demonstrated to play significant roles in lung injury. For example, IL-33-mediated ST2+ Tregs have been found to secrete IL-13 to control inflammatory response following lung injury (97), whereas Faustino et al. (96) found that by promoting the expression of Epstein-Barr virus-induced gene 3 (Ebi3), a component of IL-35, ST2+ Tregs can act as early negative regulators of innate γδ T cells, thus reducing allergen-induced lung inflammation. Furthermore, lung Tregs expressing tumor necrosis factor receptor (TNFR2) are recognized as a suppressive and proliferative subset (95). In lungs infected with pneumococcus, TNFR2+ Tregs inhibit γδ T cells by reducing their secretion of the pro-inflammatory cytokine IL-17A, thereby preventing excessive pulmonary inflammation (157). Moreover, Tregs expressing CD103 represent a unique subset that specifically suppress Th2 responses, driving the resolution of Th2-mediated allergic airway inflammation via elevated levels of IL-10 expression (98). On the basis of these “classical” immunosuppressive mechanisms, lung Tregs thus contributes to mediating the resolution of inflammation during the acute injury phase.
4.1.2 The role of lung Tregs in chronic inflammatory responses
Although antigen-specific lung Tregs generated in response to acute injury dampen the immune response to pathogens and limit inflammation-related damage, they may also contribute to the chronic persistence of inflammation. In ongoing inflammatory situations, lung Tregs not only limit inflammation but also interact with diverse non-immune cells via direct interactions and indirect effects via other immune/non-immune cells to promote tissue repair and regeneration (Figure 1). Moreover, by promoting neutrophil infiltration and upregulating the release of TGF-β1, CD103+ lung Tregs have also been demonstrated to promote AT2 cell proliferation in a CD103-dependent manner (100, 101, 158). In contrast, in response to the release of IL-18 and IL-33 from damaged tissues, lung Tregs produce significant amounts of AREG (159), a cytokine that facilitates tissue repair by mediating EGFR-induced inhibition of the pro-apoptotic effects of TNF-α on AT2 cells (80). AREG also stimulates the proliferation and differentiation of AT2 cells (158), as exemplified by Treg-derived AREG stimulation of a population of Col14a1+EGFR+ mesenchymal cells, which mediates the regeneration of AT2 cells during influenza-induced lung injury in mice (87). However, Tregs also express certain inhibitory factors that can contribute to diminishing the activity of AREG, as illustrated by the expression of NOTCH4 on Tregs, which dynamically suppresses AREG-dependent tissue repair, leading to elevated levels of pulmonary inflammation (160). Similarly, the type III intermediate filament protein vimentin has been established to suppress the IL18R- mediated increase in AREG, thereby impairing lung tissue repair (119). This dynamic regulation of AREG accordingly highlights its therapeutic implications for related diseases. Additionally, by secreting KGF, lung Tregs have been demonstrated to promote AT2 cell proliferation (86). Moreover, the repair of alveolar endothelial cells is necessary for restoring gas exchange following lung injury. It has been demonstrated in mice that lung Tregs are essential for lung angiogenesis (99), although the precise underlying mechanisms have yet to be established.
4.1.3 The role of lung Tregs in fibrosis
The proliferation and activation of alveolar epithelial cells promote tissue repair through regeneration (161). In contrast, tissue repair based on the activation of fibroblasts is often considered detrimental because it significantly contributes to fibrosis and organ dysfunction (162). Moreover, the proportion and quantity of lung Tregs produced during pulmonary fibrosis can either increase or decrease (133), thereby complicating the elucidation of the specific roles played by lung Tregs in this condition. For example, the lungs and blood of patients with connective tissue disease-associated interstitial pneumonia (CTD-IP) are typically characterized by elevated levels of cytotoxic T cells and lower levels of Tregs (163). In contrast, elevated levels of Tregs have been detected in the blood and lungs of patients with advanced fibrosis (164). A commonly used model for studying pulmonary fibrosis in mice is bleomycin (BLM)-induced acute lung inflammation (165), which subsequently leads to fibrosis, and the findings of studies using this model have revealed that the role of Tregs in the pathogenesis of pulmonary fibrosis differs depending on the stage of the disease (166). Moreover, studies that have involved the transfer or depletion of Tregs, indicate that these cells can have both protective or harmful effects during different phases of BLM-induced lung injury (167, 168). In summary, there remain considerable challenges with respect to determining whether the observed changes in lung Tregs during pulmonary fibrosis are a “cause” or a “consequence” of this disorder; that is, whether lung Tregs drive the progression of pulmonary fibrosis or react in response to counteract fibrosis. Nevertheless, research to date tends to indicate that lung Tregs play a dual role in both preventing and contributing to the development of pulmonary fibrosis (131) (Figure 1).
Lung Tregs contribute to pulmonary fibrosis via multiple mechanisms, among which they play roles in influencing the Th1/Th2 balance, generating a fibrosis-conducive cytokine environment, promoting epithelial-mesenchymal transition (EMT), and facilitating the proliferation and differentiation of fibroblasts, as well as collagen deposition. For example, in a mouse model of silica-induced pulmonary fibrosis, the depletion of lung Tregs has been found to promote an enhanced Th1 response and disrupt the Th1/Th2 balance, thereby resulting in a shift toward a Th2 phenotype (131). Lung Tregs have also been established to promote the progression of pulmonary fibrosis by secreting factors such as platelet-derived growth factor (PDGF) and TGF-β, specifically targeting lung fibroblasts. TGF-β has been identified as a key mediator in the fibrotic process, inducing the proliferation of fibroblasts and their subsequent transformation to myofibroblasts (134). Similarly, the PDGF-induced promotion of fibroblast proliferation contributes to an excessive production of extracellular matrix components (133). Furthermore, in cases of radiation-induced pulmonary fibrosis, it has been established that lung Tregs facilitate the accumulation of fibrocytes in the irradiated lungs, and in epithelial cells promote β-catenin-mediated EMT (137). Collectively, the crosstalk among lung Tregs, other infiltrating T cells, epithelial cells, and fibroblasts, contribute to the activation of myofibroblasts, thereby promoting the deposition of collagen, and ultimately leading to the destruction of the typical lung structure.
Contrastingly, lung Tregs can also play a protective role in pulmonary fibrosis. Lung Tregs can help prevent fibrosis by resolving inflammatory responses. An example is the activation of the AhR signaling pathway, which boosts Tregs numbers and reduces inflammatory T cell subsets, thereby decreasing pulmonary fibrosis in the BLM model (169). Specifically, CD69highCD103high Tregs represent a protective subset in lung inflammation and fibrosis. In a fungal antigen-induced pulmonary fibrosis model, CD103low resident memory T cells selectively express profibrotic cytokine genes Il5 and Il1. In contrast, CD69highCD103highFoxp3+ Tregs exhibit elevated expression of Itgae and Foxp3, effectively suppressing the profibrotic and inflammatory responses driven by CD103low resident memory T cells (132). Lung Tregs expressing trefoil factor family 1 (Tff1) can prevent the worsening of BLM-induced pulmonary fibrosis. They achieve this by inhibiting macrophage pro-inflammatory responses and reducing the quantity and activity of inflammatory myeloid cells (129). In addition, lung Tregs inhibit fibroblast proliferation, helping to prevent the progression of pulmonary fibrosis. It has been demonstrated that by reducing chemokine C-X-C motif ligand 12 (CXCL12) and C-X-C motif receptor 4 (CXCR4) signaling (135), as well as suppressing CXCL10 (131) and CCL2 (136), lung Tregs can play roles in controlling the recruitment of fibroblasts, thereby alleviating pulmonary fibrosis (135).
4.2 Skin Tregs
4.2.1 The role of skin Tregs in acute tissue injury
Skin Tregs facilitate early wound healing after acute injury by recruiting monocytes and macrophages to injury sites. Single-cell sequencing reveals that injury triggers preferential expression of integrin αvβ8 in skin Tregs, which activates latent TGF-β, enhancing CXCL5 production and neutrophil recruitment (170). Additionally, skin Tregs interact with keratinocytes through Jag1-Notch signaling, inducing the release of chemokines by keratinocytes that attracts monocytes and neutrophils to the site of injury (103). Although these mechanisms may slightly delay epidermal regeneration, they provide essential protection against infection, demonstrating the important role of Tregs in acute tissue damage.
Conversely, skin Tregs also prevent excessive immune responses by suppressing immune cells. They not only regulate immune responses by suppressing the activity of Teffs, but also promote the polarization of macrophages towards the M2 phenotype (171). In addition to mitigating inflammation, EGFR signaling and CD103 expression support the migration and survival of Tregs at injury sites (102, 172). The ligand AREG for EGFR can be expressed by Tregs infiltrating the injured tissue (159), while the ligand E-cadherin for CD103 is mainly expressed by epithelial cells (173). EGFR expression on Tregs reduces IFN-γ production and limits the accumulation of pro-inflammatory macrophages (102). Studies have shown that the specific removal of EGFR+ skin Tregs results in delayed re-epithelialization and altered rates of wound closure (174), underscoring their essential roles in maintaining immune balance and wound healing. Moreover, CD103+ Tregs suppress inflammation by downregulating the pro-inflammatory function of dendritic cells (DCs) through contact-dependent mechanisms, such as CTLA-4-CD80/86 and PD-L1/PD-1 axis (175).
4.2.2 The role of skin Tregs in chronic inflammatory responses
Skin Tregs facilitate the regeneration and repair of epithelial cells during the chronic inflammation phase through various mechanisms. The skin contains a substantial number of type 2 polarized Tregs that are programmed by Th2-related transcription factors, including GATA-3 and IRF4, which are important for tissue repair (176). GATA-3+ Tregs in the skin have been established to express receptors for alarm signals, such as TSLP, IL-33, and IL-18, which are released during tissue damage, thereby enabling these Tregs to sense local injuries (177). Similar to lung Tregs, skin Tregs also participate in tissue repair by directly secreting different repair mediators, among which, both IL-18 and IL-33 can stimulate the expansion of skin Tregs that produce the repair-associated cytokine AREG in the absence of TCR stimulation (174). In addition to promoting the growth of keratinocytes (104), AREG also contributes to the restoration of vascular integrity by enhancing TGF-β activation in pericytes. In a model of ultraviolet B radiation (UVB)-induced skin damage, healing-associated skin Tregs have been observed to proliferate in response to UVB exposure and secrete proenkephalin (PENK), a precursor of opioid-like substances, that promotes the growth of epidermal keratinocytes (105). Furthermore, by activating progenitor cells, skin Tregs can facilitate the regeneration of skin (71). For example, research has shown that Tregs promote the differentiation of hair follicle stem cells (HFSCs) into epithelial cells during the skin barrier repair process (106).
4.2.3 The role of skin Tregs in fibrosis
The fibrosis of skin is a defining characteristic of systemic sclerosis (SSc) (178, 179), and research in this regard has revealed increases in the levels of Tregs in peripheral blood and skin lesions during the inflammatory and fibrotic phases of the disease (180). However, these Tregs are often dysfunctional and have a reduced suppressive capacity (181), and the findings of some studies have indicated that compared with healthy skin or psoriatic skin lesions, skin Tregs are less prevalent in SSc, and that this reduction is correlated with reductions in the levels of TGF-β and IL-10 (182). The findings of a further study have indicated that compared with late-stage SSc patients and healthy controls, skin Tregs are more numerous in the skin epidermis and dermis of early SSc patients (183), whereas in patients with limited and diffuse SSc, the Tregs in skin lesions have been found to produce pro-fibrotic Th2 cytokines, such as IL-13 and IL-4 (138). Consequently, these dysfunctional skin Tregs may contribute to an exacerbation of the disease. Collectively, the findings of these studies provide evidence of an association between the quantitative reduction and/or qualitative dysfunction of skin Tregs and the occurrence SSc. However, there is currently a lack of consensus in this regard.
By interacting with dermal fibroblasts, skin Tregs contribute to the occurrence of pathological skin fibrosis (138). These Tregs secrete TGF-β, a well-known profibrotic factor (140). Moreover, while AREG promotes tissue repair, it can also promote fibrosis. It has been established that the AREG-EGFR-MEK (mitogen-activated protein kinase/extracellular receptor-stimulated kinase) signaling axis plays a central role in mediating the development of skin fibrosis. For example, using models of BLM-induced skin fibrosis, Zhang et al. (141) have shown that AREG is upregulated throughout the fibrogenesis process and is associated with an enhanced proliferation of dermal cells. Conversely, dermal cells proliferation induced by BLM does not occur in mice that lack the AREG gene. Moreover, trametinib, which inhibits MEK (a downstream effector of AREG), has proven effective in preventing skin fibrosis in models induced by BLM.
Skin Tregs may also contribute to a reduction in fibrosis. In this regard, although skin Tregs secrete TGF-β, the amounts are relatively low, but may still potentially serve as a “TGF-β reservoir” that inhibits fibroblast activation (184). In animal models of SSc (142), both the acute depletion and chronic reduction in skin Tregs lead to the spontaneous activation of skin fibroblasts, along with an increase in the expression of pro-fibrotic genes, and subsequent dermal fibrosis, thereby highlighting their key roles in the pathology of skin diseases. Additionally, skin Tregs have been shown to be characterized by elevated levels of GATA-3 expression, which is assumed to be associated with Th2 polarization (177). Conversely, in the absence of GATA-3, there are larger numbers of Th2 cells and increases in fibroblast activation, thus tending to indicate that the GATA-3 in skin Tregs has certain beneficial effects that contribute to the prevention of skin fibrosis (139, 182).
4.3 The functions of other Tregs in tissue repair and fibrosis
In addition to the lungs and skin, the findings of numerous studies have provided evidence to indicate that by interacting with immune/non-immune cells, tissue Tregs play roles in the repair and fibrosis of other tissues.
4.3.1 Liver Tregs
In the liver, Tregs have been established to play roles in the response to liver injury and in managing chronic inflammation. In the acute phase of liver injury, immune cells trigger inflammation, thereby leading to a rapid apoptosis-induced reduction in the population of liver Helios+ Tregs, and this contributes to the progression of inflammation and tissue damage (185). During the healing phase, inflammation subsides, wound healing is initiated, and immune homeostasis is restored. Hepatic stellate cells (HSCs) promote regeneration of the Helios+ Tregs subset via matrix metalloproteinase (MMP) 9/13-dependent TGF-β activation, which is essential for terminating inflammation and facilitating wound healing (185), thus, emphasizes the important role played by Helios+ Tregs as a “repair” subset in liver injury.
Liver Tregs exhibit a dual role in the onset and progression of liver fibrosis across various liver injury models. In the context of non-alcoholic steatohepatitis, liver ST2+ Tregs significantly contribute to liver tissue repair and fibrosis regulation by secreting AREG (14). Conversely, in carbon tetrachloride (CCl4)-induced liver inflammation and fibrosis, liver Tregs expand preferentially, helping to prevent fibrosis by limiting the abnormal activation of pre-fibrotic immune cells, such as Th2 cells and Ly-6Chigh inflammatory monocytes/macrophages (15). Furthermore, liver Tregs-expressed CD39 has been demonstrated to be associated with the suppression of the CD8+ T cell proliferation and their production of TNF-α and osteopontin, thereby alleviating biliary fibrosis (186).
Recent studies have highlighted the impact of liver Tregs interaction with various non-immune cells in liver fibrosis progression. First, liver Tregs have been shown to directly activate hepatic stellate cells (HSCs), which can differentiate into myofibroblast-like cells, producing extracellular matrix and cytokines (187) that promote fibrosis (188). Conversely, activated HSCs secrete matrix metalloproteinases (MMPs) that degrade the extracellular matrix, potentially inhibiting fibrosis (189–191). Second, natural killer (NK) cells regulate liver fibrosis by targeting activated HSCs (192), while Tregs can indirectly modulate HSC activity by suppressing NK cells (144) through direct cell contact (CTLA-4 signaling) (193) and cytokine release (IL-8 and TGF-β) (194). Therefore, by modulating the interaction between NK cells and HSCs, liver Tregs can alter the progression of liver fibrosis. Additionally, liver Tregs can prevent HSC activation by suppressing monocyte chemoattractant protein-1 (MCP-1) and inhibiting the IFN-γ-secretory activity of CD4+ T cells (146), thereby conferring liver protection (143). In summary, the regulatory mechanisms employed by liver Tregs on HSCs are crucial to the progression of liver fibrosis.
In turn, HSCs can mutually influence liver Tregs by promoting an IL-2-dependent increase in the numbers of these Tregs (186). In vitro experiments have revealed that a proliferation of allogeneic Tregs promoted by mature HSCs is dependent on both dose and cell contact, and enhances the Tregs-mediated suppression of Teff proliferation (186). Furthermore, by modulating the balance between Treg and Th17 cell responses, it has been demonstrated that the transfer of HSC-activated Tregs can contribute to a significant reduction in liver injury in animal models of autoimmune hepatitis (AIH). This highlights the importance of HSC regulation on Tregs in the pathology of liver injury.
In addition to HSCs, liver Tregs have been shown to inhibit the secretion of MMPs by Kupffer cells in vivo via the TGF-β pathway (146), thereby preventing fibrosis regression. Moreover, these Tregs can modulate human amniotic mesenchymal stem cells (hAMSCs) to enhance their tissue repair functions via TGF-β and indoleamine 2,3-dioxygenase, thereby promoting the hAMSC-mediated inhibition of fibrosis (147).
4.3.2 Cardiac Tregs
Cardiac Tregs play key roles in the healing process following various cardiac injury diseases, such as myocardial infarction (MI) (107). After tissue damage, cardiac Tregs initially interact with immune cells to control local inflammation. Cardiac Tregs suppress the pro-inflammatory M1 phenotype of macrophages by secreting IL-10 and TGF-β, promoting their transformation into anti-inflammatory/repair M2 phenotypes. In a mouse model of myocardial ischemia-reperfusion, the absence of Tregs leads to sustained secretion of TNF-α and IL-6 from macrophages, exacerbating myocardial injury (195). Additionally, Tregs suppress macrophage CD80/CD86 co-stimulatory signals through a CTLA-4-dependent pathway, limiting excessive inflammatory responses (107). Cardiac Tregs also promote neutrophil apoptosis by secreting lipoxin A4 (LXA4) and inhibit the formation of neutrophil extracellular traps (NETs). In a myocardial infarction-induced fibrosis model, Treg-deficient mice show prolonged neutrophil infiltration and abnormal collagen deposition (108). Tregs inhibit the differentiation of Th17 cells through cell-cell contact, such as the PD-1/PD-L1 pathway, thereby reducing IL-17-mediated myocardial fibrosis. They further regulate CD8+ T cell activation to prevent toxic damage to surviving cardiomyocytes (196).
Moreover, cardiac Tregs directly interact with parenchymal cells, including cardiomyocytes (CMs) and endothelial cells. ATP released by damaged CMs activates the P2X7 receptor on Tregs, enhancing their immunosuppressive function. Conversely, IGF-1 secreted by Tregs inhibits CMs apoptosis through the PI3K/Akt pathway, promoting their survival (197). Additionally, Tregs promote the regeneration of CMs by secreting regenerative factors including CCL24 (which stimulates proliferation through ERK1/2 signaling), AREG (an EGFR pathway activator), and GAS6 (a mediator of efferocytic clearance) (109). Tregs also promote endothelial cell proliferation and angiogenesis by secreting VEGF-A while inhibiting ICAM-1 expression, which reduces leukocyte adhesion and vascular leakage. In atherosclerosis models, Tregs expansion significantly improves endothelial function (197).
Fibroblasts play essential roles in preserving the integrity of the injured heart. Their activation facilitates effective repair and stable collagen deposition following cardiac injury (110). Within the infarct area of MI, the accumulation of fibroblasts in the hearts of mice has been found to reduce the risk of cardiac rupture after MI, primarily by enhancing collagen III production by fibroblasts (148). However, an excessive activation of fibroblasts or insufficient apoptosis of myofibroblasts following cardiac injury often contributes to poor repair-associated responses. Tregs regulate fibroblast activity and exhibit a dual role depending on the disease stage. During the acute repair phase, Tregs suppress fibroblast differentiation into myofibroblasts by secreting IL-10, thereby mitigating excessive collagen deposition. In a mouse model of myocarditis, the adoptive transfer of Tregs has been shown to lower the activation of the TGF-β/Smad3 pathway (107), reflecting their protective effect against fibrosis. In the chronic fibrosis phase, Tregs also exhibit a dual role in regulating fibrosis. In models of long-term stress overload, Tregs promote fibroblast proliferation and extracellular matrix (ECM) remodeling by secreting AREG, which activates the EGFR/ERK pathway, potentially leading to excessive fibroblast activation and aggravated fibrosis (197). However, in diabetic cardiomyopathy fibrosis, Tregs improve fibrosis by competitively absorbing glutamine, inhibiting fibroblast mitochondrial oxidative phosphorylation, and reducing their anabolic activity (108). A unique population of ST2+ Tregs has been identified that produces secreted protein acidic and rich in cysteine (SPARC) (148). In vitro studies have indicated that co-culturing fibroblasts with cardiac Tregs expressing SPARC inhibits the excessive activation of fibroblasts, which accordingly provide evidence to indicate a protective role of cardiac Tregs in fibrosis.
However, given the complexity of the multiple interactions between cardiac Tregs and the surrounding tissue cells, further studies are necessary to better understand the roles played by this Treg subset.
4.3.3 Muscle and bone Tregs
Studies show that Tregs rapidly migrate to skeletal muscle injury sites, driven by T cell receptor (TCR) signaling and IL-33 released by bone marrow-derived mesenchymal stem cells (MuSCs) (50, 62, 198, 199). At the injury site, Tregs release anti-inflammatory factors such as IL-10 and TGF-β, which help modulate the inflammatory response. The upregulation of TGF-β also enhances Treg functionality and promotes their migration to the injury site (200). Furthermore, Tregs promote macrophage polarization towards the anti-inflammatory M2 phenotype (201). By secreting growth factors like AREG (111), Tregs activate and expand muscle progenitor cells (MPCs), aiding in their differentiation into muscle cells.
Tregs play a crucial role in bone healing, a complex process that requires coordinated interactions among osteoblasts, osteoclasts, and immune cells. The dynamics of Tregs, including their numbers and functionality, are critical for healing outcomes, particularly in vulnerable populations like diabetic and elderly patients (112). Tregs foster a conducive microenvironment for bone healing by secreting anti-inflammatory cytokines, including IL-10 and TGF-β (202, 203), and directly enhancing osteoblast proliferation and differentiation (204). During fracture healing, Tregs secrete amino acids and growth factors, such as amphiregulin (AREG), to stimulate the proliferation and differentiation of osteoblast progenitor cells, thereby promoting bone formation (203).
The activity of Tregs is related to bone damage and synovial fibrosis in rheumatoid arthritis (RA). A deficiency in functional Tregs results in excessive osteoclast activation and further bone destruction (205). In RA patients, the numbers and functions of Tregs are frequently compromised, resulting in a loss of immune tolerance and heightened autoimmune responses. This not only exacerbates bone destruction but also worsens synovial fibrosis (206). In synovial fibrosis associated with RA, Tregs help regulate immune responses and exert immunosuppressive effects that inhibit Teff activation, thereby alleviating synovial inflammation and fibrosis (207). In addition, Tregs significantly influence the activity of synovial fibroblasts (SFs). Induced Treg cells (iTregs) have demonstrated inhibitory effects on SFs through cytokines such as IL-10 and TGF-β, which inhibit SF proliferation and inflammatory responses (208).
4.3.4 Intestinal Tregs
Intestinal Tregs have been established to promote tissue repair and contribute to maintaining the integrity of the gut epithelial barrier. They secrete anti-inflammatory factor IL-10 to suppress excessive inflammation, promote the regeneration of intestinal epithelial cells (IECs), and maintain barrier integrity (209). IL-10 alleviates endoplasmic reticulum stress and protects the epithelial barrier by suppressing IECs fucosylation and Fas-mediated apoptosis (210).
Intestinal Tregs also express repair-related markers, such as AREG and ST2, with the IL-33/ST2 signaling pathway drives their accumulation in the intestine, which alleviates colitis injury by enhancing Foxp3 expression (113, 211). Knockdown of the IL-33/ST2 pathway aggravates tissue damage. Studies suggest that although repair subsets of intestinal Tregs increase among HIV-infected individuals, defects in AREG secretion leads to impaired epithelial repair (212), highlighting the importance of AREG in gut epithelial restoration. Additionally, within the gut mucosa, human CD161+ Tregs, which are regulated by retinoic acid, have been demonstrated to facilitate wound repair (213). Furthermore, by contributing to the renewal of epithelial stem cells, intestinal Tregs have been found to promote homeostasis in intestinal epithelial cells (113). Intestinal Tregs can enhance crypt stem cell activity and promote epithelial renewal through the Wnt/β-catenin pathway (214). Studies have shown that the absence of Tregs in colitis models is associated with a decline in crypt stem cell function. In the intestinal crypts, Tregs also maintain the stem cell microenvironment homeostasis by regulating the levels of local cytokines (such as IL-22), thereby maintaining the balance between stem cell proliferation and differentiation (215).
4.3.5 Brain Tregs
Brain Tregs play essential roles in the repair of brain tissues following neuroinflammation and injury (216). Accumulating evidence indicates that these Tregs play a protective role during the acute phase of stroke and contribute to recovery in the chronic phase. Brain Tregs target a range of cell types, including immune and central nervous system cells, on which they have beneficial effects via their influence on intercellular interactions and the release of soluble factors (115, 217). For example, these Tregs secrete cytokines such as AREG, which modulate astrocyte responses and thereby contribute to reducing neurological damage, and also express neuron-specific genes, such as the serotonin receptor (Htr7), and respond to serotonin, which leads to an increase in Tregs numbers and an amelioration of neurological symptoms (84).
Collectively, the findings of these studies highlight the pivotal roles played by tissue Tregs in immune regulation and tissue repair among different organs, and thus, gaining a more comprehensive understanding of the mechanisms underlying the activity of these cells in different immune microenvironments will be essential for developing effective therapies for the treatment of fibrosis.
5 Conclusion
Tissue Tregs have been established to play multiple complex roles in injured and fibrotic tissues. Recent research has provided compelling evidence to indicate their essential function in promoting tissue repair. During the acute phase of injury, by interacting with other immune cells, tissue Tregs primarily contribute to the control of inflammation, whereas in the chronic phase of inflammation, they extend their role beyond immune modulation by engaging with non-immune cells to promote tissue repair. However, the precise role of Tregs in fibrosis has sparked considerable debate, which can partially be explained by their dual regulatory effects on fibroblasts. It has also been found that different subsets of tissue Tregs that express distinct suites of functional molecules may have certain tissue-specific roles, thereby emphasizing the need to study the diversity of tissue Tregs and the potential for targeted therapy via molecular regulation. Future research should focus on adjusting tissue Tregs in their local environments to balance their roles in tissue repair and the prevention of fibrosis. In summary, a comprehensive understanding of the regulatory functions of tissue Tregs in tissue repair and fibrosis, as well as their specific activities in the context of differing physiological and pathological states, will provide vital insights and practical guidance for future research and clinical applications.
Author contributions
PYZ: Conceptualization, Writing – original draft, Writing – review & editing. JW: Resources, Visualization, Writing – review & editing. JM: Supervision, Writing – review & editing. PZ: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Key Research and Development Program of Shaanxi (NO.2024SF-GJHX-46).
Acknowledgments
Table was created with Microsoft Word and figure was created with Adobe Illustrator 2024.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Martin P, Pardo-Pastor C, Jenkins RG, Rosenblatt J. Imperfect wound healing sets the stage for chronic diseases. Science. (2024) 386:eadp2974. doi: 10.1126/science.adp2974
2. Younesi FS, Miller AE, Barker TH, Rossi FMV, Hinz B. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat Rev Mol Cell Biol. (2024) 25:617–38. doi: 10.1038/s41580-024-00716-0
3. Fang D, Chen B, Lescoat A, Khanna D, Mu R. Immune cell dysregulation as a mediator of fibrosis in systemic sclerosis. Nat Rev Rheumatol. (2022) 18:683–93. doi: 10.1038/s41584-022-00864-7
4. Avanoglu Guler A, Rossi FW, Bellando-Randone S, Prevete N, Tufan A, Manetti M, et al. The role of endogenous eicosapentaenoic acid and docosahexaenoic acid-derived resolvins in systemic sclerosis. Front Immunol. (2020) 11:1249. doi: 10.3389/fimmu.2020.01249
5. Du Y, Fang Q, Zheng S. Regulatory T cells: concept, classification, phenotype, and biological characteristics. Adv Exp Med Biol. (2021) 1278:1–31. doi: 10.1007/978-981-15-6407-9_1
6. Raugh A, Allard D, Bettini M. Nature vs. nurture: FOXP3, genetics, and tissue environment shape Treg function. Front Immunol. (2022) 13:911151. doi: 10.3389/fimmu.2022.911151
7. Dikiy S, Rudensky AY. Principles of regulatory T cell function. Immunity. (2023) 56:240–55. doi: 10.1016/j.immuni.2023.01.004
8. Shao Q, Gu J, Zhou J, Wang Q, Li X, Deng Z, et al. Tissue Tregs and maintenance of tissue homeostasis. Front Cell Dev Biol. (2021) 9:717903. doi: 10.3389/fcell.2021.717903
9. Panduro M, Benoist C, Mathis D. Tissue Tregs. Annu Rev Immunol. (2016) 34:609–33. doi: 10.1146/annurev-immunol-032712-095948
10. Lui PP, Cho I, Ali N. Tissue regulatory T cells. Immunology. (2020) 161:4–17. doi: 10.1111/imm.13208
11. Nayer B, Tan JL, Alshoubaki YK, Lu Y, Legrand JMD, Lau S, et al. Local administration of regulatory T cells promotes tissue healing. Nat Commun. (2024) 15:7863. doi: 10.1038/s41467-024-51353-2
12. Li J, Xia N, Li D, Wen S, Qian S, Lu Y, et al. Aorta regulatory T cells with a tissue-specific phenotype and function promote tissue repair through Tff1 in abdominal aortic aneurysms. Adv Sci (Weinh). (2022) 9:e2104338. doi: 10.1002/advs.202104338
13. Bansal SS, Ismahil MA, Goel M, Zhou G, Rokosh G, Hamid T, et al. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation. (2019) 139:206–21. doi: 10.1161/CIRCULATIONAHA.118.036065
14. Savage TM, Fortson KT, de-Los-Santos-Alexis K, Oliveras-Alsina A, Rouanne M, Rae SS, et al. Amphiregulin from regulatory T cells promotes liver fibrosis and insulin resistance in non-alcoholic steatohepatitis. Immunity. (2024) 57:303–18. doi: 10.1016/j.immuni.2024.01.009
15. Ikeno Y, Ohara D, Takeuchi Y, Watanabe H, Kondoh G, Taura K, et al. Foxp3+ Regulatory T cells inhibit CCl(4)-induced liver inflammation and fibrosis by regulating tissue cellular immunity. Front Immunol. (2020) 11:584048. doi: 10.3389/fimmu.2020.584048
16. Nik Tavakoli N, Hambly BD, Sullivan DR, Bao S. Forkhead box protein 3: essential immune regulatory role. Int J Biochem Cell Biol. (2008) 40:2369–73. doi: 10.1016/j.biocel.2007.10.004
17. Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol. (2015) 16:276–85. doi: 10.1038/ni.3085
18. Burton OT, Bricard O, Tareen S, Gergelits V, Andrews S, Biggins L, et al. The tissue-resident regulatory T cell pool is shaped by transient multi-tissue migration and a conserved residency program. Immunity. (2024) 57:1586–602. doi: 10.1016/j.immuni.2024.05.023
19. Munoz-Rojas AR, Mathis D. Tissue regulatory T cells: regulatory chameleons. Nat Rev Immunol. (2021) 21:597–611. doi: 10.1038/s41577-021-00519-w
20. Guo F, Hancock B, Griffith A, Lin H, Howard K, Keegan J, et al. Distinct injury responsive regulatory T cells identified by multi-dimensional phenotyping. Front Immunol. (2022) 13:833100. doi: 10.3389/fimmu.2022.833100
21. Delacher M, Imbusch CD, Weichenhan D, Breiling A, Hotz-Wagenblatt A, Trager U, et al. Genome-wide DNA-methylation landscape defines specialization of regulatory T cells in tissues. Nat Immunol. (2017) 18:1160–72. doi: 10.1038/ni.3799
22. Delacher M, Schmidl C, Herzig Y, Breloer M, Hartmann W, Brunk F, et al. Rbpj expression in regulatory T cells is critical for restraining T(H)2 responses. Nat Commun. (2019) 10:1621. doi: 10.1038/s41467-019-09276-w
23. Huehn J, Hamann A. Homing to suppress: address codes for Treg migration. Trends Immunol. (2005) 26:632–36. doi: 10.1016/j.it.2005.10.001
24. Mackay LK, Kallies A. Transcriptional regulation of tissue-resident lymphocytes. Trends Immunol. (2017) 38:94–103. doi: 10.1016/j.it.2016.11.004
25. Vanderleyden I, Fra-Bido SC, Innocentin S, Stebegg M, Okkenhaug H, Evans-Bailey N, et al. Follicular regulatory T cells can access the germinal center independently of CXCR5. Cell Rep. (2020) 30:611–19. doi: 10.1016/j.celrep.2019.12.076
26. Wei S, Kryczek I, Zou W. Regulatory T-cell compartmentalization and trafficking. Blood. (2006) 108:426–31. doi: 10.1182/blood-2006-01-0177
27. Pabbisetty SK, Rabacal W, Volanakis EJ, Parekh VV, Olivares-Villagomez D, Cendron D, et al. Peripheral tolerance can be modified by altering KLF2-regulated Treg migration. Proc Natl Acad Sci U S A. (2016) 113:E4662–70. doi: 10.1073/pnas.1605849113
28. Liu X, Liu K, Wang Y, Meng X, Wang Q, Tao S, et al. SWI/SNF chromatin remodeling factor BAF60b restrains inflammatory diseases by affecting regulatory T cell migration. Cell Rep. (2024) 43:114458. doi: 10.1016/j.celrep.2024.114458
29. Graves DT, Milovanova TN. Mucosal immunity and the FOXO1 transcription factors. Front Immunol. (2019) 10:2530. doi: 10.3389/fimmu.2019.02530
30. Ren B, Xia H, Liao Y, Zhou H, Wang Z, Shi Y, et al. Endothelial SIRPalpha signaling controls VE-cadherin endocytosis for thymic homing of progenitor cells. Elife. (2022) 11:e69219. doi: 10.7554/eLife.69219
31. Yoshie O, Matsushima K. CCR4 and its ligands: from bench to bedside. Int Immunol. (2015) 27:11–20. doi: 10.1093/intimm/dxu079
32. Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. (2007) 204:1335–47. doi: 10.1084/jem.20070081
33. Ghosh S, Roy K, Rajalingam R, Martin S, Pal C. Cytokines in the generation and function of regulatory T cell subsets in leishmaniasis. Cytokine. (2021) 147:155266. doi: 10.1016/j.cyto.2020.155266
34. Zhao L, Hu S, Davila ML, Yang J, Lin Y, Albanese JM, et al. Coordinated co-migration of CCR10(+) antibody-producing B cells with helper T cells for colonic homeostatic regulation. Mucosal Immunol. (2021) 14:420–30. doi: 10.1038/s41385-020-0333-3
35. Jin S, Wan S, Xiong R, Li Y, Dong T, Guan C. The role of regulatory T cells in vitiligo and therapeutic advances: a mini-review. Inflammation Res. (2024) 73:1311–32. doi: 10.1007/s00011-024-01900-w
36. Swaminathan G, Nguyen LP, Namkoong H, Pan J, Haileselassie Y, Patel A, et al. The aryl hydrocarbon receptor regulates expression of mucosal trafficking receptor GPR15. Mucosal Immunol. (2021) 14:852–61. doi: 10.1038/s41385-021-00390-x
37. Xiong L, Dean JW, Fu Z, Oliff KN, Bostick JW, Ye J, et al. Ahr-Foxp3-RORgammat axis controls gut homing of CD4(+) T cells by regulating GPR15. Sci Immunol. (2020) 5(48):eaaz7277. doi: 10.1126/sciimmunol.aaz7277
38. Huang M, Ke Z, Lyu M, Masarova L, Sadeghi T, Flowers CR, et al. CXCR4-enriched T regulatory cells preferentially home to bone marrow and resolve inflammation. Iscience. (2024) 27:110830. doi: 10.1016/j.isci.2024.110830
39. Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol. (2010) 176:2177–87. doi: 10.2353/ajpath.2010.090759
40. de Oliveira CE, Gasparoto TH, Pinheiro CR, Amor NG, Nogueira MRS, Kaneno R, et al. CCR5-dependent homing of T regulatory cells to the tumor microenvironment contributes to skin squamous cell carcinoma development. Mol Cancer Ther. (2017) 16:2871–80. doi: 10.1158/1535-7163.MCT-17-0341
41. Akhtar S, Sagar K, Roy A, Hote MP, Arava S, Sharma A. CCR5-mediated homing of regulatory T cells and monocytic-myeloid derived suppressor cells to dysfunctional endothelium contributes to early atherosclerosis. Immunology. (2024) 173:712–29. doi: 10.1111/imm.13859
42. Singh M, Thakur M, Mishra M, Yadav M, Vibhuti R, Menon AM, et al. Gene regulation of intracellular adhesion molecule-1 (ICAM-1): A molecule with multiple functions. Immunol Lett. (2021) 240:123–36. doi: 10.1016/j.imlet.2021.10.007
43. Klaus T, Wilson A, Fichter M, Bros M, Bopp T, Grabbe S. The role of LFA-1 for the differentiation and function of regulatory T cells-lessons learned from different transgenic mouse models. Int J Mol Sci. (2023) 24(7):6331. doi: 10.3390/ijms24076331
44. Wohler J, Bullard D, Schoeb T, Barnum S. LFA-1 is critical for regulatory T cell homeostasis and function. Mol Immunol. (2009) 46:2424–28. doi: 10.1016/j.molimm.2009.04.004
45. Gultner S, Kuhlmann T, Hesse A, Weber JP, Riemer C, Baier M, et al. Reduced Treg frequency in LFA-1-deficient mice allows enhanced T effector differentiation and pathology in EAE. Eur J Immunol. (2010) 40:3403–12. doi: 10.1002/eji.201040576
46. Yuan M, Yang Y, Li Y, Yan Z, Lin C, Chen J. Mucin-like domain of mucosal address in cell adhesion molecule-1 facilitates integrin alpha4beta7-mediated cell adhesion through electrostatic repulsion. Front Cell Dev Biol. (2020) 8:603148. doi: 10.3389/fcell.2020.603148
47. Mehta P, Gouirand V, Boda DP, Zhang J, Gearty SV, Zirak B, et al. Layilin anchors regulatory T cells in skin. J Immunol. (2021) 207:1763–75. doi: 10.4049/jimmunol.2000970
48. Nakonechnaya TO, Moltedo B, Putintseva EV, Leyn S, Bolotin DA, Britanova OV, et al. Convergence, plasticity, and tissue residence of regulatory T cell response via TCR repertoire prism. Elife. (2024) 12:RP89382. doi: 10.7554/eLife.89382
49. Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. (2014) 513:564–68. doi: 10.1038/nature13577
50. Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, et al. Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity. (2016) 44:355–67. doi: 10.1016/j.immuni.2016.01.009
51. Peligero-Cruz C, Givony T, Sebe-Pedros A, Dobes J, Kadouri N, Nevo S, et al. IL18 signaling promotes homing of mature Tregs into the thymus. Elife. (2020) 9:e58213. doi: 10.7554/eLife.58213
52. Shao Y, Yang WY, Saaoud F, Drummer CT, Sun Y, Xu K, et al. IL-35 promotes CD4+Foxp3+ Tregs and inhibits atherosclerosis via maintaining CCR5-amplified Treg-suppressive mechanisms. JCI Insight. (2021) 6(19):e152511. doi: 10.1172/jci.insight.152511
53. Raeber ME, Caspar DP, Zurbuchen Y, Guo N, Schmid J, Michler J, et al. Interleukin-2 immunotherapy reveals human regulatory T cell subsets with distinct functional and tissue-homing characteristics. Immunity. (2024) 57:2232–50. doi: 10.1016/j.immuni.2024.07.016
54. Hsu PS, Lai CL, Hu M, Santner-Nanan B, Dahlstrom JE, Lee CH, et al. IL-2 enhances gut homing potential of human naive regulatory T cells early in life. J Immunol. (2018) 200:3970–80. doi: 10.4049/jimmunol.1701533
55. Appleton BD, Palmer SA, Smith HP, Stephens LE, Major AS. Oxidized phospholipid oxPAPC alters regulatory T-cell differentiation and decreases their protective function in atherosclerosis in mice. Arterioscler Thromb Vasc Biol. (2023) 43:2119–32. doi: 10.1161/ATVBAHA.123.319674
56. Bi H, Wasnik S, Baylink DJ, Liu C, Tang X. In vivo augmentation of gut-homing regulatory T cell induction. J Vis Exp. (2020) 155:10.3791/60585. doi: 10.3791/60585
57. Van NT, Zhang K, Wigmore RM, Kennedy AI, DaSilva CR, Huang J, et al. Dietary L-Tryptophan consumption determines the number of colonic regulatory T cells and susceptibility to colitis via GPR15. Nat Commun. (2023) 14:7363. doi: 10.1038/s41467-023-43211-4
58. Ugalde V, Contreras F, Prado C, Chovar O, Espinoza A, Pacheco R. Dopaminergic signalling limits suppressive activity and gut homing of regulatory T cells upon intestinal inflammation. Mucosal Immunol. (2021) 14:652–66. doi: 10.1038/s41385-020-00354-7
59. Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. (2008) 28:546–58. doi: 10.1016/j.immuni.2008.02.017
60. Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. (2001) 194:629–44. doi: 10.1084/jem.194.5.629
61. Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. (2007) 27:635–46. doi: 10.1016/j.immuni.2007.08.014
62. Lykhopiy V, Malviya V, Humblet-Baron S, Schlenner SM. IL-2 immunotherapy for targeting regulatory T cells in autoimmunity. Genes Immun. (2023) 24:248–62. doi: 10.1038/s41435-023-00221-y
63. Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. (2007) 204:1257–65. doi: 10.1084/jem.20062512
64. Tekguc M, Wing JB, Osaki M, Long J, Sakaguchi S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc Natl Acad Sci U S A. (2021) 118(30):e2023739118. doi: 10.1073/pnas.2023739118
65. Huang C, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, et al. Role of LAG-3 in regulatory T cells. Immunity. (2004) 21:503–13. doi: 10.1016/j.immuni.2004.08.010
66. Lippens C, Duraes FV, Dubrot J, Brighouse D, Lacroix M, Irla M, et al. IDO-orchestrated crosstalk between pDCs and Tregs inhibits autoimmunity. J Autoimmun. (2016) 75:39–49. doi: 10.1016/j.jaut.2016.07.004
67. Astarita JL, Dominguez CX, Tan C, Guillen J, Pauli ML, Labastida R, et al. Treg specialization and functions beyond immune suppression. Clin Exp Immunol. (2023) 211:176–83. doi: 10.1093/cei/uxac123
68. Zhang C, Li L, Feng K, Fan D, Xue W, Lu J. [amp]]lsquo;Repair’ Treg cells in tissue injury. Cell Physiol Biochem. (2017) 43:2155–69. doi: 10.1159/000484295
69. Luznik Z, Anchouche S, Dana R, Yin J. Regulatory T cells in angiogenesis. J Immunol. (2020) 205:2557–65. doi: 10.4049/jimmunol.2000574
70. Sharma A, Rudra D. Emerging functions of regulatory T cells in tissue homeostasis. Front Immunol. (2018) 9:883. doi: 10.3389/fimmu.2018.00883
71. Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong H, Lai K, et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell. (2017) 169:1119–29. doi: 10.1016/j.cell.2017.05.002
72. Hyodo T, Ito Y, Hosono K, Uematsu S, Akira S, Majima M, et al. The role of mPGES-1 in promoting granulation tissue angiogenesis through regulatory T-cell accumulation. In Vivo. (2022) 36:2061–73. doi: 10.21873/invivo.12932
73. Horsley V, Naik S. T(regs) expand the skin stem cell niche. Dev Cell. (2017) 41:455–56. doi: 10.1016/j.devcel.2017.05.020
74. Cohen JN, Gouirand V, Macon CE, Lowe MM, Boothby IC, Moreau JM, et al. Regulatory T cells in skin mediate immune privilege of the hair follicle stem cell niche. Sci Immunol. (2024) 9:eadh152. doi: 10.1126/sciimmunol.adh0152
75. Kakiuchi M, Hirata Y, Robson SC, Fujisaki J. Transfer of stem cell niche-residential regulatory T cells prevents post-irradiation bone marrow injury. Haematologica. (2021) 106:891–93. doi: 10.3324/haematol.2019.221820
76. Jarosch S, Kohlen J, Ghimire S, Orberg ET, Hammel M, Gaag D, et al. Multimodal immune cell phenotyping in GI biopsies reveals microbiome-related T cell modulations in human GvHD. Cell Rep Med. (2023) 4:101125. doi: 10.1016/j.xcrm.2023.101125
77. Mori M, Bogdan A, Balassa T, Csabai T, Szekeres-Bartho J. The decidua-the maternal bed embracing the embryo-maintains the pregnancy. Semin Immunopathol. (2016) 38:635–49. doi: 10.1007/s00281-016-0574-0
78. Torres SV, Man K, Elmzzahi T, Malko D, Chisanga D, Liao Y, et al. Two regulatory T cell populations in the visceral adipose tissue shape systemic metabolism. Nat Immunol. (2024) 25:496–511. doi: 10.1038/s41590-024-01753-9
79. Zhang R, Xu K, Shao Y, Sun Y, Saredy J, Cutler E, et al. Tissue Treg secretomes and transcription factors shared with stem cells contribute to a Treg niche to maintain Treg-ness with 80% Innate immune pathways, and functions of immunosuppression and tissue repair. Front Immunol. (2020) 11:632239. doi: 10.3389/fimmu.2020.632239
80. Li J, Tan J, Martino MM, Lui KO. Regulatory T-cells: potential regulator of tissue repair and regeneration. Front Immunol. (2018) 9:585. doi: 10.3389/fimmu.2018.00585
81. Becker M, Joseph SS, Garcia-Carrizo F, Tom RZ, Opaleva D, Serr I, et al. Regulatory T cells require IL6 receptor alpha signaling to control skeletal muscle function and regeneration. Cell Metab. (2023) 35:1736–51. doi: 10.1016/j.cmet.2023.08.010
82. Jovisic M, Mambetsariev N, Singer BD, Morales-Nebreda L. Differential roles of regulatory T cells in acute respiratory infections. J Clin Invest. (2023) 133(14):e170505. doi: 10.1172/JCI170505
83. Knoedler S, Knoedler L, Kauke-Navarro M, Rinkevich Y, Hundeshagen G, Harhaus L, et al. Regulatory T cells in skin regeneration and wound healing. Mil Med Res. (2023) 10:49. doi: 10.1186/s40779-023-00484-6
84. Ito M, Komai K, Nakamura T, Srirat T, Yoshimura A. Tissue regulatory T cells and neural repair. Int Immunol. (2019) 31:361–69. doi: 10.1093/intimm/dxz031
85. Berasain C, Avila MA. Amphiregulin. Semin Cell Dev Biol. (2014) 28:31–41. doi: 10.1016/j.semcdb.2014.01.005
86. Dial CF, Tune MK, Doerschuk CM, Mock JR. Foxp3(+) regulatory T cell expression of keratinocyte growth factor enhances lung epithelial proliferation. Am J Respir Cell Mol Biol. (2017) 57:162–73. doi: 10.1165/rcmb.2017-0019OC
87. Kaiser KA, Loffredo LF, Santos-Alexis KDL, Ringham OR, Arpaia N. Regulation of the alveolar regenerative niche by amphiregulin-producing regulatory T cells. J Exp Med. (2023) 220(3):e20221462. doi: 10.1084/jem.20221462
88. Meulenbroeks C, van Weelden H, Schwartz C, Voehringer D, Redegeld FAM, Rutten VPMG, et al. Basophil-derived amphiregulin is essential for UVB irradiation-induced immune suppression. J Invest Dermatol. (2015) 135:222–28. doi: 10.1038/jid.2014.329
89. Tong X, Kim SH, Che L, Park J, Lee J, Kim T. Foxp3(+) Treg control allergic skin inflammation by restricting IFN-gamma-driven neutrophilic infiltration and NETosis. J Dermatol Sci. (2024) 115:2–12. doi: 10.1016/j.jdermsci.2024.05.002
90. Li L, Liu Z, Tian L, Yao S, Feng L, Lai F, et al. Single-cell proteomics delineates murine systemic immune response to blast lung injury. Commun Biol. (2024) 7:1429. doi: 10.1038/s42003-024-07151-z
91. D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. (2009) 119:2898–913. doi: 10.1172/JCI36498
92. Proto JD, Doran AC, Gusarova G, Yurdagul AJ, Sozen E, Subramanian M, et al. Regulatory T cells promote macrophage efferocytosis during inflammation resolution. Immunity. (2018) 49:666–77. doi: 10.1016/j.immuni.2018.07.015
93. Huynh MN, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. (2002) 109:41–50. doi: 10.1172/JCI11638
94. Fan X, Xu Z, Li C, Zhang H, Peng Y, He B, et al. Mesenchymal stem cells regulate type 2 innate lymphoid cells via regulatory T cells through ICOS-ICOSL interaction. Stem Cells. (2021) 39:975–87. doi: 10.1002/stem.3369
95. Ahmad S, Hatmal MM, Lambuk L, Al-Hatamleh MAI, Alshaer W, Mohamud R. The role of TNFR2(+) Tregs in COVID-19: An overview and a potential therapeutic strategy. Life Sci. (2021) 286:120063. doi: 10.1016/j.lfs.2021.120063
96. Faustino LD, Griffith JW, Rahimi RA, Nepal K, Hamilos DL, Cho JL, et al. Interleukin-33 activates regulatory T cells to suppress innate gammadelta T cell responses in the lung. Nat Immunol. (2020) 21:1371–83. doi: 10.1038/s41590-020-0785-3
97. Liu Q, Dwyer GK, Zhao Y, Li H, Mathews LR, Chakka AB, et al. IL-33-mediated IL-13 secretion by ST2+ Tregs controls inflammation after lung injury. JCI Insight. (2019) 4(6):e123919. doi: 10.1172/jci.insight.123919
98. Tagkareli S, Salagianni M, Galani I, Manioudaki M, Pavlos E, Thanopoulou K, et al. CD103 integrin identifies a high IL-10-producing FoxP3(+) regulatory T-cell population suppressing allergic airway inflammation. Allergy. (2022) 77:1150–64. doi: 10.1111/all.15144
99. D’Alessio FR, Zhong Q, Jenkins J, Moldobaeva A, Wagner EM. Lung angiogenesis requires CD4(+) forkhead homeobox protein-3(+) regulatory T cells. Am J Respir Cell Mol Biol. (2015) 52:603–10. doi: 10.1165/rcmb.2014-0278OC
100. Tan W, Zhang B, Liu X, Zhang C, Liu J, Miao Q. Interleukin-33-dependent accumulation of regulatory T cells mediates pulmonary epithelial regeneration during acute respiratory distress syndrome. Front Immunol. (2021) 12:653803. doi: 10.3389/fimmu.2021.653803
101. Mock JR, Garibaldi BT, Aggarwal NR, Jenkins J, Limjunyawong N, Singer BD, et al. Foxp3+ regulatory T cells promote lung epithelial proliferation. Mucosal Immunol. (2014) 7:1440–51. doi: 10.1038/mi.2014.33
102. Nosbaum A, Prevel N, Truong H, Mehta P, Ettinger M, Scharschmidt TC, et al. Cutting edge: regulatory T cells facilitate cutaneous wound healing. J Immunol. (2016) 196:2010–14. doi: 10.4049/jimmunol.1502139
103. Lui PP, Xu JZ, Aziz H, Sen M, Ali N. Jagged-1+ skin Tregs modulate cutaneous wound healing. Sci Rep. (2024) 14:20999. doi: 10.1038/s41598-024-71512-1
104. Yu Z, Yu Q, Xu H, Dai X, Yu Y, Cui L, et al. IL-17A promotes psoriasis-associated keratinocyte proliferation through ACT1-dependent activation of YAP-AREG axis. J Invest Dermatol. (2022) 142:2343–52. doi: 10.1016/j.jid.2022.02.016
105. Shime H, Odanaka M, Tsuiji M, Matoba T, Imai M, Yasumizu Y, et al. Proenkephalin(+) regulatory T cells expanded by ultraviolet B exposure maintain skin homeostasis with a healing function. Proc Natl Acad Sci U S A. (2020) 117:20696–705. doi: 10.1073/pnas.2000372117
106. Luan J, Truong C, Vuchkovska A, Guo W, Good J, Liu B, et al. CD80 on skin stem cells promotes local expansion of regulatory T cells upon injury to orchestrate repair within an inflammatory environment. Immunity. (2024) 57:1071–86. doi: 10.1016/j.immuni.2024.04.003
107. Weiss E, Ramos GC, Delgobo M. Myocardial-Treg crosstalk: how to tame a wolf. Front Immunol. (2022) 13:914033. doi: 10.3389/fimmu.2022.914033
108. Wang Y, Wang C, Shen L, Xu D. The role of regulatory T cells in heart repair after myocardial infarction. J Cardiovasc Transl Res. (2023) 16:590–97. doi: 10.1007/s12265-022-10290-5
109. Li J, Yang KY, Tam RCY, Chan VW, Lan HY, Hori S, et al. Regulatory T-cells regulate neonatal heart regeneration by potentiating cardiomyocyte proliferation in a paracrine manner. Theranostics. (2019) 9:4324–41. doi: 10.7150/thno.32734
110. Fung THW, Yang KY, Lui KO. An emerging role of regulatory T-cells in cardiovascular repair and regeneration. Theranostics. (2020) 10:8924–38. doi: 10.7150/thno.47118
111. Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, et al. A special population of regulatory T cells potentiates muscle repair. Cell. (2013) 155:1282–95. doi: 10.1016/j.cell.2013.10.054
112. Wang YN, Wu X, Jia TT, Feng Y, Liu SY, Xu X, et al. Effect of type 2 diabetes mellitus on mandibular bone regeneration and the expression of T helper cell 17/regulat-ory T cell-related factors in mice. Hua XI Kou Qiang Yi Xue Za Zhi. (2021) 39:642–50. doi: 10.7518/hxkq.2021.06.004
113. Cosovanu C, Neumann C. The many functions of Foxp3(+) regulatory T cells in the intestine. Front Immunol. (2020) 11:600973. doi: 10.3389/fimmu.2020.600973
114. Smigiel KS, Parks WC. Macrophages, wound healing, and fibrosis: recent insights. Curr Rheumatol Rep. (2018) 20:17. doi: 10.1007/s11926-018-0725-5
115. Wang H, Ye J, Cui L, Chu S, Chen N. Regulatory T cells in ischemic stroke. Acta Pharmacol Sin. (2022) 43:1–09. doi: 10.1038/s41401-021-00641-4
116. Neupane AS, Kubes P. Imaging reveals novel innate immune responses in lung, liver, and beyond. Immunol Rev. (2022) 306:244–57. doi: 10.1111/imr.13040
117. Haertel E, Joshi N, Hiebert P, Kopf M, Werner S. Regulatory T cells are required for normal and activin-promoted wound repair in mice. Eur J Immunol. (2018) 48:1001–13. doi: 10.1002/eji.201747395
118. Weinberg SE, Singer BD. Toward a paradigm to distinguish distinct functions of FOXP3(+) regulatory T cells. Immunohorizons. (2021) 5:944–52. doi: 10.4049/immunohorizons.2100046
119. Ma R, Prigge AD, Ortiz Serrano TP, Cheng Y, Davis JM, Lou KF, et al. Vimentin modulates regulatory T cell receptor-ligand interactions at distal pole complex, leading to dysregulated host response to viral pneumonia. Cell Rep. (2024) 43:115056. doi: 10.1016/j.celrep.2024.115056
120. Tan W, Zhang C, Liu J, Miao Q. Regulatory T-cells promote pulmonary repair by modulating T helper cell immune responses in lipopolysaccharide-induced acute respiratory distress syndrome. Immunology. (2019) 157:151–62. doi: 10.1111/imm.13060
121. Yan D, Yu F, Chen L, Yao Q, Yan C, Zhang S, et al. Subconjunctival injection of regulatory T cells potentiates corneal healing via orchestrating inflammation and tissue repair after acute alkali burn. Invest Ophthalmol Vis Sci. (2020) 61:22. doi: 10.1167/iovs.61.14.22
122. Zaiss DM, Minutti CM, Knipper JA. Immune- and non-immune-mediated roles of regulatory T-cells during wound healing. Immunology. (2019) 157:190–97. doi: 10.1111/imm.13057
123. Mock JR, Dial CF, Tune MK, Gilmore RC, O’Neal WK, Dang H, et al. Impact of regulatory T cells on type 2 alveolar epithelial cell transcriptomes during resolution of acute lung injury and contributions of IFN-gamma. Am J Respir Cell Mol Biol. (2020) 63:464–77. doi: 10.1165/rcmb.2019-0399OC
124. Wu J, Ren B, Wang D, Lin H. Regulatory T cells in skeletal muscle repair and regeneration: recent insights. Cell Death Dis. (2022) 13:680. doi: 10.1038/s41419-022-05142-8
125. Mathur AN, Zirak B, Boothby IC, Tan M, Cohen JN, Mauro TM, et al. Treg-cell control of a CXCL5-IL-17 inflammatory axis promotes hair-follicle-stem-cell differentiation during skin-barrier repair. Immunity. (2019) 50:655–67. doi: 10.1016/j.immuni.2019.02.013
126. Mata R, Yao Y, Cao W, Ding J, Zhou T, Zhai Z, et al. The dynamic inflammatory tissue microenvironment: signality and disease therapy by biomaterials. Res (Wash D C). (2021) 2021:4189516. doi: 10.34133/2021/4189516
127. Borthwick LA, Wynn TA, Fisher AJ. Cytokine mediated tissue fibrosis. Biochim Biophys Acta. (2013) 1832:1049–60. doi: 10.1016/j.bbadis.2012.09.014
128. Weiskirchen R, Weiskirchen S, Tacke F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol Aspects Med. (2019) 65:2–15. doi: 10.1016/j.mam.2018.06.003
129. Okamoto M, Kuratani A, Okuzaki D, Kamiyama N, Kobayashi T, Sasai M, et al. Tff1-expressing Tregs in lung prevent exacerbation of Bleomycin-induced pulmonary fibrosis. Front Immunol. (2024) 15:1440918. doi: 10.3389/fimmu.2024.1440918
130. Moye S, Bormann T, Maus R, Sparwasser T, Sandrock I, Prinz I, et al. Regulatory T cells limit pneumococcus-induced exacerbation of lung fibrosis in mice. J Immunol. (2020) 204:2429–38. doi: 10.4049/jimmunol.1900980
131. Seyran M, Melanie S, Philip S, Amiq G, Fabian B. Allies or enemies? The effect of regulatory T cells and related T lymphocytes on the profibrotic environment in bleomycin-injured lung mouse models. Clin Exp Med. (2023) 23:1075–88. doi: 10.1007/s10238-022-00945-7
132. Ichikawa T, Hirahara K, Kokubo K, Kiuchi M, Aoki A, Morimoto Y, et al. CD103(hi) T(reg) cells constrain lung fibrosis induced by CD103(lo) tissue-resident pathogenic CD4 T cells. Nat Immunol. (2019) 20:1469–80. doi: 10.1038/s41590-019-0494-y
133. Lo Re S, Lecocq M, Uwambayinema F, Yakoub Y, Delos M, Demoulin J, et al. Platelet-derived growth factor-producing CD4+ Foxp3+ regulatory T lymphocytes promote lung fibrosis. Am J Respir Crit Care Med. (2011) 184:1270–81. doi: 10.1164/rccm.201103-0516OC
134. Wang F, Xia H, Yao S. Regulatory T cells are a double-edged sword in pulmonary fibrosis. Int Immunopharmacol. (2020) 84:106443. doi: 10.1016/j.intimp.2020.106443
135. Garibaldi BT, D’Alessio FR, Mock JR, Files DC, Chau E, Eto Y, et al. Regulatory T cells reduce acute lung injury fibroproliferation by decreasing fibrocyte recruitment. Am J Respir Cell Mol Biol. (2013) 48:35–43. doi: 10.1165/rcmb.2012-0198OC
136. Kamio K, Azuma A, Matsuda K, Usuki J, Inomata M, Morinaga A, et al. Resolution of bleomycin-induced murine pulmonary fibrosis via a splenic lymphocyte subpopulation. Respir Res. (2018) 19:71. doi: 10.1186/s12931-018-0783-2
137. Guo T, Zou L, Ni J, Zhou Y, Ye L, Yang X, et al. Regulatory T cells: an emerging player in radiation-induced lung injury. Front Immunol. (2020) 11:1769. doi: 10.3389/fimmu.2020.01769
138. Saigusa R, Asano Y, Taniguchi T, Hirabayashi M, Nakamura K, Miura S, et al. Fli1-haploinsufficient dermal fibroblasts promote skin-localized transdifferentiation of Th2-like regulatory T cells. Arthritis Res Ther. (2018) 20:23. doi: 10.1186/s13075-018-1521-3
139. Kalekar LA, Cohen JN, Prevel N, Sandoval PM, Mathur AN, Moreau JM, et al. Regulatory T cells in skin are uniquely poised to suppress profibrotic immune responses. Sci Immunol. (2019) 4(39):eaaw2910. doi: 10.1126/sciimmunol.aaw2910
140. Mantel P, Schmidt-Weber CB. Transforming growth factor-beta: recent advances on its role in immune tolerance. Methods Mol Biol. (2011) 677:303–38. doi: 10.1007/978-1-60761-869-0_21
141. Zhang MY, Fang S, Gao H, Zhang X, Gu D, Liu Y, et al. A critical role of AREG for bleomycin-induced skin fibrosis. Cell Bioscience. (2021) 11:40. doi: 10.1186/s13578-021-00553-0
142. Frantz C, Auffray C, Avouac J, Allanore Y. Regulatory T cells in systemic sclerosis. Front Immunol. (2018) 9:2356. doi: 10.3389/fimmu.2018.02356
143. Feng M, Wang Q, Zhang F, Lu L. Ex vivo induced regulatory T cells regulate inflammatory response of Kupffer cells by TGF-beta and attenuate liver ischemia reperfusion injury. Int Immunopharmacol. (2012) 12:189–96. doi: 10.1016/j.intimp.2011.11.010
144. Langhans B, Alwan AW, Kramer B, Glassner A, Lutz P, Strassburg CP, et al. Regulatory CD4+ T cells modulate the interaction between NK cells and hepatic stellate cells by acting on either cell type. J Hepatol. (2015) 62:398–404. doi: 10.1016/j.jhep.2014.08.038
145. Sun X, Gu L, Deng W, Xu Q. Impaired balance of T helper 17/T regulatory cells in carbon tetrachloride-induced liver fibrosis in mice. World J Gastroenterol. (2014) 20:2062–70. doi: 10.3748/wjg.v20.i8.2062
146. Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. (2003) 9:331–38. doi: 10.1053/jlts.2003.50073
147. Deng Z, Zhou J, Mu X, Gu J, Li X, Shao Q, et al. Regulatory T cells improved the anti-cirrhosis activity of human amniotic mesenchymal stem cell in the liver by regulating the TGF-beta-indoleamine 2,3-dioxygenase signaling. Front Cell Dev Biol. (2021) 9:737825. doi: 10.3389/fcell.2021.737825
148. Xia N, Lu Y, Gu M, Li N, Liu M, Jiao J, et al. A unique population of regulatory T cells in heart potentiates cardiac protection from myocardial infarction. Circulation. (2020) 142:1956–73. doi: 10.1161/CIRCULATIONAHA.120.046789
149. Sun R, Zhao H, Gao DS, Ni A, Li H, Chen L, et al. Amphiregulin couples IL1RL1(+) regulatory T cells and cancer-associated fibroblasts to impede antitumor immunity. Sci Adv. (2023) 9:eadd7399. doi: 10.1126/sciadv.add7399
150. Gupta S, Adhikary S, Hui SP. Decoding the proregenerative competence of regulatory T cells through complex tissue regeneration in zebrafish. Clin Exp Immunol. (2021) 206:346–53. doi: 10.1111/cei.13661
151. Jang E, Nguyen QT, Kim S, Kim D, Le THN, Keslar K, et al. Lung-Infiltrating Foxp3(+) Regulatory T Cells Are Quantitatively and Qualitatively Different during Eosinophilic and Neutrophilic Allergic Airway Inflammation but Essential To Control the Inflammation. J Immunol. (2017) 199:3943–51. doi: 10.4049/jimmunol.1700211
152. Chai Y, Chen Y, Lin S, Xie K, Wang C, Yang Y, et al. Curcumin regulates the differentiation of naive CD4+T cells and activates IL-10 immune modulation against acute lung injury in mice. BioMed Pharmacother. (2020) 125:109946. doi: 10.1016/j.biopha.2020.109946
153. Noone PM, Reddy SP. Recent advances in dead cell clearance during acute lung injury and repair. Fac Rev. (2021) 10:33. doi: 10.12703/r/10-33
154. Zhu W, Zhang Y, Wang Y. Immunotherapy strategies and prospects for acute lung injury: Focus on immune cells and cytokines. Front Pharmacol. (2022) 13:1103309. doi: 10.3389/fphar.2022.1103309
155. Xie K, Chai Y, Lin S, Xu F, Wang C. Luteolin regulates the differentiation of regulatory T cells and activates IL-10-dependent macrophage polarization against acute lung injury. J Immunol Res. (2021) 2021:8883962. doi: 10.1155/2021/8883962
156. Jin H, Aziz M, Murao A, Kobritz M, Shih AJ, Adelson RP, et al. Antigen-presenting aged neutrophils induce CD4+ T cells to exacerbate inflammation in sepsis. J Clin Invest. (2023) 133(14):e164585. doi: 10.1172/JCI164585
157. Xu R, Jacques LC, Khandaker S, Beentjes D, Leon-Rios M, Wei X, et al. TNFR2(+) regulatory T cells protect against bacteremic pneumococcal pneumonia by suppressing IL-17A-producing gammadelta T cells in the lung. Cell Rep. (2023) 42:112054. doi: 10.1016/j.celrep.2023.112054
158. Poole JA, Nordgren TM, Heires AJ, Nelson AJ, Katafiasz D, Bailey KL, et al. Amphiregulin modulates murine lung recovery and fibroblast function following exposure to agriculture organic dust. Am J Physiol Lung Cell Mol Physiol. (2020) 318:L180–91. doi: 10.1152/ajplung.00039.2019
159. Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, et al. A distinct function of regulatory T cells in tissue protection. Cell. (2015) 162:1078–89. doi: 10.1016/j.cell.2015.08.021
160. Harb H, Benamar M, Lai PS, Contini P, Griffith JW, Crestani E, et al. Notch4 signaling limits regulatory T-cell-mediated tissue repair and promotes severe lung inflammation in viral infections. Immunity. (2021) 54:1186–99. doi: 10.1016/j.immuni.2021.04.002
161. Wang Y, Wang L, Ma S, Cheng L, Yu G. Repair and regeneration of the alveolar epithelium in lung injury. FASEB J. (2024) 38:e23612. doi: 10.1096/fj.202400088R
162. Wang S, Liang Y, Dai C. Metabolic regulation of fibroblast activation and proliferation during organ fibrosis. Kidney Dis (Basel). (2022) 8:115–25. doi: 10.1159/000522417
163. Liu M, Zeng X, Wang J, Fu Z, Wang J, Liu M, et al. Immunomodulation by mesenchymal stem cells in treating human autoimmune disease-associated lung fibrosis. Stem Cell Res Ther. (2016) 7:63. doi: 10.1186/s13287-016-0319-y
164. Unterman A, Zhao AY, Neumark N, Schupp JC, Ahangari F, Cosme CJ, et al. Single-cell profiling reveals immune aberrations in progressive idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. (2024) 210:484–96. doi: 10.1164/rccm.202306-0979OC
165. Liu T, De Los Santos FG, Phan SH. The bleomycin model of pulmonary fibrosis. Methods Mol Biol. (2017) 1627:27–42. doi: 10.1007/978-1-4939-7113-8_2
166. Boveda-Ruiz D, D’Alessandro-Gabazza CN, Toda M, Takagi T, Naito M, Matsushima Y, et al. Differential role of regulatory T cells in early and late stages of pulmonary fibrosis. Immunobiology. (2013) 218:245–54. doi: 10.1016/j.imbio.2012.05.020
167. Curioni AV, Borie R, Crestani B, Helou DG. Updates on the controversial roles of regulatory lymphoid cells in idiopathic pulmonary fibrosis. Front Immunol. (2024) 15:1466901. doi: 10.3389/fimmu.2024.1466901
168. Birjandi SZ, Palchevskiy V, Xue YY, Nunez S, Kern R, Weigt SS, et al. CD4(+)CD25(hi)Foxp3(+) cells exacerbate bleomycin-induced pulmonary fibrosis. Am J Pathol. (2016) 186:2008–20. doi: 10.1016/j.ajpath.2016.03.020
169. Takei H, Yasuoka H, Yoshimoto K, Takeuchi T. Aryl hydrocarbon receptor signals attenuate lung fibrosis in the bleomycin-induced mouse model for pulmonary fibrosis through increase of regulatory T cells. Arthritis Res Ther. (2020) 22:20. doi: 10.1186/s13075-020-2112-7
170. Moreau JM, Dhariwala MO, Gouirand V, Boda DP, Boothby IC, Lowe MM, et al. Regulatory T cells promote innate inflammation after skin barrier breach via TGF-beta activation. Sci Immunol. (2021) 6(62):eabg2329. doi: 10.1126/sciimmunol.abg2329
171. Yu Q, Yan Y, Huang J, Liang Q, Li J, Wang B, et al. A multifunctional chitosan-based hydrogel with self-healing, antibacterial, and immunomodulatory effects as wound dressing. Int J Biol Macromol. (2023) 231:123149. doi: 10.1016/j.ijbiomac.2023.123149
172. Norman MU, Chow Z, Hall P, Le AC, O’Sullivan KM, Snelgrove SL, et al. CD103 regulates dermal regulatory T cell motility and interactions with CD11c-expressing leukocytes to control skin inflammation. J Immunol. (2023) 211:551–62. doi: 10.4049/jimmunol.2200917
173. Jenkinson SE, Whawell SA, Swales BM, Corps EM, Kilshaw PJ, Farthing PM. The alphaE(CD103)beta7 integrin interacts with oral and skin keratinocytes in an E-cadherin-independent manner*. Immunology. (2011) 132:188–96. doi: 10.1111/j.1365-2567.2010.03352.x
174. Boothby IC, Cohen JN, Rosenblum MD. Regulatory T cells in skin injury: At the crossroads of tolerance and tissue repair. Sci Immunol. (2020) 5(47):eaaz9631. doi: 10.1126/sciimmunol.aaz9631
175. Li R, Li H, Yang X, Hu H, Liu P, Liu H. Crosstalk between dendritic cells and regulatory T cells: Protective effect and therapeutic potential in multiple sclerosis. Front Immunol. (2022) 13:970508. doi: 10.3389/fimmu.2022.970508
176. Tordesillas L, Lozano-Ojalvo D, Dunkin D, Mondoulet L, Agudo J, Merad M, et al. PDL2(+) CD11b(+) dermal dendritic cells capture topical antigen through hair follicles to prime LAP(+) Tregs. Nat Commun. (2018) 9:5238. doi: 10.1038/s41467-018-07716-7
177. Macaubas C, Holt PG. Regulation of cytokine production in T-cell responses to inhalant allergen:GATA-3 expression distinguishes between Th1- and Th2-polarized immunity. Int Arch Allergy Immunol. (2001) 124:176–79. doi: 10.1159/000053703
178. Connolly MK. Systemic sclerosis (scleroderma): remaining challenges. Ann Transl Med. (2021) 9:438. doi: 10.21037/atm-20-5449
179. Jinnin M. Mechanisms of skin fibrosis in systemic sclerosis. J Dermatol. (2010) 37:11–25. doi: 10.1111/j.1346-8138.2009.00738.x
180. Kalekar LA, Rosenblum MD. Regulatory T cells in inflammatory skin disease: from mice to humans. Int Immunol. (2019) 31:457–63. doi: 10.1093/intimm/dxz020
181. Antiga E, Quaglino P, Bellandi S, Volpi W, Del Bianco E, Comessatti A, et al. Regulatory T cells in the skin lesions and blood of patients with systemic sclerosis and morphoea. Br J Dermatol. (2010) 162:1056–63. doi: 10.1111/j.1365-2133.2010.09633.x
182. MacDonald KG, Dawson NAJ, Huang Q, Dunne JV, Levings MK, Broady R. Regulatory T cells produce profibrotic cytokines in the skin of patients with systemic sclerosis. J Allergy Clin Immunol. (2015) 135:946–55. doi: 10.1016/j.jaci.2014.12.1932
183. Kobayashi S, Nagafuchi Y, Shoda H, Fujio K. The pathophysiological roles of regulatory T cells in the early phase of systemic sclerosis. Front Immunol. (2022) 13:900638. doi: 10.3389/fimmu.2022.900638
184. Lu K, Tsai K, Hu W. Role of TGFbeta-producing regulatory T cells in scleroderma and end-stage organ failure. Heliyon. (2024) 10:e35590. doi: 10.1016/j.heliyon.2024.e35590
185. Lu L, Feng M, Gu J, Xia Z, Zhang H, Zheng S, et al. Restoration of intrahepatic regulatory T cells through MMP-9/13-dependent activation of TGF-beta is critical for immune homeostasis following acute liver injury. J Mol Cell Biol. (2013) 5:369–79. doi: 10.1093/jmcb/mjt042
186. Huang H, Deng Z. Adoptive transfer of regulatory T cells stimulated by Allogeneic Hepatic Stellate Cells mitigates liver injury in mice with concanavalin A-induced autoimmune hepatitis. Biochem Biophys Res Commun. (2019) 512:14–21. doi: 10.1016/j.bbrc.2019.02.147
187. Priya S, Sudhakaran PR. Cell survival, activation and apoptosis of hepatic stellate cells: modulation by extracellular matrix proteins. Hepatol Res. (2008) 38:1221–32. doi: 10.1111/j.1872-034X.2008.00394.x
188. Ezhilarasan D, Najimi M. Intercellular communication among liver cells in the perisinusoidal space of the injured liver: Pathophysiology and therapeutic directions. J Cell Physiol. (2023) 238:70–81. doi: 10.1002/jcp.30915
189. Sabir U, Gu H, Zhang D. Extracellular matrix turnover: phytochemicals target and modulate the dual role of matrix metalloproteinases (MMPs) in liver fibrosis. Phytother Res. (2023) 37:4932–62. doi: 10.1002/ptr.7959
190. Mansell E, Sigurdsson V, Deltcheva E, Brown J, James C, Miharada K, et al. Mitochondrial potentiation ameliorates age-related heterogeneity in hematopoietic stem cell function. Cell Stem Cell. (2021) 28:241–56. doi: 10.1016/j.stem.2020.09.018
191. Zhu X, Tang Z, Li W, Li X, Iwakiri Y, Liu F. S-nitrosylation of EMMPRIN influences the migration of HSCs and MMP activity in liver fibrosis. Acta Biochim Biophys Sin (Shanghai). (2023) 55:1640–49. doi: 10.3724/abbs.2023141
192. Gao B, Radaeva S. Natural killer and natural killer T cells in liver fibrosis. Biochim Biophys Acta. (2013) 1832:1061–69. doi: 10.1016/j.bbadis.2012.09.008
193. Wing K, Yamaguchi T, Sakaguchi S. Cell-autonomous and -non-autonomous roles of CTLA-4 in immune regulation. Trends Immunol. (2011) 32:428–33. doi: 10.1016/j.it.2011.06.002
194. Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. (2005) 202:1075–85. doi: 10.1084/jem.20051511
195. Lu Y, Xia N, Cheng X. Regulatory T cells in chronic heart failure. Front Immunol. (2021) 12:732794. doi: 10.3389/fimmu.2021.732794
196. Wang Y, Dembowsky K, Chevalier E, Stuve P, Korf-Klingebiel M, Lochner M, et al. C-X-C motif chemokine receptor 4 blockade promotes tissue repair after myocardial infarction by enhancing regulatory T cell mobilization and immune-regulatory function. Circulation. (2019) 139:1798–812. doi: 10.1161/CIRCULATIONAHA.118.036053
197. Zhuang R, Meng Q, Ma X, Shi S, Gong S, Liu J, et al. CD4(+)FoxP3(+)CD73(+) regulatory T cell promotes cardiac healing post-myocardial infarction. Theranostics. (2022) 12:2707–21. doi: 10.7150/thno.68437
198. Cho J, Kuswanto W, Benoist C, Mathis D. T cell receptor specificity drives accumulation of a reparative population of regulatory T cells within acutely injured skeletal muscle. Proc Natl Acad Sci U S A. (2019) 116:26727–33. doi: 10.1073/pnas.1914848116
199. Castiglioni A, Corna G, Rigamonti E, Basso V, Vezzoli M, Monno A, et al. FOXP3+ T cells recruited to sites of sterile skeletal muscle injury regulate the fate of satellite cells and guide effective tissue regeneration. PLoS One. (2015) 10:e128094. doi: 10.1371/journal.pone.0128094
200. Huang T, Huang J, Liao Z, Lan H, Jian X, Gu R, et al. Regenerating myofiber directs Tregs and Th17 responses in inflamed muscle through the intrinsic TGF-beta signaling-mediated IL-6 production. Am J Physiol Endocrinol Metab. (2022) 323:E92–106. doi: 10.1152/ajpendo.00247.2021
201. Henrot P, Blervaque L, Dupin I, Zysman M, Esteves P, Gouzi F, et al. Cellular interplay in skeletal muscle regeneration and wasting: insights from animal models. J Cachexia Sarcopenia Muscle. (2023) 14:745–57. doi: 10.1002/jcsm.13103
202. Capobianco CA, Hankenson KD, Knights AJ. Temporal dynamics of immune-stromal cell interactions in fracture healing. Front Immunol. (2024) 15:1352819. doi: 10.3389/fimmu.2024.1352819
203. Wu T, Wang L, Jian C, Zhang Z, Zeng R, Mi B, et al. A distinct “repair” role of regulatory T cells in fracture healing. Front Med. (2024) 18:516–37. doi: 10.1007/s11684-023-1024-8
204. Wang J, Jiang H, Qiu Y, Wang Y, Sun G, Zhao J. Effector memory regulatory T cells were most effective at suppressing RANKL but their frequency was downregulated in tibial fracture patients with delayed union. Immunol Lett. (2019) 209:21–7. doi: 10.1016/j.imlet.2019.03.018
205. Huang Y, Tseng W, Clanchy FIL, Topping LM, Ogbechi J, McNamee K, et al. Pharmacological modulation of T cell immunity results in long-term remission of autoimmune arthritis. Proc Natl Acad Sci U S A. (2021) 118(19):e2100939118. doi: 10.1073/pnas.2100939118
206. Rajendeeran A, Tenbrock K. Regulatory T cell function in autoimmune disease. J Transl Autoimmun. (2021) 4:100130. doi: 10.1016/j.jtauto.2021.100130
207. Kazanova A, Rudd CE. Programmed cell death 1 ligand (PD-L1) on T cells generates Treg suppression from memory. PLoS Biol. (2021) 19:e3001272. doi: 10.1371/journal.pbio.3001272
208. Liu A, Cui Q, Yang S. Induced regulatory T cells remain suppressive capability on effector T cells and synovial fibroblasts in collagen-induced arthritis. Immunol Res. (2023) 71:628–38. doi: 10.1007/s12026-023-09370-8
209. Jacobse J, Li J, Rings EHHM, Samsom JN, Goettel JA. Intestinal regulatory T cells as specialized tissue-restricted immune cells in intestinal immune homeostasis and disease. Front Immunol. (2021) 12:716499. doi: 10.3389/fimmu.2021.716499
210. Jiang Z, Wu C. Reciprocal interactions between regulatory T cells and intestinal epithelial cells. Front Immunol. (2022) 13:951339. doi: 10.3389/fimmu.2022.951339
211. Griesenauer B, Paczesny S. The ST2/IL-33 axis in immune cells during inflammatory diseases. Front Immunol. (2017) 8:475. doi: 10.3389/fimmu.2017.00475
212. Tariq M, Gallien S, Surenaud M, Wiedemann A, Jean-Louis F, Lacabaratz C, et al. Profound defect of amphiregulin secretion by regulatory T cells in the gut of HIV-treated patients. J Immunol. (2022) 208:2300–08. doi: 10.4049/jimmunol.2100725
213. Povoleri GAM, Nova-Lamperti E, Scotta C, Fanelli G, Chen Y, Becker PD, et al. Human retinoic acid-regulated CD161(+) regulatory T cells support wound repair in intestinal mucosa. Nat Immunol. (2018) 19:1403–14. doi: 10.1038/s41590-018-0230-z
214. Hanna BS, Wang G, Galvan-Pena S, Mann AO, Ramirez RN, Munoz-Rojas AR, et al. The gut microbiota promotes distal tissue regeneration via RORgamma(+) regulatory T cell emissaries. Immunity. (2023) 56:829–46. doi: 10.1016/j.immuni.2023.01.033
215. Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. (2015) 528:560–64. doi: 10.1038/nature16460
216. Zhang C, Li Y, Yu Y, Li Z, Xu X, Talifu Z, et al. Impact of inflammation and Treg cell regulation on neuropathic pain in spinal cord injury: mechanisms and therapeutic prospects. Front Immunol. (2024) 15:1334828. doi: 10.3389/fimmu.2024.1334828
Keywords: tissue regulatory T cells, tissue repair, fibrosis, immune cells, non-immune cells
Citation: Zhang P, Wang J, Miao J and Zhu P (2025) The dual role of tissue regulatory T cells in tissue repair: return to homeostasis or fibrosis. Front. Immunol. 16:1560578. doi: 10.3389/fimmu.2025.1560578
Received: 14 January 2025; Accepted: 18 February 2025;
Published: 06 March 2025.
Edited by:
Jinfang Zhu, National Institute of Allergy and Infectious Diseases (NIH), United StatesReviewed by:
Xuguang Tai, National Cancer Institute (NIH), United StatesAgnieszka Bojarska-Junak, Medical University of Lublin, Poland
Copyright © 2025 Zhang, Wang, Miao and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Zhu, emh1cGluZ0BmbW11LmVkdS5jbg==; Jinlin Miao, bWlhb2ppbmxpbkBmbW11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
‡ORCID: Peiyan Zhang, orcid.org/0009-0008-9096-9427
 Peiyan Zhang†‡
Peiyan Zhang†‡ Jiawei Wang
Jiawei Wang Ping Zhu
Ping Zhu