
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 03 March 2025
Sec. Alloimmunity and Transplantation
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1556057
This article is part of the Research TopicInnovative Approaches to Immunogenetics and Organ TransplantationView all articles
 Wai Yen Yim1†
Wai Yen Yim1† Chenghao Li1†
Chenghao Li1† Fuqiang Tong1†
Fuqiang Tong1† Jincheng Hou1
Jincheng Hou1 Yuqi Chen1
Yuqi Chen1 Zongtao Liu1
Zongtao Liu1 Zihao Wang1
Zihao Wang1 Bingchuan Geng1*
Bingchuan Geng1* Yixuan Wang1,2*
Yixuan Wang1,2* Nianguo Dong1,2*
Nianguo Dong1,2*The innate and adaptive immune systems are intricately regulated by the circadian clock machinery. Recent clinical investigations have shed light on the influence of timing in organ procurement and transplantation on graft survival. In this review, we explore various mechanisms of immunological functions associated with the steps involved in organ transplantation, spanning from surgical harvesting to reperfusion and linking to the circadian rhythm. A deeper understanding of these processes has the potential to extend the principles of chrono-immunotherapy to the realm of organ transplantation, with the aim of enhancing graft durability and improving patient outcomes. This review concludes with some perspectives on future directions in this exciting and still evolving field of research.
Organ transplantation stands as the definitive treatment for end-stage organ failure. Despite its remarkable efficacy, a pervasive challenge persists in the field—the shortage of donor organs and the efficacy of immunosuppressive regimens (1, 2). Strategies to maximize graft utilization and mitigate post-transplantation adverse effects include optimizing organ allocation systems, donor management and selection criteria, and protecting organs during retrieval and ischemia-reperfusion, have been devised (3, 4).
The circadian rhythm has garnered significant attention due to the pronounced time-of-day dependence exhibited by our immune system (5, 6). Evidence suggests that the efficacy of therapeutic interventions, such as (immune checkpoint inhibitors, sepsis strategies, vaccinations, and anti-inflammatory therapies), is influenced by circadian timing (7–9). Despite the mechanistic similarities shared by these therapeutic approaches, the role of circadian rhythm in organ transplantation has yet to be fully explored in clinical practice.
In this review, we aim to consolidate existing literature pertaining to the timing of donor graft retrieval, surgical reperfusion, and their clinical implications. Furthermore, we seek to elucidate the fundamental concepts underlying the molecular mechanisms of circadian clocks and their influence on the immune system, particularly in the context of transplant immunology. Specifically, we will delve into the role of circadian rhythms in modulating innate immune responses, the dynamics of passenger leukocytes, and the induction of immune tolerance in transplantation settings.
Numerous studies have investigated the timing of surgical procedures, including donor graft clamping (10–12) or de-clamping time (13, 14), as well as the impact of day-night effects or working/after-work hours (15–31) on recipient outcomes. In the following section, we provide a summary of the main clinical findings (Table 1) from studies examining various aspects of timing in organ transplantation, including donor graft retrieval, surgical reperfusion, and surgery start time.
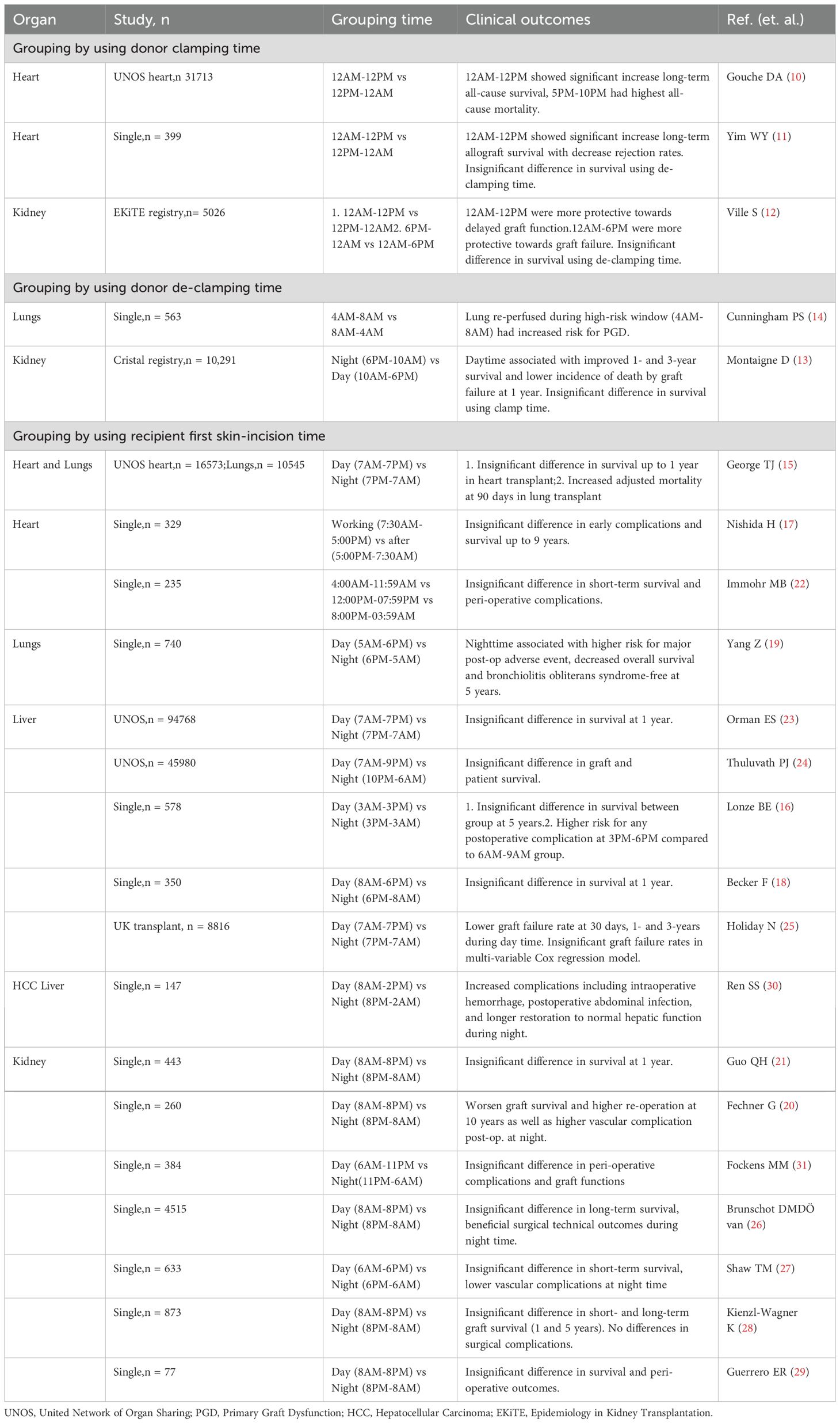
Table 1. Summary of retrospective studies using donor clamp/de-clamping and first skin-incision time (hour) to compare transplant outcome.
Organ donation occurs following stringent evaluation and selection criteria after a potential donor is declared brain dead. Subsequently, graft retrieval surgery is performed by experienced harvesting surgeons to procure different organs with obtained consent authorization. During this process, careful donor management is essential to ensure stable hemodynamics and sufficient organ perfusion. Consequently, organ retrieval can occur at any time of the day to prevent graft loss due to donor deterioration. Three studies have focused on the timing of donor graft aorta cross-clamping during organ retrieval and its impact on transplant outcomes (10–12). In a study utilizing the Epidemiology in Kidney Transplantation (EKiTE) database, donor aorta clamping between midnight-to-noon (AM) compared to noon-to-midnight (PM) was associated with superior long-term survival (>5 years) (12). This finding was consistent with a study from the United Network for Organ Sharing (UNOS) heart registry (10). In another single-center heart transplant study, with an hour-dependent increase in all-cause mortality when heart grafts were harvested between 5 PM-10 PM (11). It was demonstrated that there were more than doubled death rates after propensity score matching (PSM) and increased rejection rates when harvesting during PM. These studies primarily hypothesize a circadian-regulated immunogenicity of grafts.
Following retrieval, most grafts undergo cold or warm ischemia depending on the preservation strategy employed. Cold preservation using solutions at 4°C on ice remains the most widely used technique due to its cost-effectiveness and efficacy. Prolonged cold ischemia can lead to more severe ischemic-reperfusion injury (IRI), resulting in early graft dysfunction. Previous evidence suggests that the timing of reperfusion within a day can influence clinical outcomes (32). Analysis using the French Registry of Organ Transplantation database (Cristal registry) demonstrated superior 1-3 year graft survival for kidneys de-clamped during 6 PM-10 AM compared to 10 AM-6 PM (13). Similarly, lungs reperfused during the high-risk window (4 AM to 8 AM) showed a significantly increased risk of primary graft dysfunction (14).
As organ donation can occur at any time of the day, the timing of surgery initiation, or “first skin incision,” has been extensively studied to determine whether after-hour effects influence transplant outcomes. These studies hypothesized that surgeon’s circadian rhythms during after-hours transplants might contribute to increased medical errors and/or reduced care quality. Notably, only 3 of 18 studies reported significant differences in graft survival (15, 19, 20). Analysis from the United Network for Organ Sharing (UNOS) registry indicated that lung transplants performed at nighttime (7 PM-7 AM vs. 7 AM-7 PM) were associated with increased mortality at 90 days, but the difference was insignificant for post-operative complications and mortality at 1 year (15). However, this finding was inconsistent with another single-center cohort study of lung transplants (6 PM-5 AM vs. 5 AM-6 PM), which showed a higher risk of major post-operative adverse events, decreased 5-year survival, and a lower bronchiolitis obliterans syndrome-free rate (19). In kidney transplants, nighttime surgeries (8 PM-8 AM vs. 8 AM-8 PM) were associated with worsened graft survival at 1 year and 10 years in two different cohorts (20, 21), and were also associated with higher post-operative vascular complications and re-operation rates in one of the studies (21). No significant differences were reported in heart and liver transplants (15–18, 25).
Clinical studies examining the impact of circadian rhythms on organ transplantation outcomes have produced conflicting results. For example, some studies suggest that donor graft retrieval and reperfusion during specific times of day, such as the morning, are associated with better long-term graft survival, while others report no significant differences or even worse outcomes during certain periods. Similarly, the timing of surgery initiation has shown mixed results, with some studies indicating worse graft survival for nighttime surgeries, while others find no significant impact. These inconsistencies highlight the complexity of the relationship between circadian rhythms and transplant outcomes.
To address these conflicting results, we propose shifting the focus from merely reconciling these discrepancies to understanding the underlying mechanisms driving these variations. Specifically, we advocate for a deeper exploration of the circadian immune system, which offers a more comprehensive framework for interpreting the observed inconsistencies. By investigating the circadian regulation of immune responses, we aim to elucidate how the body’s natural rhythms can modulate the immune system’s reaction to transplanted organs. This knowledge is essential for developing evidence-based strategies that optimize graft acceptance and minimize rejection. For example, understanding the circadian variations in immune cell activity and cytokine production could inform the timing of immunosuppressive therapies to enhance their efficacy. Identifying the optimal timing for transplantation procedures based on circadian principles could also lead to improved graft survival and reduced post-transplant complications. In essence, studying the circadian immune system in transplantation is not just about resolving conflicting clinical findings; it is about uncovering fundamental biological principles that can be harnessed to improve patient outcomes.
The circadian system encompasses a central pacemaker located in the hypothalamic suprachiasmatic nucleus (SCN) and peripheral oscillators distributed throughout all organs and tissues (5). Most organisms on Earth synchronize their circadian clocks to the 24-hour solar day, with sunrise occurring around 6 AM and sunset around 6 PM across most regions. In experimental models, Zeitgeber time (ZT) serves as the standard unit of time, representing entrained time according to the light-dark cycle, with ZT0 marking the beginning of the day (light) and ZT12 marking the beginning of the night (dark). Once entrained, organisms can be isolated from environmental cues to study the endogenous free-running period of their rhythms, measured by Circadian time (CT), where CT0 marks the start of the subjective day and CT12 marks the start of the subjective night (5).
Within each cell, autonomous transcription-translation autoregulatory feedback loops form the molecular clock, generating oscillations with an almost 24-hour periodicity. Key molecular clock genes include the transcriptional activators CLOCK and BMAL1, as well as the repressor proteins known as Period (PER1, PER2, and PER3) and Cryptochrome (CRY1 and CRY2) (33). Six stages of the mammalian circadian cycle have been established based on the molecular transcription architecture (33). These stages, ranging from the poised state to the transition back into a poised state, coincide with the regulation of cell and tissue-specific functions, ultimately driving daily cyclic variations in behavioral activities such as the sleep-wake cycle, as well as systemic functions including cardiovascular, endocrine, metabolic, nervous, and digestive functions.
In recent years, advancements in understanding the circadian immune system have propelled the field of therapeutic chrono-immunology, influencing strategies such as timely delivery of vaccinations, chemotherapy, bone marrow transplantation, and treatment of diseases such as asthma, neurodegeneration, and arthritis (6). It is now recognized that the entire immune system, including innate, adaptive, and gut immunity, is regulated by the circadian clock to maintain homeostasis and execute specific immune responses in a time-dependent manner.
Various aspects of the immune system, including cellular composition, development, trafficking, and response to insults, are regulated by the circadian clock. The oscillating movement of leukocyte numbers between blood, lymphoid tissues (both peaking during the behavioral rest phase), and organ tissues occurs in a circadian manner under normal conditions (34). This rhythmic variation in leukocyte numbers is governed by circadian clock-regulated mechanisms, which control leukocyte trafficking through mobilization from the bone marrow into the blood, drainage from tissues via the lymphatic system, and homing to tissues through rhythmically expressed endothelial adhesion molecules and neutrophil clearance (34–36). Response to high doses of lipopolysaccharide (LPS), bacteria and viruses also differs at distinct time of day, resulting in more fatal conditions around the start of behavioral active phase in mice; this is associated with the circadian-mobilization of Ly6Chi monocytes, peaking between ZT0 (peak) and ZT12 (nadir) in blood and spleen (reciprocally in bone marrow) (37).
In the context of transplantation, the timing of bone marrow transplantation (BMT) into lethally irradiated mice has been shown to influence graft survival (38). Although not extensively explored in the field of organ transplantation, it is reasonable to speculate that allo-recognition of grafts by recipients is influenced by the time-of-day-dependent circadian-regulated immune system. A deeper understanding of the role of the circadian immune system in organ transplantation could uncover previously unidentified mechanisms and potentially lead to modifications in clinical practice to enhance graft durability.
Passenger leukocytes including antigen presenting cells and tissue-resident lymphocytes, encompass any immune cell components residing in the donor allograft and transferred into the recipient during transplantation. Past evidence has indicated that while circulating leukocytes are highly immunogenic, they are also pivotal in inducing graft tolerance (39, 40). This duality can be attributed to the fact that during transplantation, a highly heterogeneous population consisting of distinct cell types and states is transferred, depending on the organ. Furthermore, the composition, number, and states of immune cells residing within different organs also vary under different time and conditions (41).
Solid organ transplantation between genetically distinct individuals elicits an allo-reactive T cell response, which orchestrates cell-mediated transplant rejection (42). The activation of allo-reactive T cells relies on direct, indirect and semi-direct allo-recognition pathways mediated by antigen presenting cell (APC) (43). Nonetheless, the potency of the anti-donor response against the allograft depends on the frequency and/or number of host T cells that directly recognize allo-antigens, as well as the degree of host-derived infiltrated monocytes maturing into dendritic cells (DCs) indirectly (44). In the context of circadian biology, the rhythmic regulation of immune processes could significantly impact allo-recognition and subsequent rejection responses in transplantation. Hence, this section will explore how the circadian-regulated APC influencing the dynamics of allo-reactive T cell activation.
Donor-derived antigen-presenting cells are a major determinant of organ immunogenicity, influenced by the frequency of APCs with intact MHC molecules and their processing and/or differentiating stages during transplantation. Circadian regulation of circulating leukocyte APCs may contribute to time-of-day differences in graft survival. Under normal circumstances, professional antigen-presenting cells, including monocytes/macrophages, DCs, and B cells, exhibit rhythmic expression of intrinsic clock genes (BMAL1, CLOCK, PER1, and PER2), which can be entrained under conventional light-dark cycles (45). This suggests that the vast biological processes of these cells may be internally regulated by the central circadian machinery. Approximately 8% of genes in peritoneal macrophages are modulated by the circadian clock, covering several functional aspects, including phagocytosis, antigen presentation, immune regulation, and wound healing (46).
Subsequent studies have demonstrated that circadian clock-regulated antigen processing of DCs influences T cell responses (9, 47). Specifically, the antigen processing of DCs has been correlated with their mitochondrial BMAL1-dependent fusion-fission morphology. Clinically, greater T cell responses have been observed when mice are vaccinated during the middle of their rest phase compared to their active phase, coinciding with the highest DC antigen processing. This is consistent with the time-dependent efficacy of vaccines, which have been shown to be more effective when administered in the early morning compared to the afternoon (48, 49). Moreover, the initial time-of-day tumor engraftment has been shown to dictate tumor size in mouse cancer models (50). This phenomenon is attributed to circadian anti-tumor functions governed by both DCs and CD8+ T cells. Notably, the expression of co-stimulatory molecule CD80 by DCs, critical for generating tumor-antigen-specific CD8+ T cells, follows a circadian pattern, contributing to the time-of-day dosage-dependent tumor-killing potential. Fortin et al. demonstrated that circadian control of tumor immunosuppression significantly impacts the efficacy of immune checkpoint blockade in colorectal cancer (51). Key mechanisms include the circadian regulation of cytokine production, such as IL-2 and IFN-γ, which are essential for T cell proliferation and activation, as well as the influence of circadian rhythms on immune cell trafficking and the suppressive activity of regulatory T cells (Tregs) that secrete inhibitory cytokines like IL-10 and TGF-β. Additionally, the timing of administration of immune checkpoint inhibitors, which target molecules like PD-1 and CTLA-4, is crucial due to the circadian control of immune function. By understanding these circadian mechanisms, it may be possible to enhance the efficacy of immunosuppressive therapies in transplantation, potentially reducing graft rejection and improving patient outcomes. In clinical retrospective studies, differing outcomes have been observed when organs were harvested at distinct times of the day. Bioinformatics analyses have indicated a correlation between the expression of circadian regulators and pathways involving antigen processing and presentation in the heart (11).
Furthermore, after transplantation, donor-derived DCs (and other APC subsets) are required to migrate via graft-draining afferent lymphoid tissues to present donor MHC molecules directly to alloreactive T cells. This migration process is pivotal in driving early responses toward the graft by acute rejection, decreasing over time as donor circulating leukocytes deplete. Interestingly, the migration of DCs from the skin to lymph nodes via afferent lymphatic vessels occurs in a circadian manner (52). Various subsets of DCs preferentially migrate from the skin to lymph nodes during the rest phase of mice, regulated by the intrinsic circadian clock BMAL1 in both lymphatic endothelial cells and DCs. This migration pattern is determined by rhythmic gradients in the expression of the chemokine CCL21 and of adhesion molecules (e.g., ICAM-1 and VCAM-1).
Thus, it is speculated that mechanisms involving the circadian immune system may regulate graft immunogenicity, impacting allograft rejection according to the time of organ collection. To validate these mechanisms in transplant allo-recognition, future studies could employ murine models of heart or kidney transplantation, where donor organs are procured at different times of the day (53). Additionally, in vitro co-culture systems of donor-derived immune cells and recipient T cells could be used to assess time-of-day-dependent alloimmune responses. These approaches would help elucidate the role of circadian rhythms in graft outcomes.
Passenger Lymphocytes, in addition to the APC subsets, can also play a crucial role in priming the recipient’s adaptive allo-immunity or inducing tolerance (54). A diverse population of passenger lymphocytes has been previously studied in solid organ transplantation, each with post-transplant functional specificity. Passenger T lymphocytes including CD4+ T helper cell (TH) subsets such as TH1, TH2, TH17, T regulatory (Treg), as well as follicular T helper (TFH) cells and CD8+ T cells identified by their memory and effector functions. Organs such as the lung, liver, kidney and gut contain abundant non-migratory, tissue-resident innate-like lymphoid cells (ILC) including ILC1, ILC2 and ILC3 and tissue-resident memory T cells (TRM) (55, 56). After organ transplantation, these passenger lymphocytes may remain and proliferate in the graft, emigrate from the graft site, or be eliminated by acute rejection, while participating in the activation of the recipient allo-immunity or inducing tolerance.
Donor CD4+ T cells that evade host NK cell missing-self killing mechanism can activate the recipient’s adaptive allo-immunity and signal B cells to produce autoantibodies, leading to accelerated allograft vasculopathy (antibody-mediated rejection, AMR) (57–60). This phenomenon was further elucidated in a recent study utilizing a CD3e knockout recipient in a murine model of allogeneic heart transplantation (60). Despite lacking T cells, recipient mice exhibited a rapid, transient wave of switched alloantibodies predominantly directed against MHC I molecules (donor-specific antibodies, DSA) after transplantation, solely induced by donor T cells, thus delineating an inverted direct allo-recognition pathway.
Conversely, in another murine model of cardiac allograft vasculopathy, depletion of donor CD4+ Treg (FOXP3+ CD4+) before organ recovery resulted in markedly accelerated heart allograft rejection and augmented host effector antibody responses (61). Adoptive transfer of purified donor-strain natural Treg inhibited host humoral immunity, prolonged allograft survival, and did so more effectively than recipient Treg.
Current evidence suggests the presence of intrinsic molecular circadian clocks in both CD4+ and CD8+ subsets, regulating various functional aspects such as activation, proliferation, and differentiation capacity (9). Importantly, core clock genes participate in the differentiation of CD4+ T cells into specialized subsets such as Treg or TH17 (62, 63). However, it remains unclear whether these clock genes play a role in the time-of-day effects observed in organ transplantation. Further investigations are warranted to elucidate the potential involvement of circadian rhythms in modulating the behavior of donor lymphocytes and their impact on allorecognition and graft outcomes.
Innate lymphoid cell (ILC) represents a population of innate immune cells residing in tissue with morphology and functions resembling the CD4+ T cells but lack rearranged antigen receptors (64). Thus, ILC do not possess cytotoxic function and instead exert their functionality through robust production of cytokines and growth factors. The functional role of ILC as passenger during solid organ transplantations were not extensively studied. However, a study has shown that the proportion of ILC before and after lung transplant correlates with the occurrence of primary graft dysfunction (PGD) (65). In the study, it was found that patients without PGD exhibited significantly higher ILC1 frequencies before reperfusion, whereas the percentage of group 2 ILC (ILC2) were selectively increased after allograft perfusion, suggesting that these cells may protect against PGD. It was noted that ILC2 and ILC3 expressed high levels of circadian genes and have demonstrated an intrinsic circadian clock that can be entrained by light and are essential for maintaining immunity, and nutrient signaling and intestinal gut homeostasis (56, 66–68). The number of ILC3 cells present in the small intestinal lamina propria fluctuates according to the behavioral phases of organisms, accumulating and becoming more activated during the rest phase compared to the active phase. Similarly, graft ILCs have been replaced by substantial recipient ILCs in both the epithelium and lamina propria. However, the circadian control of ILC and their impact in solid organ transplantation remain largely unknown.
Tissue-resident memory T cells (TRM) are generated and retained in non-lymphoid tissues following site-specific infection or antigen exposure, playing a crucial role in local secondary defense through immediate cytotoxic response as well as recruitment and activation of other defense responders (69). Consequently, they are more abundant in grafts such as lungs, liver, and intestines (70). During organ transplantation, TRM cells have been shown to contribute to post-transplant outcomes. Donor TRM that migrate out of the graft can be detected in the blood for a limited time (months to years), potentially contributing to graft-versus-host disease (GvHD). Host effector T cells subsequently reject donor-derived passenger cells in both the blood and retaining tissue cells, ultimately leading to the complete replacement of the lymphocyte population. Conversely, higher persisting donor T cells have been associated with clinically protective outcomes in lung and intestinal transplantation, as evidenced by reduced rejection rates and primary graft dysfunction in lungs (55, 71). Grafts with a higher population of TRM have exhibited the lowest long-term graft survival compared to other organs such as the heart and kidney, although the underlying mechanisms remain incompletely understood. Current understanding suggests that following transplantation, distinct subsets of TRM cells may either migrate out of or be retained within the transplanted allograft. However, whether circadian rhythms impact transplant outcomes such as chimerism or GvHD through TRM remains unknown.
The intrinsic circadian clock of B lymphocytes has been less studied in the context of organ transplantation due to their low frequency in grafts (45). However, the immune system exhibits daily variations in cellular composition, trafficking, and responses to injury. For example, donor passenger leukocytes, such as dendritic cells and macrophages, may exhibit time-of-day-dependent changes in activation states or cytokine profiles, potentially influencing graft outcomes (see Table 2 for details). However, further studies are needed to uncover the potential of harnessing the circadian clock to enhance graft durability in the future.
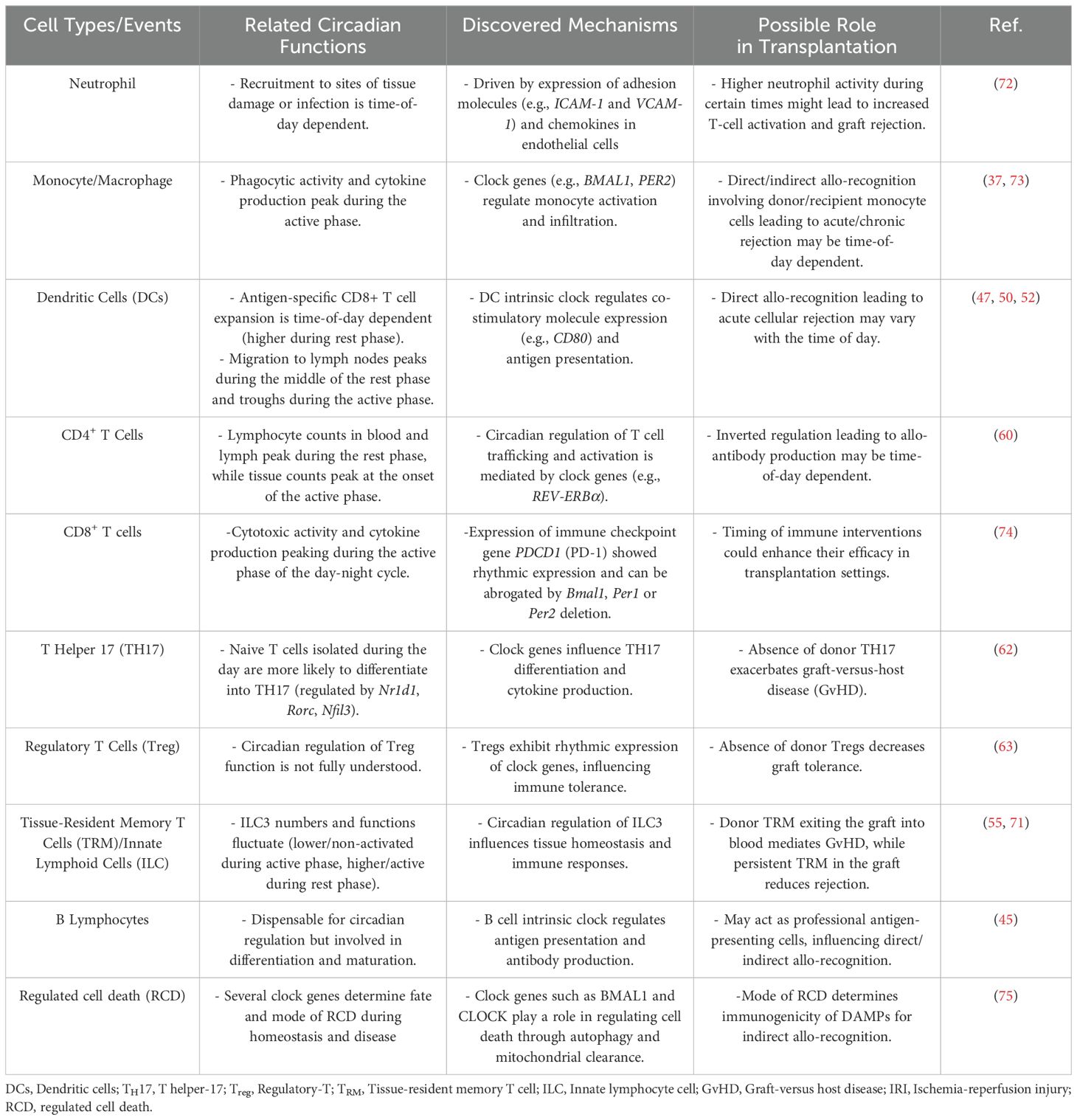
Table 2. Circadian rhythm of immune cell subsets and the role implicated in solid organ transplantation.
Organ preservation in cold preservative solutions provides protective effects by reducing cellular metabolism and maintaining osmotic pressure. However, prolonged cold ischemia in various organs can lead to increased accumulation of succinate, which upon reperfusion is rapidly re-oxidized by succinate dehydrogenase, generating extensive reactive oxidative species (ROS) through reverse electron transport at mitochondrial complex I (76, 77). It has been firmly established that tissue tolerance to IRI, especially in myocardial tissue, is strikingly time-dependent and regulated by the circadian clock (32, 75, 78, 79).
This notion is supported by several core clock genes, including PER2, which regulate oxygen sensing and metabolism within cells to mitigate the impact of ROS bursts during reperfusion (78, 79). Numerous potential therapeutic strategies exist to modify clock gene expression to enhance tissue tolerance to IRI (80, 81). These strategies include interventions targeting environmental cues, such as intense light therapy, carbon monoxide inhalation (82, 83), as well as pharmacological interventions with purinergic receptor agonists, ADORAB2 agonists, and REV-ERB agonists directly targeting clock genes (32, 79).
Severe IRI can lead to cell death, resulting in graft dysfunction due to the loss of functional cells such as tubular cells in the kidney, cardiomyocytes in the heart, and hepatocytes in the liver. Clinically, organs with higher metabolic demands, such as the heart, typically have a shorter cold ischemic time window (< 4 hours) compared to other organs like the liver or kidney (84, 85). Furthermore, the loss of cardiomyocytes can lead to irreversible injury due to their limited regenerative capacity, underscoring the importance of cardio-protection in the recovery and preservation of donor hearts (86).
Traditionally, necrosis and apoptosis have been considered the central hallmark mechanisms of IRI, with necrosis viewed as an “accidental” form of cell death and apoptosis as a programmed “suicidal” form (87). However, recent research has unveiled various subtypes of apoptosis (intrinsic, extrinsic) and regulated necrosis, as well as several novel forms of regulated cell death (RCD), including ferroptosis, necroptosis, autophagy-dependent cell death, and pyroptosis. These forms of cell death have been named according to their hallmark features and regulatory mechanisms (88–91).
Emerging evidence is gradually uncovering the roles of distinct cell death mechanisms, their occurrence stages, and consequences in transplant IRI (91–95). For example, ferroptosis can be triggered during cold stress due to upregulated mitochondrial calcium uptake regulator (MICU1) and endoplasmic reticulum stress (96), while pyroptosis is mainly described in myeloid cells, enhancing the secretion of inflammasomes and interleukins such as IL-1β and IL-18 (95, 97–99). Interestingly, circadian clock genes participate in regulating cell fates through apoptosis, necrosis, autophagy, ferroptosis, and many other forms of cell death (75, 100–102). This highlights the intricate interplay between circadian rhythms, cell death mechanisms and the activation of immune cells, suggesting potential avenues for therapeutic interventions to mitigate the detrimental effects of IRI in organ transplantation.
The release of DAMPs from stressed or dying cells represents a major source of immunogens that promote innate and adaptive immune responses (103). Several members of the DAMPs implicated in organ transplantation, such as high mobility group box 1 (HMGB1), extracellular adenosine triphosphate (eATP), and major histocompatibility complex class I chain-related proteins A and B (MICA, MICB), have been proposed (73). These DAMPs can be actively secreted by damaged allograft cells and taken up by both donor- and recipient-derived DCs, promoting immunostimulatory maturation that activates Th17 and Th1 alloreactive T cells.
While it is reasonable to speculate that time-of-day-dependent injury during IRI may result in a dose-dependent release of DAMPs mediating macrophage and/or DC maturation to enhance allograft rejection, many questions remain unexplored. For example, it was previously understood that immunogenic cell death (ICD) occurring in different scenarios may activate distinct DAMP-induced pathways and immune responses (104). Additionally, it is known that apoptotic cells that are rapidly phagocytosed can escape triggering an immune response. However, other modes of apoptosis and regulated cell deaths, such as necrosis, necroptosis, and autophagy, possess specific immunomodulatory functions, as extensively reviewed in the field of cancer (104).
However, whether time-of-day transplant IRI results in distinct forms of regulated cell death and their subsequent prognosis remains largely unexplored. Understanding the interplay between circadian rhythms, mode of regulated cell death, and the release of DAMPs could provide valuable insights into the mechanisms underlying allograft rejection and identify potential targets for therapeutic intervention in organ transplantation.
Neutrophils and monocytes/macrophages play pivotal roles in ischemia-reperfusion injury (IRI) by clearing dead cells and contributing to the inflammatory response. Recent studies reveal that these immune cells exhibit distinct responses based on their circadian rhythms, with implications for the severity of tissue damage. For instance, Sun et al. demonstrated that neutrophil infiltration in renal IRI follows a circadian pattern, suggesting that timing relative to the body’s internal clock influences the extent of damage (105). Neutrophil extracellular traps (NETs) and neutrophil-derived cytokines contribute to tissue damage during reperfusion. Given the circadian rhythmicity of neutrophil function, the timing of organ procurement and reperfusion could influence the severity of IRI (72). For example, neutrophil recruitment and NET formation might be more pronounced during certain times of the day, potentially exacerbating graft injury. Neutrophils are involved in both acute and chronic graft rejection (106). They can secrete T-cell chemo-attractants and cross-prime T cells, enhancing alloimmune responses. The circadian rhythm of neutrophils could affect their ability to initiate and propagate rejection. For instance, higher neutrophil activity during certain times might lead to increased T-cell activation and graft rejection.
Similarly, monocytes exhibit circadian rhythmicity in their functions, including migration, cytokine production, and phagocytic activity (37). The core circadian clock genes, such as BMAL1 and CLOCK, regulate the expression of clock-controlled genes that influence monocyte behavior. Monocytes show rhythmic expression of pro-inflammatory cytokines such as IL-6 and TNF-α, with peak expression occurring at specific times of the day. This rhythmicity is influenced by the circadian clock. Monocytes play a crucial role in the immune response following solid organ transplantation, particularly in IRI and graft rejection. Monocytes are rapidly recruited to the site of ischemia-reperfusion, contributing to tissue damage (73). The timing of organ procurement and reperfusion can influence the severity of IRI due to the circadian rhythmicity of monocyte recruitment and activation. Monocytes can differentiate into macrophages and dendritic cells, which are key players in initiating and propagating alloimmune responses. Dong et al. found that macrophage activation occurs in a time-dependent manner, highlighting the potential modulation of immune cell activity by circadian rhythms (107). The circadian rhythm of monocytes can affect their ability to activate T cells and contribute to graft rejection. These findings underscore the potential therapeutic value of manipulating tissue circadian rhythms to mitigate IRI-associated damage in organ transplants. Understanding and modulating these cyclic patterns could offer novel strategies for improving post-transplant outcomes and reducing complications.
Emerging research on CD8+ T cells further underscores the importance of circadian regulation in immune responses. A recent study published revealed that CD8+ T cell function is tightly regulated by circadian rhythms, with their cytotoxic activity and cytokine production peaking during the active phase of the day-night cycle (74). This rhythmicity is driven by intrinsic clock genes, which modulate metabolic pathways and effector functions in CD8+ T cells. Importantly, the study demonstrated that disrupting these circadian rhythms impairs CD8+ T cell-mediated immunity, suggesting that optimal timing of immune interventions could enhance their efficacy in transplantation settings.
These findings collectively underscore the therapeutic potential of leveraging circadian rhythms to modulate immune cell activity and mitigate IRI-associated damage in organ transplants. By understanding and manipulating these cyclic patterns, clinicians and researchers could develop novel strategies to improve post-transplant outcomes, reduce complications, and optimize immunosuppressive therapies. Future studies should explore the interplay between donor and recipient circadian rhythms, as well as the impact of circadian disruption on long-term graft survival.
Circadian rhythms—the endogenous 24-hour cycles that regulate physiological processes—play a pivotal role in immune function and transplant outcomes. Disruptions in these rhythms, whether due to organ procurement timing, recipient lifestyle, or mismatched donor-recipient cycles, can influence graft rejection, immunosuppressant pharmacokinetics, and inflammatory responses. To systematically investigate circadian interactions in transplantation, researchers must adopt standardized methodologies that account for temporal variability. Below, we outline best practices for designing and interpreting circadian-focused studies in transplant immunology, emphasizing reproducibility, mechanistic clarity, and translational relevance (see Table 3).
The optimal time for transplantation should align with the peak activity of the donor’s/recipient’s immune system. Standardizing sample collection within a 2-4-hour window during specific circadian phases (such as peak activity phases in the morning or evening) can help capture consistent data, ensuring that biological measurements reflect individualized rhythms rather than artificial or arbitrary timings. The circadian rhythm’s distinct phases suggest that individuals may have different peaks (e.g., some are most active in the morning, others in the evening), and a 2-4-hour window aligns with these key periods. This synchronization helps standardize data collection, making it easier to compare across individuals by reducing variability from external factors. The specific determination of this window likely involves empirical testing or reference to existing circadian rhythm data that identifies optimal timing for minimizing variability (108). Documentation methods, such as measuring melatonin levels or assessing sleep-wake cycles, can also provide valuable insights into individual circadian alignment, aiding in optimizing transplant timing.
Animal models, such as rodents under controlled light-dark cycles, offer a controlled environment for studying the effects of circadian rhythms on immune responses. Conditional genetic disruptions of core clock genes (e.g., Bmal1, Per2 and Nr1d1/Nr1d2) can reveal mechanistic links between circadian rhythms and immune function (109). Additionally, ex vivo systems using perfused donor organs allow isolation of tissue-specific clocks, which may differ from systemic rhythms, providing unique insights into organ-level regulation.
Advanced statistical approaches, such as cosinor analysis and mixed-effects models, are employed to identify periodic patterns in biological data and account for inter-individual variability. Replicating findings across multiple circadian cycles and validating them in different species enhances the robustness of research outcomes. This systematic approach ensures that conclusions are grounded in reliable and reproducible evidence.
In clinical trials, stratifying transplant donors and/or recipients by chronotype—assessed via validated questionnaires such as the Munich Chronotype Questionnaire—can reveal subgroups with differential susceptibility to circadian disruption (110, 111). Wearable devices (e.g., actigraphy watches) enable continuous monitoring of rest-activity cycles in patients, while telemetry in animal models tracks core body temperature or locomotor activity rhythms. Emerging technologies like single-cell RNA sequencing offer high-resolution insights into circadian gene expression within immune cell subsets. Transparent reporting of experimental conditions—including Zeitgeber Time (ZT), light intensity (lux), and synchronization protocols—is critical for reproducibility. Researchers should also disclose potential masking effects, such as anesthesia during nighttime organ procurement or timed feeding regimens.
In summary, circadian biology introduces a layer of temporal complexity to transplant immunology that, if ignored, risks obscuring critical determinants of graft survival and immune regulation. By rigorously standardizing sample timing, leveraging synchronized models, and applying rhythm-aware analytics, researchers can unravel circadian mechanisms with translational potential. Future work should prioritize bridging preclinical findings to clinical trials that test chronotherapeutic strategies, such as timed immunosuppressant delivery or donor-recipient cycle alignment. Adherence to these guidelines will enhance the validity of circadian research in transplantation and accelerate its integration into precision medicine frameworks.
While retrospective studies have provided valuable insights into the role of circadian rhythms in transplantation, several limitations must be acknowledged. First, the reliance on observational data introduces the potential for confounding by unmeasured variables. Second, experimental models often fail to fully replicate the complexity of human immune responses, limiting their translational relevance. To address these challenges, future research should prioritize prospective clinical trials and advanced in vitro models that better mimic human physiology. Additionally, the development of standardized guidelines for studying circadian rhythms in transplantation would enhance cross-study comparability and accelerate progress in this field.
Clinical studies have consistently suggested that the timing of organ procurement and transplantation may influence clinical outcomes. However, inconsistencies in findings are largely attributed to the heterogeneity of study designs, including variations in sample sizes, organ types, and methodologies. Despite these challenges, current research has identified several key mechanisms through which circadian rhythms may impact transplantation outcomes (mechanisms summarized in Figure 1):
1. Circadian-Regulated Migration and Function of Circulating Leukocyte Subsets: Emerging evidence underscores the role of circadian rhythms in governing the migration behavior and functional roles of passenger leukocyte subsets, including antigen-presenting cells and lymphocytes. Understanding how these subsets are influenced by circadian rhythms could provide insights into their contribution to allograft rejection and tolerance.
2. Ischemia-Reperfusion Injury Tolerance and Mode of Cell Death: Circadian clock mechanisms play a pivotal role in regulating tolerance to ischemia-reperfusion injury and determining the mode of cell death, which subsequently leads to the release of immunogenic DAMPs. Unraveling the interplay between circadian rhythms and these processes could shed light on novel therapeutic targets for mitigating allograft rejection and improving transplant outcomes.
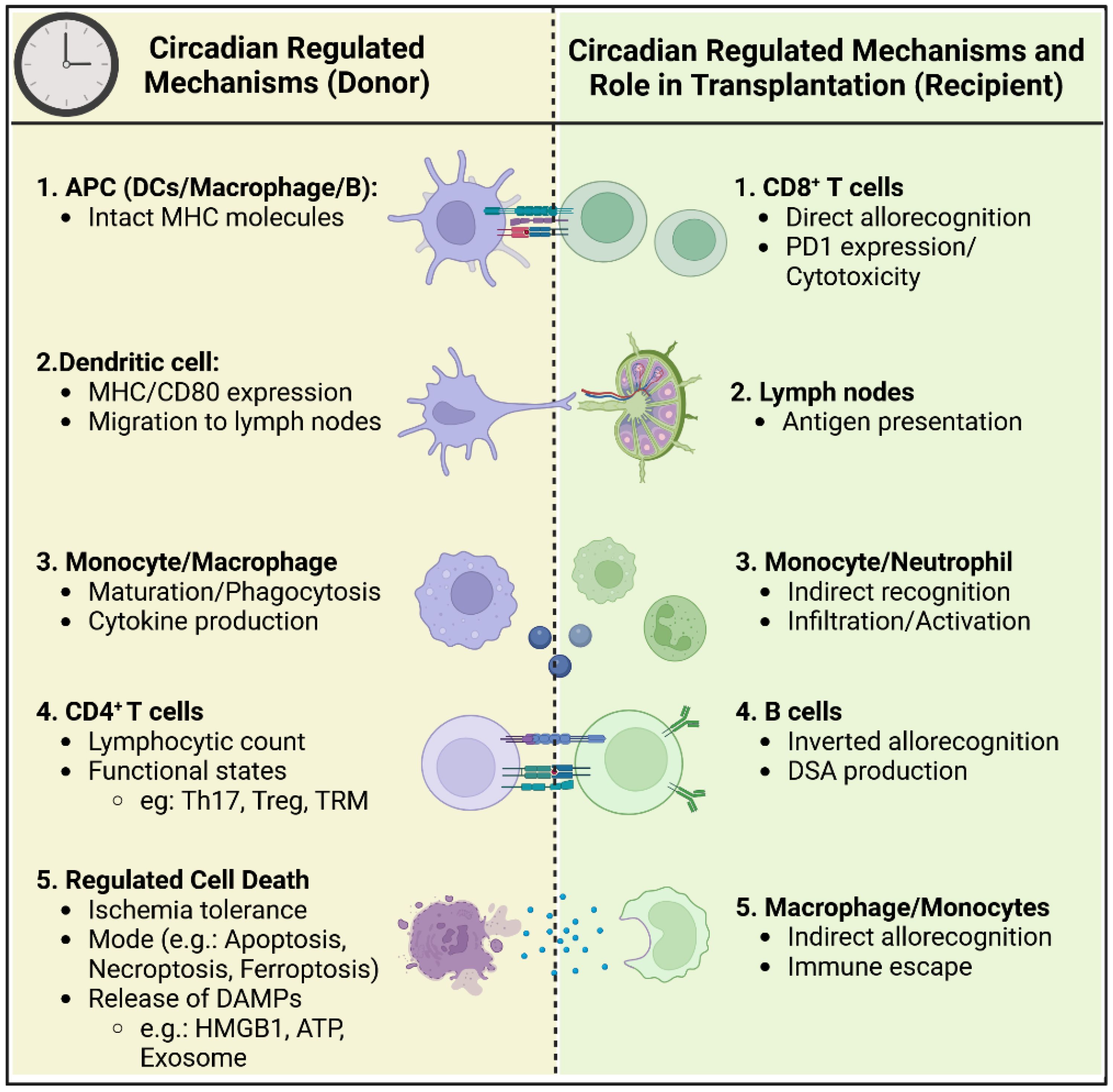
Figure 1. Circadian regulation of immune cells in donor and recipient during organ transplantation. This schematic figure illustrates the circadian-regulated mechanisms influencing the immune response in both the donor and recipient during the process of organ transplantation. The left side of the figure (Donor) details the circadian-regulated processes in antigen-presenting cells (APCs), dendritic cells, monocytes/macrophages, CD4+ T cells, and regulated cell death. The right side of the figure (Recipient) shows the corresponding circadian-regulated mechanisms and roles in CD8+ T cells, lymph nodes, monocytes/neutrophils, B cells, and macrophages/monocytes.
Despite these advances, significant gaps in knowledge remain. For instance, the relative contributions of individual circadian-regulated mechanisms to transplant outcomes are not yet fully understood. A comprehensive review of the current literature, combined with a deeper exploration of the circadian immune system, holds promise for identifying new therapeutic targets.
Developing drugs or interventions that manipulate clock genes (e.g., BMAL1, CLOCK) to optimize immune responses and reduce ischemia-reperfusion injury could enhance graft survival and patient prognosis.
Investigating whether shifting organ procurement or transplantation times aligns with the circadian rhythm of both donors and recipients to enhance graft acceptance.
Identifying biomarkers that indicate optimal timing for interventions, such as immune cell mobilization or anti-rejection therapy.
Creating personalized schedules based on individual circadian rhythms to minimize complications and improve outcomes.
Translating these findings into clinical practice will require further research, including well-designed preclinical studies and multicenter clinical trials. By addressing the identified limitations and leveraging advances in circadian rhythm research, the field has the potential to develop innovative therapeutic strategies that significantly enhance transplant success rates and patient prognoses.
WY: Conceptualization, Writing – review & editing, Writing – original draft. CL: Conceptualization, Visualization, Writing – original draft. FT: Investigation, Methodology, Writing – review & editing. JH: Methodology, Writing – review & editing. YC: Visualization, Writing – review & editing. ZL: Investigation, Writing – review & editing. ZW: Writing – review & editing. BG: Conceptualization, Writing – review & editing. YW: Conceptualization, Writing – original draft, Writing – review & editing. ND: Conceptualization, Project administration, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. National Key Research and Development Program 2106YFA0101100 (ND) National Natural Science Foundation of China 82470423, 8200701(ND) Hubei Provincial Natural Science Foundation Projects (JCZRYB202500142).
We thank Zhi Luo and Peixiang Lan for helpful suggestions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Anderson DJ, Locke JE. Progress towards solving the donor organ shortage. Nat Rev Nephrol. (2023) 19:83–4. doi: 10.1038/s41581-022-00664-y
2. Kenison JE, Stevens NA, Quintana FJ. Therapeutic induction of antigen-specific immune tolerance. Nat Rev Immunol. (2023) 24(5):338–57. doi: 10.1038/s41577-023-00970-x
3. Maciel CB, Greer DM. ICU management of the potential organ donor: state of the art. Curr Neurol Neurosci Rep. (2016) 16:86. doi: 10.1007/s11910-016-0682-1
4. Cloer CM, Givens CS, Buie LK, Rochelle LK, Lin YT, Popa S, et al. Mitochondrial transplant after ischemia reperfusion promotes cellular salvage and improves lung function during ex-vivo lung perfusion. J Heart Lung Transplant. (2023) 42:575–84. doi: 10.1016/j.healun.2023.01.002
5. Patke A, Young MW, Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol. (2020) 21:67–84. doi: 10.1038/s41580-019-0179-2
6. Wang C, Lutes LK, Barnoud C, Scheiermann C. The circadian immune system. Sci Immunol. (2022) 7:eabm2465. doi: 10.1126/sciimmunol.abm2465
7. Qian DC, Kleber T, Brammer B, Xu KM, Switchenko JM, Janopaul-Naylor JR, et al. Effect of immunotherapy time-of-day infusion on overall survival among patients with advanced melanoma in the USA (MEMOIR): a propensity score-matched analysis of a single-centre, longitudinal study. Lancet Oncol. (2021) 22:1777–86. doi: 10.1016/S1470-2045(21)00546-5
8. Deng W, Zhu S, Zeng L, Liu J, Kang R, Yang M, et al. The circadian clock controls immune checkpoint pathway in sepsis. Cell Rep. (2018) 24:366–78. doi: 10.1016/j.celrep.2018.06.026
9. Ince LM, Barnoud C, Lutes LK, Pick R, Wang C, Sinturel F, et al. Influence of circadian clocks on adaptive immunity and vaccination responses. Nat Commun. (2023) 14:476. doi: 10.1038/s41467-023-35979-2
10. Gouchoe DA, Ganapathi AM, Cui EY, Henn MC, Yim WY, Geng B, et al. Is time scheduling important? An analysis of donor heart cross-clamp times during heart transplantation. Transplant Direct. (2024) 10:e1588. doi: 10.1097/TXD.0000000000001588
11. Yim WY, Xiong T, Geng B, Xu L, Feng Y, Chi J, et al. Donor circadian clock influences the long-term survival of heart transplantation by immunoregulation. Cardiovasc Res. (2023) 119:2202–12. doi: 10.1093/cvr/cvad114
12. Ville S, Lorent M, Kerleau C, Asberg A, Legendre C, Morelon E, et al. Timing of kidney clamping and deceased donor kidney transplant outcomes. Clin J Am Soc Nephrol. (2021) 16:1704–14. doi: 10.2215/CJN.03290321
13. Montaigne D, Alhawajri N, Jacquelinet M, Coppin A, Frimat M, Bouyé S, et al. Day-time declamping is associated with better outcomes in kidney transplantation: the circarein study. J Clin Med. (2021) 10:2322. doi: 10.3390/jcm10112322
14. Cunningham PS, Maidstone R, Durrington HJ, Venkateswaran RV, Cypel M, Keshavjee S, et al. Incidence of primary graft dysfunction after lung transplantation is altered by timing of allograft implantation. Thorax. (2019) 74:413–6. doi: 10.1136/thoraxjnl-2018-212021
15. George TJ, Arnaoutakis GJ, Merlo CA, Kemp CD, Baumgartner WA, Conte JV, et al. Association of operative time of day with outcomes after thoracic organ transplant. JAMA. (2011) 305:2193–9. doi: 10.1001/jama.2011.726
16. Lonze BE, Parsikia A, Feyssa EL, Khanmoradi K, Araya VR, Zaki RF, et al. Operative start times and complications after liver transplantation. Am J Transplant. (2010) 10:1842–9. doi: 10.1111/j.1600-6143.2010.03177.x
17. Nishida H, Salerno C, Onsager D, Song T, Nguyen A, Grinstein J, et al. Comparing short-term/long-term outcomes of heart transplants that occur inside and outside of normal working hours. ESC Heart Fail. (2022) 9:2484–90. doi: 10.1002/ehf2.13947
18. Becker F, Voß T, Mohr A, Mehdorn AS, Schütte-Nütgen K, Reuter S, et al. Impact of nighttime procedures on outcomes after liver transplantation. PloS One. (2019) 14:e0220124. doi: 10.1371/journal.pone.0220124
19. Yang Z, Takahashi T, Gerull WD, Hamilton C, Subramanian MP, Liu J, et al. Impact of nighttime lung transplantation on outcomes and costs. Ann Thorac Surg. (2021) 112:206–13. doi: 10.1016/j.athoracsur.2020.07.060
20. Fechner G, Pezold C, Hauser S, Gerhardt T, Müller SC. Kidney’s nightshift, kidney’s nightmare? Comparison of daylight and nighttime kidney transplantation: impact on complications and graft survival. Transplant Proc. (2008) 40:1341–4. doi: 10.1016/j.transproceed.2008.02.072
21. Guo QH, Liu QL, Hu XJ, Li Y, Zheng J, Xue WJ. Comparison of nighttime and daytime operation on outcomes of kidney transplant with deceased donors: a retrospective analysis. Chin Med J (Engl). (2019) 132:395–404. doi: 10.1097/CM9.0000000000000056
22. Immohr MB, Mehdiani A, Oehler D, Hettlich VH, Jenkins FS, Westenfeld R, et al. Impact of circadian rhythm and daytime variation on outcome after heart transplantation. Clin Transplant. (2023) 37:e14939. doi: 10.1111/ctr.14939
23. Orman ES, Hayashi PH, Dellon ES, Gerber DA, Barritt AS. Impact of nighttime and weekend liver transplants on graft and patient outcomes. Liver Transpl. (2012) 18:558–65. doi: 10.1002/lt.23395
24. Thuluvath PJ, Amjad W, Savva Y, Thuluvath AJ, LaMattina J. Survival outcomes are not affected when liver transplant surgery is done at night, during weekends, or summer months. Transplant Direct. (2019) 5:e449. doi: 10.1097/TXD.0000000000000887
25. Halliday N, Martin K, Collett D, Allen E, Thorburn D. Is liver transplantation “out-of-hours” non-inferior to “in-hours” transplantation? A retrospective analysis of the UK Transplant Registry. BMJ Open. (2019) 9:e024917. doi: 10.1136/bmjopen-2018-024917
26. Brunschot DMDÖv, Hoitsma AJ, van der Jagt MFP, d’Ancona FC, Donders RART, van Laarhoven CJHM, et al. Nighttime kidney transplantation is associated with less pure technical graft failure. World J Urol. (2016) 34:955–61. doi: 10.1007/s00345-015-1679-0
27. Shaw TM, Lonze BE, Feyssa EL, Segev DL, May N, Parsikia A, et al. Operative start times and complications after kidney transplantation. Clin Transplant. (2012) 26:E177–183. doi: 10.1111/j.1399-0012.2012.1622.x
28. Kienzl-Wagner K, Schneiderbauer S, Bösmüller C, Schneeberger S, Pratschke J, Ollinger R. Nighttime procedures are not associated with adverse outcomes in kidney transplantation. Transpl Int. (2013) 26:879–85. doi: 10.1111/tri.12125
29. Guerrero ER, García-Baquero R, Pérez CS, Fernández-Ávila CM, Mazuecos AB, Álvarez-Ossorio JL. Nighttime kidney transplant from donor with controlled cardiac death: greater functionality at the cost of more complications? Transplant Proc. (2021) 53:2666–71. doi: 10.1016/j.transproceed.2021.07.060
30. Ren SS, Xu LL, Wang P, Schneeberger S, Pratschke J, Ollinger R. Circadian rhythms have effects on surgical outcomes of liver transplantation for patients with hepatocellular carcinoma: A retrospective analysis of 147 cases in a single center. Transplant Proc. (2019) 51:1913–9. doi: 10.1016/j.transproceed.2019.03.033
31. Fockens MM, Alberts VP, Bemelman FJ, Idu MM. Renal transplantation at night. Ned Tijdschr Geneeskd. (2014) 158:A7779.
32. Montaigne D, Marechal X, Modine T, Coisne A, Mouton S, Fayad G, et al. Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbα antagonism: a single-centre propensity-matched cohort study and a randomised study. Lancet. (2018) 391:59–69. doi: 10.1016/S0140-6736(17)32132-3
33. Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. (2017) 18:164–79. doi: 10.1038/nrg.2016.150
34. He W, Holtkamp S, Hergenhan SM, Kraus K, de Juan A, Weber J, et al. Circadian expression of migratory factors establishes lineage-specific signatures that guide the homing of leukocyte subsets to tissues. Immunity. (2018) 49:1175–1190.e7. doi: 10.1016/j.immuni.2018.10.007
35. Druzd D, Matveeva O, Ince L, Harrison U, He W, Schmal C, et al. Lymphocyte circadian clocks control lymph node trafficking and adaptive immune responses. Immunity. (2017) 46:120–32. doi: 10.1016/j.immuni.2016.12.011
36. Casanova-Acebes M, Pitaval C, Weiss LA, Weiss LA, Nombela-Arrieta C, Chèvre R, A-González N, et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. (2013) 153:1025–35. doi: 10.1016/j.cell.2013.04.040
37. Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene bmal1 regulates diurnal oscillations of ly6C hi inflammatory monocytes. Science. (2013) 341:1483–8. doi: 10.1126/science.1240636
38. Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, et al. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. (2012) 37:290–301. doi: 10.1016/j.immuni.2012.05.021
39. Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. The dichotomous functions of passenger leukocytes in solid-organ transplantation. Adv Nephrol Necker Hosp. (1995) 24:341–54.
40. Ko S, Deiwick A, Jäger MD, Dinkel A, Rohde F, Fischer R, et al. The functional relevance of passenger leukocytes and microchimerism for heart allograft acceptance in the rat. Nat Med. (1999) 5(11):1292–7. doi: 10.1038/15248
41. Domínguez Conde C, Xu C, Jarvis LB, Rainbow DB, Wells SB, Gomes T, et al. Cross-tissue immune cell analysis reveals tissue-specific features in humans. Science. (2022) 376(6594):eabl5197. doi: 10.1126/science.abl5197
42. Alegre ML, Lakkis FG, Morelli AE. Antigen presentation in transplantation. Trends Immunol. (2016) 37:831–43. doi: 10.1016/j.it.2016.09.003
43. Marino J, Babiker-Mohamed MH, Crosby-Bertorini P, Paster JT, LeGuern C, Germana S, et al. Donor exosomes rather than passenger leukocytes initiate alloreactive T cell responses after transplantation. Sci Immunol. (2016) 1:aaf8759. doi: 10.1126/sciimmunol.aaf8759
44. Abou-Daya KI, Oberbarnscheidt MH. Innate allorecognition in transplantation. J Heart Lung Transplant. (2021) 40:557–61. doi: 10.1016/j.healun.2021.03.018
45. Silver AC, Arjona A, Hughes ME, Nitabach MN, Fikrig E. Circadian expression of clock genes in mouse macrophages, dendritic cells, and B cells. Brain Behav Immun. (2012) 26:407–13. doi: 10.1016/j.bbi.2011.10.001
46. Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, et al. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A. (2009) 106:21407–12. doi: 10.1073/pnas.0906361106
47. Cervantes-Silva MP, Carroll RG, Wilk MM, Moreira D, Payet CA, O’Siorain JR, et al. The circadian clock influences T cell responses to vaccination by regulating dendritic cell antigen processing. Nat Commun. (2022) 13:7217. doi: 10.1038/s41467-022-34897-z
48. de Bree LCJ, Mourits VP, Koeken VA, Moorlag SJ, Janssen R, Folkman L, et al. Circadian rhythm influences induction of trained immunity by BCG vaccination. J Clin Invest. (2020) 130:5603–17. doi: 10.1172/JCI133934
49. Long JE, Drayson MT, Taylor AE, Toellner KM, Lord JM, Phillips AC. Morning vaccination enhances antibody response over afternoon vaccination: A cluster-randomised trial. Vaccine. (2016) 34:2679–85. doi: 10.1016/j.vaccine.2016.04.032
50. Wang C, Barnoud C, Cenerenti M, Sun M, Caffa I, Kizil B, et al. Dendritic cells direct circadian anti-tumour immune responses. Nature. (2023) 614:136–43. doi: 10.1038/s41586-022-05605-0
51. Fortin BM, Pfeiffer SM, Insua-Rodríguez J, Alshetaiwi H, Moshensky A, Song WA, et al. Circadian control of tumor immunosuppression affects efficacy of immune checkpoint blockade. Nat Immunol. (2024) 25:1257–69. doi: 10.1038/s41590-024-01859-0
52. Holtkamp SJ, Ince LM, Barnoud C, Schmitt MT, Sinturel F, Pilorz V, et al. Circadian clocks guide dendritic cells into skin lymphatics. Nat Immunol. (2021) 22:1375–81. doi: 10.1038/s41590-021-01040-x
53. Liu F, Kang SM. Heterotopic heart transplantation in mice. J Vis Exp. (2007) 6):238. doi: 10.3791/238
54. Schlitt HJ, Raddatz G, Steinhoff G, Wonigeit K, Pichlmayr R. Passenger lymphocytes in human liver allografts and their potential role after transplantation. Transplantation. (1993) 56:951–5. doi: 10.1097/00007890-199310000-00033
55. Snyder ME, Finlayson MO, Connors TJ, Dogra P, Senda T, Bush E, et al. Generation and persistence of human tissue-resident memory T cells in lung transplantation. Sci Immunol. (2019) 4:eaav5581. doi: 10.1126/sciimmunol.aav5581
56. Godinho-Silva C, Domingues RG, Rendas M, Raposo B, Ribeiro H, da Silva JA, et al. Light-entrained and brain-tuned circadian circuits regulate ILC3s and gut homeostasis. Nature. (2019) 574:254–8. doi: 10.1038/s41586-019-1579-3
57. Harper IG, Ali JM, Harper SJF, Wlodek E, Alsughayyir J, Negus MC, et al. Augmentation of recipient adaptive alloimmunity by donor passenger lymphocytes within the transplant. Cell Rep. (2016) 15:1214–27. doi: 10.1016/j.celrep.2016.04.009
58. Ali J, Harper I, Bolton E, Bradley JA, Pettigrew G. Recipient natural killer cell allorecognition of passenger donor lymphocytes and its effect on adaptive alloimmunity after transplantation. Lancet. (2015) 385 Suppl 1:S18. doi: 10.1016/S0140-6736(15)60333-6
59. Win TS, Rehakova S, Negus MC, Saeb-Parsy K, Goddard M, Conlon TM, et al. Donor CD4 T cells contribute to cardiac allograft vasculopathy by providing help for autoantibody production. Circ Heart Fail. (2009) 2:361–9. doi: 10.1161/CIRCHEARTFAILURE.108.827139
60. Charmetant X, Chen CC, Hamada S, Goncalves D, Saison C, Rabeyrin M, et al. Inverted direct allorecognition triggers early donor-specific antibody responses after transplantation. Sci Transl Med. (2022) 14:eabg1046. doi: 10.1126/scitranslmed.abg1046
61. Harper IG, Gjorgjimajkoska O, Siu JHY, Parmar J, Mulder A, Claas FHJ, et al. Prolongation of allograft survival by passenger donor regulatory T cells. Am J Transplant. (2019) 19:1371–9. doi: 10.1111/ajt.15212
62. Yu X, Rollins D, Ruhn KA, Stubblefield JJ, Green CB, Kashiwada M, et al. TH17 cell differentiation is regulated by the circadian clock. Science. (2013) 342:727–30. doi: 10.1126/science.1243884
63. Xiao T, Langston PK, Muñoz-Rojas AR, Jayewickreme T, Lazar MA, Benoist C, et al. Tregs in visceral adipose tissue up-regulate circadian-clock expression to promote fitness and enforce a diurnal rhythm of lipolysis. Sci Immunol. (2022) 7:eabl7641. doi: 10.1126/sciimmunol.abl7641
64. Klose CSN, Artis D. Innate lymphoid cells control signaling circuits to regulate tissue-specific immunity. Cell Res. (2020) 30:475–91. doi: 10.1038/s41422-020-0323-8
65. Monticelli LA, Diamond JM, Saenz SA, Tait Wojno ED, Porteous MK, Cantu E, et al. Lung innate lymphoid cell composition is altered in primary graft dysfunction. Am J Respir Crit Care Med. (2020) 201:63–72. doi: 10.1164/rccm.201906-1113OC
66. Wang Q, Colonna M. Keeping time in group 3 innate lymphoid cells. Nat Rev Immunol. (2020) 20:720–6. doi: 10.1038/s41577-020-0397-z
67. Wang Q, Robinette ML, Billon C, Collins PL, Bando JK, Fachi JL, et al. Circadian rhythm-dependent and circadian rhythm-independent impacts of the molecular clock on type 3 innate lymphoid cells. Sci Immunol. (2019) 4:eaay7501. doi: 10.1126/sciimmunol.aay7501
68. Brooks JF, Behrendt CL, Ruhn KA, Lee S, Raj P, Takahashi JS, et al. The microbiota coordinates diurnal rhythms in innate immunity with the circadian clock. Cell. (2021) 184:4154–4167.e12. doi: 10.1016/j.cell.2021.07.001
69. Yenyuwadee S, Sanchez-Trincado-Lopez JL, Shah R, Rosato PC, Boussiotis VA. The evolving role of tissue-resident memory T cells in infections and cancer. Sci Adv. (2022) 8:eabo5871. doi: 10.1126/sciadv.abo5871
70. Wang X, Tian Z, Peng H. Tissue-resident memory-like ILCs: innate counterparts of TRM cells. Protein Cell. (2020) 11:85–96. doi: 10.1007/s13238-019-0647-7
71. Zuber J, Rosen S, Shonts B, Sprangers B, Savage TM, Richman S, et al. Macrochimerism in intestinal transplantation: association with lower rejection rates and multivisceral transplants, without GVHD. Am J Transplant. (2015) 15:2691–703. doi: 10.1111/ajt.13325
72. Aroca-Crevillén A, Adrover JM, Hidalgo A. Circadian features of neutrophil biology. Front Immunol. (2020) 11:576. doi: 10.3389/fimmu.2020.00576
73. Li Q, Lan P. Activation of immune signals during organ transplantation. Signal Transduct Target Ther. (2023) 8:110. doi: 10.1038/s41392-023-01377-9
74. Wang C, Zeng Q, Gül ZM, Wang S, Pick R, Cheng P, et al. Circadian tumor infiltration and function of CD8+ T cells dictate immunotherapy efficacy. Cell. (2024) 187:2690–2702.e17. doi: 10.1016/j.cell.2024.04.015
75. Rabinovich-Nikitin I, Lieberman B, Martino TA, Kirshenbaum LA. Circadian-regulated cell death in cardiovascular diseases. Circulation. (2019) 139:965–80. doi: 10.1161/CIRCULATIONAHA.118.036550
76. Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. (2014) 515:431–5. doi: 10.1038/nature13909
77. Martin JL, Costa ASH, Gruszczyk AV, Beach TE, Allen FM, Prag HA, et al. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat Metab. (2019) 1:966–74. doi: 10.1038/s42255-019-0115-y
78. Li L, Li H, Tien CL, Jain MK, Zhang L. Kruppel-like factor 15 regulates the circadian susceptibility to ischemia reperfusion injury in the heart. Circulation. (2020) 141:1427–9. doi: 10.1161/CIRCULATIONAHA.119.041664
79. Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. (2012) 18:774–82. doi: 10.1038/nm.2728
80. Oyama Y, Walker LA, Eckle T. Targeting circadian PER2 as therapy in myocardial ischemia and reperfusion injury. Chronobiol Int. (2021) 38:1262–73. doi: 10.1080/07420528.2021.1928160
81. Ruan W, Yuan X, Eltzschig HK. Circadian rhythm as a therapeutic target. Nat Rev Drug Discovery. (2021) 20:287–307. doi: 10.1038/s41573-020-00109-w
82. Correa-Costa M, Gallo D, Csizmadia E, Gomperts E, Lieberum JL, Hauser CJ, et al. Carbon monoxide protects the kidney through the central circadian clock and CD39. Proc Natl Acad Sci U S A. (2018) 115:E2302–10. doi: 10.1073/pnas.1716747115
83. Siracusa R, Schaufler A, Calabrese V, Fuller PM, Otterbein LE. Carbon monoxide: from poison to clinical trials. Trends Pharmacol Sci. (2021) 42:329–39. doi: 10.1016/j.tips.2021.02.003
84. Saeb-Parsy K, Martin JL, Summers DM, Watson CJE, Krieg T, Murphy MP. Mitochondria as therapeutic targets in transplantation. Trends Mol Med. (2021) 27:185–98. doi: 10.1016/j.molmed.2020.08.001
85. St Peter SD, Imber CJ, Friend PJ. Liver and kidney preservation by perfusion. Lancet. (2002) 359:604–13. doi: 10.1016/S0140-6736(02)07749-8
86. Lerman JB, Agarwal R, Patel CB, Keenan JE, Casalinova S, Milano CA, et al. Donor heart recovery and preservation modalities in 2024. JACC Heart Fail. (2024) 12:427–37. doi: 10.1016/j.jchf.2023.10.012
87. Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. (2006) 43:S31–44. doi: 10.1002/hep.21062
88. Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. (2019) 29:347–64. doi: 10.1038/s41422-019-0164-5
89. Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell. (2017) 171:273–85. doi: 10.1016/j.cell.2017.09.021
90. Hadian K, Stockwell BR. The therapeutic potential of targeting regulated non-apoptotic cell death. Nat Rev Drug Discovery. (2023) 22:723–42. doi: 10.1038/s41573-023-00749-8
91. Xiang Q, Yi X, Zhu XH, Wei X, Jiang DS. Regulated cell death in myocardial ischemia–reperfusion injury. Trends Endocrinol Metab. (2024) 35:219–34. doi: 10.1016/j.tem.2023.10.010
92. Capuzzimati M, Hough O, Liu M. Cell death and ischemia-reperfusion injury in lung transplantation. J Heart Lung Transplant. (2022) 41:1003–13. doi: 10.1016/j.healun.2022.05.013
93. Linkermann A. Nonapoptotic cell death in acute kidney injury and transplantation. Kidney Int. (2016) 89:46–57. doi: 10.1016/j.kint.2015.10.008
94. Li W, Feng G, Gauthier JM, Lokshina I, Higashikubo R, Evans S, et al. Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. J Clin Invest. (2019) 129:2293–304. doi: 10.1172/JCI126428
95. Li J, Zhao J, Xu M, Li M, Wang B, Qu X, et al. Blocking GSDMD processing in innate immune cells but not in hepatocytes protects hepatic ischemia-reperfusion injury. Cell Death Dis. (2020) 11:244. doi: 10.1038/s41419-020-2437-9
96. Nakamura T, Ogawa M, Kojima K, Takayanagi S, Ishihara S, Hattori K, et al. The mitochondrial Ca2+ uptake regulator, MICU1, is involved in cold stress-induced ferroptosis. EMBO Rep. (2021) 22:e51532. doi: 10.15252/embr.202051532
97. Lucas-Ruiz F, Peñín-Franch A, Pons JA, Ramírez P, Pelegrín P, Cuevas S, et al. Emerging role of NLRP3 inflammasome and pyroptosis in liver transplantation. Int J Mol Sci. (2022) 23:14396. doi: 10.3390/ijms232214396
98. Sun P, Zhong J, Liao H, Loughran P, Mulla J, Fu G, et al. Hepatocytes are resistant to cell death from canonical and non-canonical inflammasome-activated pyroptosis. Cell Mol Gastroenterol Hepatol. (2022) 13:739–57. doi: 10.1016/j.jcmgh.2021.11.009
99. Lucas-Ruiz F, Mateo SV, Jover-Aguilar M, Alconchel F, Martínez-Alarcón L, de Torre-Minguela C, et al. Danger signals released during cold ischemia storage activate NLRP3 inflammasome in myeloid cells and influence early allograft function in liver transplantation. EBioMedicine. (2023) 87:104419. doi: 10.1016/j.ebiom.2022.104419
100. Rabinovich-Nikitin I, Rasouli M, Reitz CJ, Posen I, Margulets V, Dhingra R, et al. Mitochondrial autophagy and cell survival is regulated by the circadian Clock gene in cardiac myocytes during ischemic stress. Autophagy. (2021) 17:3794–812. doi: 10.1080/15548627.2021.1938913
101. Yang M, Chen P, Liu J, Zhu S, Kroemer G, Klionsky DJ, et al. Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci Adv. (2019) 5:eaaw2238. doi: 10.1126/sciadv.aaw2238
102. Qu M, Zhang G, Qu H, Vu A, Wu R, Tsukamoto H, et al. Circadian regulator BMAL1::CLOCK promotes cell proliferation in hepatocellular carcinoma by controlling apoptosis and cell cycle. Proc Natl Acad Sci U S A. (2023) 120:e2214829120. doi: 10.1073/pnas.2214829120
103. Land WG, Agostinis P, Gasser S, Garg AD, Linkermann A. DAMP-induced allograft and tumor rejection: the circle is closing. Am J Transplant. (2016) 16:3322–37. doi: 10.1111/ajt.14012
104. Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. (2012) 12:860–75. doi: 10.1038/nrc3380
105. Sun Q, Zeng C, Du L, Dong C. Mechanism of circadian regulation of the NRF2/ARE pathway in renal ischemia-reperfusion. Exp Ther Med. (2021) 21:190. doi: 10.3892/etm.2021.9622
106. Qu J, Jin J, Zhang M, Ng LG. Neutrophil diversity and plasticity: Implications for organ transplantation. Cell Mol Immunol. (2023) 20:993–1001. doi: 10.1038/s41423-023-01058-1
107. Dong C, Li J, Tang Q, Wang Y, Zeng C, Du L, et al. Denervation aggravates renal ischemia reperfusion injury via BMAL1-mediated Nrf2/ARE pathway. Arch Biochem Biophys. (2023) 746:109736. doi: 10.1016/j.abb.2023.109736
108. Cunningham PS, Kitchen GB, Jackson C, Papachristos S, Springthorpe T, van Dellen D, et al. ClinCirc identifies alterations of the circadian peripheral oscillator in critical care patients. J Clin Invest. (2023) 133:e162775. doi: 10.1172/JCI162775
109. Cheng B, Anea CB, Yao L, Chen F, Patel V, Merloiu A, et al. Tissue-intrinsic dysfunction of circadian clock confers transplant arteriosclerosis. Proc Natl Acad Sci U S A. (2011) 108:17147–52. doi: 10.1073/pnas.1112998108
110. Pigazzani F, Dyar KA, Morant SV, Vetter C, Rogers A, Flynn RWV, et al. Effect of timed dosing of usual antihypertensives according to patient chronotype on cardiovascular outcomes: the Chronotype sub-study cohort of the Treatment in Morning versus Evening (TIME) study. EClinicalMedicine. (2024) 72:102633. doi: 10.1016/j.eclinm.2024.102633
111. Mackenzie IS, Rogers A, Poulter NR, Williams B, Brown MJ, Webb DJ, et al. Cardiovascular outcomes in adults with hypertension with evening versus morning dosing of usual antihypertensives in the UK (TIME study): a prospective, randomised, open-label, blinded-endpoint clinical trial. Lancet. (2022) 400:1417–25. doi: 10.1016/S0140-6736(22)01786-X
Keywords: circadian rhythm, chronotherapy, transplantation, transplant immunology, antigen presentation, time-of-day, transplant surgery
Citation: Yim WY, Li C, Tong F, Hou J, Chen Y, Liu Z, Wang Z, Geng B, Wang Y and Dong N (2025) Circadian immune system in solid organ transplantation: a review article. Front. Immunol. 16:1556057. doi: 10.3389/fimmu.2025.1556057
Received: 06 January 2025; Accepted: 17 February 2025;
Published: 03 March 2025.
Edited by:
Caner Süsal, Koç Üniversitesi, TürkiyeReviewed by:
Emre Arpali, Medical College of Wisconsin, United StatesCopyright © 2025 Yim, Li, Tong, Hou, Chen, Liu, Wang, Geng, Wang and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bingchuan Geng, YmluZ2NodWFuZ2VuZ0AxNjMuY29t; Yixuan Wang, d3l4MTM1NzkxbGFiQGh1c3QuZWR1LmNu; Nianguo Dong, MTk4NlhIMDY5NEBodXN0LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.