
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 01 April 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1555790
This article is part of the Research Topic Cancer Therapy Related Organ Toxicities View all 6 articles
 Guang-Qing Shi1†
Guang-Qing Shi1† Heng-Ning Lian1†
Heng-Ning Lian1† Xue Wang2
Xue Wang2 Jie-Qiang Xia3
Jie-Qiang Xia3 Huan Wang1
Huan Wang1 Li-Jie Ma1
Li-Jie Ma1 Zhen-Liang Xiao1
Zhen-Liang Xiao1 Jing Zhou1*
Jing Zhou1*The immune checkpoint inhibitors (ICIs)-induced anti-Hu antibody-associated gastrointestinal pseudo-obstruction (GIPO) is a paraneoplastic neurological syndrome related to autoantibodies. It has a very low incidence but a high mortality rate. This report presents the case of a patient with extensive-stage small-cell lung cancer who developed recurrent bowel obstruction symptoms following ICI therapy. Colonoscopy and abdominal CT tomography failed to identify the underlying cause. A definitive diagnosis of GIPO was made based on the histological findings from an exploratory laparotomy and serum levels of paraneoplastic antibodies. Despite treatment with corticosteroids, no significant improvement was detected in the symptoms, and the patient ultimately died. This case highlights the challenges of managing this rare complication. When unexplained bowel obstruction occurs during ICI therapy, antineuronal antibody testing should be performed to exclude GIPO, as early identification and intervention can reduce mortality.
Lung cancer is the most frequently diagnosed cancer worldwide. In 2022, roughly 2.48 million new lung cancer cases were diagnosed globally, resulting in approximately 1.81 million fatalities (1). Since the approval of the first programmed death protein-1 (PD-1) inhibitor in 2014, ICIs have revolutionized lung cancer therapy, with combination regimens now achieving 5-year survival rates of 15–20% in advanced lung cancer (2). However, their expanded use has unmasked previously unrecognized immune-mediated toxicities, particularly in neurological domains. A systematic review showed that among 305,879 patients treated with ICIs, 58,291 experienced immune-related adverse events (irAEs), with an incidence rate of 19.1% (3). These adverse reactions affect almost all body systems, with immune-related neurological adverse events being relatively rare complications. The overall incidence rate of neurological irAEs is approximately 3.8% for anti-CTLA-4 therapy, 6.1% for anti-PD-1 therapy, and 12% for a combination of both therapies. The incidence rate of severe neurotoxicity was <1.0% (4), including paraneoplastic neurological syndromes (PNS), as due to its low incidence, clinical awareness is generally insufficient.
The PNS encompasses a collection of disorders linked to cancer that may affect different areas of the central or peripheral nervous system. These conditions do not result from the local effects of cancer or its spread within the nervous system; rather, they stem from immune responses triggered by cancer, primarily focusing on neuronal proteins (5). The clinical manifestations and antibody expression types in PNS are diverse. High-risk neurological phenotypes include encephalomyelitis, limbic encephalitis, rapidly progressive cerebellar syndrome, opsoclonus-myoclonus syndrome, subacute sensory neuronopathy, gastrointestinal pseudo-obstruction (GIPO), and Lambert–Eaton myasthenic syndrome (6), all of which have high mortality and morbidity rates (7). The immune responses involved encompass various antibodies, among which anti-Hu antibodies (anti-neuronal nuclear antibody type 1) represent a significant subtype of paraneoplastic anti-neuronal antibodies. These autoantibodies specifically target the Hu protein family within neuronal nuclei (8). The Hu protein family, comprising HuD, HuC, and Hel-N1, is primarily localized in neurons and neuroendocrine cells, where it participates in the regulation of mRNA stability and translation processes. The production of anti-Hu antibodies is typically associated with autoimmune reactions linked to tumors, particularly small-cell lung cancer (SCLC), wherein these antibodies attack the nervous system, leading to PNS (9). Previously, we reported a case of SCLC wherein the patient who tested positive for anti-Hu antibodies following adebrelimab treatment presented with peripheral neuropathy (10). In addition, anti-Hu antibody-associated PNS can manifest as cerebellar ataxia, limbic encephalitis, as well as GIPO. GIPO is an uncommon disorder that primarily affects the extrinsic nerves associated with the gastrointestinal tract, myenteric plexus, and pacemaker cells of Cajal (11). Clinically, it presents with recurrent abdominal pain, bloating, constipation, and/or vomiting, as well as abnormal gastric emptying or increased small intestinal pressure, but with no evidence of mechanical obstruction (12). Serplulimab is an Fc-engineered humanized IgG4κ monoclonal antibody that selectively targets the PD-1 receptor with high affinity. By effectively blocking the immunosuppressive interaction between PD-1 and its ligands PD-L1/PD-L2, this therapeutic agent restores T-cell-mediated antitumor activity (13). This report presents a case of serplulimab-induced anti-Hu antibody-associated GIPO to increase awareness of ICI-induced gastrointestinal-related PNS.
A 54-year-old male patient presented with facial and dorsal hand edema in July 2023. He was diagnosed with SCLC with hilar, mediastinal lymph node, and pleural metastases (cT1N3M1a, extensive stage) and a PS score of 1. Chemotherapy commenced on August 3, 2023, with four cycles of intravenous etoposide 160 mg (days 1–3), carboplatin (400 mg) on day 1, and serplulimab 300 mg on day 1 (Q3W). Subsequently, the patient received serplulimab maintenance therapy (Q3W). Follow-up evaluations after 18 cycles showed partial response (PR). However, after two cycles of serplulimab treatment, the patient intermittently (every 2–3 months) experienced hard stools and occasional constipation. These symptoms were not considered significant at the time and were relieved with glycerin suppositories. However, after completing 18 cycles (September 9, 2024), he was admitted to our gastroenterology department due to “no bowel movement for 5 d.” Abdominal computed tomography (CT) revealed diffuse dilatation and gas accumulation in the bowel, particularly the colon, suggesting incomplete intestinal obstruction (Figure 1). Gastroscopy showed chronic atrophic gastritis (C2) with bile reflux, and colonoscopy revealed a subpedunculated polyp approximately 1.5 cm in size, 20 cm from the anus, with smooth mucosa in the sigmoid colon. The scope reached the descending colon at 40 cm, where the mucosa and colonic haustra were smooth; however, due to severe pain and copious dry feces, the procedure was terminated. Despite multiple treatments with enemas and medications, the patient continued to experience an inability to defecate; in over 2 months, he lost 20 kg in weight. On November 12, 2024, the patient underwent laparoscopic exploration and mesenteric lymph node biopsy under general anesthesia. Intraoperative findings included significant bowel dilation and gas accumulation with no evidence of small bowel tumors or inflammatory diseases. The colon and rectum appeared normal, with slight swelling of the small bowel mesentery and numerous enlarged lymph nodes (Figure 2). Pathological examination of the lymph nodes revealed reactive hyperplasia (Figure 3), suggesting a functional gastrointestinal disorder. Therefore, ICI-related GIPO was suspected. Testing for antibodies related to serum paraneoplastic syndrome using the CAN and TBA methods, we detected anti-Hu antibodies at a dilution of 1:1000. However, tests for other antibodies against Ri, CV2, Amphiphysin, Ma1, Ma2, SOX1, DNER, Zic4, Titin, Recoverin, PKC, GAD65, and Yo were negative. The family chose not to proceed with lumbar puncture for cerebrospinal fluid antibody testing. Thus, the patient was diagnosed with anti-Hu antibody-related GIPO induced by serplulimab. Following this diagnosis, serplulimab was discontinued, and the patient received intravenous methylprednisolone sodium succinate at a dosage of 40 mg daily for 5 d. No symptom relief was observed, and the family subsequently declined high-dose steroid pulse therapy (1000 mg). The patient died 22 d later, and an autopsy was not performed at the family’s request.
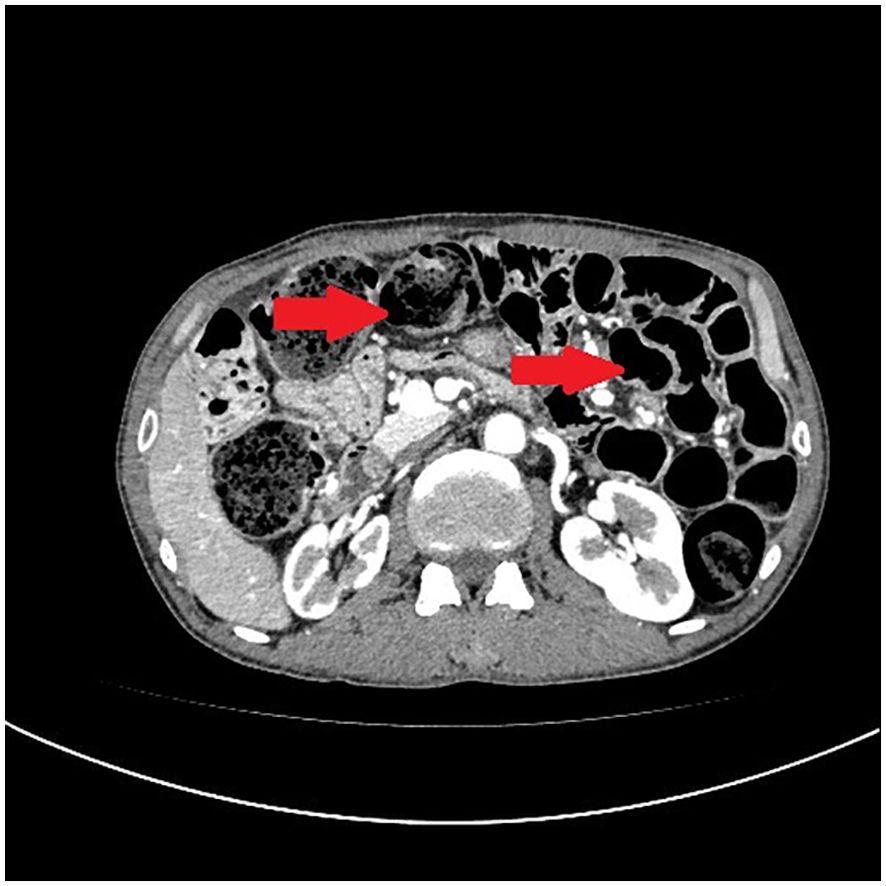
Figure 1. Abdominal computed tomography (CT) reveals diffuse dilatation and gas accumulation in the bowel, particularly the colon, suggesting incomplete intestinal obstruction.
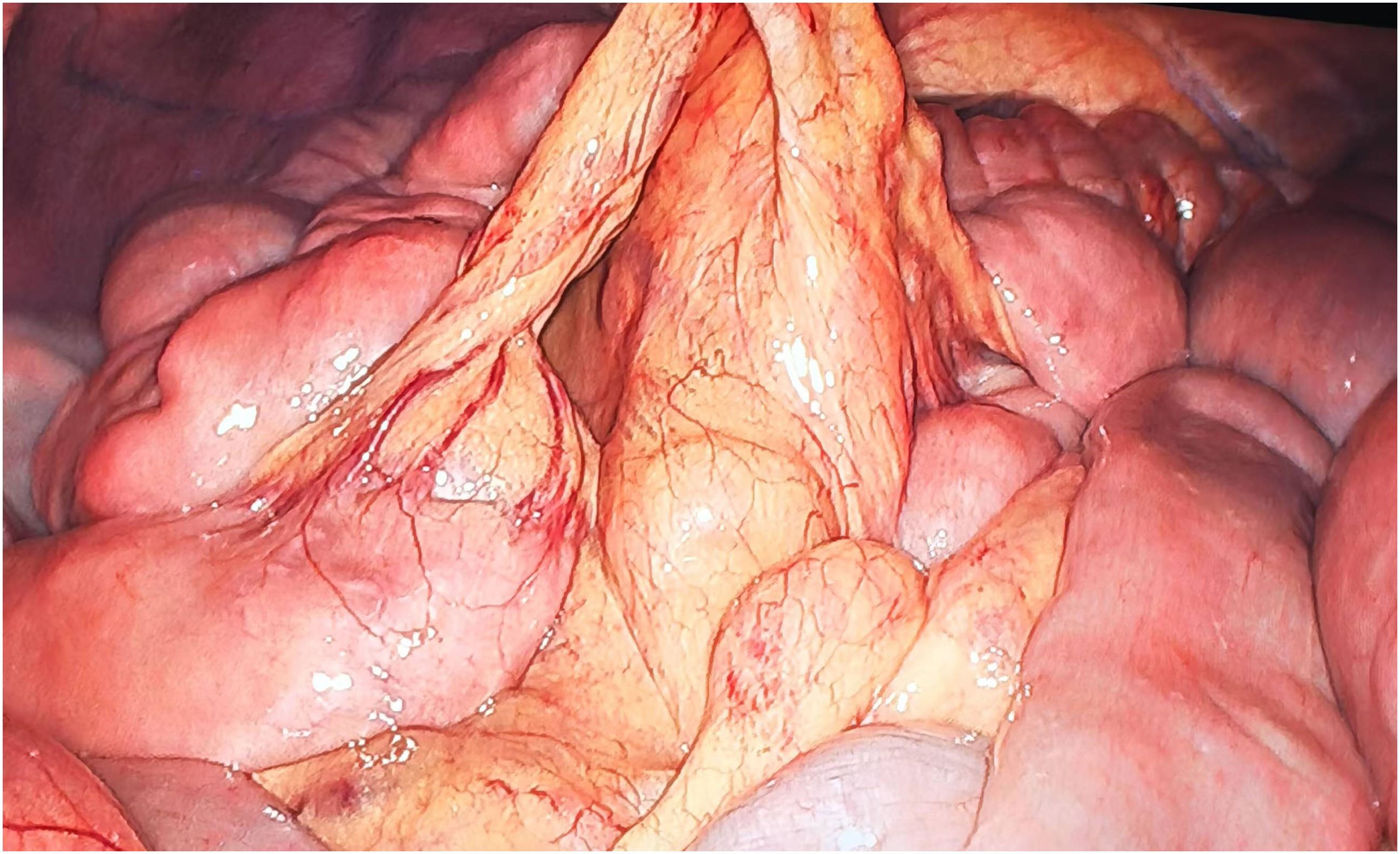
Figure 2. Intraoperative findings show significant bowel dilation and gas accumulation with no evidence of small bowel tumors or inflammatory diseases. The colon and rectum appear normal, with slight swelling of the small bowel mesentery and numerous enlarged lymph nodes.
Adverse events and toxicities related to ICIs are referred to as irAEs (14). Diarrhea and colitis are common gastrointestinal irAEs with an incidence rate of approximately 13.8%. They are easily identifiable and can be promptly managed in clinical practice (15). However, irAEs presenting as bowel obstruction are rare and can be misdiagnosed as symptoms of tumor metastasis or cachexia-induced digestive issues (16). In this case, GIPO was diagnosed in the absence of evidence of mechanical or vascular obstruction. GIPO diagnosis in patients with cancer requires excluding chemotherapy-related ileus, radiation-induced intestinal paralysis, infectious enteritis, peritoneal metastasis, and carcinomatous peritonitis, then paraneoplastic syndrome antibody testing must be performed clarify the diagnosis further (17). GIPO results from neuromuscular dysfunction of the gastrointestinal tract, which can be caused by neural inhibition, toxin stimulation, or smooth muscle pathology in the intestinal wall, leading to motility disorders. In cancer, GIPO is primarily associated with PNS in patients with lung cancer, neuroblastoma, and neuroendocrine tumors and is often linked to anti-Hu positivity. Most previous reports on GIPO in ICI-treated patients with cancer do not include antibody testing due to insufficient disease comprehension (18–22). Only four studies reported anti-Hu antibody testing, two each with positive and negative results (Table 1) (23–26). This suggests that when ICI-treated patients with cancer develop neurological symptoms, autoantibody testing and a comprehensive PNS assessment should be performed to confirm the diagnosis. According to the PNS diagnostic criteria proposed by Graus et al. (6), a scoring system (PNS-Care Score) has been designed based on the patient’s clinical phenotype, presence of neuronal antibodies, and association with cancer. A score of ≥8, along with excluding other possible diseases, confirms PNS diagnosis.
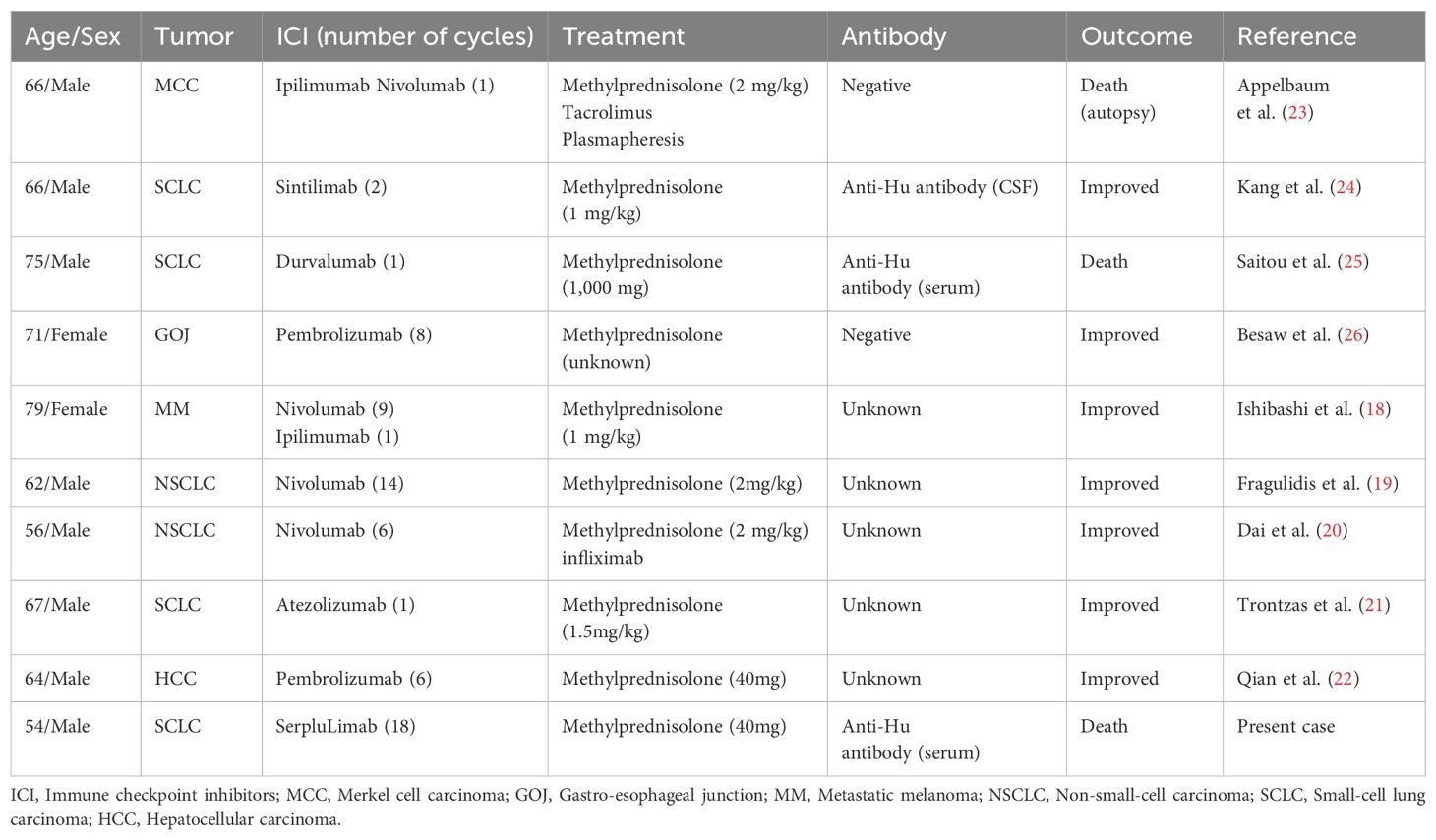
Table 1. Literature review of immune checkpoint inhibitor-induced gastrointestinal pseudo-obstruction.
The challenge in GIPO diagnosis is further highlighted in epidemiologic data. An Italian epidemiological study conducted between 2009 and 2017 showed that none of the 89 patients with PNS presented with GIPO (27). Similarly, among 264 patients with SCLC in the UK, 24 developed PNS between 2005 and 2010; however, none had GIPO (28). To the best of our knowledge, only two reported cases of ICI-induced anti-Hu-associated GIPO have been documented (24, 25). In the present study, three instances were reported, of which, all developed during SCLC treatment, which may be related to the higher proportion of patients with SCLC-developing PNS (29). In known cases of Hu-Ab-associated PNS, the condition typically progresses rapidly over weeks to months, leading to a poor prognosis, with more than half of the patients becoming bedridden or requiring a wheelchair and only 5–7% showing improvement, with a median survival time is less than a year (30, 31). Reports have suggested that age, cancer-related frailty due to advanced disease, and severe neurological manifestations are associated with Hu-Ab syndrome-related mortality following ICI treatment (30). Unlike other neurological irAEs, Hu-Ab-positive patients often show no symptom relief even after discontinuing the ICI and receiving immunosuppressive treatment, suggesting a unique immunopathological mechanism that urgently requires elucidation (32). The clinical features and treatment course, in this case, provide key clues for understanding ICI-related GIPO. Gastrointestinal endoscopy and abdominal exploration were performed to eliminate other causes. The patient had a high anti-Hu antibody titer and PNS-Care score of 10 points (≥8 points can be diagnosed). In contrast to previous cases, our patient presented with only mild constipation at the beginning, which was relieved briefly after symptomatic treatment, delaying GIPO diagnosis.
Comparison of treatment strategies further revealed key differences in clinical decision-making. Primary therapies for ICI-induced PNS include corticosteroids, intravenous immunoglobulins (IVIG), and plasmapheresis. Second-line treatment options include rituximab, cyclophosphamide, and mycophenolate (33). Plasmapheresis has also been used to accelerate ICI clearance, but the appropriate timing for this therapy remain unclear (34). An autopsy report by Appelbaum et al. reported a severe reduction in the number of sympathetic ganglia throughout the gastrointestinal tract, with no sympathetic ganglia present in the myenteric plexus and only a few remaining in the submucosa, suggesting that treatment may be ineffective after the disease has reached the “burned-out” stage (23). Therefore, treatment should be initiated immediately upon the onset of symptoms. In another previous report by Kang et al., three patients received methylprednisolone treatment, but only one survived (24). This patient had developed encephalitis and GIPO sequentially following the use of sintilimab. Since the patient had been treated with methylprednisolone for encephalitis before being diagnosed with GIPO, it was speculated that the early therapeutic intervention with corticosteroids may have contributed to the improvement in GIPO (24). While corticosteroids effectively mitigate inflammation, they cannot repair necrotic tissue or regenerate neurons, which might explain poor response to cortisol therapy. Furthermore, aggressive immunosuppressive therapy has demonstrated limited utility in severely disabled patients with PNS and antineuronal antibodies (35). Delayed administration may also permit inflammatory cascades, such as cytokine storms and oxidative stress, inflicting irreversible damage. Additionally, corticosteroids predominantly suppress innate immunity and humoral responses, rendering them insufficient for targeting T-cell-driven pathologies. In this case, the nerve damage was irreversible due to delayed treatment and suboptimal corticosteroid dose.
IVIG administered at 2 g/kg divided over 2–5 d can alleviate steroid-refractory GIPO. The primary mechanisms involve the neutralization of autoantibodies and immunomodulatory effects, thereby ameliorating impaired neuronal transmission (36). Jacob et al. (23) reported the successful management of a case of GIPO induced by ipilimumab plus nivolumab combination therapy through plasmapheresis, a strategy aimed at clearing circulating autoantibodies and pro-inflammatory mediators. Second-line treatment options for refractory autoimmune GIPO include rituximab and vedolizumab (37), while the use of cyclophosphamide and mycophenolate mofetil has not yet been reported. In the cases shown in Table 1, all patients were treated with methylprednisolone at a single dose of approximately 1–2 mg/kg. Only one patient received pulse steroid therapy (1000 mg/d). One case was managed with a combination of tacrolimus and plasmapheresis; however, the patient did not survive. In this case, due to a long-standing digestive system disease, the patient developed significant cachexia. The family refused high-dose corticosteroid pulse therapy and second-line biological treatment, and the patient eventually died.
Previous studies have indicated that the prognosis of ICI-induced GIPO is closely associated with the antibody profiles and dynamic changes in titers, although the findings are inconsistent. Vogrig et al. demonstrated that elevated titers of anti-Hu/Yo antibodies (>1:320) positively correlate with severe intestinal dysmotility, glucocorticoid resistance, and an increased risk of disease recurrence (9).In serum AQP4 antibody-positive patients with NMOSD, the neuronal autoantibody titers decreased, sometimes even reaching negative values, following high-dose glucocorticoid pulse and immunosuppressive therapies during the acute phase, especially in patients with low pre-treatment titers (<32). Similarly, in patients with high pre-treatment titers (>320), immunoadsorption treatment reduced the autoantibody titers gradually (38, 39). During maintenance immunotherapy, antibody titers gradually decline and may even turn negative, but some patients who have unchanged titers have a higher relapse rate compared to those who do (40). Lower titers of classic paraneoplastic syndrome-related antibodies may indicate a lower risk of associated tumors and better prognosis (41). In refractory patients with NMDAR encephalitis, no significant effect is depicted when first-line therapy is administered during the acute phase, whereas early use of second-line therapy is significantly important for reducing antibody titers (42, 43). Notably, in the present case, the high anti-Hu antibody titer (1:1000) was associated with an unfavorable clinical outcome.
This case report provides critical insights into the clinical management of ICI-induced GIPO, further underscoring the necessity for early recognition and risk stratification of irAEs in cancer immunotherapy. Research indicates that specific ICI regimens and baseline patient characteristics may significantly influence GIPO risk. Cuzzubbo et al. demonstrated through large-scale clinical data analysis that, compared to PD-1/PD-L1 monotherapy, CTLA-4 inhibitors (e.g., ipilimumab) or CTLA-4/PD-1 combination therapy substantially increase the incidence of severe irAEs (including GIPO) (4). Patients with pre-existing autoimmune neuropathies (e.g., myasthenia gravis) or gastrointestinal motility disorders appear more susceptible to such complications, though this remains speculative, with no confirmatory studies to date.
Effective prevention systems should be established for these high-risk populations in clinical practice via (1) baseline risk assessment with systematic screening for autoimmune history and anti-neuronal antibody testing in suspected cases before treatment initiation. (2) Personalized dose adjustment by considering extended dosing intervals or reduced doses for high-risk patients, as CTLA-4 inhibitors exhibit dose-dependent irAE profiles. For instance, ipilimumab dose escalation from 3 mg/kg to 10 mg/kg increases irAE incidence from 29% to 46% (44). (3) Dynamic monitoring and tiered intervention: Clinicians should remain vigilant for early symptoms such as abdominal distension, refractory constipation, and vomiting, implementing severity-based management. Mild cases warrant temporary ICI suspension with oral corticosteroids, whereas moderate-to-severe cases require hospitalization for intravenous steroid pulse therapy. Second-line interventions (e.g., IVIG or plasmapheresis) should be initiated if no response occurs within 72 hours. Multidisciplinary team collaboration can optimize decisions regarding ICI interruption/permanent discontinuation by comprehensively evaluating intestinal motility and immune injury mechanisms, thereby balancing antitumor efficacy and safety. Notably, GIPO profoundly impacts both patients’ quality of life and mental health. The HRQoL scores in patients with GIPO were significantly lower than those in general cancer populations, correlating strongly with symptom frequency and often triggering anxiety/depression (45).
This case is a unique presentation of GIPO associated with anti-Hu antibodies in patients with EC-SCLC treated with the PD-1 inhibitor serplulimab. Early diagnosis of enteric neuropathy related to PNS is challenging and can be easily confused with tumor-induced gastrointestinal adverse events. Therefore, increasing the awareness of anti-Hu antibody-associated GIPO is crucial for identifying this rare condition and initiating early intervention. If the condition is severe, it is essential to promptly discontinue ICIs and consider the use of corticosteroids or other immunosuppressants based on the clinical situation to improve patient outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The Ethics Committee of the General Hospital of Western Theater Command. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
G-QS: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. H-NL: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. XW: Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. J-QX: Conceptualization, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing. HW: Conceptualization, Investigation, Project administration, Resources, Writing – review & editing. L-JM: Funding acquisition, Investigation, Resources, Visualization, Writing – original draft, Writing – review & editing. Z-LX: Formal analysis, Funding acquisition, Project administration, Resources, Validation, Writing – original draft, Writing – review & editing. JZ: Conceptualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Chengdu Medical Research Project (No. 2024011).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. (2019) 37:537–46. doi: 10.1200/JCO.18.00149
3. Jayathilaka B, Mian F, Cockwill J, Franchini F, Au-Yeung G, IJzerman M. Analysis of risk factors for immune-related adverse events induced by immune checkpoint inhibitor treatment in cancer: a comprehensive systematic review. Crit Rev Oncol Hematol. (2025) 207:104601. doi: 10.1016/j.critrevonc.2024.104601
4. Cuzzubbo S, Javeri F, Tissier M, Roumi A, Barlog C, Doridam J, et al. Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur J Cancer. (2017) 73:1–8. doi: 10.1016/j.ejca.2016.12.001
5. Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. (2003) 349:1543–54. doi: 10.1056/NEJMra023009
6. Graus F, Vogrig A, Muñiz-Castrillo S, Antoine JG, Desestret V, Dubey D, et al. Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm. (2021) 8(4):e1014. doi: 10.1212/NXI.0000000000001014
7. Bar Mucha S, Rozenberg A, Gutter Kapon L, Gorenshtein A, Ganelin-Cohen E, Ben Hayun R, et al. Long term survival and outcomes in patients with paraneoplastic neurologic syndromes. Front Immunol. (2024) 15:1466704. doi: 10.3389/fimmu.2024.1466704
8. Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol. (2008) 7:327–40. doi: 10.1016/S1474-4422(08)70060-7
9. Vogrig A, Muñiz-Castrillo S, Joubert B, Picard G, Rogemond V, Skowron F, et al. Cranial nerve disorders associated with immune checkpoint inhibitors. Neurology. (2021) 96:e866–e75. doi: 10.1212/WNL.0000000000011340
10. Shi GQ, Lian HN, Wang H, Xia JQ, Ma LJ, Zhou J. Case report: Immune checkpoint inhibitor-induced paraneoplastic neurological syndrome in two patients: a case series. Front Oncol. (2024) 14:1404829. doi: 10.3389/fonc.2024.1404829
11. Panganamamula KV, Parkman HP. Chronic intestinal pseudo-obstruction. Curr Treat Options Gastroenterol. (2005) 8:3–11. doi: 10.1007/s11938-005-0046-4
12. Lee HR, Lennon VA, Camilleri M, Prather CM. Paraneoplastic gastrointestinal motor dysfunction: clinical and laboratory characteristics. Am J Gastroenterol. (2001) 96:373–9. doi: 10.1111/j.1572-0241.2001.03454.x
13. Cheng Y, Han L, Wu L, Chen J, Sun H, Wen G, et al. Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the ASTRUM-005 randomized clinical trial. Jama. (2022) 328:1223–32. doi: 10.1001/jama.2022.16464
14. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncol. (2016) 2:1346–53. doi: 10.1001/jamaoncol.2016.1051
15. Bonanno L, Lorenzi M, Massa D, De Nuzzo M, Angerilli V, Zingone F, et al. Immune-related diarrhea and colitis in non-small cell lung cancers: impact of multidisciplinary management in a real-world setting. Oncologist. (2024) 29:e118–e30. doi: 10.1093/oncolo/oyad238
16. Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. (2021) 9(6):e002435. doi: 10.1136/jitc-2021-002435
17. Bharucha AE, Lacy BE. Mechanisms, evaluation, and management of chronic constipation. Gastroenterology. (2020) 158:1232–49.e3. doi: 10.1053/j.gastro.2019.12.034
18. Ishibashi M, Ishida Y, Otsuka A, Yamamoto S, Kabashima K. Acute colonic pseudo-obstruction following nivolumab and ipilimumab combination therapy for metastatic melanoma. Trends Immunother. (2021) 5(2):32–5. doi: 10.24294/ti.v5.i2.1297
19. Fragulidis G, Pantiora E, Michalaki V, Kontis E, Primetis E, Vezakis A, et al. Immune-related intestinal pseudo-obstruction associated with nivolumab treatment in a lung cancer patient. J Oncol Pharm Pract. (2019) 25:487–91. doi: 10.1177/1078155217738325
20. Dai C, Huang YH. Treatment of steroid-refractory immune checkpoint inhibitor-induced intestinal pseudo-obstruction with infliximab. Rev Esp Enferm Dig. (2024) 116:383–4. doi: 10.17235/reed.2023.9796/2023
21. Trontzas IP, Rapti VE, Syrigos NK, Kounadis G, Perlepe N, Kotteas EA, et al. Enteric plexus neuropathy associated with PD-L1 blockade in a patient with small-cell lung cancer. Immunotherapy. (2021) 13:1085–92. doi: 10.2217/imt-2020-0350
22. Qian Y, Zhi Z, Ai J, Kang L, Qiu G, Huang X, et al. Immune-related intestinal pseudo-obstruction caused by immune checkpoint inhibitors: case report. Front Oncol. (2024) 14:1415117. doi: 10.3389/fonc.2024.1415117
23. Appelbaum J, Wells D, Hiatt JB, Steinbach G, Stewart FM, Thomas H, et al. Fatal enteric plexus neuropathy after one dose of ipilimumab plus nivolumab: a case report. J Immunother Cancer. (2018) 6:82. doi: 10.1186/s40425-018-0396-9
24. Kang K, Zheng K, Zhang Y. Paraneoplastic encephalitis and enteric neuropathy associated with anti-hu antibody in a patient following immune-checkpoint inhibitor therapy. J Immunother. (2020) 43:165–8. doi: 10.1097/CJI.0000000000000314
25. Saitou A, Shioya M, Nagahisa Y, Haseyama A, Niwa R, Tsuchimoto J, et al. Small-cell lung carcinoma with gastrointestinal pseudo-obstruction as a paraneoplastic neurological syndrome elicited by an immune checkpoint inhibitor. Intern Med. (2024) 63:2059–62. doi: 10.2169/internalmedicine.2648-23
26. Besaw RJ, Smith MP, Zerillo JA, Bullock AJ. Chronic intestinal pseudo-obstruction in a patient with metastatic gastro-oesophageal junction cancer receiving treatment with pembrolizumab. BMJ Case Rep. (2019) 12(12):e232388. doi: 10.1136/bcr-2019-232388
27. Vogrig A, Gigli GL, Segatti S, Corazza E, Marini A, Bernardini A, et al. Epidemiology of paraneoplastic neurological syndromes: a population-based study. J Neurol. (2020) 267:26–35. doi: 10.1007/s00415-019-09544-1
28. Giometto B, Grisold W, Vitaliani R, Graus F, Honnorat J, Bertolini G. Paraneoplastic neurologic syndrome in the PNS Euronetwork database: a European study from 20 centers. Arch Neurol. (2010) 67:330–5. doi: 10.1001/archneurol.2009.341
29. Senties-Madrid H, Vega-Boada F. Paraneoplastic syndromes associated with anti-Hu antibodies. Isr Med Assoc J. (2001) 3:94–103.
30. Farina A, Villagrán-García M, Ciano-Petersen NL, Vogrig A, Muñiz-Castrillo S, Taillandier L, et al. Anti-hu antibodies in patients with neurologic side effects of immune checkpoint inhibitors. Neurol Neuroimmunol Neuroinflamm. (2023) 10(1):e200058. doi: 10.1212/NXI.0000000000200058
31. Bastiaansen AEM, de Jongste AHC, de Bruijn M, Crijnen YS, Schreurs MWJ, Verbeek MM, et al. Phase II trial of natalizumab for the treatment of anti-Hu associated paraneoplastic neurological syndromes. Neurooncol Adv. (2021) 3:vdab145. doi: 10.1093/noajnl/vdab145
32. Plaçais L, Michot JM, Champiat S, Romano-Martin P, Baldini C, Joao MS, et al. Neurological complications induced by immune checkpoint inhibitors: a comprehensive descriptive case-series unravelling high risk of long-term sequelae. Brain Commun. (2021) 3:fcab220. doi: 10.1093/braincomms/fcab220
33. Jean MJ, Samkoff L, Mohile N. Management of paraneoplastic syndromes in the era of immune checkpoint inhibitors. Curr Treat Options Oncol. (2024) 25:42–65. doi: 10.1007/s11864-023-01157-1
34. Katsumoto TR, Wilson KL, Giri VK, Zhu H, Anand S, Ramchandran KJ, et al. Plasma exchange for severe immune-related adverse events from checkpoint inhibitors: an early window of opportunity? Immunother Adv. (2022) 2:ltac012. doi: 10.1093/immadv/ltac012
35. Keime-Guibert F, Graus F, Fleury A, René R, Honnorat J, Broet P, et al. Treatment of paraneoplastic neurological syndromes with antineuronal antibodies (Anti-Hu, anti-Yo) with a combination of immunoglobulins, cyclophosphamide, and methylprednisolone. J Neurol Neurosurg Psychiatry. (2000) 68:479–82. doi: 10.1136/jnnp.68.4.479
36. Haanen J, Ernstoff M, Wang Y, Menzies A, Puzanov I, Grivas P, et al. Rechallenge patients with immune checkpoint inhibitors following severe immune-related adverse events: review of the literature and suggested prophylactic strategy. J Immunother Cancer. (2020) 8(1):e000604. doi: 10.1136/jitc-2020-000604
37. Vilaseca A, Arranz P, Llaurado A, Zabalza A, Fonseca EG, Medina I, et al. Novel approaches to treatment of immune-mediated chronic intestinal pseudo-obstruction. Neurol Neuroimmunol Neuroinflamm. (2025) 12:e200369. doi: 10.1212/NXI.0000000000200369
38. Chen B, Qin C, Chen M, Yu HH, Tao R, Chu YH, et al. Dynamic changes in AQP4-IgG level and immunological markers during protein-A immunoadsorption therapy for NMOSD: A case report and literature review. Front Immunol. (2021) 12:650782. doi: 10.3389/fimmu.2021.650782
39. Naganuma T, Furusawa Y, Hanaoka A, Takemoto Y, Uchida J. A case of anti-aquaporin-4 antibody-positive optic neuritis treated by selective immunoadsorption. Transfus Apher Sci. (2021) 60:102969. doi: 10.1016/j.transci.2020.102969
40. Hyun JW, Woodhall MR, Kim SH, Jeong IH, Kong B, Kim G, et al. Longitudinal analysis of myelin oligodendrocyte glycoprotein antibodies in CNS inflammatory diseases. J Neurol Neurosurg Psychiatry. (2017) 88:811–7. doi: 10.1136/jnnp-2017-315998
41. Seluk L, Taliansky A, Yonath H, Gilburd B, Amital H, Shoenfeld Y, et al. A large screen for paraneoplastic neurological autoantibodies; diagnosis and predictive values. Clin Immunol. (2019) 199:29–36. doi: 10.1016/j.clim.2018.12.007
42. Hara M, Nakajima H. Anti-NMDAR encephalitis with poor recovery on steroid pulse and IVIg: practical approach to intensive immunotherapy. Brain Nerve. (2022) 74:433–42. doi: 10.11477/mf.1416202061
43. Leypoldt F, Armangue T, Dalmau J. Autoimmune encephalopathies. Ann N Y Acad Sci. (2015) 1338:94–114. doi: 10.1111/nyas.2015.1338.issue-1
44. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. (2018) 36:1714–68. doi: 10.1200/JCO.2017.77.6385
Keywords: anti-Hu antibodies, gastrointestinal pseudo-obstruction, immune checkpoint inhibitors, serplulimab, paraneoplastic neurological syndrome
Citation: Shi G-Q, Lian H-N, Wang X, Xia J-Q, Wang H, Ma L-J, Xiao Z-L and Zhou J (2025) Immune checkpoint inhibitor-induced anti-Hu antibody-associated gastrointestinal pseudo-obstruction: a case report and literature review. Front. Immunol. 16:1555790. doi: 10.3389/fimmu.2025.1555790
Received: 05 January 2025; Accepted: 17 March 2025;
Published: 01 April 2025.
Edited by:
Sergei Kusmartsev, University of Florida, United StatesReviewed by:
Frank Ward, University of Aberdeen, United KingdomCopyright © 2025 Shi, Lian, Wang, Xia, Wang, Ma, Xiao and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Zhou, emhvdTgxMTEwMjdAZm94bWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.