
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol. , 05 February 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1554256
This article is part of the Research Topic Immunotherapy Resistance and Advancing Adaptive Cell Therapeutics View all 10 articles
Immunotherapy has revolutionized cancer treatment, offering hope for patients with otherwise treatment-resistant tumors. Among the most promising approaches are cellular therapies, particularly chimeric antigen receptor T-cell (CAR-T) therapy, which has shown remarkable success in hematologic malignancies. However, the application of these therapies to solid tumors, such as lung and colorectal cancers, has faced significant challenges. Tumor resistance mechanisms—ranging from immune evasion, antigen loss, and immune checkpoint upregulation, to tumor microenvironment immunosuppression—remain major obstacles. This mini-review highlights the latest advancements in tumor immunotherapy, with a focus on cellular therapies, and addresses the resistance mechanisms that hinder their effectiveness in lung and colorectal cancers. We examine the evolution of CAR-T cell therapy, as well as the potential of engineered natural killer (NK) cells and macrophages in solid tumor treatment. The review also explores cutting-edge strategies aimed at overcoming resistance, including combination therapies, gene editing technologies, and nanotechnology for targeted drug delivery. By discussing the molecular, cellular, and microenvironmental factors contributing to resistance, we aim to provide a comprehensive overview of how these challenges can be overcome, paving the way for more effective, personalized immunotherapies in lung and colorectal cancer treatment.
Immunotherapy has revolutionized cancer treatment, providing novel approaches that harness the body’s immune system to fight cancer. Among these, cellular immunotherapies, particularly chimeric antigen receptor T-cell (CAR-T) therapy, have shown remarkable success, particularly in hematologic malignancies. The ability to genetically modify T-cells to express receptors that recognize specific tumor antigens has enabled significant advances, leading to durable responses in cancers such as leukemia and lymphoma (1). However, the application of CAR-T therapy to solid tumors, including lung and colorectal cancers, has faced considerable challenges. Despite these challenges, recent innovations in cellular therapies have opened new avenues for overcoming resistance and improving the effectiveness of treatments for solid tumors.
Resistance to immunotherapies in solid tumors is multifactorial and presents a significant hurdle in the successful application of CAR-T therapy. The tumor microenvironment (TME) in solid cancers is often immunosuppressive, consisting of regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages that inhibit immune responses (2). These immune cells, along with the presence of immunosuppressive factors like transforming growth factor-beta (TGF-β), create a hostile environment that limits the ability of T-cells to effectively target and kill tumor cells. Moreover, tumors often exhibit antigen loss or downregulation, which prevent CAR-T cells from recognizing their targets. Immune checkpoint molecules, such as PD-L1, are also upregulated in these tumors, allowing them to evade immune detection by inhibiting T-cell function (3).
Despite these challenges, significant strides have been made in enhancing cellular therapies to overcome the resistance mechanisms that hinder their effectiveness in solid tumors. Development of dual-CAR-T cell and the application of “off-the-shelf” cell therapies, such as allogeneic CAR-T cells or natural killer (NK) cells provide an alternative to autologous cell therapies, thereby improving accessibility and reducing the time required for treatment preparation. NK cell-based therapies are gaining traction due to the innate ability of NK cells to recognize and kill tumor cells without prior sensitization. NK cells can be genetically engineered to enhance their tumor-killing properties and, when combined with immune checkpoint inhibitors, have shown potential for overcoming the immunosuppressive TME. Similarly, macrophages, when reprogrammed into anti-tumor phenotypes, play a crucial role in targeting and eradicating solid tumors. These alternative cellular therapies offer promising strategies for addressing the limitations faced by CAR-T cells in treating lung and colorectal cancers.
This review explores the latest advancements in cellular immunotherapies, particularly CAR-T cells, NK cells, and macrophage-based therapies, with a focus on overcoming the resistance mechanisms in solid tumors. By understanding and addressing the unique challenges posed by lung and colorectal cancers, these emerging therapies offer new hope for improving the efficacy of immunotherapy and providing long-lasting responses for patients with advanced or resistant cancers.
Resistance to immunotherapy remains a significant challenge, particularly in solid tumors. The TME, immune escape mechanisms, and cellular dysfunctions are key factors contributing to the failure of immunotherapies (Figure 1). In this section, we will explore seven key resistance mechanisms that hinder the effectiveness of tumor immunotherapy, providing examples from both lung and colorectal cancers to illustrate how these mechanisms manifest in different tumor types.
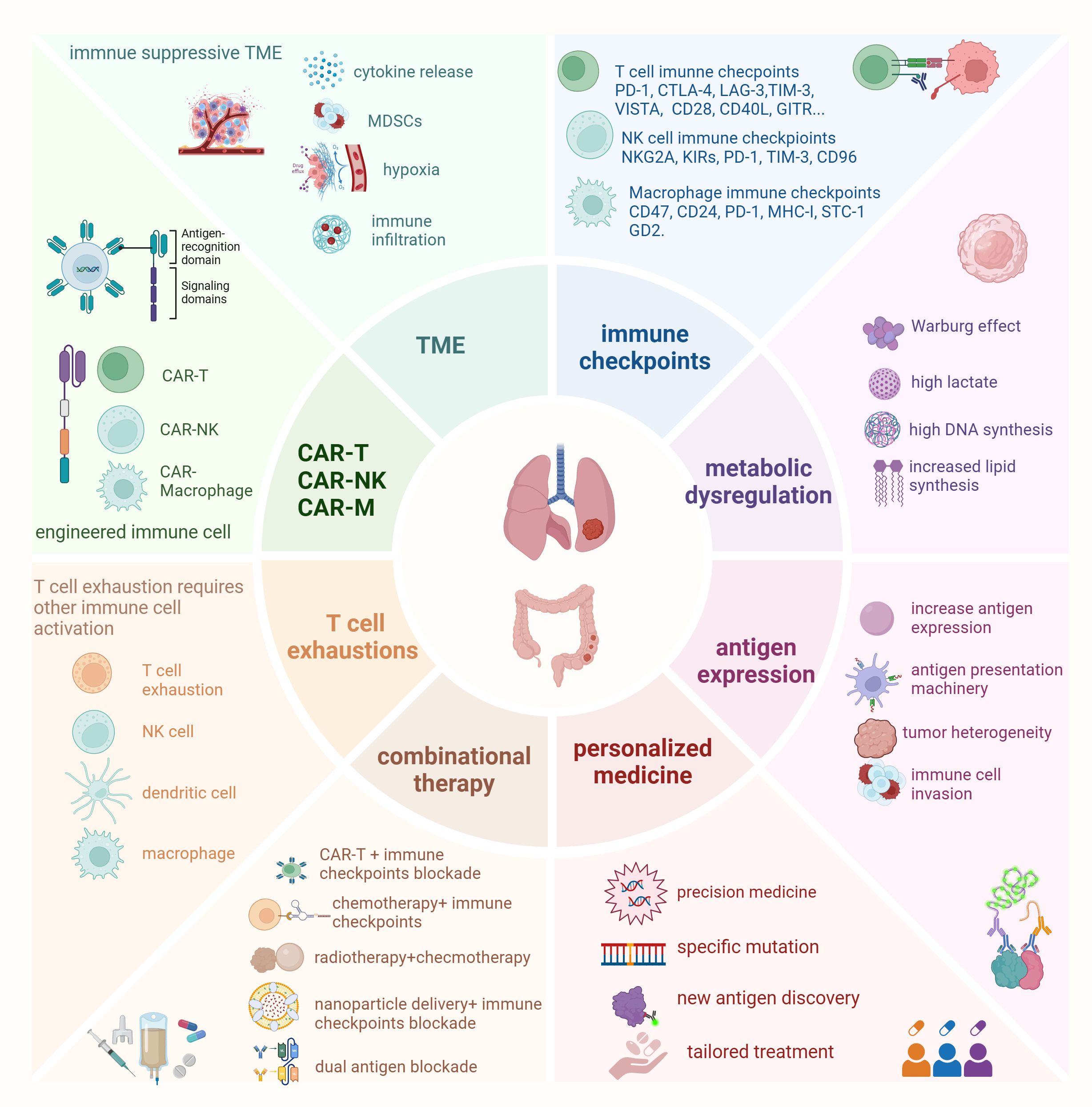
Figure 1. Main factors affect immune cell therapy in lung cancer and colorectal cancer Resistance to immunotherapy remains a significant challenge in lung cancer and colorectal cancer. Key factors contributing to the failure of immunotherapies include the tumor microenvironment (TME), immune escape mechanisms, and cellular dysfunctions. One emerging approach to overcome these challenges is targeting immune checkpoints on different immune cells, which has led to the development of new immunotherapies. In addition to CAR-T therapy, CAR-NK (chimeric antigen receptor natural killer cells) and CAR-Macrophages have garnered attention in preclinical research due to their specific advantages over CAR-T cells. However, T cell exhaustion and antigen loss represent new obstacles to cancer immunotherapy. Reversing T cell exhaustion requires not only the direct activation of T cells but also the activation of other immune cells, such as dendritic cells, NK cells, and macrophages, to modulate the overall immune response effectively. Moreover, metabolic dysfunctions in both immune cells and tumor cells play a critical role in affecting the efficacy of immune cell therapies. To address these challenges, combinational therapies and personalized medicine strategies are increasingly being encouraged. These approaches have shown promising results, offering hope for improving therapeutic outcomes in cancer immunotherapy.
TME plays a critical role in mediating resistance to immunotherapies (4). The TME is often characterized by factors that suppress immune activity and promote tumor survival. Hypoxia is a common feature of solid tumors, which leads to a lack of oxygen in the TME and induces the production of immunosuppressive cytokines (e.g., TGF-β, IL-10) and upregulation of immune checkpoint molecules (5). This hypoxic condition not only supports tumor growth but also impairs the function of immune cells. Additionally, the TME harbors a variety of immunosuppressive cell populations such as Tregs and MDSCs, which actively suppress the anti-tumor immune responses. These cells inhibit the activation of effector T-cells, promote tumor cell survival, and prevent immune cell infiltration into the tumor. The presence of these immune-suppressive cells and factors creates a protective niche for the tumor, hindering immune-based therapies to succeed.
The TME in non-small cell lung cancer (NSCLC) is often hypoxic, hypoxia promote cancer cell stemness and invasion by promoting glycolysis via lactylation of SOX9 (6), and it increased PD-L1 to suppress T-cell activation and function, Metabolic intervention that alleviates hypoxia and reduces PD-L1 expression enhances lung cancer radio-immunotherapy (7). Furthermore, NSCLC tumors are typically infiltrated with immunosuppressive cells like Tregs and MDSCs, which contribute to immune evasion by preventing effective anti-tumor immunity (8). High PD-L1 expression in NSCLC correlates with resistance to immune checkpoint inhibitors, such as pembrolizumab or nivolumab, highlighting the suppressive effects of the TME (9). In colorectal cancer (CRC), especially in microsatellite stable (MSS) tumors and metastatic tumors, the TME is also immunosuppressive (10). Tumors secrete TGF-β and IL-10, which promote immune suppression and contribute to therapy resistance. The presence of MDSCs and Tregs in CRC tumors prevents the activation of anti-tumor T-cells and inhibits the effectiveness of immunotherapies such as immune checkpoint inhibitors (11). MSS CRC tumors show poor response to anti-PD-1 therapy due to an immune-suppressive microenvironment, blocking IL-17A potentiates tumor response to anti-PD-1 immunotherapy in MSS CRC (12).
Tumor cells evade immune detection through antigen loss or downregulation. The genetic instability leads to the generation of new mutant antigens, many of which are immunogenic. However, tumors often adapt by downregulating or losing the expression of tumor-associated antigens (TAAs) or major histocompatibility complex (MHC) molecules, which are essential for the presentation of these antigens to immune cells. Loss of antigen expression enables the tumor to escape immune surveillance. In CAR-T therapy, where the engineered T-cells target a specific antigen on tumor cells, the loss of that antigen lead to therapeutic resistance, as the T-cells no longer recognize the tumor cells as targets (13). The alteration of surface antigens or clonal evolution in tumor cells complicates treatment, as therapies targeting a single antigen may become ineffective after these changes.
In NSCLC, tumor antigen loss is a significant issue, particularly for EGFR-targeted therapies. Tumors may lose or downregulate EGFR expression after prolonged treatment with EGFR tyrosine kinase inhibitors (TKIs) (14), leading to resistance. Additionally, PD-L1 downregulation hinders the effectiveness of checkpoint inhibitors. In NSCLC patients, the loss of HER2 or EGFR expression following targeted therapy leads to acquired resistance to treatments and targeting HER2 and EGFR exhibits positive immunotherapy results (15). CRC tumors with KRAS mutations or loss of MHC expression fail to present antigens effectively, reducing the ability of T-cells to recognize and attack tumor cells (16).
Tumor cells use immune checkpoint molecules to inhibit immune function, effectively “braking” immune responses (17). Checkpoints like PD-1, CTLA-4, CD47 have been studied a lot in different cancers (18). In NSCLC, tumors often increase PD-L1 expression in response to inflammation, helping them evade immune detection by binding to PD-1 receptors on T-cells. Other checkpoint molecules, such as CTLA-4 and TIM-3, also contribute to resistance to PD-1/PD-L1 therapies. High PD-L1 levels in NSCLC are linked to resistance to PD-1/PD-L1 blockers, with relapse occurring after initial response. In CRC, especially MSS tumors, upregulated PD-L1 and CTLA-4 limit checkpoint inhibitor effectiveness. Even in microsatellite instability-high (MSI-H) CRC, resistance can arise if PD-L1 expression doesn’t increase, hindering an immune response.
T-cell exhaustion is another critical mechanism of resistance to immunotherapy, particularly in chronic or persistent tumor environments. As T-cells persistently encounter tumor antigens in the TME, they undergo a process of functional decline, characterized by the upregulation of inhibitory receptors like PD-1, TIM-3, and LAG-3 (19). Exhausted T-cells exhibit reduced cytokine production, impaired proliferative capacity, and diminished cytotoxic function, making them less effective at killing tumor cells. This phenomenon is particularly pronounced in tumors that have a high degree of immune infiltration and antigen persistence. In advanced stages of NSCLC, T-cells often show exhaustion markers such as PD-1, TIM-3, and LAG-3, impairing their function (20). This is particularly problematic for patients treated with checkpoint inhibitors, as exhausted T-cells fail to mount an effective anti-tumor response. In NSCLC, patients treated with anti-PD-1 therapy who later relapse often exhibit increased PD-1 expression on T-cells, signaling exhaustion and reduced therapeutic efficacy. Similarly, in CRC, T-cell exhaustion is driven by chronic antigen exposure, particularly in tumors that are immune-infiltrated. The presence of exhausted T-cells in the TME contributes to resistance to therapies, including immune checkpoint inhibitors (21). T cell exhaustion in CRC is regulated by many infactors, cholesterol induces CD8+ T cell exhaustions by regulating endoplasmic reticulum-mitochondria contact (22); MGP promotes CD8+ T cell exhaustion by activating the NF-kB pathway and leading to cancer metastasis (23).
The metabolic landscape of tumors plays a significant role in immune resistance. Tumor cells often undergo metabolic reprogramming, such as enhanced glycolysis (the Warburg effect), to support their rapid growth. This reprogramming not only promotes tumor progression but also affects immune cell function. Tumor metabolism in NSCLC often involves aerobic glycolysis, which not only provides energy for rapid tumor growth but also generates byproducts like lactate that acidify the TME. The Warburg effect enhanced by AKR1B10 promotes acquired resistance to pemetrexed in lung cancer-derived brain metastasis (24). High lactate level induces tumor-associated fibroblast activation and IL-8 mediated macrophage recruitment to potentiate lung cancer progression and compromise the immunotherapy (25). MCT-4-mediated lactate secretion inhibits antitumor immunity in LKB1-deficient lung cancer (26). This acidic environment suppresses T-cell function and promotes immune evasion. In addition, in lung adenocarcinoma, harnessing lipid metabolism modulation indicates improved immunotherapy outcomes (27). Mitochondrial networks and biogenetics also influence cancer cell immunotherapy in lung cancer (28). H3K18 lactylation enhances immune escape by activating the POM121/MYC/PD-L1 pathway in NSCLC (29). In CRC, lactate production from glycolysis and arginine depletion inhibit the function of effector T-cells, making tumors less responsive to immunotherapies. High lactate upregulates lactylation, the lactylation-driven METTL3-mediated RNA m6A modification promotes immunosuppression of tumor-infiltrating myeloid cells in colon cancer (30). Circulating L-arginine predicts the survival of cancer patients treated with immune checkpoint inhibitors in colon cancer (31). Moreover, the fusobacterium nucleatum-derived succinic acid induces tumor resistance to immunotherapy in colorectal cancer (32).
Immunotherapy resistance remains a major obstacle in the effective treatment of cancers, especially in solid tumors like lung and colorectal cancer. Overcoming this resistance requires a multifaceted approach, involving a combination of strategies targeting different mechanisms of immune evasion. Key strategies include enhancing immune cell efficacy, overcoming immune suppressive factors in TME, and leveraging combination therapies.
NK cells and macrophages are emerging as valuable alternatives to T-cell-based therapies like CAR-T (33, 34). NK cells target tumors without prior sensitization, they are less vulnerable to antigen loss and TME immunosuppression (35, 36). NK cell-based immunotherapies including combined cytokine, CDC and ADCC, NK-92, KIR mismatch and CAR approaches. CAR-NK cell therapy results in reduced toxicity, lower cost, and broader accessibility compared to CAR-T cells (37). Specifically, CAR-NK cells have a lower risk of graft-versus-host disease (GVHD), enabling “off-the-shelf” allogeneic therapies, and possess both CAR-mediated and innate antitumor activity, making them effective against heterogeneous tumors. They have a better safety profile with reduced risks of cytokine release syndrome (CRS) and neurotoxicity and a shorter lifespan, minimizing long-term side effects. Their scalability from various sources, like cord blood or iPSCs, further enhances accessibility. In a phase I/II trial, CD19 CAR-NK cells achieved a 73% response rate in patients with relapsed/refractory B-cell malignancies, without CRS or neurotoxicity (38). NK cells prime cancer cells for mt-apoptosis and combining them with BH3 mimetics enhances cancer cell death and tumor suppression. BH3 profiling helps identify the most effective mimetic, offering a precision strategy to improve NK-based and T cell-based immunotherapies (39). Rocaglamide enhances NK cell infiltration and antitumor immunity by activating the cGAS-STING signaling pathway in non-small cell lung cancer (40). NK-cell mediated therapy in lung cancer and CRC has been reviewed (41, 42).
Similarly, macrophages can be reprogrammed to exhibit anti-tumor properties, helping to eliminate resistant tumor cells and reprogram the TME for enhanced immune responses (43). CAR-macrophages have emerged as a new cancer immunotherapy to target solid tumors (44), offering several advantages over CAR-T cells. Unlike CAR-T cells, which rely primarily on direct cytotoxicity, CAR-macrophages utilize their natural phagocytic ability to engulf tumor cells and present tumor antigens, thereby activating downstream immune responses (45, 46). Their ability to infiltrate dense tumor microenvironments makes them particularly effective against solid tumors, a challenge for CAR-T therapies. The tandem CD3ζ-TIR dual signaling CAR design enables induced pluripotent stem cell-derived macrophages (iMACs) to engage targets, promote M1 polarization, resist immunosuppressive M2 polarization, and modulate the tumor microenvironment through an NF-κB-dependent mechanism (47). Furthermore, CAR-macrophages are less prone to exhaustion and can reprogram the immunosuppressive TME, enhancing the recruitment and activation of T cells and other immune effectors (44, 48). Preclinical studies of anti-HER2 CAR-macrophages demonstrated significant tumor growth inhibition in HER2+ xenograft models, emphasizing their ability to overcome antigen escape and address TME immunosuppression (49). These properties make CAR-macrophages a powerful alternative to CAR-T cells in tackling the unique challenges of solid tumors.
Enhancing antigen targeting or using alternative antigens overcome this challenge. In NSCLC, novel antigen targets such as mesothelin or specific mutations like EGFR are being explored for CAR-T therapy, providing potential solutions to antigen loss and improving the targeting of resistant tumors (9, 50). Engineering CAR-T cells to target multiple antigens or incorporate novel features, such as cytokine secretion or checkpoint inhibition, could overcome issues of antigen loss. Moreover, IL-10-expressing CAR T cells maintain mitochondrial structure and function in the tumor microenvironment, IL-10 secretion boosted CAR T cell proliferation and effector functions, resulting in the complete regression of established solid tumors and metastatic cancers in multiple cancer types including colon in both syngeneic and xenograft mouse models (51). Dual-targeted CAR-T cells and “armored” CAR-T cells that resist the immunosuppressive TME represent exciting new avenues to enhance efficacy in solid tumors (52). In lung cancer, CAR-T cells with dual targets on HER2 and HLA-A02 enhance tumor specificity and address on-target off-tumor toxicity in HER2+ lung cancer cell lines with HLA-A02 loss of heterozygosity (53). Clinical trials of lung cancer targeting different antigens are listed in Table 1.
Tumor cells often exploit immune checkpoints such as PD-1/PD-L1, CTLA-4, and others to inhibit T-cell function. Combining checkpoint inhibitors with CAR-T therapy overcomes resistance by blocking these inhibitory signals, restoring immune cell function, and enhancing the anti-tumor response. Immunogenic chemotherapy enhances the recruitment of CAR-T cells to lung tumors, thereby improving the overall antitumor efficacy (54). When combined with checkpoint blockade therapy (anti-PD-1), this approach further boosts the therapeutic response, potentially leading to more effective treatment outcomes (55). In CRC, combining PD-1/PD-L1 inhibitors with CAR-T cells or NK cell therapy has demonstrated enhanced anti-tumor effects, particularly in tumors that express immune checkpoint molecules. The dual CAR-T cells with anti-PD-L1 scFv were capable of eradicating established tumors in mouse models of peritoneal metastasis of colorectal cancer (56).
Some of the advanced clinical trials have exhibited good results of chemotherapy in lung cancer. For instance, the phase III RATIONALE 303 trial (NCT03358875) demonstrated that Tislelizumab significantly extended overall survival in patients with advanced NSCLC who had progressed after platinum-based chemotherapy (57). Additionally, the ADRIATIC study showed that Duvatinib as a consolidation therapy after chemoradiotherapy significantly improved three-year survival rates in patients with limited-stage small cell lung cancer (SCLC) (58). However, for the cell therapies, they are still in the process.
T-cell exhaustion is a major cause of immune resistance (59). Immune checkpoint blockade has been used to prevent T-cell exhaustion, thereby enhancing CAR-T cell function. Additionally, NK cell therapy has shown potential to circumvent T-cell exhaustion, providing an alternative for overcoming resistance. Senescent T cells require NK cell to promote antitumor immunity (60). Combining anti-PD-1 inhibitors with CAR-T therapy or NK cells helps rejuvenate exhausted T cells and enhance immune responses against CRC. Additionally, improving the persistence of engineered immune cells is a focus of ongoing research in CRC. Clearance of senescent macrophages ameliorates tumorigenesis in KRAS-driven lung cancer (61).
Combination therapies have emerged as one of the most effective strategies to overcome resistance to immunotherapy (62). By simultaneously targeting different immune pathways, combination approaches address the multiple layers of resistance mechanisms that tumors use to evade immune attacks. This multi-pronged strategy aims to enhance the overall efficacy of treatment and improve patient outcomes. In NSCLC, combinations of CAR-T cells, NK cells, and immune checkpoint inhibitors have demonstrated synergistic effects, enhancing anti-tumor activity (58). This approach is particularly valuable in addressing various resistance mechanisms, such as immune evasion and immunosuppression within TME. In CRC, combining CAR-T cells with immune checkpoint inhibitors or chemotherapy has shown promising potential to improve responses, particularly in patients who are resistant to monotherapies. For instance, the combination of nivolumab and ipilimumab, with or without chemotherapy, has been shown to offer long-term, durable clinical benefits in metastatic NSCLC patients with tumor PD-L1 expression below 1% (63). This combination supports its use as a first-line treatment option, especially in populations with high unmet needs. Furthermore, adding toripalimab to perioperative chemotherapy significantly improved event-free survival in patients with resectable stage III NSCLC, while maintaining a manageable safety profile (64). These findings underscore the potential of combination therapies to offer substantial clinical benefits by overcoming resistance mechanisms and providing lasting therapeutic effects in cancers.
Given the significant variability in immune responses across patients, personalized and adaptive immunotherapies are increasingly recognized as essential for optimizing cancer treatment (65). Precision medicine tailors treatments to the specific genetic and molecular characteristics of a patient’s cancer (66). Identifying tumor-specific neoantigens, which are unique to cancer cells and not present in normal tissues, can be used to develop vaccines or engineered immune cells, such as CAR-T,CAR-NK, CAR-Macrophage therapies (67). Moreover, tailoring therapies based on genetic and immune profiling has the potential to significantly enhance treatment outcomes. By analyzing both the genetic mutations of the tumor and the immune system’s response to it, specific targets will be discovered. This allows for more targeted therapies that minimize side effects while maximizing antitumor efficacy (68). For instance, lung cancer patients with specific genetic alterations, like EGFR mutations, may benefit from targeted therapies combined with immunotherapy, whereas others may respond better to conventional treatments or personalized CAR-T therapies. Ultimately, integrating these personalized and adaptive strategies into clinical practice will be crucial for improving the success of cellular therapies, especially in complex cancers like lung and colorectal cancer, where variability in patient responses is high.
Lung cancer and colorectal cancer exhibit distinct resistance mechanisms to immunotherapy and cell-based therapies due to differences in their tumor microenvironments and molecular characteristics. In lung cancer, particularly NSCLC, resistance often arises through the upregulation of immune checkpoint pathways like PD-1/PD-L1, which suppresses T-cell activity. Despite a high tumor mutational burden (TMB) in smokers’ lung cancers, resistance can still occur due to antigen loss or altered antigen presentation (9). Additionally, CAR-T therapies face resistance from poor T-cell infiltration or downregulation of target antigens like EGFR. In contrast, CRC often involves mismatch repair deficiency (dMMR) and microsatellite instability (MSI), leading to an elevated mutational burden. However, resistance in CRC is marked by immune exclusion, where T-cells fail to infiltrate the tumor. This is particularly evident in mismatch repair-proficient (pMMR) tumors. CRC also has an immunosuppressive tumor microenvironment, which limits the effectiveness of immunotherapies. Resistance to cell-based therapies in CRC arises from antigen heterogeneity and downregulation of target antigens. While both cancers share immune evasion and antigen loss mechanisms, lung cancer is more affected by checkpoint resistance and poor immune cell penetration, whereas CRC faces challenges with immune exclusion and an immunosuppressive microenvironment. These differences underscore the need for tailored treatment strategies.
Significant advancements have been made in the field of immunotherapy, particularly in the application of cellular therapies like CAR-T, NK cells, and macrophages for treating solid tumors, including lung and colorectal cancers. Despite the promising results, resistance to immunotherapies remains a major challenge. The complexity of TME, antigen escape, immune checkpoint overexpression, and T-cell exhaustion are key mechanisms of resistance. While CAR-T cell therapies have shown notable success in hematologic malignancies, their application in solid tumors like lung and colorectal cancers is still limited due to the unique obstacles posed by these cancers. Additionally, the development of alternative therapies, such as NK cell and macrophage-based approaches, offers exciting new avenues to overcome these barriers and broaden the scope of cellular immunotherapies.
Looking forward, a multi-pronged approach combining different immunotherapeutic strategies will likely be essential for overcoming resistance in solid tumors. Enhancing the efficacy of CAR-T cells through better tumor targeting, engineering of immune cells, and modulation of the TME holds significant promise. Moreover, leveraging combination therapies, which include immune checkpoint inhibitors, cytokines, and alternative cell-based therapies like NK cells and reprogrammed macrophages, may provide synergistic effects to overcome resistance mechanisms. Understanding the mechanisms of resistance at a molecular level and further optimizing therapeutic designs will be key to improving outcomes for lung and colorectal cancer patients. The future of cancer immunotherapy depends on combining these new approaches, which could transform treatment and greatly improve patient survival and quality of life.
LQ: Writing – original draft, Writing – review & editing, Resources. YL: Resources, Visualization, Writing – original draft. JL: Conceptualization, Investigation, Supervision, Writing – review & editing. XA: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fenis A, Demaria O, Gauthier L, Vivier E, Narni-Mancinelli E. New immune cell engagers for cancer immunotherapy. Nat Rev Immunol. (2024) 24:471–86. doi: 10.1038/s41577-023-00982-7
2. Huang R, Li X, He Y, Zhu W, Gao L, Liu Y, et al. Recent advances in CAR-T cell engineering. J Hematol Oncol. (2020) 13:86. doi: 10.1186/s13045-020-00910-5
3. Hirschhorn D, Budhu S, Kraehenbuehl L, Gigoux M, Schroder D, Chow A, et al. T cell immunotherapies engage neutrophils to eliminate tumor antigen escape variants. Cell. (2023) 186:1432–1447 e1417. doi: 10.1016/j.cell.2023.03.007
4. Wang M, Zhu L, Yang X, Li J, Liu Y, Tang Y. Targeting immune cell types of tumor microenvironment to overcome resistance to PD-1/PD-L1 blockade in lung cancer. Front Pharmacol. (2023) 14:1132158. doi: 10.3389/fphar.2023.1132158
5. Shiri AM, Zhang T, Bedke T, Zazara DE, Zhao L, Lucke J, et al. IL-10 dampens antitumor immunity and promotes liver metastasis via PD-L1 induction. J Hepatol. (2024) 80:634–44. doi: 10.1016/j.jhep.2023.12.015
6. Yan F, Teng Y, Li X, Zhong Y, Li C, Yan F, et al. Hypoxia promotes non-small cell lung cancer cell stemness, migration, and invasion via promoting glycolysis by lactylation of SOX9. Cancer Biol Ther. (2024) 25:82304161. doi: 10.1080/15384047.2024.2304161
7. Wang S, Zhou Z, Hu R, Dong M, Zhou X, Ren S, et al. Metabolic intervention liposome boosted lung cancer radio-immunotherapy via hypoxia amelioration and PD-L1 restraint. Adv Sci (Weinh). (2023) 10:e2207608. doi: 10.1002/advs.202207608
8. Madeddu C, Donisi C, Liscia N, Lai E, Scartozzi M, Maccio A. EGFR-mutated non-small cell lung cancer and resistance to immunotherapy: role of the tumor microenvironment. Int J Mol Sci. (2022) 23(12):6489. doi: 10.3390/ijms23126489
9. Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. (2023) 22:40. doi: 10.1186/s12943-023-01740-y
10. Shin AE, Giancotti FG, Rustgi AK. Metastatic colorectal cancer: mechanisms and emerging therapeutics. Trends Pharmacol Sci. (2023) 44:222–36. doi: 10.1016/j.tips.2023.01.003
11. Liu YJ, Tang B, Wang FC, Tang L, Lei YY, Luo Y, et al. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics. (2020) 10:5225–41. doi: 10.7150/thno.43716
12. Liu C, Liu R, Wang B, Lian J, Yao Y, Sun H, et al. Blocking IL-17A enhances tumor response to anti-PD-1 immunotherapy in microsatellite stable colorectal cancer. J Immunother Cancer. (2021) 9(1):e001895. doi: 10.1136/jitc-2020-001895
13. Tang L, Pan S, Wei X, Xu X, Wei Q. Arming CAR-T cells with cytokines and more: Innovations in the fourth-generation CAR-T development. Mol Ther. (2023) 31:3146–62. doi: 10.1016/j.ymthe.2023.09.021
14. Grant C, Nagasaka M. Neoadjuvant EGFR-TKI therapy in Non-Small cell lung cancer. Cancer Treat Rev. (2024) 126:102724. doi: 10.1016/j.ctrv.2024.102724
15. Meric-Bernstam F, Makker V, Oaknin A, Oh DY, Banerjee S, Gonzalez-Martin A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-panTumor02 phase II trial. J Clin Oncol. (2024) 42:47–58. doi: 10.1200/JCO.23.02005
16. Chen Y, Wang D, Li Y, Qi L, Si W, Bo Y, et al. Spatiotemporal single-cell analysis decodes cellular dynamics underlying different responses to immunotherapy in colorectal cancer. Cancer Cell. (2024) 42:1268–1285 e1267. doi: 10.1016/j.ccell.2024.06.009
17. Zemek RM, Anagnostou V, Pires da Silva I, Long GV, Lesterhuis WJ. Exploiting temporal aspects of cancer immunotherapy. Nat Rev Cancer. (2024) 24:480–97. doi: 10.1038/s41568-024-00699-2
18. Liu Y, Weng L, Wang Y, Zhang J, Wu Q, Zhao P, et al. Deciphering the role of CD47 in cancer immunotherapy. J Adv Res. (2024) 63:129–58. doi: 10.1016/j.jare.2023.10.009
19. Yang R, Sun L, Li CF, Wang YH, Yao J, Li H, et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat Commun. (2021) 12:832. doi: 10.1038/s41467-021-21099-2
20. Liu X, Si F, Bagley D, Ma F, Zhang Y, Tao Y, et al. Blockades of effector T cell senescence and exhaustion synergistically enhance antitumor immunity and immunotherapy. J Immunother Cancer. (2022) 10(10):e005020. doi: 10.1136/jitc-2022-005020
21. Bell HN, Huber AK, Singhal R, Korimerla N, Rebernick RJ, Kumar R, et al. Microenvironmental ammonia enhances T cell exhaustion in colorectal cancer. Cell Metab. (2023) 35:134–149 e136. doi: 10.1016/j.cmet.2022.11.013
22. Shuwen H, Yinhang W, Jing Z, Qiang Y, Yizhen J, Quan Q, et al. Cholesterol induction in CD8(+) T cell exhaustion in colorectal cancer via the regulation of endoplasmic reticulum-mitochondria contact sites. Cancer Immunol Immunother. (2023) 72:4441–56. doi: 10.1007/s00262-023-03555-8
23. Rong D, Sun G, Zheng Z, Liu L, Chen X, Wu F, et al. MGP promotes CD8(+) T cell exhaustion by activating the NF-kappaB pathway leading to liver metastasis of colorectal cancer. Int J Biol Sci. (2022) 18:2345–61. doi: 10.7150/ijbs.70137
24. Duan W, Liu W, Xia S, Zhou Y, Tang M, Xu M, et al. Warburg effect enhanced by AKR1B10 promotes acquired resistance to pemetrexed in lung cancer-derived brain metastasis. J Transl Med. (2023) 21:547. doi: 10.1186/s12967-023-04403-0
25. Gu X, Zhu Y, Su J, Wang S, Su X, Ding X, et al. Lactate-induced activation of tumor-associated fibroblasts and IL-8-mediated macrophage recruitment promote lung cancer progression. Redox Biol. (2024) 74:103209. doi: 10.1016/j.redox.2024.103209
26. Qian Y, Galan-Cobo A, Guijarro I, Dang M, Molkentine D, Poteete A, et al. MCT4-dependent lactate secretion suppresses antitumor immunity in LKB1-deficient lung adenocarcinoma. Cancer Cell. (2023) 41:1363–1380 e1367. doi: 10.1016/j.ccell.2023.05.015
27. Chen Y, Zhou Y, Ren R, Chen Y, Lei J, Li Y. Harnessing lipid metabolism modulation for improved immunotherapy outcomes in lung adenocarcinoma. J Immunother Cancer. (2024) 12(7):e008811. doi: 10.1136/jitc-2024-008811
28. Han M, Bushong EA, Segawa M, Tiard A, Wong A, Brady MR, et al. Spatial mapping of mitochondrial networks and bioenergetics in lung cancer. Nature. (2023) 615:712–9. doi: 10.1038/s41586-023-05793-3
29. Zhang C, Zhou L, Zhang M, Du Y, Li C, Ren H, et al. H3K18 lactylation potentiates immune escape of non-small cell lung cancer. Cancer Res. (2024) 84:3589–601. doi: 10.1158/0008-5472.CAN-23-3513
30. Xiong J, He J, Zhu J, Pan J, Liao W, Ye H, et al. Lactylation-driven METTL3-mediated RNA m(6)A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol Cell. (2022) 82:1660–1677 e1610. doi: 10.1016/j.molcel.2022.02.033
31. Peyraud F, Guegan JP, Bodet D, Nafia I, Fontan L, Auzanneau C, et al. Circulating L-arginine predicts the survival of cancer patients treated with immune checkpoint inhibitors. Ann Oncol. (2022) 33:1041–51. doi: 10.1016/j.annonc.2022.07.001
32. Jiang SS, Xie YL, Xiao XY, Kang ZR, Lin XL, Zhang L, et al. Fusobacterium nucleatum-derived succinic acid induces tumor resistance to immunotherapy in colorectal cancer. Cell Host Microbe. (2023) 31:781–797 e789. doi: 10.1016/j.chom.2023.04.010
33. Tang F, Li J, Qi L, Liu D, Bo Y, Qin S, et al. A pan-cancer single-cell panorama of human natural killer cells. Cell. (2023) 186:4235–4251 e4220. doi: 10.1016/j.cell.2023.07.034
34. Dagher OK, Posey AD Jr. Forks in the road for CAR T and CAR NK cell cancer therapies. Nat Immunol. (2023) 24:1994–2007. doi: 10.1038/s41590-023-01659-y
35. Gong Y, Klein Wolterink RGJ, Wang J, Bos GMJ, Germeraad WTV. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol. (2021) 14:73. doi: 10.1186/s13045-021-01083-5
36. Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. (2021) 18:85–100. doi: 10.1038/s41571-020-0426-7
37. Li W, Wang X, Zhang X, Aziz AUR, Wang D. CAR-NK cell therapy: A transformative approach to overcoming oncological challenges. Biomolecules. (2024) 14(8):1035. doi: 10.20944/preprints202406.1007.v1
38. Marin D, Li Y, Basar R, Rafei H, Daher M, Dou J, et al. Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19(+) B cell tumors: a phase 1/2 trial. Nat Med. (2024) 30:772–84. doi: 10.1038/s41591-023-02785-8
39. Pan R, Ryan J, Pan D, Wucherpfennig KW, Letai A. Augmenting NK cell-based immunotherapy by targeting mitochondrial apoptosis. Cell. (2022) 185:1521–1538 e1518. doi: 10.1016/j.cell.2022.03.030
40. Yan X, Yao C, Fang C, Han M, Gong C, Hu D, et al. Rocaglamide promotes the infiltration and antitumor immunity of NK cells by activating cGAS-STING signaling in non-small cell lung cancer. Int J Biol Sci. (2022) 18:585–98. doi: 10.7150/ijbs.65019
41. Pockley AG, Vaupel P, Multhoff G. NK cell-based therapeutics for lung cancer. Expert Opin Biol Ther. (2020) 20:23–33. doi: 10.1080/14712598.2020.1688298
42. Della Chiesa M, Setti C, Giordano C, Obino V, Greppi M, Pesce S, et al. NK cell-based immunotherapy in colorectal cancer. Vaccines (Basel). (2022) 10(7):1033. doi: 10.3390/vaccines10071033
43. Liu Y, Wang Y, Yang Y, Weng L, Wu Q, Zhang J, et al. Emerging phagocytosis checkpoints in cancer immunotherapy. Signal Transduct Target Ther. (2023) 8:104. doi: 10.1038/s41392-023-01365-z
44. Zhang L, Tian L, Dai X, Yu H, Wang J, Lei A, et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J Hematol Oncol. (2020) 13:153. doi: 10.1186/s13045-020-00983-2
45. Li J, Chen P, Ma W. The next frontier in immunotherapy: potential and challenges of CAR-macrophages. Exp Hematol Oncol. (2024) 13:76. doi: 10.1186/s40164-024-00549-9
46. Shi Y, Li X, Dong Y, Yuan H, Wang Y, Yang R. Exploring the potential of CAR-macrophage therapy. Life Sci. (2025) 361:123300. doi: 10.1016/j.lfs.2024.123300
47. Lei A, Yu H, Lu S, Lu H, Ding X, Tan T, et al. Author Correction: A second-generation M1-polarized CAR macrophage with antitumor efficacy. Nat Immunol. (2024) 25:576. doi: 10.1038/s41590-023-01734-4
48. Chen Y, Yu Z, Tan X, Jiang H, Xu Z, Fang Y, et al. CAR-macrophage: A new immunotherapy candidate against solid tumors. BioMed Pharmacother. (2021) 139:111605. doi: 10.1016/j.biopha.2021.111605
49. Chen Y, Zhu X, Liu H, Wang C, Chen Y, Wang H, et al. The application of HER2 and CD47 CAR-macrophage in ovarian cancer. J Transl Med. (2023) 21:654. doi: 10.1186/s12967-023-04479-8
50. Patil NS, Nabet BY, Muller S, Koeppen H, Zou W, Giltnane J, et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung cancer. Cancer Cell. (2022) 40:289–300 e284. doi: 10.1136/jitc-2022-SITC2022.0435
51. Zhao Y, Chen J, Andreatta M, Feng B, Xie YQ, Wenes M, et al. IL-10-expressing CAR T cells resist dysfunction and mediate durable clearance of solid tumors and metastases. Nat Biotechnol. (2024) 42:1693–704. doi: 10.1038/s41587-023-02060-8
52. Timpanaro A, Piccand C, Dzhumashev D, Anton-Joseph S, Robbi A, Moser J, et al. CD276-CAR T cells and Dual-CAR T cells targeting CD276/FGFR4 promote rhabdomyosarcoma clearance in orthotopic mouse models. J Exp Clin Cancer Res. (2023) 42:293. doi: 10.1186/s13046-023-02838-3
53. Bassan D, Weinberger L, Yi J, Kim T, Weist MR, Adams GB, et al. HER2 and HLA-A*02 dual CAR-T cells utilize LOH in a NOT logic gate to address on-target off-tumor toxicity. J Immunother Cancer. (2023) 11(12):e007426. doi: 10.1136/jitc-2023-007426
54. Ye F, Dewanjee S, Li Y, Jha NK, Chen ZS, Kumar A, et al. Advancements in clinical aspects of targeted therapy and immunotherapy in breast cancer. Mol Cancer. (2023) 22:105. doi: 10.1186/s12943-023-01805-y
55. Srivastava S, Furlan SN, Jaeger-Ruckstuhl CA, Sarvothama M, Berger C, Smythe KS, et al. Immunogenic chemotherapy enhances recruitment of CAR-T cells to lung tumors and improves antitumor efficacy when combined with checkpoint blockade. Cancer Cell. (2021) 39:193–208 e110. doi: 10.1016/j.ccell.2020.11.005
56. Jiang G, Ng YY, Tay JCK, Du Z, Xiao L, Wang S, et al. Dual CAR-T cells to treat cancers co-expressing NKG2D and PD1 ligands in xenograft models of peritoneal metastasis. Cancer Immunol Immunother. (2023) 72:223–34. doi: 10.1007/s00262-022-03247-9
57. Zhou C, Huang D, Fan Y, Yu X, Liu Y, Shu Y, et al. Tislelizumab versus docetaxel in patients with previously treated advanced NSCLC (RATIONALE-303): A phase 3, open-label, randomized controlled trial. J Thorac Oncol. (2023) 18:93–105. doi: 10.1016/j.jtho.2022.09.217
58. Megyesfalvi Z, Gay CM, Popper H, Pirker R, Ostoros G, Heeke S, et al. Clinical insights into small cell lung cancer: Tumor heterogeneity, diagnosis, therapy, and future directions. CA Cancer J Clin. (2023) 73:620–52. doi: 10.3322/caac.21785
59. Chen ACY, Jaiswal S, Martinez D, Yerinde C, Ji K, Miranda V, et al. The aged tumor microenvironment limits T cell control of cancer. Nat Immunol. (2024) 25:1033–45. doi: 10.1038/s41590-024-01828-7
60. Kakuda T, Suzuki J, Matsuoka Y, Kikugawa T, Saika T, Yamashita M. Senescent CD8(+) T cells acquire NK cell-like innate functions to promote antitumor immunity. Cancer Sci. (2023) 114:2810–20. doi: 10.1111/cas.15824
61. Haston S, Gonzalez-Gualda E, Morsli S, Ge J, Reen V, Calderwood A, et al. Clearance of senescent macrophages ameliorates tumorigenesis in KRAS-driven lung cancer. Cancer Cell. (2023) 41:1242–1260.e1246. doi: 10.1016/j.ccell.2023.05.004
62. Plana D, Palmer AC, Sorger PK. Independent drug action in combination therapy: implications for precision oncology. Cancer Discovery. (2022) 12:606–24. doi: 10.1158/2159-8290.CD-21-0212
63. Peters S, Paz-Ares LG, Reck M, Carbone DP, Brahmer JR, Borghaei H, et al. Long-term survival outcomes with first-line nivolumab plus ipilimumab-based treatment in patients with metastatic NSCLC and tumor programmed death-ligand 1 lower than 1%: A pooled analysis. J Thorac Oncol. (2024) 20(1):94–108. doi: 10.1016/j.jtho.2024.09.1439
64. Lu S, Zhang W, Wu L, Wang W, Zhang P, Neotorch I, et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the neotorch randomized clinical trial. JAMA. (2024) 331:201–11. doi: 10.1001/jama.2023.24735
65. Ho D, Quake SR, McCabe ERB, Chng WJ, Chow EK, Ding X, et al. Enabling technologies for personalized and precision medicine. Trends Biotechnol. (2020) 38:497–518. doi: 10.1016/j.tibtech.2019.12.021
66. Dohner H, Wei AH, Lowenberg B. Towards precision medicine for AML. Nat Rev Clin Oncol. (2021) 18:577–90. doi: 10.1038/s41571-021-00509-w
67. Pan K, Farrukh H, Chittepu V, Xu H, Pan CX, Zhu Z. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J Exp Clin Cancer Res. (2022) 41:119. doi: 10.1186/s13046-022-02327-z
Keywords: immunotherapy, CAR-T cells, lung cancer, colorectal cancer, resistance mechanisms
Citation: Qin L, Li Y, Liu J and An X (2025) Advancements in cellular immunotherapy: overcoming resistance in lung and colorectal cancer. Front. Immunol. 16:1554256. doi: 10.3389/fimmu.2025.1554256
Received: 01 January 2025; Accepted: 17 January 2025;
Published: 05 February 2025.
Edited by:
Yu’e Liu, Harvard Medical School, United StatesReviewed by:
Jin-Ming Zhang, University of Texas Health Science Center at Houston, United StatesCopyright © 2025 Qin, Li, Liu and An. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoqin An, YW54aWFvcWluMTBAMTYzLmNvbQ==; Juan Liu, bGl1anVhbjgyOTFAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.