
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol., 25 February 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1548452
This article is part of the Research TopicEndocrine Dysfunctions and Immunometabolic Pathways in Autoimmune-Related CancersView all 3 articles
Thyroid dysfunction is a common immune-related adverse event (irAE) associated with immune checkpoint inhibitors (ICIs) that target PD-1, PD-L1, and CTLA-4. Nevertheless, the incidence of severe cases, defined as grade 3 or higher, remains rare. This report presents a detailed case study of severe thyroiditis in a patient with non-small cell lung cancer (NSCLC) who developed grade 3 thyroiditis following a single cycle of sintilimab monotherapy. The clinical presentation in this patient was remarkable for its early onset, occurring one week after the initiation of sintilimab therapy, and for its severe manifestations. During hospitalization, a prompt and accurate differential diagnosis was performed. Sintilimab treatment was discontinued, and the patient was promptly started on high-dose glucocorticoids, with a tapering schedule implemented as the condition improved or reached Common Terminology Criteria for Adverse Events (CTCAE) grade 1 or lower. The patient subsequently developed overt hypothyroidism, necessitating the initiation of thyroxine replacement therapy. Furthermore, we provide a comprehensive review of the mechanisms and risk factors associated with thyroid dysfunction immune-related adverse events (TD-irAEs). It is imperative for clinicians to meticulously monitor the clinical symptoms exhibited by patients. For those presenting with symptoms, prompt diagnosis and appropriate symptomatic management are essential. Additionally, regular thyroid function testing is recommended for high-risk patients, and we advocate for the assessment of baseline levels of thyroid peroxidase antibodies (TPOAb) and thyroglobulin antibodies (TGAb) prior to initiating ICI treatment.
Immune checkpoint inhibitors (ICIs), targeting the programmed cell death 1 receptor (PD-1)/programmed cell death ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) pathways, have transformed cancer treatment. These antibodies are increasingly used alone or with other therapies for metastatic and locally advanced cancers, particularly lung cancer (1, 2). However, they can cause a variety of adverse events (AE), some life-threatening, if not quickly identified and managed (3).
Thyroid disorders are common immune-related adverse events (irAEs), but severe cases (grade 3 or higher) are rare (4). Sintilimab, a PD1-directed IgG4 monoclonal antibody, is approved in China for lung, gastric/gastroesophageal adenocarcinomas, esophageal squamous cell carcinoma, and liver cancer (5). Trials combining sintilimab with chemotherapy reported no severe thyroid toxicity (6–9).
We present a case of grade 3 thyroiditis in a patient with non-small cell lung cancer (NSCLC) after one cycle of sintilimab monotherapy, following the CARE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2449).
A 76-year-old male patient, who is an active smoker, underwent a thoracoscopic right lower lobectomy with lymph node dissection on July 15,2020. Postoperative pathological analysis confirmed a diagnosis of lung adenocarcinoma with pathological staging IB2, pT1bN0M0. During a follow-up examination on March 29, 2023, a chest computed tomography (CT) scan identified a space-occupying lesion in the left upper lobe (Figure 1A). A subsequent 18-FDG positron emission tomography/computed tomography (PET/CT) scan indicated increased FDG uptake in the lesion, with obstructive pneumonia in the left upper lobe. No hilar or mediastinal lymphadenopathy was observed (Figure 1B). A bronchoscopic biopsy on April 4, 2023, indicated adenocarcinoma, with immunohistochemistry showing cytokeratin (CK)7(+), CK5/6 (–), thyroid transcription factor (TTF)-1(+), NapsinA (–), p40 (–),and a Ki67 index of about 40% (Figure 1C). Imaging and pathology confirmed stage IIIA adenocarcinoma in the left upper lung (cT4N0M0). Next-generation sequencing (NGS) foundno driver gene mutations for targeted therapy. PD-L1 expression analysis using the 22C3 antibody (DAKO) showed a TPS of 55%.
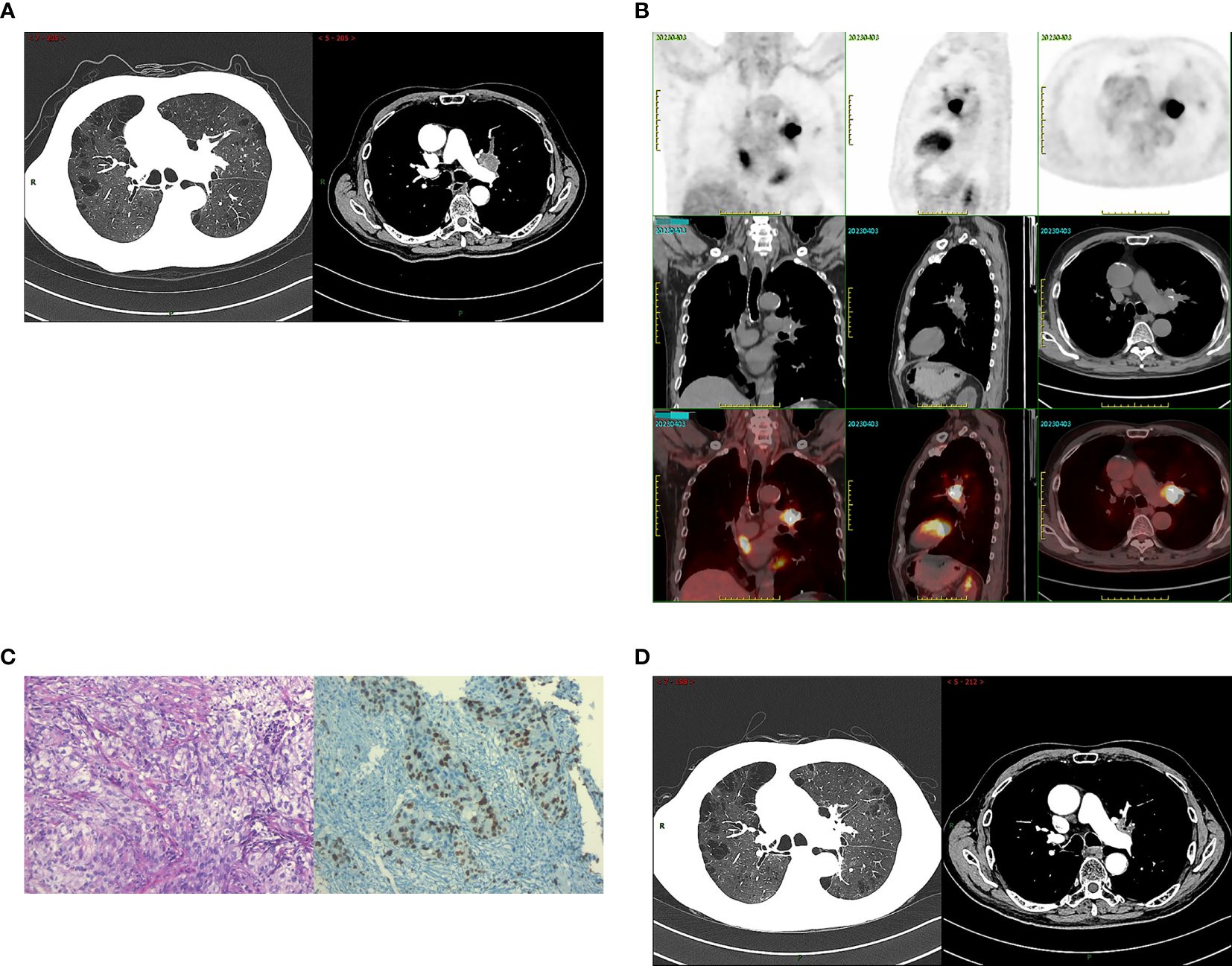
Figure 1. The radiological data at the initial diagnosis of patients and the evaluation of radiotherapy efficacy. (A) The patient’s chest CT on March 29, 2023: a space-occupying lesion in the left upper lobe. (B) The patient’s PET/CT on April 3, 2023: increased FDG uptake in the lesion, with obstructive pneumonia in the left upper lobe. No hilar or mediastinal lymphadenopathy was observed. (C) Tumor tissues were observed under light microscope (HE*200) and the immunohistochemical results: Ki67 index of about 40%(IHC*200). (D) The patient’s chest CT on June 29, 2023: the tumor showed a marked decrease in size. Radiotherapy efficacy was assessed as a partial response (PR).
The patient received radical radiotherapy targeting the left lung tumor region, with a total dose of 66 Gy administered in 33 fractions. Based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, the treatment efficacy was assessed as a partial response (PR) (Figure 1D). Immunotherapy with sintilimab at a dosage of 200 mg every three weeks commenced on June 20, 2023.
The patient was admitted on July 3, 2023, with a week-long history of palpitations, dyspnea, fatigue, and a 6kg weight loss, worsening over the last day. Tachycardia was observed during the physical exam. A chest CT scan showed no interstitial lung inflammation (Figure 2A), ruling out radiation and immune checkpoint inhibitor-related pneumonitis. Myocardial enzymes, B-type natriuretic peptide, and creatine kinase levels were within normal ranges, and myocarditis related to ICIs was ruled out. Serum thyroid function tests revealed a thyroid-stimulating hormone (TSH) level of less than 0.005mIU/L (reference range 0.27-4.2mIU/L) and free thyroxine (FT4) values exceeding 100.000pmol/L (reference value 12.0-22.0 pmol/L). Additionally, thyroglobulin antibodies (TGAb) were measured at 167.5IU/ml (reference value 0.0-115.0 IU/ml) and thyroid peroxidase antibodies (TPOAb) were greater than 600 IU/ml (reference value 0.0-34.0 IU/ml). Thyroid ultrasound demonstrated diffuse lesions within the thyroid. The Iodine-131(I-131) thyroid uptake test showed 6-hour and 24-hour uptake values of 1.2% (reference value 7-40%) and 0.7% (reference value 17.0-60.0%), respectively. Furthermore, Technetium-99m (Tc-99m) imaging of the thyroid gland indicated reduced uptake of the imaging agent (Figure 2B). The results of both tests indicated that the probable diagnosis for the patient was subacute thyroiditis. Considering the clinical and laboratory context, along with the patient’s prior history of ICI therapy, the diagnosis of ICI-induced thyroiditis was established, initially manifesting as thyroiditis (hashitoxicosis) due to the release of thyroid hormones from the inflamed thyroid gland. According to the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0, the thyroiditis was classified as grade 3. Formulate a treatment plan in accordance with the NCCN guidelines. Following a three-day course of beta-blockers and supportive care, the patient’s symptoms did not resolve. However, symptom relief was achieved after administering 60 mg of intravenous methylprednisolone daily for five days, followed by gradual tapering and discontinuation by day 43. Serum thyroid function tests performed on August 3, 2023, indicated a TSH level of 15.75 mIU/L and an FT4 level of 8.18 pmol/L. The variations in serum TSH and FT4/FT3 levels are depicted in Figure 2C. The patient subsequently developed overt hypothyroidism and commenced thyroxine replacement therapy. Following this, the patient did not undergo any antitumor treatment. At the most recent follow-up in September 2024, no disease progression was observed. CT imaging conducted at various time points is presented in Figures 3A, B. The timeline with relevant data from the episode of care is presented in Figure 3C.
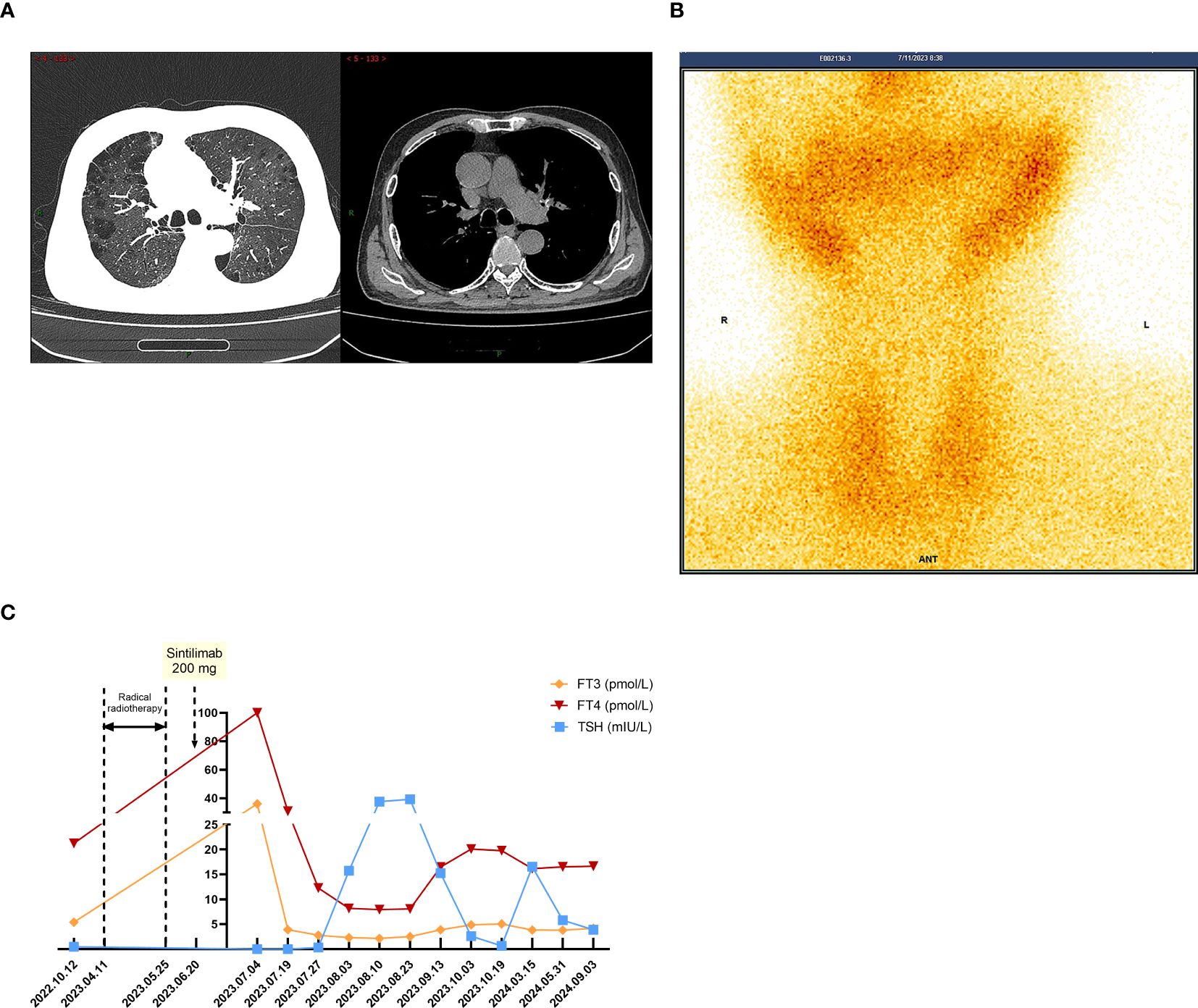
Figure 2. When TD-irAEs occur, the imaging and laboratory examinations of the patient. (A) The patient’s chest CT on July 3, 2023: no interstitial lung inflammation. (B) The patient’s SPECT/CT on July 11, 2023: thyroid technetium uptake function significantly reduced. (C) Time-course changes in the levels of TSH and FT3/FT4 in this case.
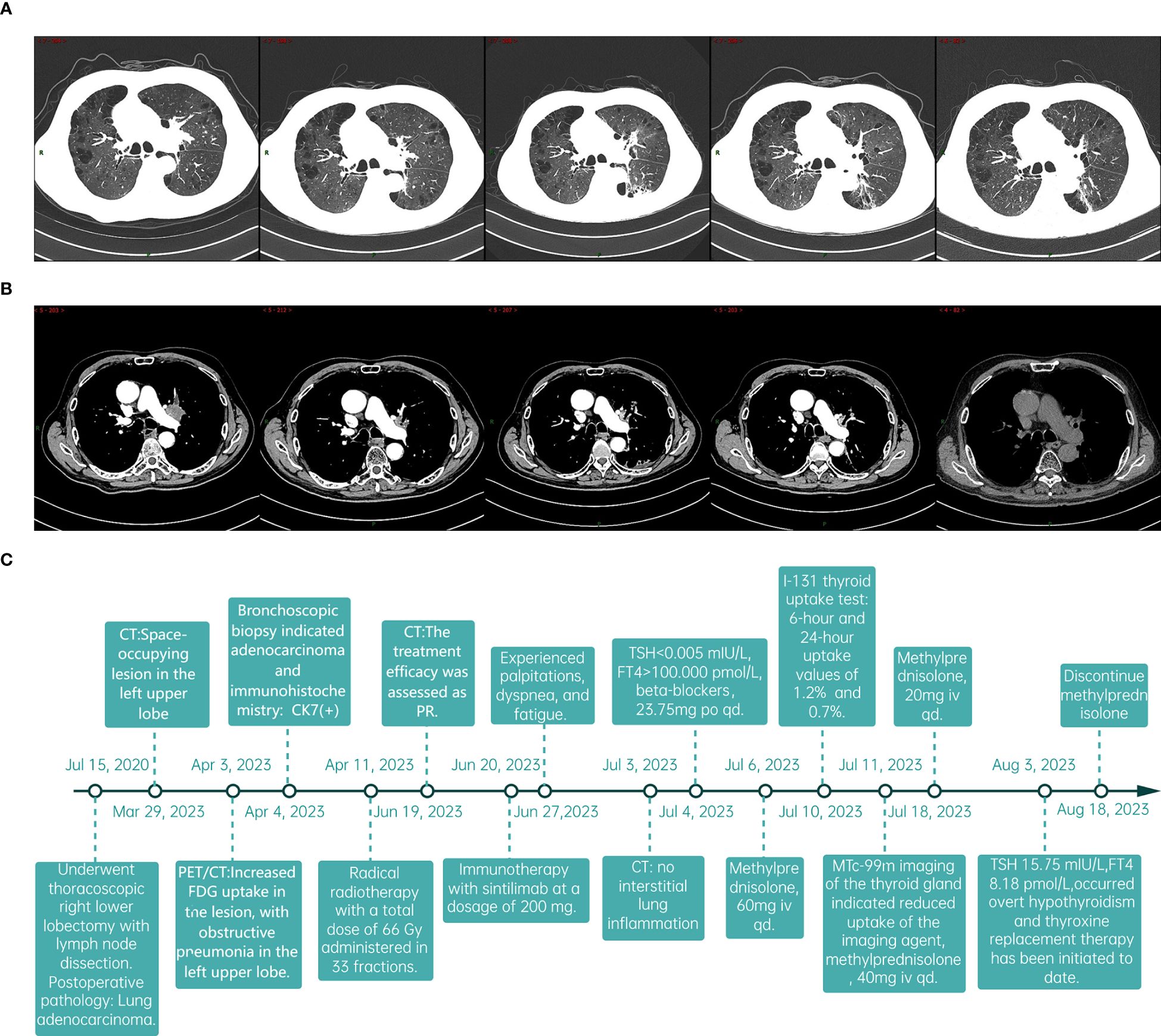
Figure 3. The follow-up data of patients and the timeline. (A, B) Sequentially from left to right, the dates indicate the patient’s CT scans: March 29, 2023; June 19, 2023; September 13, 2023; January 25, 2024; and September 3, 2024. (C) The timeline with relevant data from the episode of care.
Previous clinical studies have shown that thyroid dysfunction (TD) is among the most common irAE, with most cases being asymptomatic or mild (grade 1 or 2) (10, 11). The two most common patterns of ICI-related TD are thyroiditis followed by hypothyroidism with an incidence rate of 2-15% (12), and isolated hypothyroidism. Graves’ disease and thyroid eye disease are rare occurrences (13). Thyroiditis typically manifests relatively early, within 3 to 6 weeks after the initiation of treatment (14). In this patient, the clinical presentation was characterized by early onset, occurring one week following the initiation of sintilimab therapy, and severe manifestations. Prompt and accurate differential diagnosis was crucial to prevent the development of a thyroid crisis. The differential diagnosis for ICI-related thyroiditis in this patient was extensive. Initially, disease progression needed to be excluded. Subsequently, it was necessary to rule out radiation pneumonitis. Additionally, ICI-related myocarditis and myositis had to be considered and excluded. Imaging and blood tests ruled out the initial diagnoses. The patient’s thyroid wasn’t in the radiation area, and only sintilimab was used for treatment, excluding radioactive and drug-induced thyroiditis. Hashimoto’s thyroiditis and Graves’ disease were also excluded via the I-131 thyroid uptake test.
The destruction of the thyroid gland is recognized as the main mechanism behind ICI-related thyroiditis (13, 15), though the exact process remains unclear and may involve multiple factors. Experiments show that cytotoxic memory CD4+ T cells, activated by anti-PD-1 antibodies, are crucial in TD-irAEs development (16). Additionally, nivolumab may induce TD-irAEs by promoting IFN-γ secretion from thyroid cells, attracting CD8+ T cells, and increasing their lethality (17).
However, besides the mechanism of occurrence, the risk factors contributing to its occurrence also remain uncertain. ICI-related thyroiditis is associated with the specific type of ICIs and the combinations of treatments employed. Research indicated that combination therapy was associated with a higher incidence of adverse effects compared to monotherapy (4). The administration of combined PD-1/CTLA-4 therapy had been shown to result in thyroid dysfunction (TD) in approximately 15-20% of patients. In contrast, TD was observed in about 10% of patients receiving anti-PD-1/PD-L1 monotherapy and in 5% of those treated with ipilimumab monotherapy (4, 18). Moreover, the probability of developing hyperthyroidism was significantly greater with the use of PD-1 inhibitors compared to PD-L1 inhibitors (4). We have systematically gathered data on the incidence rates of hyperthyroidism observed in clinical trials for lung cancer involving commonly utilized PD-1 and PD-L1 inhibitors as presented in Table 1. The incidence rate for PD-1 inhibitors varies approximately from 0% to 11%, whereas for PD-L1 inhibitors, it ranges from approximately 1.2% to 16%. Notably, within the PD-1 inhibitor category, the incidence rates of hyperthyroidism differ among specific drugs. Patients receiving pembrolizumab demonstrated a higher incidence of hyperthyroidism, aligning with the findings of a meta-analysis conducted by Barroso-Sousa R and colleagues (4). Consistent with other PD-1 inhibitors, the occurrence of ICI-related hypothyroidism was more prevalent than hyperthyroidism, with the majority of cases being asymptomatic or mild (12, 54). Notably, in previous clinical trials (ORIENT 03, ORIENT 11, ORIENT 12, ORIENT 15, ORIENT 16, ORIENT 32, CONTINUUM, NCT04304209), no grade 3 or 4 thyroid dysfunction immune-related adverse events (TD-irAEs) were reported. In the ORIENT 31 study, the combination of sintilimab, the bevacizumab biosimilar IBI305, and chemotherapy (pemetrexed and cisplatin) was found to significantly enhance progression-free survival (PFS) in patients. However, this regimen was also associated with a higher incidence of grade III or higher TD-irAEs (Table 2).TD has been observed with antiangiogenesis treatments, especially with tyrosine kinase inhibitors (TKIs) like sunitinib, apatinib, and sorafenib (58, 60–64). One study noted a higher risk of immune checkpoint inhibitor (ICI)-induced TD after previous TKI treatment (59). Therefore, patients should have their thyroid function closely monitored when on combined antiangiogenic and anti-PD1/PD-L1 therapies.
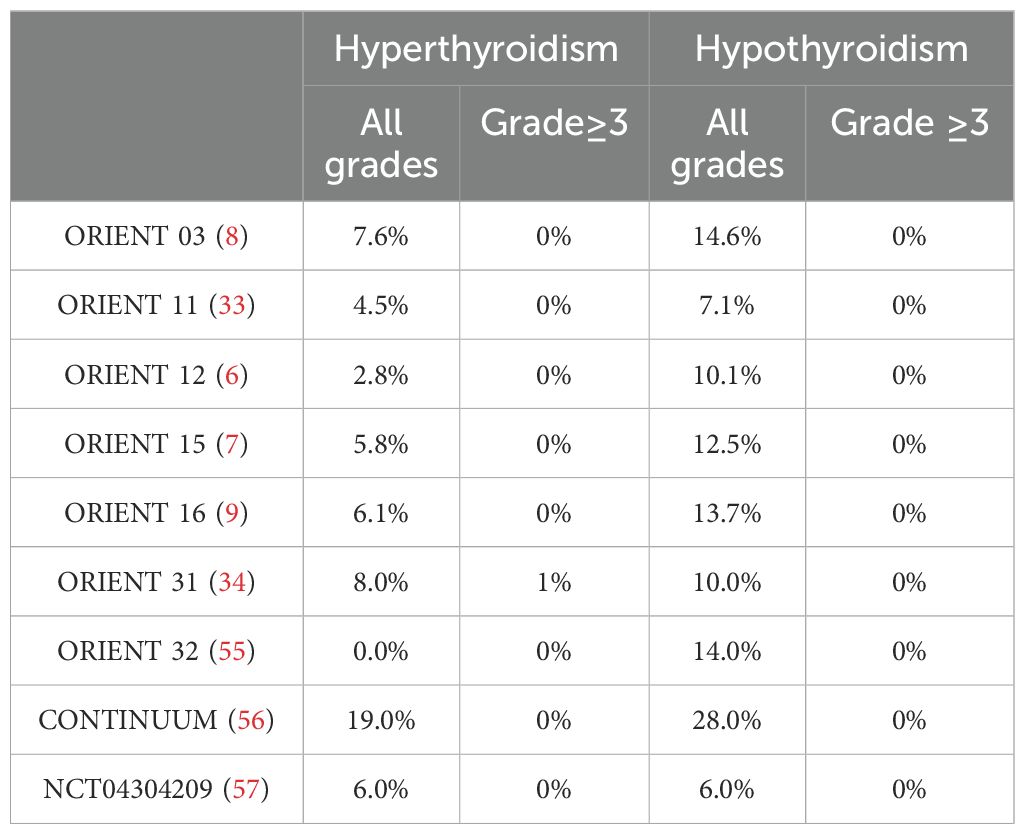
Table 2. Incidence rates of all grades and grade≥3 TD-irAEs in phase III clinical trials of sintilimab.
Patients with a history of autoimmune diseases may be at an increased risk for immune-related adverse events, such as thyroiditis (65). Studies indicate that TD-irAEs may arise from the activation of pre-existing subclinical thyroiditis prior to immune checkpoint inhibitor (ICI) therapy (13, 66, 67). In this case, the patient showed elevated TGAb and TPOAb levels in 2022 without symptoms, suggesting pre-existing latent chronic autoimmune thyroiditis. Previous research has not identified elevated TPOAb levels as a causative factor for TD-irAEs (66, 68). However, a recent prospective study stratified the risk of TD-irAEs induced by anti-PD-1 antibodies based on the presence of TGAb and TPOAb prior to treatment. The study found that patients who were positive for TGAb alone, as well as those positive for both TGAb and TPOAb at baseline, exhibited the highest risk, with an increased incidence of thyroiditis and hypothyroidism observed in both groups (69). These findings indicate that TGAb and TPOAb levels might predict the risk of TD-irAEs, and further study is needed. Patients with high TSH levels were also more prone to TD-irAEs (70). When conducting regular thyroid function tests, stratify based on baseline TPOAb and TGAb levels. For patients without abnormalities, follow guidelines to test TSH and FT4 every 4-6 weeks during ICI treatment and every 6-12 months afterward (71). For patients with elevated TPOAb and TGAb, test thyroid indicators before each medication dose and monitor for symptoms like palpitations, tremors, and weight loss during ICI treatment. Other factors like cancer subtype, sex, age (4, 11, 72, 73), and certain genetic predispositions, such as specific HLA haplotypes (73–76), might also influence the risk of ICI-induced thyroiditis.
Previous comprehensive meta-analyses examining PD-L1 expression in patients with NSCLC and its prognostic implications have demonstrated that elevated tumor PD-L1 expression is associated with reduced survival durations (77–79). Concurrently, other studies have suggested that increased PD-L1 expression in tumor tissues correlates with improved therapeutic outcomes when treated with checkpoint inhibitor therapy (20). Furthermore, some research has indicated that high levels of PD-L1 expression may increase the risk of irAEs (80), and another study has identified a link between the occurrence of checkpoint inhibitor pneumonitis (CIP) and elevated PD-L1 expression (81).In a study focusing on immune-related adverse events in patients with gastrointestinal malignancies, it was found that patients with grade 3-5 irAEs exhibited significantly higher serum PD-L1 levels compared to those with grade 0-2 irAEs. Additionally, an increase in serum PD-L1 levels was associated with the occurrence of rash (82). However, there are currently no studies that definitively clarify the relationship between PD-L1 levels and the severity of TD-irAEs. Further large-scale clinical investigations are warranted to elucidate these potential relationships.
We recommend an endocrine consultation to develop a treatment plan. For thyrotoxicosis symptoms like tachycardia and tremor, use beta-blockers (83). For grade 3 and 4 irAEs, start high-dose glucocorticoids promptly, tapering them as the condition improves or reaches CTCAE grade 1 or lower (84). Thyrotoxicosis often leads to hypothyroidism, so repeat thyroid tests in 4 to 6 weeks. If TSH is over 10 mIU/L, begin thyroid supplementation (15).
In summary, we report a case of grade 3 thyroiditis in a patient with NSCLC following a single cycle of sintilimab monotherapy. While TD-irAEs are not uncommon, the underlying mechanisms and predictive biomarkers associated with these events remain insufficiently understood. Therefore, it is crucial for clinicians to meticulously monitor the clinical symptoms exhibited by patients. For those presenting with symptoms, prompt diagnosis and appropriate symptomatic management are essential. Additionally, regular thyroid function testing is recommended for high-risk patients, and we advocate for the assessment of baseline levels of TPOAb and TGAb prior to initiating immune checkpoint inhibitor treatment.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Institutional Review Board of Central Hospital affiliated to Shandong First Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
XZ: Investigation, Writing – original draft, Writing – review & editing. XW: Investigation, Writing – original draft. SL: Project administration, Writing – review & editing. PC: Data curation, Writing – original draft. JC: Data curation, Writing – original draft. JL: Conceptualization, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Shandong Medical and Health Science and Technology Development Project (grant no. 202303110816), and China foundation for the promotion of health.
We thank the patient and their family for agreeing to the publication of case details and images.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Maritaz C, Broutin S, Chaput N, Marabelle A, Paci A. Immune checkpoint-targeted antibodies: a room for dose and schedule optimization. J Hematol Oncol. (2022) 15:6. doi: 10.1186/s13045-021-01182-3
2. Wu M, Huang Q, Xie Y, Wu X, Ma H, Zhang Y, et al. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation. J Hematol Oncol. (2022) 15:24. doi: 10.1186/s13045-022-01242-2
3. Esfahani K, Meti N, Miller WH Jr, Hudson M. Adverse events associated with immune checkpoint inhibitor treatment for cancer. CMAJ. (2019) 191:E40–40E46. doi: 10.1503/cmaj.180870
4. Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: A systematic review and meta-analysis. JAMA Oncol. (2018) 4:173–82. doi: 10.1001/jamaoncol.2017.3064
5. Liu H, Bai Y, Wang X, Zhang Y, Fu N. A retrospective analysis of post-marketing adverse drug reactions of sindili-zumab domestic PD-1 antibody drug. Chin Pharm J. (2024) 59:1748–56.
6. Zhou C, Wu L, Fan Y, Wang Z, Liu L, Chen G, et al. Sintilimab plus platinum and gemcitabine as first-line treatment for advanced or metastatic squamous NSCLC: results from a randomized, double-blind, phase 3 trial (ORIENT-12). J Thorac Oncol. (2021) 16:1501–11. doi: 10.1016/j.jtho.2021.04.011
7. Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ. (2022) 377:e068714. doi: 10.1136/bmj-2021-068714
8. Shi Y, Wu L, Yu X, Xing P, Wang Y, Zhou J, et al. Sintilimab versus docetaxel as second-line treatment in advanced or metastatic squamous non-small-cell lung cancer: an open-label, randomized controlled phase 3 trial (ORIENT-3). Cancer Commun (Lond). (2022) 42:1314–30. doi: 10.1002/cac2.12385
9. Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L, et al. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: the ORIENT-16 randomized clinical trial. JAMA. (2023) 330:2064–74. doi: 10.1001/jama.2023.19918
10. Iyer PC, Cabanillas ME, Waguespack SG, Hu MI, Thosani S, Lavis VR, et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid. (2018) 28:1243–51. doi: 10.1089/thy.2018.0116
11. Muir CA, Clifton-Bligh RJ, Long GV, Scolyer RA, Lo SN, Carlino MS, et al. Thyroid immune-related adverse events following immune checkpoint inhibitor treatment. J Clin Endocrinol Metab. (2021) 106:e3704–3704e3713. doi: 10.1210/clinem/dgab263
12. Quandt Z, Young A, Perdigoto AL, Herold KC, Anderson MS. Autoimmune endocrinopathies: an emerging complication of immune checkpoint inhibitors. Annu Rev Med. (2021) 72:313–30. doi: 10.1146/annurev-med-050219-034237
13. Husebye ES, Castinetti F, Criseno S, Curigliano G, Decallonne B, Fleseriu M, et al. Endocrine-related adverse conditions in patients receiving immune checkpoint inhibition: an ESE clinical practice guideline. Eur J Endocrinol. (2022) 187:G1–1G21. doi: 10.1530/EJE-22-0689
14. Chieng J, Htet ZW, Zhao JJ, Tai ES, Tay SH, Huang Y, et al. Clinical presentation of immune-related endocrine adverse events during immune checkpoint inhibitor treatment. Cancers (Basel). (2022) 14:2687. doi: 10.3390/cancers14112687
15. Network NCC. Management of Immunotherapy-Related Toxicities (2024.V2) (2024). Available online at: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf (Accessed November 28, 2024).
16. Yasuda Y, Iwama S, Sugiyama D, Okuji T, Kobayashi T, Ito M, et al. CD4(+) T cells are essential for the development of destructive thyroiditis induced by anti-PD-1 antibody in thyroglobulin-immunized mice. Sci Transl Med. (2021) 13:eabb7495. doi: 10.1126/scitranslmed.abb7495
17. Wu Y, Li J, Yang X, Hou B, Qiao H. Immunosensitivity mediated by downregulated AKT1-SKP2 induces anti-PD-1-associated thyroid immune injury. Int Immunopharmacol. (2023) 121:110452. doi: 10.1016/j.intimp.2023.110452
18. Wright JJ, Powers AC, Johnson DB. Endocrine toxicities of immune checkpoint inhibitors. Nat Rev Endocrinol. (2021) 17:389–99. doi: 10.1038/s41574-021-00484-3
19. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
20. Mok T, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. (2019) 393:1819–30. doi: 10.1016/S0140-6736(18)32409-7
21. O’Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. (2022) 23:1274–86. doi: 10.1016/S1470-2045(22)00518-6
22. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. (2018) 379:2040–51. doi: 10.1056/NEJMoa1810865
23. Boyer M, Şendur M, Rodríguez-Abreu D, Park K, Lee DH, Çiçin I, et al. Pembrolizumab plus ipilimumab or placebo for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%: randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol. (2021) 39:2327–38. doi: 10.1200/JCO.20.03579
24. Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Gao S, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med. (2023) 389:491–503. doi: 10.1056/NEJMoa2302983
25. Yang JC, Lee DH, Lee JS, Fan Y, de Marinis F, Iwama E, et al. Phase III KEYNOTE-789 study of pemetrexed and platinum with or without pembrolizumab for tyrosine kinase inhibitor-Resistant, EGFR-mutant, metastatic nonsquamous non-small cell lung cancer. J Clin Oncol. (2024) 42:4029–39. doi: 10.1200/JCO.23.02747
26. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. (2015) 373:123–35. doi: 10.1056/NEJMoa1504627
27. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
28. Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok T, et al. Nivolumab versus docetaxel in a predominantly chinese patient population with previously treated advanced NSCLC: checkMate 078 randomized phase III clinical trial. J Thorac Oncol. (2019) 14:867–75. doi: 10.1016/j.jtho.2019.01.006
29. Mok T, Nakagawa K, Park K, Ohe Y, Girard N, Kim HR, et al. Nivolumab plus chemotherapy in epidermal growth factor receptor-mutated metastatic non-small-cell lung cancer after disease progression on epidermal growth factor receptor tyrosine kinase inhibitors: final results of checkMate 722. J Clin Oncol. (2024) 42:1252–64. doi: 10.1200/JCO.23.01017
30. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. (2022) 386:1973–85. doi: 10.1056/NEJMoa2202170
31. Cascone T, Awad MM, Spicer JD, He J, Lu S, Sepesi B, et al. Perioperative nivolumab in resectable lung cancer. N Engl J Med. (2024) 390:1756–69. doi: 10.1056/NEJMoa2311926
32. Cheng Y, Han L, Wu L, Chen J, Sun H, Wen G, et al. Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the ASTRUM-005 randomized clinical trial. JAMA. (2022) 328:1223–32. doi: 10.1001/jama.2022.16464
33. Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Efficacy and Safety of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC: a Randomized, Double-Blind, Phase 3 Study (Oncology pRogram by InnovENT anti-PD-1-11). J Thorac Oncol. (2020) 15:1636–46. doi: 10.1016/j.jtho.2020.07.014
34. Lu S, Wu L, Jian H, Cheng Y, Wang Q, Fang J, et al. Sintilimab plus chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer with disease progression after EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): second interim analysis from a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med. (2023) 11:624–36. doi: 10.1016/S2213-2600(23)00135-2
35. Wang Z, Wu L, Li B, Cheng Y, Li X, Wang X, et al. Toripalimab plus chemotherapy for patients with treatment-naive advanced non-small-cell lung cancer: A multicenter randomized phase III trial (CHOICE-01). J Clin Oncol. (2023) 41:651–63. doi: 10.1200/JCO.22.00727
36. Lu S, Zhang W, Wu L, Wang W, Zhang P, Neotorch Investigators, et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the neotorch randomized clinical trial. JAMA. (2024) 331:201–11. doi: 10.1001/jama.2023.24735
37. Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. (2021) 397:592–604. doi: 10.1016/S0140-6736(21)00228-2
38. Gogishvili M, Melkadze T, Makharadze T, Giorgadze D, Dvorkin M, Penkov K, et al. Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: a randomized, controlled, double-blind phase 3 trial. Nat Med. (2022) 28:2374–80. doi: 10.1038/s41591-022-01977-y
39. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med. (2021) 9:305–14. doi: 10.1016/S2213-2600(20)30365-9
40. Ren S, Chen J, Xu X, Jiang T, Cheng Y, Chen G, et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-sq): A phase 3 trial. J Thorac Oncol. (2022) 17:544–57. doi: 10.1016/j.jtho.2021.11.018
41. Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, et al. Tislelizumab plus chemotherapy as first-line treatment for locally advanced or metastatic nonsquamous NSCLC (RATIONALE 304): A randomized phase 3 trial. J Thorac Oncol. (2021) 16:1512–22. doi: 10.1016/j.jtho.2021.05.005
42. Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: A phase 3 randomized clinical trial. JAMA Oncol. (2021) 7:709–17. doi: 10.1001/jamaoncol.2021.0366
43. Yue D, Wang W, Liu H, Chen Q, Chen C, Liu L, et al. Perioperative tislelizumab plus neoadjuvant chemotherapy for patients with resectable non-small-cell lung cancer (RATIONALE-315): an interim analysis of a randomised clinical trial. Lancet Respir Med. (2024) 13(2):119–29. doi: 10.1016/S2213-2600(24)00269-8. S2213-600(24)00269-8.
44. Heymach JV, Harpole D, Mitsudomi T, Taube JM, Galffy G, Hochmair M, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. (2023) 389:1672–84. doi: 10.1056/NEJMoa2304875
45. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. (2017) 377:1919–29. doi: 10.1056/NEJMoa1709937
46. Johnson ML, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Laktionov K, et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the phase III POSEIDON study. J Clin Oncol. (2023) 41:1213–27. doi: 10.1200/JCO.22.00975
47. Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. (2021) 398:1344–57. doi: 10.1016/S0140-6736(21)02098-5
48. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. (2020) 383:1328–39. doi: 10.1056/NEJMoa1917346
49. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2019) 20:924–37. doi: 10.1016/S1470-2045(19)30167-6
50. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. (2018) 378:2288–301. doi: 10.1056/NEJMoa1716948
51. Neal J, Pavlakis N, Kim SW, Goto Y, Lim SM, Mountzios G, et al. CONTACT-01: A randomized phase III trial of atezolizumab + Cabozantinib versus docetaxel for metastatic non-small cell lung cancer after a checkpoint inhibitor and chemotherapy. J Clin Oncol. (2024) 42:2393–403. doi: 10.1200/JCO.23.02166
52. Zhou Q, Chen M, Jiang O, Pan Y, Hu D, Lin Q, et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. (2022) 23:209–19. doi: 10.1016/S1470-2045(21)00630-6
53. Zhou C, Wang Z, Sun Y, Cao L, Ma Z, Wu R, et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol. (2022) 23:220–33. doi: 10.1016/S1470-2045(21)00650-1
54. Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. (2015) 4:560–75. doi: 10.3978/j.issn.2218-6751.2015.06.06
55. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. (2021) 22:977–90. doi: 10.1016/S1470-2045(21)00252-7
56. Liu X, Zhang Y, Yang KY, Zhang N, Jin F, Zou GR, et al. Induction-concurrent chemoradiotherapy with or without sintilimab in patients with locoregionally advanced nasopharyngeal carcinoma in China (CONTINUUM): a multicentre, open-label, parallel-group, randomised, controlled, phase 3 trial. Lancet. (2024) 403:2720–31. doi: 10.1016/S0140-6736(24)00594-4
57. Chen G, Jin Y, Guan WL, Zhang RX, Xiao WW, Cai PQ, et al. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: an open-label, single-centre phase 2 study. Lancet Gastroenterol Hepatol. (2023) 8:422–31. doi: 10.1016/S2468-1253(22)00439-3
58. Soni S, Rastogi A, Prasad KT, Behera D, Singh N. Thyroid dysfunction in non-small cell lung cancer patients treated with epidermal growth factor receptor and anaplastic lymphoma kinase inhibitors: Results of a prospective cohort. Lung Cancer. (2021) 151:16–9. doi: 10.1016/j.lungcan.2020.11.007
59. Kobayashi T, Iwama S, Yamagami A, Yasuda Y, Okuji T, Ito M, et al. Elevated TSH level, tgAb, and prior use of ramucirumab or TKIs as risk factors for thyroid dysfunction in PD-L1 blockade. J Clin Endocrinol Metab. (2022) 107:e4115–4115e4123. doi: 10.1210/clinem/dgac467
60. Tamaskar I, Bukowski R, Elson P, Ioachimescu AG, Wood L, Dreicer R, et al. Thyroid function test abnormalities in patients with metastatic renal cell carcinoma treated with sorafenib. Ann Oncol. (2008) 19:265–8. doi: 10.1093/annonc/mdm483
61. Khouri C, Jean Bart E, Logerot S, Decker-Bellaton A, Bontemps H, Mallaret M. Dysthyroidism with anti-VEGF treatment, a class effect? about one case report. Therapie. (2014) 69:521–4. doi: 10.2515/therapie/2014063
62. Pani F, Atzori F, Baghino G, Boi F, Tanca L, Ionta MT, et al. Thyroid dysfunction in patients with metastatic carcinoma treated with sunitinib: is thyroid autoimmunity involved. Thyroid. (2015) 25:1255–61. doi: 10.1089/thy.2015.0170
63. Walko CM, Aubert RE, La-Beck NM, Clore G, Herrera V, Kourlas H, et al. Pharmacoepidemiology of clinically relevant hypothyroidism and hypertension from sunitinib and sorafenib. Oncologist. (2017) 22:208–12. doi: 10.1634/theoncologist.2016-0233
64. Xiao J, Liang J, Zhang W, Li Y. Clinical observation of apatinib-related hypothyroidism in patients with advanced Malignancies. Exp Ther Med. (2020) 20:1961–6. doi: 10.3892/etm.2020.8937
65. Lee HJ, Manavalan A, Stefan-Lifshitz M, Schechter C, Maity A, Tomer Y. Permanent hypothyroidism following immune checkpoint inhibitors induced thyroiditis may be associated with improved survival: results of an exploratory study. Front Endocrinol (Lausanne). (2023) 14:1169173. doi: 10.3389/fendo.2023.1169173
66. García-Goñi M, Vázquez Gutiérrez B, Sanmamed MF, Martín-Algarra S, Luis-Pérez-Gracia J, Olmedo M, et al. Thyroid dysfunction caused by immune checkpoint inhibitors improves cancer outcomes. Endocr Relat Cancer. (2024) 31:e240064. doi: 10.1530/ERC-24-0064
67. Muir CA, Tsang V, Menzies AM, Clifton-Bligh RJ. Immune related adverse events of the thyroid - A narrative review. Front Endocrinol (Lausanne). (2022) 13:886930. doi: 10.3389/fendo.2022.886930
68. Jannin A, Penel N, Ladsous M, Vantyghem MC, Do Cao C. Tyrosine kinase inhibitors and immune checkpoint inhibitors-induced thyroid disorders. Crit Rev Oncol Hematol. (2019) 141:23–35. doi: 10.1016/j.critrevonc.2019.05.015
69. Zhou X, Iwama S, Kobayashi T, Ando M, Arima H. Risk of thyroid dysfunction in PD-1 blockade is stratified by the pattern of tgAb and TPOAb positivity at baseline. J Clin Endocrinol Metab. (2023) 108:e1056–1056e1062. doi: 10.1210/clinem/dgad231
70. Luongo C, Morra R, Gambale C, Porcelli T, Sessa F, Matano E, et al. Higher baseline TSH levels predict early hypothyroidism during cancer immunotherapy. J Endocrinol Invest. (2021) 44:1927–33. doi: 10.1007/s40618-021-01508-5
71. Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. (2021) 9:e002435. doi: 10.1136/jitc-2021-002435
72. Chennamadhavuni A, Abushahin L, Jin N, Presley CJ, Manne A. Risk factors and biomarkers for immune-related adverse events: A practical guide to identifying high-risk patients and rechallenging immune checkpoint inhibitors. Front Immunol. (2022) 13:779691. doi: 10.3389/fimmu.2022.779691
73. Jing Y, Yang J, Johnson DB, Moslehi JJ, Han L. Harnessing big data to characterize immune-related adverse events. Nat Rev Clin Oncol. (2022) 19:269–80. doi: 10.1038/s41571-021-00597-8
74. Kotwal A, Gustafson MP, Bornschlegl S, KottsChade L, Delivanis DA, Dietz AB, et al. Immune checkpoint inhibitor-induced thyroiditis is associated with increased intrathyroidal T lymphocyte subpopulations. Thyroid. (2020) 30:1440–50. doi: 10.1089/thy.2020.0075
75. Inaba H, Ariyasu H, Iwakura H, Kurimoto C, Takeshima K, Morita S, et al. Distinct clinical features and prognosis between persistent and temporary thyroid dysfunctions by immune-checkpoint inhibitors. Endocr J. (2021) 68:231–41. doi: 10.1507/endocrj.EJ20-0371
76. Sung C, An J, Lee S, Park J, Lee KS, Kim IH, et al. Integrative analysis of risk factors for immune-related adverse events of checkpoint blockade therapy in cancer. Nat Cancer. (2023) 4:844–59. doi: 10.1038/s43018-023-00572-5
77. Pan ZK, Ye F, Wu X, An HX, Wu JX. Clinicopathological and prognostic significance of programmed cell death ligand1 (PD-L1) expression in patients with non-small cell lung cancer: a meta-analysis. J Thorac Dis. (2015) 7:462–70. doi: 10.3978/j.issn.2072-1439.2015.02.13
78. Wang A, Wang HY, Liu Y, Zhao MC, Zhang HJ, Lu ZY, et al. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol. (2015) 41:450–6. doi: 10.1016/j.ejso.2015.01.020
79. Zhou ZJ, Zhan P, Song Y. PD-L1 over-expression and survival in patients with non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res. (2015) 4:203–8. doi: 10.3978/j.issn.2218-6751.2015.03.02
80. Suazo-Zepeda E, Bokern M, Vinke PC, Hiltermann T, de Bock GH, Sidorenkov G. Risk factors for adverse events induced by immune checkpoint inhibitors in patients with non-small-cell lung cancer: a systematic review and meta-analysis. Cancer Immunol Immunother. (2021) 70:3069–80. doi: 10.1007/s00262-021-02996-3
81. Zhou P, Zhao X, Wang G. Risk factors for immune checkpoint inhibitor-related pneumonitis in cancer patients: A systemic review and meta-analysis. Respiration. (2022) 101:1035–50. doi: 10.1159/000526141
82. Wang Y, Zou J, Li Y, Jiao X, Wang Y, Zhuo N, et al. Serological biomarkers predict immune-related adverse events and clinical benefit in patients with advanced gastrointestinal cancers. Front Immunol. (2022) 13:987568. doi: 10.3389/fimmu.2022.987568
83. Kennedy LB, Salama A. A review of cancer immunotherapy toxicity. CA Cancer J Clin. (2020) 70:86–104. doi: 10.3322/caac.21596
Keywords: sintilimab, irAEs, thyroiditis, NSCLC, case report
Citation: Zhao X, Wang X, Liu S, Cheng P, Chen J and Liu J (2025) Severe thyroiditis induced by sintilimab monotherapy in a patient with non-small cell lung cancer: a case report and literature review. Front. Immunol. 16:1548452. doi: 10.3389/fimmu.2025.1548452
Received: 19 December 2024; Accepted: 06 February 2025;
Published: 25 February 2025.
Edited by:
Tao Zhang, West China Hospital, ChinaReviewed by:
Dmitry Aleksandrovich Zinovkin, Gomel State Medical University, BelarusCopyright © 2025 Zhao, Wang, Liu, Cheng, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Liu, c2RqbmxqamllQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.