
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 03 March 2025
Sec. Microbial Immunology
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1547365
This article is part of the Research Topic Bidirectional Gut-Brain Interactions in Modulating Central Nervous System Diseases View all 3 articles
Post-stroke depression (PSD) is one of the most common and devastating neuropsychiatric complications in stroke patients, affecting more than one-third of survivors of ischemic stroke (IS). Despite its high incidence, PSD is often overlooked or undertreated in clinical practice, and effective preventive measures and therapeutic interventions remain limited. Although the exact mechanisms of PSD are not fully understood, emerging evidence suggests that the gut microbiota plays a key role in regulating gut-brain communication. This has sparked great interest in the relationship between the microbiota-gut-brain axis (MGBA) and PSD, especially in the context of cerebral ischemia. In addition to the gut microbiota, another important factor is the gut barrier, which acts as a frontline sensor distinguishing between beneficial and harmful microbes, regulating inflammatory responses and immunomodulation. Based on this, this paper proposes a new approach, the microbiota-immune-barrier axis, which is not only closely related to the pathophysiology of IS but may also play a critical role in the occurrence and progression of PSD. This review aims to systematically analyze how the gut microbiota affects the integrity and function of the barrier after IS through inflammatory responses and immunomodulation, leading to the production or exacerbation of depressive symptoms in the context of cerebral ischemia. In addition, we will explore existing technologies that can assess the MGBA and potential therapeutic strategies for PSD, with the hope of providing new insights for future research and clinical interventions.
Ischemic stroke (IS) is a prevalent central nervous system (CNS) disorder, ranking as the second leading cause of death and the third leading cause of disability worldwide (1). According to the statistics from 2013, there are approximately 25.7 million stroke survivors worldwide, with 71% being patients with IS (2). By 2020, the data showed that IS accounted for about 87% of all stroke cases (3), and the etiology involves a thrombotic or embolic event that leads to impaired blood flow to a region of the brain (4). Post-stroke depression (PSD) is the most common neuropsychiatric comorbidity, affecting more than one-third of IS survivors (5). Patients experiencing PSD often endure cognitive impairment, reduced quality of life, suicidal tendencies, and an increased risk of mortality (6). Despite substantial evidence indicating that PSD is one of the most severe complications following IS, it is frequently overlooked or inadequately treated.
PSD is considered to be a consequence of multiple interactions among biological, psychosocial, and multifactorial factors (7). Cerebrovascular diseases may serve as an initiating or exacerbating factor for depression (8, 9). While the exact pathogenesis of PSD is still not fully understood, its complexity should not be underestimated. According to the gut-brain axis(GBA) theory, alterations in the microbiota are closely linked to changes in brain structure, function, and behavior, and are associated with the pathogenesis of neuropsychiatric disorders (10). In recent years, research has increasingly recognized the gut microbiota as an important modulator of brain development, physiology, and host behavior. The gastrointestinal tract is a major organ for immune response, rich in immune cells, and accounts for over 70% of overall immune system activity (11). The Microbiota alters the intestinal barrier by interacting with immune cells (12), and in certain cases, influences the host by crossing the Blood-Brain Barrier(BBB) through the release of cytokines and metabolites, playing a crucial role in modulating stress-related behaviors, such as depression (13). Therefore, it is essential to explore the impact of the microbiota-gut-brain axis(MGBA) on PSD.
A meta-analysis revealed significant changes in the microbiota composition at the genus, family, and phylum levels in PSD patients compared to healthy individuals (14). Recent studies on animals have demonstrated that adjusting the microbiota can enhance neurological function and alleviate depressive symptoms in PSD rats, simultaneously reinforcing the integrity of the BBB (15). To fully grasp the interactions between the host and its symbiotic partners, it is essential to consider cellular barriers. Increasing evidence indicates that various cellular barriers within the MGBA act as novel conduits linking the microbiota to the brain (16). Traditionally, barriers were viewed as rigid and impenetrable, but it is now acknowledged that cellular barriers are dynamic and meticulously regulated communication interfaces. Consequently, this review will initially explore the interactions among the microbiota, intestinal barrier, and BBB within the MGBA, focusing on immune regulation and inflammatory responses. Next, we will delve into the potential mechanisms by which the microbiota-immune-barrier axis, this MGBA “high-speed pathway,” influences PSD following IS. Finally, to more effectively apply theory to clinical practice, we have thoroughly summarized various detection techniques for the MGBA and potential treatment methods for PSD.
The gut and brain barriers are fundamentally composed of epithelial or endothelial layers that, under physiological conditions, exhibit varying degrees of permeability. This characteristic is pivotal to their barrier function (17). Nevertheless, it is important to note that this barrier function is not static but rather undergoes dynamic changes. Under physiological circumstances, the primary role of the intestinal barrier is to delineate the body from the external environment, specifically the contents within the gastrointestinal lumen, while simultaneously facilitating the absorption of nutrients. On one hand, the intestinal mucosa acts as a formidable defense, preventing microorganisms from invading the host (18). On the other hand, it also permits symbiotic relationships with certain microorganisms, fostering a harmonious coexistence (19, 20).
The initial line of defense within the gastrointestinal tract is provided by a specialized coating on the exterior of the intestinal epithelium—the mucus layer. This layer is predominantly made up of mucins, notably Muc2, a glycoprotein featuring a network-like structure (21, 22). The mucus layer is divided into an inner and outer layer. It serves as a barrier, preventing large particles and microorganisms from making direct contact with the epithelium, a critical function in minimizing the exposure of intestinal epithelial cells (IEC) to potentially harmful agents (23, 24). Furthermore, the mucus layer is abundant in immunoglobulin A (IgA), secreted by plasma cells (25, 26). IgA facilitates the release of secretory IgA (sIgA) onto the intestinal surface through a complex mechanism known as transcytosis (27, 28). which neutralizes pathogens and aids in sustaining the equilibrium of the symbiotic microbiota (27, 29). It is important to note that the mucus barrier not only serves as a source of nutrients for the microbiota but also offers an ecological niche for their colonization.
The second line of defense is the intestinal epithelial barrier (IEB), which comprises a single layer of columnar epithelial cells (30). This barrier is dependent on cell-cell junctions, where neighboring intestinal cells are linked via junctional complexes, primarily made up of tight junctions (TJs) and adherens junctions, as well as desmosomes (31, 32). TJs are primarily composed of transmembrane proteins, such as claudins and occludins (33), and intracellular proteins, including zonula occludens(ZOs) (34). These structures restrict the diffusion of microorganisms and solutes through the paracellular pathway and dynamically modulate intestinal permeability, which is crucial for preserving the integrity of the epithelial barrier. Additionally, the intestinal epithelium houses several specialized cell types:Goblet cells secrete mucins to sustain the mucus barrier and transport soluble intestinal antigens to dendritic cells (DCs) (35, 36). Microfold cells (M cells), predominantly situated above Peyer’s patches (PPs) in the small intestine (37), facilitate a close antigen-sampling mechanism with DCs (38). Enteroendocrine cells (EECs) secrete various hormones and signaling molecules, acting as a communication bridge between the central and enteric nervous systems (39). Paneth cells produce antimicrobial peptides (AMPs), which regulate both symbiotic and pathogenic bacteria, aiding in the limitation of bacterial resistance and the maintenance of microbial equilibrium (40, 41). Intestinal stem cells (ISCs), located at the base of the crypts, proliferate and differentiate, migrating upwards to replenish various types of IEC (42). IEC can detect microbial stimuli and respond by bolstering their barrier function and coordinating appropriate immune responses, shifting from tolerance to pathogen-specific immunity (43). Consequently, IEC play a pivotal role in the development and homeostasis of mucosal immune cells. In concert with the mucus layer, the IEB controls the ingress of harmful “external” microorganisms into deeper tissues and their dissemination into the circulation.
The third line of defense is the intestinal vascular barrier (IVB), which is comprised of endothelial cells, pericytes, and enteric glial cells (EGCs). These endothelial cells create TJs analogous to those found in epithelial cells, and the IVB serves to shield the body from the passage of harmful molecules through both the IEB and other vascular barriers (44, 45). In contrast to epithelial barriers, intestinal endothelial cells possess a porous structure characterized by small pores delineated by the pore membrane, which enables selective permeability. The creation of these pores is contingent upon a specific endothelial membrane glycoprotein known as plasmalemma vesicle-associated protein-1 (PV-1), which is encoded by the PLVAP gene. PV-1 plays a pivotal role in maintaining endothelial homeostasis and regulating permeability (44, 46).
The gut-associated lymphoid tissues(GALT) is the largest collection of lymphoid tissues in the body, consisting of both organized lymphoid tissues, such as mesenteric lymph nodes and Peyer’s patches (PPs), and more diffusely scattered lymphocytes in the intestinal lamina propria (LP) and epithelium, including large numbers of IgA plasma blasts (47). GALT contains immune cells that coordinate the host’s local and systemic defense against intestinal insults. The LP is a thin layer of loose, non-cellular connective tissue beneath the epithelial layer, rich in immune cells and nerve endings. PPs are dome-shaped structures along the antimesenteric border of the small intestine, featuring lymphoid follicles surrounded by antigen-presenting cells and lymphocytes (predominantly IgA-producing plasma cells). The follicle-associated epithelium of PPs has a thin mucus layer and M cells that facilitate the transport of luminal antigens to the LP (48, 49). T follicular helper cells (Tfh) assist B cells in differentiating into plasma cells, which subsequently produce and secrete sIgA, a classical method (50, 51). These immune cells, including DCs, macrophages(Macs), T cells, and B cells, which are widely distributed in the LP, along with specialized IEC, rapidly respond to the invasion of foreign substances and work together to neutralize inflammation.
In summary, the mucus layer, IEB, and IVB collectively constitute an intestinal barrier. This barrier possesses chemical, mechanical, and immune properties that interact synergistically to maintain intestinal homeostasis (Figure 1).
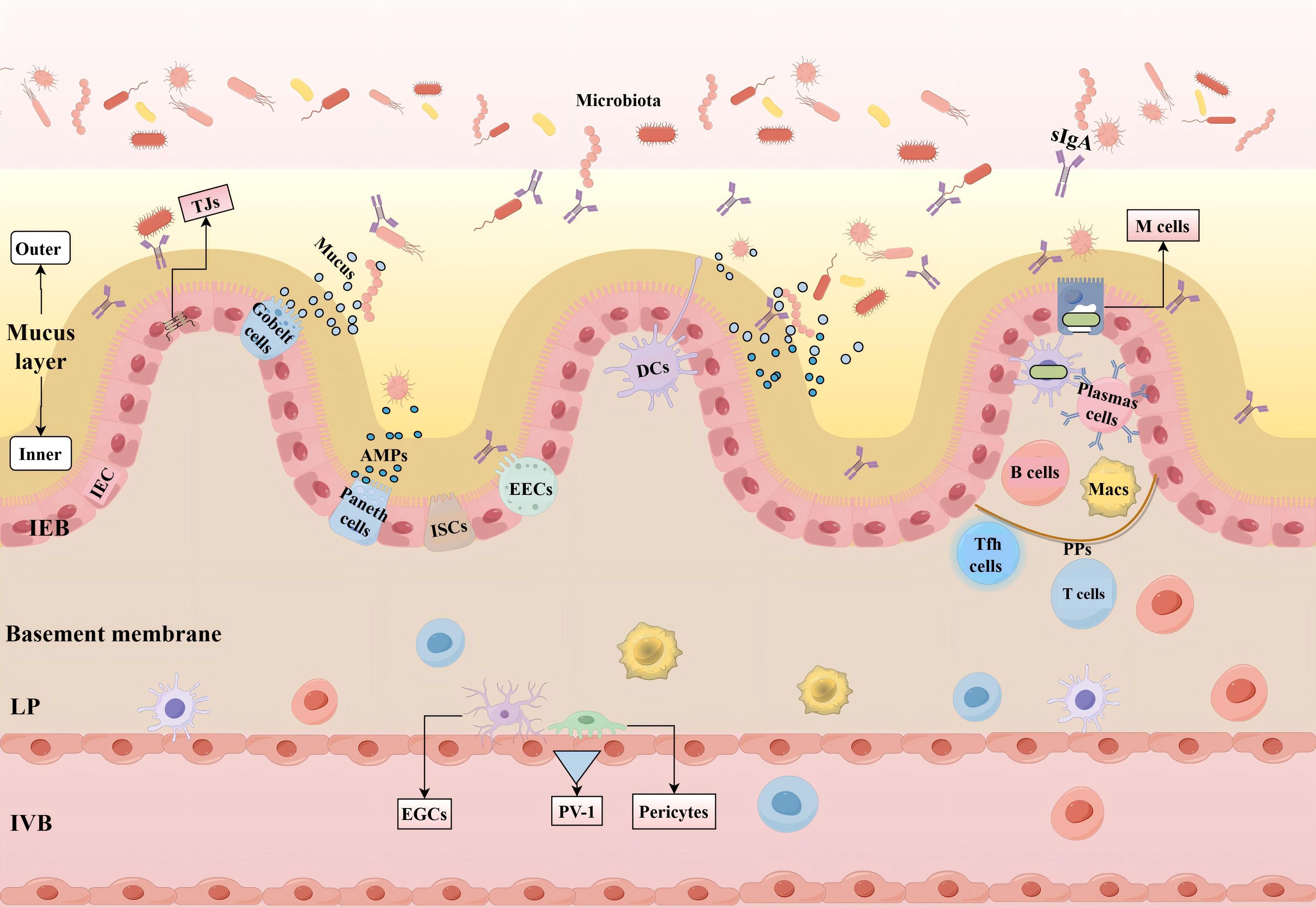
Figure 1. Intestinal barrier and its function. The mucus layer, primarily composed of mucin, is divided into an inner and outer layer and effectively blocks the entry of large particles and microorganisms. The IEB consists of a single layer of columnar IEC, which include various specialized cell types such as goblet cells that secrete mucin, EECs that secrete hormones, Paneth cells that secrete AMPs, and ISCs responsible for regeneration. Adjacent intestinal cells are connected through junctional complexes, including TJs that regulate intestinal permeability. The IVB is composed of endothelial cells, pericytes, and EGCs, which modulate the permeability of the vascular barrier and protect the intestine from harmful molecules. The formation of pores in endothelial cells is regulated by PV-1. GALT includes the LP and PPs, both of which are rich in immune cells, with M cells responsible for transporting antigens and bacteria to DCs. Tfh cells assist B cells in producing and releasing sIgA. IEC interact with DCs, Macs, T cells, and B cells to form a multi-layered defense system that maintains intestinal homeostasis. (IEB, Intestinal Epithelial Barrier; IEC, Intestinal Epithelial Cells; EECs, Enteroendocrine Cells; AMPs, Antimicrobial Peptides; ISCs, Intestinal Stem Cells; TJs, Tight junctions; IVB, Intestinal Vascular Barrier; EGCs, Enteric glial cells; PV-1, Plasmalemma Vesicle-Associated Protein-1; GALT, Gut-Associated Lymphoid Tissue; LP, Lamina Propria; PPs, Peyer’s Patches; M cells, Microfold Cells; DCs, Dendritic Cells; Tfh, T Follicular Helper Cells; sIgA, Secretory Immunoglobulin A; Macs, Macrophages.).
The gut microbiota consists of various microorganisms, including bacteria, viruses, fungi, and archaea, which coexist symbiotically within the human digestive tract and form a critical part of the gut barrier (52, 53). The microbiota educates the immune system to balance tolerance and defense, thereby maintaining gut homeostasis (54), and affecting distant organs such as the brain (55). In this section, we will delve into the interactions within the Gut Microbiota-Immune Cells-IEC-BBB pathway, as well as the direct and indirect effects of the Gut Microbiota on the barrier.
The importance of interactions between the microbiota and IEC in maintaining the structure and function of the gut barrier has been extensively studied. Specific microbes, such as Clostridia and Bacillus species, have been shown to effectively induce P-glycoprotein(P-gp) expression in murine IEC, helping to mitigate excessive inflammation and thus maintain gut homeostasis. A positive correlation has also been observed between microbial metabolites, short-chain fatty acids (SCFAs), and P-gp expression (56). Additionally, the microbiota regulates the development and maintenance of EGCs, which are a key component of the IVB (57). EGCs, which are similar to astrocytes in the brain, release soluble factors like S-nitrosoglutathione to regulate the integrity of TJs and support barrier function (58). Studies have shown that capillary network formation is stalled in adult germ-free mice but can resume and develop fully within 10 days of colonization with a complete microbiota or Bacteroides (59). Therefore, the microbiota may also influence the IVB directly or through interactions with EGCs. Experiments involving the oral administration of Lactobacillus casei and Lactobacillus paracasei have shown that these probiotics can increase Paneth cell numbers and enhance the secretion of AMPs, thus bolstering the antimicrobial activity of the intestinal barrier (60). Furthermore, Common SCFAs, including acetate, propionate, and butyrate, serve as energy sources and influence epithelial and immune host cell functions (61, 62). Acetate produced by protective bifidobacteria enhances intestinal defense mediated by epithelial cells, thereby protecting the host against lethal infection (63), and in vitro studies have shown that bifidobacterial strains use acetate to enhance TJs integrity, preventing Tumor Necrosis Factor-alpha(TNF-α)-induced epithelial barrier disruption (64). Acetate also facilitates the production of butyrate by cross-feeding other bacteria (65).
IEC expresses a range of pattern recognition receptors (PRRs), which are a diverse and well-characterized class of receptors. These include Toll-like receptors, NOD-like receptors, and RIG-I-like receptors, all of which are integral to innate immunity (66, 67). These receptors have the capacity to recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) (68). While IEC primarily engage in innate immunity, they also play crucial roles in the initiation and modulation of adaptive immune responses. Through the production of cytokines and chemokines, IEC interact with immune cells within the LP and deeper lymphoid tissues. This interaction is indispensable for maintaining immune homeostasis, ensuring a balanced and effective immune response against potential threats.
The intestinal epithelial tissue is inhabited by a distinct category of resident immune cells, known as intraepithelial lymphocytes (IELs), which are primarily T lymphocytes. IELs are intimately linked with the intestinal barrier. Initially, the gut microenvironment is abundant in transforming growth factor-beta (TGF-β), which augments the expression of CD103 (αEβ7 integrin) on the surface of IELs. CD103 serves as a pivotal marker for IEL adhesion to E-cadherin, an epithelial cell adhesion molecule, and facilitates the enduring residence of IELs within the epithelial tissue (69, 70). This mechanism facilitates the rapid acquisition of critical signals by IELs from the epithelial tissue and surrounding environment, thereby promoting their homing, maturation, and functional activation. Secondly, interleukin(IL)-15 secreted by IEC fosters the proliferation and survival of IELs and modulates their cytotoxic capabilities. The sustenance of IELs is contingent upon signals emanating from MyD88 and Toll-like receptor 2 within IEC, which are essential for IL-15 production (71). Moreover, chemokine CCL25, produced by IEC, lures CCR9-positive IELs to migrate towards the intestinal epithelium (72). CD8αβ+ IEL located in the epithelial layer of the small intestine have been confirmed to secrete α-defensins, which may serve as an important supplement to the α-defensins produced by Paneth cells. The synergistic effect of CD8αβ+ IEL and Paneth cells can effectively prevent bacterial invasion (73). IELs oversee and preserve the epithelial barrier, engaging in innate immune responses against pathogens. Owing to the absence of genetic tools targeting specific IELs subpopulations, the exact characteristics and mechanisms of IEL functions are not yet fully elucidated.
Innate lymphoid cells (ILCs) are a type of innate immune cells that play a crucial role in regulating the barrier function of various tissues, including the gastrointestinal tract (74). The cytokines secreted by IEC encourage the proliferation and activation of ILCs, encompassing natural killer cells as well as the ILC1, ILC2, and ILC3 subsets. Specifically, ILC1 responds to co-stimulatory signals from IEC, which are mediated by the microbiota, and produces interferon-gamma (IFN-γ) (75), In contrast, ILC2 secretes IL-13 upon infection, targeting crypt ISCs to promote the differentiation of goblet cells (76). Both ILC1 and ILC2 augment goblet cell mucus secretion, thereby aiding in the preservation of intestinal barrier integrity. The activation of ILC2 is contingent upon cytokines derived from epithelial cells, such as IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) (77, 78). ILC3 generates IL-22 (79), which stimulates IEC to produce AMPs, playing a vital role in sustaining epithelial barrier function (80). Additionally, IL-17, produced by ILC3, also contributes to maintaining barrier integrity during intestinal inflammation (81).
IEC actively contribute to the recruitment of Neutrophils(Neuts) by secreting chemokines. These chemokines, which encompass CXCL7 (82) and CCL20 (83, 84), aid in the migration and infiltration of neuts. Furthermore, matrix metalloproteinase (MMP)-3, produced by IEC, amplifies the bioactivity of CXCL7 (82). During inflammatory episodes, for instance, IL-6, IL-8, and IL-33 derived from IEC play a crucial role in the recruitment and migration of neuts (85–88). Notably, IEC recruit not only neuts but also support their functions by secreting the aforementioned cytokines. Studies have revealed that IL-6 and its soluble receptor sIL-6Rα can regulate the transition of neuts to monocyte infiltration at inflammatory sites by modulating chemokines. In the context of acute inflammation, IL-6 promotes the resolution of neuts, aiding in inflammation resolution; whereas in chronic inflammation, IL-6 increases monocyte infiltration, contributing to disease progression (89). IL-8 not only acts as a direct chemoattractant for neutrophils but also activates neutrophils to release secondary chemokines stored within their granules (90). Furthermore, IL-33 can also induce functional polarization of neuts (91). In summary, IEC play a critical role in regulating neuts recruitment, trans-epithelial migration, cell death, and clearance (92).
As the most abundant type of white blood cells, neuts, like other immune cells, regulate the development and function of IEC. Under physiological conditions, neutrophil-derived IL-22 has been shown to enhance the production of AMPs by IEC, contributing to barrier defense (93). Research has confirmed that neuts also enhance epithelial protection by inducing the production of amphiregulin in IEC through the secretion of TGF-β (94). Under pathological conditions, neutrophil-derived prosecretory factors are closely associated with goblet cell depletion, a histological hallmark of intestinal inflammation (95). It is well-established that the uncontrolled accumulation of overactivated neuts leads to crypt architectural distortion and crypt abscess formation, accompanied by excessive enzymatic reactions, the production of pro-inflammatory cytokines such as TNF-α and IL-1β, and the release of non-cytokine inflammatory mediators such as α-defensins and calprotectin (96, 97). which may be closely linked to the pathogenesis of inflammatory bowel disease.
IEC detect microbial signals and secrete cytokines, including IL-33, TGF-β, and TSLP, which modulate the development of DCs and Macs (98–100). Within the small intestine, IEC generate TGF-β and retinoic acid (RA), facilitating the migration of CD103+ DCs to epithelial cells and expanding the functional repertoire of gut DCs (101). CD103+ DCs reciprocally affect the differentiation of Foxp3+ Tregs by secreting TGF-β and RA (102, 103). Macs can alternate between pro-inflammatory (M1) and anti-inflammatory (M2) states in response to various stimuli (104, 105). M1 macs typically secrete high levels of pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-12. In contrast, M2 macs produce anti-inflammatory cytokines, such as IL-10, which directly or indirectly affect the function of intestinal epithelial cells (106), Additionally, IL-10 helps to promote the expression of Foxp3+ Tregs (107).
Effector T cells are typically the dominant lymphocytes in the gut, responsible for mediating a range of host immune defenses and preserving homeostasis. Within the LP, the two most prevalent types are T helper (Th) 17 cells and regulatory T cells (Tregs). These subtypes exhibit heterogeneity. Generally, Th17 cells foster inflammatory immune responses, whereas Tregs suppress excessive or unnecessary immune activation and commonly exhibit anti-inflammatory properties. The functional antagonism between these two subsets is crucial for maintaining immune homeostasis in the LP (108). Th17 cells secrete IL-17, which is pivotal in regulating the integrity of intestinal epithelial and the intestinal mucosal barrier. It influences the cellular distribution of the TJs occludin in IEC (109–111). Furthermore, IL-22 produced by Th17 cells can promote epithelial proliferation and mucosal repair (112). It is important to note that Tfh cells secrete IL-21 which promotes the differentiation of B cells into plasma cells that produce IgA and secrete sIgA (113, 114), thereby fortifying the protection of the epithelial barrier (Figure 2).
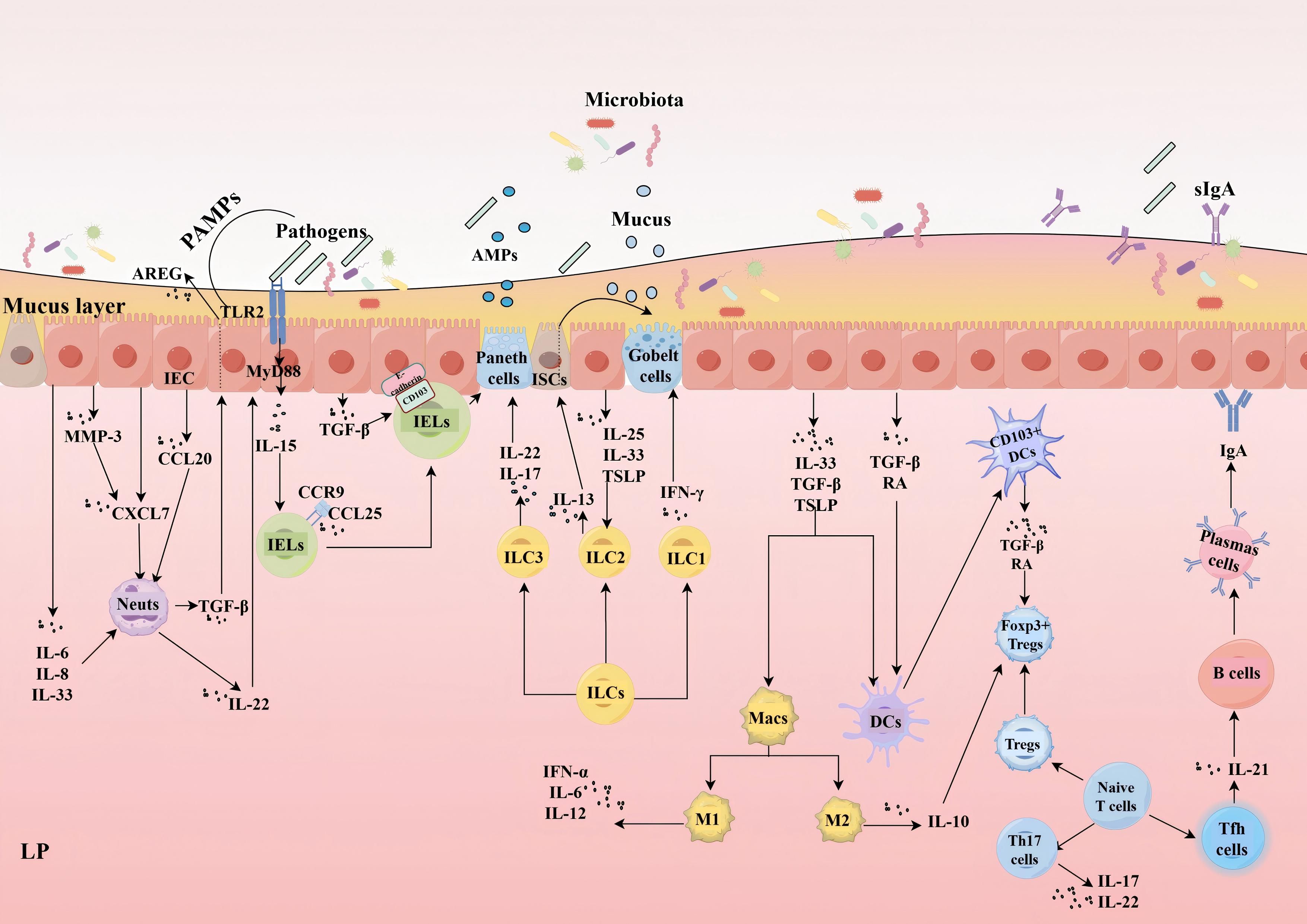
Figure 2. Interactions between IEC and immune cells. IEC promote neuts migration and infiltration by secreting CXCL7, CCL20, and MMP-3, with MMP-3 enhancing CXCL7 activity. During inflammation, IL-6, IL-8, and IL-33 produced by IEC regulate Neuts recruitment and function. Neuts enhance epithelial barrier protection by secreting IL-22 to promote the production of AMPs by IEC and by inducing the generation of AREG in IEC through TGF-β. IELs depend on TGF-β secreted by IEC to induce CD103 binding to E-cadherin, facilitating long-term residency. IL-15, derived from IEC, stimulates the proliferation, survival, and cytotoxicity of IELs; the maintenance of IELs relies on IEC MyD88/TLR2 signaling for the production of IL-15. Additionally, chemokine CCL25, produced by IEC, attracts CCR9-positive IELs to migrate towards the intestinal epithelium. IELs can also work synergistically with Paneth cells to release AMPs.ILC1 responds to signals from IEC to produce IFN-γ; ILC2 secretes IL-13 during infection, promoting crypt stem cell differentiation into goblet cells, both enhancing mucus secretion. ILC2 activation depends on IL-25, IL-33, and TSLP derived from IEC. ILC3-produced IL-22 stimulates IEC to secrete AMPs, while IL-17 maintains barrier function during inflammation. IEC detect microbial signals and secrete IL-33, TGF-β, and TSLP to regulate DCs and Macs. TGF-β and RA promote CD103+ DC migration to epithelial cells; CD103+ DCs reciprocally influence Foxp3+ Tregs differentiation by secreting TGF-β and RA. M1 macs secrete high levels of pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-12. M2 macs produce anti-inflammatory cytokines, such as IL-10, which directly or indirectly affect the function of IEC. Additionally, IL-10 aids in promoting the expression of Foxp3+ Tregs. Th17 cells secrete IL-17 and IL-22, which are vital for maintaining the integrity of the intestinal epithelium. Tfh cells secrete IL-21, which promotes the differentiation of B cells into plasma cells that produce IgA and secrete sIgA. (IEC, Intestinal Epithelial Cells; MMP, Matrix Metalloproteinase; IL, Interleukin; AMPs, Antimicrobial Peptides; Neuts, Neutrophils; AREG, Amphiregulin; TGF-β, Transforming Growth Factor Beta; IELs, Intraepithelial Lymphocytes; ILC, Innate Lymphoid Cell; IFN-γ, Interferon-gamma; TSLP, Thymic Stromal Lymphopoietin; DCs, Dendritic Cells; Macs, Macrophages; RA, Retinoic Acid; Th17,T helper 17 cells; Tregs, Regulatory T cells; TNF-α, Tumor Necrosis Factor-alpha; sIgA, Secretory Immunoglobulin A.).
The gut microbiota-IEC, immune cells-IEC, as well as gut microbiota-immune cells, and the IEC-barrier function, are all key factors affecting barrier function. In short, the stability of the gut barrier depends not only on the self-regulation of IEC but also on the continuous interaction between IEC, immune cells, and the microbiota.
As previously mentioned, the microbial community plays a crucial role through continuous interactions with a range of IEC, shaping the structure of the intestinal barrier and regulating paracellular permeability, which is essential for nutrient absorption and the reinforcement of the mucus layer. This section will explore how the microbiota indirectly modulates immune cells, thereby influencing the structure and function of the barrier, while emphasizing the indispensable role of the coordinated activity of intestinal immune cells.
The gut microbiota plays a crucial role in the maturation of the gut mucosal immune system. A review has shown that probiotics not only modulate the expression of mucins and TJs but also influence IEC apoptosis and proliferation, as well as directly or indirectly regulating immune and anti-inflammatory functions. Through these mechanisms, probiotics dynamically maintain the integrity of the intestinal barrier (115). Studies on germ-free mice have shown that they have smaller PPs, immature GALT, reduced intestinal lymphocytes, and lower IgA production (116, 117). However, these damages can be restored by re-establishing the gut microbiota (118). The microbiota’s influence extends beyond the barrier, as microbial metabolites also play critical roles in driving anti-inflammatory and barrier-protective functions, as well as impacting IEC differentiation and gene expression. For instance, butyrate, a metabolite produced by commensal microbes, induces the differentiation of colonic Tregs cells and promotes epithelial integrity (119, 120). The composition of the gut microbiota, especially the composition of Clostridium species, may affect the number and function of Tregs and promote the formation of mucosal tolerance (121). Akkermansia muciniphila (Am), known as the “intestinal sentinel,” may promote the production of mucin by goblet cells and repair intestinal barrier function (122). Immunologically, Am not only interacts with TLR4 to modify the RoR-T + regulatory T cell immunological response (123), but also activates Macs via the TLR2/NLRP3 signaling pathway both in vitro and vivo (124).
In summary, the intestinal barrier is a complex multi-layered structure that includes the microbial barrier. Within this structure, specific microbes act as probiotics; they not only directly affect the barrier but also maintain the balance of mucosal immunity through interactions with immune cells. and disruptions in microbial composition can lead to barrier dysfunction and abnormal substance release along the GBA.
The outermost layer of the brain, the meningeal barrier, is located beneath the inner surface of the skull, consisting of the dura mater, arachnoid mater, and pia mater, which encase the brain and cerebrospinal fluid. This layer allows for immune cell transport. Deeper within the brain, there are two critical barriers: BBB and the blood-cerebrospinal fluid barrier, the latter located within the choroid plexus of the brain ventricles (125). The BBB is a highly selective, semipermeable barrier composed of endothelial cells, astrocytic end-feet, and pericytes embedded in the blood vessel basement membrane (126). IVB and the BBB have several similarities, including the increased expression of PV-1 during the injury process and its regulation by the Wnt/β-catenin signaling pathway (127, 128). Furthermore, Wnt/β-catenin signaling in the intestinal endothelium regulates and maintains BBB characteristics during both embryonic and postnatal development: β-catenin enhances endothelial-specific stability to maintain barrier homeostasis, and its inactivation significantly downregulates claudin-3, upregulates vesicle-associated proteins, and leads to BBB disruption (127). A pivotal study revealed that during inflammation, IVB disruption in mice induces choroid plexus vascular barrier closure, restricting access of large molecules (129). Another study suggested that transplantation of EGCs into damaged spinal cords can accelerate the repair of the vasculature and BBB at the injury site (130). These findings suggest that the IVB and BBB are physiologically interconnected and pathologically interrelated.
The human microbiota consists of trillions of microorganisms, including over 1,000 bacterial species and approximately 3 million identified genes, a number 150 times larger than the human genome (131). The primary constituents of the microbiota are the Firmicutes, Bacteroidetes and Actinobacteria (132), When the composition of the microbiota changes, its associated functions may also change or even be compromised (133). The microbiota also plays a vital role in BBB regulation (134). Additionally, these microorganisms have the ability to convert dietary components, such as macromolecules, micronutrients, fibers, and polyphenols, into various metabolites, including SCFAs, trimethylamine, amino acid derivatives, and vitamins. These microbial-derived metabolites play essential metabolic and signaling roles in regulating the host’s internal environment, particularly in terms of their impact on the integrity of the BBB and brain function (13). These studies suggest that the microbiota is essential in regulating the intestinal and brain barriers under physiological conditions. Research indicates that a lack of microbiota is associated with increased BBB permeability and decreased expression of TJs occludin and claudin-5. Transferring fecal matter from pathogen-free mice or treating germ-free mice with SCFAs-producing bacteria can reduce BBB permeability (135). In addition to crossing the BBB and affecting the maturation of microglia, SCFAs also appear to impact neuronal function (136). therefore, SCFAs play a vital role in brain development and CNS homeostasis. An imbalance in the gut microbiota can lead to weakened intestinal barrier function, allowing endotoxins produced by Gram-negative bacteria, such as Lipopolysaccharide(LPS), and harmful substances from opportunistic pathogens to penetrate into the circulatory system. In the case of cerebral ischemia, damage to the BBB enables LPS to enter brain tissue. TLR4 plays a key role in the inflammatory response triggered by LPS, leading to neuroinflammation induced by LPS in microglia/macs, which can further exacerbate ischemic brain injury (137, 138). Furthermore, The gut microbiota plays an important role in Th17 cell differentiation, an important class of CD4+helper T cells, and their infiltration into the brain (139, 140).
In summary, the microbiota impacts BBB integrity through several mechanisms: (1) interactions with a compromised intestinal barrier and immune cells, (2) induction of inflammatory cytokine release by microbial products like LPS, (3) direct regulation of TJs expression by SCFAs or through glial cell modulation neuroinflammation, and (4) stimulation of T cell differentiation and brain infiltration. Therefore, the microbiota and their metabolites have a profound impact on the regulation of barrier function and integrity through their interactions with immune cells. Dysbiosis of the microbiota may lead to barrier dysfunction and abnormal substance release along the GBA, providing a new perspective and understanding for our comprehension of the onset and development of brain diseases.
The interaction between IS and the gut microbiota reveals the critical role of the GBA in the pathophysiology of stroke. Based on the close connection between the gut microbiota, immune cells, and integrity and function of barrier function, this section will explore the close relationship between IS and this pathway.
Previous studies have confirmed that changes in the gut microbiota can have profound effects on brain. For instance, a systematic review indicated that aging and inflammation might contribute to variations in microbial composition and predispose individuals to IS. The regulation of the Firmicutes/Bacteroidetes ratio could be a potential target for treating IS (141). Another study offers a proof-of-concept demonstrating that the gut microbiome itself is cerebroprotective in experimental stroke (142). However, whether changes in brain function also directly affect the microbiome? The following evidence on the impact of IS on the microbiome may provide an answer. The microbiota of IS patients exhibits significant dysbiosis, characterized by notable alterations in the proportion of Firmicutes and Bacteroidetes, along with a substantial increase in the abundance of opportunistic pathogens, such as Enterobacter and Desulfovibrio species (143). Simultaneously, Research has demonstrated the significant impact of brain injury on the composition of the microbiota; these effects include a reduction in the diversity of microbiota species and intestinal bacterial overgrowth, with a preferential expansion of the Bacteroidetes phylum. This phenomenon is closely linked to intestinal barrier dysfunction and decreased gut motility, which, when addressed, can lead to improved stroke outcomes (144). Beyond microbiota dysbiosis, there are also prominent changes in its metabolites. Research indicates a decrease in the abundance of SCFAs-producing bacteria, with SCFAs negatively correlated with the severity and prognosis of IS (145, 146). Another metabolite, Tryptophan (Trp), has an index—the ratio of Trp to its competing amino acids in circulation—that is inversely associated with the risk of ischemic stroke (147).
Houlden and colleagues demonstrated that, compared to the sham surgery group, Middle Cerebral Artery Occlusion(MCAO) mice exhibited alterations in the composition of the cecal microbiota, including a significant reduction in Prevotellaceae and an increase in Peptococcaceae, which correlated with the extent of brain injury and influenced the number of cecal goblet cells and mucin production (148). Beyond preclinical studies, a growing number of clinical investigations are now focusing on changes in the gut microbiota following IS and their association with stroke outcomes. Compared to healthy controls, stroke patients showed a significant increase in Aerococcaceae(f), ZB2(c), TM7-1(c), and Flavobacterium, while Mucispirillum, rc4-4, Akkermansia, Clostridiales(o), Lactobacillus, and Stenotrophomonas were significantly reduced. In terms of functional prognosis afterIS, Anaerococcus, Blautia, Dialister, Aerococcaceae(f), Propionibacterium, Microbacteriaceae(f), and Rothia were enriched in the group with good prognosis, whereas Ruminococcaceae(f) and Prevotella were enriched in the group with poor prognosis (149). Yin et al. reported that, compared to asymptomatic individuals, patients with stroke and transient ischemic attack exhibited higher α-diversity (Shannon index) in their microbiota, indicating an increased presence of opportunistic pathogens such as Enterobacter, Megasphaera, Oscillibacter, and Desulfovibrio, while symbiotic or beneficial genera like Bacteroides, Prevotella, and Faecalibacterium were relatively less abundant. Furthermore, patients with severe stroke (National Institutes of Health Stroke Scale[NIHSS] score>4) had higher α-diversity indices, more abundant Proteobacteria, and fewer Bacteroides compared to those with mild stroke (NIHSS score ≤ 4). This microbiota dysbiosis was correlated with disease severity (150). A clinical study on stroke risk stratification revealed that, compared to the low-risk group, the high-risk group exhibited a significantly higher abundance of opportunistic pathogens (e.g., Enterobacteriaceae and Veillonellaceae) and lactic acid-producing bacteria (e.g., Bifidobacterium and Lactobacillus), while butyrate-producing bacteria (e.g., Lachnospiraceae and Ruminococcaceae) were relatively reduced. This suggests that an increase in opportunistic pathogens may be associated with an elevated risk of stroke (151). A new study reveals the intricate interplay between stroke and gut microbiota imbalance. The findings suggest that IS can rapidly lead to gut ischemia and trigger an excessive production of nitrates through free radical reactions, resulting in gut microbiota imbalance. Specifically, the overexpansion and enrichment of Enterobacteriaceae exacerbate the condition of cerebral infarction by intensifying systemic inflammatory responses (152). A prospective cohort study revealed no significant differences in α-diversity indices between patients with mild stroke (NIHSS ≤ 3) and non-mild stroke (NIHSS > 4-34). However, significant differences in microbial community composition were observed. Patients with mild stroke exhibited a significant enrichment of Roseburia, while those with non-mild stroke showed an enrichment of Erysipelotrichaceae incertae sedis. Further analysis demonstrated that the relative abundance of Roseburia was significantly correlated with changes in NIHSS scores and short- and long-term functional outcomes, suggesting a potential protective role in stroke development and prognosis. In contrast, the abundance of Erysipelotrichaceae incertae sedis was positively associated with stroke severity (153). In patients with acute IS, gut microbiota comparisons between those with favorable outcomes (modified Rankin Scale [RS] score 0-2) and poor outcomes (modified RS score 3-6) at 3 months post-stroke revealed that the poor outcome group was characterized by significantly reduced α-diversity, an increased abundance of pathogenic bacteria (e.g., Enterococcaceae and Enterococcus), and a decreased abundance of SCFAs-producing bacteria(e.g., Bacteroidaceae, Ruminococcaceae, and Faecalibacterium) (154). Another study found that, compared to healthy individuals, stroke patients exhibited similar gut microbial α-diversity and overall structure. Nevertheless, significant dysbiosis was observed, primarily characterized by an increased abundance of SCFAs-producing bacteria, such as Odoribacter, Akkermansia, Ruminococcaceae_UCG_005, and Victivallis. Additionally, Christensenellaceae_R-7_group and norank_f_Ruminococcaceae were positively correlated with NIHSS1M and RS scores, whereas Enterobacter showed negative correlations with both (155) (Table 1).
Although different studies have shown variations in specific microbial changes and α-diversity, the overall trend reveals a strong link between post-stroke gut microbiota dysbiosis and disease severity as well as prognosis. Future research should further conduct large-scale, multicenter studies to validate the complex interactions between gut microbiota and IS, establish causality within specific contexts, elucidate the mechanisms of the GBA, and explore gut microbiota-based intervention strategies, thereby providing new perspectives for the prevention and treatment of IS.
It is well-established that stroke can induce neuroinflammatory responses, a process involving the activation of microglia in the brain (156) and the infiltration of leukocytes (157). The gastrointestinal immune system, a critical immune organ harboring a vast number of immune cells, serves as a significant source of immune cells recruited to ischemic brain tissue (158). Benakis et al. demonstrated that gut microbiota dysbiosis influences the outcomes of IS by suppressing the migration of effector T cells from the gut to the leptomeninges (159). Preclinical studies show that long-term invasion and activation of T cells within the brain have been observed in an experimental model of IS (160). Clinical studies have also found that activated T cells survive in the peripheral blood of IS patients and secrete pro-inflammatory cytokines (161). Further studies have shown that changes in Th1, Th2, and Th17 cells occur within 7 days after an IS. In particular, Th17 cells are associated with the exacerbation of cognitive impairment, recurrence of stroke, and increased mortality in IS patients (162). Recent research has also discovered that stroke triggers extensive lymphocyte apoptosis in intestinal mucosal tissues, particularly B cells and T cells in PPs, leading to a reduction in systemic immunoglobulin levels. Notably, this lymphocyte apoptosis is mediated by neuts extracellular traps released by activated neuts following tissue injury (163). Additionally, lower antibody concentrations in stroke patients may increase susceptibility to bacterial infections (164). Importantly, over 70% of the bacteria detected in infected patients belong to human gut commensals, suggesting that bacterial translocation may occur due to leakage of the intestinal mucosal barrier (165). In summary, impaired intestinal immune function following stroke is both a phenomenon and a critical factor contributing to infections and adverse outcomes.
In addition to neurological impairments, stroke can also trigger a variety of non-neurological complications, such as gastrointestinal dysfunction, including severe intestinal obstruction, alterations in gut microbiota, and intestinal inflammation. The overactivation of immune cells following stroke is a key factor contributing to intestinal inflammation, which increases intestinal barrier permeability, allowing the translocation of resident microbiota and potential dissemination to systemic organs, thereby predisposing to sepsis (166). Stanley et al. demonstrated that, compared to the sham surgery group, stroke mice exhibited reduced expression of ZO-1, indicating impaired gastrointestinal barrier function and increased intestinal permeability (165). In a mouse model of MCAO, after excluding surgical stress as a potential factor for infection, all mice developed spontaneous bacterial infections within three days. Moreover, over 95% of the cultured bacteria were identified as Escherichia coli (167). Another study detected LPS in ischemic brain tissue following stroke (168). A recent meta-analysis by Liu et al. revealed that stroke patients receiving enteral nutrition, including probiotics, had better prognoses and reduced rates of bacterial infections (169). Similarly, another meta-analysis involving 26 randomized controlled trials of probiotic treatment in stroke patients showed that early enteral nutrition combined with probiotics effectively modulated gut microbiota and intestinal mucosal barrier function, enhanced immune responses, and reduced the incidence of infectious complications and gastrointestinal motility disorders (170).
The gut microbiota plays a critical role in the bidirectional communication between the gut and the brain via the GBA, influencing the regulation of key immune cells (171). SCFAs through interaction with free fatty acid receptors, inhibit histone deacetylases and can cross the BBB, affecting microglial function and reducing neuroinflammation, thereby playing a key role in the GBA (172, 173). Studies have shown that SCFAs can reduce neuroinflammation by inhibiting the translocation of LPS to brain tissue, but SCFAs are significantly reduced after IS (174), which adversely affects the regulation of microglia-mediated inflammatory responses. SCFAs not only promote recovery after IS but also protect the intestinal barrier, thereby improving disease prognosis (175). Research has found that fecal transplantation of SCFAs-producing bacteria or SCFAs supplementation can enhance intestinal mucosal integrity and promote the migration of Tregs from the gut to the ischemic brain region (176), reduce neuroinflammation (177), prevent BBB breakdown, and promote neural repair (178). At the same time, SCFAs have also shown effects in improving depression (179). Additionally, Trp metabolites may also influence the occurrence and severity of cerebrovascular diseases. Studies have shown that after IS, levels of Trp and other amino acids are reduced, and a decrease in plasma Trp levels, along with an increase in the kynurenine-to-tryptophan ratio, is associated with depression (180, 181).
In summary, the interaction between IS and gut microbiota profoundly affects stroke pathophysiology and outcomes through the GBA. Dysbiosis of the microbiota, impaired intestinal barrier function, and abnormal immune responses collectively exacerbate stroke-related damage and increase the risk of complications. Notably, the IS-gut microbiota-immune cells-barrier pathway may also influence the development of PSD.
With the deepening of research on the MGBA, a variety of advanced technologies have been widely applied to unravel the complex interactions between the gut and the brain. Below are examples of commonly used techniques and their applications (1): Single-cell RNA sequencing: This technology enables the analysis of gene expression at single-cell resolution, revealing the specific roles of different cell types (182) (e.g., IEC, immune cells, and Glial cells) in the MGBA, Its strength lies in providing high-resolution, cell-type-specific information, which uncovers cellular heterogeneity within the MGBA (183–185). (2) Spatial Transcriptomics: By integrating gene expression data with spatial location information, this technique precisely maps gene expression patterns on tissue sections (186). Its advantage is the ability to reveal spatial distribution of gene expression, aiding in the elucidation of region-specific mechanisms in the MGBA. (3) Multi-omics Integration: This approach combines data from genomics, transcriptomics, proteomics, and metabolomics (187), offering a comprehensive understanding of the intricate interactions within the MGBA. For example, through multi-omics analysis, researchers can explore the interplay among microbial communities, host gene expression, and metabolites, thereby revealing how microbiota dysbiosis impacts brain mood function (188). Its core strength lies in providing a holistic systems biology perspective, facilitating the discovery of multi-level regulatory mechanisms in the MGBA. (4) Optogenetics: This technique utilizes light-sensitive proteins to precisely control the activity of specific neurons, enabling the study of neural circuit functions (189, 190). For instance, by employing these techniques, we can explore the relationship between the gut microbiome and mental illnesses such as schizophrenia (191). Its advantage is the high spatiotemporal resolution in modulating neural activity, shedding light on neural mechanisms within the MGBA. (5) Microbiota Transplantation: By transferring donor microbiota to recipients (e.g., germ-free mice or model mice), this method studies the impact of microbiota on host physiology and pathology, such as the treatment of IS and depression (192, 193). Its strength lies in directly validating the causal role of microbiota, providing a foundation for clinical interventions. (6) In Vivo Live Imaging: Utilizing fluorescent labeling and microscopy techniques (194, 195), this technology enables real-time observation of dynamic processes in the gut and brain. Its advantage is the ability to reveal spatiotemporal dynamics within the MGBA. (7) Organoid Models: These models use stem cells to cultivate organoids that mimic the structure of the gut and brain, allowing the study of their functions and interactions (196, 197).The strength of this technology is its ability to provide highly physiologically relevant experimental models, reducing ethical and technical limitations associated with animal experiments. (8) Neuroimaging Combined with Microbiome Analysis: Techniques such as Structural MRI, Functional Neuroimaging, Magnetic Resonance Spectroscopy, and Brain Iron Deposition Imaging, when integrated with microbiome analysis, could investigate the relationship between microbiota and brain microstructure, intrinsic neural activity, functional connectivity, as well as cognitive and emotional functions (198). Its advantage lies in offering non-invasive brain function assessment, combined with microbiome data to elucidate MGBA mechanisms.
The aforementioned technologies represent scientifically robust and practical approaches in current MGBA research. By integrating these techniques, researchers can explore the complex interactions between microbiota and the brain in greater depth, providing strong support for mechanistic studies and therapeutic strategies for diseases such as IS and PSD. The judicious application of these technologies not only advances fundamental research but also offers critical theoretical foundations for clinical translation.
PSD is the most prevalent neuropsychological disorder among stroke patients, characterized by persistent low mood and diminished interest (199). It is commonly used to describe depressive symptoms following IS, given the predominance of IS in related literature. As early as 2002, Whyte EM and Mulsant BH highlighted in their review that post-stroke depression is not caused by a single biological or psychological factor but rather results from the interplay of multiple factors, aligning with the biopsychosocial model of mental disorders (6). Importantly, a bidirectional relationship exists between depression and stroke: stroke increases the risk of PSD, while depression is an independent risk factor for stroke and stroke-related mortality. In stroke literature, the most consistent finding is that PSD is associated with the severity of stroke and the degree of physical and cognitive impairment (200, 201). Furthermore, studies have shown that the use of antidepressants in PSD patients can improve cognitive function (202), reduce disability (203), and increase survival rates (204).
Presently, discussions on the pathogenic mechanisms and therapeutic targets of major depressive disorder primarily focus on the imbalance of the monoamine neurotransmitter system—which includes serotonin (5-HT), norepinephrine, and dopamine—and the dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis (205). Relatively speaking, PSD is usually triggered by ischemic brain injury, often affecting the frontostriatal and limbic system circuits (206–208), and is accompanied by post-stroke neuroinflammation and impairment of neuroplasticity (209, 210), indicating the presence of structural brain damage. Notably, in studies of IS, Major depressive disorder, or PSD, gut microbial factors are gradually becoming a focal point of research. Studies have found that Major depressive disorder is closely related to changes in the baseline gut microbiota (211), which can regulate Trp metabolism through the GBA and trigger systemic inflammation, serving as significant pathogenic factors (212). PSD may be related to stroke-induced gut microbiota dysbiosis (213), increased gut permeability, and microbial-derived pro-inflammatory metabolites (such as LPS) exacerbating central nervous inflammation (144, 152, 214, 215).
PSD is widely regarded as the result of combined neurobiological dysfunction caused by ischemia and psychosocial distress. However, existing evidence suggests that neurobiological factors (rather than psychological responses to disability) are the primary contributors to PSD (5). In recent years, the gut microbiota has garnered significant attention as a key regulator of the GBA, and its importance in gut-brain communication has expanded the GBA to the MGBA (216). The significance of this axis has become increasingly prominent in research on psychiatry, neurodevelopment, and neurodegenerative diseases. The microbiota and its metabolites communicate with the brain through multiple pathways within the MGBA, thereby influencing brain function and behavior. Based on the analysis in Section 4 of this article, it is evident that IS and the gut microbiota, immune cells, and barrier integrity and functionality exhibit bidirectional interactions. Next, we will further explore how the microbiota-immune-barrier axis affects the occurrence and development of PSD.
The MGBA forms a bidirectional communication network between the microbiota and the host (217). Research has primarily focused on several aspects: neuroanatomical pathways, neuroendocrine pathways of the HPA axis, immune pathways, microbiota metabolic pathways, the intestinal mucosal barrier and BBB (218),. The role of the microbiota in this axis is critical, as various environmental factors and physiological states of the host can influence the composition of the microbiota. When this balance is disrupted, it may lead to microbiota dysbiosis, subsequently affecting the signaling function of the MGBA and adversely impacts the host’s immune, metabolic, and nervous systems (219). It is important to note that these pathways interact and influence each other.
Extensive literature exists on how the microbiota regulates host emotions through MGBA, primarily focusing on the nervous system and neurotransmitters. The brain communicates directly with the gut via parasympathetic and sympathetic fibers and indirectly through the stimulation of the enteric nervous system (220). In this process, enterochromaffin (EC) cells play an significant role. They transmit signals to the brain via the vagus nerve (221, 222). Studies have found that 5-HT synthesized and secreted by EC cells is closely related to the interaction with the microbiota, and in patients with PSD, 5-HT levels are significantly reduced (223). Additionally, γ-aminobutyric acid (GABA), as the major inhibitory neurotransmitter, plays a pivotal role in IS and depression (224). Relevant studies indicate that species such as Bacteroides, Parabacteroides and Escherichia can effectively produce GABA, and the relative abundance of Bacteroides in feces is negatively correlated with depression (225). Simultaneously, Bifidobacterium adolescentis can produce GABA to modulate the GBA response, and has an intriguing association with depression (226). Furthermore, Bacillus members have been demonstrated to boost dopamine production, whereas a rise in Bifidobacterium modifies dopamine metabolic abnormalities, improving mood after a stroke (215). The relationship between the microbiota and the HPA axis has also garnered attention. Research reveals that dysbiosis of the gut microbiota can trigger excessive activation of the HPA axis, negatively affecting the development of the prefrontal-limbic circuit. In adult experiments, the use of probiotics can normalize HPA axis activity and alleviate depressive symptoms (227).
As mentioned above, the microbiota has a significant impact on central and peripheral immune responses and plays a crucial role in maintaining the integrity of the BBB. Under pathological conditions, dysbiosis of the microbiota can further affect the physiology, behavior, and cognitive functions of the brain through the MGBA, playing a key role in PSD (214, 228). The development and function of the gut immune system largely depend on the microbiota (229), which may potentially play a role in regulating emotions and behavior (222). In the third and fourth parts, we discussed the physiological role of the microbiota, IEC, and immune cells in jointly regulating barrier function, as well as the interaction of this pathway with IS. Next, we will further explore how the microbiota, through immune regulation on the MGBA, affects barrier function post-IS and further influences the host’s emotional state (Figure 3).
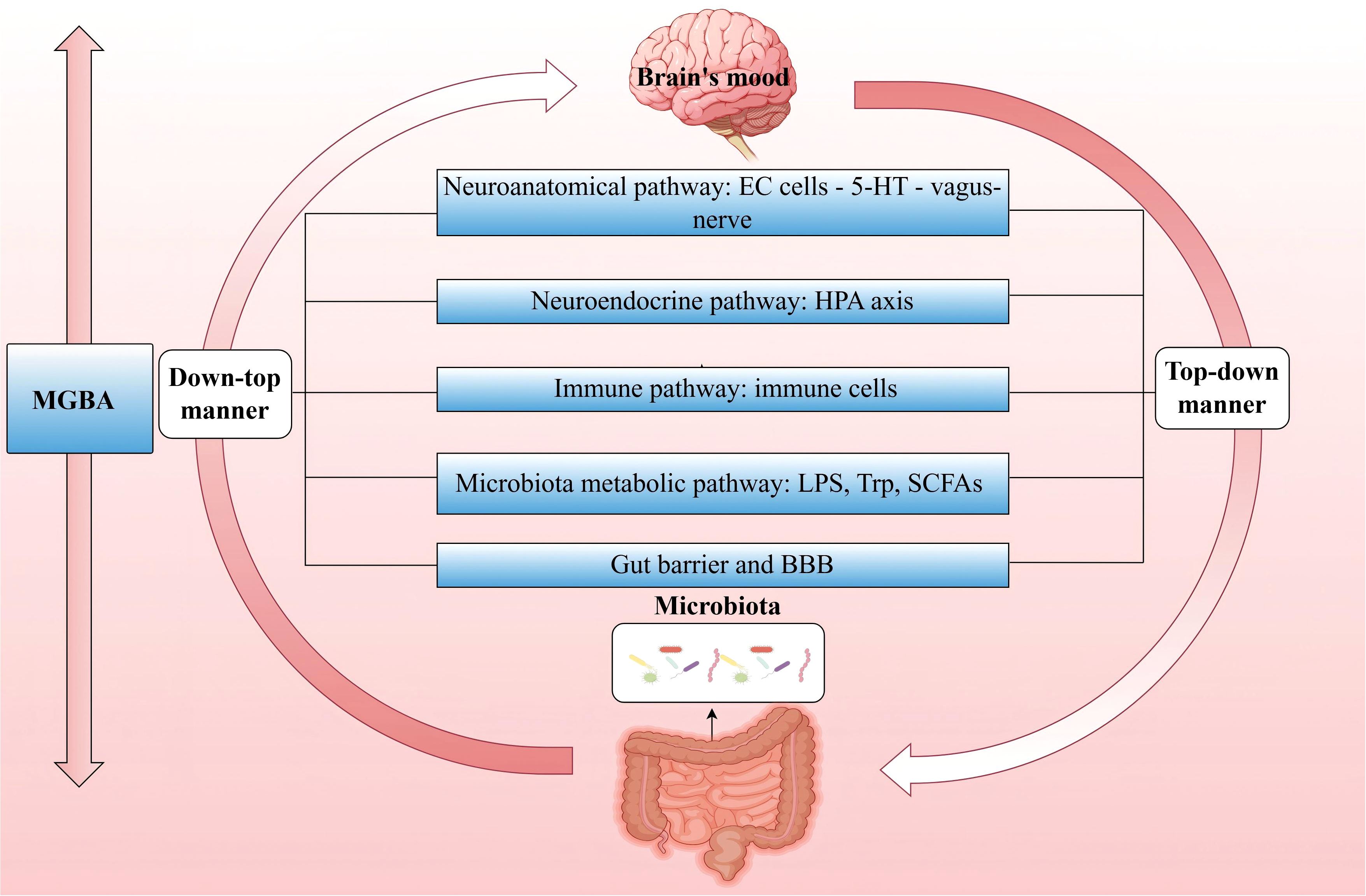
Figure 3. MGBA—Major pathways through which the microbiota regulates host mood. The MGBA represents a bidirectional communication network that interconnects the microbiota with the host. The brain exerts top-down control over the composition and diversity of the microbiota, whereas the microbiota, in turn, exerts bottom-up influence on the brain’s emotional state. (MGBA, Microbiota-Gut-Brain Axis; EC, enterochromaffin; 5-HT, serotonin; HPA, hypothalamic-pituitary-adrenal; LPS, lipopolysaccharide; Trp, Tryptophan; SCFAs, short-chain fatty acids; BBB, blood-brain barrier.).
A meta-analysis revealed that, compared to healthy individuals, patients with PSD exhibit significant differences in species diversity and microbial community structure at multiple taxonomic levels, including phylum, family, and genus (14). Another study suggested that the gut microbiota may play a role in the pathogenesis of PSD (230). Furthermore, alterations in the composition of the gut microbiota are closely associated with the severity of PSD (231).
Significant changes in the microbiota composition have been observed in PSD patients. Within the phylum Firmicutes, there is a reduction in Bifidobacterium and an increase in Enterococcus and Escherichia coli (214), the latter being recognized as an important opportunistic pathogen in the gut (232). Studies have also demonstrated distinct differences in microbiota composition and inflammatory markers between individuals with and without depressive symptoms. Compared to the non-PSD group, the PSD group exhibited higher levels of Enterococcus faecalis and Escherichia coli, along with elevated inflammatory factors, including IL-1, IL-2, IL-6, and hs-CRP(C-reactive Protein). Concurrently, the PSD group showed lower levels of Bifidobacterium. Notably, the levels of Enterococcus faecalis and Escherichia coli were positively correlated with these inflammatory cytokines, whereas Bifidobacterium levels were negatively correlated (214). Another comparative study identified similar microbiota differences between the two groups. Specifically, PSD patients had significantly higher levels of Streptococcus, Akkermansia, and Barnesiella, but lower levels of Escherichia-Shigella, Butyricicoccus, and Holdemanella compared to non-PSD patients. Correlation analysis further indicated that the abundance of Akkermansia, Barnesiella, and Pyramidobacter was positively associated with Hamilton Depression Scale (HAMD) scores, while the abundance of Holdemanella was negatively correlated with HAMD scores (213).
Interventions targeting the microbiota in PSD have been widely reported. As beneficial bacteria, Bifidobacterium species inhibit the proliferation of pathogenic bacteria and modulate the microbiota, demonstrating potential antidepressant effects (233). Additionally, Lactobacillus rhamnosus has been shown to reduce depression-related behaviors, highlighting the role of the microbiota in emotional regulation (234) (Table 2).
During the early stages of ischemic injury, DAMPs and cytokines expressed at the injury site can enter systemic circulation through the disrupted BBB. This process can trigger immune responses in primary and secondary lymphoid organs, leading to systemic inflammatory response syndrome (235). Among these, the rapid activation of immune cells plays a key role in BBB disruption following IS (236).
Neuroinflammation is known to be associated with CNS disorders, including PSD (237). Inflammatory mediators produced by immune cells play a pivotal role in shaping neuropsychiatric outcomes following stroke. The inflammatory basis of PSD is closely linked to immune cells and molecular factors, with cytokines serving as critical signaling proteins that facilitate intercellular communication. These cytokines are primarily produced by immune cells such as monocytes, macrs, and lymphocytes (238). In the context of PSD, significant elevations in pro-inflammatory cytokines, including IL-1, IL-6, and TNF-α, have been documented (238). Clinical studies have shown that serum levels of TNF-α and IL-1β are elevated in PSD patients compared to non-PSD patients (239). These cytokines can directly affect key brain regions involved in mood regulation, potentially contributing to the development of depressive symptoms. IL-6, synthesized by various cells including neurons, astrocytes, microglia, and endothelial cells, plays a crucial role in the inflammatory response associated with PSD (238, 240). Studies have further emphasized this correlation, demonstrating that higher serum IL-6 levels are independently associated with the occurrence of PSD (241).
Within the immune system, chemokines are responsible for coordinating the migration of cells to specific regions requiring an immune response (238). Particularly, chemokines such as CCL2, CCL7, CCL8, CCL12, and CCL13 have been shown to drive pro-inflammatory cells towards inflamed or injured CNS tissues, playing a significant role in the neuroinflammatory processes associated with PSD (242, 243). Reports indicate that CCL2/CCR2 signaling may be associated with depression (244). Under ischemic conditions, microglia rapidly accumulate at the injury site. They also contribute to tissue repair and remodeling by clearing debris and secreting anti-inflammatory cytokines and growth factors. Conversely, when immune regulation is imbalanced, they exacerbate tissue damage by releasing inflammatory cytokines and neurotoxic substances, highlighting their dual role in the brain’s response to injury (245). Astrocyte activation is a critical response in IS and plays a significant role in the neuroinflammatory environment (246). Following stroke, activated microglia secrete a combination of IL-1α, TNF-α, and C1q, driving astrocytes toward a neurotoxic phenotype, thereby increasing the complexity of the neuroinflammatory response (247). New research indicates that depression is associated with specific networks of the brain’s functional connectome, namely certain brain networks (248, 249). A clinical study revealed that PSD is related to increased functional connectivity strength in specific areas of the default mode network, including the contralateral angular gyrus, posterior cingulate cortex, and hippocampus (250). Further research reveals that most microglia in the PSD hippocampus exhibit both pro-inflammatory and anti-inflammatory states, with a significant negative correlation between IL-1 and PSD (251). It is evident that studying the impact of immune modulation on specific brain circuits in PSD is a field full of potential.
Imbalanced immune regulation may play a key role in the pathophysiology of PSD (252), suggesting that maintaining the homeostasis of immune cells and their mediated cytokines and chemokines in the brain’s inflammatory response is of great significance for the prevention and treatment of PSD.
Following a stroke, the microvasculature within the affected region exhibits significant inflammatory features, primarily characterized by endothelial dysfunction (253), impaired BBB (254), and the recruitment and infiltration of leukocytes (157). Barrier function impairment can lead to neurological diseases by passive means through the vascular leakage of blood-borne molecules into the CNS, and by active means through guiding inflammatory cells to migrate into the CNS. Both of these mechanisms may be directly related to changes in the molecular composition, function, and dynamics of TJs proteins (255, 256). The invasion of peripheral leukocytes can exacerbate neuronal damage (257, 258). Studies have demonstrated that the protective effects observed in PSD rats are linked to improvements in BBB permeability (259). Moreover, research on depression in mice has revealed that peripheral inflammatory factors can cross the BBB and induce depressive behaviors by modulating BBB integrity, suggesting that the BBB may play a critical role in ameliorating depression in PSD mice (260). Furthermore, in PSD rats, modulation of the gut microbiota has been shown to enhance BBB integrity, improve neurological function, and alleviate depressive symptoms (15). In summary, the destruction of BBB is not only an important pathological process of IS, but also a key factor that may trigger PSD.
After a stroke, ecological imbalance, dysregulation of intestinal immune function, and damage to the intestinal barrier become common phenomena (261). Dysbiosis of the gut microbiota not only leads to damage of the intestinal epithelium, reduced mucus secretion, and decreased expression of TJs, thereby increasing intestinal permeability, but also affects neural function and IS outcomes (262–264). Under these conditions, there may be a penetration of ectopic intestinal bacteria and pro-inflammatory cells into brain tissue through a compromised blood-brain barrier (175). It has been confirmed that inflammatory cytokines and other bacterial toxins, such as LPS, penetrate the damaged IEB and enter the circulation (265, 266). Furthermore, studies have found that immune cells, such as Neuts, DCs, Macs, and T cells, infiltrate the brain at different times (267–269). Existing evidence suggests that numerous pro-inflammatory cytokines play a critical role in the development of PSD (270). Under normal physiological conditions, T cells assist B cells in differentiating into plasma cells, which produce IgA to clear toxins and pathogens (271). However, in the MCAO model, early stress leads to significant translocation of gut bacteria and reduced IgA levels (272). Studies have shown that after IS, the host immune system is severely suppressed, and the number of B cells in the small intestine decreases. This may adversely affect the homeostasis of the intestinal and systemic immune systems, impair antimicrobial defenses, and lead to gastrointestinal complications (273). The effects of B cells crossing the damaged BBB and entering brain tissue depend on the subset, timing, and microenvironment (274). It is noteworthy that Th17 cells derived from the small intestine are considered to play a key role in the pathogenesis of depression. They affect the condition by promoting neuroinflammation in the CNS, activating microglia and astrocytes, and inducing neurotoxicity, which is closely related to the onset of depression (275–277). Another important type of T cell, Tregs, secrete anti-inflammatory cytokines to suppress the activity of pro-inflammatory cytokines, promote neurogenesis, and regulate the polarization of microglia and macs after IS (278). Furthermore, the development of depression is a dynamic, multi-stage process involving changes in the response of Tregs to different inflammatory microenvironments (279).
Following IS, the release of DAMPs and cytokines triggers the activation of microglia and astrocytes. Microglia, as the resident immune cells of the CNS, are the first to detect and respond to injury. Within the first 24 hours post-IS, anti-inflammatory M2 microglia dominate (280). During the initial phase of injury, microglia release anti-inflammatory factors to aid in neuronal repair. However, if the injury persists, microglia shift to a pro-inflammatory state, secreting factors that not only exacerbate inflammation but also further damage neurons (238). Ischemic neurons induce the polarization of M1 microglia, which secrete pro-inflammatory mediators, disrupt the BBB, and amplify harmful inflammation (281). In the CNS, astrocytes are the most abundant glial cells and can also be activated into two distinct states post-IS: A1 (pro-inflammatory) and A2 (anti-inflammatory) (282). The cascade of pro-inflammatory mediators and reactive substances released by activated M1 microglia impairs astrocyte function, reduces neurotrophic support, and hinders hippocampal neurogenesis, which is critical for brain repair and cognitive function (283). Studies suggest that the pathological mechanisms of PSD may be linked to reduced miR34b-3p levels in hippocampal neurons and enhanced microglial activation (284). Inflammatory mediators can disrupt extracellular glutamate balance by impairing the glutamate clearance capacity of microglia and astrocytes. This imbalance leads to overactivation of NMDA receptors, excitotoxicity, apoptosis, reduced neuroplasticity, and ultimately neuronal loss, potentially contributing to the development of PSD (285, 286).
In summary, following IS, microbiota dysbiosis exacerbates intestinal barrier dysfunction, leading to the excessive release of local inflammatory cytokines (287). This activates immune regulation and intensifies the inflammatory response, affecting the homeostasis of both the intestinal and brain barriers. In the context of ischemia, the disruption of the microbiota-immune-barrier axis in the MGBA further promotes the development and progression of PSD (Figure 4).
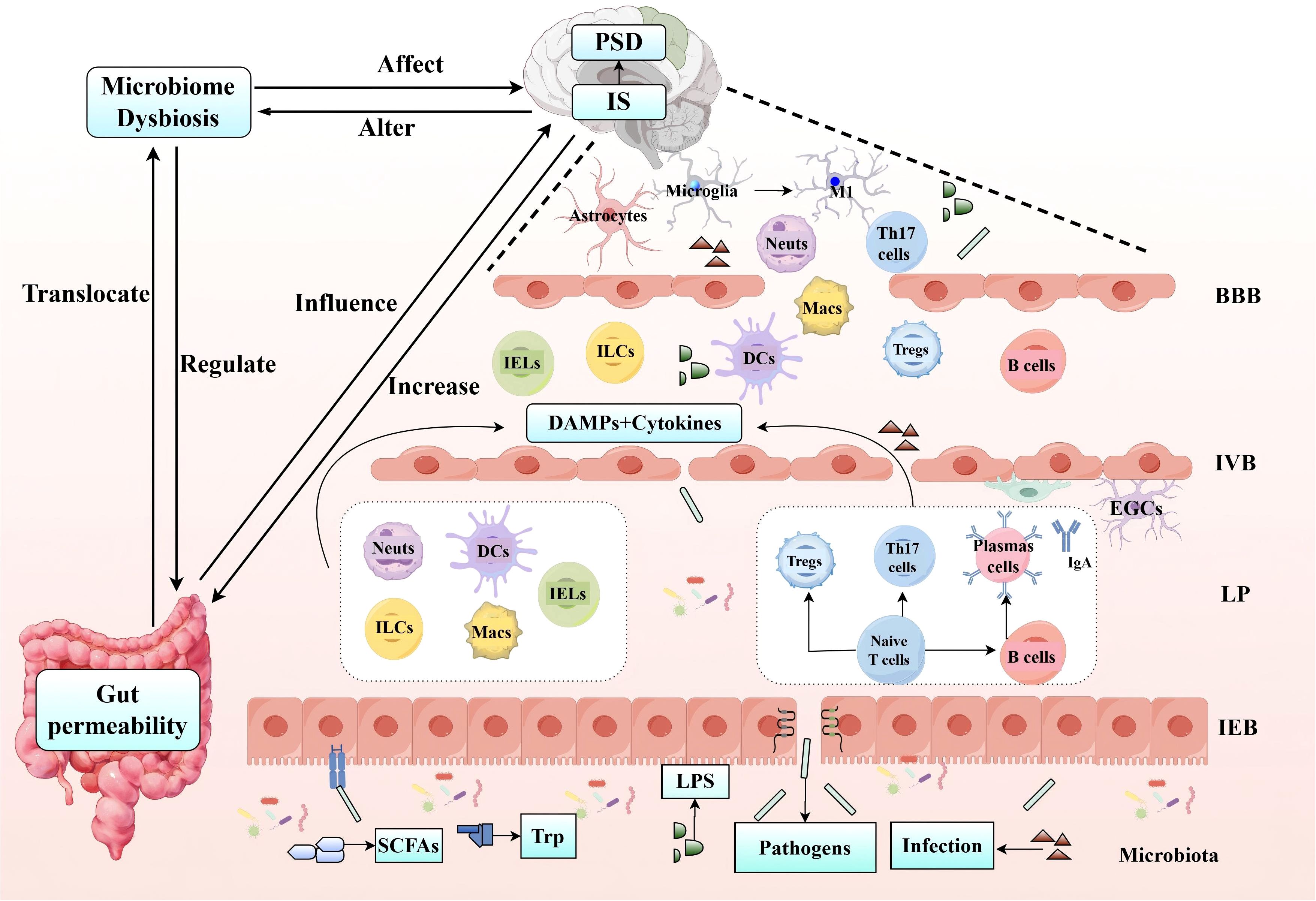
Figure 4. IS utilizes the microbiota-immune-barrier axis to influence the occurrence and development of PSD. Following IS, microbiota dysbiosis exacerbates intestinal epithelial barrier (IEB, IVB) dysfunction, resulting in the translocation of harmful substances across the intestinal barrier and the excessive release of local inflammatory cytokines. This process triggers immune regulation in the LP, involving both innate immune cells (such as Neuts, IELs, ILCs, DCs, and Macs) and adaptive immune cells (including Th17 and Tregs within the T cell population). The subsequent release of DAMPs and cytokines further amplifies the inflammatory response, leading to the activation of microglia and astrocytes, the secretion of pro-inflammatory mediators, disruption of the BBB, and the development of PSD. (IS, Ischemic Stroke; IEB, Intestinal Epithelial Barrier; IVB, Intestinal Vascular Barrier; Neuts, Neutrophils; IELs, Intraepithelial Lymphocytes; ILCs, Innate Lymphoid Cell; DCs, Dendritic Cells; Macs, Macrophages; Th17, T helper 17 cells; Tregs, Regulatory T cells; PSD, Post-Stroke Depression).
The intricate nature of PSD pathophysiology renders biological prevention and treatment approaches particularly challenging. Presently, treatment strategies for PSD predominantly encompass pharmacological therapy, neurostimulation, and psychological interventions. Although selective 5-HT reuptake inhibitors have demonstrated clinical significance, their efficacy is still debated, and they come with risks, such as the potential for bleeding (5). The prolonged use of antidepressants, the risk of dependency, and a range of side effects have steered interest towards alternative treatments. Therapies based on microbiota, which have the potential to simultaneously address the underlying condition and alleviate depressive symptoms, may emerge as a central focus in future research endeavors.
Probiotics are a class of safe microorganisms that can bring numerous benefits to the host when given to human subjects in adequate doses and at the right time (288). Reportedly, Probiotics enhance barrier function by increasing mucus production, AMPs, and sIgA levels, promoting competitive adherence against pathogens, and improving the TJs integrity of IEC (289). Preclinical studies reveal that Lactobacillus rhamnosus and Bifidobacterium breve show potential in improving neurological dysfunction caused by MCAO in rats by inhibiting neuroinflammation and modulating the GBA (290). Clinical research indicates that consuming probiotics can help enhance patients’ emotional well-being, particularly alleviating symptoms of depression and anxiety that manifest within three months following a stroke (291). Another clinical study has found that tablets containing a combination of live Bifidobacterium, Lactobacillus, Enterococcus, and Bacillus cereus can promote neurological recovery and alleviate depression in stroke patients. These effects may be attributed to the regulation of NF-κB, IL-1β, and TNF-α levels (292). Another meta-analysis showed that the combined use of probiotics with enteral nutrition significantly reduced the levels of TNF-α, IL-6, and IL-10, and statistically significantly decreased the incidence of pulmonary, gastrointestinal, and urinary tract infections, mortality, and the occurrence of intestinal dysbiosis (293).
Prebiotics show great potential in altering the gut microbiota, with different prebiotics promoting the growth of different native gut bacteria (294). Research has found that lactulose can improve neurological function after stroke by inhibiting harmful bacteria, correcting metabolic disorders, repairing damaged intestinal barriers, and suppressing inflammatory responses in mice after stroke (295). Furthermore, a fiber-rich barley variety known as BARLEYmax has been shown to increase butyrate levels in the gastrointestinal tract, thereby promoting the proliferation of beneficial bacteria (296). Similarly, dietary fiber inulin has been observed to reshape the microbiota in mice, enhancing intestinal barrier integrity through the upregulation of TJs protein expression and increasing SCFAs in feces. This nutritional intervention strategy may prevent depression symptoms by leveraging the microbiota-gut-SCFAs axis (297). In summary, the supplementation of probiotics or prebiotics can regulate the microbiota, thereby affecting the stability of the intestinal barrier and ultimately influencing brain function, offering a novel approach for the treatment of PSD.
Fecal microbiota transplantation (FMT) involves the transfer of fecal matter from a healthy donor into the gastrointestinal tract of a patient to treat specific diseases (298). The advantages of FMT have been acknowledged since the fourth century, during the Eastern Jin Dynasty in China. Research has demonstrated that FMT can prevent ischemic injury by reducing the expression of IL-17, IFN-γ, and other pro-inflammatory cytokines (299). Transplanting fecal bacteria rich in SCFAs and supplementing with butyric acid have been found to be effective treatments for IS (300). Another study indicates that FMT improves depressive-like behavior, corrects gut microbiota imbalance, and alleviates intestinal tract inflammation, intestinal mucosa disruption, and neuroinflammation in rats induced by chronic unpredictable mild stress (301). Consequently, FMT may represent a potential therapeutic approach for PSD. Further investigation into the mechanisms underlying FMT, including refining donor screening processes, optimizing fecal preparation techniques, and exploring alternative administration routes, may enhance its efficacy and safety.
Vagus nerve stimulation (VNS) is an approved method for treating epilepsy and is currently being researched for application in the treatment of other diseases, including depression, anxiety disorders, and Alzheimer’s disease (302). Previous research has reported that VNS can prevent intestinal permeability induced by traumatic brain injury. Additionally, VNS enhances enteric glial activity, potentially mediating the CNS’s regulation of intestinal permeability (303). Recent animal research has revealed that VNS ameliorates microbiota imbalance and mitigates BBB damage in rats with MCAO via the MGBA (304). Clinical studies reveal that VNS therapy can alleviate the damage to the BBB and colonic barrier after cerebral ischemia/reperfusion by modulating immune cells, and mitigate systemic inflammatory responses (305). In recent years, non-invasive transcutaneous auricular VNS (ta-VNS) has garnered interest, indicating that ta-VNS triggers anti-inflammatory pathways, restores MGBA homeostasis, and modulates psychiatric disorders (306). Additional studies have observed that ta-VNS increases the abundance of lactobacilli and bifidobacteria (307). Double-blind, randomized controlled trials have shown that the synergistic approach of combining ta-VNS with conventional treatment demonstrates remarkable efficacy and tolerability in managing PSD (308). In summary, ta-VNS represents a safe and efficacious novel therapeutic approach.
The traditional Chinese herbal extract Gastrodin (Gas), derived from the herb Tianma, has been studied extensively. Research indicates that Gas enhances intestinal barrier function by increasing the expression of TJs proteins and mucins. Additionally, it significantly reduces the secretion of pro-inflammatory cytokines in mice (309). Further studies have demonstrated its efficacy in alleviating behavioral deficits associated with depression and suggest its potential in the prevention and treatment of PSD (310). Moreover, Gas influences the gut microbiota and has been shown to improve depressive-like behaviors in mice (311). A bibliometric analysis reveals that from 2014 to 2023, numerous researchers have persistently investigated the role of acupuncture in PSD (312), corroborating its positive impact on depression (313). Acupuncture can address PSD through various mechanisms, including the protection of the intestinal mucosal barrier, immune regulation, and inflammation control, with the modulation of the gut microbiota being a common underlying theme (314). The study also found that acupuncture can alleviate depressive-like behavior in PSD by regulating the gut microbiota and inhibiting the overactivation of inflammatory mediators (315). Acupuncture can also effectively promote the rehabilitation process of PSD patients by maintaining the dynamic balance of gut microbiota, thus proving that acupuncture, as a non-pharmaceutical treatment, has significant potential in alleviating depressive symptoms (314). Moreover, acupuncture, whether administered as a standalone therapy or in conjunction with other treatments such as music therapy and repetitive transcranial magnetic stimulation, has been evidenced to effectively alleviate depressive symptoms (316–318). Despite the promising outlook for acupuncture in the treatment of PSD, further exploration is warranted to fully understand its potential mechanisms and clinical efficacy.
In the treatment of PSD, non-pharmacological interventions such as acupuncture and ta-VNS have garnered significant attention. However, their widespread application faces challenges, including unclear mechanisms and insufficient clinical evidence. Moving forward, through multidisciplinary collaboration and technological innovation, these therapies hold promise as integral components of PSD treatment, offering safer and more effective options for patients.
IS remains one of the significant global health challenges, with one of its complications-PSD-urgently requiring more attention. Currently, the exact pathogenesis of PSD, particularly its interaction mechanism with gut microbiota, is not fully understood. This review delves into how post-stroke gut microbiota dysbiosis leads to barrier dysfunction through complex immune regulation and inflammatory responses, and proposes the concept of a microbiota-immune-barrier axis based on the MGBA. The article elaborates on the connection between this pathway and IS as well as PSD, aiming to provide new insights and perspectives on the potential pathogenesis of PSD to promote the development of clinical prevention and treatment strategies for PSD. Although We have organized and summarized the potential pathogenesis of PSD, considering the heterogeneity of microbiota, immune cells, and patients, as well as the dynamic changes in the stages of PSD, it is currently impossible to establish a single definitive causal relationship. This is precisely the challenge that future research needs to further address.
JJ: Writing – original draft, Writing – review & editing. HX: Writing – review & editing. SC: Writing – review & editing. XX: Writing – original draft. JZ: Writing – original draft. QL: Writing – original draft. CD: Writing – original draft, Writing – review & editing. ML: Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Modify the grant number Hunan Provincial Science and Technology Innovation Plan (No. 2024JK2132, No. 2024RC1061), Hunan Provincial Traditional Chinese Medicine Research Program (No. C2022027), 2022 Youth Qihuang Scholars Program, State Administration of Traditional Chinese Medicine (No. Guo Zhong Yi Yao Ren Jiao Han [2022] 256), Hunan University of Chinese Medicine Acupuncture and Tuina Joint Graduate Training Base (No. Xiang Jiao Tong [2022] 357), Acupuncture Bioinformatics and Smart Health Innovation and Entrepreneurship Education Center, Hunan University of Chinese Medicine (No. Xiang Jiao Tong [2021] 356), Traditional Chinese Medicine Sub-health Graduate Training Innovation Base, Hunan University of Chinese Medicine (No. Xiao Xing Yan Zi [2020] 19).
The authors thank the editor and the reviewers for the insightful suggestions and comments. And give heartfelt thanks to all the people who have ever helped with this paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
PSD, Post-Stroke Depression; IS, Ischemic Stroke; MGBA, Microbiota-Gut-Brain Axis; CNS, Central Nervous System; GBA, Gut-Brain Axis; BBB, Blood-Brain Barrier; IEC, Intestinal Epithelial Cells; sIgA, Secretory Immunoglobulin A; IEB, Intestinal Epithelial Barrier; TJs, Tight Junctions; ZOs, Zonula Occludens; DCs, Dendritic Cells; M cells, Microfold Cells; PPs, Peyer’s Patches; LP, Lamina Propria; EECs, Enteroendocrine Cells; AMPs, Antimicrobial Peptides; ISCs, Intestinal Stem Cells; IVB, Intestinal Vascular Barrier; EGCs, Enteric Glial Cells; PV-1, Plasmalemma Vesicle-Associated Protein-1; Tfh, T Follicular Helper Cells; P-gp, P-glycoprotein; SCFAs, Short-Chain Fatty Acids; TNF-α, Tumor Necrosis Factor-alpha; PRRs, Pattern Recognition Receptors; PAMPs, Pathogen-Associated Molecular Patterns; DAMPs, Damage-Associated Molecular Patterns; IELs, Intraepithelial Lymphocytes; TGF-β, Transforming Growth Factor-beta; IL, Interleukin; ILCs, Innate Lymphoid Cells; IFN-γ, Interferon-gamma; TSLP, Thymic Stromal Lymphopoietin; Neuts, Neutrophils; MMP, Matrix Metalloproteinase; Macs, Macrophages; RA, Retinoic Acid; Th, T Helper; Tregs, Regulatory T Cells; GALT, Gut-Associated Lymphoid Tissues; Trp, Tryptophan; Am, Akkermansia muciniphila; LPS, Lipopolysaccharide; MCAO, Middle Cerebral Artery Occlusion; NIHSS, National Institutes of Health Stroke Scale; RS, Rankin Scale; TEER, Trans-Epithelial Electrical Resistance; HPA, Hypothalamic-Pituitary-Adrenal Axis; EC, Enterochromaffin; 5-HT, Serotonin; GABA, γ-Aminobutyric Acid; HAMD, Hamilton Depression Scale; CRP, C-Reactive Protein; FMT, Fecal Microbiota Transplantation; VNS, Vagus Nerve Stimulation; ta-VNS, Non-Invasive Transcutaneous Auricular Vagus Nerve Stimulation; Gas, Gastrodin.
1. Feigin VL, Brainin M, Norrving B, Martins S, Sacco RL, Hacke W, et al. World stroke organization (WSO): global stroke fact sheet 2022. Int J stroke: Off J Int Stroke Society. (2022) 17:18–29. doi: 10.1177/17474930211065917
2. Feigin VL, Krishnamurthi RV, Parmar P, Norrving B, Mensah GA, Bennett DA, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: the GBD 2013 study. Neuroepidemiology. (2015) 45:161–76. doi: 10.1159/000441085
3. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: A report from the american heart association. Circulation. (2020) 141:e139–596. doi: 10.1161/cir.0000000000000757
4. Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. (2010) 67:181–98. doi: 10.1016/j.neuron.2010.07.002
5. Villa RF, Ferrari F, Moretti A. Post-stroke depression: Mechanisms and pharmacological treatment. Pharmacol Ther. (2018) 184:131–44. doi: 10.1016/j.pharmthera.2017.11.005
6. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. (2002) 52:253–64. doi: 10.1016/s0006-3223(02)01424-5
7. Li X, Han G, Zhao J, Huang X, Feng Y, Huang J, et al. Intestinal flora induces depression by mediating the dysregulation of cerebral cortex gene expression and regulating the metabolism of stroke patients. Front Mol biosciences. (2022) 9:865788. doi: 10.3389/fmolb.2022.865788
8. Aizenstein HJ, Baskys A, Boldrini M, Butters MA, Diniz BS, Jaiswal MK, et al. Vascular depression consensus report - a critical update. BMC Med. (2016) 14:161. doi: 10.1186/s12916-016-0720-5
9. Jeon SW, Kim YK. The role of neuroinflammation and neurovascular dysfunction in major depressive disorder. J Inflammation Res. (2018) 11:179–92. doi: 10.2147/jir.S141033
10. Nagpal J, Cryan JF. Microbiota-brain interactions: Moving toward mechanisms in model organisms. Neuron. (2021) 109:3930–53. doi: 10.1016/j.neuron.2021.09.036
11. Vighi G, Marcucci F, Sensi L, Di Cara G, Frati F. Allergy and the gastrointestinal system. Clin Exp Immunol. (2008) 153 Suppl 1:3–6. doi: 10.1111/j.1365-2249.2008.03713.x
12. Kayama H, Okumura R, Takeda K. Interaction between the microbiota, epithelia, and immune cells in the intestine. Annu Rev Immunol. (2020) 38:23–48. doi: 10.1146/annurev-immunol-070119-115104
13. Parker A, Fonseca S, Carding SR. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes. (2020) 11:135–57. doi: 10.1080/19490976.2019.1638722
14. Luo F, Fang C. Association between gut microbiota and post-stroke depression in Chinese population: A meta-analysis. Heliyon. (2022) 8:e12605. doi: 10.1016/j.heliyon.2022.e12605
15. Li X, Liu Y, Deng K, Hu Y. Modulating gut microbiota improves neurological function and depressive symptoms in rats with post-stroke depression. Nan fang yi ke da xue xue bao = J South Med University. (2024) 44:405–10. doi: 10.12122/j.issn.1673-4254.2024.02.24
16. Zhuang M, Zhang X, Cai J. Microbiota-gut-brain axis: interplay between microbiota, barrier function and lymphatic system. Gut Microbes. (2024) 16:2387800. doi: 10.1080/19490976.2024.2387800
17. Aburto MR, Cryan JF. Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota-gut-brain axis. Nat Rev Gastroenterol hepatology. (2024) 21:222–47. doi: 10.1038/s41575-023-00890-0
18. Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. (2020) 69:2232–43. doi: 10.1136/gutjnl-2020-322260
19. Martens EC, Neumann M, Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol. (2018) 16:457–70. doi: 10.1038/s41579-018-0036-x
20. Etienne-Mesmin L, Chassaing B, Desvaux M, De Paepe K, Gresse R, Sauvaitre T, et al. Experimental models to study intestinal microbes-mucus interactions in health and disease. FEMS Microbiol Rev. (2019) 43:457–89. doi: 10.1093/femsre/fuz013
21. Nilsson HE, Ambort D, Bäckström M, Thomsson E, Koeck PJB, Hansson GC, et al. Intestinal MUC2 mucin supramolecular topology by packing and release resting on D3 domain assembly. J Mol Biol. (2014) 426:2567–79. doi: 10.1016/j.jmb.2014.04.027
22. Okumura R, Takeda K. The role of the mucosal barrier system in maintaining gut symbiosis to prevent intestinal inflammation. Semin immunopathology. (2024) 47:2. doi: 10.1007/s00281-024-01026-5
23. Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci United States America. (2008) 105:15064–9. doi: 10.1073/pnas.0803124105
24. Johansson ME, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol hepatology. (2013) 10:352–61. doi: 10.1038/nrgastro.2013.35
25. Cerutti A, Chen K, Chorny A. Immunoglobulin responses at the mucosal interface. Annu Rev Immunol. (2011) 29:273–93. doi: 10.1146/annurev-immunol-031210-101317
26. Corthésy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol. (2013) 4:185. doi: 10.3389/fimmu.2013.00185
27. Strugnell RA, Wijburg OL. The role of secretory antibodies in infection immunity. Nat Rev Microbiol. (2010) 8:656–67. doi: 10.1038/nrmicro2384
28. Gutzeit C, Magri G, Cerutti A. Intestinal IgA production and its role in host-microbe interaction. Immunol Rev. (2014) 260:76–85. doi: 10.1111/imr.12189
29. Johansen FE, Pekna M, Norderhaug IN, Haneberg B, Hietala MA, Krajci P, et al. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J Exp Med. (1999) 190:915–22. doi: 10.1084/jem.190.7.915
30. Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. (2017) 14:9–21. doi: 10.1038/nrgastro.2016.169
31. Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. (1963) 17:375–412. doi: 10.1083/jcb.17.2.375
32. Shen L. Tight junctions on the move: molecular mechanisms for epithelial barrier regulation. Ann New York Acad Sci. (2012) 1258:9–18. doi: 10.1111/j.1749-6632.2012.06613.x
33. González-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog biophysics Mol Biol. (2003) 81:1–44. doi: 10.1016/s0079-6107(02)00037-8
34. Beutel O, Maraspini R, Pombo-García K, Martin-Lemaitre C, Honigmann A. Phase separation of zonula occludens proteins drives formation of tight junctions. Cell. (2019) 179:923–36.e11. doi: 10.1016/j.cell.2019.10.011
35. Yang S, Yu M. Role of goblet cells in intestinal barrier and mucosal immunity. J Inflammation Res. (2021) 14:3171–83. doi: 10.2147/jir.S318327
36. McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. (2012) 483:345–9. doi: 10.1038/nature10863
37. Owen RL, Jones AL. Epithelial cell specialization within human Peyer’s patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. (1974) 66:189–203. doi: 10.1016/S0016-5085(74)80102-2
38. Lelouard H, Fallet M, de Bovis B, Méresse S, Gorvel JP. Peyer’s patch dendritic cells sample antigens by extending dendrites through M cell-specific transcellular pores. Gastroenterology. (2012) 142:592–601.e3. doi: 10.1053/j.gastro.2011.11.039
39. Latorre R, Sternini C, De Giorgio R, Greenwood-Van-Meerveld B. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol motility. (2016) 28:620–30. doi: 10.1111/nmo.12754
40. Jones DE, Bevins CL. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem. (1992) 267:23216–25. doi: 10.1016/S0021-9258(18)50079-X
41. Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. (2011) 9:356–68. doi: 10.1038/nrmicro2546
42. Umar S. Intestinal stem cells. Curr Gastroenterol Rep. (2010) 12:340–8. doi: 10.1007/s11894-010-0130-3
43. Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. (2014) 14:141–53. doi: 10.1038/nri3608
44. Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Sci (New York NY). (2015) 350:830–4. doi: 10.1126/science.aad0135
45. Brescia P, Rescigno M. The gut vascular barrier: a new player in the gut-liver-brain axis. Trends Mol Med. (2021) 27:844–55. doi: 10.1016/j.molmed.2021.06.007
46. Stan RV, Tse D, Deharvengt SJ, Smits NC, Xu Y, Luciano MR, et al. The diaphragms of fenestrated endothelia: gatekeepers of vascular permeability and blood composition. Dev Cell. (2012) 23:1203–18. doi: 10.1016/j.devcel.2012.11.003
47. Forchielli ML, Walker WA. The role of gut-associated lymphoid tissues and mucosal defence. Br J Nutr. (2005) 93 Suppl 1:S41–8. doi: 10.1079/bjn20041356
48. Honarpisheh P, Bryan RM, McCullough LD. Aging microbiota-gut-brain axis in stroke risk and outcome. Circ Res. (2022) 130:1112–44. doi: 10.1161/circresaha.122.319983
49. Acheson DW, Luccioli SJBP, Gastroenterology RC. Mucosal immune responses. Best Practice & Research Clinical Gastroenterology. (2004) 18:387–404. doi: 10.1016/j.bpg.2003.11.002
50. Deenick EK, Ma CS. The regulation and role of T follicular helper cells in immunity. Immunology. (2011) 134:361–7. doi: 10.1111/j.1365-2567.2011.03487.x
51. Seikrit C, Pabst O. The immune landscape of IgA induction in the gut. Semin immunopathology. (2021) 43:627–37. doi: 10.1007/s00281-021-00879-4
52. Sommer F, Bäckhed F. The gut microbiota–masters of host development and physiology. Nat Rev Microbiol. (2013) 11:227–38. doi: 10.1038/nrmicro2974
53. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. New Engl J Med. (2016) 375:2369–79. doi: 10.1056/NEJMra1600266
54. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. (2014) 157:121–41. doi: 10.1016/j.cell.2014.03.011
55. Delgado Jiménez R, Benakis C. The gut ecosystem: A critical player in stroke. Neuromolecular Med. (2021) 23:236–41. doi: 10.1007/s12017-020-08633-z
56. Foley SE, Tuohy C, Dunford M, Grey MJ, De Luca H, Cawley C, et al. Gut microbiota regulation of P-glycoprotein in the intestinal epithelium in maintenance of homeostasis. Microbiome. (2021) 9:183. doi: 10.1186/s40168-021-01137-3
57. Kabouridis PS, Lasrado R, McCallum S, Chng SH, Snippert HJ, Clevers H, et al. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron. (2015) 85:289–95. doi: 10.1016/j.neuron.2014.12.037
58. Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, et al. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. (2007) 132:1344–58. doi: 10.1053/j.gastro.2007.01.051
59. Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci United States America. (2002) 99:15451–5. doi: 10.1073/pnas.202604299
60. Cazorla SI, Maldonado-Galdeano C, Weill R, De Paula J, Perdigón GDV. Oral administration of probiotics increases paneth cells and intestinal antimicrobial activity. Front Microbiol. (2018) 9:736. doi: 10.3389/fmicb.2018.00736
61. Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. (1987) 28:1221–7. doi: 10.1136/gut.28.10.1221
62. Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. (2016) 165:1332–45. doi: 10.1016/j.cell.2016.05.041
63. Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. (2011) 469:543–7. doi: 10.1038/nature09646
64. Hsieh CY, Osaka T, Moriyama E, Date Y, Kikuchi J, Tsuneda S. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol Rep. (2015) 3:e12327. doi: 10.14814/phy2.12327
65. Moens F, Weckx S, De Vuyst L. Bifidobacterial inulin-type fructan degradation capacity determines cross-feeding interactions between bifidobacteria and Faecalibacterium prausnitzii. Int J Food Microbiol. (2016) 231:76–85. doi: 10.1016/j.ijfoodmicro.2016.05.015
66. Fukata M, Arditi M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. (2013) 6:451–63. doi: 10.1038/mi.2013.13
67. Lavelle EC, Murphy C, O’Neill LA, Creagh EM. The role of TLRs, NLRs, and RLRs in mucosal innate immunity and homeostasis. Mucosal Immunol. (2010) 3:17–28. doi: 10.1038/mi.2009.124
68. Zindel J, Kubes P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu Rev pathology. (2020) 15:493–518. doi: 10.1146/annurev-pathmechdis-012419-032847
69. Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. (1994) 372:190–3. doi: 10.1038/372190a0
70. Lockhart A, Mucida D, Bilate AM. Intraepithelial lymphocytes of the intestine. Annu Rev Immunol. (2024) 42:289–316. doi: 10.1146/annurev-immunol-090222-100246
71. Qiu Y, Pu A, Zheng H, Liu M, Chen W, Wang W, et al. TLR2-dependent signaling for IL-15 production is essential for the homeostasis of intestinal intraepithelial lymphocytes. Mediators inflammation. (2016) 2016:4281865. doi: 10.1155/2016/4281865
72. Marsal J, Svensson M, Ericsson A, Iranpour AH, Carramolino L, Márquez G, et al. Involvement of CCL25 (TECK) in the generation of the murine small-intestinal CD8alpha alpha+CD3+ intraepithelial lymphocyte compartment. Eur J Immunol. (2002) 32:3488–97. doi: 10.1002/1521-4141(200212)32:12<3488::Aid-immu3488>3.0.Co;2-e
73. Chen B, Ni X, Sun R, Zeng B, Wei H, Tian Z, et al. Commensal bacteria-dependent CD8αβ(+) T cells in the intestinal epithelium produce antimicrobial peptides. Front Immunol. (2018) 9:1065. doi: 10.3389/fimmu.2018.01065
74. Tait Wojno ED, Artis D. Innate lymphoid cells: balancing immunity, inflammation, and tissue repair in the intestine. Cell Host Microbe. (2012) 12:445–57. doi: 10.1016/j.chom.2012.10.003
75. Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity. (2013) 38:769–81. doi: 10.1016/j.immuni.2013.02.010
76. von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. (2016) 529:221–5. doi: 10.1038/nature16161
77. Mjösberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. (2012) 37:649–59. doi: 10.1016/j.immuni.2012.08.015
78. Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. (2010) 464:1367–70. doi: 10.1038/nature08900
79. Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. (2012) 30:647–75. doi: 10.1146/annurev-immunol-020711-075053
80. Yue R, Wei X, Hao L, Dong H, Guo W, Sun X, et al. Promoting intestinal antimicrobial defense and microbiome symbiosis contributes to IL-22-mediated protection against alcoholic hepatitis in mice. Front Immunol. (2023) 14:1289356. doi: 10.3389/fimmu.2023.1289356
81. Lee HY, Park EA, Lee KJ, Lee KH, Park SJ. Increased innate lymphoid cell 3 and IL-17 production in mouse lamina propria stimulated with giardia lamblia. Korean J parasitology. (2019) 57:225–32. doi: 10.3347/kjp.2019.57.3.225
82. Kruidenier L, MacDonald TT, Collins JE, Pender SL, Sanderson IR. Myofibroblast matrix metalloproteinases activate the neutrophil chemoattractant CXCL7 from intestinal epithelial cells. Gastroenterology. (2006) 130:127–36. doi: 10.1053/j.gastro.2005.09.032
83. Zimmerman NP, Vongsa RA, Wendt MK, Dwinell MB. Chemokines and chemokine receptors in mucosal homeostasis at the intestinal epithelial barrier in inflammatory bowel disease. Inflammatory bowel diseases. (2008) 14:1000–11. doi: 10.1002/ibd.20480
84. Sibartie S, O’Hara AM, Ryan J, Fanning A, O’Mahony J, O’Neill S, et al. Modulation of pathogen-induced CCL20 secretion from HT-29 human intestinal epithelial cells by commensal bacteria. BMC Immunol. (2009) 10:54. doi: 10.1186/1471-2172-10-54
85. Sun M, He C, Cong Y, Liu Z. Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol. (2015) 8:969–78. doi: 10.1038/mi.2015.49
86. Sitaraman SV, Merlin D, Wang L, Wong M, Gewirtz AT, Si-Tahar M, et al. Neutrophil-epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest. (2001) 107:861–9. doi: 10.1172/jci11783
87. Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J leukocyte Biol. (1994) 56:559–64. doi: 10.1002/jlb.56.5.559
88. Chen Z, Luo J, Li J, Kim G, Stewart A, Huang Y, et al. Intestinal IL-33 promotes platelet activity for neutrophil recruitment during acute inflammation. Blood. (2022) 139:1878–91. doi: 10.1182/blood.2021013474
89. Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. (2003) 24:25–9. doi: 10.1016/s1471-4906(02)00013-3
90. Taub DD, Anver M, Oppenheim JJ, Longo DL, Murphy WJ. T lymphocyte recruitment by interleukin-8 (IL-8). IL-8-induced degranulation of neutrophils releases potent chemoattractants for human T lymphocytes both in vitro and in vivo. J Clin Invest. (1996) 97:1931–41. doi: 10.1172/jci118625
91. Sun B, Zhu L, Tao Y, Sun HX, Li Y, Wang P, et al. Characterization and allergic role of IL-33-induced neutrophil polarization. Cell Mol Immunol. (2018) 15:782–93. doi: 10.1038/cmi.2017.163
92. Kang L, Fang X, Song YH, He ZX, Wang ZJ, Wang SL, et al. Neutrophil-epithelial crosstalk during intestinal inflammation. Cell Mol Gastroenterol hepatology. (2022) 14:1257–67. doi: 10.1016/j.jcmgh.2022.09.002
93. Zindl CL, Lai JF, Lee YK, Maynard CL, Harbour SN, Ouyang W, et al. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc Natl Acad Sci United States America. (2013) 110:12768–73. doi: 10.1073/pnas.1300318110
94. Chen F, Yang W, Huang X, Cao AT, Bilotta AJ, Xiao Y, et al. Neutrophils promote amphiregulin production in intestinal epithelial cells through TGF-β and contribute to intestinal homeostasis. J Immunol (Baltimore Md: 1950). (2018) 201:2492–501. doi: 10.4049/jimmunol.1800003
95. Leiper K, Campbell BJ, Jenkinson MD, Milton J, Yu LG, Democratis J, et al. Interaction between bacterial peptides, neutrophils and goblet cells: a possible mechanism for neutrophil recruitment and goblet cell depletion in colitis. Clin Sci (London England: 1979). (2001) 101:395–402. doi: 10.1042/cs1010395
96. Karmakar M, Minns M, Greenberg EN, Diaz-Aponte J, Pestonjamasp K, Johnson JL, et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nat Commun. (2020) 11:2212. doi: 10.1038/s41467-020-16043-9
97. Yui S, Nakatani Y, Mikami M. Calprotectin (S100A8/S100A9), an inflammatory protein complex from neutrophils with a broad apoptosis-inducing activity. Biol Pharm bulletin. (2003) 26:753–60. doi: 10.1248/bpb.26.753
98. Zeuthen LH, Fink LN, Frokiaer H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology. (2008) 123:197–208. doi: 10.1111/j.1365-2567.2007.02687.x
99. Tahaghoghi-Hajghorbani S, Ajami A, Ghorbanalipoor S, Hosseini-Khah Z, Taghiloo S, Khaje-Enayati P, et al. Protective effect of TSLP and IL-33 cytokines in ulcerative colitis. Auto- Immun highlights. (2019) 10:1. doi: 10.1186/s13317-019-0110-z
100. Kelsall B. Recent progress in understanding the phenotype and function of intestinal dendritic cells and macrophages. Mucosal Immunol. (2008) 1:460–9. doi: 10.1038/mi.2008.61
101. Klebanoff CA, Spencer SP, Torabi-Parizi P, Grainger JR, Roychoudhuri R, Ji Y, et al. Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. J Exp Med. (2013) 210:1961–76. doi: 10.1084/jem.20122508
102. Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. (2007) 204:1757–64. doi: 10.1084/jem.20070590
103. Konkel JE, Chen W. Balancing acts: the role of TGF-β in the mucosal immune system. Trends Mol Med. (2011) 17:668–76. doi: 10.1016/j.molmed.2011.07.002
104. Meng EX, Verne GN, Zhou Q. Macrophages and gut barrier function: guardians of gastrointestinal health in post-inflammatory and post-infection responses. Int J Mol Sci. (2024) 25:9422. doi: 10.3390/ijms25179422
105. Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. (2005) 23(17):344–6. doi: 10.1016/j.immuni.2005.10.001
106. Han X, Ding S, Jiang H, Liu G. Roles of macrophages in the development and treatment of gut inflammation. Front Cell Dev Biol. (2021) 9:625423. doi: 10.3389/fcell.2021.625423
107. Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. (2009) 10:1178–84. doi: 10.1038/ni.1791
108. Goto Y, Ivanov II. Intestinal epithelial cells as mediators of the commensal-host immune crosstalk. Immunol Cell Biol. (2013) 91:204–14. doi: 10.1038/icb.2012.80
109. Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and th17 cells. Annu Rev Immunol. (2009) 27:485–517. doi: 10.1146/annurev.immunol.021908.132710
110. Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity. (2015) 43:727–38. doi: 10.1016/j.immuni.2015.09.003
111. Song X, Dai D, He X, Zhu S, Yao Y, Gao H, et al. Growth factor FGF2 cooperates with interleukin-17 to repair intestinal epithelial damage. Immunity. (2015) 43:488–501. doi: 10.1016/j.immuni.2015.06.024
112. Zha JM, Li HS, Lin Q, Kuo WT, Jiang ZH, Tsai PY, et al. Interleukin 22 expands transit-amplifying cells while depleting lgr5(+) stem cells via inhibition of wnt and notch signaling. Cell Mol Gastroenterol hepatology. (2019) 7:255–74. doi: 10.1016/j.jcmgh.2018.09.006
113. Jogdand GM, Mohanty S, Devadas S. Regulators of tfh cell differentiation. Front Immunol. (2016) 7:520. doi: 10.3389/fimmu.2016.00520
114. Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol (Baltimore Md: 1950). (2005) 175:7867–79. doi: 10.4049/jimmunol.175.12.7867
115. Gou HZ, Zhang YL, Ren LF, Li ZJ, Zhang L. How do intestinal probiotics restore the intestinal barrier? Front Microbiol. (2022) 13:929346. doi: 10.3389/fmicb.2022.929346
116. Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. (2004) 4:478–85. doi: 10.1038/nri1373
117. Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. (2009) 9:313–23. doi: 10.1038/nri2515
118. Peh A, O’Donnell JA, Broughton BRS, Marques FZ. Gut microbiota and their metabolites in stroke: A double-edged sword. Stroke. (2022) 53:1788–801. doi: 10.1161/strokeaha.121.036800
119. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. (2013) 504:446–50. doi: 10.1038/nature12721
120. Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina CJG. The luminal short-chain fatty acid butyrate modulates NF-κB activity in a human colonic epithelial cell line. Gastroenterology. (2000) 118:724–34. doi: 10.1016/S0016-5085(00)70142-9
121. Nagano Y, Itoh K, Honda K. The induction of Treg cells by gut-indigenous Clostridium. Curr Opin Immunol. (2012) 24:392–7. doi: 10.1016/j.coi.2012.05.007
122. Cani PD, Depommier C, Derrien M, Everard A, de Vos WM. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat Rev Gastroenterol hepatology. (2022) 19:625–37. doi: 10.1038/s41575-022-00631-9
123. Liu Y, Yang M, Tang L, Wang F, Huang S, Liu S, et al. TLR4 regulates RORγt(+) regulatory T-cell responses and susceptibility to colon inflammation through interaction with Akkermansia muciniphila. Microbiome. (2022) 10:98. doi: 10.1186/s40168-022-01296-x
124. Fan L, Xu C, Ge Q, Lin Y, Wong CC, Qi Y, et al. A. Muciniphila suppresses colorectal tumorigenesis by inducing TLR2/NLRP3-mediated M1-like TAMs. Cancer Immunol Res. (2021) 9:1111–24. doi: 10.1158/2326-6066.Cir-20-1019
125. Spadoni I, Fornasa G, Rescigno M. Organ-specific protection mediated by cooperation between vascular and epithelial barriers. Nat Rev Immunol. (2017) 17:761–73. doi: 10.1038/nri.2017.100
126. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol disease. (2004) 16:1–13. doi: 10.1016/j.nbd.2003.12.016
127. Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. (2008) 183:409–17. doi: 10.1083/jcb.200806024
128. Shue EH, Carson-Walter EB, Liu Y, Winans BN, Ali ZS, Chen J, et al. Plasmalemmal vesicle associated protein-1 (PV-1) is a marker of blood-brain barrier disruption in rodent models. BMC Neurosci. (2008) 9:29. doi: 10.1186/1471-2202-9-29
129. Carloni S, Bertocchi A, Mancinelli S, Bellini M, Erreni M, Borreca A, et al. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Sci (New York NY). (2021) 374:439–48. doi: 10.1126/science.abc6108
130. Jiang S, Khan MI, Lu Y, Werstiuk ES, Rathbone MP. Acceleration of blood-brain barrier formation after transplantation of enteric glia into spinal cords of rats. Exp Brain Res. (2005) 162:56–62. doi: 10.1007/s00221-004-2119-3
131. Nam HS. Gut microbiota and ischemic stroke: the role of trimethylamine N-oxide. J stroke. (2019) 21:151–9. doi: 10.5853/jos.2019.00472
132. Zoetendal EG, Rajilic-Stojanovic M, de Vos WM. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut. (2008) 57:1605–15. doi: 10.1136/gut.2007.133603
133. Ballway JW, Song BJ. Translational approaches with antioxidant phytochemicals against alcohol-mediated oxidative stress, gut dysbiosis, intestinal barrier dysfunction, and fatty liver disease. Antioxidants (Basel Switzerland). (2021) 10(3):384. doi: 10.3390/antiox10030384
134. Al-Asmakh M, Hedin L. Microbiota and the control of blood-tissue barriers. Tissue barriers. (2015) 3:e1039691. doi: 10.1080/21688370.2015.1039691
135. Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Trans Med. (2014) 6:263ra158. doi: 10.1126/scitranslmed.3009759
136. Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front endocrinology. (2020) 11:25. doi: 10.3389/fendo.2020.00025
137. Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, et al. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J neuroscience: Off J Soc Neurosci. (2002) 22:2478–86. doi: 10.1523/jneurosci.22-07-02478.2002
138. Kurita N, Yamashiro K, Kuroki T, Tanaka R, Urabe T, Ueno Y, et al. Metabolic endotoxemia promotes neuroinflammation after focal cerebral ischemia. J Cereb Blood Flow metabolism: Off J Int Soc Cereb Blood Flow Metab. (2020) 40:2505–20. doi: 10.1177/0271678x19899577
139. Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. (2009) 139:485–98. doi: 10.1016/j.cell.2009.09.033
140. Luo A, Leach ST, Barres R, Hesson LB, Grimm MC, Simar D. The microbiota and epigenetic regulation of T helper 17/regulatory T cells: in search of a balanced immune system. Front Immunol. (2017) 8:417. doi: 10.3389/fimmu.2017.00417
141. Lee YT, Mohd Ismail NI, Wei LK. Microbiome and ischemic stroke: A systematic review. PloS One. (2021) 16:e0245038. doi: 10.1371/journal.pone.0245038
142. Singh V, Sadler R, Heindl S, Llovera G, Roth S, Benakis C, et al. The gut microbiome primes a cerebroprotective immune response after stroke. J Cereb Blood Flow metabolism: Off J Int Soc Cereb Blood Flow Metab. (2018) 38:1293–8. doi: 10.1177/0271678x18780130
143. Xia GH, You C, Gao XX, Zeng XL, Zhu JJ, Xu KY, et al. Stroke dysbiosis index (SDI) in gut microbiome are associated with brain injury and prognosis of stroke. Front neurology. (2019) 10:397. doi: 10.3389/fneur.2019.00397
144. Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J neuroscience: Off J Soc Neurosci. (2016) 36:7428–40. doi: 10.1523/jneurosci.1114-16.2016
145. Tan C, Wu Q, Wang H, Gao X, Xu R, Cui Z, et al. Dysbiosis of gut microbiota and short-chain fatty acids in acute ischemic stroke and the subsequent risk for poor functional outcomes. JPEN J parenteral enteral Nutr. (2021) 45:518–29. doi: 10.1002/jpen.1861
146. Fang Z, Chen M, Qian J, Wang C, Zhang J. The bridge between ischemic stroke and gut microbes: short-chain fatty acids. Cell Mol neurobiology. (2023) 43:543–59. doi: 10.1007/s10571-022-01209-4
147. Liu D, Hong Y, Chen Z, Ma Y, Xia S, Gu S, et al. The tryptophan index is associated with risk of ischemic stroke: A community-based nested case-control study. Nutrients. (2024) 16:1544. doi: 10.3390/nu16111544
148. Houlden A, Goldrick M, Brough D, Vizi ES, Lénárt N, Martinecz B, et al. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain behavior immunity. (2016) 57:10–20. doi: 10.1016/j.bbi.2016.04.003
149. Chang Y, Woo HG, Jeong JH, Kim GH, Park KD, Song TJ. Microbiota dysbiosis and functional outcome in acute ischemic stroke patients. Sci Rep. (2021) 11:10977. doi: 10.1038/s41598-021-90463-5
150. Yin J, Liao SX, He Y, Wang S, Xia GH, Liu FT, et al. Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc. (2015) 4(11):e002699. doi: 10.1161/jaha.115.002699
151. Zeng X, Gao X, Peng Y, Wu Q, Zhu J, Tan C, et al. Higher risk of stroke is correlated with increased opportunistic pathogen load and reduced levels of butyrate-producing bacteria in the gut. Front Cell infection Microbiol. (2019) 9:4. doi: 10.3389/fcimb.2019.00004
152. Xu K, Gao X, Xia G, Chen M, Zeng N, Wang S, et al. Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut. (2021) 70(8): 1486–94. doi: 10.1136/gutjnl-2020-323263
153. Gu M, Chen N, Sun H, Li Z, Chen X, Zhou J, et al. Roseburia abundance associates with severity, evolution and outcome of acute ischemic stroke. Front Cell infection Microbiol. (2021) 11:669322. doi: 10.3389/fcimb.2021.669322
154. Sun H, Gu M, Li Z, Chen X, Zhou J. Gut microbiota dysbiosis in acute ischemic stroke associated with 3-month unfavorable outcome. Front neurology. (2021) 12:799222. doi: 10.3389/fneur.2021.799222
155. Li N, Wang X, Sun C, Wu X, Lu M, Si Y, et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. (2019) 19:191. doi: 10.1186/s12866-019-1552-1
156. Thiel A, Heiss WD. Imaging of microglia activation in stroke. Stroke. (2011) 42:507–12. doi: 10.1161/strokeaha.110.598821
157. Wang PY, Kao CH, Mui MY, Wang SJ. Leukocyte infiltration in acute hemispheric ischemic stroke. Stroke. (1993) 24:236–40. doi: 10.1161/01.str.24.2.236
158. Zhou SY, Guo ZN, Yang Y, Qu Y, Jin H. Gut-brain axis: Mechanisms and potential therapeutic strategies for ischemic stroke through immune functions. Front Neurosci. (2023) 17:1081347. doi: 10.3389/fnins.2023.1081347
159. Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med. (2016) 22:516–23. doi: 10.1038/nm.4068
160. Xie L, Li W, Hersh J, Liu R, Yang SH. Experimental ischemic stroke induces long-term T cell activation in the brain. J Cereb Blood Flow metabolism: Off J Int Soc Cereb Blood Flow Metab. (2019) 39:2268–76. doi: 10.1177/0271678x18792372
161. Vogelgesang A, May VE, Grunwald U, Bakkeboe M, Langner S, Wallaschofski H, et al. Functional status of peripheral blood T-cells in ischemic stroke patients. PloS One. (2010) 5:e8718. doi: 10.1371/journal.pone.0008718
162. Yu S, Cui W, Han J, Chen J, Tao W. Longitudinal change of Th1, Th2, and Th17 cells and their relationship between cognitive impairment, stroke recurrence, and mortality among acute ischemic stroke patients. J Clin Lab analysis. (2022) 36:e24542. doi: 10.1002/jcla.24542
163. Tuz AA, Ghosh S, Karsch L, Ttoouli D, Sata SP, Ulusoy Ö, et al. Stroke and myocardial infarction induce neutrophil extracellular trap release disrupting lymphoid organ structure and immunoglobulin secretion. Nat Cardiovasc Res. (2024) 3:525–40. doi: 10.1038/s44161-024-00462-8
164. McCulloch L, Allan SM, Emsley HC, Smith CJ, McColl BW. Interleukin-1 receptor antagonist treatment in acute ischaemic stroke does not alter systemic markers of anti-microbial defence. F1000Research. (2019) 8:1039. doi: 10.12688/f1000research.19308.2
165. Stanley D, Mason LJ, Mackin KE, Srikhanta YN, Lyras D, Prakash MD, et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat Med. (2016) 22:1277–84. doi: 10.1038/nm.4194
166. Tuz AA, Hasenberg A, Hermann DM, Gunzer M, Singh V. Ischemic stroke and concomitant gastrointestinal complications- a fatal combination for patient recovery. Front Immunol. (2022) 13:1037330. doi: 10.3389/fimmu.2022.1037330
167. Prass K, Meisel C, Höflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. (2003) 198:725–36. doi: 10.1084/jem.20021098
168. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. (2010) 11:373–84. doi: 10.1038/ni.1863
169. Liu X, Zhang Y, Chu J, Zheng J, Cheng X, Li X, et al. Effect of probiotics on the nutritional status of severe stroke patients with nasal feeding that receive enteral nutrition: A protocol for systematic review and meta-analysis of randomized controlled trials. Medicine. (2021) 100:e25657. doi: 10.1097/md.0000000000025657
170. Chen X, Hu Y, Yuan X, Yang J, Ka L. Effect of early enteral nutrition combined with probiotics in patients with stroke: a meta-analysis of randomized controlled trials. Eur J Clin Nutr. (2022) 76:592–603. doi: 10.1038/s41430-021-00986-3
171. Mayer EA, Tillisch K. Gupta AJTJoci. Gut/brain axis microbiota. (2015) 125:926–38. doi: 10.1172/JCI76304
172. Parada Venegas D, de la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. (2019) 10:277. doi: 10.3389/fimmu.2019.00277
173. Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol hepatology. (2019) 16:461–78. doi: 10.1038/s41575-019-0157-3
174. Zou X, Wang L, Xiao L, Wang S, Zhang L. Gut microbes in cerebrovascular diseases: Gut flora imbalance, potential impact mechanisms and promising treatment strategies. Front Immunol. (2022) 13:975921. doi: 10.3389/fimmu.2022.975921
175. Hu W, Kong X, Wang H, Li Y, Luo Y. Ischemic stroke and intestinal flora: an insight into brain-gut axis. Eur J Med Res. (2022) 27:73. doi: 10.1186/s40001-022-00691-2
176. Wang T, Pan C, Xie C, Chen L, Song Z, Liao H, et al. Microbiota metabolites and immune regulation affect ischemic stroke occurrence, development, and prognosis. Mol neurobiology. (2023) 60:6176–87. doi: 10.1007/s12035-023-03473-x
177. Lee J, d’Aigle J, Atadja L, Quaicoe V, Honarpisheh P, Ganesh BP, et al. Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ Res. (2020) 127:453–65. doi: 10.1161/circresaha.119.316448
178. Li H, Sun J, Du J, Wang F, Fang R, Yu C, et al. Clostridium butyricum exerts a neuroprotective effect in a mouse model of traumatic brain injury via the gut-brain axis. Neurogastroenterol motility. (2018) 30:e13260. doi: 10.1111/nmo.13260
179. Cheng J, Hu H, Ju Y, Liu J, Wang M, Liu B, et al. Gut microbiota-derived short-chain fatty acids and depression: deep insight into biological mechanisms and potential applications. Gen Psychiatry. (2024) 37:e101374. doi: 10.1136/gpsych-2023-101374
180. Roth W, Zadeh K, Vekariya R, Ge Y, Mohamadzadeh M. Tryptophan metabolism and gut-brain homeostasis. Int J Mol Sci. (2021) 22:2973. doi: 10.3390/ijms22062973
181. Kelly JR, Borre Y, O' Brien C, Patterson E, El Aidy S, Deane J, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. (2016) 82:109–18. doi: 10.1016/j.jpsychires.2016.07.019
182. Shapiro E, Biezuner T, Linnarsson S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat Rev Genet. (2013) 14:618–30. doi: 10.1038/nrg3542
183. Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, et al. A single-cell survey of the small intestinal epithelium. Nature. (2017) 551:333–9. doi: 10.1038/nature24489
184. Papalexi E, Satija R. Single-cell RNA sequencing to explore immune cell heterogeneity. Nat Rev Immunol. (2018) 18:35–45. doi: 10.1038/nri.2017.76
185. Ding Y, Peng YY, Li S, Tang C, Gao J, Wang HY, et al. Single-cell sequencing technology and its application in the study of central nervous system diseases. Cell Biochem biophysics. (2024) 82:329–42. doi: 10.1007/s12013-023-01207-3
186. Ståhl PL, Salmén F, Vickovic S, Lundmark A, Navarro JF, Magnusson J, et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Sci (New York NY). (2016) 353:78–82. doi: 10.1126/science.aaf2403
187. Subramanian I, Verma S, Kumar S, Jere A, Anamika K. Multi-omics data integration, interpretation, and its application. Bioinf Biol Insights. (2020) 14:1177932219899051. doi: 10.1177/1177932219899051
188. Li Z, Lai J, Zhang P, Ding J, Jiang J, Liu C, et al. Multi-omics analyses of serum metabolome, gut microbiome and brain function reveal dysregulated microbiota-gut-brain axis in bipolar depression. Mol Psychiatry. (2022) 27:4123–35. doi: 10.1038/s41380-022-01569-9
189. Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci. (2015) 18:1213–25. doi: 10.1038/nn.4091
190. Han X. In vivo application of optogenetics for neural circuit analysis. ACS Chem Neurosci. (2012) 3:577–84. doi: 10.1021/cn300065j
191. Patrono E, Svoboda J, Stuchlík A. Schizophrenia, the gut microbiota, and new opportunities from optogenetic manipulations of the gut-brain axis. Behav Brain functions: BBF. (2021) 17:7. doi: 10.1186/s12993-021-00180-2
192. Chen D, Xie J, Chen X, Qin B, Kong D, Luo J. Fecal microbiota transplantation alleviates neuronal Apoptosis, necroptosis and reactive microglia activation after ischemic stroke. Neuroscience. (2025) 564:299–305. doi: 10.1016/j.neuroscience.2024.10.053
193. Hu B, Das P, Lv X, Shi M, Aa J, Wang K, et al. Amelioration of depressive symptoms on Fawn-Hooded rats through the’healthy’fecal microbiota transplantation. Research Square. (2021). doi: 10.21203/rs.3.rs-917357/v1
194. Ritsma L, Ellenbroek SIJ, Zomer A, Snippert HJ, de Sauvage FJ, Simons BD, et al. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature. (2014) 507:362–5. doi: 10.1038/nature12972
195. Kerr JN, Denk W. Imaging in vivo: watching the brain in action. Nat Rev Neurosci. (2008) 9:195–205. doi: 10.1038/nrn2338
196. Clevers H. Modeling development and disease with organoids. Cell. (2016) 165:1586–97. doi: 10.1016/j.cell.2016.05.082
197. Moradian H, Gabriel T, Barrau M, Roblin X, Paul S. New methods to unveil host-microbe interaction mechanisms along the microbiota-gut-brain-axis. Gut Microbes. (2024) 16:2351520. doi: 10.1080/19490976.2024.2351520
198. Liu P, Peng G, Zhang N, Wang B, Luo B. Crosstalk between the gut microbiota and the brain: an update on neuroimaging findings. Front neurology. (2019) 10:883. doi: 10.3389/fneur.2019.00883
199. Guo J, Wang J, Sun W, Liu X. The advances of post-stroke depression: 2021 update. J neurology. (2022) 269:1236–49. doi: 10.1007/s00415-021-10597-4
200. Shi Y, Yang D, Zeng Y, Wu W. Risk factors for post-stroke depression: A meta-analysis. Front Aging Neurosci. (2017) 9:218. doi: 10.3389/fnagi.2017.00218
201. Kutlubaev MA, Hackett ML. Part II: predictors of depression after stroke and impact of depression on stroke outcome: an updated systematic review of observational studies. Int J stroke: Off J Int Stroke Soc. (2014) 9:1026–36. doi: 10.1111/ijs.12356
202. Kimura M, Robinson RG, Kosier JT. Treatment of cognitive impairment after poststroke depression: a double-blind treatment trial. Stroke. (2000) 31:1482–6. doi: 10.1161/01.str.31.7.1482
203. Mikami K, Jorge RE, Adams HP Jr., Davis PH, Leira EC, Jang M, et al. Effect of antidepressants on the course of disability following stroke. Am J geriatric psychiatry: Off J Am Assoc Geriatric Psychiatry. (2011) 19:1007–15. doi: 10.1097/JGP.0b013e31821181b0
204. Jorge RE, Robinson RG, Arndt S, Starkstein S. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. (2003) 160:1823–9. doi: 10.1176/appi.ajp.160.10.1823
205. Jiang Y, Zou D, Li Y, Gu S, Dong J, Ma X, et al. Monoamine neurotransmitters control basic emotions and affect major depressive disorders. Pharm (Basel Switzerland). (2022) 15(10):1203. doi: 10.3390/ph15101203
206. Vataja R, Pohjasvaara T, Leppävuori A, Mäntylä R, Aronen HJ, Salonen O, et al. Magnetic resonance imaging correlates of depression after ischemic stroke. Arch Gen Psychiatry. (2001) 58:925–31. doi: 10.1001/archpsyc.58.10.925
207. Terroni L, Amaro E, Iosifescu DV, Tinone G, Sato JR, Leite CC, et al. Stroke lesion in cortical neural circuits and post-stroke incidence of major depressive episode: a 4-month prospective study. World J Biol psychiatry: Off J World Fed Societies Biol Psychiatry. (2011) 12:539–48. doi: 10.3109/15622975.2011.562242
208. Shi Y, Liu W, Liu R, Zeng Y, Wu L, Huang S, et al. Investigation of the emotional network in depression after stroke: A study of multivariate Granger causality analysis of fMRI data. J Affect Disord. (2019) 249:35–44. doi: 10.1016/j.jad.2019.02.020
209. Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD. Global brain inflammation in stroke. Lancet Neurology. (2019) 18:1058–66. doi: 10.1016/s1474-4422(19)30078-x
210. Wijeratne T, Sales C. Understanding why post-stroke depression may be the norm rather than the exception: the anatomical and neuroinflammatory correlates of post-stroke depression. J Clin Med. (2021) 10(8):1674. doi: 10.3390/jcm10081674
211. Barandouzi ZA, Starkweather AR, Henderson WA, Gyamfi A, Cong XS. Altered composition of gut microbiota in depression: A systematic review. Front Psychiatry. (2020) 11:541. doi: 10.3389/fpsyt.2020.00541
212. Jeon SW, Kim YK. Inflammation-induced depression: Its pathophysiology and therapeutic implications. J neuroimmunology. (2017) 313:92–8. doi: 10.1016/j.jneuroim.2017.10.016
213. Yao S, Xie H, Wang Y, Shen N, Chen Q, Zhao Y, et al. Predictive microbial feature analysis in patients with depression after acute ischemic stroke. Front Aging Neurosci. (2023) 15:1116065. doi: 10.3389/fnagi.2023.1116065
214. Kang Y, Yang Y, Wang J, Ma Y, Cheng H, Wan D. Correlation between intestinal flora and serum inflammatory factors in post-stroke depression in ischemic stroke. J Coll Physicians Surgeons–Pakistan: JCPSP. (2021) 31:1224–7. doi: 10.29271/jcpsp.2021.10.1224
215. Shi M, Li Z, Tang Z, Zhou H, Huang X, Wei Y, et al. Exploring the pathogenesis and treatment of PSD from the perspective of gut microbiota. Brain Res bulletin. (2024) 215:111022. doi: 10.1016/j.brainresbull.2024.111022
216. Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. (2019) 99:1877–2013. doi: 10.1152/physrev.00018.2018
217. Luan H, Wang X, Cai Z. Mass spectrometry-based metabolomics: Targeting the crosstalk between gut microbiota and brain in neurodegenerative disorders. Mass spectrometry Rev. (2019) 38:22–33. doi: 10.1002/mas.21553
218. Wang J, Zhang H, He J, Xiong X. The role of the gut microbiota in the development of ischemic stroke. Front Immunol. (2022) 13:845243. doi: 10.3389/fimmu.2022.845243
219. Zhu X, Han Y, Du J, Liu R, Jin K, Yi W. Microbiota-gut-brain axis and the central nervous system. Oncotarget. (2017) 8:53829–38. doi: 10.18632/oncotarget.17754
220. Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann gastroenterology. (2015) 28:203–9.
221. Spencer NJ, Kyloh MA, Travis L, Hibberd TJ. Mechanisms underlying the gut-brain communication: How enterochromaffin (EC) cells activate vagal afferent nerve endings in the small intestine. J Comp neurology. (2024) 532:e25613. doi: 10.1002/cne.25613
222. Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol hepatology. (2009) 6:306–14. doi: 10.1038/nrgastro.2009.35
223. Zhang X, Wang CB, Duan LH, Long JJ, Xiao P, Wang YL, et al. Correlation research of serum substance P, CCK-8, and 5-HT values with depression levels in stroke survivors. Eur Rev Med Pharmacol Sci. (2023) 27:1248–54. doi: 10.26355/eurrev_202302_31357
224. Vieira DS, Naffah-Mazacoratti MG, Zukerman E, Senne Soares CA, Alonso EO, Faulhaber MH, et al. Cerebrospinal fluid GABA levels in chronic migraine with and without depression. Brain Res. (2006) 1090:197–201. doi: 10.1016/j.brainres.2006.03.051
225. Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J, et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. (2019) 4:396–403. doi: 10.1038/s41564-018-0307-3
226. Duranti S, Ruiz L, Lugli GA, Tames H, Milani C, Mancabelli L, et al. Bifidobacterium adolescentis as a key member of the human gut microbiota in the production of GABA. Sci Rep. (2020) 10:14112. doi: 10.1038/s41598-020-70986-z
227. Freimer D, Yang TT, Ho TC, Tymofiyeva O, Leung C. The gut microbiota, HPA axis, and brain in adolescent-onset depression: Probiotics as a novel treatment. Brain behavior Immun - Health. (2022) 26:100541. doi: 10.1016/j.bbih.2022.100541
228. Ling Y, Gu Q, Zhang J, Gong T, Weng X, Liu J, et al. Structural change of gut microbiota in patients with post-stroke comorbid cognitive impairment and depression and its correlation with clinical features. J Alzheimer’s disease: JAD. (2020) 77:1595–608. doi: 10.3233/jad-200315
229. Rescigno M. Intestinal microbiota and its effects on the immune system. Cell Microbiol. (2014) 16:1004–13. doi: 10.1111/cmi.12301
230. Jiang W, Gong L, Liu F, Ren Y, Mu J. Alteration of gut microbiome and correlated lipid metabolism in post-stroke depression. Front Cell infection Microbiol. (2021) 11:663967. doi: 10.3389/fcimb.2021.663967
231. Ye X, Wang D, Zhu H, Wang D, Li J, Tang Y, et al. Gut microbiota changes in patients with major depressive disorder treated with vortioxetine. Front Psychiatry. (2021) 12:641491. doi: 10.3389/fpsyt.2021.641491
232. Sapountzis P, Segura A, Desvaux M, Forano E. An overview of the elusive passenger in the gastrointestinal tract of cattle: the shiga toxin producing escherichia coli. Microorganisms. (2020) 8(6):877. doi: 10.3390/microorganisms8060877
233. Tian P, O’Riordan KJ, Lee YK, Wang G, Zhao J, Zhang H, et al. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol stress. (2020) 12:100216. doi: 10.1016/j.ynstr.2020.100216
234. Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci United States America. (2011) 108:16050–5. doi: 10.1073/pnas.1102999108
235. Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics: J Am Soc Exp NeuroTherapeutics. (2016) 13:661–70. doi: 10.1007/s13311-016-0483-x
236. Qiu YM, Zhang CL, Chen AQ, Wang HL, Zhou YF, Li YN, et al. Immune cells in the BBB disruption after acute ischemic stroke: targets for immune therapy? Front Immunol. (2021) 12:678744. doi: 10.3389/fimmu.2021.678744
237. Cacabelos R, Torrellas C, Fernández-Novoa L, Aliev G. Neuroimmune crosstalk in CNS disorders: the histamine connection. Curr Pharm design. (2016) 22:819–48. doi: 10.2174/1381612822666151209150954
238. Feng X, Ma X, Li J, Zhou Q, Liu Y, Song J, et al. Inflammatory pathogenesis of post-stroke depression. Aging disease. (2024) 16:209–38. doi: 10.14336/ad.2024.0203
239. Kim JM, Kang HJ, Kim JW, Bae KY, Kim SW, Kim JT, et al. Associations of tumor necrosis factor-α and interleukin-1β Levels and polymorphisms with post-stroke depression. Am J geriatric psychiatry: Off J Am Assoc Geriatric Psychiatry. (2017) 25:1300–8. doi: 10.1016/j.jagp.2017.07.012
240. Kang HJ, Bae KY, Kim SW, Kim JT, Park MS, Cho KH, et al. Effects of interleukin-6, interleukin-18, and statin use, evaluated at acute stroke, on post-stroke depression during 1-year follow-up. Psychoneuroendocrinology. (2016) 72:156–60. doi: 10.1016/j.psyneuen.2016.07.001
241. Spalletta G, Cravello L, Imperiale F, Salani F, Bossù P, Picchetto L, et al. Neuropsychiatric symptoms and interleukin-6 serum levels in acute stroke. J neuropsychiatry Clin neurosciences. (2013) 25:255–63. doi: 10.1176/appi.neuropsych.12120399
242. Stuart MJ, Singhal G, Baune BT. Systematic review of the neurobiological relevance of chemokines to psychiatric disorders. Front Cell Neurosci. (2015) 9:357. doi: 10.3389/fncel.2015.00357
243. Wohleb ES, Franklin T, Iwata M, Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci. (2016) 17:497–511. doi: 10.1038/nrn.2016.69
244. Cazareth J, Guyon A, Heurteaux C, Chabry J, Petit-Paitel A. Molecular and cellular neuroinflammatory status of mouse brain after systemic lipopolysaccharide challenge: importance of CCR2/CCL2 signaling. J neuroinflammation. (2014) 11:132. doi: 10.1186/1742-2094-11-132
245. Ma Y, Wang J, Wang Y, Yang GY. The biphasic function of microglia in ischemic stroke. Prog neurobiology. (2017) 157:247–72. doi: 10.1016/j.pneurobio.2016.01.005
246. Suo Q, Deng L, Chen T, Wu S, Qi L, Liu Z, et al. Optogenetic activation of astrocytes reduces blood-brain barrier disruption via IL-10 in stroke. Aging disease. (2023) 14:1870–86. doi: 10.14336/ad.2023.0226
247. Tao X, Wu S, Tang W, Li L, Huang L, Mo D, et al. Alleviative effects of foraging exercise on depressive-like behaviors in chronic mild stress-induced ischemic rat model. Brain injury. (2022) 36:127–36. doi: 10.1080/02699052.2022.2034949
248. Chai Y, Sheline YI, Oathes DJ, Balderston NL, Rao H, Yu M. Functional connectomics in depression: insights into therapies. Trends Cogn Sci. (2023) 27:814–32. doi: 10.1016/j.tics.2023.05.006
249. Padmanabhan JL, Cooke D, Joutsa J, Siddiqi SH, Ferguson M, Darby RR, et al. A human depression circuit derived from focal brain lesions. Biol Psychiatry. (2019) 86:749–58. doi: 10.1016/j.biopsych.2019.07.023
250. Wu X, Xu K, Li T, Wang L, Fu Y, Ma Z, et al. Abnormal intrinsic functional hubs and connectivity in patients with post-stroke depression. Ann Clin Trans neurology. (2024) 11:1852–67. doi: 10.1002/acn3.52091
251. Wei L, Guo J, Yu X, Chen H, Du Y, Ji Z, et al. Role and characteristics of hippocampal region microglial activation in poststroke depression. J Affect Disord. (2021) 291:270–8. doi: 10.1016/j.jad.2021.05.022
252. Su JA, Chou SY, Tsai CS, Hung TH. Cytokine changes in the pathophysiology of poststroke depression. Gen Hosp Psychiatry. (2012) 34:35–9. doi: 10.1016/j.genhosppsych.2011.09.020
253. Kleeberg A, Luft T, Golkowski D, Purrucker JC. Endothelial dysfunction in acute ischemic stroke: a review. J neurology. (2025) 272:143. doi: 10.1007/s00415-025-12888-6
254. Sifat AE, Vaidya B, Abbruscato TJ. Blood-brain barrier protection as a therapeutic strategy for acute ischemic stroke. AAPS J. (2017) 19:957–72. doi: 10.1208/s12248-017-0091-7
255. Zheng X, Ren B, Gao Y. Tight junction proteins related to blood-brain barrier and their regulatory signaling pathways in ischemic stroke. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie. (2023) 165:115272. doi: 10.1016/j.biopha.2023.115272
256. Coisne C, Engelhardt B. Tight junctions in brain barriers during central nervous system inflammation. Antioxidants Redox Signaling. (2011) 15:1285–303. doi: 10.1089/ars.2011.3929
257. Juli C, Heryaman H, Nazir A, Ang ET, Defi IR, Gamayani U, et al. The lymphocyte depletion in patients with acute ischemic stroke associated with poor neurologic outcome. Int J Gen Med. (2021) 14:1843–51. doi: 10.2147/ijgm.S308325
258. Kumar AD, Boehme AK, Siegler JE, Gillette M, Albright KC, Martin-Schild S. Leukocytosis in patients with neurologic deterioration after acute ischemic stroke is associated with poor outcomes. J stroke cerebrovascular diseases: Off J Natl Stroke Assoc. (2013) 22:e111–7. doi: 10.1016/j.jstrokecerebrovasdis.2012.08.008
259. Qi X, Tang Z, Shao X, Wang Z, Li M, Zhang X, et al. Ramelteon improves blood-brain barrier of focal cerebral ischemia rats to prevent post-stroke depression via upregulating occludin. Behav Brain Res. (2023) 449:114472. doi: 10.1016/j.bbr.2023.114472
260. Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, et al. Social stress induces neurovascular pathology promoting depression. Nat Neurosci. (2017) 20:1752–60. doi: 10.1038/s41593-017-0010-3
261. Muhammad M, Muchimapura S, Wattanathorn J. Microbiota-gut-brain axis impairment in the pathogenesis of stroke: implication as a potent therapeutic target. Bioscience microbiota Food Health. (2023) 42:143–51. doi: 10.12938/bmfh.2022-067
262. Zhang W, Dong XY, Huang R. Gut microbiota in ischemic stroke: role of gut bacteria-derived metabolites. Trans stroke Res. (2023) 14:811–28. doi: 10.1007/s12975-022-01096-3
263. Zhao L, Xiao J, Li S, Guo Y, Fu R, Hua S, et al. The interaction between intestinal microenvironment and stroke. CNS Neurosci Ther. (2023) 29 Suppl 1:185–99. doi: 10.1111/cns.14275
264. Ye D, Hu Y, Zhu N, Gu W, Long G, Tao E, et al. Exploratory investigation of intestinal structure and function after stroke in mice. Mediators inflammation. (2021) 2021:1315797. doi: 10.1155/2021/1315797
265. Błaż M, Natorska J, Bembenek JP, Członkowska A, Ząbczyk M, Polak M, et al. Elevated lipopolysaccharide level is largely driven by time since symptom onset in acute ischemic stroke: the impact on clinical outcomes. J Thromb haemostasis: JTH. (2024) 22:3161–71. doi: 10.1016/j.jtha.2024.06.028
266. Dziedzic T. Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev neurotherapeutics. (2015) 15:523–31. doi: 10.1586/14737175.2015.1035712
267. Chen R, Zhang X, Gu L, Zhu H, Zhong Y, Ye Y, et al. New insight into neutrophils: A potential therapeutic target for cerebral ischemia. Front Immunol. (2021) 12:692061. doi: 10.3389/fimmu.2021.692061
268. Kostulas N, Li HL, Xiao BG, Huang YM, Kostulas V, Link H. Dendritic cells are present in ischemic brain after permanent middle cerebral artery occlusion in the rat. Stroke. (2002) 33:1129–34. doi: 10.1161/hs0402.105379
269. Beuker C, Strecker JK, Rawal R, Schmidt-Pogoda A, Ruck T, Wiendl H, et al. Immune cell infiltration into the brain after ischemic stroke in humans compared to mice and rats: a systematic review and meta-analysis. Trans stroke Res. (2021) 12:976–90. doi: 10.1007/s12975-021-00887-4
270. Zhang Y, Yang Y, Li H, Feng Q, Ge W, Xu X. Investigating the potential mechanisms and therapeutic targets of inflammatory cytokines in post-stroke depression. Mol neurobiology. (2024) 61:132–47. doi: 10.1007/s12035-023-03563-w
271. Suzuki K, Nakajima A. New aspects of IgA synthesis in the gut. Int Immunol. (2014) 26:489–94. doi: 10.1093/intimm/dxu059
272. Caso JR, Hurtado O, Pereira MP, García-Bueno B, Menchén L, Alou L, et al. Colonic bacterial translocation as a possible factor in stress-worsening experimental stroke outcome. Am J Physiol Regulatory Integr Comp Physiol. (2009) 296:R979–85. doi: 10.1152/ajpregu.90825.2008
273. Oyama N, Winek K, Bäcker-Koduah P, Zhang T, Dames C, Werich M, et al. Exploratory investigation of intestinal function and bacterial translocation after focal cerebral ischemia in the mouse. Front neurology. (2018) 9:937. doi: 10.3389/fneur.2018.00937
274. Malone MK, Ujas TA, Britsch DRS, Cotter KM, Poinsatte K, Stowe AM. The immunopathology of B lymphocytes during stroke-induced injury and repair. Semin immunopathology. (2023) 45:315–27. doi: 10.1007/s00281-022-00971-3
275. Slyepchenko A, Maes M, Köhler CA, Anderson G, Quevedo J, Alves GS, et al. T helper 17 cells may drive neuroprogression in major depressive disorder: Proposal of an integrative model. Neurosci Biobehav Rev. (2016) 64:83–100. doi: 10.1016/j.neubiorev.2016.02.002
276. Cui M, Dai W, Kong J, Chen H. Th17 cells in depression: are they crucial for the antidepressant effect of ketamine? Front Pharmacol. (2021) 12:649144. doi: 10.3389/fphar.2021.649144
277. Beurel E, Lowell JA. Th17 cells in depression. Brain behavior immunity. (2018) 69:28–34. doi: 10.1016/j.bbi.2017.08.001
278. Wang HY, Ye JR, Cui LY, Chu SF, Chen NH. Regulatory T cells in ischemic stroke. Acta pharmacologica Sinica. (2022) 43:1–9. doi: 10.1038/s41401-021-00641-4
279. Gao X, Tang Y, Kong L, Fan Y, Wang C, Wang R. Treg cell: Critical role of regulatory T-cells in depression. Pharmacol Res. (2023) 195:106893. doi: 10.1016/j.phrs.2023.106893
280. Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J neuroinflammation. (2014) 11:98. doi: 10.1186/1742-2094-11-98
281. Chen AQ, Fang Z, Chen XL, Yang S, Zhou YF, Mao L, et al. Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death disease. (2019) 10:487. doi: 10.1038/s41419-019-1716-9
282. Shen XY, Gao ZK, Han Y, Yuan M, Guo YS, Bi X. Activation and role of astrocytes in ischemic stroke. Front Cell Neurosci. (2021) 15:755955. doi: 10.3389/fncel.2021.755955
283. Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, et al. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry. (2014) 19:699–709. doi: 10.1038/mp.2013.155
284. Ke X, Deng M, Wu Z, Yu H, Yu D, Li H, et al. miR-34b-3p inhibition of eIF4E causes post-stroke depression in adult mice. Neurosci bulletin. (2023) 39:194–212. doi: 10.1007/s12264-022-00898-7
285. Yu D, Cheng Z, Ali AI, Wang J, Le K, Chibaatar E, et al. Down-expressed GLT-1 in PSD astrocytes inhibits synaptic formation of NSC-derived neurons in vitro. Cell Cycle (Georgetown Tex). (2019) 18:105–14. doi: 10.1080/15384101.2018.1560201
286. Feng C, Fang M, Liu XY. The neurobiological pathogenesis of poststroke depression. TheScientificWorldJournal. (2014) 2014:521349. doi: 10.1155/2014/521349
287. Pu B, Zhu H, Wei L, Gu L, Zhang S, Jian Z, et al. The involvement of immune cells between ischemic stroke and gut microbiota. Trans stroke Res. (2024) 15:498–517. doi: 10.1007/s12975-023-01151-7
288. Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Adv Nutr (Bethesda Md). (2019) 10:S49–s66. doi: 10.1093/advances/nmy063
289. Ohland CL, Macnaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointestinal liver Physiol. (2010) 298:G807–19. doi: 10.1152/ajpgi.00243.2009
290. Rahman Z, Padhy HP, Dandekar MP. Cell-Free Supernatant of Lactobacillus rhamnosus and Bifidobacterium breve Ameliorates Ischemic Stroke-Generated Neurological Deficits in Rats. Probiotics antimicrobial Proteins. (2024) 1–17. doi: 10.1007/s12602-024-10256-w
291. Liu Y, Kong C, Gong L, Zhang X, Zhu Y, Wang H, et al. The association of post-stroke cognitive impairment and gut microbiota and its corresponding metabolites. J Alzheimer’s disease: JAD. (2020) 73:1455–66. doi: 10.3233/jad-191066
292. Wang Y, Zhang X, Wang Y, Lu L, Wei R, Xu B, et al. Effects of combined live bifidobacterium, lactobacillus, enterococcus and bacillus cereus tablets on post-stroke depression and serum inflammatory factorse. Discovery Med. (2023) 35:312–20. doi: 10.24976/Discov.Med.202335176.32
293. Zhong DY, Li L, Ma RM, Deng YH. The effect of probiotics in stroke treatment. Evidence-Based complementary Altern medicine: eCAM. (2021) 2021:4877311. doi: 10.1155/2021/4877311
294. Markowiak P, Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. (2017) 9:1021. doi: 10.3390/nu9091021
295. Yuan Q, Xin L, Han S, Su Y, Wu R, Liu X, et al. Lactulose improves neurological outcomes by repressing harmful bacteria and regulating inflammatory reactions in mice after stroke. Front Cell infection Microbiol. (2021) 11:644448. doi: 10.3389/fcimb.2021.644448
296. Akagawa S, Akagawa Y, Nakai Y, Yamagishi M, Yamanouchi S, Kimata T, et al. Fiber-rich barley increases butyric acid-producing bacteria in the human gut microbiota. Metabolites. (2021) 11:559. doi: 10.3390/metabo11080559
297. Zou H, Gao H, Liu Y, Zhang Z, Zhao J, Wang W, et al. Dietary inulin alleviated constipation induced depression and anxiety-like behaviors: Involvement of gut microbiota and microbial metabolite short-chain fatty acid. Int J Biol macromolecules. (2024) 259:129420. doi: 10.1016/j.ijbiomac.2024.129420
298. Khoruts A, Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol hepatology. (2016) 13:508–16. doi: 10.1038/nrgastro.2016.98
299. Hediyal TA, Vichitra C, Anand N, Bhaskaran M, Essa SM, Kumar P, et al. Protective effects of fecal microbiota transplantation against ischemic stroke and other neurological disorders: an update. Front Immunol. (2024) 15:1324018. doi: 10.3389/fimmu.2024.1324018
300. Chen R, Xu Y, Wu P, Zhou H, Lasanajak Y, Fang Y, et al. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol Res. (2019) 148:104403. doi: 10.1016/j.phrs.2019.104403
301. Rao J, Xie R, Lin L, Jiang J, Du L, Zeng X, et al. Fecal microbiota transplantation ameliorates gut microbiota imbalance and intestinal barrier damage in rats with stress-induced depressive-like behavior. Eur J Neurosci. (2021) 53:3598–611. doi: 10.1111/ejn.15192
302. Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev. (2005) 29:493–500. doi: 10.1016/j.neubiorev.2005.01.004
303. Bansal V, Costantini T, Ryu SY, Peterson C, Loomis W, Putnam J, et al. Stimulating the central nervous system to prevent intestinal dysfunction after traumatic brain injury. J trauma. (2010) 68:1059–64. doi: 10.1097/TA.0b013e3181d87373
304. Wang Y, Tan Q, Pan M, Yu J, Wu S, Tu W, et al. Minimally invasive vagus nerve stimulation modulates mast cell degranulation via the microbiota-gut-brain axis to ameliorate blood-brain barrier and intestinal barrier damage following ischemic stroke. Int immunopharmacology. (2024) 132:112030. doi: 10.1016/j.intimp.2024.112030
305. Neren D, Johnson MD, Legon W, Bachour SP, Ling G, Divani AA. Vagus nerve stimulation and other neuromodulation methods for treatment of traumatic brain injury. Neurocritical Care. (2016) 24:308–19. doi: 10.1007/s12028-015-0203-0
306. Zhang S, He H, Wang Y, Wang X, Liu X. Transcutaneous auricular vagus nerve stimulation as a potential novel treatment for polycystic ovary syndrome. Sci Rep. (2023) 13:7721. doi: 10.1038/s41598-023-34746-z
307. Liu J, Dai Q, Qu T, Ma J, Lv C, Wang H, et al. Ameliorating effects of transcutaneous auricular vagus nerve stimulation on a mouse model of constipation-predominant irritable bowel syndrome. Neurobiol disease. (2024) 193:106440. doi: 10.1016/j.nbd.2024.106440
308. Liu C, Tang H, Liu C, Ma J, Liu G, Niu L, et al. Transcutaneous auricular vagus nerve stimulation for post-stroke depression: A double-blind, randomized, placebo-controlled trial. J Affect Disord. (2024) 354:82–8. doi: 10.1016/j.jad.2024.03.005
309. Li J, Jia J, Teng Y, Xie C, Li C, Zhu B, et al. Gastrodin alleviates DSS-induced colitis in mice through strengthening intestinal barrier and modulating gut microbiota. Foods (Basel Switzerland). (2024) 13:2460. doi: 10.3390/foods13152460
310. Wang S, Yu L, Guo H, Zuo W, Guo Y, Liu H, et al. Gastrodin ameliorates post-stroke depressive-like behaviors through cannabinoid-1 receptor-dependent PKA/rhoA signaling pathway. Mol Neurobiol. (2024) 62(1):366–85. doi: 10.1007/s12035-024-04267-5
311. Zhao Y, Qin S, Yang Z, Lu Y, Ma Z, Ping X, et al. Gastrodin ameliorates depressive-like behaviors via modulating gut microbiota in CUMS-induced mice. Behav Brain Res. (2024) 465:114968. doi: 10.1016/j.bbr.2024.114968
312. Li D, Tao L, Yang J, Cai W, Shen W. Global research trends in acupuncture treatment for post-stroke depression: A bibliometric analysis. Complementary therapies Med. (2024) 84:103070. doi: 10.1016/j.ctim.2024.103070
313. Yang NN, Lin LL, Li YJ, Li HP, Cao Y, Tan CX, et al. Potential mechanisms and clinical effectiveness of acupuncture in depression. Curr neuropharmacology. (2022) 20:738–50. doi: 10.2174/1570159x19666210609162809
314. Jiang H, Deng S, Zhang J, Chen J, Li B, Zhu W, et al. Acupuncture treatment for post-stroke depression: Intestinal microbiota and its role. Front Neurosci. (2023) 17:1146946. doi: 10.3389/fnins.2023.1146946
315. Cai W, Wei XF, Zhang JR, Tao L, Li D, Zhang K, et al. Acupuncture ameliorates depression-like behavior of poststroke depression model rats through the regulation of gut microbiota and NLRP3 inflammasome in the colon. Neuroreport. (2024) 35:883–94. doi: 10.1097/wnr.0000000000002076
316. Lam Ching W, Li HJ, Guo J, Yao L, Chau J, Lo S, et al. Acupuncture for post-stroke depression: a systematic review and network meta-analysis. BMC Psychiatry. (2023) 23:314. doi: 10.1186/s12888-023-04749-1
317. Zhang J, Zhao Y, Li H, Yang Y, Tang Q. Effectiveness of acupuncture plus music therapy for post-stroke depression: Systematic review and meta-analysis. Medicine. (2024) 103:e39681. doi: 10.1097/md.0000000000039681
Keywords: post-stroke depression, ischemic stroke, gut microbiota, immune regulation, barrier integrity and function, microbiota-gut-brain axis, inflammatory response, microbiota-immune-barrier axis
Citation: Jiang J, Xie H, Cao S, Xu X, Zhou J, Liu Q, Ding C and Liu M (2025) Post-stroke depression: exploring gut microbiota-mediated barrier dysfunction through immune regulation. Front. Immunol. 16:1547365. doi: 10.3389/fimmu.2025.1547365
Received: 18 December 2024; Accepted: 17 February 2025;
Published: 03 March 2025.
Edited by:
Yiming Meng, China Medical University, ChinaReviewed by:
Panida Sittipo, Burapha University, ThailandCopyright © 2025 Jiang, Xie, Cao, Xu, Zhou, Liu, Ding and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changsong Ding, ZGluZ2NzMTk3NUBobnVjbS5lZHUuY24=; Mi Liu, bmV3bWVhbkBobnVjbS5lZHUuY24=
†ORCID: Jia Jiang, orcid.org/0000-0002-5425-305X
Mi Liu, orcid.org/0000-0003-4860-7533
Qianyan Liu, orcid.org/0000-0003-2818-8511
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.