
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol., 19 March 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1546886
Immune checkpoint inhibitors (ICIs) have revolutionized hepatocellular carcinoma (HCC) treatment, while immune-related adverse events (IRAEs) pose significant challenges. We report a 60-year-old male with unresectable HCC who developed Guillain-Barré syndrome (GBS), a rare but severe neurologic complication, after three cycles of sintilimab plus bevacizumab biosimilar and conventional transarterial chemoembolization (c-TACE). The patient presented with progressive ascending weakness, reaching symmetric quadriparesis with proximal muscle strength of 2/5 in upper limbs and 1/5 in lower limbs. Following sintilimab discontinuation, treatment with intravenous immunoglobulin (2 g/kg) and oral prednisone (30 mg/day) achieved complete neurological recovery within one month. Given the patient’s favorable initial tumor response and strong request, immunotherapy was cautiously reinstated using tislelizumab after thorough clinical evaluation. Following four cycles of treatment, significant tumor response enabled successful conversion surgery with major pathological response (necrosis rate >70%). With 26-month survival and no evidence of recurrence, this case demonstrates the potential feasibility of ICI rechallenge with an alternative PD-1 inhibitor following sintilimab-induced GBS. Our experience suggests that ICI-related neurological adverse events may be drug-specific rather than class-specific, potentially providing valuable treatment options for patients showing favorable tumor response despite experiencing severe IRAEs, though larger studies are needed for validation.
Hepatocellular carcinoma (HCC) ranks as the fourth most common malignancy and represents the second leading cause of cancer-related mortality in China (1). In 2022, China reported 368,000 novel HCC cases, representing 42.4% of the global incidence, with 317,000 associated fatalities, accounting for 41.7% of worldwide mortality in this malignancy (2). Despite a substantial improvement in overall cancer survival rates from 30.9% to 40.5% between 2003-2015, HCC patients experienced only a marginal increase from 10.1% to 12.1% (3). Benefiting from comprehensive screening strategies, approximately 70% of patients in Japan and Taiwan are diagnosed at an early stage, with a high proportion meeting curative resection criteria. In contrast, 64% of patients in China are diagnosed at Barcelona Clinic Liver Cancer (BCLC) stages B or C (4). Curative surgical resection remains the gold standard for HCC treatment, with a 5-year survival rate of up to 64% (5). Consequently, conversion therapy, which reduces tumor size, downstaging, and creates conditions for subsequent surgery, is crucial to the patient’s long-term survival (6, 7).
With the ongoing advancement of interventional treatment techniques such as transcatheter arterial chemoembolization (TACE) and hepatic arterial infusion chemotherapy (HAIC), along with the emergence of novel targeted drugs and immune checkpoint inhibitors (ICIs), multiple conversion therapy options have become available for intermediate and advanced HCC (8). Numerous studies have shown that monotherapy generally offers limited efficacy and seldom results in long-term benefits. The integration of multiple therapeutic modalities combined with personalized treatment protocols represents the current predominant trend in medical practice (9).
As ICIs gain widespread adoption in clinical practice, the recognition and management of (immune-related adverse events) IRAEs have become critical clinical challenges. These events can impact any organ system, predominantly due to enhanced T cell activation, autoimmune responses, and dysregulated inflammatory reactions (10). Although most IRAEs are mild and manageable, certain severe complications pose life-threatening risks, requiring immediate intervention and standardized management.
Guillain-Barré syndrome (GBS) represents an exceedingly rare, yet severe neurologic IRAEs characterized by progressive ascending paralysis. Clinical manifestations typically include symmetric muscle weakness, loss of deep tendon reflexes, and sensory abnormalities (11). The pathogenesis of ICI-induced GBS remains incompletely understood but likely involves multiple mechanisms, including loss of peripheral immune tolerance, regulatory T cell dysfunction, and molecular mimicry between tumor antigens and neural tissues (12).
Current guidelines advocate for the permanent discontinuation of ICIs upon the occurrence of severe neurologic adverse events (NAEs). Nevertheless, this presents a therapeutic conundrum for patients who have demonstrated substantial antitumor responses. In contexts with limited therapeutic alternatives, the potential for a rechallenge with alternate ICIs has not been adequately investigated (13). This report describes a case of successful tislelizumab rechallenge in a patient with unresectable HCC following sintilimab-induced GBS, providing valuable clinical insights into the management of patients who demonstrate therapeutic response to immunotherapy despite experiencing NAEs.
A 60-year-old male patient, with a height of 172 cm and weight of 65 kg, had been managing chronic hepatitis B for 20 years and was consistently taking Tenofovir (Hepsera®) 300 mg daily for antiviral therapy. A large space-occupying lesion in the right hepatic lobe was incidentally discovered during a routine medical screening examination. The patient presented with an (Eastern Cooperative Oncology Group) ECOG performance status of 1 and Child-Pugh class B liver function, Laboratory tests revealed elevated (serum alpha-fetoprotein) AFP (2,356 ng/mL) and (protein induced by vitamin K absence or antagonist-II)PIVKA-II (35,680 mAU/mL) levels, with HBV-DNA less than 100 IU/mL. Integration of laboratory and imaging findings established the diagnosis of HCC, BCLC stage C. Our multidisciplinary team (MDT) determined tumor unresectability based on three key factors: elevated (indocyanine green retention rate at 15 minutes) ICG-R15 (18.5%), insufficient remnant liver volume and inadequate surgical margins due to tumor proximity (5 mm) to the middle hepatic vein. Therefore, a combination therapy regimen was established, consisting of conventional transarterial chemoembolization (c-TACE) plus sintilimab (TYVYT®) and bevacizumab biosimilar (BYVASDA®).
Initially, the patient underwent c-TACE with a mixture of epirubicin (40 mg) and lipiodol (20 mL), followed by embolization with two vials of CalliSpheres® blank microspheres (100-300 μm). Intraoperative angiography demonstrated a significant reduction in tumor vascularity with satisfactory lipiodol retention. The procedure was completed successfully without complications. Six days following c-TACE, Sintilimab immunotherapy was initiated with a standard three-week cycle regimen. One week after completing the third cycle of sintilimab, the patient developed progressive, symmetrical ascending weakness without sensory loss. Three weeks later, the weakness progressed to inability to stand and upper limb weakness, accompanied by mild dysphagia and dyspnea, but without ptosis, neck weakness, or respiratory compromise.
Upon emergency admission, physical examination revealed symmetric quadriparesis with proximal muscle strength of 2/5 in upper limbs and 1/5 in lower limbs, and distal strength of 3/5 and 2/5, respectively. Deep and superficial reflexes were absent bilaterally with negative Babinski signs and stocking-glove sensory deficit. Cerebrospinal fluid (CSF) analysis showed elevated pressure (145 mmH2O) and protein (128 mg/dL) with normal cell count (3×106/L), glucose (3.6 mmol/L), and chloride (120 mmol/L), demonstrating albuminocytologic dissociation. Nerve conduction studies revealed reduced conduction velocities in motor nerves (median: 32 m/s; common peroneal: 28 m/s) and sensory nerves (median: 35 m/s; sural: 30 m/s), with prolonged F-wave latencies (median nerve: 35 ms; peroneal nerve: 38 ms), indicating peripheral neuropathy.
The patient was diagnosed with sintilimab-induced GBS. Sintilimab was discontinued, and treatment was initiated with intravenous immunoglobulin (IVIG) at 2 g/kg (total 130 g divided into 26 g daily for 5 days) and oral prednisone (30 mg/day, divided into 15 mg in the morning and afternoon). The treatment regimen also included twice-daily bedside rehabilitation (30 minutes per session), high-protein diet supplementation with B vitamins, and prophylactic subcutaneous enoxaparin (4,000 U/day). The patient showed gradual improvement. After one week of GBS treatment, muscle strength improved to 3/5 proximally and 4/5 distally in the upper extremities, and 2/5 proximally and 3/5 distally in the lower extremities, with significant improvement in swallowing function. At discharge (day 14), muscle strength reached 4/5 in all extremities, enabling assisted standing with a walker, and swallowing function had largely recovered. Discharge instructions included continued prednisone (20 mg/day) with dose adjustment after two weeks, and ongoing rehabilitation.
Following complete neurological recovery and approximately two months after the onset of GBS, immunotherapy was reinstated upon the patient’s strong request and thorough clinical evaluation, Sintilimab was substituted with tislelizumab, which was administered for four cycles over a two-month period. Following the standard treatment course, imaging evaluation demonstrated significant tumor response, enabling surgical intervention. Pathological examination revealed a major pathological response (MPR) with tumor necrosis rate >70%. The complete treatment course is illustrated in Figure 1, and radiological changes are shown in Figure 2. As of December 15, 2024, the patient remains alive with an overall survival (OS) of 26 months. Serum tumor markers showed a sustained decrease. No evidence of recurrence or metastasis was observed during follow-up.
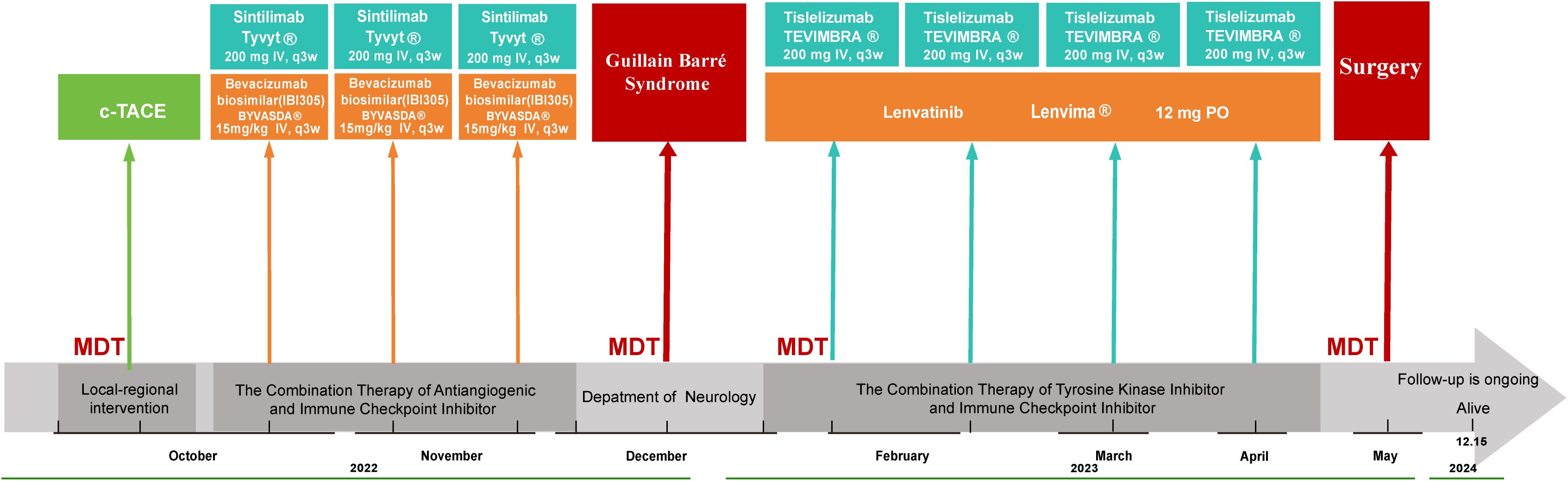
Figure 1. Schematic illustration of the treatment timeline. c-TACE Conventional Transarterial Chemoembolization, MDT Multidisciplinary Team, IV Intravenous.
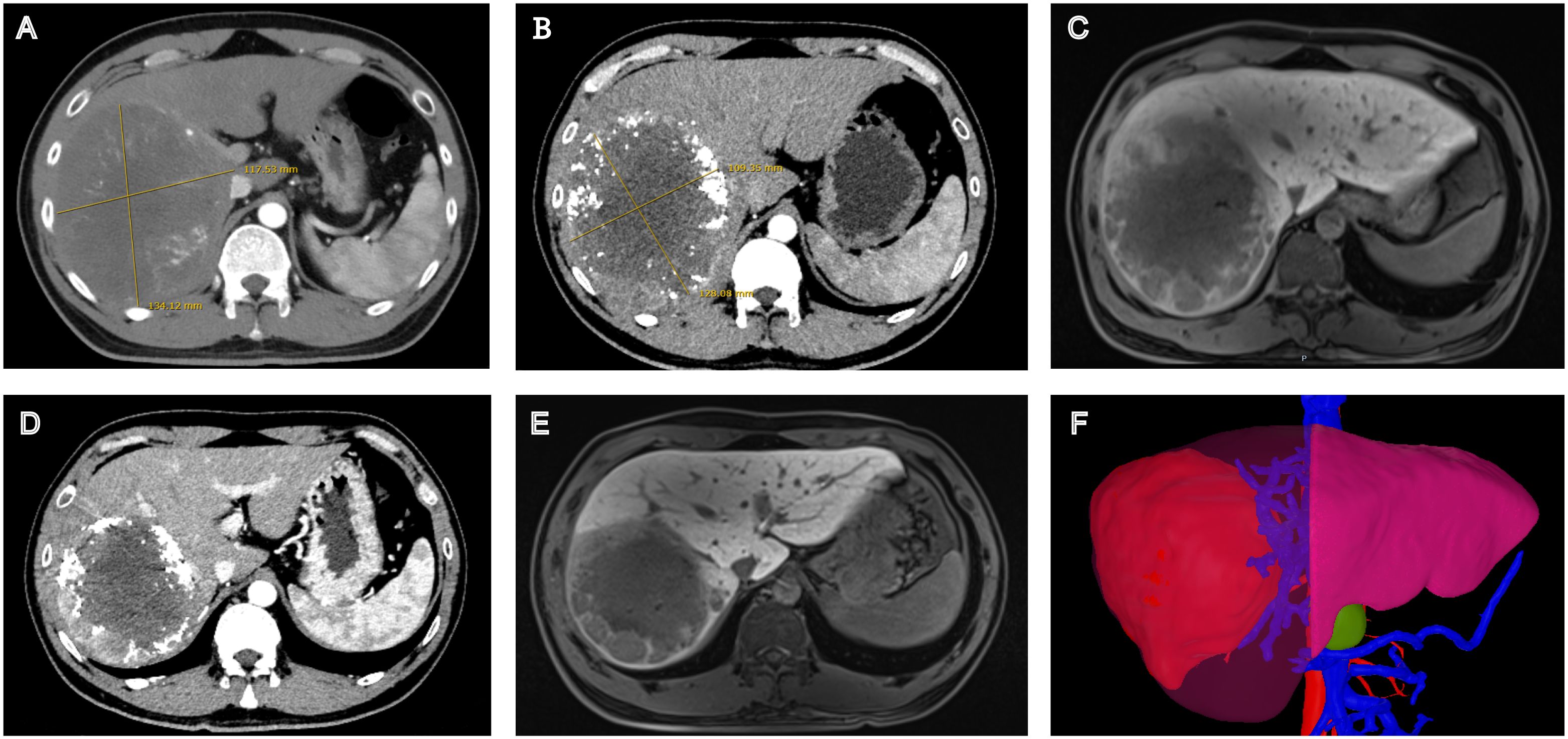
Figure 2. Radiological changes during the patient’s treatment course. (A) Contrast-enhanced CT at baseline. (B) Contrast-enhanced CT After c-TACE therapy. (C) Gd-EOB-DTPA-enhanced MR after c-TACE therapy. (D) Contrast-enhanced CT after sintilimab (3 cycles) and Tislelizumab (4 cycles). (E) Gd-EOB-DTPA-enhanced MR after sintilimab (3 cycles) and Tislelizumab (4 cycles). (F) Three-dimensional volumetric analysis based on contrast-enhanced CT before surgery.
Due to the heterogeneity of HCC and divergent treatment paradigms between Eastern and Western regions, official treatment guidelines often differ (14). For instance, the Chinese guidelines recommend TACE, hepatic resection, and radiotherapy for selected advanced HCC patients, while these options are not endorsed in Western guidelines. Mounting clinical evidence demonstrates that locoregional therapies achieve significantly higher local control rates for intrahepatic lesions compared to systemic treatment alone. In Chinese clinical practice, combination strategies incorporating both locoregional and systemic therapies are predominantly employed, while monotherapy with either tyrosine kinase inhibitors (TKIs) or ICIs is rarely utilized (15).
For unresectable hepatocellular carcinoma (uHCC), TACE is widely accepted as a first-line treatment modality. This approach is also recommended for advanced HCC patients with portal vein tumor thrombosis (PVTT) in Korea, Japan, and other Asian countries (16, 17). TACE combined with molecular targeted therapy is based on TKIs’ ability to suppress hypoxia-induced angiogenesis post-TACE. This synergistic approach enhances therapeutic efficacy through multiple mechanisms. TACE-induced tumor necrosis releases tumor antigens, enhancing antitumor immune responses, creating a pro-vascular environment for targeted therapy, and augmenting CD8+ T cell responses to tumor-associated antigens (TAAs). Immune checkpoint inhibitors complement this effect by blocking CTLA-4 and PD-1 pathways (18). A multicenter prospective randomized study in Japan and Korea reported complete or partial response rates of 73% and 2-year OS rates of 75% with TACE treatment (19). The CHANCE001 study demonstrated that TACE combined with targeted immunotherapy significantly improved outcomes compared to TACE alone (20). In contrast to previous studies incorporating various ICIs and targeted agents, the CHANCE2211 trial streamlined the therapeutic approach by evaluating the combination of carrelizumab and apatinib with TACE. Results demonstrated that this combination therapy significantly improved OS, progression-free survival (PFS), and objective response rate (ORR) compared to TACE monotherapy (21).
Recent advances in systemic therapy have not only created opportunities for surgical resection in initially unresectable patients but also effectively reduced postoperative recurrence and metastasis rates, thereby improving long-term survival benefits. The global phase III IMbrave150 trial demonstrated that atezolizumab plus bevacizumab significantly prolonged median OS and PFS compared to sorafenib, reducing the risk of death by 34% and disease progression by 35%. In the Chinese subgroup analysis, the combination therapy showed even more pronounced clinical benefits, with a 47% reduction in mortality risk and a 40% reduction in disease progression risk compared to sorafenib (22). The ORIENT-32 trial demonstrated that sintilimab plus bevacizumab biosimilar significantly outperformed sorafenib, with a 43% reduction in mortality risk and a 44% reduction in disease progression risk. This combination therapy has been approved in China as a first-line treatment for patients with unresectable or metastatic HCC who have not received prior systemic therapy (23). The BGB-A317-211 trial evaluated tislelizumab plus lenvatinib as first-line therapy for advanced HCC. The combination demonstrated statistical superiority in ORR compared to historical data on lenvatinib monotherapy. Notably, tumor shrinkage was observed in over 70% of patients, representing significant clinical implications from a surgical perspective (24). The global phase III CARES-310 trial, evaluating Camrelizumab plus apatinib as first-line therapy for advanced uHCC, achieved the longest median overall survival (25). Additionally, the HIMALAYA, which first reported 5-year overall survival data, led to regulatory approval from the Food and Drug Administration(FDA), European Medicines Agency(EMA), and Pharmaceuticals and Medical Devices Agency(PMDA) for the STRIDE regimen (tremelimumab plus durvalumab) in the treatment of uHCC (26).
The therapeutic landscape of HCC has entered the immunotherapy era, marked by the broad implementation of ICIs across diverse clinical settings, encompassing palliative, adjuvant, neoadjuvant, and multimodal treatment strategies (27). While providing clinical benefits, ICIs can disrupt immune homeostasis, resulting in IRAEs with an incidence rate of 66-72%. These events commonly affect target organs including skin, gastrointestinal tract, lungs, liver, and endocrine systems. Although NAEs are relatively rare, accounting for 1.0-12.0% of all reported IRAEs, complications such as encephalitis, GBS, and myasthenia gravis can potentially progress to severe or fatal outcomes (28–30). The Common Terminology Criteria for Adverse Events (CTCAE) provides a standardized grading system for irAE severity. Moderate (grade 2) to severe (grades 3-4) IRAEs can result in substantial organ dysfunction, deterioration of quality of life, and potential mortality, underscoring the critical importance of early recognition and appropriate therapeutic intervention (31, 32).
ICI-associated NAEs can manifest in various forms, including myasthenia gravis, facial nerve palsy, meningitis, hypophysitis, meningoradiculoneuritis, cerebellitis, transverse myelitis, and GBS (33). GBS is a particularly concerning neurological complication that can progress to life-threatening muscle weakness and autonomic dysfunction. While classical GBS is typically triggered by known or unknown immune stimuli such as infections, surgery, vaccination, or trauma, certain medications, including ICIs, can also precipitate this condition (11). Electrophysiological findings suggest that immune-related GBS subtypes primarily present as widespread sensorimotor polyneuropathy characterized by acute inflammatory demyelinating polyneuropathy (AIDP) or acute motor axonal neuropathy (AMAN). Overall, the clinical course, severity, and outcomes of GBS demonstrate substantial heterogeneity (34).
The exact pathogenic mechanism of ICI-induced GBS remains unclear, studies suggest that ICIs promote T-cell proliferation and activation, leading to immune-mediated neuronal injury and subsequent autoimmune neuropathy. Research has demonstrated that both human and murine neurons express PD-L1 molecules, with significantly increased expression during neuronal injury. Moreover, neurons, like tumor cells, can express antigens recognizable by T cells. During ICI therapy, particularly with dual ICI combinations (anti-CTLA-4 and anti-PD-1 antibodies), effector T cells may directly target neurons, triggering autoimmune neurological disorders. Currently, available CTLA-4-targeted ICIs, such as the humanized monoclonal antibody ipilimumab, block the CTLA-4-B7 pathway, enhancing effector T-cell proliferation and activation, thereby augmenting antitumor immune responses and tumor cell destruction (35, 36). The potential mechanisms of IRAEs induced by anti-PD-1 antibodies (nivolumab and pembrolizumab) or anti-PD-L1 antibodies (atezolizumab) are associated with PD-1/PD-L1 pathway inhibition, leading to excessive T-cell activation and reduced regulatory T-cell function. This results in enhanced toxicity of macrophages and neutrophils, release of interferon-gamma and tumor necrosis factor, and antibody production by B cells (37, 38).
The literature comparing the propensity of different PD-1 inhibitors to induce IRAEs remains limited. However, emerging evidence suggests that structural and pharmacological differences between PD-1 inhibitors may influence their safety profiles. Sintilimab, tislelizumab, and other PD-1 inhibitors differ in their binding epitopes and affinities, which could explain variations in their immunological effects and adverse event profiles. Large-scale clinical trial data demonstrates that in the RATIONALE-301 study, tislelizumab showed a grade 3-4 IRAE incidence rate of 18.2%, while sintilimab in the ORIENT-32 study had a corresponding rate of 24.4% (23, 39). Specifically regarding neurological adverse events, the CheckMate-459 study showed that nivolumab led to a 2.3% incidence of neurological IRAEs, while pembrolizumab in the KEYNOTE-240 study had an incidence rate of 1.8% (40, 41). In this case, the patient did not experience NAEs after switching to tislelizumab. This may be related to differences in immune system activation patterns between the two drugs or individual patient variations. Tislelizumab’s binding region differs slightly from other PD-1 inhibitors, potentially leading to unique characteristics in its immune system activation (42).
The literature on ICIs-induced GBS and experiences of ICI rechallenge is limited, as only a small number of cases have been reported, summarized in Table 1. In the case of sintilimab, research has not revealed any unexpected side effects or off-target reactions, with most adverse events being grade 1-2 and not requiring specialized treatment, apart from thyroid dysfunction, colitis, and hepatitis, most complications are rare, Notable reported cases of these rare IRAEs include ICI-associated myocarditis, toxic epidermal necrolysis (TEN), severe erosive hemorrhagic gastritis, and pyloric obstruction (43–45).
To our knowledge, this report describes the first case of a successful transition from sintilimab-related GBS to tislelizumab, with a successful immune rechallenge leading to conversion surgery. Treatment decisions, in this case, were facilitated by the patient’s high educational background and favorable socioeconomic circumstances, with therapeutic strategies determined through a shared decision-making process incorporating both multidisciplinary team (MDT) recommendations and patient preferences factors that merit careful consideration in clinical practice. The diagnosis of GBS in this case was established based on clinical history and examination, supported by ancillary investigations including cerebrospinal fluid analysis and electrophysiological studies. Upon high clinical suspicion, ICI therapy was immediately discontinued, and treatment with immunoglobulin and corticosteroids was initiated. According to the American Society of Clinical Oncology (ASCO) Clinical Practice Guidelines and National Comprehensive Cancer Network (NCCN) Guidelines, Corticosteroids and IVIG represent conventional therapeutic approaches for ICI-associated GBS. This combination therapy has demonstrated clinical improvement in 73% of patients (46, 47). Methylprednisolone at 1-2 mg/kg may be considered, particularly in GBS patients with cerebrospinal fluid pleocytosis exceeding expected levels (48). Plasma exchange (PE) may be employed as second-line therapy when initial treatments prove ineffective. However, its efficacy as a first-line treatment remains unclear (49). Physical therapy (PT) constitutes an essential component in the rehabilitation and management of GBS. In our case, the patient underwent one month of physical therapy, achieving complete neurological recovery (50).
Another critical consideration in clinical practice is the safety of resuming ICI therapy after adverse event resolution. Due to limited case numbers, literature specifically addressing cancer-specific therapy reinitiation following checkpoint inhibitor-induced GBS is scarce. Most protocols recommend discontinuation of ICI therapy in cases of severe adverse events. The NCCN guidelines further specify that severe irAEs from one class of immunotherapy necessitate permanent discontinuation of the same class, with moderate irAEs warranting cautious consideration. A retrospective study in melanoma patients suggests that toxicity may be treatment-specific rather than universal across different types of immune checkpoint blockade (51). A study investigating tremelimumab/durvalumab as an ICI rechallenge option after initial atezolizumab/bevacizumab therapy demonstrated satisfactory early safety and efficacy profiles (52). According to CTCAE criteria, this case was classified as grade 2 (53). Following discontinuation of sintilimab (anti-PD-1), the patient underwent a successful immune rechallenge with an alternative anti-PD-1 agent, tislelizumab. This conversion therapy ultimately transformed unresectable disease to surgically resectable status, achieving long-term survival benefits without recurrence of neurological symptoms.
This study offers novel therapeutic insights for managing NAEs associated with ICI therapy. For patients who initially respond well to immunotherapy, switching to an alternative PD-1 inhibitor may be a safe and viable option, even in cases of ICIs-induced GBS. This approach, implemented through systematic MDT discussions, provided timely and scientifically sound treatment decisions for patients with limited therapeutic alternatives.
ICIs have demonstrated significant efficacy in the treatment of HCC, but the management of IRAEs remains challenging. This study presents a rare but clinically significant case of a patient with sindilimab-induced GBS. After receiving standard treatment and fully recovering, cautiously selecting another PD-1 inhibitor for rechallenge was a potentially feasible treatment option, which at least brought long-term survival benefits to this patient. Moreover, this case successfully achieved a conversion from unresectable to radical resection, fully reflecting the value of individualized treatment strategies. Although the experience of a single case has certain limitations, it is a worthwhile attempt in the absence of standardized treatment regimens. Moving ahead, several crucial research directions need to be explored, including large-scale multicenter studies to validate the safety and efficacy of ICI rechallenge, investigations of differential IRAE profiles among PD-1 inhibitors, and development of predictive biomarkers for patient selection. Additionally, comprehensive assessment of patient-reported outcomes during rechallenge will be essential for clinical decision-making. We anticipate that future research in these areas will help establish evidence-based guidelines for ICI rechallenge in clinical practice.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Ethics Committee of Guilin Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
LY: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. WY: Writing – review & editing, Data curation, Formal analysis. HS: Data curation, Formal analysis, Writing – review & editing. JL: Data curation, Formal analysis, Writing – review & editing. YQ: Funding acquisition, Project administration, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Guilin City Scientific Research and Technology Development Plan Project ([2024] No. 17) and the Guangxi Medical and Health Key Discipline Construction Project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. (2024) 4:47–53. doi: 10.1016/j.jncc.2024.01.006
3. Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. (2018) 6:e555–67. doi: 10.1016/S2214-109X(18)30127-X
4. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. (2015) 35:2155–66. doi: 10.1111/liv.12818
5. Fan J, Gao Q, Huang R. Research frontiers in precision therapy for liver cancer. Zhonghua GanZangBingZaZhi. (2020) 28:897–900. doi: 10.3760/cma.j.cn501113-20201103-00596
6. Zhu XD, Huang C, Shen YH, Ji Y, Ge NL, Qu XD, et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti-PD-1 antibody combinations. Liver Cancer. (2021) 10:320–9. doi: 10.1159/000514313
7. Zhu XD, Huang C, Shen YH, Xu B, Ge NL, Ji Y, et al. Hepatectomy after conversion therapy using tyrosine kinase inhibitors plus anti-PD-1 antibody therapy for patients with unresectable hepatocellular carcinoma. Ann Surg Oncol. (2023) 30:2782–90. doi: 10.1245/s10434-022-12530-z
8. Yang C, Zhang H, Zhang L, Zhu AX, Bernards R, Qin W, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. (2023) 20:203–22. doi: 10.1038/s41575-022-00704-9
9. Wu TK, Hui RW, Mak LY, Fung J, Seto WK, Yuen MF. Hepatocellular carcinoma: Advances in systemic therapies. F1000Res. (2024) 13:104. doi: 10.12688/f1000research.145493.2
10. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. (2019) 16:563–80. doi: 10.1038/s41571-019-0218-0
11. Shahrizaila N, Lehmann HC, Kuwabara S. Guillain-Barré syndrome. Lancet. (2021) 397:1214–28. doi: 10.1016/S0140-6736(21)00517-1
12. van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. (2014) 10:469–82. doi: 10.1038/nrneurol.2014.121
13. Zhao Q, Zhang J, Xu L, Yang H, Liang N, Zhang L, et al. Safety and efficacy of the rechallenge of immune checkpoint inhibitors after immune-related adverse events in patients with cancer: A systemic review and meta-analysis. Front Immunol. (2021) 12:730320. doi: 10.3389/fimmu.2021.730320
14. Yen YH, Cheng YF, Wang JH, Lin CC, Chen YY, Yong CC, et al. Real world clinical practice in treating advanced hepatocellular carcinoma: When East meets West. PloS One. (2020) 15:e0230005. doi: 10.1371/journal.pone.0230005
15. Xie D, Shi J, Zhou J, Fan J, Gao Q. Clinical practice guidelines and real-life practice in hepatocellular carcinoma: A Chinese perspective. Clin Mol Hepatol. (2023) 29:206–16. doi: 10.3350/cmh.2022.0402
16. Korean Liver Cancer Association (KLCA), National Cancer Center (NCC). 2018 Korean liver cancer association-national cancer center Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol. (2019) 20:1042–113. doi: 10.3348/kjr.2019.0140
17. Kudo M, Kitano M, Sakurai T, Nishida N. General rules for the clinical and pathological study of primary liver cancer, nationwide follow-up survey and clinical practice guidelines: the outstanding achievements of the liver cancer study group of Japan. Dig Dis. (2015) 33:765–70. doi: 10.1159/000439101
18. Tan J, Fan W, Liu T, Zhu B, Liu Y, Wang S, et al. TREM2+macrophages suppress CD8+T-cell infiltration after transarterial chemoembolisation in hepatocellular carcinoma. J Hepatol. (2023) 79:126–40. doi: 10.1016/j.jhep.2023.02.032
19. Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. (2017) 11:317–70. doi: 10.1007/s12072-017-9799-9
20. Zhu HD, Li HL, Huang MS, Yang WZ, Yin GW, CHANCE001 Investigators. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther. (2023) 8:58. doi: 10.1038/s41392-022-01235-0
21. Jin ZC, Zhong BY, Chen JJ, Zhu HD, Sun JH, Yin GW, et al. Real-world efficacy and safety of TACE plus camrelizumab and apatinib in patients with HCC (CHANCE2211): a propensity score matching study. Eur Radiol. (2023) 33:8669–81. doi: 10.1007/s00330-023-09754-2
22. Kudo M, Tsuchiya K, Shao YY, Finn RS, Galle PR, Ducreux M, et al. Impact of Bevacizumab Being Skipped due to Adverse Events of Special Interest for Bevacizumab in Patients with Unresectable Hepatocellular Carcinoma Treated with Atezolizumab plus Bevacizumab: An Exploratory Analysis of the Phase III IMbrave150 Study. Liver Cancer. (2023) 13:401–12. doi: 10.1159/000535501
23. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. (2021) 22:977–90. doi: 10.1016/S1470-2045(21)00252-7
24. Xu L, Chen J, Liu C, Song X, Zhang Y, Zhao H, et al. Efficacy and safety of tislelizumab plus lenvatinib as first-line treatment in patients with unresectable hepatocellular carcinoma: a multicenter, single-arm, phase 2 trial. BMC Med. (2024) 22:172. doi: 10.1186/s12916-024-03356-5
25. Qin S, Chan SL, Gu S, Bai Y, Ren Z, Lin X, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. (2023) 402:1133–46. doi: 10.1016/S0140-6736(23)00961-3
26. Sangro B, Chan SL, Kelley RK, Lau G, Kudo M, Sukeepaisarnjaroen W, et al. Four-year overall survival update from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann Oncol. (2024) 35:448–57. doi: 10.1016/j.annonc.2024.02.005
27. Yu SJ. Immunotherapy for hepatocellular carcinoma: Recent advances and future targets. Pharmacol Ther. (2023) 244:108387. doi: 10.1016/j.pharmthera.2023.108387
28. Diamanti L, Picca A, Bini P, Gastaldi M, Alfonsi E, Pichiecchio A, et al. Characterization and management of neurological adverse events during immune-checkpoint inhibitors treatment: an Italian multicentric experience. Neurol Sci. (2022) 43:2031–41. doi: 10.1007/s10072-021-05561-z
29. Fan S, Ren H, Zhao L, Yin J, Feng G, Wang J, et al. Neurological immune-related adverse events associated with immune checkpoint inhibitors: A review of the literature. Asia Pac J Clin Oncol. (2020) 16:291–8. doi: 10.1111/ajco.13375
30. Marini A, Bernardini A, Gigli GL, Valente M, Muñiz-Castrillo S, Honnorat J, et al. Neurologic adverse events of immune checkpoint inhibitors: A systematic review. Neurology. (2021) 96:754–66. doi: 10.1212/WNL.0000000000011795
31. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. (2018) 36:1714–68. doi: 10.1200/JCO.2017.77.6385
32. Keam S, Turner N, Kugeratski FG, Rico R, Colunga-Minutti J, Poojary R, et al. Toxicity in the era of immune checkpoint inhibitor therapy. Front Immunol. (2024) 15:1447021. doi: 10.3389/fimmu.2024.1447021
33. Kao JC, Brickshawana A, Liewluck T. Neuromuscular complications of programmed cell death-1 (PD-1) inhibitors. Curr Neurol Neurosci Rep. (2018) 18:63. doi: 10.1007/s11910-018-0878-7
34. Bellanti R, Rinaldi S. Guillain-Barré syndrome: a comprehensive review. Eur J Neurol. (2024) 31:e16365. doi: 10.1111/ene.16365
35. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. (2016) 39:98–106. doi: 10.1097/COC.0000000000000239
36. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. (2017) 541:321–30. doi: 10.1038/nature21349
37. Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. (2016) 13:473–86. doi: 10.1038/nrclinonc.2016.58
38. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. (2016) 54:139–48. doi: 10.1016/j.ejca.2015.11.016
39. Qin S, Finn RS, Kudo M, Meyer T, Vogel A, Ducreux M, et al. RATIONALE 301 study: tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol. (2019) 15:1811–22. doi: 10.2217/fon-2019-0097
40. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. (2022) 23:77–90. doi: 10.1016/S1470-2045(21)00604-5
41. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J Clin Oncol. (2020) 38:193–202. doi: 10.1200/JCO.19.01307
42. Zhang L, Geng Z, Hao B, Geng Q. Tislelizumab: A modified anti-tumor programmedDeath receptor 1 antibody. Cancer Control. (2022) 29:10732748221111296. doi: 10.1177/10732748221111296
43. Ji H, Wen Z, Liu B, Chen H, Lin Q, Chen Z. Sintilimab induced ICIAM in the treatment of advanced HCC: A case report and analysis of research progress. Front Immunol. (2022) 13:995121. doi: 10.3389/fimmu.2022.995121
44. Yang H, Ma Q, Sun Y, Zhang K, Xing Y, Li H. Case Report: Toxic epidermal necrolysis associated with sintilimab in a patient with relapsed thymic carcinoma. Front Oncol. (2022) 12:1065137. doi: 10.3389/fonc.2022.1065137
45. Ai Q, Chen W, Li Y, Li G. Upper gastrointestinal tract irAEs: A case report about sintilimab-induced acute erosive hemorrhagic gastritis. Front Immunol. (2022) 13:840916. doi: 10.3389/fimmu.2022.840916
46. Janssen JBE, Leow TYS, Herbschleb KH, Gijtenbeek JMM, Boers-Sonderen MJ, Gerritsen WR, et al. Immune checkpoint inhibitor-related Guillain-Barré Syndrome: A case series and review of the literature. J Immunother. (2021) 44:276–82. doi: 10.1097/CJI.0000000000000364
47. Hughes RA, Brassington R, Gunn AA, van Doorn PA. Corticosteroids for Guillain-Barré syndrome. Cochrane Database Syst Rev. (2016) 10:CD001446. doi: 10.1002/14651858.CD001446.pub5
48. Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. NCCN guidelines insights: management of immunotherapy-related toxicities, version 1.2020. J Natl Compr Canc Netw. (2020) 18:230–41. doi: 10.6004/jnccn.2020.0012
49. Ding M, Deng C, Liu X, Jiang S, Gao Y, Fan D, et al. Case Report: ICIs-induced Guillain-Barré syndrome recovered from mycophenolate mofetil. Front Immunol. (2023) 14:1132692. doi: 10.3389/fimmu.2023.1132692
50. Gumusay O, Callan J, Rugo HS. Immunotherapy toxicity: identification and management. Breast Cancer Res Treat. (2022) 192:1–17. doi: 10.1007/s10549-021-06480-5
51. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. (2018) 378:158–68. doi: 10.1056/NEJMra1703481
52. Sho T, Suda G, Ohara M, Kohya R, Sasaki T, Yoshida S, et al. Efficacy and safety of durvalumab/tremelimumab in unresectable hepatocellular carcinoma as immune checkpoint inhibitor rechallenge following atezolizumab/bevacizumab treatment. Target Oncol. (2024) 19:769–78. doi: 10.1007/s11523-024-01092-7
Keywords: hepatocellular carcinoma, immune checkpoint inhibitor, immune-related adverse events, Guillain-Barré syndrome, neurologic adverse events, ICI rechallenge
Citation: Ye L, Yue WR, Shi H, Li JR and Qun YY (2025) Case Report: Successful immune checkpoint inhibitor rechallenge after sintilimab-induced Guillain-Barré syndrome. Front. Immunol. 16:1546886. doi: 10.3389/fimmu.2025.1546886
Received: 17 December 2024; Accepted: 27 February 2025;
Published: 19 March 2025.
Edited by:
Rui Liu, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaCopyright © 2025 Ye, Yue, Shi, Li and Qun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Ya Qun, eXlxMDEyOUBnbG1jLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.