- 1Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Department of Critical Care Medicine, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
The pathogenesis of autoimmune hemolytic anemia (AIHA) remains incompletely understood, typically associated with immune dysfunction and the production of autoantibodies. Zuberitamab, a novel anti-CD20 monoclonal antibody, represents an important therapeutic strategy for managing autoimmune diseases. Here, we present the first case of a patient diagnosed with AIHA who developed severe immunosuppression, lymphopenia, and B-cell depletion following zuberitamab treatment, ultimately resulting in severe Pneumocystis jirovecii pneumonia(PJP). This case highlights the complexities of B-cell-targeted immunotherapy and underscores the necessity of close monitoring of immune status in patients receiving zuberitamab or other targeted immunotherapies to mitigate the risk of severe immune-related adverse events.
1 Introduction
AIHA is a condition involving the production of antibodies against one’s own red blood cells (1–3). Autoimmune diseases are characterized by the recognition of self‐antigens by the immune system, which leads to inflammation and tissue damage. B cells are directly and indirectly involved in the pathophysiology of autoimmunity through antigen presentation to T cells and the production of proinflammatory cytokines and/or autoantibodies. Consequently, B lineage cells have been identified as therapeutic targets in autoimmune diseases (4). Anti-CD20 antibody therapy specifically targets the CD20 antigen on B cell surfaces and has been demonstrated as an effective treatment for various autoimmune diseases (5–7). Zuberitamab, a novel humanized chimeric anti-CD20 monoclonal antibody, selectively binds to CD20 antigens on B-cell surfaces, inducing B-cell depletion through antibody-dependent cellular cytotoxicity (ADCC) and complement-mediated cytotoxicity (8). While zuberitamab has shown promise in managing autoimmune diseases, its immunosuppressive effects may predispose patients to opportunistic infections.
This report presents a case of severe PJP following zuberitamab treatment in a patient with AIHA, aiming to provide practical insights and reference for evaluating the safety profile of this therapeutic approach.
2 Case presentation
A 65-year-old female was admitted to the hospital on May 20, 2025, due to “fatigue and dizziness for 3 days”. Laboratory tests revealed hemoglobin levels of 65 g/L (reference range: 115-150), reticulocyte percentage of 16.11% (reference range: 0.5-1.5), total bilirubin of 38.8 μmol/L (reference range: 0-21), conjugated bilirubin of 12 μmol/L (reference range: 0-8), unconjugated bilirubin of 26.8 μmol/L (reference range: 0-17), and lactate dehydrogenase (LDH) of 428 U/L (reference range: 120-250). A direct antiglobulin test (DAT) was positive, confirming the diagnosis of AIHA on May 23, 2024. Initial glucocorticoid theraphy showed limited efficacy. Subsequently, she received zuberitamab(600 mg) on July 4, July 10, July 18, and July 25, 2024, at an external hospital. The patient had no significant medical history and was previously in good health.
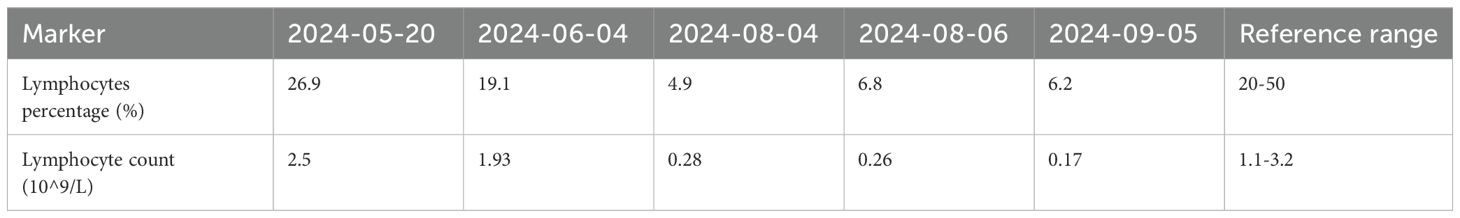
On August 4, 2024, at 10:26 AM, the female was hospitalized with a 4-day history of fever. At 7:05 PM the same day, the female developed respiratory distress and dyspnea after using the restroom, with oxygen saturation dropping to 77% under mask oxygen therapy. The patient was subsequently transferred to the intensive care unit (ICU) for further monitoring. Arterial blood gas analysis revealed pH 7.35 (reference range: 7.35-7.45), partial pressure of oxygen (PO2) 76.24 mmHg (reference range: 83-108), partial pressure of carbon dioxide (PCO2) 47.3 mmHg (reference range: 32-48), and oxygen index (OI) 108.92 mmHg(reference range: 400-500). Immediate endotracheal intubation and mechanical ventilation were initiated. Compared to the chest Computed Tomography (CT) on May 24 (Figure 1A), the chest CT on August 4 showed multiple infectious lesions in both lungs (Figure 1B). Given the patient’s history of oral glucocorticoid therapy and immunosuppressed state, and the potential for B-cell depletion induced by zuberitamab (9), the risk of opportunistic pathogen infection was significantly elevated. Broad-spectrum antimicrobial therapy was commenced, including meropenem, sulfamethoxazole, voriconazole, and ganciclovir. On August 6, respiratory distress progressively worsened, with the oxygen index declining to 34.24 mmHg. Repeated Chest CT revealed an expanded range of pulmonary infectious lesions and increased consolidation areas (Figure 1C). Emergency veno-venous extracorporeal membrane oxygenation (VV-ECMO) support was implemented. On August 7, metagenomic next-generation sequencing (mNGS) of bronchoalveolar lavage fluid identified Pneumocystis jirovecii (sequence count: 30,899, relative abundance: 98.88%). Subsequent antifungal treatment with caspofungin was initiated, with the antimicrobial treatment timeline illustrated in Figure 2. On August 18, urine culture revealed Enterococcus faecalis, thus combination therapy with vancomycin was initiated for infection management. On August 24, mNGS of bronchoalveolar lavage fluid detected Enterobacter cloacae complex (sequence count: 791),Pneumocystis jirovecii (sequence count: 5), fine cyclic virus(sequence count: 2599) and Cytomegalovirus (sequence count: 918). Consequently, vancomycin and caspofungin were discontinued, and combination therapy with imipenem and amikacin was started for infection control. The patient exhibited persistent immunosuppression, abnormal cellular immune markers, B-cell depletion, and low helper/inducer T lymphocyte counts. Immunoenhancement therapies, including intravenous immunoglobulin and thymosin, were administered sequentially, but with unsatisfactory outcomes (Tables 1, 2). Follow-up chest CT scans on August 12 and August 19 revealed an increased number of lesions in both lungs and progressively increased pulmonary parenchymal density (Figures 1D, E). On August 27, under deep analgesia and sedation, the patient received high-flow ECMO and invasive mechanical ventilation. The patient’s vital signs were relatively stable, but the tidal volume was low, and pulmonary imaging did not show significant improvement. Lung parenchymal density demonstrated an increasing trend, with bronchiectasis and notable traction signs observed (Figure 1F). Comprehensively considering the potential inflammatory proliferative phase of the patient’s disease, a lung tissue pathological diagnosis was performed to understand the disease progression and potential long-term treatment outcomes. The mNGS of the left upper lung tissue revealed a potential circovirus infection, with no concurrent fungal or bacterial pathogens identified. Pathological examination on August 30 revealed lung parenchyma with alveolar collapse, widened alveolar septa accompanied by scattered lymphoplasmacytic infiltration, fibrous tissue hyperplasia, and focal foam-like cellular deposits within alveolar spaces (Figure 3). Considering the possibility of organizing pneumonia, high-dose corticosteroid pulse therapy was initiated. Repeated Chest CT on September 2 demonstrated diffuse pulmonary consolidation, honeycomb-like changes, and prominent bronchial signs (Figure 1G).
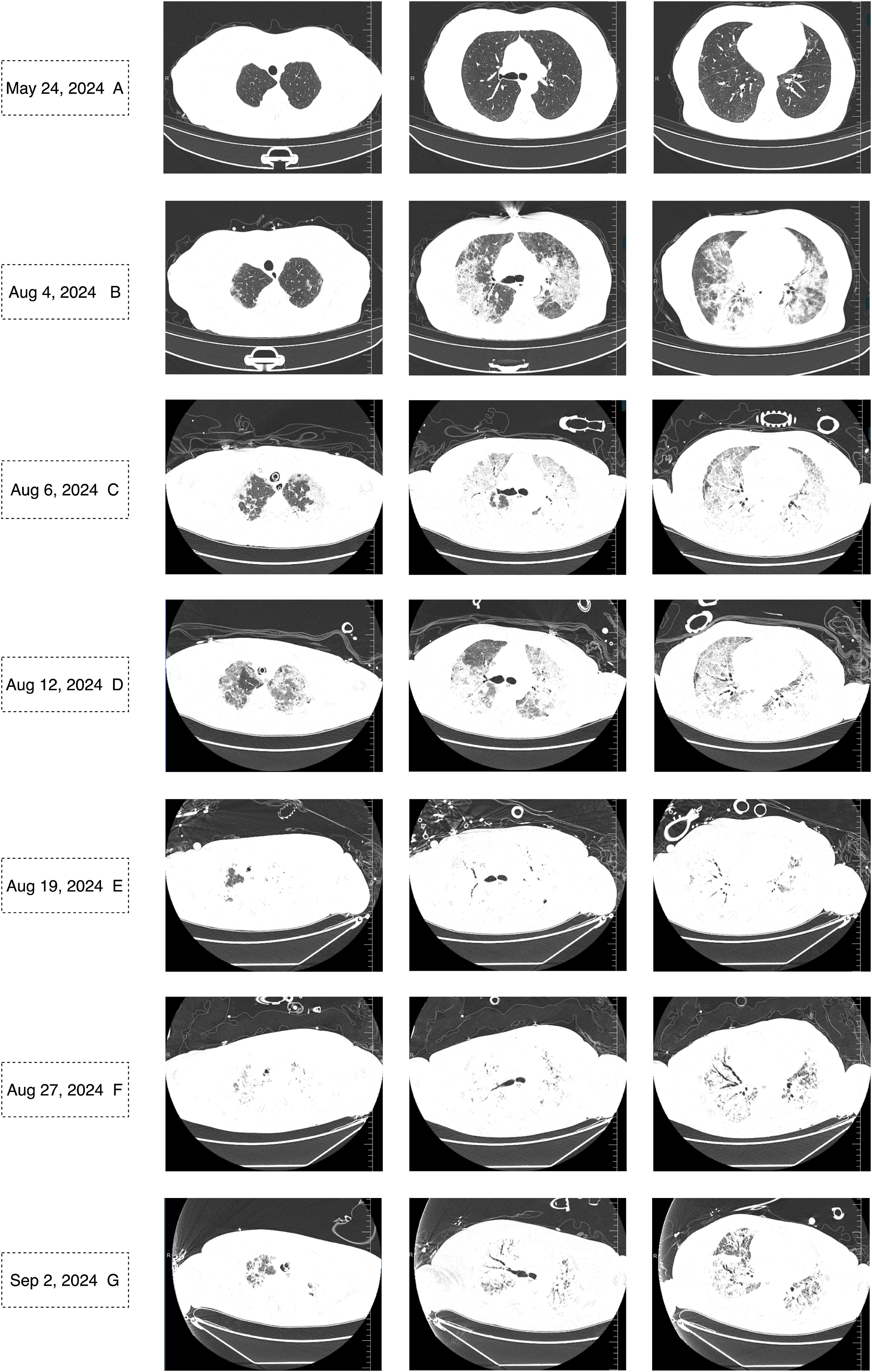
Figure 1. Chest CT on May 24, 2024, showed no signs of infection (A). Chest CT scans from August 4 to September 2, 2024, revealed a gradually expanding infectious focus in the lungs, progressively increasing lung parenchymal density, and bronchiectasis with traction signs (B-G).
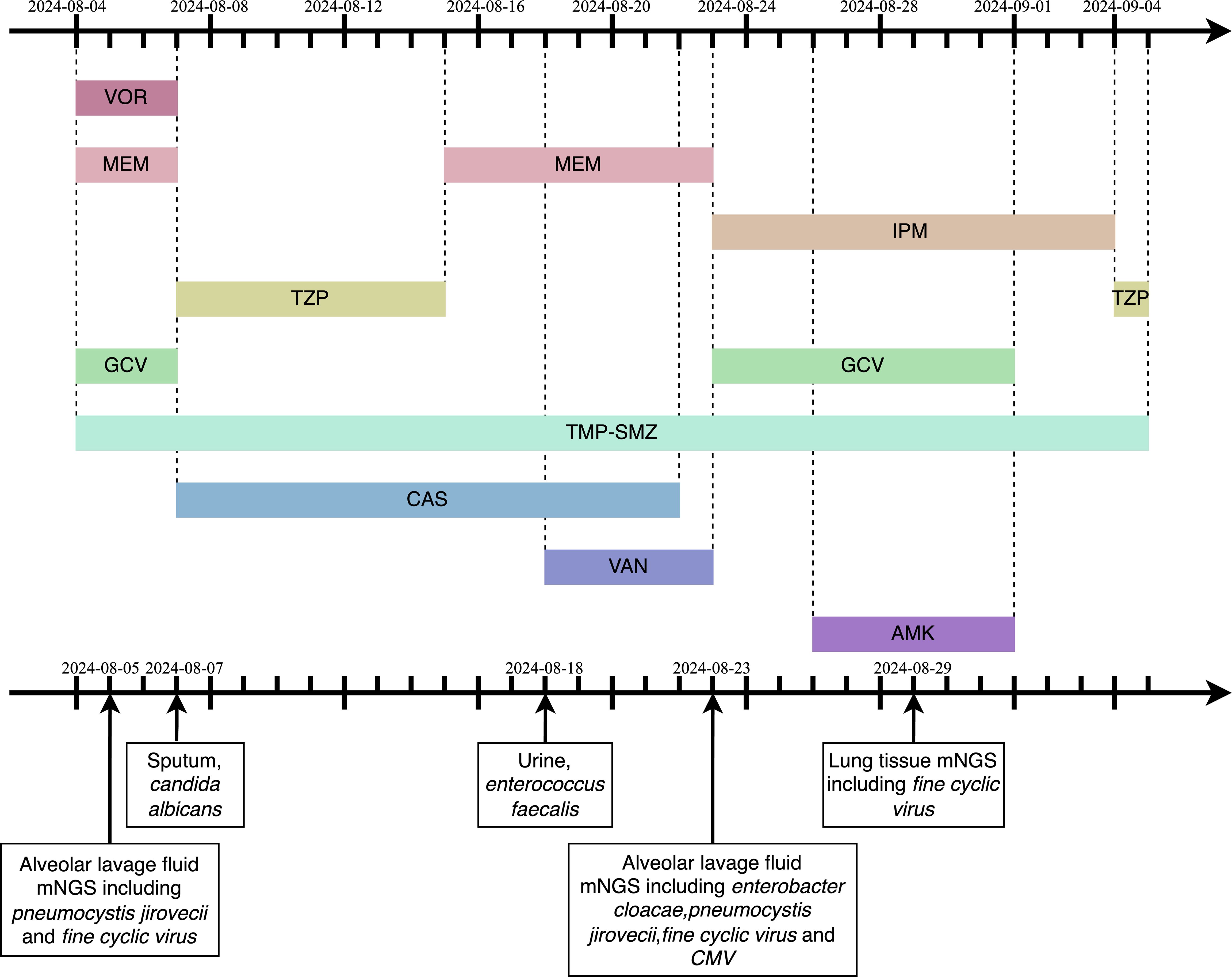
Figure 2. The timeline of antimicrobial medication. VOR,Voriconazole;MEM,Meropenem;IPM,Imipenem;TZP,Piperacillin/Tazobactam;GCV,Ganciclovir;mNGS,metagenomic next-generation sequencing;TMP-SMZ,trimethoprim-sulfamethoxazole;CAS,Caspofungin;VAN,Vancomycin;AMK,Amikacin; CMV, Cytomegalovirus.
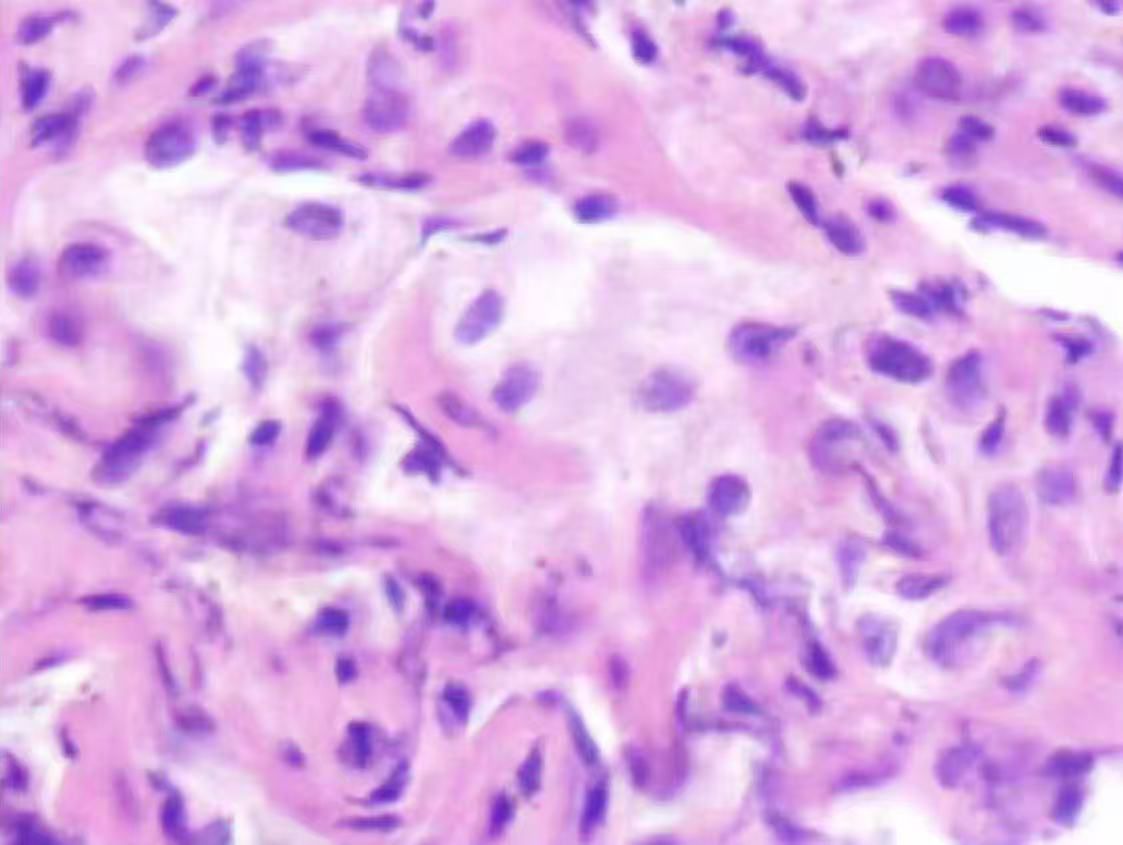
Figure 3. Pathological examination revealed lung parenchyma with alveolar collapse, widened alveolar septa accompanied by scattered lymphoplasmacytic infiltration, fibrous tissue hyperplasia, and focal foam-like cellular deposits within alveolar spaces.
Despite aggressive comprehensive treatment, the patient continued to require VV-ECMO, with no lung function recovery and an extremely poor long-term prognosis. On September 6 at 09:27 AM, the patient’s family decided to withdraw treatment, and clinical death was pronounced at 10:22 AM. The cause of death was acute respiratory distress syndrome (ARDS).
3 Discussion
This case reports a patient with autoimmune hemolytic anemia who developed B lymphocyte depletion and severe immunodeficiency following treatment with zuberitamab, ultimately resulting in severe PJP. Despite the medical team’s aggressive treatment measures, the patient unfortunately succumbed to the condition. This case reveals the potential risks of severe immune-related adverse events (irAEs) associated with zuberitamab theraphy in patients with AIHA.
AIHA is a complex autoimmune disorder characterized by immune dysfunction, where autoreactive antibodies target erythrocytes, resulting in their progressive destruction (10–12). Anti-CD20 monoclonal antibodies have emerged as an effective therapeutic strategy for AIHA (2). Zuberitamab, a humanized chimeric IgG1 anti-CD20 monoclonal antibody, effectively eliminates B cells through ADCC and complement-mediated cytotoxicity (8). Relevant research demonstrated that a single 100 mg dose of zuberitamab can rapidly and completely eliminate B cells within one week, with B-cell depletion sustained for at least 24 weeks (13). While this mechanism is highly effective in suppressing aberrant immune responses, it can also severely compromise immune defenses, leaving patients vulnerable to opportunistic infections such as PJP. Notably, PJP in non-HIV patients is often more severe, with higher mortality rates (14, 15). Emerging evidence has underscored the critical role of B cells in clearing Pneumocystis infections. Beyond their role as antibody producers, B cells are vital for antigen presentation, T-cell proliferation, and overall immune defense, constituting a critical component of the immune system (16–21).
Current guidelines do not explicitly mandate PJP prophylaxis for anti-CD20 antibody monotherapy. However, initiating preventive treatment becomes necessary when high-dose corticosteroids (e.g., prednisone dose exceeding 20 mg/day for more than one month), absolute lymphocyte reduction, or other immunosuppressive conditions (22). A prospective study revealed that the majority of PJP patients admitted to intensive care units had not received prophylactic antibiotics, with up to 88% lacking preventive treatment, significantly increasing their risk of infection (23). Infection remains a well-documented complication of AIHA and a leading cause of mortality, yet there are no specific guidelines for infection prevention in this patient population (24). Prophylactic anti-PJP medication, such as trimethoprim-sulfamethoxazole (TMP-SMX), may represent an effective intervention strategy (25). Antibiotic prevention for PJP represents a validated intervention, with prevention deficiency recognized as a well-established risk factor for severe PJP. For immunocompromised patients, prophylactic antibiotic treatment should be considered to reduce the morbidity and mortality associated with PJP (26–28).
Here, this case demonstrates the potential risk of severe immunodeficiency and opportunistic infections associated with zuberitamab treatment in AIHA. While zuberitamab effectively controls the aberrant immune response in AIHA through B-cell depletion, the concomitant weakening of immune defense mechanisms can lead to fatal consequences. B cells play a pivotal role not only in antibody production and antigen presentation but also in maintaining the immune system's overall integrity and infection-fighting capacity. Moreover, there are currently no established guidelines for infection prophylaxis in AIHA patients, and the lack of specific strategies for PJP prevention may further increase the risk of infections. One limitation of this case is the lack of further characterization of the AIHA subtype, which could provide more clarity for targeted treatment strategies. We recommend conducting rigorous and comprehensive assessments of patients undergoing B cell-targeted immunotherapy. These measures are essential to enhancing therapeutic efficacy while ensuring patient safety and quality of life. However, further research and additional cases are required to validate these observations and guide clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The study involving humans were approved by Ethics Committee of Affiliated Hospital of Chengdu University of Traditional Chinese Medicine. The study was conducted in accordance with the local legislation and institutional requirements. The study was conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the legal guardian of the participant for the publication of any potentially identifiable images or data included in this article, in accordance with institutional ethical requirements.
Author contributions
QY: Writing – original draft, Writing – review & editing. PD: Writing – original draft, Writing – review & editing. Y-XL: Writing – original draft. K-CZ: Writing – review & editing. P-YG: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Michel M, Crickx E, Fattizzo B, Barcellini W. Autoimmune haemolytic anaemias. Nat Rev Dis Prime. (2024) 10:82. doi: 10.1038/s41572-024-00566-2
2. Loriamini M, Cserti-Gazdewich C, Branch DR. Autoimmune hemolytic anemias: classifications, pathophysiology, diagnoses and management. Int J Mol Sci. (2024) 25:4296. doi: 10.3390/ijms25084296
3. Red Blood Cell Disease (Anemia) Group, Chinese Society of Hematology, Chinese Medical Association. Chinese guideline for the diagnosis and treatment of adult autoimmune hemolytic anemia (2023). Chin J Hematol. (2023) 44:12. doi: 10.3760/cma.j.issn.0253-2727.2023.01.003
4. Merino-Vico A, Frazzei G, van Hamburg JP, Tas SW. Targeting B cells and plasma cells in autoimmune diseases: From established treatments to novel therapeutic approaches. Eur J Immunol. (2023) 53:e2149675. doi: 10.1002/eji.202149675
5. Lee DSW, Rojas OL, Gommerman JL. B cell depletion therapies in autoimmune disease: advances and mechanistic insights [published correction appears in Nat Rev Drug Discov. 2024 Nov 26. doi: 10.1038/s41573-024-01103-2. Nat Rev Drug Discovery. (2021) 20:179–99. doi: 10.1038/s41573-020-00092-2
6. Kaegi C, Wuest B, Crowley C, Boyman O. Systematic review of safety and efficacy of second- and third-generation CD20-targeting biologics in treating immune-mediated disorders. Front Immunol. (2022) 12:788830. doi: 10.3389/fimmu.2021.788830
7. Crickx E, Weill JC, Reynaud CA, Mahévas M. Anti-CD20-mediated B-cell depletion in autoimmune diseases: successes, failures and future perspectives. Kidney Int. (2020) 97:885–93. doi: 10.1016/j.kint.2019.12.025
8. Crescioli S, Kaplon H, Chenoweth A, Wang L, Visweswaraiah J, Reichert JM. Antibodies to watch in 2024. MAbs. (2024) 16:2297450. doi: 10.1080/19420862.2023.2297450
9. Approval of Zuberitamab Injection by NMPA. National Medical Products Administration. Available online at: https://www.nmpa.gov.cn/zhuanti/cxylqx/cxypxx/20230517152922112.html (Accessed December 17, 2024).
10. Jäger U, Barcellini W, Broome CM, Gertz MA, Hill A, Hill QA, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: Recommendations from the First International Consensus Meeting. Blood Rev. (2020) 41:100648. doi: 10.1016/j.blre.2019.100648
11. Hill QA, Hill A, Berentsen S. Defining autoimmune hemolytic anemia: a systematic review of the terminology used for diagnosis and treatment. Blood Adv. (2019) 3:1897–906. doi: 10.1182/bloodadvances.2019000036
12. Berentsen S, Barcellini W. Autoimmune hemolytic anemias. N Engl J Med. (2021) 385:1407–19. doi: 10.1056/NEJMra2033982
13. Xu M, Shu J, Qian S, Guo J, Gong Y, Huang R, et al. Zuberitamab, an innovative anti-CD20 monoclonal antibody, for patients with primary immune thrombocytopenia in China: a randomized, double-blind, placebo-controlled, phase 2 study. Lancet Reg Health West Pac. (2024) 47:101096. doi: 10.1016/j.lanwpc.2024.101096
14. Salzer HJF, Schäfer G, Hoenigl M, Günther G, Hoffmann C, Kalsdorf B, et al. Clinical, diagnostic, and treatment disparities between HIV-infected and non-HIV-infected immunocompromised patients with pneumocystis jirovecii pneumonia. Respiration. (2018) 96:52–65. doi: 10.1159/000487713
15. Roux A, Canet E, Valade S, Gangneux-Robert F, Hamane S, Lafabrie A, et al. Pneumocystis jirovecii pneumonia in patients with or without AIDS, France. Emerg Infect Dis. (2014) 20:1490–7. doi: 10.3201/eid2009.131668
16. Kolls JK. An emerging role of B cell immunity in susceptibility to pneumocystis pneumonia. Am J Respir Cell Mol Biol. (2017) 56:279–80. doi: 10.1165/rcmb.2016-0360ED
17. Lund FE, Hollifield M, Schuer K, Lines JL, Randall TD, Garvy BA. B cells are required for generation of protective effector and memory CD4 cells in response to Pneumocystis lung infection. J Immunol. (2006) 176:6147–54. doi: 10.4049/jimmunol.176.10.6147
18. Lund FE, Schuer K, Hollifield M, Randall TD, Garvy BA. Clearance of Pneumocystis carinii in mice is dependent on B cells but not on P carinii-specific antibody. J Immunol. (2003) 171:1423–30. doi: 10.4049/jimmunol.171.3.1423
19. Opata MM, Hollifield ML, Lund FE, Randall TD, Dunn R, Garvy BA, et al. B lymphocytes are required during the early priming of CD4+ T cells for clearance of pneumocystis infection in mice. J Immunol. (2015) 195:611–20. doi: 10.4049/jimmunol.1500112
20. Opata MM, Ye Z, Hollifield M, Garvy BA. B cell production of tumor necrosis factor in response to Pneumocystis murina infection in mice. Infect Immun. (2013) 81:4252–60. doi: 10.1128/IAI.00744-13
21. Sassi M, Curran SJ, Bishop LR, Liu Y, Kovacs JA. CD40 expression by B cells is required for optimal immunity to murine pneumocystis infection. J Infect Dis. (2024) 230:1033–41. doi: 10.1093/infdis/jiae133
22. Caplan AS, Mecoli CA, Micheletti RG. Prophylaxis against pneumocystis pneumonia. JAMA. (2023) 330:1908–9. doi: 10.1001/jama.2023.18862
23. Kamel T, Janssen-Langenstein R, Quelven Q, Chelly J, Valette X, Le MP, et al. Pneumocystis pneumonia in intensive care: clinical spectrum, prophylaxis patterns, antibiotic treatment delay impact, and role of corticosteroids. A French multicentre prospective cohort study. Intensive Care Med. (2024) 50:1228–39. doi: 10.1007/s00134-024-07489-2
24. Mulder FVM, Evers D, de Haas M, Cruijsen MJ, Bernelot Moens SJ, Barcellini W, et al. Severe autoimmune hemolytic anemia; epidemiology, clinical management, outcomes and knowledge gaps. Front Immunol. (2023) 14:1228142. doi: 10.3389/fimmu.2023.1228142
25. Park JW, Curtis JR, Jun KI, Kim TM, Heo DS, Ha J, et al. Primary prophylaxis for pneumocystis jirovecii pneumonia in patients receiving rituximab. Chest. (2022) 161:1201–10. doi: 10.1016/j.chest.2021.11.007
26. Nseir S, Valadas E, Leone M. Severe Pneumocystis jirovecii pneumonia: time to reassess our practices. Intensive Care Med. (2024) 50:1310–2. doi: 10.1007/s00134-024-07547-9
27. Ghembaza A, Vautier M, Cacoub P, Pourcher V, Saadoun D. Risk factors and prevention of pneumocystis jirovecii pneumonia in patients with autoimmune and inflammatory diseases. Chest. (2020) 158:2323–32. doi: 10.1016/j.chest.2020.05.558
Keywords: autoimmune hemolytic anemia, autoimmune diseases, zuberitamab, B-cell depletion, severe Pneumocystis jirovecii pneumonia, case report
Citation: Yang Q, Ding P, Liu Y-x, Zhang K-c and Gao P-y (2025) Case Report: Severe Pneumocystis jirovecii pneumonia following zuberitamab treatment in autoimmune hemolytic anemia. Front. Immunol. 16:1546571. doi: 10.3389/fimmu.2025.1546571
Received: 18 December 2024; Accepted: 17 February 2025;
Published: 11 March 2025.
Edited by:
Marta Garcia-Recio, Hospital Clinic of Barcelona, SpainReviewed by:
Sigbjørn Berentsen, Fonna Hospital Trust, NorwayMd Al Hasibuzzaman, Ningbo First Hospital, China
Copyright © 2025 Yang, Ding, Liu, Zhang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pei-yang Gao, Z2FvcHk5MzBAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Qian Yang
Qian Yang Peng Ding
Peng Ding Yu-xiang Liu
Yu-xiang Liu Kai-chen Zhang
Kai-chen Zhang Pei-yang Gao
Pei-yang Gao