
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 14 February 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1545304
This article is part of the Research Topic The Application of Immune Checkpoint Inhibitors Combined with Chemotherapy in Tumor Immunotherapy View all 10 articles
Background: To evaluate the efficacy and safety of sintilimab in combination with trastuzumab and chemotherapy for HER2-positive advanced gastric/gastroesophageal junction cancer (GC/GEJC).
Methods: HER2-positive advanced GC/GEJC patients admitted to our department between January 2018 and October 2024 were included in this study. Patients who received sintilimab in combination with trastuzumab and chemotherapy were assigned to cohort A, while patients who received trastuzumab and chemotherapy alone were assigned to cohort B. The primary endpoints were progression-free survival (PFS) and overall survival (OS), while the secondary endpoints included disease control rate (DCR), objective response rate (ORR), and safety.
Results: A total of 103 patients were analyzed, with 46 in cohort A and 57 in cohort B. The ORR was 65.2% in cohort A compared to 40.4% in cohort B, while the DCR was 87.0% in cohort A and 70.2% in cohort B. The median follow-up duration was 14.0 months. Median PFS (mPFS) was 9.4 months (95% CI: 5.6–13.2) for cohort A and 7.4 months (95% CI: 6.1–8.7) for cohort B (p = 0.089). Median OS (mOS) was 16.4 months (95% CI: 11.5–21.3) in cohort A versus 14.2 months (95% CI: 11.2–17.2) in cohort B (p = 0.069). Adverse events were predominantly mild, and no treatment-related deaths occurred.
Conclusion: Sintilimab combined with trastuzumab and chemotherapy showed promising efficacy and acceptable safety in HER2-positive advanced GC/GEJC. However, no statistically significant improvement in survival outcomes was observed compared to trastuzumab and chemotherapy alone.
Gastric cancer (GC) is the fifth most common cancer and the fifth leading cause of cancer-related mortality worldwide (1). In China, GC ranks as the third leading cause of cancer-related mortality and accounts for 44% of global GC cases, with more than 80% of these cases diagnosed as advanced carcinomas (2–4). In patients with advanced GC, conventional chemotherapy approaches have been reported to yield median overall survival (mOS) ranging from 10 to 12 months (5).
Immunotherapy has emerged as a breakthrough in the treatment landscape of gastric and gastroesophageal junction adenocarcinoma. Based on the KEYNOTE-059 (6) and ATTRACTION-02 (7) trials, nivolumab and pembrolizumab have been successively approved for third-line treatment of recurrent or metastatic gastric/GEJ adenocarcinoma. Results from the CheckMate 649 and ATTRACTION-4 trials demonstrated the efficacy of immunotherapy combined with chemotherapy in the first-line treatment of GC, with prolonged patient survival (8, 9). In GC patients exhibiting HER2 overexpression, observed in 7.3% to 20.2% of cases, standard chemotherapy regimens are typically associated with a poor prognosis (10). Trastuzumab is a monoclonal antibody that targets the HER2 receptor and can interact with natural killer (NK) cells to upregulate PD-L1 expression in GC cells (11). Trastuzumab combined with chemotherapy can significantly improve the survival of HER2-positive advanced GC patients and has been recommended as the first-line treatment for these patients (12–14). In addition, anti-programmed death-1 receptor (PD-1) antibodies can enhance trastuzumab-induced T-cell-specific immune responses, thereby producing synergistic effects when combined with trastuzumab. A phase III trial indicated that the combination of pembrolizumab with trastuzumab and chemotherapy improved progression-free survival (PFS) and overall survival (OS) in GC patients, particularly in those with a PD-L1 combined positive score (CPS) ≥1 (15–17).
Sintilimab is a recombinant humanized anti-PD-1 antibody with higher affinity and a slower dissociation rate for PD-1 compared to pembrolizumab and nivolumab. It demonstrates high PD-1 occupancy both in vitro and in vivo. The slower dissociation rate allows Sintilimab to maintain binding site occupancy for extended periods even as plasma concentrations decline, potentially leading to prolonged drug activity. Additionally, Sintilimab exhibits a lower risk of immunogenicity relative to pembrolizumab and nivolumab (18). One study demonstrated that sintilimab exhibited a satisfactory efficacy and safety profile in advanced GC/GEJC when combined with chemotherapy (mOS, 15.2 vs. 12.3 months) (19). However, the efficacy and safety of sintilimab combined with trastuzumab and chemotherapy remain unknown. Thus, we conducted a retrospective cohort study to evaluate the efficacy and safety profiles of sintilimab in combination with trastuzumab and chemotherapy for HER2-positive advanced GC patients. Additionally, this study aimed to determine the factors influencing the efficacy for GC patients, such as PD-L1 CPS scores and H. pylori infection, among others (20, 21).
This is a retrospective cohort study conducted at The Affiliated Yantai Yuhuangding Hospital of Qingdao University. The study was conducted from January 2018 to October 2024. HER2-positive advanced GC/GEJC patients who received either sintilimab in combination with trastuzumab and chemotherapy or trastuzumab and chemotherapy alone were included in this study and were defined as Cohort A and Cohort B, respectively. The inclusion criteria were as follows: age ≥18 years, HER2 immunohistochemistry (IHC) score 3+ or 2+ with fluorescence in situ hybridization (FISH)+, microsatellite instability-low/microsatellite stability/proficient mismatch repair, Epstein-Barr virus negative, Eastern Cooperative Oncology Group (ECOG) performance status ≤2, and measurable lesions according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The exclusion criteria were as follows: previous receipt of any anti-tumor therapy except for adjuvant or neoadjuvant treatment, and presence of serious or uncontrollable systemic diseases.
Patients received 200 mg of sintilimab intravenously once every three weeks. Trastuzumab was also administered intravenously, starting with a loading dose of 8 mg/kg, followed by a maintenance dose of 6 mg/kg every three weeks. First-line chemotherapy was administered according to the following regimen: patients received capecitabine (1000 mg/m², twice daily for 14 days, followed by a 7-day rest period) or tegafur (40 mg/m², twice daily for 14 days, followed by a 7-day rest period), in combination with oxaliplatin (130 mg/m² on day 1). The chemotherapy cycle was repeated every three weeks. After six to eight cycles of treatment, patients underwent maintenance therapy with sintilimab combined with trastuzumab and capecitabine or tegafur until disease progression.
Efficacy was assessed every two cycles based on RECIST 1.1. Response to treatment was categorized as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The primary endpoints were PFS and OS. PFS was defined as the time from initial treatment to disease progression or death, while OS was defined as the time from initial treatment to death from any cause. Secondary endpoints included the objective response rate (ORR), disease control rate (DCR), and safety. ORR was defined as the proportion of patients achieving a PR or CR as their best response. DCR was defined as the proportion of patients achieving a PR, CR, or SD as their best response. Adverse events (AEs) were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
All data were analyzed using SPSS version 27.0 (IBM, New York, USA). Categorical variables were compared using χ² tests and Fisher’s exact tests. PFS and OS were calculated using the Kaplan-Meier method, and results were presented with the median and 95% confidence intervals (CIs). Univariate Cox regression was used to calculate hazard ratios (HR). To identify factors affecting efficacy, we created several subgroups based on PD-L1 CPS scores, ECOG status, and H. pylori infection, among others. Survival analyses between cohorts or subgroups were performed using the log-rank test. All tests were two-sided, with a significance level of p<0.05.
A total of 103 patients were enrolled in this study from January 2018 to October 2024. Of these, 46 patients received sintilimab in combination with trastuzumab and chemotherapy and were assigned to cohort A, while 57 patients who received trastuzumab and chemotherapy alone were assigned to cohort B. The recruitment process of our study is shown in Figure 1. The median follow-up durations for cohort A and cohort B were 14.3 months and 13.0 months, respectively. The patients in cohort A and cohort B were comparable in terms of age distribution, ECOG performance status, sex distribution, and other factors (Table 1). A total of 15 patients discontinued therapy due to adverse events (AEs), poor health, or personal reasons. The median treatment cycle was 6 cycles.
The follow-up cutoff date was 31 October 2024. The median progression-free survival (mPFS) for cohort A and cohort B were 9.4 months (95% CI, 5.6–13.2 months) and 7.4 months (95% CI, 6.1–8.7 months), respectively (Figure 2A). The mOS for cohort A and cohort B were 16.4 months (95% CI, 11.5–21.3 months) and 14.2 months (95% CI, 11.2–17.2 months), respectively (Figure 2B). Among the 46 patients in cohort A, one (2.2%) achieved a complete response (CR), 29 (63.0%) achieved a partial response (PR), 10 (21.7%) had stable disease (SD), and six (13.0%) had progressive disease (PD). Of the 57 patients in cohort B, 23 (40.4%) achieved PR, 17 (29.8%) had SD, and 17 (29.8%) had PD. Cohort A showed a higher overall response rate (ORR) of 65.2% compared to 40.4% in cohort B (P=0.012), and a higher disease control rate (DCR) of 87.0% compared to 70.2% in cohort B (P=0.042) (Table 2). Figure 3 shows typical and clear computed tomography (CT) images of a CR patient from Cohort A before and after treatment.
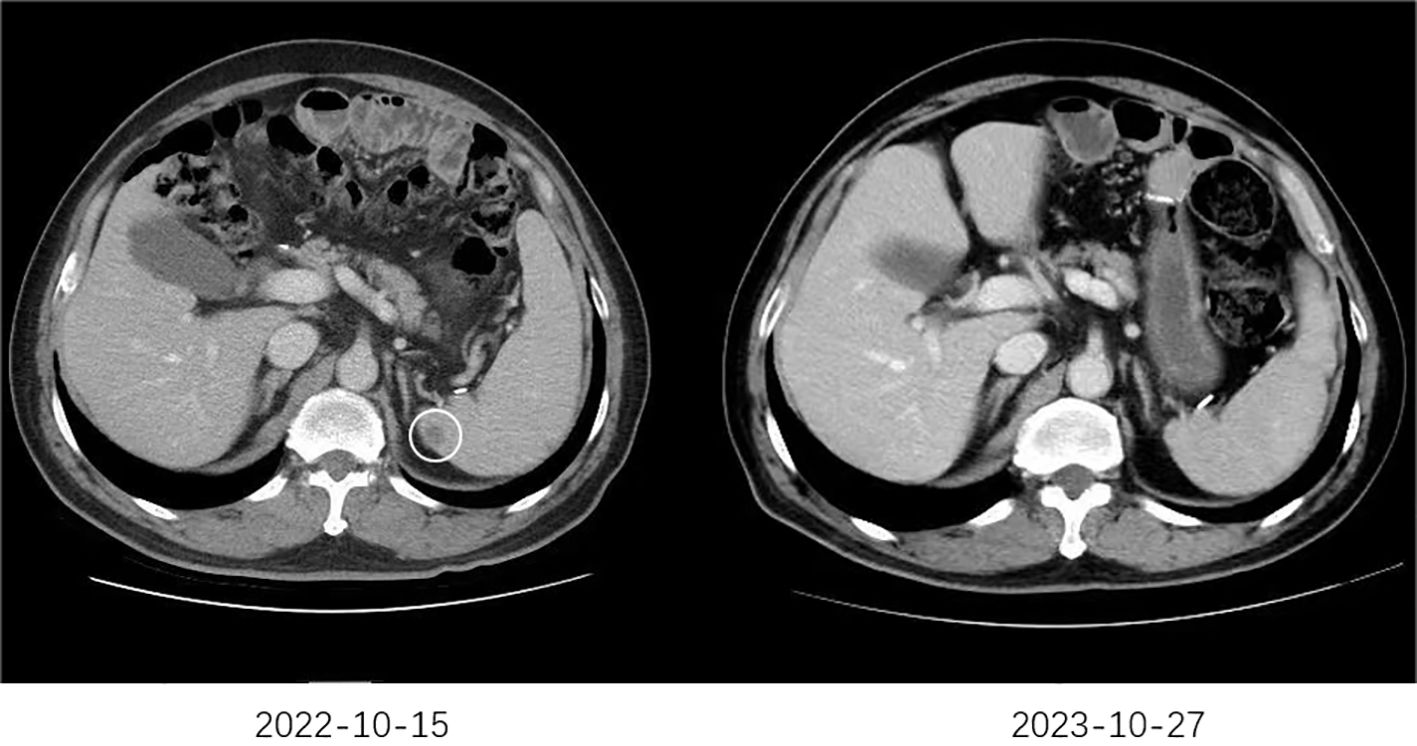
Figure 3. The typical CT images of a CR patient. The CT scan performed on October 15, 2022, revealed newly developed splenic metastases. Subsequently, the patient began first-line treatment on October 18, 2022, consisting of six cycles of sintilimab combined with trastuzumab and chemotherapy. Maintenance therapy with sintilimab, trastuzumab, and chemotherapy was continued thereafter. A follow-up CT scan on October 27, 2023, demonstrated the disappearance of the splenic metastases.
Subgroup analysis showed that gender, age, HER2 status, and nutritional status were not associated with patient survival. Among patients with ECOG ≤1, liver metastases, and PD-L1 CPS ≥1, those who received sintilimab had better OS. In patients with peritoneal metastases and H. pylori-positive status, sintilimab provided significant benefits in both PFS and OS (Figures 4, 5, Table 3).
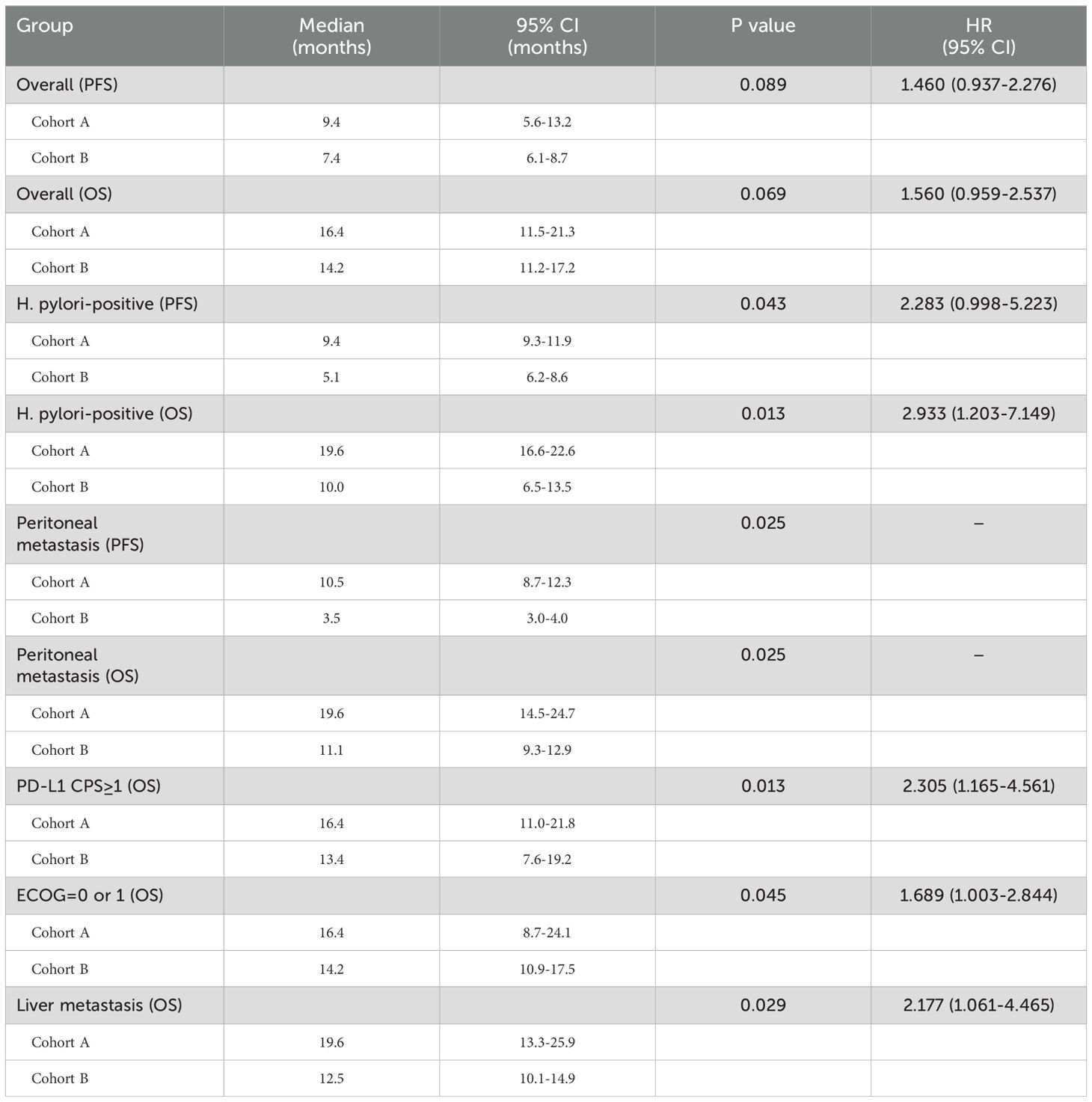
Table 3. Summary of Median Progression-Free Survival (mPFS), Median Overall Survival (mOS), and Hazard Ratios (HR) for Cohorts A and B.
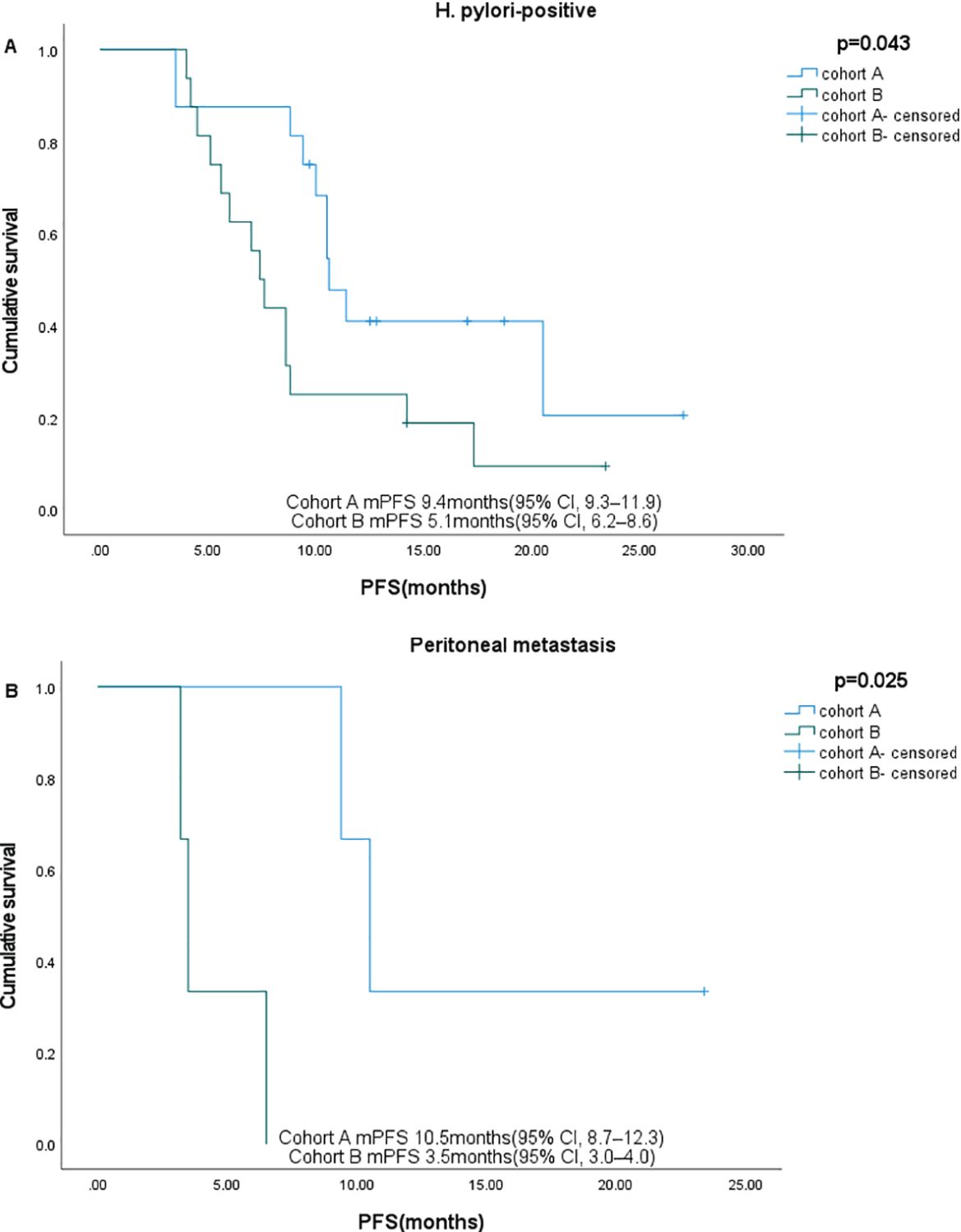
Figure 4. Subgroup analysis of PFS in different subgroups. (A) H pylori-positive, (B) Peritoneal metastases.
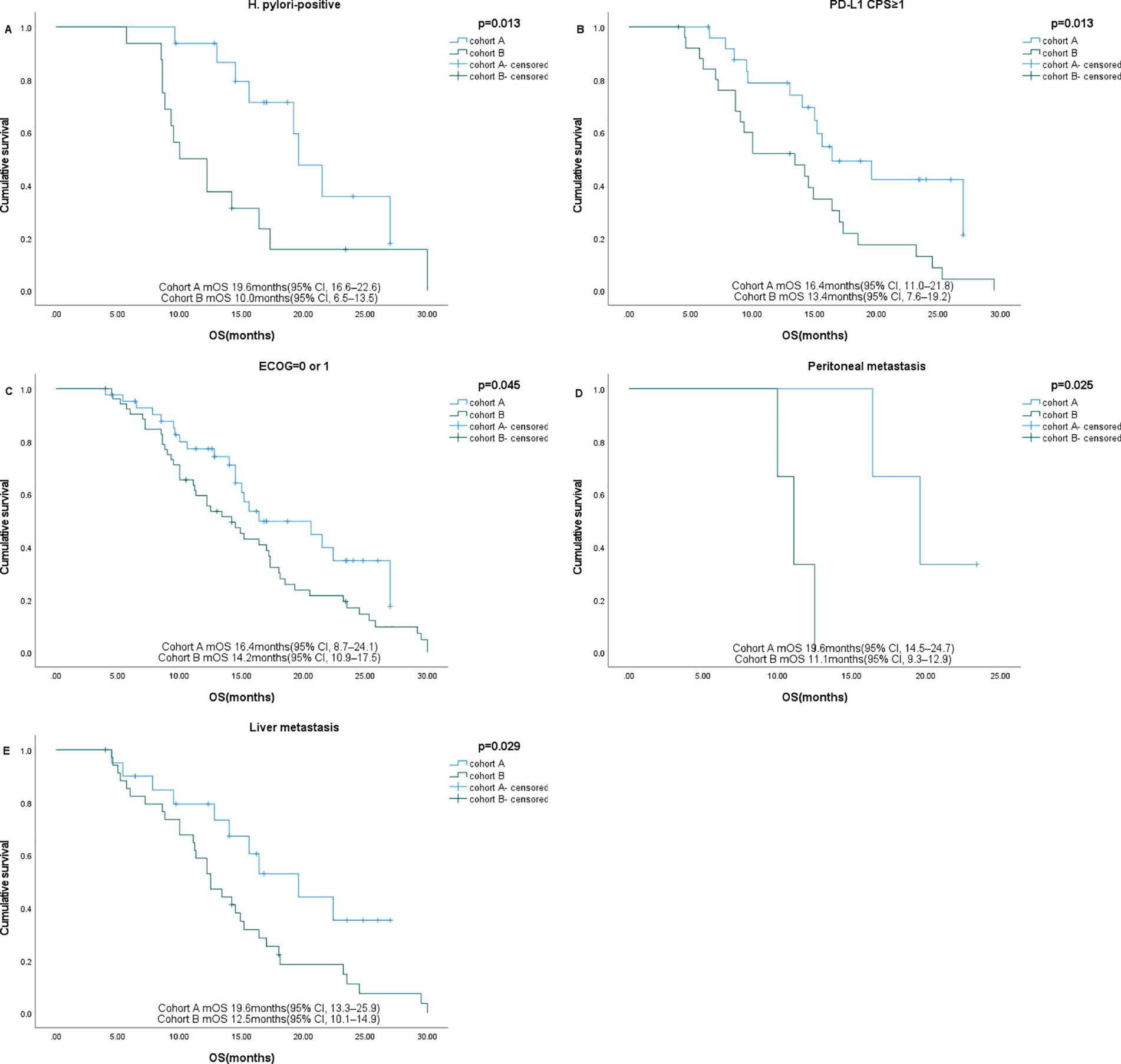
Figure 5. Subgroup analysis of OS in different subgroups. (A) H pylori-positive, (B) PD-L1 CPS ≥1, (C) ECOG=0-1, (D) Peritoneal metastases (E) Liver metastases.
In cohort A, the most common treatment-related adverse events (AEs) were bone marrow suppression (18/46, 39.1%) and abnormal liver function (12/46, 26.1%). The most common grade 3-4 AE was bone marrow suppression (6/46, 13.0%). In cohort B, the most common treatment-related AEs were bone marrow suppression (19/57, 33.3%), abnormal liver function (10/57, 17.5%), and diarrhea (10/57, 17.5%). The most common grade 3-4 AE was bone marrow suppression (4/57, 7.0%). No treatment-related deaths were observed, and there was no statistically significant difference in the incidence of treatment-related AEs between the two cohorts. AEs that occurred during treatment in cohort A and B were summarized in Table 4.
Camrelizumab and pembrolizumab, have been shown to generate longer survival periods for HER2-positive advanced GC patients when combined with trastuzumab and chemotherapy (22, 23). This is, to our knowledge, the first study to explore the efficacy of sintilimab in combination with trastuzumab and chemotherapy for the treatment of HER2-positive advanced GC patients.
At the European Society for Medical Oncology (ESMO) annual meeting in September 2024, the KEYNOTE-811 trial presented final data. The mOS was prolonged by 3.2 months in the pembrolizumab group compared to the control group (20.0 months vs. 16.8 months, HR=0.80, 95% CI 0.67–0.94, P=0.004). In patients with PD-L1 CPS ≥1, the mOS was 20.1 months in the pembrolizumab group and 15.7 months in the control group (HR=0.79, 95% CI 0.66–0.95, P=0.006) (15). Our study yielded similar results and further confirmed the satisfactory efficacy of anti-PD-1/PD-L1 therapy combined with anti-HER2 therapy and chemotherapy in patients with PD-L1 CPS ≥1. However, the comparison of overall survival (OS) results between the two cohorts in our study differed from those of the KEYNOTE-811 trial, possibly due to the differing proportions of patients with PD-L1 CPS ≥1 in the two studies (49.5% vs. 84.7%).
In subgroup analysis, sintilimab prolonged PFS in patients with peritoneal metastases or H. pylori positivity and prolonged OS in patients with ECOG ≤1, peritoneal metastases, liver metastases, H. pylori positivity, or PD-L1 CPS ≥1. 15 patients (32.6%) in Cohort A and 20 patients (35.1%) in Cohort B received subsequent therapy, including new chemotherapy, targeted therapies, or immunologic agents. These patients had better survival outcomes than those who did not receive subsequent therapy. DS8201 has been approved for the treatment of advanced GC, providing a promising therapeutic option for this patient population. Based on current evidence-based medical guidelines and drug availability, we consider DS8201 as a third-line and subsequent treatment for HER2-positive advanced GC patients who have previously experienced failure with trastuzumab therapy. The efficacy of DS8201 in this setting will be further evaluated through ongoing data collection and analysis in future studies.
Patients with PD-L1 CPS ≥1 demonstrated better therapeutic efficacy. Trastuzumab increased HER2 internalization and enhanced cross-presentation in dendritic cells, a process specifically mediated by its Fc receptor interaction. This mechanism subsequently increased antigen-specific antigen-specific cytotoxic T lymphocytes generation both in vitro and in vivo through improved cross-presentation by dendritic cells (24). Second, CD4+ T cells must recognize peptide-histocompatibility complex class II (MHC-II) complexes to become activated. Trastuzumab upregulates major MHC-II molecules in the tumor microenvironment, increasing antigen availability for CD4+ T-cell activation (11, 25). In particular, it enhances the expression of PD-1/PD-L1, thereby potentiating the efficacy of anti-PD-1 antibodies (25). Specifically, trastuzumab upregulates PD-L1 levels in HER2-overexpressing cancer cells by activating the extrinsic pathway, where immune effector cells release IFNγ. Moreover, IFN-γ can help create an environment that conducive to tumor-specific adaptive immunity. These effects are amplified in patients with higher PD-L1 CPS, as trastuzumab enhances PD-1/PD-L1 expression, further synergizing with anti-PD-1 antibodies like sintilimab (26, 27). Through these mechanisms, sintilimab and trastuzumab promote the expansion of peripheral memory T-cells, synergistically prolonging the OS of patients (28).
Our study is the first to demonstrate that among HER2-positive patients with advanced GC, those who are H. pylori-positive achieve improved survival outcomes following combined treatment with sintilimab, trastuzumab, and chemotherapy. H. pylori CagA can elevate PD-L1 expression in exosomes derived from GC cells, resulting in overall higher PD-L1 levels in H. pylori-positive versus H. pylori-negative patients (29, 30). In line with these findings, Jia et al. observed that H. pylori–positive GC may exhibit elevated densities of PD-L1+ and PD-1+ T cells, increased PDCD1 and CD274 gene expression, and a tumor microenvironment enriched with more nonexhausted CD8+ T cells. Moreover, gene set variation analysis (GSVA) indicates that H. pylori-positive tumors have a heightened proliferation-related score and a reduced stroma-related score, both of which have been linked to enhanced responses to immunotherapy (31). These findings collectively suggest that H. pylori infection fosters a tumor microenvironment conducive to immunotherapeutic efficacy, and potential biomarkers such as PD-1/PD-L1 (PDCD1/CD274) expression levels and proliferation/stroma-associated signatures may help guide treatment strategies in this patient population. Moreover, Their study demonstrates that H. pylori infection enhances the therapeutic benefit of immunotherapy in patients with PD-L1 CPS <1 or <5. Notably, the advantage of immunotherapy persists in H. pylori-positive GC patients, even in the absence of PD-L1 expression (32). Jia et al. therefore concluded that H. pylori infection is a favorable prognostic indicator for immunotherapy in GC patients, a conclusion that is consistent with our own results.
Patients with liver and peritoneal metastases had better survival after receiving the combined therapy, which is consistent with other studies (33). Subgroup analyses in the CheckMate-649 and KEYNOTE-859 trials showed statistically significant improvements in median OS in patients with liver metastases (34, 35). However, the molecular mechanisms remain to be further elucidated.
However, several limitations must be acknowledged. First, as a retrospective analysis, the study was hindered by inherent challenges, including incomplete patient treatment records that restricted the breadth of available data. Beyond the clinical factors included in our study, other variables such as Tumor Mutation Burden (TMB) may influence patient survival outcomes. However, due to economic constraints and other factors, we were unable to obtain sufficient TMB data for comprehensive analysis in our retrospective cohort. Second, the relatively small sample sizes in both cohorts limit the generalizability of our results and may introduce potential biases in subgroup analyses. Furthermore, the specific design of our subgroup analyses, which involved comparing survival outcomes within distinct patient subsets (e.g., H. pylori-positive patients) between Cohort A and Cohort B, precluded the feasibility of performing multivariate Cox regression analyses, as such analyses would require overlapping or larger populations that are not available in our current sample. This constraint underscores the necessity for larger, more diverse patient populations and more comprehensive data collection in future research. We are currently planning a prospective, multicenter trial that will not only validate our findings but also clarify the underlying mechanisms by which clinical factors influence immunotherapy responses. Finally, the current investigation did not explore the underlying mechanisms of how clinical factors influence immunotherapy responses, representing a critical avenue for future inquiry.
In conclusion, there were no statistically significant differences between sintilimab in combination with trastuzumab and chemotherapy and trastuzumab with chemotherapy for overall HER2-positive advanced GC/GEJC patients. However, sintilimab in combination with trastuzumab and chemotherapy may result in better OS in patients with PD-L1 CPS ≥1, liver metastases, or ECOG ≤1. Additionally, in patients with peritoneal metastases and H. pylori-positive status, sintilimab led to better PFS and OS.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Ethics Committee of The Affiliated Yantai Yuhuangding Hospital of Qingdao University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Due to the retrospective nature of the study, informed consent was waived. The CT images used in the manuscript were obtained with the patient’s consent.
ZL: Data curation, Formal analysis, Investigation, Writing – original draft. AL: Conceptualization, Writing – review & editing. ML: Data curation, Investigation, Writing – original draft. JX: Writing – original draft. GY: Writing – review & editing. PS: Conceptualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by Wu Jieping Medical Foundation Research Special Fund (320.6750.2020-02-45).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Fan X, Qin X, Zhang Y, Li Z, Zhou T, Zhang J, et al. Screening for gastric cancer in China: Advances, challenges and visions. Chin J Cancer Res. (2021) 33:168–80. doi: 0.21147/j.issn.1000-9604.2021.02.05
3. Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Center. (2024) 4:47–53. doi: 10.1016/j.jncc.2024.01.006
4. He F, Wang S, Zheng R, Gu J, Zeng H, Sun K, et al. Trends of gastric cancer burdens attributable to risk factors in China from 2000 to 2050. Lancet Regional Health - Western Pacific. (2024) 44:101003. doi: 10.1016/j.lanwpc.2023.101003
5. Digklia A. Advanced gastric cancer: Current treatment landscape and future perspectives. World J Gastroenterol. (2016) 22(8):2403–14. doi: 10.3748/wjg.v22.i8.2403
6. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, MaChado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer. JAMA Oncol. (2018) 4(5):e180013. doi: 10.1001/jamaoncol.2018.0013
7. Kang Y-K, Boku N, Satoh T, Ryu M-H, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2017) 390:2461–71. doi: 10.1016/S0140-6736(17)31827-5
8. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
9. Boku N, Ryu MH, Kato K, Chung HC, Minashi K, Lee KW, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol. (2019) 30:250–8. doi: 10.1093/annonc/mdy540
10. Zhu Y, Zhu X, Wei X, Tang C, Zhang W. HER2-targeted therapies in gastric cancer. Biochim Biophys Acta (BBA) - Rev Cancer. (2021) 1876(1):188549. doi: 10.1016/j.bbcan.2021.188549
11. Yamashita K, Iwatsuki M, Yasuda-Yoshihara N, Morinaga T, Nakao Y, Harada K, et al. Trastuzumab upregulates programmed death ligand-1 expression through interaction with NK cells in gastric cancer. Br J Cancer. (2020) 124:595–603. doi: 10.1038/s41416-020-01138-3
12. Bang Y-J, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. (2010) 376:687–97. doi: 10.1016/S0140-6736(10)61121-X
13. Qin S, Ji J, Xu R-H, Wang W, Tang Y, Bi F, et al. Treatment patterns and outcomes in chinese patients with gastric cancer by HER2 status: A noninterventional registry study (EVIDENCE). Oncologist. (2021) 26:e1567–e80. doi: 10.1002/onco.13826
14. Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. (2021) 41:747–95. doi: 10.1002/cac2.12193
15. Janjigian YY, Kawazoe A, Bai Y, Xu J, Lonardi S, Metges JP, et al. 1400O Final overall survival for the phase III, KEYNOTE-811 study of pembrolizumab plus trastuzumab and chemotherapy for HER2+ advanced, unresectable or metastatic G/GEJ adenocarcinoma. Ann Oncol. (2024) 35:S877–S8. doi: 10.1016/j.annonc.2024.08.1466
16. Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. (2021) 600:727–30. doi: 10.1038/s41586-021-04161-3
17. Janjigian YY, Kawazoe A, Bai Y, Xu J, Lonardi S, Metges JP, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet. (2023) 402:2197–208. doi: 10.1016/S0140-6736(23)02033-0
18. Wang J, Fei K, Jing H, Wu Z, Wu W, Zhou S, et al. Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. mAbs. (2019) 11:1443–51. doi: 10.1080/19420862.2019.1654303
19. Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L, et al. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: the ORIENT-16 randomized clinical trial. JAMA. (2023) 330:2064–74. doi: 10.1001/jama.2023.19918
20. Wu X, Jian A, Tang H, Liu W, Liu F, Liu S, et al. A multi-omics study on the effect of helicobacter pylori-related genes in the tumor immunity on stomach adenocarcinoma. Front Cell Infection Microbiol. (2022) 12. doi: 10.3389/fcimb.2022.880636
21. Bos J, Groen-van-Schooten TS, Brugman CP, Jamaludin FS, van Laarhoven HWM, Derks S. The tumor immune composition of mismatch repair deficient and Epstein-Barr virus-positive gastric cancer: A systematic review. Cancer Treat Rev. (2024) 127:102737. doi: 10.1016/j.ctrv.2024.102737
22. Xu M, Meng X, Lu Y, Wang F. Efficacy and safety of camrelizumab in combination with trastuzumab and chemotherapy as the first-line treatment for patients with HER2-positive advanced gastric cancer. J Gastrointestinal Oncol. (2022) 13:548–58. doi: 10.21037/jgo-21-897
23. Janjigian YY, Maron SB, Chatila WK, Millang B, Chavan SS, Alterman C, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. (2020) 21:821–31. doi: 10.1016/S1470-2045(20)30169-8
24. Gall VA, Philips AV, Qiao N, Clise-Dwyer K, Perakis AA, Zhang M, et al. Trastuzumab increases HER2 uptake and cross-presentation by dendritic cells. Cancer Res. (2017) 77:5374–83. doi: 10.1158/0008-5472.CAN-16-2774
25. Triulzi T, Regondi V, De Cecco L, Cappelletti MR, Di Modica M, Paolini B, et al. Early immune modulation by single-agent trastuzumab as a marker of trastuzumab benefit. Br J Cancer. (2018) 119:1487–94. doi: 10.1038/s41416-018-0318-0
26. Chaganty BKR, Qiu S, Gest A, Lu Y, Ivan C, Calin GA, et al. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNgamma secretion. Cancer Lett. (2018) 430:47–56. doi: 10.1016/j.canlet.2018.05.009
27. Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A. (2011) 108:7142–7. doi: 10.1073/pnas.1016569108
28. Ferrando-Díez A, Felip E, Pous A, Bergamino Sirven M, Margelí M. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Cancers. (2022) 14(14):3305. doi: 10.3390/cancers14143305
29. Wang J, Deng R, Chen S, Deng S, Hu Q, Xu B, et al. Helicobacter pylori CagA promotes immune evasion of gastric cancer by upregulating PD-L1 level in exosomes. iScience. (2023) 26(12):108414. doi: 10.1016/j.isci.2023.108414
30. Zhu Y, Zhu F, Ba H, Chen J, Bian X. Helicobacter pylori infection and PD-L1 expression in gastric cancer: A meta-analysis. Eur J Clin Invest. (2022) 53(2):e13880. doi: 10.1111/eci.13880
31. Janjigian YY, Shitara K, Ajani J, Moehler M, Yao J, Shen L, et al. Abstract CT037: Nivolumab plus ipilimumab vs chemotherapy as first-line treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma: CheckMate 649 biomarker analyses. Cancer Res. (2023) 83:CT037–CT. doi: 10.1158/1538-7445.AM2023-CT037
32. Jia K, Chen Y, Xie Y, Wang X, Hu Y, Sun Y, et al. Helicobacter pylori and immunotherapy for gastrointestinal cancer. Innovation. (2024) 5(2):100561. doi: 10.1016/j.xinn.2023.100561
33. Dai Y, Liu Y, Gong Z, He L, Wang L, Yang W, et al. Revalidation of the ATTRACTION-4 study in a real-world setting: a multicenter, retrospective propensity score matching study in China. Front Immunol. (2023) 14. doi: 10.3389/fimmu.2023.1264929
34. Janjigian YY, Ajani JA, Moehler M, Shen L, Garrido M, Gallardo C, et al. First-line nivolumab plus chemotherapy for advanced gastric, gastroesophageal junction, and esophageal adenocarcinoma: 3-year follow-up of the phase III checkMate 649 trial. J Clin Oncol. (2024) 42:2012–20. doi: 10.1200/JCO.23.01601
35. Rha SY, Oh D-Y, Yañez P, Bai Y, Ryu M-H, Lee J, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. (2023) 24:1181–95. doi: 10.1016/S1470-2045(23)00515-6
Keywords: HER2-positive, sintilimab, anti-PD-1 antibody, trastuzumab, gastric/gastroesophageal junction cancer, Helicobacter pylori
Citation: Liu Z, Liu A, Li M, Xiang J, Yu G and Sun P (2025) Efficacy and safety of sintilimab combined with trastuzumab and chemotherapy in HER2-positive advanced gastric or gastroesophageal junction cancer. Front. Immunol. 16:1545304. doi: 10.3389/fimmu.2025.1545304
Received: 14 December 2024; Accepted: 27 January 2025;
Published: 14 February 2025.
Edited by:
Bianca Mostert, Erasmus Medical Center, NetherlandsReviewed by:
Tao Li, People’s Liberation Army General Hospital, ChinaCopyright © 2025 Liu, Liu, Li, Xiang, Yu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guohua Yu, Z3VvaHVheXVAcWR1LmVkdS5jbg==; Ping Sun, c3VucGluZzIwMDM5QGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.