- 1Department of Urology, Yantai Affiliated Hospital of Binzhou Medical University, The Second Clinical Medical College of Binzhou Medical University, Yantai, Shandong, China
- 2Department of Urology, Haiyang City People’s Hospital, Yantai, Shandong, China
In recent years, there has been a growing trend towards the utilization of immunotherapy techniques for the treatment of cancer. Some malignancies have acquired significant progress with the use of cancer vaccines, immune checkpoint inhibitors, and adoptive cells therapy. Scholars are exploring the aforementioned methods as potential treatments for advanced prostate cancer (PCa) due to the absence of effective adjuvant therapy to improve the prognosis of metastatic castration-resistant prostate cancer (mCRPC). Immunotherapy strategies have yet to achieve significant advancements in the treatment of PCa, largely attributed to the inhibitory tumor microenvironment and low mutation load characteristic of this malignancy. Hence, researchers endeavor to address these challenges by optimizing the design and efficacy of immunotherapy approaches, as well as integrating them with other therapeutic modalities. To date, studies have also shown potential clinical benefits. This comprehensive review analyzed the utilization of immunotherapy techniques in the treatment of PCa, assessing their advantages and obstacles, with the aim of providing healthcare professionals and scholars with a comprehensive understanding of the progress in this field.
1 Introduction
Among elderly males, prostate cancer (PCa) is a prevalent form of cancer, coming in second in terms of occurrence globally among male malignancies and ranking as the fifth leading cause of death worldwide (1). In 2020, the number of new PCa cases worldwide has exceeded 1.4 million (2). For the clinical treatment of organ-localized PCa, radical prostatectomy combined with chemoradiotherapy and other therapeutic strategies are commonly used, and the therapeutic effect is excellent, and majority patients can even be cured (3). Androgen deprivation therapy (ADT) is the primary treatment for locally advanced and metastatic PCa. Although testosterone levels decrease post-treatment, many patients eventually develop resistance to the drugs and advance to castration-resistant prostate cancer (CRPC) (4). A portion of these individuals will eventually develop metastatic castration-resistant prostate cancer (mCRPC), with a 5-year survival rate of less than 30% for these patients (5).
Currently, there is rapid progress in research on immunotherapy as an adjuvant therapy for the treatment of mCRPC. Scholars endeavored to utilize immunotherapy, specifically immune checkpoint inhibitors (ICIs), tumor vaccines, and chimeric antigen receptor T cells (CAR-T), in the treatment of patients diagnosed with mCRPC. Currently, the majority of clinical studies have demonstrated that the aforementioned strategies exhibit favorable safety profiles, with certain studies also indicating notable clinical effectiveness. The FDA has authorized sipuleucel-T (targeting prostate acid phosphatase) as the initial immunotherapy treatment for asymptomatic or mild symptomatic mCRPC (5, 6). Furthermore, the researchers attempted to enhance patient prognosis by employing immunotherapy strategies in conjunction with chemoradiotherapy and other interventions, resulting in promising initial outcomes (7). This review summarizes the immunosuppression mechanism of PCa, and comprehensively summarizes the application of immunotherapy in PCa treatment in recent years, and discusses its existing value and difficulties, aiming at providing convenience for relevant clinicians and researchers to understand the progress in this field.
2 Immunosuppressive mechanism of PCa
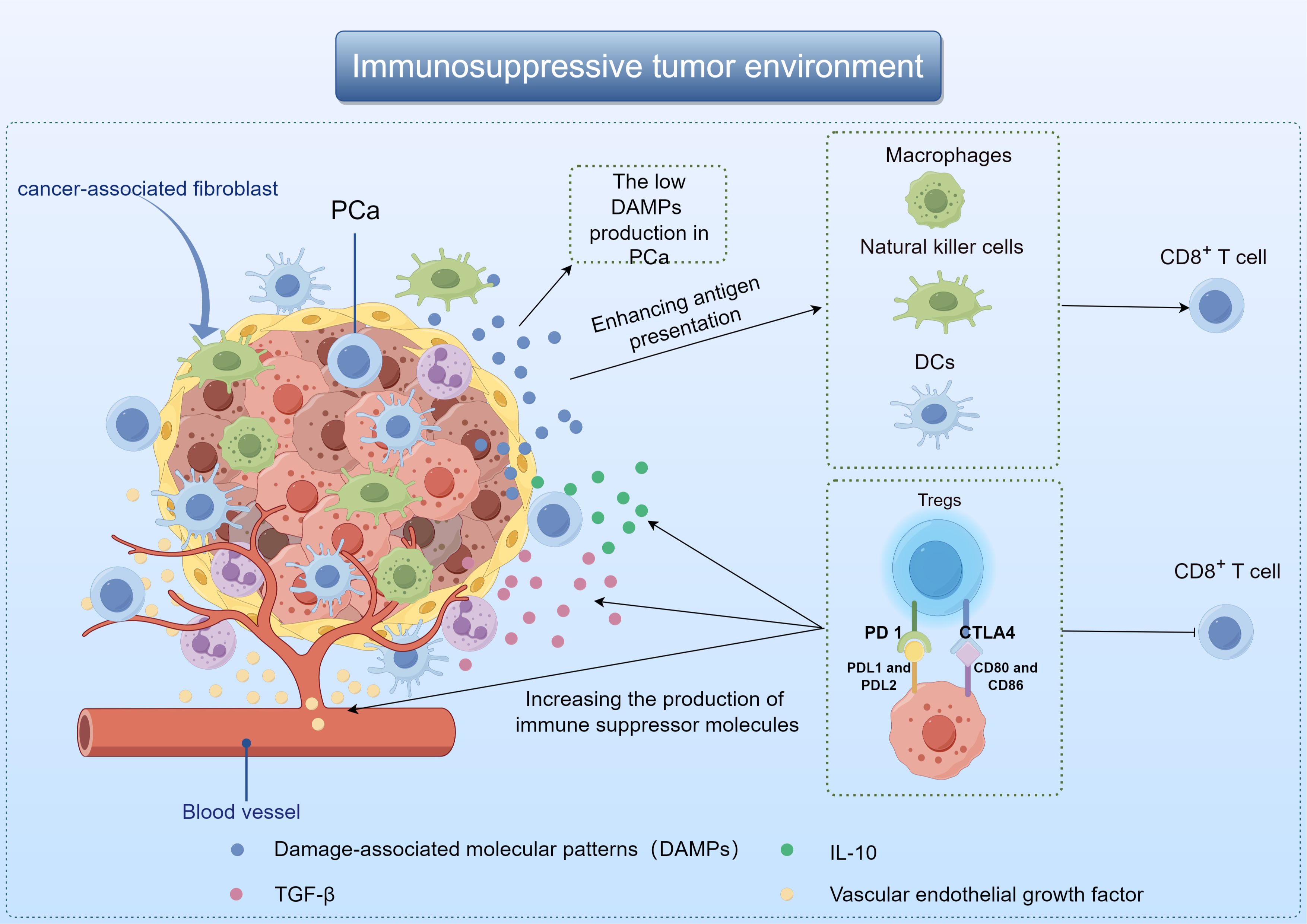
The poor immunotherapy response of PCa may be related to TME. Composed of immune cells, blood vessels and extracellular matrix components, TME plays the most important role in tumor progression and therapeutic response (8). It influences tumor behavior through interaction with surrounding macromolecules and tissues, such as inhibiting or stimulating tumor growth (9, 10). When tumor cells die, they release damage-associated molecular patterns (DAMPs) that activate macrophages, dendritic cells (DCs), and natural killer cells. These immune cells then release anti-tumor cytokines, and promote DCs maturation and antigen presentation (11, 12). The low DAMPs production in PCa results in the affected activation of DCs, which in turn reduces the production of immune-related chemokines and cytokines, and ultimately leads to the loss of T cells around the tumor (13). Besides, PCa TME also promotes the growth of immunosuppressive cells, especially regulatory T cells (Tregs). Cytotoxic T lymphocyt-associated protein 4 (CTLA4), programmed death receptor 1 (PD-1) and other inhibitory molecules were present on the cell membrane of Tregs. These compounds have the ability to block T cell activity, increase the production of immune suppressor molecules like transforming growth factor-β(TGF-β), vascular endothelial growth factor, and interleukin-10(IL-10), leading to the eventual spread of cancer cells (Figure 1) (14, 15).
Some scholars also believe that the poor effect of PCa immunotherapy may be related to the low tumor mutation burden (TMB). TMB is the mutation rate of genes in each coding region of tumor genome, and the mutation rate is different in different tumors. PCa has a mutation rate of about 1 individual cell mutation per megabase, while lung cancer or melanoma has 10 or more somatic mutations per megabase (16). Simultaneously, the emergence of neoantigens is associated with diverse types of gene mutations, with tumors exhibiting a higher somatic mutation burden producing a greater quantity of neoantigens (17). Research has indicated that certain PCa patients exhibit an elevated mutation rate in genes associated with DNA damage repair, resulting in a higher TMB and consequently a more favorable response to immunotherapy (18–20). It can be seen that the efficacy of immunotherapy is related to TMB, and most PCa patients have low TMB, resulting in poor efficacy of PCa immunotherapy (21). The application of immunotherapy to the treatment of PCa needs to overcome the resistance associated with low TMB.
In the context of immunotherapy for PCa, another significant challenge that must be addressed is the phenomenon of tumor immune escape, a common obstacle encountered in various forms of cancer treatment. Tumor immune evasion primarily encompasses two facets: Firstly, tumor cells evade immune detection through mechanisms such as diminished immune responses to tumor antigens. Additionally, alterations in the body’s immune system function, including the inability to detect low levels of tumor-associated antigens (TAAs) in the early stages of tumor development and the ineffectiveness of antigen-presenting cells (APCs) (22). Current studies have shown that many types of tumors show good clinical response to immunotherapy (23–25). Among them, cancer vaccines, ICIs, and CAR-T cells are demonstrating promising outcomes. The aforementioned treatment modalities for PCa have demonstrated only preliminary efficacy, necessitating further advancement before widespread clinical implementation.
3 Cancer vaccines are used to treat PCa
3.1 Cancer vaccines
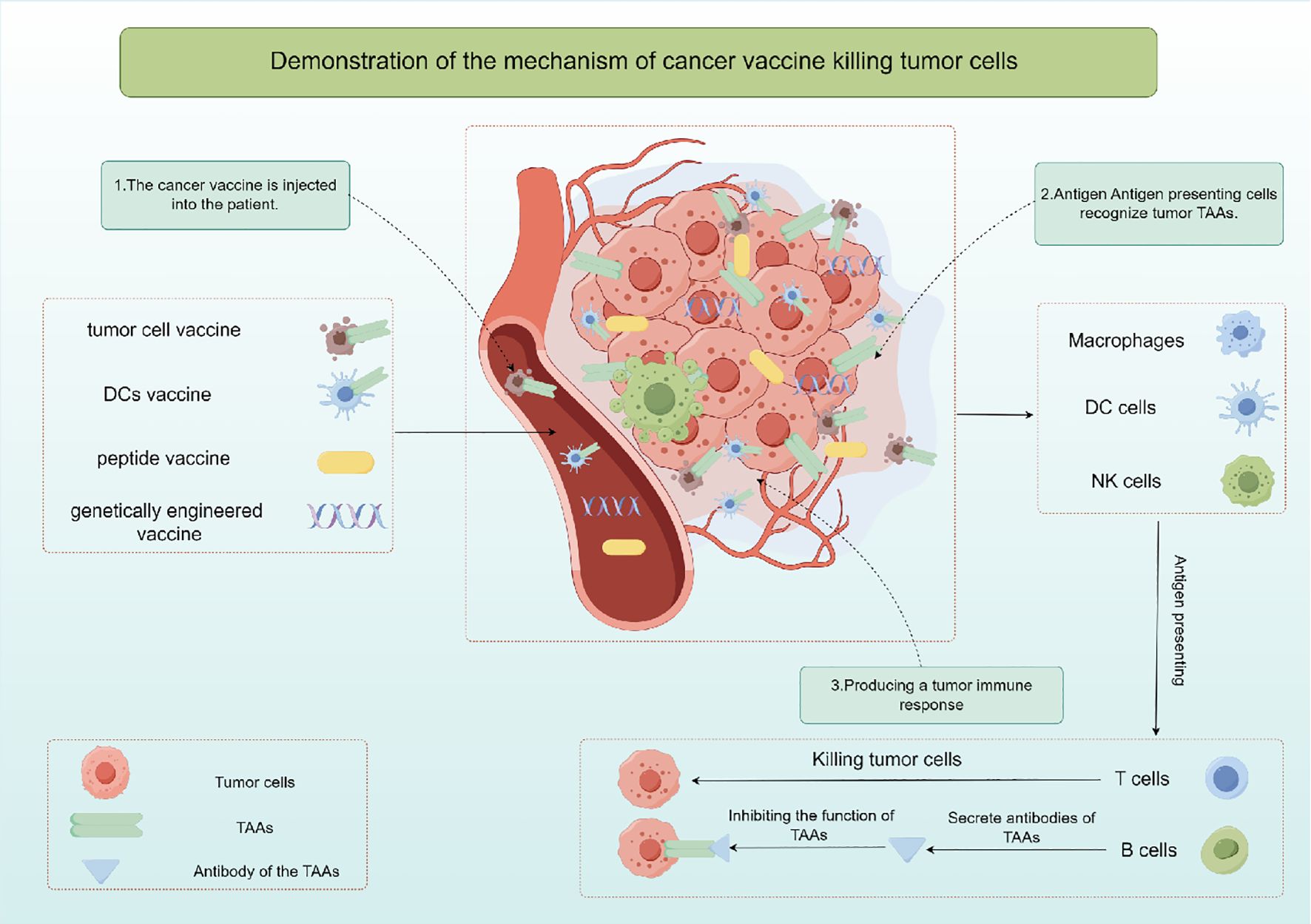
The cancer vaccine is a kind of active immunotherapy, which induces specific immune response to produce tumor killing effect by exogenous injection of human TAAs (26). It is expected to provide effective strategies for cancer prevention and treatment, and includes autologous tumor cell vaccine, DCs vaccine, peptide vaccine and genetically engineered vaccine according to the different components injected into the body. Cancer vaccine primarily works by stimulating T cells, B cells, and other immune cells using specific TAAs, resulting in the elimination of cancerous cells (Figure 2). Hence, cancer vaccine could potentially target any protein that is mutated or expressed differently in cancer cells (27, 28). Since TAAs are also expressed in normal tissue cells, they may be affected by immune tolerance, and therefore immunogenicity may be poor. Moreover, research has demonstrated that tumors generate a distinct antigen known as a tumor-specific antigen (TSAs) as a result of genetic mutations. TSAs are exclusively expressed in particular tumors and are absent on the surface of normal cells (29, 30). TSAs remain unaffected by immune tolerance mechanisms in both central and peripheral systems, enabling them to initiate targeted and potent T cell reactions against cancerous cells (31). Hence, identifying TSAs is crucial for the advancement and utilization of cancer vaccines. However, current studies have found that there are fewer TSAs on the surface of solid tumor cells, which is also a bottleneck limiting tumor immunotherapy.
Whole-cell extracts derived from malignant cells are a key strategy in cancer vaccine development. These extracts, obtained from tumor tissue or cancer cell cultures and rendered non-viable, are recognized by immune cells such as APCs like DCs, macrophages, and natural killer (NK) cells (32). Activated immune cells process the tumor antigens, present them to T cells, and trigger an immune response. CD4+ and CD8+ T cells can recognize and eliminate tumor cells presenting the specific antigen. Whole cell cancer vaccines aim to stimulate a memory response to enhance immune defense and prevent tumor recurrence. DC vaccines, derived from a patient’s peripheral blood and loaded with tumor antigens, are another type of cellular vaccine. These DCs, matured and activated with immune-stimulating agents or TSAs, migrate to lymphatic tissues, where they engage with immune cells, presenting cancer antigens to CD4+ helper T cells and CD8+ cytotoxic T lymphocytes (CTLs), leading to their activation and subsequent elimination of cancer cells (32).
Additionally, recent studies have explored the use of immunohybrids, such as dendritic-tumor cell hybridomas, in prostate cancer immunotherapy. Chowdhury et al. (33) found that the survival of castration-resistant prostate cancer patients treated with these hybridomas was negatively correlated with changes in peripheral blood CD56brightCD16− natural killer cells. This highlights the potential of hybrid approaches in enhancing immune responses against prostate cancer. Peptide vaccines and genetically engineered vaccines function similarly in delivering TAAs fragments to B and T lymphocytes through APCs to trigger an adaptive immune response and activated CD8+ T cells start the process of releasing apoptotic factors like perforin, Fas ligand, and granase, leading to tumor cell death (34). Successful therapeutic cancer vaccines rely on providing ample highly immunogenic antigens to the APCs, leading to a robust and lasting CD4+ T helper cell and CD8+ CTLs reaction. Specifically, efforts to enhance APCs activation and maturation focus on improving antigen presentation to generate the best possible T cell reactions. The focus of these methods is on finding, choosing, and confirming new substances that can boost and improve the duration of immune responses from T and B cells that target specific antigens (35).
Despite some advancements in cancer vaccine research, there are still limitations stemming from current research findings. The effectiveness of cancer vaccines is significantly hindered by tumor diversity, immunosuppressive TME, and mechanisms of immune tolerance. Identifying appropriate TAAs and selecting the best adjuvant remain critical for the development and success of cancer vaccines (36). Moreover, the efficacy of this treatment could be constrained by variations in patients’ immune responses and their capacity to identify antigens, necessitating a customized approach for its implementation.
3.2 Different types of cancer vaccines are used in the clinical study of PCa treatment
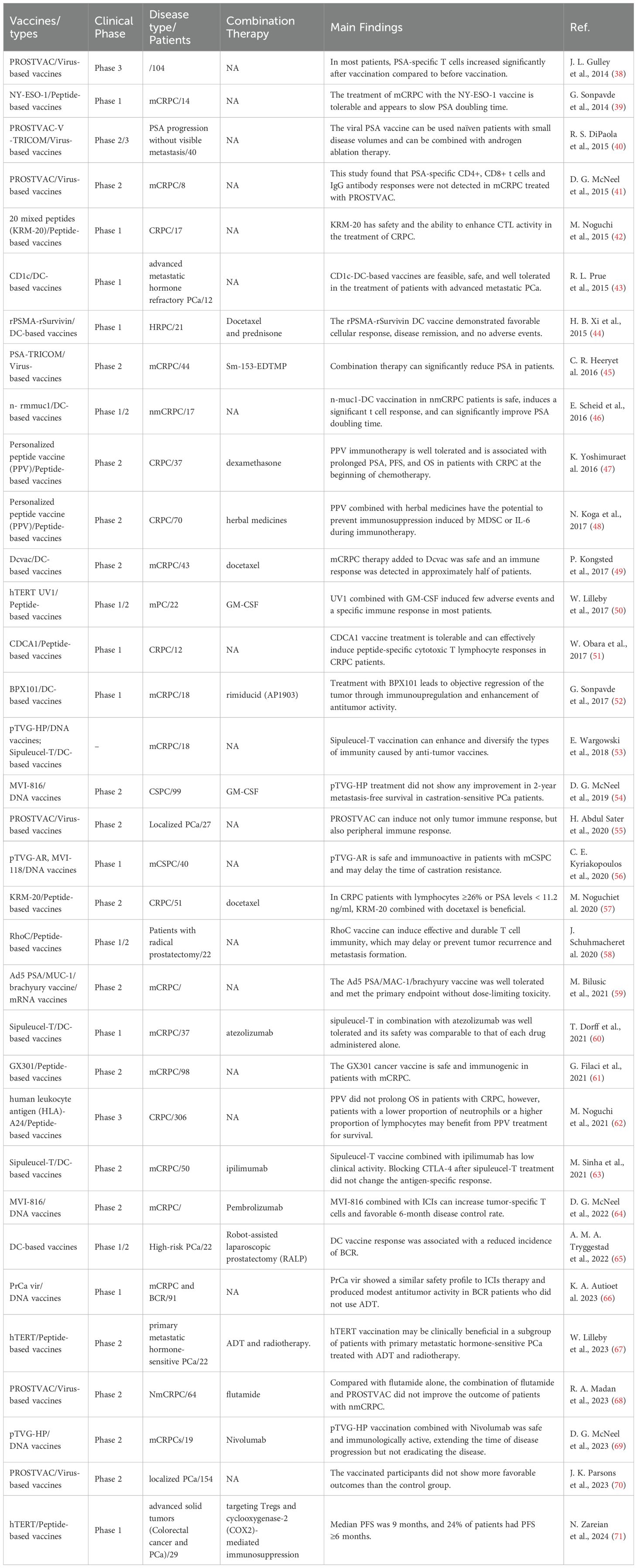
The cancer vaccine plays a crucial role in immunotherapy and holds a distinct position in the treatment of PCa. The FDA gave the green light to sipuleucel-T in April 2010, marking the approval of the initial autologous cell immunotherapy medication utilizing DCs as the primary effector cells. This drug is intended for individuals with asymptomatic or mild mCRPC (37). Sipuleucel-T significantly contributes to the advancement of immunotherapy for PCa. Since then, researchers have developed monocyte vaccines, DCs vaccines, viral vaccines, polypeptide vaccines and DNA/mRNA vaccines for PCa, and achieved initial clinical efficacy (Table 1).
3.2.1 DCs vaccines
In recent years, researchers have endeavored to utilize cancer vaccine strategies for the treatment of mCRPC, resulting in promising initial efficacy. The FDA approved Sipuleucel-T (Provenge) in April 2010 as the initial tumor immunotherapy medication, offering a ray of hope for individuals with mCRPC (72). Sipuleucel-T is an autologous cell immunotherapy based on DCs. A multicenter Phase 3 clinical trial has shown that Sipuleucel-T can significantly prolong survival in patients with mCRPC (73). The study included 512 patients, 341 of whom received Sipuleucel-T for 1 month and 171 of whom received a placebo control. The study revealed that individuals receiving Sipuleucel-T treatment experienced a 22% decrease in mortality risk and a median survival increase of 4.1 months. Following this study, Sipuleucel-T was granted FDA authorization for managing asymptomatic or mild mCRPC, marking the beginning of a fresh period of immunotherapy for PCa.
The safety and efficacy of other types of DCs vaccines against mCRPC have also been preliminatively explored. R. L. Prue et al. (43) conducted a phase I clinical study to evaluate the safety of blood-derived DCs (BDC) purified with tumor-specific peptide CD1c in patients with mCRPC. Finally, the CD1c BDC vaccine was administered to 12 mCRPC patients. The vaccine was well tolerated in all patients and no serious adverse events were detected. Similarly, BPX101 is a second-generation antigen-targeted autologous DCs vaccine, and its safety and activity were also reported in a Phase I trial (52). Eighteen patients with mCRPC were enrolled and given three doses of BPX101 subcutaneously. The results of this study found no dose-limiting toxicity of BPX101. Immunoupregulation and significant antitumor activity were observed in patients with decreased prostate-specific antigen (PSA), while objective tumor regression was also observed. Moreover, H. B. Xi et al. (44) applied DCs vaccine loaded with recombinant prostata-specific membrane antigen (rPSMA) and recombinant surviving (rSurvivin) peptide to 21 patients with hormone-refractory prostate cancer (HRPC). Patients were randomized into two groups, with docetaxel treatment as a control. The study also found that the DCs vaccine was well tolerated. The DCs vaccine induced delayed immune responses in all patients. Compared with the control group, the response rate was 72.7% vs 45.4%, and the immune-related response was 54.5% vs 27.2%. Therefore, the above studies have shown that DCs vaccine is not only well tolerated, but also has a good immune response.
Additionally, researchers are endeavoring to employ the DCs vaccine in patients with localized PCa. MUC1 is a surface glycoprotein expressed in ductal epithelial cells. Tumors undergo malignant transformation resulting in increased levels of MUC1, categorized as T or Tn tumor antigens based on the type of carbohydrate present. Tn-MUC1, containing Tn glycans, is a promising candidate for immunotherapy (74). E. Scheid et al. (46) applied autologous DCs loaded with Tn-MUC1 to non-mCRPC and discussed its safety and efficacy. The Tn-MUC1-DCs vaccine was tested on 17 patients with nmCRPC in a Phase I/II clinical trial. There was a significant improvement in PSA doubling time in 11 out of 16 patients who were evaluated (P = 0.037). Five of the seven patients had a significant immune response. Tn-MUC1-DCs vaccine in patients with nmCRPC appears to be safe and able to induce a significant T cell response. In addition, one researcher also tried to apply this technology to the adjuvant treatment of PCa patients. Furthermore, A. M. A. Tryggestad et al. (65) discussed the value of personalized DCs vaccine in reducing biochemical recurrence (BCR) in patients after robot-assisted laparoscopic prostatectomy. Twenty individuals diagnosed with high-risk PCa and undetectable prostate-specific antigen levels were administered the DCs vaccine for a period of three years or until experiencing biochemical recurrence. Out of the 20 individuals, 11 were determined to have no evidence of BCR after a median follow-up of 96 months (ranging from 84 to 99 months). All patients who developed BCR remained stable for a median of 99 months. The study is the first to use DCs vaccine as an adjunct postoperative treatment for high-risk PCa, and the results have been encouraging. Therefore, attempts should be made to apply the DCs vaccine in a larger cohort of high-risk PCa patients to further investigate its value for BCR treatment.
In order to better exert the efficacy of DCs vaccine in the treatment of PCa, some researchers have also tried to use a combination of other tumor treatment strategies. P. Kongsted et al. (49) investigated whether adding DCs vaccine to docetaxel treated mCRPC patients induced an immune response. A total of 43 patients were randomly allocated to either receive docetaxel by itself or in combination with DCs vaccine. PSA, progression-free survival (PFS), and disease-specific survival (DSS) responses did not show any notable variations. TAAs-specific or vaccine-specific immune responses were 50% and 78% in the combination treatment group respectively (75, 76). Therefore, more clinical studies are needed in the future to explore the clinical efficacy of docetaxel combined with DCs vaccine. Moreover, the researchers propose that the concurrent administration of ICIs and cancer vaccines has the potential to modulate the immune system, leading to sustained activation of T cells and a durable immunotherapeutic effect. T. Dorff et al. (60) enrolled 37 patients with asymptomatic or mild symptoms of mCRPC and conducted an initial clinical trial (NCT03024216). Random assignment determined which enrolled patients would receive sequential treatment with either atezolizumab + sipuleucel-T or sipuleucel-T + atezolizumab. The safety and tolerability of atezolizumab when combined with sipuleucel-T in various sequences was confirmed, with immunosurveillance studies indicating the potential benefits of this combination. Similarly, M. Sinha et al. (63) investigated whether administration of ipilimumab following sipuleucel-T treatment could alter immune and clinical response to this treatment. A total of 50 mCRPC patients enrolled in a clinical trial (NCT01804465) who were treated with ipilimumab either immediately after completing sipleul-T or with a delay of 3 weeks. The integrated therapy approach was determined to be well received with no unforeseen negative effects. Six patients exhibited positive clinical responses, with three of them maintaining the response for over 3 months. The use of multiple treatments did lead to the activation of CD4 and CD8 T cells, especially in the early treatment schedule. The above studies have conducted a preliminary exploration of DCs vaccine combined with other therapeutic measures. Further exploration of the cancer vaccine’s mechanism of action in the TME is necessary for widespread translation into clinical practice.
3.2.2 Peptide-based vaccines
3.2.2.1 Simple peptide targeting
Cancer vaccines based on peptides can be used in cancer treatment, especially against tumor growth and the tumor recurrence (77). Over the past decade, peptide cancer vaccines have been evaluated in multiple tumor models as a component of research in cancer immunotherapy (78). Cancer vaccines utilize two primary categories of peptides: TAAs and TSAs, which are capable of being processed by APCs and displayed to immune cells. The activation and proliferation of CD8+ cytotoxic T cells occur when they identify the presented peptides on MHC Class I molecules, leading to the destruction of tumor cells. CD4+ helper T cells identify peptides on MHC Class II molecules and transmit signals to other immune cells to aid in inhibiting tumor growth. B cells are activated by the peptides presented, resulting in the production of specific antibodies against TAAs, which ultimately induce a memory response to enhance immune protection (79).
NY-ESO-1, a tumor antigen found in testicular cancer, is present in both tumors and normal testis, but is not found in other normal tissues. NY-ESO-1 is the most potent antigen among the testicular antigens identified so far, stimulating both antibody production and robust cellular immune reactions in patients (80, 81). G. Sonpavde et al. (39) conducted a Phase I clinical trial to assess the efficacy of the NY-ESO-1 peptide vaccine in mCRPC. The study comprised nine patients, four of whom had received prior treatment with docetaxel. A higher frequency of specific T cell response was found in docetaxel pretreated patients in the NY-ESO-1 treatment group (4/4 vs 2/5). The research demonstrates that mCRPC patients can tolerate the NY-ESO-1 peptide, and it elicits antigen-specific T cell responses more frequently in patients undergoing chemotherapy. CDCA1 is upregulated as an oncogene in PCa. W. Obara et al. (51) assessed the effectiveness of a CDCA1 peptide vaccine for treating CRPC in a phase I (NCT01225471). This study included 12 patients with CRPC who had failed docetaxel chemotherapy. Patients received subcutaneous injections of CDCA1 peptide in a dose-increasing manner. The CDCA1 peptide vaccine was determined to be well received with no significant negative effects observed. Patients who had a longer survival time showed a specific CTL response to CDCA1 peptide, with a median Overall survival (OS) of 11.0 months. This study demonstrated that CDCA1-derived peptide vaccines can effectively induce peptide-specific CTL responses in patients with CRPC. RhoC, a member of the Ras homologous gene family C (RhoC), demonstrates increased expression levels in advanced solid tumors, metastatic tumors, and cancer stem cells. J. Schuhmacher et al. (58) examined the safety, tolerability, and impact of RhoC peptide vaccine on the immune system in individuals with PCa (NCT03199872). The study included 22 individuals who had previously received radical prostatectomy, with a treatment period lasting 30 weeks. Most patients (18 out of 21) exhibited a robust CD4 T cell reaction to the vaccine, which persisted for a minimum of 10 months following the final dose, as per the findings. Furthermore, the vaccine was well received with no severe treatment-related adverse events reported. According to this research, immunization against RhoC peptide vaccine could potentially postpone or hinder the reappearance and spread of tumors. Homogeneously, G. Filaci et al. (61) developed GX301, a cancer vaccine that targets telomerase. The safety and immune response of the GX301 vaccine were evaluated in patients with metastatic castration-resistant PCa (NCT02293707). Ninety-eight patients were randomly vaccinated. 54% of patients exhibited an immune response overall, with 95% demonstrating at least one immune response specific to the vaccine. The data suggest that the GX301 cancer vaccine is well-tolerated and induces an immune response in individuals with advanced castration-resistant PCa. These studies indicate that the use of fetal fragments of tumor antigens as cancer vaccines has good safety and specific immune response in the treatment of PCa. Further clinical studies are expected to investigate its value in improving the clinical prognosis of patients.
In recent years, researchers have developed a human telomerase reverse transcriptase(hTERT) cancer vaccine, which is widely used in the immunotherapy of tumors (50, 82, 83). W. Lilleby et al. (50) assessed the safety and immune reaction of the new hTERT peptide vaccine (UV1) in patients with recently diagnosed metastatic hormone-naïve PCa. Twenty-two recently diagnosed patients were included in the study, receiving three different doses of UV1 administered intradermally along with granulocyte macrophage stimulating factor (GM-CSF). By the conclusion of the nine-month observation period, a total of 17 patients showed signs of clinical stability. UV1 and GM-CSF therapy had minimal side effects and triggered a targeted immune reaction in most patients who were not HLA type-selected. The moderate amount of UV1 led to the most significant and quickest UV1-targeted immune reaction while maintaining a safe profile. More recently, W. Lilleby et al. (67) evaluated the long-term immune system response and effectiveness of patients in this study. Nine out of the 22 patients were alive at the most recent check-in. There was no progression in 6 cases, castration-resistant disease in 2 cases and castration-refractory disease in 1 case. PSA progression had a median time of 21 months, while OS and PCa-specific survival had median times of 62 and 84 months, respectively. Absence of immune reaction is a separate factor that increases the risk of death from PCa. The connection implies a potential medical advantage of hTERT immunization in a specific group of individuals with initial metastatic hormone-responsive PCa who have undergone androgen deprivation therapy and radiotherapy. Furthermore, N. Zareian et al. (71) utilized hTERT peptide in conjunction with treatment aimed at inhibiting Tregs and COX2-induced immunosuppression. In the Phase 1 study, a 7-peptide library derived from hTERT was utilized, along with oral low-dose cyclophosphamide for Tregs regulation and the COX2 inhibitor celecoxib. 29 patients with advanced PCa patients were included in the study. Median PFS was 9 weeks, and 24% of patients had PFS≥6 months. The immunophenotype indicated an increase in CD4(+) and CD8(+) T cells following inoculation, displaying characteristics of effector cells. Diminished numbers of PD-1 positive CTLs are increasing in individuals who have received vaccinations. This vaccine combination regimen is safe and associated with an antigen-specific immune response. Due to its robust and enduring capacity to stimulate tumor immune response, the hTERT peptide vaccine shows promising potential for the treatment of PCa in clinical settings.
3.2.2.2 Mixed peptide targeting
The variability within the human T cell population and the varied expression of TAAs suggest that effective cancer vaccines should elicit a broad spectrum of cytotoxic T lymphocyte responses, a strategy that can be achieved by targeting multiple TAAs with vaccination. M. Noguchi et al. (42) examined the safety and immune responses of a cancer vaccine containing 20 assorted peptides (KRM-20) aimed at stimulating CTL against 12 various TAAs in individuals with CRPC. Seventeen patients were given three different doses of KRM-20 that were randomly assigned. The findings indicated that there were no significant adverse effects from the medication. Clinical reactions to levels of prostate-specific antigen showed partial response in 2 instances, stability in 5 instances, and worsening condition in 10 instances. This study confirmed the safety of KRM-20 in PCa treatment and its ability to enhance CTL activity, however, its efficacy was not ideal, the authors attributed to the dose of treatment. The absence of further research on the dosage could also stem from the lack of precision or weak immune response of the target associated with KRM-20. Since then, the efficacy of KRM-20 in combination with docetaxel has been investigated. M. Noguchi et al. (57) explored if the addition of KRM-20 to docetaxel could improve the effectiveness against CRPC. Random assignment was used to allocate eligible patients to two groups: one receiving KRM-20 with docetaxel (n=25) and the other receiving a placebo with docetaxel (n=26). This study demonstrated that the addition of KRM-20 to CRPC therapy is safe, and the decrease in PSA and the increase in HLA-matched peptide-specific CTL and IgG responses suggest the potential of KRM-20 in the treatment of CRPC. These studies indicate that the mixed peptide vaccine has a good safety profile in the treatment of PCa, but does not show an advantage in improving patient outcomes. Due to the characteristics of targeting multiple antigens, this vaccine can solve the problem of differential expression of target antigens between individuals, and has great economic value. Therefore, more research is needed to explore the mechanism of action of this vaccine in PCa patients.
3.2.2.3 Individualized peptide
Due to the variability in the expression of target antigens among individuals, the implementation of immunotherapy necessitates the screening of appropriate candidates. Consequently, each immunotherapy approach is tailored to the individual, with ongoing efforts by researchers to develop personalized cancer vaccines specifically for the treatment of PCa. K. Yoshimura et al. (47) evaluated the safety and clinical efficacy of immunotherapy with personalized peptide vaccine (PPV). A phase 2 randomized controlled trial was conducted to assess the efficacy of combining PPV immunotherapy with dexamethasone in the treatment of chemotherapy-naive patients with CRPC. Four HLA-matched peptides were chosen according to the reaction of existing immunoglobulin G to 24 stored peptides and administered biweekly. Peptide vaccine was administered to thirty-seven patients, while thirty-five patients were treated with dexamethasone alone. PFS in inoculation group was significantly longer than dexamethasone group (22.0 months vs 7.0 months; P = 0.0076). The vaccinated group had a notably longer median survival time compared to the non-vaccinated group (73.9 months vs 34.9 months; P = 0.00084). PPV immunotherapy is well tolerated and is associated with prolonged PFS and OS in patients with CRPC at the beginning of chemotherapy. Moreover, M. Noguchi et al. (62) conducted a Phase III randomized trial of a PPV in HLA-A24 positive CRPC patients who had failed docetaxel chemotherapy. Participants were assigned randomly to either receive PPV or placebo at a ratio of 2:1. Four out of the 12 peptides stored were chosen based on existing levels of peptide-specific immunoglobulin G or a placebo and were administered subcutaneously six times per week until the disease advanced. A total of 306 patients were included in the final analysis. Analysis of intergroup effectiveness indicated that PPV did not extend overall survival in individuals with CRPC who experienced disease progression following docetaxel chemotherapy and tested positive for HLA-A24. Analysis of subgroups indicated that individuals with a low neutrophil ratio or high lymphocyte ratio at the beginning of the study may experience improved survival outcomes with PPV therapy. The findings indicate that personalized peptide vaccines may necessitate the identification of suitable candidates. Additionally, characterizing the tumor immune microenvironment at various stages of tumor progression could aid in the identification of suitable candidates for personalized peptide vaccines.
Furthermore, initial efforts have been made to explore the efficacy of combining herbal medicines (HM) with personalized vaccines in the treatment of PCa. N. Koga et al. (48) explored the immunological efficacy of HM combined with personalized peptide vaccine (PPV) in the treatment of CRPC through a phase II clinical study. Seventy patients with CRPC were divided into PPV + HM and PPV alone groups. Based on the human leukocyte antigen type and the level of antigen specific IgG titer in patients prior to receiving PPV treatment, 2-4 peptides were chosen from a pool of 31 peptides derived from cancer antigens, followed by 8 injections of PPV. The results of this study showed that monocyte medullary derived suppressor cell (Mo-MDSC) frequency and serum IL-6 level were stable during PPV combined with HM treatment. In contrast, Mo-MDSC frequency and IL-6 levels were significantly increased in the PPV group alone. The findings indicate that the mixture of HM could have the ability to inhibit immunosuppression caused by Mo-MDSC or IL-6 in the course of immunotherapy. Further investigation is required to validate the findings of this research. The study indicates that the integration of personalized vaccines with immune modulators, such as HM, could potentially serve as a novel approach for enhancing PCa immunotherapy.
3.2.3 Nucleic acid-based vaccines
DNA and RNA cancer vaccines work by delivering DNA or RNA molecules that contain instructions for TAAs. Cells in the body absorb the introduced DNA or RNA, leading to the production of TAAs. APCs cells take up TAAs and use MHC molecules to present it on their surface. CD8+ CTLs identify TAAs on MHC class I molecules, resulting in their stimulation and proliferation. CD4+ T helper cells identify TAAs on MHC Class II molecules and transmit activation signals to other cells of the immune system. B cells can be activated by TAAs presented by DCs, resulting in the production of antibodies against TAAs. Antibodies can bind directly to TAAs of tumor cells, promote their destruction, and induce a memory response to enhance immune protection.
Prostate acid phosphatase (PAP) serves as a prostate tumor antigen and is the specific target of sipuleucel-T, the sole anti-cancer vaccine approved by the FDA. E. Wargowski et al. (53) sought to assess whether a DNA vaccine encoding PAP (pTVG-HP) could enhance specific immunity in patients with mCRPC. Eighteen patients were randomly assigned to two groups: one group received monocyte T therapy alone, while the other group received intradermal pTVG-HP DNA vaccine afterwards. The treatment protocol was completed by 11 out of 18 patients. No adverse events above Grade 2 were detected in relation to treatment. Specific T cell responses to PAP with a Th1 bias were found in 11 of the 18 individuals, and there was no significant difference between the groups in the study. A high titer antibody response to PAP was detected in patients receiving pTVG-HP booster immunization. These findings suggest that from the perspective of T cell and humoral immunity, DNA as a pre-booster vaccination can enhance and diversify the types of immunity induced by anti-cancer vaccines. However, previous study has shown that pTVG-HP does not improve 2-year metastasis-free survival (MFS) in castration-sensitive PCa (54). Subsequently, D. G. McNeel et al. (64) found that the combination of ICIs with pTVG-HP increased tumor-specific T cells in patients with mCRPC and resulted in a favorable 6-month disease control rate. Recently, D. G. McNeel et al. (69) reported the efficacy of DNA vaccine pTVG-HP combined with pembrolizumab in mCRPC (NCT03600350). Of the 19 patients enrolled, PSA decreased by >50% in 4/19(21%) patients. In this population, pembrolizumab combined with pTVG-HP vaccination was safe and immunologically active, extending the time of disease progression but not eradicating the disease.
Studies conducted before clinical trials have indicated that a DNA vaccine (pTVG-AR) containing the ligand-binding domain of the androgen receptor (AR LBD) boosts CD8+ cells activization that target specific antigens, slows down the advancement of PCa and the development of castration-resistant disease, and extends the lifespan of mice with tumors. A multicenter Phase I trial assessed the effectiveness of the vaccine (56). Patients diagnosed with metastatic castration-sensitive prostate cancer (mCSPC) received treatment with pTVG-AR; 27 individuals successfully finished the treatment regimen, and 11 patients (28%) experienced a PSA progression event prior to reaching 18 months. Individuals possessing T cell immunity exhibited notably extended PFS compared to those lacking immunity (HR = 0.01; 95% CI, 0.0-0.21; P = 0.003). The research showed that pTVG-AR is both well-tolerated and stimulates the immune system in individuals with mCSPC. The correlation of immune response with PFS indicates that therapies could potentially extend the time before developing resistance to castration. The research shows how pTVG-AR could enhance results for individuals with mCSPC, but additional prospective randomized controlled trials are necessary for validation.
3.2.4 Virus-based vaccines
A viral vaccine is a viral vector that delivers recombinant genes targeting TAAs. These viral vectors expressing recombinant genes play a role by infecting epithelial cells. After cell death, the cell fragments containing target antigens are recognized and processed by APCs and presented to CD4+ and CD8+ T cells to induce immune response, and then the activated immune system can kill tumors.
PROSTVAC is a therapeutic vaccine based on two PSA-encoding recombinant poxvirus vectors, in which vaccinia virus vectors serve as initial immunization, followed by avian pox virus booster immunization, and the two recombinant viruses simultaneously encode three co-stimulatory molecules (B71, ICAM-1, and AF-3), also known as TRICOM. PROSTVAC can trigger an immune response against tumors and in the surrounding area, leading to immune cells entering the tumor environment and destroying cancer cells (55). J. L. Gulley et al. (38) investigated the efficacy of the PROSTVAC vaccine for PCa through the implementation of a Phase III clinical trial. Among the cohort of 104 individuals studied for T cell response, 57% (59/104) demonstrated a greater than twofold increase in PSA-specific T cells one month after vaccination compared to pre-vaccination levels. Additionally, 68% (19/28) of individuals exhibited an immune response to tumor-related antigens not present in the vaccine post-vaccination. These findings suggest that PSA antibodies do not influence changes in PSA levels following vaccination. Assessing the systemic immune reaction to PSA might not accurately reflect the actual therapeutic immune response. However, studies by other investigators have not found a tumor-specific immune response induced by the PROSTVAC vaccine in PCa treatment. J. K. Parsons et al. (70) explored clinical indicators for active monitoring of immune response to PROSTVAC vaccine and disease progression in patients with localized PCa. The research involved 154 males diagnosed with low to moderate-risk PCa. Included patients were randomly divided (2:1) into treatment and control groups, receiving PROSTVAC vaccine and empty fowlpox vector (EV) respectively. The study results showed that PROSTVAC did not elicit a better response in prostate tissue or peripheral T cells compared to EV. Similarly, D. G. McNeel et al. (41) assessed the effectiveness of the vaccine in CRPC patients undergoing docetaxel chemotherapy. Eight patients with mCRPC received treatment, but no immune responses targeting PSA-specific CD4+ and CD8+ T cells or PSA-specific IgG antibodies were observed. Further research is required to validate the effectiveness of the PROSTVAC vaccine for treating PCa.
Multi-target viral vaccines for PCa treatment have also been developed and used in clinical studies. Researchers have also developed a multi-target viral vaccine for PCa treatment. M. Bilusic et al. (59) developed an innovative viral vaccine utilizing adenovirus 5 (Ad5) carriers to target three TAAs: PSA, brachyury, and MUC-1. These transgenes have epitope alterations that enhance the activation of CD8+ T cells. Safety and efficacy were determined through a Phase 1 clinical study (NCT03481816). A total of eighteen individuals diagnosed with mCRPC participated in the study and were administered at least one dose of the vaccine. The participants responded positively to the Ad5 PSA/MUC-1/brachyury vaccine. Primary endpoint was met without dose-limiting toxicity. The vaccine showed clinical activity, including partial responses and PSA responses in five patients. Three patients with prolonged PSA response received palliative radiotherapy. Additional research is required to assess the therapeutic advantages and immune response of this vaccine when used alongside other immuno-oncology medications.
In addition, PROSTVAC is being tested in combination with other strategies for PCa. The E9802 trial is a Phase 2 study involving multiple institutions, aiming to assess the safety and effectiveness of PROSTVAC in combination with ADT for patients without significant metastasis but experiencing PSA progression (40). Of the 27 patients, 20 achieved complete response at 7 months. This study confirms that the viral PSA vaccine can be used in patients with smaller disease volumes and that combined with ADT may result in better disease response rates. This information provides evidence for the potential benefits of implementing vaccine treatment at an early stage of PCa in future research, aiming to lessen the impact of the disease. Samarium-153-ethyleneenetetramethylene phosphonate (Sm-153-EDTMP) is a radiopharmaceutical that binds to osteoblast lesions and releases beta particles that can cause local tumor cell destruction. C. R. Heery et al. (45) tried Sm-153-EDTMP combined with PROSTVAC for the treatment of mCRPC. In this multicenter trial’s 2 phase, patients were randomly assigned to receive either Sm-153-EDTMP by itself or in conjunction with the PROSTVAC vaccine. The study findings indicated that the median PFS for Sm-153-EDTMP was 1.7 months for the Sm-153-EDTMP group and 3.7 months for the combination group, with a statistically significant difference (P = 0.041). None of the patients in the Sm-153-EDTMP group experienced PSA reductions greater than 30%, whereas three out of four patients in the combined group had PSA reductions exceeding 50%. The findings offer a theoretical foundation for utilizing novel radiopharmaceuticals in conjunction with PROSTVAC for treating PCa. However, a recent study confirmed that androgen receptor antagonist (ARAs) flurtamide combined with PROSTVAC does not improve outcomes in patients with nmCRPC (68). Therefore, more RCT studies are needed in the future to confirm the value of the viral vaccine combined with anti-male treatment strategy in the treatment of PCa.
PF-06753512(PrCa VBIR) is a hybrid viral vaccine based for the treatment of PCa. The PrCa VBIR combines the functions of a vaccine and an immune checkpoint inhibitor, consisting primarily of an adenovirus vector that expresses PSA, PSMA, prostatic stem cell antigen (PSCA), and tremelimumab. K. A. Autio et al. (66) investigated the efficacy of PF-06753512 in mCRPC through Phase I clinical trial. The study involved 91 participants, with PSMA eliciting a response from 88.0% of antigen-specific T lymphocytes, PSA from 84.0%, and PSCA from 80.0%. PrCa VBIR generally showed similar safety signals as other ICIs combination tests. Significant side effects were found in some patients with biochemical relapse. It triggered immune response against specific antigens in all groups and showed some effectiveness in fighting tumors in patients experiencing biochemical relapse without ADT. This study suggests that hybrid virus vaccines have potential in the treatment of PCa.
4 Immune checkpoint inhibitors are used in the treatment of PCa
4.1 ICIs
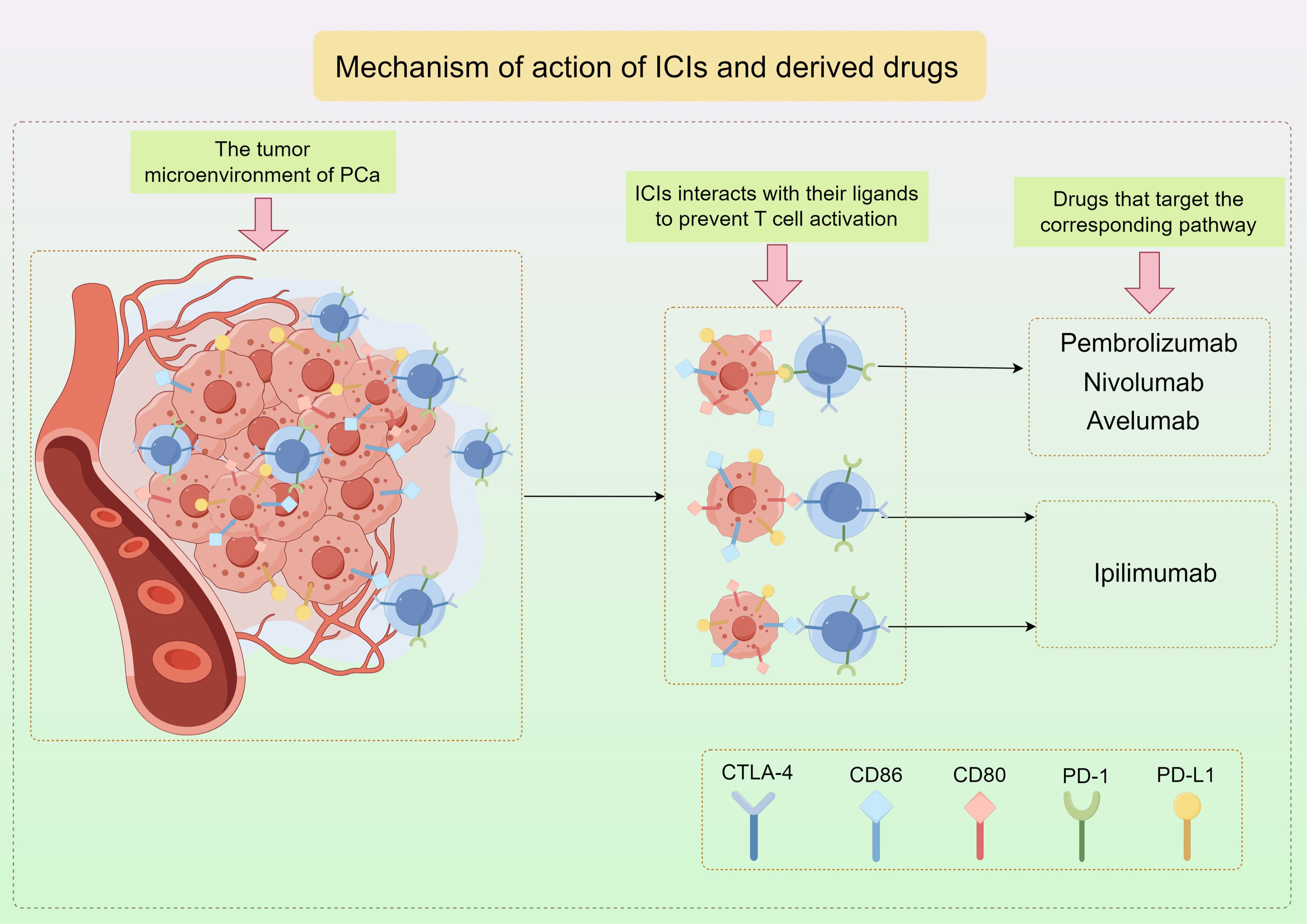
PD-1 is found on activated T cells and NK cells, and when it binds to PD-L1, it can block T cell activation, converting naive T cells into Tregs, which helps prevent the immune system from attacking normal cells in a specific immune response (84–86). Tumor cells that have PD-L1 utilize this pathway in order to evade the immune response against tumors by T cells. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), a protein found on T cells similar to PD-1, interacts with CD80 and CD86 ligands to prevent T cell activation (Figure 3) (87). Consequently, therapies targeting these pathways have become more prevalent in contemporary cancer treatment. Immunotherapy targeting checkpoints has greatly enhanced the management of numerous solid tumors, notably lung cancer, melanoma, and renal cell carcinoma (88–93).
4.2 Clinical study of ICIs in PCa
The utilization of ICIs in PCa presents a significant challenge due to the inherent weakness of the immune response in PCa and the low TMB (94). Tumors characterized by a high mutation burden are more likely to exhibit a favorable response to ICIs due to the presence of neoantigens that can be recognized by reactivated T cells, whereas tumors with a low mutation burden typically demonstrate restricted clinical efficacy. This means that fewer immune cells, including T cells, enter the tumor tissue. In addition, the hypoxic region of the TME in PCa leads to poor infiltration ability of T cells into tumor tissue. These hypoxic regions inhibit T cell function through multiple mechanisms, including acidic pH, nutrient depletion, increased adenosine expression, and PD-L1 (95, 96), and also promotes phenotypic transformation of immature myeloid cells into myeloid suppressor cells (MDSCs) and tumor-related macrophages, which made the immunosuppressive characteristics of the TME more obvious (95). However, recent studies have suggested that therapy directed at PD-L1 may hold potential for the treatment of PCa. The expression of PD-L1 in PCa tissues plays a crucial role in determining the suitability of immune checkpoint inhibitors for PCa therapy. Several preclinical research studies have examined the expression of PD-L1 in PCa samples to assess the effectiveness of checkpoint inhibitor treatment in individuals with PCa. A study utilizing immunohistochemistry (IHC) to evaluate tumor scores on 402 prostatectomy samples found that 92% (371/402) exhibited positive PD-L1 staining in tumor epithelial cells, while 59% (236/402) had high intensity PD-L1 scores. While there was a strong correlation between high-density PD-1 + lymphocytes and reduced clinical survival, this research did not discover a significant link between PD-L1 expression and the prognosis of PCa (97). A separate research study utilizing primary PCa samples from two distinct cohorts revealed that, 50 to 60 percent of cases exhibited moderate to high levels of PD-L1 expression in immunohistochemistry staining. The presence of PD-L1 positivity was determined to be a prognostic indicator for biochemical recurrence through multivariate Cox analysis (P= 0.007) (98). In conclusion, ICIs pose distinct challenges and hold considerable potential in the management of PCa. Recent research endeavors have focused on the application of ICIs targeting PD-1/PD-L1 and CTLA-4 in the treatment of PCa, demonstrating encouraging preliminary outcomes (Table 2).
4.2.1 CTLA4 inhibitors
Ipilimumab is a monoclonal antibody targeting CTLA-4, which can effectively block the binding of CTLA-4 to its ligand and inhibit the tumor-killing effect of T cells. E. D. Kwon et al. (100) examined the efficacy of ipilimumab following radiotherapy in individuals with advanced CRPC who experienced progression following docetaxel treatment (NCT00861614). A total of 799 patients were assigned in a random manner, with 399 receiving ipilimumab and 400 receiving a placebo. Following a brief monitoring period, the ipilimumab group did not show a significant difference in median OS compared to the placebo group (11.2 months vs. 10.0 months, P= 0.053). Immune-related adverse events were the most frequent grade 3-4 occurrences, with 101 patients (26%) experiencing them in the ipilimumab group compared to 11 patients (3%) in the placebo group. The toxic effects of the study drug resulted in four deaths (1%), all of which were in the ipilimumab group. Preliminary analyses showed no significant difference in OS between the ipilimumab and placebo groups. Subsequently, Fizazi et al. (102) conducted a comprehensive study on this group over an extended period of time. The ipilimumab group had a higher overall survival rate compared to the placebo group after 2 years (25.2% vs 16.6%) and 3 years (15.3% vs 7.9%). Comparing the rates at four years (10.1% vs 3.3%) and five years (7.9% vs 2.7%). The primary cause of death in seven patients (1.8%) in the ipilimumab group and one patient (0.3%) in the placebo group was reported to be drug toxicity during the study. After an extended period of observation, individuals who received ipilimumab exhibited improved survival outcomes, showing a 2-3 fold increase in OS rates at 3 years and beyond. Furthermore, T. M. Beer et al (101) evaluated the efficacy of ipilimumab in the treatment of mCRPC at the initial stage of chemotherapy without visceral metastasis. Patients in this Phase III trial were randomly allocated to receive either ipilimumab or a placebo in multiple centers. The study included 399 patients who were administered ipilimumab and 199 patients who were given a placebo. The results revealed that there was no significant difference in the short-term median OS between the group receiving ipilimumab and the control group (28.7 months vs 29.7 months, P=0.3667). However, the median PFS in the ipilimumab group was found to be significantly higher than in the placebo group (5.6 months vs 3.8 months, P < 0.05). In the ipilimumab group, 9 patients (2%) died due to treatment-related adverse events. No fatalities were reported in the control group. Grade 3-4 adverse events associated with immunity were observed in 31% and 2% of patients, respectively. Research indicates that ipilimumab may enhance OS in the short term for individuals with mCRPC, while also potentially increasing long-term survival rates. However, the incidence of treatment-related adverse events is higher.
Previous studies have not shown a significant benefit of Ipilimumab monotherapy in the treatment of mCRPC. Researchers have explored the potential synergistic effects of combining CTLA-4 and PD-1 monoclonal antibodies for the treatment of mCRPC. P. Sharma et al. (103) reported the largest trial of ipilimumab combined with nivolumab for mCRPC treatment (NCT02985957). The cohort had a median follow-up time of 11.9-13.5 months with 45 participants, and achieved an objective response rate of 10%-25%. Metastatic PCa that expresses the androgen receptor variant 7 (AR-V7) is associated with a poor prognosis, limited therapeutic options, and reduced survival rates. E. Shenderov et al. (104) combined ICIs in the treatment of AR-V7-expressed mCRPC. This study was a cohort, non-randomized phase 2 study. Ipilimumab and nivolumab were administered to a total of thirty patients. The study results indicated that the combination of nivolumab and ipilimumab had limited effectiveness in PCa patients with AR-V7 expression, leading to a decision not to pursue further investigation. Therefore, the combination of ICIs in the treatment of mCRPC has not achieved significant efficacy, which may be due to the complex immunosuppressive microenvironment of PCa.
Ipilimumab in combination with the sipuleucel-T strategy for mCRPC was also evaluated. Investigators explored whether administration of ipilimumab following sipuleucel-T treatment could alter immune and/or clinical response to this treatment (63). A total of 50 mCRPC patients enrolled in this clinical trial (NCT01804465) received ipilimumab immediately after completion of sipleul-T or a 3-week delay. The research discovered that the mixture was easily endured without any unforeseen negative occurrences. Clinical responses were observed in 6 of the 50 patients, 3 of which lasted longer than 3 months. There was no significant correlation between the length of time ipilimumab was used and the clinical response or toxicity. The use of multiple treatments did lead to the activation of CD4 and CD8 T cells, especially in the early treatment schedule. Similarly, C. Jochems et al. (99) reported the clinical results of a Phase I trial that combined ipilimumab with a PROSTVAC vaccine in patients with mCRPC. Ipilimumab and PROSTVAC vaccine were administered to a total of thirty patients. PSA levels decreased in 58% of the 24 patients who had not received chemotherapy before. The concurrent therapy did not worsen immune-related side effects linked to ipilimumab. The middle OS duration was 2.63 years, ranging from 1.77 to 3.45 years. This confirms that the use of ICIs in combination with cancer vaccines has the potential to improve patient outcomes. However, larger clinical trials of immunotherapy are needed for further evaluation.
4.2.2 PD-1/PD-L1 inhibitors
4.2.2.1 Pembrolizumab
Pembrolizumab, a monoclonal antibody of the IgG4 class, blocks the PD-1 receptor to treat cancer through immunotherapy. As a PD-1 inhibitor, it has shown obvious antitumor efficacy in several solid tumors. Pembrolizumab has shown antitumor activity against PD-L1-positive mCRPC. J. N. Graff et al. (106) reported surprising effectiveness against tumors in patients with mCRPC who were given pembrolizumab. Patients were administered pembrolizumab, resulting in a rapid decrease in PSA to ≤ 0.2 ng/ml for three out of the initial 10 patients. Two of the three responders had baseline tumor biopsies. Immunohistochemistry showed leukocyte infiltration and PD-L1 expression of CD3+, CD8+ and CD163+. This study suggests that pembrolizumab applied to mCRPC can induce a favorable tumor-specific immune response and inhibit tumor progression. Similarly, E. S. Antonarakis et al. (107) evaluated the antitumor activity and safety of pembrolizumab in mCRPC, and obtained good curative effect. In the KEYNOTE-199 Phase II trial, three cohorts of patients with mCRPC were treated with docetaxel along with one or multiple targeted hormonal treatments. All patients received pembrolizumab every 3 weeks for a total of 35 cycles. A total of 258 patients were included. Disease control rates ranged from 10%-22%, and median survival ranged from 7.9-14.1 months. In patients with bone-dominant mCRPC who had prior treatment with doxetaxel and targeted endocrine therapy for solid tumors, Pembrolizumab alone demonstrated effectiveness against tumors and was deemed safe, according to the research. The observed response appears to be persistent, and OS estimates are encouraging. The surprising and powerful response seen in the above study should be re-examined for PD-1 inhibition of PCa.
A study demonstrated that patients with PCa who showed resistance to the novel androgen receptor inhibitor enzalutamide displayed elevated levels of PD-L1 expression on circulating immune cells (119). The researchers theorized that introducing PD-1 inhibition to these individuals may trigger a significant cancer reaction. In a Phase II study conducted by J. N. Graff et al. (108), the combination of enzalutamide and pembrolizumab was assessed in a cohort of 28 patients with mCRPC. In 5 of the 28 patients (18%), PSA decreased by 50% or more. Three of the 12 patients (25%) with measurable disease at baseline achieved an objective response. Median OS was 21.9 months for all patients (95% CI: 14.7 to 28.4 months) and 41.7 months for responders. This study showed that pembrolizumab was active in mCRPC when enzalutamide was added. The response is deep and long-lasting, and does not require defects in tumor PD-L1 expression or DNA repair. Besides, A. E. Ross et al. (109) evaluated the safety and feasibility of combination pembrolizumab and androgen deprivation in the treatment of metastatic hormone-sensitive PCa. The study included 12 patients with newly diagnosed minimally metastatic PCa. The individual received a complete prostate cryoablation along with temporary ADT and pembrolizumab. The findings indicated that 42% (5 out of 12) of individuals exhibited PSA levels below 0.6 ng/mL after one year, with only 2 of them showing normalized testosterone levels at that time. The median duration of PFS was 14 months, while the median duration of survival without systemic therapy was 17.5 months. Immunohistochemistry did not detect PD-L1 expression in patients with assessable tissue. Patients with metastatic PCa can tolerate total prostate cryoablation along with short-term androgen deprivation and pembrolizumab without any safety issues. While the localized disease appears to be treated effectively in most men, the regimen only leads to sustained disease control in a few cases after testosterone recovery.
In the clinical setting, docetaxel is frequently selected for patients with mCRPC who have become resistant to abiraterone or enzalutamide and need a more potent therapy. E. Y. Yu et al. (110) assessed the effectiveness and safety of pembrolizumab in combination with docetaxel for treating patients with mCRPC. Among the 104 treated patients, the confirmed PSA response rate was 34% and the confirmed ORR was 23%. Median radiologic progression-free survival (rPFS) and OS were 8.5 months and 20.2 months, respectively. The combination demonstrated a manageable safety profile in this patient population. The combination of pembrolizumab and docetaxel demonstrated effectiveness against tumors in mCRPC patients who had not received chemotherapy and were being treated with abiraterone or enzalutamide. To determine whether pembrolizumab therapy after treatment with (177) Lu-Prostate-specific membrane antigen (PSMA)-617 is safe and induces lasting clinical benefit, R. Aggarwal et al. (111) conducted a Phase 1 study. An objective response was confirmed in 25 of the 43 patients included. Two of the 43 patients (5%) had grade 3 or more severe treatment-related adverse events. After pembrolizumab maintenance therapy, a sole administration of (177) Lu-PSMA-617 was well-tolerated and showed promising early effects in individuals with metastatic castration-resistant PCa. The above study suggests the potential value of pembrolizumab in combination with chemoradiotherapy for mCRPC treatment, and more randomized controlled studies are needed to confirm their efficacy.
Pembrolizumab and olaparib showed single-agent activity in previously treated mCRPC patients. E. Y. Yu et al. (114) evaluated the effectiveness and safety of combining pembrolizumab with olaparib for treating mCRPC. The study ended with 102 patients receiving treatment. The average duration from initial administration to the end of data collection was 24 months. The effective rate of diagnosed PSA was 15%. Among patients with detectable disease, the verified overall response rate was 8.5%, with 5 cases of partial remission. The median PFS rate was 4.5 months (95%CI: 4.0-6.5), while the median OS was 14 months (95%CI: 10.4-18.2). Consistency was observed in the clinical activity of subgroups with PD-L1 positivity and mutations in homologous recombination repair. Ninety-three patients (91%) experienced adverse events associated with treatment. The research indicated that the safety profile of pomerzumab combined with olaparib was similar to that of a standalone treatment and exhibited anti-cancer effects in mCRPC patients who had received prior docetaxel treatment without molecular selection. Similarly, E. S. Antonarakis et al. (112) evaluated the efficacy of pembrolizumab in combination with olaparib and a new generation hormone agent (NHA) for mCRPC without selected biomarkers in a Phase III clinical study. Subjects were assigned randomly (2:1) to receive either pembrolizumab combined with olaparib or NHA (abiraterone or enzalutamide). Pembrolizumab + olaparib was given to 529 participants through random assignment, while 264 participants received NHA. In the final rPFS and OS analysis, there was no difference between the two groups. This study showed that pembrolizumab combined with olaparib did not significantly improve rPFS or OS in heavily pretreated mCRPC patients with no biomarker selection compared to NHA. Besides, U. N. Vaishampayan et al. (113) conducted a Phase II study to evaluate the safety and efficacy of the combination of HER2 bispecific antibodies (HER2Bi) armed activated T cells (HER2 BAT) and pembrolizumab. Out of the total of 14 patients, five achieved the main goal of PFS at the 6-month mark, representing 38.5% (95%CI: 19.5% - 76.5%). The middle PFS was 5 months with a median survival of 31.6 months. The safety and good efficacy of the combination deserve further study.
Pembrolizumab in combination with the cancer vaccine is also being tested for mCRPC with promising results. D. G. McNeel et al. (64) reported a trial of MVI-816 administered simultaneously or sequentially to pembrolizumab over 12 weeks in patients with mCRPC. Of the 25 patients with measurable disease, a partial response was confirmed in 1 patient with microsatellite instability tumors. In 4/40(10%) patients, PSA decreased by >50%. The overall radiological PFS rate at 6 months was 47.2%. Thirty-two percent of patients did not progress in the trial beyond six months. The average survival time was 22.9 months with a confidence interval of 95% ranging from 16.2 to 25.6 months. The occurrence of immune-related adverse events was significantly associated with longer treatment duration (HR=0.42, P=0.003). The results of this study suggest that the combination of programmed cell death 1 blocking with MVI-816 is safe, increases tumor-specific T cells, and can lead to favorable 6-month disease control rates.
4.2.2.2 Nivolumab
In recent years, nivolumab, a monoclonal antibody of the human immunoglobulin G4 class, has been utilized in tumor immunotherapy by binding to the PD-1 receptor, disrupting its interaction with PD-L1 and PD-L2, and inhibiting the immunosuppressive effects of the PD-1 pathway. K. Fizazi et al. (116) investigated docetaxel combined with nivolumab in the treatment of mCRPC in a non-randomized, multi-cohort phase II trial (NCT03338790). The study included eighty-four patients with mCRPC who were starting chemotherapy. The ORR was confirmed at 40.0% (95% CI: 25.7-55.7) and the PSA response rate was confirmed at 46.9% (95% CI: 35.7-58.3). The median rPFS and OS were 9.0 months (95% CI: 8.0-11.6) and 18.2 months (95%CI: 14.6-20.7), respectively. Nivolumab in combination with docetaxel is clinically active in chemotherapy-naive mCRPC patients. In addition, nivolumab combined with rucaparib was found to be effective in patients with homologous recombination defect positive post-chemotherapy or chemotherapy-naive mCRPC, especially those with BRCA1/2 mutations (117). Similarly, in a study conducted by Z. Yuan et al. (115), a Phase I/II trial was undertaken to assess the safety and potential synergistic effects of combining brachytherapy with immunotherapy for PCa. The study aimed to investigate the feasibility, safety, and benefits of administering nivolumab in conjunction with high dose rate (HDR) brachytherapy and androgen deprivation therapy (ADT) in patients with PCa. A Phase I trial included six patients who were monitored for a minimum of 3 months following administration of Nivolumab. Overall, the combination of Nivolumab with ADT and HDR therapy was well received by patients. At one month after receiving four cycles of nivolumab and HDR brachytherapy, three patients (50%) exhibited an initial positive reaction, with no remaining tumors found. In early responders, there was an increase in CD8+ and FOXP3+/CD4+ T cells in tissues, while CD4+ effector T cells were elevated in the peripheral blood. The combination of Nivolumab, ADT, and HDR was well received and led to enhanced immune infiltration and anti-cancer effects.
Bipolar androgen therapy (BAT), a form of high-dose testosterone treatment given at intervals, is used as a treatment approach for patients with mCRPC. More recently, M. C. Markowski et al. (118) reported the results of a multicenter, single-arm Phase 2 study (NCT03554317) that included 45 heavily pretreated mCRPC patients. The patients received nivolumab following 3 cycles of BAT monotherapy. Following a typical observation period of 17.9 months, the median time to recurrence or progression was 5.6 months (95% CI 5.4-6.8) and the median OS was 24.4 months (95% CI 17.6-31.1). The study findings indicated that BAT/nivolumab was well received, with only five (11%) severe adverse events related to the drug. The data indicates that BAT could boost the immune response against tumors, especially when combined with ICIs. Furthermore, Neel et al. (69) evaluated the efficacy of nivolumab combined with pTVG-HP in patients with early recurrent PCa. PSA levels decreased by more than 50% in four out of 19 patients, representing 21% of the total. The median PSA doubling time was 5.9 months prior to treatment, increased to 25.6 months post-treatment (P=0.001), and then decreased to 9.0 months one year after treatment. The research validated the safety and immune-boosting effects of combining nivolumab with pTVG-HP vaccine, leading to a longer period before disease progression.
4.2.2.3 Avelumab
Avelumab, a PD-L1 antibody that is fully humanized, effectively inhibits the interaction between PD-L1 and PD-1, thus boosting the ability of T cells to kill tumors by counteracting the immunosuppressive effects of the TME. A. Rodriguez-Vida et al. (105) investigated the safety and efficacy of avelumab plus carboplatin in A single-arm Phase Ib study of mCRPC. The study consisted of 26 patients in total. 7.7% of patients had PSA response rate≥50%. The objective effective rate was 17.6%, and the complete effective rate was 1 case (5.9%). The middle radiological PFS was 6.6 months (95% CI: 4.28 and 9.01), while the middle OS was 10.6 months (95% CI: 6.68 and not reached). 73% of grade 3-4 adverse events related to treatment. The research showed that the combination of avelumab and carboplatin was safe and led to extended OS in a group of patients who had undergone extensive prior treatments. In addition, E. M. Kwan et al. (120) evaluated the effectiveness and safety of avelumab, when used alongside stereotactic ablative radiotherapy for managing mCRPC. This prospective Phase 2 study enrolled 31 patients with progressive mCRPC who had received at least one previous androgen receptor-directed therapy. The median follow-up was 18.0 months. The rate of disease control was 48% (95% CI: 30-67%), while the overall response rate was 31% with a confidence interval of 11-59%. The ORR of unirradiated lesions was 33% (95% CI: 10-65%). The median PFS rate was 8.4 months with a confidence interval of 95% from 4.5 to not reached, while the median OS rate was 14.1 months with a confidence interval of 95% from 8.9 to not reached. These studies indicate that Avelumab in combination with other oncology therapies has great potential in mCRPC, and more clinical studies are needed to confirm their efficacy and safety.
5 CAR-T cells in the treatment of PCa
5.1 CAR-T cells technology
While advancements have been made in the treatment of blood cancers, the efficacy of CAR-T technology in addressing solid tumor treatments remains limited. Hematologic malignancies commonly exhibit consistent target antigens across the majority of tumor cells, in contrast to solid tumors which display heterogeneous tumor antigens that can vary both within the same tumor location and between primary tumors and metastatic sites. It is difficult to select the ideal target antigen of solid tumor, which directly limits the specificity and effectiveness of CAR-T cells. Furthermore, the TME within solid tumors create a barrier that hinders the infiltration of CAR-T cells and the recognition of tumor antigens. Furthermore, numerous challenges remain for CAR-T cells to effectively penetrate tumor tissue and induce immune responses. On the one hand, metabolic disorders, including oxidative stress in TME, nutrient deprivation, hypoxia, and abnormal metabolic accumulation of acidic pH and metabolites, etc. Moreover, there are also factors that suppress the immune system, like molecules that suppress the immune response, such as TGF-β, IL-10, and other cytokines (121). Ultimately, the intrinsic inhibitory processes within T cells, including the enhancement of immune-suppressing receptors (PD-1, LAG-3, and TIM-3) or molecules, result in the suppression of T cell stimulation and potential functional depletion (122). These factors contribute to the limited success of CAR-T cell therapy in treating solid tumors and the lack of positive results in clinical trials.
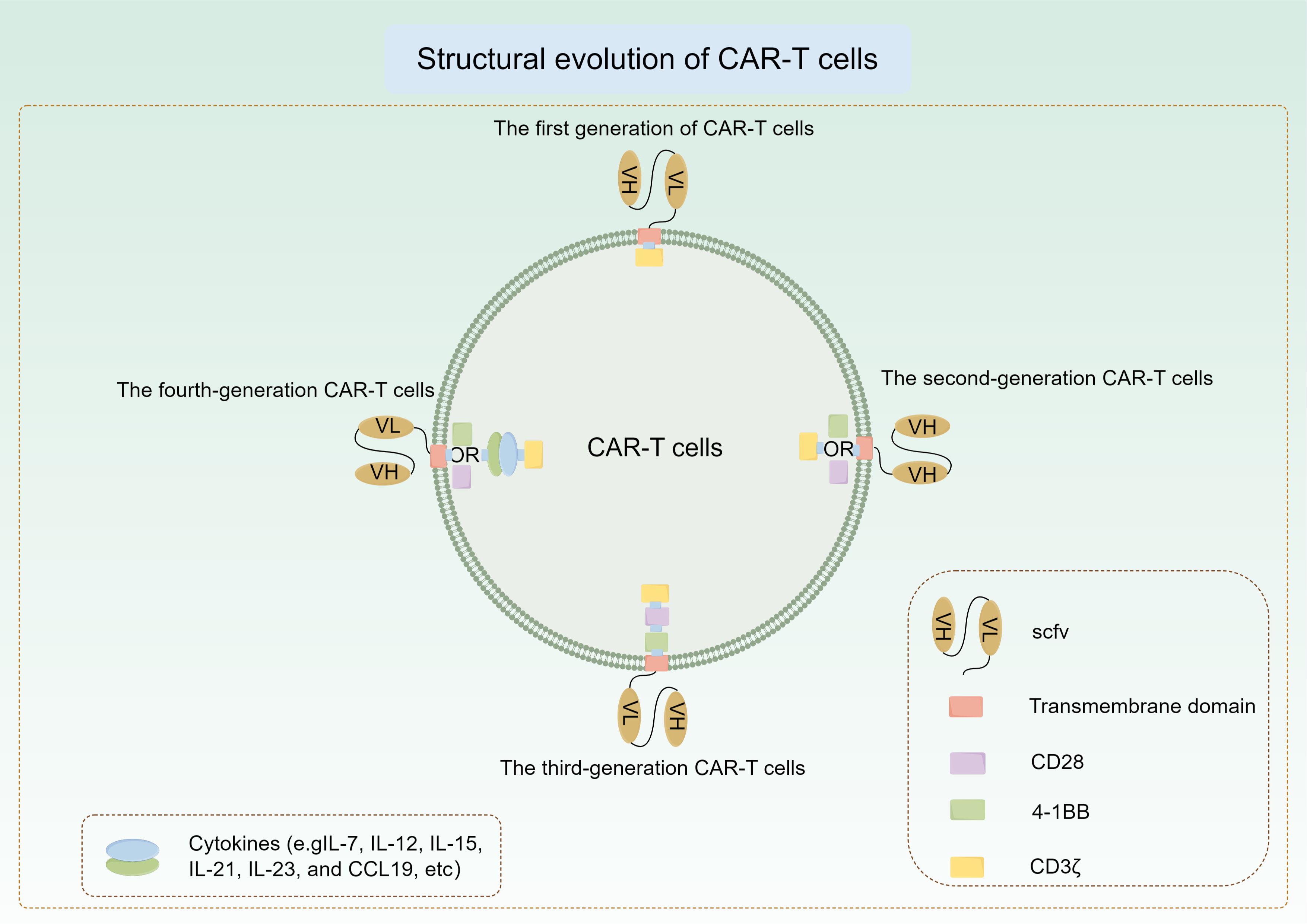
The first generation of CAR-T relies on CD3ζ to mediate the activation of T cells. This kind of CAR-T lacks intracellular costimulatory signal and cannot provide long-term T cell expansion signal, so the clinical efficacy is limited (123). The second-generation CAR-T cells were created by incorporating the co-stimulatory molecule CD28 into the intracellular domain of the initial CAR-T cells, resulting in a notable rise in the production of IL-2 and other cytokines when compared to the first-generation CAR-T cells (124). After that, the researchers added a co-stimulatory molecule(4-1BB) to the second generation to produce the third generation of CAR-T cells. Research has shown that the expansion, extended viability, cytokine release, and elimination of tumors by advanced CAR-T cells containing extra co-stimulatory regions are greatly enhanced following antigen activation (125). In recent years, in order to deal with the problems encountered in CAR-T therapy, researchers have made a series of modifications to its structure. Specific cytokine gene fragments are inserted into the intracellular region of CAR-T cells to enable the production and release of particular cytokines upon activation. This process aims to boost T cell proliferation and activation, enhance non-specific anti-tumor immune responses, promote CAR-T cell infiltration into tumors, and ultimately enhance treatment outcomes. Cytokines like IL-7, IL-12, IL-15, IL-21, IL-23, and CCL19 are examples of these molecules (126). These CAR-T cells are also known as fourth-generation CAR-T cells (Figure 4). Continuous enhancements in structural optimization and functional modifications have increased the effectiveness and safety of CAR-T cells, making the tailored design of CAR-T cells for various types of tumors a current focus of research.
5.2 Potential targets and related research
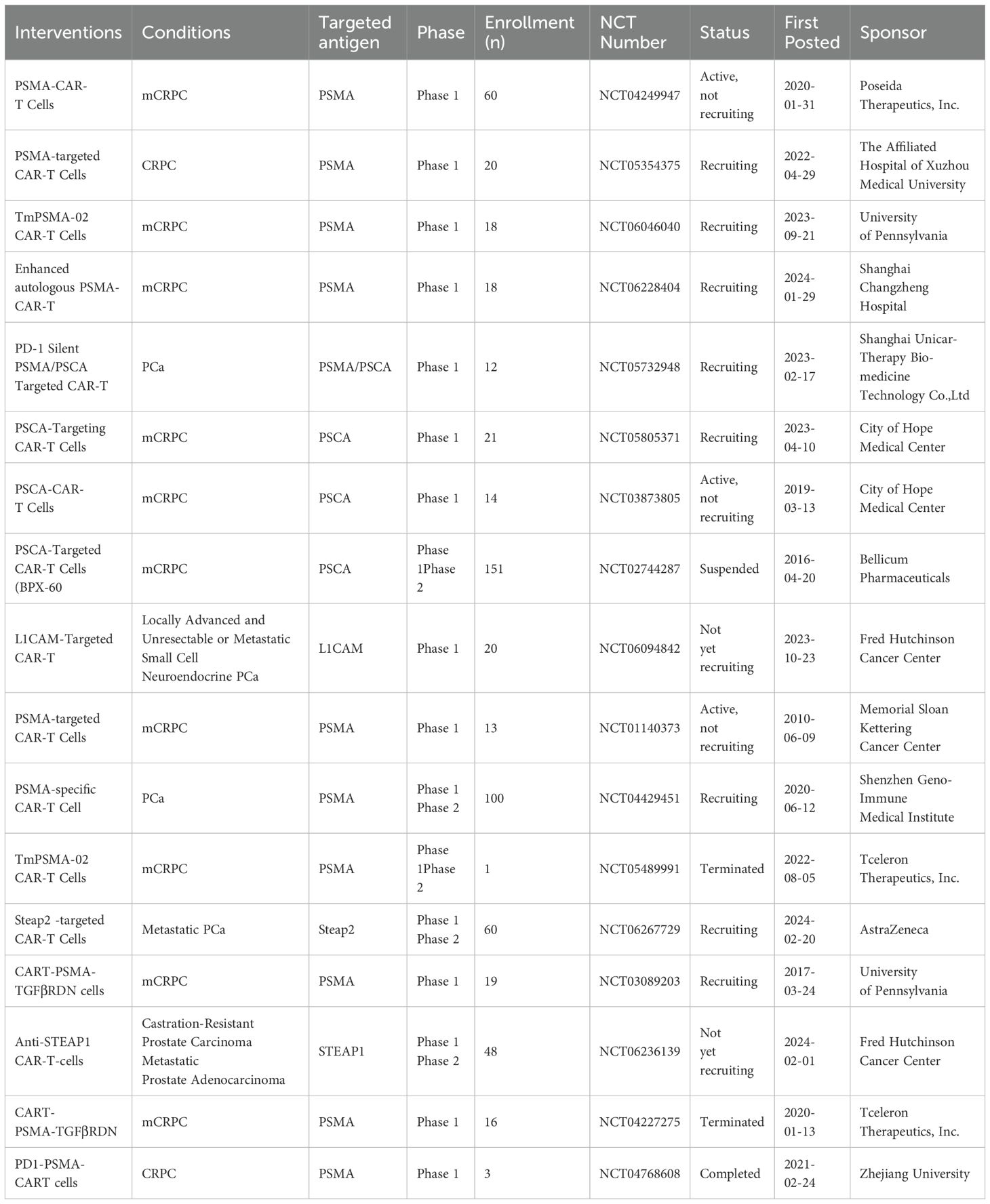
The notable efficacy of CAR-T cells in preclinical studies targeting various antigens has prompted an increase in clinical trials aimed at treating PCa (Table 3). The clinical trials published so far on CAR-T cells targeting PSMA and PSCA for PCa treatment have primarily concentrated on these specific targets, but have not achieved significant advancements (127). At present, the main problems are the inhibition effect of PCa microenvironment on CAR-T cells and the lack of specific targets. To address this dilemma, researchers try to optimize the function of CAR-T cells by using gene modification strategies, in order to achieve significant success in the treatment of PCa. Furthermore, researchers are currently exploring the development of novel targets using proteomics technology to keep pace with the swift integration of CAR-T cells in the treatment of PCa. Among them, STEAP1, STEAP2 and F77 have been used as new targets for CAR-T therapy of PCa, and clinical studies of CAR-T cells targeting STEAP1 and STEAP2 have also begun.
5.2.1 PSCA
PSCA, a protein found on the surface of prostate epithelial cells, as well as in primary and metastatic PCa cells, presents a potential target for immunotherapeutic interventions in PCa. V. Hillerdal et al. (128) developed a third generation CAR targeting PSCA, including the CD28, OX-40, and CD3ζ signal domains. It was confirmed that PSCA-CAR-T cells exhibited targeted secretion of IFN-γ and IL-2. Furthermore, PSCA-CAR-T cells efficiently eliminated cancer cells that displayed PSCA in a laboratory setting, and the use of PSCA-CAR-T cells notably slowed the progression of tumors implanted under the skin and extended the lifespan of mice. The PD-1/PD-L1 pathway is widely recognized as a crucial mechanism used by cancer cells to evade destruction by CAR-T cells through immunosuppression. J. E. Zhou et al. (129) developed third-generation PSCA-CAR-T cells with PD-1 suppression through shRNA gene silencing, aiming to boost CAR-T cells’ ability to fight tumors by inhibiting the PD-1/PD-L1 pathway. PD-1-silenced PSCA-CAR-T cells exhibited superior cytotoxicity and cytokine secretion compared to regular CAR-T cells at an effector-to-target cell ratio of 8:1. Within one week, tumor volume was significantly decreased in tumor models that received treatment with PSCA-CAR-T cells with PD-1 silenced. The research demonstrates that using shRNA to silence PD-1 is a successful approach in inhibiting the PD-1/PD-L1 immune suppression pathway and improving the effectiveness of CAR-T cells in subcutaneous xenograft treatment. Current adjuvant treatment strategies for bone mCRPC have been largely unsuccessful. J. S. Frieling et al. (130) investigated the efficacy of PSCA-CAR-T cells in the treatment of bone mCRPC. PSCA-CAR-T cells in preclinical mouse models of bone mCRPC led to quick and substantial tumor regression, as well as improved survival and decreased bone disease associated with cancer. The data provided evidence for the effectiveness of PCA-CAR-T cell therapy in managing mCRPC.
5.2.2 PSMA
PSMA, a transmembrane glycoprotein found on the cell membrane, is highly expressed in PCa. As a molecular target in PCa, it has been extensively utilized and researched for the last twenty years. Similarly, it is a potential target for the application of CAR-T cell technology in PCa treatment. J. Alzubi et al. (131) designed a CAR that recognizes PSMA and applied this CAR-T cell to a preclinical PCa model. PSMA CAR-T cells were administered via local injection in a preclinical mouse model, leading to the eradication of established human PCa xenografts in vivo. Moreover, the co-administration of systemic intravenous CAR-T cells with non-ablative low-dose docetaxel chemotherapy demonstrated significant efficacy in inhibiting tumor growth, the outcome that was not observed with either docetaxel or CAR-T cells as standalone treatments. To enhance the performance of PSMA CAR-T cells, D. Wang and colleagues developed a group of PSMA CAR-T cells that were genetically altered to include IL23 (IL23-PSMA-CAR-T) and examined their performance in a mouse experiment (132).During the co-culture trial, the IL23-PSMA-CAR-T cells exhibited a notably greater proliferation rate compared to the control CAR-T cells. IL23-PSMA-CAR-T cells exhibited a markedly elevated production of cytokines compared to the other CAR-T cell groups. IL23-PSMA-CAR-T cells demonstrated superior functionality in NSG mouse models compared to other CAR-T cell groups, leading to tumor eradication starting on day 14 post T cell infusion, with immediate weight recovery observed. There was a notable increase in CD45RO+ CD8+ T cells and CD127+ CD4+ CAR-T cells in IL23-PSMA-CAR-T mice. The results indicate that the role of PSMA-CAR-T modified with IL-23 has great potential in the eradication of PCa.
CAR-T cells face difficulties in solid tumors due to the presence of immunosuppressive TME that contain elevated amounts of various inhibitors, such as TGF-β, T cells immunoglobulin, and mucin domain 3 (TIM3). L. Tang et al. (133) constructed PSMA-CAR-T cells targeting both TIM3 and TGFβ in order to improve their tumor-killing ability. In vitro and animal experiments were conducted to assess the cytotoxicity of TIM3/TGF-PSMA-CAR-T cells. Exogenous TGF-β and TIM3-activating antibodies were discovered, leading to the successful elimination of PSMA-positive PCa cells by TIM3/TGF-PSMA-CAR-T cells. Furthermore, when transplanted into immunodeficient mouse tumor models in vitro, these cells demonstrated the capacity to eradicate tumor tissue and extend survival without causing notable toxic reactions. Similarly, V. Narayan et al. (134) reported the results of a phase 1 clinical trial of PSMA-CAR-T cells with TGF-β receptor targeting function (NCT03089203) for PCa treatment. Thirteen patients were eventually treated with TGF-β PSMA-CAR-T cells. In one patient, there was significant expansion of cloned CAR-T cells and a >98% reduction in PSA. In the other three patients, PSA decreased by≥30% and CAR-T cell failure was accompanied by the upregulation of multiple TME location-inhibiting molecules after adoptive cell metastasis. Research indicates that it is possible and promising to use gene editing techniques to enhance the ability of PSMA-CAR-T cells to destroy tumors in PCa.
5.2.3 NKG2D
NKG2D ligand is a receptor that activates NK cells and is predominantly found on numerous cancer cells, such as PCa, while typically being absent or expressed minimally in normal tissues (135, 136). Numerous CAR utilizing NKG2D have been created and researched for their diverse therapeutic benefits in combating different types of tumors (137). C. He et al. (138) developed NKG2D-CAR-T cells that co-express IL-7 and utilized them in the treatment of PCa to enhance therapeutic efficacy by leveraging the co-expression of IL-7. The findings indicated that NKG2D-CAR-T cells exhibited markedly enhanced cytotoxicity against PCa in both laboratory and animal studies compared to T cells that had not been genetically modified. Furthermore, IL7-NKG2D-CAR-T cells exhibited superior tumor-fighting capabilities and effectively suppressed tumor growth in xenograft models. The findings suggest that targeting NKG2D with CAR-T cells holds promise for treating PCa, and the inclusion of IL-7 may enhance the effectiveness of NKG2D-based CAR-T cell therapy, offering a novel approach for adoptive cell therapy in PCa.
5.2.4 B7-H3
B7-H3, an immune-suppressing molecule, is found at increased levels in various cancer types such as pancreatic ductal adenocarcinoma, ovarian cancer, lung cancer, clear cell kidney cancer, and PCa (139, 140). Elevated B7-H3 expression levels in PCa are associated with high gleason scores, advanced stages, metastases, and poor patient outcomes (141). Crucially, B7-H3 is expressed very little in healthy tissues (142). New research has uncovered compelling proof that B7-H3 controls immune responses mediated by T cells and that blocking B7-H3 could result in the proliferation of T cells (142). S. Li et al. (143) developed CAR-T cells specifically targeting B7-H3 to explore their tumor-killing potential against PCa in vitro and in vivo. B7-H3 CAR-T cells efficiently suppress PCa growth through antigen-specific mechanisms. Furthermore, tumor cells have the ability to stimulate the growth of CAR-T cells in a laboratory setting and produce elevated amounts of IFN-γ and TNF-α cytokines. The findings suggest that B7-H3 could be a promising focus for treating PCa, backing the advancement of B7-H3-targeted CAR-T cells for PCa treatment. New research indicates that radiation treatment (RT) increases the levels of B7-H3 in PCa stem cells (PCSCs). Y. Zhang et al. (144) investigated whether B7-H3-CAR-T cells could target anti-RT PCSCs in vitro and in vivo. B7-H3 expression was discovered to be higher on PCSCs compared to PCa cells, resulting in B7-H3 CAR-T cells exhibiting greater cytotoxicity towards PCSCs than PCa cells. Furthermore, RT markedly increased the levels of B7-H3 in both PCSCs and PCa cell+s. The RT and B7-H3 CAR-T cells combination proved to be more potent in suppressing the growth of hormone-insensitive PCa xenografts in immunodeficient mice compared to using RT or CAR-T cells individually. The above studies indicate that utilizing CAR-T cells directed at B7-H3 could serve as a valuable supplementary treatment for late-stage PCa.
5.2.5 STEAP1/2
Prostatic six transmembrane epithelial antigen 1 (STEAP1) was first identified more than 20 years ago and is thought to be highly expressed in PCa. In more than 80% of mCRPC cases invading the bone or lymph nodes, as well as in numerous other types of cancer, there is a high level of expression of STEAP1 (145). STEAP1 belongs to the STEAP family of metal reductases and can form auto trimers or heterotrimers with other STEAP proteins (146). The functional role of STEAP1 in enhancing cancer cell proliferation, invasion, and epithelial-to-mesenchymal cell transformation is well established (147–149). The limited expression of STEAP1 in normal tissues makes it a very attractive target for cancer therapy. STEAP1 is a cell surface antigen used in the targeted therapy of PCa. CAR-T cells that target STEAP1 demonstrated responsiveness even at low levels of antigen and exhibited effectiveness against tumors in models of metastatic PCa (150). Besides, the large-scale genomics and proteomics research have identified prostatic six transmembrane epithelial antigen 2 (STEAP2) also can be used as a superior PCa treatment target antigen (151, 152). Consistent with this finding, the study showed that STEAP2 is richly expressed in all stages of PCa and can be used as a prognostic biomarker due to its association with the gleason score (153–155). P. Zanvit et al. (156) prepared STEAP2-targeting CAR-T cells (AZD0754) for the treatment of PCa. AZD0754 showed potential effectiveness in mouse models with patient-derived xenografts expressing STEAP2, along with promising preclinical safety results. The data highlight the value of STEAP1 and STEAP2 in the treatment of PCa and their potential application as adjunctive therapies for advanced PCa.
5.2.6 F77
F77, a distinctive carbohydrate antigen found on both androgen-dependent and androgen-independent PCa cells, could serve as a promising target for immunotherapy. Immunohistological research indicated the absence of F77 expression in healthy colon, kidney, cervix, pancreas, lung, skin, and bladder tissues, validating the restricted overexpression of the F77 antigen in PCa (157). P. Grover et al. (158) constructed F77 targeting CAR-T cells with CD28 and 4-1BB co-stimulatory signals. F77-CAR-T cells eliminate tumor cells by releasing cytokines in a manner that relies on F77 expression. The F77-CAR-T cells specifically targeted and eliminated PCa in a human xenograft model with PC3 cells. The results validate F77 as a potential target for immunotherapy in PCa and other cancers characterized by this unusual carbohydrate pattern.
6 The dilemma and promise of PCa immunotherapy
In recent years, immunotherapy has significantly altered prevailing perspectives on cancer treatment and fostered a profound comprehension of tumor immunotherapy among the general populace. Cancer vaccines, ICIs, and CAR-T therapy function by stimulating the body’s adaptive immune response to recognize tumor antigens, leading to the eradication of malignant cells and the potential for tumor remission. Numerous basic and clinical studies have been undertaken to investigate the efficacy of the aforementioned strategies in the treatment of PCa, resulting in notable advancements. Cancer vaccines represented by sipuleucel-T are approved by the US FDA for the treatment of asymptomatic or mild mCRPC (37). Secondly, ICIs combined with other therapeutic strategies have achieved initial efficacy in the treatment of mCRPC, which is manifested in effective disease control rate and better OS. In addition, CAR-T cells has shown good tumor-killing efficacy in preclinical studies of PCa. At present, more clinical studies have been carried out, and most of the reported research results have shown good tolerance, and found the tumor immune response induced by CAR-T cells. However, no complete cure for PCa has been found.
Immunotherapy strategies in the treatment of advanced PCa offer promise but also face difficulties. The treatment of solid tumors should not only target cancer cells, but also focus on TME. TME in solid tumors first constitutes a physical barrier, affecting the infiltration of immune cells and the recognition of tumor antigens. In addition, immune cells need to overcome immunosuppressive TME to exert tumor immune effect (117). Secondly, PCa is called “cold tumor” because of its weak immune response function and low TMB (94). This means that fewer immune cells enter the tumor tissue. This may be the reason why cancer vaccines and ICIs have not shown complete efficacy in most clinical studies of PCa treatment (39, 71, 100, 101). Due to the tumor heterogeneity of different individuals with solid tumors, immunotherapy strategies applied to PCa should select an adaptive patient population, and then optimize the dose of drugs to achieve the best clinical response rate. Therefore, tumor immunotherapy strategies need to pay more attention to the patient population to which they are adapted while optimizing function. In addition, the combination of tumor immunotherapy strategies and other therapeutic methods is also a direction of the adjuvant therapy of PCa, such as the combination of cancer vaccine and ICIs applied to the treatment of PCa (60, 63), and the research results also confirm that the combination therapy is beneficial. The above studies made a preliminary exploration of cancer vaccine combined with other therapeutic measures, and laid a theoretical foundation for the next widespread application of cancer vaccine in the treatment of PCa.
Furthermore, the choice of a TSAs is crucial in the implementation of tumor immunotherapy. CD19 is a specific membrane protein of B cells, so CAR-T cells targeting CD19 have been successful in the treatment of B-cell origin lymphoma and leukemia (159). CAR-T cell technology has not made a breakthrough in the treatment of solid tumors. One of the main problems is that the TSAs of tumors are not found. At present, the basic and clinical research targets used in the treatment of PCa are TAAs (PSCA, PSMA, NKG2D, B7-H3, etc) (160–166), which are also expressed in other normal tissues and organs. Similarly, this is the dilemma that limits the use of cancer vaccines for tumor treatment. Target antigens are expressed in normal tissues and organs, and the tumor immune response activated by immunotherapy strategies will inevitably damage normal tissues and organs. This will lead to the problem of side effects of immunotherapy, and most of the current clinical studies of solid tumor immunotherapy have shown a high incidence of side effects. Therefore, it is necessary to screen TSAs of PCa with the help of proteomics and transcriptomics to lay a foundation for the widespread application of immunotherapy in the treatment of PCa. CAR-T is also the future direction of PCa immunotherapy, and PCa is expected to become the first solid cancer to receive FDA approval for the use of CAR-T.
In addition, immunotherapy strategies can activate not only tumor-specific immune responses, but also adaptive immune responses. However, immunotherapy for tumors was originally developed to treat progressive PCa. The majority of the research focused on males with advanced PCa. Is it feasible to combine immunotherapy with surgery to achieve radical treatment in the early stages of the tumor? A. M. A. Tryggestad et al. (65) found DCs vaccine is of great value in reducing BCR in patients after robot-assisted laparoscopic prostatectomy. Therefore, immunotherapy strategies can be applied to the treatment of early and middle stage PCa to slow down the progression of PCa as much as possible. By further exploring the mechanism of immunotherapy strategies in the microenvironment of early PCa, it may be possible to find a breakthrough for the immunotherapy of advanced PCa.
In conclusion, tumor immunotherapy has been extensively explored in research on PCa treatment, encompassing cancer vaccines, ICIs, and CAR-T cells therapy. The therapeutic approaches employed vary from single-agent treatments to combination regimens, with certain studies demonstrating modest clinical efficacy, albeit insufficient for broad clinical application. Reversing the inhibitory TME and screening out meaningful targets play a decisive role in immunotherapy of PCa. With the widespread application of transcriptomics and proteomics in PCa screening, human understanding of the inhibitory TME is gradually deepening, and immunotherapy strategies will certainly bring extensive benefits to the treatment of PCa.
Author contributions
JC: Writing – original draft, Writing – review & editing. YuL: Writing – original draft, Writing – review & editing. YaL: Writing – original draft, Writing – review & editing. JS: Data curation, Formal Analysis, Writing – review & editing. HC: Methodology, Software, Writing – review & editing. DF: Data curation, Investigation, Validation, Writing – review & editing. AT: Funding acquisition, Visualization, Writing – original draft. ZZ: Writing – original draft, Writing – review & editing. YX: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by Shandong Province Medical and Health Science and Technology Development Plan Project (NO. 202304051613) and Science and Technology Program of Yantai Affiliated Hospital of Binzhou Medical University (NO. YTFY2024KYQD01).
Acknowledgments
We would like to thank all the authors involved in writing this review and the Yantai Affiliated Hospital of Binzhou Medical College for facilitating the publication of this manuscript. Also, thanks for the convenience of FIGDRAW’s online photo making site.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rebello RJ, Oing C, Knudsen KE, Loeb S, Johnson DC, Reiter RE, et al. Prostate cancer. Nat Rev Dis Primers. (2021) 7(1):9. doi: 10.1038/s41572-021-00249-2
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Staniszewska M, Iking J, Lückerath K, Hadaschik B, Herrmann K, Ferdinandus J, et al. Drug and molecular radiotherapy combinations for metastatic castration resistant prostate cancer. Nucl Med Biol. (2021) 96-97:101–11. doi: 10.1016/j.nucmedbio.2021.03.009
4. Sumanasuriya S, De Bono J. Treatment of advanced prostate cancer-A review of current therapies and future promise. Cold Spring Harb Perspect Med. (2018) 8:1–15. doi: 10.1101/cshperspect.a030635
5. Dong L, Zieren RC, Xue W, de Reijke TM, Pienta KJ. Metastatic prostate cancer remains incurable, why? Asian J Urol. (2019) 6:26–41. doi: 10.1016/j.ajur.2018.11.005
6. Daly MB, Pal T, Berry MP, Buys SS, Dickson P, Domchek SM, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:77–102. doi: 10.6004/jnccn.2021.0001
7. Meng L, Yang Y, Mortazavi A, Zhang J. Emerging immunotherapy approaches for treating prostate cancer. Int J Mol Sci. (2023) 24:1–17. doi: 10.3390/ijms241814347
8. Xu L, Hu Y, Liu W. Tumor microenvironment-mediated immune profiles characterized by distinct survival outcome and immunotherapeutic efficacy in breast cancer. Front Genet. (2022) 13:840348. doi: 10.3389/fgene.2022.840348
9. Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. (2020) 30:R921–r925. doi: 10.1016/j.cub.2020.06.081
10. Arneth B. Tumor microenvironment. Medicina (Kaunas). (2019) 56:1–15. doi: 10.3390/medicina56010015
11. Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. (2012) 12:860–75. doi: 10.1038/nrc3380
12. Nair SS, Weil R, Dovey Z, Davis A, Tewari AK. The tumor microenvironment and immunotherapy in prostate and bladder cancer. Urol Clin North Am. (2020) 47:e17–54. doi: 10.1016/j.ucl.2020.10.005
13. Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, et al. Cold tumors: A therapeutic challenge for immunotherapy. Front Immunol. (2019) 10:168. doi: 10.3389/fimmu.2019.00168
14. Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. (2020) 19:116. doi: 10.1186/s12943-020-01234-1
15. Kim JH, Kim BS, Lee SK. Regulatory T cells in tumor microenvironment and approach for anticancer immunotherapy. Immune Netw. (2020) 20:e4. doi: 10.4110/in.2020.20.e4
16. Zhu Y, Duong L, Lu X, Lu X. Cancer-cell-intrinsic mechanisms shaping the immunosuppressive landscape of prostate cancer. Asian J Androl. (2023) 25:171–8. doi: 10.4103/aja202283
17. Jiang T, Shi T, Zhang H, Hu J, Song Y, Wei J, et al. Tumor neoantigens: from basic research to clinical applications. J Hematol Oncol. (2019) 12:93. doi: 10.1186/s13045-019-0787-5
18. Abeshouse A, Ahn J, Akbani R, Ally A, Amin S, Andry CD, et al.The molecular taxonomy of primary prostate cancer. Cell. (2015) 163:1011–25. doi: 10.1016/j.cell.2015.10.025
19. Mei P, Freitag CE, Wei L, Zhang Y, Parwani AV, Li Z. High tumor mutation burden is associated with DNA damage repair gene mutation in breast carcinomas. Diagn Pathol. (2020) 15:50. doi: 10.1186/s13000-020-00971-7
20. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. (2017) 357:409–13. doi: 10.1126/science.aan6733
21. Maleki Vareki S. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J Immunother Cancer. (2018) 6:157. doi: 10.1186/s40425-018-0479-7
22. Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. (2015) 35 Suppl:S185–s198. doi: 10.1016/j.semcancer.2015.03.004
23. Tawbi HA, SChadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutiérrez E, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. (2022) 386:24–34. doi: 10.1056/NEJMoa2109970
24. Niu J, Maurice-Dror C, Lee DH, Kim DW, Nagrial A, Voskoboynik M, et al. First-in-human phase 1 study of the anti-TIGIT antibody vibostolimab as monotherapy or with pembrolizumab for advanced solid tumors, including non-small-cell lung cancer(☆). Ann Oncol. (2022) 33:169–80. doi: 10.1016/j.annonc.2021.11.002
25. Kaptein P, Jacoberger-Foissac C, Dimitriadis P, Voabil P, de Bruijn M, Brokamp S, et al. Addition of interleukin-2 overcomes resistance to neoadjuvant CTLA4 and PD1 blockade in ex vivo patient tumors. Sci Transl Med. (2022) 14:eabj9779. doi: 10.1126/scitranslmed.abj9779
26. Watson HA, Durairaj RRP, Ohme J, Alatsatianos M, Almutairi H, Mohammed RN, et al. L-selectin enhanced T cells improve the efficacy of cancer immunotherapy. Front Immunol. (2019) 10:1321. doi: 10.3389/fimmu.2019.01321
27. Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. (1999) 10:673–9. doi: 10.1016/s1074-7613(00)80066-7
28. Finn OJ, Gantt KR, Lepisto AJ, Pejawar-Gaddy S, Xue J, Beatty PL. Importance of MUC1 and spontaneous mouse tumor models for understanding the immunobiology of human adenocarcinomas. Immunol Res. (2011) 50:261–8. doi: 10.1007/s12026-011-8214-1
29. Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. (2019) 4:7. doi: 10.1038/s41541-019-0103-y
30. Tagliamonte M, Petrizzo A, Tornesello ML, Buonaguro FM, Buonaguro L. Antigen-specific vaccines for cancer treatment. Hum Vaccin Immunother. (2014) 10:3332–46. doi: 10.4161/21645515.2014.973317
31. Xie N, Shen G, Gao W, Huang Z, Huang C, Fu L. Neoantigens: promising targets for cancer therapy. Signal Transduct Target Ther. (2023) 8:9. doi: 10.1038/s41392-022-01270-x
32. Lei W, Zhou K, Lei Y, Li Q, Zhu H. Cancer vaccines: platforms and current progress. Mol BioMed. (2025) 6:3. doi: 10.1186/s43556-024-00241-8
33. Haque Chowdhury H, Hawlina S, Gabrijel M, Trkov Bobnar S, Kreft M, Lenart G, et al. Survival of castration-resistant prostate cancer patients treated with dendritic-tumor cell hybridomas is negatively correlated with changes in peripheral blood CD56(bright) CD16(-) natural killer cells. Clin Transl Med. (2021) 11:e505. doi: 10.1002/ctm2.505
34. Halle S, Halle O, Förster R. Mechanisms and dynamics of T cell-mediated cytotoxicity in vivo. Trends Immunol. (2017) 38:432–43. doi: 10.1016/j.it.2017.04.002
35. Pulendran B, S Arunachalam P, O'Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discovery. (2021) 20:454–75. doi: 10.1038/s41573-021-00163-y
36. Morse MA, Gwin WR 3rd, Mitchell DA. Vaccine therapies for cancer: then and now. Target Oncol. (2021) 16:121–52. doi: 10.1007/s11523-020-00788-w
37. Apostolopoulos V, Thalhammer T, Tzakos AG, Stojanovska L. Targeting antigens to dendritic cell receptors for vaccine development. J Drug Delivery. (2013) 2013:869718. doi: 10.1155/2013/869718
38. Gulley JL, Madan RA, Tsang KY, Jochems C, Marté JL, Farsaci B, et al. Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol Res. (2014) 2:133–41. doi: 10.1158/2326-6066.Cir-13-0108
39. Sonpavde G, Wang M, Peterson LE, Wang HY, Joe T, Mims MP, et al. HLA-restricted NY-ESO-1 peptide immunotherapy for metastatic castration resistant prostate cancer. Invest New Drugs. (2014) 32:235–42. doi: 10.1007/s10637-013-9960-9
40. DiPaola RS, Chen YH, Bubley GJ, Stein MN, Hahn NM, Carducci MA, et al. A national multicenter phase 2 study of prostate-specific antigen (PSA) pox virus vaccine with sequential androgen ablation therapy in patients with PSA progression: ECOG 9802. Eur Urol. (2015) 68:365–71. doi: 10.1016/j.eururo.2014.12.010
41. McNeel DG, Chen YH, Gulley JL, Dwyer AJ, Madan RA, Carducci MA, et al. Randomized phase II trial of docetaxel with or without PSA-TRICOM vaccine in patients with castrate-resistant metastatic prostate cancer: A trial of the ECOG-ACRIN cancer research group (E1809). Hum Vaccin Immunother. (2015) 11:2469–74. doi: 10.1080/21645515.2015.1062190
42. Noguchi M, Arai G, Matsumoto K, Naito S, Moriya F, Suekane S, et al. Phase I trial of a cancer vaccine consisting of 20 mixed peptides in patients with castration-resistant prostate cancer: dose-related immune boosting and suppression. Cancer Immunol Immunother. (2015) 64:493–505. doi: 10.1007/s00262-015-1660-1
43. Prue RL, Vari F, Radford KJ, Tong H, Hardy MY, D’Rozario R, et al. A phase I clinical trial of CD1c (BDCA-1)+ dendritic cells pulsed with HLA-A*0201 peptides for immunotherapy of metastatic hormone refractory prostate cancer. J Immunother. (2015) 38:71–6. doi: 10.1097/cji.0000000000000063
44. Xi HB, Wang GX, Fu B, Liu WP, Li Y. Survivin and PSMA loaded dendritic cell vaccine for the treatment of prostate cancer. Biol Pharm Bull. (2015) 38:827–35. doi: 10.1248/bpb.b14-00518
45. Heery CR, Madan RA, Stein MN, Stadler WM, Di Paola RS, Rauckhorst M, et al. Samarium-153-EDTMP (Quadramet®) with or without vaccine in metastatic castration-resistant prostate cancer: A randomized Phase 2 trial. Oncotarget. (2016) 7:69014–23. doi: 10.18632/oncotarget.10883
46. Scheid E, Major P, Bergeron A, Finn OJ, Salter RD, Eady R, et al. Tn-MUC1 DC vaccination of rhesus macaques and a phase I/II trial in patients with nonmetastatic castrate-resistant prostate cancer. Cancer Immunol Res. (2016) 4:881–92. doi: 10.1158/2326-6066.Cir-15-0189
47. Yoshimura K, Minami T, Nozawa M, Kimura T, Egawa S, Fujimoto H, et al. A phase 2 randomized controlled trial of personalized peptide vaccine immunotherapy with low-dose dexamethasone versus dexamethasone alone in chemotherapy-naive castration-resistant prostate cancer. Eur Urol. (2016) 70:35–41. doi: 10.1016/j.eururo.2015.12.050
48. Koga N, Moriya F, Waki K, Yamada A, Itoh K, Noguchi M. Immunological efficacy of herbal medicines in prostate cancer patients treated by personalized peptide vaccine. Cancer Sci. (2017) 108:2326–32. doi: 10.1111/cas.13397
49. Kongsted P, Borch TH, Ellebaek E, Iversen TZ, Andersen R, Met Ö, et al. Dendritic cell vaccination in combination with docetaxel for patients with metastatic castration-resistant prostate cancer: A randomized phase II study. Cytotherapy. (2017) 19:500–13. doi: 10.1016/j.jcyt.2017.01.007
50. Lilleby W, Gaudernack G, Brunsvig PF, Vlatkovic L, Schulz M, Mills K, et al. Phase I/IIa clinical trial of a novel hTERT peptide vaccine in men with metastatic hormone-naive prostate cancer. Cancer Immunol Immunother. (2017) 66:891–901. doi: 10.1007/s00262-017-1994-y
51. Obara W, Sato F, Takeda K, Kato R, Kato Y, Kanehira M, et al. Phase I clinical trial of cell division associated 1 (CDCA1) peptide vaccination for castration resistant prostate cancer. Cancer Sci. (2017) 108:1452–7. doi: 10.1111/cas.13278
52. Sonpavde G, McMannis JD, Bai Y, Seethammagari MR, Bull JMC, Hawkins V, et al. Phase I trial of antigen-targeted autologous dendritic cell-based vaccine with in vivo activation of inducible CD40 for advanced prostate cancer. Cancer Immunol Immunother. (2017) 66:1345–57. doi: 10.1007/s00262-017-2027-6
53. Wargowski E, Johnson LE, Eickhoff JC, Delmastro L, Staab MJ, Liu G, et al. Prime-boost vaccination targeting prostatic acid phosphatase (PAP) in patients with metastatic castration-resistant prostate cancer (mCRPC) using Sipuleucel-T and a DNA vaccine. J Immunother Cancer. (2018) 6:21. doi: 10.1186/s40425-018-0333-y
54. McNeel DG, Eickhoff JC, Johnson LE, Roth AR, Perk TG, Fong L, et al. Phase II trial of a DNA vaccine encoding prostatic acid phosphatase (pTVG-HP [MVI-816]) in patients with progressive, nonmetastatic, castration-sensitive prostate cancer. J Clin Oncol. (2019) 37:3507–17. doi: 10.1200/jco.19.01701
55. Abdul Sater H, Marté JL, Donahue RN, Walter-Rodriguez B, Heery CR, Steinberg SM, et al. Neoadjuvant PROSTVAC prior to radical prostatectomy enhances T-cell infiltration into the tumor immune microenvironment in men with prostate cancer. J Immunother Cancer. (2020) 8:e10021. doi: 10.1136/jitc-2020-000655
56. Kyriakopoulos CE, Eickhoff JC, Ferrari AC, Schweizer MT, Wargowski E, Olson BM, et al. Multicenter phase I trial of a DNA vaccine encoding the androgen receptor ligand-binding domain (pTVG-AR, MVI-118) in patients with metastatic prostate cancer. Clin Cancer Res. (2020) 26:5162–71. doi: 10.1158/1078-0432.Ccr-20-0945
57. Noguchi M, Arai G, Egawa S, Ohyama C, Naito S, Matsumoto K, et al. Mixed 20-peptide cancer vaccine in combination with docetaxel and dexamethasone for castration-resistant prostate cancer: a randomized phase II trial. Cancer Immunol Immunother. (2020) 69:847–57. doi: 10.1007/s00262-020-02498-8
58. Schuhmacher J, Heidu S, Balchen T, Richardson JR, Schmeltz C, Sonne J, et al. Vaccination against RhoC induces long-lasting immune responses in patients with prostate cancer: results from a phase I/II clinical trial. J Immunother Cancer. (2020) 8:1–10. doi: 10.1136/jitc-2020-001157
59. Bilusic M, McMahon S, Madan RA, Karzai F, Tsai YT, Donahue RN, et al. Phase I study of a multitargeted recombinant Ad5 PSA/MUC-1/brachyury-based immunotherapy vaccine in patients with metastatic castration-resistant prostate cancer (mCRPC). J Immunother Cancer. (2021) 9. doi: 10.1136/jitc-2021-002374
60. Dorff T, Hirasawa Y, Acoba J, Pagano I, Tamura D, Pal S, et al. Phase Ib study of patients with metastatic castrate-resistant prostate cancer treated with different sequencing regimens of atezolizumab and sipuleucel-T. J Immunother Cancer. (2021) 9:e002931. doi: 10.1136/jitc-2021-002931
61. Filaci G, Fenoglio D, Nolè F, Zanardi E, Tomasello L, Aglietta M, et al. Telomerase-based GX301 cancer vaccine in patients with metastatic castration-resistant prostate cancer: a randomized phase II trial. Cancer Immunol Immunother. (2021) 70:3679–92. doi: 10.1007/s00262-021-03024-0
62. Noguchi M, Fujimoto K, Arai G, Uemura H, Hashine K, Matsumoto H, et al. A randomized phase III trial of personalized peptide vaccination for castration−resistant prostate cancer progressing after docetaxel. Oncol Rep. (2021) 45:159–68. doi: 10.3892/or.2020.7847
63. Sinha M, Zhang L, Subudhi S, Chen B, Marquez J, Liu EV, et al. Pre-existing immune status associated with response to combination of sipuleucel-T and ipilimumab in patients with metastatic castration-resistant prostate cancer. J Immunother Cancer. (2021) 9. doi: 10.1136/jitc-2020-002254
64. McNeel DG, Eickhoff JC, Wargowski E, Johnson LE, Kyriakopoulos CE, Emamekhoo H, et al. Phase 2 trial of T-cell activation using MVI-816 and pembrolizumab in patients with metastatic, castration-resistant prostate cancer (mCRPC). J Immunother Cancer. (2022) 10:1–10. doi: 10.1136/jitc-2021-004198
65. Tryggestad AMA, Axcrona K, Axcrona U, Bigalke I, Brennhovd B, Inderberg EM, et al. Long-term first-in-man Phase I/II study of an adjuvant dendritic cell vaccine in patients with high-risk prostate cancer after radical prostatectomy. Prostate. (2022) 82:245–53. doi: 10.1002/pros.24267
66. Autio KA, Higano CS, Nordquist L, Appleman LJ, Zhang T, Zhu XH, et al. First-in-human, phase 1 study of PF-06753512, a vaccine-based immunotherapy regimen (VBIR), in non-metastatic hormone-sensitive biochemical recurrence and metastatic castration-resistant prostate cancer (mCRPC). J Immunother Cancer. (2023) 11:1–12. doi: 10.1136/jitc-2022-005702
67. Lilleby W, Seierstad T, Inderberg EM, Hole KH. Impact of human telomerase reverse transcriptase peptide vaccine combined with androgen deprivation therapy and radiotherapy in de novo metastatic prostate cancer: Long-term clinical monitoring. Int J Cancer. (2023) 152:2166–73. doi: 10.1002/ijc.34448
68. Madan RA, Bilusic M, Stein MN, Donahue RN, Arlen PM, Karzai F, et al. Flutamide with or without PROSTVAC in non-metastatic castration resistant (M0) prostate cancer. Oncologist. (2023) 28:642–e561. doi: 10.1093/oncolo/oyad058
69. McNeel DG, Emamekhoo H, Eickhoff JC, Kyriakopoulos CE, Wargowski E, Tonelli TP, et al. Phase 2 trial of a DNA vaccine (pTVG-HP) and nivolumab in patients with castration-sensitive non-metastatic (M0) prostate cancer. J Immunother Cancer. (2023) 11:1–14. doi: 10.1136/jitc-2023-008067
70. Parsons JK, Pinto PA, Pavlovich CP, Uchio E, Nguyen MN, Kim HL, et al. A phase 2, double-blind, randomized controlled trial of PROSTVAC in prostate cancer patients on active surveillance. Eur Urol Focus. (2023) 9:447–54. doi: 10.1016/j.euf.2022.12.002
71. Zareian N, Eremin O, Pandha H, Baird R, Kwatra V, Funingana G, et al. A phase 1 trial of human telomerase reverse transcriptase (hTERT) vaccination combined with therapeutic strategies to control immune-suppressor mechanisms. Exp Biol Med (Maywood). (2024) 249:10021. doi: 10.3389/ebm.2024.10021
72. Sutherland SIM, Ju X, Horvath LG, Clark GJ. Moving on from sipuleucel-T: new dendritic cell vaccine strategies for prostate cancer. Front Immunol. (2021) 12:641307. doi: 10.3389/fimmu.2021.641307
73. Tanimoto T, Hori A, Kami M. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. (2010) 363:1–11. doi: 10.1056/NEJMc1009982
74. Gabba A, Attariya R, Behren S, Pett C, van der Horst JC, Yurugi H, et al. MUC1 glycopeptide vaccine modified with a galNAc glycocluster targets the macrophage galactose C-type lectin on dendritic cells to elicit an improved humoral response. J Am Chem Soc. (2023) 145:13027–37. doi: 10.1021/jacs.2c12843
75. Wenner CA, Martzen MR, Lu H, Verneris MR, Wang H, Slaton JW. Polysaccharide-K augments docetaxel-induced tumor suppression and antitumor immune response in an immunocompetent murine model of human prostate cancer. Int J Oncol. (2012) 40:905–13. doi: 10.3892/ijo.2011.1292
76. Mason K, Staab A, Hunter N, McBride W, Petersen S, Terry N, et al. Enhancement of tumor radioresponse by docetaxel: Involvement of immune system. Int J Oncol. (2001) 18:599–606. doi: 10.3892/ijo.18.3.599
77. Kalita P, Tripathi T. Methodological advances in the design of peptide-based vaccines. Drug Discovery Today. (2022) 27:1367–80. doi: 10.1016/j.drudis.2022.03.004
78. Kumai T, Yamaki H, Kono M, Hayashi R, Wakisaka R, Komatsuda H. Antitumor peptide-based vaccine in the limelight. Vaccines (Basel). (2022) 10:1–22. doi: 10.3390/vaccines10010070
79. Buonaguro L, Tagliamonte M. Peptide-based vaccine for cancer therapies. Front Immunol. (2023) 14:1210044. doi: 10.3389/fimmu.2023.1210044
80. Gnjatic S, Atanackovic D, Jäger E, Matsuo M, Selvakumar A, Altorki NK, et al. Survey of naturally occurring CD4+ T cell responses against NY-ESO-1 in cancer patients: correlation with antibody responses. Proc Natl Acad Sci U.S.A. (2003) 100:8862–7. doi: 10.1073/pnas.1133324100
81. Kayser S, Boβ C, Feucht J, Witte KE, Scheu A, Bülow HJ, et al. Feuchtinger: Rapid generation of NY-ESO-1-specific CD4(+) T(HELPER)1 cells for adoptive T-cell therapy. Oncoimmunology. (2015) 4:e1002723. doi: 10.1080/2162402x.2014.1002723
82. Ellingsen EB, Mangsbo SM, Hovig E, Gaudernack G. Telomerase as a target for therapeutic cancer vaccines and considerations for optimizing their clinical potential. Front Immunol. (2021) 12:682492. doi: 10.3389/fimmu.2021.682492
83. Aamdal E, Inderberg EM, Ellingsen EB, Rasch W, Brunsvig PF, Aamdal S, et al. Combining a universal telomerase based cancer vaccine with ipilimumab in patients with metastatic melanoma - five-year follow up of a phase I/IIa trial. Front Immunol. (2021) 12:663865. doi: 10.3389/fimmu.2021.663865
84. Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. (2018) 48:434–52. doi: 10.1016/j.immuni.2018.03.014
85. Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. (2010) 236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x
86. Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. (2009) 206:3015–29. doi: 10.1084/jem.20090847
87. Muenst S, Läubli H, Soysal SD, Zippelius A, Tzankov A, Hoeller S. The immune system and cancer evasion strategies: therapeutic concepts. J Intern Med. (2016) 279:541–62. doi: 10.1111/joim.12470
88. Comiskey MC, Dallos MC, Drake CG. Immunotherapy in prostate cancer: teaching an old dog new tricks. Curr Oncol Rep. (2018) 20:75. doi: 10.1007/s11912-018-0712-z
89. Madan RA, Gulley JL. Prospects for the future of prostate cancer vaccines. Expert Rev Vaccines. (2016) 15:271–4. doi: 10.1586/14760584.2015.1118348
90. Reimers MA, Slane KE, Pachynski RK. Immunotherapy in metastatic castration-resistant prostate cancer: past and future strategies for optimization. Curr Urol Rep. (2019) 20:64. doi: 10.1007/s11934-019-0931-3
91. Marshall CH, Antonarakis ES. Emerging treatments for metastatic castration-resistant prostate cancer: Immunotherapy, PARP inhibitors, and PSMA-targeted approaches. Cancer Treat Res Commun. (2020) 23:100164. doi: 10.1016/j.ctarc.2020.100164
92. Prokhnevska N, Emerson DA, Kissick HT, Redmond WL. Immunological complexity of the prostate cancer microenvironment influences the response to immunotherapy. Adv Exp Med Biol. (2019) 1210:121–47. doi: 10.1007/978-3-030-32656-2_7
93. Laccetti AL, Subudhi SK. Immunotherapy for metastatic prostate cancer: immuno-cold or the tip of the iceberg? Curr Opin Urol. (2017) 27:566–71. doi: 10.1097/mou.0000000000000433
94. Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, et al. The genomic complexity of primary human prostate cancer. Nature. (2011) 470:214–20. doi: 10.1038/nature09744
95. Chouaib S, Noman MZ, Kosmatopoulos K, Curran MA. Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer. Oncogene. (2017) 36:439–45. doi: 10.1038/onc.2016.225
96. Jayaprakash P, Ai M, Liu A, Budhani P, Bartkowiak T, Sheng J, et al. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J Clin Invest. (2018) 128:5137–49. doi: 10.1172/jci96268
97. Ness N, Andersen S, Khanehkenari MR, Nordbakken CV, Valkov A, Paulsen EE, et al. The prognostic role of immune checkpoint markers programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) in a large, multicenter prostate cancer cohort. Oncotarget. (2017) 8:26789–801. doi: 10.18632/oncotarget.15817
98. Gevensleben H, Dietrich D, Golletz C, Steiner S, Jung M, Thiesler T, et al. The immune checkpoint regulator PD-L1 is highly expressed in aggressive primary prostate cancer. Clin Cancer Res. (2016) 22:1969–77. doi: 10.1158/1078-0432.Ccr-15-2042
99. Jochems C, Tucker JA, Tsang KY, Madan RA, Dahut WL, Liewehr DJ, et al. A combination trial of vaccine plus ipilimumab in metastatic castration-resistant prostate cancer patients: immune correlates. Cancer Immunol Immunother. (2014) 63:407–18. doi: 10.1007/s00262-014-1524-0
100. Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. (2014) 15:700–12. doi: 10.1016/s1470-2045(14)70189-5
101. Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. (2017) 35:40–7. doi: 10.1200/jco.2016.69.1584
102. Fizazi K, Drake CG, Beer TM, Kwon ED, Scher HI, Gerritsen WR, et al. Final analysis of the ipilimumab versus placebo following radiotherapy phase III trial in postdocetaxel metastatic castration-resistant prostate cancer identifies an excess of long-term survivors. Eur Urol. (2020) 78:822–30. doi: 10.1016/j.eururo.2020.07.032
103. Sharma P, Pachynski RK, Narayan V, Fléchon A, Gravis G, Galsky MD, et al. Nivolumab plus ipilimumab for metastatic castration-resistant prostate cancer: preliminary analysis of patients in the checkMate 650 trial. Cancer Cell. (2020) 38:489–499.e3. doi: 10.1016/j.ccell.2020.08.007
104. Shenderov E, Boudadi K, Fu W, Wang H, Sullivan R, Jordan A, et al. Nivolumab plus ipilimumab, with or without enzalutamide, in AR-V7-expressing metastatic castration-resistant prostate cancer: A phase-2 nonrandomized clinical trial. Prostate. (2021) 81:326–38. doi: 10.1002/pros.24110
105. Rodriguez-Vida A, Maroto P, Font A, Martin C, Mellado B, Corbera A, et al. Safety and efficacy of avelumab plus carboplatin in patients with metastatic castration-resistant prostate cancer in an open-label Phase Ib study. Br J Cancer. (2023) 128:21–9. doi: 10.1038/s41416-022-01991-4
106. Graff JN, Alumkal JJ, Drake CG, Thomas GV, Redmond WL, Farhad M, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. (2016) 7:52810–7. doi: 10.18632/oncotarget.10547
107. Antonarakis ES, Piulats JM, Gross-Goupil M, Goh J, Ojamaa K, Hoimes CJ, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol. (2020) 38:395–405. doi: 10.1200/jco.19.01638
108. Graff JN, Beer TM, Alumkal JJ, Slottke RE, Redmond WL, Thomas GV, et al. A phase II single-arm study of pembrolizumab with enzalutamide in men with metastatic castration-resistant prostate cancer progressing on enzalutamide alone. J Immunother Cancer. (2020) 8:52810–17. doi: 10.1136/jitc-2020-000642
109. Ross AE, Hurley PJ, Tran PT, Rowe SP, Benzon B, Neal TO, et al. A pilot trial of pembrolizumab plus prostatic cryotherapy for men with newly diagnosed oligometastatic hormone-sensitive prostate cancer. Prostate Cancer Prostatic Dis. (2020) 23:184–93. doi: 10.1038/s41391-019-0176-8
110. Yu EY, Kolinsky MP, Berry WR, Retz M, Mourey L, Piulats JM, et al. Pembrolizumab plus docetaxel and prednisone in patients with metastatic castration-resistant prostate cancer: long-term results from the phase 1b/2 KEYNOTE-365 cohort B study. Eur Urol. (2022) 82:22–30. doi: 10.1016/j.eururo.2022.02.023
111. Aggarwal R, Starzinski S, de Kouchkovsky I, Koshkin V, Bose R, Chou J, et al. Single-dose (177)Lu-PSMA-617 followed by maintenance pembrolizumab in patients with metastatic castration-resistant prostate cancer: an open-label, dose-expansion, phase 1 trial. Lancet Oncol. (2023) 24:1266–76. doi: 10.1016/s1470-2045(23)00451-5
112. Antonarakis ES, Park SH, Goh JC, Shin SJ, Lee JL, Mehra N, et al. Pembrolizumab plus olaparib for patients with previously treated and biomarker-unselected metastatic castration-resistant prostate cancer: the randomized, open-label, phase III KEYLYNK-010 trial. J Clin Oncol. (2023) 41:3839–50. doi: 10.1200/jco.23.00233
113. Vaishampayan UN, Thakur A, Chen W, Deol A, Patel M, Dobson K, et al. Phase II trial of pembrolizumab and anti-CD3 x anti-HER2 bispecific antibody-armed activated T cells in metastatic castration-resistant prostate cancer. Clin Cancer Res. (2023) 29:122–33. doi: 10.1158/1078-0432.Ccr-22-1601
114. Yu EY, Piulats JM, Gravis G, Fong PCC, Todenhöfer T, Laguerre B, et al. Pembrolizumab plus olaparib in patients with metastatic castration-resistant prostate cancer: long-term results from the phase 1b/2 KEYNOTE-365 cohort A study. Eur Urol. (2023) 83:15–26. doi: 10.1016/j.eururo.2022.08.005
115. Yuan Z, Fernandez D, Dhillon J, Abraham-Miranda J, Awasthi S, Kim Y, et al. Proof-of-principle Phase I results of combining nivolumab with brachytherapy and external beam radiation therapy for Grade Group 5 prostate cancer: safety, feasibility, and exploratory analysis. Prostate Cancer Prostatic Dis. (2021) 24:140–9. doi: 10.1038/s41391-020-0254-y
116. Fizazi K, González Mella P, Castellano D, Minatta JN, Rezazadeh Kalebasty A, Shaffer D, et al. Nivolumab plus docetaxel in patients with chemotherapy-naïve metastatic castration-resistant prostate cancer: results from the phase II CheckMate 9KD trial. Eur J Cancer. (2022) 160:61–71. doi: 10.1016/j.ejca.2021.09.043
117. Fizazi K, Retz M, Petrylak DP, Goh JC, Perez-Gracia J, Lacombe L, et al. Nivolumab plus rucaparib for metastatic castration-resistant prostate cancer: results from the phase 2 CheckMate 9KD trial. J Immunother Cancer. (2022) 10:61–71. doi: 10.1136/jitc-2022-004761
118. Markowski MC, Taplin ME, Aggarwal R, Sena LA, Wang H, Qi H, et al. Bipolar androgen therapy plus nivolumab for patients with metastatic castration-resistant prostate cancer: the COMBAT phase II trial. Nat Commun. (2024) 15:14. doi: 10.1038/s41467-023-44514-2
119. Bishop JL, Sio A, Angeles A, Roberts ME, Azad AA, Chi KN, et al. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget. (2015) 6:234–42. doi: 10.18632/oncotarget.2703
120. Kwan EM, Spain L, Anton A, Gan CL, Garrett L, Chang D, et al. Avelumab combined with stereotactic ablative body radiotherapy in metastatic castration-resistant prostate cancer: the phase 2 ICE-PAC clinical trial. Eur Urol. (2022) 81:253–62. doi: 10.1016/j.eururo.2021.08.011
121. Rodriguez-Garcia A, Lynn RC, Poussin M, Eiva MA, Shaw LC, O’Connor RS, et al. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat Commun. (2021) 12:877. doi: 10.1038/s41467-021-20893-2
122. Poorebrahim M, Melief J, Pico de Coaña Y, L Wickström S, Cid-Arregui A, Kiessling R. Counteracting CAR T cell dysfunction. Oncogene. (2021) 40:421–35. doi: 10.1038/s41388-020-01501-x
123. Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. (1998) 161:2791–7. doi: 10.4049/jimmunol.161.6.2791
124. Jayaraman J, Mellody MP, Hou AJ, Desai RP, Fung AW, Pham AHT, et al. CAR-T design: Elements and their synergistic function. EBioMedicine. (2020) 58:102931. doi: 10.1016/j.ebiom.2020.102931
125. Miao L, Zhang Z, Ren Z, Tang F, Li Y. Obstacles and coping strategies of CAR-T cell immunotherapy in solid tumors. Front Immunol. (2021) 12:687822. doi: 10.3389/fimmu.2021.687822
126. Zhang Z, Miao L, Ren Z, Tang F, Li Y. Gene-edited interleukin CAR-T cells therapy in the treatment of Malignancies: present and future. Front Immunol. (2021) 12:718686. doi: 10.3389/fimmu.2021.718686
127. Gorchakov AA, Kulemzin SV, Kochneva GV, Taranin AV. Challenges and prospects of chimeric antigen receptor T-cell therapy for metastatic prostate cancer. Eur Urol. (2020) 77:299–308. doi: 10.1016/j.eururo.2019.08.014
128. Hillerdal V, Ramachandran M, Leja J, Essand M. Systemic treatment with CAR-engineered T cells against PSCA delays subcutaneous tumor growth and prolongs survival of mice. BMC Cancer. (2014) 14:30. doi: 10.1186/1471-2407-14-30
129. Zhou JE, Yu J, Wang Y, Wang H, Wang J, Wang Y, et al. ShRNA-mediated silencing of PD-1 augments the efficacy of chimeric antigen receptor T cells on subcutaneous prostate and leukemia xenograft. BioMed Pharmacother. (2021) 137:111339. doi: 10.1016/j.biopha.2021.111339
130. Frieling JS, Tordesillas L, Bustos XE, Ramello MC, Bishop RT, Cianne JE, et al. [amp]]gamma;δ-Enriched CAR-T cell therapy for bone metastatic castrate-resistant prostate cancer. Sci Adv. (2023) 9:eadf0108. doi: 10.1126/sciadv.adf0108
131. Alzubi J, Dettmer-Monaco V, Kuehle J, Thorausch N, Seidl M, Taromi S, et al. PSMA-directed CAR T cells combined with low-dose docetaxel treatment induce tumor regression in a prostate cancer xenograft model. Mol Ther Oncolytics. (2020) 18:226–35. doi: 10.1016/j.omto.2020.06.014
132. Wang D, Shao Y, Zhang X, Lu G, Liu B. IL-23 and PSMA-targeted duo-CAR T cells in Prostate Cancer Eradication in a preclinical model. J Transl Med. (2020) 18:23. doi: 10.1186/s12967-019-02206-w
133. Tang L, Shao H, Wu Y, Wang J, Qian X, He L, et al. Dominant negative TGFβ receptor II and truncated TIM3 enhance the antitumor efficacy of CAR-T-cell therapy in prostate cancer. Int Immunopharmacol. (2023) 124:110807. doi: 10.1016/j.intimp.2023.110807
134. Narayan V, Barber-Rotenberg JS, Jung IY, Lacey SF, Rech AJ, Davis MM, et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat Med. (2022) 28:724–34. doi: 10.1038/s41591-022-01726-1
135. Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol. (2013) 31:413–41. doi: 10.1146/annurev-immunol-032712-095951
136. Sentman CL, Meehan KR. NKG2D CARs as cell therapy for cancer. Cancer J. (2014) 20:156–9. doi: 10.1097/ppo.0000000000000029
137. Demoulin B, Cook WJ, Murad J, Graber DJ, Sentman ML, Lonez C, et al. Exploiting natural killer group 2D receptors for CAR T-cell therapy. Future Oncol. (2017) 13:1593–605. doi: 10.2217/fon-2017-0102
138. He C, Zhou Y, Li Z, Farooq MA, Ajmal I, Zhang H, et al. Co-expression of IL-7 improves NKG2D-based CAR T cell therapy on prostate cancer by enhancing the expansion and inhibiting the apoptosis and exhaustion. Cancers (Basel). (2020) 12:1–23. doi: 10.3390/cancers12071969
139. Du H, Hirabayashi K, Ahn S, Kren NP, Montgomery SA, Wang X, et al. Antitumor responses in the absence of toxicity in solid tumors by targeting B7-H3 via chimeric antigen receptor T cells. Cancer Cell. (2019) 35:221–237.e8. doi: 10.1016/j.ccell.2019.01.002
140. Benzon B, Zhao SG, Haffner MC, Takhar M, Erho N, Yousefi K, et al. Correlation of B7-H3 with androgen receptor, immune pathways and poor outcome in prostate cancer: an expression-based analysis. Prostate Cancer Prostatic Dis. (2017) 20:28–35. doi: 10.1038/pcan.2016.49
141. Chavin G, Sheinin Y, Crispen PL, Boorjian SA, Roth TJ, Rangel L, et al. Expression of immunosuppresive B7-H3 ligand by hormone-treated prostate cancer tumors and metastases. Clin Cancer Res. (2009) 15:2174–80. doi: 10.1158/1078-0432.Ccr-08-2262
142. Kontos F, Michelakos T, Kurokawa T, Sadagopan A, Schwab JH, Ferrone CR, et al. B7-H3: an attractive target for antibody-based immunotherapy. Clin Cancer Res. (2021) 27:1227–35. doi: 10.1158/1078-0432.Ccr-20-2584
143. Li S, Zhang M, Wang M, Wang H, Wu H, Mao L, et al. B7-H3 specific CAR-T cells exhibit potent activity against prostate cancer. Cell Death Discovery. (2023) 9:147. doi: 10.1038/s41420-023-01453-7
144. Zhang Y, He L, Sadagopan A, Ma T, Dotti G, Wang Y, et al. Targeting radiation-resistant prostate cancer stem cells by B7-H3 CAR T cells. Mol Cancer Ther. (2021) 20:577–88. doi: 10.1158/1535-7163.Mct-20-0446
145. Moreaux J, Kassambara A, Hose D, Klein B. STEAP1 is overexpressed in cancers: a promising therapeutic target. Biochem Biophys Res Commun. (2012) 429:148–55. doi: 10.1016/j.bbrc.2012.10.123
146. Oosterheert W, Gros P. Cryo-electron microscopy structure and potential enzymatic function of human six-transmembrane epithelial antigen of the prostate 1 (STEAP1). J Biol Chem. (2020) 295:9502–12. doi: 10.1074/jbc.RA120.013690
147. Jiao Z, Huang L, Sun J, Xie J, Wang T, Yin X, et al. Six-transmembrane epithelial antigen of the prostate 1 expression promotes ovarian cancer metastasis by aiding progression of epithelial-to-mesenchymal transition. Histochem Cell Biol. (2020) 154:215–30. doi: 10.1007/s00418-020-01877-7
148. Huo SF, Shang WL, Yu M, Ren XP, Wen HX, Chai CY, et al. STEAP1 facilitates metastasis and epithelial-mesenchymal transition of lung adenocarcinoma via the JAK2/STAT3 signaling pathway. Biosci Rep. (2020) 40. doi: 10.1042/bsr20193169
149. Gomes IM, Rocha SM, Gaspar C, Alvelos MI, Santos CR, Socorro S, et al. Knockdown of STEAP1 inhibits cell growth and induces apoptosis in LNCaP prostate cancer cells counteracting the effect of androgens. Med Oncol. (2018) 35:40. doi: 10.1007/s12032-018-1100-0
150. Bhatia V, Kamat NV, Pariva TE, Wu LT, Tsao A, Sasaki K, et al. Targeting advanced prostate cancer with STEAP1 chimeric antigen receptor T cell and tumor-localized IL-12 immunotherapy. Nat Commun. (2023) 14:2041. doi: 10.1038/s41467-023-37874-2
151. Hubert RS, Vivanco I, Chen E, Rastegar S, Leong K, Mitchell SC, et al. STEAP: a prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc Natl Acad Sci U.S.A. (1999) 96:14523–8. doi: 10.1073/pnas.96.25.14523
152. Lee JK, Bangayan NJ, Chai T, Smith BA, Pariva TE, Yun S, et al. Systemic surfaceome profiling identifies target antigens for immune-based therapy in subtypes of advanced prostate cancer. Proc Natl Acad Sci U.S.A. (2018) 115:E4473–e4482. doi: 10.1073/pnas.1802354115
153. Ylitalo EB, Thysell E, Jernberg E, Lundholm M, Crnalic S, Egevad L, et al. Subgroups of castration-resistant prostate cancer bone metastases defined through an inverse relationship between androgen receptor activity and immune response. Eur Urol. (2017) 71:776–87. doi: 10.1016/j.eururo.2016.07.033
154. Burnell SEA, Spencer-Harty S, Howarth S, Bodger O, Kynaston H, Morgan C, et al. Utilisation of the STEAP protein family in a diagnostic setting may provide a more comprehensive prognosis of prostate cancer. PloS One. (2019) 14:e0220456. doi: 10.1371/journal.pone.0220456
155. Hasegawa H, Li C, Alba BM, Penny DM, Xia Z, Dayao MR, et al. Membrane cholesterol modulates STEAP2 conformation during dynamic intracellular trafficking processes leading to broad subcellular distribution. Exp Cell Res. (2018) 370:208–26. doi: 10.1016/j.yexcr.2018.06.022
156. Zanvit P, van Dyk D, Fazenbaker C, McGlinchey K, Luo W, Pezold JM, et al. Antitumor activity of AZD0754, a dnTGFβRII-armored, STEAP2-targeted CAR-T cell therapy, in prostate cancer. J Clin Invest. (2023) 133. doi: 10.1172/jci169655
157. Zhang G, Zhang H, Wang Q, Lal P, Carroll AM, de la Llera-Moya M, et al. Suppression of human prostate tumor growth by a unique prostate-specific monoclonal antibody F77 targeting a glycolipid marker. Proc Natl Acad Sci U.S.A. (2010) 107:732–7. doi: 10.1073/pnas.0911397107
158. Grover P, Nunez-Cruz S, Leferovich J, Wentz T, Bagchi A, Milone MC, et al. F77 antigen is a promising target for adoptive T cell therapy of prostate cancer. Biochem Biophys Res Commun. (2023) 680:51–60. doi: 10.1016/j.bbrc.2023.09.018
159. Hong M, Clubb JD, Chen YY. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell. (2020) 38:473–88. doi: 10.1016/j.ccell.2020.07.005
160. Nayerpour Dizaj T, Doustmihan A, Sadeghzadeh Oskouei B, Akbari M, Jaymand M, Mazloomi M, et al. Significance of PSCA as a novel prognostic marker and therapeutic target for cancer. Cancer Cell Int. (2024) 24:135. doi: 10.1186/s12935-024-03320-6
161. Wang JH, Kiess AP. PSMA-targeted therapy for non-prostate cancers. Front Oncol. (2023) 13:1220586. doi: 10.3389/fonc.2023.1220586
162. Dhar P, Wu JD. NKG2D and its ligands in cancer. Curr Opin Immunol. (2018) 51:55–61. doi: 10.1016/j.coi.2018.02.004
163. Zhou WT, Jin WL. B7-H3/CD276: an emerging cancer immunotherapy. Front Immunol. (2021) 12:701006. doi: 10.3389/fimmu.2021.701006
164. Baciarello G, Gizzi M, Fizazi K. Advancing therapies in metastatic castration-resistant prostate cancer. Expert Opin Pharmacother. (2018) 19:1797–804. doi: 10.1080/14656566.2018.1527312
165. Bansal D, Reimers MA, Knoche EM, Pachynski RK. Immunotherapy and immunotherapy combinations in metastatic castration-resistant prostate cancer. Cancers (Basel). (2021) 13:1–18. doi: 10.3390/cancers13020334
Keywords: prostate cancer, immunotherapy, TMEs, cancer vaccines, immune checkpoint inhibitors, adoptive cell therapy, CAR-T cells
Citation: Che J, Liu Y, Liu Y, Song J, Cui H, Feng D, Tian A, Zhang Z and Xu Y (2025) The application of emerging immunotherapy in the treatment of prostate cancer: progress, dilemma and promise. Front. Immunol. 16:1544882. doi: 10.3389/fimmu.2025.1544882
Received: 13 December 2024; Accepted: 25 February 2025;
Published: 12 March 2025.
Edited by:
Jorge Adrián Ramírez De Arellano Sánchez, University of Guadalajara, MexicoReviewed by:
Simon Hawlina, University Medical Centre Ljubljana, SloveniaKenneth Sooi, National University Cancer Institute, Singapore
Sobia Wasim, Gachon University, Republic of Korea
Copyright © 2025 Che, Liu, Liu, Song, Cui, Feng, Tian, Zhang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yankai Xu, Nzg0ODg0NTZAcXEuY29t; Zhengchao Zhang, Ynl5dGZ5enpjQDE2My5jb20=
†These authors have contributed equally to this work
 Jizhong Che1†
Jizhong Che1† Zhengchao Zhang
Zhengchao Zhang