
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol., 26 February 2025
Sec. Viral Immunology
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1544622
A correction has been applied to this article in:
Corrigendum: Case Report: HLA-DRB1 04:01 found in a child with adenovirus type 2 -linked hepatitis
 Yoshiki Katsumi1*
Yoshiki Katsumi1* Yui Nishimura1
Yui Nishimura1 Sachiko Goto1
Sachiko Goto1 Seiichiro Ozawa1
Seiichiro Ozawa1 Tomoko Nishiura2
Tomoko Nishiura2 Akira Kotera3
Akira Kotera3 Yoshiyuki Kawahara3
Yoshiyuki Kawahara3 Shiori Higashikawa3
Shiori Higashikawa3 Rina Iwasaki3
Rina Iwasaki3 Yutaka Toriiminami3
Yutaka Toriiminami3 Norio Asai3
Norio Asai3 Naohisa Fujita3
Naohisa Fujita3Since 2022, cases of hepatitis of unknown origin have been reported in children worldwide. Adeno-associated virus type 2 (AAV2) was identified as a cause, with most affected children having the HLA-DRB1 04:01 genotype. In this study, we hypothesized that HLA-DRB1 04:01 in the host may also be a potential predisposing factor of acute hepatitis caused by other viruses. We report a case that met the definition of severe hepatitis of unknown cause in a child; adenovirus type 2 (AV2) was detected in her specimens. The patient was a 1-year-old girl who visited a doctor because of fever occurring 1–2 days per week, respiratory symptoms, and diarrhea. One month later, the patient was referred to our hospital because of prolonged elevated liver enzyme concentrations. Two weeks after the initial visit, her aspartate aminotransferase (AST) and alanine aminotransferase (ALT) concentrations increased to 1558 and 1843 IU/L, respectively. The patient’s liver enzyme concentrations decreased markedly with only observation and intravenous hydration during hospitalization within a few days. Thereafter, hepatic enzymes were transiently elevated with each common cold, but all recovered spontaneously. The adenovirus (AV) antibody levels increased substantially 2 weeks after admission. The patient’s human leukocyte antigen (HLA) was determined to be of the DRB1 04:01 genotype. The presence of HLA-DRB1 04:01 is consistent with that reported in pediatric patients with AAV2 hepatitis in the United Kingdom, indicating that it may have been involved in the host immune response and acute hepatitis in this child. HLA-DRB1 04:01 may predispose children to acute hepatitis from various viruses, including AV2, AAV2, and possibly respiratory viruses, which requires clinical attention.
Since the initial reports of children with acute hepatitis of unknown origin in Scotland (1, 2), similar cases have been identified globally. Although adenovirus type 41 (AV41) or adeno-associated virus type 2 (AAV2) was suspected to be the cause of hepatitis in Europe and the USA (3, 4), no evidence was found.
The human leukocyte antigen (HLA) type has been suggested to affect hepatitis susceptibility via immune-modifying effects in hosts, and HLA-DRB1 04:01 has been reported as a host factor responsible for autoimmune hepatitis (AIH) in adults (5). Here, we report a case of acute severe hepatitis in which adenovirus type 2 (AV2) was identified and HLA-DRB1 04:01 was detected in the host; this genotype was suspected to be the immunological background for factors contributing to the development of hepatitis following the coronavirus disease 2019 (COVID-19) pandemic.
A 1-year-old girl first visited a local doctor due to recurrent fever (1–2 days a week), coughing, runny nose, and diarrhea, which persisted for 2 weeks after starting nursery school. The doctor consulted us due to a prolonged elevation of liver enzymes for a month, with aspartate aminotransferase (AST) ranging from 86 to 101 IU/L and alanine aminotransferase (ALT) from 110 to 185 IU/L. Upon arriving at our hospital, the patient’s body temperature was recorded to be 37.1°C, with no jaundice or hepatosplenomegaly observed. The patient appeared healthy and had no family history of hepatitis.
The liver enzyme concentrations changed negligibly until two weeks after the first visit to our hospital, when the patient’s AST and ALT concentrations suddenly increased to 1558 IU/L and 1843 IU/L, respectively, and gamma-glutamyl transpeptidase concentration was 188 U/L (Table 1). She was admitted as a case of severe hepatitis of unknown cause, many of which had been reported at that time. The patient’s temperature remained in the range of 37 to 38°C, and there was no jaundice, somnolence, vomiting, or abdominal pain. Abdominal computed tomography indicated mild hepatomegaly. We administered intravenous hydration to the patient after examination. Four days after admission, the patient was discharged from the hospital after spontaneous recovery and reduction of enzyme concentrations to 124 IU/L (for AST), 484 IU/L (for ALT), and 100 U/L (for gamma-glutamyl transpeptidase).
When the patient was admitted to our hospital, pharyngeal swabs and blood, stool, and urine specimens were collected to identify the causative pathogen. Specimens were used for viral isolation, polymerase chain reaction (PCR), neutralizing antibody testing, and metagenomic analysis. All specimens were identified as AV2 by sequencing. Other hepatitis-causing viruses were not detected in PCR of the blood, stool, and throat swab specimens (Supplementary Table 1). The AV antibody level was less than four-fold at the time of admission and increased 64-fold subsequently, confirming acute adenovirus infection at the time of hepatitis. In the metagenomic analyses, human mastadenovirus C was detected in all specimens, which was consistent with the results of sequencing. No other causative bacteria or viruses, including AAV2, were detected (Supplementary Figure 1). Based on these results, the patient was diagnosed with hepatitis and acute AV2 infection. There was no history of vaccination or illness with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the result of the SARS-CoV-2 antibody test was negative.
The patient’s HLA-DRB1 genotype was 04:01, which is consistent with the result of a previously-reported case of childhood hepatitis involving AAV2. Two weeks after discharge, the patient presented with symptoms of coughing, runny nose, fever, and blisters in the throat, similar to those seen in patients with herpangina but with no diarrhea. The patient presented elevated liver enzymes (highest AST 635 IU/L and highest ALT 1029 IU/L), and was again diagnosed as severe hepatitis of unknown cause, but the child recovered after a few days without hospitalization (Figure 1). At that time, only viral culture and PCR tests were performed for AV2 on blood samples. Cytopathic effects were observed in the third passage and AV2 was confirmed by sequencing. However, it was difficult to determine whether the result was truly AV2 infection or viral contamination. Another month later, fever and respiratory symptoms re-appeared, and elevated liver enzymes (highest AST 791 IU/L and highest ALT 991 IU/L) again met the definition of severe hepatitis of unknown cause. This child recovered without hospitalization. Only blood was used for viral culture and PCR testing, and nothing viral (including AV2) was detected. We did not perform a metagenomic analysis.
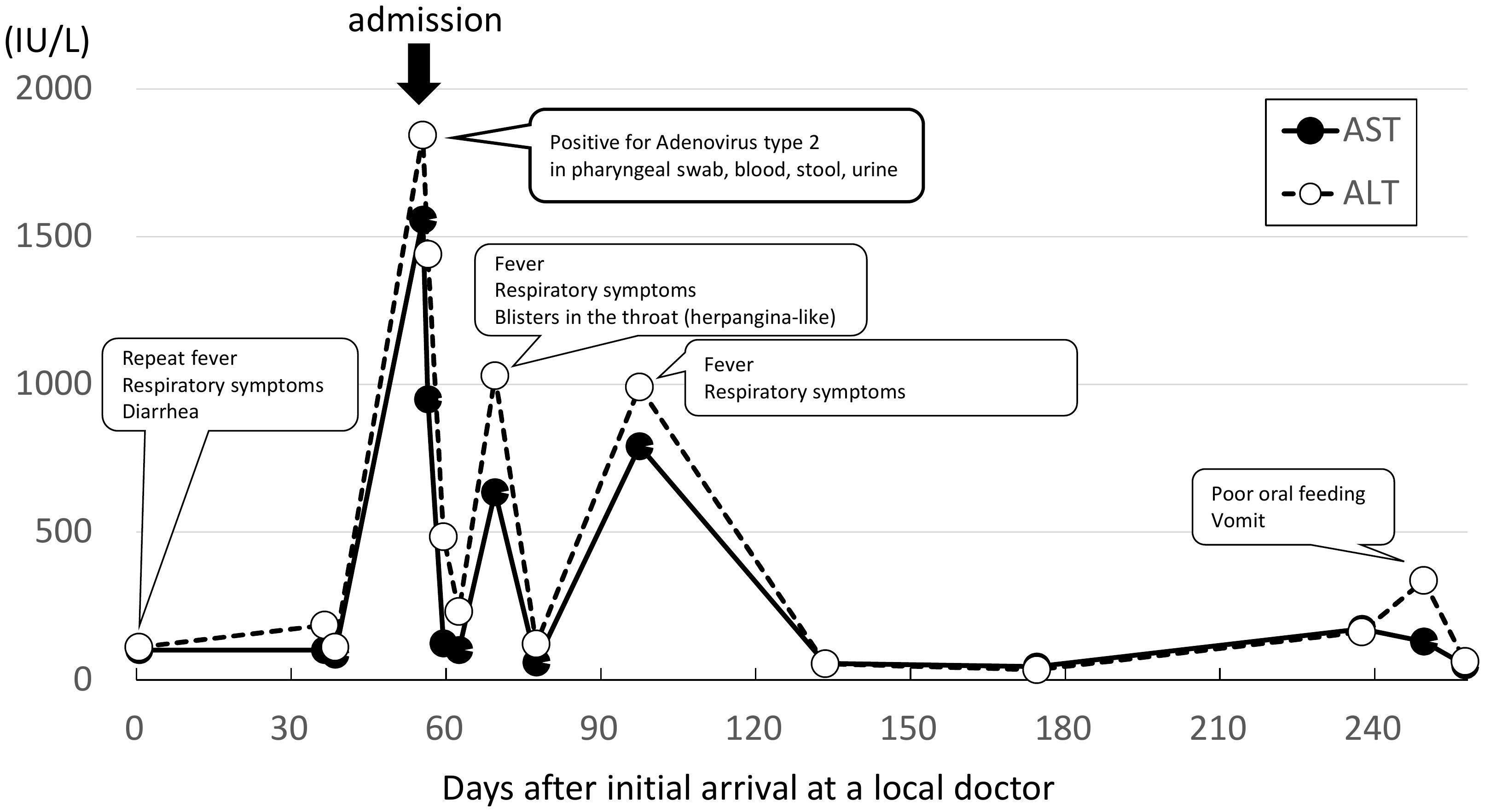
Figure 1. Symptoms and changes in liver enzyme concentrations. The patient went to a nursery school when she felt good and did not have a fever, suggesting that she might possibly have been infected with other pathogens after the adenovirus type 2 infection.
From the present case, we inferred that the HLA-DRB1 04:01 genotype may be a factor that predisposes to acute hepatitis caused by AV2. Pediatric cases of acute hepatitis of unknown cause have been reported worldwide, including in Europe and the USA, starting with a report from Scotland in 2022 (1, 3, 6). AV41 and AAV2 are suspected to cause acute hepatitis (1, 3, 4). However, no definitive conclusions have been made regarding the causative viruses and underlying mechanism. A Japanese survey could not detect any trend that may suggest a causal relationship with AV41 or AAV2 (7). Fifty pediatric cases of hepatitis of unknown cause were analyzed from 2017–2023 in Japan (including the pre-COVID-19 pandemic period). The number of AAV2-positive cases was 12% (6 out of 49 cases) from PCR using blood samples. Co-infection with AV, human herpes virus 6, cytomegalovirus, or Epstein–Barr virus as helper viruses was also observed among AAV2-positive cases (half of the cases had co-infections). Hepatitis due to AAV2 appeared to have occurred sporadically before the COVID-19 pandemic (8).
Studies have shown that the whole blood of 90% children with acute hepatitis of unknown etiology was positive for adenovirus (3, 6). However, the number of AV-positive cases with acute hepatitis that harbored AV2 in this analysis was not clear. AV2 was the most frequent (26%) isolated AV in a Japanese survey in which all reported adenovirus infections were investigated from 2008–2017 (9), whereas AV2 was detected in very few cases of hepatitis after 2022 (10).
Does any host element increase the predisposition to hepatitis? Reports show that the HLA type is involved in the induction of hepatitis as a host factor in patients with AIH (5). AIH is a chronic form of hepatitis that is common in middle-aged women. The disease begins with fatigue, jaundice, and anorexia. Viral infections and some drugs are considered triggers of AIH; however, a genetic predisposition based on HLA is also thought to be involved. Reports from Japan, England, China, and Iraq suggest that HLA-DRB1 04:01 is associated with AIH type 1 in adults (5, 11–17). In Japan, 154 cases of AIH in adults aged 48–66 years were reported (201 cases in healthy subjects). The liver histopathology of adenovirus-associated hepatitis resembles that observed in AIH, suggesting the involvement of immune-mediated processes (18). Since various viral infections are proposed to trigger AIH (19), AV infection could also potentially trigger AIH. In the current case, a liver biopsy was not performed; it was therefore difficult to identify the cause of hepatitis as AV2 infection in the liver. Based on the acute course and the patient’s age, AIH was excluded from the diagnosis in this case.
HLA-DRB1 04:01 is found in less than 1% of the Japanese population. HLA-DRB1 04:01 has been reported in pediatric patients with AAV2-induced hepatitis in the United Kingdom (4). In this study, of the 27 cases of hepatitis with AAV2 infection in children, 25 (93%) had the HLA-DRB1 04:01 genotype. In contrast, 10 (15.6%) of the 64 controls (including 12 adenovirus-infected patients without elevated liver enzyme concentrations and 33 patients with elevated liver enzyme concentrations but without adenovirus infection) had the HLA-DRB1 04:01 genotype. According to the British Biobank, the frequency of HLA-DRB1 04:01 in the United Kingdom is 2942/29379 (10%).
Studies suggest that HLA class II genes, such as HLA-DPB1 and HLA-DRB1, which are involved in presenting antigens to T cells, may influence the immune response to viruses and viral vaccines (20–23).
In the current patient’s last hepatitis episode, AV2 was not detected, but we could not rule out AV2 as the cause. The patient presented with upper respiratory tract inflammatory symptoms consistent with the common cold, suggesting a possible infection with a respiratory virus, such as rhinovirus. Human rhinovirus, which is the causative virus in 50% of common colds, infects vascular endothelium and initiates an antiviral and inflammatory innate response (24, 25). In Australia, the association between picornaviruses and respiratory syncytial virus and pediatric hepatitis was suspected before the COVID-19 pandemic, and the association between picornaviruses and pediatric hepatitis became stronger after the pandemic (26). Both human rhinovirus and enterovirus, which might have caused a common cold in the current case, are picornaviruses. This risk may be heightened when these viruses are present in larger quantities in the blood, due to reduced immunity against them caused by COVID-19 measures. It may not be necessary for the respiratory virus to infect hepatocytes; infection of endothelial cells within the liver can also cause liver damage. The liver damage observed in the current patient, associated with the upper respiratory tract infection, could be linked to the HLA-DRB1 04:01 genotype.
This was a single case report, and the possibility that the HLA-DRB1 04:01 genotype was incidentally detected in a host with hepatitis caused by AV2 cannot be ruled out. However, HLA-DRB1 04:01 is rare in Japan, found in less than 1% of the population. Based on this proposed mechanism, we assumed that host HLA-DRB1 04:01 was a possible predisposing factor for acute hepatitis caused by various viruses, including AV2, AAV2, and possibly respiratory viruses, although the causal relationship could not be confirmed.
In the future, it is expected that the immunological mechanism will be verified by basic research using HLA-DRB1 04:01 transgenic mice and viral infection of these mice; the causal relationship can then be elucidated using clinical multi-case studies of acute hepatitis in children analyzed for HLA and liver tissue histopathology.
To analyze the metagenome in pharyngeal swabs and blood, stool, and urine specimens, we extracted RNA using the QIAamp Viral RNA Mini Kit (QIAGEN, Redwood City, CA) and prepared the RNA library according to the protocol of the Zymo-Seq RiboFree Total RNA Library Kit (Zymo Research, Irvine, CA). Libraries were sequenced on the iSeq100 system or MiSeq system (Illumina, San Diego, CA) with 2 × 150 bp paired ends. The output FASTQ files were trimmed, and human-derived reads were removed using kneaddata v0.12.0 (https://github.com/biobakery/kneaddata). Using kraken2 v2.1.3 (27), the data were classified. The report was then visualized using krona v2.8.1 (28) to search for pathogens. HLA-DRB1 four-digit allele typing was performed using the Luminex 200 system (Luminex, Austin, TX) and the WAKFlow HLA Typing Kit (Wakunaga, Hiroshima, Japan). HLA alleles were automatically assigned using WAKFlow typing software (Wakunaga, Hiroshima, Japan). The probe sequences of the WAKFlow Typing kit are specifically designed to make allele determination easier in the Japanese population. By default, the analysis with WAKFlow typing software is based on the allele frequencies of the donors registered with Japan Marrow Donor Program (JMDP; https://www.bs.jrc.or.jp/bmdc/). This method can determine alleles with frequencies of ≥0.1% in the Japanese population.
Acute hepatitis in children with unknown causes should be evaluated not only from the perspective of the virus, but should also host factors, including the HLA type.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving humans were approved by the ethics review board of Kyoto Saiseikai Hospital, Kyoto Saiseikai Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
YKat: Conceptualization, Data curation, Investigation, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. YN: Conceptualization, Project administration, Writing – review & editing. SG: Project administration, Writing – review & editing. SO: Project administration, Writing – review & editing. TN: Conceptualization, Project administration, Writing – review & editing. AK: Data curation, Investigation, Project administration, Supervision, Validation, Writing – review & editing. YKaw: Data curation, Investigation, Project administration, Supervision, Validation, Writing – review & editing. SH: Investigation, Writing – review & editing. RI: Investigation, Writing – review & editing. YT: Data curation, Investigation, Project administration, Supervision, Validation, Writing – review & editing. NA: Data curation, Investigation, Project administration, Supervision, Validation, Writing – review & editing. NF: Conceptualization, Data curation, Investigation, Project administration, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The metagenomic analysis in this study was supported by AMED under Grant Number, JP22fk0108636.
We thank the medical staff and nurses of the Pediatric Department of Saiseikai Kyoto Hospital for patient care. We thank the staff of the Otokuni Health Center for delivering patient specimens. We thank the doctors at the National Institute of Infectious Diseases of Japan for the results of the viral tests.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1544622/full#supplementary-material
AV, adenovirus; AAV, adeno-associated virus; HLA, human leukocyte antigen; AIH, autoimmune hepatitis; COVID-19, coronavirus disease 2019; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
1. World Health Organization. Severe acute hepatitis of unknown aetiology in children – multi-country (2022). Available online at: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON400 (Accessed January 26, 2025).
2. World Health Organization. Acute hepatitis of unknown aetiology (2022). The United Kingdom of Great Britain and Northern Ireland. Available online at: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON368 (Accessed January 26, 2025).
3. Gutierrez Sanchez LH, Shiau H, Baker JM, Saaybi S, Buchfellner M, Britt W, et al. A case series of children with acute hepatitis and human adenovirus infection. N Engl J Med. (2022) 387:620–30. doi: 10.1056/NEJMoa2206294
4. Ho A, Orton R, Tayler R, Asamaphan P, Herder V, Davis C, et al. Adeno-associated virus 2 infection in children with non-A-E hepatitis. Nature. (2023) 617:555–63. doi: 10.1038/s41586-023-05948-2
5. Seki T, Ota M, Fukushima H, Kondo T, Hino K, et al. HLA class II molecules and autoimmune hepatitis susceptibility in Japanese patients. Gastroenterology. (1992) 103:1041–7. doi: 10.1016/0016-5085(92)90041-v
6. Kelgeri C, Couper M, Gupte GL, Brant A, Patel M, Johansen L, et al. Clinical spectrum of children with acute hepatitis of unknown cause. N Engl J Med. (2022) 387:611–9. doi: 10.1056/NEJMoa2206704
7. Otake S, Ikenoue C, Sudani N, Kobayashi M, Takahashi K, Shimada T, et al. National surveillance of pediatric acute hepatitis of unknown etiology, Japan, October 2021-December 2022. Emerg Infect Dis Japan: October. (2023) 29:1288–91. doi: 10.3201/eid2906.221579
8. Iwata KI, Torii Y, Sakai A, Fukuda Y, Haruta K, Yamaguchi M, et al. Association between adeno-associated virus 2 and severe acute hepatitis of unknown etiology in Japanese children. J Infect Chemother. (2024) S1341-321X:00180–6. doi: 10.1016/j.jiac.2024.07.002
9. National Institute of Infectious Disease. Adenovirus infections, 2008 to June 2017. Japan (2017). Available online at: https://www.niid.go.jp/niid/en/iasr-vol38-e/865-iasr/7390-449te.html (Accessed January 26, 2025).
10. Rodriguez-Frias F, Rando-Segura A, Quer J. Solved the enigma of pediatric severe acute hepatitis of unknown origin? Front Cell Infect Microbiol. (2023) 13:1175996. doi: 10.3389/fcimb.2023.1175996
11. Yoshizawa K, Ota M, Katsuyama Y, Ichijo T, Matsumoto A, Tanaka E, et al. Genetic analysis of the HLA region of Japanese patients with type 1 autoimmune hepatitis. J Hepatol. (2005) 42:578–84. doi: 10.1016/j.jhep.2004.12.019
12. Umemura T, Katsuyama Y, Yoshizawa K, Kimura T, Joshita S, Komatsu M, et al. Human leukocyte antigen class II haplotypes affect clinical characteristics and progression of type 1 autoimmune hepatitis in Japan. PloS One. (2014) 9:e100565. doi: 10.1371/journal.pone.0100565
13. Doherty DG, Donaldson PT, Underhill JA, Farrant JM, Duthie A, Mieli-Vergani G, et al. Allelic sequence variation in the HLA class II genes and proteins in patients with autoimmune hepatitis. Hepatology. (1994) 19:609–15. doi: 10.1002/hep.1840190311
14. Strettell MD, Donaldson PT, Thomson LJ, Santrach PJ, Moore SB, Czaja AJ, et al. Allelic basis for HLA-encoded susceptibility to type 1 autoimmune hepatitis. Gastroenterology. (1997) 112:2028–35. doi: 10.1053/gast.1997.v112.pm9178696
15. Czaja AJ, Strettell MD, Thomson LJ, Santrach PJ, Moore SB, Donaldson PT, et al. Associations between alleles of the major histocompatibility complex and type 1 autoimmune hepatitis. Hepatology. (1997) 25:317–23. doi: 10.1002/hep.510250211
16. Yang F, Zhou L, Shen Y, Zhao S, Zheng Y, Men R, et al. Metabolic heterogeneity caused by HLA-DRB1*04:05 and protective effect of inosine on autoimmune hepatitis. Front Immunol. (2022) 13:982186. doi: 10.3389/fimmu.2022.982186
17. Ibraheem MF, Ahmed SJ. The association between juvenile autoimmune hepatitis and HLA-DRB1 alleles: Iraqi tertiary center experience. Clin Exp Hepatol. (2021) 7:178–82. doi: 10.5114/ceh.2021.106865
18. Liang J, Kelly DR, Pai A, Gillis LA, Sanchez LHG, Shiau HH, et al. Clinicopathologic features of severe acute hepatitis associated with adenovirus infection in children. Am J Surg Pathol. (2023) 47:977–89. doi: 10.1097/PAS.0000000000002084
19. Czaja AJ. Examining pathogenic concepts of autoimmune hepatitis for cues to future investigations and interventions. World J Gastroenterol. (2019) 25:6579–606. doi: 10.3748/wjg.v25.i45.6579
20. Nishida N, Ohashi J, Khor SS, Sugiyama M, Tsuchiura T, Sawai H, et al. Understanding of HLA-conferred susceptibility to chronic hepatitis B infection requires HLA genotyping-based association analysis. Sci Rep. (2016) 19:24767. doi: 10.1038/srep24767
21. Setoyama H, Nishida N, Nagashima S, Ko K, Yamazoe T, Tanaka Y, et al. Dried blood spot-based host genome analysis technique targeting pathological associations with hepatitis B: Development and clinical application in the Cambodian population. Hepatol Res. (2023) 53:1147–55. doi: 10.1111/hepr.13949
22. Laporte GB, Auckland K, Noor Z, Kabir M, Alam M, Carstensen T, et al. Targeting hepatitis B vaccine escape using immunogenetics in Bangladeshi infants. medRxiv. (2023) 29. doi: 10.1101/2023.06.26.23291885. Preprint.
23. Yao Y, Yang H, Shi L, Liu S, Li C, Chen J, et al. HLA class II genes HLA-DRB1, HLA-DPB1, and HLA-DQB1 are associated with the antibody response to inactivated Japanese encephalitis vaccine. Front Immunol. (2019) 10:428. doi: 10.3389/fimmu.2019.00428
24. Chałubiński M, Szulc A, Pawełczyk M, Gajewski A, Gawrysiak M, Likońska A, et al. Human rhinovirus 16 induces antiviral and inflammatory response in the human vascular endothelium. APMIS. (2021) 129:143–51. doi: 10.1111/apm.13103
25. Winther B. Rhinoviruses. In: Heggenhougen HK, editor. International Encyclopedia of Public Health. Academic Press, Cambridge, MA (2008). p. 577–81. doi: 10.1016/B978-012373960-5.00610-9
26. Sawires R, Osowicki J, Clothier H, Fahey M, Buttery J. Pediatric hepatitis and respiratory viruses: a spatiotemporal ecologic analysis. Pediatr Infect Dis J. (2023) 42:276–80. doi: 10.1097/INF.0000000000003828
27. Wood DE, Salzberg SL. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. (2014) 15:R46. doi: 10.1186/gb-2014-15-3-r46
Keywords: hepatitis, child, HLA-DR, adenovirus, common cold
Citation: Katsumi Y, Nishimura Y, Goto S, Ozawa S, Nishiura T, Kotera A, Kawahara Y, Higashikawa S, Iwasaki R, Toriiminami Y, Asai N and Fujita N (2025) Case Report: HLA-DRB1 04:01 found in a child with adenovirus type 2 -linked hepatitis. Front. Immunol. 16:1544622. doi: 10.3389/fimmu.2025.1544622
Received: 13 December 2024; Accepted: 10 February 2025;
Published: 26 February 2025.
Edited by:
Marcelo A. Soares, National Cancer Institute (INCA), BrazilReviewed by:
Daisuke Miyazawa, Miyazawa Clinic, JapanCopyright © 2025 Katsumi, Nishimura, Goto, Ozawa, Nishiura, Kotera, Kawahara, Higashikawa, Iwasaki, Toriiminami, Asai and Fujita. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshiki Katsumi, cHJvc3RhZmZAa290by5rcHUtbS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.