
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Immunol., 03 March 2025
Sec. Immunological Tolerance and Regulation
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1543495
Stem cells are an important compartment supporting tissues with differentiating cells and responding to regeneration demand (1–4). There are interesting evidences that subpopulations of stem cells migrate to developing organs and tissues during embryogenesis, but do not directly contribute to development (5–10). They persist to provide support as stem cells of the adult organism. If more cells would have proliferative and differentiation potential of stem cells, it would be good for regeneration. However, it should be balanced with risks of mutations and oncogenesis. A self-maintaining, highly proliferative cell would need fewer changes to become a cancer cell. The quiescence of stem cells with high proliferative potential could be a solution to place them evolutionarily further away from cancer cells (11). Slow dividing cells also have a lower mutation potential associated with the number of divisions (11, 12). Lower mutational potential is associated with resistance to oncogenesis, as well as with a lower number of neoantigens and consequently lower autoimmunity (13). These reasons are important, but in the context of long-living strategies, could there be benefits from keeping stem cells quiescent in the short-term? The quiescence of stem cells, coupled with their metabolic processes, enables them to survive in severely damaged tissues, thereby facilitating regeneration (14, 15). Given their role in regeneration, an increase in the number of stem cells would be expected to enhance the regenerative potential. Stem cells from stroma of adult tissues are maintained at the proportion 10-5-10-4 (16–21). There should be reasons to maintain this stem cell number at a relatively low level. An interesting note that an increased number of stem cells suggests a lower number of divisions for each, this way significantly reducing a chance of a random cooperation of oncogenic mutations in a single cell, thereby lowering cancer risk (11). A potential explanation is that it is a matter of energy consumption efficiency. However, a tenfold change in the number of stem cells results in a mere 0.1% alteration in the total value. More firm reasons could be derived from the immunomodulatory properties of stem cells. Pronounced and diverse immune modulation of mesenchymal stem cells (MSCs) earlier led to their identification as agents of the immune system (22, 23). However, demonstrated later immune rejection of mesenchymal cells in transplantations ceased that idea (24, 25). Recently, the immune privileges (IPs) of stem cells, including MSCs, have been demonstrated (20, 26–28). It was previously suggested that the IPs of stem cells are associated with their quiescent state and relate to regeneration and inflammation regulation (26, 29). A number of molecular mechanisms are demonstrated to contribute to immune modulation exerted by MSCs, qualifying them as immune modulatory stem cells (IMSCs) (30). That way, IMSCs are cells demonstrating active participation in immune regulation and capability of IPs. I propose a generalized model that functionally links the newly demonstrated IPs to other attributes of IMSCs.
The functional significance of IMSCs is of particular evolutionary importance with respect to the stem and immune systems (29). The reports indicate that MSCs not only evade cytotoxic immune action (31), but also actively attract immune cells and can activate or reprogram them depending on the molecular context (32–36). Immune modulation of stem cells is employed in the context of solid organ transplantation and is utilized in the treatment of autoimmune pathology (32–35). This gives ground to mark MSCs as baring functional of immune suppression and as IMSCs. The activation of MSCs and the subsequent induction of the regenerative program results in the suppression of the inflammatory program (34, 37). MSCs have been shown to express a range of immunosuppressing molecules, including PGE2, TGF‐β, HLA-G5, IL‐10, HGF, galectins, CD73, CD39, PD-L1, HLA-G1 and other (30, 34, 37, 38). Immunomodulatory capabilities are more pronounced in IMSCs than in other differentiated cells (39, 40). It is challenging to determine where the immune or other functions of IMSCs are lost during differentiation to their progeny, particularly in light of the potential for dedifferentiation (41, 42). The existing mutual integration of stem and immune systems highlights the evolutionary significance of this integration, as an additional mechanism may potentially act as a break point. This underscores the necessity for evolutionary coordination with respect to the attributes of immune and stem cells involved in this integration. The construction of a comprehensive model is hindered by the vast number of elements and the incomplete knowledge about their connections. Therefore, I propose a functional model (Figure 1).
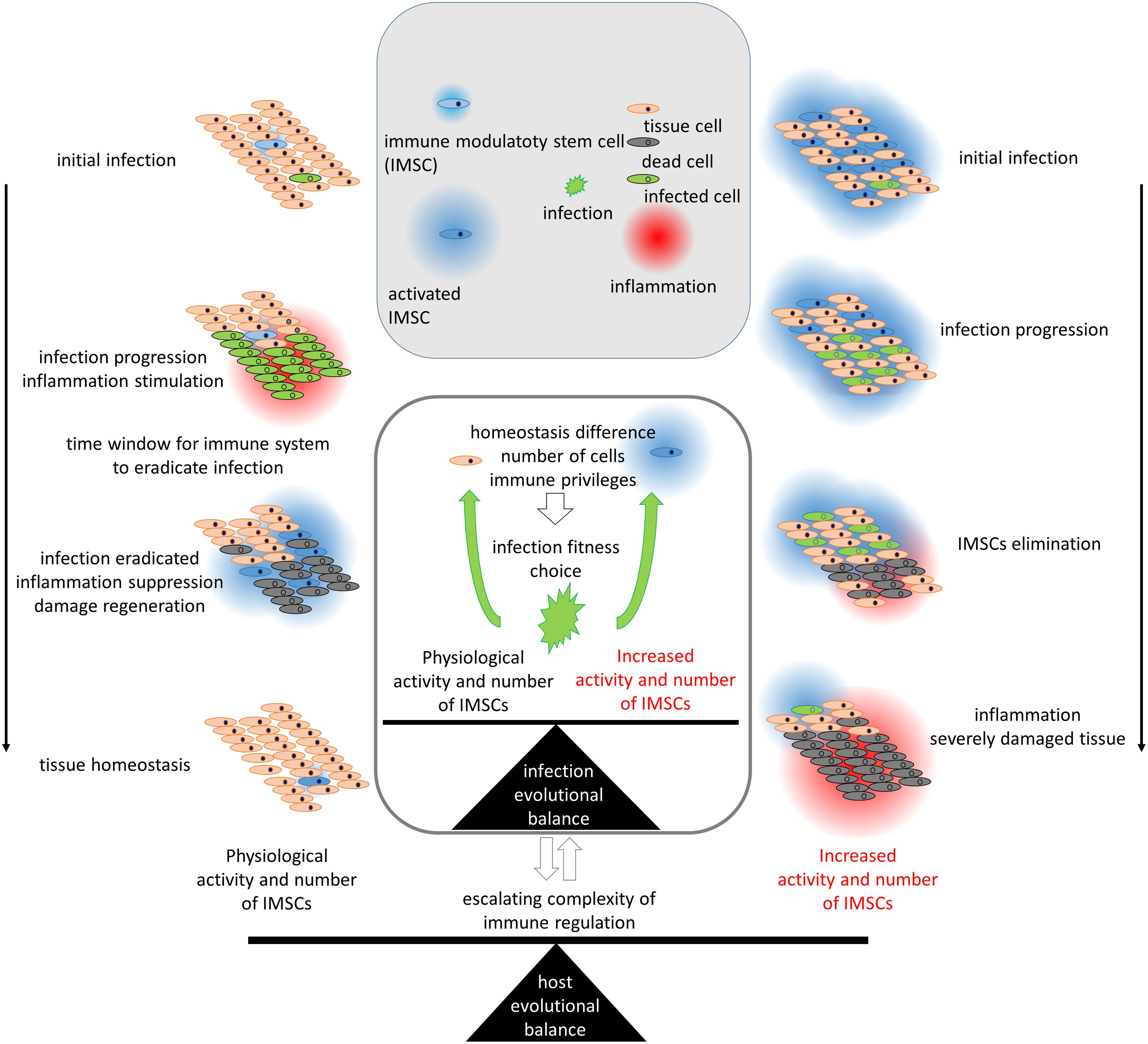
Figure 1. The evolutionary regulation of the activity and number of immune modulatory stem cells (IMSCs) according to their role in the immune system. The left part represents the process of infection under physiological conditions, while the right part represents a hypothetical scenario with increased activity and number of IMSCs. Infection of tissue cells leads to cell death and inflammation of the area. Rare IMSCs, which are able to survive and proliferate in damaged areas due to their homeostatic adaptation, are attracted to the inflamed area. Activated IMSCs suppress inflammation and drive regeneration. Suppressive immune modulation provides protection from the immune system and could be exploited by invading pathogens. The differences in homeostasis of IMSCs protect them from infections. An increase in the number or activity of IMSCs would provide an evolutionary opportunity for infections to adapt to the IMSC phenotype and support the spread of infection, leading to severe tissue damage. Thus, IMSCs and infections are interdependent in the evolutionary equilibrium. The evolutionary competition between host and infection leads to an escalation in the complexity of immune regulation.
As IMSCs provide immune suppression upon activation (32, 34, 37), they should remain inactive. Otherwise, their immune suppression could potentially compromise the immune protection of a tissue from invading pathogens. A higher concentration of IMSCs results in a more pronounced immune suppression, so reasoning their limited numbers (Figure 1). In this manner, IMSCs serve as an activating special agent in the periphery, suppressing the potentially destructive actions of an overactive immune system. This model offers an evolutionary rationale for the maintenance of IMSC quiescence and their low numbers. The traumas and infections have higher risks during life than cancer, so provide possibly stronger selective pressure for long-term living strategies and stay actual for even short-term living strategies.
In the event of infection, resident cells signal to attract immune cells. MSCs are among the cells that signal for immune activation (35, 36, 43). The relatively limited number of IMSCs induces suppressing signals at a slower rate than the initial proinflammatory reaction. This allows the necessary time for an acute inflammatory reaction to occur (Figure 1). Upon activation, IMSCs migrate to sites of damage (17, 19, 44, 45), where they exert their immunosuppressive effects. Over time, the inflammatory response stimulates the stem system, thereby inducing its regenerative and anti-inflammatory functions. As a result, the initially inflamed area becomes an area of active regeneration, with the inflammatory response polarized toward a regenerative subtype.
Immune suppression functions can be exploited by invading pathogens (43, 46, 47). The immune system is also responsible for protecting against oncogenesis (48), this emphasizes the risks associated with IPs (46, 49, 50). That way immune suppression should be presented by complex and enigmatic regulation, which serves as a natural barrier against hijacking. Furthermore, the regulatory mechanisms must be robust. The additional protection is provided by a strong connection of this function to a small subpopulation of IMSCs. If pathogens target IMSCs and their immunosuppression, it would be necessary for infection to evolve in order to fit the specific conditions of the stem niche. The physiology and energy exchange of stem cells enable their survival and resistance to infection (51, 52). The fitness of a pathogen to a small subgroup would render it ineffective for the infection of other cells, thereby exerting selection pressure against such fitness (Figure 1). The isolation of immune suppression to a small, specific subpopulation of stem cells provides a robust form of protection from infection. The coevolution of immune regulation and infection represents a dynamic and interdependent relationship (46). It is important to note that IMSCs lack absolute protection and may be infected (43, 47). When infected, stem cells can suppress an infection by direct antiviral action and by reducing the number of stem cells through apoptosis (53, 54).
This model also provides a rationale for the seeding of IMSCs to developing tissues during embryogenesis (5). The functional rationale for differentiating between stem cells in adult and embryonic contexts may be attributed to the heightened risk of pathogens invasions in adult tissues during the lifespan. Given the pivotal role of IMSCs in immune function, the divergence in immune status preceding and following labor may provide a potential explanation for the evolutionary adaptation.
The metabolic differences that distinguish stem cells enable them to survive in conditions that would otherwise be lethal for the majority of other cells (15, 51). This enables the regeneration of severely damaged tissues. The model, where IMSCs possess IPs, implies an additional potential for the restoration of areas afflicted by excessive inflammation. As different physiology and a paucity of IMSCs provide evolutionary protection from infection, the risk associated with migration of IMSCs to contaminated tissue is diminished.
The model proposes an evolutionary perspective for IMSCs, including MSC, which have been identified in various tissues of the human body (55). MUSE and VSELs are also stem cells with pronounced immune modulation, derived from a mesenchymal subpopulation of different organs (28, 56, 57). The similarities of functions and molecular mechanisms with other quiescent and immune-privileged stem cells, such as hair follicle stem cells, muscle stem cells or hematopoietic stem cells, require further definition (26, 27). The proposed model does not align with the organizational structure of all tissues and their stem cells. There are examples of stem cell organizations that do not align with the proposed model and that may require significant adjustments (58). The esophageal epithelium is an illustrative example of a tissue wherein 65% of cells are engaged in proliferation, self-maintaince, and repair-related processes, thereby fulfilling the stem cells functions (59). Lgr5+ stem cells of the colon and small intestine demonstrate sustained proliferative activity throughout the lifespan (60–62). These cycling stem cells illustrate different evolutionary solutions for tissue-specific mutational processes, in addition to quiescence, to protect against mutations (63). Proliferating Lgr5+ stem cells do not exhibit the same IPs as a subpopulation of quiescent Lgr5+ stem cells (26). In this manner, the cells also exhibit disparate patterns of immune regulation. The regeneration of acute liver damage is mediated by hepatocytes and biliary epithelial cells. In the context of liver homeostasis, hepatocytes and biliary epithelial cells are in a state of quiescence, yet they undergo activation in response to an acute damage event (64). They are differentiated parenchymal cells of the liver and are the primary contributors to cellular restoration (65, 66). Wound regeneration or inflammation not only activates quiescent cells, but also upregulates dedifferentiation (67). Dedifferentiation may serve to regulate the stem cell pool (68). The number of stem cells is also subject to negative feedback, whereby stem cells inhibit dedifferentiation and reduce the number of surrounding stem cells (69, 70). Further studies are required to elucidate the role of dedifferentiation in immune and stem cell regulation. Further experimental study is required to elucidate the strong functional distinction of quiescent, immune-privileged stem cells. More experiments are required to elucidate the nuances of immune modulation function across stem cells derived from different tissues.
The model provides a logical explanation for the difficulties in expanding stem cells and their immunomodulatory properties used in the clinic to protect against pathological inflammation and immunotoxicity (28, 32, 34, 71–75). The model could be extended to elucidate the IPs of cancer stem cells as an attribute of the stem state (49, 50). The model can also elucidate the role of non-cancerous stroma in the protection of cancer cells by conceptualizing cancerous tissue as a region of active regeneration, wherein the immunomodulatory function of the stem system is activated (67, 76, 77). This provides a natural explanation for the stimulation of immune modulation from non-cancer stroma in response to therapy that damages cancer tissue, thereby further stimulating the function of regeneration (78, 79). The immunomodulatory properties of MSCs are significant and well recognized in the scientific community (32, 34, 71, 73). The principal objective of this article is to designate MSCs or IMSCs as a component of the immune system. It is proposed that IMSCs should be acknowledged as part of the immune system, with a role in the peripheral control of inflammation and autoimmunity, in addition to IMSCs regenerative potential.
The proposed model establishes a functional link between the attributes of IMSCs and their associated IPs and immune modulation. The model provides a functional analysis, eschewing a detailed examination of the underlying mechanisms. A particular mechanism may contribute to different functions simultaneously, thereby forming a complex network. However, it should also exhibit functional robustness beyond this. Additional restrictions imposed on IMSC attributes enhance the overall robustness and offer a compelling explanation for their observed values. To provide a rationale for the links in the model, I present an evolutionary perspective, but with the support of data from experiments that are not necessarily context-specific to evolutionary theory. Nevertheless, the existing deep mutual integration of immune and stem functions provides a robust foundation for the model. It is important to note that the evolutionary link between functions is not necessarily realized by an actual molecular mechanism. Alternatively, functions could be adjusted by independent shifts, which would provide advantages in subsequent generations. The model proposes evolutionary links for the IMSCs attributes. This presentation does not provide a detailed account of the evolutionary process that led to this state or an analysis of the specific mechanisms involved. Nevertheless, these issues warrant further investigation.
KD: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The author thanks Inna Zhurova for editing the language.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation of this work the author used DeepL in order to polish the language. After using this tool/service, the author reviewed and edited the content as needed and takes full responsibility for the content of the publication.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cancedda R, Mastrogiacomo M. The phoenix of stem cells: pluripotent cells in adult tissues and peripheral blood. Front bioengineering Biotechnol. (2024) 12:1414156. doi: 10.3389/FBIOE.2024.1414156
2. Aprile D, Patrone D, Peluso G, Galderisi U. Multipotent/pluripotent stem cell populations in stromal tissues and peripheral blood: exploring diversity, potential, and therapeutic applications. Stem Cell Res Ther. (2024) 15:139. doi: 10.1186/S13287-024-03752-X
3. Tian Z, Yu T, Liu J, Wang T, Higuchi A. Introduction to stem cells. Prog Mol Biol Trans Sci. (2023) 199:3–32. doi: 10.1016/BS.PMBTS.2023.02.012
4. Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. (2019) 10:68. doi: 10.1186/S13287-019-1165-5
5. Isern J, García-García A, Martín AM, Arranz L, Martín-Pérez D, Torroja C, et al. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. eLife. (2014) 3:3696. doi: 10.7554/ELIFE.03696
6. Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M. A hypothesis for an embryonic origin of pluripotent oct-4(+) stem cells in adult bone marrow and other tissues. Leukemia. (2007) 21:860–7. doi: 10.1038/SJ.LEU.2404630
7. Ratajczak MZ, Ratajczak J, Suszynska M, Miller DM, Kucia M, Shin DM. A novel view of the adult stem cell compartment from the perspective of a quiescent population of very small embryonic-like stem cells. Circ Res. (2017) 120:166. doi: 10.1161/CIRCRESAHA.116.309362
8. Dumont NA, Wang YX, Rudnicki MA. Intrinsic and extrinsic mechanisms regulating satellite cell function. Dev (Cambridge England). (2015) 142:1572–81. doi: 10.1242/DEV.114223
9. Neo WH, Lie-A-Ling M, Fadlullah MZH, Lacaud G. Contributions of embryonic HSC-independent hematopoiesis to organogenesis and the adult hematopoietic system. Front Cell Dev Biol. (2021) 9:631699. doi: 10.3389/FCELL.2021.631699
10. Gleiberman AS, Michurina T, Encinas JM, Roig JL, Krasnov P, Balordi F, et al. Genetic approaches identify adult pituitary stem cells. Proc Natl Acad Sci United States America. (2008) 105:6332. doi: 10.1073/PNAS.0801644105
11. López-Lázaro M. The stem cell division theory of cancer. Crit Rev oncology/hematology. (2018) 123:95–113. doi: 10.1016/J.CRITREVONC.2018.01.010
12. Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Sci (New York N.Y.). (2015) 347:78–81. doi: 10.1126/SCIENCE.1260825
13. Mustelin T, Andrade F. Autoimmunity: the neoantigen hypothesis. Front Immunol. (2024) 15:1432985. doi: 10.3389/FIMMU.2024.1432985
14. Dias IB, Bouma HR, Henning RH. Unraveling the big sleep: molecular aspects of stem cell dormancy and hibernation. Front Physiol. (2021) 12:624950. doi: 10.3389/FPHYS.2021.624950
15. Cieśla J, Tomsia M. Cadaveric stem cells: their research potential and limitations. Front Genet. (2021) 12:798161. doi: 10.3389/FGENE.2021.798161
16. Maličev E, Jazbec K. An overview of mesenchymal stem cell heterogeneity and concentration. Pharm (Basel Switzerland). (2024) 17:350. doi: 10.3390/PH17030350
17. Mansilla E, Marín GH, Drago H, Sturla F, Salas E, Gardiner C, et al. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplant Proc. (2006) 38:967–9. doi: 10.1016/J.TRANSPROCEED.2006.02.053
18. Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira S, et al. and frenette, P.S. (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. (2010) 466:829–34. doi: 10.1038/nature09262
19. Sato T, Wakao S, Kushida Y, Tatsumi K, Kitada M, Abe T, et al. A novel type of stem cells double-positive for SSEA-3 and CD45 in human peripheral blood. Cell Transplant. (2020) 29:0963689720923574. doi: 10.1177/0963689720923574
20. Karpenko D, Kapranov N, Bigildeev A. Nestin-GFP transgene labels immunoprivileged bone marrow mesenchymal stem cells in the model of ectopic foci formation. Front Cell Dev Biol. (2022) 10:993056/FULL. doi: 10.3389/FCELL.2022.993056/FULL
21. Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, et al. Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci United States America. (2010) 107:8639–43. doi: 10.1073/PNAS.0911647107
22. Lombardo E, Delarosa O. Modulation of adult mesenchymal stem cells activity by toll-like receptors: implications on therapeutic potential. Mediators Inflammation. (2010) 2010:865601. doi: 10.1155/2010/865601
23. Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. (2006) 36:2566–73. doi: 10.1002/EJI.200636416
24. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. (2014) 32:252–60. doi: 10.1038/nbt.2816
25. Berglund AK, Fortier LA, Antczak DF, Schnabel LV. Immunoprivileged no more: measuring the immunogenicity of allogeneic adult mesenchymal stem cells, stem cell research and therapy. Stem Cell Res Ther. (2017) 8:288. doi: 10.1186/s13287-017-0742-8
26. Agudo J, Park ES, Rose SA, Alibo E, Sweeney R, Dhainaut M, et al. Quiescent tissue stem cells evade immune surveillance. Immunity. (2018) 48:271–285.e5. doi: 10.1016/j.immuni.2018.02.001
27. Hirata Y, Furuhashi K, Ishii H, Li HW, Pinho S, Ding L, et al. CD150 high bone marrow tregs maintain hematopoietic stem cell quiescence and immune privilege via adenosine. Cell Stem Cell. (2018) 22:445–453.e5. doi: 10.1016/j.stem.2018.01.017
28. Kuroda Y, Oguma Y, Hall K, Dezawa M. Endogenous reparative pluripotent muse cells with a unique immune privilege system: hint at a new strategy for controlling acute and chronic inflammation. Front Pharmacol. (2022) 13:1027961. doi: 10.3389/FPHAR.2022.1027961
29. Karpenko DV. Immune privileges as a result of mutual regulation of immune and stem systems. Biochem Biokhimiia. (2023) 88:1818–31. doi: 10.1134/S0006297923110123
30. Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell proliferation. (2020) 53:e12712. doi: 10.1111/CPR.12712
31. Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. (2003) 76:1208–13. doi: 10.1097/01.TP.0000082540.43730.80
32. Sergeant E, Buysse M, Devos T, Sprangers B. Multipotent mesenchymal stromal cells in kidney transplant recipients: the next big thing? Blood Rev. (2021) 45:100718. doi: 10.1016/J.BLRE.2020.100718
33. Raicevic G, Rouas R, Najar M, Stordeur P, Id Boufker H, Bron D, et al. Inflammation modifies the pattern and the function of toll-like receptors expressed by human mesenchymal stromal cells. Hum Immunol. (2010) 71:235–44. doi: 10.1016/J.HUMIMM.2009.12.005
34. López-García L, Castro-Manrreza ME. TNF-α and IFN-γ Participate in improving the immunoregulatory capacity of mesenchymal stem/stromal cells: importance of cell–cell contact and extracellular vesicles. Int J Mol Sci. (2021) 22:9531. doi: 10.3390/IJMS22179531
35. Liao Y, Li G, Zhang X, Huang W, Xie D, Dai G, et al. Cardiac nestin+ Mesenchymal stromal cells enhance healing of ischemic heart through periostin-mediated M2 macrophage polarization. Mol therapy : J Am Soc Gene Ther. (2020) 28:855–73. doi: 10.1016/J.YMTHE.2020.01.011
36. Gazdic M, Volarevic V, Arsenijevic N, Stojkovic M. Mesenchymal stem cells: A friend or foe in immune-mediated diseases. Stem Cell Rev Rep. (2015) 11:280–7. doi: 10.1007/S12015-014-9583-3
37. Shi Y, Cao J, Wang Y. Rethinking regeneration: empowerment of stem cells by inflammation. Cell Death Differentiation. (2015) 22:1891. doi: 10.1038/CDD.2015.127
38. Toh WS, Zhang BIN, Lai RC, Lim SK. Immune regulatory targets of mesenchymal stromal cell exosomes/small extracellular vesicles in tissue regeneration. Cytotherapy. (2018) 20:1419–26. doi: 10.1016/J.JCYT.2018.09.008
39. Markov A, Thangavelu L, Aravindhan S, Zekiy AO, Jarahian M, Chartrand MS, et al. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res Ther. (2021) 12:192. doi: 10.1186/S13287-021-02265-1
40. Yamada Y, Minatoguchi S, Kanamori H, Mikami A, Okura H, Dezawa M, et al. Stem cell therapy for acute myocardial infarction - focusing on the comparison between muse cells and mesenchymal stem cells. J Cardiol. (2022) 80:80–7. doi: 10.1016/J.JJCC.2021.10.030
41. Cancedda R, Mastrogiacomo M. Transit amplifying cells (TACs): A still not fully understood cell population. Front bioengineering Biotechnol. (2023) 11:1189225. doi: 10.3389/FBIOE.2023.1189225
42. Lodestijn SC, van Neerven SM, Vermeulen L, Bijlsma MF. Stem cells in the exocrine pancreas during homeostasis, injury, and cancer. Cancers. (2021) 13:3295. doi: 10.3390/CANCERS13133295
43. Lebeau G, Ah-Pine F, Daniel M, Bedoui Y, Vagner D, Frumence E, et al. Perivascular mesenchymal stem/stromal cells, an immune privileged niche for viruses? Int J Mol Sci. (2022) 23:23. doi: 10.3390/IJMS23148038
44. Yamada Y, Wakao S, Kushida Y, Minatoguchi S, Mikami A, Higashi K, et al. S1P–S1PR2 Axis Mediates Homing of Muse Cells into Damaged Heart for Long-Lasting Tissue Repair and Functional Recovery after Acute Myocardial Infarction. Circ Res. (2018) 122:1069–83. doi: 10.1161/CIRCRESAHA.117.311648/-/DC1
45. Peyvandi AA, Roozbahany NA, Peyvandi H, Abbaszadeh HA, Majdinasab N, Faridan M, et al. Critical role of SDF-1/CXCR4 signaling pathway in stem cell homing in the deafened rat cochlea after acoustic trauma. Neural regeneration Res. (2018) 13:154–60. doi: 10.4103/1673-5374.224382
46. Levi-Schaffer F, Mandelboim O. Inhibitory and coactivating receptors recognising the same ligand: immune homeostasis exploited by pathogens and tumours. Trends Immunol. (2018) 39:112. doi: 10.1016/J.IT.2017.10.001
47. Devi A, Pahuja I, Singh SP, Verma A, Bhattacharya D, Bhaskar A, et al. Revisiting the role of mesenchymal stem cells in tuberculosis and other infectious diseases. Cell Mol Immunol. (2023) 20:600–12. doi: 10.1038/S41423-023-01028-7
48. Corthay A. Does the immune system naturally protect against cancer? Front Immunol. (2014) 5:197. doi: 10.3389/FIMMU.2014.00197
49. Galassi C, Musella M, Manduca N, Maccafeo E, Sistigu A. The immune privilege of cancer stem cells: A key to understanding tumor immune escape and therapy failure. Cells. (2021) 10:2361. doi: 10.3390/CELLS10092361
50. Wu B, Shi X, Jiang M, Liu H. Cross-talk between cancer stem cells and immune cells: potential therapeutic targets in the tumor immune microenvironment. Mol Cancer. (2023) 22:1–22. doi: 10.1186/S12943-023-01748-4
51. Alessio N, Squillaro T, Özcan S, Di Bernardo G, Venditti M, Melone M, et al. Stress and stem cells: adult muse cells tolerate extensive genotoxic stimuli better than mesenchymal stromal cells. Oncotarget. (2018) 9:19328–41. doi: 10.18632/ONCOTARGET.25039
52. Wu X, Dao Thi VL, Huang Y, Billerbeck E, Saha D, Hoffmann HH, et al. Intrinsic immunity shapes viral resistance of stem cells. Cell. (2018) 172:423–438.e25. doi: 10.1016/J.CELL.2017.11.018
53. Pathak L, Gayan S, Pal B, Talukdar J, Bhuyan S, Sandhya S, et al. Coronavirus activates an altruistic stem cell-mediated defense mechanism that reactivates dormant tuberculosis: implications in coronavirus disease 2019 pandemic. Am J Pathol. (2021) 191:1255–68. doi: 10.1016/J.AJPATH.2021.03.011
54. Bhuyan S, Pal B, Pathak L, Saikia PJ, Mitra S, Gayan S, et al. Targeting hypoxia-induced tumor stemness by activating pathogen-induced stem cell niche defense. Front Immunol. (2022) 13:933329. doi: 10.3389/FIMMU.2022.933329
55. Andrzejewska A, Lukomska B, Janowski M. Mesenchymal stem cells: from roots to boost. Stem Cells (Dayton Ohio). (2019) 37:855. doi: 10.1002/STEM.3016
56. Haj-Mirzaian A, Khosravi A, Haj-Mirzaian A, Rahbar A, Ramezanzadeh K, Nikbakhsh R, et al. The potential role of very small embryonic-like stem cells in the neuroinflammation induced by social isolation stress: introduction of a new paradigm. Brain Res Bull. (2020) 163:21–30. doi: 10.1016/J.BRAINRESBULL.2020.07.006
57. Bhartiya D. Pluripotent stem cells in adult tissues: struggling to be acknowledged over two decades. Stem Cell Rev Rep. (2017) 13:713–24. doi: 10.1007/S12015-017-9756-Y
58. Clevers H, Watt FM. Defining adult stem cells by function, not by phenotype. Annu Rev Biochem. (2018) 87:1015–27. doi: 10.1146/ANNUREV-BIOCHEM-062917-012341
59. Doupé DP, Alcolea MP, Roshan A, Zhang G, Klein AM, Simons BD, et al. A single progenitor population switches behavior to maintain and repair esophageal epithelium. Sci (New York N.Y.). (2012) 337:1091–3. doi: 10.1126/SCIENCE.1218835
60. Kozar S, Morrissey E, Nicholson AM, van der Heijden M, Zecchini HI, Kemp R, et al. Continuous clonal labeling reveals small numbers of functional stem cells in intestinal crypts and adenomas. Cell Stem Cell. (2013) 13:626–33. doi: 10.1016/J.STEM.2013.08.001
61. Potten CS, Booth C, Hargreaves D. The small intestine as a model for evaluating adult tissue stem cell drug targets. Cell proliferation. (2003) 36:115–29. doi: 10.1046/J.1365-2184.2003.00264.X
62. Barker N, Van Es JH, Kuipers J, Kujala P, Van Den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene lgr5. Nature. (2007) 449:1003–7. doi: 10.1038/NATURE06196
63. Blokzijl F, De Ligt J, Jager M, Sasselli V, Roerink S, Sasaki N, et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature. (2016) 538:260–4. doi: 10.1038/NATURE19768
64. Campana L, Esser H, Huch M, Forbes S. Liver regeneration and inflammation: from fundamental science to clinical applications. Nat Rev Mol Cell Biol. (2021) 22:608–24. doi: 10.1038/S41580-021-00373-7
65. Yanger K, Knigin D, Zong Y, Maggs L, Gu G, Akiyama H, et al. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell. (2014) 15:340–9. doi: 10.1016/J.STEM.2014.06.003
66. Ying SQ, Cao Y, Zhou ZK, Luo XY, Zhang XH, Shi K, et al. Hepatocyte-derived tissue extracellular vesicles safeguard liver regeneration and support regenerative therapy. J nanobiotechnology. (2024) 22:521. doi: 10.1186/S12951-024-02790-0
67. Yanjie GUO, Weini WU, Yang X, Xiaobing FU. Dedifferentiation and in vivo reprogramming of committed cells in wound repair (Review). Mol Med Rep. (2022) 26:369. doi: 10.3892/MMR.2022.12886
68. Sun Q, Lee W, Hu H, Ogawa T, De Leon S, Katehis I, et al. Dedifferentiation maintains melanocyte stem cells in a dynamic niche. Nature. (2023) 616:774–82. doi: 10.1038/s41586-023-05960-6
69. Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, et al. A reserve stem cell population in small intestine renders lgr5-positive cells dispensable. Nature. (2011) 478:255–9. doi: 10.1038/NATURE10408
70. Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. (2013) 503:218–23. doi: 10.1038/NATURE12777
71. Li P, Ou Q, Shi S, Shao C. Immunomodulatory properties of mesenchymal stem cells/dental stem cells and their therapeutic applications. Cell Mol Immunol. (2023) 20:558. doi: 10.1038/S41423-023-00998-Y
72. Minev T, Balbuena S, Gill JM, Marincola FM, Kesari S, Lin F. Mesenchymal stem cells - the secret agents of cancer immunotherapy: promises, challenges, and surprising twists. Oncotarget. (2024) 15:793. doi: 10.18632/ONCOTARGET.28672
73. Wu X, Jiang J, Gu Z, Zhang J, Chen Y, Liu X. Mesenchymal stromal cell therapies: immunomodulatory properties and clinical progress. Stem Cell Res Ther. (2020) 11:345. doi: 10.1186/S13287-020-01855-9
74. Mishra VK, Shih HH, Parveen F, Lenzen D, Ito E, Chan TF, et al. Identifying the therapeutic significance of mesenchymal stem cells. Cells. (2020) 9:1145. doi: 10.3390/CELLS9051145
75. Miclau K, Hambright WS, Huard J, Stoddart MJ, Bahney CS. Cellular expansion of MSCs: shifting the regenerative potential. Aging Cell. (2023) 22:e13759. doi: 10.1111/ACEL.13759
76. Rivera-Cruz CM, Shearer JJ, Figueiredo Neto M, Figueiredo ML. The immunomodulatory effects of mesenchymal stem cell polarization within the tumor microenvironment niche. Stem Cells Int. (2017) 2017:4015039. doi: 10.1155/2017/4015039
77. Hass R. Role of MSC in the tumor microenvironment. Cancers. (2020) 12:1–17. doi: 10.3390/CANCERS12082107
78. Nicolas AM, Pesic M, Engel E, Ziegler PK, Diefenhardt M, Kennel KB, et al. Inflammatory fibroblasts mediate resistance to neoadjuvant therapy in rectal cancer. Cancer Cell. (2022) 40:168–184.e13. doi: 10.1016/J.CCELL.2022.01.004
Keywords: stem cells, immune modulatory stem cells, stem system, immune system, evolution, infections
Citation: Karpenko DV (2025) Immune modulatory stem cells represent a significant component of the immune system. Front. Immunol. 16:1543495. doi: 10.3389/fimmu.2025.1543495
Received: 11 December 2024; Accepted: 18 February 2025;
Published: 03 March 2025.
Edited by:
Petr O. Ilyinskii, Selecta Biosciences, United StatesReviewed by:
Philippe Lewalle, Université libre de Bruxelles, BelgiumCopyright © 2025 Karpenko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dmitriy Vladimirovich Karpenko, ZF9AbGlzdC5ydQ==
†ORCID: Dmitriy Vladimirovich Karpenko, orcid.org/0000-0002-0691-4079
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.