
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 22 April 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1543096
Ovarian cancer (OC) remains the most lethal gynecological malignancy, primarily due to its late-stage diagnosis, frequent recurrence, and resistance to conventional chemotherapy. A critical factor contributing to OC’s aggressiveness is the tumor microenvironment (TME), particularly the presence and polarization of tumor-associated macrophages (TAMs). TAMs, often skewed toward an immunosuppressive M2-like phenotype, facilitate tumor growth, angiogenesis, metastasis, and resistance to therapy. This comprehensive review delves into the multifaceted regulation of macrophage polarization in OC, highlighting key molecular pathways such as PTEN loss, Wnt/β-catenin signaling, NF-κB, Myc, STAT3, and JNK, among others. Additionally, it explores the role of chemokines, non-coding RNAs, and various proteins in modulating TAM phenotypes. Emerging evidence underscores the significance of extracellular vesicles (EVs) and ovarian cancer stem cells (CSCs) in promoting M2 polarization, thereby enhancing tumor progression and therapy resistance. The review also identifies critical biomarkers associated with macrophage polarization, including CD163, LILRB1, MUC2, and others, which hold prognostic and therapeutic potential. Therapeutic strategies targeting TAMs are extensively discussed, encompassing oncolytic viruses, engineered EVs, immunotherapies, nanoparticles, targeted therapies, and natural products. These approaches aim to reprogram TAMs from a pro-tumorigenic M2 state to an anti-tumorigenic M1 phenotype, thereby enhancing immune responses and overcoming resistance to treatments such as chemotherapy and immune checkpoint inhibitors. Furthermore, the review addresses the interplay between macrophage polarization and therapy resistance, emphasizing the need for novel interventions to modulate the TME effectively. By synthesizing current knowledge on macrophage polarization in ovarian cancer, this study underscores the potential of targeting TAMs to improve clinical outcomes and personalize treatment strategies for OC patients. Continued research in this domain is essential to develop robust therapeutic frameworks that can mitigate the immunosuppressive TME and enhance the efficacy of existing and novel cancer therapies.
Ovarian cancer (OC) remains the most lethal gynecological cancer, largely due to its late diagnosis, frequent recurrence, and resistance to chemotherapy (1). The complexity of the disease, characterized by its diverse histological subtypes and varying molecular features, presents challenges for effective treatment and prognosis (2). Standard treatment typically involves cytoreductive surgery followed by platinum-based chemotherapy, but novel approaches like PARP inhibitors, anti-angiogenic therapies, and drugs targeting the folate receptor alpha are showing potential, especially for patients with advanced or recurrent disease (3). Additional innovative treatments, such as immunotherapies, are being explored to improve outcomes. However, significant obstacles remain, including difficulties in early detection, overcoming drug resistance, and tailoring therapies to individual patients, emphasizing the need for further research to enhance survival rates and treatment personalization (4–6). Tumor-associated macrophages (TAMs) play a critical role in the immunosuppressive tumor microenvironment of ovarian cancers, which is a major reason why immunotherapies, such as immune checkpoint inhibitors, have limited success in treating this type of cancer (7, 8). A recent review has also highlighted the importance of TAMs in the ovarian cancer microenvironment, emphasizing their roles in tumor progression, chemoresistance, and immune modulation, further underscoring the significance of targeting TAMs in OC therapy (9). Targeting TAMs by reducing their recruitment, reprogramming them to an antitumor M1-like phenotype, or restoring their ability to phagocytose tumor cells can help reverse immunosuppression and improve the effectiveness of immunotherapies. Thus, TAMs are key therapeutic targets for enhancing the success of immunotherapy in ovarian cancer (10). Macrophage polarization refers to the process by which macrophages, versatile immune cells, adopt different functional states in response to signals from their environment. They can polarize into a pro-inflammatory (M1) state, which combats pathogens and promotes anti-tumor activity, or into an anti-inflammatory (M2) state, which supports tissue repair but can also aid tumor growth and metastasis (11). In cancer, TAMs are often skewed toward the M2-like phenotype due to signals from the tumor microenvironment (12). Notably, multiple studies have independently confirmed the critical role of the M1/M2 macrophage ratio in determining ovarian cancer prognosis and therapy response. For instance, analyses of tumor microenvironment composition in HGSOC have repeatedly demonstrated that higher M1/M2 ratios correlate with improved outcomes across different patient cohorts. Specifically, patients with high-grade serous ovarian cancer (HGSOC) exhibited a higher ratio of M1 macrophages (pro-inflammatory) to M2 macrophages (immunosuppressive), which was associated with longer OS, PFS, and platinum-free intervals (PFI). This positive correlation persisted regardless of the extent of cytoreductive surgery, indicating the importance of TAM polarization in patient outcomes. Additionally, patients with platinum-sensitive tumors had a higher M1/M2 ratio, suggesting that M1 polarization enhances chemotherapy effectiveness (13, 14). Analyzing 30 tissue samples from 24 ovarian cancer patients revealed that low-grade ovarian cancer had a higher M1/M2 ratio, suggesting a more immune-beneficial environment, while high-grade tumors, particularly in metastatic sites, showed increased M2 macrophage infiltration, indicating immune suppression. Treatment with platinum-based chemotherapy and bevacizumab increased M2 macrophages, especially in high-grade tumors, and also enlarged blood vessel diameters, correlating with more M2 infiltration (15). While this suggests a potential role for macrophage polarization in therapy resistance, larger patient cohorts are needed to validate these findings. These findings suggest that macrophage polarization plays a critical role in the development of malignant phenotypes in ovarian cancer cells, as well as in therapy resistance and disease progression. Targeting M2-polarized TAMs could, therefore, represent a promising therapeutic strategy to improve outcomes for ovarian cancer patients. Given the lack of specific reviews on this topic, this study aims to provide a comprehensive overview of the existing literature and findings related to macrophage polarization in ovarian cancer.
Emerging evidence indicates that the macrophage landscape in ovarian cancer is highly heterogeneous, both across different patients (intertumoral heterogeneity) and within individual tumors (intratumoral heterogeneity). Single-cell RNA sequencing (scRNA-seq) and spatial transcriptomics have uncovered distinct TAM subpopulations that vary based on anatomical location, tumor subtype, and local microenvironmental factor (16). Notably, primary tumor sites often contain a mixture of M1 and M2 macrophages, while metastatic lesions—particularly within the omentum and ascitic fluid—exhibit a dominance of immunosuppressive M2-like TAMs (17). Macrophage-driven heterogeneity is further amplified by their interactions with tumor cells and stromal components. Tumor-derived extracellular vesicles (EVs) transport miRNAs such as miR-200b and miR-221-3p, which skew macrophages toward the M2 phenotype, reinforcing immune suppression and therapy resistance (18, 19). Additionally, metabolic factors such as lactate accumulation drive TAM polarization, contributing to spatially distinct macrophage phenotypes within the tumor microenvironment (20). This heterogeneity complicates therapeutic strategies targeting TAMs, as interventions need to account for the dynamic and location-specific nature of macrophage populations.
The traditional M1/M2 classification of macrophages, while foundational for understanding macrophage function, does not fully encompass the functional and transcriptional diversity of TAMs in ovarian cancer. Although M1 (pro-inflammatory) macrophages are associated with tumor suppression and M2 (anti-inflammatory) macrophages with tumor progression, recent research highlights that TAMs often exist along a broad and dynamic spectrum of activation states (21). Insights from scRNA-seq have revealed transcriptionally distinct and functionally diverse subpopulations of TAMs within ovarian tumors and their metastases, further supporting the notion of a continuum rather than a binary M1/M2 framework. For instance, some TAM subsets exhibit markers typical of both M1-like and M2-like phenotypes, performing seemingly contradictory roles within the TME. This emerging understanding challenges the oversimplified classification of TAMs and underscores their unique adaptability in response to the TME (22, 23)
The heterogeneity of TAM subtypes in ovarian cancer has been underscored by scRNA-seq studies that unveil multiple transcriptionally distinct populations. These studies have identified TAMs with immunosuppressive phenotypes, such as those marked by high expression of CD206 (a hallmark of M2-like macrophages), which co-exist with populations producing key pro-inflammatory cytokines like TNF-α. Furthermore, TAM heterogeneity is often spatially localized within distinct regions of a tumor or its metastatic niches (e.g., ascites fluid versus primary tumor sites) (16, 24). In cancer, TAMs are often skewed toward the M2-like phenotype due to signals from the tumor microenvironment. These M2-like TAMs play a critical role in cancer metastatic progression by promoting tumor survival, angiogenesis, immune suppression, and metastasis through the secretion of factors like IL-10, TGF-β, and VEGF (25). The presence of such heterogeneous subpopulations reflects the influence of diverse signaling cues in the tumor microenvironment, highlighting TAMs’ multifaceted and dynamic nature. Their roles extend to angiogenesis, immune exhaustion, stromal remodeling, and interactions with mesothelial cells during metastasis formation (16, 24). M2 phenotype macrophages are crucial in the progression of advanced epithelial ovarian cancer. Studies have revealed that a high density of CD163-positive M2 macrophages and a high CD163/CD68 ratio are significantly associated with worse progression-free survival (PFS) and overall survival (OS) in patients with advanced ovarian cancer (26). M2 macrophages, induced by IL-4, significantly enhance the proliferation, migration, and invasion of ovarian cancer cells while inhibiting their apoptosis in vitro. In contrast, M1 macrophages, show opposing effects, reducing tumor cell proliferation and promoting apoptosis. The co-culture of macrophages with ovarian cancer cells polarized macrophages toward the M2 phenotype, indicating that the tumor microenvironment favors the tumor-promoting M2 macrophages (27). In ovarian cancer, particularly in HGSOC, M1-like macrophages are significantly enriched in tumors with high homologous recombination deficiency (HRD), while M2-like macrophages are not (28). M1 macrophages, known for their anti-tumoral and pro-inflammatory functions, release cytokines like TNF-alpha and IL-2, promoting immune responses against the tumor. The selective enrichment of M1-like macrophages in HRD-high tumors suggests a more active immune environment, which could contribute to improved patient outcomes. This enrichment of M1 macrophages correlates with increased mutation burdens and neoantigen production in HRD-high tumors, further stimulating the immune response, while the absence of significant M2 macrophage activity highlights a less immunosuppressive environment in these cases (29).Altogether, these findings demonstrate the complex interplay between TAM subsets acting simultaneously to promote or restrain tumor development.
The polarization of TAMs is far more nuanced than previously thought, as various microenvironmental drivers shape their functional states. Cytokines such as IL-4, IL-10, and TGF-β have been identified as potent inducers of anti-inflammatory TAM polarization, whereas cytokines like IFN-γ and TNF-α favor pro-inflammatory phenotypes. Beyond cytokines, metabolic factors such as tumor-derived lactate drive TAM polarization toward immunosuppressive phenotypes by activating pathways like hypoxia-inducible factor 1-alpha (HIF-1α). Lipid metabolism, glutamine utilization, and mitochondrial oxidative phosphorylation are also known to contribute to macrophage reprogramming. Additionally, cellular interactions, including crosstalk with cancer-associated fibroblasts (CAFs) and T regulatory cells, further amplify TAM functional heterogeneity. These approaches expand upon the involvement of non-genetic alterations in TAMs as they dynamically shift their roles within the cancer ecosystem (30–32). Specific subsets of emerging TAM functional states defy the traditional M1/M2 paradigm entirely and exhibit unique characteristics. For instance, macrophages marked by high expression of TREM2 represent a distinct subpopulation implicated in immune suppression and tumor progression across cancers. These TAMs have been shown to promote immune evasion by attenuating T-cell activity within the TME (33). Likewise, tissue-resident macrophages that interact with stromal compartments and extracellular matrices display unique specialization, particularly within peritoneal metastases, as compared to their counterparts in primary tumor sites. Such adaptations underscore the highly plastic and context-dependent behavior of TAMs within different anatomical and functional regions of the tumor (34).
This dynamic diversity among TAMs has profound implications for therapeutic intervention. Strategies aimed at reprogramming TAMs or inhibiting their recruitment have achieved varying success depending on the specific subset or functional state being targeted. For example, agents targeting the CCL2/CCR2 axis have shown promise in reducing the recruitment of monocyte-derived TAMs, while inhibitors of colony-stimulating factor 1 receptor (CSF1R) blunt TAM support for tumor growth (35, 36). Recent research also highlights novel combinatorial approaches, such as targeting both CSF1R and TREM2 simultaneously, which may overcome immune suppression more effectively. Emerging therapies leveraging nanocarriers, exosome-based delivery systems, or chimeric antigen receptor (CAR)-based macrophages offer exciting avenues for developing precision therapeutics. These approaches recognize the importance of subset-specific targeting to maximize therapeutic efficacy and minimize off-target immune effects in ovarian cancer treatment (37).
By shifting the focus from the classical M1/M2 paradigm to a more comprehensive view of TAM heterogeneity, researchers can better understand the transcriptional, functional, and metabolic diversity within the ovarian cancer TME. This updated classification framework provides a clearer foundation for designing therapies tailored to specific TAM subsets and microenvironmental factors. Through precision targeting of TAMs, it may be possible to optimize therapeutic approaches against immunosuppressive and tumor-promoting macrophage subpopulations, thereby improving clinical outcomes for patients with ovarian cancer. The incorporation of single-cell technologies, such as scRNA-seq, paves the way for unraveling further complexities of macrophage biology in cancer.
Macrophage polarization in cancer involves two main pathways leading to pro-inflammatory M1-like and anti-inflammatory M2-like phenotypes. M1 polarization, which promotes tumor suppression, is driven by IFN-γ, TNF-α, and TLR signaling through pathways like JAK/STAT1, NF-κB, and MAPKs (JNK, ERK), resulting in the production of pro-inflammatory cytokines and reactive oxygen species. In contrast, M2 polarization, associated with tumor promotion, is activated by signals like IL-4, IL-10, and TGF-β, primarily through the JAK/STAT6, PI3K/AKT/mTOR, and TGF-β/SMAD pathways, promoting tissue repair, immune suppression, and angiogenesis. Dual-role pathways such as NF-κB and ERK can contribute to either polarization depending on the context. Targeting these pathways therapeutically aims to shift macrophages from a tumor-promoting M2-like state to a tumor-suppressing M1-like state (38). This section reviews the pathways that interact with macrophage polarization in ovarian cancer.
PTEN loss in HGSOC significantly impacts macrophage dynamics, shaping the tumor microenvironment. PTEN deficiency activates the PI3K signaling pathway, which promotes the expansion of a specific population of resident-like macrophages in omental tumors (39). These macrophages express high levels of the enzyme heme oxygenase-1 (HMOX1), which contributes to tumor growth and correlates with poor survival outcomes. PTEN loss drives the recruitment of these HMOX1-high macrophages from peritoneal fluid macrophages rather than from monocyte-derived macrophages. These resident macrophages exhibit immunosuppressive traits, aiding tumor progression by creating a less favorable microenvironment for adaptive immune responses. Moreover, human HGSOC tumors also show a similar enrichment of HMOX1-high macrophages, further linking PI3K/mTOR pathway activation and poor prognosis. Targeting these macrophages or inhibiting HMOX1 has shown potential therapeutic value in reducing tumor growth and improving survival outcomes in preclinical models (40). PTEN deficiency in ovarian cancer leads to the recruitment and polarization of macrophages into an M2-like phenotype, which is associated with an immunosuppressive tumor microenvironment. These M2-like macrophages promote tumor progression by supporting immune evasion and reducing cytotoxic T-cell activity. PTEN-deficient tumors show increased infiltration of these M2 macrophages in the ascites and tumor stroma, contributing to more aggressive tumor behavior and decreased responsiveness to chemotherapy. Additionally, PTEN deficiency also elevates levels of immunosuppressive cytokines, such as IL-10, further reinforcing the M2-like suppressive macrophage dominance (Figure 1) (41).
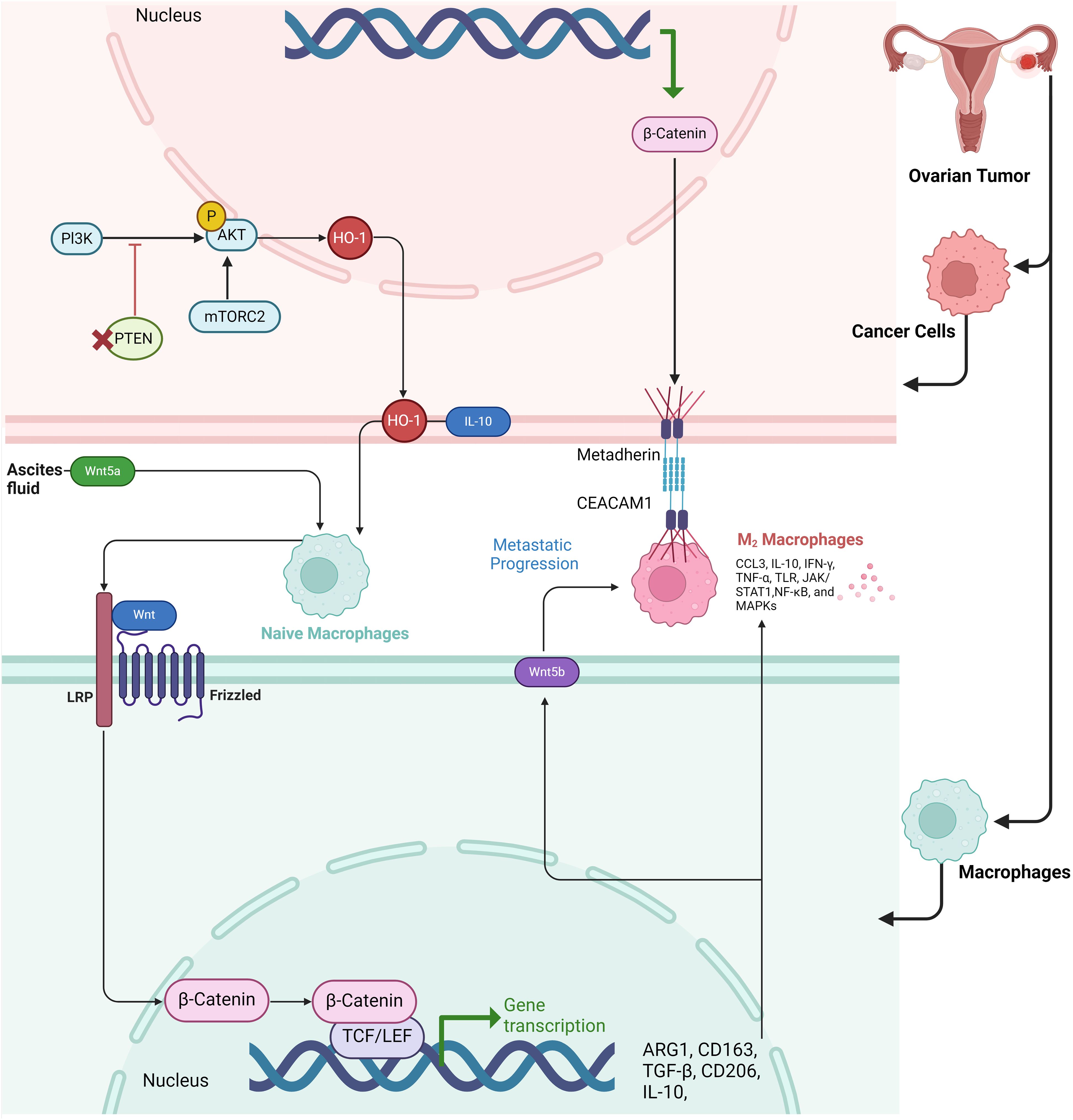
Figure 1. Schematic representation of the Wnt and PI3K/AKT pathways involved in macrophage polarization and metastatic progression in the tumor microenvironment of ovarian cancer. The figure illustrates how cancer cells in ovarian tumors release factors into the ascites fluid, including Wnt5a, which binds to Frizzled receptors on naïve macrophages, activating β-catenin signaling and promoting a shift toward an M2 macrophage phenotype. In the tumor cells, the PI3K/AKT/mTORC2 pathway upregulates HO-1, leading to IL-10 production, which further aids in immune modulation and supports the M2 macrophage polarization. The M2 macrophages, characterized by high expression of markers such as ARG1, CD163, TGF-β, CD206, and IL-10, facilitate metastatic progression by promoting cellular interactions via Metadherin and CEACAM1 receptors. The transcriptional activation of β-catenin in both macrophages and cancer cells enhances the transcription of genes associated with immune suppression and metastasis. Key signaling molecules in the M2 macrophages, including CCL3, IL-10, IFN-γ, and NF-κB, contribute to the tumor-promoting environment.
The Wnt/β-catenin signaling pathway is essential for promoting macrophage M2 polarization, which plays a significant role in ovarian cancer progression (42). Activation of this pathway leads to the nuclear translocation of β-catenin, which drives the reprogramming of macrophages from an M0 to an M2 phenotype. This polarization supports tumor growth and metastasis by enhancing cancer cell migration and invasion. Inhibiting β-catenin nuclear translocation can reverse M2 polarization, reducing its tumor-promoting effects, indicating the pathway’s critical role in ovarian cancer metastasis (43) (44). Paracrine WNT signaling loops between M2-like macrophages and ovarian cancer stem cells (CSCs) create a reciprocal interaction that promotes tumor progression. In ovarian cancer, CSCs activate macrophages, driving them to adopt an immunosuppressive M2-like phenotype, characterized by increased CD206 expression and IL-10 secretion. These macrophages, in turn, release WNT ligands, particularly WNT5B, which further enhance CSC stemness by maintaining high levels of ALDH+ cells and promoting CSC chemoresistance and invasiveness. This mutual signaling between CSCs and M2 macrophages, facilitated by WNT pathways, strengthens pro-tumoral activities, forming a feedback loop that exacerbates tumor aggressiveness and resistance to treatment. Targeting this paracrine WNT signaling could offer therapeutic opportunities to disrupt the tumor-supportive microenvironment (45). Host-derived Wnt5a, secreted by peritoneal mesothelial cells and adipocytes within the ovarian cancer microenvironment, plays a critical role in promoting ovarian cancer metastasis. High levels of Wnt5a in ascites fluid enhance the adhesion, migration, and invasion of ovarian cancer cells, driving their colonization of the peritoneum (46, 47). Wnt5a induces these prometastatic behaviors by activating the Src family kinase Fgr, which in turn accelerates cellular motility and adhesion, critical steps in metastatic progression. In addition to directly influencing cancer cell behavior, Wnt5a also shapes an immunosuppressive tumor microenvironment by skewing immune cell profiles, favoring the presence of M2 macrophages and regulatory T cells while reducing cytotoxic T cells and M1 macrophages. This results in a tumor-friendly immune landscape that further promotes metastasis. Knockout of Wnt5a in host cells dramatically reduces tumor burden and alters immune infiltration patterns, suggesting that targeting Wnt5a or its downstream effector, Fgr, may be an effective therapeutic strategy for halting ovarian cancer metastasis (46). The β-catenin-metadherin/CEACAM1-CCL3 axis plays a critical role in mediating metastatic heterogeneity by orchestrating interactions between metastatic tumor cells and TAMs. In highly metastatic (HM) ovarian cancer cells, the β-catenin signaling pathway is upregulated, leading to an increasing the expression of metadherin on the tumor cell surface. Metadherin engages with CEACAM1, a receptor on TAMs, triggering the production of CCL3, a chemokine that promotes macrophage recruitment and retention at metastatic sites. This interaction creates a positive feedback loop, wherein HM cells polarize macrophages to a pro-tumor TAM phenotype. Upon contact with macrophages, a subset of HM cells undergoes polyploidization, a process where cells fail to complete cell division, resulting in larger, multinucleated cells. These polyploid cells are more aggressive, migratory, and resistant to therapy, contributing to tumor heterogeneity and metastasis (Figure 1) (48).
Enhanced canonical NF-kappaB signaling in macrophages is enough to reduce tumor progression in syngeneic mouse models of ovarian cancer by fostering an anti-tumor immune environment (49). In a transgenic mouse model (IKFM) with inducible NF-kappaB activation in macrophages, researchers found that TAMs were reprogrammed from a pro-tumor M2 state to an anti-tumor M1 state. This reprogramming was linked to increased tumor necrosis and greater infiltration of immune cells, particularly cytotoxic CD8+ T cells, into both solid tumors and ascitic fluid. Additionally, these macrophage changes elevated the levels of the chemokine CXCL9, which attracts CD8+ T cells and further boosts the anti-tumor immune response. These effects were observed in both established tumors and early tumor growth, indicating that NF-kappaB activation in TAMs creates a more immunogenic tumor microenvironment, slowing tumor growth. This suggests that targeting macrophage NF-kappaB signaling could provide a promising new approach for improving immune-based treatments in ovarian cancer (50). Ovarian cancer stem cells (OCSCs) induce the M2 polarization of macrophages primarily through the activation of the PPARγ pathway and the suppression of the NF-κB pathway. When co-cultured with macrophages, OCSCs increase the expression of M2-associated markers such as the mannose receptor (MR), interleukin-10 (IL-10), and arginase-1 (Arg-1), while simultaneously reducing the expression of M1 markers like tumor necrosis factor-α (TNF-α), inducible nitric oxide synthase (iNOS), and CD86. This shift toward the M2 phenotype, which supports tumor growth and immune suppression, is driven by the upregulation of PPARγ, a nuclear receptor involved in anti-inflammatory responses. The suppression of NF-κB, a key transcription factor associated with inflammatory responses, further reinforces this polarization. Inhibition of PPARγ using a specific antagonist (GW9662) reverses the effects of OCSCs, restoring NF-κB activity and promoting M1 polarization. This indicates that OCSCs exploit the PPARγ/NF-κB signaling axis to skew macrophages toward a tumor-promoting M2 phenotype (51). In contrast, pro-inflammatory M1 macrophages enhance the metastatic potential of ovarian cancer cells primarily by activating the NF-κB signaling pathway. While preclinical models strongly suggest that NF-κB activation in macrophages enhances anti-tumor immunity, caution is needed when extrapolating these findings to human studies (52). Notably, studies in breast and colorectal cancer have established CXCL1 as a driver of EMT, yet direct evidence for this mechanism in ovarian cancer remains limited, warranting further validation in larger clinical datasets Secreted factors from M1 macrophages, including TNF-α and CXCL1, have been shown in other cancers such as breast and colorectal cancer to promote cancer cell migration and invasion via NF-κB activation. CXCL1, which is highly secreted by TAMs in breast cancer, has been identified as a key driver of epithelial–mesenchymal transition (EMT), increasing cancer cell motility (53). Mechanistic studies suggest that CXCL1 directly binds to the SOX4 promoter, activating its transcription via NF-κB signaling, ultimately promoting tumor progression and metastasis. Similarly, CXCL1 overexpression in colorectal cancer has been linked to enhanced tumor angiogenesis and recruitment of M2-like TAMs, further supporting an immunosuppressive tumor microenvironment (54). In ovarian cancer, the involvement of CXCL1 in NF-κB-driven metastasis remains underexplored, but these findings suggest a potential role for CXCL1 in regulating macrophage-cancer cell interactions within the ovarian tumor microenvironment. Blocking CXCL1 or its downstream NF-κB activation may provide a novel therapeutic avenue for limiting ovarian cancer metastasis.
Another key example of this NF-κB-driven interplay between ovarian cancer cells, macrophages, and the tumor microenvironment is seen with periostin (POSTN), a matrix protein overexpressed in highly invasive ovarian cancer cells (55). POSTN has been identified as a crucial mediator of macrophage recruitment and polarization through integrin-ERK-NF-κB signaling. In ovarian cancer, POSTN enhances integrin β3 and β5 signaling, which activates ERK and NF-κB pathways in tumor cells. This results in the secretion of macrophage-attracting cytokines such as MIP-1β, MCP-1, TNF-α, and RANTES, leading to increased chemotaxis of monocytes and their subsequent polarization into immunosuppressive M2 macrophages. Notably, tumors overexpressing POSTN harbored more TAMs and exhibited greater metastatic potential, further emphasizing the role of NF-κB signaling in shaping an aggressive tumor microenvironment. Additionally, POSTN-induced NF-κB activation stimulates the production of TGF-β2, which drives the differentiation of adipose-derived stromal cells into cancer-associated fibroblasts (CAFs)—another key player in ovarian cancer progression. Clinically, high POSTN expression correlates with advanced-stage disease and poor patient survival, underscoring its potential as both a biomarker and a therapeutic target (56). Together, these findings highlight the paradoxical role of NF-κB signaling in ovarian cancer. While its activation in TAMs can reprogram them toward an anti-tumor M1 phenotype, its activation in ovarian cancer cells—via CXCL1, MIP-1β, MCP-1, TNF-α, and RANTES, or other secreted factors might be involved in driving metastasis, immune suppression, and therapy resistance. Understanding this dual role is critical for developing targeted therapies that selectively inhibit NF-κB-driven tumor progression while preserving its anti-tumor immune functions.
When ovarian cancer cells are exposed to M1 macrophage-conditioned media, their migration and invasion abilities significantly increase, which are key factors in metastasis (14). M1 macrophages release pro-inflammatory cytokines, particularly TNF-α, which triggers the nuclear translocation of NF-κB subunits (p50, p65) from the cytosol to the nucleus in ovarian cancer cells. This translocation leads to an increase in NF-κB’s transcriptional activity, which drives the expression of genes that promote cancer cell motility and invasiveness. The use of an NF-κB inhibitor (TPCK) or NF-κB-specific siRNA reduced this metastatic potential, confirming that NF-κB activation is crucial for M1 macrophage-induced metastasis. Additionally, co-treatment with a TNF-α inhibitor (etanercept) reversed NF-κB activation, further demonstrating that TNF-α from M1 macrophages is the primary mediator of this process (Figure 2) (57).
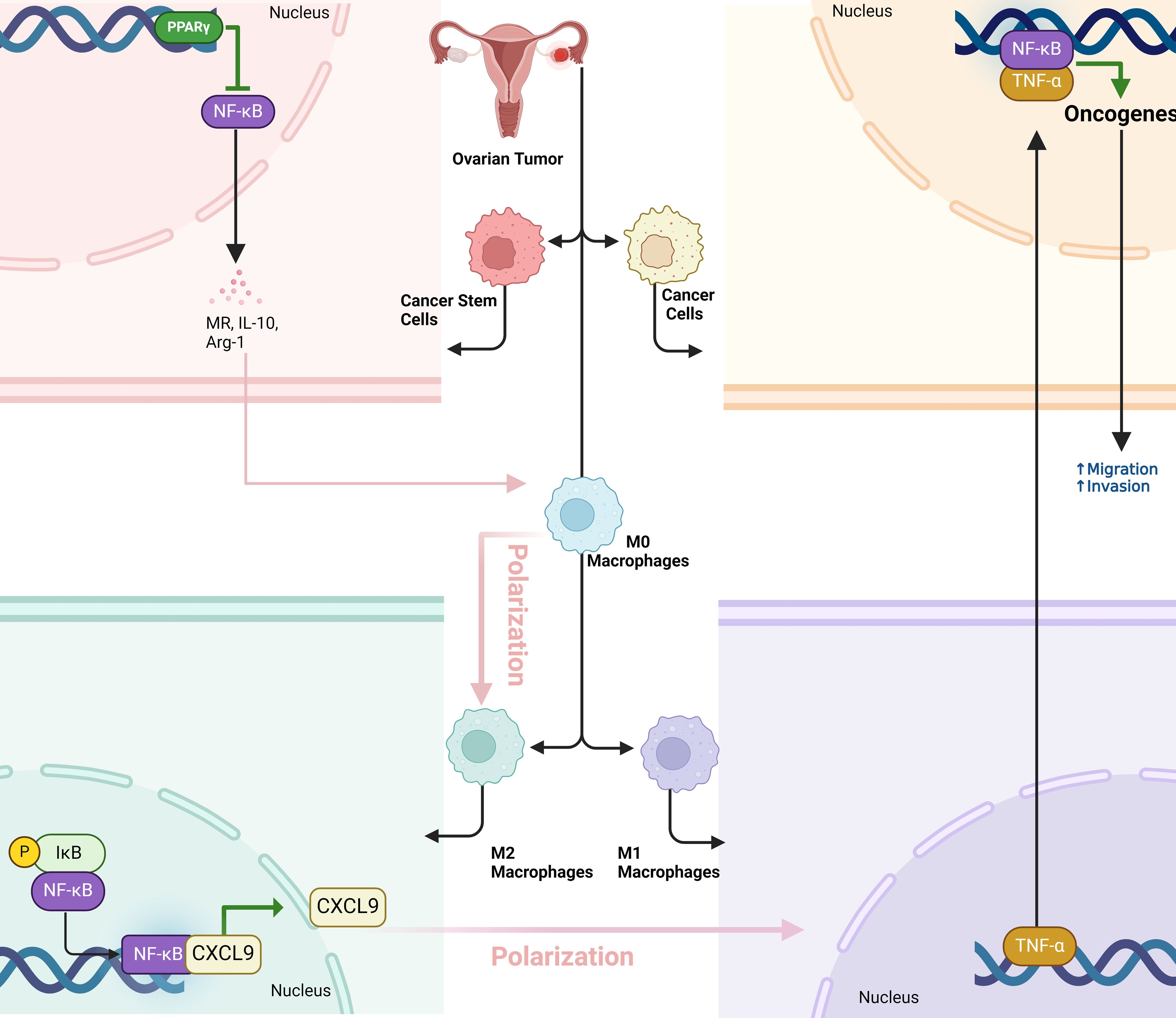
Figure 2. The dual role of NF-κB signaling in macrophage polarization and ovarian tumor progression. Cancer stem cells (OCSCs) promote a pro-tumor M2 macrophage phenotype by activating PPARγ and inhibiting NF-κB, which supports tumor growth. Conversely, NF-κB activation in macrophages reprograms them into an anti-tumor M1 phenotype, boosting immune cell infiltration (especially CD8+ T cells) and tumor necrosis. However, M1 macrophages can also enhance tumor metastasis by releasing TNF-α, which activates NF-κB in cancer cells, promoting migration and invasion. This highlights NF-κB’s dual role in tumor immunity and metastasis.
Alternatively activated macrophages (AAMs) promote the metastasis of ovarian cancer by secreting factors like FLT3L, leptin, and HB-EGF, which stimulate the spreading of HGSOC spheroids across the extracellular matrix (ECM). Each cancer cell line responds to different AAM-derived factors, but they all funnel through a common JAK2/STAT3 signaling pathway. Activation of this pathway leads to increased secretion of MMP-9, a matrix-degrading enzyme, which facilitates spheroid disaggregation and migration. This mechanism highlights JAK2/STAT3 as a central mediator of cancer cell spreading, presenting a potential therapeutic target to inhibit ovarian cancer metastasis (58). In ovarian cancer tissues, CTHRC1 is overexpressed, especially in advanced-stage tumors, and is strongly correlated with increased infiltration of M2-like TAMs, which are known to facilitate tumor growth and metastasis (59). CTHRC1 is a secreted protein that, once released into the tumor microenvironment, influences the immune landscape by activating the STAT6 pathway in macrophages (60). This activation leads to the phosphorylation of STAT6, a critical step in the polarization of macrophages toward the M2 phenotype, characterized by the expression of markers like CD206 and CD163. These M2-like TAMs, in turn, promote cancer cell migration and invasion, further contributing to tumor progression. CTHRC1-driven macrophage polarization occurs in a dose-dependent manner, both in vitro and in vivo, with higher CTHRC1 levels leading to greater activation of the STAT6 pathway and stronger M2 polarization. This indicates that CTHRC1 not only recruits macrophages but also reprograms them into an immunosuppressive M2 state, which supports cancer metastasis, making CTHRC1 a potential therapeutic target for disrupting this pro-tumorigenic interaction in ovarian cancer (59). The co-culture of ovarian cancer stem-like cells (OCSLCs) with macrophages derived from THP-1 cells promoted stemness in SKOV3 ovarian cancer cells through the IL-8/STAT3 signaling pathway. This interaction led to the polarization of macrophages into the tumor-promoting M2 phenotype, characterized by increased secretion of IL-10, VEGF, and MMP-9, alongside reduced levels of IL-12 and NO. IL-8, produced during co-culture, activated STAT3 in macrophages, which in turn enhanced the stemness of SKOV3 cells by increasing their ability to form spheres and colonies, and by upregulating cancer stem cell markers CD133 and CD44. Blocking IL-8 or inhibiting STAT3 activation disrupted these effects, indicating that the IL-8/STAT3 axis plays a crucial role in the interaction between OCSLCs and macrophages, driving tumor progression and cancer cell stemness (Figure 3) (61).
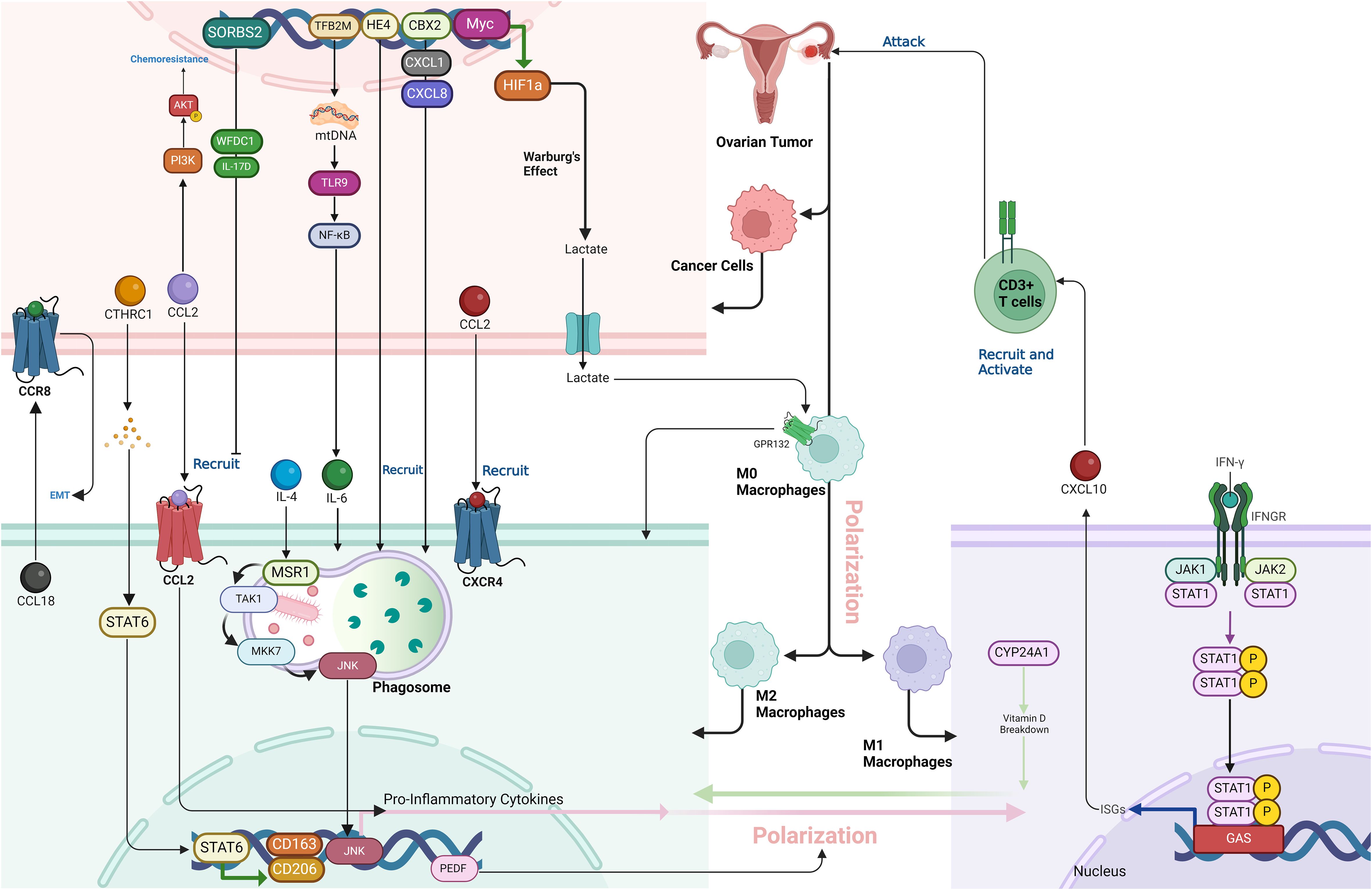
Figure 3. The complex interactions within the tumor microenvironment (TME) of ovarian cancer, emphasizing the role of key proteins, including Myc, HIF1a, GPR132, CTHRC1, STAT6, CCL2, CXCL8, CXCL10, IL-6, MSR1, TLR9, NF-κB, CYP24A1, CD206, CD163, HE4, WFDC1, IL-17D, TFB2M, PEDF, and JNK, in promoting an immunosuppressive and pro-tumorigenic landscape. These proteins collectively drive macrophage polarization, immune cell recruitment, and CD8+ T cell suppression, facilitating tumor progression and resistance to therapies in ovarian cancer.
In ovarian cancer, JNK signaling is crucial in driving macrophage polarization from an anti-inflammatory M2 state to a pro-inflammatory state (62). Triggering the macrophage scavenger receptor 1 (MSR1) in IL-4-activated M2 macrophages induces K63-linked polyubiquitylation, which recruits the TAK1/MKK7/JNK signaling complex to the phagosome. Activation of JNK leads to the production of pro-inflammatory cytokines and a shift in macrophage phenotype. This process is observed in TAMs within ovarian cancer tissue, where elevated MSR1 ubiquitylation and JNK activation contribute to the inflammatory environment, potentially promoting tumor progression and metastasis. This signaling pathway highlights the importance of macrophage plasticity in cancer progression and suggests JNK as a potential therapeutic target (Figure 3) (63).
Chemokines and their receptors play a pivotal role in the polarization of macrophages by influencing their recruitment, differentiation, and activation in response to various environmental cues (64). These small signaling proteins guide macrophages to specific tissues where they adopt different functional phenotypes based on the local microenvironment. For instance, chemokine receptors like CCR2, CCR5, and CXCR4 help recruit monocytes to inflammation or tissue damage sites, where they differentiate into macrophages. Chemokines such as CCL2, CCL18, and CXCL12 are instrumental in promoting either a pro-inflammatory M1 phenotype, which is associated with pathogen clearance and tissue damage, or an anti-inflammatory M2 phenotype, which is involved in tissue repair, fibrosis, and tumor progression (65). These chemokine-mediated signaling pathways not only determine the type of macrophage polarization but also contribute to the macrophages’ specific roles in diseases such as cancer, atherosclerosis, fibrosis, and metabolic disorders. Thus, chemokines are crucial regulators of macrophage plasticity and their function in immune responses (66). A study highlighted the critical role of the CCL2-CCR2 axis in mediating paclitaxel resistance in ovarian cancer cells through both autocrine and paracrine signaling mechanisms. Paclitaxel-resistant ovarian cancer cells were shown to overexpress CCL2, which not only promoted chemoresistance via PI3K/Akt and NF-κB signaling pathways but also attracted TAMs. These TAMs, once recruited by the CCL2 secreted by resistant cancer cells, were polarized into an M2 phenotype, which is known to promote tumor progression and further enhance resistance to paclitaxel. Inhibition of the CCL2/CCR2 axis using a CCR2 inhibitor significantly increased paclitaxel sensitivity in both in vitro and in vivo models, suggesting that targeting this signaling pathway could be a promising therapeutic strategy to overcome chemoresistance in ovarian cancer. This dual mechanism involving both the tumor cells’ autocrine signaling and macrophage recruitment contributes to the poor therapeutic response and highlights the potential benefit of CCR2 inhibitors in treating ovarian cancer (35). In ovarian cancer, CXCR2 is significantly associated with macrophage infiltration, particularly influencing both M1 and M2 macrophages in the tumor microenvironment. CXCR2 expression negatively correlates with the presence of both M1 and M2 macrophages, indicating its role in reducing macrophage infiltration. This suggests that CXCR2 may contribute to creating an immunosuppressive tumor environment by limiting macrophage activity, particularly M1 macrophages, which are typically involved in anti-tumor immune responses, and M2 macrophages, which are often associated with promoting tumor growth and immunosuppression (67). CXCL10 is crucial in M1-polarized TAMs in ovarian cancer by promoting antitumor immunity. M1-polarized TAMs, particularly in HGSOC, secrete CXCL10 in response to IFNγ signaling, which attracts T-cells into the tumor microenvironment. This enhances the immune response against the tumor, as CXCL10 helps to recruit and activate CD3+ T-cells, facilitating a coordinated attack on cancer cells. The presence of CXCL10+ M1-type TAMs is associated with better clinical outcomes, greater chemotherapy response, and improved survival in ovarian cancer patients. Multiple studies have confirmed this association in HGSOC cohorts, demonstrating a strong correlation between CXCL10 expression and improved immune infiltration. In contrast, ovarian cancer subtypes lacking CXCL10+ TAMs, such as clear cell carcinoma (CCC), exhibit poorer immune infiltration and worse prognoses. Therefore, CXCL10 in M1-polarized TAMs is a key factor in driving immune-mediated tumor suppression and enhancing therapeutic efficacy in ovarian cancer. This highlights the need for subtype-specific investigations before generalizing the role of CXCL10 across all ovarian cancer cases (68, 69). The CXCL12-CXCR4 axis in ovarian tumors promotes the recruitment and retention of M2 macrophages. This axis inhibits the presence of M1 macrophages, which are essential for antitumor immunity due to their pro-inflammatory and immune-activating properties. By blocking CXCL12-CXCR4, for example, using the antagonist AMD3100, the accumulation of M2 macrophages is reduced, leading to a shift toward M1 macrophage polarization. This shift enhances the tumor’s immune response by activating cytotoxic T cells and producing pro-inflammatory cytokines, creating a less favorable environment for tumor survival. In combination with immune checkpoint inhibitors like anti-PD-1, blocking this pathway further strengthens antitumor immune activity by facilitating M2-to-M1 macrophage reprogramming (70). M2-TAMs promote the EMT of ovarian cancer (OvCa) cells within tumor spheroids by secreting chemokine (C-C motif) ligand 18 (CCL18). CCL18 binds to its receptor, CCR8, on OvCa cells, triggering the activation of the EMT process, which transforms these cells from an epithelial to a mesenchymal state, characterized by increased invasiveness and migratory abilities. This transition is mediated by the upregulation of EMT-inducing transcription factors like ZEB1. ZEB1 not only drives EMT but also stimulates the production of macrophage colony-stimulating factor (M-CSF) in OvCa cells, which further polarizes TAMs into the pro-tumorigenic M2 subtype, creating a feedback loop that enhances the aggressiveness of OvCa spheroids and promotes metastasis (Figure 3) (71).
Recent findings by Mollaoglu et al. provide crucial insights into the role of IL-4 in shaping specific macrophage subsets in ovarian cancer. This study identified IL-4 as a key tumor-derived factor that drives macrophage polarization, particularly promoting the MARCO-expressing macrophage subset in the ovarian tumor microenvironment. These macrophages exhibit an immunosuppressive phenotype, contributing to tumor progression and resistance to immune checkpoint blockade (ICB). The study further demonstrated that IL-4 signaling operates within distinct TME neighborhoods, where even a small fraction of IL-4-producing ovarian cancer cells can exert a significant impact on the surrounding immune landscape. IL-4-mediated macrophage programming was found to be spatially restricted, emphasizing the localized effects of cytokine gradients within the tumor. These findings underscore the complexity of macrophage polarization beyond the traditional M1/M2 dichotomy, revealing that distinct factors, such as IL-4, drive specific gene expression programs within macrophage subsets that have unique immunosuppressive functions in ovarian cancer (72). IL-4, IL-10 and TGF-β are also central to macrophage-mediated immunosuppression in ovarian cancer (68). IL-10 suppresses pro-inflammatory signaling and supports the expression of CD163 and CD206, hallmark M2 markers associated with poor prognosis. TGF-β further enhances the immunosuppressive function of macrophages by promoting extracellular matrix remodeling, angiogenesis, and immune escape mechanisms (73). Conversely, pro-inflammatory cytokines such as IFN-γ and TNF-α drive M1 polarization, enhancing tumor immunity (70). IFN-γ, secreted by activated CD8+ T cells and NK cells, induces the production of CXCL10, a key chemokine that recruits additional effector T cells into the TME. High levels of CXCL10+ TAMs have been correlated with improved chemotherapy response and overall survival in ovarian cancer patients, particularly in HGSOC (68).
Collectively, these findings underscore the complex interplay between chemokines, cytokines, and macrophages in ovarian cancer. While immunosuppressive factors such as CCL2, CXCL12, IL-4, and IL-10 sustain an immune-excluded TME, pro-inflammatory mediators like IFN-γ, TNF-α, and CXCL10 counteract these effects by promoting immune cell infiltration and tumor suppression. Targeting the balance between these opposing forces offers a promising strategy for modulating macrophage polarization and enhancing the efficacy of ovarian cancer therapies.
Myc-mediated inhibition of HIF1a degradation plays a key role in fostering an immunosuppressive environment in ovarian cancer by affecting both macrophage polarization and CD8 T cell activity (74). Overexpression of Myc stabilizes HIF1a, a transcription factor that drives the Warburg effect, leading to increased lactic acid production in tumor cells (75). This lactic acid acts as a signaling molecule, inducing macrophages to polarize into the M2 phenotype, which is known for its immunosuppressive properties. This process is dependent on the activation of Gpr132, a receptor on macrophages that is activated by the elevated lactic acid levels. Once polarized, M2 macrophages release factors that inhibit CD8 T cell proliferation, lower IFN-γ secretion, and weaken the CD8 T cells’ ability to target and kill tumor cells. Thus, Myc not only promotes metabolic changes via HIF1a stabilization but also establishes an immune-suppressive tumor microenvironment by enhancing M2 macrophage polarization and directly impairing CD8 T cell function. This dual effect allows the tumor to evade immune surveillance and promotes its progression, contributing to the challenges in treating ovarian cancer (Figure 3) (74).
Chromobox 2 (CBX2) is an epigenetic regulator and a component of polycomb repressor complex 1 (PRC1), known for its role in chromatin modification and gene transcription (76). In the context of HGSOC, CBX2 is overexpressed in the majority of cases and is associated with poor prognosis and chemotherapy resistance (77). Its primary role in the tumor immune microenvironment (TIME) is to drive an immunosuppressive state by promoting the recruitment and polarization of macrophages toward a tumor-promoting M2-like phenotype. This polarization leads to decreased immune surveillance and increased tumor progression. CBX2 also regulates the expression of immune-modulatory genes, including cytokines such as CXCL1 and CXCL8, which further enhance the recruitment of TAMs. Thus, CBX2 plays a crucial role in remodeling the TIME to support tumor growth and therapy resistance, making it a potential therapeutic target to improve immune responses against tumors (Figure 3) (78).
GATA3 is a transcription factor that plays a pivotal role in immune cell differentiation and function, and it has emerged as a master regulator in the interaction between TAMs and high-grade serous ovarian carcinoma (HGSOC) (79). In HGSOC, GATA3 is highly expressed and contributes to poor clinical outcomes by promoting tumor proliferation, migration, angiogenesis, and resistance to chemotherapy, particularly in cases with mutant TP53 (80). GATA3 is abundantly released via exosomes, where it drives the polarization of macrophages to the pro-tumorigenic M2 phenotype, enhancing tumor growth and immune evasion. Moreover, GATA3 orchestrates complex cross-talk between TAMs and tumor cells, facilitating epithelial-mesenchymal transition (EMT), chemoresistance, and overall tumor progression. Targeting GATA3 has shown potential to impair these tumor-supporting interactions, making it a promising therapeutic target for treating HGSOC (Figure 4) (79).
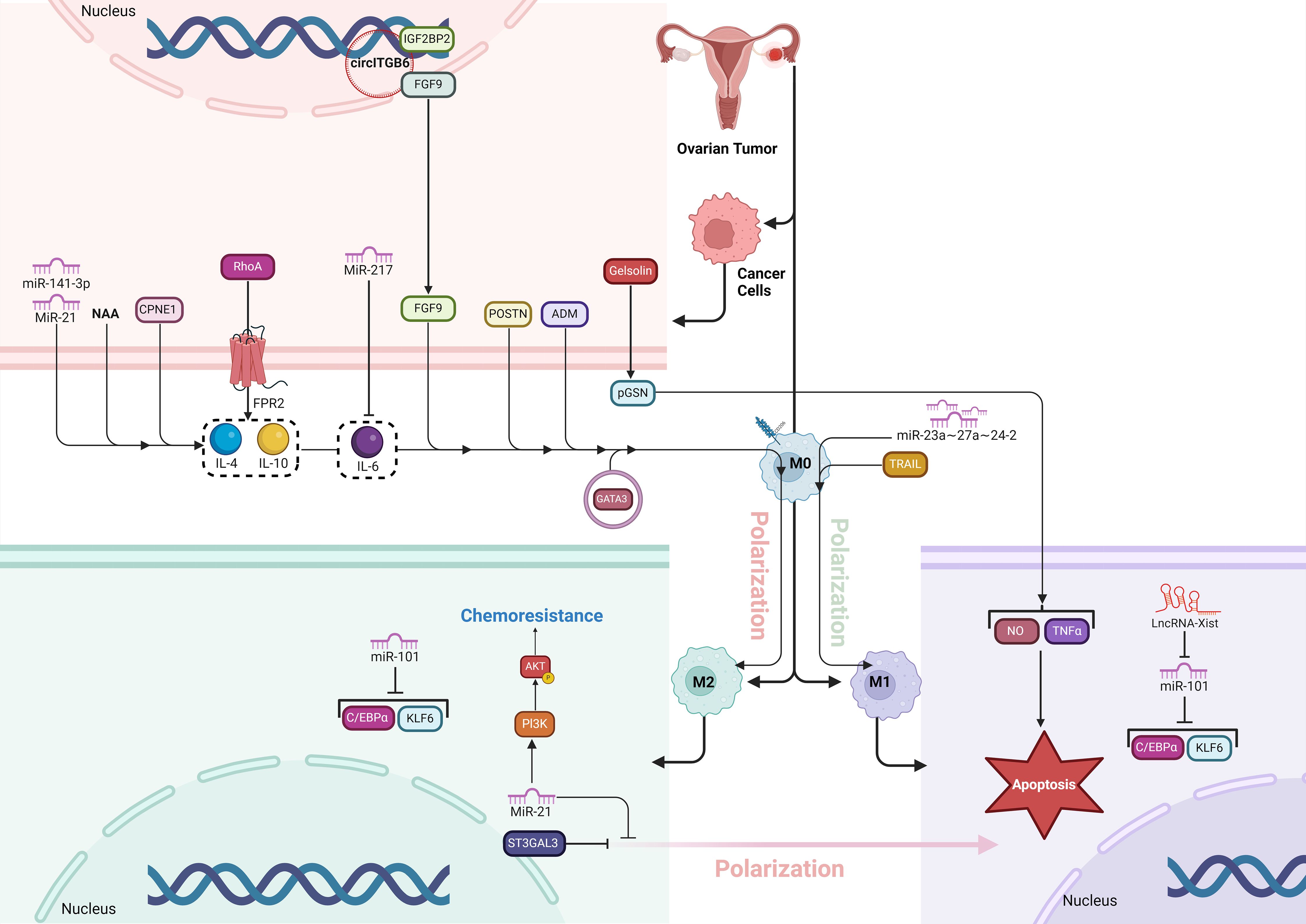
Figure 4. The figure illustrates how various molecular factors drive TAM polarization and chemoresistance in the OC microenvironment. pGSN, secreted by OC cells, promotes chemoresistance by inhibiting M1 macrophages’ anti-tumor functions and favoring M2 dominance. FPR2 activates RhoA, enhancing cell migration and fostering an immunosuppressive M2-supporting environment. GATA3, highly expressed in HGSOC, promotes M2 polarization, aiding tumor growth and chemoresistance. St3gal3 supports immune evasion by increasing M2 macrophages, while ADM drives M2 polarization through RhoA signaling. TRAIL, on the other hand, induces M1 polarization, boosting anti-tumor immunity. CPNE1 and POSTN further increase M2 prevalence, supporting tumor progression. Various ncRNAs (miR-21, miR-141-3p, miR-217, LncRNA-Xist) and circITGB6 regulate polarization and chemoresistance by targeting key pathways, with miR-21 enhancing M2-related chemoresistance via PI3K/AKT. These factors collectively establish a tumor-promoting environment, revealing potential therapeutic targets to improve OC treatment.
St3gal3 is an enzyme responsible for catalyzing the synthesis of α2,3-linked sialic acids, which play a crucial role in promoting immune evasion and tumor progression in ovarian cancer (81). Elevated St3gal3 levels are associated with poor prognosis in HGSOC by contributing to an immunosuppressive TME characterized by increased infiltration of pro-tumor macrophages and reduced CD8+ T-cell activity. Knockdown or inhibition of St3gal3 reprograms this environment by boosting immune cell infiltration, enhancing the presence of cytotoxic CD8+ T cells, and repolarizing TAMs from a tumor-promoting M2-like phenotype to an antitumor M1-like state. Additionally, blocking sialylation with pharmacological agents like ambroxol sensitizes the tumor to immune checkpoint blockade (ICB) therapies, such as anti-PD-1 and anti-CTLA4, significantly improving tumor control and extending survival in preclinical models. Thus, targeting St3gal3 and sialylation offers a promising strategy to enhance the effectiveness of immunotherapy in ovarian cancer (Figure 4) (82).
Non-coding RNAs (ncRNAs), including microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), play crucial roles in regulating macrophage polarization, a process that impacts tumor progression and immune response. NcRNAs influence this polarization by targeting key genes and pathways that either promote M1 or M2 phenotypes (83). For instance, some miRNAs like miR-16 and miR-155 drive M1 polarization, enhancing anti-tumor activity, while others like miR-145 and miR-21 promote M2 polarization, supporting tumor growth and metastasis. Similarly, lncRNAs like GAS5 favor M1 polarization, while others like NEAT1 and MALAT1 promote M2 macrophages in some cancers. By regulating these polarization states, ncRNAs are central to shaping the tumor microenvironment, offering potential therapeutic targets to modulate immune responses and combat cancer (84). MiR-21 has a key role in the polarization of macrophages, particularly by promoting the M2 macrophage phenotype. In ovarian cancer, M2 macrophages, characterized by anti-inflammatory properties, enhance the chemoresistance of cancer cells. MiR-21 influences this process by upregulating M2 markers (like CD206 and IL-10) and downregulating M1 markers (such as TNF-α and iNOS), thus driving macrophages toward the tumor-promoting M2 phenotype. The presence of M2 macrophages increases the resistance of ovarian cancer cells to chemotherapy, particularly cisplatin. This chemoresistance is mediated through the PI3K/AKT signaling pathway, where M2 macrophages with elevated miR-21 levels enhance the survival and drug resistance of ovarian cancer cells. Conversely, inhibiting miR-21 can reduce this chemoresistance by repolarizing M2 macrophages back to the M1 phenotype, making cancer cells more susceptible to treatment (85). The miR-23a∼27a∼24-2 cluster plays a critical role in promoting the inflammatory polarization of macrophages, particularly influencing the balance between pro-inflammatory (M1) and anti-inflammatory (M2) states in the context of ovarian cancer. This cluster, composed of miRs-23a, -27a, and -24-2, enhances M1 polarization, which is crucial for anti-tumor immune responses, while its deletion skews macrophages toward the immunosuppressive M2 state that supports tumor growth. In ovarian cancer models, the absence of this miRNA cluster in macrophages leads to increased tumor progression and poor outcomes, as the macrophages adopt an M2 phenotype, suppressing the immune response and aiding tumor evasion. Thus, the miR-23a cluster regulates macrophage plasticity, and targeting it could be a potential strategy to enhance tumor immunity by modulating macrophage polarization (86). Similarly, miR-141-3p accelerates OC progression by promoting cell proliferation, migration, and invasion while enhancing M2-like macrophage polarization by targeting the Keap1-Nrf2 pathway. MiR-141-3p is overexpressed in OC, leading to the downregulation of Keap1, a negative regulator of Nrf2. This, in turn, activates Nrf2, a transcription factor involved in antioxidant defense and tumor progression. The activation of Nrf2 facilitates the transformation of macrophages into the M2 phenotype, which supports tumor growth and metastasis. Inhibiting miR-141-3p disrupts this pathway, reducing M2 polarization and suppressing tumor development, making it a potential therapeutic target in OC (87). MiR-217 inhibits M2-like macrophage polarization in ovarian cancer by directly targeting and suppressing the secretion of IL-6, a key cytokine involved in promoting this immunosuppressive macrophage phenotype. In ovarian cancer tissues and cell lines, miR-217 is downregulated, which leads to increased IL-6 secretion and the activation of the JAK2/STAT3 signaling pathway, driving M2-like macrophage polarization. Overexpression of miR-217 reduces IL-6 levels, which in turn inhibits the JAK2/STAT3 pathway, decreasing M2 macrophage markers and promoting an immune environment less conducive to tumor progression. This mechanism highlights miR-217’s potential as a therapeutic target for disrupting the tumor-supportive microenvironment in ovarian cancer (88). In M1 macrophages (anti-tumor), LncRNA-Xist is upregulated, maintaining the M1 phenotype by inhibiting miR-101, which in turn allows the expression of C/EBPα and KLF6, two transcription factors that support M1 polarization. In contrast, miR-101 is highly expressed in M2 macrophages (pro-tumor) and suppresses C/EBPα and KLF6, promoting M1-to-M2 polarization. This shift enhances cancer cell proliferation and migration in ovarian cancer. Therefore, Xist acts as a competing endogenous RNA (ceRNA) by binding miR-101, preventing it from promoting the pro-tumor M2 phenotype, thereby reducing cancer progression (89). CircITGB6 contributes to cisplatin resistance in ovarian cancer by altering the tumor microenvironment, specifically by shifting TAMs toward the M2 phenotype, which is linked to tumor growth and immune suppression. Elevated levels of circITGB6 in cisplatin-resistant ovarian cancer cells form a complex with the RNA-binding protein IGF2BP2 and fibroblast growth factor 9 (FGF9) mRNA. This interaction stabilizes FGF9 mRNA, increasing its expression and secretion. Higher FGF9 levels promote the polarization of TAMs into the M2 phenotype, which supports tumor progression and limits the effectiveness of cisplatin by creating an immunosuppressive environment. This M2 macrophage-driven environment protects cancer cells from cisplatin-induced apoptosis, contributing to chemoresistance. Therapeutically, using antisense oligonucleotides to inhibit circITGB6 reduces M2 macrophage infiltration and enhances cisplatin sensitivity, positioning circITGB6 as a promising target to combat chemotherapy resistance in ovarian cancer (Figure 4) (90).
HE4 (human epididymis protein 4) is a glycoprotein commonly overexpressed in ovarian cancer, particularly in serous ovarian carcinomas, and is associated with aggressive tumor behavior and poor prognosis (91). Its overexpression creates a suppressive tumor immune microenvironment by promoting immune evasion and tumor progression. Specifically, reduces the infiltration and activation of cytotoxic T cells (CTLs) and natural killer (NK) cells, while enhancing the recruitment of M2 macrophages, which are immunosuppressive and promote tumor growth. Additionally, HE4 increases the expression of the immune checkpoint protein PD-L1 on both tumor cells and macrophages, through a novel posttranscriptional mechanism, further inhibiting immune responses against the tumor. This immune suppression driven by HE4 allows the cancer to evade immune detection, making it a potential target for therapeutic interventions, particularly in combination with PD-L1/PD-1 checkpoint inhibitors (Figure 3) (92).
Adrenomedullin (ADM) plays a pivotal role in the polarization of macrophages toward the M2 phenotype in cancers, which contributes to tumor progression (93). In ovarian cancer, ADM is secreted by tumor cells and promotes the differentiation of macrophages into M2-like TAMs. These M2 macrophages, characterized by the expression of markers like CD206 and the secretion of anti-inflammatory cytokines such as IL-10 and CCL18, have pro-tumor functions. ADM-induced M2 macrophages support tumor growth, angiogenesis, and metastasis by enhancing the migration and invasion of ovarian cancer cells. The interaction between ADM and macrophages activates the RhoA signaling pathway in cancer cells, which leads to cytoskeletal rearrangements necessary for cell migration. Thus, ADM is a key factor linking macrophage polarization and ovarian cancer cell migration, highlighting its potential as a therapeutic target to inhibit TAM-mediated cancer progression (Figure 4) (94).
Periostin (POSTN) is a secreted matricellular protein that is critical in promoting ovarian cancer metastasis (95). It enhances tumor progression by interacting with integrin receptors (specifically integrin β3 and β5) on cancer cells, which activates the ERK and NF-κB signaling pathways. This activation produces cytokines and chemokines that attract monocytes and promote their differentiation into tumor-associated M2 macrophages, known for their pro-tumor effects. POSTN also stimulates the expression of TGF-β2, which activates normal fibroblasts into cancer-associated fibroblasts (CAFs), further supporting tumor growth and invasion. These M2 macrophages and CAFs contribute to a tumor microenvironment that promotes immune evasion and metastasis, making POSTN a potential therapeutic target in ovarian cancer (Figure 4) (56).
TRAIL (TNF-related apoptosis-inducing ligand) is a member of the TNF superfamily known for its ability to induce cell death in transformed cells by binding to death receptors (DR4 and DR5) while sparing normal cells (96). TRAIL promotes the polarization of human macrophages toward a pro-inflammatory, tumor-fighting M1 phenotype by increasing the expression of M1 markers and reducing M2 markers. TRAIL enhances the cytotoxicity of macrophages against cancer cells through both DR4 and DR5 signaling pathways. Additionally, in cancer patients, high levels of TRAIL expression are associated with longer overall survival, particularly in cases with high tumor macrophage content, suggesting that TRAIL enhances anti-tumor responses by promoting M1 macrophage polarization in the tumor microenvironment (Figure 4) (97).
Prostacyclin (PGI2) plays a pivotal role in the ovarian cancer microenvironment by mediating interactions between CAFs and TAMs, significantly influencing macrophage polarization and tumor progression. CAFs, the primary producers of PGI2 due to their high expression of prostacyclin synthase (PTGIS), release this bioactive lipid into the TME, where it binds to the PGI2 receptor (PTGIR) on TAMs, which express this receptor at high levels. Upon activation, PGI2 induces a mixed polarization state in TAMs, affecting both M1 and M2 macrophage phenotypes. Typically, M1 macrophages exhibit pro-inflammatory, anti-tumorigenic properties, while M2 macrophages are associated with immunosuppressive, pro-tumorigenic behavior. However, PGI2 signaling blurs these distinctions, as it promotes M1-like markers such as CD86 but represses pro-inflammatory M1 genes like TNF and FCGRs. Simultaneously, it enhances M2-like features, including the secretion of VEGF, a key factor in angiogenesis, while inhibiting the expression of other M2 markers like CD206 (MRC1). This polarization reduces the phagocytic capacity of TAMs and suppresses immune activation by downregulating cytokines critical for T cell and NK cell recruitment, such as CXCL10 and IL12A. Consequently, PGI2 skews TAMs toward a pro-tumorigenic, immunosuppressive phenotype that supports tumor growth, metastasis, and immune evasion. By reshaping the macrophage polarization landscape, PGI2 becomes a critical factor in ovarian cancer progression, and targeting its signaling pathway offers a potential therapeutic strategy to counteract tumor-promoting macrophage functions (Figure 3) (98).
N-acetylaspartate (NAA) is a metabolite primarily known for its role in the brain but is also implicated in various cancers, including ovarian cancer (99). In glutaminolytic ovarian cancer cells, which are highly dependent on extracellular glutamine due to low glutamine synthetase (GS) levels, NAA is produced as a byproduct of altered metabolism. These cancer cells release NAA into the tumor microenvironment, where it acts in synergy with IL-10 to polarize TAMs toward an M2-like, protumoral state. NAA achieves this by inhibiting the NMDA receptor (NMDAR) on macrophages, leading to increased GS expression, which reinforces the M2-like phenotype. This metabolic crosstalk between cancer cells and macrophages supports tumor growth, immune evasion, and cancer progression (Figure 4) (100, 101).
Mitochondrial transcription factor B2 (TFB2M) is a key protein involved in the regulation of mitochondrial DNA (mtDNA) transcription and compaction. It functions as part of a mitochondrial transcription complex that includes mitochondrial transcription factors A (TFAM) and B1 (TFB1M), essential for mtDNA maintenance and the expression of genes critical for mitochondrial function (102). TFB2M specifically modulates both the transcription of mtDNA and the regulation of its copy number, playing a vital role in mitochondrial biogenesis and homeostasis. In ovarian cancer, overexpression of TFB2M has been linked to an immunosuppressive tumor microenvironment by promoting M2 macrophage infiltration. This process is mediated through cytosolic mtDNA stress, which occurs when excess mtDNA is released into the cytoplasm due to mitochondrial dysfunction or fission. The release of cytosolic mtDNA acts as a damage-associated molecular pattern (DAMP), activating the Toll-like receptor 9 (TLR9) pathway and leading to the activation of the NF-κB signaling pathway. This activation stimulates the secretion of IL-6, a pro-inflammatory cytokine known to promote the recruitment and polarization of macrophages into the M2 phenotype. M2 macrophages are associated with tumor progression due to their immunosuppressive and pro-tumorigenic activities, such as promoting angiogenesis, tissue remodeling, and suppression of cytotoxic immune responses (Figure 3) (73).
CYP24A1, an enzyme crucial in vitamin D (VD) metabolism, significantly contributes to OC progression by influencing the polarization of TAMs. Increased CYP24A1 expression accelerates the breakdown of active vitamin D, limiting its role in supporting anti-tumor immune functions. This leads to a shift in TAMs from the pro-inflammatory, cancer-fighting M1 phenotype to the tumor-supporting M2 phenotype. M2 macrophages encourage tumor growth, angiogenesis, and metastasis, thereby driving OC progression. By altering vitamin D availability and TAM polarization, CYP24A1 promotes cancer advancement, making it a promising biomarker and therapeutic target in OC treatment (Figure 3) (103).
SORBS2 is an RNA-binding protein that plays a crucial role in suppressing the metastatic colonization of cancers by stabilizing tumor-suppressive transcripts (104). It binds to the 3’ untranslated regions (UTRs) of two key immunomodulatory molecules, WFDC1 and IL-17D, which are secreted factors that inhibit cancer metastasis. By stabilizing these transcripts, SORBS2 enhances their expression, thereby limiting the invasiveness of cancer cells and promoting a tumor-suppressive immune microenvironment. This action affects immune cell polarization, reducing the recruitment of tumor-promoting myeloid cells and M2-like macrophages, ultimately creating conditions unfavorable for metastasis. Thus, SORBS2 contributes to ovarian cancer suppression by linking tumor progression and immune regulation through its post-transcriptional stabilization of key immunomodulatory transcripts (Figure 3) (92).
Pigment Epithelium-Derived Factor (PEDF) is a 50-kDa glycoprotein known for its potent anti-angiogenic and anti-tumor properties. In OC, PEDF levels are significantly reduced, and its low expression is associated with advanced disease and poor patient outcomes. PEDF delays ovarian cancer progression by modulating the immune microenvironment, specifically by influencing TAMs, which are typically polarized toward the M2 subtype that promotes tumor growth, angiogenesis, and immune suppression. PEDF overexpression shifts TAMs from the tumor-promoting M2 phenotype to the anti-tumor M1 phenotype, thereby inhibiting tumor growth and enhancing cancer cell apoptosis. This effect is mediated through the regulation of ATGL (Adipose Triglyceride Lipase) and the ERK1/2 signaling pathway. By reprogramming the macrophages in the tumor microenvironment, PEDF helps create an immune response more hostile to cancer cells, thus slowing ovarian cancer progression (Figure 3) (105).
Gelsolin is an actin-binding protein involved in the regulation of the cytoskeleton, with two forms: intracellular and extracellular (plasma gelsolin, or pGSN) (106). In ovarian cancer, plasma gelsolin (pGSN) plays a significant role in conferring chemoresistance. pGSN is secreted by ovarian cancer cells and is often found in elevated levels in chemoresistant patients. It modulates the tumor microenvironment by interacting with immune cells, specifically TAMs. Normally, M1 macrophages have anti-tumor properties, producing nitric oxide (NO) and TNFα, which promote cancer cell death. However, pGSN interferes with this process by inducing apoptosis in M1 macrophages and reducing their production of NO and TNFα, thereby inhibiting their anti-tumor function. At the same time, pGSN does not affect M2 macrophages, which are pro-tumorigenic, creating an imbalance in the M1/M2 macrophage ratio. This shift toward a tumor-promoting environment helps cancer cells evade chemotherapy, increasing tumor survival, recurrence, and chemoresistance. As a result, pGSN serves as a key factor in reducing the effectiveness of chemotherapy in ovarian cancer patients (Figure 4) (107).
Formyl peptide receptor 2 (FPR2) is a member of the G protein-coupled receptor (GPCR) family, involved in various physiological and pathological processes, including inflammation, immune responses, and cancer progression. In epithelial ovarian cancer (EOC), FPR2 significantly promotes cancer cell invasion, migration, and metastasis. It does so through activating the small GTPase RhoA, a key regulator of cytoskeletal dynamics and cellular movement. Overexpression of FPR2 in EOC cells enhances RhoA expression, leading to increased migratory capacity of the cancer cells. Additionally, FPR2 influences the tumor microenvironment by stimulating the secretion of Th2 cytokines, such as IL-4 and IL-10, which drive the polarization of macrophages into the M2 phenotype. M2 macrophages are associated with immune suppression, tumor progression, and angiogenesis, thereby facilitating EOC metastasis. In contrast, inhibiting RhoA reverses this process, promoting M1 macrophage polarization, which has anti-tumor properties. Thus, FPR2 promotes EOC progression by simultaneously enhancing cancer cell mobility through RhoA activation and fostering a pro-tumor immune environment via M2 macrophage polarization (108).
Copine 1 (CPNE1) is a calcium-dependent, membrane-binding protein that plays a critical role in promoting tumor progression in various cancers, including ovarian cancer (109). In ovarian cancer, CPNE1 is significantly upregulated and associated with poor prognosis. It regulates TAMs by promoting their polarization into the M2 phenotype, which is tumor-supportive. CPNE1 achieves this by upregulating markers such as CD163, CD206, and interleukin-10 (IL-10), which are characteristic of M2 macrophages. These M2 macrophages contribute to tumor growth and metastasis by creating a favorable tumor microenvironment, in contrast to M1 macrophages, which have anti-tumor properties. Thus, CPNE1’s role in macrophage polarization supports ovarian cancer progression and makes it a potential therapeutic target (Figure 4) (110).
Tumor-derived extracellular vesicles (EVs) significantly influence macrophage polarization in the tumor microenvironment. These EVs, released by cancer cells, transport bioactive components like miRNAs, proteins, and lipids that impact macrophage behavior. Tumor-derived EVs mainly drive macrophages to adopt the M2 phenotype, which is linked to immunosuppression, tissue repair, and the promotion of tumor growth. M2 macrophages contribute to tumor progression by supporting angiogenesis, enhancing metastasis, and dampening immune responses (111). On the other hand, M1 macrophages, which have pro-inflammatory and anti-tumor properties, are suppressed by these EVs. This capacity of tumor-derived EVs to push macrophages toward a tumor-supporting M2 state underlines their crucial role in shaping the tumor environment and advancing cancer development. By understanding these processes, new therapeutic strategies could focus on using EVs to reprogram macrophages into the M1 phenotype, potentially leading to anti-tumor outcomes. This phenomenon has been demonstrated across multiple ovarian cancer models, with findings from both in vitro and in vivo studies supporting the role of OV-EVs in immune modulation. However, the majority of these studies rely on preclinical models, and direct evidence from patient-derived EV samples remains limited, emphasizing the need for clinical validation (111). Ovarian cancer-derived extracellular vesicles (OV-EVs) promote cancer progression and angiogenesis primarily by inducing the polarization of macrophages into the M2 phenotype, which is associated with tumor growth and tissue repair. OV-EVs are taken up by macrophages, triggering their transformation into M2 macrophages. These M2 macrophages then secrete higher levels of vascular endothelial growth factor (VEGF), which binds to VEGF receptors (VEGFR) on endothelial cells, thereby stimulating angiogenesis, the formation of new blood vessels. This angiogenesis is crucial for supplying the growing tumor with nutrients and oxygen, further accelerating cancer progression. The OV-EVs also promote the infiltration of M2 macrophages into tumor tissues, enhancing their ability to support the tumor microenvironment, contributing to tumor metastasis, immune evasion, and overall disease progression (112). Cancer-associated fibroblasts (CAFs) are a key component of the tumor microenvironment and play a crucial role in cancer progression by influencing various biological processes. These fibroblasts, derived from normal stromal cells, undergo activation in the tumor context, where they secrete a variety of cytokines, growth factors, extracellular matrix, and EVs components that support tumor growth, invasion, and metastasis. CAFs can also modulate immune responses, particularly by interacting with TAMs (113). In ovarian cancer, they induce TAM polarization toward the M2-like phenotype, which is known for its immunosuppressive and pro-tumorigenic functions. This polarization is driven through the secretion of cytokines such as IL-33, IL-6, and TGF-β, which influence monocytes to differentiate into M2 macrophages. These M2 macrophages, in turn, secrete anti-inflammatory and tumor-promoting factors like IL-10 and TGF-β, further enhancing cancer cell invasion, metastasis, and immune evasion, thereby creating a tumor-promoting microenvironment (114).
Sirtuin 1 (SIRT1) is a nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase that plays a key role in regulating various cellular processes, including metabolism, aging, inflammation, and stress resistance (115, 116). In cancer, SIRT1 can influence tumorigenesis by modulating cell survival pathways and inhibiting tumor suppressor proteins. The delivery of SIRT1 by cancer-associated adipocyte-derived extracellular vesicles (CAA-EVs) significantly alters immune cell populations within the tumor microenvironment, contributing to ovarian cancer cell survival. Specifically, SIRT1 transferred via CAA-EVs promotes an increase in M2 macrophages, which are known for their immunosuppressive and tumor-promoting roles. These macrophages help create a favorable environment for tumor growth by dampening inflammatory responses and facilitating immune escape. Simultaneously, SIRT1-mediated activation of the CD24/Siglec-10 axis suppresses the activity of CD8+ T cells, crucial immune cells responsible for targeting and killing cancer cells. This dual modulation, enhancing M2 macrophages and reducing CD8+ T cell activity, creates an immune-suppressive environment that enables cancer cells to evade immune detection and survive, promoting tumor progression (117). GATA3 encapsulated within TAM-derived EVs, specifically from M2-polarized macrophages, promotes immune escape and chemotherapy resistance in OC cells by activating the CD24/Siglec-10 axis. M2 macrophages, known for their immunosuppressive and tumor-promoting roles, release EVs that transfer GATA3 to OC cells. Once inside the cancer cells, GATA3 acts as a transcription factor, upregulating CD24 expression. CD24 then interacts with Siglec-10, a receptor found on macrophages, particularly M2 TAMs, leading to the suppression of T cell-mediated immune responses. This interaction creates an immunosuppressive microenvironment, facilitating immune escape and allowing the tumor to evade immune detection. Moreover, the CD24/Siglec-10 axis enhances the tumor’s resistance to chemotherapy, particularly cisplatin, by reducing apoptosis in cancer cells (Figure 5) (118).
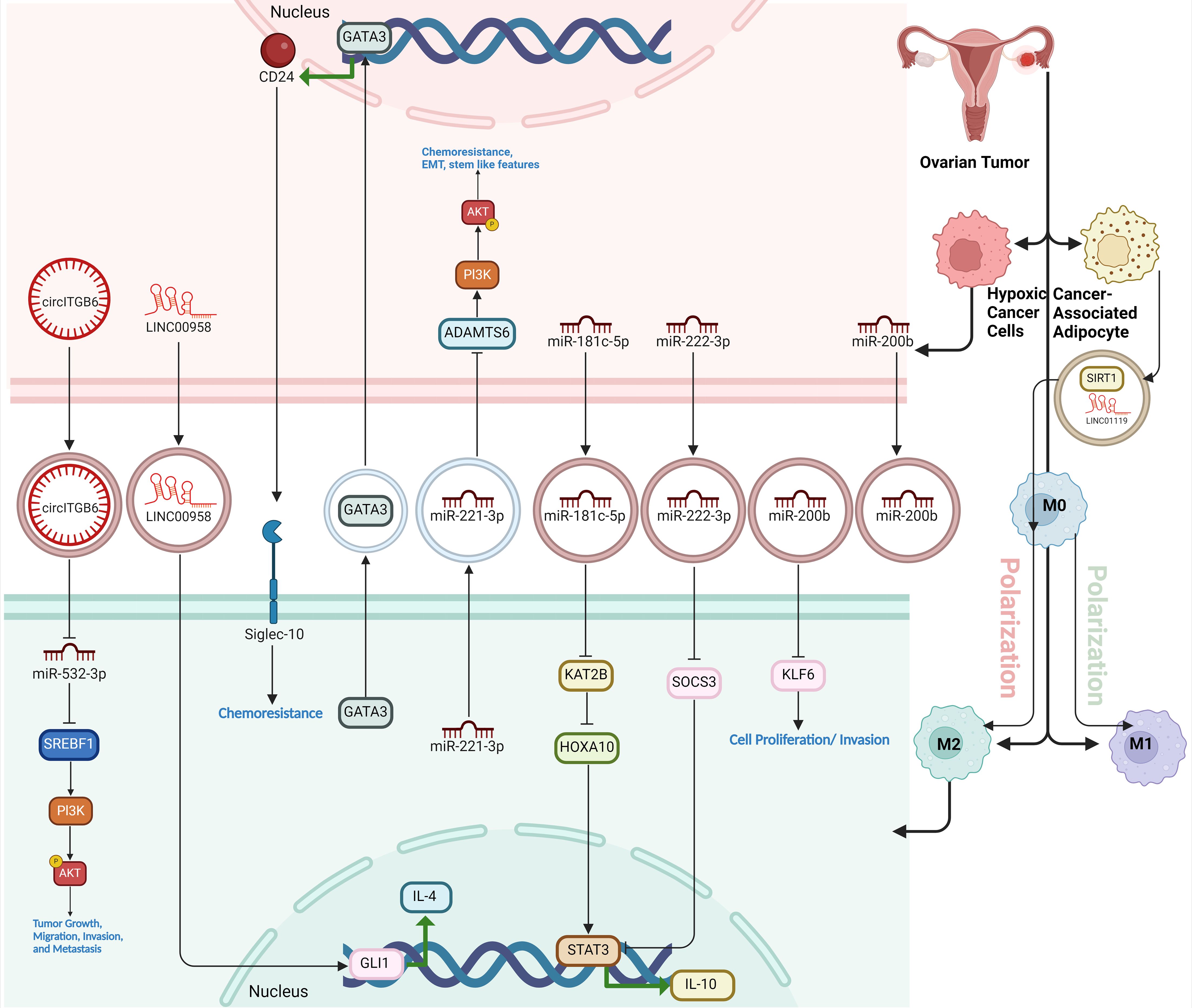
Figure 5. The role of EVs in macrophage polarization within the ovarian tumor microenvironment. Tumor-derived EVs from ovarian cancer cells and associated stromal cells (like cancer-associated adipocytes) carry various bioactive molecules, including miRNAs, circRNAs, and lncRNAs, which influence macrophage behavior. EV-encapsulated components drive macrophages toward an M2 immunosuppressive phenotype, promoting immune evasion, tumor progression, chemoresistance, and metastasis. Key pathways involved include the CD24/Siglec-10 axis and the PI3K/AKT signaling pathway, activated by transferred miRNAs and regulatory proteins, enhancing tumor-supportive functions of macrophages and contributing to a microenvironment conducive to cancer survival and invasion.
Extracellular vesicle-packaged miR-181c-5p from hypoxic EOC cells promote the M2 polarization TAMs through the KAT2B/HOXA10 axis. Specifically, miR-181c-5p is transferred to TAMs via EVs, where it targets and downregulates KAT2B, a key regulatory protein. This suppression of KAT2B increases the acetylation of HOXA10, which subsequently activates the JAK1/STAT3 signaling pathway. The activation of this pathway drives the M2 polarization of TAMs, which enhances tumor-promoting activities such as cell proliferation, migration, and invasion, contributing to the growth and metastasis of EOC (119). Likewise, miR-200b is significantly upregulated in plasma-derived exosomes of ovarian cancer patients and plays an oncogenic role by promoting macrophage M2 polarization. This polarization enhances the tumor-supportive environment, as M2 macrophages secrete anti-inflammatory cytokines that facilitate cancer cell proliferation and invasion. miR-200b achieves this by suppressing the expression of Kruppel-like factor 6 (KLF6), a tumor suppressor that normally inhibits M2 polarization. As a result, miR-200b indirectly promotes ovarian cancer progression by altering the immune landscape, making it a potential target for therapeutic intervention in ovarian cancer (18). The molecular mechanism of CD163+ TAM-derived exosome-induced cisplatin resistance in ovarian cancer ascites involves the transfer of exosomes containing miR-221-3p from TAMs to ovarian cancer cells. Upon uptake, miR-221-3p downregulates the expression of ADAMTS6, a tumor suppressor gene. This downregulation activates the AKT signaling pathway, which in turn promotes EMT, leading to the acquisition of cancer stem cell (CSC)-like characteristics and the upregulation of multidrug resistance (MDR) genes. EMT is characterized by increased levels of transcription factors such as SNAIL1 and ZEB1, as well as mesenchymal markers like Vimentin, while reducing the epithelial marker E-cadherin. This process enhances ovarian cancer cells’ resistance to cisplatin by promoting cell proliferation, migration, and survival, ultimately contributing to the poor prognosis in ovarian cancer. Overexpression of ADAMTS6 can inhibit this pathway, highlighting its potential as a therapeutic target for reversing drug resistance (19). Similarly, EOC-secreted exosomal miR-222-3p induces the polarization of macrophages into a TAM-like M2 phenotype, which supports tumor progression. Exosomes from EOC cells carry miR-222-3p, which is taken up by macrophages, leading to the downregulation of SOCS3, a negative regulator of the STAT3 signaling pathway. This suppression of SOCS3 activates STAT3, promoting M2 polarization characterized by increased expression of markers like CD206 and Arg-1, along with elevated IL-10 secretion and reduced IL-12 levels. These M2-like macrophages foster an immunosuppressive tumor microenvironment that enhances the proliferation, migration, and metastatic potential of ovarian cancer cells (120). Similarly, exosomes derived from hypoxic EOC cells are enriched with microRNA-940 (miR-940) and play a key role in inducing macrophage M2 polarization. Under hypoxic conditions, EOC cells increase the expression of miR-940, which is packaged into exosomes and delivered to nearby macrophages. Upon uptake, these exosomes drive macrophages to adopt an M2-like TAM phenotype, marked by higher expression of M2 markers such as CD163 and CD206. These M2-polarized macrophages, in turn, promote tumor progression by enhancing the proliferation and migration of EOC cells (Figure 5) (121).
LINC01119, encapsulated by exosomes derived from cancer-associated adipocytes (CAA-Exo), plays a crucial role in promoting M2 polarization of macrophages, thereby facilitating immune escape in ovarian cancer. In a 3D co-culture cell-based model, mature adipocytes co-cultured with OC cells transform into CAAs, which release exosomes carrying LINC01119. These exosomes are taken up by macrophages, triggering their polarization into the immunosuppressive M2 phenotype. M2 macrophages, in turn, suppress the proliferation and cytotoxic activity of CD3+ T cells, enhance PD-L1 expression on OC cells, and reduce the immune system’s ability to target and destroy cancer cells. This process is mediated through the upregulation of SOCS5, a target of LINC01119, which further drives macrophage polarization and immune evasion, ultimately supporting tumor progression and immune resistance in ovarian cancer (122). Exosomal LINC00958 maintains OC cell stemness and induces M2 macrophage polarization by interacting with the Hedgehog signaling pathway through the GLI1 protein. LINC00958, a long non-coding RNA, is secreted by OC cells within exosomes and transferred to other cells in the tumor microenvironment. In OC cells, LINC00958 enhances stem cell-like properties by increasing the expression of stemness-associated markers, such as ALDH and NANOG, and promoting the formation of cancer stem cell spheres. This is mediated through its interaction with GLI1, a key transcription factor in the Hedgehog signaling pathway. LINC00958 binds to GLI1, promoting its nuclear translocation and activating the transcription of GLI1 target genes, such as SOX2, which are crucial for maintaining stemness in OC cells. Additionally, LINC00958-containing exosomes induce M2 macrophage polarization, a tumor-supportive immune phenotype characterized by markers like CD206 and the expression of immunosuppressive genes such as IL-10 and Arg-1. This polarization is also mediated via the Hedgehog/GLI1 pathway, where LINC00958 facilitates GLI1 activation, driving IL-4 transcription in macrophages and enhancing the M2 phenotype. Thus, exosomal LINC00958 plays a dual role in promoting OC cell stemness and reprogramming the immune environment to support tumor progression (Figure 5) (123).
Circ_C20orf11 enhances cisplatin (DDP) resistance in ovarian cancer by acting as a competing endogenous RNA that sponges miR-527, preventing it from suppressing YWHAZ, a protein associated with drug resistance and tumor progression. This inhibition allows YWHAZ to remain active, thereby promoting cancer cell survival and DDP resistance. Additionally, circ_C20orf11 is encapsulated in EVs derived from DDP-resistant ovarian cancer cells and transferred to macrophages in the tumor microenvironment. These EVs induce macrophage polarization toward the M2 phenotype, which creates a tumor-promoting and immunosuppressive environment, further contributing to chemoresistance. Silencing circ_C20orf11 reverses these effects, reducing DDP resistance, increasing apoptosis, and decreasing macrophage M2 polarization (124). EV-packaged circATP2B4 is critical in promoting EOC metastasis by mediating M2 macrophage polarization through the miR-532-3p/SREBF1 axis. EOC cells secrete EVs containing circATP2B4, which TAMs take up in the tumor microenvironment. Once inside the macrophages, circATP2B4 acts as a competing endogenous RNA (ceRNA) by binding to and inhibiting miR-532-3p, a microRNA that normally suppresses the expression of SREBF1, a key transcription factor involved in lipid metabolism and M2 polarization. By sponging miR-532-3p, circATP2B4 allows for the upregulation of SREBF1, which subsequently activates the PI3K/AKT signaling pathway. This signaling cascade promotes the M2 polarization of macrophages, transforming them into an immunosuppressive phenotype that supports tumor growth, migration, invasion, and metastasis. M2 macrophages secrete pro-tumorigenic cytokines and growth factors that foster an environment conducive to the EMT of EOC cells, enhancing their metastatic potential. Thus, circATP2B4 facilitates a feedback loop where EVs from EOC cells modulate the tumor microenvironment by reprogramming macrophages to an M2 phenotype, thereby promoting cancer progression and metastasis. This mechanism reveals circATP2B4 as a potential therapeutic target, as its inhibition could disrupt M2 polarization and hinder EOC metastasis (Figure 5) (125).
Cancer stem cells (CSCs) play a critical role in shaping the tumor microenvironment by driving the polarization of macrophages toward the tumor-promoting M2 phenotype. CSCs achieve this by secreting chemokines, cytokines (such as CCL2, IL-6, IL-10, and TGF-β), and exosomes, which recruit macrophages and induce their differentiation into M2 TAMs (126). These M2 macrophages support tumor growth through immunosuppressive actions, angiogenesis, and extracellular matrix remodeling, while also secreting factors like IL-6 and TNF-α that reinforce CSC stemness and contribute to tumor progression and metastasis. Signaling pathways such as STAT3, TGF-β, and β-catenin/CCL2 are key mechanisms by which CSCs promote M2 polarization. This interaction creates a positive feedback loop where M2 macrophages, in turn, further enhance CSC survival, resistance to therapy, and tumor aggressiveness, making the CSC–TAM crosstalk a significant target for potential therapeutic strategies (127). OCSCs promote the polarization of macrophages into the tumor-promoting M2 phenotype through the secretion of cytokines rather than direct cell-to-cell contact. This process is primarily driven by the overexpression of COX-2 in OCSCs, which leads to an increased production of PGE2. The COX-2/PGE2 pathway activates JAK signaling pathway in macrophages, inducing their differentiation into the M2 phenotype. M2 macrophages, characterized by high levels of anti-inflammatory markers such as IL-10 and reduced pro-inflammatory factors like TNF-α, contribute to the immune suppression, tumor progression, and metastasis commonly seen in ovarian cancer. This mechanism reveals the role of OCSCs in shaping the tumor microenvironment, facilitating tumor growth and evasion of immune responses (128). OCSCs promote the M2 polarization of macrophages by modulating the PPARγ and NF-κB pathways. When OCSCs interact with macrophages, they increase the expression of M2 markers such as mannose receptor, IL-10, and arginase-1, while suppressing M1 markers like TNF-α, iNOS, and CD86. This shift is mediated by the activation of PPARγ, a nuclear receptor with anti-inflammatory properties, and the simultaneous inhibition of the pro-inflammatory transcription factor NF-κB. Blocking PPARγ with the antagonist GW9662 reverses this effect, reducing M2 marker expression and increasing M1 marker expression, indicating that PPARγ is crucial for OCSC-induced M2 polarization. This interaction helps create an immune-suppressive tumor microenvironment that favors cancer progression (51). OCSCs and macrophages engage in reciprocal interactions through the WNT signaling pathway within 3D engineered microenvironments, promoting pro-tumoral and malignant phenotypes. OCSCs drive the polarization of macrophages into an immunosuppressive M2-like phenotype, characterized by increased CD206 expression, via the secretion of WNT ligands and cytokines like IL-10. In turn, macrophages secrete WNT5B, which enhances the maintenance of the stemness properties of OCSCs, including elevated ALDH activity, chemoresistance, and invasiveness. This bidirectional WNT-mediated signaling forms a positive feedback loop, with CSC-derived WNT ligands promoting M2 macrophage activation and macrophage-derived WNT5B fostering the stemness and malignancy of OCSCs. This interaction not only supports tumor progression but also contributes to increased resistance to chemotherapy and a more invasive cancer phenotype, making the WNT pathway a potential therapeutic target in ovarian cancer (45). OCSLCs induce macrophage polarization toward the tumor-promoting M2 phenotype by altering the macrophages’ cytokine secretion profile and activating specific signaling pathways. When co-cultured with THP-1 macrophages, OCSLCs increase the expression of M2 markers such as CD163, while boosting the secretion of anti-inflammatory cytokines like IL-10 and pro-tumor factors such as VEGF and MMP-9, which are characteristic of M2 macrophages. At the same time, OCSLCs reduce the production of pro-inflammatory molecules like IL-12 and nitric oxide (NO), which are typical of the M1 macrophage phenotype. Central to this process is the secretion of interleukin-8 (IL-8) by OCSLCs, which activates the STAT3 signaling pathway in macrophages. This IL-8/STAT3 axis plays a crucial role in promoting the M2 polarization, fostering a tumor-friendly environment that supports cancer progression and stemness in ovarian cancer cells (61).
Macrophage polarization plays a critical role in therapy resistance in cancer, particularly through the actions of TAMs within the tumor microenvironment. TAMs often adopt an M2-like (pro-tumor) phenotype, which promotes tumor progression and undermines the effectiveness of cancer therapies, including chemotherapy, immunotherapy, radiotherapy, and targeted therapy. M2-like TAMs secrete immunosuppressive cytokines, express inhibitory ligands like PD-L1, and protect tumor cells from drug-induced cell death, thereby contributing to multidrug resistance. Reprogramming these macrophages to an M1-like (anti-tumor) phenotype is considered a promising strategy to enhance therapeutic responses and overcome cancer drug resistance (129).
Chemotherapy induces polarization of TAMs in ovarian cancer, shifting them from an immunosuppressive, tumor-promoting state to a more pro-inflammatory, antitumor phenotype. This polarization is characterized by a reduction in alternatively activated (M2-like) macrophages, which are typically associated with poor prognosis, and an increase in classically activated (M1-like) macrophages that support adaptive immune responses (130). After neoadjuvant chemotherapy (NACT), TAMs showed increased expression of pro-inflammatory pathways, including activation of the inflammasome and secretion of cytokines like IL-1β, which can promote T-cell activation and enhance the overall immune response against the tumor. These chemotherapy-induced changes in TAM polarization suggest that TAMs can aid adaptive immunity by fostering a more favorable tumor microenvironment, potentially enhancing the effectiveness of immunotherapies and improving clinical outcomes in ovarian cancer (28). However, it should be noted that the platinum sensitivity of ovarian cancer cells does not influence their ability to induce M2-type macrophage polarization. Both platinum-sensitive (A2780) and platinum-resistant (A2780Cis and A2780Dox) ovarian cancer cell lines were found to polarize macrophages toward an M2-like phenotype, regardless of their sensitivity to cisplatin. M2-type macrophages, which are typically associated with tumor progression and an immunosuppressive environment, were consistently induced by the cancer cells in coculture systems. This suggests that the ability of ovarian cancer cells to shape the tumor microenvironment by promoting M2-type macrophages is independent of their platinum resistance status, pointing to a robust mechanism by which ovarian cancer cells modulate the immune response to support tumor growth (131). Macrophages stimulated by paclitaxel-resistant ovarian cancer cells (OCTR) adopt an M2 phenotype, which is associated with tumor-promoting activities. These macrophages express higher levels of M2 markers, such as mannose receptor (MR) and triggering receptor expressed on myeloid cells 2 (Trem2), compared to macrophages stimulated by non-resistant ovarian cancer cells. The conditioned medium from these M2-polarized macrophages further increases the resistance of ovarian cancer cells to paclitaxel, contributing to a feedback loop where both the cancer cells and macrophages enhance each other’s survival and resistance mechanisms. This suggests that M2-polarized TAMs play a significant role in reinforcing chemoresistance in ovarian cancer (35).
Targeting the macrophage population to reprogram or enhance TAMs into an M1-polarized, CXCL10-secreting state offers a promising new therapeutic approach, particularly for ovarian cancer subtypes like clear cell carcinoma (CCC), which are often marked by immune exclusion and poor prognosis. M1-polarized TAMs have potent antitumor properties and play a key role in recruiting and activating T-cells through the production of chemokines like CXCL10. This recruitment strengthens the immune response against tumors. However, CCC typically lacks these beneficial M1 macrophages, contributing to its resistance to treatment and reduced immune cell infiltration. By reprogramming the macrophages already present in CCC tumors to shift toward an M1 phenotype, this immune-deficient environment could be transformed into one that supports a more robust immune response. This could potentially be achieved through therapies that stimulate M1 polarization, such as IFNγ or specific cytokine modulators, while simultaneously inhibiting M2 macrophage activity, which tends to promote tumor growth. This strategy could enhance the efficacy of immunotherapies by increasing tumor immunogenicity, thereby improving responsiveness to treatments such as immune checkpoint inhibitors and macrophage-targeted therapies, including CSF1R and TREM2 inhibitors. This approach offers new therapeutic prospects for challenging OC subtypes like CCC (68). IL-4 plays a crucial role in establishing an immunosuppressive TME by promoting macrophage polarization toward an M2-like phenotype, which contributes to ICB resistance in OvCa. Despite its relatively low expression in tumors, IL-4 signaling can have profound effects on TME programming, as even a small fraction of IL-4-producing cancer cells can drive significant immune suppression. Recent studies utilizing CRISPR screens and Perturb-map analysis have identified IL-4 as a key mediator of immune resistance, particularly in its ability to sustain an immunosuppressive niche that limits effector T cell activity. Notably, IL-4R blockade has shown promise in reversing these effects. While IL-4R inhibition has primarily been explored in inflammatory conditions, its potential application in cancer therapy is gaining attention. The clinically approved IL-4Rα antagonist, dupilumab, has demonstrated efficacy in modulating immune responses and is currently under clinical investigation in combination with PD-1 blockade for lung cancer. Preclinical data suggest that dual blockade of IL-4R and PD-1 leads to a more permissive immune landscape, characterized by reduced macrophage-mediated immune suppression and enhanced T cell infiltration. Given the established role of IL-4 in driving immune evasion in OvCa, IL-4R blockade may represent a promising strategy to overcome resistance to immunotherapy, particularly when combined with anti-PD-1 therapy. Future clinical trials evaluating IL-4R inhibitors in OvCa could provide critical insights into their efficacy in reversing macrophage-mediated immunosuppression and enhancing response rates to immune checkpoint inhibitors (72).
The traditional M1/M2 paradigm suggests that M1 macrophages, induced by IFN-γ, TNF-α, and TLR signaling, promote anti-tumor immunity through the production of pro-inflammatory cytokines and reactive oxygen species. In contrast, M2 macrophages, driven by IL-4, IL-10, and TGF-β, are associated with tumor progression via immunosuppression, angiogenesis, and tissue remodeling. However, TAMs within the tumor microenvironment (TME) frequently exhibit features of both M1 and M2 macrophages, demonstrating phenotypic plasticity that enables them to adapt dynamically to their microenvironment (132). This mixed polarization reflects the complexity of signaling networks and the intricate interplay of cytokines, chemokines, growth factors, and metabolites in the TME (133). As a result, the M1/M2 classification fails to fully capture the range of functional states exhibited by TAMs in ovarian cancer (134).
Recent single-cell RNA sequencing (scRNA-seq) studies have further challenged this binary M1/M2 classification by revealing diverse TAM subpopulations with distinct transcriptional profiles and functional properties (135). These studies demonstrate that TAMs may simultaneously exhibit immunosuppressive characteristics while producing pro-inflammatory mediators, emphasizing their ability to perform multiple, often contradictory roles within the TME (136). For example, some TAMs interact with regulatory T cells and tumor cells to establish an immunosuppressive TME, reinforcing the notion that macrophages do not neatly fit into M1 or M2 categories (133). This transcriptional heterogeneity underscores the necessity of moving beyond the simple M1/M2 framework toward more nuanced approaches that capture the true complexity of TAM biology. In ovarian cancer ascites, TAMs frequently display mixed-polarization phenotypes, co-expressing markers associated with both M1 and M2 macrophages (132). For instance, CD163, a marker commonly associated with M2 macrophages, correlates with ascitic levels of IL-6 and IL-10, cytokines that can mediate both pro- and anti-inflammatory responses (134). Interestingly, transcriptional profiling of TAMs indicates that CD163 expression does not always correlate with other M2 markers, and many TAMs also express M1-associated genes, further exposing the limitations of the classical M1/M2 classification. scRNA-seq analyses have identified unique TAM subpopulations with distinct transcriptional programs, revealing macrophage states that do not conform to the traditional dichotomy and that exhibit immune evasion properties (136). Given this complexity, it is imperative to redefine macrophage classification in the context of ovarian cancer. Our review will emphasize the importance of characterizing TAM phenotypes beyond the M1/M2 framework by leveraging advanced technologies such as scRNA-seq, spatial transcriptomics, and functional assays. These integrative approaches will enable a more precise understanding of TAM biology and uncover novel therapeutic strategies targeting specific TAM subpopulations, ultimately improving treatment outcomes for ovarian cancer patients. By incorporating these cutting-edge methodologies, we aim to provide a more accurate and nuanced perspective on macrophage polarization in ovarian cancer, thereby enhancing the rigor and impact of our research.
Macrophage polarization markers offer significant benefits in cancer prognosis by helping to identify the functional states of TAMs, which play key roles in tumor biology. M1 macrophages, with tumoricidal properties, support immune responses and tumor destruction, while M2 macrophages promote tumor growth, survival, and immune evasion. By identifying these subtypes through markers such as CD68 (general macrophage marker), CD163, CD204, and CD206 (M2 markers), researchers can assess the tumor microenvironment and predict patient outcomes. In general, M2 markers are associated with worse overall survival in various cancers. These markers also help in understanding the tumor’s anatomical location, invasion behavior, and the impact of macrophage presence on treatment outcomes, enabling more personalized treatment strategies (137). In this section, we have summarized the macrophage polarization-related biomarkers in ovarian cancer.
CD163 is a surface receptor primarily found on cells of the monocytic lineage, particularly macrophages, and is traditionally associated with the M2 (alternatively activated) polarization of macrophages (138). In ovarian cancer, CD163 plays a significant role in the tumor microenvironment, where it is upregulated on TAMs found in malignant ascites. While CD163 is typically regarded as a marker of M2 polarization, which is linked to anti-inflammatory and tissue repair functions, its role in ovarian cancer is more complex. The study by Reinartz et al. shows that despite high CD163 expression, TAMs in ovarian cancer exhibit a mixed-polarization phenotype that includes features of both M1 (pro-inflammatory) and M2 macrophages. CD163 expression is also correlated with elevated levels of the cytokines IL-6 and IL-10 in ascites, both of which induce CD163 expression and are associated with poorer clinical outcomes, such as early relapse. Thus, in ovarian cancer, CD163 is not strictly indicative of M2 polarization but is a marker of a more nuanced macrophage phenotype linked to disease progression and immune modulation (139).
LILRB1 (Leukocyte Immunoglobulin-Like Receptor Subfamily B1) is an inhibitory receptor involved in immune regulation by interacting with MHC class I molecules, particularly HLA-G (140). It is predominantly expressed on immune cells such as dendritic cells, macrophages, monocytes, NK cells, and certain T cell subsets, where it plays a role in suppressing immune activation and maintaining immune tolerance, often contributing to immune suppression (141). In OC, LILRB1 is primarily expressed on immune cells (ICs) rather than tumor cells (TCs) and is strongly associated with an immunosuppressive tumor microenvironment. This environment is characterized by increased infiltration of M2 macrophages, reduced dendritic cell activation, and impaired function of CD8+ T cells, which are essential for anti-tumor responses. High infiltration of LILRB1+ ICs correlates with poorer clinical outcomes, including more advanced disease stages, shorter progression-free and overall survival, and diminished responses to both chemotherapy and immune checkpoint inhibitors (ICIs) like anti-PD-1/PD-L1 therapies. Moreover, patients with higher LILRB1+ IC levels tend to show resistance to platinum-based chemotherapy. These findings suggest that LILRB1+ ICs act as independent prognostic indicators and may predict weaker responses to immunotherapy, positioning LILRB1 as a promising target for future therapeutic approaches in OC treatment (142)
MUC2 is a mucin glycoprotein that is aberrantly overexpressed in ovarian cancer, contributing to tumor progression by influencing immune cell behavior, particularly macrophages (143). In ovarian cancer, high MUC2 expression is associated with the polarization of TAMs toward the M2 phenotype, which supports tumor growth, metastasis, and immune suppression, in contrast to the M1 phenotype, which has anti-tumor effects. MUC2 interacts with TAMs, promoting the expression of COX-2 in these cells, leading to increased production of PGE2. PGE2 further polarizes macrophages toward the M2 type, creating a feedback loop that exacerbates tumor progression and is linked to poorer patient outcomes. Thus, MUC2 plays a critical role in altering the immune landscape within the tumor microenvironment, favoring cancer growth (144).
Semaphorin4D (SEMA4D) is a protein that plays a significant role in the tumor microenvironment of epithelial ovarian cancer (EOC). Overexpression of SEMA4D is associated with poor prognosis, as it correlates with advanced disease stages, chemotherapy resistance, and reduced overall and progression-free survival. In the context of EOC, SEMA4D influences the differentiation of monocytes into M2 macrophages, which are known for their pro-tumor activity. M2 macrophages promote tumor progression by fostering an environment that supports cancer growth, metastasis, and angiogenesis. Studies have shown a strong association between high levels of SEMA4D and increased counts of M2 macrophages in both primary tumors and ascites, further linking SEMA4D to the aggressive behavior of EOC and suggesting its potential as a target for prognostic and therapeutic strategies (145).
FGF18 (Fibroblast Growth Factor 18) has been identified as an important prognostic and therapeutic biomarker in high-grade serous ovarian cancer (146). Amplification of the chromosomal region 5q31-5q35.3, where FGF18 is located, is strongly associated with poor prognosis in ovarian cancer patients. FGF18 overexpression promotes tumor progression by enhancing tumor cell migration, invasion, and tumorigenicity. It modulates the tumor microenvironment by activating NF-κB signaling, which leads to the production of oncogenic cytokines and chemokines that enhance angiogenesis and recruit TAMs, particularly M2-polarized macrophages known for their tumor-supportive roles. This contributes to a more aggressive tumor phenotype. In patient samples, FGF18 overexpression correlates with increased microvessel density and TAM infiltration, confirming its involvement in driving tumor progression. Thus, FGF18 is a potential therapeutic target, and its inhibition could offer a novel treatment approach for ovarian cancer (146).
RBMS3, a RNA-binding protein with tumor-suppressing functions, shows reduced expression in epithelial ovarian cancer (EOC), and this decrease is linked to poorer outcomes for patients, such as shorter overall survival and progression-free survival (147). Research indicates that when RBMS3 is overexpressed, it can slow tumor growth, reduce the ability of cancer cells to spread, and promote cell death. Additionally, RBMS3 affects the immune environment of tumors by decreasing the presence of certain immune-suppressing cells, such as regulatory T cells, myeloid-derived suppressor cells, and M2 macrophages, which are often associated with supporting tumor growth. In contrast, it increases M1 macrophages, which aid the body’s immune response against tumors. These findings suggest that RBMS3 has a role in controlling tumor progression and shaping the immune environment, making it a potential target for new cancer therapies (148).
TRPM2 is a non-selective cationic channel that belongs to the transient receptor potential (TRP) ion channel family, known to play a role in various physiological and pathological processes, including tumor progression. In OC, TRPM2 is significantly overexpressed and is associated with poor prognosis. It is linked to immune regulation within the tumor microenvironment, particularly influencing the polarization of macrophages. TRPM2 expression is positively correlated with M2 macrophages, which are tumor-promoting and linked to immunosuppression, rather than M1 macrophages, which have tumor-suppressing functions. This suggests that TRPM2 may promote tumor progression by fostering an immunosuppressive microenvironment through M2 macrophage polarization, making it a potential target for immunotherapy in OC (149).
ALOX5AP (Arachidonate 5-Lipoxygenase Activating Protein) is an enzyme involved in the production of leukotrienes, which are immune-modulating lipid mediators. In serous ovarian cancer (SOC), ALOX5AP is highly expressed and is associated with poor prognosis and aggressive disease features, such as lymphatic invasion, resistance to platinum chemotherapy, and poor survival rates. ALOX5AP promotes tumor progression by influencing the tumor microenvironment, specifically by driving M2 macrophage polarization, which supports immunosuppression and tumor growth. Elevated ALOX5AP levels are strongly correlated with the presence of M2 macrophages, which are known to promote immune evasion, metastasis, and chemoresistance in ovarian cancer. Targeting ALOX5AP may reprogram the immune microenvironment, offering a potential therapeutic approach by combining ALOX5AP inhibitors with immunotherapy to counteract M2 macrophage-mediated immunosuppression and improve patient outcomes (150).
Interleukin-6 (IL-6) is a pro-inflammatory cytokine that plays a key role in immune responses, inflammation, and hematopoiesis (151). In the context of advanced ovarian cancer, IL-6 is heavily involved in the tumor microenvironment, where it contributes to cancer-related anemia (CRA). CRA is a form of anemia commonly seen in cancer patients, driven by inflammation and characterized by low serum iron levels and high ferritin, often resulting from the dysregulation of iron metabolism. Macrophages, which are immune cells that can polarize into two main phenotypes, M1 (pro-inflammatory) or M2 (anti-inflammatory), are central to this process. In ovarian cancer, M1 TAMs predominate and are stimulated by IL-6 to release hepcidin, a hormone that regulates iron homeostasis by restricting iron availability, which worsens anemia. This polarization of macrophages toward the M1 phenotype in the tumor microenvironment amplifies inflammation and contributes to CRA by promoting iron sequestration and reducing hemoglobin levels. Targeting IL-6 to modulate macrophage polarization and reduce hepcidin production may offer a therapeutic strategy to manage CRA in patients with advanced ovarian cancer (152).
Targeting macrophage polarization is promising in cancer therapy because macrophages, especially TAMs, play a dual role in the tumor microenvironment. While M1 macrophages exhibit antitumor properties by promoting immune responses and killing cancer cells, M2 macrophages generally support tumor growth by fostering angiogenesis, suppressing immune responses, and aiding metastasis. Since TAMs are often skewed toward the M2 phenotype in established tumors, reprogramming or repolarizing them from the tumor-promoting M2 type to the tumor-suppressing M1 type can inhibit tumor progression and enhance the efficacy of immunotherapies. This approach harnesses the natural plasticity of macrophages to shift them toward an antitumoral role, offering a novel and potent strategy for improving cancer treatment outcomes (153). In this section, we review the therapeutic potential of targeting macrophage polarization in ovarian cancer (Table 1) (Figure 6):
Figure 6. Therapeutic strategies targeting ovarian cancer macrophage polarization: Potential interventions, including oncolytic viruses, engineered vesicles, immunotherapy, nanoparticles, targeted therapy, and natural products. These approaches aim to modulate macrophage polarization, shifting from pro-tumorigenic M2 to anti-tumorigenic M1 states, enhancing the immune response against ovarian cancer.
Shaping the polarization of TAMs is emerging as a promising approach in cancer immunotherapy due to TAMs’ critical role in the TME. TAMs predominantly exhibit an M2-like phenotype, which promotes tumor progression by supporting angiogenesis, immune suppression, and metastasis (154). By reprogramming these M2-like TAMs into the pro-inflammatory, tumor-inhibiting M1 phenotype, researchers aim to enhance anti-tumor immune responses and overcome resistance to therapies such as immune checkpoint blockade (ICB) and CAR-T cell therapy. Targeting specific signaling pathways, such as JAK/STAT, PI3Kγ/AKT, and NF-κB, which regulate TAM polarization, along with innovative strategies like nanocarriers, exosomes, and CAR-macrophage therapy, could significantly improve the efficacy of cancer treatments, particularly in solid tumors where immunosuppression is a major challenge (155). In an ovarian cancer model, the combination of TLR9 agonists (CpG oligodeoxynucleotides, CpG-ODNs) and anti-PD-1 antibodies can influence macrophage polarization by shifting them toward an immunoregulatory phenotype. TLR9 agonists activate innate immune responses by engaging toll-like receptors on macrophages, stimulating them to express pro-inflammatory molecules. However, when combined with anti-PD-1 antibodies, macrophages interact with the antibody’s Fc domain through their Fc receptors, which leads to their reprogramming. This interaction drives the macrophages toward an M2b-like phenotype, characterized by increased expression of immunosuppressive markers such as CD206 and IL-10. These macrophages, instead of promoting antitumor immunity, suppress the immune response, reducing the efficacy of CpG-ODN treatment. Thus, the combination of TLR9 stimulation and PD-1 blockade reshapes macrophages into a more suppressive, tumor-promoting state, limiting the potential therapeutic benefit of this regimen in ovarian cancer (156). Murlentamab, a low-fucosylated anti-Müllerian hormone type II receptor (AMHRII) antibody, exhibits potent anti-tumor activity in ovarian cancer by reprogramming TAMs and activating T cells. The low-fucosylation of murlentamab enhances its ability to bind to Fc receptors on immune cells, which promotes antibody-dependent cell-mediated cytotoxicity (ADCC) and phagocytosis (ADCP). When murlentamab opsonizes AMHRII-expressing ovarian tumor cells, it shifts TAMs from an immunosuppressive, tumor-promoting M2 phenotype to a pro-inflammatory, anti-tumor M1-like phenotype. This reprogramming results in increased expression of M1 markers (CD80, TLR2) and the production of pro-inflammatory cytokines and chemokines, such as IL-12 and IFNγ, while reducing M2 markers (CD163, CD206). Additionally, murlentamab enhances the recruitment and activation of adaptive immune cells, specifically increasing the activation of CD8+ T cells and promoting a Th1-biased CD4+ T cell response, while reducing regulatory T cells (Tregs). This dual activation of the innate and adaptive immune systems contributes to its robust anti-tumor effects in ovarian cancer (157). Anti-CRD4-MR scFv #G11 is a human recombinant single-chain variable fragment (scFv) antibody that specifically targets the carbohydrate recognition domain 4 (CRD4) of the Mannose Receptor (MR, CD206) on macrophages. In ovarian cancer, tumor-released mesothelin, which is linked to a glycosylphosphatidylinositol (GPI) anchor, binds to MR on macrophages, driving their polarization toward the TAM phenotype, which supports tumor growth and suppresses the immune response. By blocking this interaction, anti-CRD4-MR scFv #G11 effectively inhibits the polarization of macrophages into TAMs and preserves their pro-inflammatory, anti-tumor M1-like characteristics. This reprogramming of macrophages enhances their capacity to fight the tumor, promoting a more favorable immune response against ovarian cancer (158).
PPAB001 is a bispecific antibody fusion protein designed to simultaneously target and block CD47 and CD24, two critical immune checkpoints that inhibit macrophage-mediated phagocytosis of cancer cells. Both CD47 and CD24 are overexpressed in various cancers, including ovarian cancer, where they deliver “don’t eat me” signals to macrophages, preventing the immune system from effectively eliminating tumor cells. PPAB001 disrupts the interaction between CD47 and its receptor SIRPα, as well as between CD24 and Siglec-10, thereby promoting the phagocytic activity of macrophages against cancer cells. In ovarian cancer models, PPAB001 has shown enhanced efficacy compared to therapies that block only CD47 or CD24 individually. It not only increases the proportion of tumor-infiltrating macrophages but also shifts the balance from tumor-promoting M2 macrophages to tumor-fighting M1 macrophages. This change is accompanied by an upregulation of pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), further enhancing the anti-tumor immune response. The dual blockade by PPAB001 effectively inhibits tumor growth in both mouse models and human xenograft models of ovarian cancer, suggesting its potential as a powerful immunotherapy approach (159). CAR-T cells engineered to specifically target and deplete FRβ+ TAMs in ovarian cancer effectively reprogram the TME by eliminating the immunosuppressive M2-like macrophage subset. The depletion of these FRβ+ TAMs shifts the balance toward a more pro-inflammatory state by promoting the infiltration and activation of M1-like macrophages, which are known for their antitumor properties. This reprogramming of the TME fosters a terrain that supports enhanced antitumor immunity, particularly by increasing the recruitment and activation of endogenous tumor-specific CD8+ T cells. These T cells exhibit greater infiltration into the tumor, higher expression of pro-inflammatory cytokines like IFN-γ and TNF-α, and improved tumor control. By converting the TME into a more immune-supportive environment, this approach not only suppresses tumor progression but also augments the effectiveness of other immunotherapies, enhancing overall antitumor immunity (160).
CSF1R, a receptor tyrosine kinase, plays a critical role in the differentiation and maintenance of TAMs, particularly the M2-polarized subtype, which contributes to immune suppression and tumor progression. TAMs harboring activated CSF1R release tumorigenic cytokines, creating an immunosuppressive microenvironment that hinders antitumor immunity. Pharmacological inhibition of CSF1R has therefore emerged as a promising strategy to modulate TAM function and enhance immunotherapy efficacy. Several preclinical and clinical studies have demonstrated that CSF1R inhibitors, including small-molecule tyrosine kinase inhibitors (e.g., PLX3397, GW2580, and Ki20227) and monoclonal antibodies targeting CSF1R or its ligand CSF1, can effectively deplete or reprogram TAMs, thereby improving tumor immune contexture and enhancing the efficacy of ICIs (161).
A previous study reported that CSF1 blockade in ovarian cancer models resulted in a significant reduction in CD163+ M2-like TAMs, with a concomitant increase in M1-polarized macrophages producing CXCL10, a chemokine crucial for T-cell recruitment. This shift in the TAM phenotype was associated with improved antitumor responses and enhanced T-cell infiltration. Furthermore, in high-grade serous ovarian cancer (HGSC), high CSF1 mRNA levels correlate with a dense infiltration of CD163+ TAMs, as evidenced by Nanostring gene expression analysis and immunohistochemical staining. This suggests that CSF1R blockade may be particularly beneficial for HGSC patients with high CSF1 expression (68). Clinical studies have also highlighted the therapeutic potential of CSF1R inhibitors in various cancers, including ovarian cancer. The FDA-approved CSF1R inhibitor PLX3397 (pexidartinib) has demonstrated clinical efficacy in reducing TAM-mediated immunosuppression, and early-phase trials have evaluated its combination with ICIs to further enhance immune responses (162). Additionally, GW2580, a selective CSF1R inhibitor, has been shown to normalize tumor vasculature and reduce malignant ascites in experimental ovarian cancer models by limiting M2 macrophage-mediated vascular permeability. Notably, this treatment increased the proportion of M1 macrophages expressing IL12 and IFNγ, leading to a more immunostimulatory tumor microenvironment (36). Beyond CSF1R inhibition, targeting TREM2, another macrophage-associated immunoregulatory receptor, has also emerged as a promising approach. TREM2 is expressed on CSF1R+ TAMs and modulated by CSF1, suggesting that dual inhibition of CSF1R and TREM2 may further potentiate macrophage repolarization and tumor immune surveillance. Preclinical studies have demonstrated that TREM2 blockade enhances the efficacy of ICIs, delays tumor progression, and promotes a favorable immune landscape in ovarian cancer (68).
Given the intratumoral heterogeneity of TAM polarization in ovarian cancer, a strategic combination of macrophage-targeted therapies, such as CSF1R and TREM2 inhibitors, with immunotherapies holds promise for improving clinical outcomes. By reprogramming TAMs toward an M1 phenotype, these interventions may help overcome immune exclusion, particularly in subtypes like clear cell carcinoma (CCC), where CXCL10-producing macrophages are scarce. Thus, leveraging CSF1R blockade in conjunction with ICIs or cytokine-based therapies represents a compelling avenue for enhancing immunotherapy efficacy in ovarian cancer.
Recent advancements in single-cell RNA sequencing (scRNA-seq) have significantly expanded our understanding of the complex and heterogeneous landscape of tumor-associated macrophages (TAMs) within the ovarian tumor microenvironment (TME). While our review extensively explores the molecular mechanisms and therapeutic potential of targeting macrophage polarization in ovarian cancer, the inclusion of recent scRNA-seq-based characterizations of TAM subpopulations provides critical insights into how distinct macrophage subsets contribute to tumor progression, immune evasion, and therapy resistance.
Several key scRNA-seq studies have delineated diverse TAM populations in ovarian cancer, revealing novel macrophage states and their functional implications. It has been demonstrated that ovarian tumor macrophages exhibit transcriptional diversity associated with immune evasion mechanisms, particularly through interactions with regulatory T cells and tumor cells to establish an immunosuppressive TME. This aligns with the predominance of M2-like macrophages in ovarian cancer, which foster immune suppression via IL-10, TGF-β, and VEGF signaling. These findings also reinforce the importance of identifying macrophage subtypes that exhibit differential responses to immunotherapy, suggesting that certain subsets may resist reprogramming efforts aimed at converting M2 macrophages into a pro-inflammatory M1 phenotype (163, 164). In a complementary study, researcher utilized scRNA-seq to classify macrophage populations in ovarian cancer based on their metabolic and immunoregulatory signatures. Their findings revealed that a subset of TAMs expresses high levels of oxidative stress response genes and metabolic regulators, which could contribute to their persistence in the TME and their role in therapy resistance. Our reviewer highlights similar trends, particularly in the discussion of PTEN-deficient tumors, where macrophage polarization is influenced by metabolic shifts, further reinforcing the idea that metabolism-targeted therapies could play a role in modulating TAM activity (165).
Vazquez-Garcia et al. and Hornberg et al. provided additional insights into the ontogeny of TAMs, distinguishing between monocyte-derived and tissue-resident macrophage subsets within ovarian tumors. Their findings suggest that tissue-resident macrophages may exhibit unique pro-tumor functions, distinct from infiltrating monocytes that differentiate into macrophages within the tumor. These studies provide a foundation for refining macrophage-targeting therapies by considering the origin and functional plasticity of macrophage populations. This is particularly relevant to discussion on macrophage recruitment pathways, such as CCL2-CCR2 and CXCL12-CXCR4, which influence monocyte migration into tumors and could be leveraged to alter the macrophage composition within the TME (166, 167).
Emerging spatial transcriptomics approaches have further refined our ability to map macrophage interactions within the ovarian tumor niche. The presence of distinct macrophage subtypes in different anatomical locations within tumors suggests that their functional roles may be context-dependent. The significance of the Wnt/β-catenin pathway and its role in macrophage-mediated immune suppression, and recent scRNA-seq studies suggest that these pathways may be differentially active in macrophage populations residing in peritoneal metastases compared to those in primary tumors. Moreover, focusing on immune exclusion in ovarian cancer, where tumors exhibit a low infiltration of cytotoxic T cells, is supported by findings from these scRNA-seq studies, which have identified macrophage populations that act as physical and biochemical barriers to T-cell infiltration. The data presented in Yeh et al. and Zheng et al. further emphasize the role of TAMs in modulating immune checkpoint activity, particularly through PD-L1 upregulation. This provides additional rationale for combining TAM-targeting therapies with immune checkpoint inhibitors to enhance the immune response against ovarian cancer (163, 165).
The incorporation of these recent scRNA-seq findings into our understanding of macrophage polarization in ovarian cancer underscores the complexity and adaptability of TAMs within the TME. While our review emphasizes key molecular regulators of macrophage polarization and potential therapeutic targets, these new studies highlight the need for a refined, subset-specific approach to TAM-targeted therapies. The diversity of macrophage states observed in ovarian cancer suggests that therapies aiming to reprogram TAMs should consider both their metabolic states and tissue residency status. As spatial and functional mapping of macrophages continues to evolve, integrating these insights into clinical strategies will be critical for enhancing the efficacy of existing and emerging immunotherapies.
Nanotechnology offers significant advancements in OC therapy by enabling more targeted, effective, and less toxic treatments. Nanocarriers such as liposomes, dendrimers, polymer nanoparticles, and micelles can deliver chemotherapeutic agents directly to cancer cells, minimizing damage to healthy tissue and improving drug bioavailability (168). Functionalized nanoparticles can specifically target OC cells, enhancing the efficacy of treatments like paclitaxel and cisplatin while reducing side effects. Additionally, nanosensors improve early detection of OC biomarkers, enabling timely diagnosis. Nanoparticles are also used in imaging techniques to enhance tumor localization, and gene therapy approaches, such as siRNA delivery, help overcome drug resistance. Overall, nanotechnology enhances the precision and effectiveness of OC treatment, making it a promising tool in cancer therapy (169, 170). Mannose-decorated nanoparticles (MnNPs) are a specialized drug delivery system designed to target TAMs in the tumor microenvironment, particularly in ovarian cancer. These nanoparticles are coated with mannose, a sugar molecule that binds to the CD206 mannose receptor, which is overexpressed on immunosuppressive M2 macrophages. By delivering therapeutic agents like siRNA that targets IκBα, MnNPs can effectively trigger the NF-κB signaling pathway in these macrophages, repolarizing them from a tumor-promoting M2 phenotype to a tumor-fighting M1 phenotype. This repolarization increases inflammatory responses, promoting anti-tumor immunity and enhancing the body’s ability to fight cancer. In cancer therapy, MnNPs offer a targeted approach, reducing the immunosuppressive nature of the tumor microenvironment, improving the effectiveness of immune-based therapies, and potentially decreasing tumor growth and ascites accumulation with minimal off-target effects (171). A recent study developed a bioinspired hybrid nanoparticle system, miR497/TP-HENPs, combining exosomes and liposomes to co-deliver the chemotherapy agent triptolide (TP) and microRNA-497 (miR497) for treating cisplatin-resistant OC. The hybrid nanoparticles target tumors effectively, overcoming common drug resistance mechanisms by inhibiting the PI3K/AKT/mTOR pathway, generating ROS, and depleting glutathione (GSH), leading to enhanced cancer cell apoptosis. Additionally, miR497/TP-HENPs promote macrophage polarization toward an anti-tumor M1 phenotype, supporting immune-mediated tumor suppression. In vivo, the nanoparticles exhibited efficient tumor targeting and minimized systemic toxicity, suggesting a promising approach to treating chemoresistant OC and potentially other resistant cancers (172). Targeted mRNA nanocarriers encoding IRF5 and IKKβ can effectively reprogram TAMs from an immunosuppressive M2 phenotype to a pro-inflammatory M1 phenotype, thereby promoting an anti-tumor immune response. Using biodegradable polymeric nanoparticles (NPs) engineered for TAM targeting via CD206 mannose receptor binding, researchers delivered in vitro-transcribed mRNA encoding IRF5, a transcription factor crucial for M1 polarization, and its activating kinase, IKKβ. In murine models of ovarian cancer, melanoma, and glioblastoma, the IRF5/IKKβ NPs localized within tumor sites, where they reprogrammed TAMs, reduced M2 macrophage presence, and increased inflammatory cytokine production. This polarization enhanced tumoricidal activity while minimizing systemic toxicity, as demonstrated by the absence of significant inflammatory side effects, making the treatment safe for repeated administration. Importantly, this approach effectively inhibited tumor progression, prolonged survival in treated mice, and showed potential as an adjunctive therapy in combination with other immunotherapies (173). Lastly, VSSP (Very Small Size Particles) is a nanoparticle-based immunomodulator composed of the ganglioside NAcGM3 and outer membrane vesicles derived from Neisseria meningitidis. It acts as a Toll-like receptor (TLR-2/TLR-4) agonist and has been studied for its ability to modulate immune responses within the TME. In the context of murine ovarian tumors, VSSP abrogates immune suppression driven by tumor-associated myeloid cells by reducing the accumulation of suppressive TAMs and granulocytic cells, both of which hinder effective T cell-mediated anti-tumor immunity. VSSP promotes the polarization of TAMs from an immunosuppressive M2-like state to a pro-inflammatory M1-like state, which is associated with enhanced anti-tumor immune activity. This M1 polarization boosts the production of inflammatory cytokines and reduces the TAMs’ ability to suppress CD8+ T cell responses, making the immune system more effective in combating the tumor. In addition, VSSP stimulates peritoneal inflammation by increasing the presence of inflammatory monocytes and granulocytes, further aiding the anti-tumor response. In human studies, VSSP similarly induces M1 polarization in TAMs from ovarian cancer patients, enhancing immune activation and reducing the suppressive nature of the tumor microenvironment (174).
In recent years, targeted therapies for ovarian cancer have advanced significantly, focusing on precision delivery to reduce toxicity and overcome drug resistance. Key developments include antibody-drug conjugates (ADCs), which bind to specific antigens on cancer cells to deliver cytotoxic drugs, as well as folate, peptide, and aptamer-drug conjugates that exploit ovarian cancer’s folate receptor overexpression for targeted delivery. These approaches have shown potential to increase therapeutic efficacy, reduce side effects, and target advanced, metastatic, and resistant ovarian cancers, though challenges like tumor environment and changing antigen expression require further refinement for clinical success (175, 176). Vorinostat is a histone deacetylase inhibitor (HDACi) that suppresses HDAC6 expression, thereby disrupting the ARID1A6488delG/HDAC6/IL-10 signaling pathway implicated in endometriosis-associated ovarian carcinoma (EAOC). In EAOC, the ARID1A6488delG mutation enhances HDAC6 expression, which leads to increased secretion of the anti-inflammatory cytokine IL-10, facilitating M2 macrophage polarization. M2 macrophages create an immunosuppressive environment that supports tumor growth and progression. By inhibiting HDAC6, Vorinostat reduces IL-10 levels, which decreases M2 macrophage polarization and consequently limits tumor growth. This pathway-specific effect positions Vorinostat as a promising therapeutic approach for targeting immune modulation in EAOC (177). Likewise, plinabulin is a novel microtubule-targeting agent that binds to the colchicine site of β-tubulin, destabilizing microtubules in a unique way to influence immune cells in the tumor environment. In ovarian cancer, Plinabulin promotes the polarization of TAMs toward an M1-like phenotype, which is associated with anti-tumor immunity. This M1 polarization leads to increased expression of pro-inflammatory markers (such as CD80 and CD86) and cytokines (like IL-1β, IL-6, and IL-12), while reducing levels of immunosuppressive cytokines such as IL-10. Through activation of the JNK pathway, Plinabulin not only encourages M1 polarization but also selectively boosts the proliferation of these M1-like macrophages. This shift reprograms TAMs from supporting tumor growth to actively fighting it, thereby enhancing the tumor’s vulnerability to immune attack. In co-culture experiments, plinabulin-polarized macrophages directly killed ovarian cancer cells via Fas/Fas-L signaling, demonstrating its potential as a powerful agent in anti-tumor immunotherapy (178). Similarly, erastin is a small molecule known for inducing ferroptosis, a form of cell death driven by iron-dependent lipid peroxidation, and has shown potential in cancer therapy. However, in ferroptosis-resistant OC cells, erastin paradoxically enhances metastatic potential by influencing the tumor microenvironment. Specifically, erastin promotes the M2 polarization of TAMs, a state associated with tumor progression. This effect occurs through activation of the STAT3 signaling pathway, which increases IL-8 secretion by macrophages. The elevated IL-8 levels, in turn, stimulate EMT in OC cells, facilitating invasion and migration. Blocking IL-8 or STAT3 disrupts this pathway, reducing the pro-metastatic effects induced by erastin-treated TAMs, thereby identifying the STAT3/IL-8 axis as a potential therapeutic target to counteract erastin’s unintended enhancement of metastasis in resistant OC cells (179). In addition, paclitaxel is a widely used chemotherapy drug that stabilizes microtubules, causing cell-cycle arrest in cancer cells. Beyond its cytotoxic effects, Paclitaxel has shown immune-modulatory properties, specifically reprogramming TAMs from a tumor-promoting M2 phenotype to an antitumor M1 phenotype through TLR4 (Toll-like receptor 4) signaling. In ovarian cancer, Paclitaxel’s interaction with TLR4 on TAMs activates NF-κB, shifting macrophages toward an M1 profile marked by increased production of pro-inflammatory cytokines such as TNFα and IL12. This M1 reprogramming decreases the immune-tolerant environment within tumors, reducing tumor progression and potentially enhancing the efficacy of immunotherapies. Paclitaxel’s impact on TAM polarization through TLR4 could be leveraged to strengthen antitumor immunity in ovarian cancer, offering a promising strategy for combination cancer therapies (180). In HGSOC, AZD5153, a BRD4 inhibitor, shows promising effects in reprogramming TAMs from an M2-like, immunosuppressive phenotype to an M1-like, pro-inflammatory phenotype. AZD5153 achieves this by inhibiting BRD4’s influence on MAF, a transcription factor crucial for M2 polarization. This shift increases pro-inflammatory cytokines, such as IL-12, and decreases immunosuppressive cytokines like IL-10, enhancing the immune response within the tumor microenvironment. Notably, AZD5153-activated TAMs improve CD8+ T cell activation, although not their proliferation, strengthening the antitumor immunity in HGSOC. Additionally, AZD5153 downregulates PD-L1 expression on TAMs, effectively sensitizing ovarian tumors to anti-PD-L1 therapies. This dual approach, direct tumor cell inhibition alongside immune modulation, positions AZD5153 as a potential enhancer for checkpoint inhibitor therapies, offering a promising combinatorial strategy to improve HGSOC patient outcomes (181). Digitoxin, a cardiac glycoside traditionally used to treat heart conditions, has shown potential as an anticancer agent by influencing TME in ovarian cancer. Although digitoxin does not alter macrophage polarization directly (meaning it does not shift macrophages between M1 and M2 phenotypes), it impacts macrophage-related processes within the TME. Specifically, digitoxin inhibits the migration and tube formation of endothelial cells and suppresses ovarian cancer cell migration and proliferation in response to signals from both M1- and M2-polarized macrophages. It achieves these effects by inhibiting focal adhesion kinase (FAK) phosphorylation, which is critical for cell motility and angiogenesis. This selective inhibition highlights digitoxin’s potential in targeting cancer cell interactions with the TME, without modifying the macrophage polarization itself, offering promise for its repositioning as an adjunct therapy in metastatic ovarian cancer (182). Lastly, BP1003 is a novel anticancer therapeutic that targets STAT3, a protein involved in promoting tumor growth, drug resistance, and immune evasion. It consists of a nuclease-resistant antisense oligodeoxynucleotide (ASO) encapsulated in a neutral liposome, designed to inhibit the expression of STAT3. In ovarian cancer, BP1003 has demonstrated significant anti-tumor effects by reducing STAT3 levels, which in turn decreases cell viability, migration, and spheroid formation, especially when combined with chemotherapy agents like paclitaxel and 5-fluorouracil. Additionally, BP1003 effectively disrupts the polarization of monocytes into M2 macrophages, which are typically associated with creating a pro-tumorigenic environment by suppressing immune responses. This selective inhibition of M2 macrophage polarization without affecting M1 macrophages, which are anti-tumorigenic, suggests that BP1003 has potential not only as a direct tumor inhibitor but also as an immunomodulatory agent, enhancing its overall therapeutic potential against ovarian cancer (183).
Natural products have shown potential to enhance the efficacy of immunotherapy in ovarian cancer by modulating the TME and supporting immune system function. Compounds derived from plants, marine organisms, and other natural sources, such as curcumin, resveratrol, and fucoidan, have demonstrated anti-inflammatory and immunomodulatory properties that may reduce immunosuppressive signals within the TME (184). These natural agents can inhibit regulatory T cells, myeloid-derived suppressor cells (MDSCs), and TAMs, thus alleviating immune suppression and allowing for a stronger anti-tumor immune response. Additionally, certain natural products enhance dendritic cell activity, T cell infiltration, and antigen presentation, making immunotherapy treatments, such as immune checkpoint inhibitors and CAR-T cells, more effective. By acting on both tumor cells and immune cells, these natural compounds can boost the overall effectiveness of immunotherapy and contribute to improved treatment outcomes in ovarian cancer (185).
Cardamonin, a natural chalcone compound, demonstrates potential as an anti-cancer agent by targeting TAMs in ovarian cancer. TAMs, especially in their M2-polarized form, play a significant role in tumor progression by secreting pro-tumorigenic factors that support cancer growth, angiogenesis, and metastasis. In ovarian cancer, cardamonin suppresses M2 polarization and reduces TAM-mediated tumor support by inhibiting the mTOR signaling pathway and STAT3 activation. This inhibition decreases the expression of key tumor-promoting factors like IL-6, VEGFα, MMP2, and MMP9, limiting the tumor-promoting environment created by TAMs. Cardamonin’s impact on both mTOR and STAT3 pathways emphasizes its role as a potential therapeutic agent that could disrupt TAMs’ support of ovarian cancer, offering a promising avenue for enhancing immunotherapy in this challenging cancer type (186). Corosolic acid (CA), a natural compound known for its potent inhibitory effect on STAT3 signaling, shows promise as an adjunctive treatment for epithelial ovarian cancer (EOC) by enhancing the effects of chemotherapy and disrupting tumor-promoting interactions. In ovarian cancer cell lines (SKOV3, RMG-1, and ES-2), CA inhibited cell proliferation and increased apoptosis by downregulating STAT3, a key pathway associated with tumor growth, chemoresistance, and immune suppression. Furthermore, CA increased the efficacy of chemotherapy agents like paclitaxel and cisplatin by sensitizing EOC cells to these treatments through STAT3 suppression. Additionally, CA prevented the polarization of macrophages into the M2 phenotype, a macrophage subtype that supports tumor progression through STAT3 activation in cancer cells. By inhibiting this M2 polarization, CA reduced tumorigenic interactions in the ovarian cancer microenvironment. These findings highlight CA’s potential as an effective adjuvant for EOC, leveraging its dual action on both cancer cells and TAMs to improve treatment outcomes (187).
Onionin A (ONA), a sulfur-containing natural compound derived from onions, shows promising anti-ovarian cancer activity by targeting both cancer cells and TAMs. In studies involving ovarian cancer cell lines (SKOV3, ES2, RMG1) and a murine ovarian cancer model, ONA inhibited cancer cell proliferation by suppressing STAT3 activation, a pathway crucial to cancer progression and chemoresistance. ONA also interfered with the cell-cell interactions that enhance TAMs’ protumor functions, reducing the secretion of M2 macrophage markers like IL-10 and other tumor-promoting cytokines. Additionally, ONA showed synergistic effects with chemotherapy drugs such as cisplatin (CDDP) and paclitaxel (PTX), increasing cancer cell sensitivity and enabling potential dose reductions of these agents. In vivo, ONA administration not only reduced tumor growth and prolonged survival but also decreased the infiltration of pro-tumor CD163+ macrophages in tumor tissues. These findings suggest that ONA, as an orally available compound, could be an effective adjuvant in treating advanced ovarian cancer by targeting both cancer cells and their supportive macrophage environment (188).
Triptolide (TPL), a bioactive compound from Tripterygium wilfordii, has shown promise in treating drug-resistant ovarian cancer by targeting M2 TAMs and modifying the tumor microenvironment. In studies using A2780/DDP ovarian cancer cells, TPL reduced cell proliferation, invasion, and migration by inhibiting M2 macrophage polarization through the PI3K/AKT/NF-κB signaling pathway. This effect was even more pronounced when TPL was combined with cisplatin (DDP), which enhanced anti-tumor responses by decreasing markers of tumor vascularization (CD31) and M2 polarization (CD206), resulting in significant tumor growth inhibition and increased survival in vivo. Additionally, TPL and DDP together shifted the gut microbiota composition, increasing beneficial bacteria like Akkermansia and Clostridium while reducing pathogenic Sutterella and Adlercreutzia, suggesting an indirect immune-supportive mechanism. These findings support TPL as a potential adjunctive therapy in combating chemoresistant ovarian cancer, offering a novel approach that targets immune modulation and gut microbiota for enhanced therapeutic efficacy (189). Neferine, a natural compound from Nelumbo nucifera (lotus), exhibits anti-angiogenic properties and impacts TAMs in high-grade serous ovarian carcinoma (HGSOC), potentially aiding in treatment-resistant cases. By inhibiting the mTOR/p70S6K pathway, Neferine promotes autophagy in endothelial cells, arresting cell growth and reducing blood vessel formation. Additionally, Neferine prevents macrophage polarization into the M2 phenotype, which is known to support tumor angiogenesis and growth, as M2 macrophages release pro-angiogenic factors like VEGF. In studies, treatment with Neferine showed decreased vascular formation in ovarian cancer models and a notable reduction in CD206-positive M2 macrophages, which correlated with improved overall survival in patients. This dual action on autophagy induction and M2 macrophage inhibition highlights Neferine’s potential as a complementary agent in anti-angiogenesis therapies, especially in HGSOC cases unresponsive to standard treatments (190).
Deoxyschizandrin, a primary lignan extracted from Schisandra berries, has demonstrated notable anti-cancer properties, particularly against ovarian cancer. It functions by inducing cell cycle arrest in ovarian cancer cells, specifically halting growth at the G0/G1 phase, which is achieved through the suppression of cyclin E, a protein crucial for cell cycle progression. This arrest reduces cancer cell proliferation, which is further reinforced by increased reactive oxygen species (ROS) production that disrupts the PI3K/Akt pathway, a critical pathway for cell survival and proliferation in cancer. Additionally, Deoxyschizandrin significantly inhibits the protumoural activation of TAMs, which are often reprogrammed by the tumor environment to support cancer growth. By reducing the expression of M2 macrophage markers (CD163 and CD209) and suppressing the release of tumor-promoting factors such as MMP-9, RANTES, and VEGF, Deoxyschizandrin mitigates the supportive role of TAMs in tumor progression. These combined actions highlight Deoxyschizandrin’s potential as an agent to limit both cancer cell growth and the tumor-supportive microenvironment in ovarian cancer (191).
Kaempferia parviflora (KP) extract has shown promising anti-inflammatory and anti-cancer effects in ovarian clear cell carcinoma (OCCC), which often exhibits poor responses to standard chemotherapy. In OCCC TOV-21G cells, KP extract significantly inhibited the release of inflammatory cytokines such as IL-6 and MCP-1, particularly in the presence of TNF-α stimulation. This suppression was achieved through the inhibition of the NF-κB signaling pathway by reducing NF-κB’s nuclear translocation and upregulating the inhibitory protein IκB. Furthermore, KP extract decreased the phosphorylation of ERK1/2 and AKT, key components of cell growth and survival signaling, and reduced levels of the anti-apoptotic protein MCL-1, thus promoting apoptosis. The anti-inflammatory action of KP extract extended to inhibiting the migration of monocytes (THP-1 cells) toward the tumor microenvironment by lowering MCP-1 levels, suggesting a potential reduction in TAM infiltration. This indicates KP extract’s ability to disrupt inflammatory pathways and monocyte recruitment in OCCC, positioning it as a potential adjuvant to enhance conventional therapies and improve outcomes in chemoresistant ovarian cancer (192). Verbascoside (VB), a phenylpropanoid glycoside, demonstrates significant anti-tumor activity against ovarian cancer (OC) by facilitating M1 macrophage polarization through the CCN1-AKT/NF-κB signaling pathway. Studies on OC cell lines (SKOV3 and A2780) and xenograft mouse models showed that VB suppresses OC cell proliferation, migration, and promotes apoptosis. VB induces M1 polarization of macrophages, as evidenced by increased M1 markers (CD86, IL-6, CXCL10) and reduced M2 marker expression. Mechanistically, VB directly binds and downregulates CCN1, an oncogenic factor highly expressed in OC, inhibiting the AKT/NF-κB pathway, which plays a central role in regulating tumor progression and macrophage polarization. The effect of VB on promoting M1 polarization and suppressing OC progression is enhanced by AKT inhibition (using LY294002), but reduced by CCN1 overexpression. In vivo experiments confirm that VB reduces tumor volume and weight, inhibits cell proliferation, and promotes apoptosis in OC tissues, with CCN1 overexpression diminishing VB’s effects. These findings suggest VB as a promising therapeutic agent for OC, working by altering macrophage polarization and suppressing tumorigenic pathways through CCN1-mediated AKT/NF-κB inhibition. Further studies are required to confirm the detailed mechanism of VB in macrophage polarization and its clinical efficacy in OC treatment (193).
Astragaloside IV is a natural compound derived from Radix Astragali, a traditional Chinese herb, known for its anti-inflammatory and anticancer properties. In ovarian cancer, it has shown promise by targeting the tumor microenvironment, specifically by suppressing the polarization of macrophages into the M2 pro-tumor phenotype, a shift usually triggered by interleukin IL-4 and IL-13 exposure. M2 macrophages promote cancer cell growth, invasion, and metastasis by secreting factors like TGF-β and MMP-9, which enhance the tumor’s invasive potential. Astragaloside IV interferes with this process by inhibiting the HMGB1-TLR4 signaling pathway, which is integral to M2 macrophage polarization. By blocking this pathway, Astragaloside IV reduces the expression of M2 markers and decreases the release of pro-tumor cytokines, ultimately weakening the supportive role of M2 macrophages in cancer progression and metastasis, offering a potential adjunctive strategy in ovarian cancer therapy (194).
Polyunsaturated fatty acids (PUFAs) significantly contribute to the protumor microenvironment in ovarian cancer by promoting the polarization and deposition of TAMs toward an M2-like, immunosuppressive phenotype. Within the lipid-rich ascites of ovarian cancer, PUFAs inhibit RhoA-GTPase activity, leading to the downregulation of nuclear YAP1, a critical transcription factor in the Hippo pathway, which dampens antitumor immune responses. This suppression of RhoA-YAP1 signaling reprograms macrophages toward a protumoral M2-like polarization, facilitating tumor growth and metastasis. Additionally, these PUFA-enriched TAMs exhibit heightened lipid-oxidative phosphorylation metabolism, aligning with an M2-like, immune-dampening functionality that hinders cytotoxic CD8+ T-cell infiltration, thereby impairing adaptive immune responses against the tumor. Notably, this pathway can be reversed by activating YAP1 through MST1/2 inhibition, which shifts macrophages from an M2 to an M1 phenotype, enhancing CD8+ T-cell activity and mitigating tumor progression. This pathway underscores the therapeutic potential of targeting RhoA-YAP1 signaling to modify TAM polarization and improve outcomes in ovarian cancer (195).
The ethanol extract of Solanum lyratum Thunb (SLT), a traditional Chinese medicinal herb, has demonstrated significant anticancer effects on ovarian cancer cells through its impact on cellular proliferation, apoptosis, and EMT. In vitro studies using ovarian cancer cell lines A2780 and SKOV3 reveal that SLT reduces cell viability by activating a ROS-mediated p53 pathway, resulting in G1 cell cycle arrest, enhanced apoptosis, and inhibition of cell migration and invasion. SLT also plays a crucial role in modulating the tumor immune microenvironment by influencing macrophage polarization: it promotes the shift of M0 macrophages toward the M1 phenotype, marked by increased CD86+ cells, while inhibiting the M2 phenotype, characterized by decreased CD206+ cells. This dual action not only suppresses tumor-promoting macrophage types but also enhances pro-inflammatory macrophages, which support anticancer immune responses. Overall, these findings suggest that SLT, through ROS accumulation and p53 pathway activation, could serve as a promising basis for developing new ovarian cancer treatments by directly inhibiting cancer progression and enhancing the tumor immune response (196).
9-Hydroxycanthin-6-one, a β-carboline alkaloid derived from the stem bark of Ailanthus altissima, exhibits significant anti-cancer effects against ovarian cancer cells by inducing apoptosis through caspase- and reactive oxygen species (ROS)-dependent pathways. The compound promotes apoptosis in cancer cells by activating key caspases (caspase-3, -8, and -9) and increasing intracellular ROS, which signals apoptotic cell death. Additionally, 9-hydroxycanthin-6-one plays a role in modulating the tumor microenvironment by inhibiting the recruitment and activation of TAMs. It reduces the expression of chemokines MCP-1 and RANTES, which are essential for TAM recruitment, and further suppresses TAM activation by decreasing M2 macrophage markers and tumor-promoting factors like MMP-2, MMP-9, and VEGF. This dual action, directly inducing cancer cell apoptosis and modulating TAM activity, highlights 9-hydroxycanthin-6-one’s potential as a therapeutic agent in ovarian cancer treatment (197).
Macrophage-engineered vesicles (MEVs) are bioengineered nanovesicles derived from the membranes of macrophages, particularly those polarized to the M1, or pro-inflammatory, phenotype. MEVs are created through processes like nitrogen cavitation, where macrophage cell membranes are fragmented and reassembled into small, distinct vesicular units that retain the functional properties of their parent macrophages. MEVs are designed to act as both immune modulators and therapeutic delivery vehicles. The repolarization effect of M1 MEVs works by converting M2 macrophages, which are typically associated with tumor progression and immune suppression, into M1-like macrophages that promote an anti-tumor immune response (198, 199). When M1 MEVs are introduced into the tumor microenvironment, they influence the surrounding M2 macrophages to adopt a more pro-inflammatory, tumor-fighting state by increasing the secretion of cytokines such as TNF-α and upregulating pro-inflammatory markers like CXCL8. This repolarization disrupts the immunosuppressive environment that favors tumor growth, thereby enhancing the immune system’s ability to target and destroy cancer cells. In addition to their immunomodulatory effects, M1 MEVs demonstrate direct anti-cancer activity against ovarian cancer cells by reducing cell viability when co-cultured with cancer cells, as seen in various ovarian cancer models like Caov-3 and OVCAR3. Furthermore, M1 MEVs have shown the capability to localize specifically to tumor sites in vivo. In mouse models, fluorescently labeled M1 MEVs derived from the murine macrophage cell line RAW264.7 successfully homed to ovarian cancer tumor xenografts after intravenous or intraperitoneal administration. This selective tumor localization highlights their potential for targeted cancer therapy, making them a promising tool for delivering therapeutic agents directly to the tumor site, minimizing off-target effects and enhancing treatment efficacy (200). Umbilical cord-derived macrophage exosomes loaded with cisplatin (exoCIS) show significant potential in enhancing the treatment of ovarian cancer, especially in overcoming drug resistance. Exosomes from M1 macrophages, when loaded with cisplatin, increased the cytotoxicity of the drug by 3.3 times in drug-resistant ovarian cancer cells (A2780/DDP) and by 1.4 times in drug-sensitive cells (A2780) compared to cisplatin alone. M2 macrophage-derived exosomes also increased cytotoxicity, though less effectively than M1-derived exosomes. These exosomes are naturally better suited for drug delivery due to their stability, low immunogenicity, and ability to specifically accumulate in tumor cells. Moreover, the study highlighted that the method of sonication for loading cisplatin into exosomes provided greater efficiency and resulted in a more sustained drug release. Incorporating cisplatin into M1 exosomes not only reduced the IC50 (a measure of drug potency) but also induced higher rates of apoptosis in ovarian cancer cells, indicating that this method could help overcome platinum resistance in ovarian cancer therapy (201).
Oncolytic virus (OV) therapy represents an innovative approach for treating ovarian cancer, primarily through viruses engineered to selectively infect and destroy cancer cells. The direct lysis of tumor cells triggers the release of tumor-specific antigens, which subsequently stimulate a robust anti-tumor immune response. This mechanism underpins the primary therapeutic role of OVs, while additional modulation of the tumor microenvironment (TME), including macrophage polarization, has emerged as an ancillary benefit (202–204). There is evidence suggesting that OVs can influence macrophage polarization, shifting pro-tumorigenic M2 macrophages toward an anti-tumorigenic M1 phenotype. For instance, oncolytic vaccinia viruses, like OncoVV-AVL, have been shown to increase levels of interferon-gamma (IFN-γ), a cytokine linked to M1 polarization and enhanced immune response. However, it is important to note that macrophage repolarization is not the primary mechanism of action for OVs, and the extent to which OVs alone affect macrophage polarization may be limited (205). Recent research has highlighted the potential for OVs to function synergistically with therapies that more directly target macrophages. Specifically, the use of CSF1R inhibitors has been reported to improve the efficacy of OVs by directly suppressing M2 macrophages and enhancing any macrophage-repolarizing effects OVs may initiate. Such findings suggest that OVs on their own may not efficiently remodel the immunosuppressive TME but can complement macrophage-targeted therapies or immune checkpoint inhibitors to achieve more significant therapeutic outcomes.
In ovarian cancer treatment, oncolytic virus therapy holds promise as a component of combination regimens that integrate direct macrophage-targeted therapies. While their immunomodulatory effects, including macrophage repolarization, contribute to their broader anti-tumor mechanism, future studies are needed to elucidate the specific pathways involved and to optimize strategies for combining OVs with macrophage-repolarizing therapies like CSF1R inhibitors to improve clinical outcomes (206).
Ovarian cancer continues to be the deadliest among gynecological cancers, primarily due to its subtle beginnings, diagnosis at advanced stages, frequent relapses, and inherent resistance to standard chemotherapy treatments. A significant factor contributing to the complexity and aggressiveness of OC is the intricate dynamics within TME, especially the involvement of TAMs. Macrophage polarization, the process by which macrophages adopt either a pro-inflammatory (M1) or anti-inflammatory (M2) state, is crucial in influencing tumor growth, immune evasion, and resistance to therapies in ovarian cancer. This review highlights that the dominance of M2-like TAMs in the OC TME creates an environment that supports tumor survival, promotes angiogenesis, facilitates metastasis, and suppresses adaptive immune responses through the secretion of various pro-tumorigenic factors such as IL-10, TGF-β, and VEGF. The shift of macrophages toward the M2 phenotype is driven by numerous molecular regulators and signaling pathways, including the PI3K/AKT/mTOR, NF-κB, JAK/STAT, and Wnt/β-catenin pathways. Additionally, extracellular EVs and ncRNAs play vital roles in modulating macrophage behavior, further reinforcing the immunosuppressive environment of OC. A consistent finding across multiple studies is the identification of key molecular targets that influence macrophage polarization. Factors like PTEN loss, activation of Wnt/β-catenin signaling, and overexpression of proteins such as CBX2 and HE4 are frequently linked to enhancing the M2 phenotype and poorer clinical outcomes. On the other hand, strategies aimed at reprogramming TAMs toward the M1 phenotype, using agents like oncolytic viruses and engineered nanoparticles, have shown promise in reversing immunosuppression and improving the effectiveness of immunotherapies. Natural compounds, including cardamonin and curcumin, also emerge as potential modulators of macrophage polarization, presenting opportunities for adjunctive therapies that complement existing treatment methods. Moreover, the interaction between ovarian cancer stem cells and TAMs, mediated through signaling pathways like STAT3 and Wnt, underscores the necessity for comprehensive therapeutic approaches that address both cellular and molecular aspects of tumor biology. This reciprocal relationship enhances tumor survival, resistance to treatment, and aggressiveness, highlighting the need for strategies that can disrupt these interactions effectively. Despite advancements in understanding macrophage polarization in OC, several challenges persist. Early detection of ovarian cancer remains difficult, and the heterogeneous nature of TAMs across different tumor sites complicates the development of universally effective therapies. Additionally, the plasticity of macrophages, which allows them to switch phenotypes in response to environmental changes, poses a challenge for therapeutic interventions aimed at reprogramming them.
Looking forward, future research should focus on several key areas to advance the therapeutic landscape for ovarian cancer:
● Targeted Reprogramming of TAMs: Developing more precise and effective methods to shift TAMs from the M2 to the M1 phenotype is essential. This includes advancing nanoparticle-based delivery systems and engineered EVs that can specifically target TAMs and modulate their behavior without affecting other immune cells.
● Combination Therapies: Integrating TAM-targeting agents with existing immunotherapies, such as immune checkpoint inhibitors and CAR-T cell therapies, could synergistically enhance anti-tumor immune responses and overcome resistance mechanisms.
● Biomarker Development: Identifying robust biomarkers related to macrophage polarization will be crucial for patient stratification and monitoring therapeutic responses. Markers like CD163, LILRB1, and MUC2 have shown promise and require further validation in clinical settings.
● Understanding TAM Heterogeneity: Delving deeper into the diversity of TAMs within different ovarian cancer subtypes and metastatic sites will provide insights necessary for more tailored and effective therapeutic strategies.
● Exploration of Natural Compounds: Further investigation into natural products at the clinical level that influence macrophage polarization offers a complementary approach to conventional therapies, potentially reducing toxicity and enhancing efficacy.
● Clinical Trials: Translating preclinical findings into clinical applications through well-designed clinical trials is essential to validate the efficacy and safety of TAM-targeting therapies in ovarian cancer patients.
● Mechanistic Studies: Comprehensive studies to elucidate the underlying mechanisms by which various signaling pathways and molecular factors influence macrophage polarization will aid in identifying novel therapeutic targets.
● TAMs heterogeneity: Given the significant heterogeneity of TAMs in ovarian cancer, future therapeutic strategies must consider context-specific targeting of macrophage subpopulations. Approaches such as single-cell transcriptomic-guided therapies may enable precision targeting of immunosuppressive TAM subsets while preserving or enhancing the function of pro-inflammatory macrophages. Additionally, interventions designed to alter macrophage metabolism, such as lactate blockade or glutamine inhibition, may reprogram TAMs within specific tumor niches. Therapies aimed at macrophage-TME interactions, such as inhibiting macrophage-derived EV signaling, represent another promising avenue. By incorporating a nuanced understanding of TAM heterogeneity, emerging therapies may overcome the limitations of broad-spectrum macrophage-targeting approaches, improving clinical outcomes in ovarian cancer.
In summary, macrophage polarization is a critical determinant of ovarian cancer progression and therapy resistance. By continuing to unravel the complex molecular interactions within the TME and developing innovative strategies to manipulate TAM behavior, there is significant potential to improve treatment outcomes and survival rates for patients afflicted with this formidable disease.
CX: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. JC: Writing – original draft, Writing – review & editing. MT: Writing – original draft, Writing – review & editing. QT: Project administration, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that Generative AI was used in the creation of this manuscript.
During the preparation of this work, the authors used ChatGPT 4o by Open AI to improve paper readability. After using this tool/service, the authors reviewed and edited the content as needed and took full responsibility for the publication’s content.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ortiz M, Wabel E, Mitchell K, Horibata S. Mechanisms of chemotherapy resistance in ovarian cancer. Cancer Drug Resist. (2022) 5:304. doi: 10.20517/cdr.2021.147
2. Barnes BM, Nelson L, Tighe A, Burghel GJ, Lin I-H, Desai S, et al. Distinct transcriptional programs stratify ovarian cancer cell lines into the five major histological subtypes. Genome Med. (2021) 13:1–19. doi: 10.1186/s13073-021-00952-5
3. McMullen M, Madariaga A, Lheureux S. New approaches for targeting platinum-resistant ovarian cancer. Semin Cancer Biol. (2021) 77:167–81. doi: 10.1016/j.semcancer.2020.08.013
4. Siminiak N, Czepczyński R, Zaborowski MP, Iżycki D. Immunotherapy in ovarian cancer. Arch Immunol Ther Exp (Warsz). (2022) 70:19. doi: 10.1007/s00005-022-00655-8
5. Tavares V, Marques IS, Melo IG, Assis J, Pereira D, Medeiros R. Paradigm shift: A comprehensive review of ovarian cancer management in an era of advancements. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25031845
6. Zhao C, Song W, Wang J, Tang X, Jiang Z. Immunoadjuvant-functionalized metal–organic frameworks: synthesis and applications in tumor immune modulation. Chem Commun. (2025) 61:1962–77. doi: 10.1039/D4CC06510G
7. Wang Y-Y, Choi M-J, Kim J-H, Choi J-H. Enhanced expression of TRIM46 in ovarian cancer cells induced by tumor-associated macrophages promotes invasion via the wnt/β-catenin pathway. Cells. (2025) 14. doi: 10.3390/cells14030214
8. Yin L, Wang Y. Extracellular vesicles derived from M2-polarized tumor-associated macrophages promote immune escape in ovarian cancer through NEAT1/miR-101-3p/ZEB1/PD-L1 axis. Cancer Immunol Immunother. (2023) 72:743–58. doi: 10.1007/s00262-022-03305-2
9. El-Arabey AA, Alkhalil SS, Al-Shouli ST, Awadalla ME, Alhamdi HW, Almanaa TN, et al. Revisiting macrophages in ovarian cancer microenvironment: development, function and interaction. Med Oncol. (2023) 40:142. doi: 10.1007/s12032-023-01987-x
10. Truxova I, Cibula D, Spisek R, Fucikova J. Targeting tumor-associated macrophages for successful immunotherapy of ovarian carcinoma. J Immunother Cancer. (2023) 11. doi: 10.1136/jitc-2022-005968
11. Luo M, Zhao F, Cheng H, Su M, Wang Y. Macrophage polarization: an important role in inflammatory diseases. Front Immunol. (2024) 15. doi: 10.3389/fimmu.2024.1352946
12. Huang Z, Meng F-Y, Lu L-Z, Guo Q-Q, Lv C-J, Tan N-H, et al. Calculus bovis inhibits M2 tumor-associated macrophage polarization via Wnt/β-catenin pathway modulation to suppress liver cancer. World J Gastroenterol. (2024) 30:3511–33. doi: 10.3748/wjg.v30.i29.3511
13. Macciò A, Gramignano G, Cherchi MC, Tanca L, Melis L, Madeddu C. Role of M1-polarized tumor-associated macrophages in the prognosis of advanced ovarian cancer patients. Sci Rep. (2020) 10:6096. doi: 10.1038/s41598-020-63276-1
14. Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. (2014) 7:19. doi: 10.1186/1757-2215-7-19
15. Vankerckhoven A, Wouters R, Mathivet T, Ceusters J, Baert T, Van Hoylandt A, et al. Opposite macrophage polarization in different subsets of ovarian cancer: observation from a pilot study. Cells. (2020) 9. doi: 10.3390/cells9020305
16. Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao R, et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell. (2019) 179:829–845.e20. doi: 10.1016/j.cell.2019.10.003
17. Lin S, Dai Y, Han C, Han T, Zhao L, Wu R, et al. Single-cell transcriptomics reveal distinct immune-infiltrating phenotypes and macrophage-tumor interaction axes among different lineages of pituitary neuroendocrine tumors. Genome Med. (2024) 16:60. doi: 10.1186/s13073-024-01325-4
18. Xiong J, He X, Xu Y, Zhang W, Fu F. MiR-200b is upregulated in plasma-derived exosomes and functions as an oncogene by promoting macrophage M2 polarization in ovarian cancer. J Ovarian Res. (2021) 14:74. doi: 10.1186/s13048-021-00826-9
19. Zhang X, Wang J, Liu N, Wu W, Li H, Chen J, et al. Molecular mechanism of CD163+ tumor-associated macrophage (TAM)-derived exosome-induced cisplatin resistance in ovarian cancer ascites. Ann Transl Med. (2022) 10. doi: 10.21037/atm-22-4267
20. Fang X, Zhao P, Gao S, Liu D, Zhang S, Shan M, et al. Lactate induces tumor-associated macrophage polarization independent of mitochondrial pyruvate carrier-mediated metabolism. Int J Biol Macromol. (2023) 237:123810. doi: 10.1016/j.ijbiomac.2023.123810
21. Strizova Z, Benesova I, Bartolini R, Novysedlak R, Cecrdlova E, Foley LK, et al. M1/M2 macrophages and their overlaps – myth or reality? Clin Sci. (2023) 137:1067–93. doi: 10.1042/CS20220531
22. Wu Y, Hirschi KK. Tissue-resident macrophage development and function. Front Cell Dev Biol. (2021) 8. doi: 10.3389/fcell.2020.617879
23. Cassetta L, Pollard JW. Tumor-associated macrophages. Curr Biol. (2020) 30:R246–8. doi: 10.1016/j.cub.2020.01.031
24. Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell. (2018) 174:1293–1308.e36. doi: 10.1016/j.cell.2018.05.060
25. Wang H, Yung MMH, Ngan HYS, Chan KKL, Chan DW. The impact of the tumor microenvironment on macrophage polarization in cancer metastatic progression. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22126560
26. Lan C, Huang X, Lin S, Huang H, Cai Q, Wan T, et al. Expression of M2-polarized macrophages is associated with poor prognosis for advanced epithelial ovarian cancer. Technol Cancer Res Treat. (2013) 12:259–67. doi: 10.7785/tcrt.2012.500312
27. Yi J, Lin Y, Yicong W, Chengyan L, Shulin Z, Wenjun C. Effect of macrophages on biological function of ovarian cancer cells in tumor microenvironment in vitro. Arch Gynecol Obstet. (2020) 302:1009–17. doi: 10.1007/s00404-020-05719-8
28. Heath O, Berlato C, Maniati E, Lakhani A, Pegrum C, Kotantaki P, et al. Chemotherapy induces tumor-associated macrophages that aid adaptive immune responses in ovarian cancer. Cancer Immunol Res. (2021) 9:665–81. doi: 10.1158/2326-6066.CIR-20-0968
29. Lee JJ, Kang HJ, Kim SS, Charton C, Kim J, Lee J-K. Unraveling the transcriptomic signatures of homologous recombination deficiency in ovarian cancers. Adv Biol. (2022) 6:2200060. doi: 10.1002/adbi.202200060
30. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. (2010) 11:889–96. doi: 10.1038/ni.1937
31. Colegio OR, Chu N-Q, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. (2014) 513:559–63. doi: 10.1038/nature13490
32. O’Neill LAJ, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. (2015) 213:15–23. doi: 10.1084/jem.20151570
33. Li H, Miao Y, Zhong L, Feng S, Xu Y, Tang L, et al. Identification of TREM2-positive tumor-associated macrophages in esophageal squamous cell carcinoma: implication for poor prognosis and immunotherapy modulation. Front Immunol. (2023) 14:1162032. doi: 10.3389/fimmu.2023.1162032
34. Etzerodt A, Moulin M, Doktor TK, Delfini M, Mossadegh-Keller N, Bajenoff M, et al. Tissue-resident macrophages in omentum promote metastatic spread of ovarian cancer. J Exp Med. (2020) 217. doi: 10.1084/jem.20191869
35. Yang Y-I, Wang Y-Y, Ahn J-H, Kim B-H, Choi J-H. CCL2 overexpression is associated with paclitaxel resistance in ovarian cancer cells via autocrine signaling and macrophage recruitment. BioMed Pharmacother. (2022) 153:113474. doi: 10.1016/j.biopha.2022.113474
36. Moughon DL, He H, Schokrpur S, Jiang ZK, Yaqoob M, David J, et al. Macrophage blockade using CSF1R inhibitors reverses the vascular leakage underlying Malignant ascites in late-stage epithelial ovarian cancer. Cancer Res. (2015) 75:4742–52. doi: 10.1158/0008-5472.CAN-14-3373
37. Schweer D, McAtee A, Neupane K, Richards C, Ueland F, Kolesar J. Tumor-associated macrophages and ovarian cancer: implications for therapy. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14092220
38. Kerneur C, Cano CE, Olive D. Major pathways involved in macrophage polarization in cancer. Front Immunol. (2022) 13:1026954. doi: 10.3389/fimmu.2022.1026954
39. Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T, et al. Hypoxic Tumor-Derived Exosomal miR-301a Mediates M2 Macrophage Polarization via PTEN/PI3Kγ to Promote Pancreatic Cancer Metastasis. Cancer Res. (2018) 78:4586–98. doi: 10.1158/0008-5472.CAN-17-3841
40. Spear S, Le Saux O, Mirza HB, Iyer N, Tyson K, Grundland Freile F, et al. PTEN loss shapes macrophage dynamics in high grade serous ovarian carcinoma. Cancer Res. (2024) 84:3772–87. doi: 10.1158/0008-5472.c.7541194
41. Shakfa N, Li D, Conseil G, Lightbody ED, Wilson-Sanchez J, Hamade A, et al. Cancer cell genotype associated tumor immune microenvironment exhibits differential response to therapeutic STING pathway activation in high-grade serous ovarian cancer. J Immunother Cancer. (2023) 11:e006170. doi: 10.1136/jitc-2022-006170
42. Nguyen VHL, Hough R, Bernaudo S, Peng C. Wnt/β-catenin signalling in ovarian cancer: Insights into its hyperactivation and function in tumorigenesis. J Ovarian Res. (2019) 12:122. doi: 10.1186/s13048-019-0596-z
43. Cui Z, Liu Z, Yuan X, Lu K, Li M, Xu S, et al. PFDA promotes cancer metastasis through macrophage M2 polarization mediated by Wnt/β-catenin signaling. Chemosphere. (2024) 362:142758. doi: 10.1016/j.chemosphere.2024.142758
44. Tabnak P, Ghasemi Y, Natami M, Khorram R, Ebrahimnezhad M. Role of m6A modification in dysregulation of Wnt/β-catenin pathway in cancer. BioMed Pharmacother. (2023) 157:114023. doi: 10.1016/j.biopha.2022.114023
45. Raghavan S, Mehta P, Xie Y, Lei YL, Mehta G. Ovarian cancer stem cells and macrophages reciprocally interact through the WNT pathway to promote pro-tumoral and Malignant phenotypes in 3D engineered microenvironments. J Immunother Cancer. (2019) 7:190. doi: 10.1186/s40425-019-0666-1
46. Asem M, Young AM, Oyama C, Claure de la Zerda A, Liu Y, Yang J, et al. Host wnt5a potentiates microenvironmental regulation of ovarian cancer metastasis. Cancer Res. (2020) 80:1156–70. doi: 10.1158/0008-5472.CAN-19-1601
47. Dehghani-Ghobadi Z, Sheikh Hasani S, Arefian E, Hossein G. Wnt5A and TGFβ1 Converges through YAP1 Activity and Integrin Alpha v Up-Regulation Promoting Epithelial to Mesenchymal Transition in Ovarian Cancer Cells and Mesothelial Cell Activation. Cells. (2022) 11. doi: 10.3390/cells11020237
48. To SKY, Tang MKS, Tong Y, Zhang J, Chan KKL, Ip PPC, et al. A selective β–catenin-metadherin/CEACAM1-CCL3 axis mediates metastatic heterogeneity upon tumor–macrophage interaction. Adv Sci. (2022) 9:2103230. doi: 10.1002/advs.202103230
49. Shengnan L, Jiayan X, Meng S, Li L, Shengyun C, Mingjuan X. Regulator of G protein signaling-1 facilitates ovarian cancer development by modulating NF-kB signal pathway. Sci Rep. (2025) 15:864. doi: 10.1038/s41598-024-85071-y
50. Hoover AA, Hufnagel DH, Harris W, Bullock K, Glass EB, Liu E, et al. Increased canonical NF-kappaB signaling specifically in macrophages is sufficient to limit tumor progression in syngeneic murine models of ovarian cancer. BMC Cancer. (2020) 20:970. doi: 10.1186/s12885-020-07450-8
51. Deng X, Zhang P, Liang T, Deng S, Chen X, Zhu L. Ovarian cancer stem cells induce the M2 polarization of macrophages through the PPARγ and NF-κB pathways. Int J Mol Med. (2015) 36:449–54. doi: 10.3892/ijmm.2015.2230
52. Zhang M, Liu ZZ, Aoshima K, Cai WL, Sun H, Xu T, et al. CECR2 drives breast cancer metastasis by promoting NF-κB signaling and macrophage-mediated immune suppression. Sci Transl Med. (2025) 14:eabf5473. doi: 10.1126/scitranslmed.abf5473
53. Zhuo C, Ruan Q, Zhao X, Shen Y, Lin R. CXCL1 promotes colon cancer progression through activation of NF-κB/P300 signaling pathway. Biol Direct. (2022) 17:34. doi: 10.1186/s13062-022-00348-4
54. Wang N, Liu W, Zheng Y, Wang S, Yang B, Li M, et al. CXCL1 derived from tumor-associated macrophages promotes breast cancer metastasis via activating NF-κB/SOX4 signaling. Cell Death Dis. (2018) 9:880. doi: 10.1038/s41419-018-0876-3
55. Huang Z, Byrd O, Tan S, Hu K, Knight B, Lo G, et al. Periostin facilitates ovarian cancer recurrence by enhancing cancer stemness. Sci Rep. (2023) 13:21382. doi: 10.1038/s41598-023-48485-8
56. Lin S-C, Liao Y-C, Chen P-M, Yang Y-Y, Wang Y-H, Tung S-L, et al. Periostin promotes ovarian cancer metastasis by enhancing M2 macrophages and cancer-associated fibroblasts via integrin-mediated NF-κB and TGF-β2 signaling. J BioMed Sci. (2022) 29:109. doi: 10.1186/s12929-022-00888-x
57. Cho U, Kim B, Kim S, Han Y, Song YS. Pro-inflammatory M1 macrophage enhances metastatic potential of ovarian cancer cells through NF-κB activation. Mol Carcinog. (2018) 57:235–42. doi: 10.1002/mc.22750
58. Fogg KC, Olson WR, Miller JN, Khan A, Renner C, Hale I, et al. Alternatively activated macrophage-derived secretome stimulates ovarian cancer spheroid spreading through a JAK2/STAT3 pathway. Cancer Lett. (2019) 458:92–101. doi: 10.1016/j.canlet.2019.05.029
59. Bai Y, Yin K, Su T, Ji F, Zhang S. CTHRC1 in ovarian cancer promotes M2-like polarization of tumor-associated macrophages via regulation of the STAT6 signaling pathway. Onco Targets Ther. (2020) 17:5743–53. doi: 10.2147/OTT.S250520
60. Liu Y-J, Du J, Li J, Tan X-P, Zhang Q. CTHRC1, a novel gene with multiple functions in physiology, disease and solid tumors (Review). Oncol Lett. (2023) 25:266. doi: 10.3892/ol.2023.13852
61. Ning Y, Cui Y, Li X, Cao X, Chen A, Xu C, et al. Co-culture of ovarian cancer stem-like cells with macrophages induced SKOV3 cells stemness via IL-8/STAT3 signaling. BioMed Pharmacother. (2018) 103:262–71. doi: 10.1016/j.biopha.2018.04.022
62. Dou Y, Jiang X, Xie H, He J, Xiao S. The Jun N-terminal kinases signaling pathway plays a “seesaw” role in ovarian carcinoma: a molecular aspect. J Ovarian Res. (2019) 12:99. doi: 10.1186/s13048-019-0573-6
63. Guo M, Härtlova A, Gierliński M, Prescott A, Castellvi J, Losa JH, et al. Triggering MSR1 promotes JNK-mediated inflammation in IL-4-activated macrophages. EMBO J. (2019) 38:e100299. doi: 10.15252/embj.2018100299
64. Bose S, Saha P, Chatterjee B, Srivastava AK. Chemokines driven ovarian cancer progression, metastasis and chemoresistance: Potential pharmacological targets for cancer therapy. Semin Cancer Biol. (2022) 86:568–79. doi: 10.1016/j.semcancer.2022.03.028
65. Qin R, Ren W, Ya G, Wang B, He J, Ren S, et al. Role of chemokines in the crosstalk between tumor and tumor-associated macrophages. Clin Exp Med. (2023) 23:1359–73. doi: 10.1007/s10238-022-00888-z
66. Ruytinx P, Proost P, Van Damme J, Struyf S. Chemokine-induced macrophage polarization in inflammatory conditions. Front Immunol. (2018) 9. doi: 10.3389/fimmu.2018.01930
67. Ji H, Ren M, Liu T, Sun Y. Prognostic and immunological significance of CXCR2 in ovarian cancer: A promising target for survival outcome and immunotherapeutic response assessment. Dis Markers. (2021) 2021:5350232. doi: 10.1155/2021/5350232
68. Ardighieri L, Missale F, Bugatti M, Gatta LB, Pezzali I, Monti M, et al. Infiltration by CXCL10 secreting macrophages is associated with antitumor immunity and response to therapy in ovarian cancer subtypes. Front Immunol. (2021) 12:690201. doi: 10.3389/fimmu.2021.690201
69. Rainczuk A, Rao JR, Gathercole JL, Fairweather NJ, Chu S, Masadah R, et al. Evidence for the antagonistic form of CXC-motif chemokine CXCL10 in serous epithelial ovarian tumours. Int J Cancer. (2014) 134:530–41. doi: 10.1002/ijc.28393
70. Zeng Y, Li B, Liang Y, Reeves PM, Qu X, Ran C, et al. Dual blockade of CXCL12-CXCR4 and PD-1–PD-L1 pathways prolongs survival of ovarian tumor–bearing mice by prevention of immunosuppression in the tumor microenvironment. FASEB J. (2019) 33:6596–608. doi: 10.1096/fj.201802067RR
71. Long L, Hu Y, Long T, Lu X, Tuo Y, Li Y, et al. Tumor-associated macrophages induced spheroid formation by CCL18-ZEB1-M-CSF feedback loop to promote transcoelomic metastasis of ovarian cancer. J Immunother Cancer. (2021) 9:e003973. doi: 10.1136/jitc-2021-003973
72. Mollaoglu G, Tepper A, Falcomatà C, Potak HT, Pia L, Amabile A, et al. Ovarian cancer-derived IL-4 promotes immunotherapy resistance. Cell. (2024) 187:7492–7510.e22. doi: 10.1016/j.cell.2024.10.006
73. Wu W, Zhou S, Liu T, Liang D. Mitochondrial transcription factor B2 overexpression increases M2 macrophage infiltration via cytosolic mitochondrial DNA-stimulated Interleukin-6 secretion in ovarian cancer. Bioengineered. (2022) 13:12211–23. doi: 10.1080/21655979.2022.2074615
74. Liu X, Wang X, Zhang J, Tian T, Ning Y, Chen Y, et al. Myc-mediated inhibition of HIF1a degradation promotes M2 macrophage polarization and impairs CD8 T cell function through lactic acid secretion in ovarian cancer. Int Immunopharmacol. (2024) 141:112876. doi: 10.1016/j.intimp.2024.112876
75. Podar K, Anderson KC. A therapeutic role for targeting c-Myc/Hif-1-dependent signaling pathways. Cell Cycle. (2010) 9:1722–8. doi: 10.4161/cc.9.9.11358
76. Brubaker L, Yamamoto T, Jordan K, Mitra S, Bitler B. Defining the impact of Chromobox 2 on the immune tumor microenvironment of high grade serous ovarian carcinoma (092). Gynecol Oncol. (2022) 166:S62–3. doi: 10.1016/S0090-8258(22)01318-X
77. Ma Y, Liu L, Wei Z, Zhu M, Huang L, Wang S, et al. Loss of CBX2 causes genomic instability and Wnt activation in high grade serous ovarian carcinoma cells. Mol Carcinog. (2023) 62:479–92. doi: 10.1002/mc.23500
78. Iwanaga R, Yamamoto TM, Gomez K, Nguyen LL, Woodruff ER, Post MD, et al. Tumor-intrinsic activity of chromobox 2 remodels the tumor microenvironment in high-grade serous carcinoma. Cancer Res Commun. (2024) 4:1919–32. doi: 10.1158/2767-9764.CRC-24-0027
79. El-Arabey AA, Denizli M, Kanlikilicer P, Bayraktar R, Ivan C, Rashed M, et al. GATA3 as a master regulator for interactions of tumor-associated macrophages with high-grade serous ovarian carcinoma. Cell Signal. (2020) 68:109539. doi: 10.1016/j.cellsig.2020.109539
80. El-Arabey AA, Abdalla M. GATA3 as a regulator for naughty cancer-associated fibroblasts in the microenvironment of high-grade serous ovarian cancer. Hum Cell. (2021) 34:1934–6. doi: 10.1007/s13577-021-00598-w
81. Wu X, Zhao J, Ruan Y, Sun L, Xu C, Jiang H. Sialyltransferase ST3GAL1 promotes cell migration, invasion, and TGF-β1-induced EMT and confers paclitaxel resistance in ovarian cancer. Cell Death Dis. (2018) 9:1102. doi: 10.1038/s41419-018-1101-0
82. Cao K, Zhang G, Yang M, Wang Y, He M, Zhang C, et al. Attenuation of sialylation augments antitumor immunity and improves response to immunotherapy in ovarian cancer. Cancer Res. (2023) 83:2171–86. doi: 10.1158/0008-5472.CAN-22-3260
83. Entezari M, Sadrkhanloo M, Rashidi M, Asnaf SE, Taheriazam A, Hashemi M, et al. Non-coding RNAs and macrophage interaction in tumor progression. Crit Rev Oncol Hematol. (2022) 173:103680. doi: 10.1016/j.critrevonc.2022.103680
84. Mohapatra S, Pioppini C, Ozpolat B, Calin GA. Non-coding RNAs regulation of macrophage polarization in cancer. Mol Cancer. (2021) 20:24. doi: 10.1186/s12943-021-01313-x
85. An Y, Yang Q. MiR-21 modulates the polarization of macrophages and increases the effects of M2 macrophages on promoting the chemoresistance of ovarian cancer. Life Sci. (2020) 242:117162. doi: 10.1016/j.lfs.2019.117162
86. Boucher A, Klopfenstein N, Hallas WM, Skibbe J, Appert A, Jang SH, et al. The miR-23a∼27a∼24-2 microRNA Cluster Promotes Inflammatory Polarization of Macrophages. J Immunol. (2021) 206:540–53. doi: 10.4049/jimmunol.1901277
87. Zhao J, Liu L, Zhao W, Lv C, Zhang N, Jia X, et al. miR-141-3p accelerates ovarian cancer progression and promotes M2-like macrophage polarization by targeting the Keap1-Nrf2 pathway. Open Medicine (2023) 18(1):20230729. doi: 10.1515/med-2023-0729
88. Jiang B, Zhu S-J, Xiao S-S, Xue M. MiR-217 inhibits M2-like macrophage polarization by suppressing secretion of interleukin-6 in ovarian cancer. Inflammation. (2019) 42:1517–29. doi: 10.1007/s10753-019-01004-2
89. Zhao Y, Yu Z, Ma R, Zhang Y, Zhao L, Yan Y, et al. lncRNA-Xist/miR-101-3p/KLF6/C/EBPα axis promotes TAM polarization to regulate cancer cell proliferation and migration. Mol Ther Acids. (2021) 23:536–51. doi: 10.1016/j.omtn.2020.12.005
90. Li H, Luo F, Jiang X, Zhang W, Xiang T, Pan Q, et al. CircITGB6 promotes ovarian cancer cisplatin resistance by resetting tumor-associated macrophage polarization toward the M2 phenotype. J Immunother Cancer. (2022) 10:e004029. doi: 10.1136/jitc-2021-004029
91. Anastasi E, Farina A, Granato T, Colaiacovo F, Pucci B, Tartaglione S, et al. Recent insight about HE4 role in ovarian cancer oncogenesis. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms241310479
92. Zhao L, Wang W, Huang S, Yang Z, Xu L, Yang Q, et al. The RNA binding protein SORBS2 suppresses metastatic colonization of ovarian cancer by stabilizing tumor-suppressive immunomodulatory transcripts. Genome Biol. (2018) 19:35. doi: 10.1186/s13059-018-1412-6
93. Jailani ABA, Bigos KJA, Avgoustou P, Egan JL, Hathway RA, Skerry TM, et al. Targeting the adrenomedullin-2 receptor for the discovery and development of novel anti-cancer agents. Expert Opin Drug Discovery. (2022) 17:839–48. doi: 10.1080/17460441.2022.2090541
94. Pang X, Shang H, Deng B, Wen F, Zhang Y. The interaction of adrenomedullin and macrophages induces ovarian cancer cell migration via activation of rhoA signaling pathway. Int J Mol Sci. (2013) 14:2774–87. doi: 10.3390/ijms14022774
95. Akinjiyan FA, Dave RM, Alpert E, Longmore GD, Fuh KC. DDR2 expression in cancer-associated fibroblasts promotes ovarian cancer tumor invasion and metastasis through periostin-ITGB1. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14143482
96. Wang Y, Qian X, Wang Y, Yu C, Feng L, Zheng X, et al. Turn TRAIL into better anticancer therapeutic through TRAIL fusion proteins. Cancer Med. (2025) 14:e70517. doi: 10.1002/cam4.70517
97. Gunalp S, Helvaci DG, Oner A, Bursalı A, Conforte A, Güner H, et al. TRAIL promotes the polarization of human macrophages toward a proinflammatory M1 phenotype and is associated with increased survival in cancer patients with high tumor macrophage content. Front Immunol. (2023) 14. doi: 10.3389/fimmu.2023.1209249
98. Sommerfeld L, Knuth I, Finkernagel F, Pesek J, Nockher WA, Jansen JM, et al. Prostacyclin released by cancer-associated fibroblasts promotes immunosuppressive and pro-metastatic macrophage polarization in the ovarian cancer microenvironment. Cancers. (2022) 14(24):6154. doi: 10.3390/cancers14246154
99. Zand B, Previs RA, Zacharias NM, Rupaimoole R, Mitamura T, Nagaraja AS, et al. Role of increased n-acetylaspartate levels in cancer. J Natl Cancer Inst (2016) 18(6):djv426. doi: 10.1093/jnci/djv426
100. Menga A, Favia M, Spera I, Vegliante MC, Gissi R, De Grassi A, et al. N&x2010;acetylaspartate release by glutaminolytic ovarian cancer cells sustains protumoral macrophages. EMBO Rep. (2021) 22:e51981. doi: 10.15252/embr.202051981
101. Han L, Xu S, Zhou D, Chen R, Ding Y, Zhang M, et al. Unveiling the causal link between metabolic factors and ovarian cancer risk using Mendelian randomization analysis. Front Endocrinol (Lausanne). (2024) 15. doi: 10.3389/fendo.2024.1401648
102. Tan BG, Gustafsson CM, Falkenberg M. Mechanisms and regulation of human mitochondrial transcription. Nat Rev Mol Cell Biol. (2024) 25:119–32. doi: 10.1038/s41580-023-00661-4
103. Lin Y, Chen J, Xin S, Lin Y, Chen Y, Zhou X, et al. CYP24A1 affected macrophage polarization through degradation of vitamin D as a candidate biomarker for ovarian cancer prognosis. Int Immunopharmacol. (2024) 138:112575. doi: 10.1016/j.intimp.2024.112575
104. Deng Y, Dong Y, Wu L, Zhang Q, Yang L. ARID5B promoted the histone demethylation of SORBS2 and hampered the metastasis of ovarian cancer. Pathol - Res Pract. (2023) 252:154911. doi: 10.1016/j.prp.2023.154911
105. Ma R, Chu X, Jiang Y, Xu Q. Pigment epithelium-derived factor, an anti-VEGF factor, delays ovarian cancer progression by alleviating polarization of tumor-associated macrophages. Cancer Gene Ther. (2022) 29:1332–41. doi: 10.1038/s41417-022-00447-4
106. Jiang S, Ye M, Wan J, Ye Q, Qiu S, Yang Y, et al. Significance of gelsolin superfamily genes in diagnosis, prognosis and immune microenvironment regulation for endometrial cancer. Cancer Med. (2025) 14:e70584. doi: 10.1002/cam4.70584
107. Asare-Werehene M, Tsuyoshi H, Zhang H, Salehi R, Chang C-Y, Carmona E, et al. Plasma gelsolin confers chemoresistance in ovarian cancer by resetting the relative abundance and function of macrophage subtypes. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14041039
108. Xie X, He J, Wang Q, Liu Y, Chen W, Shi K. FPR2 participates in epithelial ovarian cancer (EOC) progression through RhoA-mediated M2 macrophage polarization. J Ovarian Res. (2021) 14:177. doi: 10.1186/s13048-021-00932-8
109. Khvotchev M, Soloviev M. Copines, a family of calcium sensor proteins and their role in brain function. Biomolecules. (2024) 14. doi: 10.3390/biom14030255
110. Sheng B, Zhao B, Dong Y, Zhang J, Wu S, Ji H, et al. Copine 1 predicts poor clinical outcomes by promoting M2 macrophage activation in ovarian cancer. Carcinogenesis. (2023) 44:748–59. doi: 10.1093/carcin/bgad067
111. Wang L, Wang W, Hu D, Liang Y, Liu Z, Zhong T, et al. Tumor-derived extracellular vesicles regulate macrophage polarization: role and therapeutic perspectives. Front Immunol. (2024) 15. doi: 10.3389/fimmu.2024.1346587
112. Tang X, Ma C, Wu Q, Yu M. Ovarian cancer derived extracellular vesicles promote the cancer progression and angiogenesis by mediating M2 macrophages polarization. J Ovarian Res. (2024) 17:172. doi: 10.1186/s13048-024-01497-y
113. Raskov H, Orhan A, Gaggar S, Gögenur I. Cancer-associated fibroblasts and tumor-associated macrophages in cancer and cancer immunotherapy. Front Oncol. (2021) 11. doi: 10.3389/fonc.2021.668731
114. Feng C, Kou L, Yin P, Jing Y. Excessive activation of IL−33/ST2 in cancer−associated fibroblasts promotes invasion and metastasis in ovarian cancer. Oncol Lett. (2022) 23:158. doi: 10.3892/ol.2022.13278
115. Yang J, Wang H, Li B, Liu J, Zhang X, Wang Y, et al. Inhibition of ACSS2 triggers glycolysis inhibition and nuclear translocation to activate SIRT1/ATG5/ATG2B deacetylation axis, promoting autophagy and reducing Malignancy and chemoresistance in ovarian cancer. Metabolism. (2025) 162:156041. doi: 10.1016/j.metabol.2024.156041
116. Liu N, Li J, Dai H, Liang X, Fan H. Involvement of SIRT1-mediated cellular immune response in cancer. BioMed Pharmacother. (2024) 180:117482. doi: 10.1016/j.biopha.2024.117482
117. Zheng Q, Du X, Zhang J, Liu Y, Dong W, Dai X, et al. Delivery of SIRT1 by cancer-associated adipocyte-derived extracellular vesicles regulates immune response and tumorigenesis of ovarian cancer cells. Clin Transl Oncol. (2024) 26:190–203. doi: 10.1007/s12094-023-03240-3
118. Chen C, Zhang L, Ruan Z. GATA3 encapsulated by tumor-associated macrophage-derived extracellular vesicles promotes immune escape and chemotherapy resistance of ovarian cancer cells by upregulating the CD24/siglec-10 axis. Mol Pharm. (2023) 20:971–86. doi: 10.1021/acs.molpharmaceut.2c00557
119. Yang S, Zhao H, Xiao W, Shao L, Zhao C, Sun P. Extracellular vesicle-packaged miR-181c-5p from epithelial ovarian cancer cells promotes M2 polarization of tumor-associated macrophages via the KAT2B/HOXA10 axis. J Gene Med. (2022) 24:e3446. doi: 10.1002/jgm.3446
120. Ying X, Wu Q, Wu X, Zhu Q, Wang X, Jiang L, et al. Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget. (2016) 7:43076. doi: 10.18632/oncotarget.v7i28
121. Chen X, Ying X, Wang X, Wu X, Zhu Q, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer deliver microRNA-940 to induce macrophage M2 polarization. Oncol Rep. (2017) 38:522–8. doi: 10.3892/or.2017.5697
122. Zheng Q, Zhang J, Liu Y, Dong W, Dai X, Du X, et al. LINC01119 encapsulated by cancer-associated adipocytes-derived exosomes promotes M2 polarization of macrophages to induce immune escape in ovarian cancer in a 3D co-culture cell-based model. Clin Transl Oncol. (2023) 25:3174–87. doi: 10.1007/s12094-023-03185-7
123. Yan X, Yang Y, Guan H, Zhang X, Li L, Yu P. Exosomal LINC00958 maintains ovarian cancer cell stemness and induces M2 macrophage polarization via Hedgehog signaling pathway and GLI1 protein. Int J Biol Macromol. (2024) 279:135080. doi: 10.1016/j.ijbiomac.2024.135080
124. Yin J, Huang H-Y, Long Y, Ma Y, Kamalibaike M, Dawuti R, et al. circ_C20orf11 enhances DDP resistance by inhibiting miR-527/YWHAZ through the promotion of extracellular vesicle-mediated macrophage M2 polarization in ovarian cancer. Cancer Biol Ther. (2021) 22:440–54. doi: 10.1080/15384047.2021.1959792
125. Wang F, Niu Y, Chen K, Yuan X, Qin Y, Zheng F, et al. Extracellular Vesicle–Packaged circATP2B4 Mediates M2 Macrophage Polarization via miR-532-3p/SREBF1 Axis to Promote Epithelial Ovarian Cancer Metastasis. Cancer Immunol Res. (2023) 11:199–216. doi: 10.1158/2326-6066.CIR-22-0410
126. Raggi C, Mousa HS, Correnti M, Sica A, Invernizzi P. Cancer stem cells and tumor-associated macrophages: a roadmap for multitargeting strategies. Oncogene. (2016) 35:671–82. doi: 10.1038/onc.2015.132
127. Luo S, Yang G, Ye P, Cao N, Chi X, Yang W-H, et al. Macrophages are a double-edged sword: molecular crosstalk between tumor-associated macrophages and cancer stem cells. Biomolecules. (2022) 12. doi: 10.3390/biom12060850
128. Zhang Q, Cai D, Li B. Ovarian cancer stem-like cells elicit the polarization of M2 macrophages. Mol Med Rep. (2015) 11:4685–93. doi: 10.3892/mmr.2015.3323
129. Ding Y, Cao Q, Yang W, Xu J, Xiao P. Macrophage: hidden criminal in therapy resistance. J Innate Immun. (2024) 16:188–202. doi: 10.1159/000538212
130. Dallavalasa S, Beeraka NM, Basavaraju CG, Tulimilli SV, Sadhu SP, Rajesh K, et al. The role of tumor associated macrophages (TAMs) in cancer progression, chemoresistance, angiogenesis and metastasis - current status. Curr Med Chem. (2021) 28:8203–36. doi: 10.2174/0929867328666210720143721
131. Mlynska A, Povilaityte E, Zemleckaite I, Zilionyte K, Strioga M, Krasko J, et al. Platinum sensitivity of ovarian cancer cells does not influence their ability to induce M2-type macrophage polarization. Am J Reprod Immunol. (2018) 80:e12996. doi: 10.1111/aji.12996
132. Tan Q, Liu H, Xu J, Mo Y, Dai F. Integrated analysis of tumor-associated macrophage infiltration and prognosis in ovarian cancer. Aging (Albany NY). (2021) 13:23210–32. doi: 10.18632/aging.203613
133. Yan C, Li K, Meng F, Chen L, Zhao J, Zhang Z, et al. Integrated immunogenomic analysis of single-cell and bulk tissue transcriptome profiling unravels a macrophage activation paradigm associated with immunologically and clinically distinct behaviors in ovarian cancer. J Adv Res. (2023) 44:149–60. doi: 10.1016/j.jare.2022.04.006
134. Wang S, Wang X, Xia X, Zhang T, Yi M, Li Z, et al. Identification of the immune subtype of ovarian cancer patients by integrated analyses of transcriptome and single-cell sequencing data. Sci Rep. (2022) 12:13296. doi: 10.1038/s41598-022-17645-7
135. Wu K, Lin K, Li X, Yuan X, Xu P, Ni P, et al. Redefining tumor-associated macrophage subpopulations and functions in the tumor microenvironment. Front Immunol. (2020) 11:1731.
136. Cao M, Deng Y, Deng Y, Wu J, Yang C, Wang Z, et al. Characterization of immature ovarian teratomas through single-cell transcriptome. Front Immunol. (2023) 14:1131814. doi: 10.3389/fimmu.2023.1131814
137. López-Janeiro Á, Padilla-Ansala C, de Andrea CE, Hardisson D, Melero I. Prognostic value of macrophage polarization markers in epithelial neoplasms and melanoma. A systematic review and meta-analysis. Mod Pathol. (2020) 33:1458–65. doi: 10.1038/s41379-020-0534-z
138. Hu JM, Liu K, Liu JH, Jiang XL, Wang XL, Chen YZ, et al. CD163 as a marker of M2 macrophage, contribute to predicte aggressiveness and prognosis of Kazakh esophageal squamous cell carcinoma. Oncotarget. (2017) 8:21526–38. doi: 10.18632/oncotarget.15630
139. Reinartz S, Schumann T, Finkernagel F, Wortmann A, Jansen JM, Meissner W, et al. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: Correlation of CD163 expression, cytokine levels and early relapse. Int J Cancer. (2014) 134:32–42. doi: 10.1002/ijc.28335
140. Zhao J, Zhong S, Niu X, Jiang J, Zhang R, Li Q. The MHC class I-LILRB1 signalling axis as a promising target in cancer therapy. Scand J Immunol. (2019) 90:e12804. doi: 10.1111/sji.12804
141. Deng M, Chen H, Liu X, Huang R, He Y, Yoo B, et al. Leukocyte immunoglobulin-like receptor subfamily B: therapeutic targets in cancer. Antib Ther. (2021) 4:16–33. doi: 10.1093/abt/tbab002
142. Xu X, Yin S, Wang Y, Zhu Q, Zheng G, Lu Y, et al. LILRB1+ immune cell infiltration identifies immunosuppressive microenvironment and dismal outcomes of patients with ovarian cancer. Int Immunopharmacol. (2023) 119:110162. doi: 10.1016/j.intimp.2023.110162
143. Wang Y, Liu L, Yu Y. Mucins and mucinous ovarian carcinoma: Development, differential diagnosis, and treatment. Heliyon. (2023) 9. doi: 10.1016/j.heliyon.2023.e19221
144. He Y, Zhang M, Wu X, Sun X, Xu T, He Q, et al. High MUC2 expression in ovarian cancer is inversely associated with the M1/M2 ratio of tumor-associated macrophages and patient survival time. PloS One. (2013) 8:e79769. doi: 10.1371/journal.pone.0079769
145. Chen Y, Zhang L, Lv R, Zhang W-Q. Overexpression of Semaphorin4D indicates poor prognosis and prompts monocyte differentiation toward M2 macrophages in epithelial ovarian cancer. Asian Pacific J Cancer Prev. (2013) 14:5883–90. doi: 10.7314/APJCP.2013.14.10.5883
146. Wei W, Mok SC, Oliva E, Kim S, Mohapatra G, Birrer MJ. FGF18 as a prognostic and therapeutic biomarker in ovarian cancer. J Clin Invest. (2013) 123:4435–48. doi: 10.1172/JCI70625
147. Wang M, Fu X, Wang W, Zhang Y, Jiang Z, Gu Y, et al. Comprehensive bioinformatics analysis confirms RBMS3 as the central candidate biological target for ovarian cancer. Med Eng Phys. (2022) 110:103883. doi: 10.1016/j.medengphy.2022.103883
148. Yin T, Zhang Y, Zhao Y, Zhang X, Han S, Wang Y, et al. Tumor suppressor function of RBMS3 overexpression in EOC associated with immune cell infiltration. Heliyon. (2024) 10:e30603. doi: 10.1016/j.heliyon.2024.e30603
149. Huang W, Wu Y, Luo N, Shuai X, Guo J, Wang C, et al. Identification of TRPM2 as a prognostic factor correlated with immune infiltration in ovarian cancer. J Ovarian Res. (2023) 16:169. doi: 10.1186/s13048-023-01225-y
150. Ye X, An L, Wang X, Zhang C, Huang W, Sun C, et al. ALOX5AP predicts poor prognosis by enhancing M2 macrophages polarization and immunosuppression in serous ovarian cancer microenvironment. Front Oncol. (2021) 11. doi: 10.3389/fonc.2021.675104
151. Wu S, Cao Z, Lu R, Zhang Z, Sethi G, You Y. Interleukin-6 (IL-6)-associated tumor microenvironment remodelling and cancer immunotherapy. Cytokine Growth Factor Rev. (2025). doi: 10.1016/j.cytogfr.2025.01.001
152. Madeddu C, Gramignano G, Kotsonis P, Coghe F, Atzeni V, Scartozzi M, et al. Microenvironmental M1 tumor-associated macrophage polarization influences cancer-related anemia in advanced ovarian cancer: key role of interleukin-6. Haematologica. (2018) 103:e388–91. doi: 10.3324/haematol.2018.191551
153. Duan Z, Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct Target Ther. (2021) 6:127. doi: 10.1038/s41392-021-00506-6
154. Zhou L, Zhao T, Zhang R, Chen C, Li J. New insights into the role of macrophages in cancer immunotherapy. Front Immunol. (2024) 15. doi: 10.3389/fimmu.2024.1381225
155. Gao J, Liang Y, Wang L. Shaping polarization of tumor-associated macrophages in cancer immunotherapy. Front Immunol. (2022) 13. doi: 10.3389/fimmu.2022.888713
156. Camelliti S, Le Noci V, Bianchi F, Storti C, Arnaboldi F, Cataldo A, et al. Macrophages impair TLR9 agonist antitumor activity through interacting with the anti-PD-1 antibody fc domain. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13164081
157. Prat M, Salon M, Allain T, Dubreuil O, Noël G, Preisser L, et al. Murlentamab, a low fucosylated anti-müllerian hormone type II receptor (AMHRII) antibody, exhibits anti-tumor activity through tumor-associated macrophage reprogrammation and T cell activation. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13081845
158. Dangaj D, Abbott KL, Mookerjee A, Zhao A, Kirby PS, Sandaltzopoulos R, et al. Mannose receptor (MR) engagement by mesothelin GPI anchor polarizes tumor-associated macrophages and is blocked by anti-MR human recombinant antibody. PloS One. (2011) 6:e28386. doi: 10.1371/journal.pone.0028386
159. Yang Y, Wu H, Yang Y, Kang Y, He R, Zhou B, et al. Dual blockade of CD47 and CD24 signaling using a novel bispecific antibody fusion protein enhances macrophage immunotherapy. Mol Ther - Oncolytics. (2023) 31. doi: 10.1016/j.omto.2023.100747
160. Rodriguez-Garcia A, Lynn RC, Poussin M, Eiva MA, Shaw LC, O’Connor RS, et al. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat Commun. (2021) 12:877. doi: 10.1038/s41467-021-20893-2
161. Wen J, Wang S, Guo R, Liu D. CSF1R inhibitors are emerging immunotherapeutic drugs for cancer treatment. Eur J Med Chem. (2023) 245:114884. doi: 10.1016/j.ejmech.2022.114884
162. Yu M, Wu Y, Li Q, Hong W, Yang Y, Hu X, et al. Colony-stimulating factor-1 receptor inhibition combined with paclitaxel exerts effective antitumor effects in the treatment of ovarian cancer. Genes Dis. (2024) 11:100989. doi: 10.1016/j.gendis.2023.04.023
163. Yeh CY, Aguirre K, Laveroni O, Kim S, Wang A, Liang B, et al. Mapping spatial organization and genetic cell-state regulators to target immune evasion in ovarian cancer. Nat Immunol. (2024) 25:1943–58. doi: 10.1038/s41590-024-01943-5
164. Lin X, Yu T, Zhang L, Chen S, Chen X, Liao Y, et al. Silencing op18/stathmin by RNA interference promotes the sensitivity of nasopharyngeal carcinoma cells to taxol and high-grade differentiation of xenografted tumours in nude mice. Basic Clin Pharmacol Toxicol. (2016) 119:611–20. doi: 10.1111/bcpt.12633
165. Zheng X, Wang X, Cheng X, Liu Z, Yin Y, Li X, et al. Single-cell analyses implicate ascites in remodeling the ecosystems of primary and metastatic tumors in ovarian cancer. Nat Cancer. (2023) 4:1138–56. doi: 10.1038/s43018-023-00599-8
166. Vázquez-García I, Uhlitz F, Ceglia N, Lim JLP, Wu M, Mohibullah N, et al. Ovarian cancer mutational processes drive site-specific immune evasion. Nature. (2022) 612:778–86. doi: 10.1038/s41586-022-05496-1
167. Hornburg M, Desbois M, Lu S, Guan Y, Lo AA, Kaufman S, et al. Single-cell dissection of cellular components and interactions shaping the tumor immune phenotypes in ovarian cancer. Cancer Cell. (2021) 39:928–944.e6. doi: 10.1016/j.ccell.2021.04.004
168. Kaur P, Singh SK, Mishra MK, Singh S, Singh R. Nanotechnology for boosting ovarian cancer immunotherapy. J Ovarian Res. (2024) 17:202. doi: 10.1186/s13048-024-01507-z
169. Zhang J, Ding H, Zhang F, Xu Y, Liang W, Huang L. New trends in diagnosing and treating ovarian cancer using nanotechnology. Front Bioeng Biotechnol. (2023) 11. doi: 10.3389/fbioe.2023.1160985
170. Dong Q, Jiang Z. Platinum–iron nanoparticles for oxygen-enhanced sonodynamic tumor cell suppression. Inorganics. (2024) 12:331. doi: 10.3390/inorganics12120331
171. Glass EB, Hoover AA, Bullock KK, Madden MZ, Reinfeld BI, Harris W, et al. Stimulating TAM-mediated anti-tumor immunity with mannose-decorated nanoparticles in ovarian cancer. BMC Cancer. (2022) 22:497. doi: 10.1186/s12885-022-09612-2
172. Li L, He D, Guo Q, Zhang Z, Ru D, Wang L, et al. Exosome-liposome hybrid nanoparticle codelivery of TP and miR497 conspicuously overcomes chemoresistant ovarian cancer. J Nanobiotechnology. (2022) 20:50. doi: 10.1186/s12951-022-01264-5
173. Zhang F, Parayath NN, Ene CI, Stephan SB, Koehne AL, Coon ME, et al. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat Commun. (2019) 10:3974. doi: 10.1038/s41467-019-11911-5
174. Khan ANMNH, Emmons TR, Magner WJ, Alqassim E, Singel KL, Ricciuti J, et al. VSSP abrogates murine ovarian tumor-associated myeloid cell-driven immune suppression and induces M1 polarization in tumor-associated macrophages from ovarian cancer patients. Cancer Immunol Immunother. (2022) 71:2355–69. doi: 10.1007/s00262-022-03156-x
175. Wang Z, Meng F, Zhong Z. Emerging targeted drug delivery strategies toward ovarian cancer. Adv Drug Delivery Rev. (2021) 178:113969. doi: 10.1016/j.addr.2021.113969
176. Wu N, Zhang X, Fang C, Zhu M, Wang Z, Jian L, et al. Progesterone enhances niraparib efficacy in ovarian cancer by promoting palmitoleic-acid-mediated ferroptosis. Res (Washington DC). (2024) 7:371. doi: 10.34133/research.0371
177. Hsieh T-H, Hsu C-Y, Wu C-W, Wang S-H, Yeh C-H, Cheng K-H, et al. Vorinostat decrease M2 macrophage polarization through ARID1A6488delG/HDAC6/IL-10 signaling pathway in endometriosis-associated ovarian carcinoma. BioMed Pharmacother. (2023) 161:114500. doi: 10.1016/j.biopha.2023.114500
178. Natoli M, Herzig P, Pishali Bejestani E, Buchi M, Ritschard R, Lloyd GK, et al. Plinabulin, a distinct microtubule-targeting chemotherapy, promotes M1-like macrophage polarization and anti-tumor immunity. Front Oncol. (2021) 11. doi: 10.3389/fonc.2021.644608
179. Cang W, Wu A, Gu L, Wang W, Tian Q, Zheng Z, et al. Erastin enhances metastatic potential of ferroptosis-resistant ovarian cancer cells by M2 polarization through STAT3/IL-8 axis. Int Immunopharmacol. (2022) 113:109422. doi: 10.1016/j.intimp.2022.109422
180. Wanderley CW, Colón DF, Luiz JPM, Oliveira FF, Viacava PR, Leite CA, et al. Paclitaxel reduces tumor growth by reprogramming tumor-associated macrophages to an M1 profile in a TLR4-dependent manner. Cancer Res. (2018) 78:5891–900. doi: 10.1158/0008-5472.CAN-17-3480
181. Li X, Fu Y, Yang B, Guo E, Wu Y, Huang J, et al. BRD4 inhibition by AZD5153 promotes antitumor immunity via depolarizing M2 macrophages. Front Immunol. (2020) 11. doi: 10.3389/fimmu.2020.00089
182. Trenti A, Boscaro C, Tedesco S, Cignarella A, Trevisi L, Bolego C. Effects of digitoxin on cell migration in ovarian cancer inflammatory microenvironment. Biochem Pharmacol. (2018) 154:414–23. doi: 10.1016/j.bcp.2018.06.008
183. Gagliardi M, Kean R, Dai B, Augustine JJ, Roberts M, Fleming J, et al. BP1003 decreases STAT3 expression and its pro-tumorigenic functions in solid tumors and the tumor microenvironment. Biomedicines. (2024) 12. doi: 10.3390/biomedicines12081901
184. Deng L-J, Qi M, Li N, Lei Y-H, Zhang D-M, Chen J-X. Natural products and their derivatives: Promising modulators of tumor immunotherapy. J Leukoc Biol. (2020) 108:493–508. doi: 10.1002/JLB.3MR0320-444R
185. Yang C, Xia B-R, Zhang Z-C, Zhang Y-J, Lou G, Jin W-L. Immunotherapy for ovarian cancer: adjuvant, combination, and neoadjuvant. Front Immunol. (2020) 11. doi: 10.3389/fimmu.2020.577869
186. Chen H, Huang S, Niu P, Zhu Y, Zhou J, Jiang L, et al. Cardamonin suppresses pro-tumor function of macrophages by decreasing M2 polarization on ovarian cancer cells via mTOR inhibition. Mol Ther - Oncolytics. (2022) 26:175–88. doi: 10.1016/j.omto.2022.06.009
187. Fujiwara Y, Takaishi K, Nakao J, Ikeda T, Katabuchi H, Takeya M, et al. Corosolic acid enhances the antitumor effects of chemotherapy on epithelial ovarian cancer by inhibiting signal transducer and activator of transcription 3 signaling. Oncol Lett. (2013) 6:1619–23. doi: 10.3892/ol.2013.1591
188. Tsuboki J, Fujiwara Y, Horlad H, Shiraishi D, Nohara T, Tayama S, et al. Onionin A inhibits ovarian cancer progression by suppressing cancer cell proliferation and the protumour function of macrophages. Sci Rep. (2016) 6:29588. doi: 10.1038/srep29588
189. Le F, Yang L, Han Y, Zhong Y, Zhan F, Feng Y, et al. TPL inhibits the invasion and migration of drug-resistant ovarian cancer by targeting the PI3K/AKT/NF-κB-signaling pathway to inhibit the polarization of M2 TAMs. Front Oncol. (2021) 11. doi: 10.3389/fonc.2021.704001
190. Zhang Q, Li Y, Miao C, Wang Y, Xu Y, Dong R, et al. Anti-angiogenesis effect of Neferine via regulating autophagy and polarization of tumor-associated macrophages in high-grade serous ovarian carcinoma. Cancer Lett. (2018) 432:144–55. doi: 10.1016/j.canlet.2018.05.049
191. Lee K, Ahn J-H, Lee K-T, Jang DS, Choi J-H. Deoxyschizandrin, isolated from schisandra berries, induces cell cycle arrest in ovarian cancer cells and inhibits the protumoural activation of tumour-associated macrophages. Nutrients. (2018) 10. doi: 10.3390/nu10010091
192. Thaklaewphan P, Ruttanapattanakul J, Monkaew S, Buatoom M, Sookkhee S, Nimlamool W, et al. Kaempferia parviflora extract inhibits TNF-α-induced release of MCP-1 in ovarian cancer cells through the suppression of NF-κB signaling. BioMed Pharmacother. (2021) 141:111911. doi: 10.1016/j.biopha.2021.111911
193. Ren Y, He J, Zhao W, Ma Y. The anti-tumor efficacy of verbascoside on ovarian cancer via facilitating CCN1-AKT/NF-κB pathway-mediated M1 macrophage polarization. Front Oncol. (2022) 12. doi: 10.3389/fonc.2022.901922
194. Wang X, Gao S, Song L, Liu M, Sun Z, Liu J. Astragaloside IV antagonizes M2 phenotype macrophage polarization-evoked ovarian cancer cell Malignant progression by suppressing the HMGB1–TLR4 axis. Mol Immunol. (2021) 130:113–21. doi: 10.1016/j.molimm.2020.11.014
195. Wang H, Yung MMH, Xuan Y, Chen F, Chan W, Siu MKY, et al. Polyunsaturated fatty acids promote M2-like TAM deposition via dampening RhoA-YAP1 signaling in the ovarian cancer microenvironment. Exp Hematol Oncol. (2024) 13:90. doi: 10.1186/s40164-024-00558-8
196. Zhang C, Li Z, Wang J, Jiang X, Xia M, Wang J, et al. Ethanol Extracts of Solanum lyratum Thunb Regulate Ovarian Cancer Cell Proliferation, Apoptosis, and Epithelial-to-Mesenchymal Transition (EMT) via the ROS-Mediated p53 Pathway. J Immunol Res. (2021) 2021:5569354. doi: 10.1155/2021/5569354
197. Jeong M, Kim HM, Ahn J-H, Lee K-T, Jang DS, Choi J-H. 9-Hydroxycanthin-6-one isolated from stem bark of Ailanthus altissima induces ovarian cancer cell apoptosis and inhibits the activation of tumor-associated macrophages. Chem Biol Interact. (2018) 280:99–108. doi: 10.1016/j.cbi.2017.12.011
198. Wang S, Yang Y, Li S, Chen H, Zhao Y, Mu J. Recent advances in macrophage-derived exosomes as delivery vehicles. Nano TransMed. (2022) 1:e9130013. doi: 10.26599/NTM.2022.9130013
199. Lin W, Shen C, Li M, Ma S, Liu C, Huang J, et al. Programmable macrophage vesicle based bionic self-adjuvanting vaccine for immunization against monkeypox virus. Adv Sci. (2025) 12:2408608. doi: 10.1002/advs.202408608
200. Schweer D, Anand N, Anderson A, McCorkle JR, Neupane K, Nail AN, et al. Human macrophage-engineered vesicles for utilization in ovarian cancer treatment. Front Oncol. (2023) 12. doi: 10.3389/fonc.2022.1042730
201. Zhang X, Liu L, Tang M, Li H, Guo X, Yang X. The effects of umbilical cord-derived macrophage exosomes loaded with cisplatin on the growth and drug resistance of ovarian cancer cells. Drug Dev Ind Pharm. (2020) 46:1150–62. doi: 10.1080/03639045.2020.1776320
202. Hoare J, Campbell N, Carapuça E. Oncolytic virus immunotherapies in ovarian cancer: Moving beyond adenoviruses. Porto BioMed J. (2018) 3:e7. doi: 10.1016/j.pbj.0000000000000007
203. Pampeno C, Opp S, Hurtado A, Meruelo D. Sindbis virus vaccine platform: A promising oncolytic virus-mediated approach for ovarian cancer treatment. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25052925
204. Yin D, Zhong Y, Ling S, Lu S, Wang X, Jiang Z, et al. Dendritic-cell-targeting virus-like particles as potent mRNA vaccine carriers. Nat BioMed Eng. (2025) 9:185–200. doi: 10.1038/s41551-024-01208-4
205. Zhang G, Wang Q, Yuan R, Zhang Y, Chen K, Yu J, et al. Oncolytic vaccinia virus harboring aphrocallistes vastus lectin exerts anti-tumor effects by directly oncolysis and inducing immune response through enhancing ROS in human ovarian cancer. Biochem Biophys Res Commun. (2024) 730:150355. doi: 10.1016/j.bbrc.2024.150355
206. Shi G, Shi P, Yu Y, Xu J, Ma J, Zhang Y, et al. Oncolytic adenovirus inhibits Malignant ascites of advanced ovarian cancer by reprogramming the ascitic immune microenvironment. Mol Ther - Oncolytics. (2021) 23:488–500. doi: 10.1016/j.omto.2021.11.008
Keywords: ovarian cancer, tumor-associated macrophages, macrophage polarization, tumor microenvironment, therapy resistance, biomarkers, immunotherapy, extracellular vesicles
Citation: Xu C, Chen J, Tan M and Tan Q (2025) The role of macrophage polarization in ovarian cancer: from molecular mechanism to therapeutic potentials. Front. Immunol. 16:1543096. doi: 10.3389/fimmu.2025.1543096
Received: 10 December 2024; Accepted: 21 March 2025;
Published: 22 April 2025.
Edited by:
Prakash Radhakrishnan, University of Nebraska Medical Center, United StatesReviewed by:
Mara P. Steinkamp, University of New Mexico, United StatesCopyright © 2025 Xu, Chen, Tan and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingqing Tan, Y3p0YW5xcUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.