
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol., 07 April 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1542795
This article is part of the Research TopicImmunological Aspects and Immunotherapy in Gynecologic CancersView all 10 articles
 Ge Jin1
Ge Jin1 Jun Wang2*
Jun Wang2*Background: The therapeutic landscape for recurrent or metastatic cervical cancer remains limited, with few options available. According to National Comprehensive Cancer Network (NCCN) guidelines, pembrolizumab combined with chemotherapy, with or without bevacizumab, is recommended for affected patients. Despite these guidelines, recurrence rates remain elevated, and survival outcomes following standard interventions are unsatisfactory. Furthermore, real-world management of recurrent or metastatic cervical cancer presents inherent complexities, often requiring an integrative, multidimensional treatment approach to enhance long-term survival. The pressing need to refine and adopt multimodal therapeutic strategies is evident in addressing the persistent challenges associated with disease recurrence and progression.
Case description: The case involved a 40-year-old female diagnosed with advanced cervical cancer who underwent radical hysterectomy. Postoperative pathology identified high-risk features, including lymph node involvement, necessitating adjuvant chemoradiotherapy. However, disease progression occurred during treatment, manifesting as metastases in the left supraclavicular and axillary lymph nodes. Subsequent local radiotherapy and systemic therapy led to a favorable response. By November 2024, overall survival (OS) had surpassed 72 months, with toripalimab administered for 65 months, during which no immunotherapy-related adverse events occurred.
Conclusion: This case offers clinical insight into the efficacy and safety of integrating chemotherapy, immunotherapy, and radiotherapy in recurrent or metastatic cervical cancer. The multimodal approach contributes to prolonged survival in this patient. Further clinical trials are essential to substantiate the therapeutic benefits of this regimen in broader patient cohorts.
Cervical cancer (CC) ranks as the fourth most prevalent malignancy worldwide and remains a major contributor to cancer-related mortality among females, with approximately 604,000 new cases and 342,000 deaths reported in 2020 (1, 2). Despite a gradual increase in incidence, CC continues to impose a substantial global disease burden, second only to breast cancer. A negative correlation exists between CC burden and the socio-demographic index (SDI), with lower-income regions disproportionately affected due to insufficient public health resources (3). Once recurrence or metastasis occurs, prognosis is generally poor, particularly for those experiencing disease progression during treatment. Current guidelines from the National Comprehensive Cancer Network (NCCN) and the European Society of Gynecologic Oncology designate palliative chemotherapy as the standard of care for recurrent or metastatic cases (4, 5). However, its therapeutic efficacy remains suboptimal, as most patients derive limited clinical benefit, with only approximately one-fourth achieving a treatment response (6). The GOG-240 clinical trial demonstrated that the addition of bevacizumab to chemotherapy extended overall survival (OS) by 3.5 months, yielding modest improvements (7). Presently, pembrolizumab in combination with chemotherapy, with or without bevacizumab, is the recommended first-line treatment for recurrent or metastatic CC in patients with programmed death ligand 1 (PD-L1) positivity, defined by a combined positive score (CPS) of ≥1, as supported by findings from the KEYNOTE-826 study (8).
Lymph node metastasis (LNM) represents a significant independent prognostic determinant in CC, markedly affecting patient survival and serving as a primary conduit for tumor dissemination. Among metastatic sites, the parametrial and obturator foramen lymph nodes exhibit the highest incidence, followed by the internal, external, and common iliac nodes, as well as the para-aortic, supraclavicular, and infraclavicular lymph nodes. In contrast, axillary lymph node involvement remains uncommon. Existing research on LNM in CC predominantly centers on pelvic and para-aortic lymph nodes, with limited investigations addressing supraclavicular and axillary involvement. In recent oncological discourse, the classification of sync-oligometastases, metachronous oligometastases (meta-oligometastases), and oligo-recurrence has gained increased attention, emphasizing the prospect of prolonged survival or potential cure in patients with restricted metastatic burden when managed with appropriate local and systemic interventions.
Oligometastases are characterized by the presence of 1-5 metastatic or recurrent lesions, independent of the primary tumor’s status. Oligo-recurrence specifically denotes cases where 1-5 metastatic or recurrent lesions remain amendable to local therapy, provided the primary tumor is controlled, thereby representing a metachronous form of oligometastases. In contrast, sync-oligometastases involve concurrent metastatic disease with an active primary lesion. This report details a patient diagnosed with stage IIIC2p CC, initially treated with radical hysterectomy. During postoperative adjuvant radiotherapy, meta-oligometastases emerged, yet durable survival was achieved through a multimodal approach incorporating local radiotherapy, systemic chemotherapy, and immunotherapy.
The patient, a 40-year-old female, was admitted to the Fourth Hospital of Hebei Medical University on November 26, 2018, with a primary complaint of “contact bleeding for two weeks.” Obstetric history included four pregnancies, consisting of two induced abortions and two spontaneous deliveries. Physical examination identified no remarkable abnormalities, and her Eastern Cooperative Oncology Group (ECOG) performance status was assessed as 0. No underlying medical conditions, smoking history, or notable familial predispositions were reported. Pelvic magnetic resonance imaging (MRI) revealed a cervical mass with a maximum diameter of approximately 4 cm, alongside an enlarged right iliac vascular lymph node measuring 2.7 cm in short diameter (Figure 1). Chest X-ray showed no evident neoplastic lesions. The squamous cell carcinoma antigen (SCCA) level was elevated at 61.10 ng/ml. Based on the 2018 International Federation of Gynecology and Obstetrics (2018 FIGO) staging system, the diagnosis was classified as IIIC1r. Despite the NCCN guidelines recommending concurrent chemoradiotherapy, the patient declined treatment.
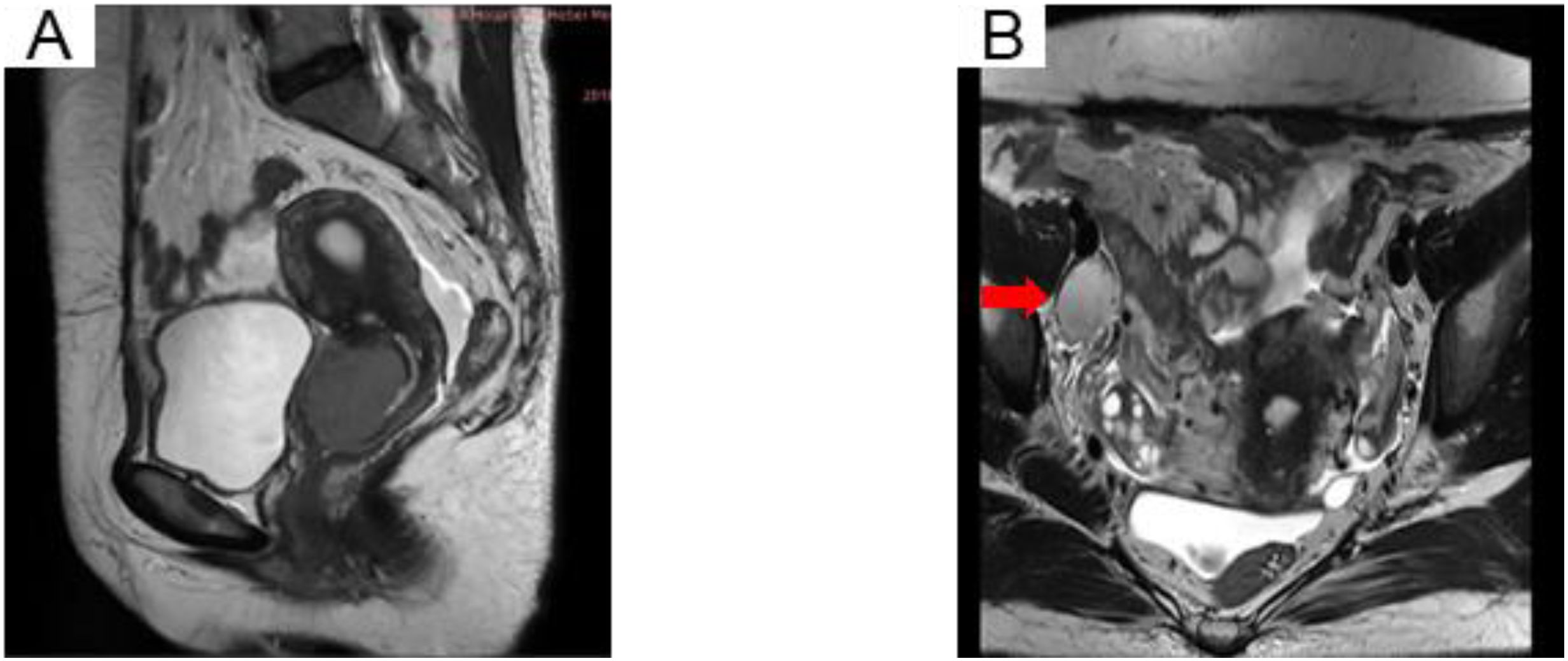
Figure 1. MRI pelvis showing an irregular uterine cervix soft tissue mass (A) and the right iliac vascular enlarged lymph node (B) in T2-weighted images.
On November 30, 2018, the patient underwent transabdominal radical hysterectomy, bilateral salpingo-oophorectomy, and pelvic and para-aortic lymph node dissection under general anesthesia, performed by the gynecologic oncology team. Histopathological evaluation confirmed squamous cell carcinoma of the cervix, with a tumor dimension of 3.5 × 3.5 × 2.5 cm, exhibiting deep stromal invasion exceeding half of the cervical stroma and clear evidence of vascular infiltration. Lymph node analysis demonstrated extensive metastatic dissemination: (1) left pelvic region: metastases detected in one of seven lymph nodes; (2) right pelvic region: five of ten lymph nodes positive for metastases; (3) right common iliac region: five of seven lymph nodes involved; and (4) para-aortic region: all 16 lymph nodes exhibiting metastatic infiltration. Based on these pathological findings, the disease was classified as 2018 FIGO stage IIIC2p (Table 1).
In alignment with NCCN guidelines, the patient’s high-risk profile for recurrence necessitated postoperative adjuvant therapy. The radiation oncology team implemented a regimen comprising three cycles of paclitaxel liposome (175 mg/m², d1) and lobaplatin (40 mg, d1) chemotherapy, administered every 21 days (q21d) from December 2018 to January 2019. Subsequently, intensity-modulated radiotherapy (IMRT) was employed to target both pelvic and extended para-aortic fields, delivering a cumulative dose of 48.6 Gy across 27 fractions beginning in January 2019. Due to the patient’s compromised physical status, concurrent chemotherapy was not pursued. Following the completion of external beam radiation, high-dose-rate brachytherapy with iridium-192 was introduced, delivering a prescribed dose of 7 Gy per fraction on a weekly basis for two fractions. SCCA levels were systematically monitored throughout the course of treatment (Figure 2).
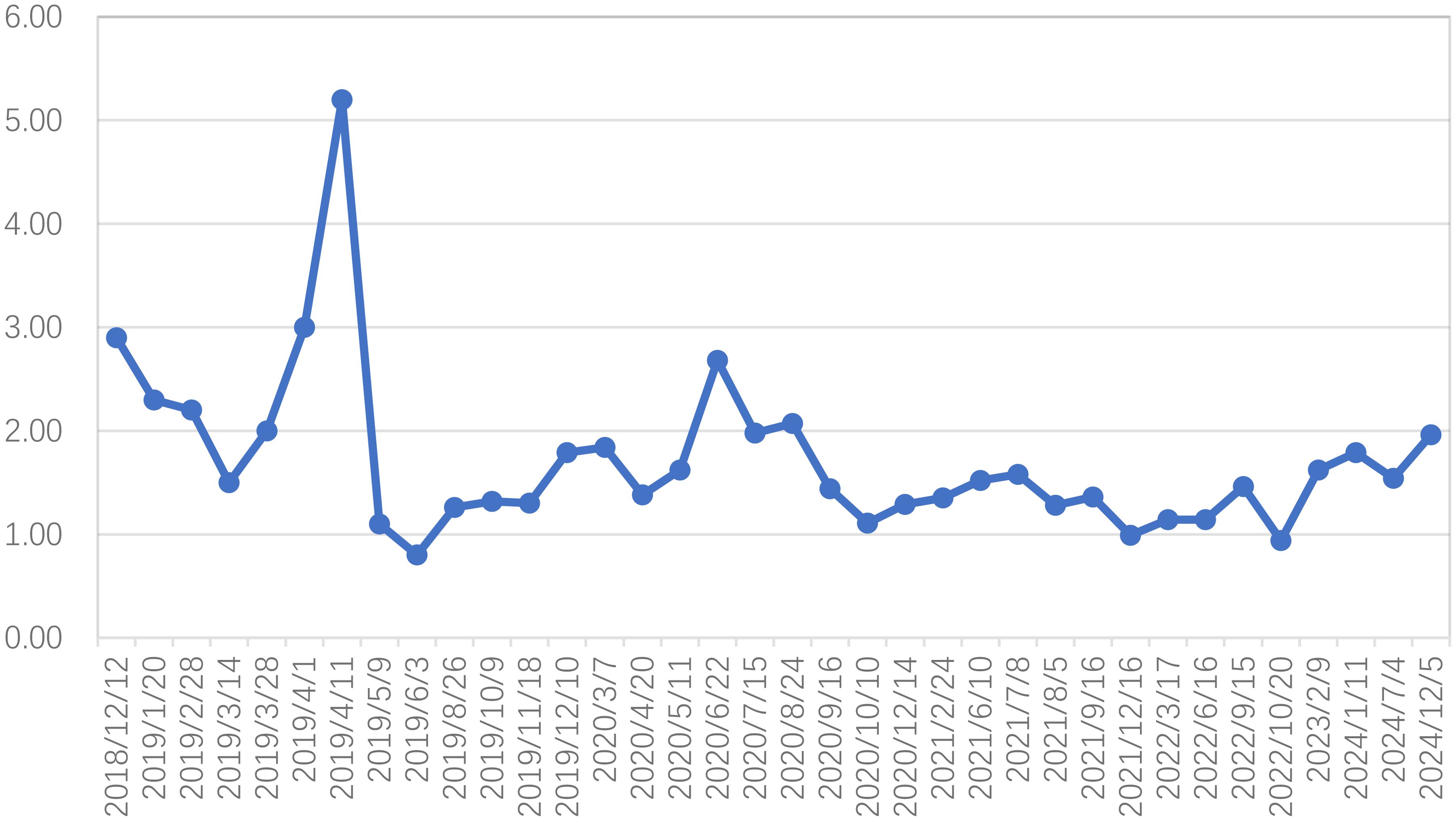
Figure 2. Squamous cell carcinoma antigen (SCCA) fluctuations from the period after radical hysterectomy to the last follow-up (reference value <1.5ng/ml before August 2019, reference value <2.7ng/ml after August 2019).
In February 2019, an SCCA level of 2.2 ng/ml was detected. Subsequent computed tomography (CT) imaging on March 29 identified mild enlargement of the left axillary lymph nodes, while no abnormalities were evident in the neck or lungs (Figures 3A, B). To further investigate, an ultrasound-guided biopsy of the left axillary lymph node was performed on April 3, confirming poorly differentiated carcinoma. However, immunohistochemical analysis was not performed due to the patient’s personal circumstances. To evaluate the extent of metastatic involvement, positron emission tomography-CT (PET-CT) imaging on April 4 demonstrated multiple enlarged lymph nodes exhibiting abnormal glucose hypermetabolism in the left posterior triangle, clavicular region, and axilla, indicating a high probability of LNM (Figures 3C, D).
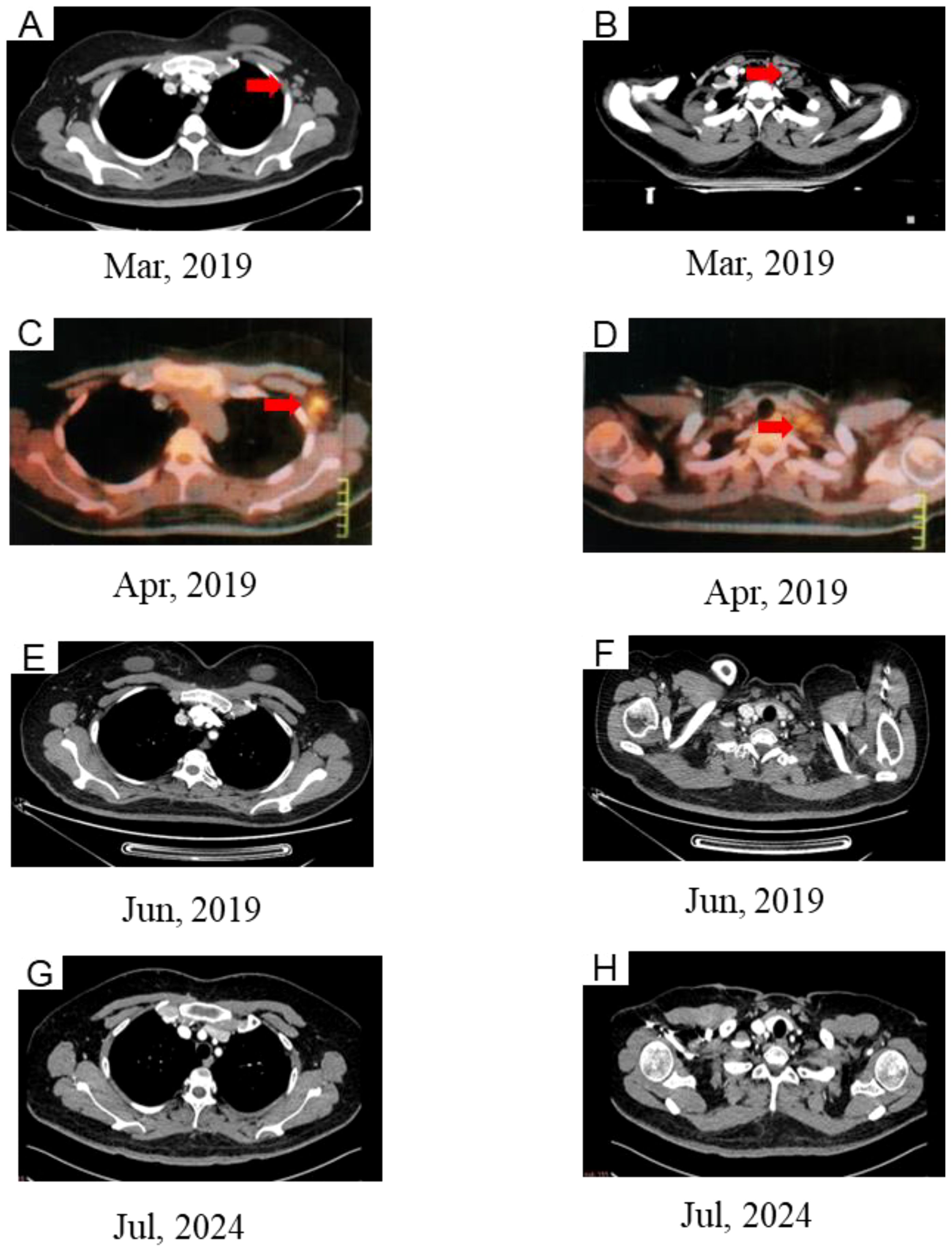
Figure 3. CT (A, B) and PET/CT (C, D) images showing new lesion in left supraclavicular and left axillary. (E, F) Complete response after chemoradiotherapy. (G, H) CT images at the last follow-up.
On April 8, the patient received chemotherapy with the TP regimen, comprising albumin-bound paclitaxel (175 mg/m², d1) and cisplatin (50 mg/m², d2-3; q21d). Throughout treatment, SCCA levels remained abnormal, exhibiting a gradual rise from 2.2 to 5.2 ng/ml. IMRT was initiated on April 15, 2019, delivering a total dose of 50.4 Gy to the cervical region over 28 fractions at five sessions per week. Additionally, the supraclavicular and axillary metastatic lymph nodes received boost doses up to 60.2 Gy. However, severe bone marrow suppression and gastrointestinal toxicity rendered concurrent chemotherapy intolerable, leading to its discontinuation at the patient’s request. Subsequently, toripalimab, an immune checkpoint inhibitor (ICI), was introduced at 240 mg intravenously every three weeks. Complete remission was achieved by the end of treatment. At the 72-month follow-up, no evidence of recurrence or metastasis was detected (Figures 3E-H), and no immune-related adverse events (AEs) were observed.
Early-stage CC is associated with a favorable prognosis, with a five-year survival rate exceeding 90% for localized lesions. However, recurrence or metastasis significantly limits therapeutic options, leading to poor clinical outcomes. The five-year survival rate declines to approximately 17%, with a median survival duration of 8-13 months and OS ranging from 13 to 17 months (4, 9). In this case, stage IIIC2p CC was diagnosed following standard radical hysterectomy. According to NCCN guidelines, patients presenting with high-risk factors post-hysterectomy require concurrent chemoradiotherapy (CCRT) with cisplatin-based regimens. Notably, the STARS study, a randomized clinical trial conducted in China, indicated that sequential chemoradiation (SCRT) yielded superior disease-free survival (DFS) rates and a lower risk of cancer-related mortality compared to radiotherapy alone in females with CC (10).
Following this clinical trial, the patient completed three cycles of TP chemotherapy before undergoing IMRT. However, concurrent chemotherapy was withheld during radiotherapy due to myelosuppression. Despite postoperative adjuvant therapy, supraclavicular and axillary LNM emerged. Research suggests that even after pelvic lymph node dissection, 10-15% of patients initially classified as node-negative (N0) may later develop lymphatic recurrence or metastasis (11). Although less frequent in advanced CC, hematogenous metastasis remains a concern, with the lungs (36.3%), bones (16.3%), liver, and brain identified as common metastatic sites (12). A study by Jung Ho Im et al. compared treatment outcomes between patients with distant LNM and those with visceral organ metastases, demonstrating significantly improved progression-free survival (PFS) and OS in cases of isolated distant LNM (13). Informed by these observations, a proactive strategy was implemented to address the patient’s disease progression.
In clinical practice, palliative chemotherapy remains a primary therapeutic approach for recurrent or metastatic CC. However, in this case, severe chemotherapy-related toxicity limited the patient to a single course of the TP regimen. Prospective studies have investigated the efficacy of PD-1 inhibitors in this patient population, reporting an objective response rate (ORR) ranging from 12.2% to 55.6% (13–16). Based on this evidence, pembrolizumab received approval from the United States Food and Drug Administration (FDA) for PD-L1-positive, locally advanced CC progressing during or after chemotherapy.
The introduction of immunotherapeutic agents has markedly reshaped the treatment paradigm for recurrent or metastatic CC, a historically challenging malignancy. However, real-world treatment complexities persist. In 2019, the high cost and restricted accessibility of pembrolizumab imposed considerable limitations on its clinical use. Toripalimab, a recombinant humanized IgG4 monoclonal antibody, serves as an alternative by inhibiting PD-1 interactions with its ligands, PD-L1 and PD-L2. Its therapeutic potential has been observed across multiple malignancies, including melanoma, urothelial carcinoma, renal cell carcinoma, nasopharyngeal carcinoma, and other solid tumors (17–20). A study involving 24 patients assessed the efficacy and safety of toripalimab in combination with bevacizumab and platinum-based chemotherapy as a first-line regimen for refractory recurrent or metastatic CC. With a median follow-up of 18.6 months (range, 3.3-28.5), the ORR reached 83.3%, while the disease control rate (DCR) was 95.8%. Among participants, 9 (37.5%) achieved a complete response, 11 (45.8%) showed a partial response, and 3 (12.5%) maintained stable disease. The median PFS was 22.6 months, whereas the median OS remained unreached (21). Additionally, in an exploratory trial assessing the efficacy of ICIs in combination with CCRT for locally advanced cervical cancer, the addition of the anti-PD-1 agent toripalimab exhibited a favorable safety profile and achieved an ORR of 100%, with PD-L1 expression on tumor cells deemed non-essential for eligibility (22). In this case, following pre-immunotherapy assessments, toripalimab treatment commenced in May 2019 at a dosage of 240 mg every three weeks. Notably, no immune-related AEs were observed throughout the treatment course. Despite disease progression, including supraclavicular and axillary LNM during postoperative adjuvant therapy, the patient obtained exceptional long-term survival exceeding five years. This outcome, attributed to the integration of immunotherapy, chemotherapy, and radiotherapy in managing metastatic lesions, marks a substantial advancement in immunotherapeutic approaches, achieving results previously considered unattainable. However, the substantial role of radiotherapy in this success must not be overlooked. A comprehensive, multimodal therapeutic strategy may offer more sustained benefits for complex and refractory malignancies such as recurrent or metastatic CC.
Survival outcomes following radiotherapy for patients with supraclavicular LNM have shown considerable variation. Matthew S et al. evaluated 38 patients undergoing definitive irradiation to oligometastatic sites of CC, reporting a median OS of 50.7 months from the completion of irradiation, with 2- and 3-year OS rates of 74% and 65%, respectively. Median PFS reached 21.7 months, with 1- and 2-year PFS rates of 63% and 48% (23). Another study reported 3- and 5-year OS rates of 75.0% and 56.3%, alongside PFS rates of 66.7% and 41.7% for the same intervals (24). Cao et al. analyzed 60 patients with stage IVB CC presenting with oligometastases at initial diagnosis, demonstrating superior PFS and OS outcomes in those receiving definitive irradiation to primary and oligometastatic sites compared to chemotherapy combined with pembrolizumab, with or without bevacizumab (25). Variability in survival rates across studies may be attributed to differences in sample size, prescription dose, and radiotherapy techniques. In this case, radiotherapy was integral to managing distant metastases, with the patient receiving 60.2 Gy in 28 fractions via IMRT at five fractions per week. Lee et al. reported effective control of supraclavicular LNM with prescription doses below 66 Gy, though their analysis was constrained by a limited sample size of seven patients (24). Another study identified an association between doses below 50 Gy and reduced PFS, advocating for a minimum dose of 50 Gy to enhance disease control (26). Cao et al. administered radiation doses between 60 and 74 Gy to metastatic lymph nodes, demonstrating favorable outcomes in metastasis management (25).
In the immunotherapy era, radiotherapy enhances treatment efficacy by enhancing antigen presentation, increasing T-cell infiltration, and reshaping the immune microenvironment. When integrated with ICIs, radiotherapy intensifies immune activation, leading to improved clinical outcomes (27–31). The KEYNOTE-A18 trial, a randomized, double-blind, placebo-controlled phase 3 study, demonstrated that pembrolizumab combined with chemoradiotherapy significantly prolonged OS in patients with LACC, establishing this regimen as the new standard of care (32). Similarly, a meta-analysis indicated that CCRT with ICIs led to superior PFS and OS compared to CCRT alone. The combination group exhibited higher ORR than the control group, while grade ≥3 treatment-related AEs occurred at comparable rates. However, immunotherapy-related AEs of any grade were more frequently observed in the combination cohort (33).
In this case, the patient received immunotherapy and local radiotherapy but, due to personal medical considerations, did not undergo systemic chemotherapy. Despite this, a complete response was achieved, with sustained long-term survival free from treatment-related or immune-related AEs. Such outcomes remain uncommon yet promising, emphasizing the therapeutic potential of integrated treatment strategies. Further investigation through prospective randomized controlled trials is warranted to refine and validate the efficacy of radiotherapy and immunotherapy combinations in recurrent or metastatic CC.
The therapeutic strategy in this case aligns with evidence-based protocols in recurrent or metastatic CC management, integrating data from clinical trials. This approach plays a key role in achieving prolonged survival. Additionally, the case offers clinically relevant evidence on the effectiveness and safety of combining immunotherapy, chemotherapy, and radiotherapy in treating recurrent or metastatic CC.
Given recent advancements in the management of recurrent or metastatic CC, several key questions arise:
Patients with oligometastases, particularly those with lymph node involvement, generally exhibit a comparatively favorable prognosis. However, uncertainty remains regarding the optimal approach for local irradiation, specifically in choosing between conventional fractionated and hypofractionated radiotherapy. The KROG 14-11 study assessed local control and survival outcomes in patients with recurrent or oligometastatic uterine CC treated with stereotactic body radiotherapy (SBRT) using CyberKnife. A total of 85 patients received a median dose of 39 Gy in three fractions, corresponding to a biologically effective dose (BED) of 90 Gy, with toxicity levels remaining within acceptable limits. Local PFS rates at 2 and 5 years were 82.5% and 78.8%, respectively (34). Additionally, a phase II study involving 50 patients with recurrent gynecologic cancer and single or multiple (≤4) metastases reported a 96% target response rate (48 of 50 patients) following robotic-assisted CyberKnife SBRT (24 Gy in three daily fractions). Furthermore, 68% (34 of 50 patients) achieved clinical benefit for at least six months (95% CI, 53.2–80.1), with no subsequent need for systemic therapy due to disease progression (35). In comparison, Cao et al. employed IMRT or volumetric modulated arc therapy (VMAT) for oligometastatic lymph node irradiation, an approach consistent with the one applied in the present case (25). The radiation oncologist managing the oligometastatic case utilized 3D-printed templates for interstitial brachytherapy targeting inguinal LNM, delivering a prescription dose of 25 Gy (36). Additionally, large-scale analyses, including those derived from the Surveillance, Epidemiology, and End Results (SEER) database, indicate that radiotherapy may contribute to improved survival in metastatic CC. Careful patient selection for radiotherapy remains essential, considering prior treatments, lesion distribution, and overall health status (37). In clinical practice, treatment strategies should be tailored to individual patient profiles to optimize therapeutic outcomes.
What is the optimal combination model for radiotherapy and immunotherapy in recurrent or metastatic CC?
Radiotherapy remains a cornerstone in the management of locally advanced CC. For patients with 2018 FIGO stage IB3-IVA, cisplatin-based chemoradiotherapy followed by image-guided adaptive brachytherapy (IGABT) is established as the gold standard (38). The integration of CCRT with ICIs has been shown to enhance immune activation, increasing both central and effector memory T-cell populations, indicative of immune modulation (39). Multiple studies have investigated the role of ICIs in LACC, demonstrating their antitumor potential. The KEYNOTE-A18 randomized, double-blind, phase 3 trial, for instance, revealed that the addition of pembrolizumab to CCRT significantly improved PFS and reduced mortality by 33% in LACC patients (32), broadening the role of immunotherapy in CC and influencing clinical practice. Moreover, clinical evidence supports the efficacy of combining immunotherapy with chemotherapy, with or without bevacizumab, in recurrent or metastatic CC (40, 41). However, key uncertainties remain: Can the integration of immunotherapy with chemoradiotherapy, with or without bevacizumab, yield additional benefits for patients with oligometastatic CC? Is sustained immunological maintenance necessary after achieving disease control? Addressing these uncertainties requires large-scale, high-quality clinical trials to refine treatment strategies.
With ICIs now integrated into frontline therapy, subsequent disease relapse presents limited treatment alternatives. Tissue factor, a critical component of the blood coagulation cascade, has been identified as a transmembrane protein involved in the proliferation and invasion of certain cancer cells, positioning it as a potential therapeutic target (42–44). The Innovate Tisotumab Vedotin 301 (innovaTV 301) trial recently demonstrated the efficacy and safety profile of tisotumab vedotin (2.0 mg/kg) compared with physician-selected chemotherapy in 502 patients (median overall survival: 11.5 months vs. 9.5 months; hazard ratio for death from any cause: 0.70; 95% CI, 0.54–0.89; P = 0.004), leading to FDA approval on April 29, 2024. Notably, the drug’s effectiveness was independent of tissue factor expression in tumor cells (45).
HER2 expression was observed in approximately 5% of all CC cases (46). Trastuzumab deruxtecan (T-DXd), a HER2-directed ADC, has been evaluated for CC treatment within the phase II DESTINY-PanTumor02 basket trial (47). This study assessed T-DXd at 5.4 mg/kg every three weeks across seven cohorts comprising patients with various HER2-positive (IHC 2-3+) advanced solid tumors that had progressed following at least one prior systemic therapy, with ORR as the primary endpoint. Between 2020 and 2022, 267 patients received treatment, including 40 with CC. Among the CC cohort, 6 of the 8 women achieved an objective response.
The STING signaling pathway has garnered significant interest in tumor immunotherapy, particularly in combination with immune checkpoint inhibitors, emerging as a focal point in clinical research (48). By detecting DNA damage signals in tumor cells, such as cytoplasmic DNA accumulation, STING activation triggers the secretion of type I interferons (IFN-I) and chemokines, promoting CD8+ T cell and NK cell infiltration into the tumor microenvironment. This process facilitates the transition from an immunologically “cold” to “hot” tumor phenotype, thereby enhancing ICI efficacy. MK-1454, a structurally modified CDNs STING agonist developed by Merck, exhibits high affinity for STING and, when administered via intratumoral injection, induces complete tumor regression. Its combination with pembrolizumab in patients with advanced solid tumors or lymphoma has demonstrated a favorable safety profile and improved therapeutic efficacy. Currently, MK-1454 is being evaluated in a Phase II clinical trial for its efficacy and safety in head and neck squamous cell carcinoma (NCT04220866). The STING agonist MSA-2 functions as a non-covalent dimer binding to STING. Both oral and subcutaneous administration of MSA-2 have demonstrated safety and tolerability in mouse models, exhibiting sustained anti-tumor immunoefficacy either as monotherapy or in combination with PD-1 inhibitors (49). Despite significant advancements in research on the STING signaling pathway and its modulators, several challenges remain, primarily concerning (1) potential adverse reactions and (2) limited clinical efficacy.
The original contributions presented in the study are included in the article/supplementary files. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by The Ethics Committee of the Fourth Hospital of Hebei Medical University, China. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
GJ: Writing – original draft. JW: Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. (2019) 8:e191–203. doi: 10.1016/S2214-109X(19)30482-6
3. Li T, Zhang H, Lian M, He Q, Lv M, Zhai L, et al. Global status and attributable risk factors of breast, cervical, ovarian, and uterine cancers from 1990 to 2021. J Hematol Oncol. (2025) 18:5. doi: 10.1186/s13045-025-01660-y
4. Cibula D, Pötter R, Planchamp F, Avall-Lundqvist E, Fischerova D, Haie Meder C, et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother Oncol. (2018) 127:404–16. doi: 10.1016/j.radonc.2018.03.003
5. Cancer. Ngvc. NCCNguidelines version 1.2022 cervical cancer. Available online at: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (Accessed 20 Dec 2022).
6. Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. (2009) 27:4649–55. doi: 10.1200/JCO.2009.21.8909
7. Tewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis ofa randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. (2017) 390:1654–63. doi: 10.1016/S0140-6736(17)31607-0
8. Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. New Engl J Med. (2021) 385:1856–67. doi: 10.1056/NEJMoa2112435
9. van Meir H, Kenter GG, Burggraaf J, Kroep JR, Welters MJ, Melief CJ, et al. The need for improvement of the treatment of advanced and metastatic cervical cancer, the rationale for combined chemo-immunotherapy. Anti-Cancer Agent Me. (2014) 14:190–203. doi: 10.2174/18715206113136660372
10. Huang H, Feng YL, Wan T, Zhang YN, Cao XP, Huang YW, et al. Effectiveness of sequential chemoradiation vs concurrent chemoradiation or radiation alone in adjuvant treatment after hysterectomy for cervical cancer: the STARS phase 3 randomized clinical trial. JAMA Oncol. (2021) 7:361–9. doi: 10.1001/jamaoncol.2020.7168
11. Balaya V, Mathevet P, Magaud L, Bonsang-Kitzis H, Delomenie M, Montero Macias R, et al. Predictive factors of unexpected lymphatic drainage pathways in early-stage cervical cancer. Gynecol Oncol. (2019) 154:102–9. doi: 10.1016/j.ygyno.2019.04.008
12. Li H, Wu X, Cheng X. Advances in diagnosis and treatment of metastatic cervical cancer. J Gynecol Oncol. (2016) 27:e43. doi: 10.3802/jgo.2016.27.e43
13. Im JH, Yoon HI, Kim S, Nam EJ, Kim SW, Yim GW, et al. Tailored radiotherapeutic strategies for disseminated uterine cervical cancer patients. Radiat Oncol. (2015) 10:77. doi: 10.1186/s13014-015-0373-0
14. Chung HC, Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. (2019) 37:1470–8. doi: 10.1200/JCO.18.01265
15. Naumann RW, Hollebecque A, Meyer T, Devlin MJ, Oaknin A, Kerger J, et al. Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II checkMate 358 trial. J Clin Oncol. (2019) 37:2825–34. doi: 10.1200/JCO.19.00739
16. Lan C, Shen J, Wang Y, et al. Camrelizumab plus apatinib in patients with advanced cervical cancer (CLAP): A multicenter, open-label, single-arm, phase II trial. J Clin Oncol. (2020) 38:4095–106. doi: 10.1200/JCO.20.01920
17. Keam SJ. Toripalimab: first global approval. Drugs. (2019) 79:573–8. doi: 10.1007/s40265-019-01076-2
18. Tang B, Yan X, Sheng X, Si L, Cui C, Kong Y, et al. Safety and clinical activity with an anti-PD-1 antibody JS001 in advanced melanoma or urologic cancer patients. J Hematol Oncol. (2019) 12:7. doi: 10.1186/s13045-018-0693-2
19. Xu R, Wang F, Feng F, Li Q, Xu N, Hu X, et al. Recombinant humanized anti-PD-1 monoclonal antibody (JS001) in patients with refractory/metastatic nasopharyngeal carcinoma: Preliminary results of an open-label phase II clinical study. Ann Oncol. (2018) 29:viii409. doi: 10.1093/annonc/mdy288.023
20. Wang F, Ren C, Zhang Y, Yao S, Feng H, Wu H, et al. Phase Ia study of a humanized anti-PD-1 monoclonal antibody (JS001) in Chinese patients with refractory solid tumors. Ann Oncol. (2017) 28:v420–1. doi: 10.1093/annonc/mdx376.051
21. Li C, Liu S, He Y, Yao H, Yuan Z, et al. Toripalimab combined with bevacizumab plus chemotherapy as first-line treatment for refractory recurrent or metastatic cervical cancer: a single-arm, open-label, phase II study (JS001-ISS-CO214). J Gynecol Oncol. (2023). doi: 10.3802/jgo.2025.36.e44
22. Ou D, Cai R, Qi WX, Cui C, Cao L, Wang SB, et al. Toripalimab combined with definitive chemoradiotherapy for locally advanced cervical squamous cell carcinoma patients (TRACE): A single-arm, phase I/II trial. Cancer Immunol Immun. (2023) 73:244. doi: 10.1007/s00262-024-03823-1
23. Ning MS, Ahobila V, Jhingran A, Stecklein SR, Frumovitz M, Schmeler KM, et al. Outcomes and patterns of relapse after definitive radiation therapy for oligometastatic cervical cancer. Gynecol Oncol. (2017) 148:132–8. doi: 10.1016/j.ygyno.2017.10.017
24. Lee S, Lee S, Lee K, Shin J, Lee K, Park C, et al. The results of radiation therapy with chemotherapy for patients with cervical cancer and supraclavicular lymph node involvement. Int J Radiat Oncol. (2010) 78:S403–4. doi: 10.1016/j.ijrobp.2010.07.950
25. Li J, Wang Y, Huo L, Huang X, Shi L, Huang L, et al. Definitive irradiation as a first treatment strategy for primary and metastatic sites of newly diagnosed IVB cervical cancer that presented with synchronous oligometastases. Radiat Oncol. (2023) 18:159. doi: 10.1186/s13014-023-02320-6
26. Mukai Y, Yokota NR, Sugiura M, Mizushima T, Taniuchi R, Imai Y, et al. Outcome of radiation therapy for stage IVB uterine cervical cancer with distant lymph nodes metastases; sequential irradiation for distant lymph nodes metastases. In Vivo. (2021) 35:1169–76. doi: 10.21873/invivo.12365
27. Demaria S, Coleman CN, Formenti SC. Radiotherapy: changing the game in immunotherapy. Trends Cancer. (2016) 2:286–94. doi: 10.1016/j.trecan.2016.05.002
28. Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. (2017) 545:60–5. doi: 10.1038/nature22079
29. Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. (2015) 1:1325–32. doi: 10.1001/jamaoncol.2015.2756
30. Mondini M, Levy A, Meziani L, Milliat F, Deutsch E. Radiotherapy-immunotherapy combinations - perspectives and challenges. Mol Oncol. (2020) 14:1529–37. doi: 10.1002/1878-0261.12658
31. Kang K, Wu Y, Yao Z, Lu Y. Tackling the current dilemma of immunotherapy in extensive-stage small cell lung cancer: A promising strategy of combining with radiotherapy. Cancer Lett. (2023) 565:216239. doi: 10.1016/j.canlet.2023.216239
32. Lorusso D, Xiang Y, Hasegawa K, Scambia G, Leiva M, Ramos-Elias P, et al. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2024) 404:1321–32. doi: 10.1016/S0140-6736(24)01808-7
33. Zhao Z, Ruan J, Fang M, Liu J, Liao G. Efficacy and safety of chemoradiotherapy plus immune checkpoint inhibitors for the treatment of locally advanced cervical cancer: a systematic review and meta-analysis. Front Immunol. (2024) 15:1459693. doi: 10.3389/fimmu.2024.1459693
34. Park HJ, Chang AR, Seo Y, Cho CK, Jang WI, Kim MS, et al. Stereotactic body radiotherapy for recurrent or oligometastatic uterine cervix cancer: A cooperative study of the Korean radiation oncology group (KROG 14-11). Anticancer Res. (2015) 35:5103–10.
35. Kunos CA, Brindle J, Waggoner S, Zanotti K, Resnick K, Fusco N, et al. Phase II clinical trial of robotic stereotactic body radiosurgery for metastatic gynecologic Malignancies. Front Oncol. (2012) 2:181. doi: 10.3389/fonc.2012.00181
36. Qin Y, Guan P, Li D, He H, He W, Tan L, et al. Successful inguinal interstitial brachytherapy in metastatic cervical carcinoma: a case report. Front Oncol. (2024) 13:1330681. doi: 10.3389/fonc.2023.1330681
37. Huang K, Jia M, Li P, Han J, Zhang R, Li Q, et al. Radiotherapy improves the survival of patients with metastatic cervical cancer: A propensity-matched analysis of SEER database. Int J Gynecol Cancer. (2018) 28:1360–8. doi: 10.1097/IGC.0000000000001313
38. Cibula D, Pötter R, Planchamp F, Avall-Lundqvist E, Fischerova D, Haie Meder C, et al. The European society of gynaecological oncology/European society for radiotherapy and oncology/European society of pathology guidelines for the management of patients with cervical cancer. Int J Gynecol Cancer. (2018) 28:641–55. doi: 10.1097/IGC.0000000000001216
39. Da Silva DM, Enserro DM, Mayadev JS, Skeate JG, Matsuo K, Pham HQ, et al. Immune activation in patients with locally advanced cervical cancer treated with ipilimumab following definitive chemoradiation (GOG-9929). Clin Cancer Res. (2020) 26:5621–30. doi: 10.1158/1078-0432.CCR-20-0776
40. Lorusso D, Colombo N, Dubot C, Cáceres MV, Hasegawa K, Shapira-Frommer R, et al. Pembrolizumab plus chemotherapy for advanced and recurrent cervical cancer: final analysis according to bevacizumab use in the randomized KEYNOTE-826 study. Ann Oncol. (2024) 36(1):65–75. doi: 10.1016/j.annonc.2024.10.002
41. Wu X, Sun Y, Yang H, Wang J, Lou H, Li D, et al. Cadonilimab plus platinum-based chemotherapy with or without bevacizumab as first-line treatment for persistent, recurrent, or metastatic cervical cancer (COMPASSION-16): a randomised, double-blind, placebo-controlled phase 3 trial in China. Lancet. (2024) 404:1668–76. doi: 10.1016/S0140-6736(24)02135-4
42. Zhao X, Cheng C, Gou J, Yi T, Qian Y, Du X, et al. Expression of tissue factor in human cervical carcinoma tissue. Exp Ther Med. (2018) 16:4075–81. doi: 10.3892/etm.2018.6723
43. Yu YJ, Hou XD, Li YM. Effect of tissue factor knockdown on the growth, invasion, chemoresistance and apoptosis of human gastric cancer cells. Exp Ther Med. (2014) 7:1376–82. doi: 10.3892/etm.2014.1591
44. Eisenreich A, Bolbrinker J, Leppert U. Tissue factor: A conventional or alternative target in cancer therapy. Clin Chem. (2016) 62:563–70. doi: 10.1373/clinchem.2015.241521
45. Vergote I, González-Martín A, Fujiwara K, Kalbacher E, Bagaméri A, Ghamande S, et al. Tisotumab vedotin as second- or third-line therapy for recurrent cervical cancer. New Engl J Med. (2023) 391:44–55. doi: 10.1056/NEJMoa2313811
46. Yan M, Schwaederle M, Arguello D, Millis SZ, Gatalica Z, Kurzrock R. HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metast Rev. (2015) 34:157–64. doi: 10.1007/s10555-015-9552-6
47. Meric-Bernstam F, Makker V, Oaknin A, Oh DY, Banerjee S, González-Martín A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-panTumor02 phase II trial. J Clin Oncol. (2023) 42:47–58. doi: 10.1200/JCO.23.02005
48. Lou F, Zheng M, Chen K, Zhang S. Research progress of cGAS-STING signaling pathway modulators in immunotherapy. J China Pharm Univ. (2024) 55:15–25. doi: 10.11665/j.issn.1000-5048.2023112402
Keywords: cervical cancer, case report, long-term survival, radiotherapy, toripalimab
Citation: Jin G and Wang J (2025) Both complete response and long-term survival after combination therapy with toripalimab in a patient with meta-oligometastases cervical cancer: a case report. Front. Immunol. 16:1542795. doi: 10.3389/fimmu.2025.1542795
Received: 10 December 2024; Accepted: 21 March 2025;
Published: 07 April 2025.
Edited by:
Bastian Czogalla, LMU Munich University Hospital, GermanyReviewed by:
Tianye Li, Zhejiang University School of Medicine, ChinaCopyright © 2025 Jin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Wang, d2FuZ2p1bjA4MThAaGVibXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.