- 1West China School of Medicine, Sichuan University, Chengdu, Sichuan, China
- 2Department of Obstetrics and Gynecology, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
- 3Key Laboratory of Birth Defects and Related Diseases of Women and Children, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
Introduction: Gynecologic tract melanoma (GTM) is a rare and aggressive malignancy with limited treatment options and poor prognosis. This study aims to evaluate the outcomes of immune checkpoint inhibitors (ICIs) in patients with GTM and identify prognostic factors influencing survival.
Methods: A retrospective analysis was conducted on 45 patients diagnosed with GTM at West China Second University Hospital from January 2019 to September 2024. Data on demographics, clinical characteristics, treatments, and outcomes were collected. Progression-free survival (PFS) and overall survival (OS) were analyzed using Kaplan-Meier curves and Cox proportional-hazards models.
Results: Among 45 patients, 24 had vaginal melanoma, 18 had vulvar melanoma, and 3 had cervical melanoma. ICIs were administered to 21 patients, but no significant survival benefit was observed. The 1-, 3-, and 5-year survival rates were 87%, 63%, and 31%, respectively. Univariate analysis revealed that patients with a family history of cancer (FHC) and those with lactate dehydrogenase (LDH) levels ≤230 had better PFS. Additionally, FHC, American Joint Committee on Cancer (AJCC) stage I-II, absence of pelvic lymph node metastasis, and LDH levels ≤230 were associated with improved OS. However, in multivariate analysis, only LDH was significantly associated with OS.
Conclusion: This single-center study suggests that ICIs have limited efficacy in treating GTM, emphasizing the need for further investigation through larger, multicenter clinical trials. Prognostic factors such as FHC, AJCC stage, lymph node involvement, and LDH levels may aid in risk stratification and personalized treatment planning. However, due to the nature of this study, external cohorts are still needed for validation.
1 Introduction
Gynecologic tract melanoma (GTM) is a rare and highly aggressive form of cancer, accounting for only 3% to 7% of all mucosal melanomas (MM) (1). GTM primarily includes melanomas originating in the vulva, vagina, and cervix, all of which are associated with poor prognoses (2–4). Approximately 18-40% of MM arise in the vulvar region (5). Epidemiological data indicate that the annual incidence of vulvar malignant melanoma is around 0.136 cases per 100,000 people, with 5-year survival rates ranging from 10% to 63% (6). Vaginal malignant melanoma is even rarer, with an incidence of 0.046 cases per 100,000 and a 5-year survival rate of about 15% (7). Cervical malignant melanoma is the rarest subtype, with only 149 reported cases worldwide and a 5-year survival rate of approximately 29% (8).
Treatment for GTM typically involves a multimodal approach, combining surgery, radiotherapy, and chemotherapy (9). However, due to the rarity and aggressive nature of GTM, treatment options are limited, and outcomes remain poor (10). GTM differs significantly from cutaneous melanoma (CM) in clinical presentation, pathological features, treatment response, and molecular characteristics, highlighting the need for tailored therapeutic approaches (11).
In recent years, the advent of targeted therapies and immunotherapies has begun to reshape the treatment landscape for GTM. Traditionally, surgery has been the cornerstone of treatment (6). However, advances in molecular research on cutaneous melanoma has led to the identification of key mutations, such as those in the BRAF, NRAS, and NF1 genes (12). Similarly, mutations in BRAF and KIT have also been discovered in vulvar and vaginal melanomas, opening the door for the application of targeted therapies (6). At the same time, immunotherapy has also made significant progress, particularly with inhibitors targeting programmed cell death protein 1 (PD-1)/Programmed death-ligand 1 (PD-L1) and Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitors, which have shown promising results in the treatment of cutaneous melanomas (13).
Despite these advances, the use of immunotherapy for GTM remains in its early stages and is primarily documented in case reports. Given the rarity and aggressive nature of this disease, there is a critical need for further research and well-designed clinical trials to assess the efficacy and safety of immune checkpoint inhibitors (ICIs) in this patient population. To contribute to the expanding body of knowledge, we conducted a retrospective analysis of patients with newly diagnosed or recurrent GTM treated at West China Second University Hospital from January 2019 to September 2024. By evaluating treatment outcomes and patient responses to ICIs, this study aims to provide preliminary evidence that may inform future large-scale clinical trials and guide the development of more effective therapeutic strategies for GTM.
2 Materials and methods
2.1 Study population
This retrospective study included all female patients diagnosed with GTM between January 2019 and September 2024 at West China Second University Hospital. The inclusion criteria were as follows: (1) histologically confirmed diagnosis of female genital malignant melanoma, and (2) receipt of surgery or any other therapeutic intervention. Patients were excluded if they met any of the following criteria: (1) age under 18 years, (2) a history of autoimmune disease, or (3) did not receive treatment.
Data on patient demographics, clinical examinations, tumor characteristics, surgical details, pathology results, and systemic treatments were collected from the hospital’s electronic medical record system. Tumor staging was standardized for all patients according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system to ensure consistency in the analysis. Follow-up information was obtained either from the outpatient medical record system or through telephone interviews. Progression-free survival (PFS) was defined as the time from diagnosis to the first clinically confirmed recurrence or death from the disease, while overall survival (OS) was calculated from the date of diagnosis to either the date of death or the last follow-up.
2.2 Statistical analysis
Kaplan-Meier survival curves were used to estimate hazard ratios for PFS and OS. Multivariate analysis was performed using Cox proportional-hazards regression models to identify significant predictors of PFS and OS. All statistical tests were two-sided, with a p-value of <0.05 considered statistically significant. Data management was conducted using Microsoft Excel, and statistical analyses were performed using SPSS software, version 25.0 (IBM Corp., Armonk, NY, USA).
2.3 Ethics approval
The study was approved by the Ethics Committee of West China Second University Hospital. All procedures adhered to the ethical guidelines and regulations of the committee. Due to the retrospective nature of the study, informed consent was waived.
3 Results
3.1 Patient characteristics
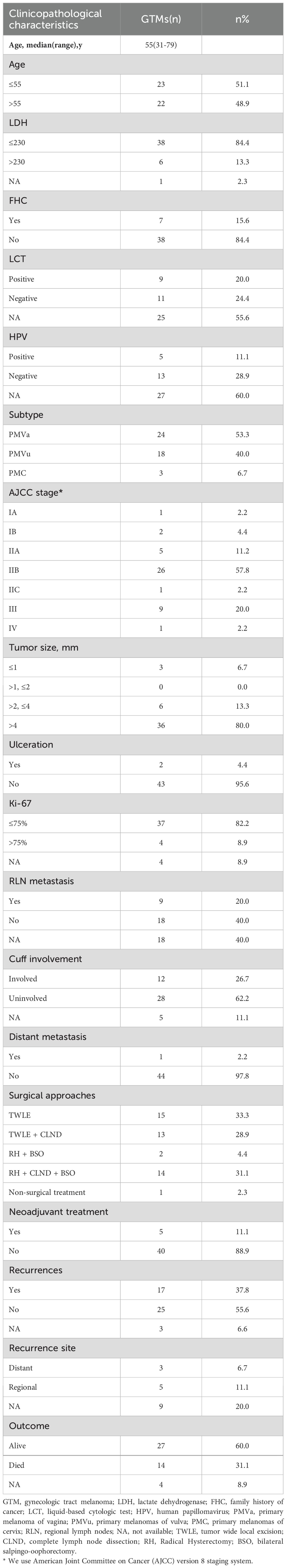
This study included a total of 45 patients (Table 1), with a median age of 55 years. Seven patients had a documented family history of malignancies among first-degree relatives, including liver cancer (2 cases), lung cancer (1 case), esophageal cancer (1 case), gastric cancer (1 case), rectal cancer (1 case), and small-cell neuroendocrine carcinoma (1 case). Cervical liquid-based cytology testing (LCT) was performed in 20 patients, of whom 9 exhibited pathological abnormalities. Cervical human papillomavirus (HPV) testing was conducted in 18 patients, revealing no infection in 13 cases. Among the 5 HPV-positive patients (3 vulvar melanoma and 2 vaginal melanoma), 2 tested positive for HPV56, and 1 each for HPV16, HPV52, and HPV58. Among the 45 patients, 24 (53.3%) were diagnosed with vaginal melanoma, 18 (40.0%) with vulvar melanoma, and 3 (6.7%) with cervical melanoma. At the time of initial diagnosis, 3 patients (6.5%) were classified as AJCC stage I, 32 (71.1%) as stage II, 9 (20.0%) as stage III, and 1 (2.2%) as stage IV.
3.2 Treatment approaches for new diagnosed GTM
Four patients received neoadjuvant chemotherapy prior to surgery. Of the total cohort, 44 patients (97.8%) underwent surgery as their initial treatment. Tumor wide local excision (TWLE) served as the standard procedure for vulvar melanoma, with complete lymph node dissection (CLND) performed selectively based on tumor size and imaging findings indicating lymph node enlargement. Similar to vulvar malignant melanoma, vaginal malignant melanoma in the lower one-third of the vagina was treated with TWLE or TWLE combined with CLND. In contrast, vaginal malignant melanoma in the upper one-third of the vagina was managed using the same surgical approach as cervical malignant melanoma, namely radical hysterectomy (RH) and bilateral salpingo-oophorectomy (BSO), with or without CLND. Specifically, 15 patients (33.3%) underwent TWLE alone, 13 (28.9%) received TWLE combined with CLND, 2 (4.4%) had RH and BSO due to poor general condition, and 14 (31.1%) underwent RH in combination with both CLND and BSO.
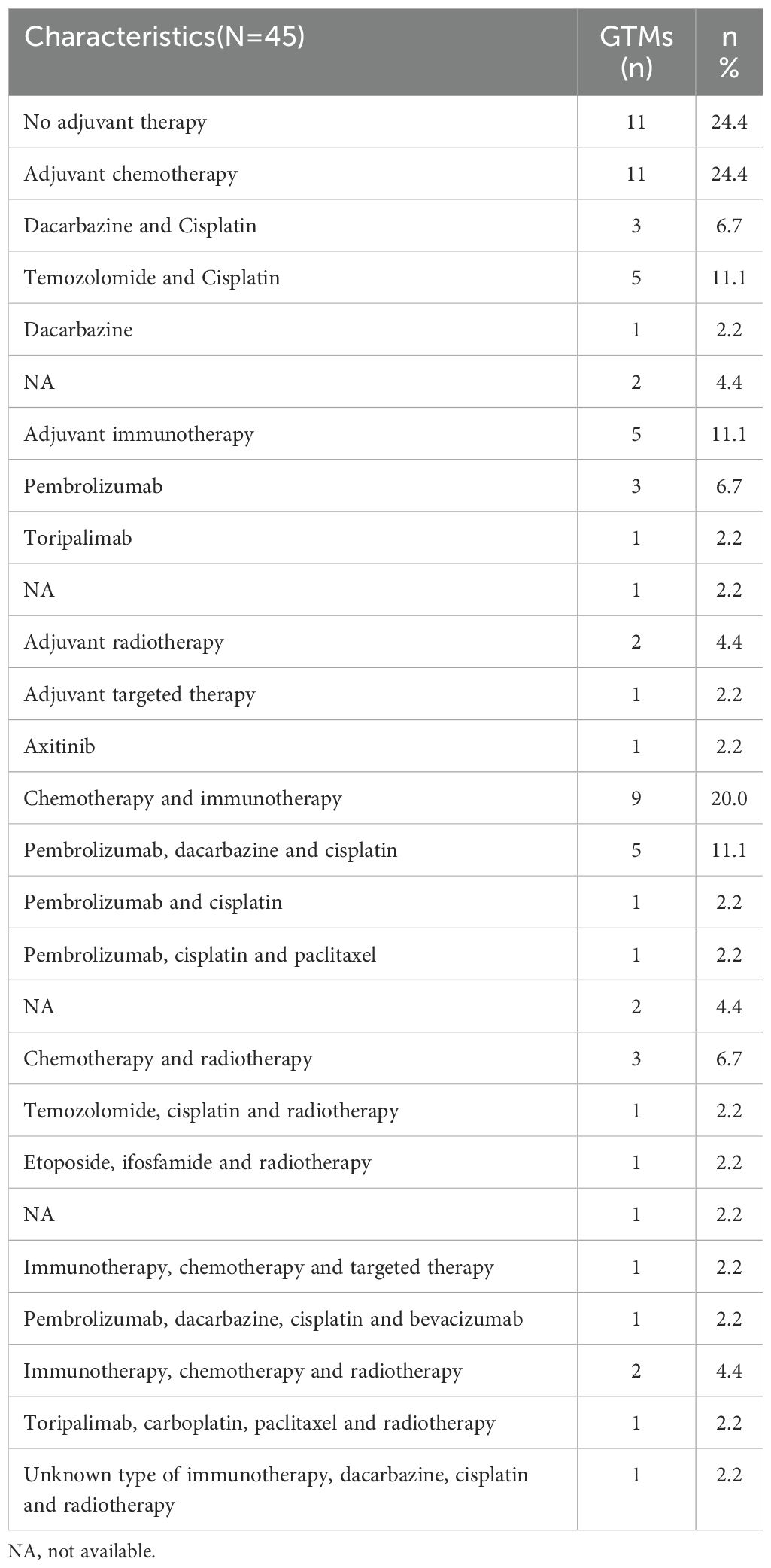
Postoperatively, 34 patients (75.6%) received adjuvant therapy (Table 2). Among them, 11 patients (24.4%) underwent chemotherapy, with regimens including dacarbazine combined with cisplatin, temozolomide with cisplatin, and dacarbazine monotherapy. Five patients (11.1%) received adjuvant immunotherapy, with single-agent pembrolizumab or toripalimab. Additionally, 2 patients (4.4%) received adjuvant radiotherapy, while 1 patient (2.2%) was treated with targeted therapy using axitinib. Combination therapies were also administered, including chemotherapy with ICIs in 9 patients (20.0%), chemotherapy with radiotherapy in 3 patients (6.7%), ICIs combined with chemotherapy and targeted therapy in 1 patient (2.2%), and ICIs combined with chemotherapy and radiotherapy in 2 patients (4.4%).
3.3 Clinical outcomes
In a cohort of 45 patients, 14 patients died within five years of diagnosis, with a median survival time of 24.00 months (IQR: 5.50–36.75). Among the 27 surviving patients, the median follow-up time was 23.00 months (IQR: 13.00–47.00). The 1-, 3-, and 5-year survival rates were 87%, 63%, and 31%, respectively. Regarding disease progression, 21 out of 45 patients experienced progression within five years, with a median PFS of 9.00 months (IQR: 3.50–15.50). The remaining 21 patients had a median follow-up time of 22.00 months (IQR: 12.50–47.50). The 1-, 3-, and 5-year progression rates were 32%, 49%, and 72%, respectively.
3.4 Prognostic factors
In this small-sample study, ICIs did not significantly improve PFS or OS compared to non-ICIs treatment. (Figure 1). However, other prognostic factors were identified (Figure 2). Table 3 summarizes clinicopathological characteristics associated with PFS and OS in GTMs. Patients with a family history of cancer (FHC) had longer PFS than those without (52.5 months vs. 25.3 months, p = 0.022). Similarly, initial lactate dehydrogenase (LDH) level ≤230 U/L were associated with improved PFS compared to >230 U/L (32.6 months vs. 14.3 months, p = 0.025). Though not statistically significant, trends indicated better PFS in patients with AJCC stage I-II compared to stage III-IV (34.0 months vs. 19.5 months, p = 0.079) and in those with tumor diameter ≤4 mm compared to >4 mm (27.4 months vs. 48.2 months, p = 0.084). However, multivariate analysis did not identify FHC (p = 0.064) or LDH level (p = 0.122) as independent prognostic factors for PFS.
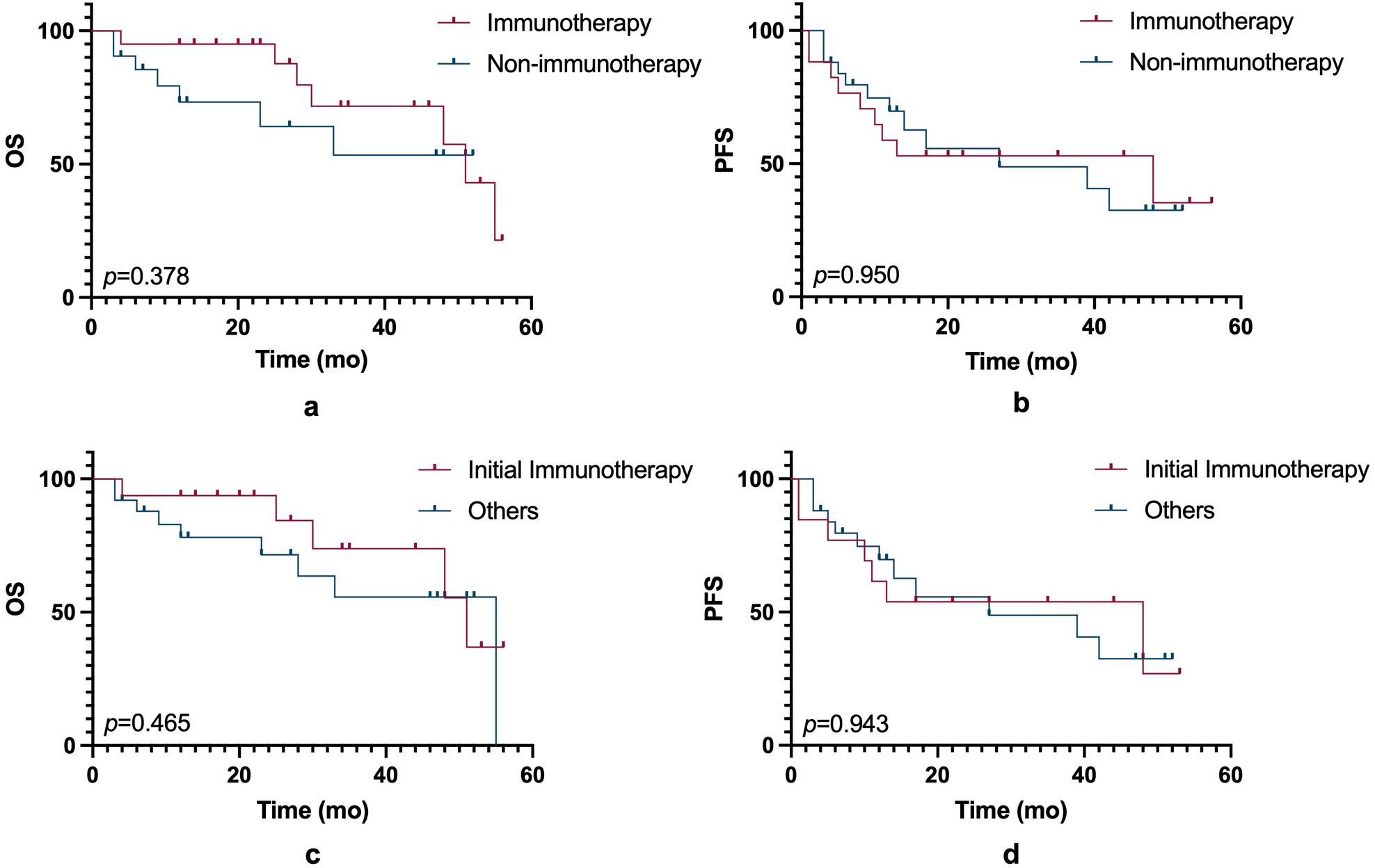
Figure 1. OS and PFS comparisons between immunotherapy and non-immunotherapy groups. (a) Kaplan-Meier curves showing OS between patients treated with immunotherapy and those without immunotherapy (p = 0.378). (b) Kaplan-Meier curves of PFS between the immunotherapy and non-immunotherapy groups (p = 0.950). (c) Comparison of OS between patients receiving initial immunotherapy and those treated with other therapies (p = 0.465). (d) PFS between patients receiving initial immunotherapy and other treatment groups (p = 0.943). Statistical significance is indicated by the p-values in each panel.
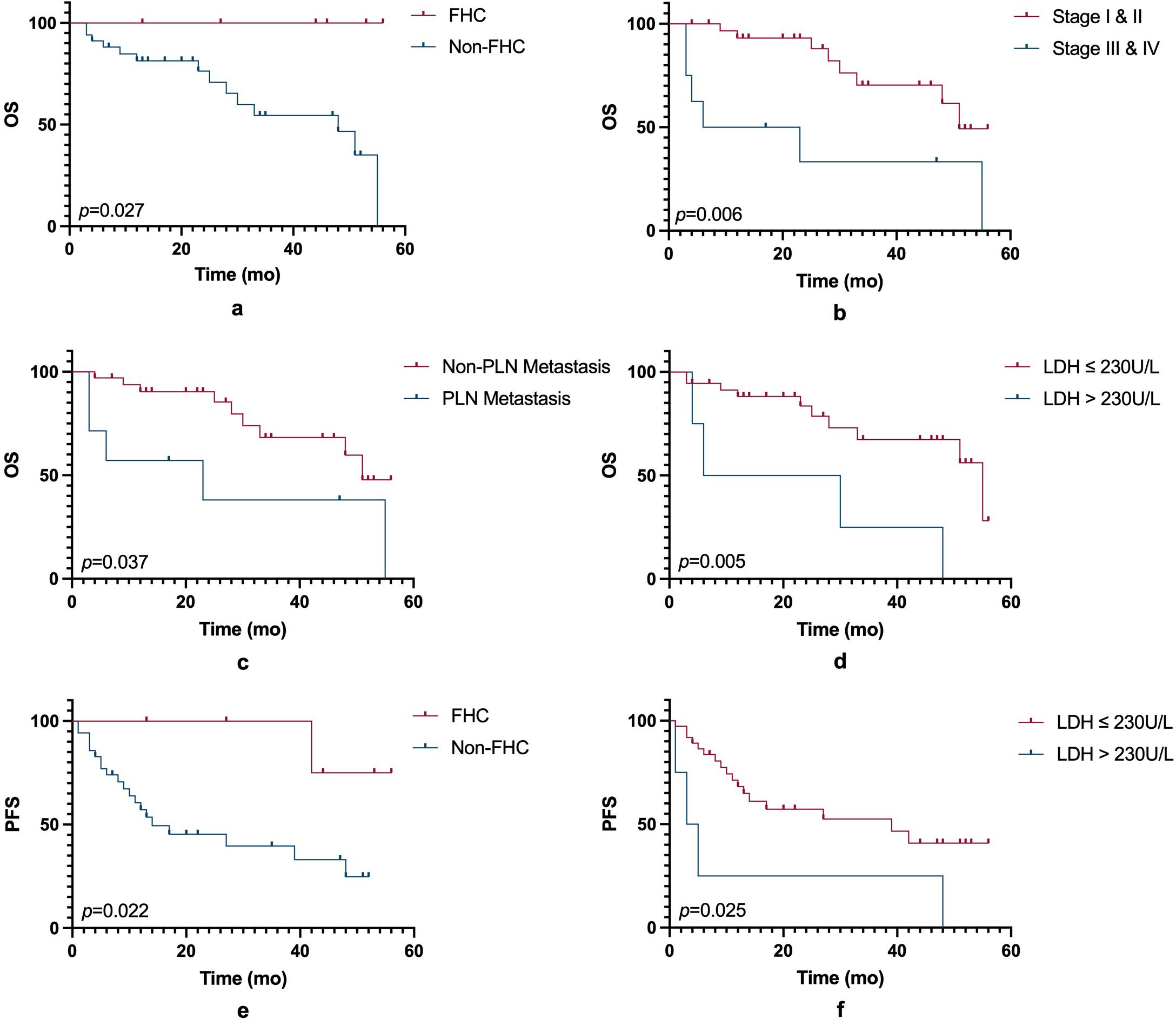
Figure 2. OS and PFS based on clinical and biological factors. (a) Kaplan-Meier curves for OS comparing patients with a family history and those without (p = 0.027). (b) OS curves comparing early-stage (Stage I & II) and advanced-stage (Stage III & IV) patients (p = 0.006). (c) OS curves comparing patients with and without regional lymph node (RLN) metastasis (p = 0.037). (d) OS curves comparing patients with serum LDH levels ≤230 U/L and >230 U/L (p = 0.005). (e) PFS curves comparing patients with a family history and those without (p = 0.022). (f) PFS curves comparing patients with LDH levels ≤230 U/L and >230 U/L (p = 0.025). Statistical significance is indicated by the p-values in each panel. FHC, family history of cancer; PLN, pelvic lymph node; LDH, lactate dehydrogenase.
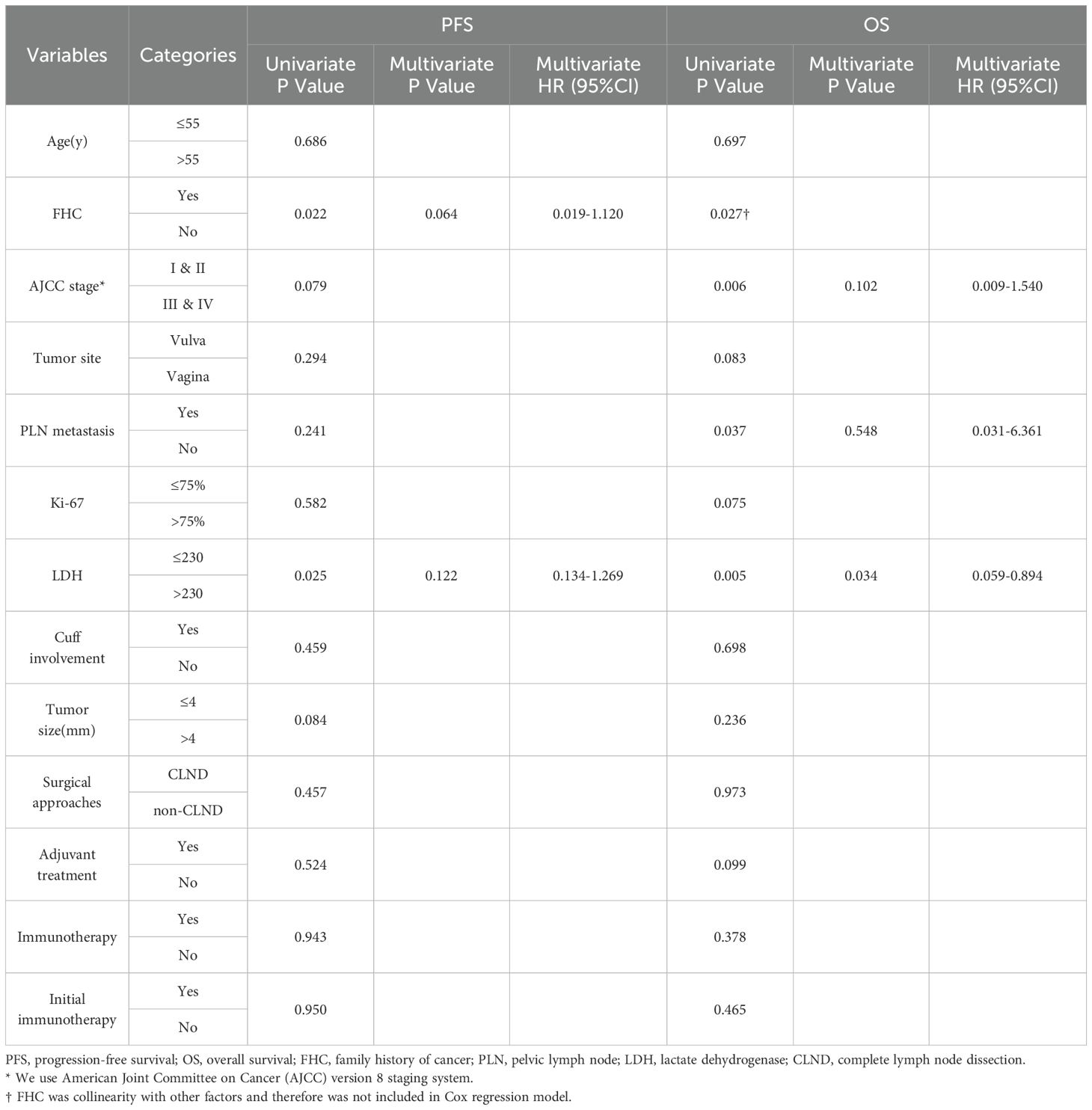
Table 3. Univariate and multivariate survival analysis of associated clinicopathological characteristics with PFS and OS in GTMs.
For OS, patients with FHC had better OS compared to those without (p = 0.027). OS was also prolonged in AJCC stage I-II compared to stage III-IV (45.4 months vs. 24.2 months, p = 0.006) and in those without pelvic lymph node (PLN) metastasis (44.2 months vs. 27.0 months, p = 0.037).An initial serum LDH level ≤230 U/L was strongly associated with improved OS (43.4 months 22.0 months, p = 0.005). Although not statistically significant, trends suggested longer OS in patients with vulvar melanoma compared to vaginal melanoma (47.7 months vs. 35.1 months, p = 0.083), and those receiving adjuvant therapy compared to those who did not (44.6 months vs. 29.3 months, p = 0.099). Subgroup analysis further indicated improved OS in AJCC stage I-II vulvar melanoma patients (p = 0.017). Due to collinearity, FHC was excluded from the multivariate analysis of OS. The analysis revealed that AJCC stage (p = 0.102) and PLN metastasis (p = 0.548) were not independent prognostic factors for OS, whereas LDH level (p = 0.034) was identified as an independent prognostic factor.
3.5 Therapy for recurrent GTMs
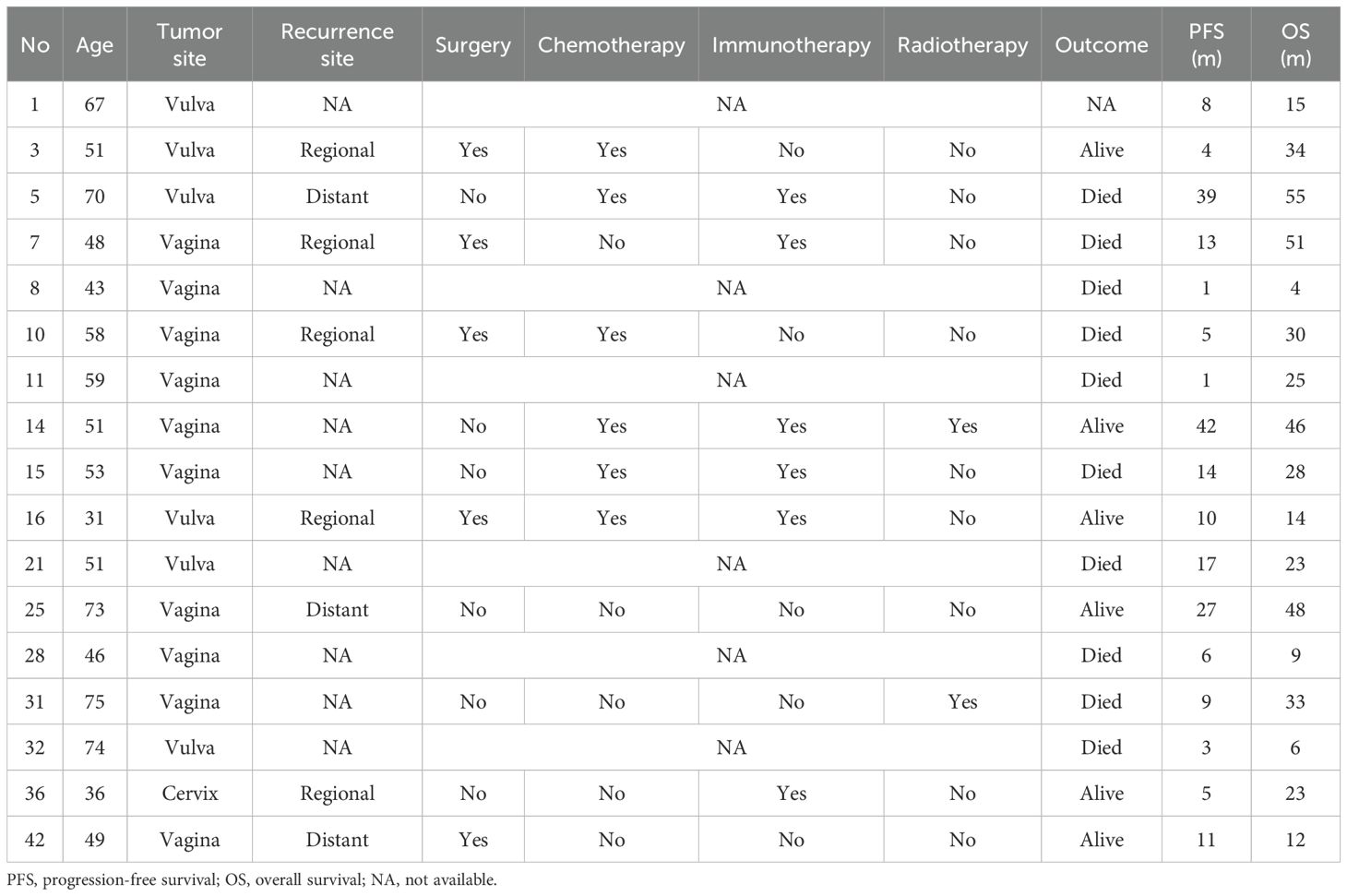
Seventeen patients experienced recurrence during follow-up, including 6 with vulvar melanoma, 10 with vaginal melanoma, and 1 with cervical melanoma (Table 4). Among them, 5 patients had local recurrence, 3 had distant recurrence, and recurrence details were unavailable for 9 patients.
Regarding treatment for recurrence, 1 patient received surgery, another received ICIs, and another received radiotherapy as part of their relapse treatment; 2 patients underwent surgery and chemotherapy; 1 patient received surgery and ICIs; and 2 patients received chemotherapy andICIs. Additionally, 1 patient received surgery, chemotherapy, andICIs, while another underwent multiple courses of radiotherapy, chemotherapy, and ICIs. One patient did not receive any treatment, and the specific treatment details for 6 patients were unknown.
During the follow-up period, 1 patient was lost to follow-up, and 10 patients died. The median PFS for patients with recurrence was 9 months (IQR: 4.5–15.5), and the median OS was 25 months (IQR: 13.0–40.0).
3.6 ICIs for GTM patients
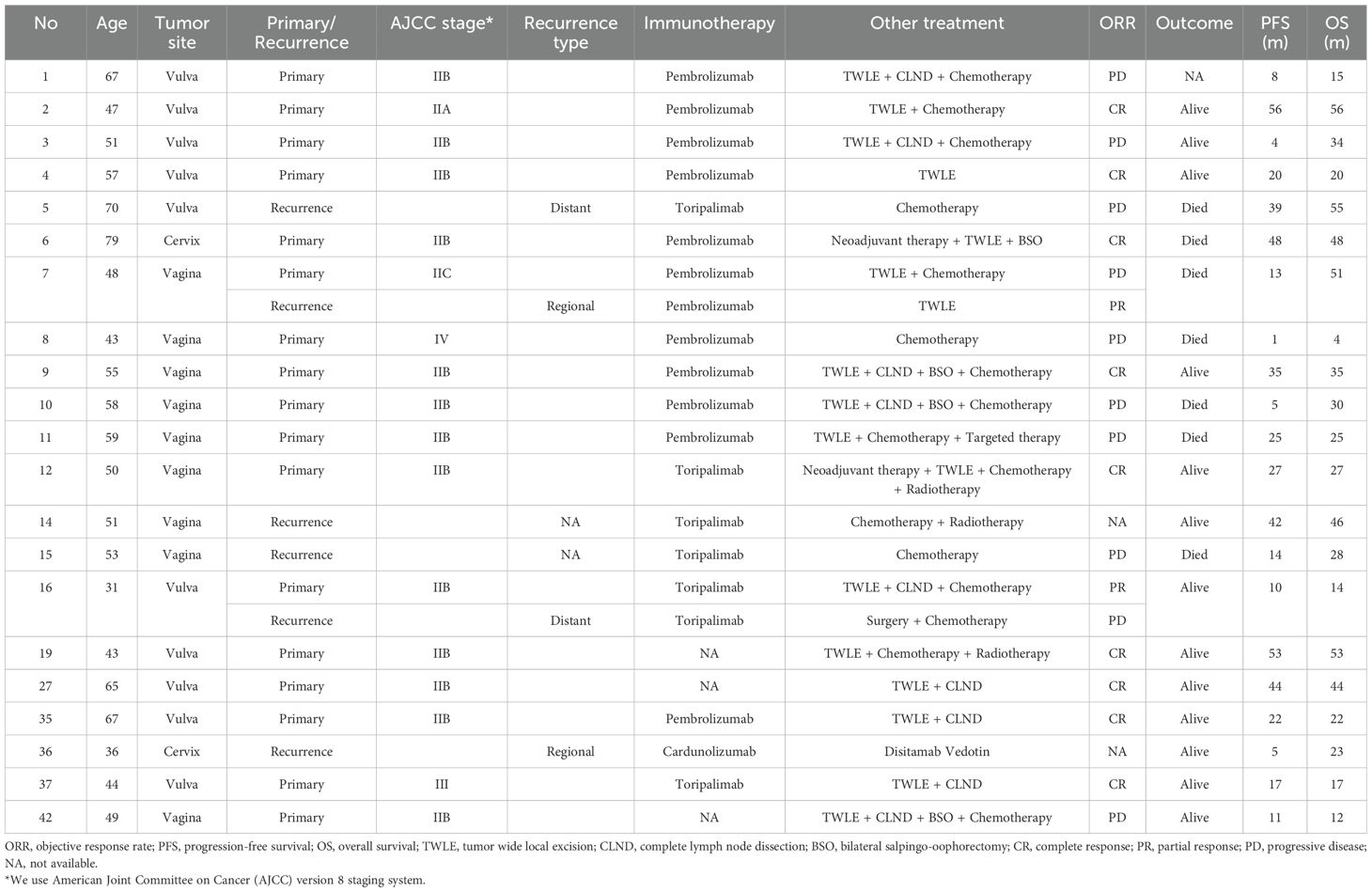
A total of 21 patients received ICIs (Table 5), including 10 with vulvar melanoma, 7 with vaginal melanoma, and 4 with cervical melanoma. Seventeen patients received postoperative adjuvant therapy, while 6 had recurrent disease, including 2 who underwent ICIs during both initial and recurrent treatments. Among the ICIs regimens, 11 patients received pembrolizumab, 6 received toripalimab, 1 received camrelizumab, and the specific immunotherapy agents were unknown in 3 cases.
Among these 21 patients, excluding two recurrent cases for whom tumor size changes could not be tracked during follow-up, the remaining 19 had evaluable responses. Of these, 9 achieved complete response (CR) and 2 achieved partial response (PR), resulting in an overall objective response rate (ORR) of 52.4%. Additionally, 1 patient lacked survival data, and 7 patients had died, leading to a mortality rate of 35% among GTM patients who received ICIs. When stratified by treatment setting, among the 17 patients who received postoperative adjuvant immunotherapy, 9 achieved CR and 1 achieved PR, yielding an ORR of 58.8%. In contrast, among the 6 patients treated for recurrent disease, 1 achieved PR, 3 experienced progressive disease (PD), and 2 were lost to follow-up, resulting in an ORR of 25%.
4 Discussion
4.1 Limitations of ICIs in GTM
This study did not observe a significant survival benefit of ICIs in patients with GTM compared to non-ICIs treatment. Although ICIs have demonstrated efficacy in cutaneous melanoma (CM) and some MM (14–16), their role in GTM remains limited. Among the 21 patients who received ICIs in our cohort, 17 were treated in the initial setting, and 6 received ICIs after recurrence. However, the median OS among ICI-treated patients was only 25 months, with more than half eventually succumbing to the disease. This finding aligns with an analysis of the Surveillance, Epidemiology, and End Results (SEER) database, which included 1,863 melanoma patients and found no clear survival benefit of ICIs in GTM (15). While Boer et al. reported improved survival with ICIs in unresectable melanomas (17), other studies have yielded inconsistent results (18). Furthermore, despite evidence suggesting that radiotherapy combined with immunotherapy may exert a synergistic anti-tumor effect (17), only two patients in our study received this combination, making it difficult to evaluate its efficacy.
The limited efficacy of ICIs in GTM may be attributed to its distinct biological characteristics. Previous studies, including EORTC 18071, CheckMate-238, and KEYNOTE-054, have demonstrated the benefit of adjuvant ICIs in improving recurrence-free survival (RFS) in stage III CM patients (19). Notably, anti-CTLA-4 drugs have not been shown to improve ORR in GTM, unlike their efficacy in melanomas of the naso-oral mucosa (20). This discrepancy may stem from the unique immune microenvironment of GTM, which differs significantly from that of CM (21). Moreover, due to its rarity and the lack of prospective randomized controlled trials (RCTs), current knowledge about ICIs in GTM is primarily derived from case reports and small retrospective studies, highlighting the need for larger, multicenter studies (13, 22).
4.2 Exploring novel immunotherapy approaches
Recent advances in immunotherapy have introduced promising strategies for melanoma treatment. For example, mRNA vaccines combined with PD-1 inhibitors may enhance RFS in high-risk melanoma patients. The KEYNOTE-942 study demonstrated that mRNA-4157 (V940) plus pembrolizumab significantly prolonged RFS compared to pembrolizumab monotherapy (23). Additionally, tumor-infiltrating lymphocyte (TIL) therapy has shown efficacy in certain melanoma subtypes, with studies indicating a higher degree of TIL infiltration is associated with better prognosis (24, 25). Furthermore, as presented by Grigoleit et al. at the 2023 ESMO (European Society for Medical Oncology) Congress in Madrid, results from the C-144-01 study involving 15 patients with mucosal melanoma treated with lifileucel were encouraging, demonstrating an ORR of 50%, with the median duration of response not yet reached at the time of analysis. Although these therapies have shown potential in CM, their role in GTM remains largely unexplored, warranting further investigation.
4.3 The role of neoadjuvant therapy
In our study, only four patients received neoadjuvant therapy, limiting the ability to assess its effectiveness. Similarly, a meta-analysis of eight RCTs found its efficacy in stage III and IV melanoma remains uncertain, with low-quality evidence for OS and DFS improvement (26). Historically, data did not confirm the superiority of neoadjuvant therapy over surgery followed by adjuvant therapy.
However, recent landmark trials have begun to shift this perspective. The SWOG S1801 trial (NEJM, 2023) demonstrated that in resectable stage III–IV melanoma, neoadjuvant pembrolizumab followed by surgery and adjuvant therapy significantly improved event-free survival (EFS) compared to adjuvant therapy alone (2-year EFS: 72% vs. 49%) (27). More strikingly, the phase 3 NADINA trial (NEJM, 2024) showed that two cycles of neoadjuvant nivolumab plus ipilimumab, followed by surgery and response-adapted adjuvant therapy, led to a 12-month EFS of 83.7% compared to 57.2% in the adjuvant-only group, with a major pathological response observed in 59% of patients (28). These findings highlight the immunological advantages of administering ICIs in the neoadjuvant setting, where the presence of the intact tumor may help prime a more effective systemic anti-tumor immune response. For advanced GTM, these results underscore the urgent need for prospective studies to explore the feasibility, safety, and potential efficacy of neoadjuvant immunotherapy or rational combination regimens. Such approaches may hold promise for improving outcomes in this challenging disease.
4.4 Challenges in treating recurrent GTM
Recurrent GTM remains a major therapeutic challenge, with limited treatment options. In our cohort, 17 patients experienced recurrence, and among the 6 who received ICIs with or without chemotherapy or radiotherapy, ORR was only 25%. This suggests that ICIs alone or in combination with chemotherapy or radiotherapy have limited efficacy. Novel immunotherapeutic approaches such as mRNA vaccines and TIL therapy, or the combination of ICIs with targeted therapy, may offer more promising strategies to improve outcomes in recurrent GTM.
In our study, two patients underwent immune rechallenge after disease recurrence, with one surviving 38 months and the other still under follow-up. Research suggests that switching to a different ICI may restore anti-tumor response, whereas continuing the same ICI post-progression may increase immune-related adverse events and reduce ORR (29–31). While current data are primarily derived from non-small cell lung cancer and renal cell carcinoma (29), further studies are needed to evaluate immune rechallenge strategies in GTM.
4.5 Prognostic factors in GTM
In this study, LDH levels were significant prognostic factors. Patients with LDH levels below 230 U/L had improved OS, suggesting that LDH could serve as a prognostic biomarker for GTM, similar to its role in metastatic melanoma (32, 33). Additionally, Patients with early-stage disease (AJCC I-II) had significantly better OS than those with advanced-stage disease, particularly in vulvar melanoma, which is consistent with previous studies (19). Lymph node metastasis also strongly influenced survival outcomes. A study of 1,863 GTM patients demonstrated that lymph node status is an independent predictor of survival (19, 34). Although we did not observe a significant impact of AJCC stage and PLN status on PFS, likely due to the limited sample size, comprehensive lymph node assessment remains crucial for staging and treatment planning.
Interestingly, FHC was associated with longer PFS, suggesting potential genetic or immunological influences. Previous studies have shown that patients with FHC may derive greater benefit from PD-1/PD-L1 therapy, possibly due to genetic mutations enhancing immune recognition (35). Further large-scale studies are needed to validate this hypothesis.
5 Limitation
This study has several limitations. As a single-center retrospective study with a small sample size, the findings may be influenced by selection bias and may not be fully generalizable. The heterogeneous treatment regimens and short follow-up period further limit the ability to assess the true impact of ICIs and long-term survival outcomes. Additionally, the lack of molecular and immune profiling data restricts insight into potential biomarkers that could predict treatment response. Finally, the absence of randomized controlled trials (RCTs) prevents definitive conclusions about the efficacy of ICIs in GTM. Despite these limitations, this study provides valuable preliminary data, emphasizing the need for larger, multicenter studies to optimize treatment strategies.
6 Conclusion
Our study indicates that despite the widespread use of ICIs in GTM patients, their survival benefit remains unclear. The rarity of GTM and the lack of prospective studies limit our understanding of optimal treatment strategies. Future research should focus on large-scale, multicenter trials to refine therapeutic approaches. Additionally, novel treatments such as mRNA vaccines, TIL therapy, and neoadjuvant immunotherapy have shown promise in CM and warrant further evaluation in GTM. LDH levels, AJCC stage, and lymph node status remain key prognostic factors, emphasizing the importance of individualized treatment strategies for this aggressive malignancy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of West China Second University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because of the retrospective nature of the study.
Author contributions
JW: Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. KL: Data curation, Investigation, Writing – review & editing. RL: Data curation, Investigation, Writing – review & editing. JZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Resources, Writing – original draft, Writing – review & editing. RY: Funding acquisition, Project administration, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 81902649), the Horizontal Science and Technology Project of Sichuan University (23H1223) and the Open Project of the Key Laboratory of Cancer Invasion and Metastasis, Ministry of Education (grant no. 2024KFKT009).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pang Y, Yuan H, Ren A, Zhang S, Liu P. Primary Malignant melanoma of the female genital tract synchronously involving the vulva and uterine cervix: a case report. Med (Baltimore). (2019) 98:e16366. doi: 10.1097/MD.0000000000016366
2. Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Am Coll Surgeons Commission Cancer Am Cancer Society. Cancer. (1998) 83:1664–78. doi: 10.1002/(SICI)1097-0142(19981015)83:8<1664::AID-CNCR23>3.0.CO;2-G
3. Vaysse C, Pautier P, Filleron T, Maisongrosse V, Rodier J-F, Lavoue V, et al. A large retrospective multicenter study of vaginal melanomas: implications for new management. Melanoma Res. (2013) 23:138–46. doi: 10.1097/CMR.0b013e32835e590e
4. Mert I, Semaan A, Winer I, Morris RT, Ali-Fehmi R. Vulvar/vaginal melanoma: an updated surveillance epidemiology and end results database review, comparison with cutaneous melanoma and significance of racial disparities. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. (2013) 23:1118–25. doi: 10.1097/IGC.0b013e3182980ffb
5. Nassar KW, Tan AC. The mutational landscape of mucosal melanoma. Semin Cancer Biol. (2020) 61:139–48. doi: 10.1016/j.semcancer.2019.09.013
6. Boer FL, Ten Eikelder MLG, Kapiteijn EH, Creutzberg CL, Galaal K, Van Poelgeest MIE. Vulvar Malignant melanoma: pathogenesis, clinical behaviour and management: review of the literature. Cancer Treat Rev. (2019) 73:91–103. doi: 10.1016/j.ctrv.2018.12.005
7. Guzik P, Łukasiewicz M, Harpula M, Zając P, Żmuda M, Śniadecki M, et al. Survival and treatment modalities in primary vaginal melanoma-case report and a narrative review. J Clin Med. (2024) 13:3771. doi: 10.3390/jcm13133771
8. Min A, Fu A, Huang M, Wang H, Chen H. Primary Malignant melanoma of the cervix: an integrated analysis of case reports and series. Front Oncol. (2022) 12:913964. doi: 10.3389/fonc.2022.913964
9. Dobrică E-C, Vâjâitu C, Condrat CE, Crețoiu D, Popa I, Gaspar BS, et al. Vulvar and vaginal melanomas—the darker shades of gynecological cancers. Biomedicines. (2021) 9:758. doi: 10.3390/biomedicines9070758
10. Mitra D, Farr M, Nagarajan P, Ho J, Bishop AJ, Jhingran A, et al. Gynecologic tract melanoma in the contemporary therapeutic era: High rates of local and distant disease progression. Gynecol Oncol. (2022) 167:483–9. doi: 10.1016/j.ygyno.2022.09.026
11. Hou JY, Baptiste C, Hombalegowda RB, Tergas AI, Feldman R, Jones NL, et al. Vulvar and vaginal melanoma: a unique subclass of mucosal melanoma based on a comprehensive molecular analysis of 51 cases compared with 2253 cases of nongynecologic melanoma. Cancer. (2017) 123:1333–44. doi: 10.1002/cncr.30473
12. Moran JMT. Identification of fusions with potential clinical significance in melanoma. Mod Pathol. (2022) 35:1837–47. doi: 10.1038/s41379-022-01138-z
13. Indini A, Di Guardo L, Cimminiello C, Lorusso D, Raspagliesi F, Del Vecchio M. Investigating the role of immunotherapy in advanced/recurrent female genital tract melanoma: a preliminary experience. J Gynecol Oncol. (2019) 30:e94. doi: 10.3802/jgo.2019.30.e94
14. Ostby SA, Daniel S, Kalogera E, De Vitis L, Fought AJ, McGree ME, et al. Treatment outcomes of vulvar and vaginal melanoma at an NCCN institution between 1993 and 2021. Gynecol Oncol Rep. (2024) 55:101483. doi: 10.1016/j.gore.2024.101483
15. Wohlmuth C, Wohlmuth-Wieser I. Vulvar melanoma: molecular characteristics, diagnosis, surgical management, and medical treatment. Am J Clin Dermatol. (2021) 22:639–51. doi: 10.1007/s40257-021-00614-7
16. Ladwa A, Elghawy O, Kaur V, Hernandez E. Single institution experience with immune checkpoint inhibitors in vulvar and vaginal melanomas. Obstet Gynecol Int. (2024) 2024:7327692. doi: 10.1155/2024/7327692
17. Boer FL, Ten Eikelder MLG, Van Geloven N, Kapiteijn EH, Gaarenstroom KN, Hughes G, et al. Evaluation of treatment, prognostic factors, and survival in 198 vulvar melanoma patients: implications for clinical practice. Gynecol Oncol. (2021) 161:202–10. doi: 10.1016/j.ygyno.2021.01.018
18. Albert A, Lee A, Allbright R, Vijayakumar S. Vulvar melanoma: an analysis of prognostic factors and treatment patterns. J Gynecol Oncol. (2020) 31:e66. doi: 10.3802/jgo.2020.31.e66
19. Wohlmuth C, Wohlmuth-Wieser I, May T, Vicus D, Gien LT, Laframboise S. Malignant melanoma of the vulva and vagina: A US population-based study of 1863 patients. Am J Clin Dermatol. (2020) 21:285–95. doi: 10.1007/s40257-019-00487-x
20. Dimitriou F, Namikawa K, Reijers ILM, Buchbinder EI, Soon JA, Zaremba A, et al. Single-agent anti-PD-1 or combined with ipilimumab in patients with mucosal melanoma: an international, retrospective, cohort study. Ann Oncol. (2022) 33:968–80. doi: 10.1016/j.annonc.2022.06.004
21. Falcone I, Conciatori F, Bazzichetto C, Ferretti G, Cognetti F, Ciuffreda L, et al. Tumor microenvironment: implications in melanoma resistance to targeted therapy and immunotherapy. Cancers. (2020) 12:2870. doi: 10.3390/cancers12102870
22. Postow MA, Luke JJ, Bluth MJ, Ramaiya N, Panageas KS, Lawrence DP, et al. Ipilimumab for patients with advanced mucosal melanoma. Oncologist. (2013) 18:726–32. doi: 10.1634/theoncologist.2012-0464
23. Weber JS, Carlino MS, Khattak A, Meniawy T, Ansstas G, Taylor MH, et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): a randomised, phase 2b study. Lancet. (2024) 403:632–44. doi: 10.1016/S0140-6736(23)02268-7
24. Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. (2012) 30:2678–83. doi: 10.1200/JCO.2011.37.8539
25. Minowa T, Murata K, Mizue Y, Murai A, Nakatsugawa M, Sasaki K, et al. Single-cell profiling of acral melanoma infiltrating lymphocytes reveals a suppressive tumor microenvironment. Sci Transl Med. (2024) 16:eadk8832. doi: 10.1126/scitranslmed.adk8832
26. Gorry C, McCullagh L, O’Donnell H, Barrett S, Schmitz S, Barry M, et al. Cochrane Skin Group, editor. Neoadjuvant treatment for stage III and IV cutaneous melanoma. Cochrane Database Syst Rev. (2023) 17:CD012974. doi: 10.1002/14651858.CD012974.pub2
27. Patel SP, Othus M, Chen Y, Wright GP, Yost KJ, Hyngstrom JR, et al. Neoadjuvant–adjuvant or adjuvant-only pembrolizumab in advanced melanoma. N Engl J Med. (2023) 388:813–23. doi: 10.1056/NEJMoa2211437
28. Blank CU, Lucas MW, Scolyer RA, Van De Wiel BA, Menzies AM, Lopez-Yurda M, et al. Neoadjuvant nivolumab and ipilimumab in resectable stage III melanoma. N Engl J Med. (2024) 391:1696–708. doi: 10.1056/NEJMoa2402604
29. Inno A, Roviello G, Ghidini A, Luciani A, Catalano M, Gori S, et al. Rechallenge of immune checkpoint inhibitors: A systematic review and meta-analysis. Crit Rev Oncol Hematol. (2021) 165:103434. doi: 10.1016/j.critrevonc.2021.103434
30. Ribas A, Puzanov I, Dummer R, SChadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. (2015) 16:908–18. doi: 10.1016/S1470-2045(15)00083-2
31. Inno A, Lo Russo G, Salgarello M, Corrao G, Casolino R, Galli G, et al. The evolving landscape of criteria for evaluating tumor response in the era of cancer immunotherapy: From Karnofsky to iRECIST. Tumori. (2018) 104:88–95. doi: 10.1177/0300891618766173
32. Bustos MA, Gross R, Rahimzadeh N, Cole H, Tran LT, Tran KD, et al. A pilot study comparing the efficacy of lactate dehydrogenase levels versus circulating cell-free microRNAs in monitoring responses to checkpoint inhibitor immunotherapy in metastatic melanoma patients. Cancers. (2020) 12:3361. doi: 10.3390/cancers12113361
33. Yamashita C, Otsuka A, Nomura M, Honda T, Kabashima K. Successful treatment of metastatic mucosal melanoma with a Del579 c-KIT mutation by imatinib after treatment of anti-PD-1 antibody. J Eur Acad Dermatol Venereol JEADV. (2019) 33:e92–3. doi: 10.1111/jdv.15246
34. Yu Y, Tse K-Y, Lee HHY, Chow K-L, Tsang H-W, Wong RWC, et al. Predictive biomarkers and tumor microenvironment in female genital melanomas: a multi-institutional study of 55 cases. Mod Pathol Off J U S Can Acad Pathol Inc. (2020) 33:138–52. doi: 10.1038/s41379-019-0345-2
Keywords: melanoma, gynecological, immunotherapy, immune checkpoint inhibitors, prognostic factors
Citation: Wang J, Li K, Li R, Zeng J and Yin R (2025) Immune checkpoint inhibitor outcomes and prognostic factors in gynecologic tract melanoma: a single-center analysis. Front. Immunol. 16:1542293. doi: 10.3389/fimmu.2025.1542293
Received: 09 December 2024; Accepted: 28 March 2025;
Published: 15 April 2025.
Edited by:
Bastian Czogalla, LMU Munich University Hospital, GermanyReviewed by:
George Au-Yeung, Peter MacCallum Cancer Centre, AustraliaWei Wei, Sun Yat-sen University Cancer Center (SYSUCC), China
Copyright © 2025 Wang, Li, Li, Zeng and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Zeng, amluZ3plbmcwNDAyQDE2My5jb20=
 Jianzhang Wang1
Jianzhang Wang1 Kemin Li
Kemin Li Rui Li
Rui Li Jing Zeng
Jing Zeng Rutie Yin
Rutie Yin