
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol. , 22 January 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1540192
 Jing-Jing Du1†
Jing-Jing Du1† Ru-Yan Zhang1†
Ru-Yan Zhang1† Shangchi Jiang1
Shangchi Jiang1 Shanshan Xiao1
Shanshan Xiao1 Yiting Liu1
Yiting Liu1 Yongheng Niu1
Yongheng Niu1 Wen-Xiang Zhao1
Wen-Xiang Zhao1 Dongyuan Wang2,3*
Dongyuan Wang2,3* XianShi Ma4*
XianShi Ma4*Cell penetrating peptides (CPPs) are usually positive charged peptides and have good cell membrane permeability. Meanwhile, CPPs are facile to synthesize, and can be functionalized to satisfy different demands, such as cyclization, incorporating unnatural amino acids, and lipid conjugation. These properties have made them as efficient drug-delivery tools to deliver therapeutic molecules to cells and tissues in a nontoxic manner, including small molecules, DNA, siRNA, therapeutic proteins and other various nanoparticles. However, the poor serum stability and low tumor targeting ability also hindered their broad application. Besides, inappropriate chemical modification can lead to membrane disruption and nonspecific toxicity. In this paper, we first reviewed recent advances in the CPP applications for cancer therapy via covalent or non-covalent manners. We carefully analyzed the advantages and disadvantages of each CPP modifications for drug delivery. Then, we concluded the recent progress of their clinical trials for different diseases. Finally, we discussed the challenges and opportunities CPPs met to translate into clinical applications. This review presented a new insight into CPPs for drug delivery, which could provide advice on the design of clinically effective systemic delivery systems using CPPs.
Due to the hydrophobic nature of the therapeutic molecules and their low bioavailability in vivo, many promising therapeutic drugs, especially those targeting intracellular therapeutic molecules, face challenges in fully realizing their therapeutic efficacy (1, 2). Enabling these molecules to cross the cell membrane so that they can have a therapeutic effect is a huge challenge.
In the late 1980s, while studying the human immunodeficiency virus (HIV), Green’s group discovered that the protein-transduction domains (PTD) of the transcription-activating protein Tat was able to penetrate cell membranes in vitro, and further studies revealed that the amino acid sequence corresponding to residues 48-60 of Tat (RKKRRQRRR) played a key role in cellular uptake (3–5). Subsequently, peptides found to penetrate cell membranes were commonly defined as cell-penetrating peptides (CPPs). CPPs have short sequences (typically less than 40 amino acids) and are usually cationic peptides, such as the two earliest identified CPPs (Tat and the Antp) (3, 4, 6), which have been shown to be capable of transporting a large number of cargoes into the intracellular environment. In addition to naturally derived sequences, many chimeric and synthetic peptides have been designed for drug delivery. Based on the type and arrangement of amino acids, CPPs are categorized as cationic, anionic, amphipathic and hydrophobic peptides (7). CPPs are usually structurally composed of 5-30 amino acids, which are divided into natural peptides and pure synthetic peptides depending on their source. They are able to pass through cells and tissues through various mechanisms, for example, cationic CPPs interact electrostatically with negatively charged carboxyl and phosphate groups on the cell membrane, whereas hydrophobic peptides may be transported by interacting with hydrophobic regions of the cell membrane (8–10). To date, CPPs have been widely used to deliver cargoes such as small molecule drugs, proteins, nucleic acids, etc. However, the specific mechanism of cellular uptake of CPPs is not yet fully understood, which may depend on the type and concentration of cargoes and the temperature (11). In conclusion, the discovery of CPPs provides an opportunity to deliver molecules with intracellular therapeutic activity.
This review will focus on the study of CPPs in facilitating intracellular delivery of a variety of cargo molecules, including the strategies of covalent conjugation and self-assembly. We will also present recent advances in the use of CPPs for clinical trials targeting different diseases and summarize the barriers to the translation of CPPs into clinical drugs.
As a class of peptides with special functions, CPPs are able to carry a variety of biomolecules across the cell membrane (Figure 1), including small molecule drugs (12–14), proteins (15, 16), nanomaterials (17), and nucleic acids (13, 18, 19), which provide novel delivery strategies for molecules that are impermeable to cells. In recent years, covalent coupling of vehicles to therapeutic molecules has been one of the hot topics in drug delivery system researches, such as antibody drug conjugates and peptide drug conjugates (20, 21). ADC is formed by connecting monoclonal antibodies and potent cytotoxic payloads through chemical linkers, while PDC is formed by combining cargo peptides and cytotoxic payloads through linkers, both of which enhance their tumor selectivity and permeability. Covalent coupling involves the formation of a chemical bond to maintain the integrity of the CPP and the cargo while forming a stable complex. This approach ensures that the cargo binds tightly to the CPP during circulation, which keeps its free from enzymatic degradation and increasing its half-life and bioavailability (1). In addition, covalent conjugation avoids non-specific distribution of cargoes, improves drug accumulation in the target cells and reduces off-target effects to a certain extent (22).
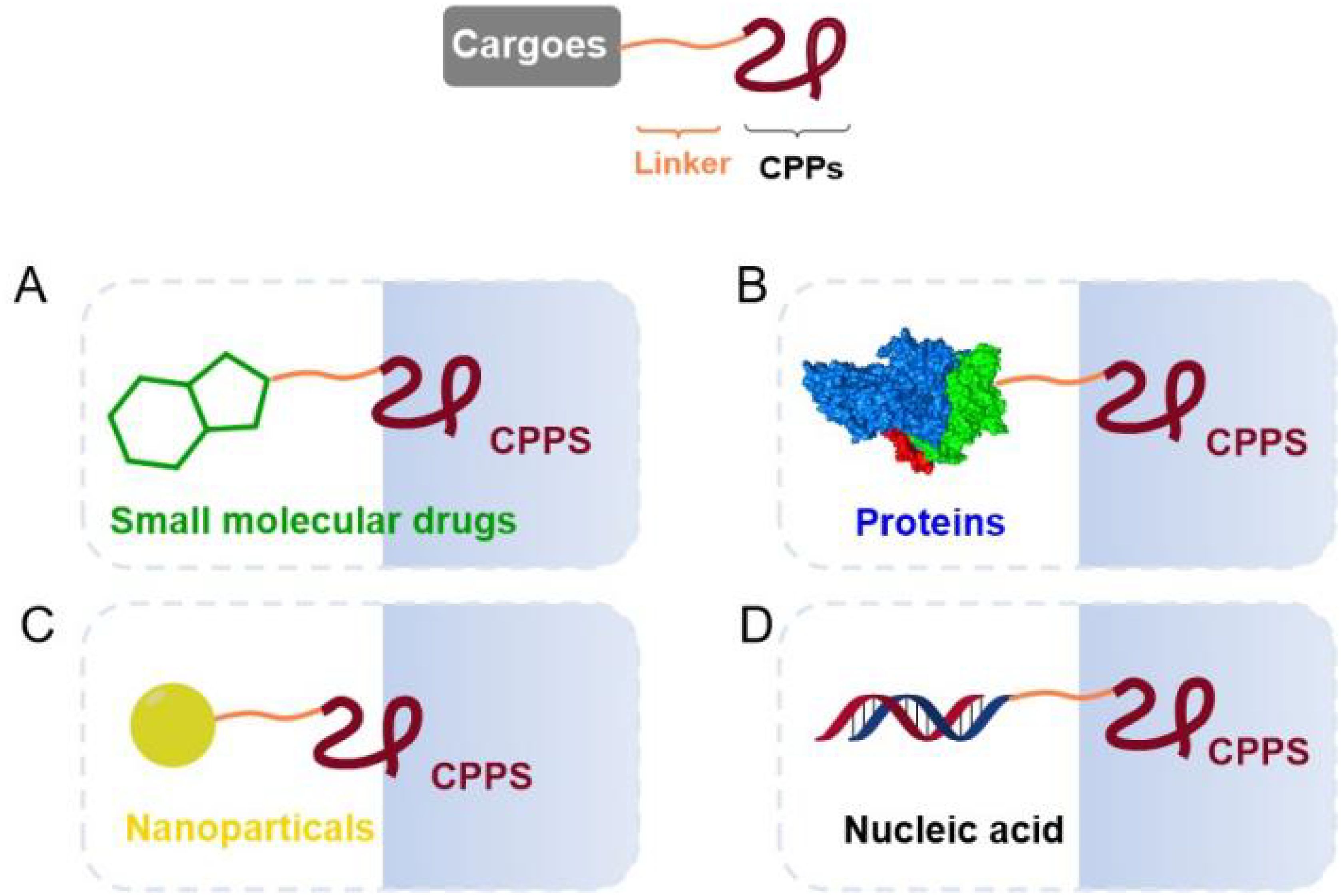
Figure 1. Covalent conjugation strategies based on CPPs and biomolecules in drug delivery system (12–19).
Small molecule drugs have been widely used in the treatment of cancer, bacterial and viral infections (23–25). However, the half-life of small molecule drugs is too short and their poor membrane permeability restricts their ability to reach the treatment site effectively. Therefore, an excessive amount of small molecule drugs is usually required to achieve therapeutic effects. Additionally, some small molecule drug candidates exhibit off-target cytotoxicity. In recent years, more studies have focused on how to effectively combine peptides with anticancer drugs for targeted delivery (26).
The Hristova group has reported a cellular transmembrane peptide, called CL peptide, containing a helical motif (RLLRLLR) and a polyarginine tail (r7). The peptide-cargo coupling increased the transport of small-molecule cargoes by approximately 10-fold and efficiently released cargoes (27). In 2012, the Qi group developed a conjugate (ACPP-DOX) of activatable cell-penetrating peptide (ACPP) with the antitumor drug adriamycin (DOX) for tumor-targeted therapy. Cellular uptake of ACPP-DOX was enhanced upon enzyme-triggered activation, and the internalized DOX was effective in inhibiting the proliferation of HT-1080 cells (28). Singh et al. designed and synthesized an octreotide-oxaliplatin concatenate (pcp-oxal). This coupling rapidly delivers oxaliplatin into cells, thereby not only improving intracellular delivery efficiency, but also enhancing passive targeting in vivo. The internalized oxaliplatin effectively inhibited tumor growth and exhibited considerable antitumor activity, indicating that the conjugate demonstrates good efficacyin vivo (29). Backlund et al. conjugated CPP with 10 peptides representing neoantigens, tumor virus antigens, and the tumor associated antigens in their study, and concluded that nine of the peptides exhibited enhanced T cell primers, demonstrating that conjugating CPP with antigens in vaccine administration could enhance therapeutic control of tumors (30). In 2024, Shi’s group developed a cell penetrating peptide induced chimeric conjugates (cp-PCCs) and used them to induce degradation of palmitoyltransferase DHHC3. This method disrupted the immunosuppressive function of PD-L1 by reducing its palmitoylation and membrane retention, thereby enhancing the immune response to tumors (31). In conclusion, delivery of small molecule drugs by covalently coupled CPPs is a promising research area that not only expands the possibilities of drug delivery but also brings new hope for the treatment of diseases.
The lipophilic components of the plasma membrane can act as a barrier, preventing proteins from easily reaching intracellular targets. Peptide-based delivery systems offer various advantages such as low immunogenicity, a high safety profile, and controlled dosage of administration (32, 33). The “YGRKKRRQRRR” sequence from the HIV Tat protein, commonly referred to as the Tat peptide, has been widely utilized. In 1994, Fawell et al. chemically crosslinked Tat peptides to a variety of proteins, including β-galactosidase, horseradish peroxidase, and the structural domain III of Pseudomonas exotoxin A (PE). The Tat successfully translocated different types of proteins into the cell, indicating that Tat-mediated uptake might allow delivery of macromolecules previously thought to be impermeable to living cells (34). Since then, researchers have developed various covalent conjugates to CPPs.
CPP-protein fusions with recombinant DNA technology have been used for many cargo proteins, including enzymes (35, 36), antibodies (37, 38), and antigens (39–42). Berne’s group constructed recombinant fusion proteins by fusing five different CPPs to the antibody, respectively, and demonstrated that the CPP-antibody fusions significantly enhanced antibody penetration into cells. This study offers new insights for further exploration of therapeutic antibodies against intracellular targets (38). Jiang’s group genetically constructed a GFP-Tat fusion protein that incorporates sequencing enzyme-mediated protein cyclization and cell-penetrating peptide (CPP)-mediated intracellular delivery improved the efficiency and stability of intracellular protein delivery (43). Cyclized GFP-Tat (cGFP-Tat) highly retained the photophysical properties of the protein and significantly improved stability in vitro with better intracellular delivery efficiency and tumor retention in vivo.
With the advancement of click chemistry, azide-functionalized CPPs were chemically coupled to alkyne-functionalized proteins by copper-catalyzed azide-alkyne cycloaddition (CuAAC) in order to construct stable site-specific structures. Christian’s group utilized the azide-functionalized polyphosphorylated adenosine and alkyne-functionalized polyphosphorylated adenosine to obtain cyclic and linear conjugates (44). The resulting cyclic CPP-GFP coupling was efficiently internalized into living cells, whereas the linear CPP analogue failed to facilitate GFP transduction. Kulkarni’s group constructed a coupling consisting of a growth factor receptor-binding protein 7 (GRB7) inhibitor, fitc-labeled penetrant peptide (CPP), and nuclear localization signal (NLS). The resulting GRB7-CPP-NLS structure greatly enhanced cellular uptake and localization to the cytoplasm and nucleus of breast cancer cells (45).
The covalent conjugation of CPP with cargo peptides or proteins could be achieved through chemical means. This involves the use of specific linkers to conjugate disulfide and amine bonds, ensuring the inherent proximity of the CPP to its cargo, thereby promoting cargo release once the linker is internalized into the cell. Through this method, CPP could be used as a carrier for peptide and protein delivery, and applied to target cancer (46–49). Currently, protein/peptide therapy is mainly used for regulating diseases in the extracellular space. In 2023, Zhao et al. developed peptides with pH dependent membrane perturbation activity by replacing Arg/Lys residues in cationic CPP with histidine, which facilitated intracellular escape of chromosomes in the context of CPP. They found in their study that the fusion of trastuzumab hsLMWP BID with 16 residue peptide (hsLMWP) and pro apoptotic protein BID (BH3 interacting domain death agonist) had potent anti-tumor efficacy, and demonstrated minimal side effects (50).
Nanoparticles are materials with sizes between 1-1000 nm, including metals, polymers, vesicles (e.g., micelles and liposomes), and carbon-based materials (e.g., nanotubes, fullerenes, and nanodiamonds) (51). In recent decades, nanomaterials have received increasing attention in the medical field due to their large surface area and favorable properties for controlled absorption and release. The diversity of nanomaterial types influences the mechanisms and effectiveness of their conjugation with CPPs. Based on these distinct categories, nanomaterials can be classified into several groups, including inorganic nanomaterials (e.g., gold and silver) (52) magnetic nanoparticles (53), polymer-based nanoparticles (54), liposomes, and vesicular systems such as micelles. Owing to their unique physicochemical properties, these various classes of nanomaterials exhibit specific advantages and application potentials when conjugated with CPPs, thereby providing a range of solutions for nanomedicine.
Because of their superior internalization and transmembrane transport capabilities, CPPs are considered an effective carrier for moving NPs through cell membranes. In 1999, Weissleder et al. (55) made the first attempt to produce CPP sequence-derived particles, which showed a 100-fold higher rate of internalization of the conjugate in lymphocytes compared to unmodified particles. Since then, researchers have continuously sought to bind CPPs to NP surfaces using covalent coupling techniques. Among metal nanoparticles, gold nanoparticles (GNPs) currently attract the most attention in drug delivery systems (53, 56–59). GNPs are suitable for targeted delivery, bioimaging, and theranostics due to their reduced toxicity, ease of modification, and excellent biodistribution when conjugated with CPPs. CPP-conjugated GNPs have enhanced cellular internalization and are suitable for various biomedical applications as nano-conjugates.
Additionally, polymer nanoparticles are attractive options for the delivery of cargoes. Among various polymer nanoparticles (54, 60–63), poly(lactic-co-glycolic acid) (PLGA) NPs have proved to be remarkably successful in combating a wide range of conditions including infectious diseases and cancer. Researchers successfully attached CPP to the surface of NPs using different surface-modification chemistries such as avidin and DSPE-PEG. Compared with unmodified NPs, CPP-modified NPs greatly improved cellular internalization, offering a promising delivery option for NP applications (62, 64, 65).A PROTAC strategy utilizing a covalent nanobody (GlueBody), known as GlueTAC, has been proposed for the targeted degradation of membrane proteins. Zhang et al. developed GlueBody through a mass spectrometry-based screening platform and successfully constructed a GlueTAC chimera that is covalently linked to cell-penetrating peptides and lysosomal sorting sequences (66). This chimera effectively triggers the internalization and degradation of programmed death ligand 1 (PD-L1), thereby providing a novel approach for targeting and degrading cell surface proteins. Leveraging the tunable chemical and physical properties of nanoparticles, along with surface functionalization strategies, allows for enhanced cell specificity. The integration of cell-penetrating peptides (CPPs) with nanoparticles (NPs) shows significant potential for enhancing cellular uptake, facilitating targeted drug delivery, and advancing anti-cancer therapies (54). As targeted delivery systems undergo continuous refinement, advancements in the CPP-NPs domain are poised to further enhance the application of CPPs in cancer research.
Due to their high internalization efficiency, low cytotoxicity, and flexible design, CPPs are a promising strategy for delivering nucleic acid drugs, including genes, short oligonucleotides, and small interfering RNAs (67, 68).
As a promising gene therapy strategy, specific gene silencing by RNAi has required the delivery of RNA into the cytoplasm. However, since RNA is negatively charged, it is difficult to cross the cell membrane due to strong repulsion by the negatively charged plasma membrane (69, 70). CPPs are cationic peptides capable of delivering oligonucleotides, which makes them highly promising delivery vectors.
Jagrosse et al. reported a structure-function study of CPPs-RNA conjugation using a series of modified cyclic amphipathic cell-penetrating peptides (CAPs), which have been shown to effectively deliver RNA. The researchers examined the effects of different peptide sequences on siRNA binding efficiency, cellular delivery and knockdown efficiency, and endocytosis uptake mechanisms (71). The results demonstrate that the strong cationic character and the aromatic residues capable of participating in CH-π interactions make CAP sequences the most effective in interacting with siRNA. The cyclic cationic CAP has exhibited a high siRNA translocation efficiency, contributing to the efficient knockdown of siRNA targets. Most CAP-siRNA complexes achieved siRNA delivery by clathrin- and caveolin-mediated endocytosis (72).
CPPs not only easily penetrate cell membranes but also enter specific organelles, improving the accuracy of targeted therapy. Targeting specific organelles is critical when treating cancer, as targeted delivery of anticancer drugs to specific intracellular targets improves therapeutic efficacy and reduces drugs toxicity. Current research shows that CPPs, combined with nanomicelles, liposomes and nanoparticles loaded with anticancer drugs, enhance drug transport across the blood-brain barrier and improve targeted treatment of tumor cells, offering greater control over drug delivery (Figure 2) (73). Due to the inherent and easily modifiable properties of most CPPs, they are particularly suitable for CPPs assembly and drug delivery applications to achieve higher therapeutic efficacy (74–77). In conclusion, CPPs are promising tools for improving cellular uptake, and CPPs binding lipid nanoparticles loaded with anticancer drugs have promising applications in cancer therapy (78).
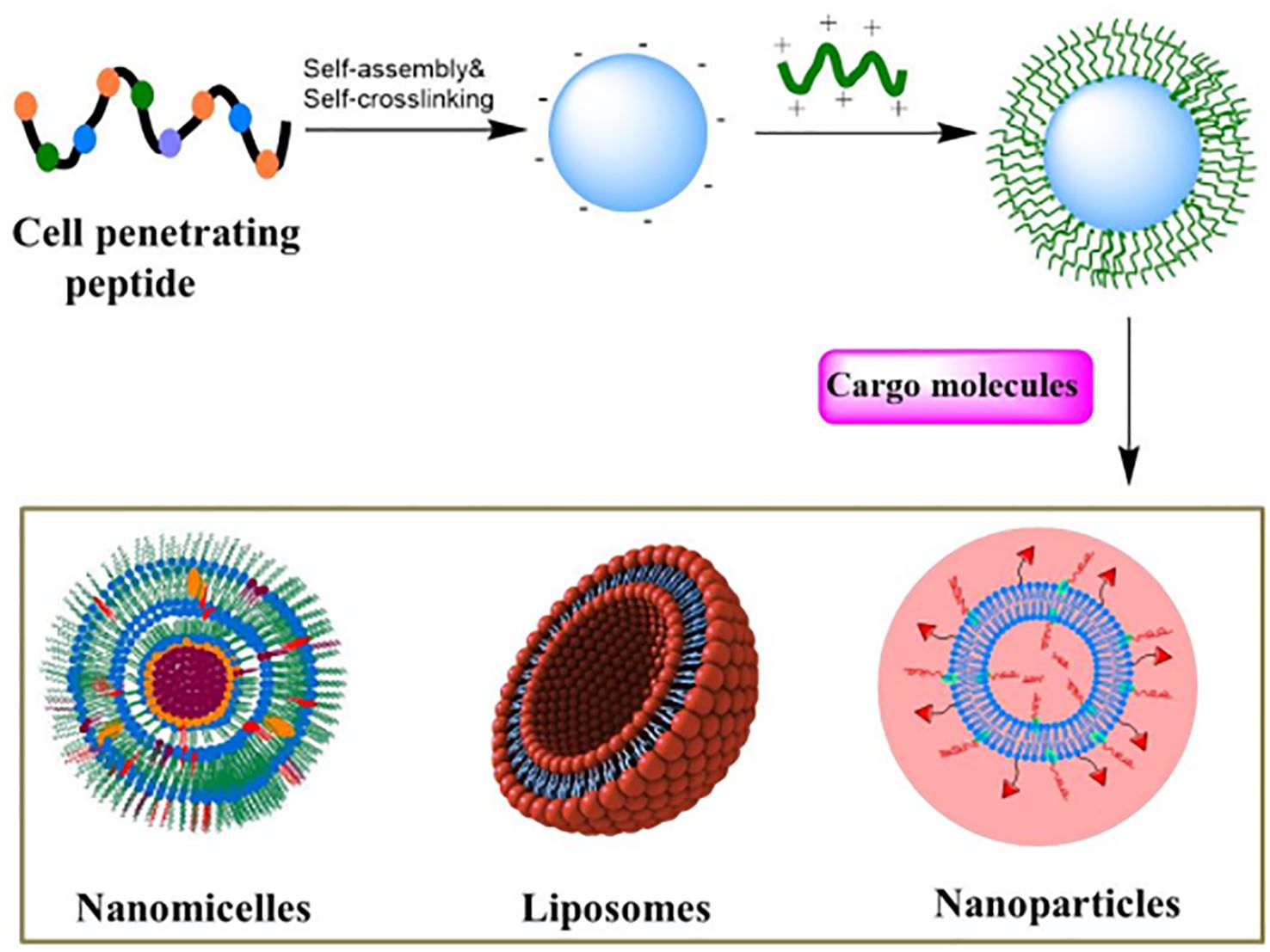
Figure 2. The main carrier system based on CPP functionalization is used for delivering therapeutic agents, which are loaded in their respective carrier systems.
Nanomicelles, with their outstanding biocompatibility, low toxicity, high drug carrying capacity, tiny size, and prolonged drug release time, and can be employed to reduce anticancer medication toxicity and avoid drug resistance (79). So far, nanosystems based on micelles for diagnosis and therapy have been extensively studied, and nano-drugs derived from micelles have emerged as a major treatment option for cancer. These micelles have a nanoscale structure, and their size (10-100 nm) allows them to penetrate deep into vascular tissues, such as tumors, by utilizing a porous vascular system. The effectiveness of CPP assembled micelles can be regulated by various external triggering factors, such as local arginine concentration, pH value, and temperature. Scientists have developed a series of strategies, including modified block copolymers coupled with cell penetrating peptides that better assemble into micelles, form stable complexes with siRNA, or load anticancer drugs and effectively delivering them to brain tissue via intranasal delivery (80).
In recent years, various studies on the combination of nanomicelles have been conducted, with the goal of providing new cancer therapy alternatives. Tat, a cell-penetrating peptide derived from HIV Tat (GRKKRRQRRRCG), and MPEG-PCL Tat are synthesized by Tat using disulfide bonds. They first self-assemble into micelles and then form stable complexes with siRNA, or can be loaded with anticancer drugs for effective delivery. Kanazawa et al. demonstrated the efficiency of drug/siRNA co-delivery and nasal brain delivery using MPEG-PCL-Tat nanomicelles in the treatment of glioblastoma and also exhibited its potential for treating brain and central nervous system-related diseases (81). In another work, Weinberger et al. designed drug molecules to self-assemble into spherical micelles, and then decorated soft nanospheres with an arginine-rich CPP (Tat) sequence on the membrane to restore the binding between CPP and lipid bilayers, improving the efficiency of CPP in delivering drugs in vivo and providing a more effective method for CPP-based cancer treatment (82).
To provide more targeted treatment for glioblastoma, efforts have been made to enhance the specificity of nanomicelle-related drugs for glioblastoma. For example, Zhu et al. proposed tandem nanomicelles co-functionalized with glioblastoma-targeting and cell-penetrating peptides, Angiopep-2 and Tat. Tandem nanomicelles with 20 mol% Angiopep-2 and 10 mol% Tat highly enhanced the specificity of anti-glioma therapy, improved survival rates, and had minimal side effects (83). Another study showed that, RRR-a-tocopheryl succinate-grafted-ϵ-polylysine conjugate (VES-g-ϵ-PLL) self-assembled ultra small micelles (NMs) served as delivery vehicles for chemotherapy, such as the hydrophobic model drug docetaxel (DTX). This study demonstrated that DTX micelles (DTX NMs) with a drug loading of 3.08% combined with ultrasound-targeted microbubble disruption (UTMD) could induce more significant apoptosis in C6 tumor cells, effectively overcoming the blood-brain barrier and treating glioblastoma (84).
The delivery of anti-tumor drugs from the systemic circulation to tumor sites is impeded by various physiological and biological barriers, including the blood-brain barrier (BBB) and the limited permeability of these drugs within tumors. To address this challenge, Pirhaghi et al. demonstrated experimentally that covalent coupling or modification of various peptides with liposomes loaded with anti-tumor agents can significantly enhance the capacity of these liposomes to traverse the blood-brain barrier (85). Given their relatively high cellular permeability, liposomes are extensively used to improve drug entry efficiency into cells (86, 87).
For instance, poly-L-arginine acts as a cell-penetrating peptide (CPP), significantly improving the endocytosis efficiency of the liposomes. Sharma et al. investigated a novel dual-ligand liposome carrier incorporating transferrin and poly-L-arginine to enhance the efficacy of drug delivery across the blood-brain barrier. Specifically, transferrin served as a targeting ligand, covalently conjugated to specific chemical moieties on the liposome surface, such as amide bonds or thiol groups, thus facilitating targeted delivery to the brain (88). Furthermore, Liu et al. developed a dual-mediated targeted liposome called transferrin-cell-penetrating peptide-electrostatically stabilized liposome (TF-CPP-SSL). This system integrated transferrin receptors (TF-R) and cell-penetrating peptides (CPP), promoting efficient drug delivery to gliomas. During this process, the researchers employed PEG modification to enhance the stability of the liposomes in circulation and optimized both the concentrations and modification densities of TF and CPP to ensure optimal cellular targeting and endocytosis efficiency. The findings indicated that TF-CPP-SSL could effectively traverse the blood-brain barrier (BBB), undergo endocytosis in C6 glioma cells, escape lysosomal degradation, and release drug components, such as doxorubicin into the cytoplasm for pharmacological action (89). In a subsequent study, Lakkadwala et al. developed a dual-functional liposome delivery system that used transferrin (Tf) to modify the surface of liposomes for enhanced receptor-mediated transport while concurrently introducing a cell-penetrating peptide (Pen) to improve cellular uptake efficiency. This formulation effectively encapsulated the chemotherapeutic agents doxorubicin (Dox) and erlotinib (Erlo), enabling precise traversal across the blood-brain barrier and targeted delivery to glioblastoma. Experimental results indicated that Tf-Pen liposomes significantly increased drug accumulation in brain tumors, with approximately 12-fold enhancement for Dox and approximately 3.3-fold for Erlo, while demonstrating favorable anti-tumor effects—over 90% tumor regression—and extending the median survival time of mice to 36 days (90, 91).
In further investigations, Shi et al. developed paclitaxel-loaded liposomes (PTX-TR-Lip) by combining TR peptide with pH-responsive and integrin αvβ3-specific carriers, effectively promoting penetration through the blood-brain barrier to target gliomas (92). Additionally, Li et al. constructed paclitaxel-loaded liposomes by incorporating the cell-penetrating peptide dNP2 along with pH-sensitive folic acid (FA), significantly enhancing cellular permeability and augmented the tumor-targeting efficacy of chemotherapeutic agents (93). Furthermore, Shi et al. developed a dual-functionalized thermosensitive lipid system (DOX@P1NS/TNC-FeLP) that integrates a glioma-specific cell-penetrating peptide (P1NS) with an anti-glioma antibody (TN-C), enabling precise traversal of the blood-brain barrier (94). Subsequently, Li et al. found that the co-utilization of transport protein (TP) peptides significantly enhanced the cellular uptake efficiency of liposomes. As an amphipathic cell-penetrating peptide, TP peptides facilitate paracellular uptake of liposomes via specific receptors and demonstrate high sensitivity to inhibitors targeting macrophage phagocytosis pathways. This finding establishes a foundation for the novel applications of TP peptides in cancer therapy (95).
CPPs, as a class of polycationic molecules, effectively facilitate the intracellular uptake of nanoscale cargo. The CPP-nanoparticle hybrid system represents an innovative approach in the fields of drug delivery and molecular biology This hybrid system integrates the cell-penetration capabilities of CPPs with the versatility of nanoparticles to enhance the delivery efficiency of therapeutic agents and genetic materials, effectively targeting tumor cells, reducing side effects, and improving therapeutic outcomes. To further explore the applications of CPPs, Moataz Dowaidar et al. conducted studies on their classification, absorption mechanisms, and hybrid carrier systems involving nanoparticles, highlighting the potential of CPPs for transporting siRNA and other cargo (96).
Researchers have extensively utilized nanoparticles modified with CPPs to deliver anticancer chemotherapeutic drugs to the brain for glioma treatment. For instance, Lakkadwala et al. developed a dual-functional liposome delivery system by combining CPPs with transferrin lipid nanoparticles while loading 5-fluorouracil (5-FU), successfully crossing the blood-brain barrier and significantly increasing 5-FU accumulation in tumor cells, along with its antitumor efficacy (97). Additionally, Kang et al. described a novel CPP characterized by an amino acid sequence comprising serine-isoleucine-tyrosine-valine (SIWV), which demonstrated significant homing ability toward glioblastoma brain tumors both in vitro and in vivo. They also investigated the potential combination of this CPP with porous silicon nanoparticles (psiNPs), which markedly enhanced selectivity and therapeutic efficacy in glioblastoma mouse models (98). Besides, tumor imaging is also a crucial step in the process of tumor treatment In 2021, Dai et al. explained that combining highly active aggregation-induced emission nanoparticles with PEG-polymers enhanced the biological activity of nanoparticles, which was more beneficial for tumor imaging and increased the accuracy of tumor diagnosis (99).
Furthermore, Silva et al. performed functionalization experiments on well-characterized nanolipid carriers (NLCs) using a straightforward and efficient adsorption method with three distinct peptide sequences. Zeta potential analysis confirmed successful peptide adsorption and indicated that various non-covalent interactions may be involved in this process. Computer simulations revealed a substantial interaction between CPP MAP and the NPY Y1 receptor, suggesting its potential significance in biological applications (100). Lastly, in 2023, Sugimoto et al. developed a highly functional KK-(EK)4 lipid and assessed its efficacy as a novel CPP-modified lipid for enhancing intracellular nanoparticle transport. They found that, compared to unmodified exosomes (EVs) and mRNA-encapsulated lipid nanoparticles (mRNA-LNPs), KK-(EK)4-lipid-modified carriers exhibited significantly improved cell-binding capacity and enhanced in vitro protein expression levels—further underscoring the promise of CPPs for intracellular delivery applications (101). Collectively, these studies underscore that the integration of CPPs with nanoparticles provides critical support for advances in modern medicine and biotechnology.
CPPs have demonstrated significant potential for clinical applications in oncology, particularly in cancer prevention and treatment, garnering increasing attention from researchers (102). These peptides not only exhibit a remarkable capacity to efficiently traverse cell membranes but also possess the ability to selectively target specific cellular organelles with large biomolecules, thereby greatly enhancing drug delivery efficiency while minimizing side effects. This advancement offers novel insights and directions for cancer prevention and treatment (103, 104). Some studies have shown that some CPPs have been used in clinical studies to inhibit tumor growth. For example, based on the evidence provided by clinical pharmacological research that there is no significant toxicity or immunogenicity, p28 has entered phase I clinical trials. In clinical practice, the combination of CPP DTS-108 and the antirectal cancer drug irinotecan significantly reduces gastrointestinal cytotoxicity compared to using irinotecan alone (105). Fifteen patients received intravenous injections of p28, which showed good tolerability and safety, indicating that p28 appears to have anti-tumor activity in advanced cancer patients (106). These findings underscore the extensive clinical applicability of CPPs in tumor management. Previous preclinical investigations indicate that CPP-based therapeutic strategies not only yield promising outcomes in oncology but also offer fresh perspectives on treating various other diseases. As clinical research progresses, it is anticipated that CPPs will assume an increasingly pivotal role in oncological treatments.
ACPPs are a novel class of in vivo targeted drugs, formed by a CPP that binds to a polyanion through a cleavable linker. Jiang et al. proposed the mechanism of ACPPs, where cleaving the linker to break down its structure, allowing the cationic peptide and its cargo to attach or enter the cell. Then, matrix metalloproteinases (MMPs) were used to cleave ACPP and combine with fluorescent groups for tumor imaging. This could concentrate molecules on cells and in areas adjacent to extracellular lytic activity within cells, ACPP became a new strategy for selectively delivering molecules to tumor cells (107). Subsequently, various studies were conducted on ACPP. For example, in 2009, Olson et al. demonstrated through their study of the structure and in vivo effects of ACPPs that ACPPs have the advantages of high resolution, enzyme specificity, and in vivo tumor imaging. Additionally, due to their elevated permeability, ACPPs can serve as an effective sensor for in vivo proteases. At the same time, they also showed that ACPP could target numerous xenograft tumor models from different cancer sites, including spontaneous breast cancer transgenic models (108). These studies indicate that ACPP has great potential in tumor research.
p28 is an effective therapeutic agent that can serve as a tumor-targeting carrier molecule to preferentially penetrate cancer cells (109). It is highly water-soluble and stable, and no significant side effects or immunogenicity were observed in clinical treatment. The primary objective of the study through the Phase I clinical trial was to determine the level of no observed adverse effects (NOAEL) and maximum tolerated dose (MTD) of p28 in adult patients with advanced solid tumors. These patients had advanced tumors that did not respond to conventional treatments and were expected to survive for approximately six months in this setting. Fifteen patients received p28 intravenously under an accelerated titration 3 + 3 dose escalation design. p28 was well tolerated with no significant adverse events, suggesting that it appeared to have antitumor activity in patients with advanced tumors (106). Another Phase I trial of p28 as a single agent in children with central nervous system (CNS) tumors was conducted. Children with recurrent or progressive CNS tumors received p28 intravenously at a dose of 4.16 mg/kg/dose (the recommended Phase II dose for adults) using a rolling 6 study design. While adult p28 doses were tolerated in adolescents, similar results were observed, further suggesting that p28-based treatments could be administered in all age groups. Results from these trials established that p28 was safe and well tolerated at the recommended Phase II dose (RP2D). Although p28 shows preliminary efficacy, further development of the drug in combination with other agents may prove more promising (110).
ST101 is a leucine zipper peptide with the ability to penetrate cells, and it is expected to be used for clinical treatment of cancer (106). ST101 is currently undergoing clinical trials for brain cancer and other solid tumors. In particular, ST101 has shown impressive anti-tumor activity in subcutaneous xenograft models. According to a report by ClinicalTrials.gov in July 2020, a “ Phase I-II study of ST101 in advanced solid tumor patients” (NCT04478279) was conducted. The abstract presented for the first time at the November 2022 meeting of the Society for Neuro-Oncology reported the results of a Phase II study of the first class peptide antagonist ST101 in recurrent glioblastoma. This study recruited adult cancer patients who relapsed after a standard treatment regimen. The treatment with ST101 involves intravenous injections of 500 mg per week. After 18 weeks of observation, only 1 out of 7 patients showed a partial response according to mRANO criteria. Although the study is still in its early phase, the apparent safety and efficacy of the drug are currently encouraging (111, 112).
Over the past decade, numerous studies have elucidated that cell-penetrating peptides (CPPs), acting as carriers for therapeutic agents, hold significant promise in the treatment of various cancers by efficiently delivering multiple biologically active cargos into cells, particularly in the context of tumor therapy. CPPs not only exhibit low cytotoxicity and high transduction efficiency but also facilitate the selective delivery of anticancer drugs, thereby reducing toxic effects on normal tissues. Although CPPs have extensive application potential in both fundamental research and clinical trials, CPPs still face challenges such as insufficient biochemical stability, short half-life, and the tendency to form cleaved peptides upon modification with drug molecules. Consequently, covalently coupling CPPs with biomolecules to form stable chemical bonds, ensuring the integrity of both the CPP and cargo, or non-covalently assembling of CPPs with nanocarriers, liposomes or micelles to significantly enhance delivery efficiency, while utilizing non-natural amino acids to improve pharmacokinetic properties, has become a focus of future research.
J-JD: Writing – original draft. R-YZ: Writing – original draft. SJ: Writing – review & editing. SX: Writing – review & editing. YL: Writing – review & editing. YN: Writing – review & editing. W-XZ: Writing – review & editing. DW: Writing – review & editing. XM: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Natural Science Foundation of China (22007033, 22207033), Wuhan Science and Technology Bureau of Hubei Province of China (2023020201020236) and the Project of the Outstanding Young and Middle-Aged Scientific Innovation Team of Universities in Hubei Province (T2023033), the Talent Introduction Project at Hubei Polytechnic University (24xjz30R).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Koo J-H, Kim G-R, Nam K-H, Choi J-M. Unleashing cell-penetrating peptide applications for immunotherapy. Trends Mol Med. (2022) 28:482–96. doi: 10.1016/j.molmed.2022.03.010
2. Miwa A, Kamiya K. Cell-penetrating peptide-mediated biomolecule transportation in artificial lipid vesicles and living cells. Molecules. (2024) 29:3339. doi: 10.3390/molecules29143339
3. Green M, Loewenstein PM. 1988 Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell. (1988) 55:1179–88. doi: 10.1016/0092-8674(88)90262-0
4. Green M, Ishino M, Loewenstein PM. Mutational analysis of HIV-1 Tat minimal domain peptides: Identification of trans-dominant mutants that suppress HIV-LTR-driven gene expression. Cell. (1989) 58:215–23. doi: 10.1016/0092-8674(89)90417-0
5. Vivès E, Brodin P, Lebleu B. A truncated HIV-1 tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. (1997) 272:16010–7. doi: 10.1074/jbc.272.25.16010
6. Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. Cell internalization of the third helix of the antennapedia homeodomain is receptor-independent. J Biol Chem. (1996) 271:18188–93. doi: 10.1074/jbc.271.30.18188
7. Kardani K, Milani A H, Shabani S, Bolhassani A. Cell penetrating peptides: the potent multi-cargo intracellular carriers. Expert. Opin Drug Deliv. (2019) 16:1227–58. doi: 10.1080/17425247.2019.1676720
8. Kurrikoff K, Gestin M, Langel Ü. Recent in vivo advances in cell-penetrating peptide-assisted drug delivery. Expert Opin Drug Deliv. (2016) 13:373–87. doi: 10.1517/17425247.2016.1125879
9. Falanga A, Galdiero M, Galdiero S. Membranotropic cell penetrating peptides: the outstanding journey. Int J Mol Sci. (2015) 16:25323–37. doi: 10.3390/ijms161025323
10. Henriques ST, Costa J, Castanho MARB. Translocation of β-galactosidase mediated by the cell-penetrating peptide pep-1 into lipid vesicles and human heLa cells is driven by membrane electrostatic potential. Biochemistry. (2005) 44:10189–98. doi: 10.1021/bi0502644
11. Kotadiya DD, Patel P, Patel HD. Cell-penetrating peptides: a powerful tool for targeted drug delivery. Curr Drug Deliv. (2024) 21:368–88. doi: 10.2174/1567201820666230407092924
12. Heh E, Allen J, Ramirez F, Lovasz D, Fernandez L, Hogg T, et al. Peptide drug conjugates and their role in cancer therapy. Int J Mol Sci. (2023) 24:829. doi: 10.3390/ijms24010829
13. Hee S, Joonhyuck N, Heebeom P. Recent advances in selective and targeted drug / gene delivery systems using cell - penetrating peptides. Arch Pharm Res. (2023) 46:18–34. doi: 10.1007/s12272-022-01425-y
14. Faheem S, Rizvi A, Zhang L, Zhang H, Fang Q. Peptide-drug conjugates: design, chemistry, and drug delivery system as a novel cancer theranostic. ACS Pharmacol Transl Sci. (2024) 7:309–34. doi: 10.1021/acsptsci.3c00269
15. Behzadipour Y, Hemmati S. Covalent conjugation and non-covalent complexation strategies for intracellular delivery of proteins using cell-penetrating peptides. Biomed Pharmacother. (2024) 176:116910. doi: 10.1016/j.biopha.2024.116910
16. Dinca A, Chien W, Chin MT. Intracellular delivery of proteins with cell-penetrating peptides for therapeutic uses in human disease. Int J Mol Sci. (2016) 17:263. doi: 10.3390/ijms17020263
17. Dowaidar M. Mitochondrion cell-penetrating peptides with nanoparticles hybrid delivery vectors and their uptake pathways. Mitochondrion. (2024) 78:101906. doi: 10.1016/j.mito.2024.101906
18. Tsylents U, Siekierska I, Trylska J. Peptide nucleic acid conjugates and their antimicrobial applications - a mini - review. Eur Biophys J. (2023) 52:533–44. doi: 10.1007/s00249-023-01673-w
19. Anwar S, Mir F, Yokota T. Enhancing the effectiveness of oligonucleotide therapeutics using cell-penetrating peptide conjugation, chemical modification, and carrier-based delivery strategies. Pharmaceutics. (2023) 15:1130. doi: 10.3390/pharmaceutics15041130
20. Tsuchikama K, Anami Y, Ha SYY, Yamazaki CM. Exploring the next generation of antibody–drug conjugates. Nat Rev Clin Oncol. (2024) 21:203–23. doi: 10.1038/s41571-023-00850-2
21. Cooper BM, Iegre J, O'Donovan DH, Ölwegård Halvarsson M, Spring DR. Peptides as a platform for targeted therapeutics for cancer: Peptide–drug conjugates (PDCs). Chem Soc Rev. (2021) 50:1480–94. doi: 10.1039/D0CS00556H
22. Kardani K, Milani A, H Shabani S, Bolhassani A. Cell penetrating peptides: the potent multi-cargo intracellular carriers. Expert Opin Drug Deliv. (2019) 16:1227–58. doi: 10.1080/17425247.2019.1676720
23. Park SE, El-sayed NS, Shamloo K, Lohan S, Kumar S, Sajid MI, et al. Targeted delivery of cabazitaxel using cyclic cell-penetrating peptide and biomarkers of extracellular matrix for prostate and breast cancer therapy. Bioconjug Chem. (2021) 32:1898–914. doi: 10.1021/acs.bioconjchem.1c00319
24. Tamatam R, Mohammed A. Small molecule anticancer drugs approved during 2021-2022: Synthesis and clinical applications. Eur J Med Chem. (2024) 272:116441. doi: 10.1016/j.ejmech.2024.116441
25. Bai YR, Seng DJ, Xu Y, Zhang YD, Zhou WJ, Jia YY, et al. A comprehensive review of small molecule drugs approved by the FDA in 2023: advances and prospects. Eur J Med Chem. (2024) 276:116706. doi: 10.1016/j.ejmech.2024.116706
26. Xiao Q, Du W, Dong X, Du S, Ong SY, Tang G, et al. Cell-penetrating mitochondrion-targeting ligands for the universal delivery of small molecules, proteins and nanomaterials. Chemistry. (2021) 27:12207–14. doi: 10.1002/chem.202101989
27. Alexander K, Maxim IB, Ran L, Honggang C, Peter CS, Kalina H. A peptide for transcellular cargo delivery: Structure-function relationship and mechanism of action. J Control Release. (2020) 324:633–43. doi: 10.1016/j.jconrel.2020.05.030
28. Shi N, Gao W, Xiang B, Qi X. Enhancing cellular uptake of activable cell-penetrating peptide–doxorubicin conjugate by enzymatic cleavage. Int J Nanomedicine. (2012) 7:1613–21. doi: 10.2147/IJN.S30104
29. Singh T, Kang DH, Kim TW, Kong HJ, Ryu JS, Jeon S, et al. Intracellular delivery of oxaliplatin con- jugate via cell penetrating peptide for the treatment of colorectal carcinoma. Vitro vivo Int J Pharm. (2021) 606:120904. doi: 10.1016/j.ijpharm.2021.120904
30. Backlund CM, Holden RL, Moynihan KD, Garafola D, Farquhar C, Mehta NK, et al. Cell-penetrating peptides enhance peptide vaccine accumulation and persistence in lymph nodes to drive immunogenicity. Proc Natl Acad Sci U S A. (2022) 119:e2204078119. doi: 10.1073/pnas.2204078119
31. Shi YY, Fan G, Tan R, Li S, Sun HB, Li R, et al. Treating ICB-resistant cancer by inhibiting PD-L1 via DHHC3 degradation induced by cell penetrating peptide-induced chimera conjugates. Cell Death Dis. (2024) 15:701. doi: 10.1038/s41419-024-07073-y
32. Bolhassan A, Jafarzade BS, Mardani G. In vitro and in vivo delivery of therapeutic proteins using cell penetrating peptides. Peptides. (2017) 87:50–63. doi: 10.1016/j.peptides.2016.11.011
33. Wu J, Roesger S, Jones N, Hu CJ, Li S. D. Cell-penetrating peptides for transmucosal delivery of proteins. J Control Release. (2024) 366:864–78. doi: 10.1016/j.jconrel.2024.01.038
34. Fawell S, Seery JOE, Daikh Y, Moore C, Chen LL, Pepinsky B. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci USA. (1994) 91:664–8. doi: 10.1073/pnas.91.2.664
35. Allen JK, Sutherland TC, Prater AR, Geoffroy CG, Pellois JP. In vivo peptide-based delivery of a gene-modifying enzyme into cells of the central nervous system. Sci Adv. (2022) 8:eabo2954. doi: 10.1126/sciadv.abo2954
36. He H, Sun L, Ye J, Liu E, Chen S, Liang Q, et al. Enzyme-triggered, cell penetrating peptide-mediated delivery of anti-tumor agents. J Control Release. (2016) 240:67–76. doi: 10.1016/j.jconrel.2015.10.040
37. Itakura S, Hama S, Ikeda H, Mitsuhashi N, Majima E. Effective capture of proteins inside living cells by antibodies indirectly linked to a novel cell-penetrating polymer-modified protein a derivative. FEBS J. (2015) 282:142–52. doi: 10.1111/febs.2014.282.issue-1
38. Gaston J, Maestrali N, Lalle G, Ga M, Masiero A, Dumas B, et al. Intracellular delivery of therapeutic antibodies into specific cells using antibody-peptide fusions. Sci Rep. (2019) 9:18688. doi: 10.1038/s41598-019-55091-0
39. Bae HD, Lee J, Jin XH, Lee K. Potential of translationally controlled tumor protein-derived protein transduction domains as antigen carriers for nasal vaccine delivery. Mol Pharm. (2016) 13:3196–205. doi: 10.1021/acs.molpharmaceut.6b00408
40. Rádis-Baptista G, Campelo IS, Morlighem JRL, Melo LM, Freitas VJF. Cell-penetrating peptides (CPPs): From delivery of nucleic acids and antigens to transduction of engineered nucleases for application in transgenesis. J Biotechnol. (2017) 252:15–26. doi: 10.1016/j.jbiotec.2017.05.002
41. Shahbazi S, Bolhassani A. Comparison of six cell penetrating peptides with different properties for in vitro and in vivo delivery of HPV16 E7 antigen in therapeutic vaccines. Int Immunopharmacol. (2018) 62:170–80. doi: 10.1016/j.intimp.2018.07.006
42. Kim Y, Tran T, Duong T, Jung S, Kim Y, Moon K, et al. Feasibility of dendritic cell-based vaccine against glioblastoma by using cytoplasmic transduction peptide (CTP) -fused protein antigens combined with anti-PD1. Hum Vaccin Immunother. (2020) 16:2840–8. doi: 10.1080/21645515.2020.1732165
43. Shi J, Hu J, Yuan Y, Zhang B, Guo W, Wu Y, et al. Genetic fusion of transacting activator of transcription peptide to cyclized green fluorescence protein improves stability, intracellular delivery, and tumor retention. ACS Omega. (2021) 6:7931–40. doi: 10.1021/acsomega.1c00532
44. Nischan N, Herce HD, Natale F, Bohlke N, Budisa N, Cardoso MC, et al. Covalent attachment of cyclic TAT peptides to GFP results in protein delivery into live cells with immediate bioavailability. Angew. Chem Int Ed Engl. (2015) 54:1950–3. doi: 10.1002/anie.201410006
45. Sang J, Kulkarni K, Watson GM, Ma X, Craik DJ, Henriques ST, et al. Evaluation of cyclic peptide inhibitors of the Grb7 breast cancer target: small change in cargo results in large change in cellular activity. Molecules. (2019) 24:3739. doi: 10.3390/molecules24203739
46. Virès E, Granier C, Prevot P, Lebleu B. Structure-activity relationship study of the plasma membrane translocating potential of a short peptide from HIV-1 Tat protein. Lett Pept Sci. (1997) 4:429–36. doi: 10.1007/BF02442912
47. Herce HD, Deng W, Helma J, Leonhardt H, Cardoso MC. Visualization and targeted disruption of protein interactions in living cells. Nat Commun. (2013) 4:1–8. doi: 10.1038/ncomms3660
48. Hao M, Zhang L, Chen P. Membrane internalization mechanisms and design strategies of arginine-rich cell-penetrating peptides. Int J Mol Sci. (2022) 23:9038. doi: 10.3390/ijms23169038
49. Liang JF, Yang VC. Insulin-cell penetrating peptide hybrids with improved intestinal absorption efficiency. Biochem Biophys Res Commun. (2005) 335:734–8. doi: 10.1016/j.bbrc.2005.07.142
50. Zhao Y, Jiang H, Yu J, Wang L, Du J. Engineered histidine-rich peptides enhance endosomal escape for antibody-targeted intracellular delivery of functional proteins. Angew Chem Int Ed Engl. (2023) 62:e202304692. doi: 10.1002/anie.202304692
51. Yang J, Firdaus F, Azuar A, Khalil ZG, Marasini N, Capon RJ, et al. Cell-penetrating peptides-based liposomal delivery system enhanced immunogenicity of peptide-based vaccine against group a streptococcus. Vaccines. (2021) 9:499. doi: 10.3390/vaccines9050499
52. Gessner I, Klimpel A, Neundorf I. Synthesis of cell-penetrating peptide coated silica nanoparticles and their physicochemical and biological characterization. Methods Mol Biol. (2022) 2383:105–17. doi: 10.1007/978-1-0716-1752-6_7
53. Nica V, Marino A, Pucci C, Şen Ö, Emanet M, De Pasquale D, et al. Cell-membrane-coated and cell-penetrating peptide-conjugated trimagnetic nanoparticles for targeted magnetic hyperthermia of prostate cancer cells. ACS Appl Mater Interfaces. (2023) 15:30008–28. doi: 10.1021/acsami.3c07248
54. Gessner I, Neundorf I. Nanoparticles modified with cell-penetrating peptides: Conjugation mechanisms, physicochemical properties, and application in cancer diagnosis and therapy. Int J Mol Sci. (2020) 21:2536. doi: 10.3390/ijms21072536
55. Josephson L, Tung CH, Moore A, Weissleder R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-Tat peptide conjugates. Bioconjug Chem. (1999) 10:186–91. doi: 10.1021/bc980125h
56. Damani M, Jadhav M, Joshi R, Singh BP, Momin MM, Ningthoujam RS, et al. Advances in gold nanoparticles: synthesis, functionalization strategies, and theranostic applications in cancer. Crit Rev Ther Drug Carrier Syst. (2024) 41:1–56. doi: 10.1615/CritRevTherDrugCarrierSyst.2024046712
57. Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold nanoparticles for biology and medicine. Angew Chem Int Ed. (2010) 49:3280–94. doi: 10.1002/anie.200904359
58. Munnilal P, Eroglu E, Subhash S, Vig K, Miller ME, Pillai S, et al. Biomaterials enhanced intracellular translocation and biodistribution of gold nanoparticles functionalized with a cell-penetrating peptide ( VG-21 ) from vesicular stomatitis virus. Biomaterials. (2014) 35:9484–94. doi: 10.1016/j.biomaterials.2014.07.032
59. Khamehchian S, Nikkhah M, Madani R, Hosseinkhani S. Enhanced and selective permeability of gold nanoparticles functionalized with cell penetrating peptide derived from maurocalcine animal toxin. J BioMed Mater Res A. (2016) 104:2693–700. doi: 10.1002/jbm.a.v104.11
60. Asai T, Tsuzuku T, Takahashi S, Okamoto A, Dewa T, Nango M, et al. Cell-penetrating peptide-conjugated lipid nanoparticles for siRNA delivery. Biochem Biophys Res Commun. (2014) 444:599–604. doi: 10.1016/j.bbrc.2014.01.107
61. Mesken J, Iltzsche A, Mulac D, Langer K. Modifying plasmid-loaded HSA-nanoparticles with cell penetrating peptides - Cellular uptake and enhanced gene delivery. Int J Pharm. (2017) 522:198–209. doi: 10.1016/j.ijpharm.2017.03.006
62. Uhl P, Grundmann C, Sauter M, Storck P, Tursch A, Özbek S, et al. Coating of PLA-nanoparticles with cyclic, arginine-rich cell penetrating peptides enables oral delivery of liraglutide. Nanomedicine. (2020) 24:102132. doi: 10.1016/j.nano.2019.102132
63. Yang YY, Zhang W, Liu H, Jiang JJ, Wang WJ, Jia ZY. Cell-penetrating peptide-modified graphene oxide nanoparticles loaded with rictor siRNA for the treatment of triple-negative breast cancer. Drug Des Devel Ther. (2021) 15:4961–72. doi: 10.2147/DDDT.S330059
64. Galindo-Camacho RM, Haro I, Gómara MJ, Espina M, Fonseca J, Martins-Gomes C, et al. Cell penetrating peptides-functionalized Licochalcone-A-loaded PLGA nanoparticles for ocular inflammatory diseases: Evaluation of in vitro anti-proliferative effects, stabilization by freeze-drying and characterization of an in-situ forming gel. Int J Pharm. (2023) 639:122982. doi: 10.1016/j.ijpharm.2023.122982
65. Steinbach JM, Seo Y-E, Saltzman WM. Cell penetrating peptide-modified poly(lactic-co-glycolic acid) nanoparticles with enhanced cell internalization. Acta Biomaterialia. (2016) 30:49–61. doi: 10.1016/j.actbio.2015.11.029
66. Zhang H, Han Y, Yang Y, Lin F, Li K, Kong L, et al. Covalently engineered nanobody chimeras for targeted membrane protein degradation. J Am Chem Soc. (2021) 143:16377–82. doi: 10.1021/jacs.1c08521
67. Nakase I, Akita H, Kogure K, Gräslund A, Langel U, Harashima H, et al. Efficient intracellular delivery of nucleic acid pharmaceuticals using cell-penetrating peptides. Acc Chem Res. (2012) 45:1132–9. doi: 10.1021/ar200256e
68. Hoyer J, Neundorf I. Peptide vectors for the nonviral delivery of nucleic acids. Acc Chem Res. (2012) 457:1048–56. doi: 10.1021/ar2002304
69. Beloor J, Zeller S, Choi CS, Lee SK, Kumar P. Cationic cell-penetrating peptides as vehicles for siRNA delivery. Ther Deliv. (2015) 6:491–507. doi: 10.4155/tde.15.2
70. Welch JJ, Swanekamp RJ, King C, Dean DA, Nilsson BL. Functional delivery of siRNA by disulfide-constrained cyclic amphipathic peptides. ACS Med Chem Lett. (2016) 7:584–9. doi: 10.1021/acsmedchemlett.6b00031
71. Jagrosse ML, Baliga UK, Jones CW, Russell JJ, García CI, Najar RA, et al. Impact of peptide sequence on functional siRNA delivery and gene knockdown with cyclic amphipathic peptide delivery agents. Mol Pharm. (2023) 20:6090–103. doi: 10.1021/acs.molpharmaceut.3c00455
72. Faghirabadi F, Abuei H, Malekzadeh MH, Mojiri A, Farhadi A. Intracellular delivery of antiviral shRNA using penetratin-based complexes effectively inhibits respiratory syncytial virus replication and host cell apoptosis. Virol J. (2024) 21:235. doi: 10.1186/s12985-024-02519-3
73. Wu Y, Angelova A. Recent uses of lipid nanoparticles, cell-penetrating and bioactive peptides for the development of brain-targeted nanomedicines against neurodegenerative disorders. Nanomaterials. (2023) 13:3004. doi: 10.3390/nano13233004
74. Chen J, Zhang W, Yang W, Xi F, He H, Liang M, et al. Separation of benzene and toluene associated with vapochromic behaviors by hybrid[4]arene-based co-crystals. Nat Commun. (2024) 15:1260. doi: 10.1038/s41467-024-45592-6
75. Yan M, Wang Y, Chen J, Zhou J. Potential of nonporous adaptive crystals for hydrocarbon separation. Chem Soc Rev. (2023) 52:6075–119. doi: 10.1039/D2CS00856D
76. Wang Y, Wu S, Wei S, Wang Z, Zhou J. Selectivity separation of ortho-chlorotoluene using nonporous adaptive crystals of hybrid[3]arene. Chem Mater. (2024) 36:1631–8. doi: 10.1021/acs.chemmater.3c02967
77. Dai Y, Sun J, Zhang X, Zhao J, Yang W, Zhou J, et al. Supramolecular assembly boosting the phototherapy performances of BODIPYs. Coord Chem Rev. (2024) 517:216054. doi: 10.1016/j.ccr.2024.216054
78. Morshed RA, Muroski ME, Dai Q, Wegscheid ML, Auffinger B, Yu D, et al. Cell-penetrating peptide-modified gold nanoparticles for the delivery of doxorubicin to brain metastatic breast cancer. Mol Pharm. (2016) 13:1843–54. doi: 10.1021/acs.molpharmaceut.6b00004
79. Tawfik SM, Azizov S, Elmasry MR, Sharipov M, Lee YI. Recent advances in nanomicelles delivery systems. Nanomaterials (Basel). (2020) 11:70. doi: 10.3390/nano11010070
80. Sikder A, Vambhurkar G, Amulya E, Bagasariya D, Famta P, Shah S, et al. Advancements in redox-sensitive micelles as nanotheranostics: A new horizon in cancer management. J Control Release. (2022) 349:1009–30. doi: 10.1016/j.jconrel.2022.08.008
81. Kanazawa T, Morisaki K, Suzuki S, Takashima Y. Prolongation of life in rats with Malignant glioma by intranasal siRNA/drug codelivery to the brain with cell-penetrating peptide-modified micelles. Mol Pharm. (2014) 11:1471–8. doi: 10.1021/mp400644e
82. Weinberger A, Walter V, MacEwan SR, Schmatko T, Muller P, Schroder AP, et al. Cargo self-assembly rescues affinity of cell-penetrating peptides to lipid membranes. Sci Rep. (2017) 7:43963. doi: 10.1038/srep43963
83. Zhu Y, Jiang Y, Meng F, Deng C, Cheng R, Zhang J, et al. Highly efficacious and specific anti-glioma chemotherapy by tandem nanomicelles co-functionalized with brain tumor-targeting and cell-penetrating peptides. J Control Release. (2018) 278:1–8. doi: 10.1016/j.jconrel.2018.03.025
84. Mao K, Jiang Q, Jiang Y, Fu Z, Hu J, Sun H, et al. Ultra-small micelles together with UTMD enhanced the therapeutic effect of docetaxel on glioblastoma. Eur J Pharm Sci. (2023) 187:106468. doi: 10.1016/j.ejps.2023.106468
85. Pirhaghi M, Mamashli F, Moosavi-Movahedi F, Arghavani P, Amiri A, Davaeil B, et al. A. Cell-penetrating peptides: promising therapeutics and drug-delivery systems for neurodegenerative diseases. Mol Pharm. (2024) 21:2097–117. doi: 10.1021/acs.molpharmaceut.3c01167
86. Du J-J, Zhou S-H, Liu J, Zhong X-Y, Zhang R-Y, Zhao W-X, et al. Diphtheria toxoid-derived T-helper epitope and α-galactosylceramide synergistically enhance the immunogenicity of glycopeptide antigen. ACS Pharmacol Transl Sci. (2024) 7:3889–901. doi: 10.1021/acsptsci.4c00437
87. Du JJ, Su Z, Yu H, Qin S, Wang D. From design to clinic: Engineered peptide nanomaterials for cancer immunotherapy. Front Chem. (2022) 10:1107600. doi: 10.3389/fchem.2022.1107600
88. Sharma G, Modgil A, Layek B, Arora K, Sun C, Law B, et al. Cell penetrating peptide tethered bi-ligand liposomes for delivery to brain in vivo: Biodistribution and transfection. J Control Release. (2013) 167:1–10. doi: 10.1016/j.jconrel.2013.01.016
89. Liu C, Liu XN, Wang GL, He Y, Meng S, Yang LF, et al. A dual-mediated liposomal drug delivery system targeting the brain: rational construction, integrity evaluation across the blood-brain barrier, and the transporting mechanism to glioma cells. Int J Nanomedicine. (2017) 12:2407–25. doi: 10.2147/IJN.S131367
90. Lakkadwala S, Dos, Santos, Rodrigues B, Sun C, Singh J. Dual functionalized liposomes for efficient co-delivery of anti-cancer chemotherapeutics for the treatment of glioblastoma. J Control Release. (2019) 307:247–60. doi: 10.1016/j.jconrel.2019.06.033
91. Lakkadwala S, Singh J. Co-delivery of doxorubicin and erlotinib through liposomal nanoparticles for glioblastoma tumor regression using an in vitro brain tumor model. Colloids Surf B Biointerfaces. (2019) 173:27–35. doi: 10.1016/j.colsurfb.2018.09.047
92. Shi K, Long Y, Xu C, Wang Y, Qiu Y, Yu Q, et al. Liposomes Combined an Integrin αvβ3-Specific Vector with pH-Responsible Cell-Penetrating Property for Highly Effective Antiglioma Therapy through the Blood-Brain Barrier. ACS Appl Mater Interfaces. (2015) 7:21442–54. doi: 10.1021/acsami.5b06429
93. Li M, Shi K, Tang X, Wei J, Cun X, Chen X, et al. pH-sensitive folic acid and dNP2 peptide dual-modified liposome for enhanced targeted chemotherapy of glioma. Eur J Pharm Sci. (2018) 124:240–8. doi: 10.1016/j.ejps.2018.07.055
94. Shi D, Mi G, Shen Y, Webster TJ. Glioma-targeted dual functionalized thermosensitive Ferri-liposomes for drug delivery through an. Vitro blood-brain barrier Nanoscale. (2019) 11:15057–71. doi: 10.1039/c9nr03931g
95. Li YX, Wang N, Hasan MM, Pang HB. Co-administration of transportan peptide enhances the cellular entry of liposomes in the bystander manner both. Vitro vivo Mol Pharm. (2022) 19:4123–34. doi: 10.1021/acs.molpharmaceut.2c00537
96. Dowaidar M. Cell-penetrating peptides with nanoparticles hybrid delivery vectors and their uptake pathways. Mitochondrion. (2024) 78:101906. doi: 10.1016/j.mito.2024.101906
97. Lakkadwala S, Singh J. Dual functionalized 5-fluorouracil liposomes as highly efficient nanomedicine for glioblastoma treatment as assessed in an. Vitro Brain tumor Model J Pharm Sci. (2018) 107:2902–13. doi: 10.1016/j.xphs.2018.07.020
98. Kang RH, Jang JE, Huh E, Kang SJ, Ahn DR, Kang JS, et al. A brain tumor-homing tetra-peptide delivers a nano-therapeutic for more effective treatment of a mouse model of glioblastoma. Nanoscale Horiz. (2020) 5:1213–25. doi: 10.1039/D0NH00077A
99. Dai J, Dong X, Wang Q, Lou X, Xia F, Wang S. PEG-polymer encapsulated aggregation-induced emission nanoparticles for tumor theranostics. Adv Healthc Mater. (2021) 10:e2101036. doi: 10.1002/adhm.202101036
100. Silva S, Marto J, Gonçalves LM, Fernandes HS, Sousa SF, Almeida AJ, et al. Development of neuropeptide Y and cell-penetrating peptide MAP adsorbed onto lipid nanoparticle surface. Molecules. (2022) 27:2734. doi: 10.3390/molecules27092734
101. Sugimoto Y, Suga T, Umino M, Yamayoshi A, Mukai H, Kawakami S. Investigation of enhanced intracellular delivery of nanomaterials modified with novel cell-penetrating zwitterionic peptide-lipid derivatives. Drug Deliv. (2023) 30:2191891. doi: 10.1080/10717544.2023.2191891
102. Guidotti G, Brambilla L, Rossi D. Cell-penetrating peptides: from basic research to clinics. Trends Pharmacol Sci. (2017) 38:406–24. doi: 10.1016/j.tips.2017.01.003
103. Bottens RA, Yamada T. Cell-penetrating peptides (CPPs) as therapeutic and diagnostic agents for cancer. Cancers (Basel). (2022) 14:5546. doi: 10.3390/cancers14225546
104. Greene LA, Zhou Q, Siegelin MD, Angelastro JM. Targeting transcription factors ATF5, CEBPB and CEBPD with cell-penetrating peptides to treat brain and other cancers. Cells. (2023) 12:581. doi: 10.3390/cells12040581
105. Meyer-Losic F, Nicolazzi C, Quinonero J, Ribes F, Michel M, Dubois V, et al. DTS-108, a novel peptidic prodrug of SN38: in vivo efficacy and toxicokinetic studies. Clin Cancer Res. (2008) 14:2145–53. doi: 10.1158/1078-0432.CCR-07-4580
106. Warso MA, Richards JM, Mehta D, Christov K, Schaeffer C, Rae Bressler L, et al. A first-in-class, first-in-human, phase I trial of p28, a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in patients with advanced solid tumours. Br J Cancer. (2013) 108:1061–70. doi: 10.1038/bjc.2013.74
107. Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci USA. (2004) 101:17867–72. doi: 10.1073/pnas.0408191101
108. Olson ES, Aguilera TA, Jiang T, Ellies LG, Nguyen QT, Wong EH, et al. In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr Biol (Camb). (2009) 1:382–93. doi: 10.1039/b904890a
109. Razzak M. Targeted therapies: One step closer to drugging p53. Nat Rev Clin Oncol. (2013) 10:246. doi: 10.1038/nrclinonc.2013.43
110. Lulla RR, Goldman S, Yamada T, Beattie CW, Bressler L, Pacini M, et al. Phase I trial of p28 (NSC745104, a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in pediatric patients with recurrent or progressive central nervous system tumors: a pediatric brain tumor consortium study. Neuro Oncol. (2016) 18:1319–25. doi: 10.1093/neuonc/now047
111. Darvishi E, Ghamsari L, Leong SF, Ramirez R, Koester M, Gallagher E, et al. A. Anticancer activity of ST101, a novel antagonist of CCAAT/enhancer binding protein β. Mol Cancer Ther. (2022) 21:1632–44. doi: 10.1158/1535-7163.MCT-21-0962
112. Iwamoto F, Gondi V, Butowski N, Falchook G, Williams A, Peters K, et al. CTNI-49. Early signal of activity from a phase 2 study of ST101, a first-in-class peptide antagonist of CCAAT/enhancerbinding protein β (C/EBPβ), in recurrent glioblastoma (GBM). Neuro-Oncol. (2022) 24:vii83. doi: 10.1093/neuonc/noac209.314
Keywords: cell penetration peptide, covalent conjugation, non-covalent delivery, cancer immunotherapy, clinical application
Citation: Du J-J, Zhang R-Y, Jiang S, Xiao S, Liu Y, Niu Y, Zhao W-X, Wang D and Ma X (2025) Applications of cell penetrating peptide-based drug delivery system in immunotherapy. Front. Immunol. 16:1540192. doi: 10.3389/fimmu.2025.1540192
Received: 05 December 2024; Accepted: 06 January 2025;
Published: 22 January 2025.
Edited by:
Paulo Rodrigues-Santos, University of Coimbra, PortugalReviewed by:
Jiong Zhou, Northeastern University, ChinaCopyright © 2025 Du, Zhang, Jiang, Xiao, Liu, Niu, Zhao, Wang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongyuan Wang, d2FuZ2R5MjAxOUBodXN0LmVkdS5jbg==; XianShi Ma, bWF4czk1MTExMkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.